- School of Civil Engineering, Vellore Institute of Technology, Vellore, Tamil Nadu, India
Polyamide microplastics, which originate from textiles, industrial processes, and everyday consumer products, contaminate waterways, threatening aquatic life, human health, and the environment, emphasizing the urgent need for effective removal technology and sustainable mitigation strategies. This study explores the removal of polyamide microplastics using a coagulation-flocculation- sedimentation (CFS) approach, comparing the effectiveness of natural coagulants (Strychnos potatorum and Cicer arietinum) with the synthetic coagulant alum. The methodology involved optimizing coagulant dosages (50–300 mg/L) and applying a CFS process with rapid mixing (100 rpm, 1 min), slow mixing (30 rpm, 30 min), and sedimentation (30 min). Two sizes of polyamide MPs (<500 μm and >500 μm) were evaluated. The results showed that Strychnos potatorum and Cicer arietinum achieved higher removal efficiencies (up to 92.6% ± 3.21%) for smaller polyamide microplastics (<500 μm) than did alum (up to 87.6% ± 6.5%). Conversely, alum demonstrated superior removal efficiency (up to 91.33% ± 4.16%) for larger polyamide microplastics (>500 μm) compared with Cicer arietinum and Strychnos potatorum (up to 79.6% ± 4.72%). To assess real-world applicability, the method was tested using natural water from Muttukadu Lake. In this more complex matrix, Strychnos potatorum maintained high removal efficiencies, achieving 91.33% ± 3.05% for <500 μm MP and 86.66% ± 3.05% for >500 μm MP, outperforming alum, which showed reduced performance (82.3% ± 9.07% and 78.6% ± 9.50% for small and large MPs, respectively). Cicer arietinum demonstrated moderate efficiency (79.33% ± 4.16% and 76.66% ± 8.6%, respectively), with some sensitivity to natural matrix interference. This study reveals the potential of natural coagulants as viable and sustainable alternatives to synthetic coagulants for the removal of polyamide microplastics, particularly those of smaller sizes. These findings can inform green infrastructure design, enhance environmental sustainability, reduce microplastic pollution, and protect public health, paving the way for innovative, eco-friendly treatment technologies.
1 Introduction
Plastics have become an integral part of daily life, owing to their unique combination of physical and chemical properties, which make them incredibly versatile and widely applicable across various industries and aspects of human life (Nguyen et al., 2022). The demand for plastic has increased significantly over the past few decades because of its low cost, high resistance, and flexibility. Global plastic production has experienced explosive growth, increasing from 1.5 million tons in 1950 to a forecasted 445.25 million tons by 2025 (Rout et al., 2022). However, their durable nature and slow degradation rate make plastics highly resistant to breakdown, leading to widespread environmental contamination when improperly disposed of, including in lakes, groundwater, rivers, estuaries, and oceans (Schwarz et al., 2019). As hydrophobic materials with high physical and chemical durability, plastics are transferred from terrestrial environments to aquatic environments (Wang et al., 2021). Once in aquatic environments, plastics undergo physical damage, weathering, oxidation, and ultraviolet degradation resulting in the formation of minute plastic particles smaller than 5 mm in size, known as microplastics (Sun et al., 2023).
The detection of these tiny particles can be traced back to the 1970s when pioneering research revealed the presence of plastic particles as predominant constituents of ocean floor waste (Waldschläger et al., 2022). However, the introduction of the term 'microplastics' by Thompson et al. (2004) marked a paradigm shift in the scientific community’s understanding of these particles, catalyzing an exponential increase in research efforts and concerns regarding their profound environmental implications. Microplastic pollution is a critical global threat, contaminating water, harming ecosystems and human health, and undermining environmental sustainability and human wellbeing (Ghosh et al., 2023). The unique features of the large surface area and hydrophobic nature of microplastics lead to the adsorption of hazardous substances such as cyanide, pesticides, and polycyclic aromatic hydrocarbon compounds (PAHs) (Amaro-Medina et al., 2022; Li et al., 2021; Mai et al., 2018). Therefore, the detrimental effects of microplastics on the environment, particularly aquatic ecosystems, are being increasingly recognized, underscoring the urgent need for effective solutions to mitigate their presence and promote their removal from these sensitive environments (Khan et al., 2023).
Polyamide microplastics, a type of synthetic polymer, have become ubiquitous in modern life, and have widespread applications in textiles, packaging, and industrial processes (Periyasamy and Tehrani-Bagha, 2022). However, the persistence and minuscule size and density of polyamide microplastics raise alarming concerns about their ecological and health impacts (Kamani et al., 2024; Mejías et al., 2023). Interestingly, polyamide microplastics have unique physicochemical characteristics that can affect their behavior in the environment and interactions with pollutants, such as greater polarity and hydrophilicity when compared to other widely researched polymers like polyethylene and polypropylene (Mejías et al., 2023). Moreover, polyamide fibers are frequently reported as a dominant microplastic type in freshwater and estuarine environments, particularly in regions impacted by urban wastewater discharge (Ramakrishnan et al., 2024). Therefore, developing effective methods for removing polyamide microplastics from aquatic environments is crucial to mitigate their harmful environmental and health impacts.
Several technologies exist for removing microplastics from aquatic environments, including membrane filtration, biodegradation, adsorption, thermal degradation, coagulation, electrocoagulation, and photocatalytic degradation (Le et al., 2023; Miranda Zoppas et al., 2023; Shen et al., 2022; Sridhar et al., 2024; Wang et al., 2021). However, their effectiveness varies depending on the type of microplastic and the method used. While these technologies show promise, they have limitations, such as low removal rates and high energy costs (Khan et al., 2023). To address the limitations of existing technologies, researchers are developing innovative methods for microplastic removal from water, prioritizing enhanced efficiency, reduced energy consumption, and sustainability. One promising approach is coagulation, a simple and effective technique for removing suspended particles from effluents. Its benefits include a low carbon footprint, reduced operational costs, and eco-friendly features, making it an attractive solution for removing microplastics from water and wastewater treatment plants (Zhang et al., 2022).
Coagulation and flocculation are proven techniques for removing microplastics from wastewater and aquatic ecosystems, wherein particles aggregate to form larger clusters that can be efficiently separated from the aqueous phase (Osman et al., 2023). Synthetic coagulants, such as polyaluminum chloride (PACl) and polyacrylamide (PAM), are commonly utilized in these processes because of their high efficiency and ease of application (Ahmad et al., 2008). However, the use of synthetic coagulants is associated with several limitations, including residual toxicity, elevated operational costs, environmental persistence, and potential health risks attributed to excessive aluminium levels in water (Diver et al., 2023). Therefore, there is a growing need to explore alternative, sustainable, and eco-friendly approaches for the removal of microplastics from water, including the utilization of natural coagulants and innovative treatment technologies. Plant-derived materials, particularly seeds from select plant species, have emerged as promising and eco-friendly alternatives for water and wastewater treatment applications (Agarwal and Saini, 2022). By adopting plant-derived coagulants, we can mitigate ecological and health risks associated with synthetic coagulants, fostering a more sustainable and circular water treatment approach. Moreover, natural coagulants have been shown to reduce lifecycle impacts across various treatment stages (Ugural et al., 2024). By leveraging locally sourced plant materials, water treatment facilities can reduce their reliance on external resources, decrease their carbon footprint, and promote regional sustainability (Agarwal et al., 2024).
This study explores the novel application of plant-based materials, Strychnos potatorum and Cicer arietinum, as natural coagulants for removing polyamide microplastics from aquatic media. Strychnos potatorum, a tree native to southern and central India, has been traditionally used for water purification due to its unique composition of colloids and alkaloids (Alenazi et al., 2020; Yadav et al., 2014). Cicer arietinum, commonly known as chickpea, belongs to the Leguminosae family and is a pulse crop that grows in arid and semiarid zones (Koul et al., 2022), rich in various monosaccharides, including glucose, ribose, galactose, and fructose. Notably, this study takes a significant step forward by testing the coagulation process not only in controlled laboratory settings but also in natural environment water samples from Muttukadu Lake. Due to its ecological relevance, anthropogenic influence, and proximity to urban and industrial activities. The lake receives runoff from nearby residential and industrial zones, making it a representative site for studying microplastic pollution in real-world aquatic environments (Ramakrishnan and Sathiyamoorthy, 2024). This approach allows for a realistic assessment of the natural coagulants' efficacy in complex matrices, bridging the gap between laboratory findings and real-world applications. A comparative evaluation with alum, a chemical coagulant, highlights the potential of Strychnos potatorum and Cicer arietinum as effective and sustainable alternatives for microplastic removal. By demonstrating the feasibility of natural coagulant-based solutions in both laboratory and natural water settings, this research contributes to achieving Sustainable Development Goal 6, ensuring clean water and sanitation for all while promoting environmental sustainability.
2 Material and methods
2.1 Sample preparation of microplastics
The polyamide microplastics employed in this investigation were produced through the mechanical comminution of a nylon cable tie to a minute size using a commercial blender (Model: MX201). The blender was operated at a moderate speed to ensure efficient fragmentation of the cable ties into smaller microplastic particles (Azizi et al., 2023). The resulting microplastic fragments were then subjected to a sieving process with a mesh size of 35 (500 µm pore size) to isolate microplastics within the two desired size ranges of smaller microplastics (<500 µm) and larger microplastics (>500 µm). The sieved polyamide microplastics were stored in a dark, sealed container to prevent alterations in their physical properties and molecular weights. Polyamide microplastics were subjected to attenuated total reflectance-Fourier transform infrared (ATR-FTIR) spectroscopy analysis to verify their chemical composition. The resulting ATR-FTIR spectra displayed distinct absorption peaks characteristic of polyamide functional groups, confirming the presence of polyamide microplastics.
2.2 Synthetic and natural coagulants
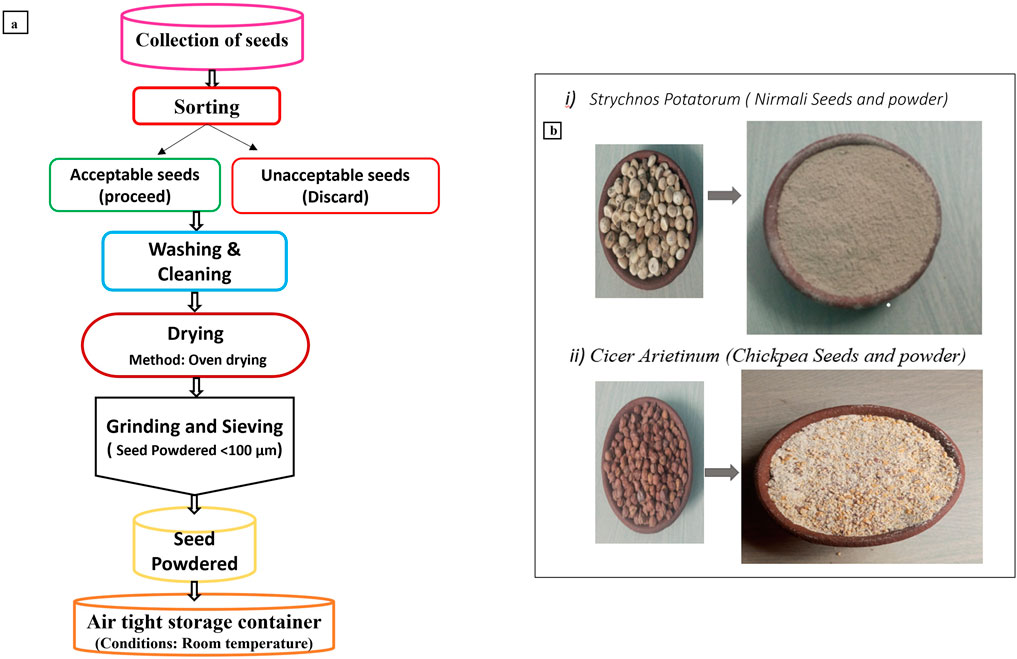
Alum was obtained from Merck Company in Bangalore. The seeds of Strychnos potatorum and Cicer arietinum were collected from a local Ayurvedic herbal market in Vellore District, Tamil Nadu, India. The seeds were cleaned and sun-dried to facilitate natural coagulant extraction (Agarwal and Saini, 2022). The dried seeds were ground into a medium-fine powder using a domestic blender and sieved through a 50-micron sieve to ensure a uniform particle size. The powdered seeds were then stored in airtight containers to preserve freshness. The step-by-step extraction process of natural coagulants from Strychnos potatorum (SP) and Cicer arietinum (CA) seeds is schematically represented in Figure 1. This figure illustrates the methodology used to prepare the natural seed powders, including seed collection, cleaning, drying, and powderization, providing a clear visual representation of the process.
2.3 Experimental design
The jar test is the most widely used and suitable technique for coagulation and flocculation processes. The jar test experimental apparatus consisted of a 1-L capacity jar with six paddle stirrers. All the experiments were conducted in 1-L glass jars with 500 mL of tap water throughout. To ensure the tap water was not contaminated with microplastics, we conducted a preliminary analysis using a 0.45 µm membrane filter and stereomicroscope examination. Only after verifying the absence of background microplastic contamination, we proceed with the experiments. The efficiency of microplastic removal was evaluated on the basis of various criteria, including the size and concentration of microplastics, coagulant dosage, and contact time. The concentration of the polyamide microplastics was uniformly maintained at 50 mg/L in all the tests. Polyamide microplastic concentrations were quantified using optical microscopy before and after treatment to assess removal efficiency. The polyamide microplastics were categorized into two size ranges (<500 µm and >500 µm). Coagulation experiments were conducted using three different coagulants: alum (at dosages of 30–150 mg/L) and a natural coagulant: Strychnos potatorum and Cicer arietinum (at dosages of 50–300 mg/L). The dosages of natural and synthetic coagulant were determined based on the preliminary optimization experiments. Noting that higher doses of natural coagulants were required to compared to alum due to difference in their chemical properties. The jar testing protocol involved a three-stage mixing process, consisting of rapid mixing at 100 rpm for 1 min, slow mixing at 30 rpm for 30 min, and sedimentation for 30 min without mixing, following the method outlined previously (Adib et al., 2022).
2.4 Application in environmental water samples
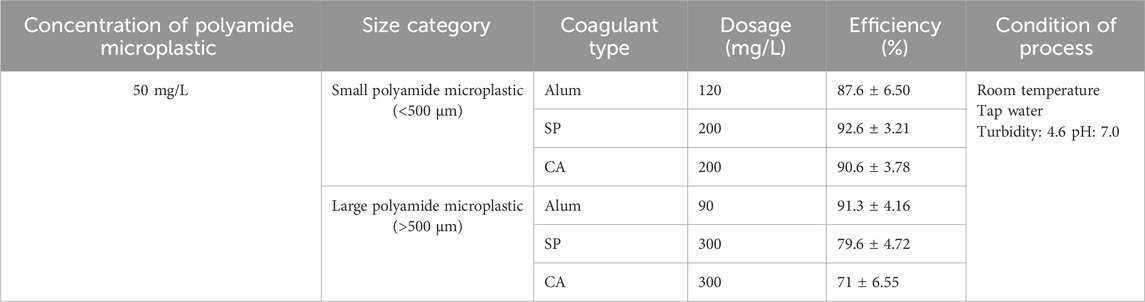
To evaluate the practical applicability of the proposed method, surface water samples were collected from Muttukadu Lake, located in Kanchipuram District, Tamil Nadu, India, on 20 December 2024. These samples were used to represent natural aquatic environment. Key water quality parameters including pH, turbidity, total suspended solids (TSS), and total hardness were analyzed, as presented in Table 1. The initial concentration of microplastics in the natural water was quantified prior to treatment. Following the procedure described by Li et al. (2021) the water samples were first filtered using a 20 μm pore size membrane to remove any existing microplastics. This ensured that all test experiments started with a known and controlled concentration of polyamide microplastics. Subsequently, the CFS process was applied using each coagulant at a consistent dosage optimized from previous trials. Microplastic removal efficiencies were calculated for each coagulant, and the treatment impact on water quality parameters was also assessed. This evaluation provided valuable insights into the effectiveness of both natural and synthetic coagulants in removing microplastics from natural water matrices under environmentally relevant conditions.
2.5 Supernatant separation and analysis
After sedimentation, the clear supernatant was extracted using a 50 mL syringe, avoiding disturbance to the settled particles. The supernatant was filtered through 0.45 µm Whatman filter paper to capture the remaining microplastics. The retained microplastics were rinsed into a 50 mL Petri dish and dried in an air-circulating oven at 60°C for 2 h. The dried microplastics were then weighed. Scanning electron microscopy (SEM) analysis (acceleration voltage: 10 kV, resolution: 10 nm) revealed spherical-shaped polyamide particles (mean diameter: 20 µm) trapped within the formed flocs (Figure 2b).
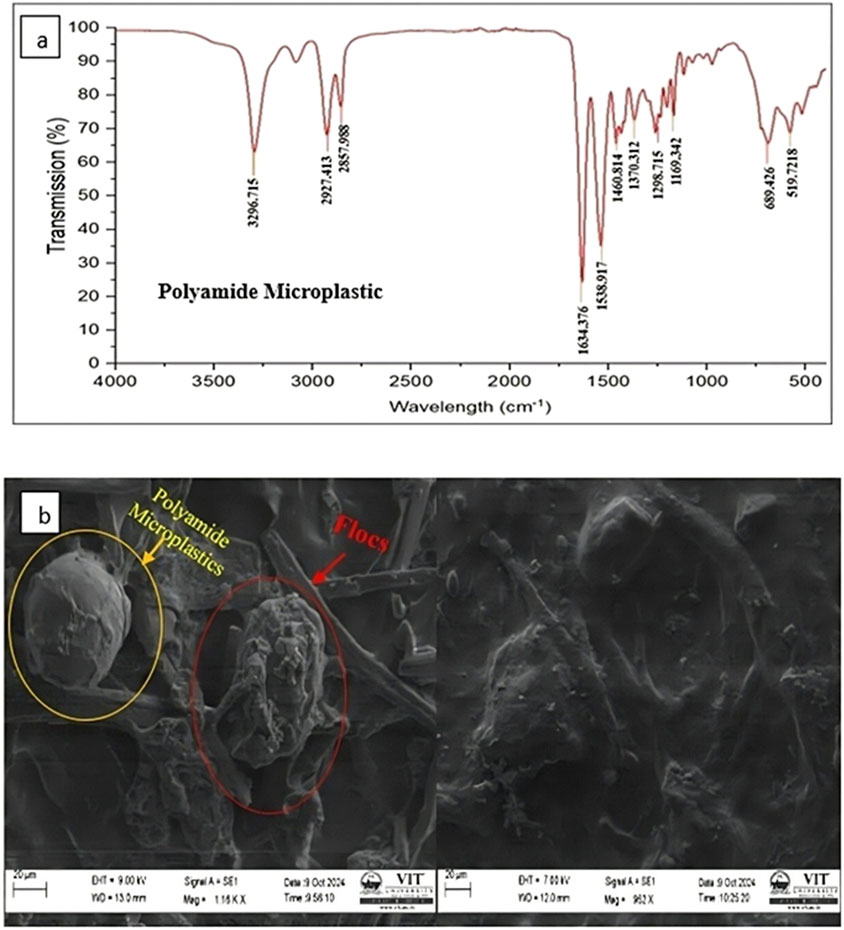
Figure 2. (a) FTIR spectrum of polyamide microplastics and (b) SEM images of the polyamide microplastic and flocs formed.
2.6 Measurement of microplastic removal efficiency
Various methods exist for identifying and quantifying microplastic removal, and a standard technique for evaluating microplastic removal efficiency remains scarce (Li et al., 2018). Recent studies (Bhola and Chakraborty, 2024; Wiśniowska et al., 2024; Zhang et al., 2024) have employed microscopic and infrared approaches, which offer accurate results. However, weighting methods are considered to provide more reliable outcomes (Ma et al., 2019a). The removal efficiency of polyamide microplastics can be determined using the weighting method, which is represented by formula.
In the above formula.
W1 represents the initial weight of the microplastic (mg/L) before removal and,
W2 represents the final the weight of microplastics (mg/L) remaining on the Whatman filter paper after the removal process.
The experiment was conducted in triplicate to ensure accuracy, and the removal efficiency (%) was reported as the mean ± standard deviation (SD).
2.7 Quality assurance and contamination control
Careful measures were taken to avoid external microplastic contamination, hence ensuring the validity of the test results for the coagulant. All the experiments were conducted in a closed environment to minimize the amount of external airborne plastic. Researchers were allowed to clot with personal protective equipment such as laboratory coats during the experiments. In addition, every test trial included an open blank control to confirm the accurate removal efficiency of microplastics, and samples after sedimentation and before supernatant analysis, all glass jars were wrapped with aluminum foil to avoid any post-sedimentation contamination.
3 Results
3.1 Characterization of microplastics
Experiments were conducted to evaluate the removal efficiency of polyamide microplastics of two size fractions (<500 µm and >500 µm) using the chemical coagulant alum and natural coagulants (Strychnos potatorum and Cicer arietinum) at various dosages. A known concentration of microplastics was treated with each coagulant, and the resulting removal efficiencies were compared to determine the optimal dosage and coagulant type for maximum microplastic removal (Table 2). Fourier transform infrared spectroscopy (FTIR) analysis verified the presence of the polyamide microplastics, confirming their chemical composition through characteristic transmission bands. The FTIR spectrum of the polyamide microplastic reveals characteristic transmission bands that provide insight into its molecular structure. The peaks at 2927.413 cm-1 and 2857.98 cm-1 correspond to C-H stretching vibrations of methylene groups, indicating aliphatic chains (Fernández-González et al., 2021). The peak at 1634.376 cm-1 is attributed to C=O stretching of amide groups, while the peak at 1538.917 cm-1 corresponds to N-H bending and C-N stretching vibrations, confirming the polyamide structure. Additional peaks at 1298.715 cm-1 and 1169.342 cm-1 relate to C-N stretching and skeletal vibrations (Tummino et al., 2023). The peaks at 689.426 cm-1 and 519.72 cm-1 may correspond to out-of-plane bending vibrations and skeletal modes as illustrated in Figure 2A. Scanning electron microscopy (SEM) imaging further revealed the effective trapping of flocs around the spherical-shaped polyamide microplastics, as exemplified in Figure 2b. The SEM micrographs illustrate the formation of dense aggregates, with polyamide microplastics encapsulated a network of flocs.
3.2 Removal efficiency of coagulants
Figure 3 compares the removal efficiency of polyamide microplastics. When alum, Strychnos potatorum, and Cicer arietinum are used as coagulants. For smaller polyamide microplastic (<500 µm), the alum removal efficiency increased in a dose-dependent manner from 30˗120 mg/L, peaking at 87.6% ± 3.51% at 120 mg/L, before decreasing at 150 mg/L. In contrast, Strychnos potatorum achieved a maximum removal of 92.6% ± 3.21% at 200 mg/L, with no further enhancement beyond this dosage. Cicer arietinum showed a similar trend, reaching 90.3% ± 3.78% removal at 200 mg/L, followed by decreased efficiency at dosages exceeding 250 mg/L. Notably, Strychnos potatorum outperformed alum by 5% and Cicer arietinum by 2.3% (Figure 3). This superior removal efficiency can be attributed to Strychnos potatorum unique combination of polysaccharides and proteins, facilitating electrostatic, hydrophobic, and bridging interactions (Nagaraja et al., 2022).
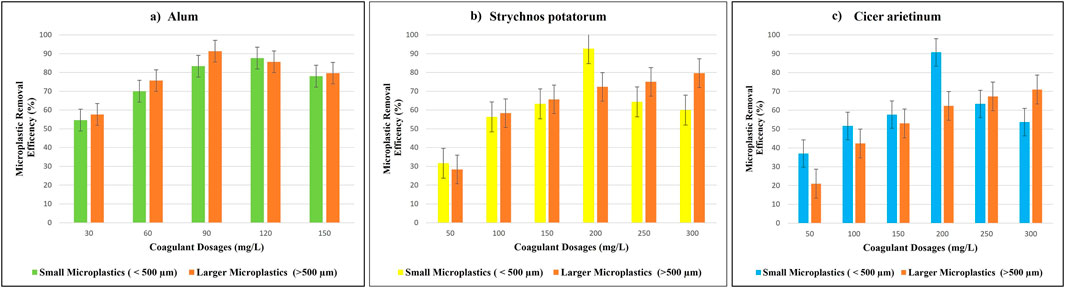
Figure 3. Polyamide microplastic removal efficiency at different dosages of coagulant (a) Alum (b) Strychnos potatorum (c) Cicer arietinum.
3.3 Comparison of removal efficiency
For large polyamide microplastics (>500 µm), alum coagulant demonstrated a dose-dependent increase in removal efficiency from 30–90 mg/L, reaching a peak of 91.3% ± 4.16% at 90 mg/L, before decreasing at higher dosages (120–150 mg/L). Conversely, the natural coagulants Strychnos potatorum and Cicer arietinum achieved relatively lower removal efficiencies of 79.6% ± 4.72% and 71% ± 6.5%, respectively, at 300 mg/L. Notably, increasing the coagulant dose increased the removal efficiency for polyamide microplastics, with Strychnos potatorum exhibiting a minimum removal efficiency of 28.3% ± 1.07% at 50 mg/L, outperforming Cicer arietinum (25.6% ± 6.5%) at the same dosage. Overall, alum surpassed natural coagulants in removing larger polyamide microplastics with Strychnos potatorum and Cicer arietinum showing 11.7% and 20.3% lower removal efficiencies, respectively. Figure 3 illustrate the removal efficiency of polyamide microplastics at varying dosages of Alum, Strychnos potatorum, and Cicer arietinum. Alum shows increasing removal efficiency up to an optimal dosage, beyond which efficiency decreases. Strychnos potatorum achieves high removal efficiency at lower dosages, outperforming Alum in some cases. Cicer arietinum exhibits moderate efficiency, with an optimal dosage range. These trends indicate that each coagulant has a specific dosage range for maximum removal efficiency, informing optimal treatment strategies. This finding aligns with previous studies (Girish et al., 2023; Li et al., 2022; Tang et al., 2022) that attributed the effectiveness of synthetic coagulants to charge neutralization, adsorption bridging, and sweep flocculation.
3.4 Evaluation of coagulant effectiveness from natural water
The removal efficiency of polyamide microplastics using three coagulants alum, Strychnos potatorum, and Cicer arietinum were evaluated in natural water samples from Muttukadu Lake. Strychnos potatorum achieved the highest removal efficiency of 86.66% ± 3.05% at 250 mg/L for large microplastics, outperforming alum (78.6% ± 9.50% at 150 mg/L) and Cicer arietinum (76.66% ± 8.6% at 250 mg/L). Notably, Strychnos potatorum consistently showed superior performance for both small and large microplastics, likely due to its bioactive compounds and polysaccharides enhancing bioflocculation and adsorption (Aragaw and Bogale, 2023). In contrast, alum’s efficiency decreased in lake water compared to tap water, possibly due to interference from natural organic matter. For smaller microplastics (<500 µm), Strychnos potatorum excelled with 91.33% removal at 200 mg/L, indicating a strong affinity for fine microplastics. Furthermore, Strychnos potatorum outperformed alum for larger microplastics (>500 µm) in natural water, suggesting better adaptability of bio-coagulants in high-turbidity environments.
Figure 4 illustrates the superior performance of Strychnos potatorum in removing polyamide microplastics from natural lake water, achieving removal efficiencies of 86.66% for larger microplastics at 250 mg/L and 91.33% for smaller microplastics at 200 mg/L. In comparison, alum and Cicer arietinum showed lower removal efficiencies for both larger (78.6% and 76.66%, respectively) and smaller microplastics (82.33% and 79.33%, respectively). These results indicate Strychnos potatorum’s potential as an effective coagulant for microplastic removal, with optimal dosages varying depending on microplastic size.
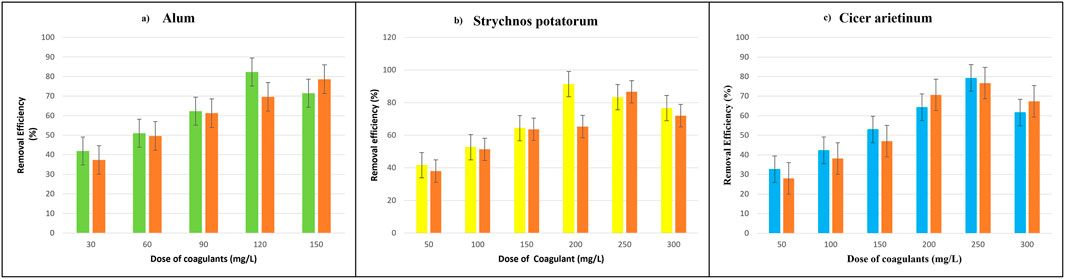
Figure 4. Removal efficiency of polyamide microplastic in natural lake water (a) Alum (b) Strychnos potatorum (c) Cicer arietinum.
4 Discussion
4.1 Efficiency of synthetic coagulant in microplastic removal
In the present study, the maximum removal of polyamide microplastic (>500 µm) was 91.3% ± 4.16% at 90 mg/L alum. Previous studies have demonstrated the effectiveness of various coagulants in removing microplastics (MPs). For example, (Ma et al., 2019a), achieved 90.91% ± 1.01% removal of polyethylene MPs using 540 mg/L ferric chloride (2 mM) with 15 mg/L polyacrylamide as a coagulant aid. Notably, smaller particles resulted in higher removal yields when AlCl3·6H2O and FeCl3·6H2O were used as coagulants, with efficiencies increasing from <40% to >60% upon the addition of anionic polyacrylamide (15 mg/L) to particles <500 μm (Ziembowicz et al., 2023). Similarly, (Zhang et al., 2021a), reported a 91.45% removal efficiency for polyethylene terephthalate (PET) MPs using 200 mg/L polyaluminum chloride and 100 mg/L polyacrylamide (PAM).
4.2 Natural coagulants as sustainable alternatives
The efficacy of Strychnos potatorum and Cicer arietinum as natural coagulants in removing small polyamide microplastics (<500 µm) was notable, achieving remarkable removal efficiencies of 92.6% ± 3.21% and 90.6% ± 3.78%, respectively. Previous studies have highlighted the potential of Strychnos potatorum as a natural coagulant (Sheeba et al., 2023), attributing its effectiveness in reducing turbidity from washing machine effluents to its anionic polyelectrolyte content. Moreover, (Rajin et al., 2023), successfully utilized Strychnos potatorum seeds as natural coagulants for purifying and producing potable water in flood-prone areas of Bangladesh. Recent research by (Avazpour and Noshadi, 2024) revealed that the coagulation performance of Al2(SO4)3 can be enhanced by the separate addition of anionic polyacrylamide (APAM) and naturally derived Moringa oleifera, with Al2(SO4)3 in combination with APAM showing better performance than Al2(SO4)3 or Moringa Oleiferous alone. The anionic polyelectrolyte properties of Strychnos potatorum seeds, characterized by carboxylic acid (-COOH) and hydroxyl (-OH) groups, facilitate coagulation through charge neutralization and adsorption bridging, effectively removing contaminants from water (Sharma et al., 2023). Similarly, Cicer arietinum has been employed as a natural coagulant for reducing turbidity in wastewater and sewage treatment, achieving a notable 95.89% reduction in turbidity and underscoring its potential as a sustainable alternative for treatment processes (Mohammed et al., 2021).
4.3 Influence of microplastic size and type of removal efficiency
The present study revealed that compared with larger microplastics (over 500 µm), smaller microplastics (less than 500 µm) are more efficiently removed, which is in line with the findings of (Ma et al., 2019b) that smaller sizes facilitate better removal. However, the efficacy of coagulation removal is contingent upon the type and size of microplastics, as noted by (Zhang et al., 2021b), who reported that larger polystyrene microplastics are removed more efficiently. A recent review by (Azizi et al., 2023) reported that the size of polyethylene and polystyrene microplastic particles significantly affects their removal efficiency. A study (Shahi et al., 2020) predicts that shape, size and surface morphology of microplastics play an important role in the removal of microplastic in drinking water treatment plants. Another study (Ziembowicz et al., 2023) revealed the critical importance of microplastic size, type, and coagulant selection in determining removal efficiency, with the Al3+ coagulant demonstrating 95% greater effectiveness than Fe3+ for polyethylene and polyvinyl chloride microplastics.
4.4 Application of removal efficiency in natural environment water
To evaluate the real-world applicability of the CFS process, experiments were conducted using surface water samples collected from Muttukadu Lake, which represents a typical natural aquatic environment with complex water chemistry. These samples contained naturally occurring organic matter, suspended solids, and other potential interfering substances that can impact treatment performance. Among the tested coagulants, Strychnos potatorum demonstrated superior removal efficiency, achieving up to 91.33% ± 3.05% for small polyamide microplastics (<500 µm). Notably, it also outperformed the synthetic coagulant alum for larger microplastics (>500 µm), with a removal efficiency of 86.66% ± 3.05%, compared to 78.6% ± 9.50% for alum.
These findings indicate that natural coagulants, particularly Strychnos potatorum, retain high removal efficiency even in complex natural water matrices, where conventional coagulants often experience reduced effectiveness due to interference from dissolved organic compounds and variable ionic composition (Badawi et al., 2023). The robustness of natural coagulants in such environments highlights their potential as sustainable and eco-friendly alternatives for microplastic remediation in surface waters (Facchino et al., 2025). Their biodegradability, local availability, and low toxicity further support their applicability in decentralized or low cost water treatment systems, especially in regions with limited access to advanced infrastructure (Obaideen et al., 2022). These results contribute to the growing evidence supporting the integration of green coagulants into practical water treatment strategies aimed at mitigating microplastic pollution and enhancing environmental sustainability.
4.5 Effect of coagulation on water quality matrix
The impact of coagulation on water quality parameters was evaluated using surface water samples collected from Muttukadu lake and treated with CFS using Strychnos potatorum, Cicer arietinum, and alum. Parameters such as pH, turbidity, total hardness, and total suspended solids (TSS) were measured before and after treatment to access the effect of each coagulant on the natural water matrix (Table 1). The initial water sample was slightly alkaline (pH 7.85), with elevated turbidity (18 NTU), total hardness (4,850 mg/L), and TSS (185 mg/L), consistent with natural surface water characteristics. After treatment, a reduction in pH was observed across all coagulants, with the average final value reaching 6.80. This slight decrease is attributed to the presence of acidic functional groups in the natural coagulants, such as carboxyl (-COOH) and hydroxyl (-OH), which are known to interact with cationic species during charge neutralization (Alazaiza et al., 2022).
A notable improvement in turbidity was recorded, with levels decreasing from 18 NTU to 12.5 NTU, representing a 30.6% reduction. This indicates moderate but effective removal of colloidal particles from the water matrix. Similarly, the TSS concentration declined from 185 mg/L to 105 mg/L, achieving a 43.2% reduction, which highlights the ability of both natural and synthetic coagulants to aggregate and settle suspended particulates including polyamide microplastics. In contrast, only a marginal decrease in total hardness was observed, dropping from 4,850 mg/L to 4,560 mg/L (∼6% reduction). This minimal change aligns with expectations, as coagulation primarily targets particulate and colloidal matter rather than dissolved ionic species (Tahraoui et al., 2024). Overall, the results confirm that natural coagulants are effective in improving key water quality parameters, particularly turbidity and TSS, even in complex natural matrices. These outcomes also suggest the potential for these plant-based coagulants to be integrated into existing water treatment systems, especially in regions with high particulate loads and limited access to conventional chemical coagulants.
4.6 Influence of natural water matrix on coagulant performance
The performance of coagulants in removing polyamide microplastics was notably influenced by the complex composition of natural water collected from Muttukadu Lake. Compared to tap water, a slight decline in removal efficiency was observed for all three coagulants, particularly for alum. This reduction is attributed to the presence of natural organic matter (NOM), suspended solids, and inorganic ions, which compete with microplastic particles for interaction with the coagulant (Tahraoui et al., 2024). Alum, a conventional chemical coagulant, exhibited greater variability and lower efficiency in the natural water matrix (78.6% ± 9.50% for large MPs and 82.3% ± 9.07% for small MPs), suggesting that its coagulation mechanism primarily based on charge neutralization is more sensitive to interference from competing ions and colloids (Yang et al., 2025).
In contrast, Strychnos potatorum, a natural coagulant, demonstrated more stable and effective performance in Muttukadu lake water, removing 86.66% ± 3.05% of large and 91.33% ± 3.05% of small microplastics. The effectiveness of Strychnos potatorum in real water can be attributed to its polysaccharide and protein content, which enhances adsorption and bioflocculation, making it less susceptible to matrix interferences (Makani et al., 2025). Cicer arietinum showed moderate efficiency in both size categories, though it was less effective than Strychnos potatorum. Overall, the results indicate that natural coagulants are more adaptable in real-water conditions and could serve as sustainable alternatives to traditional coagulants for microplastic remediation.
4.7 Microplastic particle size effects on removal efficiency
Particle size had a significant impact on removal efficiency across all tested coagulants. For microplastics smaller than 500 μm, Strychnos potatorum exhibited the highest removal efficiency (91.33% ± 3.05%), followed by alum (82.3% ± 9.07%) and Cicer arietinum (79.33% ± 4.16%). The superior performance of Strychnos potatorum for smaller particles (<500 μm) can be attributed to its bioactive components, which enhance surface interactions and promote the formation of finer flocs capable of entrapping microplastics more effectively. In contrast, for microplastics larger than 500 μm, the removal efficiency of alum (78.6% ± 9.50%) decreased significantly compared to its performance in tap water, likely due to impaired floc formation caused by competing from natural particulates present in the environment water matrix. Interestingly, Strychnos potatorum maintained high efficiency (86.66% ± 3.05%) even for larger microplastics, demonstrating its versatility across different particles size ranges. These findings indicate that natural coagulants are not only effective across a range of microplastic sizes but are also more resilient to environmental variability. The consistently high performance of Strychnos potatorum supports its potential as a biodegradable and environmentally friendly coagulant suitable for diverse water treatment applications.
4.8 Environment and health consideration of coagulant use
The use of synthetic coagulants raises profound environmental and health concerns, including the release of xenobiotic compounds, the generation of nonbiodegradable sludge, and economic burdens. Furthermore, excessive aluminum in water poses significant health risks. Unlike to synthetic coagulants, nature-based products, exemplified by the seed powder utilized in this study, present a viable, sustainable, cost-effective, and low-tech alternative. This eco-friendly approach not only minimizes environmental impacts but also enhances accessibility for underdeveloped and developing communities, thereby promoting environmental justice. Notably, this research initiates the application of natural coagulants for microplastic (MP) removal, addressing an emerging contaminant of global concern. The findings of this study are anticipated to catalyze further research, driving innovations in sustainable solutions to combat MP pollution and informing evidence-based policies.
4.9 Implications and future research directions
This study represents a major advancement in the use of natural coagulants to remove polyamide microplastics, a new class of contaminants with an impact on the environment worldwide. The encouraging outcomes with Cicer arietinum and Strychnos potatorum pave the way for additional study into dosage optimization, treatment conditions, and combination treatment approaches. Future research should also examine integration with current water treatment systems, regeneration potential, and large-scale applicability. The results could influence policy development and innovation in microplastic mitigation methods, and they add to the increasing amount of evidence that supports the use of sustainable materials in environmental restoration.
4.10 Limitations and optimal solution
Despite achieving higher than 90% removal of polyamide microplastics with Strychnos potatorum and Cicer arietinum in bench-scale tests face several limitations that must be addressed for real-world application. Pilot-scale trials in continuous-flow systems are necessary to replicate realistic hydraulic and mixing conditions and to optimize automated dosing via real-time turbidity or zeta-potential feedback; standardized extraction and quality-control assays (e.g., protein content, charge density) or mild enzymatic pre-treatments can reduce batch-to-batch variability in seed quality; sludge valorization pathways such as composting or anaerobic digestion and investigation of coagulant regeneration techniques will minimize disposal impacts and fresh-seed demand; testing across diverse real water matrices (municipal, industrial, agricultural) and blending with low doses of synthetic flocculants can ensure robust performance under high organic loads or competing ions; and a simplified life-cycle and cost analysis comparing natural versus conventional coagulants accounting for transport, processing energy, sludge handling, and biosludge credits will clarify economic viability and inform policy incentives.
5 Conclusion
This experimental study conclusively demonstrated the remarkable efficacy of natural coagulants, Strychnos potatorum and Cicer arietinum, in removing polyamide microplastics from water. In controlled experiments, Strychnos potatorum and Cicer arietinum achieved removal efficiencies of 92.6% and 90.6% for smaller microplastics (<500 µm), respectively, outperforming synthetic alum (87.6%). However, in environmental water samples from Muttukadu Lake, Strychnos potatorum exhibited superior performance, achieving removal efficiencies of up to 91.33% ± 3.05% for small microplastics (<500 µm) and 86.66% ± 3.05% for larger microplastics (>500 µm). These findings highlight the potential of natural coagulants as sustainable alternatives for microplastic removal, even in complex water matrices. The unique properties of Strychnos potatorum contribute to its effectiveness. This study paves the way for developing eco-friendly water treatment technologies, mitigating microplastic pollution, and ensuring a cleaner environment. Future research should focus on scaling up natural coagulant-based systems, evaluating their economic and environmental feasibility, and exploring their applicability in diverse water treatment scenarios. Moreover, pilot-scale trials, diverse microplastic types, long-term assessments, and real-world implementation are essential for further development. By advancing sustainable natural coagulant-based technologies, we can move toward a more environmentally sustainable future.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
DR: Investigation, Writing – original draft, Formal Analysis, Methodology. MS: Supervision, Writing – review and editing, Methodology, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors acknowledge Vellore Institute of Technology, Vellore for providing VIT SEED grant (Sanction Order No: SG20230092) for carrying out this research work.
Acknowledgments
The authors are grateful for access to the “FTIR and SEM” facilities provided by School of Advanced Sciences, Vellore Institute of Technology, Vellore.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adib, D., Mafigholami, R., Tabeshkia, H., and Walker, T. R. (2022). Optimization of polypropylene microplastics removal using conventional coagulants in drinking water treatment plants via response surface methodology. J. Environ. Health Sci. Eng. 20, 565–577. doi:10.1007/s40201-022-00803-4
Agarwal, P., Prakash, S., and Saini, G. (2024). Natural coagulants (Moringa oleifera and Benincasa hispida) based removal of microplastics. Clean. Water 1, 100010. doi:10.1016/j.clwat.2024.100010
Agarwal, P., and Saini, G. (2022). Use of natural coagulants (Moringa oleifera and Benincasa hispida) for volume reduction of waste drilling slurries. Mater Today Proc. 49, 3274–3278. doi:10.1016/j.matpr.2020.12.924
Ahmad, A., Wong, S., Teng, T., and Zuhairi, A. (2008). Improvement of alum and PACl coagulation by polyacrylamides (PAMs) for the treatment of pulp and paper mill wastewater. Chem. Eng. J. 137, 510–517. doi:10.1016/j.cej.2007.03.088
Alazaiza, M., Albahnasawi, A., Ali, G., Bashir, M., Nassani, D., Al Maskari, T., et al. (2022). Application of natural coagulants for pharmaceutical removal from water and wastewater: a review. Water (Basel) 14, 140. doi:10.3390/w14020140
Alenazi, M., Hashim, K. S., Hassan, A. A., Muradov, M., Kot, P., and Abdulhadi, B. (2020). Turbidity removal using natural coagulants derived from the seeds of strychnos potatorum: statistical and experimental approach. IOP Conf. Ser. Mater Sci. Eng. 888, 012064. doi:10.1088/1757-899X/888/1/012064
Amaro-Medina, B. M., Martinez-Luevanos, A., Soria-Aguilar, Ma, de, J., Sanchez-Castillo, M. A., Estrada-Flores, S., et al. (2022). Efficiency of adsorption and photodegradation of composite TiO2/Fe2O3 and industrial wastes in cyanide removal. Water (Basel) 14, 3502. doi:10.3390/w14213502
Aragaw, T. A., and Bogale, F. M. (2023). Role of coagulation/flocculation as a pretreatment option to reduce colloidal/bio-colloidal fouling in tertiary filtration of textile wastewater: a review and future outlooks. Front. Environ. Sci. 11. doi:10.3389/fenvs.2023.1142227
Avazpour, S., and Noshadi, M. (2024). Enhancing the coagulation process for the removal of microplastics from water by anionic polyacrylamide and natural-based Moringa oleifera. Chemosphere 358, 142215. doi:10.1016/j.chemosphere.2024.142215
Azizi, N., Pirsaheb, M., Jaafarzadeh, N., and Nabizadeh Nodehi, R. (2023). Microplastics removal from aquatic environment by coagulation: selecting the best coagulant based on variables determined from a systematic review. Heliyon 9, e15664. doi:10.1016/j.heliyon.2023.e15664
Badawi, A. K., Salama, R. S., and Mostafa, M. M. M. (2023). Natural-based coagulants/flocculants as sustainable market-valued products for industrial wastewater treatment: a review of recent developments. RSC Adv. 13, 19335–19355. doi:10.1039/D3RA01999C
Bhola, S., and Chakraborty, S. (2024). Morphology and polymeric composition-based source apportionment of microplastics in surface water and sediment of drinking water supply reservoirs in ranchi, India. Environ. Process. 11, 32. doi:10.1007/s40710-024-00708-4
Diver, D., Nhapi, I., and Ruziwa, W. R. (2023). The potential and constraints of replacing conventional chemical coagulants with natural plant extracts in water and wastewater treatment. Environ. Adv. 13, 100421. doi:10.1016/j.envadv.2023.100421
Facchino, M., Pietrelli, L., Menegoni, P., Capocelli, M., Limiti, E., Trombetta, M., et al. (2025). Greener microplastics removal: progressive replacement of iron-based coagulants with sodium alginate and chitosan to enhance sustainability. Chempluschem 90, e202400736. doi:10.1002/cplu.202400736
Fernández-González, V., Andrade, J. M., Ferreiro, B., López-Mahía, P., and Muniategui-Lorenzo, S. (2021). Monitorization of polyamide microplastics weathering using attenuated total reflectance and microreflectance infrared spectrometry. Spectrochim. Acta A Mol. Biomol. Spectrosc. 263, 120162. doi:10.1016/j.saa.2021.120162
Ghosh, S., Sinha, J. K., Ghosh, S., Vashisth, K., Han, S., and Bhaskar, R. (2023). Microplastics as an emerging threat to the global environment and human health. Sustainability 15, 10821. doi:10.3390/su151410821
Girish, N., Parashar, N., and Hait, S. (2023). Coagulative removal of microplastics from aqueous matrices: recent progresses and future perspectives. Sci. Total Environ. 899, 165723. doi:10.1016/j.scitotenv.2023.165723
Kamani, H., Ghayebzadeh, M., Azari, A., and Ganji, F. (2024). Characteristics of microplastics in a hospital wastewater treatment plant effluent and hazard risk assessment. Environ. Process. 11, 15. doi:10.1007/s40710-024-00694-7
Khan, M. T., Ahmad, M., Hossain, M. F., Nawab, A., Ahmad, I., Ahmad, K., et al. (2023). Microplastic removal by coagulation: a review of optimizing the reaction conditions and mechanisms. Water Emerg. Contam. and Nanoplastics 2. doi:10.20517/wecn.2023.39
Koul, B., Bhat, N., Abubakar, M., Mishra, M., Arukha, A. P., and Yadav, D. (2022). Application of natural coagulants in water treatment: a sustainable alternative to chemicals. Water (Basel) 14, 3751. doi:10.3390/w14223751
Le, L.-T., Bui, X.-B., Tran, C.-S., Chiemchaisri, C., and Pandey, A. (2023). “Membrane and filtration processes for microplastic removal,” in Current developments in biotechnology and bioengineering (Elsevier), 203–220. doi:10.1016/B978-0-443-19180-0.00019-5
Li, H., Wang, F., Li, J., Deng, S., and Zhang, S. (2021). Adsorption of three pesticides on polyethylene microplastics in aqueous solutions: kinetics, isotherms, thermodynamics, and molecular dynamics simulation. Chemosphere 264, 128556. doi:10.1016/j.chemosphere.2020.128556
Li, J., Dagnew, M., and Ray, M. B. (2022). Effect of coagulation on microfibers in laundry wastewater. Environ. Res. 212, 113401. doi:10.1016/j.envres.2022.113401
Li, J., Liu, H., and Paul Chen, J. (2018). Microplastics in freshwater systems: a review on occurrence, environmental effects, and methods for microplastics detection. Water Res. 137, 362–374. doi:10.1016/j.watres.2017.12.056
Ma, B., Xue, W., Ding, Y., Hu, C., Liu, H., and Qu, J. (2019a). Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 78, 267–275. doi:10.1016/j.jes.201.10.006
Ma, B., Xue, W., Ding, Y., Hu, C., Liu, H., and Qu, J. (2019b). Removal characteristics of microplastics by Fe-based coagulants during drinking water treatment. J. Environ. Sci. 78, 267–275. doi:10.1016/j.jes.2018.10.006
Mai, L., Bao, L.-J., Shi, L., Liu, L.-Y., and Zeng, E. Y. (2018). Polycyclic aromatic hydrocarbons affiliated with microplastics in surface waters of Bohai and Huanghai Seas, China. Environ. Pollut. 241, 834–840. doi:10.1016/j.envpol.2018.06.012
Makani, I., Marenga, W., Lekgoba, T., Rantong, G., Sithole, N. T., Ntuli, F., et al. (2025). The characteristics and efficiency of Strychnos potatorum as a medicinal plant and water purification agent: a review. Int. J. Environ. Sci. Technol. 22, 9781–9792. doi:10.1007/s13762-025-06398-1
Mejías, C., Martín, J., Santos, J. L., Aparicio, I., and Alonso, E. (2023). Role of polyamide microplastics as vector of parabens in the environment: an adsorption study. Environ. Technol. Innov. 32, 103276. doi:10.1016/j.eti.2023.103276
Miranda Zoppas, F., Sacco, N., Soffietti, J., Devard, A., Akhter, F., and Marchesini, F. A. (2023). Catalytic approaches for the removal of microplastics from water: recent advances and future opportunities. Chem. Eng. J. Adv. 16, 100529. doi:10.1016/j.ceja.2023.100529
Mohammed, H., and Marey, A. (2021). Usage of Cicer Arietinum as a local and eco-friendly natural coagulant in sewage treatment and its ability to increase the formation of floc process. Bionatura 3 (03.9), 1939–1943. doi:10.21931/rb/2021.06.03.9
Nagaraja, K., Rao, K. M., Hemalatha, D., Zo, S., Han, S. S., and Rao, K. S. V. K. (2022). Strychnos potatorum L. Seed polysaccharide-based stimuli-responsive hydrogels and their silver nanocomposites for the controlled release of chemotherapeutics and antimicrobial applications. ACS Omega 7, 12856–12869. doi:10.1021/acsomega.2c00131
Nguyen, H. V., Le, M. T. T., and Do, L. T. (2022). Intrinsic motivation for reducing single-use plastics: the compensation effects of basic psychological needs. Resour. Conserv. Recycl 185, 106482. doi:10.1016/j.resconrec.2022.106482
Obaideen, K., Shehata, N., Sayed, E. T., Abdelkareem, M. A., Mahmoud, M. S., and Olabi, A. G. (2022). The role of wastewater treatment in achieving sustainable development goals (SDGs) and sustainability guideline. Energy Nexus 7, 100112. doi:10.1016/j.nexus.2022.100112
Osman, A. I., Hosny, M., Eltaweil, A. S., Omar, S., Elgarahy, A. M., Farghali, M., et al. (2023). Microplastic sources, formation, toxicity and remediation: a review. Environ. Chem. Lett. 21, 2129–2169. doi:10.1007/s10311-023-01593-3
Periyasamy, A. P., and Tehrani-Bagha, A. (2022). A review on microplastic emission from textile materials and its reduction techniques. Polym. Degrad. Stab. 199, 109901. doi:10.1016/j.polymdegradstab.2022.109901
Rajin, S., Nasif, A., Tahsan, K., and Syed Tamjid, T. (2023). Using strychnos potatorum seeds as a natural coagulant in flood affected areas of Bangladesh. Int. J. Water Wastewater Treat. 9. doi:10.16966/2381-5299.188
Ramakrishnan, D., Loganathan, S., Sathiyamoorthy, M., and Azamathulla, H. M. (2024). Microplastic pollution – a rising threat along an urban lake in the Vellore district of Tamil Nadu, India: abundance and risk exposure. Water Qual. Res. J. 60, 89–108. doi:10.2166/wqrj.2024.133
Ramakrishnan, D., and Sathiyamoorthy, M. (2024). Seasonal distribution, source apportionment and risk exposure of microplastic contaminants along the Muttukadu backwater estuary, Tamil Nadu, India. Results Eng. 23, 102776. doi:10.1016/j.rineng.2024.102776
Rout, P. R., Mohanty, A., Sharma, A., Miglani, M., Liu, D., and Varjani, S. (2022). Micro- and nanoplastics removal mechanisms in wastewater treatment plants: a review. J. Hazard. Mater. Adv. 6, 100070. doi:10.1016/j.hazadv.2022.100070
Schwarz, A. E., Ligthart, T. N., Boukris, E., and van Harmelen, T. (2019). Sources, transport, and accumulation of different types of plastic litter in aquatic environments: a review study. Mar. Pollut. Bull. 143, 92–100. doi:10.1016/j.marpolbul.2019.04.029
Shahi, N. K., Maeng, M., Kim, D., and Dockko, S. (2020). Removal behavior of microplastics using alum coagulant and its enhancement using polyamine-coated sand. Process Saf. Environ. Prot. 141, 9–17. doi:10.1016/j.psep.2020.05.020
Sharma, N., Yadav, Y., and Sharma, K. C. (2023). A systemic review on Ayurvedic and modern pharmacology of Strychnos potatorum Linn. to determine its therapeutic potential. Natl. J. Pharmacol. Ther. 1, 125–132. doi:10.4103/NJPT.NJPT_32_23
Sheeba, N. L., Esakki, E. S., Sarathi, R., Esaiarasi, A., and Sundar, S. M. (2023). Investigation on the removal of contaminants from washing machine discharge using Strychnos potatorum (clearing nut) – a potential purifying agent. Heliyon 9, e19869. doi:10.1016/j.heliyon.2023.e19869
Shen, M., Zhang, Y., Almatrafi, E., Hu, T., Zhou, C., Song, B., et al. (2022). Efficient removal of microplastics from wastewater by an electrocoagulation process. Chem. Eng. J. 428, 131161. doi:10.1016/j.cej.2021.131161
Sridhar, S., Murugesan, N., Gopalakrishnan, M., Janjoren, D., and Ganesan, S. (2024). Removal of microplastic for a sustainable strategy by microbial biodegradation. Sustain. Chem. Environ. 6, 100088. doi:10.1016/j.scenv.2024.100088
Sun, N., Shi, H., Li, X., Gao, C., and Liu, R. (2023). Combined toxicity of micro/nanoplastics loaded with environmental pollutants to organisms and cells: role, effects, and mechanism. Environ. Int. 171, 107711. doi:10.1016/j.envint.2022.107711
Tahraoui, H., Toumi, S., Boudoukhani, M., Touzout, N., Sid, A. N. E. H., Amrane, A., et al. (2024). Evaluating the effectiveness of coagulation–flocculation treatment using aluminum sulfate on a polluted surface water source: a year-long study. Water (Basel) 16, 400. doi:10.3390/w16030400
Tang, W., Li, H., Fei, L., Wei, B., Zhou, T., and Zhang, H. (2022). The removal of microplastics from water by coagulation: a comprehensive review. Sci. Total Environ. 851, 158224. doi:10.1016/j.scitotenv.2022.158224
Thompson, R. C., Olsen, Y., Mitchell, R. P., Davis, A., Rowland, S. J., John, A. W. G., et al. (2004). Lost at sea: where is all the plastic? Science 304, 838. doi:10.1126/science.1094559
Tummino, M. L., Chrimatopoulos, C., Bertolla, M., Tonetti, C., and Sakkas, V. (2023). Configuration of a simple method for different polyamides 6.9 recognition by ATR-FTIR analysis coupled with chemometrics. Polym. (Basel) 15, 3166. doi:10.3390/polym15153166
Ugural, M. N., Ozyilmaz, M. R., and Burgan, H. I. (2024). Life cycle assessment analysis based on material selection in sustainable airport buildings. Buildings 14, 2728. doi:10.3390/buildings14092728
Waldschläger, K., Brückner, M. Z. M., Carney Almroth, B., Hackney, C. R., Adyel, T. M., Alimi, O. S., et al. (2022). Learning from natural sediments to tackle microplastics challenges: a multidisciplinary perspective. Earth Sci. Rev. 228, 104021. doi:10.1016/j.earscirev.2022.104021
Wang, J., Sun, C., Huang, Q.-X., Chi, Y., and Yan, J.-H. (2021). Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard Mater 419, 126486. doi:10.1016/j.jhazmat.2021.126486
Wiśniowska, E., Moraczewska-Majkut, K., Nocoń, W., and Popenda, A. (2024). Microplastics removal from natural surface water by coagulation process. Desalination Water Treat. 319, 100462. doi:10.1016/j.dwt.2024.100462
Yadav, K., Kadam, P., Patel, J., and Patil, M. (2014). Strychnos potatorum: phytochemical and pharmacological review. Pharmacogn. Rev. 8, 61. doi:10.4103/0973-7847.125533
Yang, B., Zhou, P., Tian, L., Graham, N., Li, G., Su, Z., et al. (2025). The nanoscale explanation of metal cations differences in enhancing the Fe(III) coagulation performance. Water Res. 280, 123524. doi:10.1016/j.watres.2025.123524
Zhang, Y., Fu, S., and Chen, L. (2024). Enhanced removal of polystyrene microplastics through coagulation using polyaluminum ferric chloride with Opuntia Milpa Alta particles. J. Environ. Chem. Eng. 12, 113802. doi:10.1016/j.jece.2024.113802
Zhang, Y., Wang, X., Li, Y., Wang, H., Shi, Y., Li, Y., et al. (2022). Improving nanoplastic removal by coagulation: impact mechanism of particle size and water chemical conditions. J. Hazard Mater 425, 127962. doi:10.1016/j.jhazmat.2021.127962
Zhang, Y., Zhou, G., Yue, J., Xing, X., Yang, Z., Wang, X., et al. (2021a). Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 800, 149589. doi:10.1016/j.scitotenv.221.149589
Zhang, Y., Zhou, G., Yue, J., Xing, X., Yang, Z., Wang, X., et al. (2021b). Enhanced removal of polyethylene terephthalate microplastics through polyaluminum chloride coagulation with three typical coagulant aids. Sci. Total Environ. 800, 149589. doi:10.1016/j.scitotenv.2021.149589
Keywords: Alum, cicer Arietinum, natural Coagulants, polyamide Microplastic, removal Efficiency, strychnos Potatorum
Citation: Ramakrishnan D and Sathiyamoorthy M (2025) Enhancing the remediation of polyamide microplastics: A comparative study of natural and synthetic coagulants. Front. Environ. Sci. 13:1620074. doi: 10.3389/fenvs.2025.1620074
Received: 30 April 2025; Accepted: 30 May 2025;
Published: 13 June 2025.
Edited by:
Amin Mojiri, Arizona State University, United StatesReviewed by:
Vinoth Kumar Ponnusamy, Kaohsiung Medical University, TaiwanHalil Ibrahim Burgan, Akdeniz University, Türkiye
Copyright © 2025 Ramakrishnan and Sathiyamoorthy. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mahenthiran Sathiyamoorthy, bWFoZW50aGlyYW4uc0B2aXQuYWMuaW4=
 Devananth Ramakrishnan
Devananth Ramakrishnan Mahenthiran Sathiyamoorthy
Mahenthiran Sathiyamoorthy