- 1Department of Bio Sciences, School of Bio Sciences and Technology, Vellore Institute of Technology, Vellore, Tamil Nadu, India
- 2Department of Plant Pathology and Entomology, VIT-School of Agricultural Innovation and Advanced Learning, Vellore Institute of Technology, Vellore, Tamil Nadu, India
Pollution caused by heavy metals (HMs) poses a significant threat to environmental and agricultural sustainability. The current study emphasizes on the isolation and characterization of lead (Pb)- and cadmium (Cd)-resistant bacteria from lake soil sediment. Among all the six isolates obtained, VITLLJ4 was capable of tolerating Cd and Pb up to concentrations of 600 ppm and 1,200 ppm, respectively. It also exhibited strong biofilm formation under metal stress with specific biofilm formation (SBF) values ranging at 0.32–1.06 for Pb and 0.38–0.91 for Cd, facilitating the sequestration of metals. Growth profiling of VITLLJ4 showed steady exponential growth under metal stress conditions, and response surface methodology (RSM) confirmed the optimization of pH, carbon source, and nitrogen source for efficient bioremoval of Cd and Pb. Scanning electron microscopy (SEM) revealed the presence of pleomorphism in cells upon exposure to HMs. Furthermore, VITLLJ4 exhibited plant growth-promoting rhizobacterial (PGPR) traits, revealing its ability to produce indole acetic acid, siderophore, and ammonia, and the isolate was also capable of solubilizing insoluble phosphate. A pilot-scale study on pot culture showed an increase in the phenotypic characteristics of Spinacia oleracea augmented with VITLLJ4 (rhizoremediation) than that in untreated plants (phytoremediation). The bioaccumulation of Pb and Cd was found to be higher in the roots than in the shoots of S. oleracea, confirming the plant to be a root accumulator for heavy metals. The maximum removal efficiency of Pb and Cd was found to be higher in rhizoremediation treatments, i.e. 80% and 75%, than in phytoremediation, i.e., 59% and 50%, upon supplementation of 150 ppm of Cd and Pb. These findings highlighted that microbe-assisted phytoremediation is an effective strategy in the bioremoval of Pb and Cd from polluted sites.
1 Introduction
The growing global population has significantly increased the need for products and has led to rapid industrialization, contributing to the discharge of heavy metals (HMs) (Chowdhury and Rahman, 2024). Lead (Pb) and Cadmium (Cd) are two naturally occurring metals that are used and discharged in effluents from various industries such as batteries, paints, pesticides, smelting process, mining activities, and emission of harmful fumes from the industries (Mishra and De, 2024; Raj and Das, 2023). These metals accumulate in the environment, resulting in the contamination of air, water, and soil; furthermore, it surpasses the food chain and causes severe ill-effects on humans by damaging various organs (Dewangan and Bhatia, 2023). Despite their widespread use, long-term exposure to these metals can result in neurological, cardiovascular, and kidney damage (Lentini et al., 2017). Due to their chronic toxicity, various agencies have set permissible limits for the usage of these HMs. The permissible limit provided by the Environmental Protection Agency (EPA) for Pb and Cd in drinking water is 0.015 mg/L and 0.005 mg/L, respectively, and in soil, it is 400 mg/kg and 3 mg/kg–5 mg/kg, respectively. Similarly, the World Health Organization (WHO) has set guidelines for Pb and Cd levels in drinking water, i.e. 0.01 mg/L and 0.003 mg/L, while the maximum concentration of Cd permitted in soil is 0.01–1 mg/kg.
Several conventional methods such as chemical precipitation, ion exchange, and reverse osmosis are employed for the removal of HMs; however, these chemical processes are expensive, can generate excess amounts of sludge and chemical waste, and may not be effective for removal of all HMs (Qasem et al., 2021). Phytoremediation is an eco-friendly and cost-effective approach that uses plants such as hyperaccumulators to stabilize, transform, and remove toxic pollutants from the polluted environments (Priya et al., 2023). Plants are easily cultivable and possess high absorption capacity to resist and take up HMs. Some of the mechanisms involved in HM uptake include phytoextraction, phytostabilization, and phytovolatilization. Spinacia oleracea is commonly known as spinach and has been used as a potential hyperaccumulator for the bioremediation of HMs due to its high biomass production capacity and fast growing ability under varying environmental conditions, which makes it a suitable candidate for the uptake of HMs. It also has a deep root system that aids in the absorption of HMs from the sub-soil zone.
Among the various methods used in the bioremoval of HMs, the application of microbes has emerged as a promising technology due to its minimal cost and environmental safety. Certain beneficial microbes such as the plant growth-promoting bacteria increase the phytoremediation efficiency when supplemented to the rhizosphere region of the plant by enhancing the pollutant bioavailability in the soil through various mechanisms such as transformation, chelation, and solubilization (Bhanse et al., 2022), which, in turn, is referred to as rhizoremediation. These mechanisms could increase the uptake of HMs as microbes exhibit resistance toward HMs by forming biofilms or by synthesizing certain biomolecules that could provide resistance and enhance plant growth. This plant- and microbe-mediated uptake is often referred to as effective microbe (EM) technology. Bacteria use mechanisms such as biosorption, bioaccumulation, and efflux pump to resist HMs, which can be effective in the bioremoval of HMs. However, Pb and Cd are often sequestered through an energy-dependent process (active transport), and in certain instances, the binding of Pb and Cd to the cell surface has also been reported (Pal et al., 2022). Bacteria can also synthesize enzymes that can reduce HM ions to their less toxic form. Klebsiella sp. is known to possess various HM resistance mechanisms that could aid in their bioremoval (Aransiola et al., 2017; Yetunde Mutiat et al., 2018). Bacteria such as Bacillus megaterium and Serratia liquefaciens (Han et al., 2020) and Bacillus xiamenensis (Wagh et al., 2024) are used for the removal of Pb and Cd from soil. Similarly, studies reported Lysinibacillus fusiformis (Chen et al., 2025) and Bacillus paramycoides (Kaur et al., 2024) for Cd removal and Lactobacillus fermentum (Liu et al., 2024) and Paenibacillus dendritiformis (Dawwam et al., 2023) for the bioremoval of Pb. Various microbe-assisted phytoremediation strategies have also been used for the remediation of HMs. A study conducted by Mohammadzadeh et al. (2017) revealed bioremoval of Cd using co-culture of Bacillus safensis and Kocuria rosea upon augmentation to the rhizosphere region of Helianthus annuus. Das et al. (2017) also showed bioremoval of Pb by Enterobacter cloacae while supplemented to the rhizosphere region of Pennisetum purpureum. In another study, Lemna minor was supplemented with Cupriavidus taiwanensis and Pseudomonas putida for the bioremoval of Cd (Feng et al., 2022).
Though there are several reports on Cd and Pb removal, studies on the application of the rhizoremediation strategy are limited. Bioremoval potential of microbe-assisted plant based remediation reported 95% removal for single-metal supplementation (Wagh et al., 2024; Das et al., 2017; Vais et al., 2025). To our knowledge, there are limited studies on the survivability of effective microbes in the natural environment, and the mechanisms involved in the bioremoval of Cd and Pb are also not explored. Therefore, the current study focused on the bioremoval of Cd and Pb using bacteria obtained from polluted soil sediments. The indigenous bacteria obtained was screened for its ability to resist Cd and Pb along with its potential to develop biofilms for efficient bioremoval of HMs. Optimization of parameters such as carbon, nitrogen, and pH was carried out for enhanced bioremoval. The ability of an organism to resist and grow in the presence of Cd and Pb stress was also studied along with its plant growth-promoting (PGP) traits. Furthermore, the effective bacterial strain obtained was augmented to the rhizosphere of S. oleracea for the bioremoval of Cd and Pb on a pilot-scale pot culture-based study (Chamam et al., 2013).
2 Materials and methods
2.1 Chemicals and culture media
Cadmium chloride (CdCl2) and lead nitrate (Pb(NO3)2) were obtained from HiMedia, Chennai, India. MSM consisting of di-potassium hydrogen phosphate (K2HPO4, 1.2 g/L), potassium dihydrogen phosphate (KH2PO4, 0.6 g/L), ammonium chloride (NH4Cl, 0.5 g/L), dextrose (C6H12O6, 5 g/L), magnesium sulfate (MgSO4, 0.2 g/L), zinc sulfate (ZnSO4, 0.02 g/L), calcium chloride (CaCl2, 0.025 g/L), and sodium molybdate (Na2MoO4, 0.001 g/L) was obtained from SRL.
2.2 Sampling and isolation of lead- and cadmium-resistant bacteria
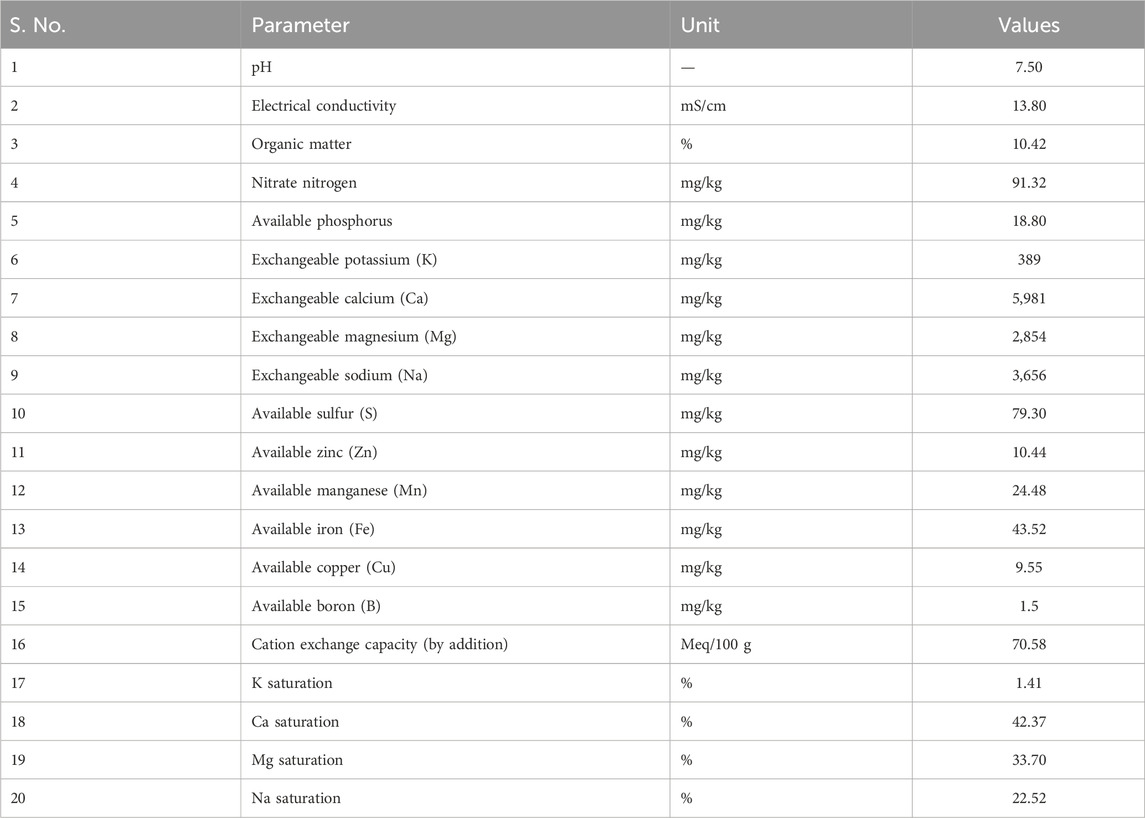
HM-resistant bacteria (HMRB) were obtained from sediment samples collected from Puliyanthangal Lake, Ranipet, Tamil Nadu, India (latitude, 12.96480 N; longitude, 79.29370 E). Samples were collected from six different locations using the random sampling method, the distance between two sampling locations was 100 m, and the depth at which the samples were collected was 5–10 cm. Furthermore, the samples obtained were transported to the laboratory upon transferring into sterile polyethylene bags (Babu et al., 2013). Subsequently, the soil samples were further analyzed for their physico-chemical parameters (Table 1).
2.2.1 Direct and indirect method of bacterial isolation
For the direct method of isolation, the soil samples were pooled, and 1 g of the soil sample was added to the test tube containing 10 mL distilled water, followed by serially diluting the samples under sterile conditions. MSM plates were supplemented with 50 ppm of Pb and Cd, and spread plating was performed from 10−2 to 10−4 dilutions. The plates were further incubated at 28 °C ± 2 °C for 48 h, and the plates were enumerated. The isolated colonies were purified by using the quadrant streak method and stored in glycerol stock at −80 °C (Wei et al., 2009).
For the indirect method (enrichment), MSM medium amended with 50 ppm Pb and Cd was prepared, and 5 g of the pooled soil sample was transferred into the flask. Upon incubation at 28 °C for 5 days at 120 rpm, 1 mL of the samples was serially diluted, and plating was performed from 10−4 to 10−6 dilutions. The colonies were differentiated based on its morphology and was further purified and maintained in glycerol stock (Wagh et al., 2024).
2.3 Screening for the effective isolate
2.3.1 Assessment of the maximum tolerable concentration (MTC) by plate and broth assays
The maximum tolerable concentrations of the isolates were assessed by the plate and broth assays. In plate assay, the concentration ranging at 200 mg/L–1,200 mg/L for Pb and 100 mg/L–600 mg/L for Cd was prepared in MSM agar medium. Furthermore, the isolates were inoculated onto the MSM medium and incubated for 48 h at 28 °C ± 2 °C. In broth assay, varying concentrations of Pb (200 mg/L–1,200 mg/L) and Cd (100 mg/L–600 mg/L) were prepared in test tubes, and 2% of v/v of the seed culture with 0.257 OD (at 620 nm) was added into the test tubes and incubated for 48 h. Upon incubation, droplet assay was performed, where 10 µL from each test tube was added to the nutrient agar medium to assess the viability of the cells (Itusha et al., 2019).
2.3.2 Biofilm formation assay
2.3.2.1 Qualitative analysis of biofilm formation by test tube assay
Test tubes were prepared with 10 mL of sterile MSM supplemented with various Pb and Cd concentrations ranging 200 mg/L–1,200 mg/L and 100 mg/L–600 mg/L, respectively. Bacterial seed culture (2%) of the effective strain with (0.257 OD, 3*108 cfu/mL) was inoculated into the respective test tubes and further incubated at 28 °C ± 2 °C for 48 h in a rotary shaker at 120 rpm. MSM broth without inoculation served as the control. Upon incubation, the broth was decanted, and 0.1% of crystal violet (CV) was added to the respective test tubes and vortexed for 5 min to ensure the complete exposure of the biofilm to the indicator; then, the tubes were incubated in static condition for 10 min, the stain was removed, and the tubes were washed with 1X phosphate-buffered saline (PBS) to eliminate the loosely bound cells and excess dye. Furthermore, the tubes were placed in an inverted position for drying, and the biofilm formation was characterized as strong, moderate or weak based on the intensity of the color formed on the walls of the test tubes (Wagh et al., 2024).
2.3.2.2 Quantitative analysis of biofilm formation by microtiter plate assay
Biofilm formation was quantitatively estimated using a microtiter plate (MTP) by preparing various concentrations of Pb, Cd, and mixed metals (Pb and Cd) ranging 50 mg–150 mg/L in test tubes containing MSM broth. For the preparation of seed culture, the isolate VITLLJ4 was inoculated in 50 mL of MSM broth and incubated under shaking condition till the OD reached 0.257 (3*108) cfu/mL. Seed culture with 2% inoculum size was transferred into the test tubes and kept overnight for incubation at 28 °C ± 2 °C. Upon incubation, 200 µL of the cell suspension of the respective concentration was transferred to the wells and was incubated for 48 h. Upon incubation, the broth was discarded, and 1% of CV was added to each well and incubated for 10 min. Furthermore, the CV was discarded from the wells and washed with 1X PBS. Upon air drying, 95% EtOH (ethanol) was added to dissolve the cells adherent to the wells. Furthermore, the matrix with cells was transferred into a fresh microtiter plate, and OD was measured at 570 nm using a microtiter plate reader (INNO-S, LTEK Co., Ltd. instruments, Republic of Korea) for the identification of the specific biofilm formation (SBF). The following formula was used for calculating SBF:
Here, AB is the OD of the stained cells recorded at 570 nm, CW is the OD of the control wells recorded at 570 nm, and G is the growth of the isolate recorded at 620 nm. Using the following SBF values, the biofilm was categorized as strong (≥1.10), moderate (0.70–1.09) or weak (0.35–0.69) (Milton et al., 2023).
2.4 Growth profiling of the effective isolate
Growth curve of the effective isolate was monitored with and without the supplementation of HMs (Cd and Pb). MSM broth inoculated with effective isolates with and without the addition of HMs was used to assess the difference in the growth profile. Furthermore, to monitor the growth of the effective isolate under stress conditions, 100 mL of MSM was prepared in two 250-mL Erlenmeyer flasks amended with 50 ppm and 100 ppm of mixed HMs (Pb and Cd) and inoculated with effective isolates. The un-inoculated broth served as the control. Upon inoculation, the flasks were incubated on a rotary shaker at 120 rpm at 28 °C ± 2 °C. At each 2-h interval, the OD was recorded at 620 nm till the organism reached the decline phase, and a graph with time versus OD was plotted (Wagh et al., 2024; Das et al., 2017; Itusha et al., 2019; Chatterjee et al., 2012).
2.5 Media optimization for the bioremoval of lead and cadmium using RSM
Statistical software (response surface methodology (RSM)) was used for optimizing and modeling the required factors to obtain maximum bioremoval. To obtain effective bacterial growth for the efficient removal of HMs (Pb and Cd), parameters such as carbon and nitrogen sources and pH were optimized (Vais et al., 2025). The carbon sources such as sucrose, glucose, and starch were amended in the concentration range of 0%–2%. Similarly, nitrogen sources such as ammonium chloride, urea, and yeast extract were tested in the range of 0%–2% with varying pH values of 6, 7, and 8 for designing the study in the central composite design (CCD) model using Design-Expert software. Upon obtaining the experimental design, various combinations were experimentally tested. The results obtained were analyzed using Design-Expert software to obtain the optimal parameters and the associated ANOVA and 3D contour plots. The obtained parameters were experimentally validated with statistical significance (Zhang and Yi, 2024).
2.6 Scanning electron microscopy of the effective isolate with and without HM (Pb and Cd) stress
The effective strain was assessed for variations in the morphology upon treatment with HMs (Pb and Cd). Bacterial cell suspensions (10 mL) grown overnight containing HMs were centrifuged at 10,000 rpm for 10 min at 4 °C. The pellet obtained was washed with 1X PBS, and a thin smear of bacterial cells was prepared on a sterile coverslip and air-dried. The cells were fixed with 2.5% glutaraldehyde and kept undisturbed overnight for dehydration. Upon incubation, the cells were washed with ethanol gradients at the range of 20%–90%. Furthermore, the samples were air-dried and subjected to scanning electron microscopy (SEM) (SEM, Carl Zeiss, EVO 18 SEM, Munich, Bavaria, Germany) analysis for obtaining micrographs at ×10,000 magnification. The samples were fixed on to a stub using a carbon tape in sterile conditions, and further, in an argon atmosphere, the samples were sputter-coated with gold under vacuum conditions (Wagh et al., 2024; Das et al., 2017).
2.7 Screening for plant growth-promoting rhizobacterial (PGPR) traits
The isolates obtained were screened for PGPR traits in the absence and presence of HMs (Cd and Pb). All the HM-resistant bacteria were tested for PGPR traits in the absence of HMs, and the isolates that tested positive for all the PGPR traits were further tested for their activity upon supplementation of Cd and Pb at a concentration of 50 ppm.
2.7.1 Ammonia production
For the estimation of ammonia, the effective bacteria was inoculated into 10 mL of peptone water (peptone, 10 g/L; NaCl, 5 g/L) and incubated for 48 h. After incubation, the cell suspension was centrifuged, and 0.5 mL of Nessler’s reagent was added to 2 mL of the cell-free supernatant (CFS). The change in color from yellow to reddish-brown indicated a positive result (Abdelwahed et al., 2022). For the quantification of ammonia, 2 mL of the supernatant was mixed with 0.04 mL of Nessler’s reagent and sodium potassium tartarate, and the absorbance was recorded at 450 nm using UV-VIS Spectrophotometer Model no. S-924 (Systonic).
2.7.2 Phosphate solubilization
National Botanical Research Institute of phosphates (NBRIP) agar plates were prepared by the supplementation of tricalcium phosphate, which acted as the source of insoluble phosphate. A loopful of bacterial culture was patch-inoculated onto NBRIP agar plates, which was then incubated for 7 days at 28 °C ± 2 °C. The presence of halozone indicated the solubilization of insoluble phosphate to its soluble form (Mehta and Nautiyal, 2001).
The phosphate solubilization index was determined using the above formula (Morales et al., 2011).
2.7.3 IAA production
Indole acetic acid (IAA) is a phytohormone that is crucial for plant growth and development as it enhances cell elongation, division, and differentiation. For assessing IAA production, MSM broth (KH2PO4, 1.36 g/L; Na2HPO4, 2.13 g/L; MgSO4, 0.2 g/L; and glucose, 1 g/L) supplemented with 5 mM of tryptophan was prepared. The broth was inoculated with 2% seed culture and incubated for 48 h. To 1 mL of CFS, 2 mL of Salkowski’s reagent and 2 mL of orthophosphoric acid were added, and the tubes were further incubated in dark for 30 min. Development of pink coloration upon incubation indicated IAA production, which was further quantified by measuring the absorbance at 540 nm using a microtiter plate reader (INNO-S, LTEK Co., Ltd. instruments, Republic of Korea) (Mukherjee et al., 2017).
2.7.4 Siderophore production
The effective isolate was inoculated into FISS minimal medium (KH2PO4, 0.5 g/L; L-asparagine, 0.5 g/L; MgSO4, 0.4 g/L; MnSO4, 0.01 g/L; ZnCl2, 0.05 g/L; FeSO4, 0.02 g/L; and glucose, 5 g/L), and the tubes were incubated for 48 h in dynamic conditions in a rotary shaker at 120 rpm at 28 °C ± 2 °C. The CFS was added into wells of Chrome Azurol S (CAS) agar plates and incubated overnight at 28 °C ± 2 °C. Siderophore production was indicated by the appearance of a halo zone around the wells added with CFS (Schwyn and Neilands, 1987).
2.7.5 HCN production
Hydrogen cyanide (HCN) production by use of an effective strain was assessed using King’s B broth amended with 4.4 g/L of glycine, and an overnight suspension of the effective strain was inoculated and incubated for 10 days at 28 °C ± 2 °C. To detect the presence of HCN, filter strips were soaked with a mixture of 0.05% of picric acid and 0.01% of sodium bicarbonate. Brown coloration on the filter paper strips indicated HCN production (Wagh et al., 2024).
2.8 Pot culture study experimental set-up
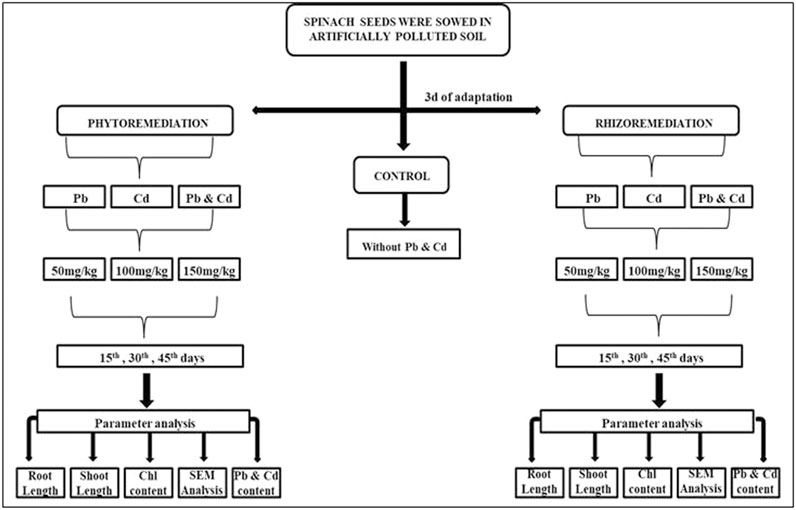
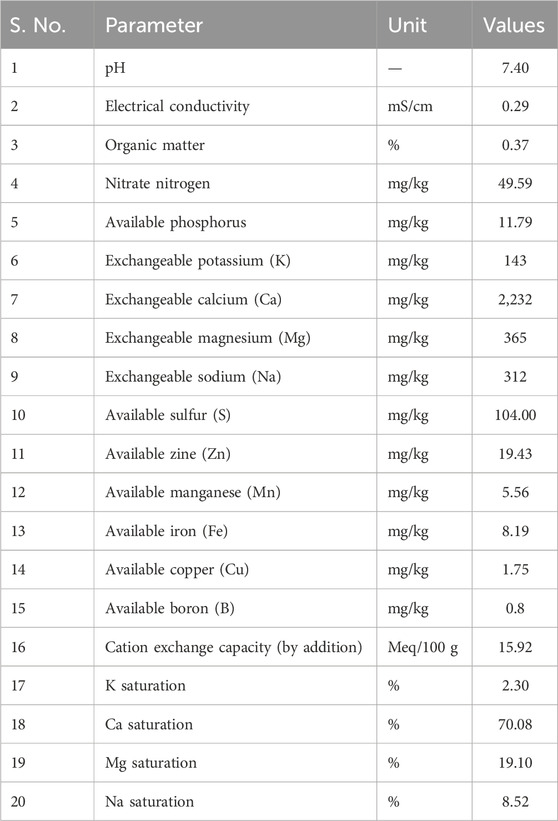
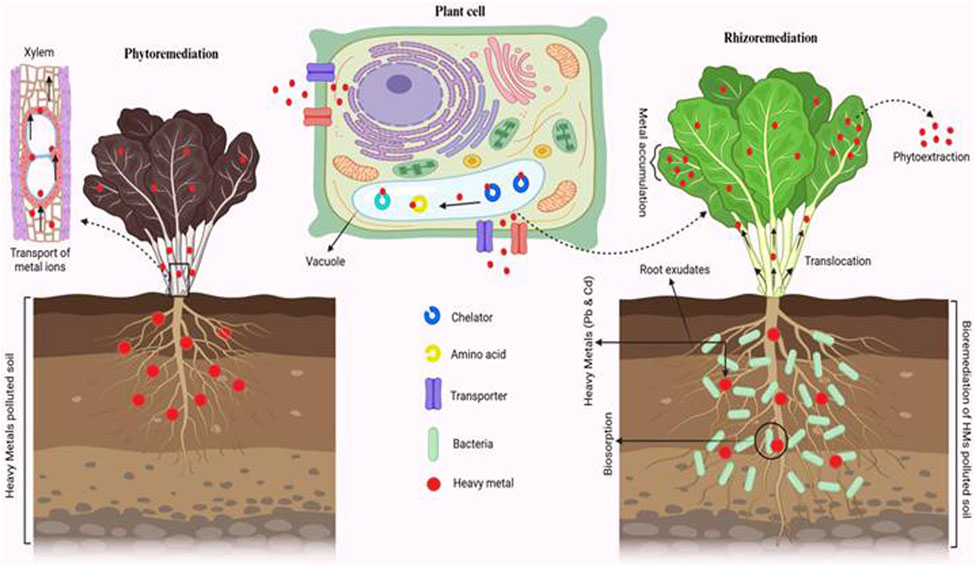
The pot culture study was carried out with two sets of treatments that included phytoremediation and rhizoremediation. The concentrations of Cd and Pb amended to the soil in both the treatments were 50, 100, and 150 ppm and were selected based on the studies carried out by Wagh et al. (2024) and Das et al. (2017) for phytoremediation and rhizoremediation treatments (Figure 1). The treatment without any pollutant, i.e., only soil, served as the control. Red loamy soil used for the study was obtained from the nursery of the Vellore Institute of Technology (VIT). The soil was sieved (2-mm sieve) before being packed into LDPE bags, and the physico-chemical parameters of the experimental soil such as pH, conductivity, carbon, and nitrogen were studied (Table 2). The rhizoremediation treatment was augmented with the use of the effective microbe at an interval of 10 days till the 45th day at the rhizosphere region to enhance the plant growth, which thereby could increase Pb and Cd uptake. The seeds of S. oleracea were subjected to a water test to assess their viability, and viable seeds were sown for the respective treatments. The various treatments used for the pot culture study are as follows:
After 3 days of acclimatization, the effective bacterial culture (LCRB) was mass-multiplied to obtain a cell density ranging from 2.0 × 1010 to 2.0 × 1011 cfu/mL. The cells were augmented to the rhizosphere region of all the rhizoremediation treatments at every 10th-day interval to maintain a consistent microbial load throughout the experiment.
The plants were uprooted at an interval of 15, 30, and 45 days for the assessment of parameters such as root length, shoot length, and chlorophyll content (SPAD-502 m (Konica, Minolta, Japan)) (Limantara et al., 2015). Furthermore, the concentrations of Pb and Cd in all the treatments were analyzed upon acid digestion (70% nitric acid and 30% hydrochloric acid) using an atomic absorption spectrophotometer (AAS) (Varian SpectrAA 220 model) (Sardans et al., 2010). Upon analysis, the translocation factor (TF) was also studied by estimating the concentrations of Cd and Pb in the root, shoot, and soil. The following formula was used to determine the TF (Marchiol et al., 2004):
2.8.1 Scanning electron microscope analysis of plant tissues and root biofilm
After uprooting the plant, the root and shoot samples were subjected to SEM to observe the colonization/development of biofilms by the effective strain on the rhizoplane of S. oleracea. The samples were processed by drying at 45 °C for 48 h to remove the moisture present in the root and shoot to avoid charging of particles during imaging. The samples were placed on a stub and attached using a carbon tape. Micrographs were obtained at a magnification of ×10,000 using an SEM (Carl Zeiss, EVO 18 SEM, Germany). The presence of Cd and Pb was analyzed using energy-dispersive X-ray analysis (EDAX) (Henagamage et al., 2022).
2.8.2 Statistical analysis
All the experiments were carried out in triplicate. All the statistical analyses were conducted with GraphPad InStat software. The data were analyzed by one-way ANOVA to identify substantial differences among treatments, with a significant threshold value of p < 0.05.
3 Results and discussion
3.1 Isolation of lead- and cadmium-resistant bacteria
From the lake sediments, a total of six bacterial isolates were obtained, which were found to be resistant to both Pb and Cd at a concentration of 50 ppm when supplemented in MSM. Several studies have also reported the isolation of Pb- and Cd-resistant bacteria from lake sediments (Sowmya et al., 2014; Chihomvu et al., 2014; Bowman et al., 2018; Abid et al., 2020; Ra et al., 2022), which were further used for the bioremoval of HMs. Vais et al. (2025) isolated the strain VITVJ3 from Puliyanthangal lake, Ranipet, Tamil Nadu, which was capable of bioremediating Pb.
3.2 Assessment of the maximum tolerable concentration (MTC) by plate and broth assays
In plate assay, VITLLJ4 was capable of resisting Pb and Cd up to a concentration of 1,200 ppm and 600 ppm, respectively, among all the tested isolates (Supplementary Figure S1). This could be because bacteria possess resistance mechanisms such as bioaccumulation, biosorption, and bioprecipitation against metals. Bacteria selectively work on an efflux pump system and extra- and intracellular sequestration for Pb (Tiquia-Arashiro, 2018). Meanwhile, Cd concentrations can be resisted through cell wall binding and extracellular sequestration. In certain cases, low-molecular weight cysteine-rich proteins such as metallothioneins can aid in the resistance to Cd (Sharma et al., 2024). A study by Wagh et al. (2024) reported the MTC for Pb and Cd to be 1,000 ppm and 200 ppm, respectively, in plate assay. Similarly, several studies conducted on the bioremoval of Cd exhibited resistance up to 500 ppm (Itusha et al., 2019). Additionally, Das et al. (2017) also showed organisms capable of resisting 1,000 ppm of Pb. A study by Feruke-Bello et al. (2023) also revealed the isolate’s resistance to Pb and Cd at concentrations of 2,000 ppm and 1,200 ppm, respectively. The ability of VITLLJ4 to thrive under stress induced by Cd and Pb makes it a potent organism for the bioremoval of HMs.
3.3 Screening for biofilm formation by test tube and microtiter plate assays
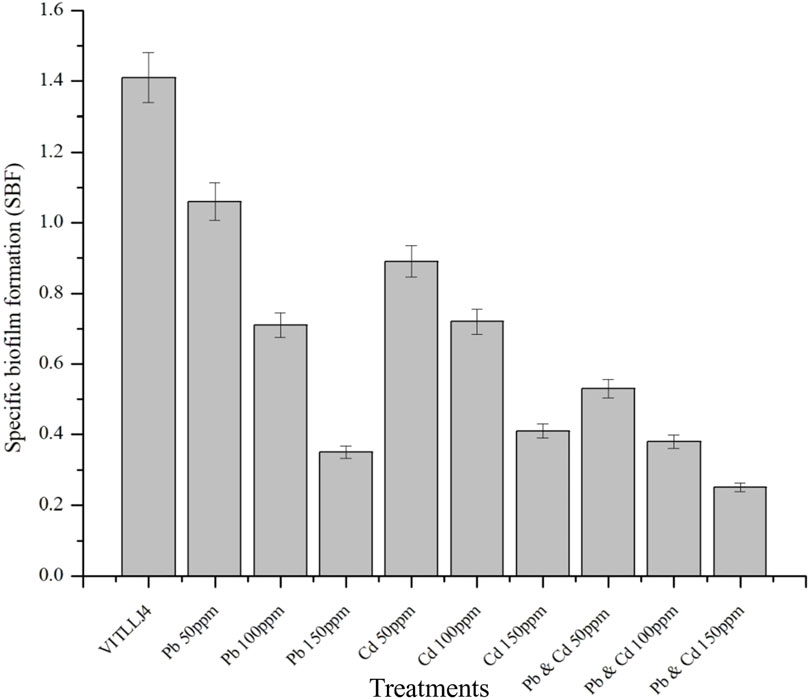
Bacterial biofilms are mixed- or single-species specific populations of bacteria that can form a self-produced EPS matrix. These biofilms are reported to show resistance toward Cd and Pb by forming a complex with the components of the biofilm, such as protein, lipids, and polysaccharides, and they can also have negatively charged functional groups, which can bind to positively charged metal ions such as Pb2+ and Cd2+ (Pan et al., 2023). The isolate VITLLJ4 showed a maximum tolerable potential for Cd and Pb up to concentrations of 600 and 1,200 ppm, respectively. However, there strong biofilm formation was observed in the presence of Cd up to 300 ppm, and moderate biofilm formation was observed above it. Meanwhile, cells treated with Pb showed strong biofilm formation up to 1,200 ppm in qualitative analysis by test tube assay (Supplementary Figure S2). Several studies have shown the ability of bacteria in sustaining the biofilm formed with Pb and Cd exposure. Bacteria such as Staphylococcus epidermidis, Proteus myxofaciens, and Bacillus xiamenensis have proven to be effective in forming biofilms upon exposure to Pb and Cd (Wagh et al., 2024; Wu et al., 2015; Vais et al., 2025). Quantitative analysis by microtiter plates also supported the results of the qualitative studies by showing the development of a strong biofilm formation with an SBF value of 1.06, 0.68, and 0.32 for 50, 100, and 150 ppm of Pb, respectively. The concentrations for the MTP assay were considered based on the concentration used for the pot culture studies. A similar trend was also observed in this study, i.e., an increase in the concentration led to a gradual decrease in biofilm formation for Cd with SBF values of 0.91, 0.71, and 0.38 for 50, 100, and 150 ppm, respectively. However, when VITLLJ4 was treated with both Cd and Pb, there was a slight decrease in the biofilm formation, with the SBF value being 0.5, 0.33, and 0.28 for 50, 100, and 150 ppm, respectively, of mixed metals (Figure 2). This could be due to the combined toxic effect of Cd and Pb on the bacterial cells, which could have interrupted the expression of certain factors involved in biofilm formation. In a similar study carried out by Wagh et al. (2024), the isolate VITMSJ3 also showed reduction in biofilm formation with an increase in the concentrations of Pb, Ni, and Cd. There are other reports on the reduction of biofilm formation with an increase in the concentrations of HMs inferred through SBF values (Itusha et al., 2019). Therefore, the characterization of the effective strain by 16S rRNA gene-sequencing showed close resemblance to Klebsiella africana, and the sequence has been submitted to GenBank with accession no. PP818866.
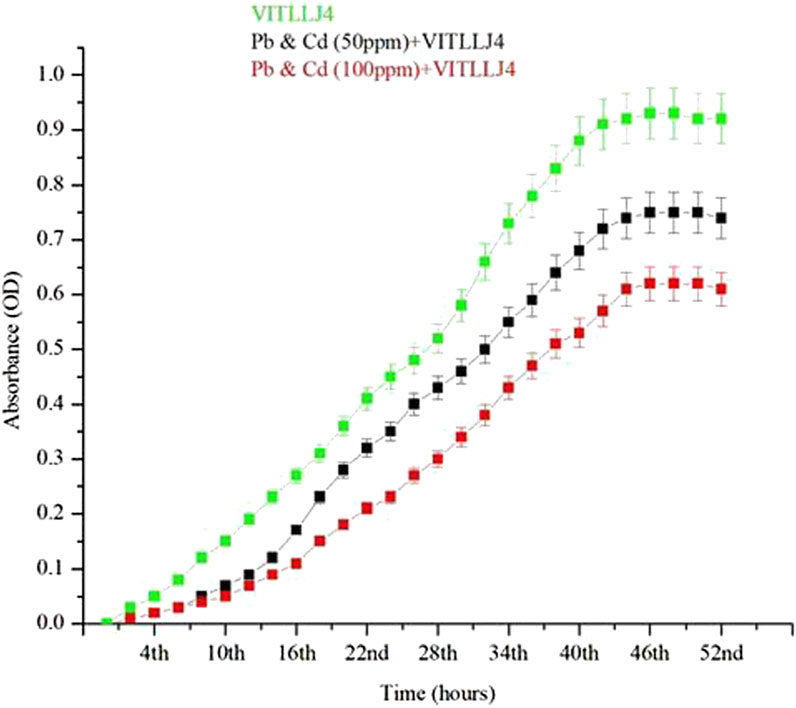
3.4 Growth profiling of VITLLJ4
Growth profiling of the effective strain VITLLJ4 was carried out with varied concentrations of Cd and Pb. Cells with and without treatment revealed a significant increase in the lag phase of the cells treated with Cd and Pb (Figure 3). MSM supplemented with VITLLJ4 was considered the positive control, with cells reaching the exponential phase at 6 h, which extended till 44 h. However, in the presence of 50 ppm and 100 ppm of mixed HMs, the cells reached the log phase after an extended lag phase till the 13th h, which extended up to the 42nd h. Furthermore, the cells were found to be in a stationary phase from the 42nd to the 52nd h, and with supplementation of 100 ppm of Cd and Pb, cells reached the log phase at the 16th h, which extended up to the 44th h. The increase in the lag phase with cells treated with both 50 and 100 ppm of Pb and Cd was due to the acclimatization of cells to the supplemented metals. A slight increase in the log phase in cells treated with 100 ppm could result because of the increased time taken by the organism to adapt to higher concentrations of mixed metals. The study was carried out with mixed metals since the application of effective organism (VITLLJ4) during remediation was done with both Pb and Cd. A study by Wagh et al. (2024) reported the extended lag phase when bacterial cells were amended with mixed metals such as Pb, Ni, and Cd. In another study, effective strains such as P. myxofaciens were capable of surviving and growing in a cocktail of HMs, which includes chromium, Pb, and zinc (Vais et al., 2025). The adaptability of VITLLJ4 under metal stress proved to be an effective isolate in surviving under high concentrations of HMs. Some existing studies also show the increase in the time period of the lag phase, which could be due to the stress proteins synthesized by bacteria, which eventually provide resistance toward toxic HMs (Pal et al., 2022).
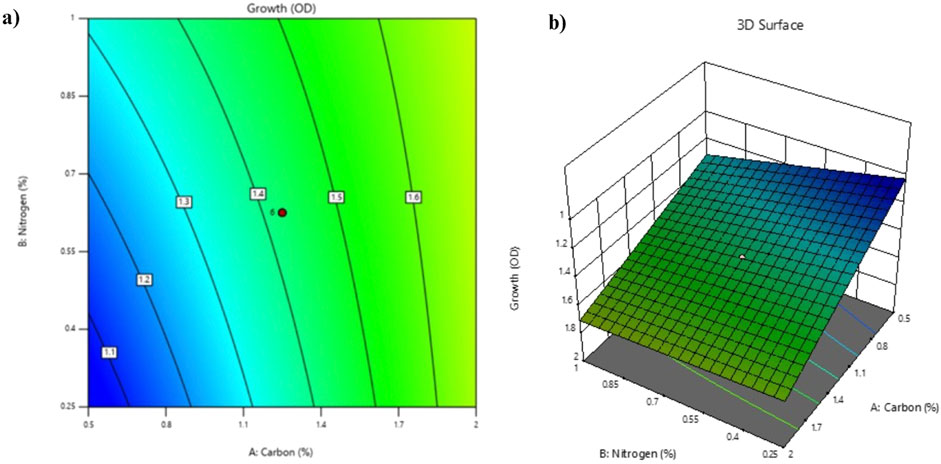
3.5 Media optimization for the bioremoval of lead and cadmium by the RSM
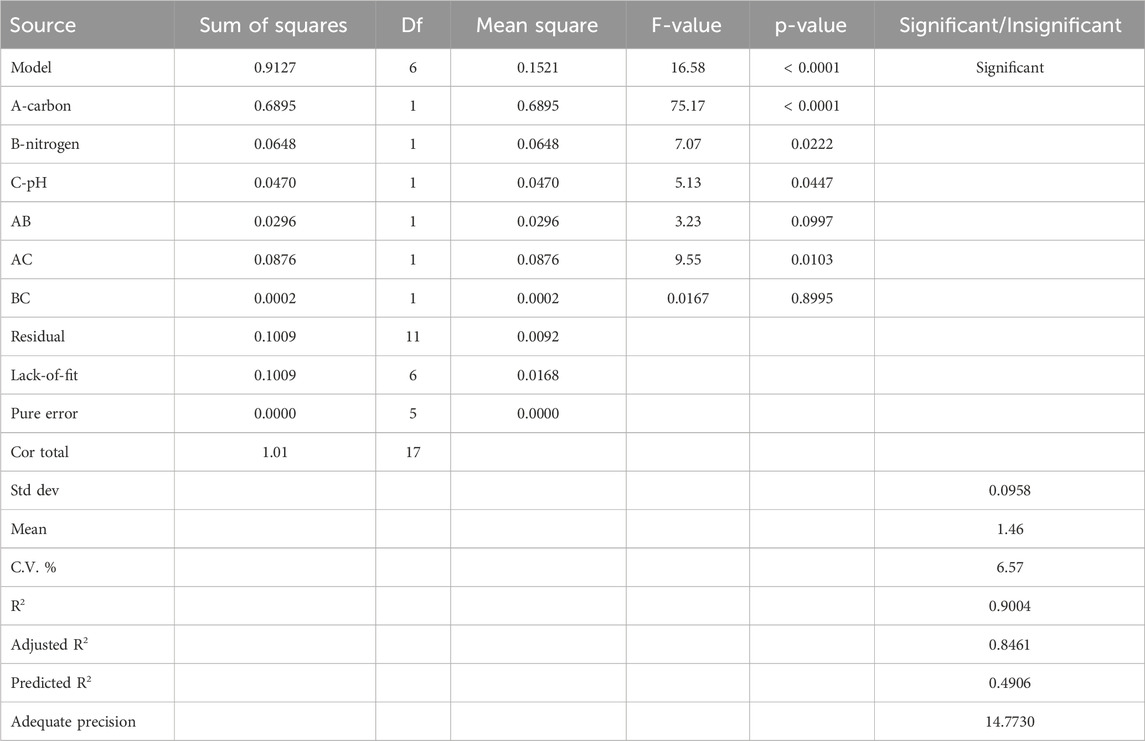
The CCD of RSM aided in the identification of parameters that could effectively aid in bioremoval of HMs (Pb and Cd) using VITLLJ4. Parameters such as carbon source (1.25%), nitrogen source (0.625), and pH 7 were found to be the optimal parameters for effective removal of Pb and Cd by VITLLJ4 (Supplementary Figure S3). A similar study by Anaukwu et al. (2024) showed the importance of RSM in identifying the optimum carbon source, nitrogen source, and pH, which were used in the bioremoval of HMs (Pb and Cd). Contour plots and surface diagrams facilitated the visualization of factor interactions and their effect on HM (Pb and Cd) removal (Figure 4). Zhang and Yi (2024) emphasized the bioremoval of Pb from industrial wastewater using engineered yeast with parameters that were optimized by RSM. Similarly, studies were also carried out for the optimization of various parameters using RSM for the efficient bioremoval of Pb (Dawwam et al., 2023) and on the optimization of pH, contact time, and adsorbent dose in the removal of Cd from an aqueous solution using the RSM model based on CCD. Mohseni et al. (2024) optimized conditions such as pH, contact time, and adsorbent dose for the remediation of Cd from an aqueous solution.
CCD in RSM was used for the development of a prediction model, which was further validated through ANOVA. The results showed p-values less than 0.05 (0.0001, 0.022, and 0.044), which indicated the statistical significance of the data obtained from RSM. The adequate precision of the model was confirmed by a signal-to-noise ratio of 14.773 (Table 3).
Three-dimensional diagrams and contour plots revealed the optimal input variable values that can be used for the bioremoval of Pb and Cd. A CCD equation was derived to predict bioremoval efficiency based on three factors.
Here, A is carbon, B is nitrogen, and C is the pH.
The RSM design encompasses 20 experimental runs (Supplementary Table S1), and RSM models also confirmed the reliability of the observed responses and statistical values, exhibiting effective and enhanced removal of Cd and Pb by VITLLJ4.
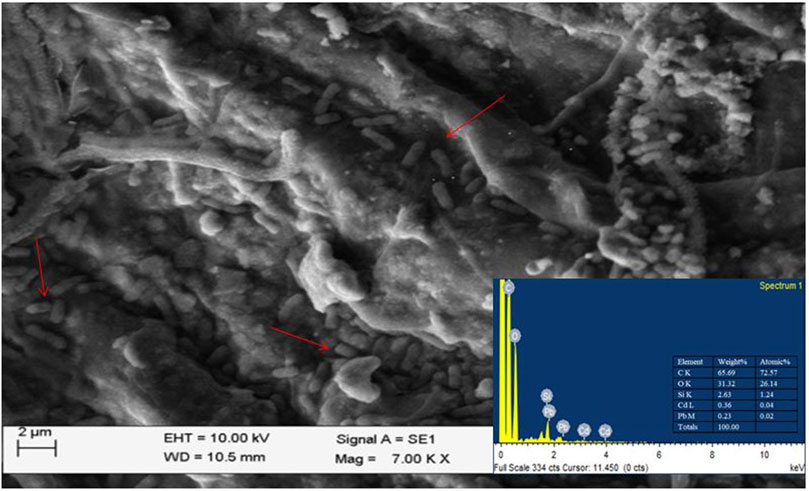
3.6 Scanning electron microscopy (SEM) analysis of the isolate VITLLJ4 with and without HM stress
SEM was performed to analyze the variation in the morphology of cells treated with and without Pb and Cd (Supplementary Figure S4). The micrographs of cells treated with Pb and Cd revealed the presence of pleomorphic cells, which could be due to the synthesis of proteins that could aid in the resistance toward Pb and Cd (Figure 5). A study by El-Beltagi et al. (2024) revealed through SEM analysis that exposure to lead results in cell deformation. Another study involving SEM analysis of bacteria showed that exposure to Cd results in morphological changes in the cell wall and shape of the cell (Ra et al., 2022). The presence of peaks for Cd and Pb on the bacterial cell surface of VITLLJ4 was confirmed with EDAX. A similar study by Wagh et al. (2024) also revealed the presence of HMs such as Pb, Cd, and Ni on the cell surface of bacteria detected by EDAX. Another study by Rahman et al. (2019) revealed biosorption of HM Pb on the bacterial cell surface upon analysis with SEM-EDAX.
3.7 Screening for plant growth-promoting rhizobacterial traits
Among all the tested isolates (Supplementary Table S2), VITLLJ4 tested positive for all PGPR traits in the presence of HMs (Supplementary Figure S5). Ammonia production was performed to assess the bacteria capable of utilizing atmospheric nitrogen, and VITLLJ4 tested positive for ammonia production, which was evident due to the color change from yellow to brownish-orange. The maximum ammonia production was recorded at 72 h with a concentration of 4.9 μmol/mL. Wagh et al. (2024) also showed ammonia production of 5.99 μmol/mL after 72 h. In soil, phosphates are often present in its insoluble form, which inhibits the plant in its uptake and is an essential nutrient required for plant growth. VITLLJ4 was also found to solubilize insoluble phosphate, which was further confirmed by the halo zone formation due to the production of organic acids with the maximum solubility efficiency (SE). Studies also reported the solubilization of phosphate under HMs (Pb and Cd) stress (Lal et al., 2019). IAA is a phytohormone that is essential in stimulating root elongation. VITLLJ4 was able to produce indole-3-acetic acid, which was confirmed by the appearance of pink coloration upon addition of Salkowski’s reagent. Quantitative analysis revealed that the maximum IAA produced was 33.25 μg/mL. A study by Pal and Sengupta (2019) reported the ability of bacteria to produce 20 μg/mL–22 μg/mL of IAA under exposure to Pb and Cd. Siderophores are low-molecular weight iron chelators; when the intracellular iron content is low, bacteria release siderophores that bind to extracellular Fe and restore into the cell. The formation of a halo zone around the well by the VITLLJ4 colony on Chrome Azurol S agar plates confirmed the production of siderophores. A report by Li et al. (2023) also showed the production of siderophores under Cd stress. Hydrogen cyanide (HCN) has antimicrobial properties, which aid in controlling plant pathogens. VITLLJ4 also tested positive for HCN production, as indicated by the brown coloration on the filter paper immersed in picric acid.
Studies were also found to show positive results for HCN, IAA, and phosphate solubilization under Pb and Cd stress (Abdollahi et al., 2020). Another study by Pal and Sengupta (2019) also revealed bacteria that tested positive for PGPR traits under HM stress. VITLLJ4 was also positive for all PGPR traits, underscoring its potential as a potent PGPR strain that can enhance the plant growth under metal stress and also aid in the bioremoval of HMs (Pb and Cd).
3.8 Pot culture study
The concentration of Cd and Pb used in the study is higher than the permissible limits provided by the agencies as industries such as battery and paper discharge higher concentrations of these metals in their effluents or the increase in concentration in the soil could be due to the constant discharge of Cd and Pb in the same discharge site.
3.8.1 Analysis of morphological parameters and chlorophyll content of the plant
The supplementation of specific HMs (Pb and Cd) led to variations in the phenotypic characteristics of plant growth and morphological characteristics. The plant (S. oleracea) in the control study exhibited normal growth since it was without the augmentation of Cd and Pb. In the non-bioaugmented (phytoremediation) treatment amended with Cd and Pb, a gradual reduction was observed in the root length and shoot height, with an increase in the concentration of the tested metals till the 45th day, indicating the interference of metals in plant growth. In contrast, when the effective strain was augmented to the rhizosphere region, a significant and gradual increase was observed in the root length and shoot height till the 45th day (Figure 6). HMs can interfere in the plant growth by disrupting certain essential metabolic pathways, thereby inhibiting the synthesis of enzymes, signaling hormones, membrane transporters, etc. Interference of HMs in these mechanisms could lead to the disruption in photosynthetic efficiency and protein synthesis. It also generates reactive oxygen species, which can cause oxidative damage such as lipid peroxidation, protein oxidation, and DNA damage in plant cells (Gill et al., 2021). A study by Zainab et al. (2021) also revealed enhanced root and shoot growth upon augmentation of Bacillus gibsonii and B. xiamenensis to the rhizosphere region of Sesbania sesban plant under HM (Cu, Zn, Ni, and Cr) stress. A significant decrease in the chlorophyll content was observed in the phytoremediation set-up when plants were exposed to Pb and Cd, which was similar to a study carried out by Tripathi et al. (2005), where there was a significant reduction in the chlorophyll content of plants treated with Pb and Cd. Meanwhile, in the rhizoremediation set-up augmented with VITLLJ4, a significant and gradual increase in the chlorophyll content was observed, which was similar to a study carried out by Madhogaria et al. (2024) where Vigna radiata augmented with Pseudomonas geniculata showed improved chlorophyll content as compared to the phytoremediation study. The increase in the rhizoremediation set-up could be due to the PGP traits possessed by VITLLJ4 (Supplementary Figure S6). In rhizoremediation, the uptake efficiency was 80% for Pb and 75% for Cd; however, in phytoremediation, the uptake efficiency 65% for Pb and 59% for Cd. The rate of decline in uptake was 21.33% for Pb and 18.75% for Cd as compared to rhizoremediation. The reduction in the concentration of uptake in the rhizoremediation set-up at higher concentration could be due to the reduced biofilm-formation potential of VITLLJ4, as discussed earlier in Section 3.3.
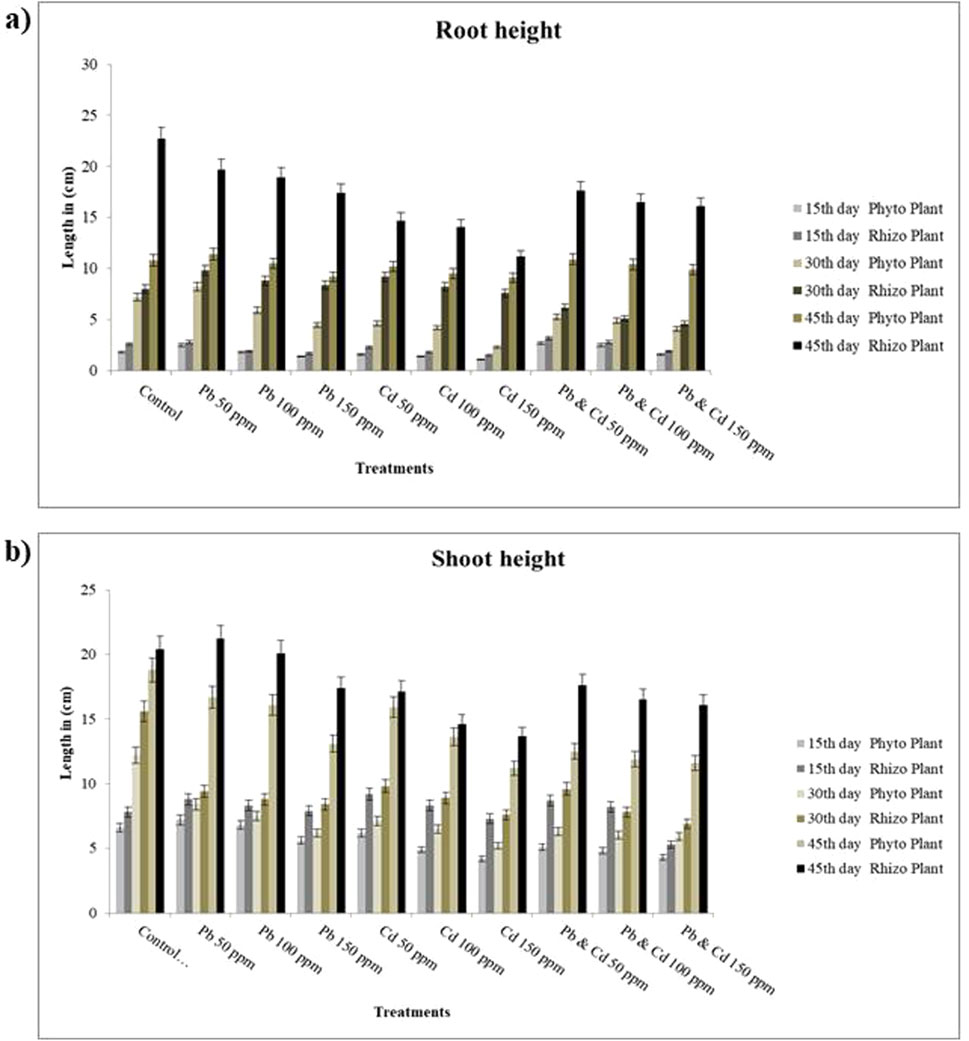
Figure 6. (a) Root height (b) Shoot length of Spinacia oleracea after 15, 30, and 45 days. Data represent the mean and standard error, and statistical significance showed p-value <0.05.
3.8.2 Estimation of Pb and Cd in the root and shoot
The uptake of Pb and Cd by S. oleracea was assessed under both phytoremediation and microbe-assisted plant-based rhizoremediation treatment over a period of 45 days. In the phytoremediation treatment, Pb uptake was observed to be higher in 50,100, and 150 ppm, showing an uptake percentage of 56%, 51%, and 58%, respectively, in the plant biomass compared to that of the rhizoremediation treatments at the 45th day with an uptake percentage of 86%, 81%, and 83.33%. Similarly, in plants treated (phytoremediation) with 50, 100, and 150 ppm of Cd, the uptake percentage was observed to be 64%, 48%, and 50%, respectively, in the plant biomass compared to that of the rhizoremediation treatments at the 45th day with an uptake percentage of 80%, 87%, and 78% (Figures 7, 8). The uptake percentage was found to be less in the 15th day of phytoremediation and rhizoremediation treatments as bacteria requires time to acclimatize to the rhizosphere region of the soil. The uptake of Cd and Pb increased in the 45th day of the rhizoremediation treatment due to the establishment of biofilms on the rhizoplane of S. oleracea, which could have contributed to the uptake mechanism through biosorption (passive) and bioaccumulation (active transport). In contrast, the phytoremediation treatments (without effective microbe augmentation) showed reduced uptake of Pb and Cd as compared to rhizoremediation due to the reduced efficiency of biofilm formation by the indigenous soil microbes. The uptake of Cd and Pb was found to be higher in the roots than in the shoots, confirming S. oleracea to be a root accumulator. A study by Das et al. (2017) showed the increased uptake of Pb by the root of Pennisetum purpureum augmented with Enterobacter cloacae (VITPASJ1), hence confirming it to be a root accumulator; this study aligns with the current study and suggests S. oleracea to be a root accumulator of Pb and Cd. Another study by Wagh et al. (2024) also reported increased uptake of Cd and Pb by the root of Vetiveria zizanioides upon supplementation with Bacillus xiamenensis (VITMSJ3). All the measurements showed statistically significant differences (p < 0.05) between the rhizoremediation and phytoremediation set-ups, thereby validating the positive impact of microbial augmentation on metal uptake efficiency.
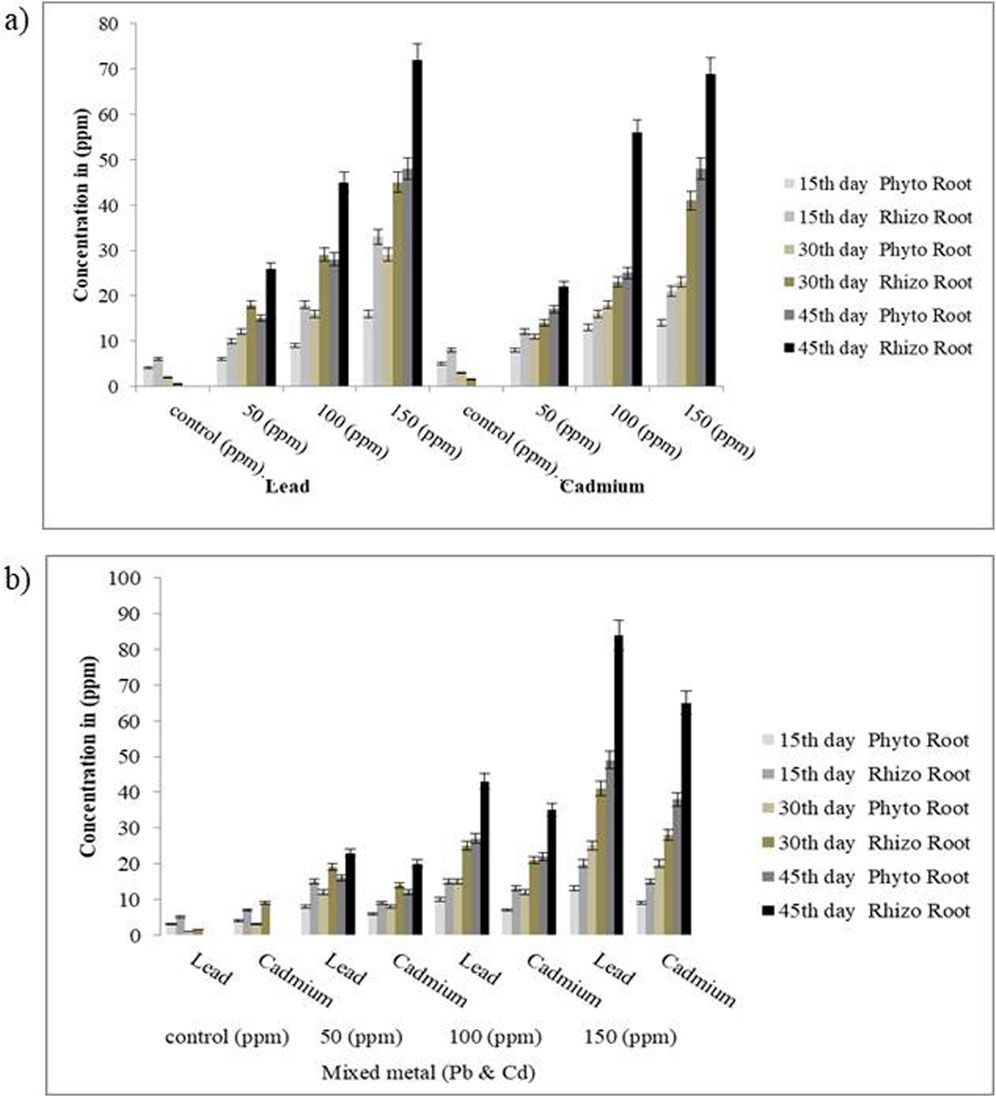
Figure 7. Effect of HMs uptake in roots: (a) individual metals and (b) mixed metals after 15, 30, and 45 days. Data represent the standard error, and statistical significance showed p-value <0.05.
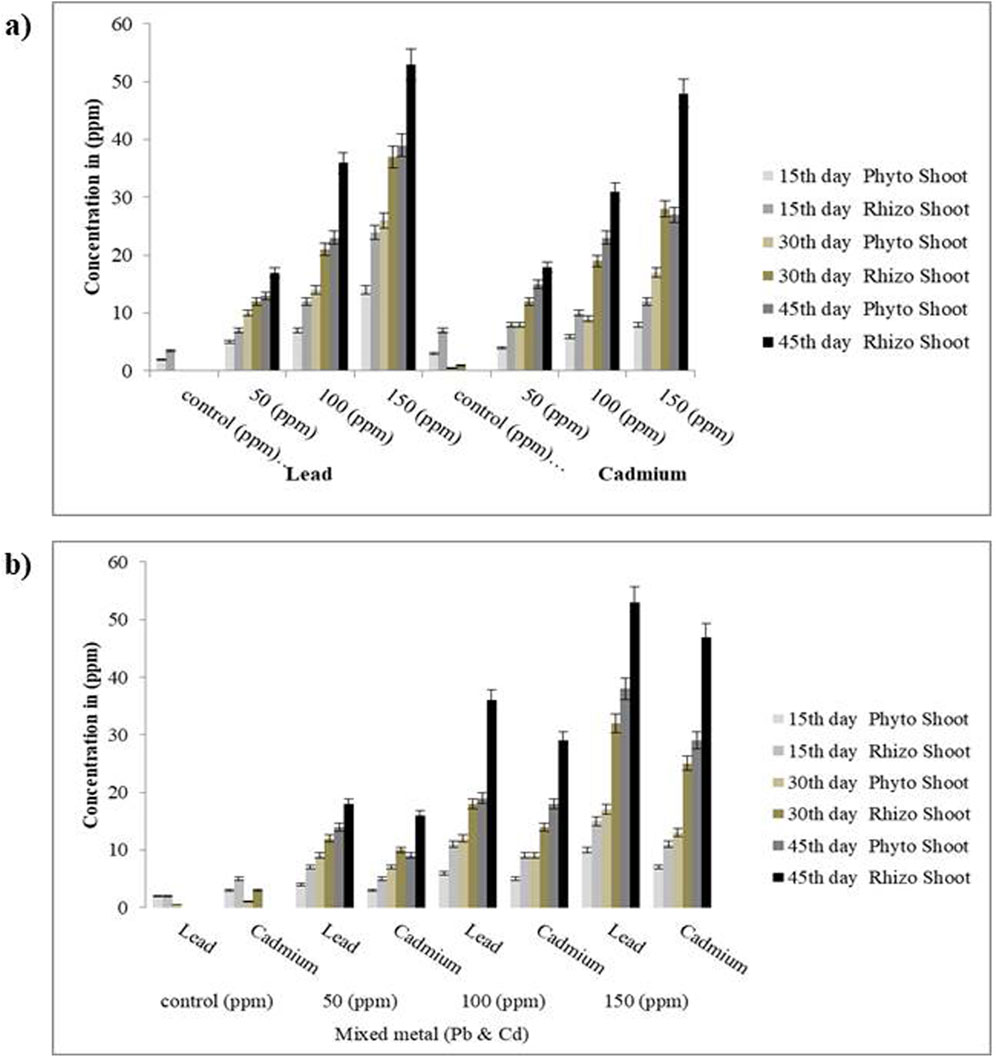
Figure 8. Effects of HMs uptake in shoots: (a) individual metals and (b) mixedmetals after 15, 30, and 45 days. Data represent the standard error, and statistical significance showed p-value <0.05.
Studies conducted on the bioremoval of Pb and Cd by the incorporation of Enterobacter bugandensis, Bacillus thuringensis, and Klebsiella michigans in the rhizosphere region of Ipomoea aquatica (forsk plant) resulted in the reduction of Cd by 59.78%–72.41% and of Pb by 43.36%–74.21% (Wang et al., 2022). A study by He et al. (2020) also reported that Bacillus sp. augmented to Solanum nigrum enhanced the uptake of Pb and Cd by 1.28–1.81 and 1.08–1.55 fold, respectively. A report by Abdollahi et al. (2020) also revealed that higher metal accumulation of 10%–60% for Pb and 5%–40% for Cd was obtained upon supplementing PGP Rhizobacteria as compared to conventional phytoremediation. The results indicated that the augmentation of the microbial strain VITLLJ4 into the rhizosphere region of spinach significantly enhanced HM uptake compared to that by phytoremediation. This finding highlights the efficacy of bacteria in combination with hyperaccumulator plant in serving as an excellent strategy for the bioremoval of HMs.
3.8.3 Estimation of Pb and Cd content in soil
To evaluate the efficiency of metal removal by phytoremediation and rhizoremediation approaches, residual metal concentrations of Pb and Cd in soil were quantified using AAS at 15-day intervals over a period of 45 days. In both the phytoremediation and rhizoremediation set-up, a significant reduction in the HM concentration was observed in soil till the 45th day (Figure 9). The slower decline suggests limited metal mobilization and uptake by S. oleracea alone, which may be attributed to the natural sequestration limits of the plant and possible metal mobilization in soil. However, in the rhizoremediation set-up, the concentration of HMs present in the soil was less as compared to the phytoremediation set-up, which could be due to the absorption or adsorption of HMs by VITLLJ4 (K. africana). The elevated removal rate can be result of multiple microbial mechanisms, such as biofilm-mediated sequestration, biosorption, bioaccumulation, and enhanced metal solubilization and mobilization. The data also showed a strong correlation (R2 value >0.9) between reduction in the metal content in soil and increased uptake by plant tissues, confirming the effective transfer of metals from soil to biomass in the rhizoremediation system.

Figure 9. Effect of HMs in soil: (a) individual metals and (b) mixed metals after 15, 30, and 45 days. Data represent the standard error, and statistical significance showed p-value <0.05.
3.8.4 SEM analysis of plant tissues and root biofilm
Scanning electronic microscopic images revealed the presence of biofilms formed on the rhizoplane region of the plants augmented with the effective strain (VITLLJ4), which could have played a major role in the uptake of Cd and Pb by S. oleracea. In contrast, the plants without supplementation with the effective strain showed the absence of biofilm formation, thereby leading to reduced uptake of Cd and Pb. The uptake of Pb and Cd was also confirmed by EDAX, which showed the presence of Pb and Cd in the field focused during capturing SEM micrographs. In the phytoremediation study, only trace amounts of Pb and Cd were detected in the plant tissues (Supplementary Figure S7). However, in the rhizoremediation set-up, SEM analysis identified rod-shaped bacteria (VITLLJ4) forming biofilms on the rhizoplane of S. oleracea, along with the presence of HMs, which was further confirmed by EDAX (Figure 10). Das et al. (2017) and Wagh et al. (2024) also reported the presence of Pb and Cd on the surface of roots, which was hypothesized to enhance the uptake of Cd and Pb.
3.8.5 Translocation factor
The movement of Pb and Cd from the rhizosphere into the plant occurs through various physiological and biochemical steps. Initially, the metal ions are absorbed through the cation transporters such as ZIP and HMA families. Once the metal ions are inside the root cells, these ions form complexes with chelators such as phyochelatins and amino acids. This detoxification aids in preventing toxicity and allows the safe transport of the metals. TF was analyzed to evaluate the presence of HMs (Pb and Cd) in the root and shoot of S. oleracea. The TF range was between 0.6 and 0.9 for Pb and 0.5 and 0.9 for Cd (Supplementary Figure S8). TF value less than 1 indicated that S. oleracea plant is a root accumulator. A large portion of Pb and Cd may be sequestered in the vacuoles of root cells, which was indicated by the low TF values. VITLLJ4 may have influenced vacuolar efflux through the secretion of growth hormones that change the root membrane permeability. Furthermore, the metals that escape vacuolar storage are transported to the xylem through the xylem parenchymal cells in a complex form. Finally, the metal ions are translocated from the roots to shoots via xylem sap flow, which is driven by transpiration. The increase in TF values under rhizoremediation showed improved plant health under the treatment with VITLLJ4. A study conducted on P. purpureum for the bioremoval of Pb revealed P. purpureum as a root accumulator with a TF value <1 (Das et al., 2017). Another study conducted by Wagh et al. (2024) on Chrysopogon zizanioides for Cd removal proved it to be a root accumulator with a TF value ranging between 0.2 and 0.9.
3.9 Mechanism of Pb and Cd uptake by Spinacia oleracea augmented with and without VITLLJ4
Figure 11 shows the reduced uptake of Pb and Cd by S. oleracea in both the root and shoot in the phytoremediation study, which infers it to be an effective phytoextracter. In phytoremediation, HMs such as Pb and Cd were absorbed by the roots of the plant. Furthermore, the uptake of metal ions is facilitated by the transporters. Once the metal ions are inside the root, they are sequestered in the vacuoles to reduce toxicity; furthermore, they are translocated to the shoots and leaves through the vascular tissue. Plants use chelators (the molecules that bind to metal ions and further detoxify and mobilize it) as transporters, which aids in the movement of metals into the vacuoles and amino acids for stabilizing the metal ions. Meanwhile, in rhizoremediation, VITLLJ4 absorbs metal ions into the cells, thereby reducing the free ion concentration in the soil. It also release chelators, acids, and enzymes, which are helpful in breaking down metal complexes, thus increasing the solubility of metal ions and converting metals into their nontoxic form, which can be used by plants. Upon augmentation of K. africana (VITLLJ4), there was an increase in the uptake of metals, confirming the plant to be a phytoextracter, and the enhanced uptake of Cd and Pb makes it a suitable root accumulator. The transformation and binding of VITLLJ4 result in enhanced phytoextraction efficiency and greater detoxification of the contaminated soil. Though K. africana has been reported to be an opportunistic pathogen in some studies, its efficiency in bioremediation has not been explored. Though the genus Klebsiella has been reported to be a pathogen, there are several reports showing its potential efficiency in the bioremoval of HMs such as Cd, Cr, Ni, and Pb (Aransiola et al., 2017; Yetunde Mutiat et al., 2018).
4 Conclusion
In the current study, bacterial isolates were obtained from lake sediments, and VITLLJ4 was screened and identified to be capable of forming biofilms in the presence and absence of Cd and Pb. The isolate VITLLJ4 was also capable of producing siderophore, IAA, ammonia, and HCN and solubilizing insoluble tricalcium phosphate. The growth kinetics showed an extended lag phase due to the acclimatization time required for the cells in presence of CD and Pb. The optimization studies using RSM provided a predictive model that was effectively used for the uptake of Cd and Pb in bioremoval studies. The SEM micrographs and EDAX of the rhizoplane region of the treatments revealed the presence of root biofilms in the rhizoremediation set-up, which enhanced the uptake potential of S. oleracea as compared to that of the phytoremediation set-up. AAS analysis revealed that rhizoremediation with VITLLJ4 led to higher accumulation and uptake of Pb and Cd in plant roots and shoots as compared to phytoremediation. The TF further confirmed that the accumulation of HMs in S. oleracea was more in the roots rather than translocating into shoots, indicating it to be an effective root accumulator. Overall, these findings highlight the potential of VITLLJ4 as an effective bioremediation agent for HM-polluted environments through S. oleracea augmented with K. africana for bacterial-assisted phytoremediation, and the combination of bacterial-assisted rhizoremediation with hyperaccumulator plants offers a promising strategy for the removal of toxic HMs from polluted soil.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material; further inquiries can be directed to the corresponding author.
Author contributions
LD: Methodology, Writing – original draft, Conceptualization, Investigation, Visualization, Validation, Data curation. JO: Methodology, Supervision, Validation, Conceptualization, Resources, Project administration, Writing – review and editing, Funding acquisition, Formal Analysis. LB: Project administration, Supervision, Validation, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
The authors are grateful to the VIT management for providing their support. They are also grateful to Dr. John from VIT-TBI for providing the AAS facility and Mr. Alwin Pattrick for the SEM facility.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1633959/full#supplementary-material
References
Abdelwahed, S., Trabelsi, E., Saadouli, I., Kouidhi, S., Masmoudi, A. S., Cherif, A., et al. (2022). A new pioneer colorimetric micro-plate method for the estimation of ammonia production by plant growth promoting rhizobacteria (PGPR). Main. Group Chem. 21 (1), 55–68. doi:10.3233/MGC-210077
Abdollahi, S., Golchin, A., and Shahryari, F. (2020). Lead and cadmium-resistant bacterial species isolated from heavy metal-contaminated soils show plant growth-promoting traits. Int. Microbiol. 23 (4), 625–640. doi:10.1007/s10123-020-00133-1
Abidin, Z. A. Z., Badaruddin, P. N. E., and Chowdhury, A. J. K. (2020). Isolation of heavy metal resistance bacteria from Lake sediment of IIUM, Kuantan. Desalination Water Treat. 188, 431–435. doi:10.5004/dwt.2020.25298
Anaukwu, C. G., Ekwealor, C. C., Anakwenze, V. N., Orji, C. C., Ogbukagu, C. M., Anyaoha, V. I., et al. (2024). Heavy metal application of response surface optimized-lipopeptide biosurfactant produced by Pseudomonas aeruginosa strain CGA-02 in low-cost substrate. Discov. Appl. Sci., 6(5), p.252. doi:10.1007/s42452-024-05821-5
Aransiola, E. F., Ige, O. A., Ehinmitola, E. O., and Layokun, S. K. (2017). Heavy metals bioremediation potential of Klebsiella species isolated from diesel polluted soil. Afr. J. Biotechnol. 16 (19), 1098–1105. doi:10.5897/AJB2016.15823
Babu, A. G., Kim, J. D., and Oh, B. T. (2013). Enhancement of heavy metal phytoremediation by Alnus firma with endophytic Bacillus thuringiensis GDB-1. J. Hazard. Mater. 250, 477–483. doi:10.1016/j.jhazmat.2013.02.014
Bhanse, P., Kumar, M., Singh, L., Awasthi, M. K., and Qureshi, A. (2022). Role of plant growth-promoting rhizobacteria in boosting the phytoremediation of stressed soils: opportunities, challenges, and prospects. Chemosphere 303, 134954. doi:10.1016/j.chemosphere.2022.134954
Bowman, N., Patel, D., Sanchez, A., Xu, W., Alsaffar, A., and Tiquia-Arashiro, S. M. (2018). Lead-resistant bacteria from saint clair river sediments and Pb removal in aqueous solutions. Appl. Microbiol. Biotechnol. 102, 2391–2398. doi:10.1007/s00253-018-8772-4
Chamam, A., Sanguin, H., Bellvert, F., Meiffren, G., Comte, G., Wisniewski-Dyé, F., et al. (2013). Plant secondary metabolite profiling evidences strain-dependent effect in the Azospirillum–oryza sativa association. Phytochemistry 87, 65–77. doi:10.1016/j.phytochem.2012.11.009
Chatterjee, S., Mukherjee, A., Sarkar, A., and Roy, P. (2012). Bioremediation of lead by lead-resistant microorganisms, isolated from industrial sample. Adv. Biosci. Biotechnol. 3 (3), 290–295. doi:10.4236/abb.2012.33041
Chen, C., Li, X., Liang, J., Yang, X., Hu, Z., Li, J., et al. (2025). The role of Lysinibacillus fusiformis S01 in cadmium removal from water and immobilization in soil. J. Hazard. Mater. 485, 136828. doi:10.1016/j.jhazmat.2024.136828
Chihomvu, P., Stegmann, P., and Pillay, M. (2014). “Identification and characterization of heavy metal resistant bacteria from the Klip river”, in Proceedings of the International Conference on Ecological, Environmental and Biological Sciences, WASET, Cape Town, South African, 2526.
Chowdhury, F. N., and Rahman, M. M. (2024). “Source and distribution of heavy metal and their effects on human health”, in Heavy metal toxicity: human health impact and mitigation strategies (Cham: Springer Nature Switzerland), 45–98. doi:10.1007/978-3-031-56642-4_3
Das, A., Belgaonkar, P., Raman, A. S., Banu, S., and Osborne, J. W. (2017). Bioremoval of lead using Pennisetum purpureum augmented with enterobacter cloacae-VITPASJ1: a pot culture approach. Environ. Sci. Pollut. Res. 24, 15444–15453. doi:10.1007/s11356-017-8988-3
Dawwam, G. E., Abdelfattah, N. M., Abdel-Monem, M. O., Jahin, H. S., Omer, A. M., Abou-Taleb, K. A., et al. (2023). An immobilized biosorbent from Paenibacillus dendritiformis dead cells and polyethersulfone for the sustainable bioremediation of lead from wastewater. Sci. Rep. 13 (1), 891. doi:10.1038/s41598-023-27796-w
Dewangan, S., and Bhatia, A. K. (2023). “Heavy metal contamination in air, groundwater, freshwater and soil”, in Heavy metals in the environment: management strategies for global pollution (American Chemical Society), 79–101. doi:10.1021/bk-2023-1456.ch006
El-Beltagi, H. S., Halema, A. A., Almutairi, Z. M., Almutairi, H. H., Elarabi, N. I., Abdelhadi, A. A., et al. (2024). Draft genome analysis for Enterobacter kobei, a promising lead bioremediation bacterium. Front. Bioeng. Biotechnol. 11, 1335854. doi:10.3389/fbioe.2023.1335854
Feng, L. A., Liang, B., Zeng, X., Shi, C., Yin, H., Feng, Y., et al. (2022). Engineered bacterium-binding protein promotes root recruitment of functional bacteria for enhanced cadmium removal from wastewater by phytoremediation. Water Res. 221, 118746. doi:10.1016/j.watres.2022.118746
Feruke-Bello, Y. M., Odeyem, O., and Babalola, G. O. (2023). Bioremediation potential of heavy metal multi-tolerant pseudomonas spp. Isolated from a municipal waste dumpsite at ile-ife, Osun state, Nigeria. J. Pet. Environ. Biotechnol. 14, 543. doi:10.4172/2157-7463.23.14.543
Gill, R. A., Ahmar, S., Ali, B., Saleem, M. H., Khan, M. U., Zhou, W., et al. (2021). The role of membrane transporters in plant growth and development, and abiotic stress tolerance. Int. J. Mol. Sci. 22 (23), 12792. doi:10.3390/ijms222312792
Han, H., Cai, H., Wang, X., Hu, X., Chen, Z., and Yao, L. (2020). Heavy metal-immobilizing bacteria increase the biomass and reduce the Cd and Pb uptake by pakchoi (Brassica chinensis L.) in heavy metal-contaminated soil. Ecotoxicol. Environ. Saf. 195, 110375. doi:10.1016/j.ecoenv.2020.110375
He, X., Xu, M., Wei, Q., Tang, M., Guan, L., Lou, L., et al. (2020). Promotion of growth and phytoextraction of cadmium and lead in Solanum nigrum L. mediated by plant-growth-promoting rhizobacteria. Ecotoxicol. Environ. Saf. 205, 111333. doi:10.1016/j.ecoenv.2020.111333
Henagamage, A. P., Peries, C. M., and Seneviratne, G. (2022). Fungal-bacterial biofilm mediated heavy metal rhizo-remediation. World J. Microbiol. Biotechnol. 38 (5), 85. doi:10.1007/s11274-022-03267-8
Itusha, A., Osborne, W. J., and Vaithilingam, M. (2019). Enhanced uptake of Cd by biofilm forming Cd resistant plant growth promoting bacteria bioaugmented to the rhizosphere of Vetiveria zizanioides. Int. J. phytoremediation 21 (5), 487–495. doi:10.1080/15226514.2018.1537245
Kaur, M., Sidhu, N., and Reddy, M. S. (2024). Removal of cadmium through biomineralization using halophilic and ureolytic bacteria under saline conditions. Int. Biodeterior. Biodegrad. 191, 105805. doi:10.1016/j.ibiod.2024.105805
Lal, S., Kumar, R., Ahmad, S., Dixit, V. K., and Berta, G. (2019). Exploring the survival tactics and plant growth promising traits of root-associated bacterial strains under Cd and Pb stress: a modelling based approach. Ecotoxicol. Environ. Saf. 170, 267–277. doi:10.1016/j.ecoenv.2018.11.100
Lentini, P., Zanoli, L., Granata, A., Signorelli, S. S., Castellino, P., and Dellaquila, R. (2017). Kidney and heavy metals-the role of environmental exposure. Mol. Med. Rep. 15 (5), 3413–3419. doi:10.3892/mmr.2017.6389
Li, Y., Wei, S., Chen, X., Dong, Y., Zeng, M., Yan, C., et al. (2023). Isolation of cadmium-resistance and siderophore-producing endophytic bacteria and their potential use for soil cadmium remediation. Heliyon 9 (7), e17661. doi:10.1016/j.heliyon.2023.e17661
Limantara, L., Dettling, M., Indrawati, R., and Brotosudarmo, T. H. P. (2015). Analysis on the chlorophyll content of commercial green leafy vegetables. Procedia Chem. 14, 225–231. doi:10.1016/j.proche.2015.03.032
Liu, G., Geng, W., Wu, Y., Zhang, Y., Chen, H., Li, M., et al. (2024). Biosorption of lead ion by lactic acid bacteria and the application in wastewater. Archives Microbiol. 206 (1), 18. doi:10.1007/s00203-023-03755-x
Madhogaria, B., Banerjee, S., Chakraborty, S., Dhak, P., and Kundu, A. (2024). Alleviation of heavy metals chromium, cadmium and lead and plant growth promotion in Vigna radiata L. plant using isolated Pseudomonas geniculata. Int. Microbiol. 28, 133–149. doi:10.1007/s10123-024-00546-2
Marchiol, L., Sacco, P., Assolari, S., and Zerbi, G., (2004). Reclamation of polluted soil: phytoremediation potential of crop-related brassica species. Water, Air, Soil Pollut., 158, 345–356. doi:10.1023/B:WATE.0000044862.51031.fb
Mehta, S., and Nautiyal, C. S., (2001). An efficient method for qualitative screening of phosphate-solubilizing bacteria. Curr. Microbiol., 43, 51–56. doi:10.1007/s002840010259
Milton, A. A. P., Srinivas, K., Lyngdoh, V., Momin, A. G., Lapang, N., Priya, G. B., et al. (2023). Biofilm-forming antimicrobial-resistant pathogenic escherichia Coli: a one health challenge in northeast India. Heliyon 9 (9), e20059. doi:10.1016/j.heliyon.2023.e20059
Mishra, S., and De, A. (2024). “Various sources of heavy metals contamination, including industrial activities, mining, agriculture, and urbanization”, in Heavy metal contamination in the environment (Boca Raton, FL: CRC Press), 71–94. Available online at: https://www.taylorfrancis.com/chapters/edit/10.1201/9781032685793-6/various-sources-heavy-metals-contamination-including-industrial-activities-mining-agriculture-urbanization-sumit-mishra-asmita-de.
Mohammadzadeh, A., Tavakoli, M., Motesharezadeh, B., and Chaichi, M. R. (2017). Effects of plant growth-promoting bacteria on the phytoremediation of cadmium-contaminated soil by sunflower. Archives Agron. Soil Sci. 63 (6), 807–816. doi:10.1080/03650340.2016.1235781
Mohseni, E., Rahmani, A., Hamdi, Z., and Ghorbanzadeh, P. (2024). Efficiency of response surface method based on central composite design in optimizing the adsorption process of cadmium metal from aqueous solutions using activated charcoal prepared from walnut shell. Desalination Water Treat. 317, 100250. doi:10.1016/j.dwt.2024.100250
Morales, A., Alvear, M., Valenzuela, E., Castillo, C. E., and Borie, F. (2011). Screening, evaluation and selection of phosphate-solubilising fungi as potential biofertiliser. J. soil Sci. plant Nutr. 11 (4), 89–103. doi:10.4067/S0718-95162011000400007
Mukherjee, A., Bhattacharjee, P., Das, R., Pal, A., and Paul, A. K. (2017). Endophytic bacteria with plant growth promoting abilities from Ophioglossum reticulatum L. AIMS Microbiol. 3 (3), 596–612. doi:10.3934/microbiol.2017.3.596
Pal, A. K., and Sengupta, C. (2019). Isolation of cadmium and lead tolerant plant growth promoting rhizobacteria: lysinibacillus varians and Pseudomonas putida from Indian agricultural soil. Soil Sediment Contam. Int. J. 28 (7), 601–629. doi:10.1080/15320383.2019.1637398
Pal, A., Bhattacharjee, S., Saha, J., Sarkar, M., and Mandal, P. (2022). Bacterial survival strategies and responses under heavy metal stress: a comprehensive overview. Crit. Rev. Microbiol. 48 (3), 327–355. doi:10.1080/1040841X.2021.1970512
Pan, H., Zhao, X., Zhou, X., Yan, H., Han, X., Wu, M., et al. (2023). Research progress on the role of biofilm in heavy metals adsorption-desorption characteristics of microplastics: a review. Environ. Pollut. 336, 122448. doi:10.1016/j.envpol.2023.122448
Priya, A. K., Muruganandam, M., Ali, S. S., and Kornaros, M. (2023). Clean-up of heavy metals from contaminated soil by phytoremediation: a multidisciplinary and eco-friendly approach. Toxics 11 (5), 422. doi:10.3390/toxics11050422
Qasem, N. A., Mohammed, R. H., and Lawal, D. U. (2021). Removal of heavy metal ions from wastewater: a comprehensive and critical review. Npj Clean. Water 4 (1), 36–15. doi:10.1038/s41545-021-00127-0
Rahman, K., Menon, U., V, S., and J, R. (2022). Removal of cadmium by heavy metal–resistant bacteria isolated from hussain sagar lake—hyderabad. Biomass Convers. Biorefinery 12, 1703–1713. doi:10.1007/s13399-021-01354-8
Rahman, Z., Thomas, L., and Singh, V. P. (2019). Biosorption of heavy metals by a lead (Pb) resistant bacterium, Staphylococcus hominis strain AMB-2. J. Basic Microbiol. 59 (5), 477–486. doi:10.1002/jobm.201900024
Raj, K., and Das, A. P. (2023). Lead pollution: impact on environment and human health and approach for a sustainable solution. Environ. Chem. Ecotoxicol. 5, 79–85. doi:10.1016/j.enceco.2023.02.001
Sardans, J., Montes, F., and Peñuelas, J. (2010). Determination of As, Cd, Cu, Hg and Pb in biological samples by modern electrothermal atomic absorption spectrometry. Spectrochim. Acta Part B At. Spectrosc. 65 (2), 97–112. doi:10.1016/j.sab.2009.11.009
Schwyn, B., and Neilands, J. (1987). Universal chemical assay for the detection and determination of siderophores. Anal. Biochem. 160 (1), 47–56. doi:10.1016/0003-2697(87)90612-9
Sharma, M., Sharma, S., Paavan, , Gupta, M., Goyal, S., Talukder, D., et al. (2024). Mechanisms of microbial resistance against Cadmium–A review. J. Environ. Health Sci. Eng. 22 (1), 13–30. doi:10.1007/s40201-023-00887-6
Sowmya, M., Rejula, M. P., Rejith, P. G., Mohan, M., Karuppiah, M., and Hatha, A. M. (2014). Heavy metal tolerant halophilic bacteria from vembanad Lake as possible source for bioremediation of lead and cadmium. J. Environ. Biol. 35 (4), 655–660.
Tiquia-Arashiro, S. M., (2018). Lead absorption mechanisms in bacteria as strategies for lead bioremediation. Appl. Microbiol. Biotechnol., 102 (13), 5437–5444. doi:10.1007/s00253-018-8969-6
Tripathi, M., Munot, H. P., Shouche, Y., Meyer, J. M., and Goel, R. (2005). Isolation and functional characterization of siderophore-producing lead-and cadmium-resistant Pseudomonas putida KNP9. Curr. Microbiol. 50, 233–237. doi:10.1007/s00284-004-4459-4
Vaishnavi, J., and Osborne, W. J. (2025). Phyto-rhizoremediation potential of C. zizanioides augmented with Bacillus infantis (VITVJ8) for the uptake of heavy metals (Cr, Pb and Zn). Front. Soil Sci. 5, 1484039. doi:10.3389/fsoil.2025.1484039
Wagh, M. S., Sivarajan, S., and Osborne, J. W., (2024). Deciphering the enhanced translocation of Pb, Ni and Cd from artificially polluted soil to Chrysopogon zizanioides augmented with Bacillus xiamenensis VITMSJ3. Biotech, 14(7), 180. doi:10.1007/s13205-024-04001-x
Wang, X., Cai, D., Ji, M., Chen, Z., Yao, L., and Han, H. (2022). Isolation of heavy metal-immobilizing and plant growth-promoting bacteria and their potential in reducing Cd and Pb uptake in water spinach. Sci. Total Environ. 819, 153242. doi:10.1016/j.scitotenv.2022.153242
Wei, G., Fan, L., Zhu, W., Fu, Y., Yu, J., and Tang, M. (2009). Isolation and characterization of the heavy metal resistant bacteria CCNWRS33-2 isolated from root nodule of Lespedeza cuneata in gold mine tailings in China. J. Hazard. Mater. 162 (1), 50–56. doi:10.1016/j.jhazmat.2008.05.040
Wu, X., Santos, R. R., and Fink-Gremmels, J. (2015). Cadmium modulates biofilm formation by Staphylococcus epidermidis. Int. J. Environ. Res. Public Health 12 (3), 2878–2894. doi:10.3390/ijerph120302878
Yetunde Mutiat, F. B., Gbolahan, B., and Olu, O. (2018). A comparative study of the wild and mutated heavy metal resistant Klebsiella variicola generated for cadmium bioremediation. Bioremediation J. 22 (1-2), 28–42. doi:10.1080/10889868.2018.1445695
Zainab, N., Khan, A. A., Azeem, M. A., Ali, B., Wang, T., Shi, F., et al. (2021). PGPR-mediated plant growth attributes and metal extraction ability of Sesbania sesban L. in industrially contaminated soils. Agronomy 11 (9), 1820. doi:10.3390/agronomy11091820
Keywords: heavy metals, phytoremediation, rhizoremediation, biofilm, PGPR, effective microbe technology
Citation: Deo L, Osborne JW and Benjamin LK (2025) Biotranslocation of lead and cadmium in Spinacia oleracea amended with Klebsiella sp. VITLLJ4: an effective microbe technology-based phyto-rhizoremediation study. Front. Environ. Sci. 13:1633959. doi: 10.3389/fenvs.2025.1633959
Received: 23 May 2025; Accepted: 02 September 2025;
Published: 26 September 2025.
Edited by:
Xuesheng Zhang, Anhui University, ChinaReviewed by:
Xiaoming Wan, Chinese Academy of Sciences (CAS), ChinaNosir Shukurov, Institute of Geology and Geophysics, Uzbekistan
Muskhazli Mustafa, Putra Malaysia University, Malaysia
Artur Banach, The John Paul II Catholic University of Lublin, Poland
Copyright © 2025 Deo, Osborne and Benjamin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jabez William Osborne, amFiZXoudml0QGdtYWlsLmNvbQ==; Lincy Kirubhadharsini Benjamin, bGluY3kuYkB2aXQuYWMuaW4=
 Loknath Deo
Loknath Deo Jabez William Osborne
Jabez William Osborne Lincy Kirubhadharsini Benjamin
Lincy Kirubhadharsini Benjamin