- 1Environmental Remediation and Nanoscience (EnviReN), Department of Geography and Environmental Sciences, Faculty of Science, Engineering and Agriculture, University of Venda, Thohoyandou, Limpopo, South Africa
- 2Water Research Group, Civil Engineering, Faculty of Engineering and Built Environment, University of Cape Town, Rondebosch, South Africa
- 3School of Chemistry and Material Sciences, Technical University of Kenya, Nairobi, Kenya
This study aims to optimize the SPE parameters for purification and preconcentration of EFV and LVG to enable optimum detection and quantification by LC-20 Prominence High-Performance Liquid Chromatography (HPLC) system. The gradient elution method was used to profile and quantify efavirenz (EFA) and levonorgestrel (LVG). The optimized parameters were solution pH, solvent type and concentration, and elution volume. The 60 mg/3 mL Hydrophilic-lipophilic balance (HLB) was used to extract the target pharmaceutical contaminants. The percentage recoveries of EFA and LVG ranged from 67% to 83% and 70% to 94.61%, respectively at an optimal pH of 2, solvent concentration and type 100% Methanol and an elution volume of 4 mL using HLB cartridges. The method’s accuracy was validated by obtaining a correlation coefficient (R2) > 0.98 from the respective calibration curves of the target contaminants. The limit of detection (LOD) and limit of quantification (LOQ) for efavirenz were 0.705 µg/L and 0.14 µg/L, respectively, and for levonorgestrel, they were 0.061 µg/L and 0.199 µg/L. The optimized SPE method was used to extract wastewater samples, and the yield results showed that the method could be applied for the simultaneous detection of efavirenz and levonorgestrel, demonstrating its potential applications in environmental research. The concentration of EFA ranged from 0.36 to 8.10 µg/L in influent samples and 2.88 to 8.11 µg/L in effluent samples. Conversely, the concentration of levonorgestrel ranged from 2.64 to 32.31 µg/L in influent samples and 2.32 to 12.35 µg/L in effluent samples. The obtained results were validated by analyzing these samples using ultra-high-performance liquid chromatography. Based on the results, the optimized Solid Phase Extraction (SPE) method can be used to pre-concentrate EFA and LVG in wastewater samples, inspiring future research.
1 Introduction
Contraceptives and antiviral drugs are among the different pharmaceutical compounds used for birth control and treating viral infections (Quirke, 2017; Tariq et al., 2019). The release of synthetic progestogens such as levonorgestrel into aquatic environments has significantly increased due to continuous human population growth over the past decades (King et al., 2016). Notably, the discharge of raw and treated wastewater has been identified as the primary source of these pharmaceutical compounds in wastewater due to their inadequacy in removing them in aqueous solution. Prolonged exposure to antiviral and contraceptive drugs such as efavirenz and levonorgestrel can disrupt the endocrine system even at low concentrations, which could affect aquatic organisms’ development, growth, and reproduction (Narváez et al., 2019; Kloas et al., 2009; O et al., 2014). The occurrence of efavirenz and levonorgestrel in wastewater and surface water has been reported in wastewater with a concentration range of ng/L to µg/L. Recently, an average concentration of 3.81–11.9 µg/L (influent) and 0.69–6.3 µg/L (effluent) and 6.2–8.09 µg/L (influent) and 4.25–20.9 µg/L (effluent) of efavirenz and levonorgestrel was reported in wastewater within the Vhembe and Mopani district, Limpopo, South Africa (Munzhelele et al., 2024; Schoeman et al., 2017). Although these compounds have been reported in surface water and wastewater streams (Schoeman et al., 2017; Golovko et al., 2018). There is data scarcity about their occurrence, mainly in African countries, due to a lack of advanced resources and analytical techniques to detect these compounds at trace levels. Furthermore, our prior investigation (Munzhelele et al., 2025) revealed that these compounds exhibit similar retention times, complicating their simultaneous profiling and quantification. Consequently, optimizing the solid-phase extraction (SPE) methodology to enhance the purification and preconcentration of these compounds would facilitate their effective separation and enable simultaneous detection. Thus, improving the detection of pharmaceutical compounds remains paramount (Fick et al., 2010; Campos et al., 2019; Oro et al., 2020; Madikizela et al., 2020).
Extraction and/or pre-concentration of analytes from different matrixes plays a crucial role in enhancing their detection by analytical equipment (Madikizela et al., 2020; Hawthorne et al., 1994; Namieśnik et al., 2005). Solid phase extraction (SPE) method is the most commonly used for pre-concentration of the analytes due to its ability to clean up, isolate the analyte, and remove interfering matrices from the extract (Furey et al., 2013; Muhammad et al., 2017). Hydrophilic and Lipophilic balance (HLB) cartridges are commonly used to extract pharmaceutical residues and other organic compounds due to the better retention capabilities towards both polar and non-polar compounds (Fatoki et al., 2018; Giebułtowicz et al., 2016). Parameters such as the pH of the solution, concentration, the type of solvent, and the volume of the eluent generally impact the extraction efficiency of the cartridge and, subsequently, the detection of the analyte. This study, therefore, is designed to optimize the solid phase extraction parameters for the simultaneous detection and quantification of efavirenz and levonorgestrel in wastewater using high-performance liquid chromatography equipped with a photodiode array detector.
2 Experimental protocol
2.1 Chemical reagents and materials
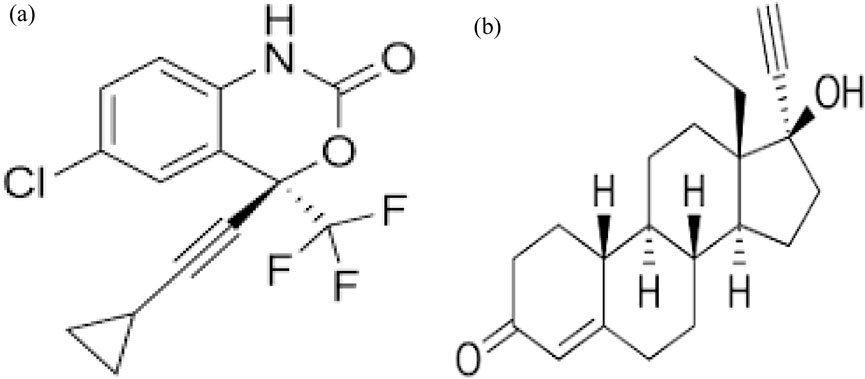
Efavirenz (EFA: C14H9ClF3NO2), levonorgestrel (LVG: C21H28O2), acetonitrile (C2H3N), methanol (CH3OH) (HPLC grade), HPLC water, and isopropanol of HPLC grade were purchased from Sigma-Aldrich, South Africa. Sodium chloride, hydrochloric acid, nylon syringe filters (0.22 µm), 1 mm syringes, insets, amber vials (2 mL), and Oasis Hydrophilic-Lipophilic Balance (HLB: 223170-AC (60 mg/3 mL)) were purchased from Stargate, South Africa. An individual stock solution of 1,000 ppm was prepared by dissolving the analyte (efavirenz and levonorgestrel) into 100 mL of 80% Methanol. The dilution method was used to prepare the respective mixed working standards of the analytes (500, 1,000, 5,000, 10,000, 15,000, 20,000, and 30,000 µg/L). All the stock solutions were prepared in amber bottles to avoid sample degradation. All chemicals used were of analytical grade. EFA and LVG chemical structures are shown in Figure 1.
2.2 Methods
2.2.1 Optimization of solid phase extraction conditions
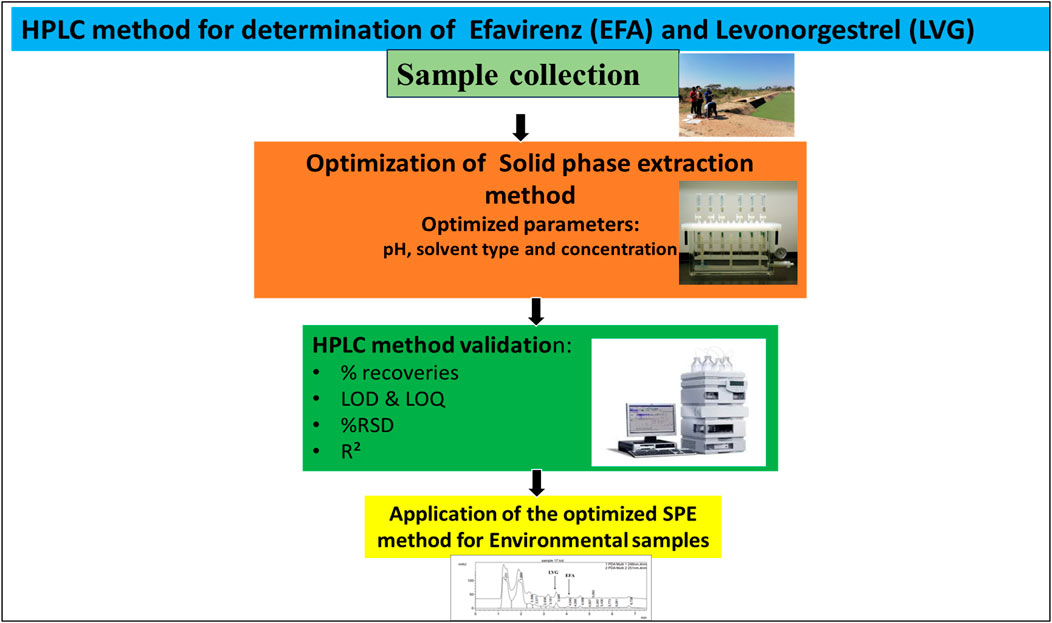
The optimum pH, solvent concentration, and elution volume for sample extraction were determined using a synthetic solution containing 1 ppm of efavirenz and levonorgestrel. The extraction was performed using an Oasis HLB cartridge. Before extraction, the cartridges were preconditioned by adding 5 mL of 10% methanol and then rinsed with 5 mL of ultra-pure water at a 1 mL/min flow rate (Abafe et al., 2018). The optimization was performed by varying one parameter and keeping others constant. Briefly, the effect of solution pH in the extraction of efavirenz and levonorgestrel was accomplished by changing the solution pH from 2 to 12 using 0.1 M of NaOH and HCl. A volume of 100 mL of the synthetic solution containing 1 ppm of the EFA and LVG was then loaded into the HLB cartridges under vacuum. After extraction, the cartridges were rinsed with 5 mL of 10% Methanol and 5 mL ultra-pure water to remove the untargeted compounds from the surface of the cartridge. Thereafter, the adsorbed analytes were eluted with 6 mL of 80% Methanol. The eluted samples were then dried under nitrogen at 50°C and reconstituted using 1 mL of Methanol. Before analysis, the samples were filtered using nylon syringe filters (0.22 µm). To elucidate the effect of elution solvent and solvent concentration, acetonitrile and Methanol were used at concentrations varying from 50, 80, and 100% to elute efavirenz and levonorgestrel. The above procedure was repeated to evaluate the effect of elution volume, except that the volume of eluent solvent varied from 3, 4, 5, and 6 mL using 100% Methanol. The summary of the method is shown in Figure 2.
2.2.2 Instrumentation and chromatography conditions
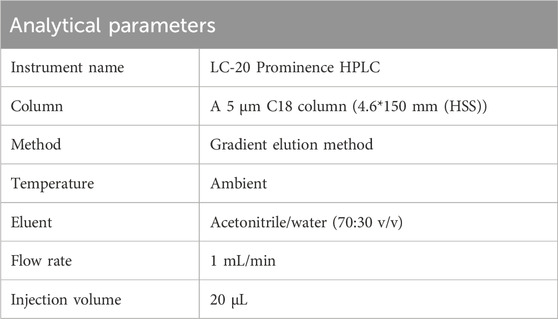
LC-20 Prominence HPLC system with a photodiode array detector (Shimadzu, Japan) was used for analysis. The column type, size, and analytical parameters are summarized in Table 1.
2.2.3 Instrument and method validation
Seven-point calibration curves were constructed for EFA and LVG development to determine the linear working range, with concentrations ranging from 0.5 to 30 ppm. The calibration curve was plotted using the linear regression peak area vs. concentrations. The respective standards were analysed in triplicates. The calibration curve’s correlation coefficient (R2) values were >0.98, implying method precision. The limit of detection and quantification were calculated using Equations 1, 2 (Nicolay et al., 2011) to evaluate the linear working range, recoveries, and method sensitivity. The lowest and highest known concentrations (0.5 and 30 ppm) and the blank matrix were analysed to evaluate the method’s accuracy.
where σ is the standard deviation of the calibration curve, and S is the slope of the calibration curve.
The analytes recoveries were calculated using Equation 3:
The chromatographic variations on the RSD, peak area, resolutions, tailing factor, and theoretical plates of the mixed standard working solution were used to assess the method’s robustness. The validated method was employed to quantify EFA and LVG in environmental samples. The concentration (
where, Cf is the final concentration (µg/L), measured concentration (µg/L), volume extracted in (L), extracted volume in (L).
2.2.4 Applicability of method in wastewater
The method was applied for the quantification of EFV and LVG in wastewater collected from Thohoyaṋdou, Malamulele, Giyani, Makhado, Nkowankowa, Tzaneen, Kgapane, and Siloam wastewater treatment plants located in Limpopo Province, South Africa (Supplementary Figure S1). For sample collection, bottles were soaked in 10% Methanol, rinsed with de-ionized water, and oven-dried for 2 h at 80°C to avoid contamination. The samples were kept in ice and transported to the laboratory. In the laboratory, samples were subjected to a solid phase extraction process using optimized conditions at pH 2, 100% Methanol, and an elution volume of 4 mL. The eluted samples were dried under nitrogen flow at 50°C. Thereafter, the samples were reconstituted with 1 mL of 100% Methanol and analysed.
2.2.5 Quality assurance
To validate the obtained results from HPLC, the EFA and LVG were analysed using liquid chromatography–quadrupole time-of-flight tandem MS instrument (LCMS-9030 qTOF, Shimadzu Corporation, Kyoto, Japan). Chromatographic separation of the analytes was done using a Shim-pack Velox C18 column (100 × 2.1 mm, 2.7 μm) (Shimadzu Corporation, Kyoto, Japan) maintained at 40°C. An injection volume of 10 μL was used. The analytes were separated using a mobile phase gradient elution method at a flow rate of 0.4 mL min−1. The mobile phase composition of A and B was 0.1% (v/v) formic acid in ultrahigh purity water and acetonitrile, respectively. The mobile phase composition was 5% mobile B from 0 to 1.5 min. At 1.5–4 min, the composition of mobile B was increased to 95% and kept constant until 4 min. The gradient was changed to 5% of mobile phase B at 5 min and maintained at this composition until 6 min. Mass spectral analysis was performed using a QqQ mass spectrometer with an electrospray interface (ESI) in positive (LVG) and negative (EFA) modes. Parameters were set as follows: nebulization 3 L min−1, heating gas, and drying gas flow 10 L/min, interface voltage of 4.0 kV, interface temperature of 300°C, dissolving temperature 526°C, DL temperature of 250°C, heat block temperature of 400°C, detector voltage of 1.8 kV and the flight tube temperature at 42°C.
3 Results and discussion
3.1 Quality assurance parameters of the method
The chromatogram of the target compounds and their respective calibration curves are presented in Figure 3. The obtained chromatogram of LVG and EFA indicated that these compounds eluted after 3.55 and 4.08 min of retention times, respectively. The two compounds are quantified at 246 and 251 nm wavelengths using HPLC with a photodiode array detector. Two distinct peaks were identified in the sample to validate the optimized SPE method’s applicability for analyte detection using HPLC, corresponding closely to those observed in the standard chromatogram. The method accuracy was further validated by a correlation (R2) of 0.99 obtained from the fitted standard calibration curve of the respective target compounds (Table 3). The instrument’s detection limit was calculated as the limit of detection (LOD) and limits of quantification (LOQ) using a statistical Equations 1, 2. The summary of the results is shown in Table 2. It was observed that the sample sensitivity for EFA and LVG increased as the detection limit was reduced. Specifically, the sensitivity for EFA decreased from 0.705 to 2.138 µg/L, while the sensitivity for LVG increased from 0.199 to 0.061 µg/L. High RSD% % further validated the accuracy and quality assurance of the method, with an accuracy of >79.9% for all the target compounds since they were within the acceptable range of >80%.
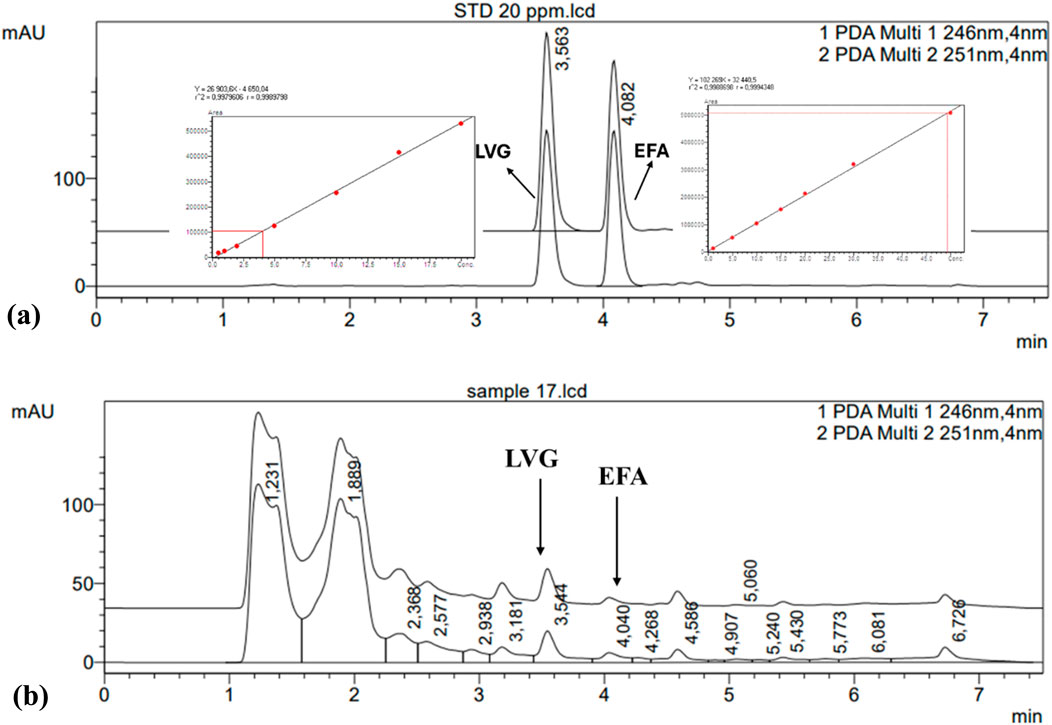
Figure 3. The chromatogram of levonorgestrel and efavirenz standards (a) with their respective calibration curves and sample (b).
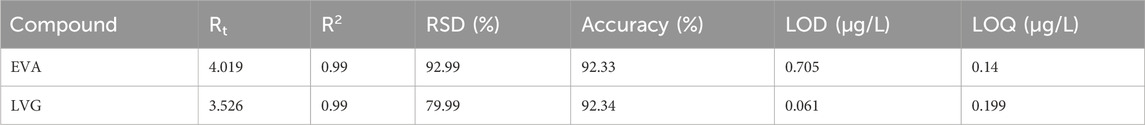
Table 2. The performance metrics the HPLC method for the detection and quantification of LVG and EFA.
Standards and samples were further analysed for quality assurance using UHPLC-QqQ-MS/MS. The chromatograms of both EFV and LVG are presented in Figure 4. The linear concentration range was 25 µg/L to 750 µg/L. The coefficient of determination (R2) was 0.99 for all analytes. The retention times for EFA and LVG were 3.50 and 3.79 min, respectively. The chromatograms of the blank samples did not show any peak of the analytes. The chromatograms of the standard were distinct from those of the sample. However, a similar resemblance between the standard and sample chromatograms and retention times was observed. EFA and LVG were profiled and quantified using 314.67 and 312.92 m/z. The product m/z for levonorgestrel 109. 91 and 245 m/z were similar to the one reported by Theron et al. (2004). Meanwhile, for EFA products, m/z were 245.1, 68.95, and 242.
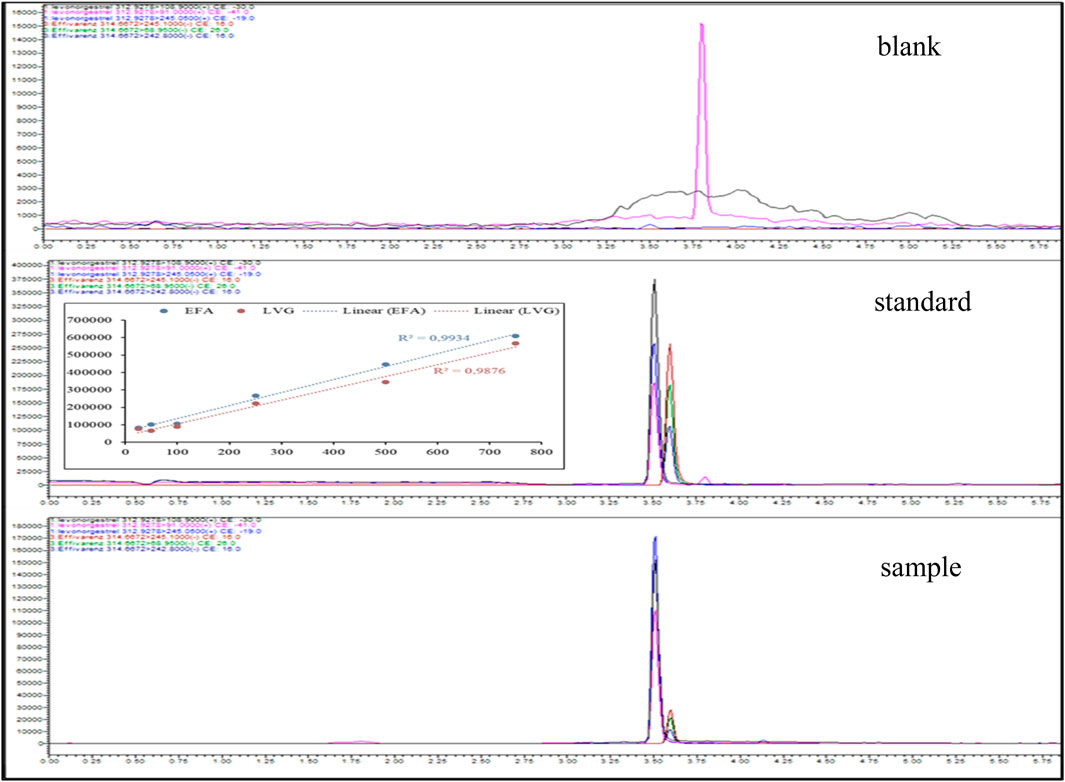
Figure 4. Multiple retention monitoring chromatograms of the blank sample, calibration standard, and environmental sample.
3.2 Optimization of solid phase extraction method
3.2.1 Effect of sample pH
Figure 5 shows the variation of % recoveries for LVG and EFA with the change in solution pH. The highest percentage recoveries for EFV and LVG were observed at pH of 2 (67.3% and 70.82%), respectively. A decrease in the respective analytes recoveries was observed with increasing pH. LVG and EFA are weak acid compounds that are easily protonated and become soluble at low pH, hence their substantial recoveries at low pH. At alkaline pH, they form strong bonds with HLB carboxylic, divinyl benzene, and N-vinylpyrrolidone, reducing their mobility. Hence, low EFA and LVG recoveries with increasing pH. Based on the obtained results, it was observed that sample pH can significantly affect the recoveries of EFA and LVG. pH of 2 was further used for subsequent experiments.
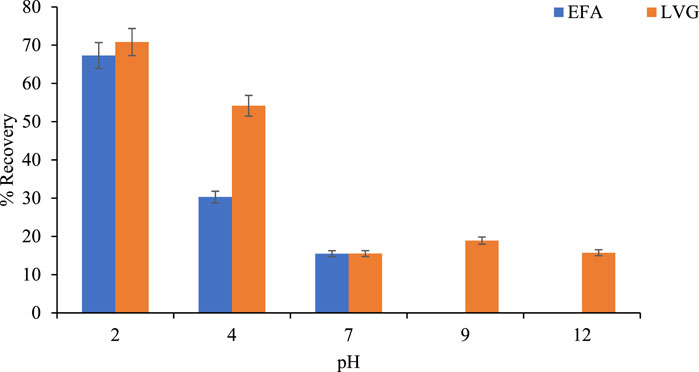
Figure 5. Variation of % recoveries for EFV and LVG with pH (extracted volume:100 mL, elution volume: 5 mL, and elution solvent MeOH: 80%).
3.2.2 Solvent type and concentration
Figure 6 depicts the effect of solvent type and concentration on the recovery of efavirenz and levonorgestrel. Methanol gave higher recoveries for EFV (71%) and LVG (84.89%) compared to acetonitrile (50.17% and 73.63%). High recoveries for Methanol could be attributed to the introduction of methoxy groups on the surface of the HLB cartridges, enhancing their reactivity (Cortés-Lagunes et al., 2024). Comparatively, methanol is a proactive solvent with high polarity compared to acetonitrile. Thus, the OH functional group dissociate from methanol through hydrogen transfer (Jordaan and Shapi, 2017; Ngwenya and Mahlambi, 2023) and infused into the HLB matrix, improving the solubility of the analytes and enhancing their leaching efficiency. Due to its strong nitrile group, acetonitrile is a non-aprotic solvent that suggests lower polarity than methanol, affecting its reactivity due to its nitrile functional group, which undergoes hydrolysis in acid pH to form carboxylic acid through protonation of nitrogen taking time to infuse the HLB matrix (Zarzycki et al., 2010; Gooßen et al., 2008). An increase in recoveries of the target compounds was observed as the solvent concentration increased for both solvents. This could be attributed to an improved reactivity of the analytes and increased solvent purity (Hu et al., 2009; Jing et al., 2020). Low recoveries at low concentrations might be attributed to reduced reactivity due to increased solvent impurity. Based on the results, 100% of Methanol achieved high EFA and LVG recoveries and was used for subsequent experiments.
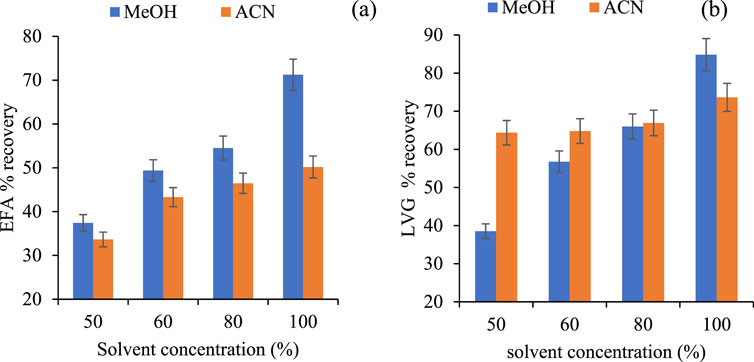
Figure 6. Effect of solvent type and concentration of EFA (a) and LVG (b) recoveries (extracted volume 100 mL, solution pH: 2, elution volume 5 mL).
3.2.3 Elution volume
Figure 7 shows the effect of elution volume towards efavirenz and levonorgestrel recoveries. EFA and LVG increased from 3.5% to 83% and 1.08% to 94.61% with an increasing volume from 3 mL to 6 mL, respectively. The higher EFA and LVG recoveries were observed with an elution volume of 3–4 mL. This could be attributed to improved dissociation from surface charges, possibly due to increased methoxy ions (Koekemoer, 2016; Sturm, 2005). This phenomenon effectively returns the pollutants to their original state. A slight decrease in the recovery of LVG with increasing elution volume could be due to the degradation of these compounds during prolonged drying. A significant reduction in EFA recoveries was observed when using an elution volume of 5–6 mL, which could be linked to the degradation of EFAs during prolonged drying periods (Yao et al., 2021). Furthermore, since methanol was used as an elution solvent, its increased volume increases the methoxy ions in a solution, which initiates the EFA leaching process from the HLB sorbent. This further increases the solution’s electronegativity, reducing the solute’s leaching activity (EFA) since it mainly comprises electronegative charges, implying less EFA recoveries.
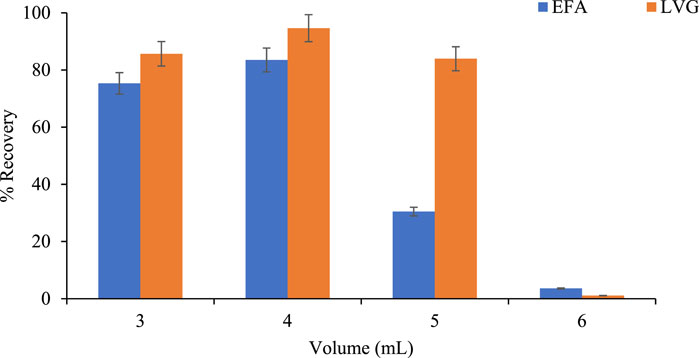
Figure 7. Effect of volume elution on recovery of LVG and EFA in wastewater (Solvent: 100% MeOH, pH of 2, Sample volume 100 mL).
3.3 Application of the method in field water samples
Wastewater samples were analysed using optimized solid-phase extraction (SPE) parameters, including a pH of 2, 100% methanol (Methanol), and an elution volume of 4 mL. This method was employed to analyze the effects of the sample matrix on the recovery of wastewater samples from the Vhembe and Mopani districts. Table 3 depicts the concentration of LVG and EFA obtained following SPE at optimized conditions. The concentrations of efavirenz and levonorgestrel ranged from LOD-33.675 µg/L and LOD- 7.195 µg/L, respectively, with Nkowankowa showing the highest concentrations of LVG and EFA. A slight increase in LVG was observed in effluent samples in most WWTP samples, which could be ascribed to immobilization in different stages of the purification process. A similar trend was observed with EFA. All the target compounds were detected in all sampling sites except LVG at Kgapane. Based on the obtained results, the optimized SPE conditions could be used for simultaneous pre-concentration to improve the detection of EFA and LVG in wastewater using HPLC. For quality assurance, samples were analysed using the UHPLC-QqQ-MS/MS technique, and the results are presented in Supplementary Table S2. The results of the UHPLC-QqQ-MS/MS were in the same range as those obtained from HPLC with less than 10% difference, validating that the conditions developed effectively enhance the simultaneous detection of EFA and LVG.
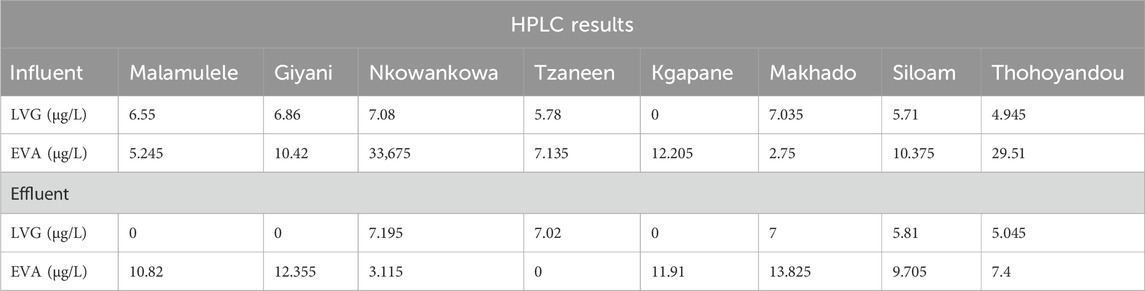
Table 3. Concentrations of levonorgestrel and efavirenz obtained using high-performance liquid chromatography (HPLC) at optimized SPE conditions.
4 Conclusion
This study successfully determined solid-phase extraction conditions for the simultaneous quantification of efavirenz and levonorgestrel by HPLC-PDA. The established conditions were a pH of 2, 100% Methanol, and an elution volume of 4 mL. Under these conditions, maximum recoveries of 83% for efavirenz and 94% for levonorgestrel were obtained. The optimized SPE method was successfully applied in the preconcentration of EFA and LVG in wastewater samples. In influent samples, the EFA concentration ranged from 0.36 to 8.10 µg/L, while in effluent samples, it ranged from 2.88 to 8.11 µg/L. The levonorgestrel concentration in influent samples ranged from 2.64 to 32.31 µg/L; in effluent samples, it ranged from 2.32 to 12.35 µg/L. The optimized SPE method for preconcentration and simultaneous analysis of EFA and LVG using HPLC was validated by UHPLC results, which showed no significant differences.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
EM: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. WA: Conceptualization, Supervision, Validation, Visualization, Writing – review and editing. WG: Conceptualization, Funding acquisition, Resources, Supervision, Validation, Visualization, Writing – review and editing. RM: Conceptualization, Funding acquisition, Resources, Supervision, Visualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. NRF-Thuthuka project (Ref: TTK2205087912), and National Research Foundation-Sasol (MND210510600480).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1539932/full#supplementary-material
References
Abafe, O. A., Späth, J., Fick, J., Jansson, S., Buckley, C., Stark, A., et al. (2018). LC-MS/MS determination of antiretroviral drugs in influents and effluents from wastewater treatment plants in KwaZulu-Natal, South Africa. Chemosphere 200, 660–670. doi:10.1016/j.chemosphere.2018.02.105
Campos, J. M., Queiroz, S. C., and Roston, D. M. (2019). Removal of the endocrine disruptors ethinyl estradiol, bisphenol A, and levonorgestrel by subsurface constructed wetlands. Sci. total Environ. 693, 133514. doi:10.1016/j.scitotenv.2019.07.320
Cortés-Lagunes, R. S., Garduño-Jiménez, A.-L., Romero-Solano, A., Zanella, R., Prado, B., Zamora, O., et al. (2024). Optimizing solid phase extraction and HPLC-MS/MS parameters for reliable quantification of COVID-19 pharmaceuticals in Mexico City’s wastewater: a design of experiments approach. Microchem. J. 200, 110493. doi:10.1016/j.microc.2024.110493
Fatoki, O. S., Opeolu, B. O., Genthe, B., and Olatunji, O. S. (2018). Multi-residue method for the determination of selected veterinary pharmaceutical residues in surface water around Livestock Agricultural farms. Heliyon 4 (12), e01066. doi:10.1016/j.heliyon.2018.e01066
Fick, J., Lindberg, R. H., Parkkonen, J., Arvidsson, B., Tysklind, M., and Larsson, D. J. (2010). Therapeutic levels of levonorgestrel detected in blood plasma of fish: results from screening rainbow trout exposed to treated sewage effluents. Environ. Sci. and Technol. 44 (7), 2661–2666. doi:10.1021/es903440m
Furey, A., Moriarty, M., Bane, V., Kinsella, B., and Lehane, M. (2013). Ion suppression; a critical review on causes, evaluation, prevention and applications. Talanta 115, 104–122. doi:10.1016/j.talanta.2013.03.048
Giebułtowicz, J., Stankiewicz, A., Wroczyński, P., and Nałęcz-Jawecki, G. (2016). Occurrence of cardiovascular drugs in the sewage-impacted Vistula River and in tap water in the Warsaw region (Poland). Environ. Sci. Pollut. Res. 23 (23), 24337–24349. doi:10.1007/s11356-016-7668-z
Golovko, O., Šauer, P., Fedorova, G., Kroupová, H. K., and Grabic, R. (2018). Determination of progestogens in surface and waste water using SPE extraction and LC-APCI/APPI-HRPS. Sci. Total Environ. 621, 1066–1073. doi:10.1016/j.scitotenv.2017.10.120
Gooßen, L. J., Rodríguez, N., and Gooßen, K. (2008). Carboxylic acids as substrates in homogeneous catalysis. Angew. Chem. Int. Ed. 47 (17), 3100–3120. doi:10.1002/anie.200704782
Hawthorne, S. B., Yang, Y., and Miller, D. J. (1994). Extraction of organic pollutants from environmental solids with sub-and supercritical water. Anal. Chem. 66 (18), 2912–2920. doi:10.1021/ac00090a019
Hu, Y., Liu, R., Zhang, Y., and Li, G. (2009). Improvement of extraction capability of magnetic molecularly imprinted polymer beads in aqueous media via dual-phase solvent system. Talanta 79 (3), 576–582. doi:10.1016/j.talanta.2009.04.029
Jing, W., Zhou, Y., Wang, J., Zhu, Y., Lv, Y., Bi, W., et al. (2020). Sorbent and solvent co-enhanced direct analysis in real time-mass spectrometry for high-throughput determination of trace pollutants in water. Talanta 208, 120378. doi:10.1016/j.talanta.2019.120378
Jordaan, M. A., and Shapi, M. (2017). Investigation of the solvent-dependent photolysis of a nonnucleoside reverse-transcriptase inhibitor, antiviral agent efavirenz. Antivir. Chem. Chemother. 25 (3), 94–104. doi:10.1177/2040206617730170
King, O. C., van de Merwe, J. P., McDonald, J. A., and Leusch, F. D. (2016). Concentrations of levonorgestrel and ethinylestradiol in wastewater effluents: is the progestin also cause for concern? Environ. Toxicol. Chem. 35 (6), 1378–1385. doi:10.1002/etc.3304
Kloas, W., Urbatzka, R., Opitz, R., Würtz, S., Behrends, T., Hermelink, B., et al. (2009). Endocrine disruption in aquatic vertebrates. Ann. N. Y. Acad. Sci. 1163 (1), 187–200. doi:10.1111/j.1749-6632.2009.04453.x
Koekemoer, S. M. (2016). Gradient high performance liquid chromatographic method for the simultaneous analysis of efavirenz, emtricitabine and tenofovir. South Africa.
Madikizela, L. M., Pakade, V. E., Ncube, S., Tutu, H., and Chimuka, L. (2020). Application of hollow fibre-liquid phase microextraction technique for isolation and pre-concentration of pharmaceuticals in water. Membranes 10 (11), 311. doi:10.3390/membranes10110311
Muhammad, N., Subhani, Q., Wang, F., Guo, D., Zhao, Q., Wu, S., et al. (2017). Application of a simple column-switching ion chromatography technique for removal of matrix interferences and sensitive fluorescence determination of acidic compounds (pharmaceutical drugs) in complex samples. J. Chromatogr. A 1515, 69–80. doi:10.1016/j.chroma.2017.07.007
Munzhelele, E., Ayinde, W., Gitari, W., Pindihama, G., and Mudzielwana, R. (2025). Occurrence of efavirenz, levonorgestrel, ibuprofen, and diclofenac in wastewaters of limpopo province, South Africa. Heliyon 11 (2), e41524. doi:10.1016/j.heliyon.2024.e41524
Munzhelele, E. P., Mudzielwana, R., Ayinde, W. B., and Gitari, W. M. (2024). Pharmaceutical contaminants in wastewater and receiving water bodies of South Africa: a review of sources, pathways, occurrence, effects, and geographical distribution. Water 16 (6), 796. doi:10.3390/w16060796
Namieśnik, J., Zabiegała, B., Kot-Wasik, A., Partyka, M., and Wasik, A. (2005). Passive sampling and/or extraction techniques in environmental analysis: a review. Anal. Bioanal. Chem. 381, 279–301. doi:10.1007/s00216-004-2830-8
Narváez, J. F., Grant, H., Gil, V. C., Porras, J., Sanchez, J. C. B., Duque, L. F. O., et al. (2019). Assessment of endocrine disruptor effects of levonorgestrel and its photoproducts: environmental implications of released fractions after their photocatalytic removal. J. Hazard. Mater. 371, 273–279. doi:10.1016/j.jhazmat.2019.02.095
Ngwenya, N., and Mahlambi, P. (2023). Methods optimization and application: solid phase extraction, ultrasonic extraction and Soxhlet extraction for the determination of antiretroviral drugs in river water, wastewater, sludge, soil and sediment. J. Pharm. Biomed. Analysis 230, 115358. doi:10.1016/j.jpba.2023.115358
Nicolay, A., Wolff, E., Vergnes, M.-F., Kaloustian, J., and Portugal, H. (2011). Rapid HPLC method for determination of parachloroaniline in chlorhexidine antiseptic agent in mouthrinses, ophthalmic and skin solution. Am. J. Anal. Chem. 2 (04), 422–428. doi:10.4236/ajac.2011.24051
Orlando, E. F., and Ellestad, L. E. (2014). Sources, concentrations, and exposure effects of environmental gestagens on fish and other aquatic wildlife, with an emphasis on reproduction. General Comp. Endocrinol. 203, 241–249. doi:10.1016/j.ygcen.2014.03.038
Oropesa, A. L., and Guimarães, L. (2020). Occurrence of levonorgestrel in water systems and its effects on aquatic organisms: a review. Rev. Environ. Contam. Toxicol. 254, 57–84. doi:10.1007/398_2020_44
Pindihama, G. K., and Gitari, M. W. (2020). Cyanobacterial toxins: an emerging threat in South African irrigation water. Water Environ. J. 34 (3), 506–516. doi:10.1111/wej.12473
Quirke, V. M. (2017). Tamoxifen from failed contraceptive pill to best-selling breast cancer medicine: a case-study in pharmaceutical innovation. Front. Pharmacol. 8, 620. doi:10.3389/fphar.2017.00620
Schoeman, C., Dlamini, M., and Okonkwo, O. (2017). The impact of a Wastewater Treatment Works in Southern Gauteng, South Africa on efavirenz and nevirapine discharges into the aquatic environment. Emerg. Contam. 3 (2), 95–106. doi:10.1016/j.emcon.2017.09.001
Sturm, S. (2005). A general unknown screening for drugs and toxic compounds in human serum. Switzerland: University_of_Basel.
Tariq, S., Wani, S., Rasool, W., Shafi, K., Bhat, M. A., Prabhakar, A., et al. (2019). A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb. Pathog. 134, 103580. doi:10.1016/j.micpath.2019.103580
Theron, H., Coetzee, C., Sutherland, F., Wiesner, J., and Swart, K. (2004). Selective and sensitive liquid chromatography–tandem mass spectrometry method for the determination of levonorgestrel in human plasma. J. Chromatogr. B 813 (1-2), 331–336. doi:10.1016/j.jchromb.2004.10.039
Yao, L., Dou, W.-Y., Ma, Y.-F., and Liu, Y.-S. (2021). Development and validation of sensitive methods for simultaneous determination of 9 antiviral drugs in different various environmental matrices by UPLC-MS/MS. Chemosphere 282, 131047. doi:10.1016/j.chemosphere.2021.131047
Keywords: wastewater, solid phase, efavirenz, levonorgestrel, high-performance liquid chromatography
Citation: Munzhelele EP, Ayinde WB, Gitari WM and Mudzielwana R (2025) Optimization of solid phase extraction for simultaneous quantification of efavirenz and levonorgestrel in wastewater using HPLC. Front. Environ. Sci. 13:1539932. doi: 10.3389/fenvs.2025.1539932
Received: 05 December 2024; Accepted: 02 June 2025;
Published: 13 June 2025.
Edited by:
Dandan Li, Beijing Technology and Business University, ChinaReviewed by:
Vimbai Mhuka, O Summit International College, South AfricaSinegugu Khulu, University of the Witwatersrand, South Africa
Idris Sanusi, Kampala International University Western Campus, Uganda
Copyright © 2025 Munzhelele, Ayinde, Gitari and Mudzielwana. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: E. P. Munzhelele, bXVuemhlbGVsZTk4QGdtYWlsLmNvbQ==, MTUwMDMyNjRAbXZ1bGEudW5pdmVuLmFjLnph; R. Mudzielwana, cmFiZWxhbmkubXVkemllbHdhbmFAdW5pdmVuLmFjLnph
 E. P. Munzhelele
E. P. Munzhelele W. B. Ayinde2
W. B. Ayinde2 W. M. Gitari
W. M. Gitari R. Mudzielwana
R. Mudzielwana