- 1Department of Life and Environmental Sciences Polytechnic University of Marche, Ancona, Marche, Italy
- 2National Biodiversity Future Centre, Palermo, Italy
Cladocora caespitosa is the only reef-building zooxanthellate coral in the Mediterranean Sea. It forms beds of colonies, sporadically aggregating in extensive reef-like structures. The carbonate deposition of C. caespitosa enhances habitat complexity, supports biodiversity, and contributes to carbon sequestration. Anthropogenic pressures have increasingly threatened this species. Due to its decline, the International Union for the Conservation of Nature (IUCN) included C. caespitosa in the endangered species list. Here we exploited the available knowledge on tropical coral reefs to develop a protocol for the propagation of this Mediterranean species. In this preliminary study, we exploited naturally occurring dislodged fragments as donor material and successfully implemented the micro-fragmentation and nursery-rearing techniques on this species, looking forward to future restoration interventions. We reported that micro-fragmented C. caespitosa has high survival and asexual reproduction rates surpassing those of naturally occurring juvenile colonies. We also report that bleached C. caespitosa fragments were able to survive and grow, although at a much lower rate than their unbleached counterparts and recover with the re-establishment of lower temperatures. One year after the start of micro-fragmentation experiment, a survival rate of 89.8% was observed, with all fragments encrusting on their artificial substrates.
1 Introduction
Cladocora caespitosa is the sole zooxanthellate, constructional coral that can form structures comparable to tropical reefs in the Mediterranean Sea. According to the fossil record, this species was able to build extensive reefs during the Pliocene (Aguirre et al., 1998), whereas today it typically forms beds of interspersed colonies that sporadically aggregate into extensive reef-like structures (Peirano et al., 2004; Azzola et al., 2022; Kersting et al., 2023).
Cladocora caespitosa typically colonizes hard bottoms at depths ranging from 5 to 35 m (Kersting et al., 2023) and exhibits diverse morphologies in response to local conditions (Kersting and Linares, 2012; Kersting et al., 2023). Furthermore, this species demonstrates notable trophic plasticity, shifting across a gradient from autotrophic to heterotrophic metabolism in response to changing environmental conditions (Hoogenboom et al., 2010; Ferrier-Pagés et al., 2011).
On occasion, C. caespitosa has been found as free-living nodules, exhibiting a distinctive spherical growth pattern (Kersting et al., 2017b; Chimienti et al., 2025 Fare clic o toccare qui per immettere il testo). This phenomenon is attributed to a periodic wave-induced turnover of loose individuals occurring within pebbles and coarse bottoms (Kersting et al., 2017b; Kersting et al., 2017a). With calcification rates exceeding 1.7 kg CaCO3 m2 year−1 (Peirano et al., 2001), C. caespitosa is a relevant bioconstructor in the Mediterranean Sea, increases habitat complexity and promotes biodiversity (Pitacco et al., 2014; Pitacco et al., 2019).
C. caespitosa is facing an increasing threat from anthropogenic pressures (Kružić and Požar-Domac, 2007; Kersting et al., 2014a; Ganias et al., 2023). Over the past 2 decades, there has been a growing body of evidence documenting mass mortality events associated with heatwaves (Kersting et al., 2013; Jiménez et al., 2016; Antoniadou et al., 2023). Since C. caespitosa is a long-lived, slowly growing, and low-dispersal species (Kružić and Benković, 2008; Kersting et al., 2013; López-Márquez et al., 2020), the natural resilience of this species is likely insufficient to cope with the current loss rates (Kersting et al., 2014b; Kersting and Linares, 2019). Accordingly, the International Union for the Conservation of Nature (IUCN) has classified C. caespitosa as an endangered species (Kersting et al., 2023).
One potential solution to offset the current decline of this and many other species and habitats is to promote active restoration (Danovaro et al., 2025). Previous attempts to restore C. caespitosa have been limited and have focused on direct translocation (Roveta et al., 2023). However, this approach implies a relevant impact on naturally occurring colonies, making it not optimally suited for large-scale intervention. Given the high similarity with tropical reef-building corals (Kružić and Benković, 2008), it may be possible to design restoration protocols for this Mediterranean species by profiting from the experience acquired from tropical reef restoration.
Cultivation approaches have been widely and successfully applied in active reef restoration, even sustaining large-scale projects with minimal to no impact on “wild” corals (Rinkevich, 2019; Rinkevich, 2021). These methods entail the generation of a coral stock through sexual or asexual reproduction of donor material (i.e., recruits, fragments or corals of opportunity), followed by transplantation on the natural substrate (Rinkevich, 2021). As recruits and small coral fragments are highly vulnerable (Rinkevich, 2005; Forsman et al., 2006; Kersting et al., 2014b), which may hinder their restoration, coral gardening requires cultivation in a controlled environment (e.g., in situ nurseries) (Forsman et al., 2006).
Coral nurseries are typically categorized into two major classifications: mid-water suspended structures and leg-fixed structures (Konh and Parry, 2019; Boström-Einarsson et al., 2020; Berman et al., 2023). Despite both designs have exhibited similar survival rates, they are optimally suited to different environmental settings (Shaish et al., 2008). Suspended nurseries are straightforward and cost-effective rearing tools, but are more suitable for sheltered areas, such as lagoons. These structures rely on disposable materials, including metal wire, plastic tubes and ropes, which are inherently susceptible to deterioration (Boström-Einarsson et al., 2020). Conversely, leg-fixed designs can be more expensive, but they can be more durable, depending on the materials selected (Shaish et al., 2008; Boström-Einarsson et al., 2020). Consequently, they are better suited for more exposed and lower maintenance scenarios.
During the cultivation phase, the micro-fragmentation technique (cutting corals into fragments of a few polyps) is used as a tool to increase stocks, relying on the high regenerative capacity of corals and their tendency to restore colony integrity through rapid asexual reproduction (Zakai et al., 2024; Forsman et al., 2015). Micro-fragmentation has been successfully implemented to propagate slow-growing massive corals (Page et al., 2018) and to generate large numbers of new colonies from limited donors (Lirman et al., 2014; Schopmeyer et al., 2017).
In this study, the micro-fragmentation of ‘corals of opportunity’ was employed for the first time for the cultivation of C. caespitosa. This technique, coupled with nursery rearing, was used to develop a protocol for future restoration interventions.
2 Materials and methods
2.1 Site selection and sample collection
Fragments of C. caespitosa were collected from two sites in the Adriatic Sea, which were characterized by the presence of loose fragments and nodules (Conero Riviera in the central Adriatic and Tremiti Island in the southern Adriatic) (Figure 1). Samples were collected from the Conero Riviera and the Tremiti Islands, whose populations were representative of encrusting and phaceloid growth, respectively. All specimens were collected by SCUBA diving at a depth of 7–8 m in June 2023 and transported to laboratory aquariums in thermally insulated containers. A nursery area was selected along the Conero Riviera (Sirolo: 43.5139973 N; 13.6265533 E; Figure 1b), which was easily accessible to SCUBA operators, thus facilitating monitoring and maintenance efforts. A permanent installation site was selected along the Conero Riviera, Portonovo (43.5595279 N; 13.6093117 E) (Figure 1b), near the Conero Riviera donor site.
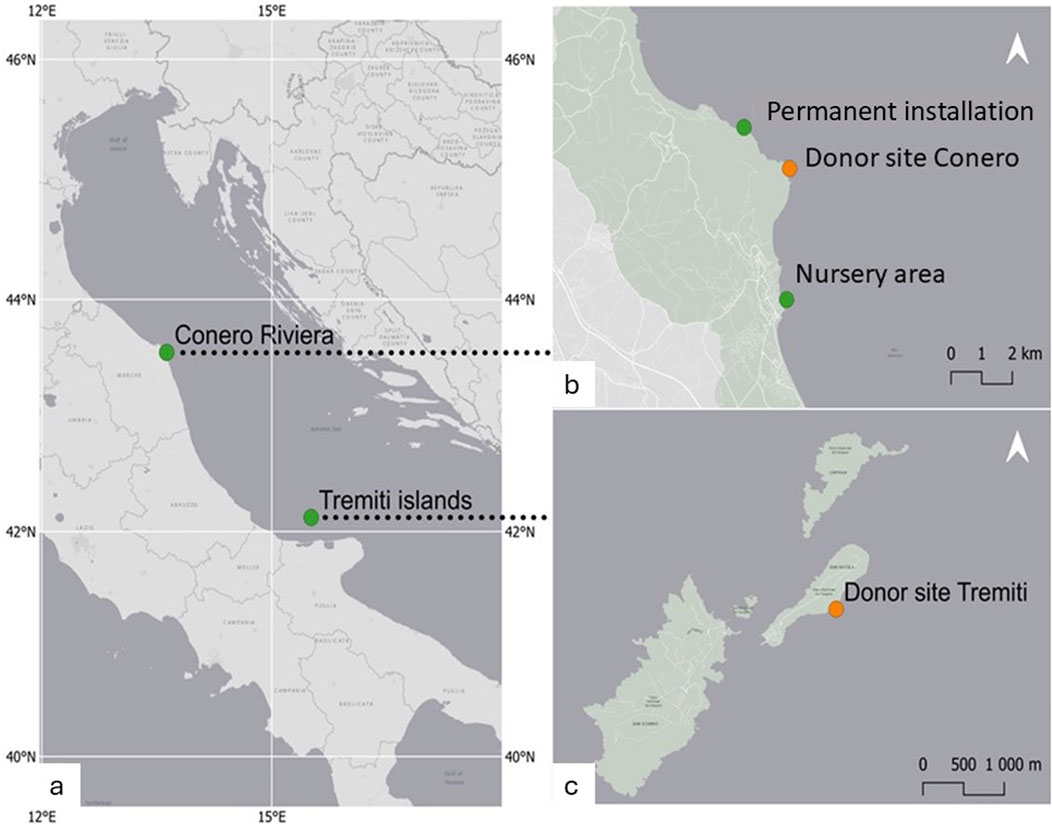
Figure 1. Sampling area and location of the restoration sites. Reported are: (a) Selected areas for the collection of donor material. Sea. (b) Conero Riviera: Donor site (43.5486466 N; 13.6277424 E), the Nursery area, Sirolo (43.5139973 N; 13.6265533 E) and the permanent installation site, Portonovo (43.5595279 N; 13.6093117 E); (c) Donor site at Tremiti islands (42.1224919 N; 15.5128281 E).
2.2 Micro-fragmentation approach
Following a preliminary acclimatization period of 1 week, C. caespitosa was meticulously divided using a dremel to obtain fragments comprising 1–20 polyps. Phaceloid specimens were cut on the exposed skeleton between corallites to avoid damaging the limited living tissue. In the case of encrusting specimens, the cutting was performed directly on the coenosarc, avoiding damage to the polyps. Consequently, the number and size of micro-fragments produced were not fully predictable.
The obtained fragments were divided into size classes according to the number of polyps: Class 1 (1–2 polyps; mean surface 0,3 cm2; n = 13), Class 2 (3–4 polyps; mean surface 0,5 cm2; n = 10), Class (5–9 polyps; mean surface 1,0 cm2 n = 13); Class 4 (10–15 polyps; mean surface 2,5 cm2; n = 8) and Class 5 (15–20 polyps; mean surface 4,0 cm2 n = 6). Micro-fragments were mounted onto ceramic substrates using cyanoacrylate and two-part epoxy. Different substrate sizes (2 cm and 7 cm in diameter) were used, depending on the size of the fragments, to minimize the exposed surface area, thereby reducing the risk of fouling and sedimentation. All corals were treated with an antibiotic coral dip (Continuum Aquatics™) and kept in aquaria for 1 week to allow for proper healing.
2.3 Experimental design and in situ rearing
Obtained fragments were assigned to one of two fragment arrays based on their original morphology (and location), namely, ‘Phaceloid’ (n = 13) and ‘Encrusting’ (n = 20). A subset of the donor material used to generate the ‘Phaceloid’ array was kept as unfragmented specimens in aquaria.
Two weeks after in situ collection, two arrays of micro-fragments, were translocated on the nursery (Sirolo; 43,5139973 N; 13,6265533 E). The leg-fixed nursery module (Figure 2a), consisting of a ballasted aluminum frame (60 cm × 60 cm x 40 cm h) holding a metal net, was previously deployed at a 6.5 m-depth on a soft bottom to facilitate operations.
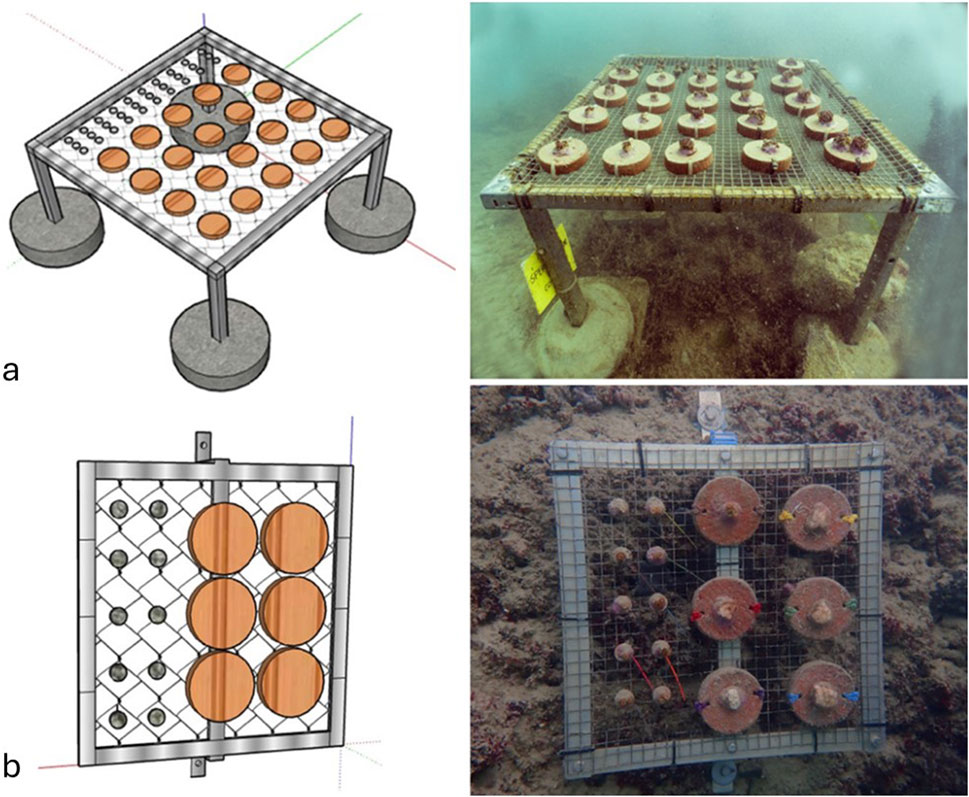
Figure 2. Artificial structures used for the cultivation of C. caespitosa. Reported are: (a) leg-fixed nursery module housing ceramic substrate (20 fragments of the ‘encrusting’ array and 29 fragments of the ‘phaceloid’ and ‘phaceloid II’ array), fixed to the metal (stainless steel) net; and (b) Rock-mounted cultivation modules used for long-term rearing, with a detail of the ceramic substrates (6 fragments of the ‘encrusting’ array and 10 fragments of the ‘phaceloid’ and ‘phaceloid II’ array) fixed to the metal net. Due to the vulnerability of small coral fragments, all structures were designed to be elevated from the natural substrate, limiting the effects of sedimentation, fouling, and competition with other benthic organisms.
During the nursery phase, the module was monitored and maintained at 2-week intervals. Maintenance procedures included resuspension of debris and removal of fouling. HD images were collected monthly from July 2023 (T0) to November 2023 (T4) using an Olympus TG6 camera to assess survival, new polyp formation, growth and any specific information (i.e., tissue necrosis, bleaching, predation, fouling). Temperatures were measured in situ by SCUBA operators.
A third array of micro-fragments, labeled as “Phaceloid II” (n = 16), was generated from the same donors as the “Phaceloid I” array. It was introduced at T2 (September 2023) to establish whether the high budding frequencies initially observed in the “Phaceloid I” array were due to high summer temperatures or a response to the micro-fragmentation. Four months after the fragmentation (T4), all fragments were relocated to the designated permanent installation site on a rocky substrate to protect the array from the winter meteorological-conditions (Portonovo; 3.5595279° N; 13.6093117° E). Specimens were collected from the nursery, placed in thermally insulated containers, transported by boat to the permanent installation site, and affixed to three rock-mounted cultivation modules. Each cultivation module (Figure 2b), comprised an aluminum and net frame (35 cm × 35 cm) (Figure 2b), was affixed to a rock formation at a depth of 6.5 m. After a year following the fragmentation (July 2024) assay, the permanent installations were monitored to assess the long-term survival of the fragments.
2.4 Survival, asexual reproduction and surface increase
The identification of dead fragments was based on the total loss of soft tissue and epiphyte overgrowth. Asexual reproduction, which occurs through the formation of new corallites (extra-tentacular budding), was tracked by in situ counting by SCUBA operators and verified through examination of the photographic material. Budding frequencies were then normalized to each fragment’s initial number of corallites.
Given that changes in surface area were anticipated to occur primarily across the orthogonal plane through bud formation and elongation, the fragment’s surface was determined from orthogonal photographs using the ImageJ™ software.
The degree of bleaching was carefully assessed on 3 coral fragments randomly collected from both the “Phaceloid” and the “Encrusting” arrays, using the Mean Intensity Grey (MIG) metrics. The colour of the images was converted to grayscale and measured using ImageJ™ software, as described in Mclachlan and Grottoli (2021). An elevated MIG value denotes a lighter colour (higher degree of bleaching), while a lower value indicates a healthier condition. All data are expressed as means ± standard deviation.
2.5 Statistical analyses
A log-rank (Mantel-Cox) test was conducted to ascertain whether there were significant differences in survival between fragment arrays and between size classes. The normality of the data distribution was checked using a Shapiro-Wilk test. To investigate differences in growth rates within size classes and time points for each fragment array, non-parametric tests (Kruskal–Wallis test and Dunn’s multiple comparisons test) were employed. All statistical analyses were conducted using GraphPad Prism 8 software.
3 Results
3.1 Survival and translocation outcomes
Following the acclimation period, at the start of the experiment (T0), all fragments displayed full polyps’ extension and no evidence of tissue loss. In the following weeks, a sudden increase in sea temperature (resulting in a rise to 28 °C) led to a bleaching event affecting all C. caespitosa fragments (100%) originating from the Conero Riviera (array: “Encrusting”). In contrast, the fragments from Tremiti Islands (array: “Phaceloid”) did not exhibit any discernible indications of bleaching (Figure 3). At T3, 80% of the bleached fragments survived (Figure 3) (Supplementary Table S3) and showed signs of recovery (Figure 4b).
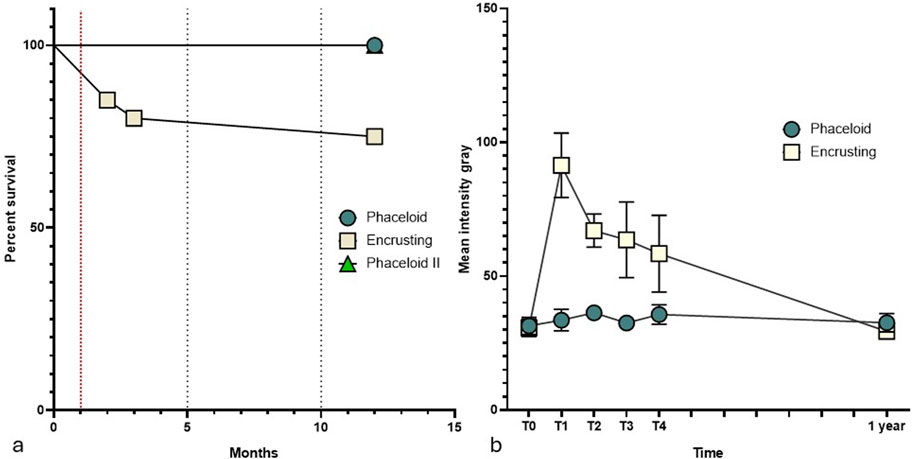
Figure 3. (a) Micro-fragments’ survival during in situ cultivation. The red line indicates the bleaching event reported in August 2023 (T1); (b) Mean Intensity Grey (MIG) trend over the observation period.
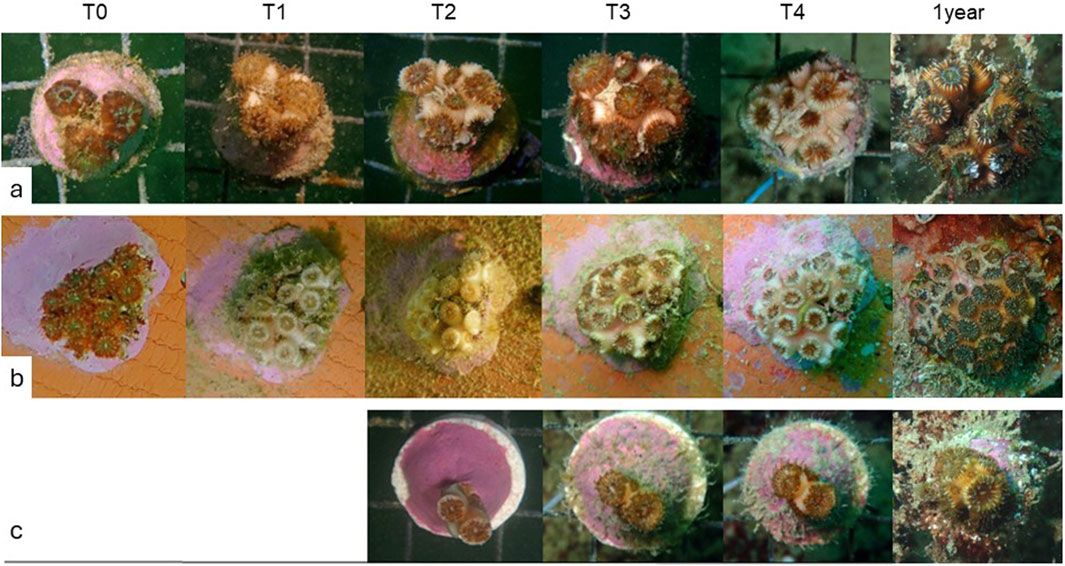
Figure 4. Time-series illustrating the growth of C. caespitosa micro-fragments from Row (a) ‘Phaceloid’, Row (b) ‘Encrusting’, and Row (c) ‘Phaceloid II’ colonies over the whole experimentation period. Note: images were collected after stimulating the polyps to retract for proper examination.
The Mean Intensity Grey (MIG) of fragments from the “encrusting” array exhibited a range from 30.62 ± 3.16 at (T0) to 91.39 ± 12.04 at (T1), the point at which bleaching was first observed, and then underwent a gradual decrease over the subsequent months. At 1 year following the fragmentation assay, the MIG had returned to normal values of 29.36 ± 1.03. Conversely, the MIG of fragments from the phaceloid array remained at a mean value of 33.66 ± 1.55.
At T4, all fragments were transferred to the rock-mounted cultivation modules, survived, and no tissue loss was observed. One year following the fragmentation trial, 89.8% of the initial fragments were alive and actively encrusting on their artificial substrates, including the “Phaceloid” array, which had converted to an encrusting growth pattern. The only fragment that was lost was dislodged from its substrate and subsequently misplaced.
3.2 Budding frequencies
In fragments of C. caespitosa that had not undergone bleaching, rapid bud formation was observed immediately following translocation on the nursery (T1), with new buds originating from the exosarc of existing corallites (see Supplementary Table S1) Figure 4a.
At the end of the assay, the budding frequencies of smaller-sized micro-fragments (class 1, 1–2 polyps) were found to be significantly higher than those of the larger-sized class (class 3, 5–9 polyps) (Dunn’s multiple comparisons test; p = 0.0074) (Figure 5a). Significant variations were observed in budding frequencies between time points (Kruskal–Wallis test; p < 0.0001) (Figure 5b). After the initial month (T1), an average of 0.9 ± 0.4 buds polyp−1 month-1 was observed across the entirety of the “Phaceloid” array. This gradually declined towards T4, reaching an average of 0.1 ± 0.08 buds polyp−1 month-1. The “Phaceloid II” array, deployed in September (T2), exhibited a budding frequency of 0.7 ± 0.4 buds polyp−1 month-1 at T3 and 0.4 ± 0.4 buds polyp−1 month-1 at T4 Figure 4c.
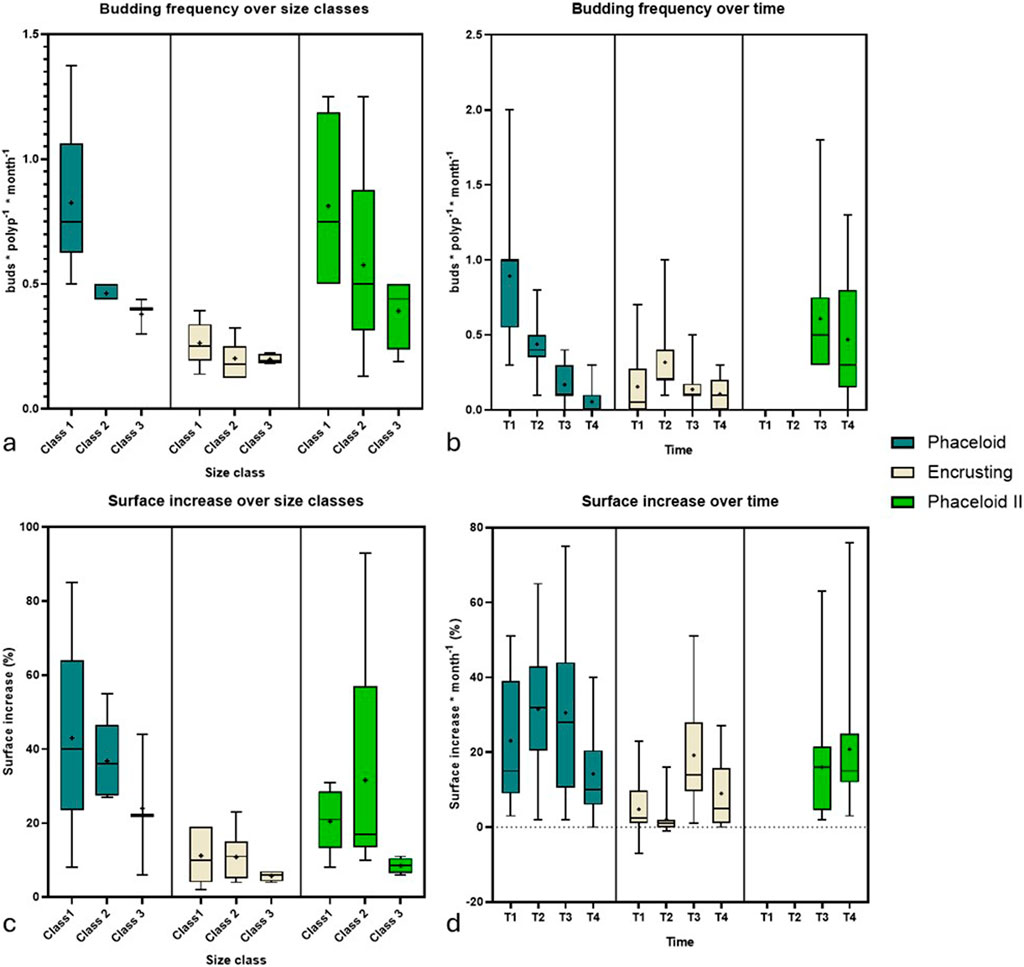
Figure 5. Micro-fragments’ growth from ‘Phaceloid’, ‘Encrusting’ and ‘Phaceloid II’ during the experimentation. Reported are: (a) Budding frequencies across size classes and (b) Temporal changes in budding frequencies. (c) Surface percentage increase across size classes; (d) Temporal changes in percentage surface increase. Please note that for array ‘Phaceloid II’ the deployment in T2 allowed the measurement of the growth rates only in T3 and T4 (as equivalent of months 1 and 2 after fragmentation).
The budding frequencies of bleached fragments were found to be significantly lower in comparison to non-bleached fragments of the “Phaceloid” array (Dunn’s multiple comparisons test; p = 0.0095) and the “Phaceloid II” array (Dunn’s multiple comparisons test; p = 0.0037). No significant differences were identified across size classes (Kruskal–Wallis test; p = 0.3183). However, significant differences were observed between time points (Kruskal–Wallis test; p < 0.0062) (Figures 5 a,b). At T1, 60% of the bleached fragments exhibited evidence of bud formation, with an average budding frequency of 0.2 ± 0.2 buds polyp−1 month-1. At T2, surviving fragments exhibited a peak in bud formation, with an average of 0.3 ± 0.2 buds polyp−1 month-1. In contrast, both T3 and T4 showed a significantly lower budding frequency, with an average of 0.1 ± 0.1 buds polyp−1 month-1.
3.3 Surface increase
At T4, the overall surface area covered by the array “Phaceloid I” exhibited an increase of 7.9 cm2, representing a 136% expansion of the initial surface area (Supplementary Table S2). No significant differences were observed between size classes (Kruskal–Wallis test; p < 0.5143) or time points (Kruskal–Wallis test; p < 0.0768). The total surface area of the array “Phaceloid II” exhibited an increase of 2.6 cm2, representing a 30% expansion relative to the initial surface area (Figures 5c,d) (Supplementary Table S2). No actual surface increase was observed for the whole “Encrusting” fragment array. However, the loss of 20% of the fragments was compensated (+0.5 cm2; +0.01%). The surface increase demonstrated significant differences among time points (Kruskal–Wallis test; p < 0.0001) with a peak in T3.
4 Discussion
The present study was designed to implement an in situ cultivational approach for the first time for the propagation of the threatened Mediterranean coral C. caespitosa. To avoid any significant impact on natural colonies, the collection of “corals of opportunity” was adopted, a method previously used for tropical (Monty et al., 2006) and for temperate corals (e.g., Astroides calycularis (Musco et al., 2017)). Loose fragments of C. caespitosa were found to be characterized by the coexistence of live, dead and partially eroded corallites, suggesting that most of these corals would otherwise be lost through abrasion and burial.
The leg-fixed nursery module used in this study proved to be an effective platform for manipulating coral fragments and collecting data during the early growth phases. Both the nursery and cultivation modules supported the survival and growth of C. caespitosa micro-fragments. Moreover, the persistence of the rock-mounted cultivation modules through the winter season highlights their potential as long-term, low-maintenance tools for rearing corals, enabling the practice of “coral gardening” within the seasonal variability of the Mediterranean Sea. Previous studies have found no significant differences in the survivorship and development of tropical corals farmed using suspended versus bottom-fixed structures. However, they noted that bottom-fixed nurseries tend to be more durable against hydrodynamic forces and easier to maintain (Shaish et al., 2008).
The modules developed in this study could be applied using a two-phase approach, initial nursery rearing followed by long-term maintenance on cultivation modules, or a direct approach using cultivation modules only. The choice depends on local seafloor topography, sedimentation, fouling, and hydrodynamic conditions. The use of rock-mounted cultivation modules ensures the persistence and rapid propagation of coral stocks, which can be exploited as a source for larval replenishment or collected and transplanted onto natural substrates.
The application of the micro-fragmentation technique did not directly result in the mortality of the specimens that underwent manipulation, irrespective of the phaceloid or encrusting morphologies. The sole factor that threatened the success of the study was the summer heatwave (temperatures up to 28 °C for more than 1 week), which caused bleaching in some individuals. It is noteworthy that this phenomenon was exclusively observed in specimens collected from the Central Adriatic region, with no such effects detected in specimens obtained from the Tremiti Islands. This finding suggests that colonies originating from lower latitudes may possess a higher degree of adaptation to elevated temperatures, which renders them a potentially valuable source for future restoration initiatives (Caruso et al., 2021; Quigley, 2023).
The possibility that the two different growth morphologies are characterized by a different vulnerability to the high summer temperatures cannot not be ruled out and deserves further investigations. At the same time, this technique allows a fast checking for the selection of the populations that are best adapted to face heatwaves without showing signs or bleaching or showing the fastest recovery after a bleaching event. This might prove to be extremely useful to maximize the success of active restoration interventions in the future, especially in areas subjected to increasing frequency and intensity of heatwaves.
Following the seasonal decrease in temperature, survived bleached fragments were successfully recovered. The evidence of growth of the bleached fragments also suggests that C. caespitosa may have the ability to enhance heterotrophy as a means of coping with the deficiency in autotrophic production (Hoogenboom et al., 2010; Ferrier-Pagés et al., 2011).
Micro-fragments exhibited elevated budding frequencies (0.2–1.4 buds polyp−1 month-1), which significantly exceeded the documented values for juvenile colonies (0.33–1.14 buds polyp−1 yr-1) (Kersting et al., 2014b). In addition, the higher budding frequencies observed in smaller micro-fragments provide further support for the hypothesis that micro-fragmentation enhances asexual reproduction in C. caespitosa. A peak in bud production was observed immediately after micro-fragmentation, with buds generating rapidly on all available exosarc (1–2 months). Subsequently, the surface of each new corallite increases, leading to the expansion of the fragment (the following 1–2 months). This, in turn, results in the formation of newly available exosarc for further bud formation, a growth pattern comparable to those reported previously (Rodolfo-Metalpa et al., 2008).
We conclude that the micro-fragmentation technique, combined with nursery rearing, represents a promising approach for the restoration of this endangered species. The method’s minimal requirements for collection, along with its capacity to sustain fast-growing coral stocks, offer a range of restoration possibilities. The approach proposed here requires limited facilities and low costs, making it scalable for broader restoration efforts. Additionally, it enables coral farming for scientific purposes with virtually zero impact on native coral populations.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
PC: Conceptualization, Data curation, Formal Analysis, Investigation, Methodology, Software, Validation, Visualization, Writing – original draft, Writing – review and editing. RD: Conceptualization, Funding acquisition, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was carried out within the framework of the European Union Horizon 2020 projects, REDRESS, and the Next-Generation EU Recovery and Resilience National Plan (PNRR), National Biodiversity Future Centre (Italy)- (NBFC), Spoke 2.
Acknowledgments
The Authors thank Domenico Sacco for the support in SCUBA diving and for contributing to the preparation of the rearing structures; the photographer Pablo Marone for the underwater image support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer (SK) declared a past co-authorship with the author (RD) to the handling editor.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1605099/full#supplementary-material
References
Aguirre, J., Nez, J., and Jime´nezjime´nez, A. P. (1998). Fossil analogues of present-day Cladocora caespitosa coral banks: sedimentary setting, dwelling community, and taphonomy (Late Pliocene, W Mediterranean). Springer-Verlag.
Antoniadou, C., Pantelidou, M., Skoularikou, M., and Chintiroglou, C. C. (2023). Mass mortality of shallow-water temperate corals in marine protected areas of the North Aegean sea (eastern Mediterranean). Hydrobiology 2, 311–325. doi:10.3390/hydrobiology2020020
Azzola, A., Bianchi, C. N., Morri, C., Oprandi, A., Peirano, A., and Montefalcone, M. (2022). Population structure change in a temperate reef coral after a quarter of century. Estuar. Coast Shelf Sci. 270, 107851. doi:10.1016/j.ecss.2022.107851
Berman, O., Levy, N., Parnas, H., Levy, O., and Tarazi, E. (2023). Exploring new frontiers in coral nurseries: leveraging 3D printing technology to benefit coral growth and survival. J. Mar. Sci. Eng. 11, 1695. doi:10.3390/jmse11091695
Boström-Einarsson, L., Babcock, R. C., Bayraktarov, E., Ceccarelli, D., Cook, N., Ferse, S. C. A., et al. (2020). Coral restoration – a systematic review of current methods, successes, failures and future directions. PLoS One 15, e0226631. doi:10.1371/journal.pone.0226631
Caruso, C., Hughes, K., and Drury, C. (2021). Selecting heat-tolerant corals for proactive Reef restoration. Front. Mar. Sci. 8, 632027. doi:10.3389/fmars.2021.632027
Chimienti, G., Tursi, A., Logrieco, A., Notarangelo, S., and Mastrototaro, F. (2025). Corallith bed of the endangered coral Cladocora caespitosa in the South Adriatic Sea. Sci. Rep. 15, 16690. doi:10.1038/s41598-025-01554-6
Danovaro, R., Aronson, J., Boström, S. C., Chen, W., Cimino, R., Corinaldesi, C., et al. (2025). Assessing the success of marine ecosystem restoration using meta-analysis. Nat. Commun. 16, 3062. doi:10.1038/s41467-025-57254-2
Ferrier-Pagés, C., Peirano, A., Abbate, M., Cocito, S., Negri, A., Rottier, C., et al. (2011). Summer autotrophy and winter heterotrophy in the temperate symbiotic coral Cladocora caespitosa. Limnol. Oceanogr. 56, 1429–1438. doi:10.4319/lo.2011.56.4.1429
Forsman, Z. H., Rinkevich, B., and Hunter, C. L. (2006). Investigating fragment size for culturing reef-building corals (Porites lobata and P. compressa) in ex situ nurseries. Aquaculture 261, 89–97. doi:10.1016/j.aquaculture.2006.06.040
Forsman, Z. H., Page, C. A., Toonen, R. J., and Vaughan, D. (2015). Growing coral larger and faster: Micro-colony-fusion as a strategy for accelerating coral cover. PeerJ 3, e1313. doi:10.7717/peerj.1313
Ganias, K., Zafeiriadou, A., Garagouni, M., and Antoniadou, C. (2023). High bycatch rate of the coral Cladocora caespitosa offsets the low discards ratio in Thermaikos Gulf gillnet fishery. Mediterr. Mar. Sci. 24, 203–210. doi:10.12681/mms.31197
Hoogenboom, M., Rodolfo-Metalpa, R., and Ferrier-Pagès, C. (2010). Co-variation between autotrophy and heterotrophy in the Mediterranean coral Cladocora caespitosa. J. Exp. Biol. 213, 2399–2409. doi:10.1242/jeb.040147
Jiménez, C., Hadjioannou, L., Petrou, A., Nikolaidis, A., Evriviadou, M., and Lange, M. A. (2016). Mortality of the scleractinian coral Cladocora caespitosa during a warming event in the Levantine Sea (Cyprus). Reg. Environ. Change 16, 1963–1973. doi:10.1007/s10113-014-0729-2
Kersting, D. K., and Linares, C. (2012). Cladocora caespitosa bioconstructions in the Columbretes Islands Marine Reserve (Spain, NW Mediterranean): distribution, size structure and growth. Mar. Ecol. 33, 427–436. doi:10.1111/j.1439-0485.2011.00508.x
Kersting, D. K., and Linares, C. (2019). Living evidence of a fossil survival strategy raises hope for warming-affected corals. Sci. Adv. 5. doi:10.1126/sciadv.aax2950
Kersting, D. K., Bensoussan, N., and Linares, C. (2013). Long-Term responses of the endemic reef-builder Cladocora caespitosa to mediterranean warming. PLoS One 8, e70820. doi:10.1371/journal.pone.0070820
Kersting, D. K., Ballesteros, E., De Caralt, S., and Linares, C. (2014a). Invasive macrophytes in a marine reserve (Columbretes Islands, NW Mediterranean): spread dynamics and interactions with the endemic scleractinian coral Cladocora caespitosa. Biol. Invasions 16, 1599–1610. doi:10.1007/s10530-013-0594-9
Kersting, D. K., Teixidó, N., and Linares, C. (2014b). Recruitment and mortality of the temperate coral Cladocora caespitosa: implications for the recovery of endangered populations. Coral Reefs 33, 403–407. doi:10.1007/s00338-014-1144-3
Kersting, D. K., Cebrian, E., Verdura, J., and Ballesteros, E. (2017a). A new Cladocora caespitosa population with unique ecological traits. Mediterr. Mar. Sci. 18, 38–42. doi:10.12681/mms.1955
Kersting, D. K., Cebrian, E., Verdura, J., and Ballesteros, E. (2017b). Rolling corals in the Mediterranean Sea. Coral Reefs 36, 245. doi:10.1007/s00338-016-1498-9
Kersting, D. K., Cefalì, M. E., Movilla, J., Vergotti, M. J., and Linares, C. (2023). The endangered coral Cladocora caespitosa in the Menorca Biosphere Reserve: distribution, demographic traits and threats. Ocean. Coast Manag. 240, 106626. doi:10.1016/j.ocecoaman.2023.106626
Konh, B., and Parry, M. (2019). Design, fabrication, installation, and population of a novel fiberglass reinforced plastic coral nursery structure off the south shore of o’ahu, Hawaii. Front. Mar. Sci. 6, 569. doi:10.3389/fmars.2019.00569
Kružić, P., and Benković, L. (2008). Bioconstructional features of the coral Cladocora caespitosa (Anthozoa, Scleractinia) in the Adriatic Sea (Croatia). Mar. Ecol. 29, 125–139. doi:10.1111/j.1439-0485.2008.00220.x
Kružić, P., and Požar-Domac, A. (2007). Impact of tuna farming on the banks of the coral Cladocora caespitosa in the Adriatic Sea. Coral Reefs 26, 665. doi:10.1007/s00338-007-0237-7
Lirman, D., Schopmeyer, S., Galvan, V., Drury, C., Baker, A. C., and Baums, I. B. (2014). Growth dynamics of the threatened caribbean staghorn coral Acropora cervicornis: influence of host genotype, symbiont identity, colony size, and environmental setting. PLoS One 9, e107253. doi:10.1371/journal.pone.0107253
López-Márquez, V., Lozano-Martín, C., Hadjioannou, L., Acevedo, I., Templado, J., Carlos Jimenez, K., et al. (2020). Asexual reproduction in bad times? The case of Cladocora caespitosa in the eastern Mediterranean Sea. Coral Reefs 40, 663–677. doi:10.1007/s00338
Mclachlan, R., and Grottoli, A. (2021). Image analysis to quantify coral bleaching using greyscale model v1. doi:10.17504/protocols.io.bx8wprxe
Monty, J. A., Gilliam, D. S., Banks, K., Stout, D. K., Dodge, R. E., Monty, J. A., et al. (2006). Coral of opportunity survivorship and the use of coral nurseries in coral Reef restoration NSUWorks citation. Available online at: https://nsuworks.nova.edu/occ_facpresentations/31.
Musco, L., Prada, F., D’Anna, G., Galasso, N. M., Pipitone, C., Vega Fernández, T., et al. (2017). Turning casualty into opportunity: fragmenting dislodged colonies is effective for restoring reefs of a Mediterranean endemic coral. Ecol. Eng. 98, 206–212. doi:10.1016/j.ecoleng.2016.10.075
Page, C. A., Muller, E. M., and Vaughan, D. E. (2018). Microfragmenting for the successful restoration of slow growing massive corals. Ecol. Eng. 123, 86–94. doi:10.1016/j.ecoleng.2018.08.017
Peirano, A., Morri, C., Bianchi, C. N., and Rodolfo-Metalpa, R. (2001). Biomass, Carbonate standing stock and production of the mediterranean coral Cladocora caespitosa (L.). Facies 44, 75–80. doi:10.1007/bf02668168
Peirano, A., Morri, C., Bianchi, C. N., Aguirre, J., Antonioli, F., Calzetta, G., et al. (2004). “The Mediterranean coral Cladocora caespitosa: a proxy for past climate fluctuations?,” in Global and planetary change (Elsevier B.V.), 195–200. doi:10.1016/S0921-8181(03)00110-3
Pitacco, V., Orlando-Bonaca, M., Mavrič, B., and Lipej, L. (2014). Macrofauna associated with a bank of Cladocora caespitosa (Anthozoa, scleractinia) in the Gulf of Trieste (northern Adriatic).
Pitacco, V., Mistri, M., and Lipej, L. (2019). Species-Area Relationship (SAR) models as tools for estimating faunal biodiversity associated with habitat builder species in sensitive areas: the case of the Mediterranean stony coral (Cladocora caespitosa). Mar. Environ. Res. 149, 27–39. doi:10.1016/j.marenvres.2019.05.016
Quigley, K. M. (2023). Breeding and selecting corals resilient to global warming. Downloaded doi:10.1146/annurev-animal-021122
Rinkevich, B. (2005). Conservation of coral reefs through active restoration measures: recent approaches and last decade progress. Environ. Sci. Technol. 39, 4333–4342. doi:10.1021/es0482583
Rinkevich, B. (2019). The active reef restoration toolbox is a vehicle for coral resilience and adaptation in a changing world. J. Mar. Sci. Eng. 7, 201. doi:10.3390/jmse7070201
Rinkevich, B. (2021). Augmenting coral adaptation to climate change via coral gardening (the nursery phase). J. Environ. Manage 291, 112727. doi:10.1016/j.jenvman.2021.112727
Rodolfo-Metalpa, R., Peirano, A., Houlbrèque, F., Abbate, M., and Ferrier-Pagès, C. (2008). Effects of temperature, light and heterotrophy on the growth rate and budding of the temperate coral Cladocora caespitosa. Coral Reefs 27, 17–25. doi:10.1007/s00338-007-0283-1
Roveta, C., Coppari, M., Calcinai, B., Di Camillo, C. G., Marrocco, T., Pulido Mantas, T., et al. (2023). What’s the key for success? Translocation, growth and thermal stress mitigation in the Mediterranean coral Cladocora caespitosa (Linnaeus, 1767). Front. Mar. Sci. 10, 1199048. doi:10.3389/fmars.2023.1199048
Schopmeyer, S. A., Lirman, D., Bartels, E., Gilliam, D. S., Goergen, E. A., Griffin, S. P., et al. (2017). Regional restoration benchmarks for Acropora cervicornis. Coral Reefs 36, 1047–1057. doi:10.1007/s00338-017-1596-3
Shaish, L., Levy, G., Gomez, E., and Rinkevich, B. (2008). Fixed and suspended coral nurseries in the Philippines: establishing the first step in the “gardening concept” of reef restoration. J. Exp. Mar. Biol. Ecol. 358, 86–97. doi:10.1016/j.jembe.2008.01.024
Keywords: Cladocora caespitosa, ecological restoration, coral gardening, bleaching, microfragmentation, Mediterranean Sea
Citation: Cardinale P and Danovaro R (2025) Restoration of the endemic hermatypic coral Cladocora caespitosa in the Mediterranean Sea: micro-fragmentation and nursery rearing. Front. Environ. Sci. 13:1605099. doi: 10.3389/fenvs.2025.1605099
Received: 02 April 2025; Accepted: 21 August 2025;
Published: 29 August 2025.
Edited by:
Ester A. Serrao, University of Algarve, PortugalReviewed by:
Stelios Katsanevakis, University of the Aegean, GreeceLucia Bongiorni, National Research Council (CNR), Italy
Copyright © 2025 Cardinale and Danovaro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: P. Cardinale, cC5jYXJkaW5hbGVAcG0udW5pdnBtLml0
 P. Cardinale
P. Cardinale R. Danovaro
R. Danovaro