- 1School of Energy and Environment, Zhongyuan University of Technology, Zhengzhou, China
- 2Institute of Chemistry Co., Ltd. Henan Academy of Sciences, Zhengzhou, China
The disposal of sludge and the treatment of phosphorus in water bodies are significant environmental challenges. This study explores the adsorption performance and mechanism of lanthanum-calcium modified sludge/wheat straw biochar (LC-SWBC). LC-SWBC was prepared through a one-step hydrothermal carbonization process and was used to remove phosphorus from water. The results indicate that La(OH)3 and Ca(OH)2 were successfully loaded onto the surface of the biochar. The adsorption of phosphates by LC-SWBC follows a pseudo-second-order kinetic model and the Langmuir model, with a maximum theoretical adsorption capacity of 80.78 mg P/g. LC-SWBC exhibits selective adsorption of phosphate under competitive anion experiments. In actual wastewater treatment, LC-SWBC can effectively remove phosphates, achieving a total phosphorus concentration of 0.77 mg/L at a dosage of 0.4 g/L, meet the discharge standard of class I B pollutants (1 mg/L) in GB 18918-2002 of China. In addition, the hydrothermal liquid of LC-SWBC is primarily composed of organic phosphorus (OP); after adsorption, the main component in the biochar LC-SWBC-P is apatite phosphate (AP), both of which provide biochemical utilization conditions for phosphorus resource recovery and recycling.
1 Introduction
With the continuous advancement of urbanization and the sustained growth of urban population, the volume of urban wastewater treatment remains steadily increasing, leading to a year-on-year rise in municipal sludge production. Municipal sludge contains a significant amount of hazardous substances, including organic pollutants, heavy metals, and pathogenic microorganisms (Overcash et al., 2005). Improper disposal can easily lead to secondary pollution (Dong et al., 2013), posing a serious threat to the ecological environment and human health. At present, the main disposal methods for municipal sludge in China include incineration (Srivastava and Chakma, 2022), sanitary landfill (Li et al., 2023), building materials (Prabhakar et al., 2022), and anaerobic digestion. But these methods have problems such as high cost, narrow application scope, and secondary pollution (Liu et al., 2023). Research has found that municipal sludge contains a large amount of carbon compounds, making it an excellent raw material for preparing biochar. Sludge biochar production not only enables proper disposal of sludge, but also achieves resource reuse (Krahn et al., 2023). The biochar prepared from sludge has the characteristics of rich pores and large specific surface area, and has good adsorption and catalytic performance. It has been widely used in water pollution control (Leng et al., 2015), air pollution control (Xu et al., 2014), electrochemistry (Yuan and Dai, 2016), and soil remediation (Khan et al., 2013).
Phosphorus is one of the most significant pollutants in domestic wastewater. It is the primary element contributing to water eutrophication and a key control parameter for water pollution. Common wastewater phosphorus removal methods include chemical, biological, electrolytic, membrane, and adsorption methods. Compared with chemical and biological methods for phosphorus removal, adsorption method has the advantages of simple operation, high efficiency, fast reaction, recyclable resources, and environmental friendliness (Bacelo et al., 2020), and has broad application prospects in the field of wastewater phosphorus removal. Biochar has a stable structure, a large specific surface area and porosity, and a large number of surface functional groups, making it an excellent adsorbent (Vikrant et al., 2018). The use of biochar for adsorbing phosphorus in wastewater has become a current research hotspot.
Using sludge to produce biochar and selecting phosphorus as the target pollutant to be removed can not only solve the problem of sludge treatment, but also achieve the recycling and utilization of phosphorus resources in sewage, achieving the goal of “treating pollution with waste.” Research has shown that sludge has a certain adsorption capacity for phosphorus, but its adsorption efficiency is not high. To enhance biochar performance, researchers often employ two primary methods: improvement of the sludge biochar’s pore structure and metal modification. Adding agricultural or wood waste to the preparation of sludge biochar can increase its carbon content and porous structure, further improving the performance of biochar (Peng et al., 2022). Yin et al. (2019) prepared biochar by co pyrolysis of sludge and walnut shell biomass. The microstructure of biochar was optimized, enriching the metal oxides and functional groups of biochar. It still had good adsorption effect on phosphate in a wide pH range, with a maximum adsorption capacity of 303.49 mg/g. In addition to improving the pore structure of sludge biochar, modification can also enhance the performance of biochar, mainly using metal compounds as modifiers. Lanthanum is a rare earth metal with exhibits strong affinity for phosphorus, demonstrating high adsorption capacity and efficiency. Elkhlifi et al. (2022) prepared La(OH)3 loaded biochar by using sewage sludge as a precursor, followed by pyrolysis and alkaline precipitation. Their research revealed a maximum phosphorus adsorption amount was 312.55 mg/g. However, single-metal modification often leads to metal accumulation and uneven distribution, diminishing the utilization efficiency of lanthanum. Fang et al. (2015) soaked biochar in MgCl2 and CaCl2 solutions, the dual-metal doping strategy effectively mitigated metal agglomeration. The phosphate adsorption capacity of the prepared biochar was greatly improved. In addition, numerous studies have shown that the addition of biomass such as wheat straw or Ca based additives during the preparation of sludge biochar can promote the conversion of OP to IP and NAIP to AP, thereby enhancing the bioavailability of sludge biochar (Zhao et al., 2018; Yang et al., 2019).
The effective recovery of phosphorus from biochar after adsorption is a key step in achieving phosphorus resource recycling. Currently, common phosphorus recovery strategies include chemical desorption and thermochemical treatment methods. The chemical desorption method uses acid, alkali, or salt solutions as desorbents to detach the adsorbed phosphates from the surface of the biochar. This method has advantages such as ease of operation and environmental friendliness, and theoretically, biochar can be reused multiple times. However, after multiple adsorption-desorption cycles, the phosphorus recovery rate of biochar typically decreases significantly, limiting its long-term application. The thermochemical treatment method usually first converts the phosphorus in biochar into soluble phosphates or volatile matter through high-temperature calcination. Subsequently, phosphorus is extracted using methods such as struvite crystallization or acid leaching. Although this method has a higher phosphorus recovery rate, the structure of the biochar is often damaged, and it also has a high energy consumption.
The potential release of metals in modified biochar is an important aspect of environmental risk assessment. On one hand, the sludge contains metal elements, particularly heavy metal elements, which can cause pollution in water bodies and soil if not disposed of properly (Alam et al., 2024; Zheng et al., 2024a); on the other hand, common modified metals such as lanthanum and calcium, if released into the environment, may lead to increased water hardness, soil alkalization, and disrupt the community structure and activity of environmental microorganisms (Zheng et al., 2024b). However, existing studies have shown that lanthanum loaded on the surface of adsorbent materials has low solubility and leaching risk. For example, numerous studies have applied lanthanum for phosphate control in river sediment, and no significant negative ecological effects have been observed (Dithmer et al., 2016). In studies on the stability of adsorbent materials, (Chen et al., 2012) found that lanthanum was not detected in the acidic and neutral desorption solutions of La(III)-loaded granular ceramic, and even after four cycles of adsorption-desorption, the leaching amount of lanthanum from La(III)-loaded granular ceramic remained below the detection limit.
Based on the above analysis, this study intends to doping sludge biochar (SBC) with lanthanum to achieve high-capacity phosphorus adsorption; doping with calcium to improve lanthanum distribution and enhance phosphorus bioavailability; incorporating wheat straw biomass to enhance the specific surface area and pore structure of the biochar. Prepare a high-performance and environmentally friendly adsorption functional material. After preparation and synthesis, using X-ray diffraction (XRD), scanning electron microscope (SEM) (Zhong et al., 2024a), Fourier transform infrared spectroscopy (FTIR) and Brunauer–Emmett–Teller (BET) to analyze the physicochemical properties of modified SBC. The study will investigate the effects of pH, concentration, temperature, reaction time, competitive anions, and SBC dosage on phosphorus removal efficiency. The adsorption mechanism, kinetics, isotherms, and thermodynamics of phosphorus removal will also be investigated.
2 Materials and methods
2.1 Materials and reagents
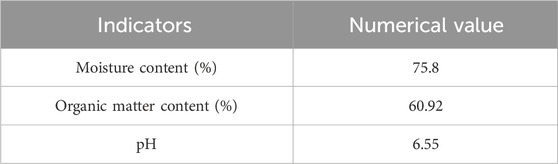
Materials: The experimental sludge was taken from the dewatered sludge after mechanical dehydration in a municipal sewage treatment plant in Zhengzhou. The various indicators of the sludge were tested, and the results are shown in Table 1. The sludge (SS) was freeze-dried, after freeze-dried sludge reaching room temperature, the sludge was pulverized. The sample was sieved through a 60-mesh sieve and collected for further use. Wheat straw was obtained from a agricultural waste processing plant in Jiangsu Province. The straw was dried and pulverized. It was then placed in an oven at 105°C until a constant weight was achieved. After cooling to room temperature in a desiccator, the straw was pulverized and sieved through a 60-mesh sieve for subsequent experiments use.
Reagents: Lanthanum chloride heptahydrate (LaCl3·7H2O), Calcium chloride anhydrous (CaCl2), Potassium dihydrogen phosphate (KH2PO4), Sulfuric acid (H2SO4), Ascorbic acid (C6H8O6), Ammonium molybdate ((NH4)2MoO4), Potassium antimonyl tartrate (C8H4K2OSb2), Sodium hydroxide (NaOH, solid pellets), Hydrochloric acid (HCl, 36.5% solution). All reagents were analytical grade and were purchased from Mclin Biochemical Technology Co., Ltd., Shanghai, China, stored in a dry and dark environment. The experimental water is deionized water, which is prepared by the laboratory system.
2.2 Preparation of adsorbent
The modified hydrothermal carbon was prepared by one-step hydrothermal method and using alkali-tuned precipitation. A certain proportion of LaCl3·7H2O and anhydrous CaCl2 (M(LaCl3):M(CaCl2) = 1:2 1:1.5 1:1 1.5:1 2:1) were weighed, and a total of 10 mmol was taken and dissolved in 50 mL of deionized water. Freeze-dried sludge and wheat straw were each weighed 0.5 g, mixed with the solution and stirred well, and then 10% NaOH solution was added drop by drop to keep the pH at about 11.0, and stirred magnetically for 15 min. Subsequently, it was put into a hydrothermal reactor and placed in a 170°C blast drying oven for 8.5 h of hydrothermal carbonization (HTC). After hydrothermal completion, the hydrothermal reactor was cooled to room temperature, the product in the kettle was pump-filtered and washed with deionized water to near neutrality, and dried at 60°C in the oven until the weight was constant. After grinding and crushing in a mortar and passing through a 60-mesh sieve, lanthanum calcium modified sludge/wheat straw hydrothermal carbon (LC-SWBC) was obtained. On this basis, separately prepared sludge hydrothermal carbon (SBC), hydrothermal carbon that sludge: wheat straw = 1: 1 (SWBC) and metal-modified sludge carbon (LC-SBC).
2.3 Characterization of sludge biochar
The surface functional groups of the sludge biochar were analyzed using a TENSOR II FTIR spectrometer from Burker, Germany, with a wave number range of 4000–500 cm−1. The samples were analyzed by XRD using a D8 Advance x-ray diffractometer from Buker, Germany, with a CuKα radiation range of 10°–90°. The microstructure and morphology of the samples were measured using a scanning electron microscope (SEM) from Hitachi su8010 (Japan). The elemental distribution on the sample surface was analyzed using an EMAX mics2 spectrometer (HORIBA, Japan). BET analysis was performed using a Belsorp-max II specific surface and pore analyzer from MicrotracBEL, Japan.
2.4 Adsorption experiments
The effects of different initial solution pH, competitive anions, dosage and reaction time on phosphorus adsorption were investigated by batch adsorption experiments. The adsorption mechanism of phosphorus by LC-SWBC adsorbent was investigated using adsorption kinetics, isotherm and thermodynamic modeling. The adsorption mechanism of LC-SWBC for phosphate removal was investigated using kinetic, isotherm, and thermodynamic models. Adsorption kinetics, isotherms and thermodynamic models were used to investigated the phosphorus adsorption mechanism of SBC adsorbent.
Weigh 0.4394 g of KH2PO4 dried at 100°C for 2 h, dissolve it with water, transfer it to a 1L volumetric flask, mix it well, and dilute it with deionized water to the mark. This solution is a 100 mg P/L stock solution. During the adsorption experiment, dilute the stock solution to the phosphorus concentration required for the experiment. For each experiment, a specific amount of LC-SWBC adsorbent was added to a beaker containing the phosphorus solution. The mixture was then subjected to adsorption at a constant temperature and shaking speed of 120 rpm. In addition to the real wastewater and competitive ion experiments, the initial pH of the phosphorus solution was adjusted to pH = 3 using 1 mol/L NaOH and HCl solutions. After the adsorption process was completed, the liquid was filtered through a 0.45 µm aqueous membrane and the concentration of phosphates in the solution was determined using the molybdenum-antimony anti-spectrophotometric method (GB 11893-89) with a DR6000 UV-visible spectrophotometer from Hach Company, United States. All phosphate concentrations are reported as P element. To ensure the accuracy of the analysis, a standard curve was plotted, and the instrument’s calibration was checked regularly. Each set of adsorption experiments was conducted in triplicate, and after checking the data results for any outliers, the average value was taken and the standard deviation was calculated. To assess the effect of initial solution pH on phosphorus adsorption, the pH was adjusted to 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, and 12 prior to adsorption and the change in pH before and after adsorption was measured. To investigate the effect of adsorbent dosage on the adsorption of phosphate in water by LC-SWBC, a series of experiments were conducted using different adsorbent dosages (0.5 g/L, 0.7 g/L, 1 g/L, 1.5 g/L, and 2 g/L) with adsorption time of 12 h, and an initial phosphorus concentration of 100 mg P/L. In order to evaluate the potential of the prepared materials for application in real water bodies, it is therefore important to study the variation of coexisting anion concentrations on the effect of adsorbents on phosphorus removal. The common inorganic-like acid radical ions in water bodies include NO3−, Cl−, SO42-, and HCO3−, and the main organic anions are humate and citrate. In this experiment, a gradient experiment was conducted for the above anions that may have a competitive adsorption relationship with phosphate to study the effect of different anions on the adsorption of phosphorus by biochar, so the solution was adjusted to pH = 7 and the gradient of the competitive ions’ concentration was set to 0 mM, 2 mM, 5 mM, and 10 mM. In the adsorption kinetics experiments, the adsorption times of phosphorus were 0.25, 0.5, 0.75, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h. In the adsorption isotherm experiments, the concentrations of phosphorus solutions were 20, 50, 100, 200, 400, and 600 mg/L, and the temperatures of the thermodynamic adsorption experiments were 15°C, 25°C, and 35°C, respectively. In the adsorption experiments of real wastewater, adsorption experiments were conducted using five different dosages (0.2 g/L, 0.3 g/L, 0.4 g/L, 0.5 g/L, and 0.6 g/L). Used for adsorption with constant temperature oscillation at 120 rpm, and the samples were collected and tested at 12 h to investigate the adsorption performance of the adsorbent on the real wastewater with low concentration.
2.5 SMT graded phosphorus extraction
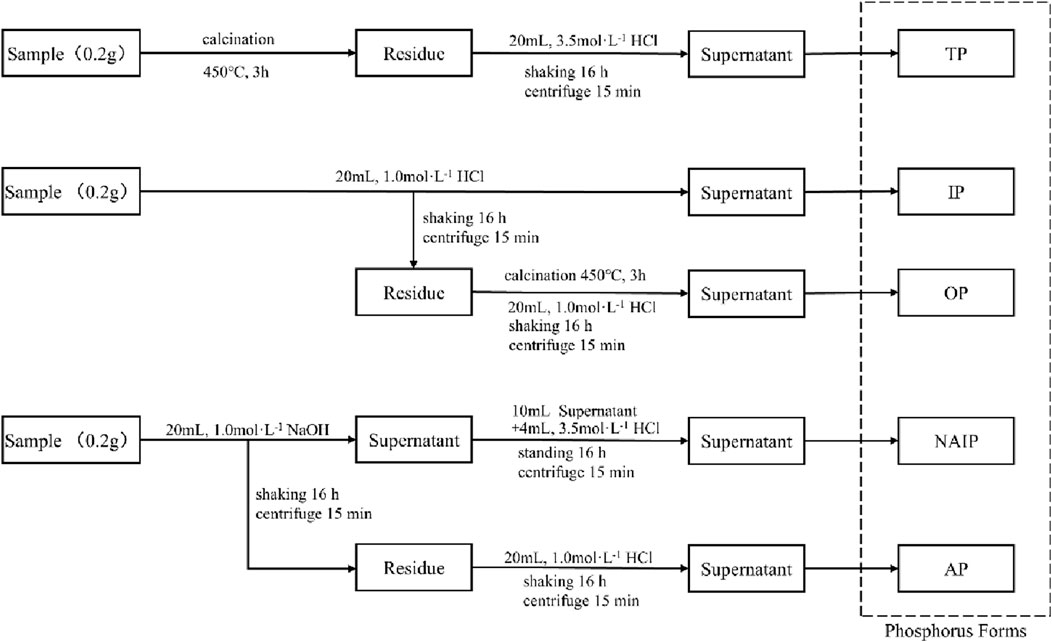
The SMT graded extraction method was utilized to separate different forms of phosphorus from sludge and hydrothermal carbon. The detailed analytical extraction process is shown in Figure 1. The SMT method classifies the extracted phosphorus into five forms, which are non-apatite inorganic phosphorus (NAIP, usually phosphorus bound to Fe, Mn, and Al oxides and their hydroxides), apatite inorganic phosphorus (AP, usually various types of phosphorus bound to Ca and Mg), inorganic phosphorus (IP), organic phosphorus (OP), and total phosphorus (TP) (Ruban et al., 2001). In this method, TP was extracted from the first sample, IP and OP from the 2nd sample and NAIP and AP from the 3rd sample.
3 Results and discussion
3.1 Effect of metal molar ratio on performance
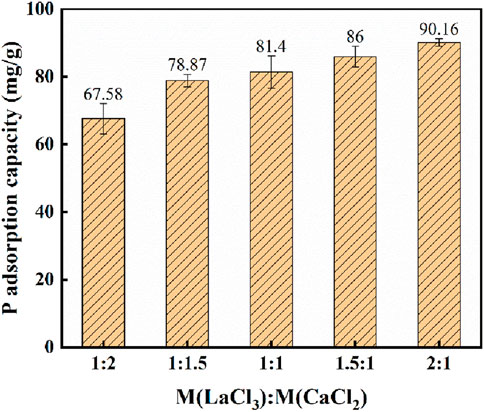
Figure 2 shows the effect of varying the La/Ca molar ratio on the phosphate unit adsorption capacity of hydrothermal carbon while keeping other experimental conditions fixed. The results indicate that the unit adsorption capacity is positively correlated with the La/Ca molar ratio. Specifically, as the La/Ca molar ratio increases, the unit adsorption capacity of hydrothermal carbon significantly increases. This phenomenon is primarily attributed to La being a metal with a high affinity for phosphorus, which has a much greater adsorption capacity for phosphorus compared to Ca. As the ratio of La to Ca in the adsorbent increases, the number of effective adsorption sites for phosphate ions also increases, making it more favorable for the removal of phosphates from aqueous solution. It is noteworthy that when the La/Ca molar ratio exceeds 1:1.5, the rate of increase in unit adsorption capacity significantly slows down. This is because when the proportion of La is too high, lanthanum metal tends to agglomerate and accumulate on the surface of the adsorbent, leading to an uneven distribution of lanthanum on the material’s surface, which prevents some adsorption sites from being fully exposed (Yu et al., 2019), thereby reducing the utilization efficiency of lanthanum metal. Additionally, an excess of lanthanum may also lead to structural changes in the material, affecting its adsorption performance.
Based on the above experimental results, considering the dual objectives of increasing unit adsorption capacity and controlling costs, a La/Ca molar ratio of 1:1.5 is deemed ideal. This ratio ensures that the adsorbent has high phosphorus adsorption performance while avoiding the issues of increased costs and insufficient exposure of adsorption sites due to excessive lanthanum. Under these conditions, LC-SWBC with the best adsorption performance was prepared. The cost is estimated according to the market price and dosage of raw materials. The market price of LaCl3·7H2O is 22 CNY/kg, and the market price of CaCl2 is 1 CNY/kg. Combined with the yield of biochar preparation, the unit cost price is about 8 CNY/kg.
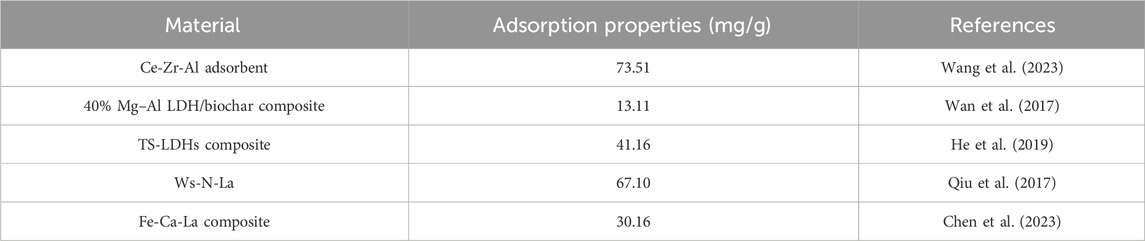
In recent years, the research of metal modified materials as phosphate adsorbents has made significant progress. So far, a variety of metals including Al, Mg, Ca, Zr and La have been successfully modified in a variety of carriers to form new adsorbents with customizable properties. Compared with other polymetallic modified adsorbents, LC-SWBC has the advantages of stronger adsorption capacity and wider pH range. Table 2 shows the comparison of the maximum adsorption capacity of phosphate by different polymetallic modified adsorbents.
3.2 The properties of biochar
3.2.1 SEM-EDS
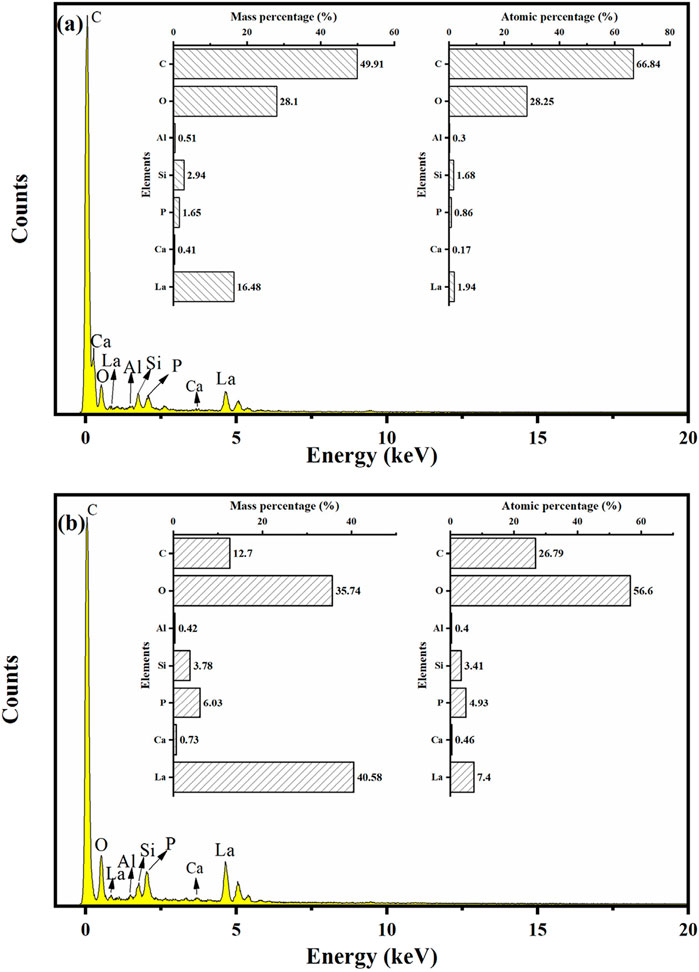
Figure 3 shows the SEM images of LC-SWBC. From Figure 3a, it can be observed that the surface of LC-SWBC exhibits a porous structure, with some pores being surrounded by a large number of nanoparticles. This rough surface morphology effectively increases the specific surface area of the material (Zhong et al., 2024b), providing abundant active sites for subsequent adsorption reactions. Figure 4a presents the EDS analysis results of LC-SWBC. The energy spectrum indicates that the mass percentages of La, Ca, and P in LC-SWBC are 16.48%, 0.41%, and 1.65%, respectively, confirming that lanthanum and calcium have been successfully loaded onto the surface of LC-SWBC, while the presence of a small amount of phosphorus is due to the high phosphorus content in the sludge.
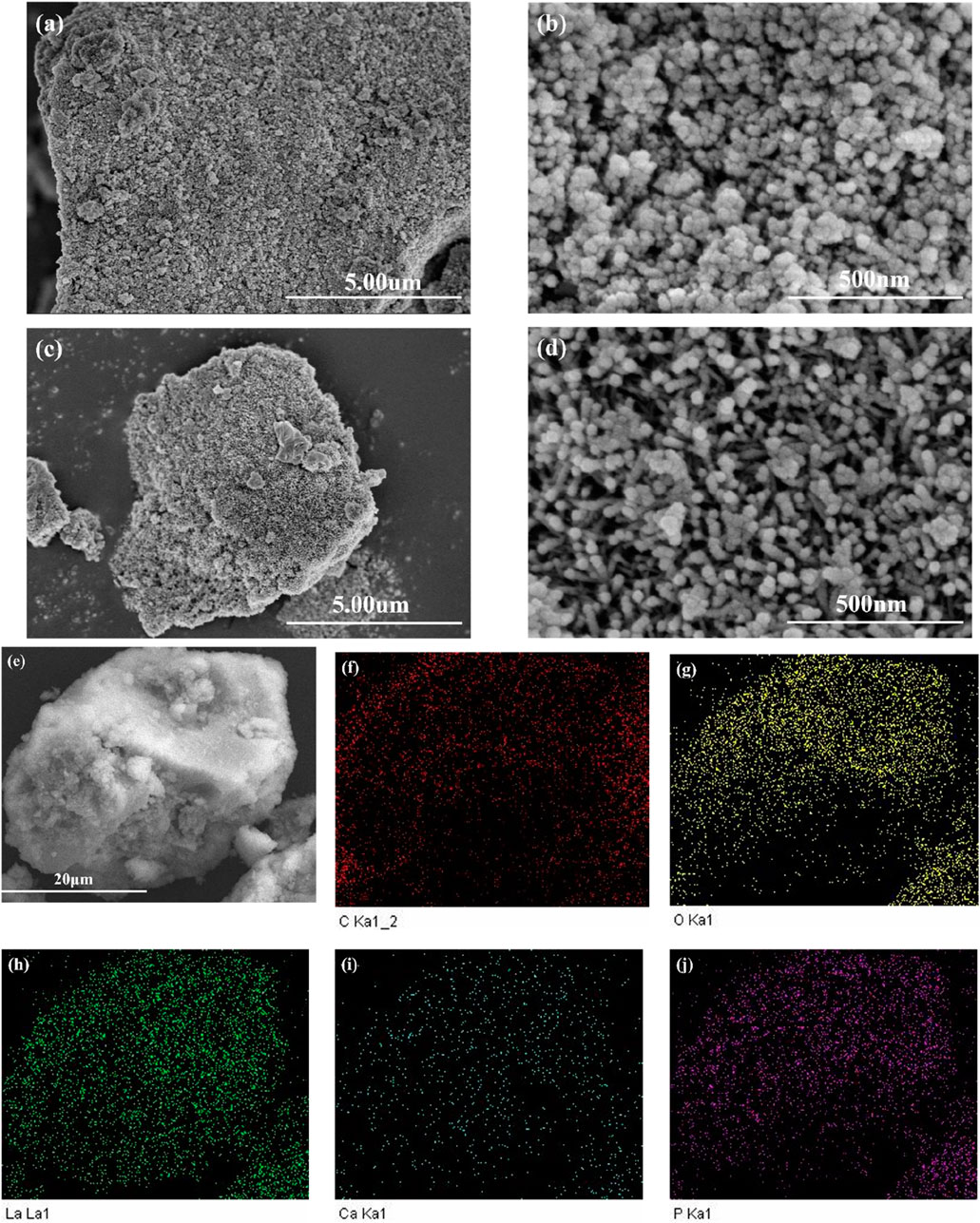
Figure 3. SEM images and EDS elemental images of the samples: (a,b) are SEM images of LC-SWBC; (c,d) are SEM images of LC-SWBC-P; (e–j) EDS elemental images of C, O, La, Ca and P, respectively.
Comparing Figures 3b,d, it is evident that the surface of LC-SWBC-P has formed a large number of clustered nanorod structures, consistent with the morphology of phosphate precipitates. The formation of these nanorod structures is primarily due to the chemical reaction between the La(OH)3 and Ca(OH)2 loaded on the biochar surface and phosphate ions, resulting in the generation of insoluble phosphate precipitates. To further verify the distribution of elements, Figures 3e–j display the EDS area-scan results of LC-SWBC-P. The results confirm that elements such as C, O, La, Ca, and P are uniformly distributed on the surface of LC-SWBC-P. The quantitative analysis results of the energy spectrum in Figure 4b indicate that compared to LC-SWBC, the mass percentage of P in LC-SWBC-P has significantly increased, reaching 6.03%. This suggests that during the adsorption process, phosphorus was effectively adsorbed onto the surface of the LC-SWBC material, confirming the phosphorus adsorption capability of this material.
3.2.2 XRD
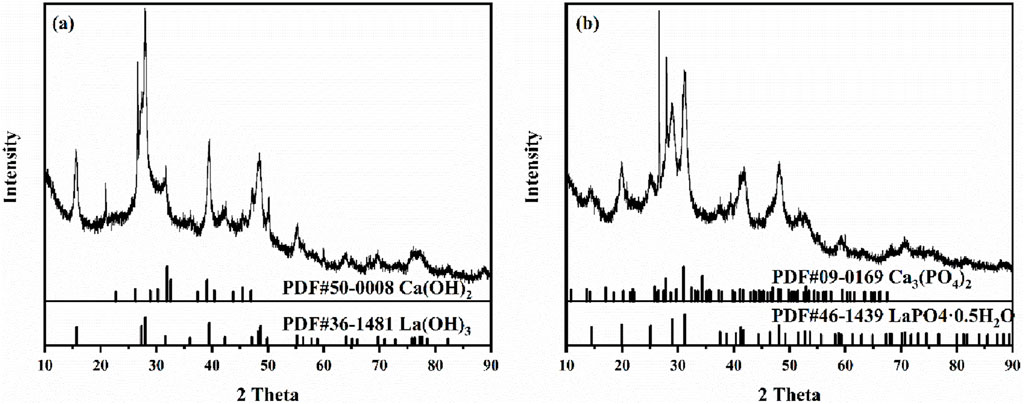
To further investigate the crystal structure of LC-SWBC, XRD analysis was conducted on the material, with a testing range of 5°–90°. Figure 5 shows the XRD test results. As shown in Figure 5a, LC-SWBC exhibits distinct diffraction peaks at diffraction angles of 15.66°, 27.31°, 27.97°, 39.47°, and 48.64°, which correspond to the standard diffraction peaks of La(OH)3 crystals (Hu et al., 2007), indicating that lanthanum hydroxide crystals have been successfully loaded onto the surface of the biochar. Additionally, LC-SWBC also displays significant diffraction peaks at diffraction angles of 31.86°, 32.57°, 39.06°, 45.47°, and 46.97°, which correspond to the standard diffraction peaks of Ca(OH)2 crystals, confirming that calcium hydroxide crystals have also been successfully loaded onto the surface of the biochar.
In order to study the changes in crystal structure of LC-SWBC after phosphorus adsorption and to explore the adsorption mechanism, XRD analysis was conducted on the samples after phosphorus adsorption (LC-SWBC-P). The XRD spectrum in Figure 5b shows that LC-SWBC-P exhibits distinct diffraction peaks at 27.76°, 31.02°, and 34.37°, which correspond to the standard diffraction peaks of Ca3(PO4)2 crystals. Additionally, new diffraction peaks were detected at 19.89°, 29.03°, and 31.15°, which correspond to the standard diffraction peaks of LaPO4·0.5H2O crystals.
3.2.3 FTIR
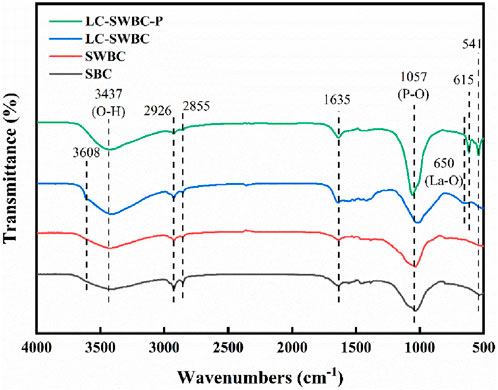
The FTIR analysis results of biochar are shown in Figure 6. SBC and SWBC are basically the same, with the characteristic peak at 3437 cm−1 attributed to the stretching vibration of the O-H bond in the adsorbed water on the material’s surface (Yu et al., 2015); the characteristic peaks at 2,855 cm−1 and 2,926 cm−1 correspond to the asymmetric and symmetric stretching vibrations of the C-H bond, respectively. The characteristic peak at 1,635 cm−1 is attributed to the adsorbed water molecules, specifically caused by the bending vibration of the H-O-H of interlayer water molecules. This indicates the presence of adsorbed water on the material’s surface, and it may also exist within the material (Regkouzas et al., 2023); the strong peak at 1,057 cm−1 originates from the asymmetric stretching vibration of P-O, which is due to the presence of phosphates in the sludge that are fixed in the solid phase during the hydrothermal carbonization process. A new sharp peak appeared at 3608 cm-1 for LC-SWBC, which is attributed to the stretching vibration of hydroxyl groups in the modified La(OH)3 and Ca(OH)2. According to the literature (Jia et al., 2020), the peak at 650 cm−1 corresponds to La-O, indicating that La(OH)3 and Ca(OH)2 are effectively loaded on the surface. The characteristic peaks at 615.5 cm−1 and 541 cm−1 correspond to the bending vibrations of the O-P-O group, indicating that phosphates are chemically bonded to the material’s surface, possibly due to the combination of phosphorus in the small amount of sludge with La(OH)3 and Ca(OH)2. The infrared spectrum of the LC-SWBC-P underwent significant changes. First, the absorption peak corresponding to O-H in La(OH)3 and Ca(OH)2 at 3608 cm−1 disappears, indicating that hydroxyl groups are consumed during the adsorption process. Meanwhile, the area of the absorption peak corresponding to the asymmetric stretching vibration of P-O at 1,057 cm−1 significantly increases, indicating the formation of a new phosphate structure. Additionally, the characteristic peaks of the bending vibrations of the O-P-O group at 615 cm−1 and 541 cm−1 are significantly enhanced compared to LC-SWBC, further confirming that phosphates are chemically bonded to the material surface.
3.2.4 BET
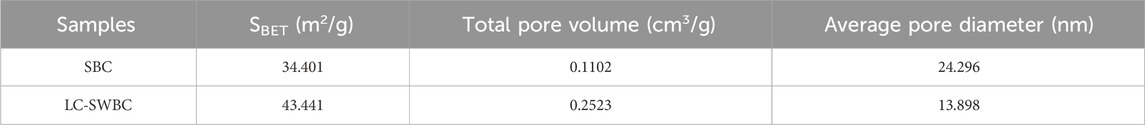
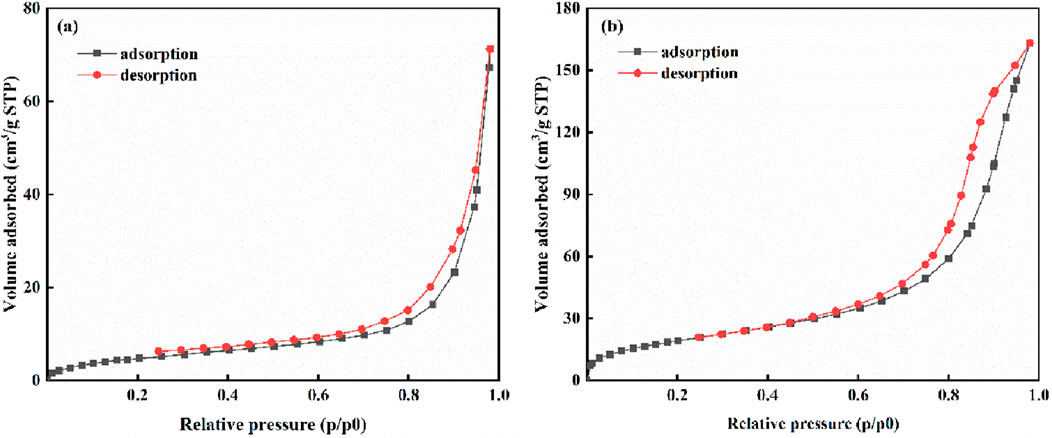
Table 3 summarizes the specific surface area and pore size distribution characteristics of raw biochar (SBC) and modified biochar (LC-SWBC). The specific surface area of LC-SWBC increased from 34.401 m2/g for SBC to 43.441 m2/g, indicating a significant enhancement in the surface area of the material after modification. Research by Sun et al. (Yin et al., 2019) suggests that biomass doping may be an important reason for optimizing the structure of LC-SWBC; additionally, metal modification can also increase the specific surface area of biochar to some extent (Sun et al., 2023). Furthermore, the alkaline conditions during the hydrothermal carbonization process of LC-SWBC may have facilitated the expansion of the biochar’s pore structure. These three factors may have a synergistic effect, collectively enhancing the specific surface area of LC-SWBC. The average pore sizes of SBC and LC-SWBC are 24.296 nm and 13.898 nm, respectively, both of which fall within the pore size range of mesoporous materials (2–50 nm), confirming that both materials are mesoporous. Figure 7 shows their nitrogen adsorption-desorption isotherms (Peng et al., 2020). From Figure 7, it can be seen that the nitrogen adsorption-desorption isotherms of SBC and LC-SWBC both exhibit a typical type IV isotherm, further indicating that both materials have significant mesoporous structural characteristics (Boateng et al., 2024). Notably, compared to the original SBC, the average pore size of LC-SWBC has significantly decreased. This may be due to some metals entering the biochar pores during the modification process, causing blockage or filling (Wan et al., 2017).
3.3 LC-SWBC phosphorus adsorption experiments
3.3.1 Effect of pH
The adsorption performance of the adsorbent was determined by exploring the phosphorus removal of the adsorbent material over a wide range of pH conditions. Figure 8a shows the results of the effect of pH on LC-SWBC and the corresponding pH values after adsorption. The adsorption performance of the adsorbent was good at pH = 2–6, and the unit adsorption was above 65 mg/L. When the pH was between 2–7.72, the surface of LC-SWBC was protonated with positive charge, at this time, H2PO4− and HPO42- were predominant in the liquid phase, and the stronger electrostatic attraction caused H2PO4− and HPO42- to be adsorbed onto the surface of LC-SWBC. In addition, Cl− introduced by the HCl solution used during low pH adjustment strengthens the adsorption sites on the surface of LC-SWBC, which leads to an increase in the adsorption capacity of LC-SWBC in a acidic environment (Akram et al., 2021). The increase in pH after adsorption may be due to the fact that at low pH, the phosphate species in solution are mainly H2PO4−, whose concentration decreases after adsorption by the adsorbent, and thus the concentration of ionized hydrogen ions decreases, resulting in a slight increase in the overall pH of the solution.
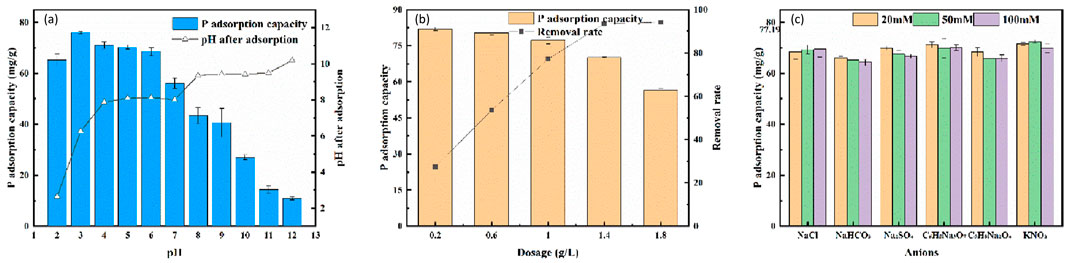
Figure 8. The effects of conditions on adsorption performances: (a) pH, (b) adsorbent dosage, (c) competitive ions.
As the pH increases, the protonation level on the surface of LC-SWBC decreases, and the amount of phosphorus adsorbed decreases. When the pH gradually increased to 12, the concentration of hydroxide ions in the solution increased, the phosphate in the liquid phase was more in the form of HPO42-, and the surface of LC-SWBC was deprotonated and negatively charged, on the one hand, the decrease in pH after adsorption indicated that OH- in the solution was involved in the competition for the adsorption of phosphorus; on the other hand, the gradual enhancement of electrostatic repulsion between the adsorbent and anion of the phosphorus solution prevented phosphorus from being adsorbed onto the surface of LC-SWBC. At this time, the phosphate species are mainly HPO42-, and its adsorption leads to a decrease in the concentration of hydrolyzed OH−, so the pH of the solution decreases after adsorption (Jung et al., 2015).
3.3.2 Effects of adsorbent dosage
The results are shown in Figure 8b. As the amount of biochar added increases, the adsorption amount decreases while the removal rate increases, but the trend of change tends to slow down. This trend is consistent with the published researches (Zhou et al., 2024; Ugural et al., 2024; Barquilha and braga, 2021; Regkouzas et al., 2023). This is because the initial increase in the amount of adsorbent provides more adsorption active sites, leading to significant changes. However, when the amount of adsorbent reaches a certain value, although the active sites will gradually be occupied by the adsorbate, the number of available active sites on the adsorbent surface is still much greater than the amount of adsorbate in the solution, resulting in no significant improvement in removal efficiency (Hasanzadeh et al., 2017). Considering both the removal effect and the control of biochar usage costs, 1 g/L is selected as the optimal addition amount for LC-SWBC.
3.3.3 Impact of competitive ions
As shown in Figure 8c, the adsorption capacity of phosphorus by LC-SWBC was 77.19 mg/g in the absence of other anions. The experimental results showed that most of the inorganic and organic acid radicals interfered more and more strongly with the phosphorus adsorption by LC-SWBC as the concentration of the competitive anions was increased from 0 to 10 mM, and the intensity of the interference was not the same for different competing ions. Overall, the interference of NO3− and citrate with the biochar was subtle, and the adsorption capacity of LC-SWBC could reach more than 70 mg/g at any concentration of these two anions. The adsorption capacity of LC-SWBC decreased to 68.45 mg/g when the solution contained 2 mM of Cl−. While the concentration of Cl− was further increased, the adsorption capacity of the material increased. This is because the presence of a large amount of Cl− has a higher promotion effect on the adsorbent than the competition for adsorption. The competition of SO42-, humate and HCO3− was stronger and the adsorption capacity of LC-SWBC was reduced to 65 mg/L or less. LC-SWBC adsorbed 64.32 mg/L at 10 mM HCO3-, which had the greatest interference effect, reducing the adsorption performance to 83.32%. In the competitive adsorption experiment, the adsorption performance of LC-SWBC was stable and anti-interference.
3.3.4 Adsorption and desorption cycle
As shown in Figure 9, when using deionized water, 0.1 M NaOH solution, 0.1 M HCl solution, and 0.1 M NaCl solution as desorbing agents, the phosphorus desorption rates of LC-SWBC were 9.01%, 103.16%, 60.55%, and 45.77%, respectively. The initial phosphorus adsorption amount of LC-SWBC was 77.19 mg/g, and the desorption amount in the 0.1 M NaOH solution reached 46.73 mg/g, indicating a good desorption effect. This is because OH− ions undergo ion exchange with phosphate ions, promoting the desorption of phosphate from the biochar. In the 0.1 M HCl solution, the desorption amount reached as high as 79.63 mg/g, with a desorption rate exceeding 100% (103.16%). This is because the precipitates formed by phosphate ions with La and Ca are dissolved by the acid, leading to the release of phosphate ions. However, the strong acidity of the HCl solution also damages the internal structure of the biochar, causing the release of sludge phosphorus from within the biochar, resulting in a desorption amount that exceeds the adsorption amount. The structural damage makes it difficult for the biochar to be reused for adsorption. The desorption effect of the 0.1 M NaCl solution was weaker than that of the 0.1 M NaOH solution, with a desorption amount of 35.33 mg/g; the desorption effect of deionized water was even lower, with only 6.95 mg/g desorbed. Based on the above experimental results, a 0.1 M NaOH solution was chosen as the desorbent to further investigate the recycling efficiency of LC-SWBC.
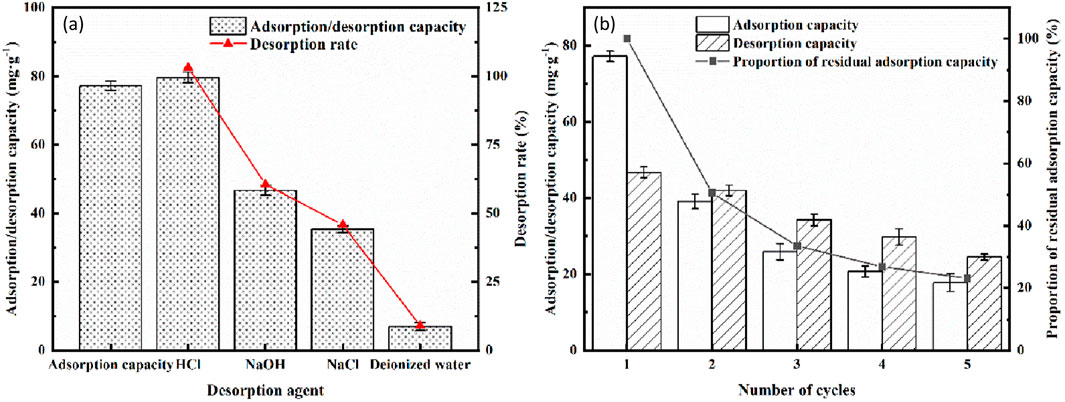
Figure 9. Adsorption desorption experiment: (a) Desorption experiment, (b) Adsorption desorption cycle experiment.
After the desorption and cyclic use with the 0.1 M NaOH solution, the phosphate adsorption capacity of LC-SWBC gradually decreased. The decline in the material’s adsorption capacity is usually due to the reduction of available adsorption sites, which may stem from two mechanisms: first, during the regeneration process, the PO43- bound to the surface of the biochar was not completely desorbed, still occupying some active adsorption sites, thereby reducing the material’s adsorption capacity; second, during the adsorption and desorption processes, a small amount of La and Ca in LC-SWBC partially dissolved, reducing the number of adsorption sites and leading to a decrease in adsorption capacity. After the second cycle, the adsorption capacity decreased from the initial 77.19 mg/g to 39.08 mg/g, still demonstrating a good phosphate adsorption effect. After five cycles, the adsorption capacity was only 20% of the initial value, but there was still an adsorption amount of 17.8 mg/g, which can meet basic water treatment needs. In summary, LC-SWBC has good regeneration and recycling value.
3.4 Adsorption kinetics
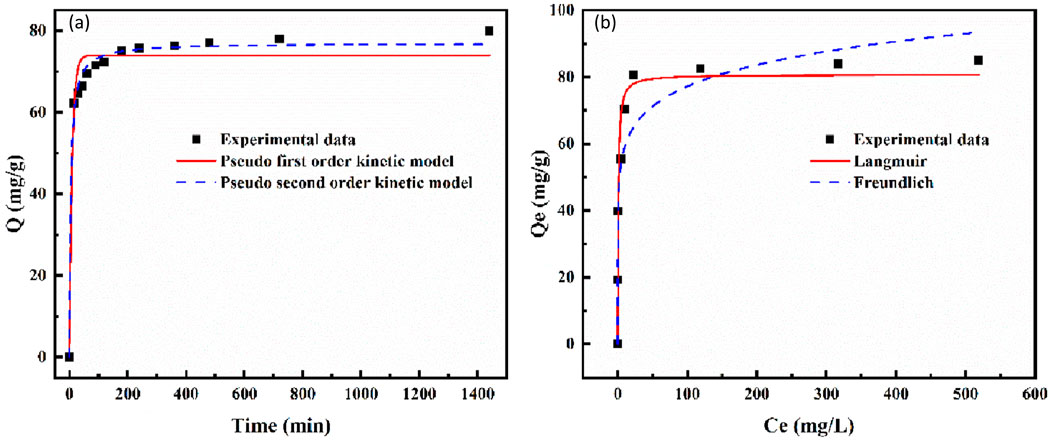
This study fitted the kinetic experimental data of phosphorus adsorption processes on LC-SWBC using pseudo-first-order and pseudo-second-order kinetic models. As shown in Figure 10a, the adsorption rate initially rises rapidly and then gradually levels off, approaching dynamic equilibrium after about 4 h, which is consistent with existing research findings (Ren et al., 2012). Numerous studies have indicated that this is related to the high adsorption affinity of lanthanum for phosphate; lanthanum is an effective phosphate binder, and lanthanum-calcium composite modification has been proven to effectively promote phosphate adsorption. In the initial stage of adsorption, phosphate ions in the solution quickly bind to the abundant La-O/La-OH coordination active sites on the LC-SWBC surface, leading to a rapid decrease in phosphorus concentration in the solution. The active sites on the LC-SWBC surface become occupied by phosphate ions and tend to become saturated. At this point, phosphate ions diffuse through the pores of the LC-SWBC and migrate to the internal active sites. In the later stages of adsorption, as the internal active sites of the adsorbent are gradually occupied, mass transfer resistance increases, limiting the diffusion of phosphorus within the LC-SWBC, resulting in a further decrease in the adsorption rate. When all the adsorption sites on the LC-SWBC are occupied by phosphorus, the adsorption process reaches dynamic equilibrium.
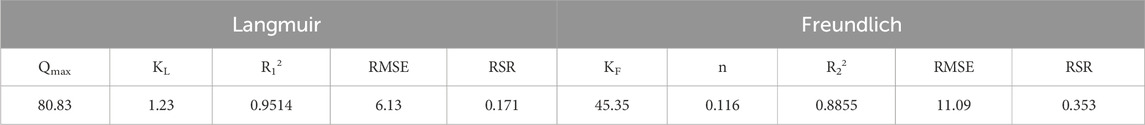
Table 4 shows the fitting results of adsorption kinetics. The correlation coefficient of the pseudo-second-order kinetic model (R2 = 0.9896) is higher than that of the pseudo-first-order kinetic model (R2 = 0.9589), and the RMSE of pseudo second-order kinetic model (1.94 mg/g) was also lower than that of pseudo first-order kinetic model (3.06 mg/g). The RSR of the pseudo second order kinetic model was only 0.093, far below the threshold of 0.5. The model had good performance and could effectively predict the adsorption performance. The theoretical value of the pseudo-second-order kinetic model (76.96 mg/g) is closer to the experimentally measured actual adsorption amount (77.19 mg/g) than the theoretical value of the pseudo-first-order kinetic model (73.97 mg/g). This indicates that chemical adsorption plays a dominant role in the process of LC-SWBC adsorbing phosphate (Lee et al., 2019).
3.5 Adsorption isotherms
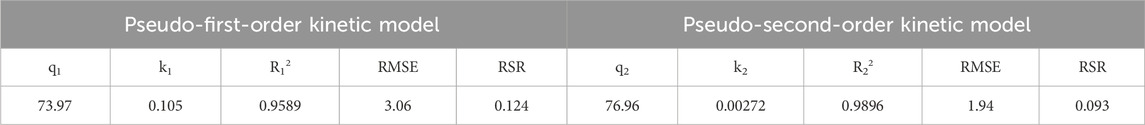
In order to explore the adsorption mechanism of LC-SWBC in depth, this study conducted a fitting analysis of the experimental data using the Langmuir and Freundlich adsorption isotherm models. The adsorption isotherm curve shown in Figure 10b illustrates the variation in the adsorption capacity of LC-SWBC for phosphate under a phosphorus concentration gradient (0–600 mg/L). The experimental results indicate that as the phosphorus concentration increases, the adsorption capacity of LC-SWBC for phosphorus rapidly increases and then gradually approaches saturation, reflecting the typical characteristics of the adsorption process. This is because, during the initial adsorption phase, the high concentration of phosphorus solution provides a greater mass transfer driving force, facilitating the diffusion of phosphate ions to the surface of the adsorbent and the progression of the adsorption process.
The Langmuir model is based on the assumption of uniform surface monolayer adsorption with no interactions between adsorption sites; in contrast, the Freundlich model is suitable for describing the adsorption of heterogeneous active sites on the adsorbent surface, where the adsorbate can form multilayer adsorption and interactions between adsorption sites are considered. The fitting results in Table 5 indicate that the fitting correlation coefficient for the Langmuir model is R2 = 0.9514, significantly higher than that of the Freundlich model (R2 = 0.8855), which suggests that the Langmuir model can better describe the adsorption process of phosphates by LC-SWBC. The RMSE and RSR of Langmuir model are also better than Freundlich model. This indicates that the adsorption of phosphorus by LC-SWBC is primarily monolayer adsorption, mainly through chemical adsorption (Yao et al., 2011). According to the fitting results of the Langmuir model, the maximum adsorption capacity of this adsorbent material is 80.83 mg/g.
3.6 Adsorption thermodynamics
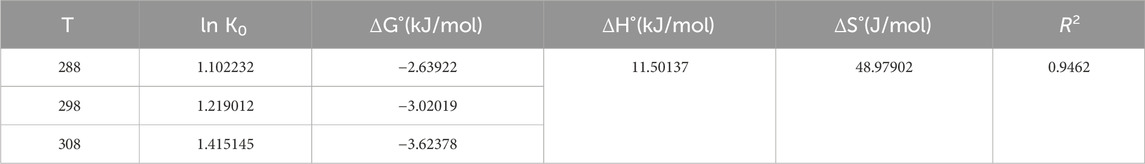
The thermodynamic study of adsorption (Table 6) can further reveal the adsorption mechanism of phosphorus on LC-SWBC. The magnitude of the adsorption enthalpy change (ΔH°) can be used to distinguish between physical adsorption processes (2.1–20.9 kJ/mol) and chemical adsorption processes (20.9–418.4 kJ/mol). Similarly, the value of ΔG° can be used to differentiate between physical adsorption processes (−20–0 kJ/mol) and chemical adsorption processes (−400∼−80 kJ/mol). In this study, the negative ΔG° values indicate that the adsorption reaction can occur spontaneously without external force conditions.
The thermodynamic parameters ΔH° and ΔS° are both positive, with ΔH° being 11.50 kJ/mol and ΔS° being 48.98 J/mol. The ΔG° ranges from −20 to 0 kJ/mol, indicating that the adsorption of phosphorus by LC-SWBC involves a physical adsorption process. The positive ΔH° indicates that the phosphate adsorption process on LC-SWBC is an endothermic reaction, meaning that higher reaction temperatures are more favorable for the process, enhancing the material’s adsorption efficiency, which is consistent with the experimental results (Karadag et al., 2006). This is because an increase in temperature leads to a gradual decrease in the ΔG° value, thereby accelerating the effective contact between phosphate ions and the adsorption sites on LC-SWBC and improving mass transfer efficiency, resulting in enhanced phosphorus adsorption by LC-SWBC and making the adsorption of phosphate ions increasingly spontaneous, thus making the adsorption effect more pronounced. ΔS°>0 indicates that the adsorption reaction is an entropy-increasing reaction, where the degree of molecular disorder increases, which is favorable for the adsorption of phosphorus (Yuan et al., 2021).
3.7 Mechanism of LC-SWBC phosphorus adsorption
Combining the previous XRD analysis results, the LC-SWBC shows a high degree of correspondence with the PDF cards of Ca(OH)2 and La(OH)3. LC-SWBC-P exhibits distinct diffraction peaks at 27.76°, 31.02°, and 34.37°, which match the standard diffraction peaks of Ca3(PO4)2 crystals. Additionally, new diffraction peaks were detected at 19.89°, 29.03°, and 31.15°, corresponding to the standard diffraction peaks of LaPO4·0.5H2O crystals. It can be concluded that La(OH)3 and Ca(OH)2 in LC-SWBC are transformed into LaPO4·0.5H2O and Ca3(PO4)2 precipitates during the adsorption of phosphorus, effectively fixing phosphate ions from the water and forming stable phosphate precipitates, thereby achieving effective removal of phosphorus from the water body. Surface precipitation is an important mechanism for LC-SWBC in adsorbing phosphates. Further analysis through FTIR shows that, compared to LC-SWBC, the O-H groups in La(OH)3 and Ca(OH)2 at 3608 cm−1 are consumed in LC-SWBC-P. Meanwhile, the area of the absorption peak corresponding to the P-O asymmetric stretching vibration at 1,057 cm−1 significantly increases, and the characteristic peaks of the O-P-O bending vibrations at 615 cm−1 and 541 cm−1 are also significantly enhanced, indicating that during the phosphate adsorption process, hydroxyl groups exchange with phosphate ions, and phosphates bond to the material surface through chemical bonds. The adsorption mechanism of the ligand exchange forming La-O-P bonds is consistent with the mechanism of lanthanum adsorption of phosphorus reported in the literature (Wu et al., 2017), and it also indicates that lanthanum plays a key role in phosphate adsorption. In the experiments concerning the pH factor of the solution, there is a significant increase in the pH of the solution after phosphate adsorption, and under low pH conditions, the amount of phosphate adsorption is more pronounced. This is because the hydroxyl groups on the surface of LC-SWBC become positively charged through protonation, which increases the electrostatic attraction between phosphate in the solution and La3+ and Ca+, enhancing the adsorption of phosphate on the LC-SWBC surface. In summary, combined with the fitting results of various models, the adsorption behavior of LC-SWBC for phosphorus is a spontaneous adsorption process that involves both physical and chemical adsorption, with chemical adsorption being dominant, primarily as monolayer chemical adsorption. The main adsorption mechanisms include surface precipitation, ligand exchange, and electrostatic adsorption.
3.8 Efficacy of LC-SWBC in treating real wastewater
3.8.1 Effect of adsorbent dosage on real wastewater
Table 7 lists the water quality indicators of the actual wastewater in this study. Figure 11a shows the removal rates of phosphates, inorganic phosphorus (IP), chemical oxygen demand (COD), ammonium nitrogen (NH4+-N), and nitrate nitrogen (NO3−-N) in actual wastewater as a function of the amount of LC-SWBC added. The experimental results indicate that LC-SWBC exhibits a certain adsorption effect on the aforementioned pollutants, but there are significant differences in adsorption efficiency for different pollutants.
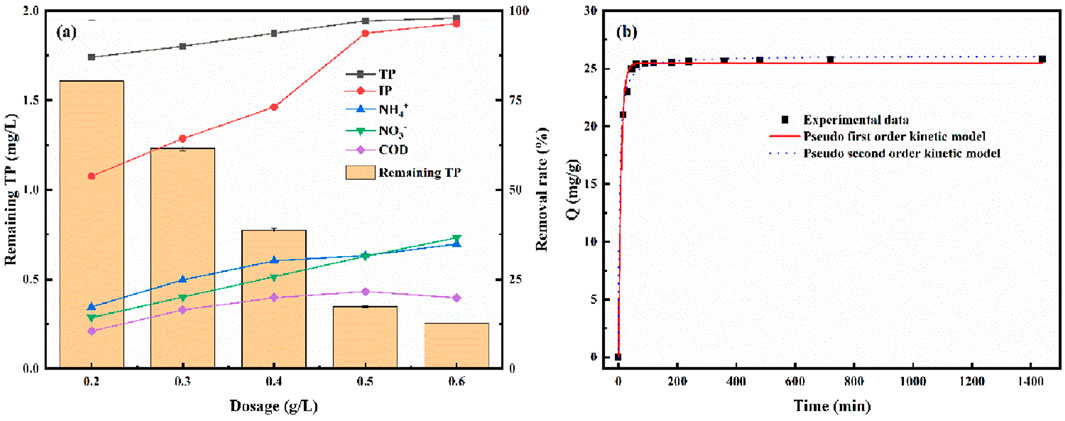
Figure 11. (a) Effect of adsorbent dosage on real wastewater, (b) Adsorption kinetics of real wastewater.
As the amount of LC-SWBC added increases, the removal rate of phosphates in the wastewater significantly improves, which is consistent with the results of previous optimization experiments. When the dosage reaches 0.4 g/L, the phosphate removal rate can reach 95%, with a total phosphorus (TP) effluent concentration of 0.77 mg/L, meet the discharge standard of class I B pollutants (1 mg/L) in GB 18918-2002 of China. Further increasing the LC-SWBC dosage to 0.6 g/L, the phosphate removal rate gradually increases from 87.03% to about 97.95%; the COD removal rate also shows a slight increase, rising from 10.45% to 19.83%; while the adsorption removal rates of NH4+-N and NO3−-N remain stable at around 35%. Overall, LC-SWBC shows the most significant adsorption effect on total phosphorus and inorganic phosphorus, followed by NH4+-N and NO3−-N, with the lowest adsorption efficiency for COD.
3.8.2 Adsorption kinetics of real wastewater
As shown in Figure 11b, the adsorption kinetics characteristics of LC-SWBC for phosphorus in wastewater are consistent with existing literature reports, exhibiting a rapid initial decrease in adsorption rate followed by a gradual slowdown. The fitting results of the kinetic models indicate that the correlation coefficients R2 for the pseudo-first-order and pseudo-second-order kinetic models of LC-SWBC are 0.9622 and 0.9450, respectively, suggesting that both models can adequately describe the adsorption process, but the pseudo-first-order kinetic model more accurately represents the adsorption process. This indicates that the adsorption mechanism of LC-SWBC for phosphates in wastewater is not singular but rather the result of multiple mechanisms acting together, with chemical adsorption still being the dominant factor.
In actual wastewater treatment, the fitted maximum adsorption capacity of LC-SWBC is only 29.62 mg/g, which is significantly lower than its maximum adsorption capacity of 76.96 mg/g in phosphate solutions. The reasons for this significant difference are mainly twofold: on one hand, the phosphorus concentration in actual wastewater is relatively low, and the pH is high, which is unfavorable for the adsorption efficiency and capacity of LC-SWBC; on the other hand, the presence of other pollutants in actual wastewater, such as organic matter, suspended solids, and other anions, may occupy the active sites on the biochar surface and block the pores inside the biochar, leading to a significant decrease in phosphorus adsorption capacity.
3.9 Analysis of phosphorus migration and transformation pathways
3.9.1 Distribution of phosphorus forms in hydrothermal carbonization solid-phase products
Figure 12a shows the composition ratio and unit content of phosphorus forms in the solid phase under different treatment conditions. The phosphorus component analysis of SS indicates that OP accounts for 12.73%, while IP accounts for 88.5%, with a unit content reaching 18.13 mg/g. Notably, the ratio of NAIP to AP is high at 5.5:1, indicating that the bioavailability of phosphorus in SS is relatively low. After hydrothermal carbonization (SBC), the reduction of sludge caused by hydrothermal carbonization leads to an increase in the unit content of TP. The proportion of OP decreases to 2.54%, which is due to the hydrolysis of organic matter in the sludge and the conversion of organic phosphorus to orthophosphate (Huang et al., 2017); the proportion of IP is 96.80%, and the ratio of NAIP to AP decreases to 1.33:1, with an increase in the proportion of AP. This indicates that NAIP is an unstable compound, and under high-temperature conditions, it may promote the conversion of NAIP to AP, although the conversion is not complete (Xu et al., 2018). The SWBC with added wheat straw shows two significant changes: on one hand, the increase in biochar yield generally leads to a decrease in the unit content of various phosphorus forms; on the other hand, the biomass contains rich mineral elements such as Ca, Mg, Si, Al, and Cl, which can promote the conversion of phosphorus during the thermal treatment of sludge (Ren and Li, 2015). The ratio of NAIP to AP further decreases to 0.27:1, representing an 80% reduction compared to the SBC group, significantly improving the bioavailability of phosphorus. The modification treatment has a particularly prominent regulatory effect on phosphorus forms. In LC-SBC, the proportion of OP is 1.22%, while the proportion of IP reaches 99.59%. The ratio of NAIP/AP is reduced to 0.048:1, which is significantly lower compared to SBC and SWBC. The biochemical availability is significantly enhanced. This demonstrates the significant promoting effect of the addition of La and Ca modifiers on the conversion pathways from OP to IP and from NAIP to AP. It reflects the dominant role of Ca and La in phosphorus capture, as they lead to the directional transformation of phosphorus forms by forming stable compounds with phosphorus species. In LC-SWBC, the total phosphorus (TP) is primarily composed of IP, accounting for 97.65% of TP, with a content of 6.65 mg/g, most of which is AP, with a content of 6.52 mg/g, accounting for 95.79% of TP. The ratio of NAIP to AP is the lowest among the various preparation schemes, at 0.004:1. The contents of OP and NAIP are as low as 0.04 and 0.03 mg/g, respectively, indicating strong biochemical properties.
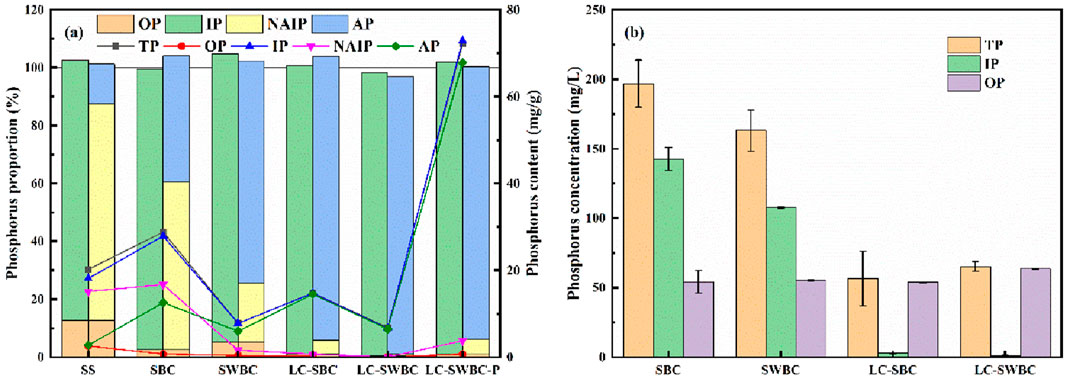
Figure 12. (a) Phosphorus forms in solid-phase products, (b) Phosphorus forms in hydrothermal fluids.
In the LC-SWBC-P material formed after adsorption, the total phosphorus content significantly increased, mainly due to the increase in the AP component, which accounted for 95.79%. This is a phosphorus-containing mineral phase that is available for plant use (Peplinski et al., 2009), and it also confirms the important role of La-Ca in the adsorption process. The ratio of NAIP to AP is 0.056:1, indicating that LC-SWBC-P has strong biochemical availability, which provides good biochemical conditions for the subsequent treatment of biochar and the utilization of phosphorus resources.
3.9.2 The morphological composition of phosphorus in hydrothermal liquids
Figure 12b shows the morphological composition of phosphorus in hydrothermal liquid. The total phosphorus concentration in the hydrothermal liquid of the sludge reached 197 mg/L, of which 142.71 mg/L was inorganic phosphorus (IP). Organic phosphorus (OP) was 54.29 mg/L, accounting for only 27.56% of the total phosphorus. Under hydrothermal conditions with sludge and biomass, the OP content remained relatively unchanged at 55.46 mg/L, but due to a decrease in IP, the proportion of OP in total phosphorus increased slightly to 34.00%. In the hydrothermal liquid produced from the preparation of LC-SBC, the IP content significantly decreased to 2.75 mg/L, with OP becoming the main form of phosphorus present in the hydrothermal liquid at this time. Under the combined influence of biomass and metal modification, the IP content in the hydrothermal liquid further decreased, with only 0.83 mg/L of IP in 65.35 mg/L of total phosphorus (TP). The proportion of OP in TP reached 97.20%.
Overall, the addition of biomass and modified metals leads to a decrease in IP content in the hydrothermal liquid, while changes in OP content are significantly smaller than those in TP and IP. The proportion of OP in TP increases due to the decrease in TP and IP content, with 97.20% of TP in the hydrothermal liquid under LC-SWBC preparation conditions being OP. This is because OP in the hydrothermal liquid is difficult to convert to IP and is also challenging to enrich in the hydrothermal solid phase.
3.9.3 The migration and transformation pathways of phosphorus in the hydrothermal carbonization process
In the hydrothermal process of SWBC, although the addition of wheat straw promotes the conversion of IP in the hydrothermal liquid to the solid phase, the content of IP and total phosphorus TP in the hydrothermal liquid decreases. However, the content of various forms of phosphorus units in the solid phase actually decreases. This is due to the increased yield of biochar caused by the incorporation of wheat straw, which dilutes the phosphorus unit content in the solid phase. The increase in yield has a significantly stronger dilutive effect on the phosphorus content in the solid phase than the positive gain from the migration of liquid phase IP to the solid phase, ultimately leading to a decrease in both IP and TP content in the hydrothermal liquid and solid phase. This change in the interphase balance reveals the key regulatory role of product increment on the phosphorus distribution between the two phases.
In the hydrothermal carbonization process of LC-SBC, due to the significant phosphorus affinity of the La-Ca modifier, most of the IP in the hydrothermal liquid is enriched into the solid phase LC-SBC through chemical interactions. The mechanism involves the formation of stable compounds such as LaPO4·nH2O and Ca3(PO4)2 between the modified metals and phosphates. This chemical adsorption effectively reduces the content of IP and TP in the hydrothermal liquid while significantly increasing the proportion of IP in LC-SBC, achieving selective enrichment of liquid phase IP into the solid phase. Therefore, the modified metals significantly alter the migration and transformation patterns of phosphorus during the hydrothermal carbonization process.
The preparation of LC-SWBC, through the synergistic enhancement of wheat straw doping and metal modification, can effectively transfer and fix phosphorus from hydrothermal liquid into the solid phase product while increasing the carbon yield. This allows for the effective recovery of phosphorus resources in solid form, providing favorable biochemical conditions for the subsequent treatment of biochar and the utilization of phosphorus resources. The adsorbed LC-SWBC-P also has potential for resource utilization. This study provides a theoretical basis and technical pathway for the use of auxiliary phosphorus fertilizers or for soil improvement, which has significant practical value in promoting the resource utilization of sludge and sustainable phosphorus management. A large number of studies have treated hydrothermal liquid as an organic carbon source for water treatment, utilizing biochemical processes. The OP in the hydrothermal liquid is effectively treated during biochemical reactions while serving as an organic carbon source. The OP in the hydrothermal liquid of LC-SWBC is also retained, providing good conditions for biochemical disposal as an organic carbon source. These aspects are significant for achieving resource recovery and harmlessness in the hydrothermal carbonization process. The migration and transformation pathways of phosphorus during the preparation of LC-SWBC through hydrothermal carbonization are shown in Figure 13.
4 Conclusion
This study focuses on sludge as the research object and employs alkaline precipitation and a one-step hydrothermal method to prepare LC-SWBC. Characterization techniques confirm that La and Ca are successfully loaded onto the surface of hydrothermal carbon and effectively adsorb phosphates from water. Mechanistic studies indicate that the adsorption behavior of LC-SWBC towards phosphorus exhibits both physical and chemical adsorption characteristics, with chemical adsorption mechanisms such as surface precipitation, ligand exchange, and electrostatic adsorption being predominant. Langmuir fitting results show that the maximum monolayer adsorption capacity of LC-SWBC for phosphorus reaches 80.78 mg/g. At an addition amount of 0.4 g/L, the phosphorus concentration in urban wastewater can be significantly reduced from 12.39 mg/L to 0.77 mg/L, meet the discharge standard of class I B pollutants (1 mg/L) in GB 18918-2002 of China. Notably, the phosphorus in the hydrothermal liquid produced during the preparation of LC-SWBC is primarily OP, while the main phosphorus composition of the adsorbed LC-SWBC-P is AP, providing favorable process conditions for the subsequent resource utilization and harmless treatment of hydrothermal liquid and biochar. As an adsorption material prepared based on the resource utilization of sludge, LC-SWBC shows promising prospects in terms of adsorption performance and biodegradability. It not only has application value in wastewater treatment but also has development potential as a new type of slow-release phosphorus fertilizer or soil amendment, promoting sustainable development. Additionally, the by-product hydrothermal liquid has potential for resource utilization as a carbon source and other applications.
This study prepared LC-SWBC and revealed its PO43- removal efficiency and mechanism of action, but there is still much work to be done for improvement and further exploration. Firstly, this study produced LC-SWBC with optimal adsorption performance, but did not consider the adsorption performance under the unit cost of the material. Based on the market price of the raw materials used for preparation, the estimated unit cost is about 8 CNY/kg. Future research could further investigate the economic preparation conditions of LC-SWBC, specifically the maximum adsorption performance at the unit cost in actual wastewater, to facilitate its application in industrial production and promotion. Secondly, although this study theoretically analyzed the biological effectiveness of the phosphorus components, the actual efficacy of biochemical utilization still needs to be validated, for example, by conducting seed germination experiments to further confirm it. Additionally, potential risks during long-term use (such as metal release) also need to be assessed to ensure its environmental friendliness.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WG: Supervision, Writing – review and editing. CT: Data curation, Writing – original draft. ZT: Investigation, Writing – review and editing. ZH: Writing – review and editing. HL: Conceptualization, Writing – original draft. CQ: Formal Analysis, Writing – review and editing. ZY: Visualization, Writing – review and editing. LG: Software, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the Natural Science Foundation of Zhongyuan University of Technology (Project No. K2022YY0007).
Conflict of interest
Authors ZT and ZH were employed by Institute of Chemistry Co., Ltd. Henan Academy of Sciences.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
A, M., X, X., G, B., Wang, S., Khan, R., Yue, Q., et al. (2021). Highly efficient removal of phosphate from aqueous media by pomegranate peel co-doping with ferric chloride and lanthanum hydroxide nanoparticles. J. Clean. Prod. 292, 125311. doi:10.1016/j.jclepro.2020.125311
A, O., Z, X., Du, D., Qiao, X., Dai, L., Li, J., et al. (2024). A critical review on advances in remediation of toxic heavy metals contaminated solids by chemical processes. J. Environ. Chem. Eng. 12 (4), 113149. doi:10.1016/j.jece.2024.113149
B, H., P, A. M. A., S, S. C. R., Boaventura, R. A., and Botelho, C. M. (2020). Performance and prospects of different adsorbents for phosphorus uptake and recovery from water. Chem. Eng. J. 381, 122566. doi:10.1016/j.cej.2019.122566
B, C. E. R., and B, M. C. B. (2021). Adsorption of organic and inorganic pollutants onto biochars: challenges, operating conditions, and mechanisms. Bioresour. Technol. Rep. 15, 100728. doi:10.1016/j.biteb.2021.100728
B, I. D., Y, X.-M., Y, H., and Liu, W. (2024). Separation and purification of polyprenols from Ginkgo biloba leaves by silver ion anchored on imidazole-based ionic liquid functionalized mesoporous MCM-41 sorbent. Food Chem. 450, 139284. doi:10.1016/j.foodchem.2024.139284
Chen, N., Feng, C., Zhang, Z., Liu, R., Gao, Y., Li, M., et al. (2012). Preparation and characterization of lanthanum(III) loaded granular ceramic for phosphorus adsorption from aqueous solution. J. Taiwan Inst. Chem. Eng. 43 (5), 783–789. doi:10.1016/j.jtice.2012.04.003
Chen, N., Zhao, T., Li, Z., Yue, X., Li, G., Zhang, J., et al. (2023). Synthesis of ternary Fe-Ca-La oxide composite as highly effective adsorbents to remove phosphate from aqueous solution. Environ. Technol. and Innovation 32, 103326. doi:10.1016/j.eti.2023.103326
Dithmer, L., Nielsen, U. G., LüRLING, M., Spears, B. M., Yasseri, S., Lundberg, D., et al. (2016). Responses in sediment phosphorus and lanthanum concentrations and composition across 10 lakes following applications of lanthanum modified bentonite. Water Res. 97, 101–110. doi:10.1016/j.watres.2016.02.011
Dong, B., Liu, X., Dai, L., and Dai, X. (2013). Changes of heavy metal speciation during high-solid anaerobic digestion of sewage sludge. Bioresour. Technol. 131, 152–158. doi:10.1016/j.biortech.2012.12.112
Elkhlifi, Z., Sellaoui, L., Zhao, M., Ifthikar, J., Jawad, A., Shahib, I. I., et al. (2022). Lanthanum hydroxide engineered sewage sludge biochar for efficient phosphate elimination: echanism interpretation using physical modelling. Sci. Total Environ. 803, 149888. doi:10.1016/j.scitotenv.2021.149888
F, C., Zhang, T., Li, P., Jiang, R., Wu, S., Nie, H., et al. (2015). Phosphorus recovery from biogas fermentation liquid by Ca–Mg loaded biochar. J. Environ. Sci. 29, 106–114. doi:10.1016/j.jes.2014.08.019
Hasanzadeh, R., Moghadam, P. N., Bahri-Laleh, N., and Sillanpää, M. (2017). Effective removal of toxic metal ions from aqueous solutions: 2-Bifunctional magnetic nanocomposite base on novel reactive PGMA-MAn copolymer@Fe(3)O(4) nanoparticles. J. Colloid Interface Sci. 490, 727–746. doi:10.1016/j.jcis.2016.11.098
He, H., Zhang, N., Chen, N., Lei, Z., Shimizu, K., and Zhang, Z. (2019). Efficient phosphate removal from wastewater by MgAl-LDHs modified hydrochar derived from tobacco stalk. Bioresour. Technol. Rep. 8, 100348. doi:10.1016/j.biteb.2019.100348
H, C. G., Liu, H., Dong, W. T., Zhang, Y., Bao, G., Lao, C., et al. (2007). La(OH)3 and La2O3 nanobelts—ynthesis and hysical roperties. Adv. Mater. 19 (3), 470–474. doi:10.1002/adma.200601300
Huang, R., F, C., L, X., Jiang, R., and Tang, Y. (2017). Transformation of hosphorus during (Hydro)thermal reatments of olid biowastes: eaction echanisms and implications for P reclamation and ecycling. Environ. Sci. Technol. 51 (18), 10284–10298. doi:10.1021/acs.est.7b02011
Jia, Z., Zeng, W., X, H., Li, S., and Peng, Y. (2020). Adsorption removal and reuse of phosphate from wastewater using a novel adsorbent of lanthanum-modified platanus biochar. Process Saf. Environ. Prot. 140, 221–232. doi:10.1016/j.psep.2020.05.017
Jung, K. W., Jeong, T. U., Hwang, M. J., Kim, K., and Ahn, K. H. (2015). Phosphate adsorption ability of biochar/Mg-Al assembled nanocomposites prepared by aluminum-electrode based electro-assisted modification method with MgCl(2) as electrolyte. Bioresour. Technol. 198, 603–610. doi:10.1016/j.biortech.2015.09.068
Karadag, D., Koc, Y., Turan, M., and Armagan, B. (2006). Removal of ammonium ion from aqueous solution using natural Turkish clinoptilolite. J. Hazard Mater 136 (3), 604–609. doi:10.1016/j.jhazmat.2005.12.042
Khan, S., Chao, C., Waqas, M., Arp, H. P. H., and Zhu, Y. G. (2013). Sewage ludge iochar nfluence upon ice (oryza sativa L) ield, etal bioaccumulation and greenhouse gas emissions from cidic paddy oil. Environ. Sci. and Technol. 47 (15), 8624–8632. doi:10.1021/es400554x
Krahn, K. M., Cornelissen, G., Castro, G., Arp, H. P. H., Asimakopoulos, A. G., Wolf, R., et al. (2023). Sewage sludge biochars as effective PFAS-sorbents. J. Hazard. Mater. 445, 130449. doi:10.1016/j.jhazmat.2022.130449
Lee, S. Y., Choi, J.-W., Song, K. G., Jeong, D. W., Lee, J. G., Yi, Y. H., et al. (2019). The association between obesity phenotypes and arly renal unction ecline in adults without hypertension, dyslipidemia, and diabetes. Compos. Part B Eng. 40, 176–181. doi:10.4082/kjfm.18.0139
Leng, L., Yuan, X., Huang, H., Shao, J., Wang, H., Chen, X., et al. (2015). Bio-char derived from sewage sludge by liquefaction: haracterization and application for dye adsorption. Appl. Surf. Sci. 346, 223–231. doi:10.1016/j.apsusc.2015.04.014
Li, J., H, X., Shen, Z., Wu, Y., and van Loosdrecht, M. C. (2023). Low-temperature drying of waste activated sludge enhanced by agricultural biomass towards self-supporting incineration. Sci. Total Environ. 888, 164200. doi:10.1016/j.scitotenv.2023.164200
Liu, Z., Liu, H., Zhang, Y., and Lichtfouse, E. (2023). Efficient phosphate recycling by adsorption on alkaline sludge biochar. Environ. Chem. Lett. 21 (1), 21–30. doi:10.1007/s10311-022-01527-5
Overcash, M., Sims, R. C., Sims, J. L., and Nieman, J. K. C. (2005). Beneficial euse and sustainability: he ate of rganic ompounds in land-pplied aste. J. Environ. Qual. 34 (1), 29–41. doi:10.2134/jeq2005.0029
Peng, B., Liu, Q., Li, X., Zhou, Z., Wu, C., and Zhang, H. (2022). Co-pyrolysis of industrial sludge and rice straw: ynergistic effects of biomass on reaction characteristics, biochar properties and heavy metals solidification. Fuel Process. Technol. 230, 107211. doi:10.1016/j.fuproc.2022.107211
Peng, G., Jiang, S., W, Y., Zhang, Q., Cao, Y., Sun, Y., et al. (2020). Synthesis of Mn/Al double oxygen biochar from dewatered sludge for enhancing phosphate removal. J. Clean. Prod. 251, 119725. doi:10.1016/j.jclepro.2019.119725
Peplinski, B., Adam, C., Michaelis, M., Kley, G., Emmerling, F., and Simon, F. G. (2009). Reaction sequences in the thermochemical treatment of sewage sludge ashes revealed by X-ray powder diffraction A contribution to the European project SUSAN. Z. für Kristallogr. Suppl. 2009 (30), 459–464. doi:10.1524/zksu.2009.0068
Prabhakar, A. K., Krishnan, P., Lee, S. S.-C., Lim, C. S., Dixit, A., Mohan, B. C., et al. (2022). Sewage sludge ash-based mortar as construction material: echanical studies, macrofouling, and marine toxicity. Sci. Total Environ. 824, 153768. doi:10.1016/j.scitotenv.2022.153768
Qiu, H., Liang, C., Y, J., Zhang, Q., Song, M., and Chen, F. (2017). Preferable phosphate sequestration by nano-La(III) (hydr)oxides modified wheat straw with excellent properties in regeneration. Chem. Eng. J. 315, 345–354. doi:10.1016/j.cej.2017.01.043
Regkouzas, P., Sygellou, L., and Diamadopoulos, E. (2023). Production and characterization of graphene oxide-engineered biochars and application for organic micro-pollutant adsorption from aqueous solutions. Environ. Sci. Pollut. Res. 30 (37), 87810–87829. doi:10.1007/s11356-023-28549-y
Ren, Q., and Li, L. (2015). Co-ombustion of gricultural traw with unicipal ewage ludge in a fluidized ed: ole of hosphorus in Potassium ehavior. Energy and Fuels 29 (7), 4321–4327. doi:10.1021/acs.energyfuels.5b00790
Ren, Z., Shao, L., and Zhang, G. (2012). Adsorption of hosphate from queous olution sing an iron–zirconium inary xide orbent. Water, Air, and Soil Pollut. 223 (7), 4221–4231. doi:10.1007/s11270-012-1186-5
Ruban, V., Lopez-Sanchez, J. F., Pardo, P., Rauret, G., Muntau, H., and Quevauviller, P. (2001). Harmonized protocol and certified reference material for the determination of extractable contents of phosphorus in freshwater sediments--a synthesis of recent works. Fresenius J. Anal. Chem. 370 (2-3), 224–228. doi:10.1007/s002160100753
Srivastava, A. N., and Chakma, S. (2022). Bioavailability reduction of heavy metals through dual mode anaerobic Co-landfilling of municipal solid waste and industrial organic sludge. Chem. Eng. J. 439, 135725. doi:10.1016/j.cej.2022.135725
Sun, F., Chen, Y., He, L., Tang, J., and Li, Y. (2023). Comparative study of sediment phosphorus immobilization via the addition of lanthanum-modified and thermal-modified drinking water treatment sludge. Environ. Sci. Pollut. Res. Int. 30 (30), 76227–76245. doi:10.1007/s11356-023-27960-9
Ugural, M. N., Aghili, S., and Burgan, H. I. (2024). Adoption of ean onstruction and AI/IoT technologies in Iran’s ublic onstruction sector: ixed-ethods approach sing fuzzy logic. Buildings 14 (10), 3317. doi:10.3390/buildings14103317
Vikrant, K., Kim, K.-H., Ok, Y. S., Tsang, D. C., Tsang, Y. F., Giri, B. S., et al. (2018). Engineered/designer biochar for the removal of phosphate in water and wastewater. Sci. Total Environ. 616-617, 1242–1260. doi:10.1016/j.scitotenv.2017.10.193
W, Y., Liu, G.-H., Yuan, J., Li, Q., Qi, L., and Wang, H. (2023). Performance and mechanism of phosphorus adsorption removal from wastewater by a Ce-Zr-Al composite adsorbent. Environ. Sci. Pollut. Res. 30 (32), 79258–79268. doi:10.1007/s11356-023-27894-2
Wan, S., W, S., Li, Y., and Gao, B. (2017). Functionalizing biochar with Mg–Al and Mg–Fe layered double hydroxides for removal of phosphate from aqueous solutions. J. Industrial Eng. Chem. 47, 246–253. doi:10.1016/j.jiec.2016.11.039
W, B., F, L., Fortner, J. D., Guan, X., and Lo, I. M. (2017). Highly efficient and selective phosphate removal from wastewater by magnetically recoverable La(OH)(3)/Fe(3)O(4) nanocomposites. Water Res. 126, 179–188. doi:10.1016/j.watres.2017.09.034
X, X., C, X., Zhao, L., and Sun, T. (2014). Comparison of sewage sludge- and pig manure-derived biochars for hydrogen sulfide removal. Chemosphere 111, 296–303. doi:10.1016/j.chemosphere.2014.04.014
X, Y., Y, F., Zhang, L., Wang, X., Sun, Y., Liu, Q., et al. (2018). Migration and transformation of phosphorus in municipal sludge by the hydrothermal treatment and its directional adjustment. Waste Manag. 81, 196–201. doi:10.1016/j.wasman.2018.10.011
Y, F., Chen, J., Y, M., Wang, X., Sun, Y., Xu, Y., et al. (2019). Phosphorus recovery from sewage sludge via incineration with chlorine-based additives. Waste Manag. 95, 644–651. doi:10.1016/j.wasman.2019.06.029
Y, Y., G, B., Inyang, M., Zimmerman, A. R., Cao, X., Pullammanappallil, P., et al. (2011). Removal of phosphate from aqueous solution by biochar derived from anaerobically digested sugar beet tailings. J. Hazard Mater 190 (1-3), 501–507. doi:10.1016/j.jhazmat.2011.03.083
Yin, Q., Liu, M., and Ren, H. (2019). Biochar produced from the co-pyrolysis of sewage sludge and walnut shell for ammonium and phosphate adsorption from water. J. Environ. Manag. 249, 109410. doi:10.1016/j.jenvman.2019.109410
Yuan, S.-J., and Dai, X.-H. (2016). An efficient sewage sludge-derived bi-functional electrocatalyst for oxygen reduction and evolution reaction. Green Chem. 18 (14), 4004–4011. doi:10.1039/c5gc02729b
Yuan, J., Zhu, Y., W, J., Gan, L., He, M., Zhang, T., et al. (2021). Preparation and application of Mg–Al composite oxide/coconut shell carbon fiber for effective removal of phosphorus from domestic sewage. Food Bioprod. Process. 126, 293–304. doi:10.1016/j.fbp.2021.01.004
Y, J., Xiang, C., Zhang, G., Wang, H., Ji, Q., and Qu, J. (2019). Activation of lattice xygen in LaFe (Oxy)hydroxides for fficient hosphorus emoval. Environ. Sci. and Technol. 53 (15), 9073–9080. doi:10.1021/acs.est.9b01939
Yu, Q., Zheng, Y., Wang, Y., Shen, L., Wang, H., Zheng, Y., et al. (2015). Highly selective adsorption of phosphate by pyromellitic acid intercalated ZnAl-LDHs: assembling hydrogen bond acceptor sites. Chem. Eng. J. 260, 809–817. doi:10.1016/j.cej.2014.09.059
Zhao, Y., Ren, Q., and Na, Y. (2018). Phosphorus ransformation from unicipal ewage ludge ncineration with iomass: ormation of patite hosphorus with igh ioavailability. Energy and Fuels 32 (10), 10951–10955. doi:10.1021/acs.energyfuels.8b01915
Z, X., A, O., Zhou, Y., Du, D., Li, G., and Zhu, W. (2024a). Heavy metals detection and removal from contaminated water: critical review of adsorption methods. J. Environ. Chem. Eng. 12 (6), 114366. doi:10.1016/j.jece.2024.114366
Z, X., L, H., Du, D., Alam, O., Cheng, Z., et al. (2024b). Remediation of heavy metals polluted soil environment: critical review on biological approaches. Ecotoxicol. Environ. Saf. 284, 116883. doi:10.1016/j.ecoenv.2024.116883
Zhong, S., Dai, L., X, H., Yang, X. C., Zheng, X., and Wang, S. (2024a). Highly porous carbon with selective transformation of nitrogen groups boosts the capacitive performance through boric acid template assisted strategy. J. Energy Storage 97, 112867. doi:10.1016/j.est.2024.112867
Zhong, S., Xu, H., Zheng, X., Li, G., and Wang, S. (2024b). High-value conversion of invasive plant into nitrogen-doped porous carbons for high-performance supercapacitors. J. Anal. Appl. Pyrolysis 183, 106814. doi:10.1016/j.jaap.2024.106814
Keywords: hydrothermal carbonization, sewage sludge, phosphorus adsorption, biochar, wheat straw
Citation: Gong W, Tao C, Tian Z, Huang Z, Lin H, Qi C, Yu Z and Guo L (2025) Characterization and mechanism of phosphorus adsorption from wastewater by lanthanum calcium doped sludge/wheat straw biochar. Front. Environ. Sci. 13:1604542. doi: 10.3389/fenvs.2025.1604542
Received: 02 April 2025; Accepted: 28 May 2025;
Published: 12 June 2025.
Edited by:
Shuai Wang, Southwest University, ChinaCopyright © 2025 Gong, Tao, Tian, Huang, Lin, Qi, Yu and Guo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weijin Gong, OTc2Nzc1NEBxcS5jb20=
 Weijin Gong
Weijin Gong Chenhan Tao1
Chenhan Tao1