- School of Molecular and Life Sciences, Curtin University, Perth, WA, Australia
Innovative and practical technological solutions are needed to support the restoration of degraded terrestrial ecosystems. Seed coating technology applied to native species is a promising solution to improve the efficiency of seed-based ecological restoration. Seed pelleting is a type of seed coating that increases the size and standardises the shape of small seeds, facilitating precision seeding. Such technology has been tested on native species, but in previous studies, the pelleting process was performed with one species at a time (Single Species Pelleting, SSP). Although effective, such an approach might be too costly when applied to the many species required to restore highly diverse ecosystems. Here, we present a novel method for seed pelleting multiple small-seeded species (MSP) in a single pass. Seeds of different species are mixed and then sorted by size through a series of sieves. The pelleting process starts with the smallest seeds. When the pellet reaches the size of the second-smallest seeds, those are added to the mix until they all reach the size of the third-smallest seeds. The process is repeated until all seeds are added and all pellets reach a uniform target size. To assess the efficacy of this new method, the time required to perform the pelleting and the associated cost on two batches of 13 and 19 species were compared to the SSP. The MSP allowed for pelleting times 5.6 to 10.2 times faster and a 3.1 to 3.9-fold reduction in cost compared to the SSP approach. The MSP approach is an effective method for increasing the efficiency of seed pelleting on diverse restoration mixes. Ultimately, the MSP technology combined with broad-acre seeding agricultural equipment has the potential to drastically increase the scale and effectiveness of seed-based restoration of degraded terrestrial ecosystems.
1 Introduction
A majority of terrestrial ecosystems have been damaged, degraded or destroyed to some degree by the direct and indirect impact of human activities such as land clearing, deforestation, unsustainable land use, introduced invasive species, and climate change (Nellemann and Corcoran, 2010). Such degradation directly impacts human wellbeing by eroding the natural capital and services that healthy ecosystems provide (Bai et al., 2013; Dean et al., 2021; Hasan et al., 2020) and threatens biodiversity (Ceballos et al., 2015; López-Bedoya et al., 2022).
Reducing societal impact and conserving the remaining healthy ecosystems are hence priorities (WWF, 2020); however, to reverse the degradation trend, it is necessary to restore degraded ecosystems (Holl and Aide, 2011). Ecological restoration is defined as the process of assisting the recovery of an ecosystem that has been degraded, damaged, or destroyed. The Society for Ecological Restoration (SER) developed a set of standards to guide the planning, implementation, monitoring and evaluation of restoration projects and provide clear definitions of what should be considered ecologically sound restoration (Gann et al., 2019) and in 2021, the United Nations launched the global initiative “UN Decade for Ecosystem Restoration” to promote international collaboration and set ambitious global restoration goals (United Nations, 2021).
Ecological restoration often relies on the vegetation recolonising degraded areas from neighbouring healthy ecosystems in a process known as natural regeneration (Chazdon et al., 2020). However, when degradation is too advanced, and remnant vegetation is too distant or fragmented, natural regeneration alone might not be enough, and it is often supported by the active reintroduction of vegetal material, namely, nursery-grown seedlings and seeds (Di Sacco et al., 2021).
1.1 Seed-based restoration
Seed-based ecological restoration is becoming an increasingly popular and cost-effective approach to re-establish native vegetation in a degraded site (Larson A. J. S. et al., 2023). It relies on using species-rich seed mixes that represent the vegetation community of a reference native ecosystem (Gann et al., 2019). For seed-based restoration to be performed at scale, the local native seed supply chain should be able to provide seed in the quantity, quality and species diversity required (Pedrini and Dixon, 2020). Unfortunately, in most scenarios, seed availability is not enough to meet demand, the quality is poor or unknown (Gibson-Roy et al., 2021), and in areas where the supply is overreliant on direct seed collection from natural populations, the cost can be prohibitive (Brancalion et al., 2019; Pedrini et al., 2022). Even when seeds are available, seed delivery to the site needs careful planning, site preparation, and impeccable timing to maximise the chance of successful seedling emergence and plant establishment (Shaw et al., 2020).
1.2 Direct seeding
Various seeding approaches have been tested for delivering seeds to site (Masarei et al., 2019). Precision seed delivery (seed drill), whereby seeds are incorporated into the soil, has usually proved more effective than broadcasting seeds on the soil surface (Svejcar et al., 2022). Precision seeding equipment and methods are often derived from agricultural equipment developed to handle a specific type of seeds (e.g., wheat, canola) and, therefore, struggle to handle the high variability in size and morphology of native seed mixes. A high degree of customisation of seeding machines can account for such complexities, but that often comes at the expense of seeding speed and scale (Shaw et al., 2020). Moreover, accommodating the variability of a native seed mix in a single seeding device necessitates compromises that might favour seeds with certain traits over others.
For example, during transport and seeding, vibrations can cause seeds to be separated (segregation) based on size and density, with smaller and denser seeds ending in the lower part of the hopper (Liu et al., 2013; Rosato et al., 2002). This would then result in an uneven distribution of species across the site. Using bulking agents, such as vermiculite or sand, could overcome this issue but further increase logistical complexities, including sourcing the material, delivering it to the site and ensuring constant mixing with the seeds, which would slow down seeding operations.
1.3 Seed enhancement technologies
Another approach to improving seeding efficiency is directly modifying seed shape and size to reach a more homogenous mix. This is often achieved via seed processing by removing (Barberis et al., 2023) or reducing (Berto et al., 2020; Guzzomi et al., 2016) appendages (e.g., awns and hairs in grass florets) that can impact seed flow (e.g., bridging). A further step involves applying fine powders (fillers) and binders to the seed to modify its shape, size and density via seed coating (Brown et al., 2021; Halmer, 2008; Pedrini et al., 2017; Taylor et al., 1998). There are different kinds of seed coatings, and the one that modifies the shape and size of the seed unit the most, seed pelleting, can be effective in improving seed delivery efficiency, especially in small-seeded species. (Gornish et al., 2019; Hoose et al., 2019).
Like seeding equipment, such technology originated in the crop and horticulture seed industry and has been developed to pellet one species at a time (Afzal et al., 2020). When applied to native species for ecological restoration, most studies have performed seed pelleting on individual species (Beveridge et al., 2020; Hoose et al., 2019; Madsen et al., 2014; Pearson et al., 2019; Pedrini et al., 2023; Turner et al., 2006; Westbrook et al., 2023). This allows for species-specific customisation of pelleting recipes, accounting for factors such as germination requirements, which may improve establishment outcomes. However, this approach presents some practical drawbacks when applied to diverse seed mixes. A recent study by Pedrini et al. (2023) highlights that the time required to pellet seeds of 15 small-seeded Myrtaceae species ranges between 37 and 188 min per species, with an average of 98 min. This means that the more diverse a mix is, the longer and more expensive the pelleting will be, making the process economically non-viable, thus nullifying any potential increased efficiency of seeding with pelleted seeds.
This paper describes a novel approach that overcomes such logistical issues by pelleting seeds of multiple small-seeded species in a single pass while maintaining singulation (one seed per pellet), thus potentially reducing the time and cost associated with seed pelleting.
The first part of the methods section (4. 1 Multispecies pelleting method) provides a detailed explanation of the process.
In the second part of the methods section (4.2 Method testing and validation) and the results section, two case studies of the multispecies pelleting approach (MSP), of 13 and 19 species, are presented and compared with a model that estimates the time and cost that would have been required if those species were pelleted using the traditional single-species method (SSP). Such a model was built using data from 32 pelleted seed batches belonging to 28 species of comparable size and traits to the ones tested with the MSP.
This preliminary analysis comparing the time and cost associated with both methods provides a general indication of the potential efficacy and impact of the MSP approach.
2 Materials and equipment
2.1 Equipment selection
Seed pelleting, defined as the process of adding material to a seed until its original shape is no longer evident (Pedrini et al., 2017), is generally performed by alternatively applying a liquid binder and a fine powder (filler) until the seed is covered, the predetermined amount of pelleting material is fully used, or the desired pellet size is reached.
The most commonly used equipment for pellet seeds are rotating pans (Scott et al., 1997) and rotary coaters (Halmer, 2008).
As the name suggests, a rotating pan consists of a circular pan with raised sides to retain seeds and material inside the pan, similar to a cement mixer. The rotating motion allows the seed mass to be constantly mixed while sitting on the lower part of the pan, ensuring even distribution of pelleting material among all the seeds. The friction exercised by the pellets tumbling on each other results in the compaction and shaping of pellets into approximately spherical shapes (Bennett and Lloyd, 2015; Gregg and Billups, 2010).
A rotary coater (or rotor-stator) consists of a vertical metal drum (stator) with a rotating concave disk (rotor) at its bottom. Seeds are placed in the drum, and the bottom disk’s rotation causes the seeds’ mass to rapidly move along the lower side of the wall. Material is then added to the mass of moving seeds. Deflectors mounted on the inward-looking side of the drum ensure proper mixing of seed and material. A constant flow of air is usually forced in the gap between the edge of the disk and the wall of the drum to avoid material and seeds falling through the gap.
The rotary coater is often preferred for native seeds (Pedrini et al., 2020) as it allows for faster operations and is generally easier to operate. However, it can present problems with very small-seeded species, as they can either get stuck in the small gap between the drum or be blown out of the pan by the airflow. A rotating pan was preferred in this study because we focused on pelleting small-seeded species.
2.2 Setup and material
The machine used was a PC-S rotating 340 mm segmented pan (Hoopman, Aalten, NL), connected to a peristaltic pump and compressed air line for precision delivery of liquid binder via spray gun mounted on an adjustable arm (Figure 1). Pan rotation speed and liquid feed rate from the peristaltic pump can be adjusted from the control panel. Spray intensity and liquid delivery rate can be fine-tuned directly on the spray gun by rotating the front nozzle and nob on the back of the gun, respectively.

Figure 1. Seed pelleting station setup: (A) rotating pan, (B) control panel, (C) peristaltic pump, (D) liquid binders, (E) spray gun mounted on an adjustable arm, (F) fine powders, paintbrush and strainer, (G) set of sieves, (H) graduate volume cylinder (I), precision balance and, (J) larger replacement rotating pan. A list of items and costs is provided in Supplementary Material S1.
The liquid binder used across all batches was an 8% solution of Polyvinyl alcohol (Glow Paint Industries, Urangan, QLD, Australia) without colour for the internal layer and with a dye (SATEC, Elmshorn, Germany) for the external layer. The powder used for the internal layer was micronised Azomite® (Dr Greenthumbs, Bellambi, NSW, Australia) and talcum powder was used for the external finishing layer. Azomite was chosen for ease of pelleting (regular pellet shape), relatively high density, and limited impact on germination (Pedrini et al., 2023), while finishing the talc layer was selected to improve pellets’ mechanical resistance, reduce azomite dust-off and allow even and easier colouring of the final layer. The powder was manually dusted on the seed with a paintbrush or strainers. A set of sieves of mesh size ranging from 0.25 mm to 2.36 mm was kept next to the pelleting station for sorting pellets during and after pelleting operations. A dehydrator (Sunbeam, Boca Raton, FL, United States) is also needed to dry the seed after the pelleting process.
Such materials were selected based on previous experiences (Pedrini, 2019) and published protocols (Pedrini et al., 2023) but are just one of many potential material combinations. Material selection should be informed by availability, cost, the desired pellet properties (e.g., density, mechanical resistance, water permeability), and the potential effect of the material on seed germination and seedling establishment.
3 Methods
3.1 Multispecies pelleting method
This section provides a detailed explanation of the preparatory, pelleting, and post-pelleting steps. Ideally, such instruction would guide researchers and practitioners in testing the MSP method, acknowledging that customisation and improvement might be needed to address specific needs and equipment availability.
3.1.1 Preliminary seed processing and quality testing
Before pelleting, the seed batch of each species used in the mix is processed to remove appendages and inert material as far as practical (Frischie et al., 2020). Once seeds are cleaned to a satisfactory level, they are tested for purity and viability following the methodology described by Pedrini et al. (2022), thus determining the thousand pure seed unit weight (TPSU) and the quality of a seed batch, expressed as Pure Live Seed % (PLS). Such information is essential in determining how much of each seed batch should be used in the mix.
3.1.2 Seed mix composition
Species composition and seed quantity in a restoration mix should be based on the community of the reference ecosystem and the size of the site to be restored (Erickson and Halford, 2020).
Once the target for species composition and relative abundance is set, the number of seeds for each species can be determined by estimating the number of PLS to use. The PLS number can then be converted to a weight value using the results of the seed quality test, and each species comprising the mix can be weighted. Species whose seed size (diameter) is large enough (e.g., >1.6 mm) can be set aside as they do not require pelleting and will be mixed with the pelleted species before seeding. If the density of larger seeds differs from that of pelleted seeds, those can be coated lightly (encrusted) to increase homogeneity and avoid seed separation during seeding.
All species with smaller seed sizes are mixed, and the total weight and volume are recorded.
3.1.3 Seed sorting by size
The seeds in the mix are sorted by size by running them through a series of sieves or perforated screens stacked in decreasing mesh size order. This process allows the entire mix to be subset into various size classes according to the sieve size range. For example, all seeds can be grouped as size XS (0.25 mm–0.425 mm), size S (0.425 mm–0.6 mm), size M (0.6 mm–0.8 mm), size L (0.8 mm–1.0 mm) size XL (1.0 mm–1.2 mm). Each size class is kept in separate containers for later use during the pelleting process (Figure 2).
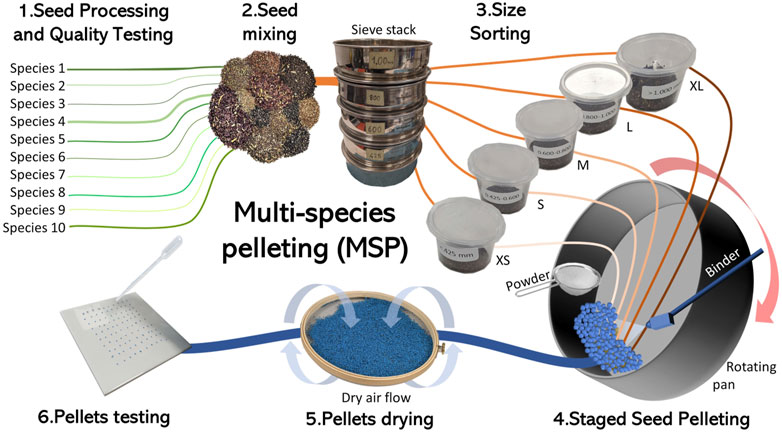
Figure 2. Step-by-step process of the Multiple Species Pelleting (MSP) approach: 1) test the quality of each seed lot to be used in the mix and, if necessary, process the low-quality lots to improve purity and viability; 2) mix all the lots in the desired relative quantities; 3) sort the seed mix according to size using a series of stack sieves and place the different seed size fractions in separate containers; 4) place the smaller seeds (XS) in the rotating pan and, by alternating the delivery of binder and filler, increase the seed size until they reach the size of the second fraction (S); then add the S fraction until it reaches the size of the M fraction. Repeat this process until all seeds are pelleted and the desired final size range is reached. During the process, pellets are routinely removed from the pan and sieved. If they have reached the desired size, they’re set aside; if not, they are placed back in the pan and the pelleting process resumed; 5) remove the pellets from the rotating pan once they’ve all reached the final size and dry them; 6) test the quality of pelleted seeds. The weight of the lines represents the number of seeds or pellets, and the line colours represent different species (green), seed mixes (oranges and brown) and pelleted seeds (blue).
3.1.4 Seed pelleting process
The seeds of the smallest size fraction (e.g., 0.25 mm–0.425 mm) are placed in the pan, and then rotation is activated. It is useful to set the pan rotation speed to low (25–30 rpm) in the early phases as it allows better control of material delivery and visual assessment of seeds. Rotation speed can be increased (30–40 rpm) as the size of the pellets increases.
The first step in the process is to gradually (slow intensity, low rate) add liquid binder to the seed mass, carefully avoiding seeds sticking to the pan or forming clumps of multiple seeds (agglomerates) due to the liquid being added too quickly. Both issues can be solved by gently pressing on the seed mass and the drum with a dry brush.
After wetting, a small amount of powder is added using a paintbrush or a fine-meshed tea strainer. If excessive powder is delivered, the powder that fails to adhere to the seed surface can clump and form pellets that contain no seeds (“dead balls”).
The early phase of pelleting build-up on the smallest seeds is the most delicate, requiring a careful balance between wetting and dusting. Constant visual assessment is required to avoid agglomerates and dead balls. By rapidly alternating small wetting and drying cycles, seeds will first become encrusted (when the shape of the seed is still apparent) and then turn into almost spherical pellets. It is good practice to remove all of the pellets periodically from the pan, run them through sieves and set aside the ones that have reached the next size class. The rest can be returned in the pan for further pelleting.
Once the majority of pellets remain in the larger mesh size sieve (e.g., 0.425 mm), it means that they have reached the desired size. The seed of the next size class (e.g., 0.425–0.6 mm) can then be added to the pelleted seeds, the mix returned to the pan, and the process repeated until the next size range is reached (Figure 2).
These steps are repeated until the seeds of all size ranges have been added. It is worth noting that as the number of seeds increases along with the size of each pellet, the process can be accelerated by raising the liquid delivery rate and providing powder with larger strainers. If available, a mechanical powder feeder can be used at this stage, and the pan’s rotation speed can also be increased (45 rpm).
Once most pellets have reached the desired diameter (e.g., 1.6–2.00 mm), a final thin layer is built using talc instead of Azomite. At this stage, the previously transparent liquid binder can be replaced with a coloured one. This final layer can help smooth the external pellet surface, improve the pellets’ mechanical integrity and reduce the loss of dry powder due to friction during transport and deployment (dust-off).
Once the pelleting process is terminated, all pellets are placed on fine mesh trays and dried at 35°C for 3 hours in a dehumidifier (Sunbeam, Boca Raton, FL, United States). After drying, pelleted seeds are weighed, and volume is recorded.
3.1.5 Pellet quality test
The total time required for the pelleting operation (excluding drying) and the quantity of material used can be recorded. Compression and crush tests (Pedrini et al., 2018) can then be performed. The compression test is performed by sampling the mechanical integrity of ten randomly selected pellets with a Digital Force Gage (Polygon Instrument Ltd., Shenzhen, China). The crush test is performed by wetting and crushing 50 randomly selected pellets to quantify the number of units that achieved singulation (one seed per pellet), agglomeration or were empty (Figure 3).
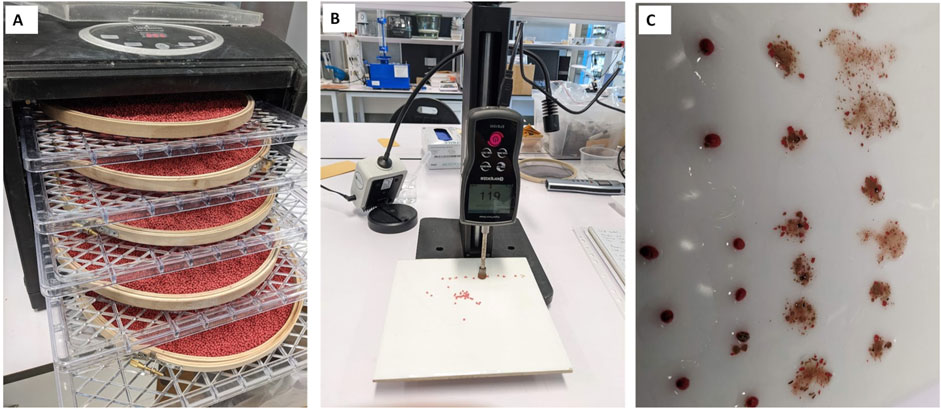
Figure 3. Post-pelleting drying and quality testing. (A) Pellet drying at 35°C in a dehumidifier. (B) Compression test performed with a force gauge on ten randomly selected pellets to record the force required to compromise the mechanical integrity of each pellet. (C) Crush test on 50 randomly selected pellets to asses how many seeds are present in each pellet: one (singulation), none (dead balls), or multiple (agglomeration). Wetting the pellet before the crush test facilitates seed extraction.
3.2 Method testing and validation
3.2.1 Seed selection, processing, quality testing and pelleting
To compare the efficacy of single-species (SSP) and multiple-species pelleting methods (MSP), 64 seed batches belonging to 56 small-seeded species native to Western Australia were used. Of the chosen species, 44 belonged to the Myrtaceae family, 5 to Chenopodiaceae, 3 to Fabaceae, 2 to Hemerocallidaceae, 1 to Asparagaceae and 1 to Proteaceae. 32 batches of 28 species were pelleted using the traditional SSP approach. Two seed mixes of 13 and 19 species were used to test the MSP approach (see Supplementary Tables S2, S3). The selection of the species used for both the SSP and MSP was based on a few considerations: 1) Seed availability: pelleting was possible for batches for which a sufficient quantity of seed was available after the seeds were cleaned. 2) Seed size: species whose seeds were already large enough (e.g., Acacia or Banksia) and would not benefit from the increase in size and volume were excluded, and just small-seeded species (seed weight <5 mg) were pelleted. 3) Need for revegetation: the list of species of interest and seeds were provided by organisations, such as mining companies, public agencies (Main Road) and Aboriginal corporations (ETNTAC) that would have used the seeds for various restoration programs.
The seeds were supplied by industry partners (mining companies and Aboriginal corporations) or purchased from local commercial seed suppliers.
Before pelleting, the seed batches that required cleaning were processed to extract seeds from the pericarp (Chenopodiacea) and remove inert material (Myrtaceae) as far as practical.
Once seeds were cleaned to a satisfactory level, they were tested for purity and viability, thus determining the thousand pure seed unit weight (TPSU) and the quality of a seed batch, expressed as Pure Live Seed % (PLS).
Seed pelleting was performed on single species (SSP) from June 2020 to December 2022, following the methodology described by Pedrini et al. (2023). Multiple species pelleting (MSP) using the methods described earlier was performed in May 2022 on a 13-species mix to be used in a restoration project in the Avon Wheatbelt (AVW) bioregion region in Western Australia and in June 2023 on 19 Myrtaceae species from the Esperance plain (ESP) bioregion region in Western Australia.
3.2.2 Pelleting time and cost estimate
Because the MSP and SSP were not performed as part of a single experiment, and different species were pelleted over several years, I could not use statistical inference to directly test a hypothesis (e.g., that MSP is cheaper and faster than SSP). However, because the data collected for each pelleted batch were standardised (e.g., type and quantity of material used, seed quantity, pelleting time), I used this information to build a linear model to explore potential cost and time outcomes across treatments. This approach can be described as a predictive modelling exercise using standardised observational data. While it does not allow for formal statistical inference due to the absence of experimental control, it provides a practical framework for estimating trends and potential differences in cost and time between MSP and SSP treatments.
Data on the material used and process duration were collected for SSP and MSP. The cost of fine powders was estimated at 15 $/kg, the cost of the liquid binder at 12 $/kg and the hourly rate of the pelleting technician at 50 $/hour. As the price of seeds was not available for all species, the average cost of a Thousand Pure Live Seeds at the family level, as quantified by Pedrini et al. (2022), was used.
To allow for a comparison of the two methods, I estimated the time that would have been required for each species in the MSP if they were pelleted as SSP. This was achieved by developing a linear model using the data from the 32 SSP batches in MS Excel. A series of pelleting parameters were considered and plotted against pelleting duration (time) to identify the one that would provide the best fit. The parameters considered were: 1) increase in batch weight (xWeight), 2) batch volume (xVolume), and 3) unit weight (xTPSU) before and after pelleting. The equation of the regression line of the parameter with the best fit was then used to estimate the pelleting duration time.
The recorded pelleting time does not take into account the time required for preliminary and post-pelleting operations, such as equipment and material seed set-up, pellets handling for drying, quality tests, and equipment clean-up. To account for such activities, 30 min for SSP and 45 min for MSP (for mixing and separating seeds in size classes) were added to the recorded and estimated pelleting duration time.
4 Results
4.1 Single species pelleting (SSP)
The average seed weight of a batch before pelleting was 3.82 ± 0.28 g and was increased to 77.45 ± 18.09 g after pelleting for an average increase of 18.58 ± 3.49 fold (xWeight). The average volume per batch was 6.31 ± 0.49 mL before pelleting and was increased by 16.38 ± 3.09 fold (xVolume) to 94.51 ± 19.89 mL. The average weight of a thousand pure seed units was increased by 18.16 ± 3.18 times (xTPSU) on average, from 0.76 ± 0.14 g to 6.27 ± 0.43 g. The average duration of the pelleting process, without accounting for 30 min of pre and post-pelleting operations, was 87.66 ± 9.06 min, ranging from a minimum of 23 min for Gastrolobium capitatum, to a maximum of 210 min for Calothamnus quadrifidus (see Supplementary Table S2).
When pelleting parameters were plotted on a linear model against the pelleting duration (min), the best fit was identified for Thousand Pure Seed Unit weight increase (xTPSU, R2: 0.702), followed by weight increase (xWeight, R2: 0.641) and volume increase (xVolume, R2: 0.583) (Figure 4).
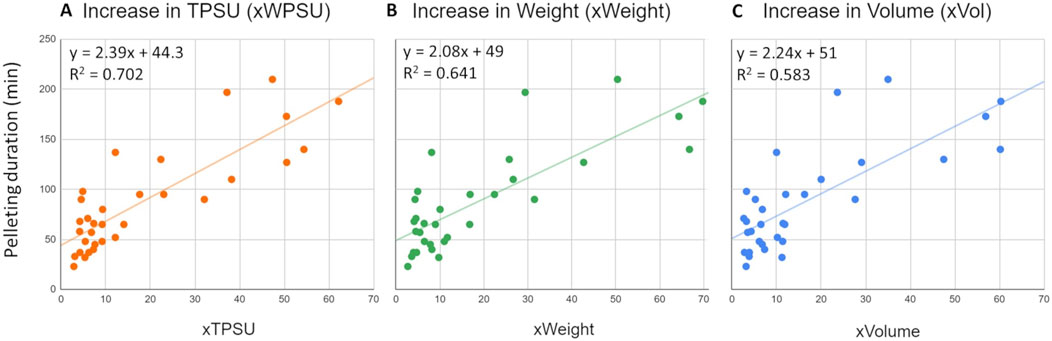
Figure 4. Linear models developed using the data from 32 SSP batches. The models relate the pelleting duration (Y-axis) to three variables: (A) the increase in Thousand Pure Seed Unit weight (xTPSU), which is the fold increase of TPSU of the seeds before pelleting and the pellets, (B) the increase in batch weight before and after pelleting and (C) the increase in volume. On the top corner of each scatter plot, equations of the trend line and R2 of the model are reported.
4.2 Multiple species pelleting (MSP)
The MSP from the Avon Wheatbelt bioregion (AVW) batch consisted of 13 species for a total seed weight of 57.08 g at an average TPSU of 1.186 ± 0.46 g and an estimated 107,750 Pure Live Seeds. Seeds were pelleted for 362 min, using 790 g of liquid binder and 1,810 g of powder until most pellets reached the targe size range of 2 mm–2.36 mm, for a total weight of 1,118 g and a TPSU of 9.376 g. When quality tested, 4% of the pellets were empty (dead balls), 76% contained one seed, and 20% contained two or more seeds (Figure 5). An estimated total of 115,616 pellets were produced, 110,994 of which were filled.
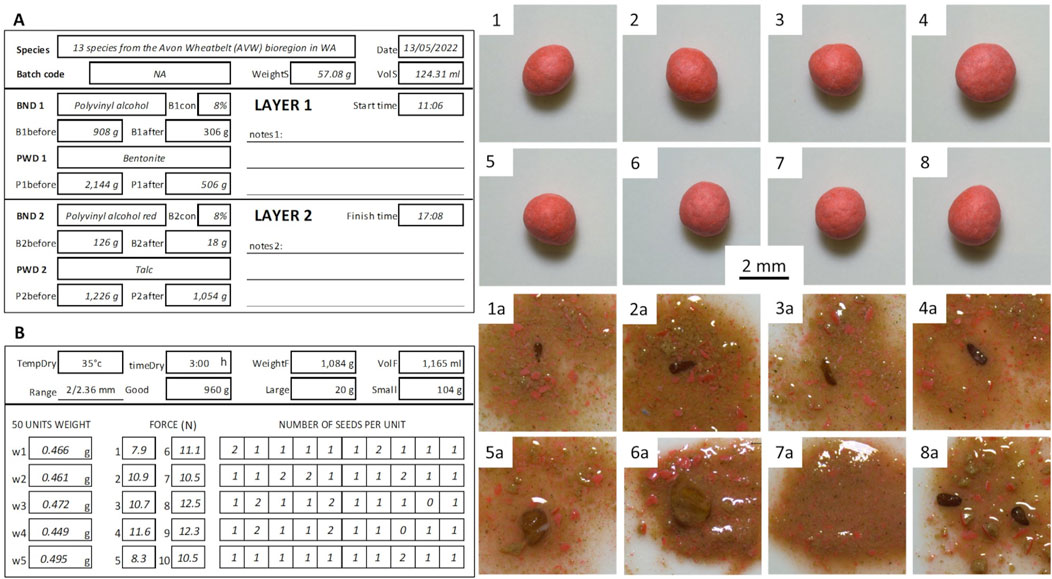
Figure 5. Forms and picture of MSP mix from the Avon Wheatbelt (AVW). (A) The front side of the pelleting form filled with information about initial seed weight and volume, start and finish time, and quantity and type of material used for the first and second layers. (B) Back of the pelleting form with information about drying temperature and duration, weight and volume of dry pellets, expected size range (e.g., 2/2.36 mm), and the fraction of pellets that fall within the interval (Good), that are bigger (Large) or smaller (Small). Data on the weight of 50 pellets used to calculate the Thousand Pure Seed Unit Weight (TPSU), results of 10 compression tests that measure the force (N) needed to crack a pellet and provide an indication of the pellet’s mechanical resistance, and the result of crush test performed on 50 randomly selected pellets, recording the number of seeds present in each pellet. (Pedrini et al., 2018). (1–8) Images of individual pellets before the crush test, and (1a-8a) images of the same pellets after the crush test. It is worth noticing that, although the pellets are similar in shape and size, the seeds they contain are of variable relative size: small (1a-4a, e.g., Kunzea glabrescens), medium (5a, e.g., Eremaea pauciflora), and large (6a, e.g., Eucalyptus drummondii). The pellets can also contain no seeds (7a dead ball), or multiple seeds (8a agglomerate).
The MSP batch from the Esperance Plains bioregion (ESP) of 68.67 g comprised 19 Myrtaceae species at an average TPSU of 0.267 ± 0.05 g and an estimated total of 200,000 PLS. The pellet size range was set between 1.6 mm and 2 mm and was reached in 318 min using 842 g of liquid binder and 1,868 g of powder. The final weight of the pelleted batch was 1,117 g at a TPSU of 5.302 g. Tests on the pellets revealed 8% of empty pellets, 78% of single-seed pellets, and 14% of agglomerates. The total number of pellets produced was 210,659, of which 193,807 were filled.
4.3 Time and cost comparison
Assuming that the material and seeds used would have been comparable in both SSP and MSP, the difference in cost between the two approaches is due to the differences in total time (preparatory, pelleting and post-pelleting operations) and associated labour costs. For the 13 species of the AVW mix, MSP lasted 403 min, and for the 19 species ESP mix, it was 363 min.
To estimate the time that would have taken to pellet each seed lot individually with the SSP method, the equation for the linear regression of xTPSU against pelleting duration (Time) was used.
The AVW mix time was estimated at an average of 177 ± 30 min per species for a combined total of 2,298 min. The average time for the ESP mix was 195 ± 21, and the sum was 3,700 min (Table 1).
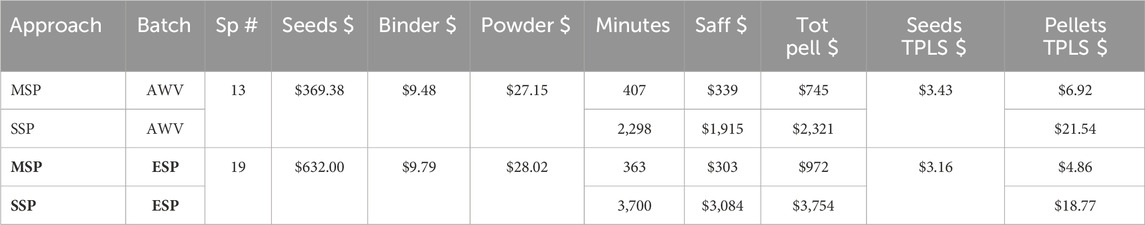
Table 1. Cost comparison of Multiple Species Pelleting approach (MSP) and Single Species Pelleting (SSP) in two mix trials from the Avon Wheatbelt bioregion (AVW) and the Esperance Plains bioregion (ESP) in Western Australia. For each site, the cost of seeds and material (binder and power) is constant, but the time required and associated staff costs are higher for SSP than MSP, resulting in a higher final cost of SSP. The cost of a Thousand Pure Live Seed (TPLS $) without pelleting is provided as a comparison of the cost of TPLS after pelleting.
In the AVW mix, the MSP approach allowed for a 5.6-time reduction in time compared to SSP, and resulted in a 3.1-fold reduction in the total cost of the pelleted seed batch. This difference was more accentuated in the ESP mix with a 10.2-fold reduction in pelleting time and consequent 3.9-fold reduction in total cost.
When considering the cost on a seed number basis, using the cost of a thousand pure live seeds (TPLS $) in the AVW mix, the cost for pelleted seeds compared to untreated seeds was doubled for MSP but 6.2 times higher for SSP. For the ESP mix, MPS had a 1.5 increase in TPLS $ compared to a 5.9 increase in cost for SSP (Table 1).
5 Discussion
The goal of pelleting in both MSP and SSP was to achieve singulation, meaning that, ideally, each pellet contains one seed. This was not entirely possible in the two MSP mixes. To some degree, empty units and agglomerates will occur, especially in the early phase of the process, on very small seeds. However, as long as the relative quantity of dead balls and agglomerates is known and limited, it might not be a problem, as seeding rates can be adjusted accordingly.
In some instances, agglomeration might also be preferred. For example, Madsen et al. (2012) proved that combining multiple seeds of the same species in a single pellet would improve the collective push power of germinating seeds, thereby improving the chance of emerging through the soil crust in the North American grass Pseudoroegneria spicata. However, agglomeration of seeds from different species in a single pellet can be problematic as the emerging seedlings would compete for the same resources in the same niche, potentially limiting their chance of successful establishment (Callaway and Walker, 1997).
Another approach that could be incorporated into the process is the inclusion of seeds and inert materials in the pellets (Hoose et al., 2019). Such a method, known as conglomeration, might be necessary when the separation of seeds and debris is not practically or economically feasible, as is sometimes the case for some batches of small-seeded species belonging to the Myrtaceae (e.g., Eucalyptus ssp.), Ericaceae (e.g., Calluna vulgaris) and Asteraceae (e.g., Artemisia tridentata) families (Hoose et al., 2019; Pedrini et al., 2023).
Although all these approaches may present limitations, seed pelleting by singulation, agglomeration, or conglomeration is often the most effective way to deliver small-seeded species that would otherwise be sown unevenly or excluded from the mix.
5.1 Cost-effectiveness
While experimental seed enhancement technology might be promising, it needs to be scalable and cost-effective for its adoption by seed suppliers and restoration practitioners (Brown et al., 2021).
The Multi-Species Pelleting method (MSP) presented in this study demonstrates potential time and cost savings compared to the traditional Single-Species Pelleting method (SSP).
Such savings become more accentuated as the number of species to be pelleted increases, as seen in the AVW mix of 13 species and the ESP mix of 19 species.
However, MSP is still responsible for a considerable cost increase compared to untreated seeds.
To address this issue, two approaches for further development and experimentation are suggested: 1) Find approaches to improve the MSP method efficiency and scalability, and 2) evaluate the benefit of pelleted seed-based restoration compared to traditional methods to ensure that the increase in seed cost is offset by better seeding efficiency and plant establishment.
5.2 Further improvement to improve MSP efficiency and scalability
The relatively long time (between 5 and 6 h per mix) required to pellet the two MSP batches was largely due to the fact that these were the first-ever mixes tested with this approach, and extra care was taken to avoid issues during the process. However, as the seed pelleting operator gains experience with this approach, the duration could be reduced to times similar to those of SSP, further enhancing the cost efficiency of this approach. This trend could already be observed, with the second attempt on the ESP mix in 2023 being 44 min faster than the first batch in 2022. Such time saving was achieved even though the number of species and quantity of seeds were higher on the second attempt. This element is particularly relevant for further scalability of the process, as the time required for pelleting is not appreciably affected by the number of species in the mix or batch size.
A method to improve speed and capacity is to couple the pan coater with a rotary coater and run a pelleting session across two machines. The rotating pan is required to pellet seeds in the first phases (<1 mm), as it allows more control of the variables and reduces the risk of accidentally losing small seeds; however, when pelleted seeds reach a relatively large size, (>1 mm), further size increase to reach the final dimension can be slow. Moving the partially pelleted seed mix to a rotary coater would allow for a much faster second accretion phase, reducing the time required to complete an MSP batch. Moreover, if the rotary coater is equipped with an automatic and programmable powder and liquid delivery system, this could be set up to autonomously complete the process, allowing the operator to commence a new batch on the rotating pan.
A limiting factor is, however, the total size of the seed mix that can be processed in one session. The pelleting pan used in this study is equipped with a 350 mm pan that allowed for the pelleting of a total estimated 200,000 PLS for the ESP mix, which was close to capacity towards the end of the process. This limit can be raised by installing a larger pan, such as a 600 mm one (Figure 1), that could increase the number of seeds that can be pelleted in one session to more than a million.
5.3 Evaluation of improved efficiency in seed-based restoration
Assuming that with the improved approach, we can obtain a 1 million pelleted PLS mix in one session, that the average seed-to-established plant conversion is 10% (James et al., 2011), and that the target plant density is 10,000 per Ha, a single batch would be sufficient to cover a 100-ha restoration site. By employing large-scale agricultural seeding equipment (e.g., canola seeder), such an area over a flat terrain could be seeded in a matter of days, rather than the weeks required with current restoration seeding methods (Pedrini et al., 2023). This would further reduce seed-based restoration costs and allow for seeding operations to occur when soil and environmental conditions are optimal for seedling emergence and establishment, maximising the chances of seed-based restoration success, especially in arid and semi-arid environments (Shackelford et al., 2021).
Clearly, such assumptions need to be field-tested, and a more comprehensive cost-efficiency analysis based on the cost per successfully established plant, other than just the cost of seeds, needs to be performed. Such studies, in different restoration scenarios and species mixes, would then allow for better-informed decisions on whether MSP is a viable option and to what extent it can improve seed-based restoration efficacy.
This study has focused exclusively on the logistical improvement afforded by seed pelleting. However, seed germination and successful seedling establishment are ultimately the key outcomes of seed technology. In this study, I did not evaluate seed performance as it was previously assessed for the SSP species and method by Pedrini et al. (2023), and, on average, pelleting showed no detrimental effect on seedling emergence. To further increase the chances of germination and successful plant establishment, active compounds, such as predator deterrents (Pearson et al., 2019; Taylor et al., 2020), protectants from diseases and herbicides (Brown et al., 2021; Hoose et al., 2022), and survival, germination, and emergence promoters (Erickson et al., 2017; Larson J. E. et al., 2023; Pedrini et al., 2021) can be incorporated with the pelleting material (Madsen et al., 2016).
New creative approaches have recently been developed to introduce seeds of native species within agroecosystems using existing agricultural seeding equipment. For example, Westbrook et al. (2023) developed an innovative multi-seed moulding method to agglomerate seeds of Common milkweed (Asclepias syriaca L.) in the shape of corn seeds (Zea mais), that can be seeded by farmers alongside the crop and support the conservation of monarch butterfly, by introducing in farmland its main food source. A similar approach was developed by Corli et al. (2024) by encrusting rice seeds with the micro-seeds of endangered Linderia procumbens, allowing farmers to reintroduce a conservation priority annual species within their crop, with no impact on crop yield.
Even though this analysis focused predominantly on cost reduction, there is a key element that is difficult to quantify but must be considered. Native seeds, when collected from a natural population, can impact the population’s reproductive capability, especially for annual and short-lived perennial species (Bucharova et al., 2023), or damage the surrounding vegetation by trampling or accidental introduction of diseases. Until native seed production in farm settings becomes a viable alternative to wild seed collection, the potential collateral ecological damages of collection should be acknowledged. Therefore, limiting seed wastage by maximising the use of such valuable resources becomes a moral imperative, even when the monetary cost for doing so might be higher.
6 Conclusion
Ultimately, the MSP method described here presents a cost-effective approach that would allow seed pelleting technology to leave the lab benches and start to be evaluated and deployed at scale by native seed suppliers and restoration practitioners. Moreover, its combination with broad-acre agricultural seeding equipment could potentially allow for the drastic improvement of efficiency and scale of seed-based ecological restoration.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
SP: Conceptualization, Data curation, Formal Analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study would not have been possible without the contribution of the Australian Research Council (ARC) Center for Mine Site Restoration (ICI150100041), which provided resources and equipment to perform the single species pelleting (SSP). This manuscript was prepared with the support of ARC Centre for Healing Country (IC210100034), Ian Potter’s Foundation project on “Research and development to build best practice into native seed production in a changing world,” and the “”Native Seed Technology and Innovation Hub,” funded by Lotterywest.
Acknowledgments
I would like to acknowledge the Whadjuk people of the Noongar nation, the traditional owner of the land where the experiments took place and where the seeds of some SSP species were collected, the people of the Yamatji Nation, where the remaining species of the SSP were collected, the Ballardong Noongar for the seeds of the Avon Wheatbelt bioregion and the Kepa Kurl Wudjari Noongar for the collection of seeds in the Esperance Plains bioregion. A special thanks goes to the director of the ARC Centre for mine Site Restoration Kingsley Dixon, for the support, and the centre’s partner Hanson and Karara who provided the seeds for single species pelleting (SSP). My gratitude goes to Tina Arya, Hayley D’Agui, Gina Lintern, Michael Sullivan, Michael Just, Shane Turner, Bradley Albert, Ashton Reid, and Zoe Webber for helping with seed processing and quality testing. The seeds for the Avon Wheatblet mix were provided by the Main Road Department of Western Australia, and special thanks are due to Martine Scheltema, Sheree Walter and Rob Howard. Crucial was the contribution of seeds for the Esperance plain mix by the Esperance Tjaltjraak Native Title Aboriginal Corporation (ETNACT), especially to Sean Hazelden and the rangers in the seed collection team.
Conflict of interest
The author declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1595530/full#supplementary-material
References
Afzal, I., Javed, T., Amirkhani, M., and Taylor, A. G. (2020). Modern seed technology: seed coating delivery systems for enhancing seed and crop performance. Agriculture 10, 526. doi:10.3390/agriculture10110526
Bai, Z., Dent, D., Wu, Y., and de Jong, R. (2013). “Land degradation and ecosystem services,” in Ecosystem services and carbon sequestration in the biosphere. Editors R. Lal, K. Lorenz, R. F. Hüttl, B. U. Schneider, and J. von Braun (Netherlands, Dordrecht: Springer), 357–381. doi:10.1007/978-94-007-6455-2_15
Barberis, D., Turner, S., and Pedrini, S. (2023). Overcoming germination constraints in seven grass species for seed-based restoration in the Australian monsoonal tropics. Restor. Ecol. n/a 32, e14064. doi:10.1111/rec.14064
Bennett, G. M., and Lloyd, J. (2015). “Seed inoculation, coating and precision pelleting: science,” in Technology and practical applications. Boca Raton FL: Taylor and Francis group.
Berto, B., Erickson, T. E., and Ritchie, A. L. (2020). Flash flaming improves flow properties of mediterranean grasses used for direct seeding. Plants 9, 1699. doi:10.3390/PLANTS9121699
Beveridge, F. C., Williams, A., and Adkins, S. W. (2020). Seed enhancement technologies to improve germination and emergence of Australian native poaceae. Seed Sci. Res. 30, 293–303. doi:10.1017/S0960258520000276
Brancalion, P. H. S., Meli, P., Tymus, J. R. C., Lenti, F. E. B., M. Benini, R., Silva, A. P. M., et al. (2019). What makes ecosystem restoration expensive? A systematic cost assessment of projects in Brazil. Biol. Conserv. 240, 108274. doi:10.1016/j.biocon.2019.108274
Brown, V. S., Erickson, T. E., Merritt, D. J., Madsen, M. D., Hobbs, R. J., and Ritchie, A. L. (2021). A global review of seed enhancement technology use to inform improved applications in restoration. Sci. Total Environ. 798, 149096. doi:10.1016/j.scitotenv.2021.149096
Bucharova, A., Bossdorf, O., Scheepens, J. F., and Salguero-Gómez, R. (2023). Sustainable seed harvesting in wild plant populations. doi:10.1101/2023.01.12.523821
Callaway, R. M., and Walker, L. R. (1997). Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology 78, 1958–1965. doi:10.1890/0012-9658(1997)078[1958:cafasa]2.0.co;2
Ceballos, G., Ehrlich, P. R., Barnosky, A. D., Garcia, A., Pringle, R. M., and Palmer, T. M. (2015). Accelerated modern human-induced species losses: entering the sixth mass extinction. Sci. Adv. 1, e1400253. doi:10.1126/sciadv.1400253
Chazdon, R. L., Lindenmayer, D., Guariguata, M. R., Crouzeilles, R., Rey Benayas, J. M., and Lazos Chavero, E. (2020). Fostering natural forest regeneration on former agricultural land through economic and policy interventions. Environ. Res. Lett. 15, 043002. doi:10.1088/1748-9326/ab79e6
Corli, A., Mondoni, A., Porro, F., Rossi, G., Vaglia, V., Orengo, M., et al. (2024). Rice encrusting with small-seeded native species for reintroduction in agroecosystems: a case study in Lindernia procumbens. Restor. Ecol. n/a 32, e14257. doi:10.1111/rec.14257
Dean, G., Rivera-Ferre, M. G., Rosas-Casals, M., and Lopez-i-Gelats, F. (2021). Nature’s contribution to people as a framework for examining socioecological systems: the case of pastoral systems. Ecosyst. Serv. 49, 101265. doi:10.1016/j.ecoser.2021.101265
Di Sacco, A., Hardwick, K. A., Blakesley, D., Brancalion, P. H. S., Breman, E., Cecilio Rebola, L., et al. (2021). Ten golden rules for reforestation to optimize carbon sequestration, biodiversity recovery and livelihood benefits. Glob. Change Biol. 27, 1328–1348. doi:10.1111/gcb.15498
Erickson, T. E., Muñoz-Rojas, M., Kildisheva, O. A., Stokes, B. A., White, S. A., Heyes, J. L., et al. (2017). Benefits of adopting seed-based technologies for rehabilitation in the mining sector: a pilbara perspective. Aust. J. Bot. 65, 646. doi:10.1071/BT17154
Erickson, V. J., and Halford, A. (2020). Seed planning, sourcing, and procurement. Restor. Ecol. Rec. 28, 13199. doi:10.1111/rec.13199
Frischie, S., Miller, A. L., Pedrini, S., and Kildisheva, O. A. (2020). Ensuring seed quality in ecological restoration: native seed cleaning and testing. Restor. Ecol. 28 (rec), 13217. doi:10.1111/rec.13217
Gann, G. D., McDonald, T., Walder, B., Aronson, J., Nelson, C. R., Jonson, J., et al. (2019). International principles and standards for the practice of ecological restoration. Second edition. Restor. Ecol. 27. doi:10.1111/rec.13035Second edition
Gibson-Roy, P., Hancock, N., Broadhurst, L., and Driver, M. (2021). Australian native seed sector practice and behavior could limit ecological restoration success: further insights from the Australian native seed report. Restor. Ecol. 29, 1–12. doi:10.1111/rec.13429
Gornish, E., Arnold, H., and Fehmi, J. (2019). Review of seed pelletizing strategies for arid land restoration. Restor. Ecol. 27, 1206–1211. doi:10.1111/rec.13045
Gregg, B. B. R., and Billups, G. (2010). Seed coating and pelletizing, NH. Enfield: Science Publishers. doi:10.1201/b10312
Guzzomi, A. L., Erickson, T. E., Ling, K. Y., Dixon, K. W., and Merritt, D. J. (2016). Flash flaming effectively removes appendages and improves the seed coating potential of grass florets. Restor. Ecol. 24, S98–S105. doi:10.1111/rec.12386
Halmer, P. (2008). Seed technology and seed enhancement. Acta Hortic. 771, 17–26. doi:10.17660/actahortic.2008.771.1
Hasan, S. S., Zhen, L., Miah, Md.G., Ahamed, T., and Samie, A. (2020). Impact of land use change on ecosystem services: a review. Environ. Dev., Resour. Use, Ecosyst. Restor. Green Dev. 34, 100527. doi:10.1016/j.envdev.2020.100527
Holl, K. D., and Aide, T. M. (2011). When and where to actively restore ecosystems? For. Ecol. Manag., Ecol. Ecosyst. Serv. Native Trees Implic. Refor. Land Restor. Mesoam. 261, 1558–1563. doi:10.1016/j.foreco.2010.07.004
Hoose, B. W., Call, R. S., Bates, T. H., Anderson, R. M., Roundy, B. A., and Madsen, M. D. (2019). Seed conglomeration: a disruptive innovation to address restoration challenges associated with small-seeded species. Restor. Ecol. 27, 959–965. doi:10.1111/rec.12947
Hoose, B. W., Geary, B. D., Richardson, W. C., Petersen, S. L., and Madsen, M. D. (2022). Improving dryland seedling recruitment using fungicide seed coatings. Ecological Solutions and Evidence 3, 1–12. doi:10.1002/2688-8319.12132
James, J. J., Svejcar, T. J., and Rinella, M. J. (2011). Demographic processes limiting seedling recruitment in arid grassland restoration. J. Appl. Ecol. 48, 961–969. doi:10.1111/j.1365-2664.2011.02009.x
Larson, A. J. S., Cartwright, M. M., Jones, W. D., Luce, K., Chen, M.-Y., Petersen, K., et al. (2023a). Slow release of GA3 hormone from polymer coating overcomes seed dormancy and improves germination. Plants 12, 4139. doi:10.3390/plants12244139
Larson, J. E., Agneray, A. C., Boyd, C. S., Bradford, J. B., Kildisheva, O. A., Suding, K. N., et al. (2023b). A recruitment niche framework for improving seed-based restoration. Restor. Ecol. n/a 31, e13959. doi:10.1111/rec.13959
Liu, C., Tong, L., Yin, S., Zhang, P., and Wang, L. (2013). Size separation of binary mixture under vibration. AIP Conf. Proc. 1542, 714–717. doi:10.1063/1.4812031
López-Bedoya, P. A., Bohada-Murillo, M., Ángel-Vallejo, M. C., Audino, L. D., Davis, A. L. V., Gurr, G., et al. (2022). Primary forest loss and degradation reduces biodiversity and ecosystem functioning: a global meta-analysis using dung beetles as an Indicator taxon. J. Appl. Ecol. 59, 1572–1585. doi:10.1111/1365-2664.14167
Madsen, M. D., Davies, K. W., Boyd, C. S., Kerby, J. D., and Svejcar, T. J. (2016). Emerging seed enhancement technologies for overcoming barriers to restoration. Restor. Ecol. 24, S77–S84. doi:10.1111/rec.12332
Madsen, M. D., Davies, K. W., Mummey, D. L., and Svejcar, T. J. (2014). Improving restoration of exotic annual grass-invaded rangelands through activated carbon seed enhancement technologies. Rangel. Ecol. Manag. 67, 61–67. doi:10.2111/REM-D-13-00050.1
Madsen, M. D., Davies, K. W., Williams, C. J., and Svejcar, T. J. (2012). Agglomerating seeds to enhance native seedling emergence and growth. J. Appl. Ecol. 49, 431–438. doi:10.1111/j.1365-2664.2012.02118.x
Masarei, M., Guzzomi, A. L., Merritt, D. J., and Erickson, T. E. (2019). Factoring restoration practitioner perceptions into future design of mechanical direct seeders for native seeds. Restor. Ecol. 27, 1251–1262. doi:10.1111/rec.13001
Nellemann, C., and Corcoran, E. (2010). Dead planet, living planet. Biodiversity and ecosystem restoration for sustainable development, challenges. Norway: United Nations Environment Programme, GRID-Arendal.
Pearson, D. E., Valliant, M., Carlson, C., Thelen, G. C., Ortega, Y. K., Orrock, J. L., et al. (2019). Spicing up restoration: can chili peppers improve restoration seeding by reducing seed predation? Restor. Ecol. 27, 254–260. doi:10.1111/rec.12862
Pedrini, S., Balestrazzi, A., Madsen, M. D., Bhalsing, K., Hardegree, S. P., Dixon, K. W., et al. (2020). Seed enhancement: getting seeds restoration-ready. Restor. Ecol. 28 (rec), 13184. doi:10.1111/rec.13184
Pedrini, S., Bhalsing, K., Cross, A. T., and Dixon, K. W. (2018). Protocol development tool (PDT) for seed encrusting and pelleting. Seed Sci. Technol. 46, 393–405. doi:10.15258/sst.2018.46.2.21
Pedrini, S., D’Agui, H. M., Arya, T., Turner, S., and Dixon, K. W. (2022). Seed quality and the true price of native seed for mine site restoration. Restor. Ecol. 30. doi:10.1111/rec.13638
Pedrini, S., and Dixon, K. W. (2020). International principles and standards for native seeds in ecological restoration. Restor. Ecol. 28, 286–303. doi:10.1111/rec.13155
Pedrini, S., Merritt, D. J., Stevens, J., and Dixon, K. (2017). Seed coating: science or marketing spin? Trends Plant Sci. 22, 106–116. doi:10.1016/j.tplants.2016.11.002
Pedrini, S., Stevens, J. C., and Dixon, K. W. (2021). Seed encrusting with salicylic acid: a novel approach to improve establishment of grass species in ecological restoration. PLOS ONE 16, e0242035. doi:10.1371/journal.pone.0242035
Pedrini, S., Webber, Z., D’Agui, H., Dixon, K., Just, M., Arya, T., et al. (2023). Customise the seeds, not the seeder: pelleting of small-seeded species for ecological restoration. Ecol. Eng. 196, 107105. doi:10.1016/j.ecoleng.2023.107105
Rosato, A. D., Blackmore, D. L., Zhang, N., and Lan, Y. (2002). A perspective on vibration-induced size segregation of granular materials. Chem. Eng. Sci., Neptis 9 - “Solids flow Mech. their Appl. 57, 265–275. doi:10.1016/S0009-2509(01)00380-3
Scott, J. M., Blair, G. J., and Andrews, A. C. (1997). The mechanics of coating seeds in a small rotating drum. Seed Sci. Technol. 25, 281–292.
Shackelford, N., Paterno, G. B., Winkler, D. E., Erickson, T. E., Leger, E. A., Svejcar, L. N., et al. (2021). Drivers of seedling establishment success in dryland restoration efforts. Nat. Ecol. Evol. 5, 1283–1290. doi:10.1038/s41559-021-01510-3
Shaw, N., Barak, R. S., Campbell, R. E., Kirmer, A., Pedrini, S., Dixon, K., et al. (2020). Seed use in the field: delivering seeds for restoration success. Restor. Ecol. 28 (rec), 13210. doi:10.1111/rec.13210
Svejcar, L. N., Kerby, J. D., Svejcar, T. J., Mackey, B., Boyd, C. S., Baughman, O. W., et al. (2022). Plant recruitment in drylands varies by site, year and seeding technique. Restor. Ecol. 15, 4–6. doi:10.1111/rec.13750
Taylor, A. G., Allen, P. S., Bennett, M. a., Bradford, K. J., Burris, J. S., and Misra, M. K. (1998). Seed enhancements. Seed Sci. Res. 8, 245–256. doi:10.1017/S0960258500004141
Taylor, J. B., Cass, K. L., Armond, D. N., Madsen, M. D., Pearson, D. E., and St. Clair, S. B. (2020). Deterring rodent seed-predation using seed-coating technologies. Restoration Ecology 28, 927–936. doi:10.1111/rec.13158
Turner, S. R., Pearce, B., Rokich, D. P., Dunn, R. R., Merritt, D. J., Majer, J. D., et al. (2006). Influence of polymer seed coatings, soil raking, and time of sowing on seedling performance in post-mining restoration. Restor. Ecol. 14, 267–277. doi:10.1111/j.1526-100X.2006.00129.x
United Nations (2021). UN decade on restoration WWW document. UN decade restor. Available online at: http://www.decadeonrestoration.org/(Accessed 27 March, 24).
Westbrook, A. S., Amirkhani, M., Taylor, A. G., Loos, M. T., Losey, J. E., and DiTommaso, A. (2023). Multi-seed zea pellets (MSZP) for increasing agroecosystem biodiversity. Weed Sci. 71, 160–171. doi:10.1017/wsc.2023.5
Keywords: native seeds, seed enhancement, seed coating, ecological restoration, direct seeding, small-seeded species
Citation: Pedrini S (2025) A novel multi-species seed pelleting method to improve the efficiency of seed-based ecological restoration. Front. Environ. Sci. 13:1595530. doi: 10.3389/fenvs.2025.1595530
Received: 18 March 2025; Accepted: 17 June 2025;
Published: 30 June 2025.
Edited by:
Vinicius Londe, Independent Researcher, Bothell, United StatesReviewed by:
Diego Souza, Ministério Público do Estado de Minas Gerais, BrazilMatthew Madsen, Brigham Young University, United States
Copyright © 2025 Pedrini. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Simone Pedrini, c2ltb25lLnBlZHJpbmlAY3VydGluLmVkdS5hdQ==
 Simone Pedrini
Simone Pedrini