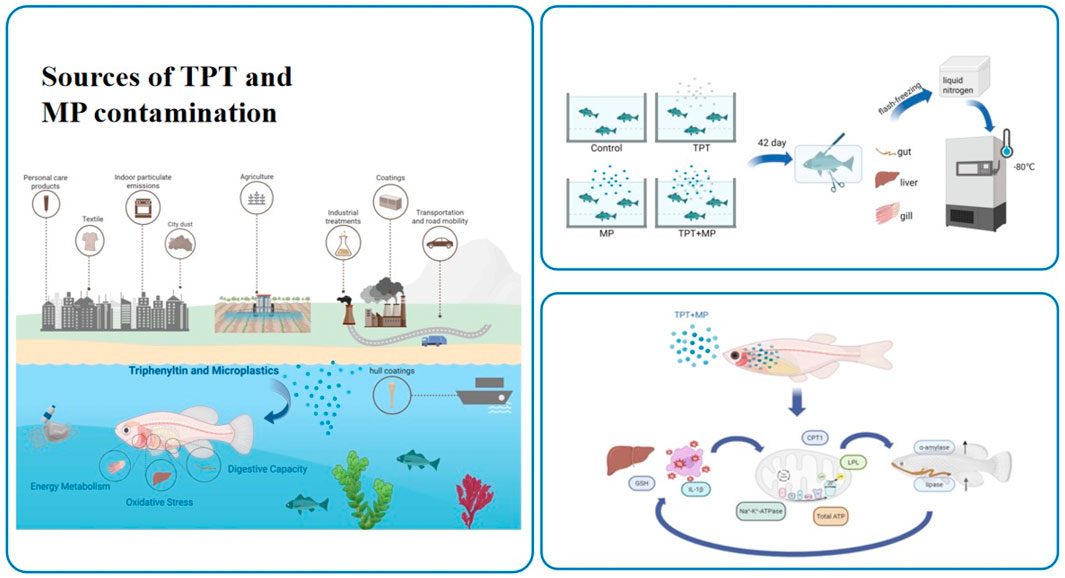
- Marine College, Shandong University, Weihai, Shandong, China
With the increasing presence of emerging pollutants in the environment, the combined toxic effects of organotin compounds and microplastics on aquatic organisms have garnered significant attention. This study investigated the individual and combined toxic effects of triphenyltin (TPT, 1 μg/L) and microplastics (MP, 0.5 mg/L) on oxidative stress, energy metabolism, and digestive function in Cyprinus carpio over a 42-day exposure period. The results demonstrated that TPT and MP combined exposure significantly reduced glutathione (GSH) activity, enhanced the activity of inflammatory factors (IL-1β) and upregulated the expression of antioxidant and inflammatory genes (gpx and il-1β) comparing with the control group in the liver, indicating severe oxidative stress. Combined exposure exhibited complex interactive effects, potentially involving adaptive or antagonistic mechanisms. Regarding energy metabolism, TPT and MP inhibited Na+/K+-ATPase activity, disrupting ion balance in the gills, whereas combined exposure promoted the expression of genes related to fatty acid metabolism (cpt1 and lpl). The combined exposure led to a partial restoration of ion pump activity, which implies the existence of a compensatory mechanism. TPT and MP exerted an inhibitory effect on the activity of digestive enzymes in the intestines, while they triggered a compensatory reaction by upregulating the expression of digestive enzyme genes. The combined exposure vividly unveiled the complex and intertwined interactions between the inhibitory and adaptive responses, highlighting the multifaceted nature of the biological impacts under such combined stressors. This study provides essential insights into the combined toxicity of TPT and MP, highlighting their ecological risks and underscoring the need for improved environmental monitoring and management strategies.
1 Introduction
Triphenyltin (TPT), an organotin compound used in pesticides and antifouling paints, is a persistent organic pollutant due to its toxicity and environmental stability (Anastasiou et al., 2016; Lee et al., 2016; Snoeij et al., 1987) and it poses severe risks to aquatic ecosystems (Liu Q. et al., 2024; Song and Huang, 2005). Despite regulations from the International Maritime Organization (IMO) and European Union limiting its use (de Castro et al., 2012), the misuse of TPT persists globally (Antes et al., 2011; Li and Li, 2021). TPT is widely detected in aquatic environments, with varying concentrations across regions. In China, concentrations average 6.9 ng Sn/L in Bohai Bay (Hu et al., 2006), while higher levels are observed in freshwater systems such as the Yangtze and Jialing Rivers, reaching up to 37.2 ng Sn/L (Gao et al., 2013). Concentrations of 3.8–11.7 ng/L have been observed in Hong Kong waters (Sham et al., 2020). TPT accumulates in aquatic organisms (Liu et al., 2011; Wen et al., 2018), primarily in the liver (Morcillo and Porte, 1997; Harino et al., 2000; Berge et al., 2004), but also in muscle, gills, heart, and brain. It disrupts endocrine systems, affecting development, reproduction, and immunity in marine life (He et al., 2021; Li et al., 2025). Recently, TPT has also been detected in Marine organisms near the Fields Peninsula in Antarctica, and it has been confirmed that it can enter high-trophic organisms such as penguins and birds through the food chain. So biomagnification through the food chain increases TPT levels in higher trophic organisms, posing risks to human health through seafood consumption (Yi et al., 2012).
Microplastics (MPs), defined as plastic particles smaller than 5 mm (Ulluwishewa et al., 2011), are a major environmental pollutant found ubiquitously in aquatic systems, from coastal waters to deep oceans (Xu et al., 2021; Wan et al., 2019; Li X. et al., 2023). Their persistence enables them to adsorb chemical pollutants, including persistent organic pollutants such as TPT, thereby enhancing their toxicity (Pei et al., 2022; Li et al., 2013). MPs originate from tire wear, textile washing, personal care products, paint, and the breakdown of larger plastics (Kelly, 1977; Zitko and Hanlon, 1991). Microplastics have been found extensively in oceans, from coastal regions to the abyssal depths, and have even been discovered in polar areas, with concentrations of around 0.3 mg/L in the North Pacific Subtropical Gyre (Goldstein et al., 2012) and 3.02 mg/L in the Central Pacific Gyre (Sun et al., 2020). MPs significantly affect aquatic organisms by accumulating within their digestive tracts, causing mucosal damage, inflammation, and metabolic disorders (Pei et al., 2022; Lu et al., 2016). They can also enter muscle tissue through gills, inhibiting growth, survival, reproduction, and increasing oxidative damage (Rainieri et al., 2018). MPs disrupt gut microbiota and metabolomes, affecting sugar, lipid, and energy metabolism (Nelms et al., 2016). Their unique surface properties, such as small size and hydrophobicity (Dong et al., 2019), enable them to act as carriers for chemical pollutants, further increasing toxicity (Zhang et al., 2023). The multifaceted toxic effects of MPs pose a substantial threat to aquatic ecosystems. Combined exposure to TPT and MPs is also toxic. It can be neurotoxic to Marine medaka (Lin et al., 2024; Liu L. et al., 2024), and it also has a toxic effect on the gut-brain axis of carp (Zhang et al., 2023).
As a major freshwater cultured fish, carp are economically valuable, easy to breed, and widely used in biological, aquaculture, and ecological research. Carp were chosen as the experimental model due to their environmental adaptability, economic value, and representation of freshwater fish exposed to pollutants. Their well-documented genome and allotetraploid nature (two sets of chromosomes) provide a robust basis for toxicological and gene expression studies. Carp’s polyploid characteristics enhance their adaptability to environmental changes and offer unique insights into polyploid organisms. Their complete genome information supports evolutionary research, while the expression divergence strategies of their A and B sub-genomes provide plasticity and tolerance, aiding gene expression regulation studies.
Despite MPs and TPT being recognized as agents capable of instigating oxidative stress, disrupting energy metabolism, and impeding digestion in fish, the combined toxicological impacts and ecological risks of their co-exposure remain underexplored. This study aims to elucidate the effects of TPT and MPs on carp under both individual and combined exposure scenarios, thereby addressing a critical gap in the existing literature.
2 Materials and methods
2.1 Reagents and experimental animals
2.1.1 Experimental reagents and reagent preparation
Triphenyltin chloride (96%, CAS 639–58-7) was obtained from Shanghai Macklin Biochemical Technology Co., Ltd. and used as the main pollutant. For the TPT stock solutions, dimethyl sulfoxide (DMSO, 99.5%, Solarbio, Beijing) was used as the solvent. Polystyrene microspheres (200 nm diameter, 0.025 g/mL stock concentration) were obtained from Wuxi RuiGe Technology Co., Ltd. Additionally, all reagents used in this study were of analytical grade to endure experimental reliability.
Preparation of TPT Exposure Solution: 30 mg of triphenyltin (TPT) powder was dissolved in 1 mL of dimethyl sulfoxide (DMSO) to produce a 30 mg/mL TPT stock solution. This stock solution was gradually diluted to achieve a concentration of 0.3 μg/μL TPT. A 1 μg/L TPT exposure solution was prepared by mixing 100 μL of the 0.3 μg/μL TPT solution with 30 L of water, and the mixture was stored at 4°C. To prevent contamination, the stock solution was dispensed into 1 mL centrifuge tubes before use. Preparation of MP Exposure Solution: 600 μL of the MP suspension stock solution (0.025 g/mL, polystyrene microspheres with a diameter of 200 nm) was added to 30 L of water to prepare a 0.5 mg/L MP exposure solution. Preparation of TPT and MP Combined Exposure Solution: The same protocol as described above was followed.
2.1.2 Experimental animals and cultivation environment
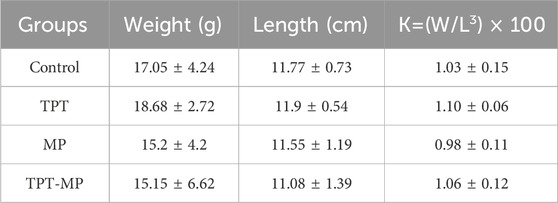
The carp utilized in this study were obtained from Tianjin Xinda Breeding Farm, and their weight and length measurements were presented (Table 1). Before the experiment, the fish underwent a 2-week acclimatization period, during which they were fed commercial bait twice daily (at 9:00 a.m. and 3:00 p.m.). Water changes were conducted every 2 days to remove excreta and uneaten bait. The water temperature was maintained at 23°C ± 1°C, and a photoperiod of 14 h of light and 10 h of darkness was implemented. The temporary rearing system comprised a temperature-controlled recirculating system equipped with multiple aeration stones to ensure sufficient dissolved oxygen levels.
2.2 Exposure experiment and sampling
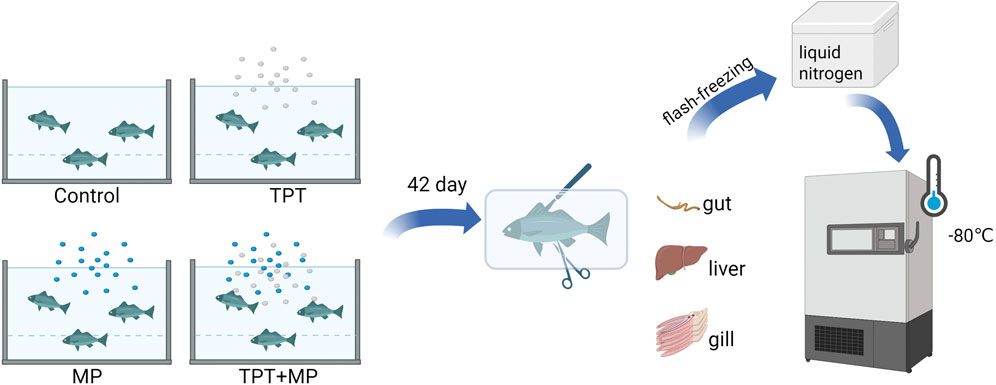
The study was conducted with a single control group alongside three distinct treatment groups. 0, 1 μg/L TPT, 0.5 mg/L MP and 1 μg/L of TPT with 0.5 mg/L of MP were adopted as the exposure concentrations. The concentration levels for these treatment groups were determined based on the environmental concentrations found in highly polluted regions and previous toxicological studies (He et al., 2022; Ding et al., 2022).
The experimental carp were housed in a total of 12 aquariums, each with a volume of 70 L, dividing them into three replicates for every group. Each aquarium contained 12 carp, and the volume of the experimental solution used was 30 L. Throughout the experiment, the carp were fed commercial feed twice daily at 9:00 a.m. and 3:00 p.m. To ensure consistent experimental solution concentrations and maintain optimal water quality, water was replaced every 2 days, and waste materials, including uneaten food, were removed. All experimental protocols complied with the guidelines of the Chinese Association for Laboratory Animal Sciences and adhered to ethical standards for animal research stipulated by Shandong University, with humanitarian principles rigorously upheld.
The fish were exposed continuously to the experimental conditions for 42 days prior to sampling. A 24-h fasting period was implemented before tissue collection. During sampling, the carp were anesthetized with MS222, and their body weight and length were measured (Table 1). Tissues from the liver, intestine, and gills were immediately preserved by freezing in liquid nitrogen, followed by transfer to a −80°C freezer for long-term storage. These samples were later used for biochemical and RT-qPCR gene expression analyses. The exposure and sampling workflow is illustrated (Figure 1).
2.3 Biochemical index detection
Tissue samples were rinsed with cold physiological saline to remove residual blood, dried using filter paper, and accurately weighed before being transferred to 5 mL homogenizing tubes. Homogenizing medium (pH 7.4, composed of 0.01 mol/L Tris-HCl, 1 mmol/L EDTA-2Na, 0.01 mol/L sucrose, and 0.8% NaCl solution) was added to the tubes at a weight-to-volume ratio of 1:9 (g/mL). The tissues were homogenized in an ice bath using an internal cutting tissue homogenizer (10 s per cycle, with 30-s intervals, for 3–5 cycles) to ensure uniformity. Following centrifugation at 2500 rpm for 10 min, the supernatant was collected and further diluted with homogenizing medium to achieve the required concentration for subsequent analyses.
Biochemical markers, including glutathione, interleukin-1β, amylase, Na+/K+-ATPase, and total ATP, were measured in accordance with the protocols provided by Nanjing Jiancheng reagent kits.
2.4 qRT-PCR detection
A SYBR Green Premix Pro Taq HS qRT-PCR Kit was used for a 10 μL qRT-PCR reaction system. The template was diluted 5 times, and the amplification efficiency of the primers was obtained according to the standard curve. The specificity of qRT-PCR amplification was judged by the presence of a single peak at the expected Tm value on the melting curve. Each sample was repeated three times. The qRT-PCR reaction system was as follows: 2 × SYBR Green Pro Taq HS Premix, 5 μL; Template, <100 ng; Prime F (10 μM), 0.2 μL; Prime R (10 μM), 0.2 μL; ROX Reference Dye (4 μM), 0.2 μL; RNase-free water, up to 10 μL. The reaction mixture was mixed and briefly centrifuged. The reaction liquid was placed at the bottom of the wells of a 96-well plate and subjected to qRT-PCR reaction in a fluorescence quantitative qRT-PCR instrument (LightCycler 96) with the following program:
Two-step qRT-PCR reaction program: Step 1: 95°C, 30 s, 1 cycle; Step 2: 95°C, 5 s, 60°C, 30 s, 40 cycles; Step 3: Melting stage.
β-actin was stably expressed throughout the experiment and used as an internal control. The relative mRNA levels of the target genes were normalized using the 2-△△CT method for relative quantification (Table 2).
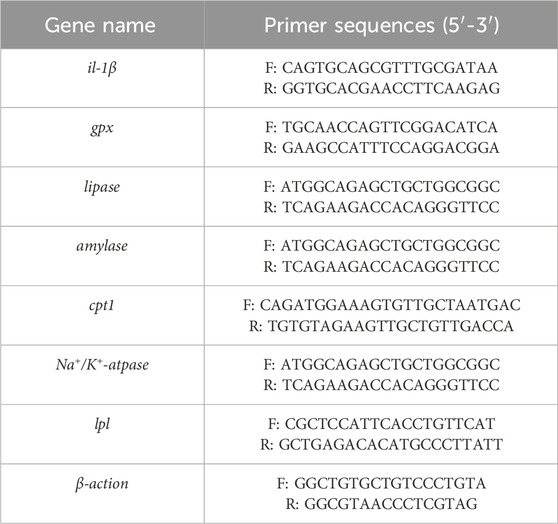
Table 2. The primers used for qRT-PCR analysis (Zhang et al., 2021b; Hoseini et al., 2020; Al-Ghanem, 2011).
2.5 Statistical analysis
Statistical analysis was conducted using SPSS 27.0. One-way analysis of variance (ANOVA) followed by Duncan’s multiple comparison tests was used to compare the significant differences between different groups. Results were presented as mean ± standard deviation (SD), with significance determined at P < 0.05. GraphPad Prism 8.0.1 was used to generate plots, while Chiplot platform was utilized for analyzing principal coordinate.
3 Results and discussion
3.1 Oxidative stress
The oxidative stress responses were illustated in carp livers under different exposure conditions, including the control group (C), TPT exposure group (TPT), microplastics exposure group (MP), and the combined exposure group (TPT + MP).
GSH activity was significantly reduced in the TPT and MP group compared to the control group, indicating that pollutants induces oxidative stress by depleting antioxidant reserves to neutralize reactive oxygen species (ROS). Interestingly, the TPT + MP group showed partial recovery in GSH activity compared to the MP group, possibly due to microplastics adsorbing TPT, reducing its bioavailability, activating Nrf2-ARE pathways to enhance antioxidant defenses, regulating mitochondrial GSH transport, and triggering compensatory physiological adaptations (Figure 2a).
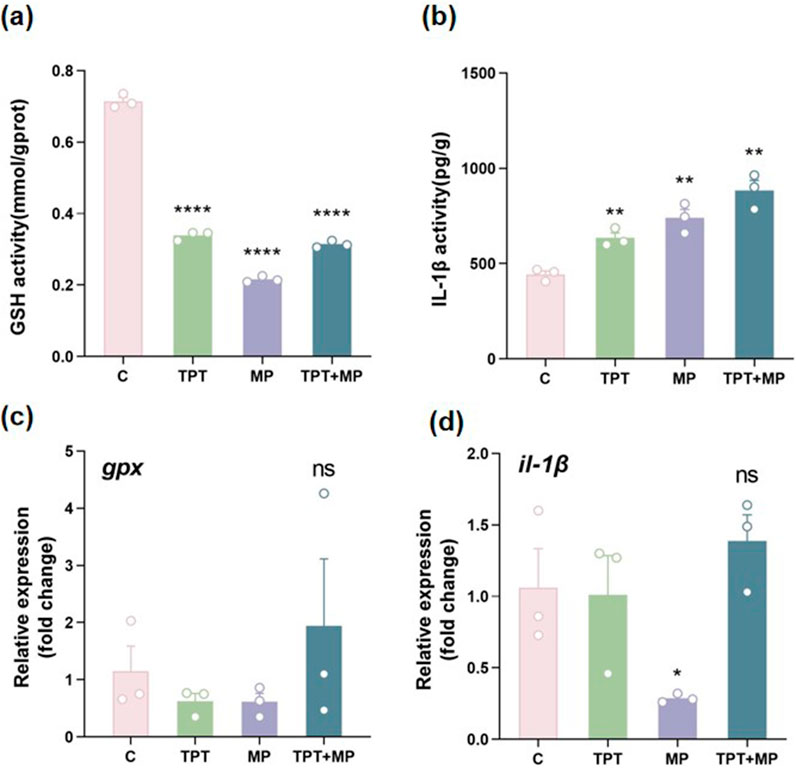
Figure 2. Comparison of biochemical and RT-PCR indicators of oxidative stress levels among the control group (C), TPT exposure group (TPT), microplastics exposure group (MP), and the combined TPT and microplastics exposure group (TPT + MP). (a) Glutathione (GSH) activity (mmol/gprot); (b) Interleukin-1β (IL-1β) activity (pg/g); (c) Relative expression of the gpx; (d) Relative expression of the il-1β. Data are presented as mean ± SEM; Significance levels: **P < 0.01, ****P < 0.0001; ns indicates no significant difference.
IL-1β, a key inflammatory marker, was elevated in the TPT, MP and TPT + MP groups (P < 0.01), with the highest levels observed in the combined exposure group. This suggests that co-exposure exacerbates the inflammatory response, likely through a synergistic interaction between TPT and MP (Figure 2b). Previous studies indicate that TPT induces inflammation by activating pro-inflammatory cytokines (Li ZH. et al., 2023), while MP increases oxidative stress, which can amplify cytokine production (Pei et al., 2022).
Relative expression of gpx gene, a critical antioxidant enzyme, showed no significant difference among groups (P > 0.05). However, the TPT + MP group exhibited the highest mean value, suggesting that co-exposure may induce stronger oxidative stress, requiring upregulation of antioxidant defenses (Figure 2c). This trend aligns with previous findings that combined pollutants often enhance oxidative damage (Wang et al., 2023).
il-1β gene expression displayed no statistically significant changes among groups (P > 0.05), but the TPT + MP group showed a upward trend. This aligns with the biochemical data for IL-1β activity, indicating that transcriptional regulation of inflammatory responses may require further investigation under chronic exposure scenarios (Figure 2d).
Overall, these results confirm that TPT exerts a pronounced impact on oxidative stress and inflammation in carp livers, as evidenced by reduced GSH activity and increased IL-1β levels. MP exposure alone causes milder effects, but co-exposure results in complex interactions, likely synergistic, that exacerbate oxidative damage and inflammatory responses. TPT disrupts mitochondrial function and generates ROS, while MP induces membrane damage and oxidative stress, collectively amplifying toxicity. This highlights the need for further research on pollutant interactions and their ecological implications (Zhang et al., 2021a; Zhang et al., 2024; Zhou et al., 2019; Usman et al., 2021).
3.2 Energy metabolism
Significant disruptions were revealed in energy metabolism caused by TPT and MP exposure (Figure 3). Na+/K+-ATPase activity decreased in the TPT group compared to the control (P < 0.05), indicating ionic regulation impairment likely due to oxidative stress-induced membrane damage. In the MP group, a decrease in Na+/K+-ATPase activity was observed, while partial recovery in the TPT + MP group suggests compensatory mechanisms or interactions between MP and TPT mitigating toxicity (Figure 3a).
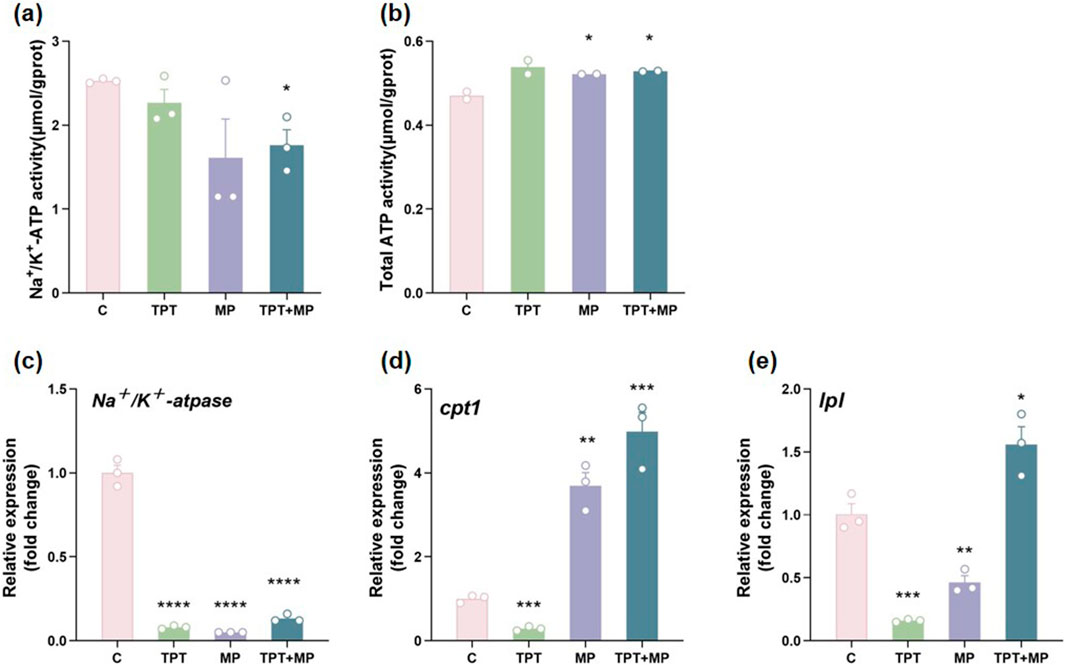
Figure 3. Comparison of biochemical and RT-PCR indicators of energy metabolism levels among the control group (C), TPT exposure group (TPT), microplastics exposure group (MP), and the combined TPT and microplastics exposure group (TPT + MP). (a) Na+/K+-ATPase activity (μmol/gprot); (b) Total ATP activity (μmol/gprot); (c) Relative expression of the Na+/K + -ATPase gene; (d) Relative expression of the CPT1 gene; (e) Relative expression of the LPL gene. Data are presented as mean ± SEM; Significance levels: *P < 0.05, ***P < 0.001, ****P < 0.0001.
Total ATP activity also increased in the TPT group and in the MP group (P < 0.05). However, in the TPT + MP group, ATP levels were restored to control-like levels, implying activation of alternative metabolic pathways to sustain energy homeostasis (Figure 3b).
qRT-PCR analysis highlights gene expression changes. cpt1, key lipid metabolism enzymes, were significantly upregulated in the TPT + MP group (P < 0.001), indicating enhanced fatty acid β-oxidation and lipolytic activity as adaptive responses. The TPT + MP group showed the highest cpt1 and lpl expression, reflecting synergistic metabolic stress from TPT and MP. Interestingly, lpl expression was suppressed in the TPT group and further reduced in the TPT + MP group, suggesting TPT-induced lipolysis inhibition (Figures 3c–e).
TPT alone disrupted Na+/K+-ATPase activity via ROS-induced lipid peroxidation and membrane damage, while MP alone caused milder ion regulation impairment. In co-exposure, MP likely mitigated TPT toxicity by adsorbing it and activating Nrf2-ARE pathways to enhance antioxidant enzymes (e.g., GPx1), thereby partially restoring ion pump function. ATP levels increased in TPT/MP groups due to glycolysis activation or mitochondrial compensatory metabolism, whereas co-exposure restored ATP to control levels through repairing mitochondrial function and regulating energy metabolic pathways, indicating MP’s multi-target intervention to maintain energy homeostasis (Zhou et al., 2019; Feng et al., 2024; Shen et al., 2021; Zhang et al., 2022). Combined exposure reveals intricate compensatory and inhibitory interactions, underscoring the need for further research on pollutant co-toxicity mechanisms.
3.3 Digestive capacity
The impact of TPT and MP on digestive enzyme activity and gene expression was illustrated in carp intestines. The α-amylase activity was the highest in the control group, with no significant differences observed between the groups (P > 0.05) (Figure 4a). This indicates that starch digestion capacity remained stable despite exposure. However, at the molecular level, a significant upregulation of the α-amylase gene expression in the MP and group (P < 0.001), suggested transcriptional compensation for potential enzymatic inhibition. The TPT + MP group exhibited the higher α-amylase gene expression than the control group, implying a synergistic effect between TPT and MP that intensifies the demand for starch metabolism (Figure 4b). For lipase, the MP exposure group upregulated lipase gene expression (P < 0.01), possibly as an adaptive response to energy deficits (Figure 4c).
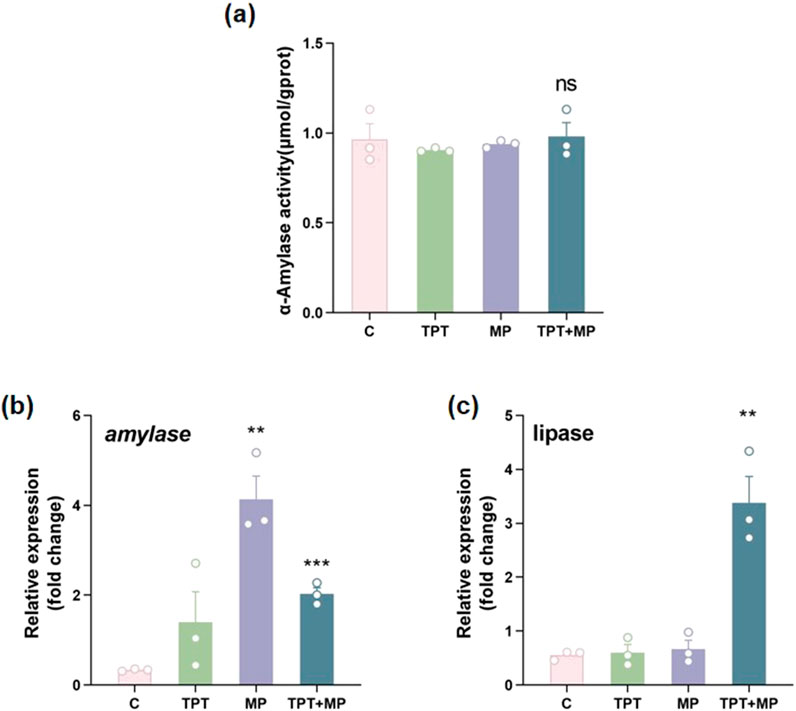
Figure 4. Comparison of biochemical and RT-PCR indicators of digestive capacity levels among the control group (C), TPT exposure group (TPT), microplastics exposure group (MP), and the combined TPT and microplastics exposure group (TPT + MP). (a) α-Amylase activity (μmol/gprot); (b) Relative expression of the α-Amylase gene; (c) Relative expression of the Lipase gene. Data are presented as mean ± SEM; Significance levels: **P < 0.01, ***P < 0.001; ns indicates no significant difference.
TPT directly inhibited α-amylase activity to disrupt starch digestion, while MP alone activated compensatory mechanisms (e.g., Nrf2-ARE pathways) to upregulate α-amylase gene expression. In co-exposure, MP likely reduced TPT bioavailability via adsorption and MP-induced mechanical damage or metabolic stress further activated compensatory demand for starch metabolism, leading to synergistic upregulation of α-amylase genes. Lipase activity decline was attributed to TPT’s direct toxicity on lipid metabolism, whereas MP alone induced lipidase gene upregulation via gut microbiota disruption or energy deficits; co-exposure exacerbated metabolic stress, sustaining high lipidase gene expression to maintain energy homeostasis (Jiang et al., 2023; Chen et al., 2020; Trestrail et al., 2021). These findings warrant further investigation into the molecular mechanisms underlying pollutant interactions in aquatic organisms.
3.4 Correlation analysis
The correlation heatmaps illustrate the complex interplay among biochemical parameters and gene expressions under pollutant exposure. Total ATP exhibits a positive correlation with IL-1β (r = 0.68), suggesting that inflammatory responses may influence cellular metabolic activity. Similarly, IL-1β is positively correlated with α-amylase (r = 0.66), highlighting the oxidative stress-mediated impact on digestive enzyme activity. Conversely, total ATP shows a negative correlation with Na+/K+-ATPase (r = −0.36), reflecting potential antagonism between energy metabolism and ion transport. The strong negative correlation between GSH and Total ATP (r = −0.86) suggests that oxidative stress significantly depletes antioxidant capacity. Hierarchical clustering reveals that GSH and Na+/K+-ATPase cluster closely, indicating oxidative stress-induced ion homeostasis disruptions, while the clustering of IL-1β and α-amylase suggests a mechanistic link between inflammation and digestive processes (Figure 5a).
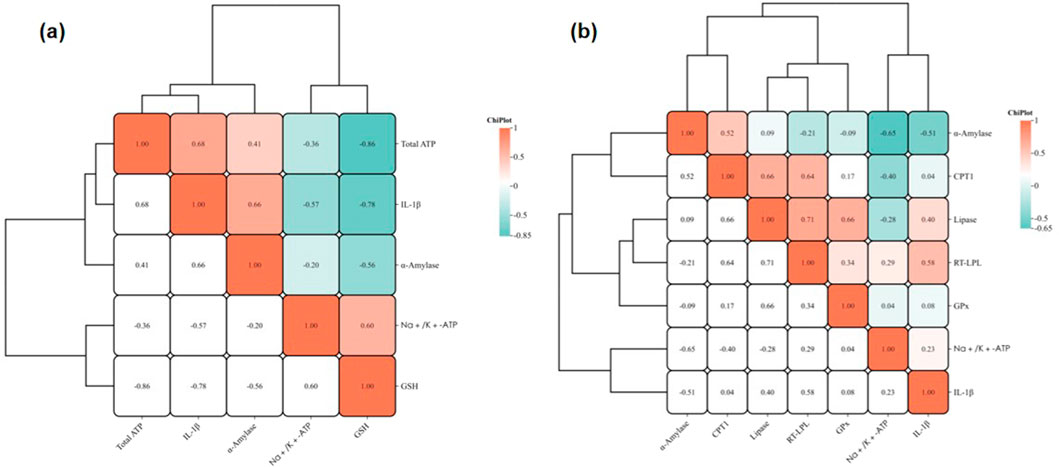
Figure 5. Correlation heatmaps of biochemical parameters and gene expression levels. (a) Correlations among biochemical parameters; (b) Correlations among gene expression levels. Colors represent the strength of the correlations, with red indicating positive correlations and blue indicating negative correlations. Hierarchical clustering demonstrates potential interaction patterns among the indicators.
qRT-PCR data show that CPT1 and Lipase are strongly correlated (r = 0.66), indicating co-regulation of lipid metabolism. LPL and lipase also exhibit a high correlation (r = 0.71), reinforcing the disruption of lipid metabolism pathways. Interestingly, α-amylase is negatively correlated with Na+/K+-ATPase (r = −0.65), suggesting reduced energy metabolism impairs digestive enzyme activity. IL-1β displays a mild positive correlation with Na+/K+-ATPase (r = 0.23), implying inflammation may modulate ion transport. GPx is positively correlated with RT-LPL (r = 0.34) and lipase (r = 0.66), linking oxidative stress with lipid metabolic pathways (Figure 5b).
TPT disrupts mitochondrial fatty acid oxidation and induces inflammation, while MP enhances oxidative stress and membrane damage through physical and chemical interactions. Combined exposure synergistically amplifies these toxic effects, disrupting energy metabolism, antioxidant defense, and lipid pathways (Capitao et al., 2017; Solomando et al., 2020; Wright and Kelly, 2017).
The PCoA plot illustrates distinct clustering of experimental groups, indicating significant biochemical variations induced by pollutant exposure. Control (purple) and exposure groups (TPT, MP, TPT + MP) are well-separated along PCoA1 (95.25%), reflecting major differences in biochemical indicators. The TPT + MP group (green) diverges on both PCoA1 and PCoA2 axes, suggesting enhanced toxicity under combined exposure. Notably, MP (orange) and TPT (red) exhibit different patterns along PCoA2 (3.95%), highlighting distinct mechanisms of toxicity (Figure 6).
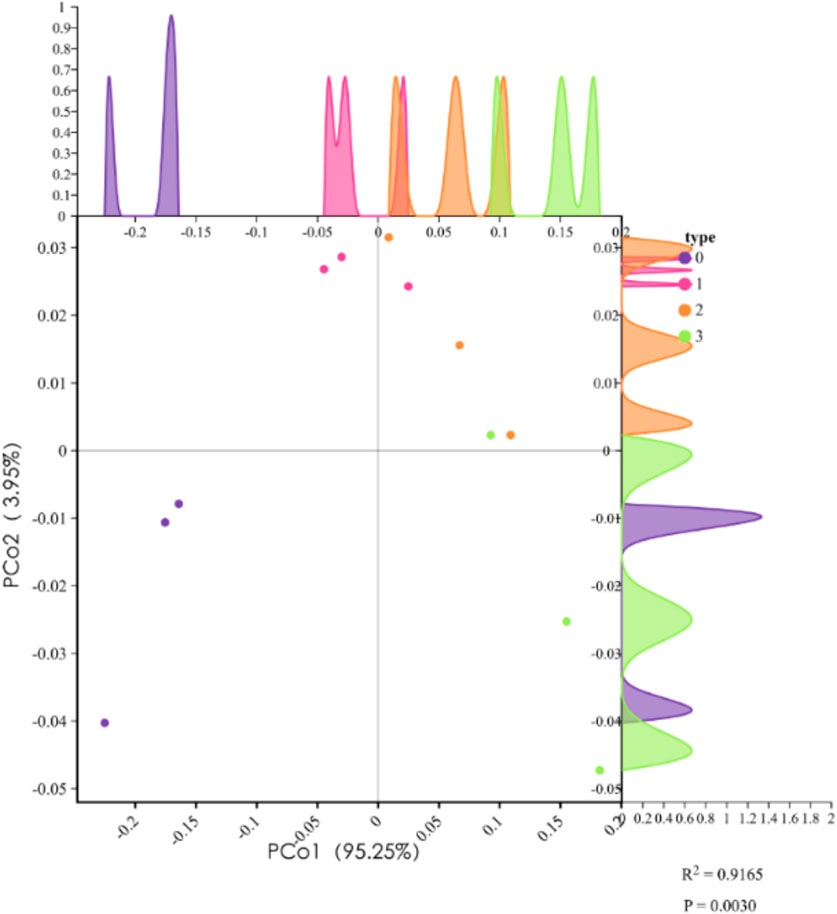
Figure 6. Principal coordinate analysis (PCoA) results of different treatment groups. PCoA1 explains 95.25% of the variance, and PCoA2 explains 3.95%. Samples include the control group (purple, type 0), TPT exposure group (pink, type 1), microplastics exposure group (orange, type 2), and the combined TPT and microplastics exposure group (green, type 3). Density curves illustrate the distribution trends of each group along PCoA1 and PCoA2 axes. The results demonstrate significant separation of treatment groups along PCoA1 (R2 = 0.9165, P = 0.0030), indicating notable differences in biochemical and gene expression parameters among groups.
Biochemical data support these findings. GSH levels were significantly reduced in all exposure groups, indicating oxidative stress. Elevated IL-1β levels confirm strong inflammatory responses, supported by increased RT-IL-1β expression in RT-PCR data. Na+/K+-ATPase activity decreased in exposure groups, reflecting ionic imbalance. Meanwhile, upregulated CPT1 and Lipase expression in TPT + MP groups indicates compensatory shifts in lipid metabolism under stress. These results underscore the synergistic effects of TPT and MP in disrupting oxidative balance, inducing inflammation, and altering energy metabolism.
4 Conclusion
This study investigated the toxic effects of triphenyltin (TPT) and microplastics (MPs) on carp (Cyprinus carpio) under single and combined exposure conditions over a 42-day period. Both TPT and MPs can trigger oxidative stress and inflammatory responses, and co-exposure has a more severe impact on the experimental subjects, characterized by reduced glutathione (GSH) activity and upregulated expression of antioxidant and inflammatory markers (GPx1 and IL-1β). As for energy metabolism, co-exposure may regulate gill ion homeostasis (restore Na+/K+-ATPase activity) through a compensation mechanism. TPT impaired both amylase and lipase activities in the intestines, suppressing starch and lipid digestion, while MP exposure upregulated the expression of digestive enzyme genes as a compensatory response. Co-exposure revealed a complex interplay, with potential adaptive adjustments in digestive metabolism.
These findings offer valuable insights into the individual and combined toxicities of triphenyltin (TPT) and microplastics (MPs), revealing their potential synergistic or antagonistic effects on aquatic organisms. Given the widespread presence of these pollutants in aquatic ecosystems, this study underscores the need for further mechanistic investigations to elucidate the molecular pathways underlying pollutant interactions. Moreover, this study was limited to a specific highly polluted environment, but its systematic analysis provided an important benchmark for understanding ecological responses under extreme pollution conditions. Subsequent research needs to further clarify the applicable scope and boundary conditions of the conclusion through multi-scale and multi-dimensional comparative experiments, thereby providing more precise scientific support for the differentiated protection of aquatic ecosystems. By enhancing our understanding of the ecological risks posed by TPT and MPs, this work provides a scientific basis for environmental risk assessment and the development of effective strategies to protect aquatic ecosystems and biodiversity.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics statement
The animal study was approved by the ethics committee of Shandong University. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
PL: Writing – review and editing, Software. YW: Writing – original draft. SZ: Writing – review and editing, Investigation. TL: Methodology, Writing – review and editing. CL: Writing – review and editing, Validation. LL: Writing – review and editing, Software. Z-HL: Supervision, Conceptualization, Writing – review and editing, Funding acquisition.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by National Natural Science Foundation of China, 42277269.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Al-Ghanem, K. A. (2011). Effect of cobalt-supplemented diets on bioaccumulation, digestive enzyme activities and growth of Cyprinus carpio. Toxicol. Environ. Chem. 93 (5), 985–995. doi:10.1080/02772248.2011.568088
Anastasiou, T. I., Chatzinikolaou, E., Mandalakis, M., and Arvanitidis, C. (2016). Imposex and organotin compounds in ports of the Mediterranean and the Atlantic: is the story over? Sci. Total Environ. 569, 1315–1329. doi:10.1016/j.scitotenv.2016.06.209
Antes, F. G., Krupp, E., Flores, E. M. M., Dressler, V. L., and Feldmann, J. (2011). Speciation and degradation of triphenyltin in typical paddy Fields and its uptake into rice plants. Environ. Sci. and Technol. 45 (24), 10524–10530. doi:10.1021/es202832g
Berge, J. A., Brevik, E. M., Bjorge, A., Folsvik, N., Gabrielsen, G. W., and Wolkers, H. (2004). Organotins in marine mammals and seabirds from Norwegian territory. J. Environ. Monit. 6 (2), 108–112. doi:10.1039/b311662j
Capitao, A., Lyssimachou, A., Castro, L. F. C., and Santos, M. M. (2017). Obesogens in the aquatic environment: an evolutionary and toxicological perspective. Environ. Int. 106, 153–169. doi:10.1016/j.envint.2017.06.003
Chen, Q., Lv, W., Jiao, Y., Liu, Z., Li, Y., Cai, M., et al. (2020). Effects of exposure to waterborne polystyrene microspheres on lipid metabolism in the hepatopancreas of juvenile redclaw crayfish, Cherax quadricarinatus. Aquat. Toxicol., 224. doi:10.1016/j.aquatox.2020.105497
de Castro, I. B., Perina, F. C., and Fillmann, G. (2012). Organotin contamination in South American coastal areas. Environ. Monit. Assess. 184 (3), 1781–1799. doi:10.1007/s10661-011-2078-7
Ding, N., Jiang, L., Wang, X., Wang, C., Geng, Y., Zhang, J. X., et al. (2022). Polyethylene microplastic exposure and concurrent effect with Aeromonas hydrophila infection on zebrafish. Environ. Sci. Pollut. Res. 29 (42), 63964–63972. doi:10.1007/s11356-022-20308-9
Dong, X. F., Zheng, M. G., Qu, L. Y., Shi, L., Wang, L., Zhang, Y., et al. (2019). Sorption of tonalide, musk xylene, galaxolide, and musk ketone by microplastics of polyethylene and polyvinyl chloride. Mar. Pollut. Bull. 144, 129–133. doi:10.1016/j.marpolbul.2019.04.046
Feng, L., Jiang, W., Wu, P., and Liu, H. (2024). The development of “Marine Ranching” requires global interdisciplinary collaboration and academic sharing. 2, 118. doi:10.1002/aro2.64
Gao, J. M., Zhang, Y., Guo, J. S., Jin, F., and Zhang, K. (2013). Occurrence of organotins in the Yangtze river and the jialing river in the urban section of chongqing, China. Environ. Monit. Assess. 185 (5), 3831–3837. doi:10.1007/s10661-012-2832-5
Goldstein, M. C., Rosenberg, M., and Cheng, L. N. (2012). Increased oceanic microplastic debris enhances oviposition in an endemic pelagic insect. Biol. Lett. 8 (5), 817–820. doi:10.1098/rsbl.2012.0298
Harino, H., Fukushima, M., and Kawai, S. (2000). Accumulation of butyltin and phenyltin compounds in various fish species. Archives Environ. Contam. Toxicol. 39 (1), 13–19. doi:10.1007/s002440010074
He, S. W., Li, P., and Li, Z. H. (2021). Review on endocrine disrupting toxicity of triphenyltin from the perspective of species evolution: aquatic, amphibious and mammalian. Chemosphere, 269. doi:10.1016/j.chemosphere.2020.128711
He, S. W., Yu, D. D., Li, P., Zhang, M., Xing, S. Y., Liu, B., et al. (2022). A new perspective on endocrine disrupting effects of triphenyltin on marine medaka: from brain transcriptome, gut content metabolome and behavior. Chemosphere, 307. doi:10.1016/j.chemosphere.2022.136190
Hoseini, S. M., Khalili, M., Rajabiesterabadi, H., Hoseinifar, S. H., and Doan, H. V. (2020). Effects of dietary monoterpene, myrcene, administration on immune- and health-related genes expression in common carp gill following exposure to copper sulfate. Fish and Shellfish Immunol. 98, 438–445. doi:10.1016/j.fsi.2020.01.027
Hu, J. Y., Zhen, H. J., Wan, Y., Gao, J. M., An, W., An, L. H., et al. (2006). Trophic magnification of triphenyltin in a marine food web of Bohai Bay, north China: comparison to tributyltin. Environ. Sci. and Technol. 40 (10), 3142–3147. doi:10.1021/es0514747
Jiang, H., Li, Q.-Y., Sun, J.-X., Mao, Y.-F., Liu, X., Que, S., et al. (2023). The adsorption and desorption behavior of bisphenol A on five microplastics under simulated gastrointestinal conditions. Water Air Soil Pollut. 234 (2), 106. doi:10.1007/s11270-023-06105-1
Kelly, R. (1977). Mycobacterium-marinum infection from tropical fish tank - treatment with trimethoprim and sulfamethoxazole. Clin. Med. 84 (11), 27. doi:10.5694/j.1326-5377.1976.tb130426.x
Lee, C. C., Hsu, Y. C., Kao, Y. T., and Chen, H. L. (2016). Health risk assessment of the intake of butyltin and phenyltin compounds from fish and seafood in Taiwanese population. Chemosphere 164, 568–575. doi:10.1016/j.chemosphere.2016.08.141
Li, P., Liu, B., He, S. W., Liu, L., and Li, Z. H. (2025). Transgenerational neurotoxic effects of triphenyltin on marine medaka: impaired dopaminergic system function. Environ. Pollut. 366, 125456. doi:10.1016/j.envpol.2024.125456
Li, X., Sun, C., and Li, F. (2023a). Transfer of polystyrene microplastics with different functional groups in the aquatic food chain. J. Phys. Conf. Ser. 2463, 012059. doi:10.1088/1742-6596/2463/1/012059
Li, Z. H., and Li, P. (2021). Effects of the tributyltin on the blood parameters, immune responses and thyroid hormone system in zebrafish. Environ. Pollut. 268 (Pt A), 115707. doi:10.1016/j.envpol.2020.115707
Li, Z. H., Xing, S. Y., Li, P., He, S. W., Cao, Z. H., Wang, X., et al. (2023b). Systematic toxicological analysis of the effect of salinity on the physiological stress induced by triphenyltin in Nile tilapia. Aquat. Toxicol. 257, 106441. doi:10.1016/j.aquatox.2023.106441
Li, Z. H., Xu, H. Y., Zheng, W. L., Lam, S. H., and Gong, Z. Y. (2013). RNA-sequencing analysis of TCDD-induced responses in zebrafish liver reveals high relatedness to in vivo mammalian models and conserved biological pathways. Plos One 8 (10), e77292. doi:10.1371/journal.pone.0077292
Lin, P. R., Liu, L., Ma, Y. Q., Du, R. Y., Yi, C. S., Li, P., et al. (2024). Neurobehavioral toxicity induced by combined exposure of micro/nanoplastics and triphenyltin in marine medaka (Oryzias melastigma). Environ. Pollut., 356. doi:10.1016/j.envpol.2024.124334
Liu, L., Du, R. Y., Jia, R. L., Wang, J. X., Chen, C. Z., Li, P., et al. (2024b). Micro(nano)plastics in marine medaka: entry pathways and cardiotoxicity with triphenyltin. Environ. Pollut. 1, 123079. doi:10.1016/j.envpol.2023.123079
Liu, L. L., Wang, J. T., Chung, K. N., Leu, M. Y., and Meng, P. J. (2011). Distribution and accumulation of organotin species in seawater, sediments and organisms collected from a Taiwan mariculture area. Mar. Pollut. Bull. 63 (5-12), 535–540. doi:10.1016/j.marpolbul.2011.02.003
Liu, Q., Wei, J., and Wang, Y. (2024a). Triphenyltin induced oxidative damage in sea urchin Glyptocidaris crenularis. Proc. SPIE 13279, 132791G–132796. doi:10.1117/12.3044595
Lu, Y. F., Zhang, Y., Deng, Y. F., Jiang, W., Zhao, Y. P., Geng, J. J., et al. (2016). Uptake and accumulation of polystyrene microplastics in zebrafish (Danio rerio) and toxic effects in liver. Environ. Sci. and Technol. 50 (7), 4054–4060. doi:10.1021/acs.est.6b00183
Morcillo, Y., and Porte, C. (1997). Interaction of tributyl- and triphenyltin with the microsomal monooxygenase system of molluscs and fish from the western Mediterranean. Aquat. Toxicol. 38 (1-3), 35–46. doi:10.1016/s0166-445x(96)00841-7
Nelms, S. E., Duncan, E. M., Broderick, A. C., Galloway, T. S., Godfrey, M. H., Hamann, M., et al. (2016). Plastic and marine turtles: a review and call for research. Ices J. Mar. Sci. 73 (2), 165–181. doi:10.1093/icesjms/fsv165
Pei, X., Heng, X., and Chu, W. H. (2022). Polystyrene nano/microplastics induce microbiota dysbiosis, oxidative damage, and innate immune disruption in zebrafish. Microb. Pathog., 163. doi:10.1016/j.micpath.2021.105387
Rainieri, S., Conlledo, N., Larsen, B. K., Granby, K., and Barranco, A. (2018). Combined effects of microplastics and chemical contaminants on the organ toxicity of zebrafish (Danio rerio). Environ. Res. 162, 135–143. doi:10.1016/j.envres.2017.12.019
Sham, R. C. T., Ho, K. K. Y., Zhou, G. J., Li, Y. Y., Wang, X. H., and Leung, K. M. Y. (2020). Occurrence, ecological and human health risks of phenyltin compounds in the marine environment of Hong Kong. Mar. Pollut. Bull., 154. doi:10.1016/j.marpolbul.2020.111093
Shen, J., Liang, B., Zhang, D., Li, Y., Tang, H., Zhong, L., et al. (2021). Effects of PET microplastics on the physiology of Drosophila. Chemosphere 283, 131289. doi:10.1016/j.chemosphere.2021.131289
Snoeij, N. J., Penninks, A. H., and Seinen, W. (1987). Biological-activity of organotin compounds - an overview. Environ. Res. 44 (2), 335–353. doi:10.1016/s0013-9351(87)80242-6
Solomando, A., Capo, X., Alomar, C., Alvarez, E., Compa, M., Valencia, J. M., et al. (2020). Long-term exposure to microplastics induces oxidative stress and a pro-inflammatory response in the gut of Sparus aurata Linnaeus, 1758. Environ. Pollut. 266 (Pt 1), 115295. doi:10.1016/j.envpol.2020.115295
Song, Z. H., and Huang, G. L. (2005). Toxic effect of triphenyltin onLemna polyrhiza. Appl. Organomet. Chem. 19 (7), 807–810. doi:10.1002/aoc.893
Sun, S. G., Shi, W., Tang, Y., Han, Y., Du, X. Y., Zhou, W. S., et al. (2020). Immunotoxicity of petroleum hydrocarbons and microplastics alone or in combination to a bivalve species: synergic impacts and potential toxication mechanisms. Sci. Total Environ., 728. doi:10.1016/j.scitotenv.2020.138852
Trestrail, C., Walpitagama, M., Miranda, A., Nugegoda, D., and Shimeta, J. (2021). Microplastics alter digestive enzyme activities in the marine bivalve, Mytilus galloprovincialis. Sci. Total Environ. 779, 146418. doi:10.1016/j.scitotenv.2021.146418
Ulluwishewa, D., Anderson, R. C., McNabb, W. C., Moughan, P. J., Wells, J. M., and Roy, N. C. (2011). Regulation of tight junction permeability by intestinal bacteria and dietary components. J. Nutr. 141 (5), 769–776. doi:10.3945/jn.110.135657
Usman, S., Razis, A. F. A., Shaari, K., Amal, M. N. A., Saad, M. Z., Isa, N. M., et al. (2021). Polystyrene microplastics exposure: an insight into multiple organ histological alterations, oxidative stress and neurotoxicity in Javanese medaka fish (Oryzias javanicus bleeker, 1854). Int. J. Environ. Res. Public Health 18 (18), 9449. doi:10.3390/ijerph18189449
Wan, Z. Q., Wang, C. Y., Zhou, J. J., Shen, M. L., Wang, X. Y., Fu, Z. W., et al. (2019). Effects of polystyrene microplastics on the composition of the microbiome and metabolism in larval zebrafish. Chemosphere 217, 646–658. doi:10.1016/j.chemosphere.2018.11.070
Wang, Y., Zhao, Y. X., Liang, H. W., Ma, C. F., Cui, N. Q., Cao, H. H., et al. (2023). Single and combined effects of polyethylene microplastics and acetochlor on accumulation and intestinal toxicity of zebrafish (Danio rerio). Environ. Pollut., 333. doi:10.1016/j.envpol.2023.122089
Wen, J., Cui, X., Gibson, M., and Li, Z. (2018). Water quality criteria derivation and ecological risk assessment for triphenyltin in China. Ecotoxicol. Environ. Saf. 161, 397–401. doi:10.1016/j.ecoenv.2018.06.012
Wright, S. L., and Kelly, F. J. (2017). Plastic and human health: a micro issue? Environ. Sci. Technol. 51 (12), 6634–6647. doi:10.1021/acs.est.7b00423
Xu, C., Guo, H. Q., Wang, R. J., Li, T. Y., Gu, L. Q., and Sun, L. W. (2021). Accumulation and distribution of fluorescent microplastics in the early life stages of zebrafish. Jove-Journal Vis. Exp. 173. doi:10.3791/62117
Yi, A. X., Leung, K. M. Y., Lam, M. H. W., Lee, J. S., and Giesy, J. P. (2012). Review of measured concentrations of triphenyltin compounds in marine ecosystems and meta-analysis of their risks to humans and the environment. Chemosphere 89 (9), 1015–1025. doi:10.1016/j.chemosphere.2012.05.080
Zhang, C. N., Jiang, D. X., Wang, J. H., and Qi, Q. (2021a). The effects of TPT and dietary quercetin on growth, hepatic oxidative damage and apoptosis in zebrafish. Ecotoxicol. Environ. Saf., 224. doi:10.1016/j.ecoenv.2021.112697
Zhang, C. N., Ma, J. S., Wang, B. K., Pu, C. C., Chang, K., Zhu, J. X., et al. (2024). Sulforaphane modulates some stress parameters in TPT-exposed Cyprinus carpio in relation to liver metabolome. Ecotoxicol. Environ. Saf., 284. doi:10.1016/j.ecoenv.2024.116882
Zhang, C. N., Wang, Q. J., Zuo, Z. H., Ding, J. H., Xu, G. H., and Zou, J. X. (2021b). Interactive effects of microplastics and tetracycline on bioaccumulation and biochemical status in jian carp (Cyprinus carpio var. Jian). Front. Environ. Sci. 9. doi:10.3389/fenvs.2021.764344
Zhang, S. Q., Li, P., He, S. W., Xing, S. Y., Cao, Z. H., Zhao, X. L., et al. (2023). Combined effect of microplastic and triphenyltin: insights from the gut-brain axis. Environ. Sci. Ecotechnol 16, 100266. doi:10.1016/j.ese.2023.100266
Zhang, Y., Yang, B., Xie, P., Hu, L., Zhao, X., Xu, S., et al. (2022). Effects of polystyrene microplastics on metabolism in liver of swordtail fish. Asian J. Ecotoxicol. 17 (3), 35–43. doi:10.7524/AJE.1673-5897.20210401001
Zhou, X., Zhao, X., Zhang, S., and Lin, J. (2019). Marine ranching construction and management in East China Sea: programs for sustainable fishery and aquaculture. Water 11 (6), 1237. doi:10.3390/w11061237
Keywords: triphenyltin, microplastics, physiological responds, combined toxicity, Cyprinus carpio
Citation: Li P, Wu Y, Zhang S, Li T, Liu C, Liu L and Li Z-H (2025) The impact of combined exposure to triphenyltin and microplastics on the oxidative stress, energy metabolism, and digestive function of common carp (Cyprinus carpio). Front. Environ. Sci. 13:1614013. doi: 10.3389/fenvs.2025.1614013
Received: 18 April 2025; Accepted: 30 May 2025;
Published: 13 June 2025.
Edited by:
Yafei Duan, South China Sea Fisheries Research Institute, ChinaReviewed by:
Sun Cuici, Chinese Academy of Sciences (CAS), ChinaJingjing Miao, Ocean University of China, China
Copyright © 2025 Li, Wu, Zhang, Li, Liu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi-Hua Li, bGl6aEBzZHUuZWR1LmNu
†These authors have contributed equally to this work
 Ping Li†
Ping Li† Zhi-Hua Li
Zhi-Hua Li