- 1Division of Soil Science and Agricultural Chemistry, ICAR-Indian Agricultural Research Institute, New Delhi, India
- 2Division of Soil Conservation, ICAR-Indian Institute of Soil and Water Conservation, Dehradun, Uttarakhand, India
- 3Division of Crop Production, ICAR-Indian Institute of Maize Research, New Delhi, India
- 4Division of Grassland and Silvipasture Management, ICAR-Indian Grassland and Fodder Research Institute, Jhansi, Uttar Pradesh, India
- 5College of Life Sciences, Northwest Normal University, Lanzhou, China
- 6Plant Production Department, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia
- 7State Key Laboratory of Desert and Oasis Ecology, Key Laboratory of Ecological Safety and Sustainable Development in Arid Lands, Xinjiang Institute of Ecology and Geography, Chinese Academy of Sciences, Urumqi, China
- 8Department of Soil Science, University of Chinese Academy of Sciences, Beijing, China
- 9Department of Agricultural Engineering, College of Food and Agriculture Sciences, King Saud University, Riyadh, Saudi Arabia
- 10Department of Botany and Microbiology, College of Science, King Saud University, Riyadh, Saudi Arabia
Despite agroforestry has large potential for soil organic carbon (SOC) sequestration, limited information is available on SOC pools and deep SOC sequestration as affected by agroforestry systems. Potential of long-term (15 years) agroforestry systems to store SOC was assessed in the foot hills of the north western Indian Himalayas. The study was carried out during 2009–2023 and soil samples were taken from four depths, viz. 0–15, 15–30, 30–45, 45–60 cm in Bhimal (Grewia optiva L.) and Mulberry (Morus alba L.) based agroforestry system under cowpea-toria based cropping systems and turmeric as ground storey crop. Results showed that in surface soil (0–15 cm), plots with mulberry + cowpea (Vigna unguiculata L.)-toria (Brassica campestris L.) (T7) had maximum C stock (21.35 Mg C ha−1) which was similar to mulberry + turmeric (Curcuma longa L.) (T6) plots. In deep soil layer (30–60 cm), plots under T7 had 33.52 Mg C ha-1 which was significantly higher than farmers’ practice cowpea-toria (T4). The results revealed that M. alba L. based agroforestry practices had 33%, 18% and 8% higher labile C concentration than (cultivated fallow land) (T9), T5 and T4 plots, respectively, in 0–15 cm soil depth. Recalcitrant C was maximum in T7 (4.49 g kg−1) plots. In the 0–30 cm layer, C accumulation rate ranged from 0.27 Mg C ha−1yr−1 to 0.99 Mg C ha−1y−1. Maximum C accumulation was found in T7 plots which was 160% and 135% higher than the farmers’ practise T4 plots and sole mulberry plantation (T5) plots. In the surface soil (0–15 cm), the treatment T7 had approximately 33% higher carbon management index (CMI) value compared with the farmers’ practise T4 plots. Thus, adopting M. alba L. based agroforestry practices has great potential for improving higher carbon stock in deep soil layer and can be recommended for sustainable management practices in the region, and it also could be considered as a strategy to restore degraded land, which is vital for food security, livelihood enhancement, and overall preserving the environmental services.
1 Introduction
Soils are integral to providing essential ecosystem services, including food production, water retention, and the sequestration of soil organic carbon (SOC). The physical structure of the soil is critically important in the processes that enable these functions. However, intensive land use and poor agricultural management practices have resulted in deterioration of soil structure, thereby diminishing agricultural productivity and the capacity of soils to stabilize SOC globally (Banwart, 2011; Montgomery, 2007). Organic soils are particularly significant, as they contain over two-thirds of the organic carbon stored in terrestrial ecosystems (Sharma et al., 2016). The carbon stored in forest soils is particularly susceptible to loss under the current climate system, with significant implications for soil biodiversity, productivity, and climate feedback mechanisms (de Deyn et al., 2008). Given that soil carbon sequestration is a vital ecosystem service linked to climate change mitigation, there is a pressing need for a more comprehensive understanding of the processes and mechanisms that govern it.
Soil organic carbon (SOC) plays a pivotal role in maintaining the physical, chemical, and biological quality of soil, making it one of the most critical indicators of soil health (Wang et al., 2003). Higher levels of organic carbon in the soil are associated with increased productivity across ecosystems. However, soil carbon levels vary significantly across different ecosystems-such as forests, grasslands, plantations, and agricultural lands-primarily due to variations in vegetation and land use practices (Awasthi et al., 1986). The significance of agroforestry as a land-use system is gaining widespread recognition, not only for its contribution to agricultural sustainability but also for its role in carbon sequestration and climate change mitigation. Agroforestry has emerged as an effective approach for mitigating and adapting to climate change (Mosquera-Losada et al., 2018). It increases carbon sinks by absorbing atmospheric carbon via photosynthesis and storing it in biomass and soil, so directly attributes in climate change mitigation (Albrecht and Kandji, 2003). In terms of India’s mitigation policies, the revised Nationally Determined Contributions (NDCs) aim to lower India’s GDP emission intensity by 45% from 2005 levels by 2030. To reach this target, the Government of India aims to expand forest and tree cover by 2030, resulting in extra carbon sinks of 2.5–3 billion tonnes of CO2 eq. Agroforestry can help achieve this goal by expanding tree cover on agricultural land (Nath et al., 2020). Researchers and policymakers around the world are facing long-term challenges in feeding a rising population while lowering greenhouse gas (GHG) emissions. Climate change is a serious threat to global food security (Babu et al., 2020). Carbon sequestration in biomass and soils has tremendous potential for reducing GHG emissions (Scholes et al., 2014). Agroforestry has the ability to boost carbon sequestration (Montagnini and Nair, 2004; Nair et al., 2009), in addition to contributing to food security and providing diverse livelihood supports such as timber and fuelwood for the household consumption (Babu et al., 2020).
There is substantial evidence demonstrating that agroforestry systems generally surpass annual cropping systems in terms of overall productivity, soil fertility enhancement, soil conservation, microclimate regulation, and carbon sequestration potential (Dhyani et al., 2009). SOC is fundamental to the carbon cycling and overall health of terrestrial ecosystems.
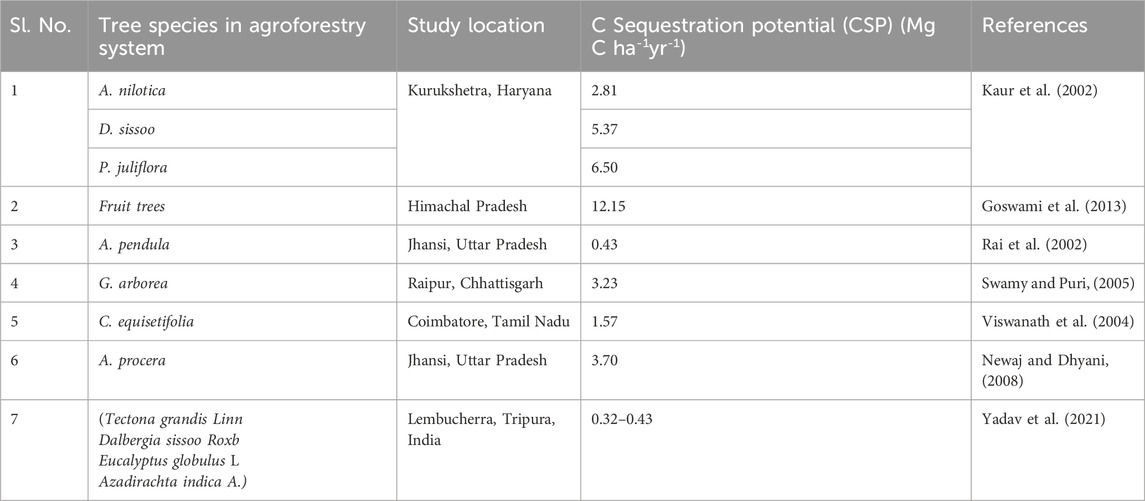
Although agroforestry systems are widely believed to have the potential to enhance SOC stocks (Table 1), relatively few studies have examined the SOC dynamics in deep soil layers. Trees have a robust root system that penetrates deeper soil strata than any annual crop or plant (Lorenz and Lal, 2014). As a result, a significant proportion of root biomass reaches deeper into the soil. In deeper soil horizons, C inputs from tree and shrub roots constitute an important source of SOC stock accumulation (Corbeels et al., 2019). The physicochemical interaction of root-generated carbon with soil particles contributes to better soil stability than C obtained from aboveground biomass (Rasse et al., 2005). This could be due to a greater SOC pool (65.3–71.6 Mg ha−1) in AFSs than in cultivated soils. Comparable results were seen, with 19.4%–46% and 21%–34% larger SOC stocks under different age groups of AFSs compared to cropland SOC stocks at other sites in India (Guillemot et al., 2018; Thangavel et al., 2018). Soil carbon sequestration, which involves the transfer of atmospheric CO2 into the soil in a form that is not readily re-emitted, is increasingly recognized as a viable strategy for mitigating climate change. Agroforestry systems, in particular, have attracted attention for their capacity to sequester carbon by capturing atmospheric CO2 and storing it in both plant biomass and soil (Nair, 2012). This process is natural, cost-effective, and environmentally friendly. Management practices such as agroforestry and interplanting, which promote the accumulation and retention of soil carbon, are gaining acceptance among farmers due to the growing interest in conserving soil organic matter (SOM) (Jose, 2009; Nair et al., 2009). Agroforestry systems, in particular, have shown promise in enhancing the potential of soils to act as SOC sinks, suggesting increased long-term storage and stabilization of SOC.
We hypothesize that differences in C stocks and hierarchy at surface and deep soil layers of SOC stocks under long-term agroforestry systems and farmers’ practice of arable cropping in the Himalayas would promote soil carbon sequestration. Therefore, this study aims to evaluate SOC pools and C sequestration as affected by long-term agroforestry system and to assess SOC sequestration in deep soil (30–60 cm) layer as affected by agroforestry practices in the foot hills of the Indian Himalayas.
2 Materials and methods
2.1 Experiment details
The study was carried out in the Selakui, ICAR-Indian Institute of Soil and Water Conservation in Dehradun (Figure 1), Uttarakhand from 2009 to 2023. The experiment is situated between 30° 20′4″N and longitude 72° 52′12″E with an average slope of 4.0%. Soil type is fine silty hyperthermic udic haplustalf with silty loam texture and study site was rainfed. It is located in the foot hills of the north-western part of the Indian Himalayas with an area 573.75 m2. There is an average annual rainfall of 1636 mm in this humid sub-tropical environment.
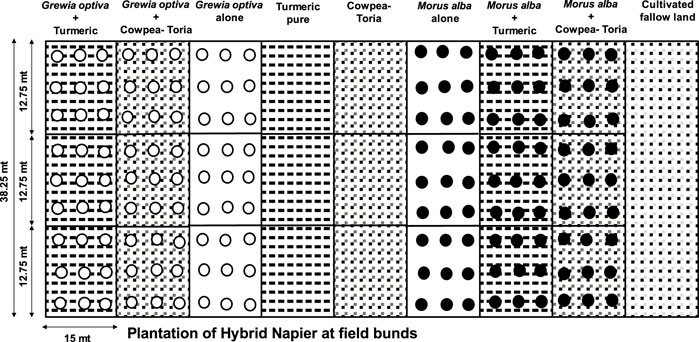
The experiment commenced in August 2009 with the establishment of 324 seedlings representing enhanced provenances of bhimal (Grewia optiva L.) and mulberry (Morus alba L.), which are important agroforestry tree species in the foothills of the Indian Himalayas (Figure 2). Short duration oilseed crop toria (Brassica campestris var. Toria) was grown uniformly in rabi (winter) season. Whereas, during kharif (rainy) season, viz., cowpea (Vigna unguiculata L.) and turmeric (Curcuma longa L.) were grown as ground storey crops. Cultivated fallow land, which is annually tilled without growing any crops. Treatment details are given in Table 2.
2.2 Soil sampling and processing
Composite soil samples were collected from four different depths (0–15, 15–30, 30–45 and 45–60 cm) under each plots. These depths are commonly used in studies total soil organic carbon (SOC). Soil samples from the depth of 0–15 cm and 15–30 cm are used for the analysis of labile carbon and recalcitrant carbon pool. Using a core sampler (3.5 cm diameter, 5.5 cm height) made of galvanized iron cylinder, undisturbed soil samples were extracted at four specified depths to assess soil bulk density.
2.3 Soil bulk density (BD)
The core sampler was driven into the soil to the desired depth, collecting the soil into the core (Veihmeyer and Hendrickson, 1948) to assess soil bulk density
2.4 Carbon pools in bulk soil
Different total soil organic C (SOC) fractions were determined under a range of oxidizing conditions using sulfuric acid (H2SO4)-aqueous solution ratios of 0.5:1, 1:1, and 2:1, which equate to 12 N, 18 N, and 24 N H2SO4, respectively (Chan et al., 2001). The 24 N H2SO4 oxidizable C method is equivalent to the classic Walkley and Black (1934) method. The concentration of organic C (OC) obtained using the three acid-aqueous solution ratios allowed TOC to be divided into four fractions with decreasing oxidizability/lability.
Fraction 1 (very labile): Organic C oxidizable under 12 N H2SO4
Fraction 2 (Labile): Difference in oxidizable organic C extracted between 18 N and 12 N H2SO4 (18 N–12 N H2SO4)
Fraction 3 (Less labile): Difference in oxidizable organic C extracted between 24 N and 18 N H2SO4
Fraction 4 (Non-labile): Difference in organic C extracted with 24 N H2SO4 and total SOC determined by CHN analyzer (total SOC–24 N H2SO4).
By using the four fractions indicated above, we can compute the active and passive C pools, which indicate the soil’s carbon oxidizability. The active carbon (C) pool comprises (Fraction 1-very labile) and (Fraction 2- labile), representing the labile and easily oxidizable portion. This was because of their very easy oxidizability using 12 and 18 N H2SO4. The passive C pool includes (Fraction 3- less labile) and (Fraction 4-non labile), which are recalcitrant and less reactive, and require more effort to extract and oxidize.
2.5 Total soil organic carbon (SOC)
Total soil organic C in the bulk soil was determined by the dry combustion method (Nelson and Sommers, 1982). For that purpose, the bulk soils were crushed and put through a 0.2 mm sieve and analysed for total C by a CHN analyzer (Foss Heraeus Elemental Analyzer CHN-O RAPID, Hanau, Germany).
2.6 Carbon stock
Soil organic carbon stock was calculated using the concentration of the total soil organic carbon (SOC) and the result of soil bulk density of each layer. Soil organic carbon stock was calculated using the following formula (Lal et al., 1998):
2.7 Soil organic carbon (SOC) accumulation rate
The SOC accumulation rate was calculated using the following formula (Bhattacharyya et al., 2015):
where, A and B indicate SOC stock (Mg ha-1) of a given treatment and control plots, in 2023 and n is no. of years of experiment (15 years). Here, the cultivated fallow land is considered as the control plot for the purpose of calculation.
2.8 Lability index (LI), C pool index (CPI), C management index (CMI)
KMnO4-C, the fraction of labile C which is obtained from chemical oxidation methods using KMnO4 (Blair et al., 1995), has since been considered as an early sensitive index for the impacts of long-term applications of fertilizers or organic resources on the dynamics of the active SOC fraction. Non-labile C can be estimated as the difference between SOC and KMnO4-C. The method used by Tirol-Padre and Ladha (2004) was used to determine KMnO4 oxidizable-C.
The calculation procedure of CMI is as follows (Blair et al., 1995):
2.9 Statistical analysis
The experimental data were statistically analyzed by completely randomized design (CRD) using analysis of variance (ANOVA). Using Microsoft Office Excel 2013 and the guidelines provided by Gomez and Gomez (1984), the analysis was carried out. Data normality was tested using the Shapiro-Wilk test. The analysis of variance (ANOVA) used the equation:
where Yij represents the jth observation (j = 1,2, … ,ni) on the ith treatment (i = 1,2, … ,k levels).
µ is the common effect for the whole experiment,
3 Result
3.1 Soil bulk density (BD)
After 15 years of long-term agroforestry practices, 0–15 cm and 15–30 cm soil layers showed significant differences in soil bulk density (BD) (Mg m-3) among the treatments (Figure 3). In the 0–15 cm soil layer, the BD ranged from 1.33 to 1.40 Mg m−3 and in 15–30 cm soil the BD ranged from 1.37 to 1.44 Mg m−3. Mulberry + cowpea-toria (T7) plots exhibited the lowest BD (1.33 Mg m−3) followed by mulberry + turmeric (T6) (1.34 Mg m−3) and bhimal + cowpea-toria (T3) (1.34 Mg m−3) and the values were significantly less than the cultivated fallow land (T9). In 15–30 cm soil also T7 plots exhibited the lowest BD (1.37 Mg m−3) among all plots. In the soil layers of 30–45 cm and 45–60 cm, there were no significant differences in BD values among different treatments (Figure 3). The result shows that bulk density values increase with increasing soil depth among all plots. The cultivated fallow land had higher values of bulk density and least bulk density values were found in agroforestry based treatments. This may be due to higher organic matter in agroforestry treated plots compared with cultivated fallow land.
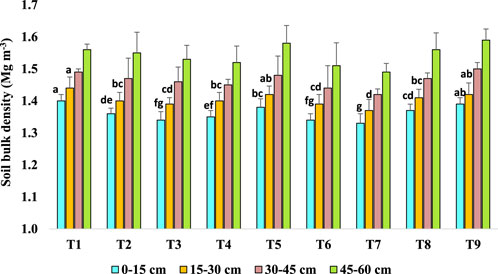
Figure 3. Effect of long-term agroforestry practices on soil bulk density (BD) (Mg m-3) in the 0–15, 15–30, 30–45, 45–60 cm soil layers. Vertical lines on the column indicate standard deviation of the mean (n = 3). Least significant difference (LSD; P < 0.05); (0–15 cm) = 0.014, (15–30 cm) = 0.021, (30–45 cm) = ns, (45–60 cm) = ns.
3.2 Effect of long-term agroforestry practices on permanganate (KMnO4) oxidizable carbon (POXC) (g kg−1)
After 15 years of long-term agroforestry practices, both surface (0–15 cm) and subsurface soil (15–30) layers showed significant differences in permanganate (KMnO4) oxidizable carbon (g kg-1) in bulk soils among the different plots (Figure 4). According to Moebius-Clune et al. (2017), the KMnO4 oxidisable C is a chemical fraction of C that reflects a biologically active pool. Despite having a significant association with total SOC, soil quality reacts slowly to changes in land and soil management (Marriott and Wander, 2006). In such cases, KMnO4 oxidisable C could be early indicators of land and soil management practices. In 0–15 cm soil, plots with T7 had ∼72%, 23% and 32% higher KMnO4 oxidizable C than T9, T5 and T4 plots, respectively. Similarly, in 15–30 cm soil layer T7 plot exhibited maximum KMnO4 oxidizable C (0.850 g kg−1) followed by T6, T2 and T3, respectively.
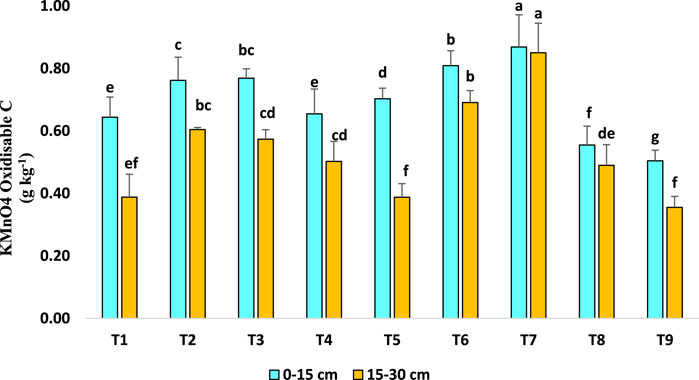
Figure 4. Effect of long-term agroforestry practices on KMnO4 oxidizable carbon (POXC) (g kg−1) in the 0–15, 15–30 cm soil layers. Vertical lines on the column indicate standard deviation of the mean (n = 3). Least significant difference (LSD; P < 0.05); (0–15 cm) = 0.044, (15–30 cm) = 0.094.
3.3 Effect of long-term agroforestry practices on labile carbon and recalcitrant carbon (g kg-1)
Labile C was found to be maximum in plots with T7 (6.20 g kg−1) which was similar to T6 and T2 plots and was 33%, 18% and 8% higher labile C concentration than T9, T5 and T4 plots, respectively, in 0–15 cm soil depth. Recalcitrant C was maximum in T7 (4.49 g kg−1) plots (Figure 5a). In the 15–30 cm soil depth, plots with T7 showed maximum concentration of labile C. T7 and T6 exhibited similar values of recalcitrant C concentration and T7 plots had 116% and 61% higher C concentration than T4 and T5 plots, respectively (Figure 5b).
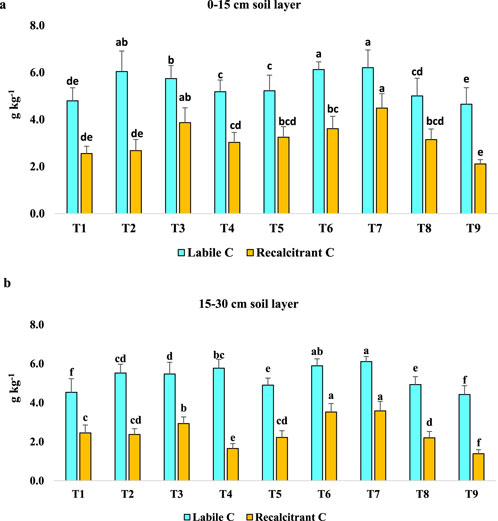
Figure 5. (a) Effect of long-term agroforestry practices on labile carbon and recalcitrant carbon (g kg-1) in the 0–15 cm soil layer. Vertical lines on the column indicate standard deviation of the mean (n = 3). Least significant difference (LSD; P < 0.05); Labile C = 0.42; Recalcitrant C = 1.15. (b) Effect of long-term agroforestry practices on labile carbon and recalcitrant carbon (g kg-1) in the 15–30 cm soil layer. Vertical lines on the column indicate standard deviation of the mean (n = 3). Least significant difference (LSD; P < 0.05); Labile C = 0.29; Recalcitrant C = 0.23.
3.4 Effect of long-term agroforestry practices on total soil organic carbon (g kg-1) in bulk soils
In surface soil (0–15 cm soil), T7 had maximum total SOC concentration (10.69 g kg−1) that was 30% and 26% higher than T4 and T5 plots, respectively (Figure 6). Agroforestry system T6 had 17% higher total SOC (9.73 g kg−1) than T4 plots. In the 15–30 cm soil, T7 (9.69 g kg−1) exhibited maximum content of total SOC which was similar toT6 and was 67%, 30% and 36% higher C content than T9, T4 and T5 plots, respectively. In the 30–45 cm soil, T7 and T6 showed highest effect followed by T3, T5 respectively.
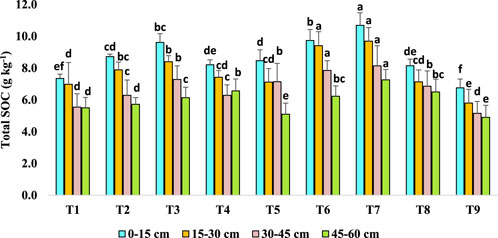
Figure 6. Effect of long-term agroforestry practices on total soil organic carbon (SOC) (g kg-1) in the 0–15, 15–30, 30–45, 45–60 cm soil layers. Vertical lines on the column indicate standard deviation of the mean (n = 3). Least significant difference (LSD; P < 0.05); (0–15 cm) = 0.95, (15–30 cm) = 0.81, (30–45 cm) = 0.55, (45–60 cm) = 0.38.
C stock in bulk soil was significantly affected by 15-year of mulberry and bhimal based agroforestry systems in the foot hills of the Indian Himalayas in the 0–15, 15–30, 30–45 and 45–60 cm soil layers (Table 3). In surface soil, plots under T7 had maximum C stock (21.35 Mg C ha−1) which was 51%, 21% and 28% higher than T9, T5 and T4 plots, respectively. Bhimal based agroforestry system (T3) also showed 16% and 25% higher C stock (19.33 Mg C ha−1) than T4 and T1 plots, respectively. In the 15–30 cm soil, T7, T6 showed highest mean values of C stocks followed by T3 and T2 plots. With increasing depth, the C stock decreased. In the 30–45 cm soil layer, T7 exhibited maximum C stock (17.34 Mg C ha−1) which was similar to T6 and T7 plots had 9% and 26% higher C stock than T5 and T4, plots respectively. Morus alba L. based T7 agroforestry system showed higher C stock (16.18 Mg C ha−1) than plots with T4 system.
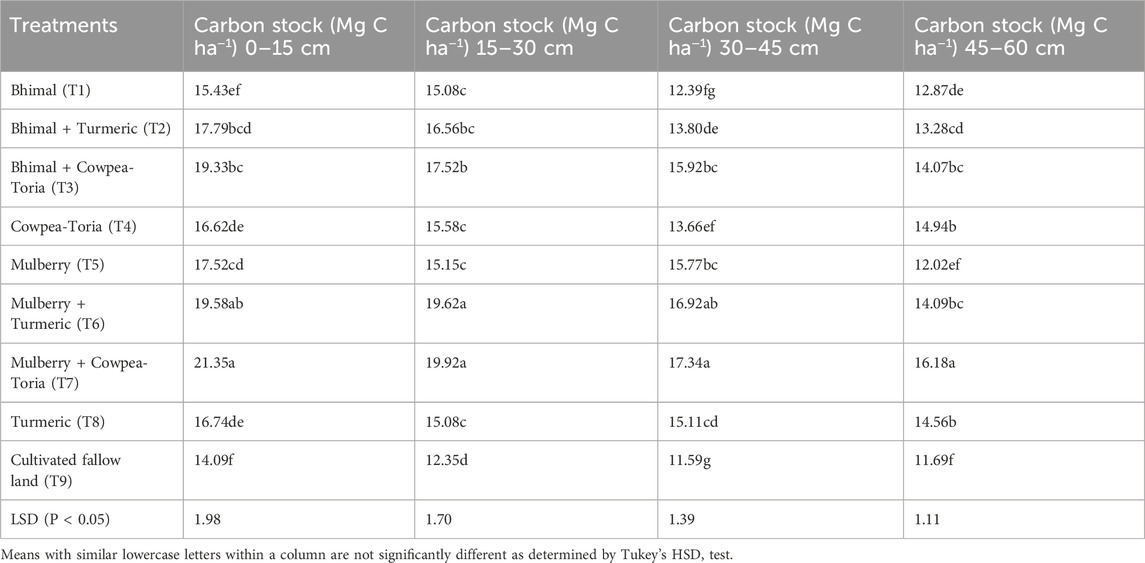
Table 3. Effect of long-term agroforestry practices on carbon stock (Mg C ha-1) in the Indian Himalayas.
Mulberry based agroforestry systems under a cowpea-toria cropping system resulted in a significant increase in total SOC storage and C accumulation rate in the surface soil (0–30 cm) layer (Table 4). Plots under T7 practices resulted in a significant improvement in the C accumulation rate compared to the cultivated fallow land treatment. In the 0–30 cm layer, C accumulation rate ranged from 0.27 Mg C ha−1yr−1 to 0.99 Mg C ha−1y−1. Maximum C accumulation was found in T7 (0.99 Mg C ha−1yr−1) plots which was 160% and 135% higher than the farmers’ practise (T4 plots) and sole mulberry plantation (T5 plots), respectively. Similarly, in subsurface soil layer, the C accumulation rate ranged from 0.13 Mg C ha−1yr−1 to 0.68 Mg C ha−1yr−1. Maximum C accumulation was found in T7 (0.68 Mg C ha−1yr−1) plots followed by T6 and T3 in subsurface soil, respectively.
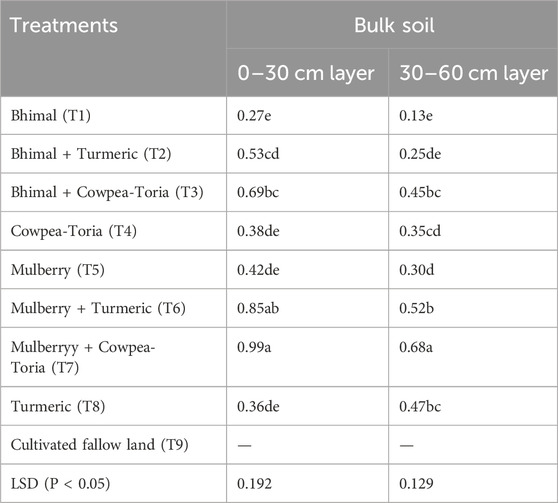
Table 4. Effect of long-term agroforestry practices on C accumulation rate (Mg C ha−1 yr−1) in the Indian Himalayas. Means with similar lowercase letters within a column are not significantly different as determined by Tukey’s HSD test.
As cultivated fallow land had the lowest total SOC in soil under T9 plots was considered as the reference soil for the calculation of C management index (CMI). Lability index (LI) of carbon and carbon pool index (CPI) were significantly affected by the agroforestry treatments in the 0–15 cm and 15–30 cm soil depths, as indicated in (Table 5). In the surface soil (0–15 cm), the treatment T7 had approximately 33% higher CMI value compared with the farmers’ practise. The plots treated with T7 had ∼30% and 59% higher CPI value compared with the T4 and T9 plots, respectively, in surface soil layer.
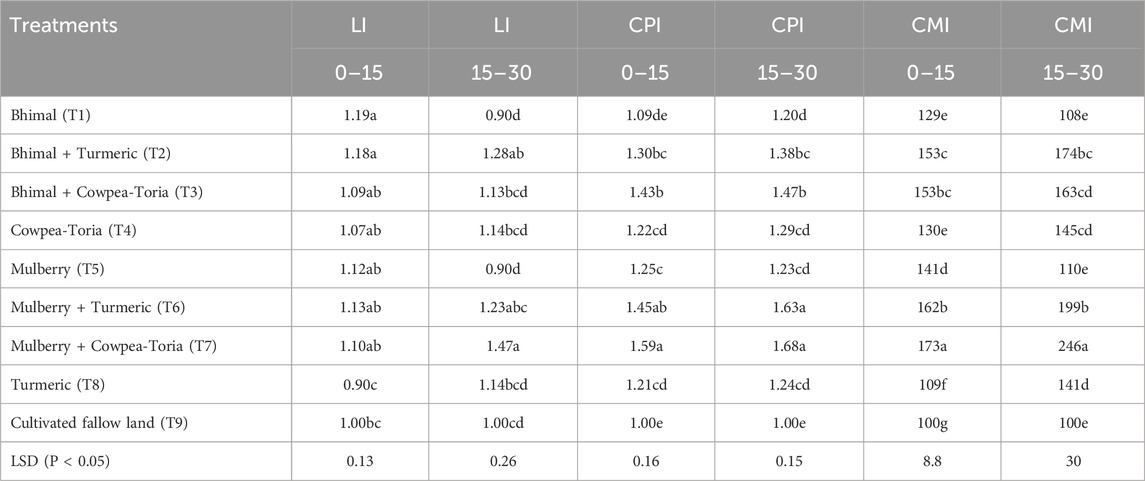
Table 5. Lability index (LI), C pool Index (CPI) and C management index (CMI) as affected by agroforestry system in the Indian Himalayas. Means with similar lowercase letters within a column are not significantly different as determined by Tukey’s HSD test.
4 Discussion
4.1 Effect of long-term agroforestry systems on soil bulk density (BD)
The findings indicate that bulk density values rose with increasing soil depth across all plots. The bulk density values of cultivated fallow land were greater, while agroforestry-based treatments had the lowest. This could be owing to increased organic matter in agroforestry-treated plots compared to cultivated fallow land. Messing et al. (1997) observed that bulk density was lower beneath trees than in grass pasture, but only in the top 15 cm of soil. This is consistent with our findings, in which T7 had decreased bulk density values due to agroforestry treatment. The presence of trees influences soil porosity and other physical properties as a result of aggregate formation. Improvements in soil aggregates result in decreased bulk density, lower resistance to penetration, a reduced surface sealing, increased soil porosity, and, hence, higher water infiltration and available water capacity (Rolo et al., 2023). The quality of litter and its location in the soil can influence its decomposition pathway, soil respiration rates, and the absorption of plant litter-C into soil microbial biomass (SMB) and soil organic C (SOC) pools (Hu et al., 2016). As a result, uneven organic matter inputs to agroforestry soils produce ubiquitous horizontal heterogeneity in soil traits (Bambrick et al., 2010; Cardinael et al., 2015; Pardon et al., 2017). Organic matter inputs decrease exponentially with depth, (Cardinael et al., 2018; Peichl et al., 2012), and total porosity decreased with increase in depth across the agroforestry practices (Syano et al., 2023) which leads to increase in soil bulk density in lower depth of the soil. Another reason of increased bulk density beneath agroforestry system or cropping system to both compression of the soil exerted by root growth, and lower soil moisture content caused by increased water uptake which leads to bulk density unaffected in all the systems, in this study in deep soil layer.
4.2 Effect of long-term agroforestry practices on permanganate (KMnO4) oxidizable carbon (POXC)
KMnO4 oxidizable carbon concentrations were significantly higher in mulberry-based agroforestry system (T7) in both the surface and subsurface soil layers than sole mulberry (T5), farmers’ practise (T4) and cultivated fallow land (T9) system. The oil palm-based agroforestry system also had a higher KMnO4- oxidizable C (0.95 g kg−1) content than did the oil palm monocultures (0.66 g kg−1) which is in line with our study. KMnO4- oxidizable C, also known as soil active C, is related with soil organic matter pools, which are critical for nutrient cycling (Blair et al., 1995; Weil et al., 2003). This C percentage is regarded as an excellent indication of soil quality since it is sensitive to the effects of soil management changes and is relatively easy to determine (Gruver, 2015; Weil et al., 2003). Furthermore, KMnO4- oxidizable C is used to calculate the soil C management index (CMI), which considers soil carbon lability and recalcitrancy to evaluate soil quality and soil organic C restoration (Blair et al., 1995). The agroforestry system studied were composed of several species that contribute to KMnO4- oxidizable C via the following two mechanisms: (1) plant material decomposition formed by litterfall and pruning (Dawoe et al., 2010; Fontes et al., 2014; Schneidewind et al., 2019) and (2) diverse root systems (root architecture, root morphology, root association with microorganisms, and chemical composition) that incorporate C into the soil by root cycling and release of exudates in different amounts and depths (Hombegowda et al., 2016) and exploit soil resources better.
4.3 Effect of long-term agroforestry practices on C pools in bulk soils
Higher mean value of the labile C pool under agroforestry practices shows the significant positive effects under its long-term adoption in the foot hills of north western Indian Himalayan soils. The trees were initially surrounded by arable crops rather than fallow, resulting in a deeper coarse root distribution and less lateral expansion. Agroforestry systems are particularly interesting because they have the ability to boost carbon sequestration while preserving agricultural production (Pandey, 2002; Montagnini and Nair, 2004; Nair et al., 2009). Although the introduction of trees into agricultural systems results in the buildup of aboveground biomass, the carbon stored in mulberry-based agroforestry system T7 is very labile and depends on the fate of woody biomass-derived products. Labile organic carbon pools are more easily affected by management practices compared to recalcitrant pools (Biederbeck et al., 1994). Roots release carbon compounds that are labile, and the high levels of labile carbon in forests are due to the year-round supply of easily decomposable leaf litter. In contrast, lower labile carbon levels in cropping systems are linked to the disruption of soil structure and increased oxidation of organic matter, especially in conventional agricultural systems (Bayer et al., 2006).
4.4 Effect of long-term agroforestry practices on total soil organic C (SOC) in bulk soils
Total SOC concentrations were higher in plots under mulberry + cowpea-toria (T7) than native forest system, sole mulberry (T5) and bhimal (T1) system and cowpea-toria cropping system (T4). Jastrow et al. (2007) reported that repeated biomass removal in agricultural fields adversely affects SOM accumulation. In contrast, forest soils, which have a large amount of dead and fallen leaves, significantly contribute to build up of SOC concentration (Sparling et al., 1998). The vast root system of trees in a mulberry-based agroforestry system (T7) may gather nutrients from a huge volume of soil, whereas litter fall concentrates nutrients near the soil surface. According to the findings of Nair et al. (2010), aboveground carbon (C) in plant residues and organic matter from aboveground biomass (AGB) return to the soil. Grewia optiva L. and Morus alba L. contributed around 0.84 Mg ha−1 and 0.94 Mg ha−1 of green leaves or 0.24 Mg ha−1 and 0.30 Mg ha−1 of air-dried leaves per year, as well as 1.1 Mg ha−1 and 1.3 Mg ha−1 of branches, respectively (Khybri et al., 1992). Litter fall and fine-root rotation could boost soil organic matter concentration. Trees may also improve the above and below-ground microclimate, while the meso and microfauna and microflora surrounding plant roots may affect soil chemical, biological, and physical properties.
4.5 Effect of long-term agroforestry practices on carbon stock in bulk soils
This long-term study indicated that mulberry + cowpea-toria system (T7) had highest C stock in all the depths and with increasing depth the C stock is decreased. The higher soil carbon stock in (T7) agroforestry systems is attributed to the significant annual input of organic matter from leaf litter, which remains in the soil due to the lack of disturbance. The cooler temperatures at higher altitudes, common in such ecosystems, likely slow down residue decomposition, contributing to increased carbon accumulation (Haynes, 2005). The unprotected SOC pool also increased with cumulative carbon inputs, primarily due to the addition of plant residues such as stubble and roots, as well as straw (Abrar et al., 2020; Tian et al., 2017). Furthermore, agroforestry plants allocate a larger proportion of their growth to root system development compared with those in monoculture systems. Several meta-analyses have already reported greater SOC stocks in 0–30 cm depth beneath agroforestry systems than in cropland (Chatterjee et al., 2018; Shi et al., 2018; Douglas et al., 2020; Guo et al., 2020). The surface soil layer may have absorbed more C-rich biomass in the form of leaves and twigs, which could contribute to the greater rise in SOC stocks in the topsoil layer (0–30 cm) compared to those in deeper layers (Albrecht and Kandji, 2003), yet fine tree roots and exudates may introduce significant amounts of organic matter into deep soil layers (Cardinael et al., 2018; Peichl et al., 2012) which improves soil carbon stock in deep soil layers in agroforestry systems than farmers’ practise (T4) and cultivated fallow land (T9) plots. Numerous other researchers worldwide have identified a similar pattern (Brahma et al., 2018; De Stefano and Jacobson, 2018; Guo et al., 2020; Hairiah et al., 2020). Possible reason of lower soil C stock in T4 and T9 could be soil erosion removes a significant amount of particulate and dissolved organic matter from soil matrixes, resulting in SOC loss. On the other hand, soil erosion coarsens soil texture because fine particles are preferentially removed with water movement (Ostovaria et al., 2018). The loss of fine-sized clay minerals reduces the production of soil aggregates and organic-mineral complexes, hastening SOC breakdown. To summarize, the decreased SOC levels on farmed fallow land are mostly due to reduced organic matter supply and extensive SOC loss induced by microbial breakdown and soil erosion. Litterfall from trees can increase soil surface rugosity and soil aggregate stability, whereas tree roots can increase soil porosity, increasing water infiltration and, ultimately, minimizing water erosion (Liu et al., 2016). The primary mechanism for transferring atmospheric carbon into the soil is through the plant’s roots, which are used for photosynthesis (Forseth, 2010). While part of this carbon is taken by the roots, majority of it is released as root exudates into the soil (Ma et al., 2022). These exudates, which are made up of different organic substances, can boost soil microbial activity and contribute to stable soil organic matter, which increases carbon inputs in soil (Panchal et al., 2022).
4.6 Effect of long-term agroforestry practices on C accumulation rate
The higher increase in SOC stocks in the surface layer (0–30 cm) than in lower layers was consistent with theoretical assumptions, as the surface soil layer may have absorbed more C-rich biomass in the form of leaves and twigs (Albrecht and Kandji, 2003). The results showed that trees had a favourable influence on total soil carbon. This could be due, in part, to increased leaf litter fall and tree roots caused by higher tree density in woodlots. Tree roots and litter fall contribute significantly to SOC (Schmidt et al., 2011). These could be possible explanations for the increased annual gain of C accumulation in the mulberry-based agroforestry system in our study. Nath et al. (2020) reported the rate of SOC sequestration rate (0.39–0.45 Mg C ha-1year-1) under different agroforestry systems in India. Tree-based systems might have added more root-derived C, which is protected by the physicochemical process and their inter action with the mineral present in soils as compared to C added by crops (Lorenz and Lal, 2014). In India, a 21-year-old block plantation of Eucalyptus have been reported to sequestrate 0.6–3.98 Mg C ha−1year−1 (Dhyani et al., 2016), 6-7-year-old agri-silviculture (Populus deltoids L.) added 1.62–2.63 Mg C ha−1year-1 (Chauhan et al., 2010) in the soils of Himalayan Indo-Gangetic plains. Block plantation (6.5 years old) of Gmelina arborea L., Ceiba pentandra L. and Acacia mangium L. sequestrated 1.09–3.4 Mg C ha−1year−1 in humid and sub-humid tropical region (Swamy et al., 2003). However, all India’s average of C sequestration of 2 to 51-year-old agroforestry systems ranged from 0.003 to 0.513 Mg C ha−1year−1 (Ajit et al., 2017).
4.7 Effect of long-term agroforestry practices on lability index (LI), C pool index (CPI) and C management index (CMI) in bulk soils
The carbon management index (CMI) encompasses both SOC pool and C lability, which can be a useful indicator to assess the ability of management practices to improve soil quality (Blair et al., 1995; Diekow et al., 2005) under different agricultural systems. Soils with higher CMI can support and sustain crop productivity and improve soil C and concentrations (West and Post, 2002; Kumar et al., 2019), and nutrient availability for crop uptake (Singh et al., 2019). CMI has no 'ideal' value. The index is a sensitive measure of the rate of change in soil C dynamics of systems in relation to a more stable reference soil. When monitored over time or when a new practice is introduced, as in this case, with increasing depth CMI increased in subsurface soil (15–30 cm) layer in T7 system, because of the relative contribution of that treatment vs. the reference plot that will dictate the lability index (LI), and the value of LI will determine CMI. LI is a sum of proportionate weightage of labile fractions of C, a higher LI value indicates healthy soil and soil with more active C (Hazra et al., 2019). The higher LI of soil under T7 system than sole cropping system T4 in the 0–15 and 15–30 cm soil depths might be attributed to a higher proportion of labile fraction of C in these treatments. It is well documented that the bhimal and mulberry based agroforestry practices enhance the carbon pool index (CPI) or SOC buildup under different cropping systems. Adoption of agroforestry systems in the present study indicated higher CPI which indicated the buildup of SOC in 0–15 cm and 15–30 cm depths. The higher value of CMI under mulberry based agroforestry systems (T7) could be due to relatively high values of LI and CPI in this treatment indicating a buildup of total SOC and labile SOC in the surface layer as a result of additional inputs of C through rhizodeposition, microbial biomass, and aboveground and belowground biomass addition.
5 Conclusion
Adopting 15 years of agroforestry practices on distribution of proportion of soil carbon (C) stocks in surface soil as well as subsurface soil showed significant differences among the treatments. Agroforestry system mulberry + cowpea-toria (T7) had maximum C stocks in all soil depths. Total soil organic carbon (SOC) was maximum in T7 plots that varied from (10.69 g kg−1) to (7.26 g kg−1) in surface soil layer to deep soil layer. The enhancement of the labile C pool (6.20 g kg−1) under mulberry based agroforestry system shows the positive effects of agroforestry under its long-term adoption in the foot hills of the Indian Himalayas. Lower bulk density was found in upper surface soil layer in agroforestry system and it is increased with increasing soil depth. C accumulation rate (0.99 Mg C ha−1 yr−1) and CMI index (173) also showed higher significant positive value in M. alba L. based agroforestry system T7. This is attributed to the increased net biomass input and more efficient utilization of available resources in M. alba L. based agroforestry system (T7) than farmer’s practice ∼ T4 system. In M. alba L. based agroforestry system (T7), the C sequestration capacity is 44.82% in deep soil layer (30–60 cm) (12.72 g kg−1) and this 15 years of agroforestry system attributes proportion of 45.63% of C sequestration under the same in the foot hills of north-western Himalayas. The pattern of carbon storage capacity in both surface and deep soil layers shows that tree-based systems significantly resulting in greater carbon storage compared to conventional agricultural systems. Thus, adopting M. alba L. based agroforestry system has excellent potential for higher carbon sequestration in surface soil layer as well as deeper soil layers, which is considered to be a key mechanism for the long-term stabilization of SOC, and, hence, could be adopted in the foothills of the Indian Himalayas and similar agro-ecosystems. Adoption of suitable agroforestry practices should be part of the National and County government policy interventions and should be factored in as a strategy for improved soil fertility, carbon credit payments to the farmers and for a green economy. To achieve this, there is need for retrospective studies on accurate evaluation of effect of different agroforestry practices on soil carbon at different types of soil.
Data availability statement
The datasets presented in this article are not readily available because ICAR does not encourage to share the original data. Requests to access the datasets should be directed to Ranjan Bhattacharyya, cmFuamFuX3Zwa2FzQHlhaG9vLmNvbQ==.
Author contributions
SB: Writing – original draft. RB: Conceptualization, Supervision, Writing – review and editing. CS: Data curation, Writing – review and editing. AR: Data curation, Writing – review and editing. VS: Writing – review and editing. DB: Writing – review and editing. NA: Supervision, Writing – review and editing. SD: Supervision, Writing – review and editing. SK: Supervision, Writing – review and editing. SJ: Data curation, Writing – review and editing. TD: Supervision, Writing – review and editing. SN: Funding acquisition, Supervision, Writing – review and editing. AG: Writing – review and editing. FU: Funding acquisition, Writing – review and editing. HE: Funding acquisition, Writing – review and editing. MN: Funding acquisition, Writing – review and editing. AF: Funding acquisition, Writing – review and editing. MR: Funding acquisition, Writing – review and editing. IM: Funding acquisition, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The work was supported by the 1. Department of Science and Technology, Government of India, through the National Mission for Sustaining Himalayan Ecosystem Taskforce on Himalayan Agriculture being implemented by the ICAR and 2. Ongoing Research Funding Program, (ORF-2025-118), King Saud University, Riyadh, Saudi Arabia.
Acknowledgments
The financial assistance provided by the ICAR in the form of Senior Research Fellowship (SRF) is gratefully acknowledged. The authors extend their deep appreciation to Ongoing Research Funding Program, (ORF-2025-118), King Saud University, Riyadh, Saudi Arabia.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor YSR declared a past co-authorship with the authors H O. E and IM.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abrar, M. M., Xu, M., Shah, S. A. A., Aslam, M. W., Aziz, T., Mustafa, A., et al. (2020). Variations in the profile distribution and protection mechanisms of organic carbon under long-term fertilization in a Chinese Mollisol. Sci. Total. Environ. 723, 138181. doi:10.1016/j.scitotenv.2020.138181
Ajit, , Dhyani, S. K., Handa, A. K., Newaj, R., Chavan, S. B., Alam, B., et al. (2017). Estimating carbon sequestration potential of existing agroforestry systems in India. Agrofor. Syst. 91 (6), 1101–1118. doi:10.1007/s10457-016-9986-z
Albrecht, A., and Kandji, S. T. (2003). Carbon sequestration in tropical agroforestry systems. Agric. Ecosyst. Environ. 99, 15–27. doi:10.1016/S0167-8809(03)00138-5
Awasthi, K. D., Singh, B. R., and Sitaula, B. K. (1986). Profile carbon and nutrient levels and management effect on soil quality indicators in the Mardi watershed of Nepal. Acta Agric. Scand. B 55, 192–204. doi:10.1080/09064710510029079
Babu, S., Mohapatra, K. P., Yadav, G. S., Lal, R., Singh, R., Avasthe, R. K., et al. (2020). Soil carbon dynamics in diverse organic land use systems in North Eastern Himalayan ecosystem of India. Catena 194, 104785. doi:10.1016/j.catena.2020.104785
Bambrick, A. D., Whalen, J. K., Bradley, R. L., Cogliastro, A., Gordon, A. M., Olivier, A., et al. (2010). Spatial heterogeneity of soil organic carbon in tree-based intercropping systems in Quebec and Ontario, Canada. Agrofor. Syst. 79, 343–353. doi:10.1007/s10457-010-9305-z
Bayer, C., Martin-Neto, L., Mielniczuk, J., Pavinato, A., and Dieckow, J. (2006). Carbon sequestration in two Brazilian Cerrado soils under no till. Soil. till. Res. 86, 237–245. doi:10.1016/j.still.2005.02.023
Bhattacharyya, R., Das, T. K., Sudhishri, S., Dudwal, B., Sharma, A. R., Bhatia, A., et al. (2015). Conservation agriculture effects on soil organic carbon accumulation and crop productivity under a rice--wheat cropping system in the western Indo-Gangetic Plains. Eur. J. Agron. 70, 11–21. doi:10.1016/j.eja.2015.06.006
Biederbeck, V. O., Janzen, H. H., Campbell, C. A., and Zentner, R. P. (1994). Labile soil organic matter as influenced by cropping practices in an arid environment. Soil Biol. biochem. 26, 1647–1656. doi:10.1016/0038-0717(94)90317-4
Blair, G. J., Lefroy, R. D., and Lisle, L. (1995). Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res. 46, 1459–1466. doi:10.1071/ar9951459
Brahma, B., Pathak, K., Lal, R., Kurmi, B., Das, M., Nath, P. C., et al. (2018). Ecosystem carbon sequestration through restoration of degraded lands in Northeast India. Land. Degrad. Dev. 29 (1), 15–25. doi:10.1002/ldr.2816
Cardinael, R., Chevallier, T., Barthès, B. G., Saby, N. P. A., Parent, T., Dupraz, C., et al. (2015). Impact of alley cropping agroforestry on stocks, forms and spatial distribution of soil organic carbon-a case study in a Mediterranean context. Geoderma 259-260, 288–299. doi:10.1016/j.geoderma.2015.06.015
Cardinael, R., Guenet, B., Chevallier, T., Dupraz, C., Cozzi, T., and Chenu, C. (2018). High organic inputs explain shallow and deep SOC storage in a long-term agroforestry system–combining experimental and modeling approaches. Biogeosciences 15, 297–317. doi:10.5194/bg-15-297-2018
Chan, K. Y., Bowman, A., and Oates, A. (2001). Oxidizable organic carbon fractions and soil quality changes in an Oxicpaleustalf under different pasture leys. Soil Sci. 166, 61–67. doi:10.1097/00010694-200101000-00009
Chatterjee, N., Nair, P. K. R., Chakraborty, S., and Nair, V. D. (2018). Changes in soil carbon stocks across the Forest-Agroforest-Agriculture/Pasture continuum in various agroecological regions: a meta-anal ysis. Agril. Ecosyst. Environ. 266, 55–67. doi:10.1016/j.agee.2018.07.014
Chauhan, S. K., Sharma, S. C., Chauhan, R., Gupta, N., and Srivastava, R. (2010). Accounting poplar and wheat productivity for carbon sequestration in agriculture system. Ind. For. 136 (9), 174–182. doi:10.36808/if2F20102Fv136i92F12701
Corbeels, M., Cardinael, R., Naudin, K., Guibert, H., and Torquebiau, E. (2019). The 4 per 1000 goal and soil carbon storage under agroforestry and conservation agriculture systems in sub-Saharan Africa. Soil till. Res. 188, 16–26. doi:10.1016/j.still.2018.02.015
Dawoe, E. K., Isaac, M. E., and Quashie-Sam, J. (2010). Litterfall and litter nutrient dynamics under cocoa ecosystems in lowland humid Ghana. Plant Soil 330, 55–64. doi:10.1007/s11104-009-0173-0
de Deyn, G. D. B., Cornelissen, J. H. C., and Bardgett, R. D. (2008). Plant functional traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 11, 516–531. doi:10.1111/j.1461-0248.2008.01164.x
De Stefano, A., and Jacobson, M. G. (2018). Soil carbon sequestration in agroforestry systems: a meta-analysis. Agrofor. Syst. 92 (2), 285–299. doi:10.1007/s10457-017-0147-9
Dhyani, S. K., Newaj, R., and Sharma, A. R. (2009). Agroforestry: its relation with agronomy, challenges and opportunities. Ind. J. Agrofor. 54, 249–266.
Dhyani, S. K., Ram, A., and Dev, I. (2016). Potential of agroforestry systems in carbon sequestration in India. Indian J. Agric. Sci. 86 (9), 1103–1112. doi:10.56093/ijas.v86i9.61348
Diekow, J., Mielniczuk, J., Knicker, H., Bayer, C., Dick, D. P., and Kogel-Knaber, I. (2005). Carbon and nitrogen stocks in physical fractions of a subtropical Acrisol as influenced by long-term no-till cropping systems and N fertilisation. Plant Soil 268, 319–328. doi:10.1007/s11104-004-0330-4
Douglas, G., Mackay, A., Vibart, R., Dodd, M., McIvor, I., and McKenzie, C. (2020). Soil carbon stocks under grazed pasture and pasture-tree systems. Sci. Total Environ. 715, 136910. doi:10.1016/j.scitotenv.2020.136910
Fontes, A. G., Gama-Rodrigues, A. C., Gama-Rodrigues, E. F., Sales, M. V. S., Costa, M. G., and Machado, R. C. R. (2014). Nutrient stocks in litterfall and litter in cocoa agroforests in Brazil. Plant Soil 383, 313–335. doi:10.1007/s11104-014-2175-9
Forseth, I. N. (2010). The ecology of photosynthetic pathways. Nat. Educ. Knowl. 3, 4. Available online at: https://www.nature.com/scitable/knowledge/library/the-ecology-of-photosynthetic-pathways-15785165/.
Gomez, K. A., and Gomez, A. A. (1984). Statistical procedures for agricultural research. 2nd Edn. New York: John Wiley and Sons, 680.
Goswami, S., Verma, K. S., and Kaushalm, R. (2013). Biomass and carbon sequestration in different agro forestry systems of a Western Himalayan watershed. Biol. Agric. Hortic. 30, 88–96. doi:10.1080/01448765.2013.855990
Gruver, J. (2015). Evaluating the sensitivity and linearity of a permanganate-oxidizable carbon method. Commun. Soil Sci. Plant Anal. 46, 490–510. doi:10.1080/00103624.2014.997387
Guillemot, J., le Maire, G., Munishamappa, M., Charbonnier, F., and Vaast, P. (2018). Native coffee agroforestry in the Western Ghats of India maintains higher carbon storage and tree diversity compared to exotic agroforestry. Agric. Ecosyst. Environ. 265, 461–469. doi:10.1016/j.agee.2018.06.002
Guo, J., Wang, B., Wang, G., Myo, S. T. Z., and Cao, F. (2020). Effects of three cropland afforestation practices on the vertical distribution of soil organic carbon pools and nutrients in eastern China. Glob. Ecol. Conserv. 22, e00913. doi:10.1016/j.gecco.2020.e00913
Hairiah, K., van Noordwijk, M., Sari, R. R., Saputra, D. D., Suprayogo, D., Kurniawan, S., et al. (2020). Soil carbon stocks in Indonesian (agro) forest transitions: compaction conceals lower carbon concentrations in standard accounting. Agric. Ecosyst. Environ. 294, 106879. doi:10.1016/j.agee.2020.106879
Haynes, R. J. (2005). Labile organic matter fractions as central components of the quality of agricultural soils: an overview. Adv. Agron. 85, 221–268. doi:10.1016/S0065-2113(04)85005-3
Hazra, K. K., Nath, C. P., Singh, U., Praharaj, C. S., Kumar, N., Singh, S. S., et al. (2019). Diversification of maize-wheat cropping system with legumes and integrated nutrient management increases soil aggregation and carbon sequestration. Geoderma 353, 308–319. doi:10.1016/j.geoderma.2019.06.039
Hombegowda, H. C., Van Straaten, O., Köhler, M., and Hölscher, D. (2016). On the rebound: soil organic carbon stocks can bounce back to near forest levels when agroforests replace agriculture in southern India. Soil 2, 13–23. doi:10.5194/soil-2-13-2016
Hu, Y. L., Zeng, D. H., Ma, X. Q., and Chang, S. X. (2016). Root rather than leaf litter input drives soil carbon sequestration after afforestation on a marginal cropland. For. Ecol. Mang 362, 38–45. doi:10.1016/j.foreco.2015.11.048
Jastrow, J. D., Amonette, J. E., and Bailey, V. L. (2007). Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Cli. Change 80, 5–23. doi:10.1007/s10584-006-9178-3
Jose, S. (2009). Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor. Syst. 76 (1), 1–10. doi:10.1007/s10457-009-9229-7
Kaur, B., Gupta, S. R., and Singh, G. (2002). Carbon storage and nitrogen cycling in silvopastoral systems on a sodic soil in northwestern India. Agrofor. Syst. 54, 21–29. doi:10.1023/A:1014269221934
Khybri, M. L., Gupta, R. K., Ram, S., and Tomar, H. P. S. (1992). Crop yields of rice and wheat grown in rotation as intercrops with three tree species in the outer hills of Western Himalaya. Agrofor. Syst. 17, 193–204. doi:10.1007/BF00054147
Kumar, N., Nath, C. P., Hazra, K. K., Das, K., Venkatesh, M. S., Singh, M. K., et al. (2019). Impact of zero-till residue management and crop diversification with legumes on soil aggregation and carbon sequestration. Soil till. Res. 189, 158–167. doi:10.1016/j.still.2019.02.001
Lal, R., Kimble, J. M., and Follett, R. F. (1998). “Land use and soil C pools in terrestrial ecosystems. p. 1–10,” in Management of carbon sequestration in soil. Editors R. Lal, J. M. Kimble, R. F. Follet, and B. A. Stewart (Boca Raton, FL: CRC Press).
Liu, W., Zhu, C., Wu, J., and Chen, C. (2016). Are rubber-based agroforestry systems effective in controlling rain splash erosion? Catena 147, 16–24. doi:10.1016/j.catena.2016.06.034
Lorenz, K., and Lal, R. (2014). Soil organic carbon sequestration in agroforestry systems. A review. Rev. Agron. Sustain. Dev. 34 (2), 443–454. doi:10.1007/s13593-014-0212-y
Ma, W., Tang, S., Dengzeng, Z., Zhang, D., Zhang, T., and Ma, X. (2022). Root exudates contribute to belowground ecosystem hotspots: a review. Front. Microbiol. 13, 937940. doi:10.3389/fmicb.2022.937940
Marriott, E. E., and Wander, M. M. (2006). Total and labile soil organic matter in organic and conventional farming systems. Soil Sci. Soc. Am. J. 70, 950–959. doi:10.2136/sssaj2005.0241
Messing, I., Alriksson, A., and Johansson, W. (1997). Soil physical properties of afforested and arable land. Soil Use Manage 13, 209–217. doi:10.1111/j.1475-2743.1997.tb00588.x
Moebius-Clune, B. N., Moebius-Clune, D., Gugino, B., Idowu, O. J., Schindelbeck, R. R., Ristow, A. J., et al. (2017). Comprehensive assessment of soil health - the Cornell Framework manual.
Montagnini, F., and Nair, P. K. R. (2004). Carbon sequestration: an underexploited environmental benefit of agroforestry systems. Agrofor. Syst. 61, 281–295. doi:10.1007/978-94-017-2424-1_20
Montgomery, D. R. (2007). Soil erosion and agricultural sustainability. Pro. Nat. Acad. Sci. U. S. A. 104, 13268–13272. doi:10.1073/pnas.0611508104
Mosquera-Losada, M. R., Santiago-Freijanes, J. J., Rois-DíAz, M., Moreno, G., den Herder, M., Aldrey-Vazquez, J. A., et al. (2018). Agroforestry in Europe: a land management policy tool to combat climate change. Land. use. Pol. 78, 603–613. doi:10.1016/j.landusepol.2018.06.052
Nair, P. K. R. (2012). Carbon sequestration studies in agroforestry systems: a reality-check. Agrofor. Syst. 86, 243–253. doi:10.1007/s10457-011-9434-z
Nair, P. K. R., Kumar, B. M., and Nair, V. D. (2009). Agroforestry as a strategy for carbon sequestration. J. Plant. Nutr. Soil Sci. 172, 10–23. doi:10.1002/jpln.200800030
Nair, P. K. R., Nair, V. D., Kumar, B. M., and Showalter, J. M. (2010). Carbon sequestration in agroforestry systems. Adv. Agron. 108, 237–307. doi:10.1016/S0065-2113(10)08005-3
Nath, A. J., Sileshi, G. W., Laskar, S. Y., Pathak, K., Reang, D., Nath, A., et al. (2020). Quantifying carbon stocks and sequestration potential in agroforestry systems under divergent management scenarios relevant to India’s Nationally Determined Contribution. J. Clean. Prod. 281, 124831. doi:10.1016/j.jclepro.2020.124831
Nelson, D. W., and Sommers, L. E. (1982). in Methods of soil analysis, Part 2: chemical and microbial properties (Madson, Wiconsin: Soil Science Society of America).
Newaj, R., and Dhyani, S. K. (2008). Agroforestry for carbon sequestration: scope and present status. Ind. J. Agrofor. 10, 1–9. Available online at: https://epubs.icar.org.in/index.php/IJA/article/view/103776.
Ostovaria, Y., Ghorbani-Dashtakia, S., Bahramib, H. A., Abbasic, M., Demattee, A. M., Arthurd, E., et al. (2018). Towards prediction of soil erodibility, SOM and CaCO3 using laboratory Vis-NIR spectra: a case study in a semi-arid region of Iran. Geoderma 314, 102–112. doi:10.1016/j.geoderma.2017.11.014
Panchal, P., Preece, C., Peñuelas, J., and Giri, J. (2022). Soil carbon sequestration by root exudates. Trends. Plant. Sci. 27, 749–757. doi:10.1016/j.tplants.2022.04.009
Pandey, D. (2002). Carbon sequestration in agroforestry systems. Cli. Pol. 2, 367–377. doi:10.3763/cpol.2002.0240
Pardon, P., Reubens, B., Reheul, D., Mertens, J., de Frenne, P., Coussement, T., et al. (2017). Trees increase soil organic carbon and nutrient availability in temperate agroforestry systems. Agril. Ecosyst. Environ. 247, 98–111. doi:10.1016/j.agee.2017.06.018
Peichl, M., Leava, N. A., and Kiely, G. (2012). Above- and belowground ecosystem biomass, carbon and nitrogen allocation in recently afforested grassland and adjacent intensively managed grassland. Plant Soil 350, 281–296. doi:10.1007/s11104-011-0905-9
Rai, A. K., Solanki, K. R., and Rai, P. (2002). Performance of Anogeissus pendula genotypes under agrisilvi culture system. Indian J. Agrofor. 4, 71–77. Available online at: https://epubs.icar.org.in/index.php/IJA/article/view/102431.
Rasse, D. P., Rumpel, C., and Dignac, M.F. (2005). Is soil carbon mostly root carbon? Mechanisms for a specific stabilization. Plant Soil 269, 341–356. doi:10.1007/s11104-004-0907-y
Rolo, V., Rivest, D., Maillard, E., and Moreno, G. (2023). Agroforestry potential for adaptation to climate change: a soil-based perspective. Soil. use. Mang 39, 1006–1032. doi:10.1111/sum.12932
Schmidt, M., Torn, M., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I., et al. (2011). Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56. doi:10.1038/nature10386
Schneidewind, U., Niether, W., Armengot, L., Schneider, M., Sauer, D., Heitkamp, F., et al. (2019). Carbon stocks, litterfall and pruning residues in monoculture and agroforestry cacao production systems. Experin. Agril. 55, 452–470. doi:10.1017/S001447971800011X
Scholes, R. J., Palm, C. A., and Hickman, J. E. (2014). “Agriculture and climate change mitigation in the developing world,” in CCAFS working Paper No. 61. CGIAR research program on climate change, agriculture and food security (CCAFS). Copenhagen, Denmark. Available online at: https://ccafs.cgiar.org/.
Sharma, R., Chauhan, S. K., and Tripathi, A. M. (2016). Carbon sequestration potential in agroforestry system in India: an analysis for carbon project. Agrofor. Syst. 90, 631–644. doi:10.1007/s10457-015-9840-8
Shi, L. L., Feng, W. T., Xu, J. C., and Kuzyakov, Y. (2018). Agroforestry sys tems: meta-analysis of soil carbon stocks, sequestration processes, and future potentials. Land deg. Dev. 29, 3886–3897. doi:10.1002/ldr.3136
Singh, V. K., Dwivedi, B. S., Mishra, R. P., Shukla, A. K., Timsina, J., Upadhyay, P. K., et al. (2019). Yields, soil health and farm profits under a rice-wheat system: long-term effect of fertilizers and organic manures applied alone and in combination. Agron 9, 1. doi:10.3390/agronomy9010001
Sparling, G., Vojvodic- Vukovic, M., and Schipper, L. A. (1998). Hot water-soluble C as a simple measure of labile soil organic matter; the relationship with microbial biomass C. Soil Biol. biochem. 30, 1469–1472. doi:10.1016/S0038-0717(03)00186-X
Swamy, S. L., Mishra, A., and Puri, S. (2003). Biomass production and root distribution of Gmelia arborea under an agri-silviculture system in sub-humid tropics of central India. N. For. 26, 167–186. doi:10.1023/A:1024478700645
Swamy, S. L., and Puri, S. (2005). Biomass production and C-sequestration of Gmelina arborea in plantation and agroforestry system in India. Agrofor. Syst. 64, 181–195. doi:10.1007/s10457-004-1999-3
Syano, N. M., Nyangito, M. M., Kironchi, G., and Wasonga, O. V. (2023). Agroforestry practices impacts on soil properties in the drylands of Eastern Kenya. Trees. Forst. Peop 14, 100437. doi:10.1016/j.tfp.2023.100437
Thangavel, R., Kanchikerimath, M., Sudharsanam, A., Ayyanadar, A., Karunanithi, R., Deshmukh, N. A., et al. (2018). Evaluating organic carbon fractions, temperature sensitivity and artificial neural network modeling of CO2 efflux in soils: impact of land use change in subtropical India (Meghalaya). Ecol. Indic. 93, 129–141. doi:10.1016/j.ecolind.2018.04.077
Tian, J., Lou, Y., Gao, Y., Fang, H., Liu, S., Xu, M., et al. (2017). Response of soil organic matter fractions and composition of microbial community to long-term organic and mineral fertilization. Biol. Fert. Soil. 53, 523–532. doi:10.1007/s00374-017-1189-x
Tirol-Padre, A., and Ladha, J. K. (2004). Assessing the reliability of permanganate-oxidizable carbon as an index of soil labile carbon. Soil Sci. Soc. Am. J. 68, 969–978. doi:10.2136/sssaj2004.9690
Veihmeyer, F. J., and Hendrickson, A. H. (1948). Soil density and root penetration. Soil Sci. 65, 487–494. doi:10.1097/00010694-194806000-00006
Viswanath, S., Peddappaiah, R. S., Subramoniam, V., Manivachakam, P., and George, M. (2004). Management of Casuarina equisetifolia in wide-row intercropping systems for enhanced productivity. Ind. J. Agrofor. 6, 19–25. Available online at: https://epubs.icar.org.in/index.php/IJA/article/view/102787.
Walkley, A., and Black, I. A. (1934). An examination of the degtjareff method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 37, 29–38. doi:10.1097/00010694-193401000-00003
Wang, J., Fu, B., Qiu, Y., and Chen, L. (2003). Analysis on soil nutrient characteristics for sustainable land use in Danangou catchment of the Loess Plateau, China. Catena 54, 17–29. doi:10.1016/S0341-8162(03)00054-7
Weil, R. R., Islam, K. R., Stine, M., Gruver, J. B., and Samson-Liebig, S. E. (2003). Estimating active carbon for soil quality assessment: a simplified method for laboratory and field use. Amr. J. Altr. Agril. 18, 3–17. doi:10.1079/AJAA2003003
West, T. O., and Post, W. M. (2002). Soil organic carbon sequestration rates by tillage and crop rotation. Soil Sci. Soc. Am. J. 66, 1930–1946. doi:10.2136/sssaj2002.1930
Keywords: agroforestry, carbon stock, total soil organic carbon (SOC), bulk density, C accumulation rate
Citation: Barman S, Bhattacharyya R, Singh C, Rathore AC, Singhal V, Biswas DR, Ahmed N, Das S, Kumar S, Jat SL, Das TK, Kumar SN, Ghosh A, Ullah F, Elansary HO, Nazim M, Fickak AA, Rashwan MA and Moussa IM (2025) Long-term agroforestry enhances soil organic carbon pools and deep soil carbon sequestration in the Indian Himalayas. Front. Environ. Sci. 13:1568564. doi: 10.3389/fenvs.2025.1568564
Received: 30 January 2025; Accepted: 30 April 2025;
Published: 12 June 2025.
Edited by:
Yashwant Singh Rawat, Federal Technical and Vocational Education and Training Institute (FTVETI), EthiopiaReviewed by:
Gaurav Mishra, Indian Council of Forestry Research and Education (ICFRE), IndiaMeraj Alam Ansari, ICAR-Indian Institute of farming system research, India
Copyright © 2025 Barman, Bhattacharyya, Singh, Rathore, Singhal, Biswas, Ahmed, Das, Kumar, Jat, Das, Kumar, Ghosh, Ullah, Elansary, Nazim, Fickak, Rashwan and Moussa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ranjan Bhattacharyya, cmFuamFuX192cGthc0B5YWhvby5jb20=
 Swarnashree Barman1
Swarnashree Barman1 Ranjan Bhattacharyya
Ranjan Bhattacharyya Sandeep Kumar
Sandeep Kumar S. L. Jat
S. L. Jat T. K. Das
T. K. Das Soora Naresh Kumar
Soora Naresh Kumar Avijit Ghosh
Avijit Ghosh Hosam O. Elansary
Hosam O. Elansary Ihab Mohamed Moussa
Ihab Mohamed Moussa