- 1Northwest Fisheries Science Center, NOAA Fisheries, Seattle, WA, United States
- 2United States Geological Survey, Western Geographic Science Center, Tucson, AZ, United States
Wet meadows are globally significant ecosystems that provide critical hydrological, ecological, and biogeochemical functions, yet their extent has declined dramatically due to land use changes and hydrologic alteration. These sedge-dominated wetlands exist at the drier end of the wetland gradient, maintained by shallow groundwater and periodic inundation. This paper is a global synthesis of the ecological, geomorphic, and hydrological dynamics of wet meadows, with an emphasis on alluvial systems, to inform effective restoration strategies. We compare wet meadows to other wetlands, classify them into palustrine, lacustrine, and alluvial types, then focus on alluvial wet meadows and discuss how their formation and persistence depend on ground and surface water interactions, sediment deposition and flow obstructions, all mediated by biological processes. In particular, we highlight the role of hydric graminoids in resisting erosion and maintaining soil cohesion, how beaver promote meadow persistence, and the significance of wet meadows as carbon sinks. We also present stratigraphic evidence demonstrating that incision, often triggered by anthropogenic activity or changing climate, is the primary mechanism of alluvial wet meadow degradation, resulting in water table decline and shifts in vegetation composition. Restoration requires reversing these incisional processes through techniques that elevate water tables, disperse flow and retain sediment—methods traditionally associated with either soil conservation or stream restoration. These include nature-based solutions that create obstructions such as beaver dams and their analogues, rock and wood-based obstructions and incision trench or gully filling and grading. Given their multifunctional value—including but not limited to flood attenuation, biodiversity support, and carbon sequestration—wet meadows warrant a focused restoration framework. This review advocates for a valley-floor scale restoration paradigm that integrates hydrological reconnection, sediment retention, and biological reinforcement to ensure long-term resilience of these systems in the face of changing climate and land use pressures.
Introduction
Wet meadows are biologically rich and highly productive ecosystems found throughout much of the world that like most types of wetlands, are becoming increasingly endangered (Wu et al., 2020; Fluet-Chouinard et al., 2023). Though once formerly abundant, particularly on alluvial valley floors (Meyer, 1936; Hewes, 1951; Christy and Alverson, 2011), wet meadows now occupy a relatively small proportion of most watersheds, usually <1% of the total land area, and are generally found on alluvium, gentle slopes, lakeshores and in isolated depressions lacking a waterbody (Patton and Judd, 1970; Cummings et al., 2023). Wet meadows are often found in mountainous terrain on valley floors or hillslope bases where groundwater emerges (Chambers and Miller, 2011; Lord et al., 2011; Pope et al., 2015). These unique ecosystems are characterized by elevated water tables, the widespread presence of hydric graminoids (e.g., sedges) and the general absence of trees and shrubs (Joyce and Wade, 1998). While the bulk of wet meadow studies are from North America and Europe, Wet meadow ecosystems have been described in diverse regions throughout much of the world, including the savannahs of Nigeria, the Highlands of Ethiopia, the mountains of Peru, the Mediterranean region of North Africa and southern Europe, the tropical regions of Central Africa, the Patagonian region of Argentina, the Atacama desert of Chile and parts of Brazil, China, India and Australia (Walker, 1968; Britton and Crivelli, 1993; Gopal and Sah, 1995; Zierholz et al., 2001; Von der Heyden, 2004; Mactaggart et al., 2008; Enriquez et al., 2015; Gao and Li, 2016; Joyce et al., 2016; Fryirs and Brierley, 2018; Tully et al., 2019; Wu et al., 2020). They are less frequently described in tropical lowlands or polar regions, where climate and hydrology favor other types of meadow-like wetlands such as floating meadows or peat lands (Junk et al., 2011; Page and Baird, 2016; Tuboi and Hussain, 2018). Wet meadows are particularly important in the arid and semi-arid areas making up 41% of the earth’s land surface and home to 2.5 billion people, as they provide moisture and humidity needed for numerous species to thrive in an otherwise xeric environment (Minckley et al., 2013; Al-Obaid et al., 2017; Gaur and Squires, 2018). In most systems today wet meadows exist as small components within more spatially extensive ecosystems, but historically, they were sufficiently vast to be referred to as ‘wet prairies’ and extended for many kilometers alongside rivers (e.g., refer to Meyer, 1936; Hewes, 1951; Christy and Alverson, 2011). Where such ecosystems are (were) widespread, increased evapotranspiration may have localized meteorological influences (Şimşek and Ödül, 2018; Zhang et al., 2022). In fire-prone regions, wet meadows, particularly those influenced by beaver, are often the only places remaining with green vegetation and cover after severe burns, and may serve as post-fire refugia for plant and animals (Fairfax and Whittle, 2020). Wet meadows are likely major source areas for seed banks, propagules and offspring that help to recolonize burned over areas. As such, they contribute substantially to the maintenance of regional biological diversity and general ecosystem health that extends well beyond the extent of wet meadows. In part because they are less likely to burn, stable wet meadows also sequester carbon through soil-building processes that accumulate peat and other organic materials, retaining 2–4 times as much carbon as their mesic or dryland meadow counterparts (Dwire et al., 2004).
While the ecological importance of wet meadows is recognized, there is limited understanding of the range of hydrogeomorphic, edaphic and ecologic conditions under which they are formed and maintained. Further, wetland inventories and classifications often do not include a wet meadow type (Cowardin et al., 1979; Gopal and Sah, 1995; Zedler and Kercher, 2005; Ramsar-Convention-Bureau, 2006; Brinson, 2009; Fagorite et al., 2019). Fundamental questions about these systems remain mostly unanswered. These include questions such as: How long do wet meadows persist? What processes form and destroy them? What role do extrinsic influences or disturbances, such as beaver, floods and landslides, play in maintaining these systems? And, what are the trends in wet meadow creation and loss in different regions of the world? Currently, there is not a unifying scientific framework that forms a basis for understanding how to restore and maintain these systems in part because they are not recognized as a distinct wetland type in many wetland classification systems and they are often described as a region-specific rather than a global wetland type (Grootjans and Verbeek, 2002; Ramsar-Convention-Bureau, 2006; Chambers and Miller, 2011; Chambers et al., 2021). Herein, we review and synthesize the science of wet meadows for the purposes of understanding how degraded wet meadows can recover and how existing wet meadows might be maintained in the context of a rapidly changing climate.
Wet meadows compared to other wetlands
Wet meadows are a type of emergent wetland dominated by hydric graminoids (grasslike plants) that are found towards the drier end of the wetland spectrum and are hydrologically transitional between dry upland meadows and wetter marshes with standing water throughout much of the year (Figure 1) (Cowardin et al., 1979; Joyce et al., 2016). Some riparian forests and shrub-scrub wetlands may have similar hydrologic characteristics as wet meadows, and wet meadow sites may transition between graminoid, shrub or tree dominated wetlands in response to subtle changes in hydrologic, geomorphic or biologic conditions (Pastor et al., 1993; Lord et al., 2011; Miller et al., 2011). Less subtle changes may lead to conversion to dry meadows or invasion by upland trees or shrubs (Darrouzet-Nardi et al., 2006; Surfleet et al., 2019; Cummings et al., 2023). Peatlands (rainwater-fed bogs and groundwater-fed fens) are similar to wet meadows in many ways but differ in that they are typically dominated by mosses (e.g., Sphagnum sp.) rather than hydric graminoids, generally have deep accumulations of undecomposed organic material (peat), indicating a consistently anaerobic environment, and typically have pockets of standing water throughout the year (Page and Baird, 2016). Wetland types are gradients more than categories and there is overlap in their features; for example, wet meadows can accumulate peat, bogs can be dominated by sedges, hydric graminoids can coexist with mosses or taller emergent marsh vegetation such as cattails (Typha sp.), bulrush (Scirpus sp.) and reeds (Phragmites sp.) can exist within a mosaic of other wetland and upland types.
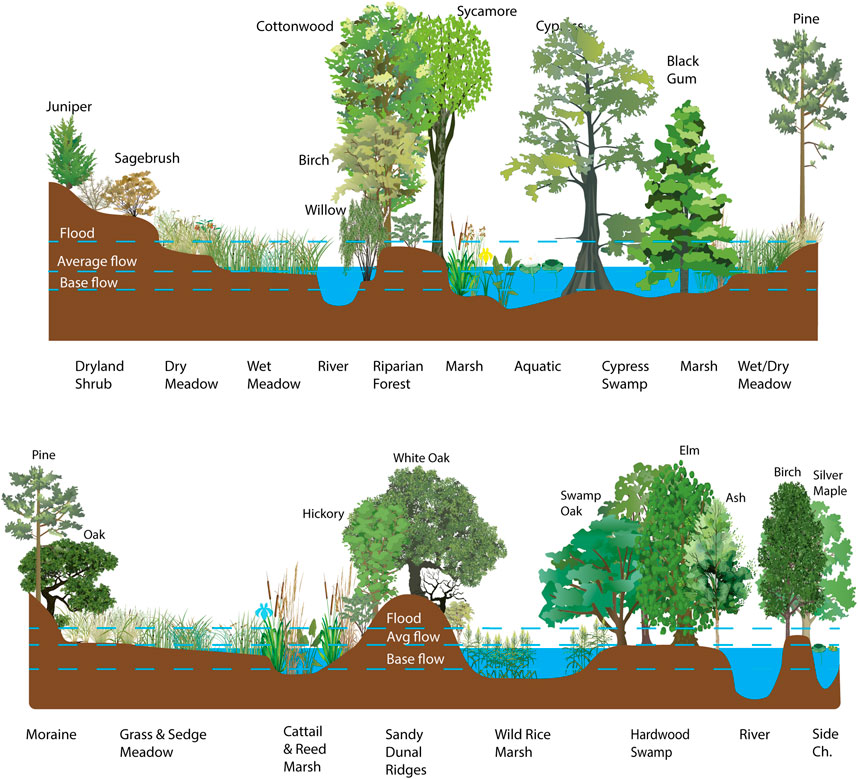
Figure 1. Hydrologic relationship of wet meadows relative to other common wetland, riparian and adjacent upland communities found in the United States. Adapted from Cowardin et al., 1979.
Characteristics of different types of wet meadows
Wet meadows are biologically defined by dominance of hydric graminoids and the general absence of trees and shrubs, but their presence is largely determined by the underlying hydrology, which is influenced by geomorphology (Chambers and Miller, 2011). Thus, wet meadows can be further characterized by their underlying hydrogeomorphology. Additionally, biological processes modify these systems, creating unique environments. Because different hydrological, geomorphological and biological processes can be operating over large areas that appear to be a largely continuous wet meadow system, the term “wet meadow complex” is used to describe such heterogenous areas that may contain a number of different community types (Chambers and Miller, 2011). While we discuss the differing wet meadow types below to give a broader understanding of these unique ecosystems, our focus is primarily on alluvial wet meadows because they are the most vulnerable to hydrologic and geomorphic changes.
Riverine, lacustrine and palustrine wet meadows
From a hydrogeomorphic perspective, we define three broad types of wet meadows: 1) Palustrine meadows, often found on hillslopes or in isolated depressions that are disconnected from any type of surface water body and are primarily groundwater fed; 2) Meadows associated with depressional land forms that contain a lake or pond (e.g., lakeshore meadows); and 3) Riverine or alluvial meadows that are associated with channel networks that are typically fed through a combination of ground and surface water sources (Figure 2) (Richardson and Brinson, 2001; Brinson, 2009; Maltby and Barker, 2009).
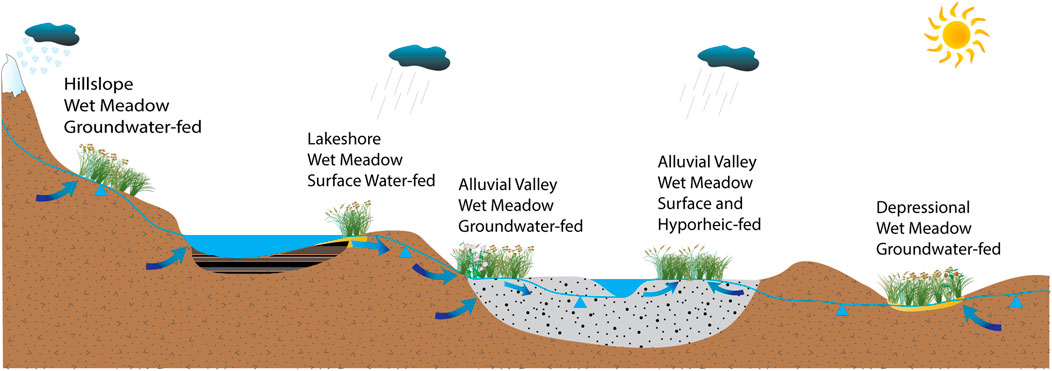
Figure 2. Examples of the variation in groundwater and surface water sources that maintain wet meadow ecosystems. From left to right: a palustrine wet meadow on a hillslope fed by groundwater; a depressional lacustrine wet meadow next to a lake, two examples of alluvial (riverine) wet meadows with different sources of water and an isolated palustrine depressional wet meadow not associated with any open water body.
In drier regions such as the Basin and Range Geologic Province of the western United State and the Sierra Nevada of California, wet meadows are often referred to as mountain meadows because they are typically found in montane areas, the only remaining place where there is sufficient groundwater to maintain them (Germanoski et al., 2011). Meadows associated with lakes and ponds are lacustrine meadows, while meadows associated with channel networks are alluvial wet meadows because they are usually found on valley floor alluvial or colluvial deposits and frequently are adjacent to an active stream channel.
Alluvial meadows
Alluvial wet meadows, inclusive of beaver meadows as described below, are dynamic systems that often encompass a variety of vegetation types and hydrogeomorphic conditions that change over time (Chambers et al., 2021). In contrast, palustrine or lacustrine wet meadows are comparatively stable and do not typically undergo dramatic changes in hydrologic conditions or sediment inputs over time and consequently have relatively stable biotic communities.
Alluvial wet meadows are dynamic communities because they are regularly subject to disturbances such as landslides, debris fans, beaver dam formation and breaches and in-channel sediment deposits and anastomosing (Johnston et al., 1995; Chambers and Miller, 2011). Such features can slow the flow of ground and surface waters, which helps support wet meadows (Chambers et al., 2021).
Rapid pulses of sediment from higher gradient streams deposit in the channels of lower-gradient streams, filling channels and causing avulsions (Schumm, 1979; Jones and Schumm, 1999). Depending on the extent of cover, type of vegetation and presence or absence of beaver dams down valley, the avulsed flow may turn into dispersed sheet flow moving through vegetation or a multi-thread channel system before eventually returning to the original channel. Alternatively, in a more poorly vegetated system, erosion down valley of the avulsion may occur, resulting in flow concentration leading to formation of rills, followed by a new single channel which may quickly incise, particularly if the alluvial substrate is fine-grained and lacking a substantial root network (Lord et al., 2011).
Beaver meadows
In the northern hemisphere alluvial wet meadows are also created and modified by the dam-building activity of beaver. Phylogenetic evidence suggests beaver have been building dams at least 7.6 Ma and possibly for more than 24 Ma (Rybczynski, 2008; Horn et al., 2011; Davies et al., 2022), while physical evidence dates dam-building back at least to the Pliocene (Davies et al., 2022). This evidence suggests that beaver-meadow complexes have long been a feature of Northern Hemisphere ecosystems. Until the past millennia, beaver were widespread throughout Eurasia and North America, numbering in the tens to hundreds of millions, and historically extended as far south as present-day Syria and northern Mexico (Pollock et al., 2003). Beaver meadows or beaver-meadow complexes are wetlands found behind active or abandoned beaver dams where fine sediment has accumulated (over centuries to millennia) sufficiently to create an elevated surface of poorly drained, fine-grained material ideal for colonization by clonal hydric graminoids such as sedges but unfavorable to the growth of shrubs and trees (Ives, 1942; Polvi and Wohl, 2012). Stratigraphic evidence suggests that in some areas of beaver meadows, substantial peat layers were able to accumulate uninterrupted by sediment deposits, which suggests a relatively stable wetland environment over long periods of time (Johnston, 2014). Strata from other beaver meadows reveal a mix of fine sediment and organic matter accumulation, indicating an environment that fluctuated between pond and wetland, characteristic of a site being intermittently inhabited and deserted by beavers, in accordance with their natural behavior (Ives, 1942; Neff, 1957; Johnston, 2000; Westbrook et al., 2011; Polvi and Wohl, 2012). Beaver build dams in alluvial environments and form a unique and dynamic wetland environment that includes wet meadows but also can include aquatic, emergent, willow-shrub wetland vegetation, with the extent of each depending on the hydrogeomorphic site conditions, particularly sediment inputs, and the length of the cycle of occupation and abandonment by beaver. Beaver wet meadows are dynamic systems and can undergo successional processes in response to beaver colonization and abandonment, but the hydric soil conditions of abandoned beaver dams seem to favor meadow establishment over shrub or tree establishment, and beaver meadows appear to be relatively stable landscape features (Neff, 1957; Johnston et al., 1995). The systems are complicated enough that they are referred to as beaver-meadow complexes (Ives, 1942; Polvi and Wohl, 2012). Beaver can build dams in headwater valleys where fine-grained alluvium would not otherwise be deposited and thus increase the upstream extent of wet-meadow complexes. Thus, there are alluvial wet meadows that would not exist without beaver dams and those with pre-existing hydrogeomorphic conditions to create wet meadows that have subsequently been modified by beaver. Understanding the difference between these two wet meadow types is important because there will be significant differences in the vegetative responses to the natural cycle of beaver occupation and abandonment.
Wet hay meadows
Primarily described from Europe, there is another type of wet meadow, often referred to as a wet grasslands, which are a novel system created by humans (Homo sapiens) draining bogs, fens and sometimes riparian forests for agriculture, usually to make hay (Joyce et al., 2016; Lennartsson et al., 2016; Straubinger et al., 2023). Because they were drained a long time ago using rudimentary techniques, these meadows could grow hay but also retained a wet character like natural wet meadows. Creation of these unique systems began over 7,000 years ago and accelerated about 1,500 years ago such that many have been in existence for over a millennium, creating a novel but stable ecosystem (Grootjans and Verbeek, 2002). These biologically diverse systems house numerous species: many of which are endangered and are considered an important type of habitat worthy of conservation and restoration (Grootjans and Verbeek, 2002; Klimkowska et al., 2007). There are active efforts to restore the agricultural practices that maintained them including undoing the recent intensive draining efforts that have occurred with the advent of more modern technologies (Grootjans and Verbeek, 2002; Poptcheva et al., 2009; Kołos and Banaszuk, 2013). Wet hay meadows also existed in the United States under similar circumstances. In the 1800s wet sedge and grass meadows were “hayed” during the dry season and rudimentary drainage efforts using draft animals and hand digging were applied towards improving drainage (Meyer, 1936). Power-driven dredge boats replaced the relatively low impact dredging methods of the past. These new boats dug deep, wide drainage canals and were accompanied by extensive tiling and channel straightening. This transformation eliminated the once-common wet meadows along U.S. rivers during the early European colonial period, converting them primarily into dry farmland (Meyer, 1936; Dahl, 1990; Valayamkunnath et al., 2020).
Palustrine and lacustrine meadows
Because palustrine and lacustrine meadows are by definition, not connected to alluvial systems they are not subject to the same sorts of disturbance regimes and cycles of incision that affect meadows connected to flowing waters. Lacustrine meadows are adjacent to lakes, and should thrive at a surface elevation relative to the lake elevation that keeps them within about a meter of the lake elevation at its low point in later summer and at a high enough elevation that they are not continuously inundated (Figure 2). Similarly palustrine meadows are often found in depressional areas or at the base of hillslopes, connected to steady groundwater sources where groundwater emerges and stays near the surface elevation even during later summer base flow conditions (Figure 2). At high elevations, palustrine wet meadows may also be found on hillslopes where snow regularly accumulates in the winter and melts in the summer. A palustrine meadow may in some cases be a lake or pond that has filled in over time.
Where there is human development, palustrine and lacustrine wetlands of all types have been destroyed through filling, rather than drainage, a situation that also exists for riverine wetlands in some cases where there has been rapid aggradation (Walter and Merritts, 2008; Dahl, 1990). Headward incision, gullying and intentional extends stream networks can connect palustrine wet meadows to stream networks, effectively converting them into riverine wet meadows. As such, they would be impacted by alluvial processes and pathways towards recovery would be essentially the same as for alluvial wet meadows.
Ecological characteristics
Globally, the term “wet meadows” is consistently defined by the domination of hydric graminoids, though no universal classification exists (Prosser and Slade, 1994; Joyce and Wade, 1998; Grootjans and Verbeek, 2002; Adam et al., 2010; Chambers and Miller, 2011; Tully et al., 2019; Wu et al., 2020). The dense shoot and root networks of wet meadow graminoids give these systems unique properties that allow them to substantially modulate water, sediment, nutrient and thermal energy fluxes. Shrubs and trees such as willow (Salix spp), alder (Alnus spp), aspen and cottonwood (Populus spp), birch (Betula spp) and other woody species may occur in elevated or recently disturbed patches within the meadows or on the fringes, but they are generally a minor vegetative component.
Water tables in wet meadows are elevated such that they are slightly above or slightly below the surface elevation throughout the year. Dominant species in wet meadows are almost entirely from the Order Poales (the grass Order) and mostly from the Cyperaceae (sedge) Juncaceae (rush) and to a lesser extent Poaceae (grass) families (Tang et al., 2017). Examples of specific genera that have a competitive advantage under such hydric conditions include Carex, Scirpus, Juncus, Eleocharis, Deschampsia, and Calamagrostis species (Cowardin et al., 1979; Chambers and Miller, 2011; Wu et al., 2020). Across the globe, species from the sedge family, of which there are nearly 7,000, are ubiquitous in wet meadow ecosystems (Tang et al., 2017). In some situations, such as when water levels are constant and elevated throughout the year, mosses (e.g., Sphagnum species) also thrive and can be abundant. Where moss dominates, the system is classified as either a bog (if precipitation is its only source of water) or a fen (if it is fed by groundwater) and is not considered a wet meadow (Lourenco et al., 2023). In North America, wetlands with shallow water more consistently above the soil surface favor a different suite of generally taller taxa such as Typha, Phragmites, Sparganium, Alisma, and Sagittaria as well as some species of Scirpus, Carex and Juncus (Cowardin et al., 1979). Permanent flooding and deeper water favors aquatic taxa such as Nuphar, Potamogeton, and Nymphaea (Cowardin et al., 1979). Conversely, where water tables are near or at the surface for part of the year but drop more deeply during summer months, mesic or dry meadows form, and these are typically dominated more by dryland grasses rather than sedges or rushes.
Properties of hydric graminoids that create resilience to disturbance
Alluvial wet meadows have specific hydrological and biological features that in combination make them unique, including a down slope/down valley obstruction to flow and sediment transport that may be biogenic in nature, a groundwater source, often a surface water source, hydric graminoids, and an accumulation of alluvium that may include organic rich strata. The morphological and physiological characteristics of wet meadow graminoids create a biological architecture with certain unique properties. In hydric soils, undisturbed wet meadows over time form a mat of a dense root networks within the upper half meter of soil that makes them highly resistant to erosive forces, which increases channel stability and reduces sediment transport (Micheli and Kirchner, 2002a; b). The root densities of sedges are some of the highest ever recorded and are among the strongest in terms of resisting the erosive force of flowing water (Gyssels et al., 2005; Vannoppen et al., 2015). Shear strengths of intact wet meadow soils (during low-flow conditions) in the root zone are in the range of 37–47 kPa, compared to shear strengths of 8 kPA for dry meadows and 6 kPa for unvegetated river bars (Micheli and Kirchner, 2002b).
The shoots of wet meadow graminoids can grow to high densities ranging from 2,000 to 10,000 shoots per m2, with shear strengths positively correlated with shoot density (Figure 3). High shoot densities are efficient at lowering velocities when water flows across the surface which reduces the potential for scour. This increases the capture efficiency of any sediment contained in the water, and helps wet meadow plants acquire nutrients needed for growth (Gorrick and Rodríguez, 2012; Yagci and Strom, 2022).
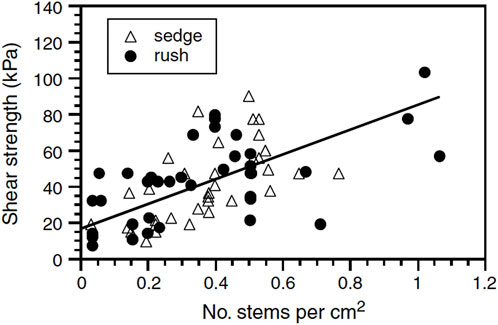
Figure 3. Shear strength versus sedge and rush stem density. The strength of vegetated bank and bar materials is correlated with stem density, with a slope of 68.4 kPa per number of stems per cm2 and a y-intercept of 16.8 kPa (From Micheli and Kirshner, 2002b).
Similar filtering processes operate that also help wet meadows capture aeolian particles (Wood, 1975; Betz et al., 2015). The capture and deposition of airborne soil particles and volcanic ejecta can be a significant source of nutrients, propagules and organic material for ecosystems around the world (McTainsh and Strong, 2007; Brahney et al., 2024).
Further, the dense root networks of hydric graminoids reduce the potential for tree and shrub seedling establishment. The absence of trees reduces the potential for disturbance from treefall from windthrow or bank erosion and the subsequent exposure of large patches of bare soils is minimal. The lack of trees also reduces low-root density patches and bare ground which are common features of soils shaded by tree canopies. This helps reduce erosion and headcutting vulnerabilities.
Carbon sequestration
Wetlands as a whole occupy only 4-6 percent of the Earth’s land area (∼0.53–0.57 Gha) (Matthews and Fung, 1987; Aselmann and Crutzen, 1989), but store an estimated 20–33 percent of the world’s soil carbon (350–650 GtC) (Gorham, 1995; Lal et al., 2007; Alhassan et al., 2018; Nag, 2019; Were et al., 2019; IPCC, 2023). This is equivalent to about 15%–27% of the total 2,400 GtC released by human activity from 1850 to 2019, much of which was originally sequestered in wetlands millions of years ago (Flores, 1986; Flores et al., 1987). Conversely, the widespread incision or intentional drainage of wetlands has created oxidative soil conditions that has released substantial amounts of soil carbon into the atmosphere (Dahl, 1990; Dwire et al., 2004). Around the world, degradation of peat-rich wetlands alone is annually releasing about 2 GtC, or about 5% of the total annual amount of GtC released by human activities (Leifeld et al., 2019; Loisel et al., 2021; UNEP, 2022).
The stratigraphic records shows that wetlands are major sites of carbon sequestration, inclusive of wet meadows (Wood, 1975; Flores, 1986; Flores et al., 1987). Wet meadows are important ecosystems for carbon sequestration due to their ability to store large amounts of organic carbon in both plant biomass and soil. Intact wet meadows have reported carbon sequestration rates ranging from 50 to 578 g C m−2 y−1, the upper end being some of the highest rates of carbon accumulation of any ecosystems outside of mangrove swamps (Reed et al., 2021). Sequestration rates are generally higher in humid colder environments with greater water availability, more elevated water tables and longer growing seasons, and lower in more arid environments where growth rates are lower because water availability is more limited during the growing season and lower water levels may increase soil carbon oxidation rates. In contrast, degraded wetlands such as those where water tables are lowering due to incision can be sources of atmospheric carbon as the soils slowly oxidize (Reed et al., 2021).
Wetlands in general are significant areas of carbon accumulation. Much of the great carbon accumulations in ancient times such as during the Carboniferous Period and later the Paleocene Epoch (prior to the Paleocene-Eocene Thermal Maximum) occurred in freshwater and estuarine wetlands (Flores, 1986; Flores et al., 1987). Wetlands that are consistently waterlogged prevent the decomposition of organic material primarily due to the lack of oxygen (anoxic conditions) and the reduced activity of decomposers that would normally oxidize organic carbon compounds into gases such as carbon dioxide (Mitsch and Gosselink, 1993). When an area is waterlogged, oxygen from the atmosphere is unable to penetrate the water and reach the organic material (Tiedje et al., 1984). Oxygen is a crucial component for aerobic decomposers, such as bacteria and fungi, which break down organic matter. In the absence of oxygen, these aerobic organisms cannot function effectively, leading to a slowdown in the decomposition process. The slower rate of anaerobic decomposition compared to aerobic decomposition is due to the lower energy yield from the reduction of alternative electron acceptors, the specialized nature of the enzymes involved, and the overall less efficient energy extraction from organic matter in the absence of oxygen (Davies, 1980; Heider and Fuchs, 1997). Further, anaerobic accumulations of organic material can also become more acidic over time, which alters the effectiveness of enzymes involved in decomposition (Mitsch and Gosselink, 1993).
The biochemistry of decomposition under aerobic and anaerobic conditions suggests that wet meadows will most effectively sequester carbon if their soils are flooded during the growing season. Wet meadows that dry out to a certain extent during the summer months when temperatures are higher and decomposition rates at their maximum seem likely to accumulate less carbon. At the same time aerobic soils such as those found in grasslands can also accumulate substantial amounts of carbon if the soil structure is ideal (Bai and Cotrufo, 2022).
Well-structured aerobic soils with good aggregation can protect organic carbon from decomposition through several mechanisms: (1) Soil aggregates are clumps of soil particles bound together by organic substances, clay, and minerals. Organic carbon can become physically protected within these aggregates, making it less accessible to soil microbes that decompose organic matter. This physical protection reduces the rate at which organic carbon is broken down and released as CO2. Within soil aggregates, especially in the interior, there can be limited oxygen availability. This microenvironment can slow down the activity of aerobic decomposers; (2) microbes, such as arbuscular mycorrhizal fungi, can form symbiotic relationships with plants, contributing to the stabilization of soil aggregates and the protection of organic carbon; (3) finally, soil aggregation contributes to soil stability and can reduce erosion (Totsche et al., 2018; Bai and Cotrufo, 2022).
These carbon sequestration mechanisms operating in oxygen-rich soils suggest how wet meadow soils, that are seasonally aerobic may be able to sequester carbon and prevent it from being converted to carbon dioxide as a byproduct of microbial metabolism.
Physical evidence of the ability of wet meadows to sequester carbon is found in the stratigraphic record of wet meadow soils (usually made visible because of incision), which show long, meters deep, multimillennial periods of organic matter accumulation (Wood, 1975; Koehler and Anderson, 1994; Zierholz et al., 2001; Pearthree and Cook, 2015). This suggests that biomass loss through oxidative microbial metabolism during the short dry season is substantially outweighed by the accumulation of organic material under anoxic metabolic pathways (e.g., glycolysis, lactic acid and alcohol fermentation) that leave undecomposed carbon-rich byproducts in the soil (Davies, 1980).
In sum, wet meadows with healthy carbon-rich organic soils that are well-structured may not release more carbon into the atmosphere even if the upper layers are exposed to oxygen for limited periods during the summer. Incision and the rapid drainage of wet meadow soils would likely result in oxidation of some of the stored soil carbon over longer time periods, depending on the pH of the organic deposits, and reduce the density of the above and below-ground vegetative biomass. Relationships between specific wet meadow hydrologic regimes, vegetation types and their synergistic effects on soil structure and carbon sequestration rates could be explored further in future research.
Hydrologic processes and effects on streamflow
Wet meadows are often directly connected to streams and rivers with surface or near-surface flow, but there is typically a groundwater component also providing flow to the system (Loheide and Gorelick, 2006; Schook et al., 2020). Groundwater sources for wet meadows include deep or regional aquifers, local or hillside aquifers, and alluvial aquifers or hyporheic flow (Tonina and Buffington, 2009; 2023). Wet meadows can also exist in isolation with a proximate groundwater connection but no surface water connection (Chambers and Miller, 2011). Less commonly, wet meadows can exist on surface or near surface water and precipitation, with no groundwater inputs.
Common hydrologic features of wet meadows include seeps, unpressurized springs, artesian springs, perched aquifers, confined or semi-confined aquifers, unconfined aquifers, sheet flow, channelized flow, areas of upwelling and downwelling and recharge and discharge areas (Figure 4) (Lord et al., 2011).
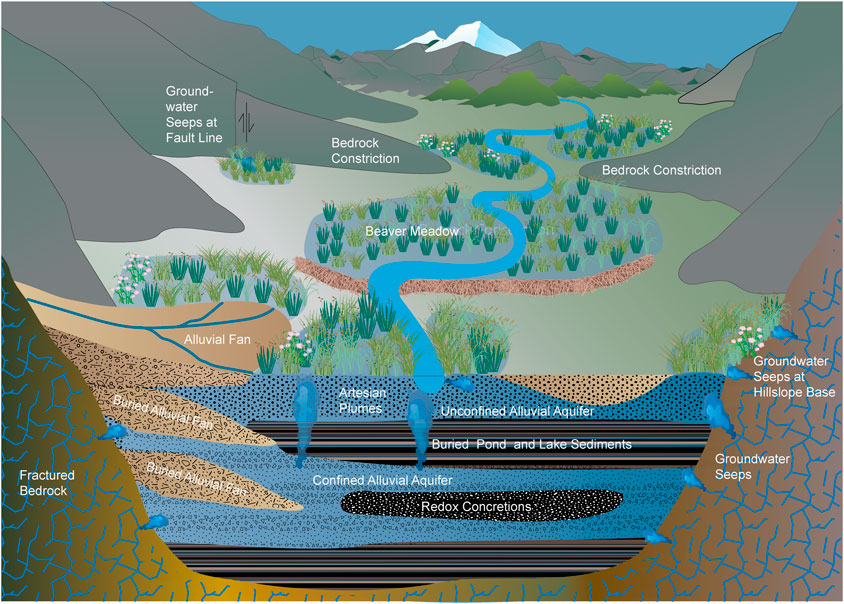
Figure 4. Examples of hydrological conditions that favor the formation of wetlands across landscapes. Here, alluvial fans, beaver dams, bedrock constrictions, fine-grained pond and lake sediments and concretions such as calcium carbonates and iron oxides form subsurface flow obstructions that cause upwelling which creates conditions favorable towards the establishment of wet meadows in alluvial landscapes. Fractured bedrock and fault lines also allow groundwater seepage that creates a gaining reach that further promotes wet meadow establishment.
Some meadows may be relatively simple and contain only a few hydrological features, while others may be extremely complex with multiple hydrological features that vary across space. The specific hydrologic features present in a wet meadow however, and their spatial arrangement relative to geological features, can significantly affect the biological structure, climate sensitivity, and resilience of the ecosystem (Ciruzzi and Lowry, 2017; Klos et al., 2023). These factors also form the foundation for the most effective approach to restoring a degraded wet meadow system.
Effects of wet meadows on downstream hydrographs
Healthy wet meadows affect hydrology by storing water, elevating water tables and attenuating flow during periods of high discharge and releasing water during periods of low flow, typically during the summer (Hammersmark et al., 2008). Stored water leaves wet meadows by direct discharge to streams, through down-gradient sub-surface drainage, plant transpiration and soil evaporation. Thus, there is a seasonal cycle of water accumulation and discharge that is strongly affected by wet meadow biota. In the mountain meadows of the Sierra Nevada of California, where many of these studies have occurred, evapotranspiration in wet meadows is about 6 mm per day or about 54 cm over the course of the 90-day growing season, while degraded wet meadows growing sagebrush, conifers or annual grasses have evapotranspiration rates about half that amount or 3 mm per day (Wood, 1975; Loheide and Gorelick, 2007).
There is scientific consensus that restoring wet meadows degraded through incision results in elevated water tables and increased water storage, but there is less consensus on whether stored water returns to the atmosphere via transpiration and evaporation or is released as stream discharge (Loheide et al., 2009; Lord et al., 2011; Hunt et al., 2018; Nash et al., 2018). Restoration practitioners may be interested in the timing and route through which water leaves these systems, and whether relative to incised wet meadows, the presence of well-vegetated, aggraded wet meadows increases downstream surface discharge during low-flow conditions, or if surface discharge decreases because wet meadow vegetation transpires the water (Loheide et al., 2009; Essaid and Hill, 2014; Hunt et al., 2020).
Empirical studies that measured flow indicate that unincised wet meadows (natural or restored) contribute to dry season baseflow usually downstream of the meadow and not necessarily within the meadow (Hammersmark et al., 2008; Tague et al., 2008; Hunsaker et al., 2015; Hunt et al., 2018; 2020). For example, Hunt et al. (2018) reconnected 0.1 km2 of wet meadow by filling an incised channel. Prior to incision trench filling, the meadow increased downstream baseflow by 5% relative to inflow, whereas afterwards, baseflow increased 35%–95% (5–15 L/s), depending on the year. Tague et al. (2008) found that filling a 3,000-m incised channel and reengineering a new sinuous stream on top of a wet meadow system increased discharge during the summer recession period by as much as 40% and elevated water tables across a wide range of flow conditions. However the restoration did not increase summer baseflow and seemed to increase evapotranspiration based on noted improvements in riparian condition. In another example, Hammersmark et al. (2008) found that a wet meadow restoration project slightly decreased the duration of flow within the meadow itself but increased baseflow below the meadow due to increased downgradient groundwater flow through the meadow alluvium.
In contrast, wet meadow hydrology models predict varying effects of wet meadows on hydrographs. Some models concluded that when a wet meadow incision trench is filled, the increased transpiration and soil evaporation resulting from elevated water tables and conversion from mesic or xeric to hydric vegetation may exceed increases in storage capacity (Loheide et al., 2009; Essaid and Hill, 2014; Nash et al., 2018). For example, Nash et al. (2018) compared baseflow of an incised and unincised (restored) wet meadow in the Middle Fork John Day River, Oregon and under their model assumptions projected that the incised meadow had a minuscule (and virtually unmeasurable) baseflow increase relative to the unincised wet meadow (3.3 mL/s v. 6.6 mL/s per km of stream). This was primarily because their model assumed under both conditions that the alluvial aquifer recharged fully but that the incised meadow drained the aquifer to a greater depth so could provide more baseflow.
Other models, using different assumptions, suggest that pristine or restored wet meadows can increase baseflows relative to incised meadows. For example Ohara et al. (2014) modeled the effects of a restored 9-mile segment on Last Chance Creek in the Feather River Basin, California and found a 10%–20% baseflow increase post restoration. Similarly, (Loheide and Gorelick, 2006), used temperature as a proxy and found that a restored 1.7 km reach in Cottonwood Creek, in Plumas National Forest, California had increased hyporheic exchange and increased baseflow relative to surrounding unrestored incised reaches, and also that the restoration decreased maximum stream temperatures by more than 3°C. Much of the difference in model outcomes appears to be differences in model assumptions about the extent to which incised streams can recharge alluvial aquifers.
Despite substantial differences in the type, location and relative abundance of groundwater and surface water sources, wet meadows share common hydrologic characteristics: Groundwater and surface water inflows are sufficiently greater than the combination of outflows, inclusive of transpiration, evaporation and surface and subsurface discharge, such that water levels are elevated throughout the growing season throughout most years. The biological result is that over the long-term, the competitive advantage shifts towards hydric graminoids that are more tolerant of anoxic and low nitrogen soil conditions created by constant flooding or saturation and away from shrubs, trees and grassland species with roots systems that are at a more competitive advantage when growing in aerobic soils (Cowardin et al., 1979; Mitsch and Gosselink, 1993; Johnston et al., 1995; Keddy, 2010; Cooper et al., 2012).
None of the hydrologic features common to wet meadows assures the formation of a wet meadow. For wet meadow vegetation to form, water levels need to remain near the surface over an extended area and this usually requires an obstruction to subsurface or surface flow such that soils stay saturated. Such obstructions may include colluvial deposits on a valley floor, alluvium with low hydraulic conductivity, e.g. high clay, silt or organic content, or a bedrock constriction or sill (Figure 5). Wet meadows terminate down valley in the absence of alluvium with a low hydraulic conductivity or the absence of alluvium altogether, for example when a stream transitions from a low-gradient alluvial reach to a high gradient bedrock reach. That is, down valley of whatever low permeability below-ground feature was keeping water tables elevated, water tables drop, and mesic vegetation adapted to better-drained soils become dominant (Chambers and Miller, 2011).
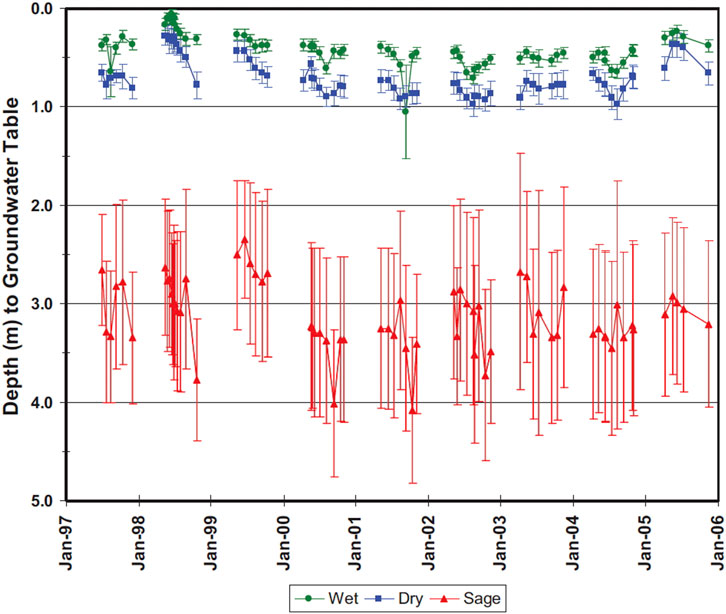
Figure 5. Average depth to groundwater (±1 SD) in piezometers located in wet, dry, and sage plant communities of the Big Creek meadow (Toiyabe Range, Nevada), January 1997 to January 2006. Water level measurements were taken monthly during growing season, five to six times per year (from Lord et al., 2011).
Response of vegetation to water table variation
Community composition shifts away from hydric wet meadow species if the water table is consistently lower than about half a meter, which places it below the rooting zone of the typically dominant herbaceous wet meadow species but not below the roots of dry meadow species, shrubs and trees. For example Lord et al. (2011) observed water table fluctuations over a 10-year period in an alluvial valley in Nevada and found that meadows generally had water table elevations less that 0.5-m deep throughout most months of most years (Figure 5). More mesic and xeric meadow communities tended to have water tables fluctuating interannually at depths of 0.5–1.0 m, while shrub communities (Artemesia spp (sagebrush)) dominated where incision had dropped water tables in the range of 1.5–4.5 m.
Similarly, Loheide and Gorelick (2007) found that xeric, mesic and hydric meadow or shrub communities could be separated by the range of depth to water table over the growing season, with wet meadow water levels generally dropping to less than 0.5 m below the surface through most of the growing season and dropping to less than 1.0 m below the surface by the end of the growing season (Figure 6).
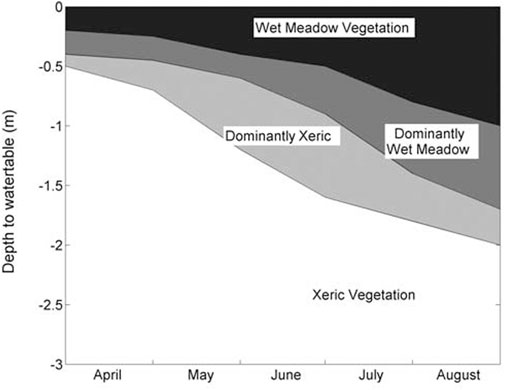
Figure 6. Wet meadow, mesic meadow and xeric shrub communities (e.g., sagebrush) are separated by depth to water table over the growing season (from Loheide and Gorelick, 2007).
In wet meadows bisected by streams, distribution of wet meadow vegetation is affected by stream hydrology. In a gaining stream, for example one fed by hillslope seeps, water levels drop closest to stream channels where hydraulic gradients are the highest. This is particularly true if the stream has experienced incision, with water levels being higher towards the valley margins. In such situations the result is the counterintuitive development of more mesic or even xeric vegetation adjacent to the stream where drainage is the greatest, with more hydric graminoids towards the valley edges where water tables remain consistently higher (Loheide and Gorelick, 2007). Conversely, in a losing stream, water is most available near the steam and least available towards the valley margins, so the vegetative pattern is reversed.
Sediment processes
While the dense shoot and root networks of wet meadow graminoids ensure that degradation of wet meadows rarely occurs from erosional processes in the vegetated portion of the meadow, degradation may still be triggered by changes within adjacent stream channels or areas of bare ground that are flooded. Incision initiated downstream can result in headcutting into a wet meadow and this can expose bank sediments below the root zone to flow. When eroded, these sediments when eroded, undermine the wet meadow “sod” and cause “calving” of sedge blocks and a widening and further lowering of the stream channel bed (Schumm et al., 1984; Micheli and Kirchner, 2002b). Wet meadow degradation in alluvial systems occurs when water tables lower, and water table lowering is almost always related to incision (Chambers and Miller, 2011). Incision is initiated by either downcutting, headcutting or avulsions as described below (Schumm et al., 1984). Understanding which of these mechanisms have contributed to wet meadow degradation is key to developing tractable restoration strategies.
Downcutting—refers to the bed lowering of an existing channel. Incision initially occurs when sediment is moved from a channel faster than it is replaced either when flows increase such that sediment can be more effectively transported, or when the sediment supply decreases (Schumm, 1973). In wet meadow systems incision often seems to result from alteration of the biological architecture that maintains these systems. In the Northern Hemisphere, the widespread removal of beaver eliminated valley spanning instream structures that maintained elevated water tables and stored sediment (Pollock et al., 2014). Simultaneously, intense livestock grazing damaged the dense root network and shoots of hydric graminoids, which both reduced resistance to flow and enabled flowing water to mobilize sediment underlying the protective root zone. Increased peak flows caused by upstream changes in land use practice such as logging, roadbuilding, grazing or increased wildfire frequency can also cause downcutting within streams flowing through wet meadows (Darby and Simon, 1999).
Headcutting—refers to the upward movement of an incision nick point and is the mechanism whereby incision trenches move upstream and deepen (Schumm et al., 1984). Headcutting is also the mechanism whereby stream channel networks expand upstream to create incised channels where previously wet meadows existed with no channelized flow.
Once incision is initiated, it can more easily propagate through headcutting, even in the absence of higher flows or lower sediment supplies (Schumm et al., 1984). Headcutting in wet meadows often occurs through erosion of a less cohesive lower stratum such as gravel or sand that is found below a highly cohesive surface layer, held together by fine roots and/or cohesive fine-grained material (silts and clays) (Micheli and Kirchner, 2002b). Particles from the less cohesive and therefore more easily erodible layer are transported downstream, leaving an overhanging layer that eventually collapses and also moves downstream. Thus, a key to preventing incision is for flowing water not to encounter easily erodible sediments, such as sands and gravels, or unconsolidated finer-grained materials.
Headward channel formation can also continue even in the absence of floods or overland flow through a process known as groundwater sapping (Germanoski et al., 2011). Groundwater flowing into a channel can entrain sediment on a particle-by-particle basis, which eventually results in an incised stream. Groundwater sapping often occurs where there is a high-permeability strata just above a low-permeability strata. This results in water flowing at the interface and removing the high-permeability strata, particle by particle. This process can expand the channel network and lead to gullying where channels form on steep hillslopes and as side-gullies within wet meadow complexes.
Although the common vision of a wet meadow ecosystem usually includes a stream flowing through it, a well-managed wet meadow may have no channel at all, with surface flow dispersed across a wide, well-vegetated surface. Where a channel does exist, the successful persistence of hydric vegetation depends in large part on how well the vegetation ‘manages’ the channel network. In turn, the length and depth of a channel network depends largely on how well sediment is managed. Channel sediment that is not protected by vegetation can be mobilized by moving water, which can lead to erosion and the formation of channels (Liu et al., 2019; Frankl et al., 2021). Channel formation can lead to yet more erosion, which can lead to incision, and incision typically leads to the lowering of water tables (Schumm et al., 1984). A water table that consistently stays below a certain elevation and beyond the root zone can cause the death of hydric vegetation (Miller et al., 2011). Incision can also cause head cutting, or gullying, that is, a headward extension of the channel network, which also can lead to the lowering of water tables and the subsequent loss of hydric and mesic vegetation (Liu et al., 2019; Frankl et al., 2021).
Avulsions—Incision can be triggered by avulsions (Jones and Schumm, 1999). Avulsions in wet meadows can occur when a large slug of sediment moves downstream from a typically higher gradient reach and loses energy when it reaches a relatively low-gradient wet meadow system, causing the sediment to drop out suspension, thereby filling a channel (Miller et al., 2011). The water then moves to a new location across the surface of the wet meadow. Depending on the density of vegetation, the water may move as sheet flow across a meadow surface and do little to no harm if vegetation shoot and root densities are high. If vegetation is more sparse, bare soil may be exposed and the potential for erosion and thus incision through downcutting is higher.
The sediment that creates avulsions in channels flowing through wet meadows often originates from sources outside of the wet meadow system, such as from landslides, fires, road failures or other land use practices that can release large amounts of sediment during precipitation events (Miller et al., 2011). Avulsion-triggering sediment plugs can also come from instream sources, such as bank erosion or beaver dam failures (Miller et al., 2011).
The potential for avulsions, natural or anthropogenic, speaks to the need to view the entirety of the valley floor as the object of restoration, rather than a simple focus on a channel. A wet meadow resilient to incision must be able to absorb the effects of avulsions that shift channel location. This suggests that elevated water tables, a continuous vegetation cover and a dense root network throughout the potential avulsion zone are required for resiliency to incision (Micheli and Kirchner, 2002a; b, Pollock et al., 2014). Examples from numerous stratigraphic studies suggest that avulsions or other mechanisms have regularly deposited coarse grained materials over wet meadows and other floodplain surfaces without triggering incision, indicating the potential for such resiliency (Wood, 1975; Elliot, 2000; Zierholz et al., 2001; Germanoski et al., 2011).
Sedimentary processes and cycles of wet meadow formation
Stratigraphic analysis of wet meadow soils indicate that meadows are just one of a number of ecosystem states that can exist at a site (Figure 7), with observed meadow lifespans ranging from thousands to tens of thousands of years and alternative states including mesic and xeric plant communities, stream channels and open water bodies (Wood, 1975; Johnston, 2000; Zierholz et al., 2001; Tully et al., 2019).
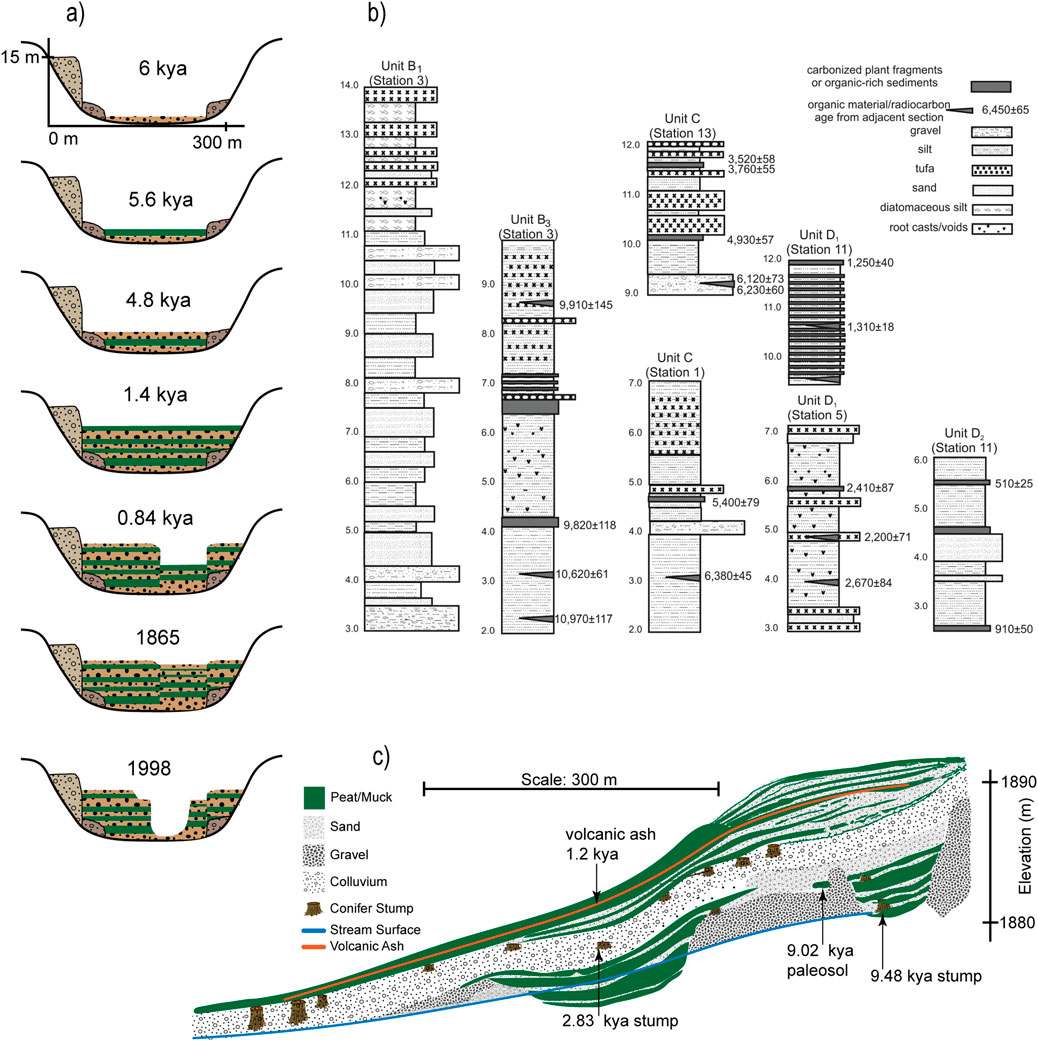
Figure 7. Holocene stratigraphies of alluvium from three different continents indicate wet meadows are just one of multiple states that can exist at a site, typically alternating between alluvial sands and gravels. Wet meadows can exist as stable communities for centuries to millennia, with common alternative states including upland forest, riparian shrubs and trees, xeric grasslands and gravel bars: (a) cross sectional stratigraphy of Wolumla Creek in the coastal plain of New South Wales, Australia showing the cut and fill sequences over the past six millenia, with rich organic layers (green) alternating with sandy alluvium (Fryirs and Brierley, 1998); (b) alluvial stratigraphy of the Rio San Salvador in the Atacama Desert, Chile, showing layers rich in organic material alternating with layers of inorganic sand and gravel (Tully et al., 2019); (c) longitudinal profile of the side bank of an incision trench in the East Meadow in Yosemite National Park, California showing alternating layers of peat or muck with sand, gravel and colluvium with remnants of trees (Wood, 1975).
Even in the absence of incision, shifts to drier climates can also shift the competitive advantage to dry meadow species (e.g., grasses) or shrubs and trees. Streams in dryland climates also undergo natural cycles of incision and aggradation, likely related to climate change, with drier climates seemingly correlated to increased incision but with imprecision in the stratigraphic record creating ambiguity with regards to this generalization (Darby and Simon, 1999; Zierholz et al., 2001; Tully et al., 2019). Stratigraphic analysis of incision events in individual watersheds across arid regions show both asynchrony and synchrony of events, suggesting timing is determined by local factors rather than regional climate shifts (Darby and Simon, 1999). Some stratigraphic analyses suggest that incision occurs during periodic regional droughts and lowered water tables that cause wet meadow vegetation to die off. This leaves systems vulnerable to erosion and incision during large flood events, which may be more local in nature, thus explaining the asynchrony (Tully et al., 2019). Whether studies that show synchrony or asynchrony is simply a by-product of limited resolution of the geologic record or temporally accurate reflections of the geologic record is not entirely clear. More recently, land-use changes in the past few centuries have also caused incision and/or headcutting that has lowered water tables and facilitated the shift to more xeric vegetation independent of climate change (Reagan, 1924; Bryan, 1925; Fryirs and Brierley, 1998).
Although the stratigraphic record clearly shows that incision and aggradation cycles have been naturally occurring for millennia, the sudden appearance of relatively high densities of humans on every continent outside of Antarctica has led to a global incision event that in terms of spatial extent is not matched anywhere in the geologic record (Darby and Simon, 1999). Incision is still an ongoing phenomenon, suggesting that today’s intact, apparently well-managed wet meadow may be tomorrow’s incised and degraded system (Renteria-Villalobos and Hanson, 2015; Schumm et al., 1984; Darby and Simon, 1999). There are some “at-risk” indicators of the potential for incision such as convexities in the longitudinal profile of the valley floor, evidence of significant reduced vegetation biomass from grazing or fires, evidence of rills and gullies in the uplands or on livestock trails, or other indicators of recent flow concentration on bare soils (Schumm et al., 1984; Lord et al., 2011). In contrast, there are indications that well-vegetated systems are quite resilient to floods, even floods of extraordinary magnitude (Tully et al., 2019).
Mechanisms of recovery after degradation
Throughout the world, humans have extensively drained wet soils by increasing the density and depth of drainage channels, including those in wet meadow systems. The effect has been that water drains more quickly from the landscape rather than being stored and slowly released. Many valley floors now have defined channels that were absent prior to human-induced drainage (Walter and Merritts, 2008). Stream channel heads begin at the point where flow is concentrated enough to lift and transport underlying sediment particles (i.e. critical shear stress is exceeded) (Montgomery and Dietrich, 1992). The dense root network of healthy wet meadow plant communities creates a tensile strength that makes them more or less unerodable from surface flow under even extreme floods (e.g., refer to Tully et al., 2019). Over time, wet meadow vegetation should be able to extend their roots into small stream channels, stabilizing exposed in-channel sediments, extinguishing channels and converting them to wet meadows. In theory, this stabilization would shift the point of channel initiation down valley, though direct observation of such phenomena is lacking. In contrast, the reverse process, whereby channel headcutting undermines sedge meadows by eroding sediment beneath the dense root network so that sedge clumps collapse and slump as blocks, is well-documented (Darby and Simon, 1999; Micheli and Kirchner, 2002b). Channel headcutting can extend the channel network back upvalley or upslope. The process of headcutting and channel “rejuvenation” or extension (aka gullying or incision) has been well studied (Schumm et al., 1984), but the reverse process, stream channel extinguishment and in particular the role of biota in this process are not well understood. Most relevant literature is found under gully restoration and generally discussed in the context of agricultural practices and erosion caused by ephemeral flow rather than stream or wet meadow systems where there is perennial flow and more erosive flooding (reviewed in Frankl et al., 2021). Nonetheless, such studies indicate that vegetation (mostly grass species) can develop dense shoot and root networks to create surfaces highly resistant to erosion in a matter of years.
The high shear strength of wet meadow root systems suggest they will not erode when exposed to flowing water (Micheli and Kirchner, 2002b). Meadow degradation would likely only occur when bare sediment is exposed to flowing water and particles can be lifted into the water column. Wet meadows most resilient to disturbance will have a thick continuous cover. Actions that create gaps in the dense shoot and root network, such as ungulate trails, ditching or avulsions create vulnerabilities where sediment may be in contact with flowing water, allowing localized erosion and subsequent headcutting and undermining of material beneath the root network (Germanoski et al., 2011).
Plants clearly possess the biomechanical properties to create and maintain unincised valley floors with elevated water tables, but beaver and the dams they build would likely create additional resiliency to degradation in these systems and aid in the recovery process if degradation did occur (Rudemann and Schoonmaker, 1938; Westbrook et al., 2011). Beaver often build dams across the entirety of small valleys and in doing so, greatly reduce the erosive potential of stream flow by reducing unit stream power (Green and Westbrook, 2009). Thus, if erosion vulnerabilities in a wet meadow system as described above exists, such vulnerabilities may be mitigated if there are beaver dams downstream. At the same time, beaver dam failures can potentially result in large, sudden increases in discharge and the downstream transport and deposition of large volumes of sediment if available. Sometimes these events are sufficient to cause avulsions, burial of wet meadow vegetation, sediment exposure and incision (Chambers et al., 2021). Cycles of beaver dam construction and abandonment adds a level of stable dynamism to wet meadow ecosystems and prove to be an important biological control on the formation, persistence and recovery of wet meadows (Pollock et al., 2007; Polvi and Wohl, 2012). Because beaver populations are continuing to recover and expand following near extirpation throughout much of their range in the late 19th century, most low-gradient wet meadow valley floors with an active channel are likely to be colonized by beaver eventually, reinitiating an important biological control on physical processes that have been operating for millions of years (Pollock et al., 2003; Davies et al., 2022). As such, wet meadow restoration efforts, at least in the Northern Hemisphere, should plan accordingly and recognize that beaver-mediated wet meadow dynamics may include transition through a few different vegetative states, including non-meadow communities, and will likely increase spatial variation in sediment transport and depositional processes.
Because wet meadow vegetation depends on water table levels at or near the surface throughout the year, wet meadow vegetation is sensitive to land use changes that directly or indirectly lower water tables. 1n alluvial environments, dredging, drain tiling, channel straightening and removing instream obstructions such as bedrock sills are all proximate causes that lower water tables (e.g., refer to Meyer, 1936). Water tables are also indirectly lowered from certain land use practices that can alter watershed hydrology such as (over)grazing, logging, beaver removal, roadbuilding and development. These activities can decrease infiltration and increase storm discharge, leading to erosion that can cause headcutting and incision. This in turn lowers water tables and promotes conversion to more mesic vegetation. Other processes that directly remove vegetation, such as grazing and fires can expose soils or reduce the root strength within soils, making them susceptible to erosion and potentially triggering headcutting and incision.
In opposition to incisional processes are aggradational processes such as clonal growth of rhizomatous hydric graminoids, landslides, progradation of alluvial and colluvial fans across valley floors, beaver dam building and large, usually storm-related sediment pulses that create avulsions. While such processes help to maintain or restore wet meadows, in some cases, depending on the length of time that such obstructions persist and the rate at which sediment and organic material builds up behind the obstruction, open water can form upstream of the obstruction, creating an environment more favorable to emergent marsh or open water hydrophytes (Duman, 2009; Pollock et al., 2014; Wang et al., 2019).
Wet meadows can be degraded by a range of land use practices that alter the interdependent sediment transport and hydrologic and biological processes. Wet meadow degradation can often be traced back to lowered water tables, though not all problems will be resolved by restoring water tables (Chambers and Miller, 2011). As examples, invasive native and non-native species may persist under a natural hydrologic regime, and continued heavy livestock grazing may disrupt soil surfaces and allow species not normally found in wet meadows to gain and maintain footholds (Pope et al., 2015; Chambers et al., 2021; Cummings et al., 2023). Many examples of recent wet meadow degradation are related to water table lowering resulting from incision, whether intentional or a by-product of other land use activities (Wolf and Cooper, 2011; Hunt et al., 2018; Chambers et al., 2021).
In the past, climate change-induced wet meadow loss may have resulted in a slightly different sequence of causative events. As a result of climate change, reduced precipitation led to lower water tables in alluvial aquifers, subsequently losing wet meadow vegetation and the cohesive strength provided by the roots. This made such systems vulnerable to erosion, headcutting and incision during high magnitude discharge events (Tully et al., 2019). Wet meadows fed by deep groundwater sources were likely less impacted but still vulnerable to climate changes.
Although widespread wet meadow loss was a natural occurrence in response to past climate fluctuations, the current widespread global losses of wet meadows seem unrelated to a specific change in climate or a specific region because the losses are occurring in both dry and humid environments and with direct observation of degradation caused by human actions (Reagan, 1924; Darby and Simon, 1999). Even if wet meadow losses due to incision were the result of natural processes, the result is still a diminishment of value in terms of ecological services provided (Cluer and Thorne, 2014). Regardless of the cause, there is growing recognition of the value of wet meadows and growing efforts to restore these ecosystems and the services they provide (Grootjans and Verbeek, 2002; Pope et al., 2015; Chambers et al., 2021). Negative effects stem primarily from water table lowering, which reduces the ability of the system to store water, accumulate carbon and diminishes the productive capacity and biological diversity of the system.
Water tables that consistently lower tend to favor dry meadow species, while water tables that create ponded water above the land surface elevation that remains throughout most of the growing season (e.g., beaver ponds), tend to favor taller emergent marsh or aquatic vegetation (Cowardin et al., 1979; Lord et al., 2011). However, if wet meadow species are well established, conversion to other community types in a hydrologic regime less favorable to wet meadows can be a slow process. That is, there is a lag time between altered hydrology and observed changes in the species composition resulting from the altered hydrology. Put differently, this suggests that degraded wet meadows that retain some proportion of wet meadow species, even if they are no longer dominant, are likely to recover more quickly than sites where the duration or magnitude of disturbance has eliminated all wet meadow propagules such as seeds and isolated patches of living vegetation.
Water table elevation is not the only necessary hydrological condition for wet meadow establishment. Although the dense roots and shoots of intact wet meadow vegetation both resists erosion and lowers unit stream power, a degraded system has no such protections and is much more vulnerable to erosive floods. Thus the rate at which a degraded system that has can recover from an incision event depends to a certain extent on whether or not there are severe floods in the years following an incision event (Tully et al., 2019).
Soil conservation techniques as restoration tools
Historically, the general problem of incision and gullying and the headward extension of drainage networks has largely been viewed as a soil conservation problem rather than a stream restoration problem, and the focus has been on preventing erosion and soil loss, and mitigating the effects of erosion on water quality (Schumm et al., 1984). Although the effects of incision on water table lowering have been understood for some time, until recently, re-elevating water tables has not been considered an indicator or desirable outcome of soil and water conservation. Much of this is attributable to the fact that soil conservation is rooted in agriculture, and Western agricultural practices are rooted in growing crops that need aerobic soils rather than anaerobic soils. Thus agriculture has a well-developed history of lowering rather than raising water tables (Dahl, 1990; Valayamkunnath et al., 2020). Nonetheless, many of the soil conservation practices initially implemented to mitigate gullying and incision should also result in increased water storage, elevation of water tables and even improved flow (Norman et al., 2015; Frankl et al., 2021). Increasingly these are seen as as explicit benefits, provided water tables do not rise so far as to interfere with crop production (Frankl et al., 2021).
Until recently, stream restoration practitioners did not consider elevating water tables to the surface to be a desirable outcome and were explicitly designed to be moderately incised so that stream adjacent terraces or floodplains were only briefly flooded once or twice every few years during large discharge events such that streams were largely in equilibrium in terms of sediment transport (Lane, 1955; Leopold et al., 1964; Rosgen, 2003). This was known as the “bankfull channel” with a “2-year floodplain” and provided the basis for stream restoration design for decades, and is still in widespread practice in many areas of the United States (Rosgen, 2003; Rosgen, 2011). Designing alluvial systems where floodplains only flood occasionally and briefly creates hydrologic conditions incompatible with the needs of wet meadow ecosystems.
Currently, there is growing scientific recognition that rivers and stream ecosystems function best when water tables are elevated, allowing floodplains to be inundated throughout much of the year (Cluer and Thorne, 2014; Wohl et al., 2021; Powers et al., 2022). Historically this was a widespread natural condition, creating valley floor ecosystems full of wetlands, lakes, rivers and swamps, collectively now called river-wetland corridors (Meyer, 1936; Sedell and Froggatt, 1983; Walter and Merritts, 2008; Christy and Alverson, 2011; Cluer and Thorne, 2014; Pollock et al., 2014; Wohl et al., 2021). There is also a growing literature on the use of restoration techniques that re-elevate, store and modulate the downvalley movement of alluvial groundwater and these techniques are applicable towards wet meadow restoration.
Restoration techniques applicable towards wet meadow restoration include beaver-based restoration (Pollock et al., 2014; Pollock et al., 2017), incision trench filling (also known as stage zero restoration) (Cluer and Thorne, 2014; Powers et al., 2018), pond and plug (Hammersmark et al., 2008; Zeedyk and Vrooman, 2017), and the myriad techniques described as natural infrastructure. These include rock detention structures, weirs and check dams (Norman, 2020; Norman et al., 2022), most of which are intended to obstruct the downstream movement of water and sediment, raise water tables, increase groundwater recharge and sustain the growth of wetland and riparian vegetation (see also Yochum, 2016). Also potentially applicable but not in widespread use are hyporheic exchange structures (HES) (Vaux, 1968), which are intended to increase downwelling and upwelling of surface waters into and out of alluvium (Herzog et al., 2016; Herzog et al., 2023). In the context of wet meadow restoration, HESs could be used to restore subsurface flow obstructions and create areas of upwelling to bring water tables closer to the surface, essentially mimicking a bedrock constriction or buried alluvial fan.
Many of these evolving stream restoration techniques intended to elevate water tables towards the surface of valley floors or floodplains have applicability to wet meadows, but alluvial wet meadows often represent a dynamic transitional zone between stream channels and uplands, therefore caution should be used when applying stream restoration techniques. A successful wet meadow restoration project may result in the elimination of concentrated channelized surface flow on an erodible bed (i.e. a stream channel) and conversion to a system of dispersed, non-channelized flow across a well-vegetated surface (i.e. a wetland). This distinction is important because stream restoration projects, even those that seek to elevate water tables, generally envision stream channels of some sort as an outcome for the restorative action.
Further upvalley, mostly on agricultural lands, the problem of gullying and incision has been treated as a soil conservation problem, with soil loss by export into the channel network being the primary concern (Castillo and Gómez, 2016; Liu et al., 2019; Frankl et al., 2021). Here, numerous restoration or conservation techniques have focused on soil (sediment) retention and are often intended to extinguish channels and ensure continuous vegetative cover. Because these techniques are usually applied on agricultural lands, soil and water conservation efforts have not focused on natural ecosystem restoration, and non-native species are often recommended for planting. Nonetheless, the conceptual frameworks for soil conservation on agricultural lands and wet meadow restoration are similar, as are the goals in many respects (Liu et al., 2019; Frankl et al., 2021). Both seek to stop the export of sediment by creating a continuous cover of plants with dense root and shoot systems that are efficient at trapping water-borne sediment and are not easily eroded. Both approaches seek to extinguish channelized flow paths underlain by easily erodible sediment (soil) that can readily incise, and both approaches recognize that gullying or incision causes landscape desiccation and seeks to mitigate the effect, though to differing degrees. Soil conservation seeks to ensure that the topsoil is arable, that is, it can grow crops that require aerobic soil condition within the root zone, whereas wet meadow restoration seeks to maintain saturated (anaerobic) soils throughout the soil column for much of the year. What they share is the recognition that extension of the drainage channel network headwards through gullying and incision is an unnatural and undesirable condition that should be reversed. As a result, many of the soil conservation techniques designed to address the problem of gullying have applicability towards wet meadow restoration. Such techniques include headcut filling, channel reshaping, armoring, check dams, living walls, vegetative barriers, grassy water ways, and revegetation. These techniques have been well described in numerous publications, though not in the context of wet meadow restoration (Haregeweyn et al., 2015; Vannoppen et al., 2015; Castillo and Gómez, 2016; Liu et al., 2019; Frankl et al., 2021).
In summary, alluvial wet meadow restoration is neither a stream restoration practice nor a soil conservation practice but involves principles from both disciplines. The primary hydrologic goal of wet meadow restoration is to elevate water tables in alluvial aquifers such that they are at or near the surface for much of the year. The primary geomorphic goal of wet meadow restoration is to eliminate vulnerabilities that could result in incision or gullying and future water table lowering. Such vulnerabilities include exposed, poorly vegetated soils subject to flooding or concentrated run-off (e.g., ungulate trails), upstream migration of downstream headcuts, excess deposition of sediment from upstream sources meadows and the general presence of any channelized flow within the wet meadow system.
Stream restoration and soil conservation practitioners may often come from different backgrounds, use a different language and lexicon used to describe their practices, and generally work in opposite ends of the channel network (Norman, 2020; Norman et al., 2022). There is also divergence on the desirability of concentrating flow to create channels and there has been little analysis or agreement as to where channelized flow should begin in a well-managed, well-vegetated watershed (Lave, 2009). Thus, we suggest that soil conservation practices seek to reduce the extent of the drainage channel network as much as possible given the current biophysical conditions, whereas stream restorationists seek to restore the existing channel network, usually working under the implicit assumption that channelized flow is desirable and a natural condition where channels currently exist.
There are many operational techniques or tools used by stream restoration and soil conservation practitioners that have applicability towards the restoration of alluvial wet meadows, such as using natural rock and wood detention structures to slow flows (Hunt et al., 2018; Silverman et al., 2019; Rondeau et al., 2024). What all applicable techniques share is that they obstruct, to varying degrees, the downvalley movement of sediment and water. In doing so, they create moisture-retaining deposits of alluvium that are conducive to the establishment of vegetation. This creates a self-sustaining positive feedback loop that encourages the persistence of these systems absent a significant change in climate (Norman et al., 2015; Norman et al., 2022).
Channelized flow will continue to exist where the frequency and magnitude of flooding prevents complete encroachment of the roots and shoots of stream-adjacent vegetation across the channel bed such that there is no longer exposure of sediment to surface flows capable of transporting sediment. Humanity’s long history of the intentional and unintentional extension of stream channel networks and the widespread extent and sheer magnitude of channel network extension suggests that many of the small usually incised streams we see widespread today likely historically existed as unchannelized wetlands, many of which were wet meadows. While in the Northern Hemisphere the creation and maintenance of alluvial wet meadows was facilitated by beaver, studies from South America, Australia and Africa demonstrate that wet meadows develop and sustain themselves without beaver (Prosser et al., 1994; Von der Heyden, 2004; Tully et al., 2019) providing evidence that recovery of wet meadows and channel extinguishment can occur in degraded, incised systems when beaver are not present. Put differently, hydric graminoids such as sedges and rushes evolved tens of millions of years before beaver evolved to build dams, suggesting wet meadow ecosystems had been thriving for millions of years without beaver (Rybczynski, 2007; Tang et al., 2017). Beaver dams simply obstruct the movement of water and sediment, creating and maintaining conditions favorable to the accumulation of fine-grained, moisture-retaining sediment on low-gradient valley floors, and such conditions generally favor the hydric graminoids that compose the biomass bulk of wet meadow species.
In some cases, vegetation alone can act as an obstruction to flow in channelized systems and facilitate accumulation of fine sediment to sustain wet meadows and the extinguishment of channelized flows. This is perhaps best illustrated by Prosser and others (1994) in the tablelands of New South Wales, Australia. Here, a sustained episode of incision initiated by land use changes when Europeans colonized the continent in the 1800s converted linear wet meadows punctuated by ponds (hence known as ‘chain of ponds’, into deeply incised continuous streams. Without any active restoration efforts to create instream obstructions and without any apparent natural formation of obstructions, vegetation is growing into the incised stream, sediment is accumulating and defined channels are beginning to disappear. Similar self-repairing wet meadows have been observed in eastern Oregon after a similar series of land use changes resulting from European colonization in the 1800s (Welcher, 1993 and Pollock, personal observation). Such evidence suggests that some systems may eventually recover without a restoration intervention. However natural processes typically proceed much slower than desired for typical management time frames (Darby and Simon, 1999).
Invasive species and wet meadow restoration
Invasive species are a global problem across virtually all ecosystems, thought the severity of the problem varies considerably (Hansen et al., 2021). While wet meadow restoration is intended to improve ecosystem condition, poorly designed restoration efforts have the potential to make matters worse by creating conditions ripe for invasion by unwanted species (Ramstead et al., 2012; Long and Pope, 2014). Of particular note is that Reed Canary Grass (Phalaris arundinacea) is often mentioned as a very problematic invasive because it is well-adapted to the hydrologic conditions of wet meadows, though not the high carbon content of wet meadow soils (Perry et al., 2004; Perry and Galatowitsch, 2006). Assessments of invasive species in wet meadow restoration projects suggest that incorrect hydrologic regimes, soil compaction, exposure of mineral soils, introduction of non-native seeds during the restoration process, increased nitrogen availability, reduced soil carbon content and creation of novel habitat types have all been implicated in facilitating expansion of invasives into wet meadow environments (Perry et al., 2004; Perry and Galatowitsch, 2006; Price et al., 2011; Long and Pope, 2014; Rojas and Zedler, 2015; Hansen et al., 2021). Plants are not the only invasives of concerns in wet meadows. Certain restoration approaches such as ‘pond and plug” create deep ponds that are a novel habitat type in wet meadow ecosystems and are ideal for invasive aquatic species such as bullfrogs (Rana catesbeiana), brook trout (Salvelinus fontinalis), amphibian chytrid fungus (Batrachochytrium dendrobatidis) and green sunfish (Lepomis cyanellus) (Ramstead et al., 2012; Pope et al., 2019; Nelson, 2020). Poorly designed restoration projects, in particular, projects that do not result in the correct hydrologic conditions, can also result in the persistence or further encroachment of native species or upland non-native species tolerant of more xeric conditions (Chambers and Miller, 2011; Chambers et al., 2021; Cummings et al., 2023; Pope and Cummings, 2023). In sum, the limited number of studies of species invasions into wet meadow ecosystems suggest that most invasive species can be precluded from wet meadow restoration sites primarily by establishing the correct hydrologic regime, and secondarily by ensuring all soils are rich in organic material material with a healthy seed bank of native species, and lastly, avoiding the creation of habitat that would normally not be found in a pristine wet meadow, such as deep water ponds, entrenched stream beds, exposed mineral soils, compact soils and soils rich in nitrogen or low in carbon.
Limitations, gaps and future research needs
While in some regions such as the “Basin and Range and Cascade-Sierra Mountain (geological) Provinces of the western United States and parts of western Europe wet meadows are recognized as unique and valuable ecosystems, they are sometimes but not always recognized in national or international wetland classification systems (Cowardin et al., 1979; Scott and Jones, 1995; Zoltai and Vitt, 1995; Ramsar-Convention-Bureau, 2006; Brinson, 2009). As such, we were able to identify a number of gaps and additional research needs that have limited the thoroughness of this review. Herein, we argue that these systems are characterized by unique hydrologic, geomorphic and biological characteristics that separate them from more hydric ecosystems such as marshes that are much more frequently inundated and more xeric ecosystems such as dry meadows lacking in hydrophytes. We also recognize that these systems are not well-characterized throughout much of the world, so caution must be applied towards any conclusions as to their nature. In particular the carefully observed hydroperiods that are characteristic of wet meadows in the Basin and Range and Cascade-Sierra Mountain Provinces may not be characteristic of wet meadows in other regions or continents, though more general observations suggest that other wet meadow systems are also characterized by a regular, if not annual flooding followed by a drying period during the growing season when water tables drop below the surface. What is less clear for all wet meadow systems are the types and nature of subsurface obstructions that force water to the surface and allow for these systems to form and persist. Understanding the differences between and abundance of wet meadows formed by faulting, dykes, alluvial fans, bedrock sills, beaver dams, fine-grained alluvial deposits, and other subsurface flow obstructions would be helpful towards understanding the potential for recovery of degraded systems and the resiliency of existing systems. Further research should also more thoroughly investigate the sources and timing of different water supplies, including deep groundwater, shallow groundwater, hyporheic flow and surface flow.
Where wet meadow ecosystems are recognized, their importance to the contribution of regional biological diversity is often noted (Chambers et al., 2011; Grootjans and Verbeek, 2002; Norman et al., 2022; Poptcheva et al., 2009). Future research should focus on identifying where wet meadows exist, how they can be identified using remote sensing tools, what are the trends in wet meadow losses and gains by region, what are the mechanisms for degradation, and in particular where is incision or groundwater upwelling occurring and how is this impacting wet meadow condition. Additional research should focus on understanding the region-specific species composition of wet meadows and in particular if they are providing critical habitat for rare or threatened ‘red book’ species. Better understanding the unique hydrological, geomorphological and climate conditions under which wet meadows are formed is essential towards understanding where they may be restored or recreated. Of particular importance is understanding where incision has affected these systems and the integral importance of certain biota in restoring degraded systems, inclusive of hydric graminoids and where applicable, beaver. In this context, understanding the successional trajectory of recovering systems, the time needed for recovery and the expected biological and physical changes that should occur as these systems move along a recovery pathway are essential towards developing meaningful region-specific strategies for the restoration and conservation of these unique ecosystems.
Conclusion
Alluvial wet meadows are biologically rich ecosystems that regulate the cycling of water and carbon in the global climate. Although once abundant in alluvial valleys throughout most continents, alluvial wet meadows have been extirpated in many places because they are ideal locations for agriculture and are now mostly confined to montane alluvial valleys. There is growing recognition of their ecological and hydrological importance and as such, there are increasing efforts to restore these systems or at least prevent them from degrading further. The scale of loss of alluvial wet meadows is hard to comprehend and difficult to quantify, but data that are available suggest alluvial wet meadows were a major component of alluvial ecosystems, at least in North America, prior to colonization by Europeans. Wet meadows rely on elevated water tables throughout much of the year and as such, the primary cause of degradation has been through intentional or unintentional drainage by channel incision and extension of the channel network. Restoration efforts have primarily focused on stream restoration within alluvial meadows, but the reality is that many streams flowing through alluvial meadows may not have existed historically, and flow in these areas would have been unchannelized sheet flow through dense vegetation. Therefore, alluvial wet meadow restoration at its basic essence relies on understanding the causes of water table lowering and channelization and developing strategies for reversing those impacts. Such underlying causative agents suggest that wet meadow restoration would likely be most successful when practiced at the scale of a valley floor or large valley floor segments with consideration of the underlying biologic and hydrogeomorphic conditions that historically caused water tables to be elevated such that they were at or near the surface for much of the year.
Author contributions
MP: Conceptualization, Formal Analysis, Writing – original draft, Writing – review and editing. LN: Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by the Northwest Fisheries Science Center of the National Oceanic and Atmospheric Administration (NOAA) with support from the Aridland Water Harvesting Study, Core Science Systems Mission Area of the U.S. Geological Survey (USGS).
Acknowledgments
We are thankful for reviews provided by Britta Timpane-Padgham, Northwest Fisheries Science Center, Michaela Lowe, Washington Department of Fish and Wildlife, and Jeffrey Ziegeweid, USGS. Their comments substantially improved the quality of this manuscript. We are also thankful to four! reviewers and their constructive comments, which were very helpful. Any use of trade, firm, or product names is for descriptive purposes only and does not imply endorsement by the U.S. Government.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adam, E., Mutanga, O., and Rugege, D. (2010). Multispectral and hyperspectral remote sensing for identification and mapping of wetland vegetation: a review. Wetl. Ecol. Manag. 18, 281–296. doi:10.1007/s11273-009-9169-z
Alhassan, A. R. M., Ma, W., Li, G., Jiang, Z., Wu, J., and Chen, G. (2018). Response of soil organic carbon to vegetation degradation along a moisture gradient in a wet meadow on the Qinghai–Tibet Plateau. Ecol. Evol. 8, 11999–12010. doi:10.1002/ece3.4656
Al-Obaid, S., Samraoui, B., Thomas, J., El-Serehy, H. A., Alfarhan, A. H., Schneider, W., et al. (2017). An overview of wetlands of Saudi Arabia: values, threats, and perspectives. Ambio 46, 98–108. doi:10.1007/s13280-016-0807-4
Aselmann, I., and Crutzen, P. (1989). Global distribution of natural freshwater wetlands and rice paddies, their net primary productivity, seasonality and possible methane emissions. J. Atmos. Chem. 8, 307–358. doi:10.1007/bf00052709
Bai, Y., and Cotrufo, M. F. (2022). Grassland soil carbon sequestration: current understanding, challenges, and solutions. Science 377, 603–608. doi:10.1126/science.abo2380
Betz, F., Halik, Ü., Kuba, M., Tayierjiang, A., and Cyffka, B. (2015). Controls on aeolian sediment dynamics by natural riparian vegetation in the eastern Tarim Basin, NW China. Aeolian Res. 18, 23–34. doi:10.1016/j.aeolia.2015.04.005
Brahney, J., Heindel, R., Gill, T., Carling, G., Gonzalez-Olalla, J., Hand, J., et al. (2024). Dust in the critical zone: North American case studies. Earth-Science Rev. 258, 104942. doi:10.1016/j.earscirev.2024.104942
Brinson, M. M. (2009). “The United States HGM (Hydrogeomorphic) approach,” in The wetlands handbook. Chichester: Wiley-Blackwell, 486–512.
Britton, R., and Crivelli, A. (1993). “Wetlands of southern Europe and North Africa: mediterranean wetlands,” in Inventory, ecology and management volume I: africa, Australia, Canada and Greenland, mediterranean, Mexico, Papua New Guinea, south Asia, tropical South America. United States: Springer, 129–194.
Bryan, K. (1925). Date of channel trenching (arroyo cutting) in the arid southwest. Science 62, 338–344. doi:10.1126/science.62.1607.338
Castillo, C., and Gómez, J. (2016). A century of gully erosion research: urgency, complexity and study approaches. Earth-Science Rev. 160, 300–319. doi:10.1016/j.earscirev.2016.07.009
J. C. Chambers, and J. R. Miller (2011). “Geomorphology, hydrology, and ecology of great basin meadow complexes—implications for management and restoration,” Gen. Tech. Rep. RMRS-GTR-258 (Fort Collins, Colorado: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station).
Chambers, J. C., Miller, J. R., Lord, M. L., Board, D. I., and Knight, A. C. (2021). “Geomorphic sensitivity and ecological resilience of great basin streams and riparian ecosystems,”. Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Research Station, 159. doi:10.2737/rmrs-gtr-426
Christy, J. A., and Alverson, E. R. (2011). Historical vegetation of the willamette valley, Oregon, circa 1850. Northwest Sci. 85, 93–107. doi:10.3955/046.085.0202
Ciruzzi, D. M., and Lowry, C. S. (2017). Impact of complex aquifer geometry on groundwater storage in high-elevation meadows of the Sierra Nevada Mountains, CA. Hydrol. Process. 31, 1863–1875. doi:10.1002/hyp.11147
Cluer, B., and Thorne, C. (2014). A stream evolution model integrating habitat and ecosystem benefits. River Res. Appl. 30, 135–154. doi:10.1002/rra.2631
Cooper, D. J., Chimner, R. A., and Merritt, D. M. (2012). “Western Mountain wetlands,” in 328 wetland habitats of North America: ecology and conservation concerns, 313.
Cowardin, L. M., Carter, V., Golet, F. C., and LaRue, E. T. (1979). “Classification of wetlands and freshwater habitats of the United States,” in United States fish and wildlife service, office of biological services (FWS/OBS-79/31). Washington, DC: United States Government Printing Office.
Cummings, A. K., Pope, K. L., and Mak, G. (2023). Resetting the baseline: using machine learning to find lost meadows. Landsc. Ecol. 38, 2639–2653. doi:10.1007/s10980-023-01726-7
Dahl, T. E. (1990). Wetlands losses in the United States 1780's to 1980's. U.S. dept of the interior, U.S. fish and wildlife service. Washington, D.C.
Darrouzet-Nardi, A., D’Antonio, C. M., and Dawson, T. E. (2006). Depth of water acquisition by invading shrubs and resident herbs in a Sierra Nevada meadow. Plant Soil 285, 31–43. doi:10.1007/s11104-005-4453-z
Davies, D. D. (1980). “Anaerobic metabolism and the production of organic acids,” in Pages 581-611 the biochemistry of plants: a comprehensive treatise, volume 2: metabolism and respiration. Chichester: Elsevier.
Davies, N. S., Gosse, J. C., Rouillard, A., Rybczynski, N., Meng, J., Reyes, A. V., et al. (2022). Wood jams or beaver dams? Pliocene life, sediment and landscape interactions in the Canadian high arctic. Palaios 37, 330–347. doi:10.2110/palo.2021.065
Duman, T. Y. (2009). The largest landslide dam in Turkey: tortum landslide. Eng. Geol. 104, 66–79. doi:10.1016/j.enggeo.2008.08.006
Dwire, K. A., Kauffman, J. B., Brookshire, E. J., and Baham, J. E. (2004). Plant biomass and species composition along an environmental gradient in montane riparian meadows. Oecologia 139, 309–317. doi:10.1007/s00442-004-1498-2
Elliot, A. H. (2000). Settling of fine sediment in a channel with emergent vegetation. J. Hydraulic Eng. 126, 570–577. doi:10.1061/(asce)0733-9429(2000)126:8(570)
Enriquez, A. S., Chimner, R. A., Cremona, M. V., Diehl, P., and Bonvissuto, G. L. (2015). Grazing intensity levels influence C reservoirs of wet and mesic meadows along a precipitation gradient in northern patagonia. Wetl. Ecol. Manag. 23, 439–451. doi:10.1007/s11273-014-9393-z
Essaid, H. I., and Hill, B. R. (2014). Watershed-scale modeling of streamflow change in incised montane meadows. Water Resour. Res. 50, 2657–2678. doi:10.1002/2013wr014420
Fagorite, V., Odundun, O., Iwueke, L., Nwaigbo, U., and Okeke, O. (2019). Wetlands; A review of their classification, significance and management for sustainable development. Wetlands 5, 24–38.
Fairfax, E., and Whittle, A. (2020). Smokey the beaver: beaver-dammed riparian corridors stay green during wildfire throughout the Western United States. Ecol. Appl. 30, e02225. doi:10.1002/eap.2225
Flores, R. M. (1986). “Styles of coal deposition in tertiary alluvial deposits, powder river basin, Montana and Wyoming,” in Pages 79-104 paleoenvironmental and tectonic controls in coal-forming basins in the United States.
Flores, R. M., Lucas, S., and Hunt, A. (1987). Sedimentology of Upper Cretaceous and tertiary siliciclastics and coals in the raton basin, New Mexico and Colorado. Northeastern New Mexico. N. M. Geol. Soc. Guideb. 38, 255–264.
Fluet-Chouinard, E., Stocker, B. D., Zhang, Z., Malhotra, A., Melton, J. R., Poulter, B., et al. (2023). Extensive global wetland loss over the past three centuries. Nature 614, 281–286. doi:10.1038/s41586-022-05572-6
Frankl, A., Nyssen, J., Vanmaercke, M., and Poesen, J. (2021). Gully prevention and control: techniques, failures and effectiveness. Earth Surf. Process. Landforms 46, 220–238. doi:10.1002/esp.5033
Fryirs, K., and Brierley, G. (1998). The character and age structure of valley fills in upper Wolumla Creek catchment, south Coast, New South Wales, Australia. Earth Surf. Process. Landforms 23, 271–287. doi:10.1002/(sici)1096-9837(199803)23:3<271::aid-esp867>3.3.co;2-x
Fryirs, K. A., and Brierley, G. J. (2018). What’s in a name? A naming convention for geomorphic river types using the river styles framework. PloS one 13, e0201909. doi:10.1371/journal.pone.0201909
Gao, J., and Li, X. (2016). Degradation of frigid swampy meadows on the Qinghai–Tibet Plateau: current status and future directions of research. Prog. Phys. Geogr. 40, 794–810. doi:10.1177/0309133316659283
Gaur, M. K., and Squires, V. R. (2018). “Geographic extent and characteristics of the world’s arid zones and their peoples,” in Pages 3-20 climate variability impacts on land use and livelihoods in drylands. London: Springer Nature.
Germanoski, D., Miller, J. R., and Lord, M. L. (2011). “Controls on meadow distribution and characteristics,” in Pages 11-23 great basin riparian ecosystems: ecology, management, and restoration. Covelo, California: Island Press.
Gopal, B., and Sah, M. (1995). Inventory and classification of wetlands in India. Classif. Inventory World’s Wetl., 39–48. doi:10.1007/978-94-011-0427-2_5
Gorham, E. (1995). “The biogeochemistry of northern peatlands and its possible responses to global warming,” in Biotic feedbacks in the global climatic system, 169–187.
Gorrick, S., and Rodríguez, J. F. (2012). Sediment dynamics in a sand bed stream with riparian vegetation. Water Resour. Res. 48. doi:10.1029/2011wr011030
Green, K. C., and Westbrook, C. J. (2009). Changes in riparian area structure, channel hydraulics, and sediment yield following loss of beaver dams. J. Ecosyst. Manag. 10, 59–64. doi:10.22230/jem.2009v10n1a412
Grootjans, A., and Verbeek, S. (2002). A conceptual model of European wet meadow restoration. Ecol. Restor. 20, 6–9. doi:10.3368/er.20.1.6
Gyssels, G., Poesen, J., Bochet, E., and Li, Y. (2005). Impact of plant roots on the resistance of soils to erosion by water: a review. Prog. Phys. Geogr. 29, 189–217. doi:10.1191/0309133305pp443ra
Hammersmark, C. T., Rains, M. C., and Mount, J. F. (2008). Quantifying the hydrological effects of stream restoration in a montane meadow, northern California, USA. River Res. Appl. 24, 735–753. doi:10.1002/rra.1077
Hansen, W., Wollny, J., Otte, A., Eckstein, R. L., and Ludewig, K. (2021). Invasive legume affects species and functional composition of Mountain meadow plant communities. Biol. Invasions 23, 281–296. doi:10.1007/s10530-020-02371-w
Haregeweyn, N., Tsunekawa, A., Nyssen, J., Poesen, J., Tsubo, M., Tsegaye Meshesha, D., et al. (2015). Soil erosion and conservation in Ethiopia: a review. Prog. Phys. Geogr. 39, 750–774. doi:10.1177/0309133315598725
Heider, J., and Fuchs, G. (1997). Anaerobic metabolism of aromatic compounds. Eur. J. Biochem. 243, 577–596. doi:10.1111/j.1432-1033.1997.00577.x
Herzog, S., Higgins, C., and McCray, J. (2016). Engineered streambeds for induced hyporheic flow: enhanced removal of nutrients, pathogens, and metals from urban streams. J. Environ. Eng. 142, 04015053. doi:10.1061/(asce)ee.1943-7870.0001012
Herzog, S. P., Galloway, J., Banks, E. W., Posselt, M., Jaeger, A., Portmann, A., et al. (2023). Combined surface-subsurface stream restoration structures can optimize hyporheic attenuation of stream water contaminants. Environ. Sci. and Technol. 57, 4153–4166. doi:10.1021/acs.est.2c05967
Hewes, L. (1951). The northern wet prairie of the United States: nature, sources of information, and extent. Ann. Assoc. Am. Geogr. 41, 307–323. doi:10.2307/2561071
Horn, S., Durka, W., Wolf, R., Ermala, A., Stubbe, A., Stubbe, M., et al. (2011). Mitochondrial genomes reveal slow rates of molecular evolution and the timing of speciation in beavers (castor), one of the largest rodent species. PloS one 6, e14622. doi:10.1371/journal.pone.0014622
Hunsaker, C., Swanson, S., McMahon, A., Viers, J., and Hill, B. (2015). Effects of meadow erosion and restoration on groundwater storage and baseflow in national forests in the Sierra Nevada, California. Vallejo, CA: USDA For Serv, 1–59.
Hunt, L. J., Fair, J., and Odland, M. (2018). Meadow restoration increases baseflow and groundwater storage in the Sierra Nevada Mountains of California. J. Am. Water Resour. Assoc. 54, 1127–1136. doi:10.1111/1752-1688.12675
Hunt, L. J., Fair, J., and Odland, M. (2020). Reply to discussion: “Meadow Restoration Increases Base Flow and Groundwater Storage in the Sierra Nevada Mountains of California”. JAWRA J. Am. Water Resour. Assoc. 56, 180–181. doi:10.1111/1752-1688.12829
IPCC (2023). Summary for Policymakers. In: Climate Change 2023: Synthesis Report. Contribution of Working Groups I, II and III to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change Core Writing Team. eds. H. Lee, and J. Romero Geneva, Switzerland: IPCC, 1–34. doi:10.59327/IPCC/AR6-9789291691647.001
Johnston, C., Pinay, G., Arens, C., and Naiman, R. (1995). Influence of soil properties on the biogeochemistry of a beaver meadow hydrosequence. Soil Sci. Soc. Am. J. 59, 1789–1799. doi:10.2136/sssaj1995.03615995005900060041x
Johnston, C. A. (2000). Wetland soil and landscape alteration by beavers. Baton Rouge, Louisiana: CRC Press, 405–422.
Johnston, C. A. (2014). Beaver pond effects on carbon storage in soils. Geoderma 213, 371–378. doi:10.1016/j.geoderma.2013.08.025
Jones, L., and Schumm, S. (1999). Causes of avulsion: an overview. In: Fluvial sedimentology VI, Special Publication Number 28 of the International Association of Sedimentologists. Editors N. D. Smith, and J. Rogers (Hoboken, NJ: Wiley-Blackwell, 169–178.
Joyce, C. B., Simpson, M., and Casanova, M. (2016). Future wet grasslands: ecological implications of climate change. Ecosyst. Health Sustain. 2, e01240. doi:10.1002/ehs2.1240
Joyce, C. B., and Wade, P. M. (1998). European wet grasslands: biodiversity, management and restoration. Chichester: John Wiley and Sons Ltd.
Junk, W. J., Piedade, M. T. F., Schöngart, J., Cohn-Haft, M., Adeney, J. M., and Wittmann, F. (2011). A classification of major naturally-occurring Amazonian lowland wetlands. Wetlands 31, 623–640. doi:10.1007/s13157-011-0190-7
Keddy, P. A. (2010). Wetland ecology: principles and conservation. Cambridge, England: Cambridge University Press.
Klimkowska, A., Van Diggelen, R., Bakker, J. P., and Grootjans, A. P. (2007). Wet meadow restoration in Western Europe: a quantitative assessment of the effectiveness of several techniques. Biol. Conserv. 140, 318–328. doi:10.1016/j.biocon.2007.08.024
Klos, P., Lucas, R., and Conklin, M. (2023). Influence of critical zone architecture and snowpack on streamflow generation processes: a mountain-meadow headwater system in a mediterranean climate. Water Resour. Res. 59, e2023WR034493. doi:10.1029/2023wr034493
Koehler, P. A., and Anderson, R. S. (1994). The paleoecology and stratigraphy of nichols meadow, Sierra national forest, California, USA. Palaeogeogr. Palaeoclimatol. Palaeoecol. 112, 1–17. doi:10.1016/0031-0182(94)90132-5
Kołos, A., and Banaszuk, P. (2013). Mowing as a tool for wet meadows restoration: effect of long-term management on species richness and composition of sedge-dominated wetland. Ecol. Eng. 55, 23–28. doi:10.1016/j.ecoleng.2013.02.008
Lal, R., Follett, R. F., Stewart, B. A., and Kimble, J. M. (2007). Soil carbon sequestration to mitigate climate change and advance food security. Soil Sci. 172, 943–956. doi:10.1097/ss.0b013e31815cc498
Lane, E. W. (1955). The importance of fluvial morphology in hydraulic engineering. Am. Soc. Civil engineers. Proc 81, 745.
Lave, R. (2009). The controversy over natural channel design: substantive explanations and potential avenues for resolution 1. J. Am. Water Resour. Assoc. 45, 1519–1532. doi:10.1111/j.1752-1688.2009.00385.x
Leifeld, J., Wüst-Galley, C., and Page, S. (2019). Intact and managed peatland soils as a source and sink of GHGs from 1850 to 2100. Nat. Clim. Change 9, 945–947. doi:10.1038/s41558-019-0615-5
Lennartsson, T., Westin, A., Iuga, A., Jones, E., Madry, S., Murray, S., et al. (2016). The meadow is the mother of the field. Comparing transformations in hay production in three European agroecosystems. Martor 21, 103–126. doi:10.57225/martor.2016.21.08
Leopold, L. B., Wolman, M. G., and Miller, J. P. (1964). Fluvial processes in geomorphology. San Francisco, California: Freeman.
Liu, X., Li, H., Zhang, S., Cruse, R. M., and Zhang, X. (2019). Gully erosion control practices in northeast China: a review. Sustainability 11, 5065. doi:10.3390/su11185065
Loheide, S. P., Deitchman, R. S., Cooper, D. J., Wolf, E. C., Hammersmark, C. T., and Lundquist, J. D. (2009). A framework for understanding the hydroecology of impacted wet meadows in the Sierra Nevada and Cascade ranges, California, USA. Hydrogeology J. 1, 229–246. doi:10.1007/s10040-008-0380-4
Loheide, S. P., and Gorelick, S. M. (2006). Quantifying stream− aquifer interactions through the analysis of remotely sensed thermographic profiles and in situ temperature histories. Environ. Sci. and Technol. 40, 3336–3341. doi:10.1021/es0522074
Loheide, S. P., and Gorelick, S. M. (2007). Riparian hydroecology: a coupled model of the observed interactions between groundwater flow and meadow vegetation patterning. Water Resour. Res. 43. doi:10.1029/2006wr005233
Loisel, J., Gallego-Sala, A. V., Amesbury, M., Magnan, G., Anshari, G., Beilman, D., et al. (2021). Expert assessment of future vulnerability of the global peatland carbon sink. Nat. Clim. Change 11, 70–77. doi:10.1038/s41558-020-00944-0
Long, J. W., and Pope, K. (2014). Wet meadows. Gen. Tech. Rep. PSW-GTR-247. Albany, CA: US Department of Agriculture, Forest Service, 341–372.
Lord, M. L., Jewett, D. G., Miller, J. R., Germanoski, D., and Chambers, J. C. (2011). “Hydrologic processes influencing meadow ecosystems,” in Great basin riparian ecosystems: ecology, management and restoration, 44–67.
Lourenco, M., Fitchett, J. M., and Woodborne, S. (2023). Peat definitions: a critical review. Prog. Phys. Geogr. Earth Environ. 47, 506–520. doi:10.1177/03091333221118353
Mactaggart, B., Bauer, J., Goldney, D., and Rawson, A. (2008). Problems in naming and defining the swampy meadow—An Australian perspective. J. Environ. Manag. 87, 461–473. doi:10.1016/j.jenvman.2007.01.030
Matthews, E., and Fung, I. (1987). Methane emission from natural wetlands: global distribution, area, and environmental characteristics of sources. Glob. Biogeochem. cycles 1, 61–86. doi:10.1029/gb001i001p00061
McTainsh, G., and Strong, C. (2007). The role of Aeolian dust in ecosystems. Geomorphology 89, 39–54. doi:10.1016/j.geomorph.2006.07.028
Meyer, A. (1936). The kankakee marsh of northern Indiana and Illinois. Mich. Acad. Sci. Arts, Lett. 21, 359–396.
Micheli, E., and Kirchner, J. (2002a). Effects of wet meadow riparian vegetation on streambank erosion. 1. Remote sensing measurements of streambank migration and erodibility. Earth Surf. Process. Landforms 27, 627–639. doi:10.1002/esp.338
Micheli, E., and Kirchner, J. (2002b). Effects of wet meadow riparian vegetation on streambank erosion. 2. Measurements of vegetated bank strength and consequences for failure mechanics. Earth Surf. Process. Landforms 27, 687–697. doi:10.1002/esp.340
Miller, J. R., Germanoski, D., and Lord, M. L. (2011). Geomorphic processes affecting meadow ecosystems. Geomorphol. Hydrology, Ecol. Gt. Basin Meadow Complexes—Implications Manag. Restor. 24. doi:10.2737/RMRS-GTR-258
Minckley, T. A., Turner, D., and Weinstein, S. (2013). The relevance of wetland conservation in arid regions: a re-examination of vanishing communities in the American southwest. J. Arid Environ. 88, 213–221. doi:10.1016/j.jaridenv.2012.09.001
Mitsch, W. J., and Gosselink, J. G. (1993). Wetlands. 2nd ed. edition. New York: Van Nostrand Reinhold.
Montgomery, D. R., and Dietrich, W. E. (1992). Channel initiation and the problem of landscape scale. Science 255, 826–830. doi:10.1126/science.255.5046.826
Nag, S. K. (2019). “Carbon sequestration potential of wetlands–An overview,”. In Perspectives on Inland Fisheries & Climate Change in India. (Barrackpore, India: ICAR- Central Inland Fisheries Research Institute), 359–372.
Nash, C. S., Selker, J. S., Grant, G. E., Lewis, S. L., and Noël, P. (2018). A physical framework for evaluating net effects of wet meadow restoration on late-summer streamflow. Ecohydrology 11, e1953. doi:10.1002/eco.1953
Neff, D. J. (1957). Ecological effects of beaver habitat abandonment in the Colorado rockies. J. Wildl. Manag. 21, 80–84. doi:10.2307/3797684
Nelson, N. L. (2020). “American bullfrog (Lithobates catesbeianus) invasion in California,” in Masters thesis. Pullman, Washington: Washington State University.
Norman, L. M. (2020). Ecosystem services of riparian restoration: a review of rock detention structures in the madrean archipelago ecoregion. Air, Soil Water Res. 13. doi:10.1177/1178622120946337
Norman, L. M., Brinkerhoff, F., Gwilliam, E., Guertin, D. P., Callegary, J., Goodrich, D. C., et al. (2015). Hydrologic response of streams restored with check dams in the chiricahua Mountains, Arizona. River Res. Appl. 32, 519–527. doi:10.1002/rra.2895
Norman, L. M., Lal, R., Wohl, E., Fairfax, E., Gellis, A. C., and Pollock, M. M. (2022). Natural infrastructure in dryland streams (NIDS) can establish regenerative wetland sinks that reverse desertification and strengthen climate resilience. Sci. total Environ. 849, 157738. doi:10.1016/j.scitotenv.2022.157738
Ohara, N., Kavvas, M., Chen, Z., Liang, L., Anderson, M., Wilcox, J., et al. (2014). Modelling atmospheric and hydrologic processes for assessment of meadow restoration impact on flow and sediment in a sparsely gauged California watershed. Hydrol. Process. 28, 3053–3066. doi:10.1002/hyp.9821
Page, S. E., and Baird, A. (2016). Peatlands and global change: response and resilience. Annu. Rev. Environ. Resour. 41, 35–57. doi:10.1146/annurev-environ-110615-085520
Pastor, J., Bonde, J., Johnson, C., and Naiman, R. J. (1993). Markovian analysis of the spatially dependent dynamics of beaver ponds. Lect. Math. Life Sci. 23, 5–27.
Patton, D. R., and Judd, B. (1970). The role of wet meadows as wildlife habitat in the southwest. Rangel. Ecol. and Management/Journal Range Manag. Archives 23, 272–275. doi:10.2307/3896220
Pearthree, P., and Cook, J. (2015). “Geology and geomorphology of the San Pedro River”, 10. southeastern Arizona. Tucson, AZ: Arizona Geological Survey Special Paper, 24.
Perry, L. G., and Galatowitsch, S. M. (2006). Light competition for invasive species control: a model of cover crop–weed competition and implications for Phalaris arundinacea control in sedge meadow wetlands. Euphytica 148, 121–134. doi:10.1007/s10681-006-5946-4
Perry, L. G., Galatowitsch, S. M., and Rosen, C. J. (2004). Competitive control of invasive vegetation: a native wetland sedge suppresses Phalaris arundinacea in carbon-enriched soil. J. Appl. Ecol. 41, 151–162. doi:10.1111/j.1365-2664.2004.00871.x
Pollock, M., Lewallen, G., Woodruff, K., Jordan, C., and Castro, J. (2017). The beaver restoration guidebook: working with beaver to restore streams, wetlands, and floodplains. Portland, Oregon: United States Fish and Wildlife Service.
Pollock, M. M., Beechie, T. J., and Jordan, C. E. (2007). Geomorphic changes upstream of beaver dams in bridge creek, an incised stream channel in the interior columbia river basin, eastern Oregon. Earth Surf. Process. Landforms 32, 1174–1185. doi:10.1002/esp.1553
Pollock, M. M., Beechie, T. J., Wheaton, J. M., Jordan, C. E., Bouwes, N., Weber, N., et al. (2014). Using beaver dams to restore incised stream ecosystems. BioScience 64, 279–290. doi:10.1093/biosci/biu036
Pollock, M. M., Heim, M., and Werner, D. (2003). “Hydrologic and geomorphic effects of beaver dams and their influence on fishes,” in The ecology and management of wood in world Rivers. Editors S. V. Gregory, K. Boyer, and A. Gurnell (Bethesda, Maryland: American Fisheries Society), 213–233.
Polvi, L. E., and Wohl, E. (2012). The beaver meadow complex revisited–the role of beavers in post-glacial floodplain development. Earth Surf. Process. Landforms 37, 332–346. doi:10.1002/esp.2261
Pope, K., Yarnell, S., and Piovia-Scott, J. (2019). How to make meadow restoration work for California’s Mountain frogs?in Proceedings of the 4th joint federal interagency sedimentation and hydrologic modeling conference.
Pope, K. L., and Cummings, A. K. (2023). Recovering the lost potential of meadows to help mitigate challenges facing California’s forests and water supply. Calif. Fish Wildl. J. 109 (1), 977–109. doi:10.51492/cfwj.109.3
Pope, K. L., Montoya, D. S., Brownlee, J. N., Dierks, J., and Lisle, T. E. (2015). Habitat conditions of montane meadows associated with restored and unrestored stream channels of California. Ecol. Restor. 33, 61–73. doi:10.3368/er.33.1.61
Poptcheva, K., Schwartze, P., Vogel, A., Kleinebecker, T., and Hölzel, N. (2009). Changes in wet meadow vegetation after 20 years of different management in a field experiment (North-West Germany). Agric. Ecosyst. and Environ. 134, 108–114. doi:10.1016/j.agee.2009.06.004
Powers, P. D., Helstab, M., and Niezgoda, S. L. (2018). A process-based approach to restoring depositional river valleys to stage 0, an anastomosing channel network. River Res. Appl. 35, 13. doi:10.1002/rra.3378
Powers, P., Staab, B., Cluer, B., and Thorne, C. (2022). Rediscovering, reevaluating, and restoring entiatqua: identifying pre-anthropocene valleys in north cascadia, USA. River Res. Appl. 38, 1527–1543. doi:10.1002/rra.4016
Price, J. N., Berney, P., Ryder, D., Whalley, R., and Gross, C. L. (2011). Disturbance governs dominance of an invasive forb in a temporary wetland. Oecologia 167, 759–769. doi:10.1007/s00442-011-2027-8
Prosser, I. P., Chappell, J., and Gillespie, R. (1994). Holocene valley aggradation and gully erosion in headwater catchments, south-eastern highlands of Australia. Earth Surf. Process. Landforms 19, 465–480. doi:10.1002/esp.3290190507
Prosser, I. P., and Slade, C. J. (1994). Gully formation and the role of valley-floor vegetation, southeastern Australia. Geology 22, 1127–1130. doi:10.1130/0091-7613(1994)022<1127:gfatro>2.3.co;2
Ramsar-Convention-Bureau (2006). The ramsar convention manual: a guide to the convention on wetlands. Ramsar, Iran: Ramsar Convention Bureau.
Ramstead, K. M., Allen, J. A., and Springer, A. E. (2012). Have wet meadow restoration projects in the Southwestern US been effective in restoring geomorphology, hydrology, soils, and plant species composition? Environ. Evid. 1, 11–16. doi:10.1186/2047-2382-1-11
Reagan, A. B. (1924). Recent changes in the Plateau region. Science 60, 283–285. doi:10.1126/science.60.1552.283
Reed, C. C., Merrill, A. G., Drew, W. M., Christman, B., Hutchinson, R. A., Keszey, L., et al. (2021). Montane meadows: a soil carbon sink or source? Ecosystems 24, 1125–1141. doi:10.1007/s10021-020-00572-x
Renteria-Villalobos, M., and Hanson, R. (2015). Capturing wetness for sustainability from climate variability and change in the rio conchos. Chihuahua, Mexico: Chihuahua.
Richardson, J., and Brinson, M. M. (2001). “Wetland soils and the hydrogeomorphic classification of wetlands,” in Wetland soils. Genesis, hydrology, landscapes and classification. Boca Raton: Lewis Publishers, 209–227.
Rojas, I. M., and Zedler, J. B. (2015). An invasive exotic grass reduced sedge meadow species richness by half. Wetl. Ecol. Manag. 23, 649–663. doi:10.1007/s11273-015-9409-3
Rondeau, R. J., Austin, G., Miller, R. S., Parker, S., Breibart, A., Conner, S., et al. (2024). Restoration of wet meadows to enhance gunnison sage-grouse habitat and drought resilience in arid rangelands. Restor. Ecol. 32, e14039. doi:10.1111/rec.14039
Rosgen, D. L. (2011). “Natural channel design: fundamental concepts, assumptions, and methods,” in Stream restoration in dynamic fluvial systems: scientific approaches, analyses, and tools 194, 69–93.
Rybczynski, N. (2007). Castorid phylogenetics: implications for the evolution of swimming and tree-exploitation in beavers. J. Mammalian Evol. 14, 1–35. doi:10.1007/s10914-006-9017-3
Rybczynski, N. (2008). Woodcutting behavior in beavers (castoridae, rodentia): estimating ecological performance in a modern and a fossil taxon. Paleobiology 34, 389–402. doi:10.1666/06085.1
Schook, D. M., Borkenhagen, A. K., McDaniel, P. A., Wagner, J. I., and Cooper, D. J. (2020). Soils and hydrologic processes drive wet meadow formation and approaches to restoration, Western USA. Wetlands 40, 637–653. doi:10.1007/s13157-019-01200-8
Schumm, S. (1973). Geomorphic thresholds and complex response of drainage systems. Fluv. Geomorphol. 6, 69–85.
Schumm, S., Harvey, M., and Watson, C. (1984). Incised channels: morphology, dynamics and control. Littleton, Colorado: Water Resources Publications.
Schumm, S. A. (1979). Geomorphic thresholds: the concept and its applications. Trans. Inst. Br. Geogr. 4, 485–515. doi:10.2307/622211
Scott, D., and Jones, T. (1995). Classification and inventory of wetlands: a global overview. Vegetatio 118, 3–16. doi:10.1007/978-94-011-0427-2_2
Sedell, J. R., and Froggatt, J. L. (1983). Importance of streamside forests to large Rivers: the isolation of the willamette river, Oregon, USA, from its floodplain by snagging and streamside forest removal; congress in France. Congress of the international association of limnology, Lyon (France) 1828–1834. (22).
Silverman, N. L., Allred, B. W., Donnelly, J. P., Chapman, T. B., Maestas, J. D., Wheaton, J. M., et al. (2019). Low-tech riparian and wet meadow restoration increases vegetation productivity and resilience across semiarid rangelands. Restor. Ecol. 27, 269–278. doi:10.1111/rec.12869
Şimşek, Ç. K., and Ödül, H. (2018). Investigation of the effects of wetlands on micro-climate. Appl. Geogr. 97, 48–60. doi:10.1016/j.apgeog.2018.05.018
Straubinger, C., Reisch, C., and Poschlod, P. (2023). Effects of historical management on the vegetation and habitat properties of wet meadows in Germany. Restor. Ecol. 31, e13839. doi:10.1111/rec.13839
Surfleet, C., Sanford, T., VanOosbree, G., and Jasbinsek, J. (2019). Hydrologic response of meadow restoration the first year following removal of encroached conifers. Water 11, 428. doi:10.3390/w11030428
Tague, C., Valentine, S., and Kotchen, M. (2008). Effect of geomorphic channel restoration on streamflow and groundwater in a snowmelt-dominated watershed. Water Resour. Res. 44. doi:10.1029/2007wr006418
Tang, C. Q., Orme, C. D. L., Bunnefeld, L., Jones, F. A., Powell, S., Chase, M. W., et al. (2017). Global monocot diversification: geography explains variation in species richness better than environment or biology. Botanical J. Linn. Soc. 183, 1–15. doi:10.1111/boj.12497
Tiedje, J., Sexstone, A., Parkin, T., Revsbech, N., and Shelton, D. R. (1984). Anaerobic processes in soil. Plant Soil 76, 197–212. doi:10.1007/978-94-009-6101-2_18
Tonina, D., and Buffington, J. M. (2009). Hyporheic exchange in Mountain Rivers II: effects of channel morphology on mechanics, scales, and rates of exchange. Geogr. Compass 3, 1038–1062. doi:10.1111/j.1749-8198.2009.00225.x
Tonina, D., and Buffington, J. M. (2023). “Physical and biogeochemical processes of hyporheic exchange in alluvial Rivers,” in 87 groundwater ecology and evolution. Elsevier, 61.
Totsche, K. U., Amelung, W., Gerzabek, M. H., Guggenberger, G., Klumpp, E., Knief, C., et al. (2018). Microaggregates in soils. J. Plant Nutr. Soil Sci. 181, 104–136. doi:10.1002/jpln.201600451
Tuboi, C., and Hussain, S. A. (2018). Plant community structure of the floating meadows of a hypereutrophic wetland in the indo-burma biodiversity hotspot. Aquat. Bot. 150, 71–81. doi:10.1016/j.aquabot.2018.06.006
Tully, C. D., Rech, J. A., Workman, T. R., Santoro, C. M., Capriles, J. M., Gayo, E. M., et al. (2019). In-stream wetland deposits, megadroughts, and cultural change in the northern atacama desert, Chile. Quat. Res. 91, 63–80. doi:10.1017/qua.2018.122
UNEP (2022). “The state of the World’s peatlands: evidence for action toward the conservation, restoration, and sustainable management of peatlands,” in Global peatlands initiative. Nairobi: United Nations Environment Programme.
Valayamkunnath, P., Barlage, M., Chen, F., Gochis, D. J., and Franz, K. J. (2020). Mapping of 30-meter resolution tile-drained croplands using a geospatial modeling approach. Nature-Scientific data 7, 257. doi:10.1038/s41597-020-00596-x
Vannoppen, W., Vanmaercke, M., De Baets, S., and Poesen, J. (2015). A review of the mechanical effects of plant roots on concentrated flow erosion rates. Earth-Science Rev. 150, 666–678. doi:10.1016/j.earscirev.2015.08.011
Vaux, W. G. (1968). Intragravel flow and interchange of water in a streambed. Fish. Bull. Fish Wildl. Serv. 66, 479–489.
Von der Heyden, C. J. (2004). The hydrology and hydrogeology of dambos: a review. Prog. Phys. Geogr. 28, 544–564. doi:10.1191/0309133304pp424oa
Walker, R. (1968). The amphibians of zaria, in the northern Guinea Savannah, Nigeria. Copeia 1968, 164–167. doi:10.2307/1441565
Walter, R. C., and Merritts, D. J. (2008). Natural streams and the legacy of water-powered mills. Science 319, 299–304. doi:10.1126/science.1151716
Wang, H., Cui, P., Liu, D., Liu, W., Bazai, N. A., Wang, J., et al. (2019). Evolution of a landslide-dammed Lake on the southeastern Tibetan Plateau and its influence on river longitudinal profiles. Geomorphology 343, 15–32. doi:10.1016/j.geomorph.2019.06.023
Welcher, K. E. (1993). Holocene channel changes of camp creek: an arroyo in eastern Oregon. Eugene, OR: University of Oregon.
Were, D., Kansiime, F., Fetahi, T., Cooper, A., and Jjuuko, C. (2019). Carbon sequestration by wetlands: a critical review of enhancement measures for climate change mitigation. Earth Syst. Environ. 3, 327–340. doi:10.1007/s41748-019-00094-0
Westbrook, C. J., Cooper, D., and Baker, B. W. (2011). Beaver assisted river valley formation. River Res. Appl. 27, 247–256. doi:10.1002/rra.1359
Wohl, E., Castro, J., Cluer, B., Merritts, D., Powers, P., Staab, B., et al. (2021). Rediscovering, reevaluating, and restoring lost river-wetland corridors. Front. Earth Sci. 9, 653623. doi:10.3389/feart.2021.653623
Wolf, E., and Cooper, D. (2011). “Restoration of geomorphic structure, hydrologic regime, and vegetation in upper halstead meadow,”Proj. Rep. Sequoia Natl. Park, Three Rivers California.
Wood, S. H. (1975). Holocene stratigraphy and chronology of Mountain meadows, Sierra Nevada. California: California Institute of Technology.
Wu, J., Wang, H., Li, G., Ma, W., Wu, J., Gong, Y., et al. (2020). Vegetation degradation impacts soil nutrients and enzyme activities in wet meadow on the Qinghai-Tibet Plateau. Sci. Rep. 10, 21271. doi:10.1038/s41598-020-78182-9
Yagci, O., and Strom, K. (2022). Reach-scale experiments on deposition process in vegetated channel: suspended sediment capturing ability and backwater effect of instream plants. J. hydrology 608, 127612. doi:10.1016/j.jhydrol.2022.127612
Yochum, S. E. (2016). “Guidance for stream restoration and rehabilitation,” in US department of agriculture, forest service, national stream and aquatic ecology center.
Zedler, J. B., and Kercher, S. M. (2005). Wetland resources: status, trends, ecosystem services, and restorability. Annu. Rev. Environ. Resour. 30, 39–74. doi:10.1146/annurev.energy.30.050504.144248
Zeedyk, W., and Vrooman, S. (2017). The plug and pond treatment: restoring sheetflow to high elevation slope wetlands in New Mexico. N. M. Environ. Dep. Surf. Water Qual. Bur. Wetl. Program. Available online at: https://www.env.nm.gov/surface-water-quality/wetlands-technical-guides/.
Zhang, Z., Chen, F., Barlage, M., Bortolotti, L., Famiglietti, J., Li, Z., et al. (2022). Cooling effects revealed by modeling of wetlands and land-atmosphere interactions. Water Resour. Res. 58, e2021WR030573. doi:10.1029/2021wr030573
Zierholz, C., Prosser, I. P., Fogarty, P. J., and Rustomji, P. (2001). In-stream wetlands and their significance for channel filling and the catchment sediment budget, jugiong creek, New South Wales. Geomorphology 38, 221–235. doi:10.1016/s0169-555x(00)00092-1
Keywords: wet meadows, incision, process-based restoration, ecosystem services, hydrology, stream restoration, wetland restoration, carbon sequestration
Citation: Pollock MM and Norman LM (2025) Wet meadow regeneration through restoration of biophysical feedbacks. Front. Environ. Sci. 13:1592036. doi: 10.3389/fenvs.2025.1592036
Received: 12 March 2025; Accepted: 02 July 2025;
Published: 28 July 2025.
Edited by:
Francisca C. Aguiar, University of Lisbon, PortugalReviewed by:
Doru Stelian Banaduc, Lucian Blaga University of Sibiu, RomaniaMatthew Orr, Oregon State University Cascades Campus, United States
Copyright © 2025 Pollock and Norman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael M. Pollock, bWljaGFlbC5wb2xsb2NrQG5vYWEuZ292
 Michael M. Pollock
Michael M. Pollock Laura M. Norman
Laura M. Norman