- 1College of Ecology and Environment, Xinjiang University, Urumqi, China
- 2Key Laboratory of Oasis Ecology, Xinjiang University, Urumqi, China
- 3Xinjiang Jinghe Observation and Research Station of Temperate Desert Ecosystem, Ministry of Education, Jinghe, China
- 4Technology Innovation Center for Ecological Monitoring and Restoration of Desert-Oasis, Urumqi, China
- 5Institute of Desert Meteorology, China Meteorological Administration, Urumqi, China
- 6Natural Resources Bureau of Kashgar Prefecture, Kashgar, China
- 7Department of Science and Technology, Xinjiang University, Urumqi, China
Fly ash and bentonite, two abundant industrial by-products in China, offer promising potential for ecological restoration in arid regions. In this study, we conducted a factorial pot experiment with 24 treatment combinations (6 fly ash levels: F0–F5 = 0–100%; 4 bentonite levels: P0–P3 = 0–70 g kg-1) to evaluate their synergistic effects on two xerophytic shrubs, Lycium ruthenicum and Ephedra intermedia. A total of 600 seeds per species were sown (5 seeds × 5 replicates × 24 treatments), and plant performance was assessed based on germination rate, survival rate, plant height, dry mass, and root morphological traits. Results showed species-specific and dosage-dependent responses. For L. ruthenicum, the highest germination rate (76%) was observed under 80% fly ash without bentonite (P0F4), but biomass and root development declined under higher fly ash or bentonite-only treatments (e.g., dry mass in P1F2: 10.40% ± 6.02%). In contrast, E. intermedia showed broader tolerance, with peak germination (88%) under moderate bentonite and 60%–80% fly ash (P1F3/P1F4), and optimal growth under P3F5 (root surface area: 10.18 ± 3.09 cm2; dry mass: 33.76% ± 26.53%). Two-way ANOVA revealed significant main effects and interactions between fly ash and bentonite on key traits (e.g., p < 0.001 for germination and survival in E. intermedia). Moderate fly ash addition (F2–F3, i.e., 40%–60%) with bentonite levels of 30–50 g kg-1 effectively enhanced soil water retention and promoted early-stage growth, especially in E. intermedia. This study highlights the importance of species-specific amendment strategies and demonstrates the ecological benefits of valorizing industrial by-products for arid-land revegetation.
1 Introduction
Soil degradation in arid and semi-arid regions poses a major threat to vegetation establishment and ecological sustainability. Poor soil structure, low fertility, and weak water retention capacity hinder plant growth and exacerbate desertification (Ayub et al., 2020; Naorem et al., 2023; Smith et al., 2016; Thomas et al., 2007; Yang et al., 2024). Traditional soil management approaches, including conservation tillage and organic amendments, often fall short in rapidly restoring severely degraded lands, particularly under extreme water scarcity (Lal, 2017; Sonderegger and Pfister, 2021; Visser et al., 2004; Yang et al., 2019). Thus, exploring cost-effective and resource-efficient solutions for soil improvement in these fragile ecosystems remains an urgent scientific and practical concern (Alotaibi and Schoenau, 2019; Jiang et al., 2019; Mganga et al., 2015; Shao et al., 2024; Zhang et al., 2019).
Industrial by-products such as fly ash and bentonite have attracted growing attention for their potential use as soil amendments in ecological restoration (Pal & Ghosh, 2014; Usman et al., 2023). Fly ash, a residue from coal combustion, is rich in minerals (e.g., SiO2, Al2O3, Fe2O3) and exhibits fine particle size and porosity, which can enhance soil aeration and provide essential nutrients (Luo et al., 2021; Cao et al., 2008; Wang et al., 2019; Pandey and Singh, 2010). Bentonite, a natural clay mineral with high swelling capacity, improves soil structure by increasing water-holding capacity and cation exchange properties (Croker et al., 2004; Mi et al., 2021). Despite their individual benefits, each material has limitations: fly ash can lead to pH imbalance and heavy metal risk at high doses, while bentonite may restrict aeration and root penetration if applied excessively.
The rationale for combining fly ash and bentonite lies in their complementary physical and chemical properties (Deka and Sekharan, 2017; Gupt et al., 2021). Fly ash improves nutrient availability and porosity, while bentonite enhances moisture retention and mitigates toxicity by immobilizing harmful ions. However, little research has evaluated the synergistic effects of these amendments on plant performance, particularly in arid ecosystems where soil limitations are multidimensional. Furthermore, few studies have addressed species-specific responses to different amendment combinations, which are critical for optimizing ecological restoration strategies.
In this study, we designed a controlled factorial pot experiment to assess the combined effects of fly ash and bentonite on two xerophytic shrubs (Lycium ruthenicum and Ephedra intermedia), focusing on early-stage germination, survival, biomass, and root traits. We hypothesize that moderate application of fly ash and bentonite will improve soil quality and promote plant growth, with optimal combinations varying between species. Our findings aim to provide practical guidance for using industrial by-products in vegetation restoration and contribute to the sustainable management of arid land resources.
2 Methods
2.1 Materials
2.1.1 Soils
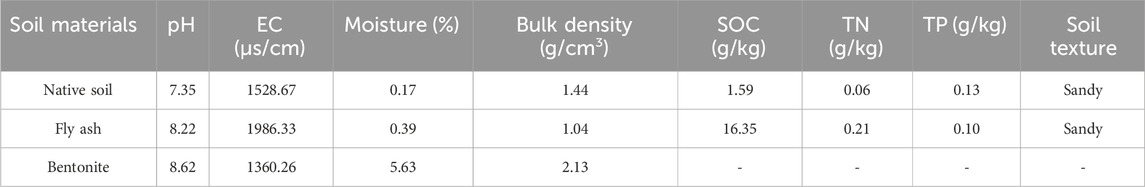
The base soil was collected from the surface layer (0–20 cm) near an abandoned fly ash dump site in Toksun County, Xinjiang (42°41′58″–42°42′07″ N, 88°38′18″–88°38′35″ E). The site is characterized by extreme aridity (annual precipitation ∼5.7 mm) and coarse-textured sandy soils. Fly ash was obtained from a local coal-fired power plant. It contains high levels of SiO2, Al2O3, and Fe2O3, with a bulk density of 1.04 g/cm3 and pH of 8.22. Bentonite (sodium-based) was purchased from Xinjiang Zhongfei Xazijie Co., with a cation exchange capacity of ∼50 meq/100g and high swelling capacity. Its pH was 8.62 and bulk density 2.13 g/cm3. The physical and chemical properties of the experimental soils are shown in Table 1.
In order to better understand the microstructural characteristics of these materials, scanning electron microscope (SEM, Hitachi SU8010, Japan) images were taken at different magnifications (1000×, 6000×, and 10000×). These high-resolution images allow for a detailed observation of the microstructure of the two materials, which can provide insights into the physical properties affecting their soil remediation effects. As shown in Figure 1A, the fly ash consists of porous spherical particles with irregular shapes and sizes. These particles have a high surface area and porosity, which enhances the ability to adsorb water and nutrients, enabling them to effectively improve soil structure and water retention. Figure 1B shows that the surface of fly ash spherical microbeads is relatively smooth with small particles attached to it. These small particles aggregated together to form larger agglomerates, which enhanced the adsorption capacity of the fly ash, and was particularly beneficial in improving the water retention and structure of the soil. Figure 1C shows the rough surface of the porous particles, which contain a large number of pores that contribute to the permeability and water-holding capacity of the soil.

Figure 1. SEM scan of fly ash. Note: (A) Porous spherical particles observed at 1000× magnification, enhancing adsorption. (B) Smooth surface with aggregated particles at 6000× magnification, improving water retention. (C) Rough surface with pores at ×10000 magnification, increasing permeability and water-holding capacity.
In contrast, Figure 2A shows bentonite particles with irregular shapes and sizes. Figure 2B further highlights the complex surface characteristics of bentonite, exhibiting significant roughness and abundant pore space. As shown in Figure 2C, the surface morphology of bentonite shows high porosity, which greatly improves its adsorption capacity and swelling capacity. These properties are critical for enhancing water retention and improving soil structure, especially in dryland environments, to promote plant growth and overall soil fertility.

Figure 2. SEM scan of bentonite. Note: (A) Bentonite particles with irregular shapes and sizes at 1000× magnification. (B) Detailed surface features of bentonite showing roughness and abundant pore spaces at 6000× magnification. (C) High porosity of bentonite surface enhancing its adsorption and swelling capacity at ×10000 magnification.
In addition, Figures 3A,B show the X-ray diffraction (XRD) patterns of fly ash and bentonite, respectively. Figure 3A shows that the fly ash exhibits obvious crystalline peaks, indicating a relatively high degree of mineral crystallinity. The main mineral constituent of fly ash is SiO2, with minor amounts of iron oxides and salts, which together contribute to the physical and chemical properties of the soil. In contrast, Figure 3B shows an XRD pattern of bentonite, which has a higher degree of crystallinity than fly ash. The dominant mineral phase in bentonite is quartz, highlighting the important role of quartz in soil amelioration, especially in improving soil water retention and fertility.
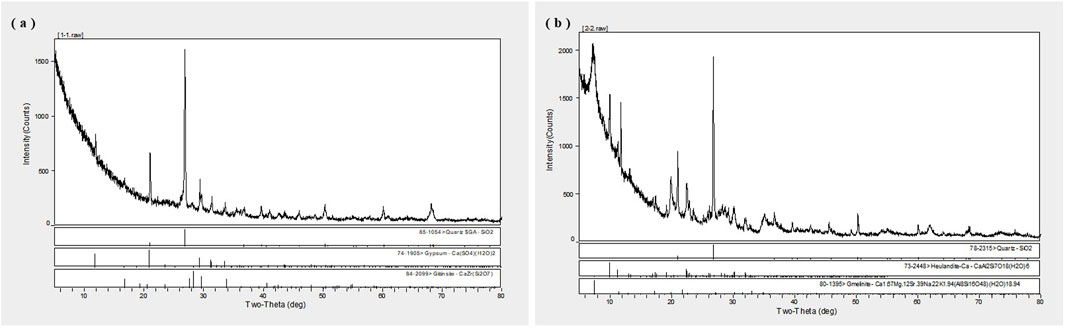
Figure 3. XRD Spectra of Fly Ash and Bentonite. Note: (A) X-ray diffraction (XRD) pattern of fly ash with high mineral crystallinity and SiO2 as the main mineral component. (B) XRD pattern of bentonite, the main mineral phase is quartz.
2.1.2 Fertilizer
Phosphorus fertilizer (monopotassium phosphate, MKP) and nitrogen fertilizer (urea) were used as basal fertilizers in the experiment. Monopotassium phosphate (MKP) fertilizer provides essential nutrients such as phosphorus, potassium and trace elements to promote plant growth and disease resistance. Urea provides nitrogen essential for photosynthesis and protein synthesis to promote plant growth. Potassium monophosphate was produced by Wal Group (USA) and urea (46.4% total nitrogen) was provided by Kuitun Jinjiang Chemical Fertilizer Co.
2.1.3 Plants
The plants selected for this study included Central Asian ephedra (Ephedra intermedia) and Lycium ruthenicum (Lycium ruthenicum). Ephedra is a perennial shrub, adapted to arid and semi-arid environments, with a deep root system and very strong resistance; while Lycium ruthenicum has high medicinal value and strong tolerance to salinity and drought. The two test plants have good ecological and economic significance, as well as the ability to grow in arid and semi-arid environments. The two test plants were selected in order to evaluate the plant growth promotion effect of bentonite and fly ash.
2.2 Experiment design
2.2.1 Experiment design and treatment setup
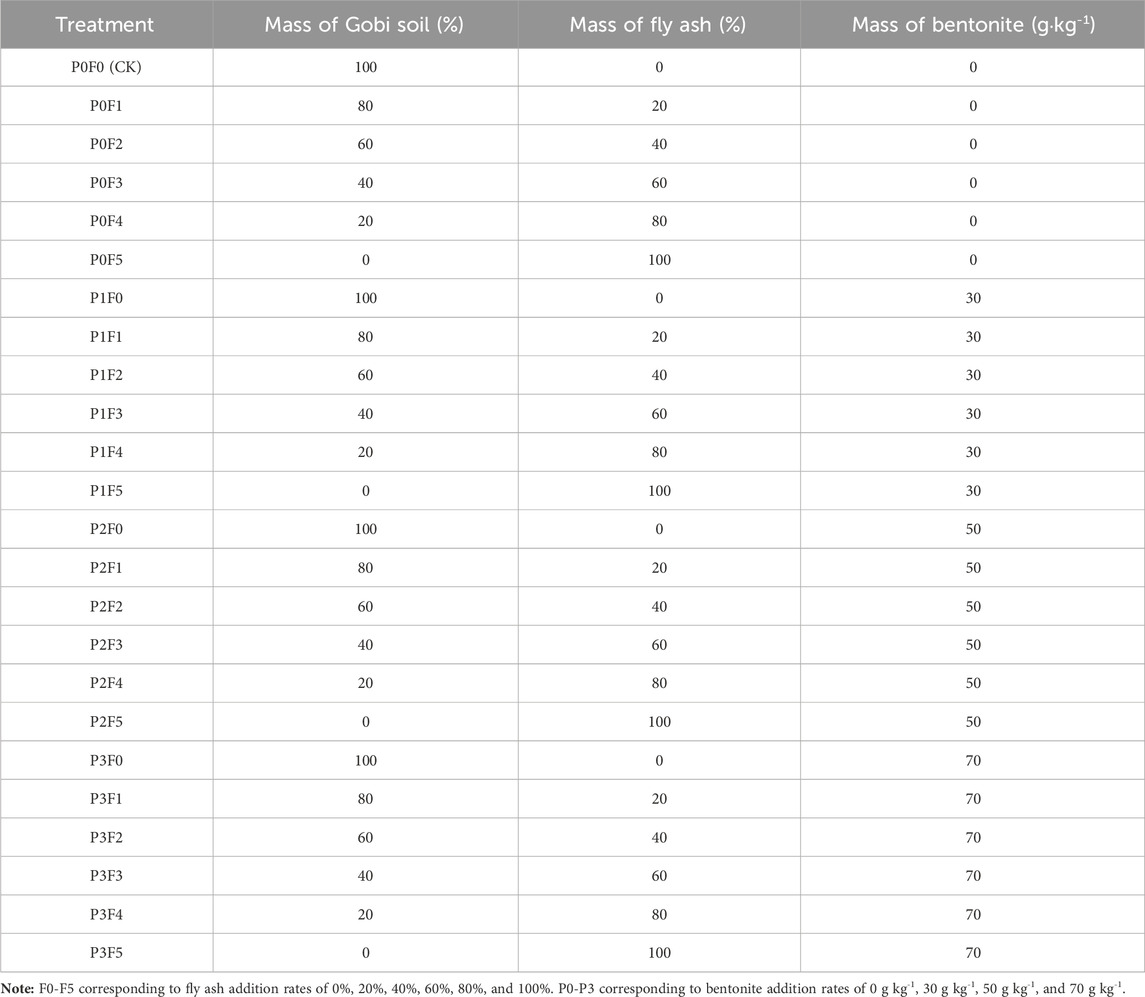
This study employed a two-factor pot experiment design to assess the effects of fly ash and bentonite on plant growth and soil properties. The experiment started on 9 April 2023, and concluded on 18 September 2023. We applied a two-factor completely randomized design with six fly ash levels (0%, 20%, 40%, 60%, 80%, 100%) and four bentonite levels (0, 30, 50, 70 g kg-1), resulting in 24 treatment combinations (Table 2). Each treatment was replicated five times (n = 5) for two plant species. Each pot (10 × 10 × 9 cm) was filled with a 1.0 kg mixture adjusted by the corresponding fly ash and bentonite ratio. Germination was conducted in trays, followed by transplantation to pots after seedling stabilization. Plants were grown in a controlled climate chamber (25/30 °C for Ephedra, 15/25 °C for Lycium, RH 75%, 12 h light/day). Water was added to maintain 80% field capacity.
2.2.2 Experimental process and procedures
The experiment was conducted in two stages. The first stage involved using seed trays for seed germination, with five seeds sown per tray, and each plant species repeated five times. Starting from the sowing date (9 April 2023), the number of germinated seeds was recorded daily, and the germination rate was calculated until it stabilized. The second stage involved transplanting the seedlings (4 July 2023) into pots (10 × 10 × 9 cm) once the germination rate had stabilized. Plant height was measured every 10 days during the cultivation phase. At the end of the pot experiment (18 September 2023), the number of surviving plants was counted, and the survival rate was calculated. All plants were harvested from the pots to measure dry matter content and assess root development. Soil moisture and plant growth conditions were monitored daily, and irrigation was applied when the surface soil (0–2 cm) showed signs of drying, such as when it could not form a cohesive clump. Irrigation was conducted to maintain soil moisture at approximately 80% of field capacity. When plants exhibited signs of nutrient deficiency, such as leaf yellowing or wilting, fertilization was applied with a 1:1000 ratio of phosphate fertilizer (monopotassium phosphate) and nitrogen fertilizer (urea), each at 15 mL per pot.
The plants were grown in a controlled environment using an artificial climate chamber, with the temperature, humidity, light duration, and sowing depth optimized for each plant species. Ephedra intermedia seeds were subjected to alternating temperatures of 25/30°C for germination, with a post-germination temperature of 20/35°C. The humidity was maintained at 75%, and plants received 12 h of light per day, with a sowing depth of 1.5–2 cm. Lycium ruthenicum seeds were pretreated at 15/25°C, with similar conditions for germination and post-germination temperatures, humidity, and light.
2.2.3 Data collection and measurement methods
Throughout the experiment, multiple growth parameters were measured periodically, including germination rate, plant height, root growth, and dry matter content. The specific measurement methods are as follows:
Germination Rate: The germination rate was determined by calculating the ratio of germinated seeds to the total number of seeds sown (Equation 1). The formula is:
Plant Height: Plant height was measured every 10 days from the collar to the tip of the highest leaf, beginning on Day 0 post-transplantation and ending on Day 120.
Root Length and Volume: Roots were carefully washed and laid flat. The root system was scanned using an HP Scanjet 8300 series scanner, and the root images were analyzed using image analysis software to calculate total root length (RL) and root volume (RV).
Dry Matter Content: At the end of the experiment, plants were harvested and dried to a constant weight in an oven. The dry matter content was calculated using the formula (Equation 2):
Soil Properties Analysis: Physical and chemical properties of the soil were measured at both the start and end of the experiment, including soil moisture (Equation 3), bulk density (Equation 4), pH, and electrical conductivity. Particle size distribution was analyzed using a laser diffraction instrument (Microtrac Bluewava), while electrical conductivity and pH were measured with an electrical conductivity meter and pH meter. Soil moisture and bulk density were determined through the drying method and calculated using the formulas:
Additionally, soil moisture retention capacity was evaluated by measuring field capacity and permanent wilting point, which are critical for understanding plant available water under different treatments.
Water Management and Fertilization: The soil moisture status was carefully monitored during the experiment. When surface soil (0–2 cm) dried or plants showed signs of water stress, irrigation was provided. Soil moisture for all treatments was maintained at around 80% of field capacity to simulate natural conditions. Field capacity (Equation 5) was calculated as:
Fertilization was applied when plants exhibited signs of nutrient deficiency (e.g., yellowing leaves), with 15 mL of a 1:1000 mixture of monopotassium phosphate and urea solution applied per pot.
2.2.4 Data statistics and analysis
To ensure statistical transparency and methodological rigor, we clarify the experimental replication structure and data size as follows. For each plant species, 24 treatment combinations (6 fly ash levels × 4 bentonite levels) were established, each with five replicate pots, resulting in 120 pots per species and 240 in total. At the germination stage, each replicate pot received five seeds (n = 25 seeds per treatment, or 600 seeds per species). Germination, survival, and subsequent growth indicators were recorded for all surviving individuals, and all analyses were based on pot-level replicates (n = 5 per treatment), unless otherwise specified. In addition, survival rate was defined as the proportion of germinated seedlings that remained alive and physiologically active at the end of the experiment (i.e., retained green leaves, no wilting or senescence).
Statistical analyses were primarily conducted using one-way and two-way analysis of variance (ANOVA) for treatment comparisons, with n = 5 replicates per treatment (unless otherwise specified), assuming normal distribution and homogeneity of variance. Where significant differences were detected, Tukey’s HSD post hoc test was applied at p < 0.05. Descriptive statistics (mean ± SE, min, max, median) are explicitly reported in the Results section for key indicators such as germination rate, plant height, root length, and dry biomass, enabling readers to assess effect sizes and variability. All data were processed using SPSS 23.0 and visualized in Origin 2024.
3 Results
3.1 Germination rate and survival rate
A total of 600 seeds per species were used for the germination experiment (25 seeds × 24 treatments), and all treatments were replicated five times (n = 25 per treatment). Germination was recorded daily, and survival was evaluated at day 162 (18 September 2023) by counting the number of seedlings that remained viable and growing.
For Lycium ruthenicum, germination rates ranged from 28% (P2F2, P2F5, and P3F5; seven seeds germinated out of 25) to 76% (P0F4; 19 out of 25), with a median value of 11.5 germinated seeds per treatment (Figure 4). The highest survival rate (100%) was observed in several moderate treatments (e.g., P0F0, P0F1, P0F3, P1F1, P1F2, etc.), indicating stable establishment under low to moderate amendment levels. In contrast, the lowest survival rate (12.5%) was recorded under P3F0 (only 1 out of 8 germinated seedlings survived). Median survival rate across all treatments was approximately 87.3%.
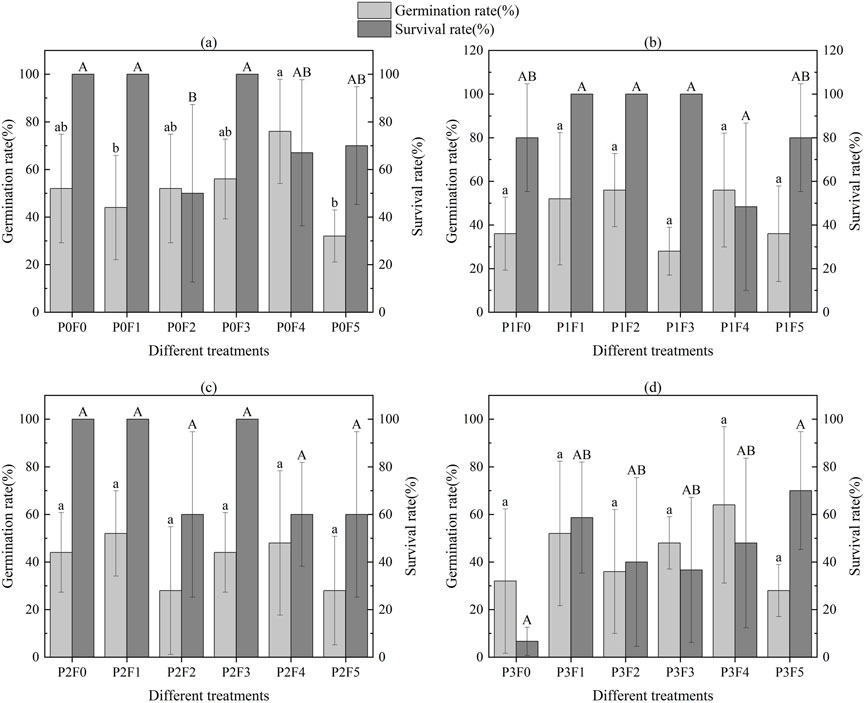
Figure 4. Effect of fly ash and bentonite on germination rate and survival rate of Lycium ruthenicum. Note: The letters “a”, “b”, “c”, “A”, “B”, etc. in the graphs indicate significant differences, and differences between different letters indicate statistical significance based on ANOVA and Tukey’s HSD test (p < 0.05). Each subplot (A–D) demonstrates the variation in germination and survival of Lycium ruthenicum at different fly ash and bentonite concentrations and their corresponding statistical results.
Statistical analysis (Table 3) showed a significant main effect of fly ash addition on germination rate (p < 0.01), and a highly significant interaction between fly ash and bentonite (p < 0.001). Survival rate was also significantly influenced by both fly ash and the interaction term (p < 0.05), while bentonite alone did not show a significant effect.
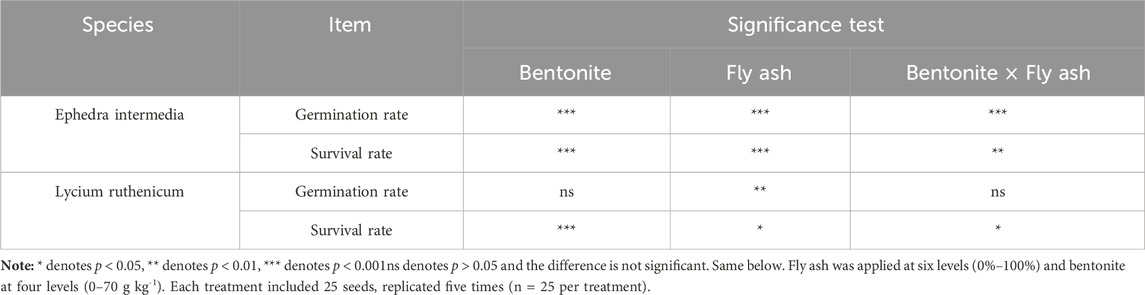
Table 3. Two-way variance analysis of fly ash and bentonite on germination rate and survival rate of plants.
For Ephedra intermedia (Figure 5), germination rates exhibited a broader distribution, ranging from as low as 8% (P1F1) to as high as 88% (P1F3 and P1F4; 22 out of 25). However, the survival rate was more variable, with values from 0% (P3F1) to 78.6% (P2F5), and a median of 42.0%. This species generally responded positively to higher fly ash concentrations, but survival was more sensitive to specific treatment combinations.
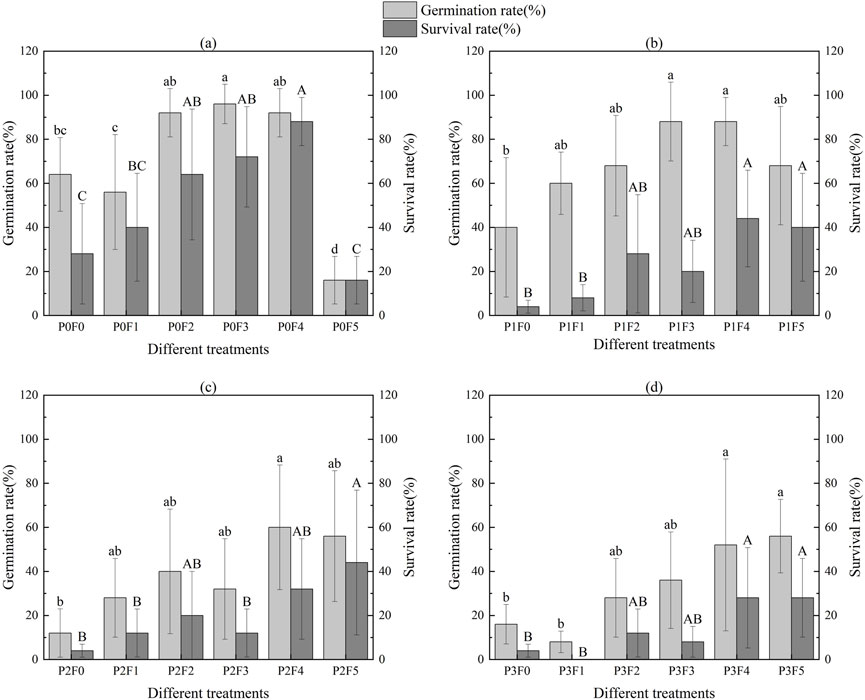
Figure 5. Effect of fly ash and bentonite on germination rate and survival rate of Ephedra intermedia. Note: Letters (a, b, c, A, B, etc.) indicate significant differences based on ANOVA and Tukey’s HSD test (p < 0.05). Each subplot (A,B,C,D) shows the variation in germination and survival of Ephedra intermedia at different fly ash and bentonite concentrations.
3.2 Plant height
Plant height was measured every 10 days post-transplantation on all surviving individuals in each treatment group. Due to differences in germination and survival rates, the number of measured plants varied across treatments, ranging from 1 to 25. Unless otherwise specified, the statistical analysis was performed using the mean value per treatment per species (n = 5 replicates).
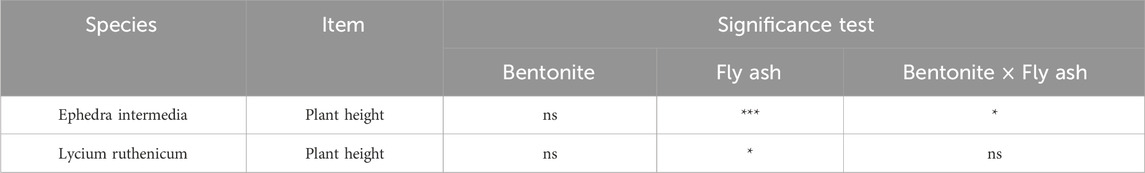
For Lycium ruthenicum, plant growth was more moderate, and the overall plant height was lower than Ephedra intermedia (Figure 6). The maximum height at day 120 was recorded under treatment P0F3 (60% fly ash, no bentonite) at 93.60 mm, followed by P0F4 (72.09 mm) and P1F4 (80.00 mm). Control treatment P0F0 resulted in a much lower height (34.55 mm). Notably, the application of bentonite alone (e.g., P1F0–P1F5) without fly ash did not yield substantial increases in plant height. Two-way ANOVA (Table 4) confirmed that fly ash had a significant effect on plant height (p < 0.01), whereas the bentonite main effect and its interaction with fly ash were not statistically significant (p > 0.05).
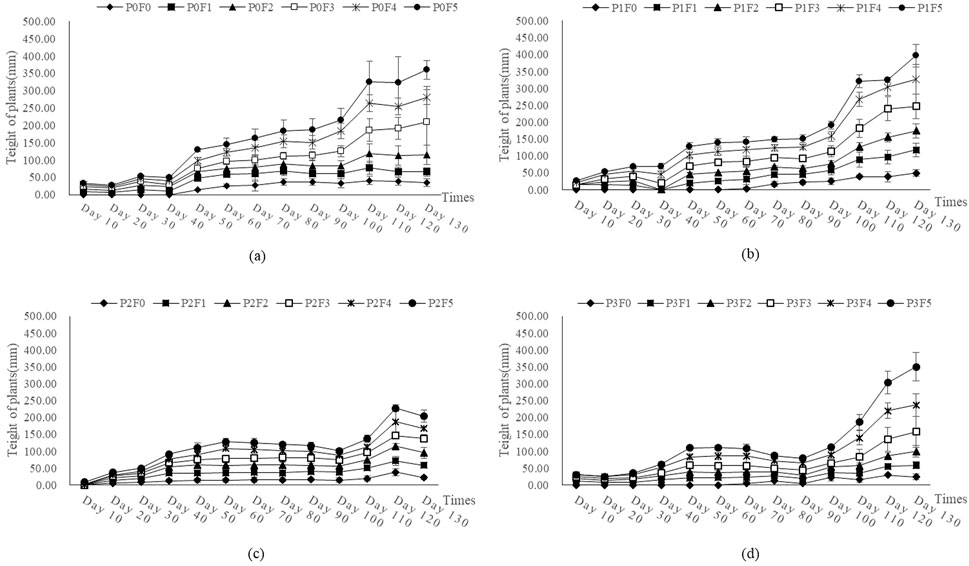
Figure 6. Effect of fly ash and bentonite on plant height of Lycium ruthenicum. Note: The x-axis represents time (days) and the y-axis represents plant height (mm). Each curve corresponds to a treatment group (e.g., P0F0, P0F1, P0F2), differentiated by symbols and line styles. Error bars show data variability, and different letters (A–D) indicate significant differences between treatments based on ANOVA.
Two-way ANOVA (Table 4) confirmed that fly ash had a significant effect on plant height (p < 0.01), whereas the bentonite main effect and its interaction with fly ash were not statistically significant (p > 0.05).
In contrast, Ephedra intermedia exhibited a steady increase in plant height over time across treatments, with marked differences due to fly ash and bentonite concentrations (Figure 7). By day 120, the tallest plants were observed under treatment P3F5 (100% fly ash and 70 g kg-1 bentonite), reaching a mean height of 138.33 mm, followed by P3F4 (125.14 mm) and P3F3 (97.00 mm). In contrast, control treatment P0F0 yielded significantly shorter plants with a final mean height of 83.86 mm. Treatments with 0% bentonite (P0F*) also showed a clear gradient, where moderate fly ash addition (e.g., P0F4, 119.76 mm) significantly enhanced plant height. Two-way ANOVA results (Table 4) indicated that fly ash had a highly significant effect on Ephedra intermedia height (p < 0.001), and the interaction with bentonite was also significant (p < 0.05), while the bentonite main effect alone was not statistically significant (p > 0.05).
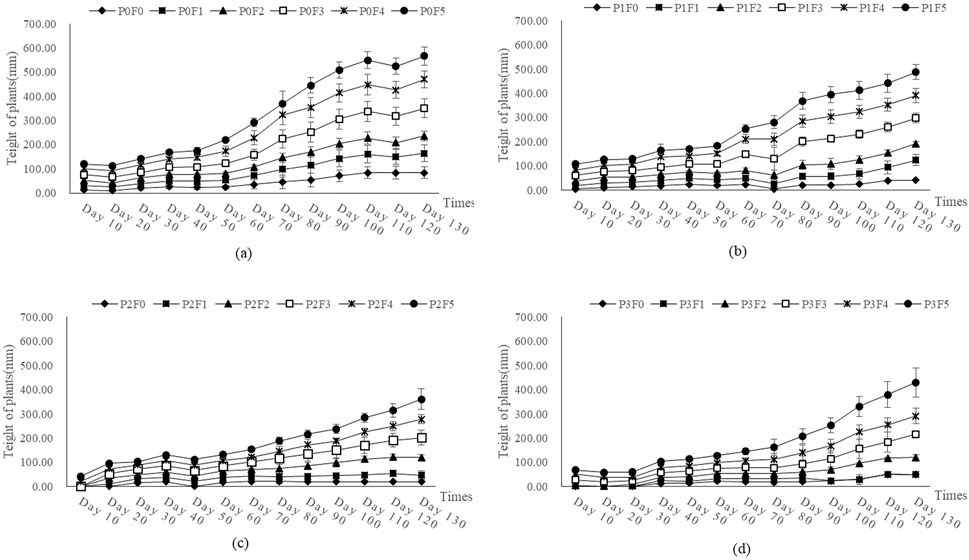
Figure 7. Effect of fly ash and bentonite on plant height of Ephedra intermedia. Note: Plant height trends over time under different treatments. Each subplot (A–D) corresponds to a different experimental group (P0, P1, P2, P3). The x-axis represents time (days), and the y-axis represents plant height (mm). Each curve represents a treatment group, with error bars indicating data variability.
3.3 Dry mass
Figure 8 shows the dry matter content (%) of Lycium ruthenicum and Ephedra intermedia under various combinations of fly ash and bentonite. For Lycium ruthenicum, dry mass varied considerably across treatments, with the highest value observed in the control group P0F0 (44.13% ± 21.7%). Compared to this, the addition of either amendment generally led to a decrease in dry matter content. In particular, high-dose treatments such as P0F5 (13.8% ± 10.74%) and P1F2 (10.4% ± 6.02%) significantly suppressed biomass accumulation, while moderate fly ash levels (e.g., P0F2: 40.22% ± 1.67%) still supported relatively high biomass. Interestingly, certain treatments under high bentonite levels (e.g., P3F1: 33.05% ± 19.88%) partially restored dry mass, suggesting a possible mitigating effect under specific conditions. However, extreme reductions were still noted under P3F0 (7.58% ± 13.12%).
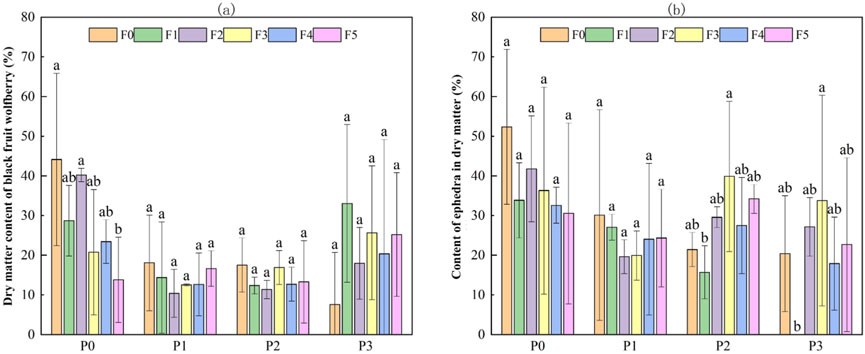
Figure 8. Effect of fly ash and bentonite on dry mass content of Lycium ruthenicum and Ephedra intermedia. Note: Bars represent the mean ± SE. Different letters indicate significant differences (ANOVA, Tukey’s HSD test, p < 0.05).
In contrast, Ephedra intermedia exhibited a more moderate response. The highest dry mass was found under the control P0F0 (52.34% ± 19.53%), followed by P0F2 (41.79% ± 13.35%) and P3F3 (33.76% ± 26.53%). Unlike Lycium, some treatments with higher bentonite levels (e.g., P2F3: 39.85% ± 18.92%, P2F5: 34.19% ± 3.63%) yielded relatively stable or even improved dry mass. Notably, bentonite-alone treatments (P1F*) consistently resulted in lower dry mass, with values ranging from 19.62% to 30.14%. Among all combinations, the extreme case of bentonite-only plus no fly ash (P1F2: 19.62% ± 4.24%) showed marked suppression, indicating that bentonite by itself was not beneficial.
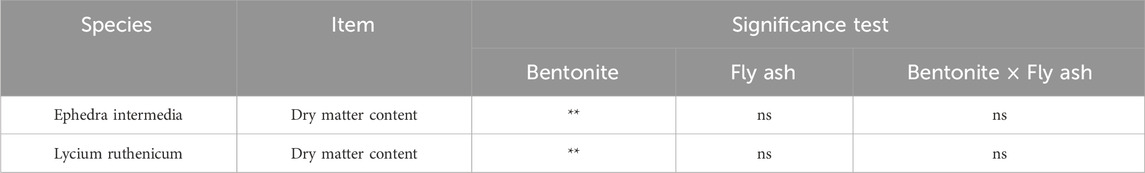
According to the two-way ANOVA (Table 5), bentonite addition had a statistically significant main effect on dry matter content for both species (p < 0.01), whereas fly ash addition alone and its interaction with bentonite were not statistically significant (p > 0.05).
3.4 Root characteristics
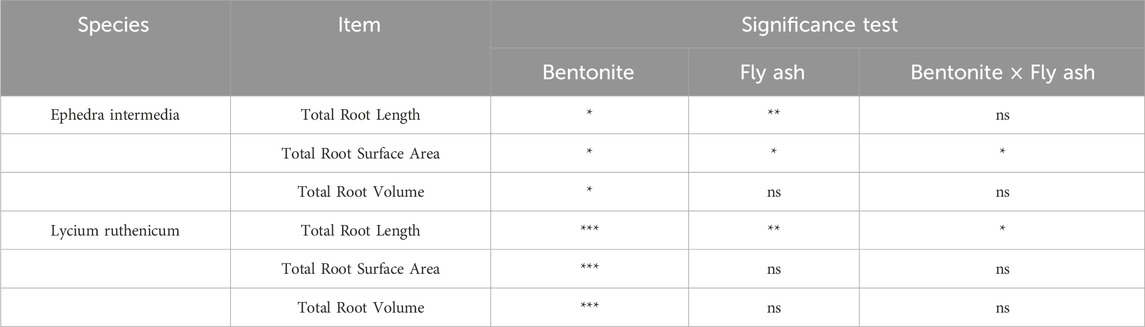
The root traits of Lycium ruthenicum exhibited notable variations across treatments with different fly ash and bentonite combinations (Figure 9). The control treatment (P0F0) showed a moderate root length of 25.41 ± 9.60 mm, surface area of 2.06 ± 0.57 cm2, and volume of 0.026 ± 0.01 cm3. Moderate fly ash additions (e.g., P0F2 and P0F4) significantly enhanced root development. Specifically, P0F2 achieved the highest values for both root surface area (14.21 ± 13.07 cm2) and root volume (0.359 ± 0.401 cm3), while P0F4 promoted the longest roots (138.53 ± 40.77 mm). However, excessive fly ash (P0F5) and high bentonite treatments (e.g., P3F0–P3F3) suppressed root traits, with minimum root length observed under P3F0 (4.45 ± 1.71 mm) and the lowest root volume (0.001 ± 0.000 cm3). In treatments combining fly ash and bentonite (e.g., P1F3 and P2F5), root traits showed moderate recovery, with P3F5 recording root length of 68.45 ± 2.92 mm, surface area of 5.53 ± 0.54 cm2, and volume of 0.082 ± 0.004 cm3.
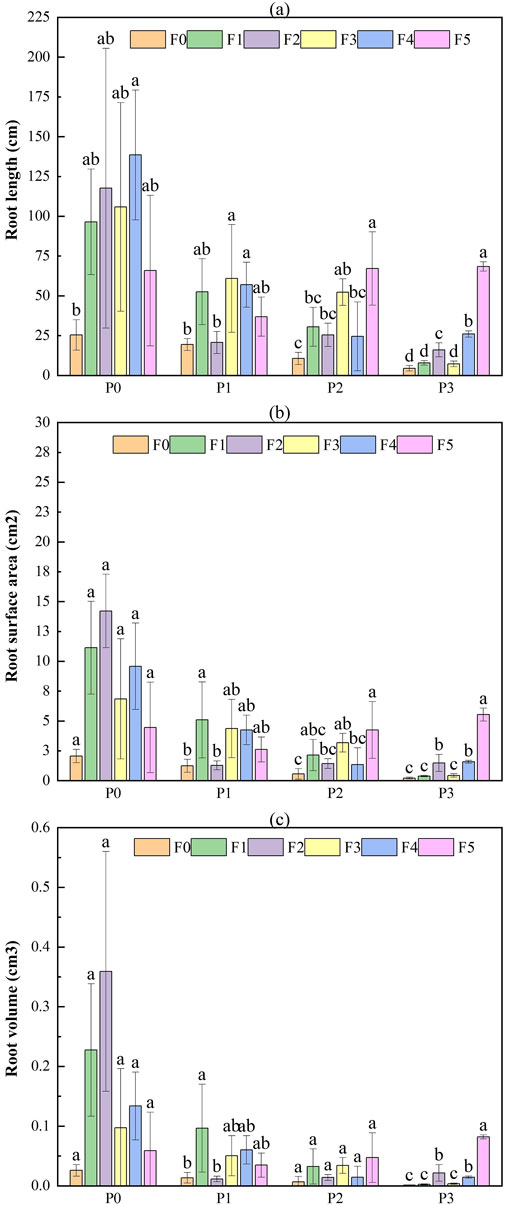
Figure 9. Effect of fly ash and bentonite on the root characteristics of Lycium ruthenicum. Note: Bars represent the mean ± SE. Different letters indicate significant differences (ANOVA, Tukey’s HSD test, p < 0.05).
Ephedra intermedia showed a more stable response across treatments, with less pronounced suppression under bentonite-only treatments (Figure 10). The control group (P0F0) had root length, surface area, and volume values of 35.24 ± 20.49 mm, 4.55 ± 4.32 cm2, and 0.099 ± 0.134 cm3, respectively. Root development generally improved with increasing fly ash concentration, especially under P0F3 (88.55 ± 82.74 mm, 7.37 ± 4.23 cm2, 0.155 ± 0.09 cm3) and P0F4 (68.10 ± 44.08 mm, 9.34 ± 8.70 cm2, 0.233 ± 0.298 cm3). The best overall performance was observed under P3F5, where root surface area and volume reached 10.18 ± 3.09 cm2 and 0.200 ± 0.130 cm3, respectively. In contrast, bentonite-only treatments (P1F0, P2F0, P3F0) completely suppressed root growth (all values 0).
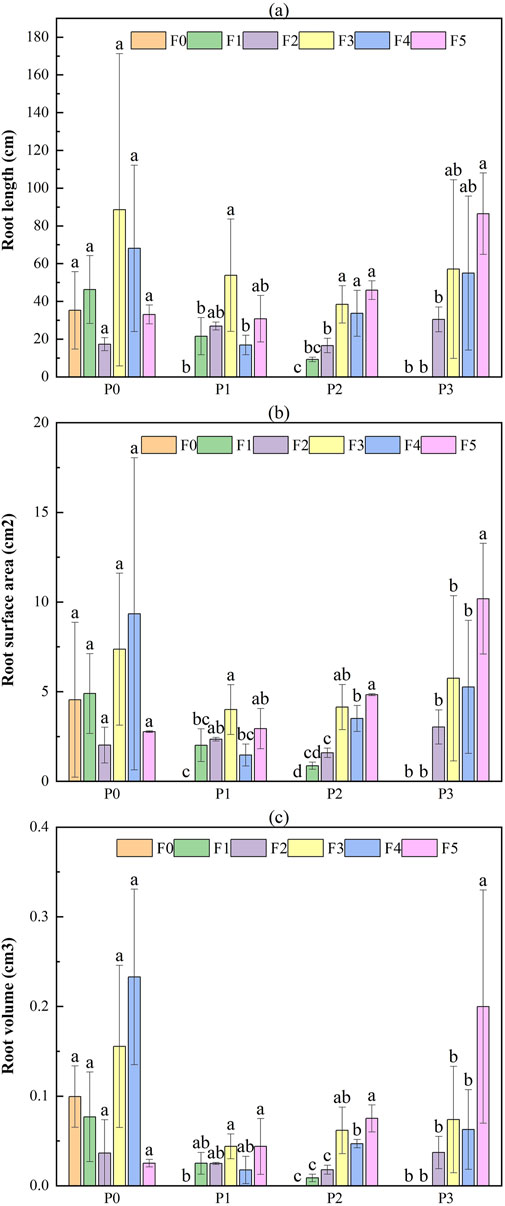
Figure 10. Effect of fly ash and bentonite on the root characteristics of Ephedra intermedia. Note: Bars represent the mean ± SE. Different letters indicate significant differences (ANOVA, Tukey’s HSD test, p < 0.05).
Two-way ANOVA results (Table 6) showed that for Lycium ruthenicum, fly ash, bentonite, and their interaction all had significant effects on root length and surface area (p < 0.01 for main effects; p < 0.05 for interaction). Root volume was mainly influenced by fly ash (p < 0.01), with bentonite and the interaction showing weaker or non-significant effects. In contrast, for Ephedra intermedia, fly ash significantly affected all root traits (length and surface area: p < 0.01; volume: p < 0.05), while bentonite had significant effects on root length and surface area (p < 0.05), but not on volume. The interaction term was not statistically significant.
4 Discussion
4.1 Effect of fly ash and bentonite on plant growth
This study revealed distinct species-specific responses to fly ash and bentonite during germination. For Lycium ruthenicum, fly ash had a significant main effect on germination rate (p < 0.01), while bentonite and its interaction with fly ash were not significant (p > 0.05; Table 3). Germination peaked at moderate fly ash levels (e.g., P0F4: 76%) but declined under high concentrations (e.g., P2F2, P2F5, P3F5: all 28%), likely due to heavy metal toxicity or pH imbalance. These results align with previous findings that moderate fly ash improves soil porosity and nutrient availability, enhancing germination (Pani et al., 2015), whereas excessive doses may induce physiological stress. Contrary to studies suggesting bentonite enhances germination via improved water retention and ion immobilization (Abdurakhimov et al., 2023), we found no significant effect of bentonite alone on L. ruthenicum. Treatments with bentonite but without fly ash (e.g., P1F0–P1F5) showed no consistent improvement over the control, suggesting that L. ruthenicum is more sensitive to fly ash than to bentonite during early growth. In contrast, Ephedra intermedia responded more favorably. Germination was significantly influenced by both amendments and their interaction (p < 0.001; Table 3), with the highest rates (88%) under combined treatments (e.g., P1F3, P1F4), indicating synergistic effects. This species appears more tolerant to high amendment levels, likely due to its inherent stress resilience. These results underscore the importance of species-specific amendment strategies.
4.2 Plant survival
The survival pattern of Lycium ruthenicum closely mirrored its germination response. Survival was significantly affected by fly ash and its interaction with bentonite (p < 0.05), while bentonite alone had no significant effect (p > 0.05; Table 3). Highest survival rates (up to 100%) occurred under moderate fly ash levels, including P0F0, P0F1, and P0F3. In contrast, excessive amendments (e.g., P3F0) drastically reduced survival, with only 1 of 8 seedlings (12.5%) surviving. These results support previous findings that moderate amendment levels can improve early seedling establishment, whereas excessive inputs may cause abiotic stress due to heavy metals or altered pH (Krzaklewski et al., 2012; Pietrzykowski et al., 2015). By contrast, Ephedra intermedia showed significant responses to all three factors: bentonite (p < 0.001), fly ash (p < 0.001), and their interaction (p < 0.01; Table 3). Survival varied widely, from 0% (P3F1) to 78.6% (P2F5). Several moderate-to-high combined treatments (e.g., P1F3, P2F5, P3F3) enhanced survival, indicating a greater tolerance to soil amendments. This may reflect adaptive traits enabling E. intermedia to exploit improved soil aeration and nutrient supply, even under higher amendment stress. Overall, these results emphasize the need for species-specific amendment strategies. L. ruthenicum requires more cautious application rates to avoid stress, while E. intermedia exhibits broader tolerance and benefits from combined treatments at higher dosages.
4.3 Plant height and dry matter accumulation
Plant height responses differed markedly between Lycium ruthenicum and Ephedra intermedia, with fly ash being the primary influencing factor (Gupta et al., 2002; Usman et al., 2023). For L. ruthenicum, two-way ANOVA showed a significant effect of fly ash on height (p < 0.01), while bentonite and its interaction were not significant (p > 0.05; Table 4). The tallest plants were observed under moderate fly ash without bentonite—P0F3 (93.60 mm) and P0F4 (72.09 mm). Treatments with bentonite alone (P1F0–P1F5) showed minimal height gains, and the control group (P0F0) had the lowest height (34.55 mm). These results suggest that moderate fly ash promotes vertical growth, while bentonite alone may hinder it. In contrast, E. intermedia exhibited greater and more consistent height increases across a broader amendment range. Plant height was significantly affected by fly ash (p < 0.001) and its interaction with bentonite (p < 0.05), but not by bentonite alone (p > 0.05; Table 4). Maximum heights were observed under high-dose combinations: P3F5 (138.33 mm), P3F4 (125.14 mm), and P3F3 (97.00 mm). Even without bentonite, height increased with fly ash concentration, peaking at 119.76 mm (P0F4), indicating synergistic effects and a higher tolerance in E. intermedia (Yu et al., 2019).
Dry mass patterns also revealed species-level differences. For L. ruthenicum, bentonite significantly affected biomass (p < 0.01), while fly ash and the interaction were not significant (Table 5). The highest biomass occurred in the control (P0F0: 44.13% ± 21.70%) and declined under bentonite-only or high fly ash treatments (e.g., P0F5: 13.80% ± 10.74%; P1F2: 10.40% ± 6.02%). Moderate fly ash doses (e.g., P0F2: 40.22% ± 1.67%) maintained relatively high biomass, suggesting tolerance to limited amendment inputs (Pandey et al., 2009). For E. intermedia, dry mass also peaked in the control (P0F0: 52.34% ± 19.53%) but remained stable across a wider treatment range. Bentonite significantly influenced biomass (p < 0.01), while fly ash and the interaction had no significant effects. High bentonite treatments (e.g., P2F3: 39.85% ± 18.92%; P2F5: 34.19% ± 3.63%) supported comparable or improved biomass, likely due to enhanced water retention and nutrient buffering. However, extreme bentonite-only treatments (e.g., P1F2: 19.62% ± 4.24%) suppressed growth, underscoring the importance of dose optimization.
Overall, E. intermedia demonstrated greater plasticity and resilience in height and biomass accumulation, while L. ruthenicum was more sensitive and favored moderate fly ash levels with minimal bentonite.
4.4 Root development and water retention
Root traits responded significantly to fly ash and bentonite amendments in a species- and treatment-specific manner (Gupta et al., 2002). For Lycium ruthenicum, two-way ANOVA showed that root length and surface area were significantly affected by both fly ash and bentonite (p < 0.01), with interaction effects also significant (p < 0.05); root volume was primarily influenced by fly ash (p < 0.01; Table 6). Moderate fly ash treatments, especially P0F2 and P0F4, enhanced root traits markedly—P0F4 achieved the greatest length (138.53 ± 40.77 mm), and P0F2 yielded the largest surface area (14.21 ± 13.07 cm2) and volume (0.359 ± 0.401 cm3). Conversely, excessive amendments (e.g., P3F0) severely inhibited root growth (length: 4.45 ± 1.71 mm, surface area: 0.22 ± 0.07 cm2, volume: 0.001 ± 0.000 cm3). Notably, partial recovery in high-dose combinations (e.g., P3F5) suggests bentonite may mitigate fly ash toxicity (He et al., 2017). Ephedra intermedia exhibited more stable root development across treatments. Root length and surface area were significantly influenced by both amendments (p < 0.05), while root volume was affected by fly ash only (p < 0.05); interaction effects were not significant (Table 6). Compared to the control (P0F0: 35.24 ± 20.49 mm, 4.55 ± 4.32 cm2, 0.099 ± 0.134 cm3), substantial improvements occurred under P0F3 and P0F4, and peaked at P3F5 (10.18 ± 3.09 cm2 surface area, 0.200 ± 0.130 cm3 volume). However, bentonite-only treatments (e.g., P1F0–P3F0) completely suppressed root development (all traits = 0), indicating that fly ash presence is essential for root formation in E. intermedia.
These results confirm that while both species benefit from moderate fly ash additions, E. intermedia is more resilient under high-dose amendments, especially when bentonite co-applies. In contrast, L. ruthenicum shows stronger sensitivity to amendment extremes and performs best under moderate, balanced applications.
4.5 Practical applications and research outlook
Although Lycium ruthenicum and Ephedra intermedia reach full maturity over several years, early-stage traits—such as germination rate, seedling height, root development, and biomass—are widely recognized as effective indicators of plant-soil interactions and adaptive potential under restoration treatments (Szerement et al., 2021; Wasilkowski et al., 2019). Our 5-month controlled experiment provides valuable insights into species’ early ecological responses to fly ash and bentonite, serving as a proxy for long-term restoration success.
The results highlight the synergistic benefits of combining fly ash and bentonite, which improved soil structure, nutrient availability, and water retention, thereby enhancing seedling establishment. These improvements have promising implications for both ecological restoration and sustainable agriculture in arid regions, potentially accelerating land recovery and reducing external inputs (Abulimiti et al., 2023). Moreover, repurposing fly ash supports circular economy goals by transforming waste into a valuable soil amendment with additional contributions to carbon sequestration and soil health.
However, the reduced plant performance at high amendment levels indicates species-specific tolerance thresholds and potential trade-offs, such as heavy metal toxicity or nutrient imbalances. This underscores the need for optimized dosage strategies and tailored applications. Future research should expand to diverse plant functional types, incorporate multi-season field trials, and assess environmental risks to ensure ecological efficacy and long-term safety.
5 Conclusion
This study examined the effects of fly ash and bentonite amendments on early-stage growth of two desert shrubs, Lycium ruthenicum and Ephedra intermedia, under controlled pot conditions. The results revealed distinct species-specific responses to amendment regimes. L. ruthenicum showed optimal germination, survival, and growth under moderate fly ash application (40%–60%) without bentonite, while higher amendment levels—particularly bentonite alone or excessive fly ash—significantly suppressed biomass and root traits. In contrast, E. intermedia exhibited greater tolerance and plasticity, maintaining stable growth and even enhanced performance under combined high-dose treatments, especially in root development. However, bentonite alone consistently inhibited root formation in both species. These findings highlight the necessity of species-specific amendment strategies: moderate fly ash rates with minimal bentonite are preferable for sensitive species like L. ruthenicum, whereas more resilient species such as E. intermedia can benefit from synergistic amendment combinations. Importantly, excessive amendment levels reduced performance in both species, underscoring the need for careful dosage calibration to optimize restoration outcomes in arid and degraded environments.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YW: Conceptualization, Funding acquisition, Methodology, Visualization, Writing – original draft, Writing – review and editing. YL: Visualization, Writing – review & editing. DX: Methodology, Visualization, Writing – review and editing. LJ: Investigation, Methodology, Writing – review & editing. ZX: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Visualization, Writing – original draft. JY: Conceptualization, Funding acquisition, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by Natural Science Foundation of Xinjiang Uygur Autonomous Region (No. 2024TSYCCX004, No. 2023E01006, No.2022D01B234), National Key Research and Development Program of China (No. 2023YFF1304200).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdurakhimov, N., Kadirova, S., and Davronova, Z. (2023). Advantages of covering seed with bentonite mud. Tex. J. Agric. Biol. Sci. 14, 108–111.
Abulimiti, M., Wang, J., Li, C., Zhang, Y., and Li, S. (2023). Bentonite could be an eco-friendly windbreak and sand-fixing material. Environ. Technol. & Innovation 29, 102981. doi:10.1016/j.eti.2022.102981
Alotaibi, K. D., and Schoenau, J. J. (2019). Addition of biochar to a sandy desert soil: effect on crop growth, water retention and selected properties. Agronomy 9 (6), 327. doi:10.3390/agronomy9060327
Ayub, M. A., Usman, M., Faiz, T., Umair, M., ul Haq, M. A., Rizwan, M., et al. (2020). Restoration of degraded soil for sustainable agriculture. Soil Health Restor. Manag., 31–81. doi:10.1007/978-981-13-8570-4_2
Cao, D.-z., Selic, E., and Herbell, J.-D. (2008). Utilization of fly ash from coal-fired power plants in China. J. Zhejiang University-SCIENCE A 9, 681–687. doi:10.1631/jzus.a072163
Croker, J., Poss, R., Hartmann, C., and Bhuthorndharaj, S. (2004). Effects of recycled bentonite addition on soil properties, plant growth and nutrient uptake in a tropical sandy soil. Plant Soil 267, 155–163. doi:10.1007/s11104-005-4641-x
Deka, A., and Sekharan, S. (2017). Contaminant retention characteristics of fly ash–bentonite mixes. Waste Manage. Res. 35 (1), 40–46. doi:10.1177/0734242x16670002
Gupt, C. B., Bordoloi, S., Sahoo, R. K., and Sekharan, S. (2021). Mechanical performance and micro-structure of bentonite-fly ash and bentonite-sand mixes for landfill liner application. J. Clean. Prod. 292, 126033. doi:10.1016/j.jclepro.2021.126033
Gupta, D. K., Rai, U. N., Tripathi, R. D., and Inouhe, M. (2002). Impacts of fly-ash on soil and plant responses. J. Plant Res. 115, 401–409. doi:10.1007/s10265-002-0057-3
He, H., Dong, Z., Peng, Q., Wang, X., Fan, C., and Zhang, X. (2017). Impacts of coal fly ash on plant growth and accumulation of essential nutrients and trace elements by alfalfa (Medicago sativa) grown in a loessial soil. J. Environ. Manage. 197, 428–439. doi:10.1016/j.jenvman.2017.04.028
Jiang, C., Liu, J., Zhang, H., Zhang, Z., and Wang, D. (2019). China’s progress towards sustainable land degradation control: insights from the northwest arid regions. Ecol. Eng. 127, 75–87. doi:10.1016/j.ecoleng.2018.11.014
Karimi, L., and Salem, A. (2011). The role of bentonite particle size distribution on kinetic of cation exchange capacity. J. Industrial Eng. Chem. 17 (1), 90–95. doi:10.1016/j.jiec.2010.12.002
Krzaklewski, W., Pietrzykowski, M., and Woś, B. (2012). Survival and growth of alders (Alnus glutinosa (L.) Gaertn. and Alnus incana (L.) Moench) on fly ash technosols at different substrate improvement. Ecol. Eng. 49, 35–40. doi:10.1016/j.ecoleng.2012.08.026
Lal, R. (2017). Soil erosion by wind and water: problems and prospects, soil erosion research methods. Routledge, 1–10.
Luo, Y., Wu, Y., Ma, S., Zheng, S., Zhang, Y., and Chu, P. K. (2021). Utilization of coal fly ash in China: a mini-review on challenges and future directions. Environ. Sci. Pollut. Res. 28, 18727–18740. doi:10.1007/s11356-020-08864-4
Ma, R., Zong, Y., and Lu, S. (2012). Reducing bioavailability and leachability of copper in soils using coal fly ash, apatite, and bentonite. Commun. Soil Sci. Plant Anal. 43 (15), 2004–2017. doi:10.1080/00103624.2012.693232
Mganga, K. Z., Musimba, N. K., and Nyariki, D. M. (2015). Combining sustainable land management technologies to combat land degradation and improve rural livelihoods in semi-arid lands in Kenya. Environ. Manage. 56 (6), 1538–1548. doi:10.1007/s00267-015-0579-9
Mi, J., Gregorich, E. G., Xu, S., McLaughlin, N. B., Ma, B., and Liu, J. (2017). Effect of bentonite amendment on soil hydraulic parameters and millet crop performance in a semi-arid region. Field Crops Res. 212, 107–114. doi:10.1016/j.fcr.2017.07.009
Mi, J., Gregorich, E. G., Xu, S., McLaughlin, N. B., Ma, B., and Liu, J. (2021). Changes in soil biochemical properties following application of bentonite as a soil amendment. Eur. J. Soil Biol. 102, 103251. doi:10.1016/j.ejsobi.2020.103251
Naorem, A., Jayaraman, S., Dang, Y. P., Dalal, R. C., Sinha, N. K., Rao, C. S., et al. (2023). Soil constraints in an arid environment—challenges, prospects, and implications. Agronomy 13 (1), 220. doi:10.3390/agronomy13010220
Pal, S. K., and Ghosh, A. (2014). Volume change behavior of fly ash–montmorillonite clay mixtures. Int. J. Geomech. 14 (1), 59–68. doi:10.1061/(asce)gm.1943-5622.0000300
Pandey, V. C., Abhilash, P., and Singh, N. (2009). The Indian perspective of utilizing fly ash in phytoremediation, phytomanagement and biomass production. J. Environ. Manage. 90 (10), 2943–2958. doi:10.1016/j.jenvman.2009.05.001
Pandey, V. C., and Singh, N. (2010). Impact of fly ash incorporation in soil systems. Agric. Ecosyst. Environ. 136 (1-2), 16–27. doi:10.1016/j.agee.2009.11.013
Pani, N. K., Samal, P., Das, R., and Sahoo, S. (2015). Effect of fly ash on growth and yield of sunflower (Helianthus annuus L.). Int. J. Agron. Agric. Res. 7 (2), 64–74.
Pietrzykowski, M., Krzaklewski, W., and Woś, B. (2015). Preliminary assessment of growth and survival of green alder (Alnus viridis), a potential biological stabilizer on fly ash disposal sites. J. For. Res. 26, 131–136. doi:10.1007/s11676-015-0016-1
Ramesh, G. P., Shah, P., Sharma, D., Kamesh, A. M. K., and Maitry, A. (2024). Impacts of fly ash on different vegetation near industrial areas: a review. Environ. Ecol. 42 (2), 470–478. doi:10.60151/envec/xeqb1441
Shao, W., Zhang, Z., Guan, Q., Yan, Y., and Zhang, J. (2024). Comprehensive assessment of land degradation in the arid and semiarid area based on the optimal land degradation index model. Catena 234, 107563. doi:10.1016/j.catena.2023.107563
Smith, P., House, J. I., Bustamante, M., Sobocká, J., Harper, R., Pan, G., et al. (2016). Global change pressures on soils from land use and management. Glob. Chang. Biol. 22 (3), 1008–1028. doi:10.1111/gcb.13068
Sonderegger, T., and Pfister, S. (2021). Global assessment of agricultural productivity losses from soil compaction and water erosion. Environ. Sci. Technol. 55 (18), 12162–12171. doi:10.1021/acs.est.1c03774
Szerement, J., Szatanik-Kloc, A., Jarosz, R., Bajda, T., and Mierzwa-Hersztek, M. (2021). Contemporary applications of natural and synthetic zeolites from fly ash in agriculture and environmental protection. J. Clean. Prod. 311, 127461. doi:10.1016/j.jclepro.2021.127461
Thomas, G., Dalal, R., and Standley, J. (2007). No-till effects on organic matter, pH, cation exchange capacity and nutrient distribution in a Luvisol in the semi-arid subtropics. Soil Tillage Res. 94 (2), 295–304. doi:10.1016/j.still.2006.08.005
Usman, M., Anastopoulos, I., Hamid, Y., and Wakeel, A. (2023). Recent trends in the use of fly ash for the adsorption of pollutants in contaminated wastewater and soils: effects on soil quality and plant growth. Environ. Sci. Pollut. Res. 30 (60), 124427–124446. doi:10.1007/s11356-022-19192-0
Visser, S., Sterk, G., and Ribolzi, O. (2004). Techniques for simultaneous quantification of wind and water erosion in semi-arid regions. J. Arid. Environ. 59 (4), 699–717. doi:10.1016/j.jaridenv.2004.02.005
Wang, H., Li, J., Tao, W., Zhang, X., Gao, X., Yong, J., et al. (2018). Lycium ruthenicum studies: molecular biology, phytochemistry and pharmacology. Food Chem. 240, 759–766. doi:10.1016/j.foodchem.2017.08.026
Wang, Y., Yang, K., and Tang, Z. (2019). In situ effect of combined utilization of fly ash and polyacrylamide on sand stabilization in North China. Catena 172, 170–178. doi:10.1016/j.catena.2018.08.022
Wang, Y.-x. (2000). Climate change in Turpan Basin and its possible impacts on the water resources. J. Desert Res. 20 (2), 207.
Wasilkowski, D., Nowak, A., Michalska, J., and Mrozik, A. (2019). Ecological restoration of heavy metal-contaminated soil using Na-bentonite and green compost coupled with the cultivation of the grass Festuca arundinacea. Ecol. Eng. 138, 420–433. doi:10.1016/j.ecoleng.2019.08.004
Yang, H., Zou, X., Wang, J. a., and Shi, P. (2019). An experimental study on the influences of water erosion on wind erosion in arid and semi-arid regions. J. Arid Land 11, 208–216. doi:10.1007/s40333-019-0097-3
Yang, M., Zhou, D., Hang, H., Chen, S., Liu, H., Su, J., et al. (2024). Effects of balancing exchangeable cations Ca, Mg, and K on the growth of tomato seedlings (Solanum lycopersicum L.) based on increased soil cation exchange capacity. Agronomy 14 (3), 629. doi:10.3390/agronomy14030629
Yin, H., Yan, X., Zhang, W., Shi, Y., Qian, C., Yin, C., et al. (2016). Geographical or ecological divergence between the parapatric species Ephedra sinica and E. intermedia? Plant Syst. Evol. 302, 1157–1170. doi:10.1007/s00606-016-1323-5
Yu, C.-L., Deng, Q., Jian, S., Li, J., Dzantor, E. K., and Hui, D. (2019). Effects of fly ash application on plant biomass and element accumulations: a meta-analysis. Environ. Pollut. 250, 137–142. doi:10.1016/j.envpol.2019.04.013
Keywords: fly ash and bentonite mixture, ecological restoration, soil amendments, plant growth, soil properties
Citation: Wang Y, Liu Y, Xu D, Jin L, Xu Z and Yang J (2025) Restoring arid ecosystems: the synergistic benefits of fly ash and bentonite soil amendments. Front. Environ. Sci. 13:1571505. doi: 10.3389/fenvs.2025.1571505
Received: 06 February 2025; Accepted: 20 June 2025;
Published: 30 July 2025.
Edited by:
Chong Jiang, Guangdong Academy of Science (CAS), ChinaReviewed by:
Nguyen Van Loc, Vietnam National University of Agriculture, VietnamChrizette Neethling, Institute of Ecology and Botany, Centre for Ecological Research, Hungarian Academy of Sciences, Hungary
Copyright © 2025 Wang, Liu, Xu, Jin, Xu and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhonglin Xu, emx4dUB4anUuZWR1LmNu; Jianjun Yang, eWpqQHhqdS5lZHUuY24=
 Yao Wang
Yao Wang Yingzhen Liu6
Yingzhen Liu6