- School of Biological, Earth and Environmental Sciences, Environmental Research Institute, University College Cork, Cork, Ireland
The meat processing industry generates a considerable amount of meat processing wastewater (MPW) that is potentially harmful when released in the natural environment. Therefore, current industry practices involve extensive MPW remediation before release of effluent into local waters. Here, it was investigated whether aquatic duckweed (Lemna minor L.) can be used to remediate and retain nitrogen and phosphorus present in MPW that had undergone primary and secondary treatment. Physicochemical analyses, as well as laboratory and glasshouse growth trials, show the suitability of MPW as a growth medium for duckweed. Quantitative analysis revealed that duckweed growth on MPW is associated with rapid removal of nitrogen and phosphorus with calculated uptake rates similar to those reported in the literature. Longer term cultivation on MPW (>6 days) led to increased salinity problems, however, short-term (3 days) remediation of MPW was found to be sufficient to achieve wastewater discharge requirements. Thus, a duckweed-based system can be used to remediate MPW. The suitability of duckweed biomass as a source of protein, bioenergy and/or fertiliser will facilitate retention of plant nutrients within the agri-feed sector in line with the principles of the circular economy and constitute a promising avenue towards more sustainable meat processing. Future work needs to focus on upscaling duckweed remediation under realistic industry conditions, while exploring technical (salinity and seasonality), economic (cost-benefit), social, regulatory and sanitary aspects.
Highlights
• Meat processing generates wastewater that is harmful for the environment
• Trials show the suitability of meat processing wastewater for duckweed growth
• Duckweed remediates wastewater and retains nitrogen and phosphorus in its biomass
• Duckweed can be used to make meat processing more sustainable
1 Introduction
The meat processing industry produces substantial amounts of by-products such as skin, bones, and hoofs, as well as a considerable volume of meat processing wastewater (MPW) (Toldrá et al., 2021). Production of by-products and MPW has increased in recent decades as a consequence of a rise in meat production and processing (Philipp et al., 2021). Many of the by-products of the meat processing industry are being utilised as feedstocks for production of feed, pet food, fertiliser, bioenergy, and fat and protein isolates, within the framework of a strict regulatory system (Toldrá et al., 2021). Valorisation of by-products improves the sustainability of the industry and limits the environmental burden of meat processing. Yet, in contrast to by-products, few attempts have been made to valorise the wastewater associated with meat processing (Bustillo-Lecompte and Mehrvar, 2015; Adekanmi et al., 2020). At present, meat processing industries in most countries operate large-scale wastewater treatment plants to treat MPW to a water quality standard that allows discharge of treated effluents into local surface waters (Bustillo-Lecompte and Mehrvar, 2015). Such remediation-focussed treatment changes an environmental burden into an economic burden for the industry, whilst valuable resources present in MPW remain unutilised.
MPW is a complex wastewater as it combines different waste streams generated along the entire pathway of meat processing. Wastewater is generated from the moment live animals enter the meat processing facility and includes mud, faecal matter and urine, slaughter and processing wastewater as well as wastewater generated during cleaning of facilities and equipment (Aziz et al., 2019). Volumes are large with the meat processing industry using an estimated 1.5 and 18 m3 of water per ton of processed meat (Philipp et al., 2021). On a global scale, this amounts to 24% of total water used by the food and beverage industry (Bustillo-Lecompte and Mehrvar, 2015).
MPW is characterised by a high content of organic matter, suspended solids, plant nutrients, oil and grease, as well as pathogenic and non-pathogenic microorganisms (Johns, 1995; Wu and Mittal, 2011; Bustillo-Lecompte and Mehrvar, 2015; Philipp et al., 2021; Shende and Pophali, 2021). Detergents and disinfectants used for factory cleaning will also be present in MPW (Shende and Pophali, 2021). The composition of MPW is quite variable and depends on a range of factors including animal species handled and the size and type of operation (e.g., slaughter vs meat processing) (Johns, 1995; Bustillo-Lecompte and Mehrval, 2015; Shende and Pophali, 2021; Ng et al., 2022). Levels of Biological Oxygen Demand (BOD), Chemical Oxygen Demand (COD), Total Suspended Solids (TSS) and nutrients in MPW are typically in excess of local water discharge levels (Philipp et al., 2021) and unprocessed MPW is harmful if released into the environment (Bustillo-Lecompte and Mehrval, 2015; Adekanmi et al., 2020; Alam et al., 2021).
Most MPW treatment plants use methods that are not dissimilar to those employed in municipal wastewater treatment. These methods reliably remove the high amount of organic matter and nutrients present in MPW (Debik and Coskun, 2009; Bustillo-Lecompte and Mehrval, 2015; Aziz et al., 2019). This commonly includes mechanical or primary treatment, biological or secondary treatment, as well as subsequent physical and chemical treatments (Bustillo-Lecompte and Mehrval, 2015; Adekanmi et al., 2020; Philipp et al., 2021; Ng et al., 2022). Operation of conventional wastewater treatment is economically costly and commonly does not generate value from waste. In this study the capture of the valuable plant nutrients nitrogen and phosphorus from MPW, that had undergone primary and secondary wastewater treatment, is explored. In a conventional wastewater treatment plant, phosphorus is chemically precipitated as a non-bioavailable salt, while nitrogen is discharged as N2 gas following a nitrification-denitrification cycle. Nitrogen is a key ingredient of fertilisers, but production of N-containing urea compounds is expensive due to the high costs of fossil fuel required for the Haber–Bosch reaction (Moghadam et al., 2024). Phosphorus is another key ingredient of fertilisers; however geological phosphorus deposits are non-renewable, and concerns have been raised about future depletion of this resource (Alewell et al., 2020). Thus, there is a strong argument to capture nitrogen and phosphorus present in wastewaters, and to re-use these elements according to the principles of the circular economy, a process that will likely generate long-term environmental and social benefits (Furness et al., 2021).
Valorisation of wastewater provides an alternative to conventional methods of wastewater treatment. Multiple studies have shown that duckweed ponds can be used as an alternative to conventional tertiary wastewater treatment, capturing plant nutrients and/or other minerals of interest (Goopy et al., 2004; Sasmaz et al., 2021; Paolacci et al., 2021). In this scenario, nitrogen and phosphorus are removed from the water column by floating duckweed plants. The duckweed (Lemnaceae) family is known for its rapid growth rate (Ziegler et al., 2015) and associated rapid uptake of macro- and micronutrients from a variety of nutrient-rich wastewaters, such as farm manure (Devlamynck et al., 2021; Stadtlander et al., 2024), dairy processing wastewater (Walsh et al., 2022; O’Mahoney et al., 2022), aquaculture effluent (Paolacci et al., 2021) and dairy soiled water (Redmond et al., 2025). The resulting duckweed biomass tends to contain high levels of protein, up to 40% on a dry weight basis, with an amino acid profile that is attractive from a nutritional perspective (Appenroth et al., 2017). Indeed, duckweed biomass can be used as an ingredient in animal feeds (e.g., Anderson et al., 2011; Minich and Michael, 2024), biofuel or fertiliser. This creates a novel waste to value cycle, whereby valuable plant nutrients in MPW are returned to the farm. However, in the case of the use of wastewater-grown duckweed, biomass intended to be used as feed needs to be assessed for the presence of contaminants such as toxic metals, pesticides, pharmaceuticals and pathogens (Sońta et al., 2019).
The aims of this study were to determine whether (1) Lemna minor can grow on MPW effluent that had undergone primary and secondary treatment, and (2) to assess wastewater remediation capacity. To do so, the study was conducted in two phases. First, L. minor was cultivated in small tanks to pioneer cultivation on MPW. Subsequently, L. minor was cultivated on MPW in a scaled-up, semi-outdoor, recirculatory system to determine nutrient uptake and remediation capacity. The data were then analysed to explore whether L. minor has the potential to be used as a part of an eco-friendly industry-based system for valorisation of MPW, in accordance with the principles of the circular economy.
2 Materials and methods
2.1 Duckweed stock cultivation
Experiments were performed using L. minor “Blarney” (MJ100). This L. minor clone was originally selected in the south of Ireland, and is characterised by consistent, rapid growth. The clone is kept at the Rutgers Duckweed Stock Cooperative database (5500). Axenic stock of L. minor “Blarney” was maintained in flasks in a growth room under controlled environmental conditions (22 °C; 16 h:8 h light:dark photoperiod; light intensity of 50 μmol m−2 s−1). Stock was cultivated on half-strength Hutner’s medium (Hutner and Johns, 1953). Stock grown under the aforementioned conditions was used for small scale, indoor experiments. In contrast, for scaled-up experiments stock of L. minor was first acclimated to semi-outdoor conditions on a commercial growth medium [pH Perfect Grow (0.25 mL L−1) and pH Perfect Micro (0.25 mL L−1)] (Advanced Nutrients, West Hollywood, CA, United States) prior to use in experiments.
2.2 Meat processing wastewater
MPW was obtained from a local meat processing abattoir (KEPAK, Watergrasshill, Co. Cork, Ireland) which processes around 65,000 cows per year. The wastewater treatment plant processes approximately 260 m3 MPW per day. Raw wastewater, including sludge, is fed into a balancing tank where it is homogenised through continuous mixing. It then passes on to the drum screen, the first element of the treatment process, where larger solids are separated from the liquid fraction. Subsequently, a dissolved air flotation unit removes suspended solids, oils, grease and associated BOD and COD. The relatively clear effluent then cycles between oxic and anoxic tanks whereby microbes in the oxic tank facilitate breakdown of remaining organic matter and oxidation of nitrogen to nitrate. In the anoxic tank nitrate undergoes denitrification to yield molecular N2 gas. Subsequently, effluent passes through a clarifier at which stage samples for duckweed growth were collected. This effluent has low BOD and COD levels but still high levels of nitrogen and phosphorus. In the wastewater treatment plant, this clarifier effluent would normally be treated through the addition of coagulants and flocculants and subsequently passed through a second clarifier to remove the remaining suspended solids and phosphorus from the wastewater.
2.3 Lemna minor cultivation on MPW under controlled conditions
In the initial, small-scale, experiments L minor was grown on MPW at various concentrations (25%, 50%, and 100% of the concentration of clarifier effluent in Magenta vessels with vented lids (7.7 cm × 7.7 cm × 9.7 cm - GA-7). Distilled water was used to dilute the MPW if required. The pH value of unamended MPW was 7.8. To avoid potential toxicity and volatilisation of un-ionised ammonia, whilst also accounting for the inherent pH increase observed when duckweed is cultivated on MPW (Redmond et al., 2025), the pH was set at pH 4.5 for half the samples. This was achieved through the addition of diluted hydrochloric acid. Half-strength Hutner’s medium at pH 4.5 was chosen as an optimised control medium (Hutner and Johns, 1953). Each experiment was replicated at least three times, and all samples were kept under a light regime of 14 h light and 10 h dark, a light intensity of 50 μmol m−2 s−1 Photosynthetically Active Radiation (PAR), and a temperature of 22 °C. Experiments were conducted for a period of 1 week.
To start each small-scale experiment, three colonies of four fronds from a sterile L. minor stock culture were placed on 100 mL of MPW effluent, or half-strength Hutner’s medium. The starting weight of L. minor was determined by calculating the average from representative samples. At the end of a 1-week growth period, the fresh weight of each plant sample was measured, and the Relative Growth Rate (RGR) (Connolly and Wayne, 1996) was calculated.
In the small-scale experiments, total colony number as well as the total number of fronds alive were counted visually at the end of each experiment (i.e., day 7). Furthermore, the weight of each sample was recorded and the RGR of each sample was calculated according to Connolly and Wayne (1996);
Whereby W1 and W2 are respectively the starting biomass and the total biomass generated by the system. ΔT is the length of the experiment in days.
The photosynthetic health of plants grown on different concentrations of MPW, at two pH values, was quantified using a modulated, imaging fluorometer equipped with ImagingWin software (WALZ Imaging fluorometer, Effeltrich, Germany) (Schreiber et al., 1986). The maximum quantum efficiency of photosystem II (PSII) (Fv/Fm) was measured on plants dark adapted for 15–20 min. A weak measuring light (<1 μmol m−2 s−1) was used to determine F0, the minimum fluorescence obtained in the dark-adapted state. The maximum fluorescence (Fm) was obtained by applying a saturating pulse of light (Schreiber et al., 1986). The steady state quantum yield (YII) of photosynthesis was obtained following 5 min of incubation under an actinic light intensity of 80 μmol m−2 s−1, an intensity similar to that in the growth room used for duckweed cultivation. The intensity of the measuring light was less than 1 μmol m−2 s−1, the intensity of the saturating pulse of white light was 2,200 μmol m−2 s−1.
2.4 Lemna minor cultivation on MPW in a semi-outdoor, recirculating system
L. minor was grown in non-sterile, semi-outdoor, recirculating systems (n = 3). For this paper, the term semi-outdoor refers to a glasshouse system where plants were exposed to ambient light conditions and temperatures. Each replicate system consisted of three tiered tanks (20 cm length × 13 cm width × 17 cm height) and a lower sump tank (27 cm × 17 cm x 13 cm) with a capacity of 9 L per system (Figure 1). Three identical systems were operated. The total surface area for duckweed growth in the tiered tanks was 780 cm2 for each replicate. A 3 W pump was placed in each system to achieve circulation. A volume of 9 L MPW, at a 50% concentration, was prepared for each replicate system, added to the sump tank and pumped at a rate of 0.13 L min−1 from the sump tank to the top-tiered tank, a slow flowrate that does not lead to disruption of the duckweed mat (Coughlan et al., 2022). The lower tanks were gravity-fed from the higher tanks, and the MPW was guided from the lowest tank back into the sump. Mesh filters placed in each tank’s outflow pipe prevented L. minor from moving between tanks while allowing the medium to continue to circulate. Sides and bottoms of all tanks were painted black to prevent light penetration below the duckweed mat and to impede algal growth. A muslin cloth covered the recirculating systems to prevent exposure to excess direct sunlight. The mean light intensity experienced by L. minor plants, cultivated under muslin cloth, was 135 μmol m−2 s−1, with a noon-time maximum of 463 μmol m−2 s−1 (Onset HOBO MX 2202 datalogger, Tempcon Instrumentation, Ford, United Kingdom). Daylength ranged from 16.25 to 16.5 h (Late June/early July in Ireland).
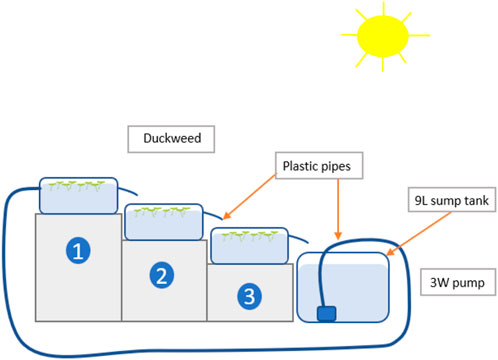
Figure 1. A schematic overview of a small recirculating system, comprised of a sump tank and three duckweed cultivation tanks, each with 260 cm2 of surface area, and with a combined volume of 9 L. An electric pump circulates wastewater from a sump to the first of three cascading growth tanks. The two lower tanks are gravity-fed from the higher tank.
In the recirculation system L. minor was grown on a 50% concentration of MPW effluent for 15 days. At the start of the experiment tanks were inoculated with 4.5 g fresh duckweed to achieve 60% surface cover. For this purpose, L. minor plants were pre-acclimated for at least 2 weeks to glasshouse conditions. At the end of each 3-day interval, plant material was harvested, excess water was removed with tissue paper, and the plants were weighed. Furthermore, water samples were taken from the sump tank at the end of each 3-day incubation period and pH, Electrical Conductivity (EC), Total Nitrogen (TN) and Total Phosphorus (TP) were determined. Subsequently, half of the volume of MPW effluent (i.e. 4.5 L per system) was replaced by fresh MPW, and a total of 13.5 g (4.5 g per tank) of the measured fresh plant biomass was returned to the three tanks for the next 3 days of growth. Nutrient uptake per square metre of initial plant surface cover, and per day, was calculated based on TN and TP concentrations which were measured every third day.
2.5 Water quality analysis
A physicochemical assessment of the MPW effluent was obtained through analysis of samples by a GLP-certified laboratory (Aquatic Services Unit, Cork, Ireland). Replicate samples were taken on three occasions, at least 4 months apart. Using standard analysis methods (Walsh et al., 2021), unfiltered wastewater samples were evaluated for concentrations of BOD, COD, total solids, TN, and TP. The effluent was filtered (0.45 µm) and examined using the Lachat Quik-Chem FIA 8000 (Zeilweger Analytics, Inc., Milwaukee, United States) to determine the dissolved contents of ammonia, nitrate, nitrite, and orthophosphate (QuikChem protocols 10-107-06-3-D, 10-107-04-1-C, 10-107-04-1-C, and 10-115-01-1-B, respectively). Sodium, potassium, calcium, magnesium, zinc, and iron concentrations were determined using a flame AAS (Varian Australia Ply Ltd., Mulgrave, Australia), whilst copper and manganese concentrations were assessed using a graphite furnace AAS (Varian Australia Ply Ltd., Mulgrave, Australia).
Water samples were also collected for analysis from the experimental, scaled-up, recirculating duckweed system. Samples were taken every third day, and the concentrations of TN and TP were determined with Hach test LCK138 Laton testing kit (which measures TN from 1 mg L−1–16 mg L−1) and Hach test LCK348 Laton testing kit (which measures TP from 0.5 mg L−1–5.0 mg L−1) using a DR3900 spectrophotometer. It was opted to measure Total Nitrogen and Total Phosphorus in order to include N and P present as amino acids and small peptides, which are bioavailable for duckweed. This also distinguishes nutrient removal from effluent by duckweed from potential microbial (i.e., algal) nutrient uptake. Where relevant, distilled water was added to compensate for water loss through evapotranspiration.
Electrical conductivity (EC), pH of MPW were assessed every 3 days, in conjunction with water quality analysis, using a HANNA Combo pH/Conductivity Tester (High Range HI98130, Hanna Instruments, City of Woonsocket RI, United States).
2.6 Data analysis
Data from both small-scale, controlled experiments and upscaled recirculatory systems were analysed using R Studio version 4.3.2. Normality of data was assessed using the Shapiro-Wilk test. Subsequently, growth data (RGR, colony and frond number, Fv/Fm and Y(II)) were analysed using Analysis of Variance (ANOVA) with either post hoc Tukey’s HSD (in case of a normal distribution) or the Kruskal–Wallis test (non-normal distribution). Data from an upscaled (recirculatory) system were also analysed using IBM SPSS 28 software. In both R Studio and SPSS, an ANOVA test was conducted on each variable, namely, RGR, pH, EC, TDS, TN, and TP. The purpose was to examine time-dependent changes and establish their statistical significance. Also, the relationship between dynamic changes in key variables was assessed by calculating the correlation coefficient.
3 Results
3.1 The composition of MPW effluent
The physicochemical composition of MPW effluent was analysed (Table 1). The average pH of the effluent was 7.8 while BOD and COD were low at 6.1 and 74.3 mg O2 L−1, respectively. The total nitrogen (TN) concentration was 14.7 mg N L−1 of which un-ionised ammonia (i.e., ammonia NH3) and nitrate (NO3) made up 0.8 mg N L−1 and 9.4 mg N L−1, respectively. The total phosphorus (TP) concentration was 7.8 mg P L−1 of which 7.0 mg P L−1 was orthophosphate. Sulphate (SO4), potassium (K), calcium (Ca) and magnesium (Mg) concentrations were modest at 26.3 mg L−1, 72.9 mg L−1, 49.3 mg L−1, 11.3 mg L−1, respectively. However, sodium (Na) and chloride (Cl) concentrations were relatively high at 361.7 mg L−1 and 420.7 mg L−1, respectively. Iron, copper, manganese, zinc and nickel are all present in concentrations of less than 1 mg L−1.
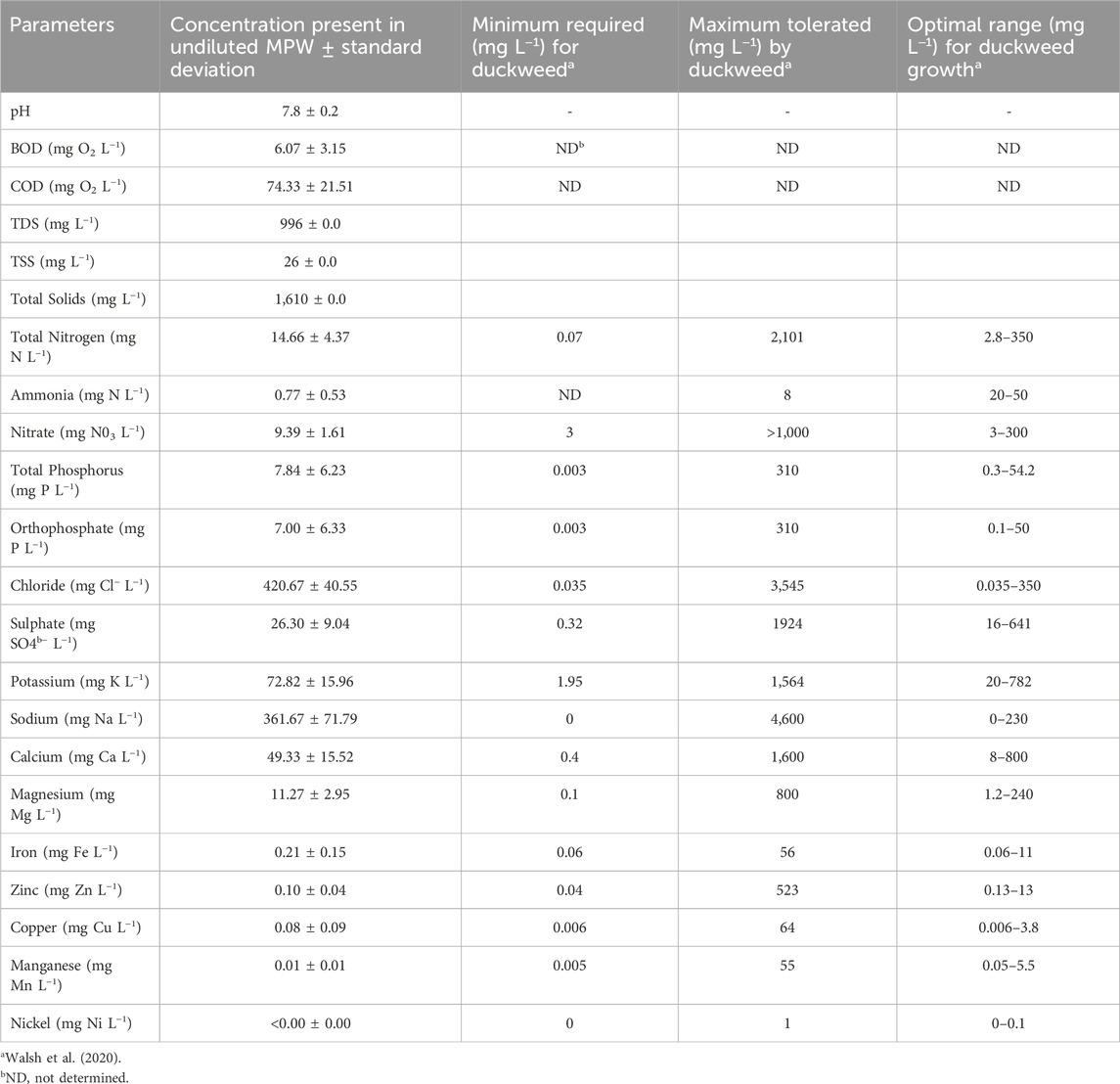
Table 1. The concentrations of minerals and organic compounds present in MPW (n = 3), as well as the required, tolerated, and optimal concentrations for duckweed according to Walsh et al. (2020).
3.2 Growth of Lemna minor on MPW effluent
In the small-scale range finding experiment, plants were cultivated on different concentrations of MPW effluent. The initial pH of MPW was 7.8, irrespective of the used MPW concentration. In comparison, the pH of standard, half-strength Hutner’s is 4.5. To compare growth of L. minor on MPW with that on half-strength Hutner’s, the pH of MPW was either retained at pH 7.8 or lowered to pH 4.5.
Experiments commenced using a total of 12 fronds per Magenta vessel. After 1 week of growth, samples on half-strength Hutner’s medium comprised some 46 fronds, while samples on MPW comprised between 40 and 59 fronds (Figure 2). Significantly (p < 0.05) more fronds were generated on 100% wastewater at pH 4.5, compared to any other MPW treatment. A large increase in the number of colonies occurred (p < 0.001) for cultures on MPW at pH 7.8. This result may indicate mild stress. The biomass relative growth rate (RGR) on MPW ranged between 0.34 and 0.39 days−1, and this rate is similar to that obtained on half-strength Hutner’s medium, under otherwise similar conditions. Overall, good growth was achieved, with no substantial differences in RGR as a result of changes in MPW concentration and/or pH.
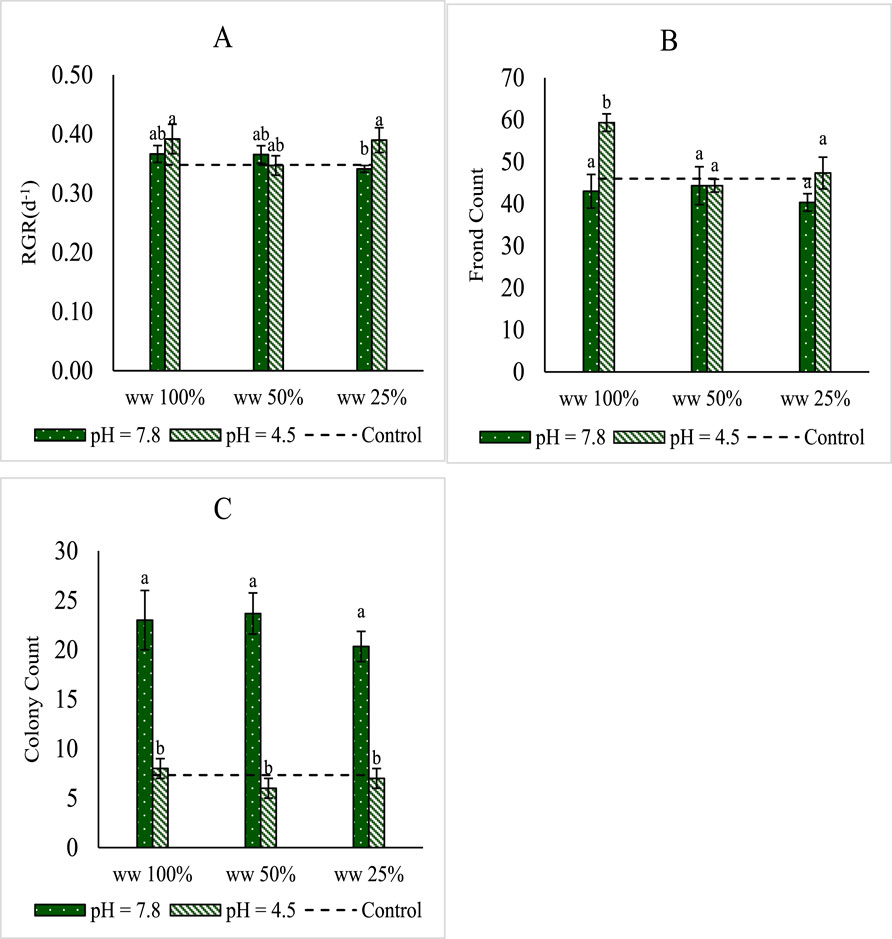
Figure 2. Growth of Lemna minor cultures grown on 100 mL MPW. Concentrations of TN and TP in wastewater (WW) at the start of the experiment were, respectively 13.8 mg N L−1, and 4.4 mg P L−1in undiluted, 100%, MPW. Shown are (A) mean RGR (d−1) (B) frond count and (C) colony count of Lemna minor grown on 25%, 50%, and 100% concentrations of MPW effluent, with a starting pH of either 4.5 or 7.8. Shown as a dotted line are the equivalent growth parameters on half-strength Hutner’s medium. Standard deviations are shown. n = 3. Bars that do not share at least one same letter are significantly different from one another for p < 0.05, as per post hoc test.
To investigate potential plant stress in more detail, chlorophyll a fluorometry was used to quantify photosynthetic performance. The maximal quantum yield of photosystem II (Fv/Fm) ranged between 0.68 and 0.75 (Figure 3). No substantial differences were found between plants on MPW and those on half-strength Hutner’s medium. Statistical analysis did not reveal any clear trend of concentration and/or pH on the maximal quantum yield. In contrast, the steady state quantum yield (Y(II)) of photosystem II was slightly lower at higher pH values (p < 0.01). Effects of different concentrations of MPW on Y(II) were not significant.
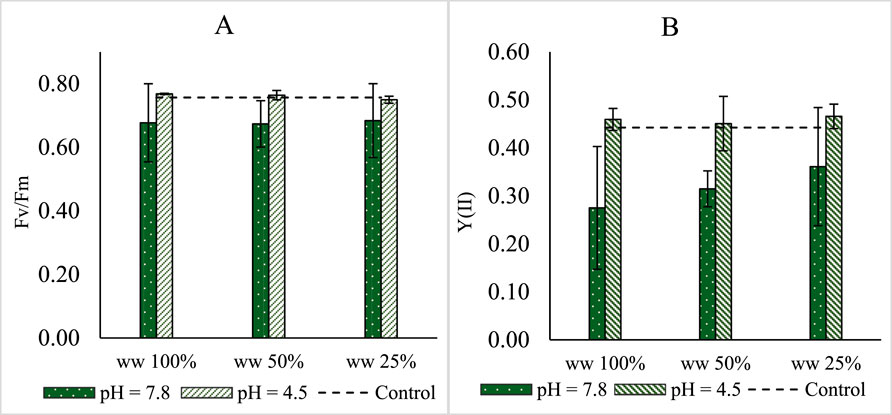
Figure 3. Growth of Lemna minor cultures grown on 100 mL MPW. Concentrations of TN and TP in wastewater (WW) at the start of the experiment were, respectively 13.8 mg N L−1, and 4.4 mg P L−1 in undiluted, 100%, MPW. Shown are (A) the mean maximal quantum yield of photosystem II (Fv/Fm) and (B) the steady state quantum yield (Y(II) of photosystem II grown on 25%, 50%, and 100% concentrations of MPW effluent, with a starting pH of either 4.5 or 7.8. Shown as a dotted line are the equivalent growth parameters on half-strength Hutner’s medium. Standard deviations are shown (n = 3). A post hoc test did not reveal significant (i.e., p < 0.05) differences between bars.
3.3 The cultivation of Lemna minor on MPW in a recirculating semi-outdoor system
In the recirculatory system, a 50% concentration of MPW was used as signs of colony break up (decreased frond to colony ratio) had been noticed on 100% MPW in the small-scale laboratory studies (Figure 2B). The batch of MPW used for these experiments was slightly more dilute than average MPW (Table 1), with initial concentrations of the 50% dilution being TN = 6.9 mg L−1 and TP = 2.1 mg L−1. Half of the medium was replaced every third day, at which stage plant biomass, TN and TP concentrations as well as pH and EC were quantified. The increase in biomass for each 3-day period varied from 15 g for the first 3 days, to just 3.2 g for the final 3-day period (Figure 4). One-way ANOVA showed that the mean increase in biomass for each 3-day period changed significantly with time (F = 91.654, df = 5, p < 0.001). RGR values varied between 0.24 days−1 for the first 3 days, to just 0.11 days−1 for the final 3 days. A one-way ANOVA revealed that the change in RGR with time (Figure 4) was similarly statistically significant (F = 86.507, df = 5, p < 0.001). Although the plants exhibited modest growth at the end of the experiment, they appeared healthy.
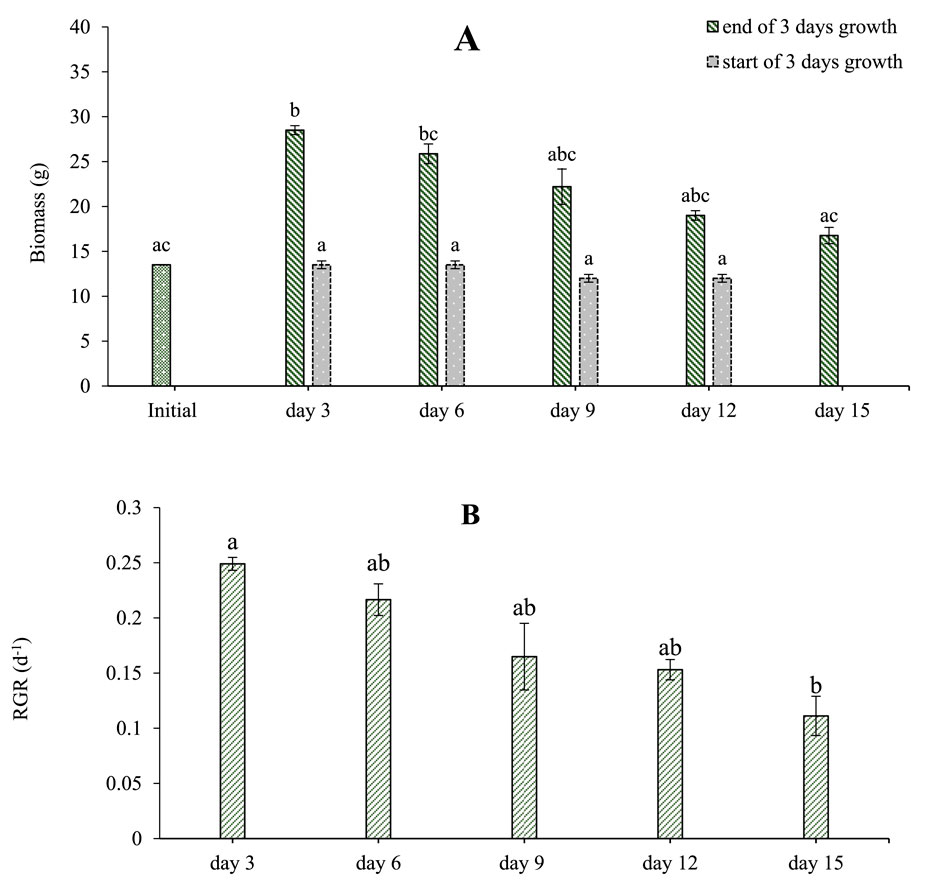
Figure 4. Growth of Lemna minor cultures grown on 50% concentrated MPW effluent over a 15-day period. Concentrations of TN and TP at the start of the experiment were, respectively 6.9 mg N L−1, and 2.1 mg P L−1 in a 50% concentration of MPW. Shown are (A) mean biomass (g) and (B) RGR (day −1). Standard deviations are shown (n = 3). Bars that do not share at least one same letter are significantly different from one another (p < 0.05) as per post hoc test.
The pH of the MPW in the semi-outdoor 15-day experiment ranged between 8.3 on the first day to 9.9 on the 15th day (Figure 5). The pH gradually increased with time. A one-way ANOVA test performed on pH values showed that the increase in mean pH was statistically significant (F = 147.648, df = 5, p < 0.001) (Figure 5). Similarly, EC values increased with time. EC levels varied between 0.87 S m−1 on the first day to 1.54 S m−1 on day 15. A one-way ANOVA test performed on EC levels showed that the increase in EC level was statistically significant (F = 81.577, df = 5, p < 0.001). A strong correlation was found between pH and EC, with a Pearson correlation coefficient of r = 0.812.

Figure 5. Growth of Lemna minor cultures grown on a 50% concentration of MPW effluent over a 15-day period. Concentrations of TN and TP at the start of the experiment were, respectively 6.9 mg N L−1, and 2.1 mg P L−1 in a 50% concentration of MPW. Shown are (A) pH and (B) EC. Standard errors are shown (n = 3). Bars that do not share at least one same letter are significantly different from one another for p < 0.05, as per post hoc test.
The concentrations of TN and TP in the MPW-medium were measured every 3 days, over a total period of 15 days. High concentrations of TN and TP were measured at the initial start of the experiment (day 0, TN = 6.9 mg L−1; TP = 2.1 mg L−1). After 3 days of growth, these concentrations had decreased to just 1.40 mg L−1 T, and 0.46 mg L−1 TP (Figure 6). Subsequently, following replacement of 50% of the MPW by fresh medium, the concentration of TN was restored to 4.15 mg L−1. From day 3 onwards, concentrations of TN remained fairly constant, about 1.4 mg L−1 at the end of each 3-day period, and about 4.2 mg L−1 after addition of fresh medium (Figure 6). No time-dependent trends were detected in TN concentration at the end of each growing period, or TN concentration after addition of fresh medium (F = 37.208, df = 5, p < 0.001).
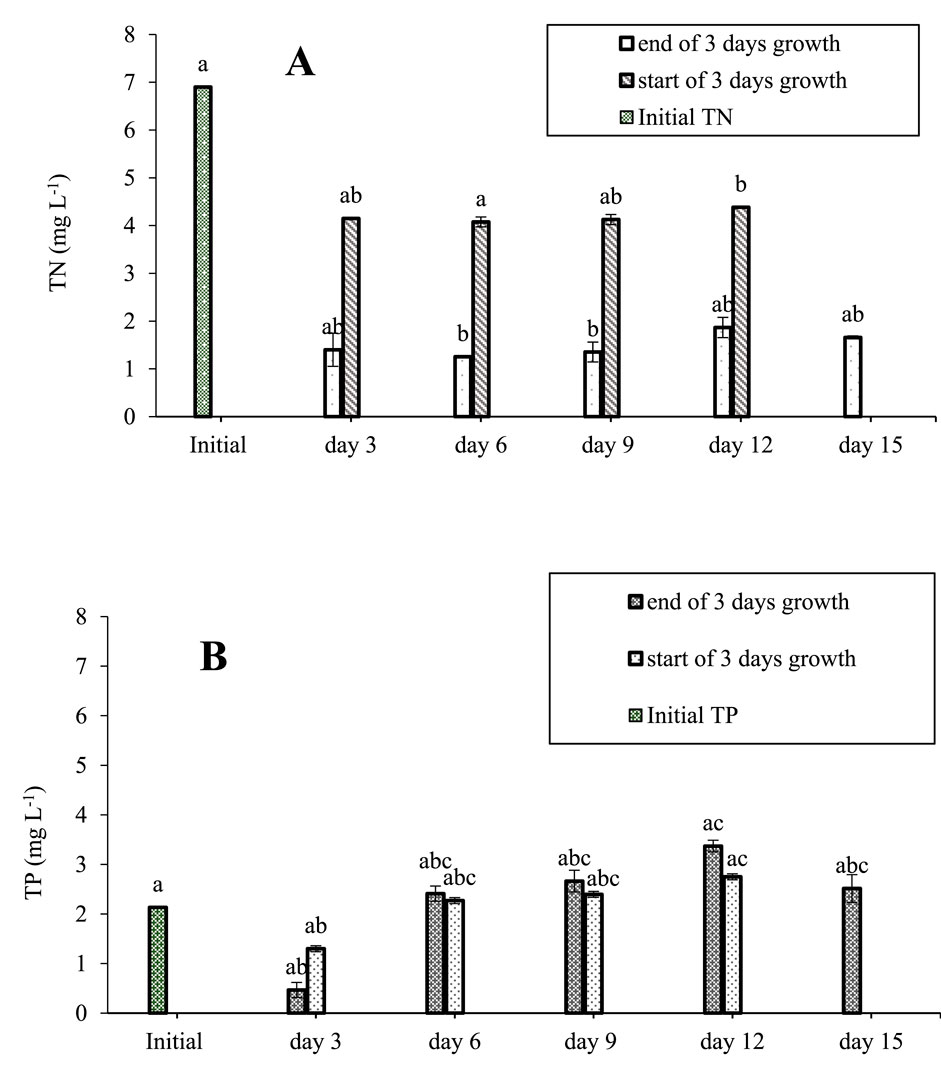
Figure 6. (A) Mean (±SD) TN (B) and TP of a 50% concentration of MPW effluent measured over 15-day period. Concentrations of TN and TP at the start of the experiment were, respectively 6.9 mg N L−1, and 2.2 mg P L−1 in a 50% concentration of MPW. Standard deviations are shown. Bars that do not share at least one same letter are significantly different from one another for p < 0.05, as per post hoc test.
The concentration of TP at the end of the first 3-day growth period was just 0.46 mg L−1 (Figure 6). Following replacement of 50% of the medium with fresh MPW, this rose to 1.3 mg L−1. From day 3 onwards, concentrations of TP gradually increased, reaching a concentration of 3.37 mg L−1 on day 12 (Figure 6). The increase in TP from day 3 onwards, is significant (F = 174.061 df = 5, p < 0.001).
The TN concentration of MPW effluent decreased from, on average, 6.9 mg L−1 on day 0–1.40 mg L−1 at the end of day 3, i.e., a decrease in TN concentration of 5.5 mg TN L−1 within the first 3 days. Given that the system volume was 9 L, this equates to the removal of 49.5 mg of TN in 3 days, or on average, 16.63 mg of TN per day. The total surface area of the experimental setup was 0.078 m2, and L. minor covered 60% of this surface area. Therefore, 353 mg of TN m−2 d−1 was removed from MPW effluent within the first 3 days (Figure 7). When the same calculation was repeated for TP removal rate, it was found that 107 mg of TP m−2 d−1 was removed within the first 3 days (Figure 7).
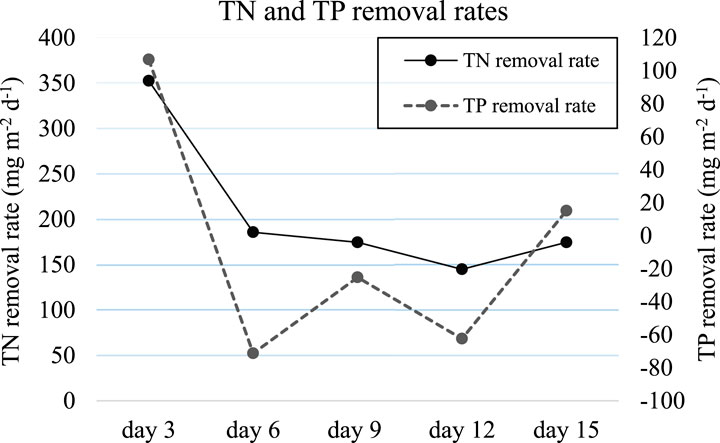
Figure 7. Mean (±SD) TN and TP removal rate by Lemna minor grown on a 50% concentration of MPW effluent over a 15-day period. Concentrations of TN and TP at the start of the experiment were, respectively 6.9 mg N L–1, and 2.1 mg P L–1 in a 50% concentration of MPW.
TN and TP removal rates were calculated for L. minor grown on MPW effluent for all timepoints (Figure 7). The TN removal rate was 353 mg TN m−2 d−1 over the first 3 days of the experiment (Figure 7) yet subsequently decreased. For example, on day 6, the removal rate was 186 mg TN m−2 d−1, while at day 15 the TN removal rate had decreased to 174 mg TN m−2 d−1. The TP removal rate over the first 3 days of the experiment was 107 mg of TP m−2 d−1, but TP removal had virtually ceased at subsequent timepoints, or even become negative, suggesting nett TP release (Figure 7).
4 Discussion
4.1 MPW is suitable for duckweed growth
Previous studies have shown that untreated, raw, MPW is not a suitable growth medium for duckweed species (Goopy et al., 2004). Therefore, the current study focussed on MPW that had undergone primary and secondary treatment. The physicochemical composition of such MPW was analysed at three time points throughout the year (Table 1). Overall, the composition was found to be relatively stable, with variations in the contents of different elements being quite modest. This lack of seasonal variation in the composition of MPW likely reflects intake of a consistent quality of livestock, consistent slaughtering and processing procedures, and consistent wastewater treatment operating procedures throughout the year. In turn, this will greatly facilitate the development and implementation of potential duckweed-based wastewater valorisation approaches.
The relatively low concentrations of BOD and COD in MPW are likely to facilitate duckweed cultivation, in so far as that the concentration of organic matter in MPW is low enough not to result in strong microbial growth. Measured concentrations of nitrogen and phosphorus are suitable for cultivation of duckweed species (Landolt and Kandeler, 1987; Walsh et al., 2020). Total nitrogen and total phosphorus are mostly bioavailable as, respectively, nitrate and ortho-phosphate, further emphasising the suitability of MPW as a duckweed growth medium. In some commercial wastewater treatment plants MPW is treated using anaerobic fermentation, which lowers BOD and COD, but this may result in accumulation of large amounts of ammonia (Goopy et al., 2004). High levels of phytotoxic, un-ionised ammonia in combination with high pH, can inhibit duckweed growth (Körner et al., 2001; Caicedo et al., 2000) and necessitate pH management (Jones et al., 2023; Redmond et al., 2025). However, the samples used in the current study had passed through an aeration tank and nitrogen was largely in the form of nitrate. In fact, the concentration of ammonia, the preferred uptake form of nitrogen for duckweed species (Fang et al., 2007; Zhou et al., 2021) is low, although MPW does contain enough nitrate nitrogen to facilitate good growth. Furthermore, given that nitrogen requirements typically exceed phosphorus demand by several fold (Paolacci et al., 2021; Pasos-Panqueva et al., 2024), the concentration of total nitrogen is somewhat low, relative to the concentration of total phosphorus (Landolt and Kandeler, 1987; Walsh et al., 2020). Therefore, it can be hypothesised that under long-term growth conditions phosphorus uptake will be limited by a deficiency of nitrogen. Concentrations of sulphate, potassium, calcium and magnesium are all suitable for the cultivation of duckweed species (Landolt and Kandeler, 1987; Walsh et al., 2020). The calcium to magnesium ratio which has been shown to be important for plant growth, is also favourable in MPW (Walsh et al., 2020). However, it is noted that both sodium and chloride concentrations are above optimal (Tkalec et al., 2001; Sree et al., 2015; Lambert et al., 2022), potentially leading to accumulation of these salts in long-term cultivation systems.
In agreement with the assessment that MPW is a suitable medium for duckweed growth, it was found that L. minor displayed good growth on different concentrations of MPW, under laboratory conditions. RGR values on MPW were similar to those obtained on optimised half-strength Hutner’s medium. An increase in colony numbers, i.e., colony splitting, observed on MPW kept at a higher pH indicates potential mild plant stress (Henke et al., 2011). A small, pH dependent decrease in the steady state yield of photosystem II also suggests mild toxicity and stress (Redmond et al., 2025). Such pH dependent toxicity resembles ammonia toxicity, but as ammonia concentrations are low, this is unlikely. Conversely, it is possible that the stress-symptoms are due to pH-dependent changes in uptake of other components of MPW such as sodium, chloride or metals (Verma and Suthar, 2015). Alternatively, the increase in pH can potentially be due to algal growth which would be associated with increased competition for nutrients such as nitrogen and phosphorus (Gerardi and Lytle, 2015). To further explore whether any of these minor effects could affect a duckweed-based remediation system, studies progressed to a scaled-up, semi-outdoors, recirculatory system.
4.2 Growth and remediation in a recirculatory system
The initial RGR (days 3 and 6) in the semi-outdoor cultivation system ranged between 0.25 days−1 and 0.22 days−1 on days 3 and 6 respectively. These RGR values compare favourably with those obtained under indoor conditions, as well as with RGR values obtained on other wastewaters under similar semi-outdoor conditions. For example, O’Mahoney et al. (2022) achieved an RGR of 0.1 d−1 in a similar circulatory system using anaerobic digestor effluent from a dairy processing factory. Using a variety of mediums, the literature reports many RGR values less than 0.2 days−1 (e.g., Al-Nozaily and Alaerts, 2002; Dinh et al., 2020). Thus, laboratory and semi-outdoor growth trials, together with physicochemical analyses, all emphasise the suitability of MPW as a growth medium for short term duckweed cultivation.
Good plant growth and nutrient removal were observed during the first 6 days of operation of the recirculatory duckweed-system. The TN removal rate during the first 3 days was 353 mg TN m−2d−1. This rate of nitrogen removal from the MPW is in line with previously published nitrogen removal rates which give values ranging between 124 and 4,400 mg TN m−2d−1 (cf. Walsh et al., 2022). The data obtained in this study place the initial (day 3) nitrogen removal rate at the lower end of the range found in the literature. Observed TN removal rates between 150 and 200 mg TN m−2d−1 can be deemed low (cf. Walsh et al., 2022). The TP removal rate of 107 mg TP m-2 d−1 for the first 3 days of remediation in the circulatory system is also consistent with reported TP removal rates, which vary between 14 and 590 mg TP m−2 d−1 (cf. Walsh et al., 2022). TP removal rates at later sampling points are either negative, or can be deemed low (cf. Walsh et al., 2022).
Beyond the first 6 days of operation of the recirculatory system, both pH and conductivity (salinity) were found to gradually increase. Increases in pH did not lead to visible precipitation of salts. Increases in salinity in a recirculating system were also reported by Lambert et al. (2022). These authors reported that salinity becomes a substantial problem in systems where salt that is not taken up by plants, gradually starts to accumulate, resulting in well documented salinity stress (Tkalec et al., 2001; Sree et al., 2015). Indeed, after 6 days of operation RGR, TN and TP removal all decreased markedly. Initial decreases in RGR, TN and TP removal are associated with a minor increase in conductivity. Major increases in conductivity are only observed from day 9. However, it remains to be seen that the initial decrease in RGR, TN and TP removal is causally linked to salinity. The source of increased conductivity of the medium in these experiments remains unclear. A possible explanation is the release of ions when solids, present in MPW (Table 1), gradually dissolve (Taylor et al., 2018). Interestingly, while TP removal virtually ceases from day 6, nitrogen removal still proceeds at a substantial rate, which is fast enough to prevent a rise in TN concentration in the medium. It is possible that biosynthesis of compatible solutes such as proline and glycine betaine, important for the prevention of salinity stress, drives continued nitrogen demand (Carillo et al., 2008). It could be speculated that luxury phosphorus uptake from the phosphorus enriched medium during the first 3 days of the experiment (Paterson et al., 2020) may have contributed to increased leaching of phosphorus into the medium at later timepoints. However, quantitative analysis showed that the amounts of phosphorus in the plant are not sufficient to explain the observed increase in phosphorus in the medium. Instead, it can be speculated that apart from release of phosphorus by plants, the gradual dissolution of solids, present in MPW (Table 1), may also have contributed to the rise in phosphorus concentration in the medium. However, this remains to be proven.
4.3 Future prospects of using a recirculatory system to valorise MPW
In this paper it is shown that a recirculatory remediation system, whereby fresh medium is added every 3 days, will cease to operate by day six. It is concluded that at this timepoint all medium needs to be replaced to avoid the build-up of salts (similarly, problematic microbial and algal growth might at this stage become prevalent). This is not necessarily a problem, as the data in this paper show that after 3 days, L. minor had reduced the average TN concentration from 6.90 mg L−1–1.40 mg L−1 as well as the TP concentration from 2.13 mg L−1 to 0.46 mg L−1. According to the EU Urban Wastewater Treatment Directive (98/15/EC), allowable limits for discharging TN into environmentally sensitive receiving waters are around 6 mg L−1 and for TP 0.5 mg L−1. Thus, it is concluded that short-term remediation of MPW effluent is sufficient to achieve wastewater discharge requirements, without resulting in the built up of salinity.
Remediation of MPW can potentially be optimised by manipulating the composition of the wastewater. MPW is a complex wastewater that combines different waste streams including faecal matter, urine, slaughter and processing wastewater, as well as cleaning and disinfection fluids. A recent study of fish-processing wastewater (Katsara et al., 2025) proposed the development of bespoke remediation treatments for the different waste streams (e.g., initial washings, filleting wastewater, cooking and canning wastewater) that make up combined fish processing wastewater. Similarly, it can be hypothesised that exclusion of some waste streams from the MPW will increase remediation efficiency. By extrapolating the data acquired in this study and using TN and TP removal rates of 353 and 107 mg m−2 d−1, respectively, it can be calculated that a recirculatory system using 3-day cycles, will remove 1,285 kg TN and 391 kg TP per hectare, per year from the MPW effluent. However, this number will strongly depend on seasonal and climatic factors. Assuming a meat processing factory producing 200 m3 of wastewater, and using calculated TN and TP removal rates of 353 and 107 mg m−2 d−1, respectively, it can be estimated that a pond area of 312 m2 is required to bring down the TN concentration from 6.9 mg L−1–1.4 mg L−1, and the TP concentration from 2.1 mg L−1 to 0.47 mg L−1. While such calculations are of theoretical interest and indicate that a duckweed-based remediation approach can be realistic, it is noted that extending calculations beyond the scale of the current study requires a careful evaluation of scaling-up challenges. Thus, future work needs to focus on upscaling duckweed remediation under realistic industry conditions, while exploring economic, social, regulatory and sanitary aspects.
5 Conclusion
Physicochemical analyses, laboratory and semi-outdoor growth trials all show the suitability of MPW as a growth medium for short term cultivation of Lemna minor. Quantitative analysis revealed rapid removal of nitrogen and phosphorus removal from MPW, with calculated uptake rates similar to those reported in the literature. In a scaled-up, semi-outdoor system short-term (3 days) remediation of MPW effluent was found to be sufficient to achieve wastewater discharge requirements. Thus, a duckweed-based system can contribute to remediation, while also leading to the production of nutrient-rich biomass that can potentially be used as animal feed, biofuel or fertiliser, thus contributing to a more sustainable circular economy. Future work needs to focus on the nutritional value of MPW raised biomass (especially starch and protein content), safety (presence of contaminants such as toxic metals, pesticides, pharmaceuticals and pathogens (Sońta et al., 2019) and upscaling duckweed remediation under realistic industry conditions, while exploring technical (salinity and seasonality), economic (cost-benefit), social, and regulatory aspects.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
MS: Investigation, Writing – review and editing, Writing – original draft, Validation, Methodology, Conceptualization, Formal Analysis, Data curation. AK: Investigation, Data curation, Formal Analysis, Writing – review and editing, Methodology, Conceptualization, Writing – original draft. CR: Methodology, Formal Analysis, Writing – review and editing, Conceptualization, Writing – original draft, Investigation. WD: Writing – review and editing, Methodology, Writing – original draft, Investigation, Formal Analysis, Data curation. MJ: Methodology, Supervision, Data curation, Conceptualization, Investigation, Validation, Formal Analysis, Resources, Funding acquisition, Writing – original draft, Writing – review and editing, Project administration.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was co-funded by the Department of Agriculture, Food and the Marine, Government of Ireland (2021R487, project Duck-Feed), The European Regional Development Fund through the Ireland - Wales Cooperation Programme (82078, project Brainwaves) and The European Union’s Horizon 2020 research and innovation programme (101084437, project IMPRESS). MJ acknowledges support by WoB.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Adekanmi, A. A., Adekanmi, A. S., and Adekanmi, U. T. (2020). Biotreatment of slaughterhouse wastewater by microalgae. United Int. J. Res. and Technol. 1 (9), 19–30.
Al-Nozaily, F. A., and Alaerts, G. (2002). Performance of duckweed-covered sewage lagoons in Sana'a, Yemen, depending on sewage strength. J. Water Supply Res. Technology—AQUA. 51 (3), 173–182. doi:10.2166/aqua.2002.0015
Alam, R., Khan, S. U., Basheer, F., and Farooqi, I. H. (2021). Nutrients and organics removal from slaughterhouse wastewater using phytoremediation: a comparative study on different aquatic plant species. Bristol, United Kingdom: IOP Conf. Ser. Mater. Sci. Eng. 1058, 1012068. doi:10.1088/1757-899x/1058/1/012068
Alewell, C., Ringeval, B., Ballabio, C., Robinson, D. A., Panagos, P., and Borrelli, P. (2020). Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 11 (1), 4546. doi:10.1038/s41467-020-18326-7
Anderson, K. E., Lowman, Z., Stomp, A. M., and Chang, J. (2011). Duckweed as a feed ingredient in laying hen diets and its effect on egg production and composition. Int. J. Poult. Sci. 10 (1), 4–7. doi:10.3923/ijps.2011.4.7
Appenroth, K. J., Sree, K. S., Böhm, V., Hammann, S., Vetter, W., Leiterer, M., et al. (2017). Nutritional value of duckweeds (Lemnaceae) as human food. Food Chem. 217, 266–273. doi:10.1016/j.foodchem.2016.08.116
Aziz, A., Basheer, F., Sengar, A., Khan, S. U., and Farooqi, I. H. (2019). Biological wastewater treatment (anaerobic-aerobic) technologies for safe discharge of treated slaughterhouse and meat processing wastewater. Sci. Total Environ. 686, 681–708. doi:10.1016/j.scitotenv.2019.05.295
Bustillo-Lecompte, C. F., and Mehrvar, M. (2015). Slaughterhouse wastewater characteristics, treatment, and management in the meat processing industry: a review on trends and advances. J. Environ. Manag. 161, 287–302. doi:10.1016/j.jenvman.2015.07.008
Caicedo, J. R., van Der Steen, N. P., Arce, O., and Gijzen, H. J. (2000). Effect of total ammonia nitrogen concentration and pH on growth rates of duckweed (Spirodela polyrrhiza). Water Res. 34 (15), 3829–3835. doi:10.1016/s0043-1354(00)00128-7
Carillo, P., Mastrolonardo, G., Nacca, F., Parisi, D., Verlotta, A., and Fuggi, A. (2008). Nitrogen metabolism in durum wheat under salinity: accumulation of proline and glycine betaine. Funct. Plant Biol. 35 (5), 412–426. doi:10.1071/fp08108
Connolly, J., and Wayne, P. (1996). Asymmetric competition between plant species. Oecologia 108, 311–320. doi:10.1007/bf00334656
Coughlan, N. E., Walsh, É., Ahern, R., Burnell, G., O’Mahoney, R., Kuehnhold, H., et al. (2022). Flow rate and water depth alters biomass production and phytoremediation capacity of Lemna minor. Plants 11 (16), 2170. doi:10.3390/plants11162170
Debik, E., and Coskun, T. (2009). Use of the Static Granular Bed Reactor (SGBR) with anaerobic sludge to treat poultry slaughterhouse wastewater and kinetic modeling. Bioresour. Technol. 100 (11), 2777–2782. doi:10.1016/j.biortech.2008.12.058
Devlamynck, R., de Souza, M. F., Leenknegt, J., Jacxsens, L., Eeckhout, M., and Meers, E. (2021). Lemna minor cultivation for treating swine manure and providing micronutrients for animal feed. Plants 10 (6), 1124. doi:10.3390/plants10061124
Dinh, T. T. U., Soda, S., Nguyen, T. A. H., Nakajima, J., and Cao, T. H. (2020). Nutrient removal by duckweed from anaerobically treated swine wastewater in lab-scale stabilization ponds in Vietnam. Sci. Total Environ. 722, 137854. doi:10.1016/j.scitotenv.2020.137854
Fang, Y. Y., Babourina, O., Rengel, Z., Yang, X. E., and Pu, P. M. (2007). Ammonium and nitrate uptake by the floating plant Landoltia punctata. Ann. Bot. 99 (2), 365–370. doi:10.1093/aob/mcl264
Furness, M., Bello-Mendoza, R., Dassonvalle, J., and Chamy-Maggi, R. (2021). Building the ‘bio-factory’: a bibliometric analysis of circular economies and life cycle sustainability assessment in wastewater treatment. J. Clean. Prod. 323, 129127. doi:10.1016/j.jclepro.2021.129127
Gerardi, M. H., and Lytle, B. (2015). “Algae, alkalinity, and pH,” in The biology and troubleshooting of facultative Lagoons. Editors M. H. Gerardi, and B. Lytle (Hoboken, New Jersey: John Wiley and Sons, Inc.). doi:10.1002/9781118981771
Goopy, J. P., Murray, P. J., Lisle, A. T., and Al Jassim, R. A. M. (2004). Use of chemical and biological agents to improve water quality of effluent discharge from abattoirs. Asian-Australasian J. Animal Sci. 17 (1), 137–145. doi:10.5713/ajas.2004.137
Henke, R., Eberius, M., and Appenroth, K. J. (2011). Induction of frond abscission by metals and other toxic compounds in Lemna minor. Aquat. Toxicol. 101 (1), 261–265. doi:10.1016/j.aquatox.2010.10.007
Hutner, S. H. (1953). Comparative physiology of heterotrophic growth in higher plants. Ames (IA): Growth and differentiation in plants. Iowa State College Press,417–447.
Johns, M. R. (1995). Developments in wastewater treatment in the meat processing industry: a review. Bioresour. Technol. 54 (3), 203–216. doi:10.1016/0960-8524(95)00140-9
Jones, G., Scullion, J., Dalesman, S., Robson, P., and Gwynn-Jones, D. (2023). Lowering pH enables duckweed (Lemna minor L.) growth on toxic concentrations of high-nutrient agricultural wastewater. J. Clean. Prod. 395, 136392. doi:10.1016/j.jclepro.2023.136392
Katsara, A., Coughlan, N. E., and Jansen, M. A. K. (2025). Characterization of seafood processing wastewater; processing procedures and physicochemical variability. Environ. Pollut. 383, 126761. doi:10.1016/j.envpol.2025.126761
Körner, S., Das, S. K., Veenstra, S., and Vermaat, J. E. (2001). The effect of pH variation at the ammonium/ammonia equilibrium in wastewater and its toxicity to Lemna gibba. Aquat. Bot. 71 (1), 71–78. doi:10.1016/s0304-3770(01)00158-9
Lambert, M., Devlamynck, R., Fernandes de Souza, M., Leenknegt, J., Raes, K., Eeckhout, M., et al. (2022). The impact of salt accumulation on the growth of duckweed in a continuous system for pig manure treatment. Plants 11 (23), 3189. doi:10.3390/plants11233189
Landolt, E., and Kandeler, R. (1987). The family of Lemnaceae—A monographic study, vol. 2. Biosystematic investigations in the family of duckweeds (Lemnaceae). Zürich, Switzerland: Des Geobotanischen Institutes der Eidgenössischen Technischen Hochschule.
Minich, J. J., and Michael, T. P. (2024). A review of using duckweed (Lemnaceae) in fish feeds. Rev. Aquac. 16, 1212–1228. doi:10.1111/raq.12892
Moghadam, M. R., Bazmandegan-Shamili, A., and Bagheri, H. (2024). “The current methods of ammonia synthesis by Haber-Bosch process,” in Progresses in ammonia: science, technology and membranes (Amsterdam, Netherlands: Elsevier), 1–32.
Ng, M., Dalhatou, S., Wilson, J., Kamdem, B. P., Temitope, M. B., Paumo, H. K., et al. (2022). Characterization of slaughterhouse wastewater and development of treatment techniques: a review. Processes 10 (7), 1300. doi:10.3390/pr10071300
O’Mahoney, R., Coughlan, N. E., Walsh, É., and Jansen, M. A. K. (2022). Cultivation of Lemna minor on industry-derived, anaerobically digested, dairy processing wastewater. Plants 11 (22), 3027. doi:10.3390/plants11223027
Paolacci, S., Stejskal, V., and Jansen, M. A. K. (2021). Estimation of the potential of Lemna minor for effluent remediation in integrated multi-trophic aquaculture using newly developed synthetic aquaculture wastewater. Aquac. Int. 29, 2101–2118. doi:10.1007/s10499-021-00736-z
Pasos-Panqueva, J., Baker, A., and Camargo-Valero, M. A. (2024). Unravelling the impact of light, temperature and nutrient dynamics on duckweed growth: a meta-analysis study. J. Environ. Manag. 366, 121721. doi:10.1016/j.jenvman.2024.121721
Paterson, J. B., Camargo-Valero, M. A., and Baker, A. (2020). Uncoupling growth from phosphorus uptake in Lemna: implications for use of duckweed in wastewater remediation and P recovery in temperate climates. Food Energy Secur. 9 (4), 244. doi:10.1002/fes3.244
Philipp, M., Masmoudi Jabri, K., Wellmann, J., Akrout, H., Bousselmi, L., and Geißen, S. U. (2021). Slaughterhouse wastewater treatment: a review on recycling and reuse possibilities. Water 13 (22), 3175. doi:10.3390/w13223175
Redmond, C., Coughlan, N. E., Purcell, A., and Jansen, M. A. K. (2025). Optimisation of dairy soiled water as a novel duckweed growth medium. Plants 14 (1), 110. doi:10.3390/plants14010110
Sasmaz, M., Uslu Senel, G., and Obek, E. (2021). Strontium accumulation by the terrestrial and aquatic plants affected by mining and municipal wastewaters (Elazig, Turkey). Environ. Geochem. Health 43, 2257–2270. doi:10.1007/s10653-020-00629-9
Schreiber, U., Schliwa, U., and Bilger, W. (1986). Continuous recording of photochemical and non-photochemical chlorophyll fluorescence quenching with a new type of modulation fluorometer. Photosynth. Res. 10, 51–62. doi:10.1007/bf00024185
Shende, A. D., and Pophali, G. R. (2021). Anaerobic treatment of slaughterhouse wastewater: a review. Environ. Sci. Pollut. Res. 28 (1), 35–55. doi:10.1007/s11356-020-10921-x
Sońta, M., Rekiel, A., and Batorska, M. (2019). Use of duckweed (Lemna L.) in sustainable livestock production and aquaculture – a review. Ann. Animal Sci. 19 (2), 257–271. doi:10.2478/aoas-2018-0048
Sree, K. S., Adelmann, K., Garcia, C., Lam, E., and Appenroth, K. J. (2015). Natural variance in salt tolerance and induction of starch accumulation in duckweeds. Planta 241, 1395–1404. doi:10.1007/s00425-015-2264-x
Stadtlander, T., Schmidtke, A., Baki, C., and Leiber, F. (2024). Duckweed production on diluted chicken manure. J. Animal Feed Sci. 33 (1), 128–138. doi:10.22358/jafs/169431/2023
Taylor, M., Elliott, H. A., and Navitsky, L. O. (2018). Relationship between total dissolved solids and electrical conductivity in Marcellus hydraulic fracturing fluids. Water Sci. Technol. 77 (8), 1998–2004. doi:10.2166/wst.2018.092
Tkalec, M., Mlinarec, J., Vidaković-Cifrek, Ž., Jelenčić, B., and Regula, I. (2001). The effect of salinity and osmotic stress on duckweed Lemna minor L. Acta Bot. Croat. 60 (2), 237–244.
Toldrá, F., Reig, M., and Mora, L. (2021). Management of meat by-and co-products for an improved meat processing sustainability. Meat Sci. 181, 108608. doi:10.1016/j.meatsci.2021.108608
Verma, R., and Suthar, S. (2015). Lead and cadmium removal from water using duckweed–Lemna gibba L.: impact of pH and initial metal load. Alexandria Eng. J. 54 (4), 1297–1304. doi:10.1016/j.aej.2015.09.014
Walsh, É., Paolacci, S., Burnell, G., and Jansen, M. A. K. (2020). The importance of the calcium-to-magnesium ratio for phytoremediation of dairy industry wastewater using the aquatic plant Lemna minor L. Int. J. Phytoremediation 22 (7), 694–702. doi:10.1080/15226514.2019.1707478
Walsh, É., Kuehnhold, H., O’Brien, S., Coughlan, N. E., and Jansen, M. A. K. (2021). Light intensity alters the phytoremediation potential of Lemna minor. Environ. Sci. Pollut. Res. 28, 16394–16407. doi:10.1007/s11356-020-11792-y
Walsh, É., Cialis, E., Dillane, E., and Jansen, M. A. K. (2022). Lemnaceae clones collected from a small geographic region display diverse traits relevant for the remediation of wastewater. Environ. Technol. and Innovation 28, 102599. doi:10.1016/j.eti.2022.102599
Wu, P., and Mittal, G. (2011). Characterization of provincially inspected slaughterhouse wastewater in Ontario, Canada. Can. Biosyst. Eng. 53 (6)–9e6.18.
Zhou, Y., Kishchenko, O., Stepanenko, A., Chen, G., Wang, W., Zhou, J., et al. (2021). The dynamics of NO 3− and NH 4+ uptake in duckweed are coordinated with the expression of major nitrogen assimilation genes. Plants 11 (1), 11. doi:10.3390/plants11010011
Keywords: Lemna minor, duckweed, remediation, nutrient recovery, meat processing wastewater, circular economy
Citation: Sasmaz Kislioglu M, Katsara A, Redmond C, Drenckhan W and Jansen MAK (2025) Meat processing wastewater; the use of Lemna minor L. to convert an environmental burden into a new resource. Front. Environ. Sci. 13:1622266. doi: 10.3389/fenvs.2025.1622266
Received: 19 May 2025; Accepted: 07 October 2025;
Published: 24 October 2025.
Edited by:
Nikolai Borisjuk, National Academy of Sciences of Ukraine, UkraineReviewed by:
Gbekeloluwa B. Oguntimein, Morgan State University, United StatesYuzhen Zhou, Huaiyin Normal University, China
Copyright © 2025 Sasmaz Kislioglu, Katsara, Redmond, Drenckhan and Jansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Marcel A. K. Jansen, bS5qYW5zZW5AdWNjLmll
†These authors have contributed equally to this work
 Merve Sasmaz Kislioglu
Merve Sasmaz Kislioglu Alexandra Katsara
Alexandra Katsara Cian Redmond
Cian Redmond Wiebke Drenckhan
Wiebke Drenckhan Marcel A. K. Jansen
Marcel A. K. Jansen