- 1Technology Center, China Tobacco Anhui Industrial Co., Ltd., Hefei, China
- 2The Industrial Crop Institute, Anhui Academy of Agricultural Sciences (AAAS), Hefei, China
- 3Anhui Wannan Tobacco Company, Xuancheng, China
- 4College of Resources and Environment, Zhongkai University of Agriculture and Engineering, Guangzhou, China
The rational application of fertilization methods and the timing of film removal are crucial for enhancing tobacco yield and quality, as well as for promoting soil health. In our study, we established fertilization methods (hole fertilization and band fertilization) and film removal timing treatments (film removal 30 days after transplanting, T1; 60 days after transplanting, T2; and no film removal, T3). We analyzed differences in soil nutrients, microbial community composition, and tobacco growth under different treatments. The results showed that, compared with other treatments, the combination of hole fertilization and T2 significantly promoted tobacco growth. Compared to band fertilization, hole fertilization increased plant height, as well as the area of middle and upper leaves, by 0.62%–5.01%, 4.71%–9.85%, and 4.71%–9.67%, respectively. In addition, root length, average diameter, root volume, number of tips, forks, and crossings all showed varying degrees of improvement with hole fertilization. Furthermore, hole fertilization combined with the T2 treatment contributed to enhanced soil pH, nutrient availability, and bacterial diversity. At the maturity stage, soil pH and soil organic matter (SOM) increased by 0.26–0.35 units and 9.09%–16.44%, respectively, with hole fertilization compared to band fertilization. Moreover, these agronomic practices influenced the relative abundance of functional microorganisms (p_Nitrospirota, p_Desulfobacterota, and p_Actinobacteriota) by regulating soil nutrient levels. In conclusion, the use of hole fertilization combined with film removal 60 days after transplanting effectively improves soil chemical properties, optimizes the microbial community structure, and increases tobacco yield in southern Anhui, representing an efficient fertilization management strategy.
1 Introduction
The unique flavor and distinctive characteristics of the tobacco planting area in southern Anhui, China, make it an important specialty tobacco production base in the country (Shen et al., 2025). An adequate supply of water and fertilizer is crucial for ensuring the normal growth and development of flue-cured tobacco, as well as for maintaining its desirable flavor characteristics (Liu et al., 2021). In recent years, the fertility of tobacco-growing soils in southern Anhui has declined due to continuous cropping, improper fertilization methods, and unreasonable timing of film removal, all of which can reduce the quality of cured tobacco leaves (Yang et al., 2021). Therefore, it is imperative to explore appropriate cultivation practices to enhance both the yield and quality of tobacco leaves in this region.
Different fertilization methods can influence soil structure and nutrient availability, thereby affecting the efficiency of nutrient uptake by crops (McLaughlin et al., 2011). In traditional farming practices, fertilizers are primarily applied to the surface soil, which often leads to significant nutrient loss due to water runoff (MacDonald et al., 2011; Li et al., 2015). Band application can prevent ‘seedling burning’ during the early growth stages and continuously supply nutrients during later stages; however, it also increases the risk of nutrient loss due to rainfall (Liu et al., 2022). Compared to traditional applications, band fertilization can enhance both fertilizers use efficiency and crop yield (Wu et al., 2021; Liu et al., 2023). Hole application ensures a sufficient nutrient supply during the transplanting stage, thereby reducing the risk of nutrient loss and water volatilization, although it may result in insufficient nutrient availability during later stages (Dekyi et al., 2024). Optimizing fertilization methods is essential for achieving higher nutrient efficiency and improved crop productivity.
Plastic film mulching is a crucial agronomic practice in tobacco cultivation, significantly contributing to drought resistance, waterlogging resistance, and low-temperature resilience during the growth period of flue-cured tobacco (Yang et al., 2022). The timing of film removal significantly impacts tobacco plant growth and the quality of cured leaves. In response to the unique climatic characteristics of southern Anhui, early film removal may lead to fertilizer loss due to rainfall, resulting in inadequate fertility in later stages (Wang et al., 2018). Conversely, delayed film removal may result in excessively high soil temperatures, causing premature root aging and accelerated ripening of tobacco plants, ultimately reducing yield and quality (Fan et al., 2019). Therefore, identifying the optimal film removal period for the ecological conditions of the Wannan tobacco-growing area is essential for regulating hydrothermal conditions and improving tobacco yield and quality. Under optimal soil moisture conditions, microbial activity and biomass can reach peak levels; however, excessively high soil moisture can negatively affect the composition and metabolic activity of soil microbial communities (Muhr et al., 2010).
The selection of fertilization methods and the timing of film removal are critical agronomic practices that significantly influence soil moisture and nutrient availability, thereby altering the composition of the rhizosphere microbial community (Peng et al., 2022). Fertilization affects soil quality, biological fertility, productivity, and the stability of farmland ecosystems by modifying the soil’s nutrient status as well as the structure and function of microbial communities (Wang et al., 2020). It alters the nutrient balance and supply status in the soil, leading to changes in the quantity, diversity, and structure of microbial communities, including bacteria and fungi (Ma et al., 2021; Molina and Matilla, 2020). The deep application of chemical fertilizers can, on the one hand, prevent volatilization losses by minimizing contact with air, and on the other hand, increase the contact area with the soil, thereby reducing runoff losses (Ibrahim et al., 2014).
The objective of this study is to explore appropriate fertilization methods and film removal timings suited to the climatic conditions of the southern Anhui tobacco-growing region. This research aims to provide a theoretical basis and practical reference for scientific fertilization practices to achieve high yield and quality in flue-cured tobacco production in this area. To achieve this objective, a field study was conducted in the tobacco-growing region of southern Anhui to investigate the effects of different film removal periods and fertilization methods on tobacco growth, soil nutrients, and microbial communities. The hypotheses to be tested are as follows: 1) hole application can reduce nutrient loss, promote the root development of flue-cured tobacco, and consequently increase crop yield; and 2) hole application combined with timely film removal can enhance soil microbial communities and improve plant yield.
2 Materials and methods
2.1 Site description and experimental design
The experiment was conducted in 2024 in Jingxian, Xuancheng City, Anhui Province (30°56′N, 118°45′E), and the region has a subtropical monsoon climate, with an average annual temperature of 16.0° and an average annual precipitation of 1429.6 mm. The soil type in this area is classified as paddy soil, and the basic physicochemical properties of the soil are presented in Supplementary Table S1. The experiment included six treatments based on a two-factor design: fertilization method (hole fertilization, H; and band fertilization) and film removal timing (30 days after transplanting, T1; 60 days after transplanting, T2; and no film removal, T3). Film removal at 30 days after transplanting combined with band fertilization represents the traditional cultivation method used in the tobacco-growing region of southern Anhui. Each treatment consisted of three replicate plots (4 m × 6 m), resulting in a total of 18 plots. The tobacco variety used in the field study was Yunyan 87, the main cultivar in Anhui Province. The fertilizers used in this study included potassium nitrate, potassium sulfate, and calcium magnesium phosphate, with fertilization occurring in early March. Fertilizers were applied at the following rates: P2O5 at 200 kg/ha, K2O at 300 kg/ha, and N at 200 kg/ha.
2.2 Sampling and analyses
Soil samples were collected at three growth stages of flue-cured tobacco-rosette stage (RS), rapid growth stage (RGS), and mature stage (MS)-and were divided into two portions for analysis. One portion was air-dried for the determination of basic soil nutrients (Bao, 2000), while the other portion was stored at −80°C for the analysis of soil microbial communities (Xia et al., 2023a). Soil pH was measured using a pH meter (Soil: H2O = 1:2.5), and soil organic matter (SOM) was determined using the dichromate oxidation–ferrous sulfate titration method. Available phosphorus (AP) and available nitrogen (AN) were measured using a continuous-flow analyzer (AA3; Bran Luebbe, Germany), and available potassium (AK) was determined by flame photometry (AP-1200, Shanghai Precision Instrument Co., China). Plant and soil samples were collected at three growth stages (RS, RGS, and MS). Agronomic traits, root growth parameters, and nutrient concentrations in different plant parts were recorded at each stage. Root growth parameters were measured using a root scanner in combination with WinRHIZO Pro software. Plant samples were oven-dried at 80°C, and nutrient concentrations were determined following H2SO4–H2O2 digestion (Ansquer et al., 2009).
Fresh soil samples were stored at −80°C, and total soil DNA was extracted using a commercial kit (Omega Bio-tek, Norcross, GA, USA). The purity and concentration of the extracted DNA were assessed by 2% agarose gel electrophoresis. The primers used for amplifying bacterial and fungi DNA were 338F–506R and ITS1F–ITS2R, respectively. Microbial data analysis was performed using a cloud platform with technical support from Shanghai Majorbio Bio-Pharm Technology Co., Ltd. (Xia et al., 2023b).
2.3 Data analysis
Experimental data were organized and statistically analyzed using Excel 2022. One-way ANOVA was conducted using SPSS 22.0, with significance set at P < 0.05. R software was used to perform joint analyses of soil microorganisms and environmental factors, including redundancy analysis (RDA), Procrustes analysis, correlation analysis, and network analysis, using the vegan package (Xia et al., 2024).
3 Results
3.1 Effects of different treatments on the agronomic traits of tobacco
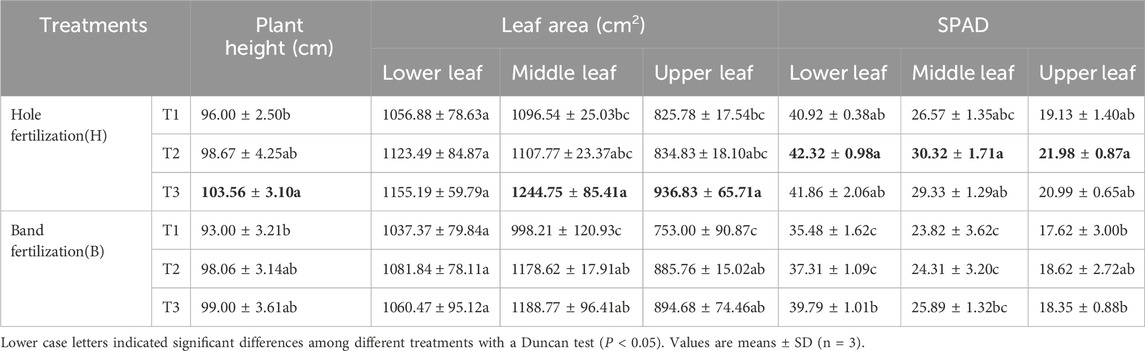
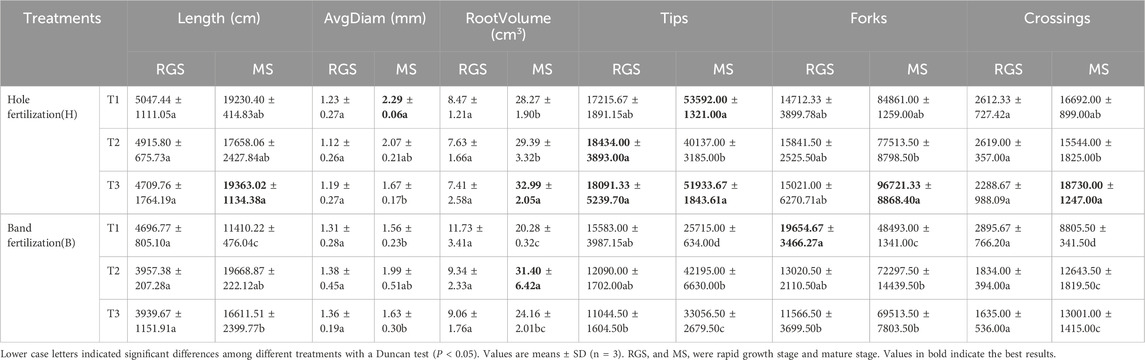
Compared to band fertilization, plant height increased by 0.62%–5.01% under hole fertilization (Table 1). The areas of the middle and upper leaves increased by 4.71%–9.85% and 4.71%–9.67%, respectively, under hole fertilization compared to band fertilization (Table 1). Furthermore, compared to band fertilization, the SPAD values of the lower, middle, and upper leaves increased by 5.20%–15.33%, 11.54%–24.72%, and 8.57%–18.05%, respectively, under hole fertilization (Table 1). Moreover, compared with the T1 treatment, the agronomic traits of tobacco were improved under other treatments with different fertilization methods. Specifically, compared to T1, plant height and the leaf area of the middle and upper leaves increased significantly by 6.41%–7.88%, 13.52%–19.09%, and 13.45%–18.81%, respectively, under the T3 treatment (Table 1). Root growth parameters were also affected by the fertilization method and the timing of film removal (Table 2). At the MS stage, compared to band fertilization, root length, average diameter, root volume, number of tips, forks, and crossings increased by 16.56%–68.54%, 2.45%–46.79%, 36.55%–39.40%, 57.11%–108.41%, 7.21%–74.99%, and 22.74%–89.56%, respectively, under hole fertilization (except for T2) (Table 2). Compared to the T1 treatment, the number of root tips significantly decreased under the T2 treatment with hole fertilization, while significantly increasing with band fertilization (Table 2). Root volume, number of forks, and crossings were significantly enhanced under the T3 treatment with both fertilization methods, compared to the T1 treatment (Table 2).
3.2 Effects of different treatments on the nutrient absorption of tobacco
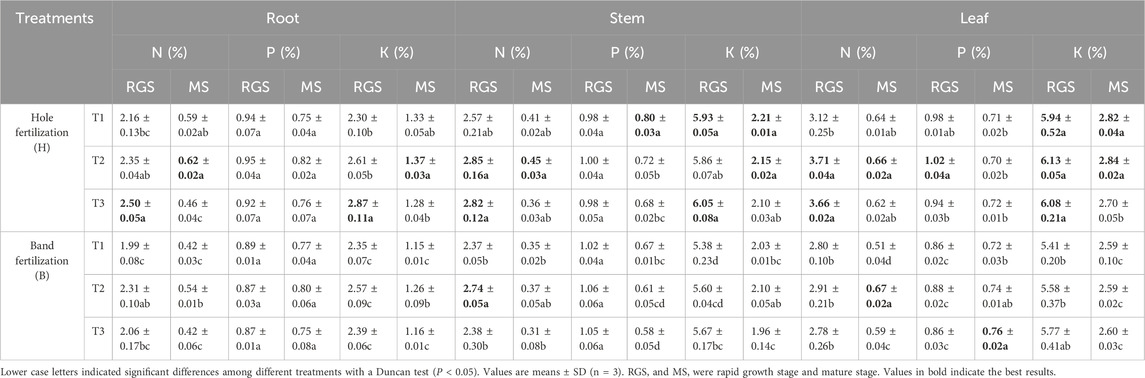
At both the RGS and MS stages, compared to band fertilization, the nutrient content in various parts of the tobacco plant increased significantly under hole fertilization, particularly in potassium (K) content (Table 3). In the MS stage, the K contents in the roots, stems, and leaves were 10.34%–15.65%, 7.14%–8.87%, and 3.85%–8.88% higher, respectively, under hole fertilization compared to band fertilization (Table 3). Among all film removal treatments, the highest nutrient contents in different parts of the plant were observed under the T2 treatment with both fertilization methods (Table 3).
3.3 Effects of different treatments on soil chemical properties and soil microorganisms
Compared to the T1 treatment, soil AN, AP, and AK levels were significantly reduced under the T2 treatment during the RGS and RS stages (Figures 1A–C). Soil pH and soil organic matter (SOM) were affected by the fertilization method and film removal timing during the MS stage (Figures 1D,E). Specifically, compared to band fertilization, soil pH increased by 0.26–0.35 units under hole fertilization. Additionally, SOM increased by 9.09%–16.44% under other treatments with both fertilization methods compared to the T1 treatment in the MS stage (Figure 1E). Compared to band fertilization, the number of bacterial OTUs increased under hole fertilization (Supplementary Figure S1A–C), while the number of fungi OTUs decreased (Supplementary Figure S1D–F). Compared to the T1 treatment, the number of fungi OTUs increased by 14.71%–43.25% (RG), 6.92%–23.79% (RGS), and 5.92%–33.81% (MS) under the T2 treatment (Supplementary Figure S1). In addition, the α-diversity of soil microorganisms was generally higher under hole fertilization across various stages compared to band fertilization (Supplementary Figure S2). The α-diversity also increased under other treatments with both fertilization methods compared to the T1 treatment, with the highest levels observed under T2 (Supplementary Figure S2). As shown in Figure 2, the β-diversity of soil microorganisms exhibited significant variation under different treatments at various growth stages of tobacco. Compared to bacteria, the β-diversity of fungi showed more pronounced differentiation across treatments (Figure 2).
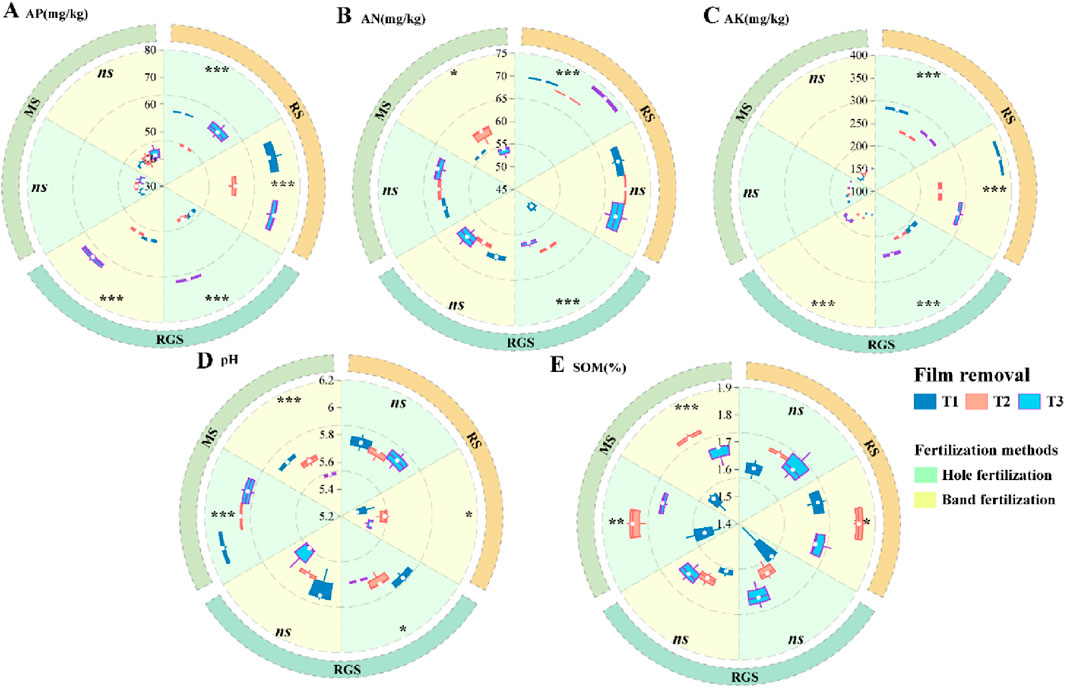
Figure 1. Effects of varieties treatments on basic soil chemical properties. The asterisk (*) was used for comparison among different treatments. *, ** and *** were P < 0.05, P < 0.01 and P < 0.001. RS, RGS and MS were rosette stage, rapid growth stage and mature stage. (A–E) were available phosphorus (AP), available nitrogen (AN), available potassium (AK), pH and soil organic matter (SOM).
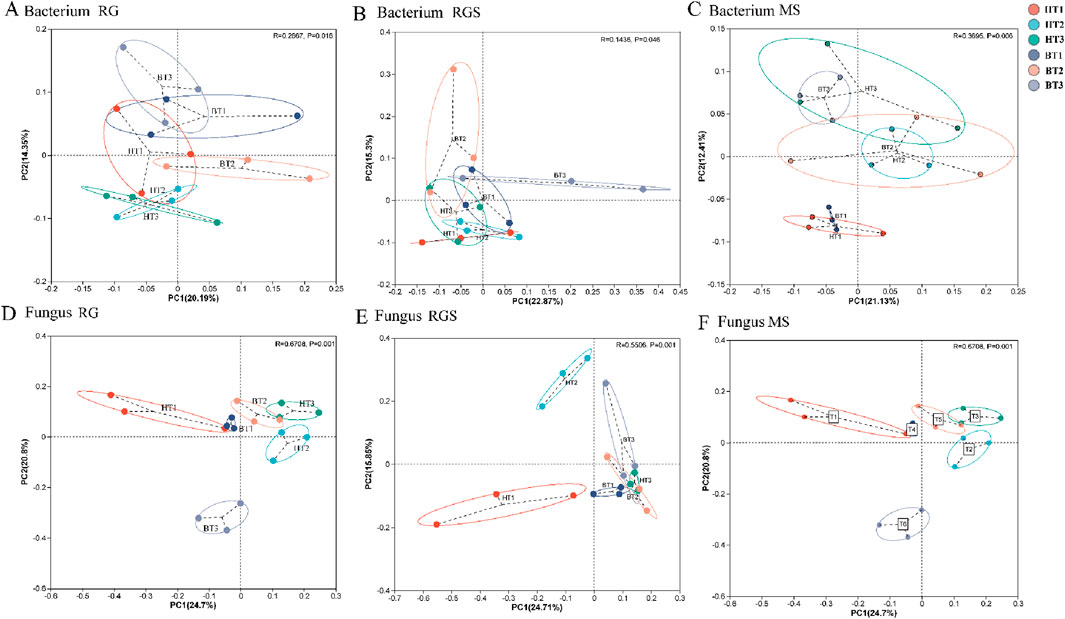
Figure 2. The PCOA analysis of soil bacterium (A–C) and fungus (D–F) between soil environment and microorganism. RS, RGS and MS were rosette stage, rapid growth stage and mature stage.
3.4 The relationship between environmental factors and microorganisms under different treatments
Compared to fungi, the correlation between bacterial α-diversity and environmental factors was more significant (Figure 3). At the RG stage, there was a significant positive correlation (P < 0.05) between soil factors (AP and AK) and the α-diversity of bacteria (Figure 3). Soil pH was a key environmental factor that exhibited a significant correlation with soil nutrients throughout all growth stages of tobacco (Figure 3). Compared to fungi, soil bacterial communities showed a closer association with soil chemical factors across various stages (Figure 4; Supplementary Table S2). Throughout all growth stages of tobacco, pH emerged as a significant environmental factor influencing the bacterial community (Figure 4; Supplementary Table S2). Additionally, as the plant developed, the relationship between soil chemical properties and fungi communities became increasingly complex (Supplementary Table S2). At the MS stage, soil pH and SOM also affected the community composition of both fungi and bacteria (Supplementary Table S2). The results of the Procrustes analysis confirmed that soil nutrients significantly affected microbial community composition across all growth stages (P < 0.05) (Supplementary Table S3). For example, at the RG stage, the relative abundances of p_Bdellovibrionota, p_Myxococcota, p_Desulfobacterota, and p_Nitrospirota were negatively correlated with soil AP and AK, while p_RCP2-54, p_Methylomirabilota, and p_Latescibacterota showed significant positive correlations (P < 0.05) (Supplementary Figure S1A). At the RGS stage, p_Desulfobacterota, p_Methylomirabilota, p_Latescibacterota, and p_Nitrospirota were significantly positively correlated with soil AK (Supplementary Figure S1A). For fungi, the relative abundance of p_Glomeromycota was significantly influenced by soil AN, AP, AK, and SOM at the MS stage (Supplementary Figure S1F). In the RG stage, soil pH and AP were key environmental factors for bacterial communities, whereas AN was the most important factor for fungi communities (Figures 5A,D). Soil pH showed a significant positive correlation with the relative abundances of p_Desulfobacterota, p_Methylomirabilota, p_Latescibacterota, and p_RCP2-54. In contrast, soil AP showed a significant negative correlation with the relative abundances of p_RCP2-54, p_Latescibacterota, p_Nitrospirota, p_Methylomirabilota, p_Desulfobacterota, and p_Myxococcota during the RG stage (Figure 5A). At the RGS stage, soil AK was a key environmental factor influencing bacterial communities such as p_Latescibacterota, p_Nitrospirota, and p_Actinobacteriota, whereas soil AN was a significant factor for fungi communities such as p_Rozellomycota and p_Basidiobolomycota (Figures 5B,E). Additionally, the relative abundances of p_Cyanobacteria and p_Glomeromycota were affected by soil factors (Figures 5C,F).
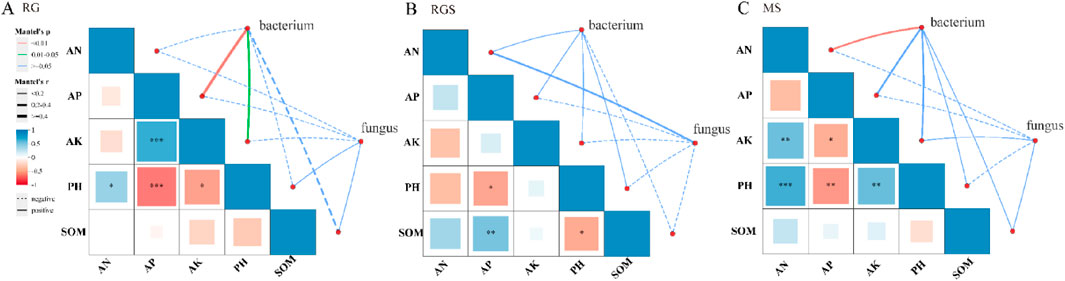
Figure 3. The Mantel test between soil environment and microorganism. (A–C) were rosette stage (RS), rapid growth stage (RGS) and mature stage (MS).
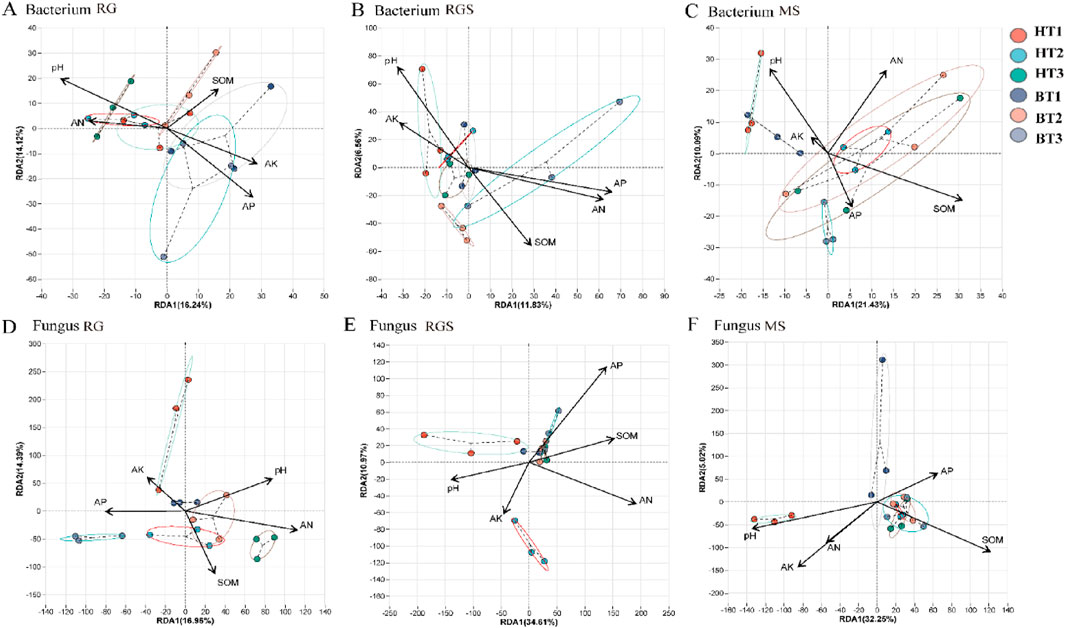
Figure 4. The RDA analysis between soil environment and microorganism [soil bacterium (A–C) and fungus (D–F)]. RS, RGS and MS were rosette stage, rapid growth stage and mature stage.
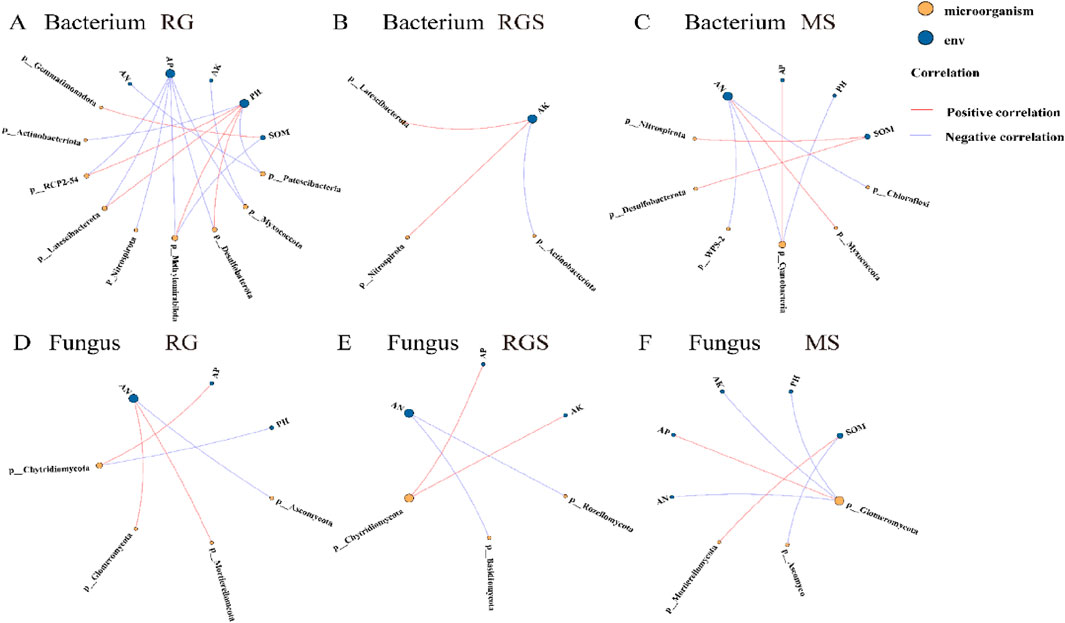
Figure 5. The network analysis between soil environment and microorganism [soil bacterium (A–C) and fungus (D–F)]. RS, RGS and MS were rosette stage, rapid growth stage and mature stage. Colors represent the correlation type (purple and red for negative and positive correlations, respectively).
4 Discussion
This study demonstrates that hole fertilization combined with plastic film removal 60 days after transplanting (T2 treatment) can synergistically enhance tobacco growth and soil environment. Compared to strip fertilization, hole fertilization significantly increased tobacco plant height and the area of upper leaves, while also optimizing root morphology parameters such as root length, root volume, and branching number. This planting pattern simultaneously improved soil physical and chemical properties, reduces acidification, and increases organic matter content. Furthermore, by regulating soil nutrients, it activated the abundance of functional microorganisms, thereby enhancing bacterial diversity. Ultimately, the synergistic effect of hole fertilization and plastic film removal 60 days after transplanting forms an efficient fertilization strategy that can optimize soil micro-ecology, strengthen nutrient supply, and significantly increase tobacco yield in the southern Anhui region.
4.1 Effects of different treatments on the agronomic traits and nutrient absorption of tobacco
The soil fertility significantly impacts plant growth and development, as well as crop yield and quality (Wang et al., 2025). Fertilization methods and film removal treatments indirectly influence the yield and quality of flue-cured tobacco by regulating soil moisture and nutrient availability (Guo et al., 2024). In our study, the combination of hole fertilization and film removal 60 days after transplantation (T2) significantly enhanced tobacco plant growth and promoted root development (Tables 1, 2). Due to the varying placement of fertilizer, different fertilization methods exert different effects on the migration and transformation of available soil nutrients, as well as on crop growth and development (Jing et al., 2012). It also increases root mass, root volume, and root surface area, while promoting deeper root growth (Yu et al., 2013). Besides, the timing of film removal is closely related to the geographical and climatic conditions of the southern Anhui tobacco-growing region. The climate in this area is characterized by low temperatures and high rainfall in the early stage, followed by high temperatures and low rainfall in the later stage (Zu et al., 2004). In the tobacco-growing region of Southern Anhui, transplanting after 60 days of film removal not only ensures a lower moisture content and higher soil temperature within the film during the early stages, which is conducive to root growth, but also guarantees the moisture required for the later growth stages of the tobacco plants (Huang et al., 2003). Biodegradable films have been shown to positively influence the growth and development of tobacco leaves by improving soil water retention and thermal insulation (He et al., 2024). Timely film removal is beneficial for the root development of tobacco plants, enhances soil permeability, regulates soil temperature, and prevents poor growth and development of tobacco plants in the later stages (Li et al., 2004). In our study, the application of hole fertilization combined with appropriate film removal timing significantly increased nutrient content in various parts of the plant, particularly potassium levels (Table 3). There are some explanations for these results: 1) Hole fertilization can enhance root vigor and development, thereby enabling crops to absorb and utilize soil nutrients more effectively (Nkebiwe et al., 2016; Chen et al., 2022); 2) Deep fertilizer application effectively reduces nutrient volatilization and runoff losses, directly targets the crop root zone, and enhances nutrient uptake by plants (Li et al., 2020). Timely membrane removal can regulate the soil temperature and moisture, which can facilitate the absorption of nutrients and water by the roots, resulting in a more balanced internal chemical composition of the tobacco leaves (Ji et al., 2017).
4.2 Effects of different treatments on soil chemical properties and microorganism
The present study found that hole fertilization combined with film removal 60 days post-transplantation (T2) increased soil pH and soil organic matter (SOM) (Table 3). This effect may be attributed to the ability of deep fertilization to promote more complete absorption of nutrients by crops, thereby reducing nitrogen loss and residue, as well as mitigating soil acidification (Zhang et al., 2010). That was the other reason the secretion of acidic substances from the roots is reduced, preventing the accumulation of acidic exudates from the roots with the washing effect of rainwater (Li et al., 2004). In addition, the deep application of fertilizer improves soil porosity and aeration, which in turn enhances the population and activity of soil microorganisms and contributes to greater stability of soil organic carbon (Jeewani et al., 2021). As the primary carrier of nutrients, water plays a crucial role in the transport and absorption of soil substances (Zhao et al., 2021). Appropriate soil moisture facilitates the decomposition of organic matter and the release of available nutrients, thereby reducing nutrient loss through volatilization and surface runoff (Yin et al., 2023).
In this study, hole fertilization combined with film removal 60 days post-transplantation (T2) was found to improve bacterial α-diversity and increase the number of operational taxonomic units (OTUs) (Supplementary Figures S1, 2). Two main factors likely contribute to these findings: 1) Chemical fertilizers and abiotic environmental factors can induce changes in soil microbial communities, thereby affecting microbial richness and community structure (Demoling et al., 2007). 2) Film can indirectly influence the composition of soil microbial communities by regulating soil moisture, thermal conditions, and soil fertility (Bandopadhyay et al., 2018; Ding et al., 2021). Studies have demonstrated that various types of mulching films can affect soil thermal and moisture regimes, indirectly altering soil enzyme activities and microbial community composition (Xia et al., 2025a). On one hand, soil temperature can influence the respiration rate and cumulative respiration of soil bacteria (Peng et al., 2021). On the other hand, soil moisture not only directly regulates the life activities of soil microorganisms but also indirectly regulates the diversity of soil bacteria (de Vries et al., 2018). Bacterial-dominated pathways typically prevail in nutrient-rich soils, which are characterized by rapid carbon turnover and high nutrient cycling rates (Ingwersen et al., 2008). Optimal soil moisture promotes microbial activity and increases nutrient availability, whereas excessive moisture can inhibit soil organic carbon mineralization, reduce carbon supply capacity, and shift microbial community composition (Sahrawat, 2003; Vanhala et al., 2021).
4.3 Relationship between environmental factors and microorganisms
Previous studies have demonstrated that soil moisture, pH, and chemical properties significantly influence the structure of microbial communities (Wu et al., 2008; Carney and Matson, 2006). In our study, fertilization methods and film removal treatments indirectly affected the composition of soil microbial communities by altering soil nutrient availability, with bacterial communities exhibiting a more pronounced response (Supplementary Figures S3, 4; Figure 4). Increases in soil nutrient availability can enhance carbon mineralization, microbial biomass, soil enzyme activity, and the diversity of microbial communities (Galicia and Garcya-Oliva, 2004). Fertilization management practices also have a significant impact on the structure of microbial communities in soil (Jangid et al., 2008). The differential responses of fungi and bacteria may be attributed to the fact that fertilization methods and film removal primarily affect soil structure and nutrient conditions, creating a nutrient-rich environment that is particularly favorable for bacterial growth (Hermans et al., 2017). Furthermore, the findings of this study reveal that changes in soil nutrients influence the relative abundance of functional microbial groups in the soil (Figure 5). For instance, the fertilization method and film removal timing can affect the relative abundance of p_Nitrospirota by regulating soil pH (Koch et al., 2015). The relative abundance of p_Desulfobacterota plays a significant role in soil sulfate reduction, as well as in the transformation and cycling of sulfur in the soil (Chu et al., 2024). Similarly, the relative abundances of p_RCP2-54, p_Latescibacterota, and other microbial taxa are influenced by the nutrient status of the soil environment, which alters habitat suitability for these organisms (Xia et al., 2024). These findings suggest that such cultivation measures are effective in alleviating soil acidification, enhancing soil quality, promoting the colonization of beneficial microbial communities, and mitigating the toxicity of sulfate compounds (Fang et al., 2024).
5 Conclusion
The present study was conducted to explore the effects of different fertilization methods and film removal treatments on tobacco growth, soil nutrients, and microbial communities. Based on the results, the following conclusions can be drawn: Compared with other treatments, plant height, leaf area, and root parameters of tobacco were significantly improved under hole fertilization combined with the T2 treatment. In addition, hole fertilization increased soil pH and the levels of available nutrients compared to band application. Furthermore, hole fertilization and T2 treatment significantly enhanced the diversity of soil bacterial communities and increased the abundance of bacteria. Fertilization methods and film removal treatments also influenced the relative abundance of soil functional microorganisms (p_Nitrospirota, p_Desulfobacterota, and p_Actinobacteriota) by regulating key environmental factors in the soil, including nutrient content, temperature, and moisture. In response to the unique climatic characteristics of the Wannan region, this study suggests the use of hole fertilization combined with plastic film removal 60 days after transplanting (T2). This approach is beneficial for improving tobacco yield and quality while simultaneously enhancing the ecological environment of the soil.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
CS: Writing – original draft, Data curation, Supervision. YY: Supervision, Writing – review and editing. ZP: Writing – original draft, Supervision. MR: Writing – review and editing. HD: Supervision, Writing – original draft. ZL: Supervision, Writing – original draft. LY: Supervision, Writing – review and editing. CJ: Writing – review and editing, Supervision. HX: Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Funding was received for the research and/or publication of this article: This work was funded by the Anhui Zhongyan Wannan Base Unit Technical Service Project (2023340000340027); Anhui Academy of Agricultural Sciences Academic Research Plan Project (2025YL030).
Conflict of interest
Authors CS, HD, and ZL were employed by China Tobacco Anhui Industrial Co., Ltd. Authors ZP and LY were employed by Anhui Wannan Tobacco Company.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1607410/full#supplementary-material
References
Ansquer, P., Duru, M., Theau, J. P., and Cruz, P. (2009). Functional traits as indicators of fodder provision over a short time scale in species-rich grasslands. Ann. Bot. 103 (1), 117–126. doi:10.1093/aob/mcn215
Bandopadhyay, S., Martin-Closas, L., Pelacho, A. M., and DeBruyn, A. M. (2018). Biodegradable plastic mulch films: impacts on soil microbial communities and ecosystem functions. Front. Microbiol. 9, 819. doi:10.3389/fmicb.2018.00819
Carney, K. M., and Matson, P. A. (2006). The influence of tropical plant diversity and composition on soil microbial communities. Microb. Ecol. 52, 226–238. doi:10.1007/s00248-006-9115-z
Chen, G. Z., Ren, L. Q., Wang, J. F., Liu, F. G., Li, H. G. X, Li, H., et al. (2022). Optimizing fertilization depth can promote sustainable development of dryland agriculture in the loess Plateau region of China by improving crop production and reducing gas emissions. Plant Soil 479 (1-2), 73–89. doi:10.1007/s11104-022-05795-6
Chu, J. C., Wang, L. H., Jia, R., Zhou, J., Zang, H. D., Wang, J. H., et al. (2024). Straw returning with no-tillage alleviates microbial metabolic carbon limitation and improves soil multifunctionality in the northeast plain. Land Degrad. Dev. 35 (17), 5149–5161. doi:10.1002/ldr.5286
Dekyi, D. M., Zeng, K., Yin, B., Li, Q., and Yao, Y. L. (2024). Eliminating NH3, NO and N2O emissions simultaneously in a wheat field by urea point placement. Soil Fertilizer Sci. China 6, 18–26.
Demoling, F., Figueroa, D., and Bååth, E. (2007). Comparison of factors limiting bacterial growth in different soils. Soil Biol. Biochem. 39 (10), 2485–2495.
de Vries, F. T., Griffiths, R. I., Mark, B., Hayley, C., Mariangela, G., Soon, G. H., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9 (1), 3033. doi:10.1038/s41467-018-05516-7
Ding, F., Flury, M., Schaeffer, S. M., Xu, Y. D., and Wang, J. K. (2021). Does long-term use of biodegradable plastic mulch affect soil carbon stock? Resour. Conserv. Recy 175, 105895. doi:10.1016/j.resconrec.2021.105895
Fan, J., Tan, J., Deng, J. Q., Peng, W. X., Zhao, X. Y., Zheng, H. Z., et al. (2019). Degradation property of degradable films and their effects on flue-cured tobacco development and soil ecological environment. Chin. Tob. Sci. 40 (04), 22–28+36.
Fang, C. Y., Li, P., Zhang, J. L., Lu, Y. H., Tang, Y. Y., Tu, N. M., et al. (2024). Soil Cd bioavailability response characteristics to microbes in paddy fields with co-incorporation of milk vetch, rice straw and amendments. Sci. Total Environ. 935, 173306. doi:10.1016/j.scitotenv.2024.173306
Galicia, L., and Garcya-Oliva, F. (2004). The effects of C, N and P additions on soil microbial activity under two remnant tree species in a tropical seasonal pasture. Appl. Soil Ecol. 26, 31–39. doi:10.1016/j.apsoil.2003.10.006
Guo, S. Q., Cui, Z. T., Gao, F., Tian, Z. H., Ren, Q. L., Zheng, J., et al. (2024). Effects of deep application of nitrogen fertilizer on maize nutrient utilization and yield under mulching farmland in semiarid region. J. Maize Sci. 32 (07), 83–91.
He, Y., Li, L. K., Gao, H. J., Wu, Z. Y., Chen, G. Y., Liu, C. K., et al. (2024). Effects of different mulching methods on soil physicochemical properties, yield and quality of cigar tobacco leaves. J. Henan Agric. Sci. 53 (12), 54–61.
Hermans, S. M., Buckley, H. L., Case, B. S., Curran-Cournane, F., Taylor, M., and Lear, G. (2017). Bacteria as emerging indicators of soil condition. Appl. Environ. Microb. 83, e02826-16–2826. doi:10.1128/aem.02826-16
Huang, Y. L., Chen, S. H., and Li, W. Q. (2003). Effects of the time of film-covering on growing, yield and quality of flue-cured tobacco leaves. Tob. Sci. Technol. 7, 33.
Ibrahim, A., Pasternak, D., and Fatondji, D. (2014). Impact of depth of placement of mineral fertilizer micro-dosing on growth, yield and partial nutrient balance in pearl millet cropping system in the Sahel. J. Agric. Sci. 153, 1412–1421. doi:10.1017/s0021859614001075
Ingwersen, J., Poll, C., Streck, T., and Kandeler, E. (2008). Micro-scale modelling of carbon turnover driven by microbial succession at a biogeochemical interface. Soil Biol. Biochem. 40 (4), 864–878. doi:10.1016/j.soilbio.2007.10.018
Jangid, K., Williams, M. A., Franzluebbers, A. J., Sanderlin, J. S., Reeves, J. H., Jenkins, M. B., et al. (2008). Relative impacts of land-use, management intensity and fertilization upon soil microbial community structure in agricultural systems. Soil Biol. Biochem. 40, 2843–2853. doi:10.1016/j.soilbio.2008.07.030
Jeewani, P. H., Van Zwieten, L., Zhu, Z. K., Ge, T. D., Guggenberger, G., Luo, Y., et al. (2021). Abiotic and biotic regulation on carbon mineralization and stabilization in paddy soils along iron oxide gradients. Soil Biol. Biochem. 160, 108312. doi:10.1016/j.soilbio.2021.108312
Ji, X. J., Meng, H. D., Zuo, X., Liu, Y. X., Wu, L., and Li, F. X. (2017). Relationships between main climatic factors and chemical components of flue-cured tobacco leaves in Henan tobacco-growing areas. Chin. Tob. Sci. 38 (1), 35.
Jing, J., Zhang, F., Rengel, Z., and Shen, J. (2012). Localized fertilization with P plus N elicits an ammonium-dependent enhancement of maize root growth and nutrient uptake. Field Crop Res. 133, 176–185. doi:10.1016/j.fcr.2012.04.009
Koch, H., Sebastian, L., Albertsen, M., Kitzinger, K., and Daims, H. (2015). Expanded metabolic versatility of ubiquitous nitrite-oxidizing bacteria from the genus nitrospira. P Natl. Acad. Sci. U. S. A. 112 (36), 11371–11376.
Li, F. M., Wang, J., Xu, J. Z., and Xu, H. L. (2004). Productivity and soil response to plasics film mulching durations for spring wheat on entisols in the semiarid loess Plateau of China. Soil Till Res. 7 (8), 9.
Li, G., Zhao, B., Dong, S., Zhang, J., Liu, P., and Lu, W. (2020). Controlled-release urea combining with optimal irrigation improved grain yield, nitrogen uptake, and growth of maize. Agr Water Manage 227, 105834. doi:10.1016/j.agwat.2019.105834
Li, H., Liu, J., Li, G., Shen, J., Bergstrom, L., and Zhang, F. (2015). Past, present, and future use of phosphorus in Chinese agriculture and its influence on phosphorus losses. Ambio 44 (S2), 274–285. doi:10.1007/s13280-015-0633-0
Liu, C., Yan, H., Wang, W., Han, R., Li, Z., Lin, X., et al. (2023). Layered application of phosphate fertilizer increased winter wheat yield by promoting root proliferation and phosphorus accumulation. Soil Till Res. 225, 105546. doi:10.1016/j.still.2022.105546
Liu, P., Yan, H., Xu, S., Lin, X., Wang, W., and Wang, D. (2022). Moderately deep banding of phosphorus enhanced winter wheat yield by improving phosphorus availability, root spatial distribution, and growth. Soil Till Res. 220, 105388. doi:10.1016/j.still.2022.105388
Liu, Y. B., Wang, J., Zhu, X. J., Jiang, W. F., Liu, T., Zhang, J. Z., et al. (2021). Effects of different water and fertilizer management modes on nutrients accumulation and quality of flue-cured tobacco leaves. J. Agric. Sci. Technol. 3 (09), 193–201.
Ma, L., Gao, W., Luan, H. A., Tang, J. W., Li, M. Y., and Huang, S. W. (2021). Soil microbial community characteristics in greenhouse vegetable production under different fertilization patterns based on metagenomic analysis. J. Plant Nutr. Fertilizers 27 (3), 403–416.
Macdonald, G. K., Bennett, E. M., Potter, P. A., and Ramankutty, N. (2011). Agronomic phosphorus imbalances across the world’s croplands. P Natl. Acad. Sci. U. S. A. 108 (7), 3086–3091. doi:10.1073/pnas.1010808108
McLaughlin, M. J., McBeath, T. M., Smernik, R., Stacey, S. P., Ajiboye, B., and Guppy, C. (2011). The chemical nature of P accumulation in agricultural Soils—Implications for fertiliser management and design: an Australian perspective. Plant Soil 349, 69–87. doi:10.1007/s11104-011-0907-7
Molina, S. C., and Matilla, M. A. (2020). Chemical fertilization: a short-term solution for plant productivity. Microb. Biotechnol. 13 (5), 1311–1313. doi:10.1111/1751-7915.13515
Muhr, J., Franke, J., and Borken, W. (2010). Drying-rewetting events reduce C and N losses from a Norway spruce forest floor. Soil Biol. Biochem. 42 (8), 1303–1312. doi:10.1016/j.soilbio.2010.03.024
Nkebiwe, P. M., Weinmann, M., Bar-Tal, A., and Torsten, M. (2016). Fertilizer placement to improve crop nutrient acquisition and yield: a review and meta-analysis. Field Crop Res. 196, 389–401. doi:10.1016/j.fcr.2016.07.018
Peng, S. L., Wu, R. J., Zhang, X., Ge, Z. W., and Yang, N. (2021). Effects of temperature and moisture treatments on microbial characteristics of arable and grassland soil in dry-hot valley. Pratacultural Sci. 38 (12), 2350–2362.
Peng, Y. L., Fei, L. J., Liu, X. G., Sun, G. Z., and Wang, X. K. (2022). Effects of reduced fertilization and regulated deficit irrigation coupling on yield and quality of mango in a dry-hot region. J. Plant Nutr. Fertilizers 28 (3), 521–531.
Sahrawat, K. L. (2003). Organic matter accumulation in submerged soils. Adv. Agron. 81 (3), 169–201. doi:10.1016/s0065-2113(03)81004-0
Shen, C., Dong, H. X., Wang, J. T., Wang, H. J., Pei, Z. Y., Zhang, F. J., et al. (2025). The influence of region and variety interaction on tobacco quality in southern Anhui province. J. Anhui Agric. Sci. 53 (03), 18–24+42.
Vanhala, P., Karhu, K., Tuomi, M., Björklöf, K., Fritze, H., Hyvärinen, H., et al. (2011). Transplantation of organic surface Horizons of boreal soils into warmer regions alters microbiology but not the temperature sensitivity of decomposition. Glob. Change Biol. 17 (1), 538–550. doi:10.1111/j.1365-2486.2009.02154.x
Wang, J., Yang, X., Huang, S., Wu, L., Cai, Z., and Xu, M. (2025). Long-term combined application of organic and inorganic fertilizers increases crop yield sustainability by improving soil fertility in maize–wheat cropping systems. J. Integr. Agric. 24 (1), 290–305.
Wang, N., Nan, H. Y., and Feng, K. Y. (2020). Effects of reduced chemical fertilizer with organic fertilizer application on soil microbial biomass, enzyme activity and cotton yield. Chin. J. Appl. Ecol. 31 (1), 173–181. doi:10.13287/j.1001-9332.202001.022
Wang, W. L., Zhou, Y. H., Liu, H., Li, J., Li, W. B., Guo, H. P., et al. (2018). The effects of different film-removing stages on growth and yield of flue-cured tobacco. J. Anhui Agric. Sci. 46 (15), 36–38+102.
Wu, P., Liu, F., Li, H., Cai, T., Zhang, P., and Jia, Z. (2021). Suitable fertilizer application depth can increase nitrogen use efficiency and maize yield by reducing gaseous nitrogen losses. Sci. Total Environ. 781, 146787. doi:10.1016/j.scitotenv.2021.146787
Wu, T., Chellemi, D. O., Graham, J. H., Martin, K. J., and Rosskopf, E. N. (2008). Comparison of soil bacterial communities under diverse agricultural land management and crop production practices. Microb. Ecol. 55, 293–310. doi:10.1007/s00248-007-9276-4
Xia, H., Jiang, C. Q., Riaz, M., Yu, F., Dong, Q., Yan, Y., et al. (2025a). Impacts of continuous cropping on soil fertility, microbial communities, and crop growth under different tobacco varieties in a field study. Environ. Sci. Eur. 37 (1), 5–13. doi:10.1186/s12302-024-01037-x
Xia, H., Riaz, M., Tang, X. F., Yan, L., El-desouki, Z., Li, X. Y., et al. (2023a). Insight into mechanisms of biochar-fertilizer induced of microbial community and microbiology of nitrogen cycle in acidic soil. J. Environ. Manage 336, 117602. doi:10.1016/j.jenvman.2023.117602
Xia, H., Muhammad, R., Saba, B., Yan, L., Li, Y. X., Wang, X. L., et al. (2023b). Assessing the impact of biochar on microbes in acidic soils: alleviating the toxicity of aluminum and acidity. J. Environ. Manage 345, 118796. doi:10.1016/j.jenvman.2023.118796
Xia, H., Shen, J., Riaz, M., Ran, F. F., Cheng, T. M., Wang, X. Y., et al. (2025b). Effects of agricultural plastic films on crop growth and soil health in tobacco fields: a comparative study. Appl. Soil Ecol. 206, 105795. doi:10.1016/j.apsoil.2024.105795
Xia, H., Shen, J., Riaz, M., Yang, H. Y., Dong, Q., Zu, C. L., et al. (2024). Microbial regulatory mechanisms of disease-resistant tobacco varieties in the prevention and control of bacterial wilt disease. Appl. Soil Ecol. 202, 105598. doi:10.1016/j.apsoil.2024.105598
Yang, D. H., Peng, R., Peng, X. C., Zhao, W. J., Ma, J. J., Yang, Y., et al. (2022). Effects of film uncovering and Re-earthing time on growth period, yield and quality of flue-cured tobaccos under membrane transplanting. J. Anhui Agric. Sci. 50 (23), 14–16+39.
Yang, P. Z., Chen, H. L., Chen, S. H., Zheng, S. H., Wang, C. T., Shen, X. S., et al. (2021). Effects of tillage and fertilization methods on the yield and nutrient utilizations of rapeseed. Soil Fertilizer Sci. China (05), 176–184.
Yin, Z. R., Ke, Y., and Cai, J. J. (2023). The combined effect of irrigation and fertilization on soil water, nutrient transport, yield and water use efficiency of greenhouse tomato. J. Irrigation Drainage 42 (06), 33–44.
Yu, X. F., Gao, J. L., Ye, J., Wang, Z. G., Sun, J. Y., Hu, S. P., et al. (2013). Effects of deep loosening with nitrogen deep placement on root growth, grain yield and nitrogen use efficiency of super high-yield spring maize. J. Maize Sci. 21 (1), 114–119.
Zhang, Y. C., Wang, J. D., Shen, M. X., Shen, Q. S., Xu, X. J., and Ning, Y. W. (2010). Effects of longterm fertilization on soil acidification in taihu Lake region, China. Acta Pedol. Sin. 47 (3), 465–472.
Zhao, F., Wu, Y., Hui, J., Sivakumar, B., Meng, X., and Liu, S. (2021). Projected soil organic carbon loss in response to climate warming and soil water content in a loess watershed. Carbon Balance Manag. 16 (1), 24. doi:10.1186/s13021-021-00187-2
Keywords: fertilization methods, film removal, tobacco, soil microorganism, soil nutrients
Citation: Shen C, Yan Y, Pei Z, Riaz M, Dong H, Liu Z, Yin L, Jiang C and Xia H (2025) Effects of fertilization methods and film removal periods on soil nutrients and microbial community structure in tobacco fields of Southern Anhui Province. Front. Environ. Sci. 13:1607410. doi: 10.3389/fenvs.2025.1607410
Received: 07 April 2025; Accepted: 10 July 2025;
Published: 24 July 2025.
Edited by:
Zhiguang Liu, Shandong Agricultural University, ChinaReviewed by:
Song Baiquan, Heilongjiang University, ChinaHong Lin, Southwest Forestry University, China
Copyright © 2025 Shen, Yan, Pei, Riaz, Dong, Liu, Yin, Jiang and Xia. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Xia, eGhhaG5reTIwMjNAMTYzLmNvbQ==
 Chen Shen1
Chen Shen1 Muhammad Riaz
Muhammad Riaz Chaoqiang Jiang
Chaoqiang Jiang Hao Xia
Hao Xia