- 1Chinese PLA Center for Disease Control and Prevention, Beijing, China
- 2School of Environment and Energy Engineering, Beijing University of Civil Engineering and Architecture, Beijing, China
The widespread application of triclosan (TCS) and triclocarban (TCC) as antimicrobial agents has raised significant concerns among environmental scientists about their environmental behavior, ecological effects, and possible human health risks. To comprehensively understand the current research landscape and developmental trends pertaining to TCS and TCC research in the environmental field, this study analyzed literature data from the Web of Science Core Collection (WOSCC) database from 2002 to 2024 using advanced visualization tools such as Bibliometrix, VOSviewer, and Citespace. The study revealed that China dominates the field of TCS and TCC in environmental research with a publication count significantly higher than other countries, while Brazil and India, as developing nations, also make substantial contributions, ranking among the top 10 in publication output. The journals Science of the Total Environment, Chemosphere, and Environmental Science and Technology featured the highest number of publications on this topic. Keywords co-occurrence and burst anlaysis revealed that contemporary research predominantly concentrates on the environmental behaviour and fate of these compounds, as well as their ecotoxicological impacts and mechanisms. Additionally, there is a focused interest in evaluating human exposure risks and investigating technologies for their degradation and removal. The interaction of these compounds with other environmental pollutants, along with their mechanisms, and the improvement of removal and degradation technologies, are expected to be key focal points in forthcoming research efforts. Based on the comprehensive bibliometric analysis, this study highlights the critical need for environmental policies to address the persistence and bioaccumulation potential of TCS and TCC, urging the development of more effective degradation technologies and strategies to mitigate their environmental and health impacts, which could guide future research and regulatory frameworks.
Highlights
• Applied bibliometric visualization tools to analyze TCS and TCC research trends from 2002 to 2024.
• Identified China as the leading contributor to global research on TCS and TCC.
• Current research focused on the environmental behavior, ecotoxicological effects, human exposure risk, and removal technologies for TCS and TCC.
• Promising avenues for future studies include investigating the co-toxicity of TCS and TCC and developing advanced degradation technologies and materials.
1 Introduction
Triclosan (TCS) and triclocarban (TCC) are two widely used antibacterial agents utilized in products like hand sanitizers, toothpastes, skincare items, and surface cleaners, making them some of the most frequently detected chemicals within the pharmaceuticals and personal care products (PPCPs) category. Globally, the annual consumption of TCS surpasses 1,500 metric tons. In Europe, TCS is primarily utilized with 85% in PPCPs, followed by 10% in food-related materials, and 5% in the textile industry (van Wijnen et al., 2018). TCS exhibits low water solubility and high lipophilicity, which facilitates its distribution and migration within both aqueous and solid environment, with a propensity for adsorption to solid-phase media (logKow>4, indicating a high potential for adsorption) (Rozaini et al., 2022). Due to their widespread application, TCS and TCC are broadly distributed across various environmental media, including wastewater (Juksu et al., 2019), sewage sludge (Barrett et al., 2022), surface waters (Wang et al., 2024), soil (Fu et al., 2016), and sediment (Chen Z. F. et al., 2018). Due to their environmental persistence and resistance to degradation, TC) and TCC have a propensity for accumulation, which has led to a consistent increase in their levels in the environment over recent years. Although the environmental concentrations of TCC and TCS are relatively low, these compounds have the potential to bioaccumulate in aquatic organisms such as fish, amphibians, and invertebrates, primarily through dietary intake. Chronic exposure to low concentrations of TCS and TCC has been shown to lead to reduced growth, diminished reproductive capabilities, and impaired immune system functions in aquatic life (Vimalkumar et al., 2019). Furthermore, these substances may undergo biomagnification within the food chain, thereby impacting higher trophic levels. Numerous studies have detected TCS and TCC in human urine (Tkalec et al., 2021), serum (Li et al., 2020), and breast milk (Chi et al., 2024), highlighting their potential adverse effects on human and animal health.
Widely used as antimicrobials, TCS and TCC not only kill bacteria but are also recognized for their ability to disrupt endocrine functions (Iyer et al., 2018). Moreover, TCS and TCC promote the proliferation and spread of antibiotic resistance genes (ARGs) and the persistence of antibiotic-resistant bacteria (ARB) by enhancing horizontal gene transfer (HGT), increasing bacterial biofilm formation, and inducing mutations that cause DNA damage or interfere with DNA repair mechanisms (Lu et al., 2020). More alarmingly, TCS and TCC can interact with strong oxidizing agents or transform into more toxic substances, such as methyl triclosan, chlorodioxins, chlorophenols, and chloroform, under photocatalytic conditions (Ding et al., 2018). In recent years, numerous research and review articles has been published that concerning the environmental behavior, analytical detection techniques, toxicological effects, human exposure, and degradation and removal technologies related to TCS and TCC (Table 1). Although these review articles primarily synthesize existing findings, there remains a significant gap in the literature that comprehensively summarizes the overall research characteristics and advancements in the environmental impact of TCS and TCC from a macroscopic perspective, as the current reviews rely heavily on reading, summarization, and critical analysis without providing a broader examination of the state and emerging trends. In contrast, bibliometrics employs a combination of mathematical and statistical methods to analyze extensive bibliographic data, facilitating the identification of research trends, key contributors, core journals, and hot topics, thereby providing a comprehensive understanding of the scientific structure and developmental dynamics of the field (Huang et al., 2024). By incorporating data mining, information analysis, scientometrics, and graphical representation, this methodology objectively and quantitatively maps the knowledge landscape of an academic discipline, significantly improving our understanding of its structure and evolution. Currently, bibliometrics has been extensively applied to the study of various emerging contaminants (ECs), including polychlorinated naphthalenes (PCNs) (Olisah et al., 2024), 6PPD and 6PPD-quinone (Babaei et al., 2024), organophosphate flame retardants (OPFRs) (Du Z. Y. et al., 2024), steroidal estrogens (SEs) (Odinga et al., 2022), and polycyclic aromatic hydrocarbons (PAHs) (Yemele et al., 2024). However, there is a notable deficiency in the analysis of the cutting-edge developments and trends of TCS and TCC in the environmental field.
This paper employed bibliometric and visualization technologies to conduct a comprehensive and systematic analysis of research findings related to TCS and TCC in the environmental field from January 2002 to December 2024 recorded in the Web of Science Core Collection (WOSCC) database. Employing advanced software tools such as Bibliometrix, VOSviewer, and Citespace, this study conducted a detailed visual analysis of publication output, publishing institutions, contributing countries/regions, research teams, and keywords co-occurrence to enhance understanding of the distribution and impact of research in the field. The objective of this study is to methodically and objectively delineate the research landscape pertaining to TCS and TCC, providing a foundational reference point for future research endeavors.
2 Methodology
2.1 Data collection and screening
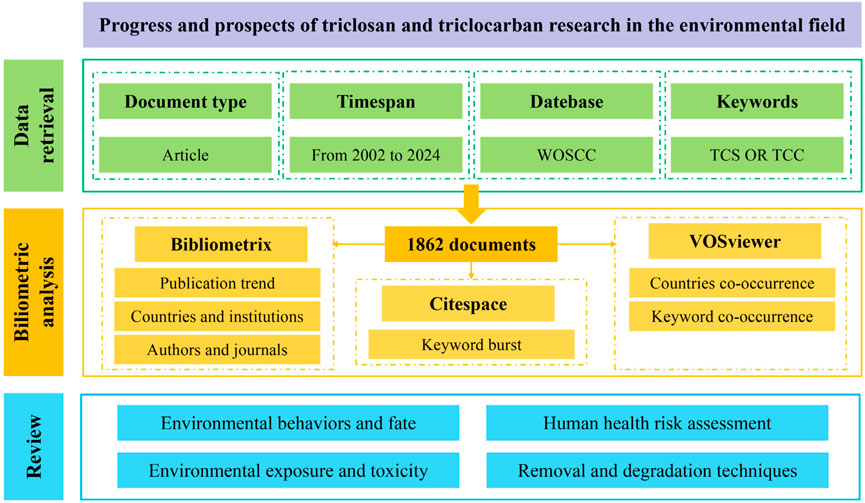
For the bibliometric analysis presented in this paper, the data were sourced from the WOSCC database, utilizing the topic keywords: “Triclosan OR Triclocarban.” The search period was from 1 January 2002 to 31 December 2024. In this study, the document type was restricted as “ARTICLE” and the language type was restricted to “English,” encompassing subject categories including “Environmental Sciences,” “Engineering Environmental,” “Public Environmental Occupational Health,” “Water Resources,” “Marine Freshwater Biology”, “Agriculture Multidisciplinary,” “Ecology,” “Soil Sciences,” “Environmental Studies,” and “Agricultural Engineering.” After a careful screening process to discard duplicates and irrelevant articles, a total of 1862 papers were identified as pertinent to investigating TCC and TCS in the environmental field. The selected documents were downloaded in plain text format as “Full Record and Cited References” to serve as the sample for subsequent analysis. The data retrieval date was 16 March 2025. The technical roadmap of the process is illustrated in Figure 1.
2.2 Scientometric analysis
This study utilized Bibliometrix, VOSviewer, and CiteSpace to analyze and summarize the publication trend, prominent contributors (countries/regions, institutions and authors), influential journals, highly cited documents, and research hotspots and trends. Bibliometrix is an R package specifically designed for bibliometric and scientometric analysis, enables the examination of literature output changes over time and helps identify developmental trends and the most prolific authors, institutions, and countries in a research field (Aria and Cuccurullo, 2017). VOSviewer, developed by Van Eck N.J. and Waltman L. at Leiden University in the Netherlands, is a freely available tool for bibliometric analysis that uses VOS clustering techniques to transform complex knowledge units into intuitive visual cluster networks (van Eck and Waltman, 2010). The “burst detection” function in CiteSpace software is essential for identifying periods of significant activity increases related to keywords, authors, institutions, or documents, thereby discerning rapidly gaining themes or concepts within specific research areas that may signal emerging trends or scientific breakthroughs (Chen, 2006).
The present study utilized Bibliometrix (version 3.1.4) to analyze annual variations in publication output and keywords associated with TCS and TCC in the environmental field, identifying contributors (countries/regions, institutions and authors), influential journals, and constructing a keyword hotspot evolution map to trace the yearly progression of research focal points. The visualization software VOSviewer (version 1.6.11) was employed to create networks of keyword co-occurrence and clustering, thereby visually representing the research content and identifying hotspots in the field. Furthermore, the software CiteSpace (version 6.3. R1) was used to investigate the burst of keywords, providing insights into the research development trends in this field.
3 Results and discussion
3.1 Analysis of publication growth trends
The trend in the number of publications over time highlights the evolving focus within a research field, facilitating a comprehensive understanding of its overall dynamics (Du C. et al., 2024). By searching the WOSCC database for literature pertaining to TCS and TCC in the environmental field, the earliest article identified dated back to 1980. Due to the limited number and lack of representativeness of early publications, this study concentrated on the literature spanning from 2002 to 2024. During this period, the total number of publications related to TCS and TCC research in the environmental field reached 1862, with an upward trend in annual publication output. This can broadly be divided into two stages (Figure 2): (1) From 2002 to 2010, publication activity was relatively subdued, resulting in 213 articles, which accounted for 11.44% of the total number of publications, with an average of 23.67 articles published annually. (2) From 2011 to 2024, the publication landscape witnessed a significant expansion, with a total of 1,649 articles being published, reflecting the increasing global emphasis on ECs.
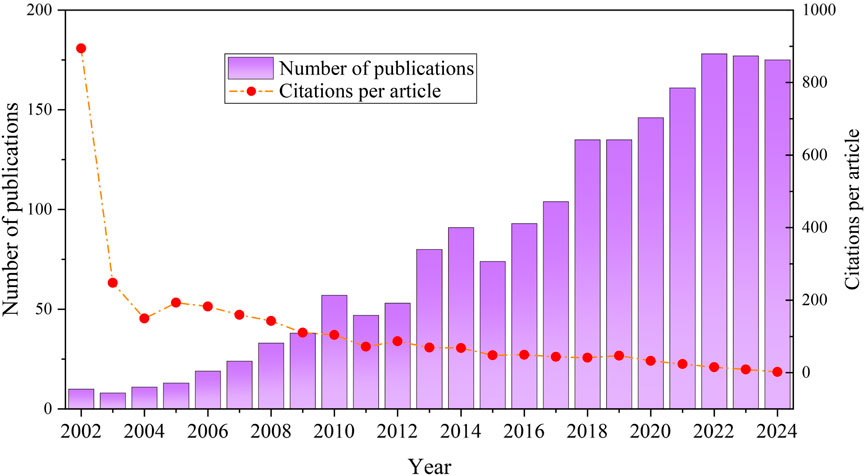
Figure 2. Statistics on the number of publications and citations per article from WOSCC database during 2002–2024.
TCS and TCC were first introduced in the 1950s and 1960s, primarily serving as antimicrobial agents in medical and personal care products. By the 1970s, the demonstrated efficacy of TCS and TCC in inhibiting bacterial growth prompted their expanded use in personal care products, including toothpaste, soap, and hand wash. With the rising application of TCS and TCC in everyday products, studies started to reveal their environmental risks, such as their tendency to persist and accumulate in water bodies, as well as their negative impacts on human health. With increasing recognition of their environmental and health impacts, TCS and TCC faced regulatory and usage constraints in multiple countries. For instance, during the 2010s, the U.S. Food and Drug Administration (FDA) and the European Union initiated reassessments of TCS’s safety and efficacy. In 2016, the FDA issued a regulation that prohibited the use of TCS and 18 other antimicrobial chemicals in over-the-counter antibacterial soaps. Subsequently, in 2017, the FDA further extended these regulations, incorporating restrictions on the application of TCC in various consumer products The promulgation of these policies and standards has also prompted a modest increase in research on TCS and TCC in the environmental field.
3.2 Geographical distribution and collaboration
The number of publications originating from a specific country or region may serve as an indicator of the intensity of research activity in a particular field (Wan et al., 2024). As depicted in Figure 3A, a total of 76 countries/regions have contributed to the literature in this field, with China, the United States, Canada, India, Spain, Germany, South Korea, Brazil, Japan, and Australia ranking as the top 10 publishing countries/regions. Notably, China and the United States together account for 53.65% of the total publications in this field, and these two countries also led in the total number of citations received. Notably, the United States led prominently in this research field, with an average of 91.92 citations per article. This study utilizes the multinational co-publication ratio (MCPR) to measure international research collaboration, revealing that Australia leads with an MCPR of 0.50, followed by Germany at 0.45 and Spain at 0.35, indicating varied levels of global scientific cooperation.
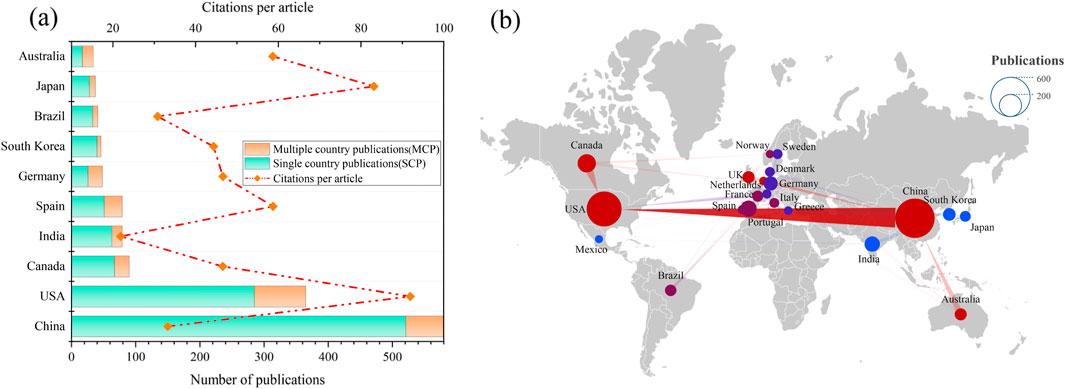
Figure 3. (a) The number of publications and citations per article of countries/regions related to TCS and TCC research in environmental field. (b) Cooperation between the prominent countries/regions.
Figure 3B depicted a network diagram illustrating international collaborations in TCC and TCS research in the environmental field from 2002 to 2024. In the figure, the size of each node corresponds to the publication output of the respective country, while the thickness of the lines denotes a higher number of co-authored publications, indicating more intensive collaboration. This figure illustrates that China, the United States, Canada, Australia, and the United Kingdom maintain notably close collaborative ties with other countries in this research field.
3.3 Analysis of productivity institutions
The publication output of research institutions not only reflects their academic caliber and research capabilities but also indicates their influence and academic dynamism (Li K. et al., 2018). In the field of environmental research related to TCS and TCC, articles have been published by 1951 research institutions. Among the top 10 institutions, seven are based in China, with the remaining three located in the United States as shown in Figure 4. The institutions exhibiting the highest publication outputs in this field include the Chinese Academy of Sciences, the University of Chinese Academy of Sciences, and Nanjing University, with respective publication counts of 119, 40, and 36 publications. Regarding citation metrics, the Chinese Academy of Sciences has accumulated a total of 8,260 citations, demonstrating substantial academic influence. Meanwhile, the Centers for Disease Control and Prevention has achieved 102.36 citations per paper, reflecting a high level of international recognition and scholarly impact.
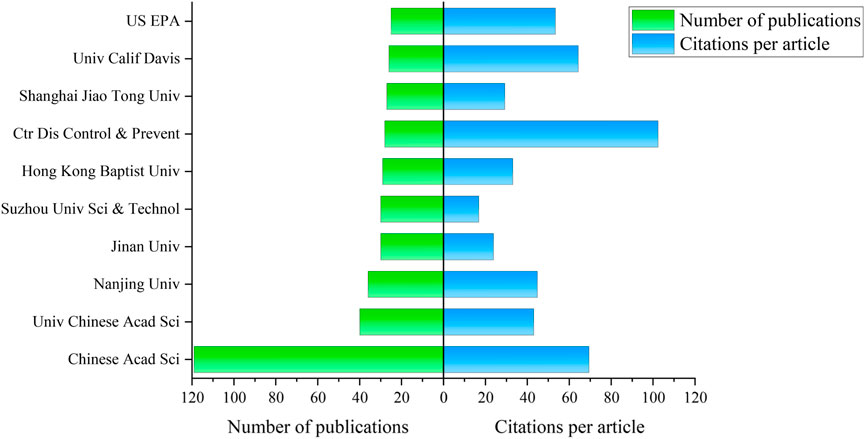
Figure 4. The number of publications and citations per article of institutions related to TCS and TCC research in environmental field.
3.4 Analysis of influential authors
Statistical analysis of literature authors provides a deeper understanding of scholarship distribution within a research field, enabling the identification of core contributors and revealing the principal forces and cutting-edge trends (Li et al., 2022). From 2002 to 2024, a total of 6,904 scholars globally have published research papers related to TCS and TCC in the environmental field. Figure 5 illustrates the top 10 authors by global publication outputs in this field, with Ying GG leading with 35 publications, followed by Calafat AM with 31 publications, and Wang XD with 28 publications. Ying GG’s publications primarily focused on investigating the distribution of TCC and TCS in the environment, alongside their associated environmental risk assessments (Juksu et al., 2019; Yao et al., 2019; Liu et al., 2018). Calafat AM’s research was focused on investigating the exposure of TCS and TCC in humans and their endocrine-disrupting effects (Aker et al., 2019; Ashrap et al., 2018; Bethea et al., 2020). While Wang XD’s research predominantly explored the toxicological effects of TCS and TCC (Wang H. L. et al., 2023; Diao et al., 2023; Wang W. W. et al., 2023). The research conducted by Halden RU, which focus on the environmental aspects and detection methods of TCS and TCC, stands out with an average of 143.17 citations per paper (Adhikari et al., 2022; Chen et al., 2019; Chen J. et al., 2018).
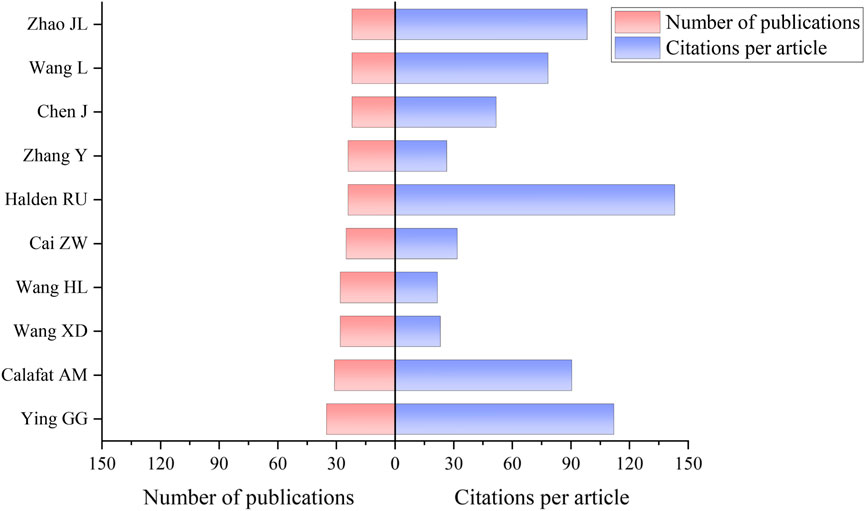
Figure 5. The number of publications and citations per article of authors related to TCS and TCC research in environmental field.
3.5 Analysis of source journals
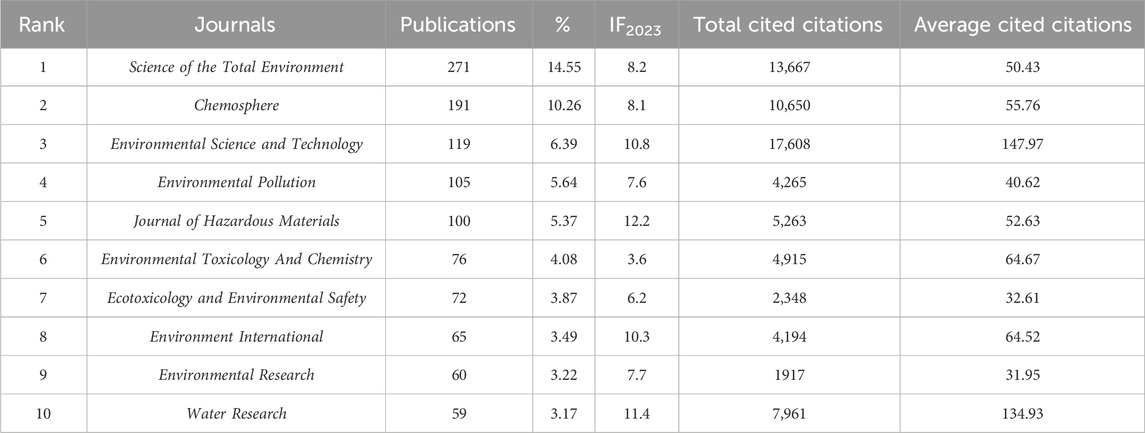
Journals serve as the primary platforms for disseminating scholarly articles and play a pivotal role in accessing the achievements within a specific research field (Du Z. Y. et al., 2024). Analyzing the number of publications within these journals facilitates the rapid identification of core literature and principal authors in the field. A comparative analysis was performed on journals, publication outputs, impact factors, citations, and average citations per paper concerning TCS and TCC research in the environmental sector from 2002 to 2024. The results are presented in Table 2. A total of 154 journals have published research related to TCC and TCS in the environment, with the top 10 journals accounting for 1,118 papers, representing 60.04% of the total number of publications in this field. The journal Science of the Total Environment led with the highest number of published articles, contributing 271 papers, which constitutes 14.55% of the total publications. It is followed by Chemosphere, with 191 publications accounting for 10.26% of the total, and Environmental Science and Technology, ranking third with 119 publications, making up 6.39% of the total. In the analysis of citations, Environmental Science and Technology emerged as the leader with the highest citations, reaching 17,608 citations. It is followed by Science of the Total Environment and Chemosphere, which have garnered 13,667 and 10,650 citations respectively. Evaluating the average citations per paper, Environmental Science and Technology led with an average of 147.97 citations per article, followed by Water Research with 134.93, and Environmental Toxicology And Chemistry with 64.67. This analysis highlights the substantial impact and scholarly contributions of these journals in the environmental research field, particularly in studies related to TCC and TCS.
3.6 Keyword analysis
3.6.1 Evolution trend of keywords
Keywords serve as nouns that encapsulate the core content or essence of a document, enable co-occurrence analysis to reveal predominant research themes and focal points within a field (Huang et al., 2023). Between 2002 and 2024, research on TCC and TCS in the environmental field involved 6,971 keywords. In this dataset, 136 keywords were identified that appeared over 25 times, indicating their high frequency and facilitating the analysis of research hotspots within the field (Figure 6). Beyond keywords directly related to the search topics, such as “triclosan” and “triclocarban,” prominent keywords included “waste water,” “surface waters,” “treatment plants,” “aquatic environment,” and “sewage sludge.” The majority of TCS and TCC from consumer products are introduced into sewer systems and subsequently reach local wastewater treatment plants. A 2015 study revealed that out of China’s annual consumption of approximately 100 tons of TCS, an estimated 66.1 tons were discharged into the environment, with 90.8% entering aquatic systems and the remaining 9.2% being deposited into soil (Zhang et al., 2015). The chemical stability of TCS and TCC complicates their elimination during wastewater treatment processes, as they are readily adsorbed by sewage sludge. Furthermore, the technologies currently employed at wastewater treatment plants rarely target such organic micropollutants, leading to the pervasive presence of TCS and TCC throughout various stages of the treatment process. Sun et al. observed that the average influent concentration of TCS was 53.3 ng ·L−1, while the effluent concentration slightly increased to 56.4 ng·L−1 in a year-long survey. For TCC, the average influent concentration was recorded at 33.8 ng·L−1, with the effluent concentration notably higher at 60.6 ng·L−1. (Sun et al., 2016). Owing to the hydrophobic and lipophilic properties of TCS and TCC, their concentrations were found to be higher in sewage sludge than in primary sludge. Lozano reported that following treatment, the total concentrations of TCS and TCC decreased by more than 97%, with about 79% of TCC and 64% of TCS being transferred to solid phases. Moreover, the highest concentrations of TCS and TCC in primary sludge were recorded at 13.1 ± 0.9 μg g−1 dry weight and 20.3 ± 0.9 μg g−1 dry weight, respectively (Lozano et al., 2013). An EPA study indicated that the concentrations of TCC and TCS in sewage sludge were 36 ± 8 and 12.6 ± 3.8 mg kg−1, respectively (McClellan and Halden, 2010). The frequent appearance of keywords such as “fate,” “toxicity,” “removal,” “exposure,” “degradation,” “risk assessment,” “bioaccumulation,” and “adsorption” highlights the prevailing research focus on the environmental behaviors and fate, toxicological effects and mechanisms, human exposure and risk assessment, and the removal and degradation of TCS and TCC.
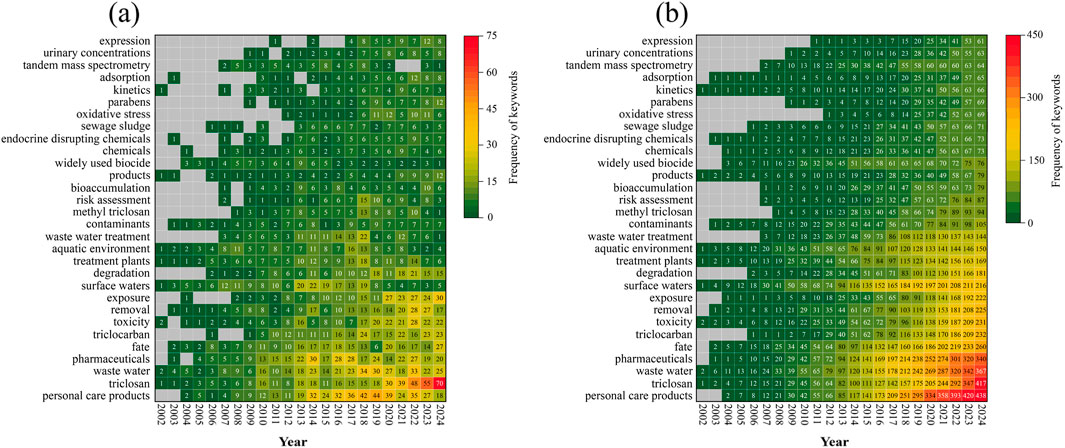
Figure 6. (a) Annual frequency and (b) Cumulative frequency of keywords for TCS and TCC research in the environmental field from 2002 to 2024.
3.6.2 Keywords co-occurrence network analysis
A co-occurrence analysis using VOSviewer software was conducted on thematic keywords found in the corpus, resulting in the production of a clustered visualization map. The VOSviewer software computed inter-keyword correlations and categorized these keywords into 4 distinct clusters, each delineated by different colors: red for Cluster I, green for Cluster II, yellow for Cluster III, and blue for Cluster IV, as depicted in Figure 7.
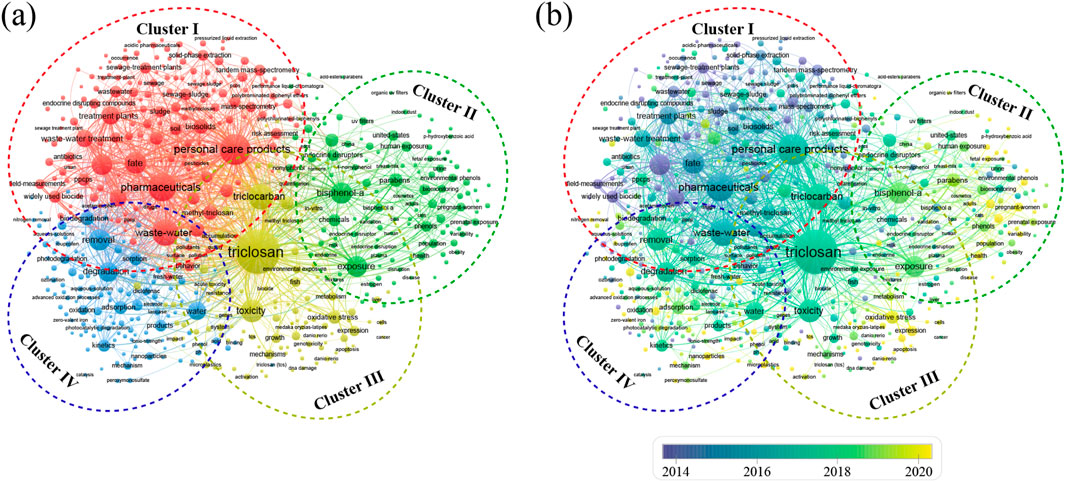
Figure 7. The (a) co-occurring distribution and (b) overlay visualization of keywords related to TCS and TCC research in environment field.
Cluster I primarily includes keywords such as “fate,” “occurrence,” “mass spectrometry,” “tandem mass spectrometry,” and “risk assessment,” focusing predominantly on the environmental behaviors and distributions of TCS and TCC. These compounds predominantly enter the environment through personal care products and other consumer goods. TCS and TCC are predominantly decomposed through photolysis and microbial activity in water environments, but the rate of these processes is considerably slow (Dar et al., 2022). Under the influence of sunlight, TCS and TCC decompose rapidly, primarily through direct photolysis and photocatalytic processes facilitated by ultraviolet light. Photodegradation of these compounds produces byproducts such as smaller organic molecules and free radicals like 2,4-dichlorophenol, despite being potentially less toxic, still pose ongoing threats to environmental and biological health due to their ecotoxicity and persistence (Tohidi and Cai, 2017). In dark environments like groundwater and soil, the decomposition rates of these compounds are extremely slow, leading to their long-term persistence. They can have half-lives that extend for many months or even years, particularly in areas with low levels of biological activity. The environmental behavior of TCS and TCC is influenced by factors such as pH, temperature, and organic matter content. Environmental pH influences their charge states and solubility, impacting adsorption, partitioning, and bioavailability. Temperature variations affect chemical reaction rates and microbial activity, thereby influencing the biodegradation of TCS and TCC. Furthermore, organic matter can modify their behavior by forming complexes or competing for adsorption sites (Ding et al., 2015). However, the migration pathways and transformation mechanisms of TCS and TCC are not fully understood, future research should conduct advanced laboratory and field experiments to simulate their behavior under various environmental conditions. Employing techniques like stable isotope labeling and high-resolution mass spectrometry will allow for detailed tracing of TCS and TCC migration, transport, and accumulation in ecosystems. Additionally, molecular biology and chemical analysis methods are essential to explore their metabolic pathways and identify key degradation products, thereby deepening our understanding of their environmental impacts.
To gain a more comprehensive understanding of these complex interactions and processes, it is crucial to utilize advanced analytical techniques for a detailed investigation of the environmental behaviors of TCS and TCC. Currently, gas chromatography (GC) and liquid chromatography (LC) are the primary methodologies employed for the detection of TCS and TCC. These techniques provide detailed quantitative and qualitative insights into the presence and behavior of these compounds, as well as their metabolic products. Generally, these techniques are enhanced by integrating them with mass spectrometry (MS) to improve their sensitivity and precision in detection, with LC-MS/MS being particularly favored for its exceptional sensitivity and selectivity in identifying TCS and TCC in environmental samples. Researchers are developing biosensors based on enzymes, antibodies, or microbes to facilitate rapid on-site detection of TCS and TCC. For instance, Safwat et al. devised an environmentally friendly potentiometric liquid sensor to monitor and quantify TCS in water samples. This sensor incorporates an internal filling solution modified with hydrophilic 2-hydroxypropyl β-cyclodextrin, which not only enhances its sensitivity but also reduces its response time to a mere 5 s (Safwat et al., 2021). Despite advancements in detecting low concentrations of TCS and TCC, challenges persist in identifying ultra-low levels, highlighting the need for improved sensitivity and selectivity in methods like GC-MS and LC-MS. The preparation of environmental samples, which includes handling sediments, sludge, and complex aquatic matrices, often demands intricate and costly pre-treatment procedures such as solid-liquid extraction and purification. There is a critical need for research into more efficient sample processing techniques that can streamline these steps, reduce costs, and decrease time consumption.
Cluster II primarily includes keywords such as “exposure,” “human exposure,” “pregnant women,” “fetal exposure,” and “population,” focusing on the human exposure and health risks associated with TCS and TCC. These compounds are ubiquitously present in various environmental media and personal care products, and can be absorbed through the gastrointestinal tract, oral mucosa, and skin. Due to the lipophilicity of TCS and TCC, even brief contact can result in its absorption through the skin or oral mucosa in humans and other animals, leading to its accumulation within the body. The half-life of TCS in the human body is approximately 21 h, significantly extending the duration of exposure (Weatherly and Gosse, 2017). The primary route of excretion for TCS and its metabolites is through urine, leading the most detailed investigations into human exposure to TCS to center on analyses of urine. TCS and TCC can be detected in biological samples across all age groups, with higher concentrations observed in the adult population. For instance, a birth cohort study in Sheyang County, Jiangsu Province, during 2009–2012 found that the detection rates of urinary TCS in 377 mothers and their 3-year-old children were 100% and 99.5%, respectively, with median concentrations of 0.65 and 0.44 μg·L−1 (Guo et al., 2020). Furthermore, the levels of exposure to TCS and TCC vary internationally. A study investigating the concentrations of TCS and TCC in the urine of populations from seven Asian countries (China, India, South Korea, Kuwait, Japan, Saudi Arabia, and Vietnam), as well as Greece and the United States, revealed that the overall geometric mean concentrations of TCS and TCC were 1.36 and 0.03 ng mL−1, respectively. Notably, the average concentration of TCS in Chinese urine was the highest at 100 ng mL−1, while the lowest was found in Vietnamese urine at 2.34 ng mL−1 (Iyer et al., 2018). The presence of TCS and TCC in the human body is extensive, with significant levels not only in urine but also in critical tissues such as the liver, adipose tissue, and brain (Geens et al., 2012).
Research into the toxicity of TCS and TCC has progressively deepened, extending from aquatic organisms and invertebrates to mammals and human cells, with a particular focus on their health impacts on humans. Studies indicated that TCS and TCC may disrupt human hormonal systems, particularly the function of thyroid hormones, potentially affecting metabolic processes and development. For instance, Wang et al. found a significant negative correlation between maternal urinary TCS levels and fetal umbilical blood FT3, as well as maternal blood FT4 concentrations during late pregnancy (Wang et al., 2017). Geens et al. observed a negative correlation between urinary TCS levels and serum FT4 among overweight and obese women who either commenced a diet plan or underwent bariatric surgery, measured at baseline and 3 months post-intervention (Geens et al., 2015). Furthermore, research is investigating the potential associations between exposure to TCS and TCC and the incidence of chronic diseases, including obesity, diabetes, and cancer. For instance, Hu et al. conducted a study on a cohort of 423 children aged 7 years from Laizhou Bay, Shandong Province, and discovered that higher levels of TCS were associated with an approximately two to threefold increase in the risk of abdominal obesity (Hu et al., 2022). Xie et al. investigated whether exposure to TCS and TCC was correlated with impaired glucose tolerance (IGT) and type 2 diabetes mellitus (T2DM), discovering a significant positive correlation between TCC exposure and T2DM in female participants (Xie et al., 2020). Ouyang et al. investigated the relationship between maternal urinary TCS levels and the risk of gestational diabetes mellitus (GDM), as well as its impact on infant birth weight. Their findings suggest that the widespread exposure to TCS is associated with an increased risk of GDM and higher birth weight in female infants, indicating a gender-specific correlation (Ouyang et al., 2018). Despite the increasing amount of research indicating potential associations between TCS, TCC, and chronic diseases, the current evidence is still limited, and the exact biological mechanisms have yet to be fully elucidated. Future studies are essential to further explore how TCS and TCC interact with metabolic and endocrine pathways, with a particular focus on clarifying their mechanisms of action in human health.
Cluster III primarily includes keywords such as “toxicity,” “environmental exposure,” “genes,” “growth,” “DNA damage,” “expression,” “metabolism,” “acute toxicity,” “mechanisms,” and “in vitro,” focusing on the ecotoxicological effects and toxicological mechanisms of TCS and TCC. The toxicity of TCS and TCC is manifested across acute, subacute, and chronic levels. The acute toxicity of TCS and TCC is particularly evident in its impacts on aquatic organisms, including fish, crustaceans, and algae. Studies have demonstrated that TCS and TCC can induce toxic effects (such as inhibition of bioluminescence, growth suppression, and mortality) within a short duration (typically between 48 and 96 h). For instance, in the luminescent bacterium Vibrio fischeri, the median lethal concentration (LC50) or median effective concentration (EC50) of TCS can range from micrograms to milligrams per liter, indicating significant acute toxicity. Research on the protozoan Tetrahymena thermophila revealed that the 24-hour LC50 was 1,063 μg·L−1 for TCS and 295 μg·L−1 for TCC (Gao et al., 2015). Similarly, in studies involving the Artemia salina, the LC50 were 171.1 μg·L−1 for TCS and 17.8 μg·L−1 for TCC (Xu et al., 2015). By conducting acute toxicity experiments, researchers can quickly determine the lethal or impactful levels of TCS and TCC for certain aquatic organisms, thus aiding in the assessment of their potential risks to water-based life forms. For instance, Farré et al. investigated the toxicological impacts of assorted mixtures of TCS, methyl triclosan, and surfactants in diverse aquatic environments (including milli-Q water, groundwater, and wastewater), with V. fischeri serving as the experimental organism. The findings revealed that TCS is a predominant toxic organic pollutant in domestic wastewater (Farré et al., 2008). Carmosini et al. studied the impact of TCS on zebrafish in environments with varying DOC levels, finding that DOC enhances survival and reduces toxicity (Carmosini et al., 2016).
Research on the subacute toxicity of TCS and TCC examines their extended effects, ranging from several days to weeks, on biological organisms, including disruptions to the endocrine system and impacts on reproductive and developmental processes. TCS and TCC which are compounds that incorporate chlorine atoms within their chemical structures share similarities with other environmentally significant compounds such as polybrominated diphenyl ethers (PBDEs), polychlorinated biphenyls (PCBs), dioxins, bisphenol A, synthetic estrogens, and thyroid hormones. Classified as organic pollutants, these substances predominantly possess potential endocrine-disrupting properties, capable of mimicking or interfering with the hormonal functions within organisms (Halden et al., 2017). Research has indicated that TCS can disrupt endocrine functions in various species, including fish, amphibians, and mammals, particularly affecting thyroid hormones. For instance, Martínez-Paz et al. discovered that exposure to TCS altered the transcriptional activity of endocrine-related genes in Chironomus riparius aquatic larvae, such as the ecdysone receptor gene (EcR), ultraspiracle gene (usp), estrogen-related receptor gene (ERR), and the early ecdysone-induced gene E74 (Martínez-Paz et al., 2017). TCS and TCC have been found to negatively impact the reproductive systems of fish, amphibians, and other aquatic species, leading to decreased reproductive success, hindered development of reproductive organs, and reduced fecundity. Qiao et al. exposed adult zebrafish to TCS concentrations of 2, 20, and 200 μg·L−1 for 150 days, observing that while fertilization rates remained stable at 2 and 20 μg·L−1, hatching rates significantly decreased across all concentrations. Notably, at 200 μg·L−1, both fertilization and hatching rates in female fish declined significantly (Qiao et al., 2022). Furthermore, TCS and TCC exposure contributes to early developmental defects, such as malformations in embryos, lowered rates of hatching, and impeded growth. Liu et al. found that TCS induced oxidative stress in zebrafish embryos and led to cell apoptosis through the ROS-p53-caspase-dependent apoptotic pathway (Liu F. et al., 2022).
Chronic toxicity studies of TCS and TCC focus on the long-term effects (ranging from several months to years) of exposure on organisms. Long-term exposure to these compounds has been shown to decrease immune system function, slow growth rates, and reduce reproductive capabilities in certain species. Furthermore, the bioaccumulative properties of TCS and TCC enable their progression and amplification up the food chain, representing a sustained risk to ecological systems. For instance, Peng et al. observed that after 7 days of exposure to 3.1 μg of TCS or sediment containing 3.1 μg g−1 dry weight, TCS accumulation in Lumbriculus variegatus reached equilibrium, achieving a biota-sediment accumulation factor (BSAF) of 2.07 (Peng et al., 2018). Researchers have demonstrated that compounds such as TCS and TCC possess the potential to cause DNA damage, exhibiting pronounced genotoxic effects. Specifically, these substances can compromise the structural integrity of cell membranes, which interferes with normal cellular functions and can lead to cell death. Additionally, TCS and TCC may prompt the production of reactive oxygen species (ROS), thereby initiating oxidative stress responses that result in cellular structural damage and DNA degradation. For instance, Gyimah observed that after 30 days of exposure to 50, 100, and 150 μg·L−1 TCS, the levels of superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx) significantly decreased in adult zebrafish brain and liver tissues (Gyimah et al., 2020). Despite considerable research revealing the influences of TCS and TCC on endocrine and reproductive health, and development, the detailed molecular mechanisms, specific targets, and long-term effects on ecosystems are yet to be fully determined. Further investigations could leverage molecular biology and ecotoxicological approaches to better understand the specific ways TCS and TCC interfere with the endocrine and reproductive systems of aquatic organisms, particularly through receptor binding, signaling pathways, and the regulation of gene expression, and their effects on hormonal processes.
TCS and TCC can facilitate the dissemination of ARGs through multiple mechanisms. Initially, environmental exposure to TCS and TCC enhances bacterial resistance to these compounds as well as to other antibiotics, primarily by increasing the levels of ARGs and facilitating the horizontal transfer of resistance plasmids. Additionally, TCS and TCC promote the survival and proliferation of bacteria that harbor ARGs through selective pressure, thereby expediting the accumulation and dissemination of ARGs in both natural and clinical environments. The spread of ARGs in bacterial communities often happens through HGT, with bacteria acquiring these ARGs through transformation, transduction, or conjugation (Jiao et al., 2017). The presence of TCS and TCC may facilitate this HGT by increasing the selective pressure for ARGs within bacterial populations. For instance, Cen et al. found that exposure to TCC significantly increased the conjugative transfer rate of plasmids by 2.17–4.31 times. The facilitation of HGT of ARGs was primarily driven by the stimulation of RpoS regulation and the SOS response induced by free radicals, increased cell membrane permeability, and the control of genes linked to conjugative transfer. (Cen et al., 2020). For instance, Lu et al. demonstrated that at concentrations between 0.02 and 20 μg·L−1, TCS significantly enhanced the transfer of multidrug resistance genes across bacterial genera. This effect was mediated by increased production of reactive oxygen species, damage to bacterial membranes, and upregulation of SOS response genes umuC, dinB, and dinD in donor bacteria (Lu et al., 2018). The specific molecular mechanisms through which TCS and TCC influence the dissemination of ARGs under different environmental conditions are not yet fully understood. Current research primarily focuses on short-term effects in laboratory settings and lacks long-term, field-based studies. Moreover, investigations have concentrated on specific bacterial types, offering limited insight into ARG dynamics within complex microbial communities. Future research should explore both the molecular and ecological processes through which TCS and TCC affect the spread of ARGs, with a focus on long-term consequences and a wider range of microbial species to comprehensively evaluate their impact on ARG distribution within ecosystems.
Cluster IV primarily includes keywords such as “degradation,” “adsorption,” “removal,” “biodegradation,” “photodegradation,” “kinetics,” “nanoparticles,” and “advanced oxidation processes.” This cluster primarily focuses on the degradation and removal technologies for TCS and TCC. The prevailing methodologies for the degradation and removal of TCS and TCC encompass biodegradation, photocatalysis, adsorption, and advanced oxidation techniques. The biological removal of TCS and TCC is primarily dependent on the degradative capabilities of particular bacterial and fungal strains. Studies have demonstrated that bacteria belonging to the genus Sphingomonas can break down TCS, transforming it into less harmful, smaller molecules through distinct metabolic processes. For instance, Kim et al. conducted comparative studies on the degradation of TCS by three strains of Sphingomonas. Although all three strains were capable of degrading biphenyl and diphenyl ether, which are structurally akin to TCS, only the strain Sphingomonas sp. PH-07 was shown to possess the capability to degrade TCS. However, its efficacy was limited, achieving a reduction of TCS from 10 to 7.5 mg·L−1 over an 8-day incubation period (Kim et al., 2011). Mulla et al. reported that strain YL-JM2C degraded up to 35% of TCC (4 mg·L−1) within 5 days, while also achieving degradation rates of 77% for 3,4-dichloroaniline and 80% for 4-chloroaniline, both starting at concentrations of 4 mg·L−1 (Mulla et al., 2016). Several studies have demonstrated the capability of bacteria from the genus Pseudomonas to effectively degrade specific compounds. For instance, Kumari et al. isolated a wastewater microbe, Pseudomonas aeruginosa KS 2002, which was capable of transforming TCS into 2,4-dichlorophenol within a period of 96 h (Kumari and Sachan, 2019). Additionally, white-rot fungi such as Pycnoporus and Trametes have demonstrated significant biodegradation potential for TCS by secreting a diverse array of efficient enzymes (such as laccases and peroxidases), that effectively disrupt the molecule’s structural integrity (Hundt et al., 2000). But due to the toxic or inhibitory impacts of TCS and TCC on the majority of microbes, the effectiveness of bacterial or fungal degradation tends to be low, thereby restricting the practical applications of these methods. Future research should persist in identifying microbes from environmental samples that can efficiently biodegrade TCS and TCC. By employing transcriptomics and proteomics, researchers can deeply explore the metabolic pathways and breakdown processes of these microbes, shedding light on the biochemical mechanisms that enable them to degrade TCS and TCC. For instance, Yin et al. utilized various biochemical and molecular biology methods to elucidate the molecular mechanisms involved in the biodegradation of TCS, facilitated by a newly identified Rieske non-heme iron-dependent dioxygenase (TcsAB) (Yin et al., 2024). Furthermore, the application of advanced genetic engineering technologies, such as the CRISPR-Cas9 gene editing system and plasmid-mediated gene transduction techniques, could significantly enhance the degradation efficiency of microbial strains.
Biological methods are widely recognized as environmentally benign and cost-effective, especially for treating large volumes of wastewater. Commonly employed biological processes in wastewater treatment facilities encompass the rotating biological contactor, trickling filter, and activated sludge processes. Thompson et al. investigated the removal efficiency of TCS through three distinct processes, finding that the removal efficiencies varied with retention time. Specifically, rotating biological contactors achieved removal efficiencies ranging from 58% to 96%, trickling filters from 95% to 98%, and activated sludge processes from 86% to 97% (Thompson et al., 2005). The degradation of TCS and TCC is influenced by various factors including the species of microorganisms, microbial diversity, environmental pH, temperature, oxygen supply, nutrient availability, presence of organic matter, initial concentrations, and cometabolic activities. For instance, Armstrong et al. demonstrated that the removal efficiency of TCS and TCC in activated sludge processes can be enhanced by increasing the hydraulic retention time (HRT), solid retention time (SRT), and temperature (Armstrong et al., 2018). The application of biofilm reactors in treating wastewater containing TCS and TCC has demonstrated high removal efficiencies, owing to their complex 3-dimensional structure that facilitates the coexistence of diverse microorganisms to degrade specific compounds. For instance, Chtourou reported that the ultrafiltration membrane bioreactor process achieved a TCS removal efficiency of 89.7% ± 8.3% (Chtourou et al., 2018). Navrozidou et al. found that bioreactor operation led to a removal rate of over 99.5% for TCS in wastewater (Navrozidou et al., 2023). Despite the effectiveness of biofilm reactors in removing TCS and TCC, optimizing operational conditions such as hydraulic retention time, membrane pore size, biofilm thickness, and nutrient supply remains crucial. Additionally, the common issue of membrane fouling necessitates frequent maintenance to ensure system efficiency.
Due to their considerable logKow, TCS and TCC exhibit significant hydrophobicity, even in their ionized forms. This characteristic suggests a strong propensity for these compounds to bind to solid or particulate surfaces. Activated carbon and clay minerals (including montmorillonite and kaolinite, etc.), have traditionally served as adsorbents for the removal of TCS and TCC. For instance, Behera et al. conducted a study on the adsorption of TCS onto activated carbon, kaolinite, and montmorillonite, analyzing the effects of pH, ionic strength, and humic acid concentration on this process. They found that TCS adsorption was enhanced in acidic conditions due to changes in the surface charge of the adsorbents and the ionization of TCS, and noted that increasing ionic strength also improved adsorption capacity (Behera et al., 2010). Ong et al. found that bentonite modified with the cationic surfactant dodecyltrimethylammonium bromide (DTAB) significantly enhanced the removal efficiency of TCS (Ong et al., 2014). Both activated carbon and clay minerals effectively remove TCS and TCC from environments, but activated carbon is more expensive to maintain due to saturation and the need for frequent regeneration, while clay minerals are limited by their lower adsorption abilities and specific selectivity. Produced from organic sources like agricultural waste and forestry residues through pyrolysis without oxygen, biochar is an effective adsorbent due to its porous structure, expansive surface area, and rich functional groups. Due to its porous structure, extensive surface area, and numerous functional groups, along with its cost-effectiveness, biochar outperforms activated carbon and clay minerals in the adsorption of TCS and TCC (Sashidhar et al., 2020). For instance, Cho et al. discovered that the adsorption capacity of kenaf-derived biochar for TCS varied with pyrolysis temperature, showing a lower capacity at 450°C. The study revealed that biochar, produced at 750°C and featuring enriched aromatic groups along with an expanded surface area, showed a markedly higher adsorption rate, peaking at 77.4 mg g−1 (Cho et al., 2021). The enhancement of adsorbents’ surface properties through methods such as chemical or physical modifications, the addition of functional groups can greatly improve their ability to selectively and effectively bind TCS and TCC. For instance, Philip et al. produced biochar from empty palm bunches (PEB) at temperatures between 250°C and 750°C. To enhance its adsorption capacity, the biochar created at 450°C (PEBC450) was treated with H2SO4, which showed a maximum adsorption capacity for TCS of 35.4 mg g−1 (Philip and Choudhary, 2022). During real-world wastewater treatment processes, various organic and inorganic materials are present along with TCS and TCC, which may compete for the same adsorption sites. To improve the selectivity of adsorbents for TCS and TCC, techniques such as adding specific functional groups and developing materials with porous structures, including mesoporous silica and modified clays, are used to greatly enhance their affinity, capacity, and selectivity for these substances. Furthermore, a comprehensive understanding of the adsorption mechanisms, encompassing both kinetics and thermodynamics, along with an exploration of the molecular interactions involved, such as van der Waals forces, hydrogen bonding, and electrostatic interactions, is essential for the design of more efficient adsorption systems. Lastly, adsorbents reach saturation with continuous use, significantly reducing their removal efficiency, necessitating the development of effective regeneration techniques such as thermal desorption, chemical regeneration, and biological methods to restore their activity.
Advanced oxidation processes (AOPs) constitute a powerful water treatment technology that utilizes catalysts activated by physical and chemical forces such as sonic, photonic, electric, and magnetic influences to generate highly reactive hydroxyl radicals (·OH), which effectively attack the chemical bonds of contaminants, facilitating their rapid mineralization into harmless smaller molecules such as water and carbon dioxide. Currently, the primary advanced oxidation technologies employed for degrading TCS and TCC include ozonation, electrochemical oxidation, sonochemical oxidation, Fenton and Fenton-like oxidations, and photocatalytic oxidation (Luo et al., 2019). Among these methods, Fenton and Fenton-like oxidations, as well as photocatalytic oxidation, have shown considerable potential for treating TCS and TCC. The Fenton reaction utilizes iron salts (typically Fe2+) and hydrogen peroxide (H2O2) under acidic conditions to generate hydroxyl radicals, which are highly reactive and effective in attacking and mineralizing TCS and TCC. Fenton-like reactions involve the use of alternative transition metals (e.g., copper, manganese) or modified reaction conditions to enhance the applicability and efficiency of the process (Singh and Kaur, 2023). Recent research has concentrated on enhancing the environmental adaptability and degradation efficiency of Fenton and Fenton-like reactions. For instance, optimization of these reactions has been achieved by carefully adjusting parameters such as pH, temperature, and the stoichiometric ratio of reactants. Studies on Fenton-like reactions have explored the potential of various metal ions and their effective operation across a broader pH range. Furthermore, significant research has been conducted on the development of immobilized and composite catalysts, aimed at enhancing catalyst stability and reusability. For instance, So et al. investigated the effects of factors such as the concentration of Fe3O4, persulfate (PMS), adsorption capacity, pH, and catalyst surface charge on the heterogeneous Fenton degradation of TCS. The system achieved optimal degradation at a TCS to PMS ratio of 1:25, with Fe3O4 concentrations ranging from 0.125 to 0.750 g L−1 enhancing performance linearly (So et al., 2019). Despite the promising results demonstrated by Fenton and Fenton-like technologies at the laboratory scale, several challenges hinder their practical application. Traditional Fenton reactions necessitate operation under acidic conditions, which constrains their use in various real-world wastewater treatments. Moreover, the effective recovery and reuse of catalysts are essential for economically viable water treatment systems. Future research should focus on developing novel catalysts that operate effectively across a broader pH spectrum and exhibit enhanced stability and recyclability. Additionally, optimizing reaction conditions through systems engineering approaches and integrating Fenton and Fenton-like technologies with other treatment methods, such as biological processes and adsorption, will improve the efficiency and sustainability of these systems.
Photocatalytic degradation of TCS relies on semiconductor materials like titanium dioxide (TiO2), which produce photo-induced electrons and holes under illumination. These electrons and holes generate reactive oxygen species, such as hydroxyl radicals, capable of disrupting the chemical structure of TCS or TCC (Solá-Gutiérrez et al., 2020). Current research is primarily centered on enhancing the activity, stability, and light utilization efficiency of photocatalysts. By employing techniques such as doping, creating composite materials, or modifying surfaces, researchers have successfully developed various modified titanium dioxide (TiO2) and other non-TiO2 based materials, including zinc oxide (ZnO) and cadmium sulfide (CdS). These advancements aim to broaden the light response range and enhance photocatalytic efficiency. Furthermore, recent studies have employed complex semiconductor architectures, such as Z-scheme and S-scheme configurations, to facilitate effective electron-hole pair separation and significantly enhance photocatalytic performance by improving charge carrier dynamics. For instance, Liu et al. developed hierarchically porous TiO2 using peanut shells and synthesized TiO2/BiFeO3 composites with varying doping levels. These catalysts, with a surface area of 153.64 m2 g−1 and a 1.92 eV bandgap, achieved an 81.2% degradation rate at a doping ratio of 1 mol mol−1 (Liu G. et al., 2022). Li et al. developed a ternary Ag/BiVO4 reduced graphene oxide (rGO) photocatalyst via hydrothermal synthesis, which completely removed TCS under visible light within 100 min at a concentration of 1 mg mL−1. The inclusion of Ag and rGO enhanced photocatalytic efficiency by promoting charge separation and reducing electron-hole recombination (Li et al., 2019). Photocatalytic oxidation degradation technology stands out among advanced oxidation processes due to its rapid kinetics, strong oxidative capacity, and high efficiency in removing TCS and TCC from water. However, it faces challenges like low light utilization and recovery rates. Future research should focus on developing innovative photocatalytic materials that improve light absorption and catalytic activity through nanotechnology and surface modifications. Additionally, enhancing the stability, safety, and sunlight utilization of photocatalysts in real-world conditions is crucial. There is also a need to develop integrated systems and optimize reactor designs to increase the scalability and economic viability of photocatalytic processes, thereby expanding their applications in wastewater treatment.
3.6.3 Keywords burst analysis
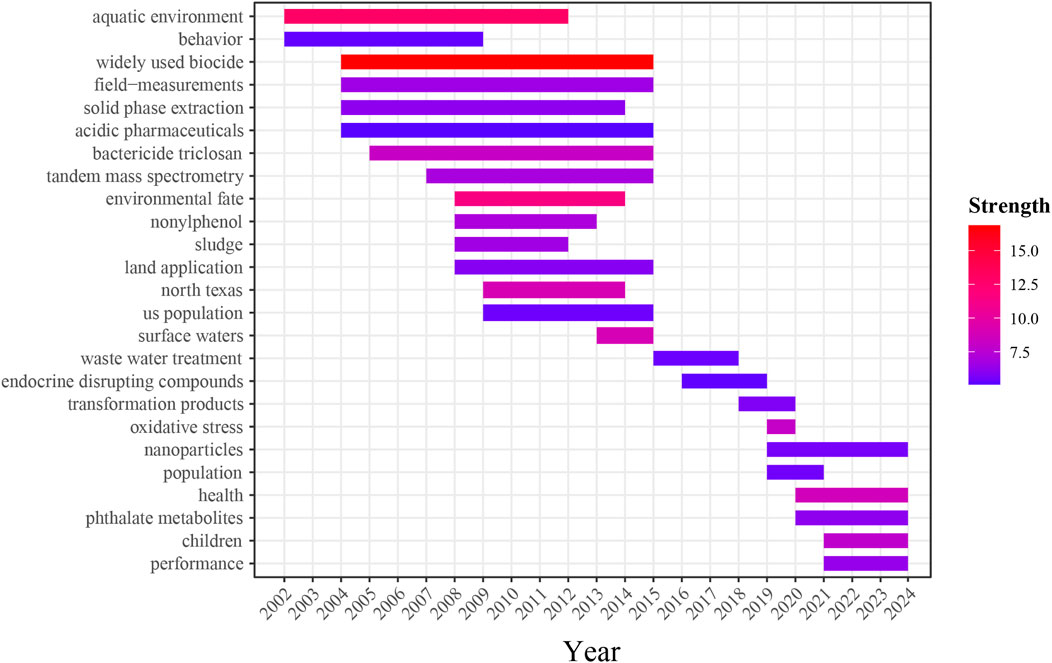
Keyword burst denotes terms that emerge as focal points of research within a specific period, acting as indicators of future trends and hotspots in scholarly inquiry (Li J. et al., 2018). By analyzing burst keywords based on their strength, the top 25 keywords with the highest burst strength were selected, as depicted in Figure 8. The distribution of these burst keywords can be categorized into two distinct stages:
(1) 2002–2015: During this period, keywords such as “behavior,” “solid phase extraction,” “tandem mass spectrometry,” and “environmental fate” exhibited high burst strength, indicating that research primarily focused on the environmental distribution, behavior, and fate of TCS and TCC. Additionally, keywords such as “sludge” and “land application” also demonstrated high burst strength. Due to their hydrophobic properties, TCS and TCC enter wastewater treatment plants through sewage after usage, where they predominantly accumulate in the sewage sludge generated during the treatment process. When sewage sludge is applied in land use, the residual TCS and TCC within it are likely to undergo adsorption and subsequently accumulate in the soil. Research by Langdon et al., has observed that during the land application of sewage sludge, concentrations of 4-nonylphenol, 4-tert-octylphenol, and bisphenol A tend to decrease according to field data, while the concentration of TCS does not show a significant decline (Langdon et al., 2012). The environmental residues of TCS and TCC can adversely affect soil ecosystems, potentially reducing the diversity and activity of soil microbes, diminishing soil fertility, and impairing crop growth. Furthermore, the accumulation of TCS and TCC in the soil may be absorbed by crops and enter the food chain, thereby posing risks to human and animal health (Higgins et al., 2011).
(2) Between 2016 and 2024, keywords such as “oxidative stress,” “population,” “health,” and “children” have demonstrated high burst strength. This trend indicated that research has primarily focused on assessing the risks associated with human exposure to TCS and TCC, particularly among vulnerable groups including children and pregnant women.
4 Future challenges and prospects
4.1 Combined toxicity and mechanisms of toxicity
The synergistic and antagonistic toxicities of TCS and TCC in conjunction with other pollutants constitute a critical field of research. Environmental pollutants usually coexist with other chemicals, rather than existing in isolation. This co-occurrence can lead to interactions that are either synergistic or antagonistic, influencing their combined ecological toxicity and associated health risks. For instance, Zhang et al. found that for 120 hpf zebrafish, the LC50 and EC50 for TCS, 2,4,6-trichlorophenol (2,4-TCP), and 2,4-dichlorophenol (2,4-DCP) were 0.51, 1.11, 2.45 mg·L−1 and 0.36, 0.74, 1.53 mg·L−1, respectively. When the concentration ratio of TCS:2,4,6-TCP:2,4-DCP was set at 1:2:4, the toxicity of TCS, 2,4,6-TCP, and 2,4-DCP peaked, and two toxicity assessment methods (toxicity units and mixed toxicity index) indicated that the interactions among these chemicals produced a partially additive toxicity (Zhang et al., 2018). Recent studies have explored the combined toxicity of TCS and TCC with other pollutants, such as heavy metals (Ding et al., 2023), perfluorooctanesulfonic acid (PFOS) (González-Doncel et al., 2020), and microplastics (MPs) (Sheng et al., 2021). MPs have garnered significant attention in recent years due to concerns regarding their combined toxicity with TCS and TCC. Defined as plastic particles with diameters less than 5 mm, MPs can adsorb organic pollutants such as TCS and TCC. Their adsorption to MPs may enhance the environmental stability and persistence of these compounds, thus extending their environmental impact. Additionally, the adsorption of TCS and TCC onto MPs may modify their bioavailability in the environment. This process can potentially prevent the release of TCS and TCC into aquatic environments, thereby mitigating their direct toxicity to aquatic organisms. For instance, Zhu et al. studied the combined effects of TCS and 4 MPs (PE, PS, PVC, and PVC800) on diatoms. They found that TCS notably hindered microalgal growth, with the inhibition strongest in PVC800, followed by PVC, PS, and PE (Zhu et al., 2019). Secondly, organisms that ingest MPs may experience an increased toxic burden if TCS desorbs and releases internally. For instance, Rubin employed the TrOCs model, focusing on TCS, and primary MPs such as polystyrene microbeads, to investigate their combined toxic effects. The study found that functionalizing the surface of polystyrene microbeads significantly increased their TCS adsorption capacity, from 2.3 mg g−1 on non-functionalized beads to 4.6 and 6.1 mg g−1 on amino and carboxy-functionalized beads, respectively. Toxicity tests revealed that TCS adsorbed on MPs was an order of magnitude more toxic than low concentrations of TCS and MPs individually on Caco-2 cells (Rubin and Zucker, 2022).
While the toxic effects of combined exposures to TCS and TCC with other pollutants have been documented, the precise toxicological mechanisms driving these interactions remain poorly understood. This gap in knowledge presents significant challenges for accurately assessing and mitigating the risks associated with such complex contaminations. Moreover, the majority of existing studies are based on in vitro experiments or animal models, which may not fully translate to human health risk assessments. To address these limitations, it is essential that future research incorporates more epidemiological studies to assess the real-world impacts of combined exposures to TCS, TCC, and other pollutants on human health, with a particular focus on long-term effects. Such an approach is crucial for developing a thorough understanding of the potential health risks posed by these chemical interactions in practical scenarios.
4.2 Advanced degradation technologies and materials
Among the various strategies to mitigate pollution from TCS and TCC, biodegradation stands out as an environmentally benign option. However, its efficacy is markedly influenced by environmental conditions, including pH, temperature, and the availability of nutrients. This method is also characterized by a relatively slow rate of degradation. Adsorption method provides a cost-effective and straightforward method but often necessitates frequent replacement of adsorbents and proper disposal of the spent materials to avoid environmental complications. AOPs such as Fenton and photo-Fenton reactions, along with photocatalysis, can effectively mineralize TCS and TCC, but are associated with high operational costs and the potential generation of secondary pollutants (Sun et al., 2023). Considering the widespread occurrence of TCS and TCC in the environment and their associated risks, it is crucial to explore more sophisticated treatment materials and technologies.
The development of innovative catalytic materials for photocatalysis and AOPs significantly enhances the efficiency and practicality of these technologies. By exploring advanced semiconductor materials, metal composites, or doping techniques can enhance catalysts’ light absorption and utilization efficiency, thereby increasing pollutant degradation rates while reducing by-product formation. For instance, Arifin et al. explored the characteristics and effectiveness of zeolite-modified TiO2 nanotubes as composite photocatalysts (MTNZC) for degrading TCC in aqueous solutions. They achieved photocatalytic degradation rates of 94.2% in the first cycle and 77.4% in the fifth cycle, demonstrating that MTNZC can be effectively reused multiple times (Arifin et al., 2022). Furthermore, advancements in nanotechnology and materials science are facilitating the development of adsorbents characterized by higher adsorption capacities, enhanced regenerative abilities, and extended lifespans. For instance, functionalized nanomaterials or porous materials can be engineered with specific surface modifications to enhance the adsorption of TCS and TCC, while simultaneously facilitating their ease of regeneration and reuse. For instance, Vidovix et al. utilized iron oxide-functionalized soy hulls (shbhf) and acai seed fibers (AcSF) to adsorb TCS, finding that at 318 K, the maximum adsorption capacities were 158.35 mg g−1 for shbhf and 155.09 mg g−1 for AcSF, respectively (Vidovix et al., 2022).
Beyond the aforementioned methods for processing TCS and TCC, electrocatalytic and photoelectrocatalytic technologies have demonstrated considerable potential in the degradation of these contaminants. Electrochemical oxidation is a technique that utilizes an electric current to generate oxidants through electrodes and has been shown to effectively degrade TCS and TCC. Recent research has concentrated on optimizing electrode materials and operational parameters to enhance both energy efficiency and degradation efficacy. For instance, Zhou et al. prepared Mn/Fe@porous carbon (PC) by simply carbonizing a Mn-doped MIL-53 (Fe) precursor and utilized it as a cathode modification material in the heterogeneous electro-Fenton (hetero-EF) process for degrading TCS. The results demonstrated complete degradation of TCS within 120 min, under conditions of a 40 mA current and an initial pH of 3 (Zhou et al., 2020). Photoelectrocatalysis combines the benefits of photocatalysis and electrocatalysis and efficiently degrade TCS and TCC under environmentally friendly conditions by harnessing light energy to stimulate electrochemical reactions on the electrodes. For instance, Zhang et al. developed a highly efficient photoelectrocatalytic system using dual-Pd/TNAs photoelectrodes that achieved a removal efficiency of 99.77% for TCS under specific conditions of pH 8, a voltage of 3.0 V, and an electrolyte concentration of 1.0 mol·L−1 (Zhang et al., 2020). Furthermore, integrating multiple treatment technologies such as biodegradation with adsorption or photocatalysis could offer a more comprehensive and efficient solution. These systems capitalize on the strengths of each method while addressing their individual limitations during the treatment process. For instance, Jagini et al. investigated the removal efficiency of TCS utilizing nano zero-valent iron (nZVI), multi-walled carbon nanotubes (MWCNTs), and a carbon filter. At a TCS concentration of 5 ppm and dosages of 0.1 g·L−1, nZVI and MWCNTs achieved removal rates of 97.07% and 100%, respectively, within 40 and 20 min (Jagini et al., 2021).
5 Conclusion
This study focused on the scholarly review of TCS and TCC in the environmental field, encompassing literature from the WOSCC database spanning the years 2002–2024. Utilizing bibliometric tools such as Bibliometrix, VOSviewer, and Citespace, the analyses provided insights into the leading authors, influential journals, prolific institutions, and countries/regions, and co-occurrence and burst analysis of keywords. Based on these analyses, several principal conclusions were drawn.
(1) From 2002 to 2024, the number of literature related to TCS and TCC in the environmental field has shown an overall upward trend. Publications predominantly published in journals related to environmental science and toxicology, such as Science of the Total Environment, Chemosphere, and Environmental Science and Technology. China led in publication output, with its research institutions playing a pivotal role, thereby indicating a significant influence in this field. Ying GG, Calafat AM, and Wang XD rank as the top three most prolific authors in this field, with each focusing on distinct aspects of the research.
(2) Based on keyword co-occurrence and burst analyses, current research in environmental field pertaining to TCS and TCC primarily focus on environmental distribution and behavior, ecological risk assessments, biotoxicity evaluations, and degradation and removal techniques. Future research must develop novel, efficient, and eco-friendly materials and technologies for removing TCS and TCC. Additionally, it is crucial to investigate their synergistic toxic effects with other pollutants (such as MPs) and explore the implications at molecular, cellular, and ecosystem levels for human health and ecological safety.
Author contributions
JH: Data curation, Formal Analysis, Funding acquisition, Methodology, Writing – original draft. HC: Formal Analysis, Software, Validation, Visualization, Writing – original draft. ZR: Data curation, Writing – original draft. XZ: Data curation, Writing – original draft. ZS: Software, Writing – original draft. QW: Conceptualization, Writing – review and editing. ZL: Conceptualization, Methodology, Software, Validation, Visualization, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adhikari, S., Kumar, R., Driver, E. M., Perleberg, T. D., Yanez, A., Johnston, B., et al. (2022). Mass trends of parabens, triclocarban and triclosan in Arizona wastewater collected after the 2017 FDA ban on antimicrobials and during the COVID-19 pandemic. Water Res. 222, 118894. doi:10.1016/j.watres.2022.118894
Aker, A. M., Ferguson, K. K., Rosario, Z. Y., Mukherjee, B., Alshawabkeh, A. N., Calafat, A. M., et al. (2019). A repeated measures study of phenol, paraben and triclocarban urinary biomarkers and circulating maternal hormones during gestation in the Puerto Rico PROTECT cohort. Environ. Health 18, 28–13. doi:10.1186/s12940-019-0459-5
Aria, M., and Cuccurullo, C. (2017). Bibliometrix: an R-tool for comprehensive science mapping analysis. J. Informetr. 11, 959–975. doi:10.1016/j.joi.2017.08.007
Arifin, S. N. H., Mohamed, R., Al-Gheethi, A. A., Wei, L. C., Yashni, G., Fitriani, N., et al. (2022). Modified TiO2 nanotubes-zeolite composite photocatalyst: characteristics, microstructure and applicability for degrading triclocarban. Chemosphere 287, 132278. doi:10.1016/j.chemosphere.2021.132278
Armstrong, D. L., Lozano, N., Rice, C. P., Ramirez, M., and Torrents, A. (2018). Degradation of triclosan and triclocarban and formation of transformation products in activated sludge using benchtop bioreactors. Environ. Res. 161, 17–25. doi:10.1016/j.envres.2017.10.048
Ashrap, P., Watkins, D. J., Calafat, A. M., Ye, X. Y., Rosario, Z., Brown, P., et al. (2018). Elevated concentrations of urinary triclocarban, phenol and paraben among pregnant women in Northern Puerto Rico: predictors and trends. Environ. Int. 121, 990–1002. doi:10.1016/j.envint.2018.08.020
Babaei, S., Reguyal, F., and Sarmah, A. K. (2024). A bibliometric analysis of global research hotspots and progress on emerging environmental pollutants 6PPD and 6PPD-quinone from 2004 to 2024. Environ. Pollut. 362, 124969. doi:10.1016/j.envpol.2024.124969
Barrett, H., Sun, J. X., Gong, Y. F., Yang, P., Hao, C. Y., Verreault, J., et al. (2022). Triclosan is the predominant antibacterial compound in Ontario sewage sludge. Environ. Sci. Technol. 56, 14923–14936. doi:10.1021/acs.est.2c00406
Behera, S. K., Oh, S. Y., and Park, H. S. (2010). Sorption of triclosan onto activated carbon, kaolinite and montmorillonite: effects of pH, ionic strength, and humic acid. J. Hazard Mater. 179, 684–691. doi:10.1016/j.jhazmat.2010.03.056
Bethea, T. N., Wesselink, A. K., Weuve, J., McClean, M. D., Hauser, R., Williams, P. L., et al. (2020). Correlates of exposure to phenols, parabens, and triclocarban in the study of environment, lifestyle and fibroids. J. Expo. Sci. Environ. Epidemiol. 30, 117–136. doi:10.1038/s41370-019-0114-9
Carmosini, N., Grandstrand, S., and King-Heiden, T. C. (2016). Developmental toxicity of triclosan in the presence of dissolved organic carbon: moving beyond standard acute toxicity assays to understand ecotoxicological risk. Zebrafish 13, 424–431. doi:10.1089/zeb.2015.1220
Cen, T. Y., Zhang, X. Y., Xie, S. S., and Li, D. (2020). Preservatives accelerate the horizontal transfer of plasmid-mediated antimicrobial resistance genes via differential mechanisms. Environ. Int. 138, 105544. doi:10.1016/j.envint.2020.105544
Chen, C. M. (2006). CiteSpace II: detecting and visualizing emerging trends and transient patterns in scientific literature. J. Am. Soc. Inf. Sci. Technol. 57, 359–377. doi:10.1002/asi.20317
Chen, J., Hartmann, E. M., Kline, J., Van den Wymelenberg, K., and Halden, R. U. (2018a). Assessment of human exposure to triclocarban, triclosan and five parabens in US indoor dust using dispersive solid phase extraction followed by liquid chromatography tandem mass spectrometry. J. Hazard Mater. 360, 623–630. doi:10.1016/j.jhazmat.2018.08.014
Chen, J., Meng, X. Z., Bergman, A., and Halden, R. U. (2019). Nationwide reconnaissance of five parabens, triclosan, triclocarban and its transformation products in sewage sludge from China. J. Hazard Mater. 365, 502–510. doi:10.1016/j.jhazmat.2018.11.021
Chen, Z. F., Wen, H. B., Dai, X. X., Yan, S. C., Zhang, H., Chen, Y. Y., et al. (2018b). Contamination and risk profiles of triclosan and triclocarban in sediments from a less urbanized region in China. J. Hazard Mater. 357, 376–383. doi:10.1016/j.jhazmat.2018.06.020
Chi, Z. H., Liu, L., Zheng, J. Y., Tian, L., Chevrier, J., Bornman, R., et al. (2024). Biomonitoring of bisphenol A (BPA) and bisphenol analogues in human milk from South Africa and Canada using a modified QuEChERS extraction method. Environ. Pollut. 348, 123730. doi:10.1016/j.envpol.2024.123730
Cho, E. J., Kang, J. K., Moon, J. K., Um, B. H., Lee, C. G., Jeong, S., et al. (2021). Removal of triclosan from aqueous solution via adsorption by kenaf-derived biochar: its adsorption mechanism study via spectroscopic and experimental approaches. J. Environ. Chem. Eng. 9, 106343. doi:10.1016/j.jece.2021.106343
Chtourou, M., Mallek, M., Dalmau, M., Mamo, J., Santos-Clotas, E., Ben Salah, A., et al. (2018). Triclosan, carbamazepine and caffeine removal by activated sludge system focusing on membrane bioreactor. Process Saf. Environ. Prot. 118, 1–9. doi:10.1016/j.psep.2018.06.019
Dar, O. I., Raouf, A., Deng, P., Sunil, S., Megha, A., Kaur, A., et al. (2022). Source, bioaccumulation, degradability and toxicity of triclosan in aquatic environments: a review. Environ. Technol. Innov. 25, 102122. doi:10.1016/j.eti.2021.102122
Diao, W. Q., Yan, J., Wang, X. D., Qian, Q. H., and Wang, H. L. (2023). Mechanisms regarding cardiac toxicity triggered by up-regulation of miR-144 in larval zebrafish upon exposure to triclosan. J. Hazard Mater. 443, 130297. doi:10.1016/j.jhazmat.2022.130297
Ding, S. L., Wang, X. K., Jiang, W. Q., Zhao, R. S., Shen, T. T., Wang, C., et al. (2015). Influence of pH, inorganic anions, and dissolved organic matter on the photolysis of antimicrobial triclocarban in aqueous systems under simulated sunlight irradiation. Environ. Sci. Pollut. R. 22, 5204–5211. doi:10.1007/s11356-014-3686-x
Ding, T. D., Lin, K. D., Bao, L. J., Yang, M. T., Li, J. Y., Yang, B., et al. (2018). Biouptake, toxicity and biotransformation of triclosan in diatom Cymbella sp and the influence of humic acid. Environ. Pollut. 234, 231–242. doi:10.1016/j.envpol.2017.11.051
Ding, T. D., Wei, L. Y., Yue, Z. M., Lin, S. Q., and Li, J. Y. (2023). Individual and combined toxicity of silver nanoparticles and triclosan or galaxolide in the freshwater algae Euglena sp. Sci. Total Environ. 887, 164139. doi:10.1016/j.scitotenv.2023.164139
Du, C., Yu, Y. X., and Fan, X. Y. (2024a). Analysis of research trends (2014-2023) on oxidative stress and male fertility based on bibliometrics and knowledge graphs. Front. Endocrinol. 15, 1326402. doi:10.3389/fendo.2024.1326402
Du, Z. Y., Ruan, Y. Y., Chen, J. B., Fang, J., Xiao, S., Shi, Y. W., et al. (2024b). Global trends and hotspots in research on the health risks of organophosphate flame retardants: a bibliometric and visual analysis. Toxics 12, 391. doi:10.3390/toxics12060391
Farré, M., Asperger, D., Kantiani, L., González, S., Petrovic, M., and Barceló, D. (2008). Assessment of the acute toxicity of triclosan and methyl triclosan in wastewater based on the bioluminescence inhibition of Vibrio fischeri. Anal. Bioanal. Chem. 390, 1999–2007. doi:10.1007/s00216-007-1779-9
Fu, Q. G., Sanganyado, E., Ye, Q. F., and Gan, J. (2016). Meta-analysis of biosolid effects on persistence of triclosan and triclocarban in soil. Environ. Pollut. 210, 137–144. doi:10.1016/j.envpol.2015.12.003
Gao, L., Yuan, T., Cheng, P., Bai, Q. F., Zhou, C. Q., Ao, J. J., et al. (2015). Effects of triclosan and triclocarban on the growth inhibition, cell viability, genotoxicity and multixenobiotic resistance responses of Tetrahymena thermophila. Chemosphere 139, 434–440. doi:10.1016/j.chemosphere.2015.07.059
Geens, T., Dirtu, A. C., Dirinck, E., Malarvannan, G., Van Gaal, L., Jorens, P. G., et al. (2015). Daily intake of bisphenol A and triclosan and their association with anthropometric data, thyroid hormones and weight loss in overweight and obese individuals. Environ. Int. 76, 98–105. doi:10.1016/j.envint.2014.12.003
Geens, T., Neels, H., and Covaci, A. (2012). Distribution of bisphenol-A, triclosan and n-nonylphenol in human adipose tissue, liver and brain. Chemosphere 87, 796–802. doi:10.1016/j.chemosphere.2012.01.002
González-Doncel, M., Torija, C. F., Pablos, M. V., Hortigüela, P. G., Arévalo, M. L., and Beltrán, E. M. (2020). The role of PFOS on triclosan toxicity to two model freshwater organisms. Environ. Pollut. 263, 114604. doi:10.1016/j.envpol.2020.114604
Guo, J. Q., Wu, C. H., Zhang, J. M., Xiao, H. X., Lv, S. L., Lu, D. H., et al. (2020). Early life triclosan exposure and neurodevelopment of children at 3 years in a prospective birth cohort. Int. J. Hyg. Environ. Health. 224, 113427. doi:10.1016/j.ijheh.2019.113427
Gyimah, E., Dong, X., Qiu, W. H., Zhang, Z., and Xu, H. (2020). Sublethal concentrations of triclosan elicited oxidative stress, DNA damage, and histological alterations in the liver and brain of adult zebrafish. Environ. Sci. Pollut. R. 27, 17329–17338. doi:10.1007/s11356-020-08232-2
Halden, R. U., Lindeman, A. E., Aiello, A. E., Andrews, D., Arnold, W. A., Fair, P., et al. (2017). The florence statement on Triclosan and Triclocarban. Environ. Health Perspect. 125, 064501. doi:10.1289/EHP1788
Higgins, C. P., Paesani, Z. J., Chalew, T. E. A., Halden, R. U., and Hundal, L. S. (2011). Persistence of triclocarban and triclosan in soils after land application of biosolids and bioaccumulation in Eisenia foetida. Environ. Toxicol. Chem. 30, 556–563. doi:10.1002/etc.416
Hu, Y., Ding, G. D., Lv, C., Zhang, Q. L., Zhang, Y., Yuan, T., et al. (2022). Association between triclosan exposure and obesity measures among 7-year-old children in northern China. Ecotoxicol. Environ. Saf. 239, 113610. doi:10.1016/j.ecoenv.2022.113610
Huang, H., Zhao, W. J., Qin, N., and Duan, X. L. (2024). Recent progress on physiologically based pharmacokinetic (PBPK) model: a review based on bibliometrics. Toxics 12, 433. doi:10.3390/toxics12060433
Huang, X. F., Zheng, J. Q., Ma, Y., Hou, M. J., and Wang, X. B. (2023). Analysis of emerging trends and hot spots in respiratory biomechanics from 2003 to 2022 based on CiteSpace. Front. Physiol. 14, 1190155. doi:10.3389/fphys.2023.1190155
Hundt, K., Martin, D., Hammer, E., Jonas, U., Kindermann, M. K., and Schauer, F. (2000). Transformation of triclosan by Trametes versicolor and Pycnoporus cinnabarinus. Appl. Environ. Microbiol. 66, 4157–4160. doi:10.1128/AEM.66.9.4157-4160.2000
Iyer, A. P., Xue, J. C., Honda, M., Robinson, M., Kumosani, T. A., Abulnaja, K., et al. (2018). Urinary levels of triclosan and triclocarban in several Asian countries, Greece and the USA: association with oxidative stress. Environ. Res. 160, 91–96. doi:10.1016/j.envres.2017.09.021
Jagini, S., Thaduri, S., Konda, S., Saranga, V. K., Dheeravath, B., and Vurimindi, H. (2021). Emerging contaminant (Triclosan) removal by adsorption and oxidation process: comparative study. Model. Earth Syst. Environ. 7, 2431–2438. doi:10.1007/s40808-020-01020-4
Jiao, Y. N., Chen, H., Gao, R. X., Zhu, Y. G., and Rensing, C. (2017). Organic compounds stimulate horizontal transfer of antibiotic resistance genes in mixed wastewater treatment systems. Chemosphere 184, 53–61. doi:10.1016/j.chemosphere.2017.05.149
Juksu, K., Zhao, J. L., Liu, Y. S., Yao, L., Sarin, C., Sreesai, S., et al. (2019). Occurrence, fate and risk assessment of biocides in wastewater treatment plants and aquatic environments in Thailand. Sci. Total Environ. 690, 1110–1119. doi:10.1016/j.scitotenv.2019.07.097
Kim, Y. M., Murugesan, K., Schmidt, S., Bokare, V., Jeon, J. R., Kim, E. J., et al. (2011). Triclosan susceptibility and co-metabolism - a comparison for three aerobic pollutant-degrading bacteria. Bioresour. Technol. 102, 2206–2212. doi:10.1016/j.biortech.2010.10.009
Kumari, R., and Sachan, S. G. (2019). Bioconversion of toxic micropollutant triclosan to 2,4-dichlorophenol using a wastewater isolate Pseudomonas aeruginosa KS2002. Int. J. Environ. Sci. Technol. 16, 7663–7672. doi:10.1007/s13762-018-2129-5
Langdon, K. A., Warne, M. S., Smernik, R. J., Shareef, A., and Kookana, R. S. (2012). Field dissipation of 4-nonylphenol, 4-t-octylphenol, triclosan and bisphenol A following land application of biosolids. Chemosphere 86, 1050–1058. doi:10.1016/j.chemosphere.2011.11.057
Li, A. J., Zhuang, T. F., Zhu, Q. Q., Song, M. Y., Liao, C. Y., and Jiang, G. B. (2020). Concentration and distribution of parabens, triclosan, and triclocarban in pregnant woman serum in China. Sci. Total Environ. 710, 136390. doi:10.1016/j.scitotenv.2019.136390
Li, J., Wang, Y., and Yan, B. B. (2018a). The hotspots of life cycle assessment for bioenergy: a review by social network analysis. Sci. Total Environ. 625, 1301–1308. doi:10.1016/j.scitotenv.2018.01.030
Li, K., Rollins, J., and Yan, E. (2018b). Web of Science use in published research and review papers 1997-2017: a selective, dynamic, cross-domain, content-based analysis. Scientometrics 115, 1–20. doi:10.1007/s11192-017-2622-5
Li, M., Wang, Y., Shen, Z. F., Chi, M. S., Lv, C., Li, C. Y., et al. (2022). RETRACTED: investigation on the evolution of hydrothermal biochar. Chemosphere 307, 135774. doi:10.1016/j.chemosphere.2022.135774
Li, M., Xu, G. H., Guan, Z. Y., Wang, Y., Yu, H. W., and Yu, Y. (2019). Synthesis of Ag/BiVO4/rGO composite with enhanced photocatalytic degradation of triclosan. Sci. Total Environ. 664, 230–239. doi:10.1016/j.scitotenv.2019.02.027
Liu, F., Zhang, Y., and Wang, F. (2022a). Environmental relevant concentrations of triclosan affected developmental toxicity, oxidative stress, and apoptosis in zebrafish embryos. Environ. Toxicol. 37, 848–857. doi:10.1002/tox.23448
Liu, G., Lin, Y. Z., Li, S. W., Shi, C. Y., and Zhang, D. H. (2022b). Mechanism and efficiency of photocatalytic triclosan degradation by TiO2/BiFeO3 nanomaterials. Water Sci. Technol. 86, 3133–3152. doi:10.2166/wst.2022.397
Liu, W. R., Yang, Y. Y., Liu, Y. S., Zhao, J. L., Zhang, Q. Q., Yao, L., et al. (2018). Biocides in the river system of a highly urbanized region: a systematic investigation involving runoff input. Sci. Total Environ. 624, 1023–1030. doi:10.1016/j.scitotenv.2017.12.225
Lozano, N., Rice, C. P., Ramirez, M., and Torrents, A. (2013). Fate of Triclocarban, Triclosan and Methyltriclosan during wastewater and biosolids treatment processes. Water Res. 47, 4519–4527. doi:10.1016/j.watres.2013.05.015
Lu, J., Wang, Y., Li, J., Mao, L. K., Nguyen, S. H., Duarte, T., et al. (2018). Triclosan at environmentally relevant concentrations promotes horizontal transfer of multidrug resistance genes within and across bacterial genera. Environ. Int. 121, 1217–1226. doi:10.1016/j.envint.2018.10.040
Lu, J., Wang, Y., Zhang, S., Bond, P., Yuan, Z. G., and Guo, J. H. (2020). Triclosan at environmental concentrations can enhance the spread of extracellular antibiotic resistance genes through transformation. Sci. Total Environ. 713, 136621. doi:10.1016/j.scitotenv.2020.136621
Luo, Z. R., He, Y. Z., Zhi, D., Luo, L., Sun, Y. Q., Khan, E., et al. (2019). Current progress in treatment techniques of triclosan from wastewater: a review. Sci. Total Environ. 696, 133990. doi:10.1016/j.scitotenv.2019.133990
Martínez-Paz, P., Morales, M., Urien, J., Morcillo, G., and Martínez-Guitarte, J. L. (2017). Endocrine-related genes are altered by antibacterial agent triclosan in Chironomus riparius aquatic larvae. Ecotoxicol. Environ. Saf. 140, 185–190. doi:10.1016/j.ecoenv.2017.02.047
McClellan, K., and Halden, R. U. (2010). Pharmaceuticals and personal care products in archived US biosolids from the 2001 EPA national sewage sludge survey. Water Res. 44, 658–668. doi:10.1016/j.watres.2009.12.032
Mulla, S. I., Hu, A. Y., Wang, Y. W., Sun, Q., Huang, S. L., Wang, H., et al. (2016). Degradation of triclocarban by a triclosan-degrading Sphingomonas sp strain YL-JM2C. Chemosphere 144, 292–296. doi:10.1016/j.chemosphere.2015.08.034
Navrozidou, E., Remmas, N., Melidis, P., Sylaios, G., and Ntougias, S. (2023). Biotreatment efficiency, degradation mechanism and bacterial community structure in an immobilized cell bioreactor treating triclosan-rich wastewater. Environ. Technol. 44, 1518–1529. doi:10.1080/09593330.2021.2007287
Odinga, E. S., Zhou, X., Mbao, E. O., Ali, Q., Waigi, M. G., Shiraku, M. L., et al. (2022). Distribution, ecological fate, and risks of steroid estrogens in environmental matrices. Chemosphere 308, 136370. doi:10.1016/j.chemosphere.2022.136370
Olisah, C., Malloum, A., Adegoke, K. A., Ighalo, J. O., Conradie, J., Ohoro, C. R., et al. (2024). Scientometric trends and knowledge maps of global polychlorinated naphthalenes research over the past four decades. Environ. Pollut. 357, 124407. doi:10.1016/j.envpol.2024.124407
Ong, L. K., Soetaredjo, F. E., Kurniawan, A., Ayucitra, A., Liu, J. C., and Ismadji, S. (2014). Investigation on the montmorillonite adsorption of biocidal compounds incorporating thermodynamical-based multicomponent adsorption isotherm. Chem. Eng. J. 241, 9–18. doi:10.1016/j.cej.2013.12.001
Ouyang, F. X., Tang, N., Zhang, H. J., Wang, X., Zhao, S. S., Wang, W. Y., et al. (2018). Maternal urinary triclosan level, gestational diabetes mellitus and birth weight in Chinese women. Sci. Total Environ. 626, 451–457. doi:10.1016/j.scitotenv.2018.01.102
Peng, F. J., Ying, G. G., Pan, C. G., Selck, H., Salvito, D., and Van den Brink, P. J. (2018). Bioaccumulation and biotransformation of Triclosan and Galaxolide in the freshwater Oligochaete Limnodrilus hoffmeisteri in a water/sediment microcosm. Environ. Sci. Technol. 52, 8390–8398. doi:10.1021/acs.est.8b02637
Philip, L., and Choudhary, V. (2022). Sustainability assessment of acid-modified biochar as adsorbent for the removal of pharmaceuticals and personal care products from secondary treated wastewater. J. Environ. Chem. Eng. 10, 107592. doi:10.1016/j.jece.2022.107592
Qiao, Y. J., He, J. Y., Han, P., Qu, J. B., Wang, X. B., and Wang, J. (2022). Long-term exposure to environmental relevant triclosan induces reproductive toxicity on adult zebrafish and its potential mechanism. Sci. Total Environ. 826, 154026. doi:10.1016/j.scitotenv.2022.154026
Rozaini, H., Saad, B., Lim, J. W., Yahaya, N., Ramachandran, M. R., Ridzuan, N. D. M., et al. (2022). Competitive removal mechanism to simultaneously incarcerate bisphenol A, triclosan and 4-tert-octylphenol within beta-cyclodextrin crosslinked citric acid used for encapsulation in polypropylene membrane protected-micro-solid-phase extraction. Chemosphere 309, 136626. doi:10.1016/j.chemosphere.2022.136626
Rubin, A. E., and Zucker, I. (2022). Interactions of microplastics and organic compounds in aquatic environments: a case study of augmented joint toxicity. Chemosphere 289, 133212. doi:10.1016/j.chemosphere.2021.133212
Safwat, N., Mahmoud, A. M., Abdel-Ghany, M. F., and Ayad, M. F. (2021). In situ monitoring of triclosan in environmental water with subnanomolar detection limits using eco-friendly electrochemical sensors modified with cyclodextrins. Environ. Sci.-Process Impacts 23, 457–466. doi:10.1039/d0em00387e
Sashidhar, P., Kochar, M., Singh, B., Gupta, M., Cahill, D., Adholeya, A., et al. (2020). Biochar for delivery of agri-inputs: current status and future perspectives. Sci. Total Environ. 703, 134892. doi:10.1016/j.scitotenv.2019.134892
Sheng, C., Zhang, S. H., and Zhang, Y. (2021). The influence of different polymer types of microplastics on adsorption, accumulation, and toxicity of triclosan in zebrafish. J. Hazard Mater. 402, 123733. doi:10.1016/j.jhazmat.2020.123733
Singh, A., and Kaur, I. (2023). Remediation of triclosan contaminated water - a comprehensive reprint. J. Water Process Eng. 55, 104149. doi:10.1016/j.jwpe.2023.104149
So, H. L., Lin, K. Y., and Chu, W. (2019). Triclosan removal by heterogeneous Fenton-like process: studying the kinetics and surface chemistry of Fe3O4 as catalyst. J. Environ. Chem. Eng. 7, 103432. doi:10.1016/j.jece.2019.103432
Solá-Gutiérrez, C., Schröder, S., San-Román, M. F., and Ortiz, I. (2020). Critical review on the mechanistic photolytic and photocatalytic degradation of triclosan. J. Environ. Manage. 260, 110101. doi:10.1016/j.jenvman.2020.110101
Sun, C., Zhang, T., Zhou, Y., Liu, Z. F., Zhang, Y., Bian, Y., et al. (2023). Triclosan and related compounds in the environment: recent updates on sources, fates, distribution, analytical extraction, analysis, and removal techniques. Sci. Total Environ. 870, 161885. doi:10.1016/j.scitotenv.2023.161885
Sun, Q., Li, M. Y., Ma, C., Chen, X. Q., Xie, X. Q., and Yu, C. P. (2016). Seasonal and spatial variations of PPCP occurrence, removal and mass loading in three wastewater treatment plants located in different urbanization areas in Xiamen, China. Environ. Pollut. 208, 371–381. doi:10.1016/j.envpol.2015.10.003
Thompson, A., Griffin, P., Stuetz, R., and Cartmell, E. (2005). The fate and removal of triclosan during wastewater treatment. Water Environ. Res. 77, 63–67. doi:10.2175/106143005X41636
Tkalec, Z., Kosjek, T., Tratnik, J. S., Stajnko, A., Runkel, A. A., Sykiotou, M., et al. (2021). Exposure of Slovenian children and adolescents to bisphenols, parabens and triclosan: urinary levels, exposure patterns, determinants of exposure and susceptibility. Environ. Int. 146, 106172. doi:10.1016/j.envint.2020.106172
Tohidi, F., and Cai, Z. W. (2017). Fate and mass balance of triclosan and its degradation products: comparison of three different types of wastewater treatments and aerobic/anaerobic sludge digestion. J. Hazard Mater. 323, 329–340. doi:10.1016/j.jhazmat.2016.04.034
van Eck, N. J., and Waltman, L. (2010). Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 84, 523–538. doi:10.1007/s11192-009-0146-3
van Wijnen, J., Ragas, A. M. J., and Kroeze, C. (2018). River export of triclosan from land to sea: a global modelling approach. Sci. Total Environ. 621, 1280–1288. doi:10.1016/j.scitotenv.2017.10.100
Vidovix, T. B., Januário, E. F. D., Araújo, M. F., Bergamasco, R., and Vieira, A. M. S. (2022). Investigation of two new low-cost adsorbents functionalized with magnetic nanoparticles for the efficient removal of triclosan and a synthetic mixture. Environ. Sci. Pollut. R. 29, 46813–46829. doi:10.1007/s11356-022-19187-x
Vimalkumar, K., Seethappan, S., and Pugazhendhi, A. (2019). Fate of Triclocarban (TCC) in aquatic and terrestrial systems and human exposure. Chemosphere 230, 201–209. doi:10.1016/j.chemosphere.2019.04.145
Wan, Y. T., Ding, J. N., Jia, Z. X., Hong, Y. H., Tian, G. J., Zheng, S. Q., et al. (2024). Current trends and research topics regarding organoids: a bibliometric analysis of global research from 2000 to 2023. Heliyon 10, e32965. doi:10.1016/j.heliyon.2024.e32965
Wang, H. L., Li, X., Wang, W. W., Xu, J. Q., Ai, W. M., Huang, H. S., et al. (2023a). Immunotoxicity induced by triclocarban exposure in zebrafish triggering the risk of pancreatic cancer. Environ. Pollut. 325, 121458. doi:10.1016/j.envpol.2023.121458
Wang, W. W., Li, X., Qian, Q. H., Yan, J., Huang, H. S., Wang, X. D., et al. (2023b). Mechanistic exploration on neurodevelopmental toxicity induced by upregulation of alkbh5 targeted by triclosan exposure to larval zebrafish. J. Hazard Mater. 457, 131831. doi:10.1016/j.jhazmat.2023.131831
Wang, X., Ouyang, F. X., Feng, L. P., Wang, X., Liu, Z. W., and Zhang, J. (2017). Maternal urinary triclosan concentration in relation to maternal and neonatal thyroid hormone levels: a prospective study. Environ. Health Perspect. 125, 067017. doi:10.1289/EHP500
Wang, Z. Y., Li, X., Li, Y., Liu, H., Lin, C. S. K., Sun, J., et al. (2024). Unveiling the occurrence and ecological risks of triclosan in surface water through meta-analysis. Environ. Pollut. 361, 124901. doi:10.1016/j.envpol.2024.124901
Weatherly, L. M., and Gosse, J. A. (2017). Triclosan exposure, transformation, and human health effects. J. Toxicol. Env. Health-Pt B-brit. Rev. 20, 447–469. doi:10.1080/10937404.2017.1399306
Xie, X., Lu, C. Y., Wu, M., Liang, J. Y., Ying, Y. T., Liu, K. L., et al. (2020). Association between triclocarban and triclosan exposures and the risks of type 2 diabetes mellitus and impaired glucose tolerance in the National Health and Nutrition Examination Survey (NHANES 2013-2014). Environ. Int. 136, 105445. doi:10.1016/j.envint.2019.105445
Xu, X. L., Lu, Y., Zhang, D. Y., Wang, Y. Y., Zhou, X. S., Xu, H. Y., et al. (2015). Toxic assessment of triclosan and triclocarban on Artemia salina. Bull. Environ. Contam. Toxicol. 95, 728–733. doi:10.1007/s00128-015-1641-2
Yao, L., Lv, Y. Z., Zhang, L. J., Liu, W. R., Zhao, J. L., Yang, Y. Y., et al. (2019). Bioaccumulation and risks of 24 personal care products in plasma of wild fish from the Yangtze River, China. Sci. Total Environ. 665, 810–819. doi:10.1016/j.scitotenv.2019.02.176
Yemele, O. M., Zhao, Z. H., Nkoh, J. N., Ymele, E., and Usman, M. (2024). A systematic review of polycyclic aromatic hydrocarbon pollution: a combined bibliometric and mechanistic analysis of research trend toward an environmentally friendly solution. Sci. Total Environ. 926, 171577. doi:10.1016/j.scitotenv.2024.171577
Yin, Y. R., Ren, H., Wu, H., and Lu, Z. M. (2024). Triclosan dioxygenase: a novel two-component rieske nonheme iron ring-hydroxylating dioxygenase initiates triclosan degradation. Environ. Sci. Technol. 58, 13833–13844. doi:10.1021/acs.est.4c02845
Zhang, Q. Q., Ying, G. G., Chen, Z. F., Zhao, J. L., and Liu, Y. S. (2015). Basin-scale emission and multimedia fate of triclosan in whole China. Environ. Sci. Pollut. R. 22, 10130–10143. doi:10.1007/s11356-015-4218-z
Zhang, X. Y., Nengzi, L. C., Li, B., Liu, L. C., and Cheng, X. W. (2020). Design and construction of a highly efficient photoelectrocatalytic system based on dual-Pd/TNAs photoelectrodes for elimination of triclosan. Sep. Purif. Technol. 235, 116232. doi:10.1016/j.seppur.2019.116232
Zhang, Y. H., Liu, M., Liu, J. F., Wang, X. D., Wang, C. H., Ai, W. M., et al. (2018). Combined toxicity of triclosan, 2,4-dichlorophenol and 2,4,6-trichlorophenol to zebrafish (Danio rerio). Environ. Toxicol. Pharmacol. 57, 9–18. doi:10.1016/j.etap.2017.11.006
Zhou, X. L., Xu, D., Chen, Y. C., and Hu, Y. Y. (2020). Enhanced degradation of triclosan in heterogeneous E-Fenton process with MOF-derived hierarchical Mn/Fe@PC modified cathode. Chem. Eng. J. 384, 123324. doi:10.1016/j.cej.2019.123324
Keywords: triclosan, triclocarban, toxicity, scientometric analysis, degradation, environmental risk
Citation: Han J, Chen H, Ren Z, Zhang X, Shao Z, Wei Q and Li Z (2025) Global progress and prospects of triclosan and triclocarban research in the environmental field: a bibliometric analysis and review. Front. Environ. Sci. 13:1545699. doi: 10.3389/fenvs.2025.1545699
Received: 15 December 2024; Accepted: 20 March 2025;
Published: 23 May 2025.
Edited by:
Nsikak U. Benson, Topfaith University, NigeriaReviewed by:
Elena Sezenna, Polytechnic University of Milan, ItalyMengjing Wang, University of California, San Francisco, United States
Copyright © 2025 Han, Chen, Ren, Zhang, Shao, Wei and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Qiuhua Wei, d2VpcWl1aHVhQHllYWgubmV0; Zhonghong Li, cGxfbGl6aGhAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jie Han1†
Jie Han1† Zhonghong Li
Zhonghong Li