- 1School of Environmental Science and Engineering, Southwest Jiaotong University, Chengdu, China
- 2School of Resources and Environment, Yili Normal University, Yining, China
After exogenous pollutants in Zhangjiayan Reservoir were effectively controlled, sediment phosphorus release became a key driver of eutrophication. Thus, exploring how sediment bacterial communities respond to environmental factors and phosphorus cycling is important. In this study, we collected Zhangjiayan sediment and overlying water samples in spring (April), summer (July), fall (November), and winter (January) from 2022–2023, measured their physical and chemical factors as well as amplified and sequenced 16S rRNA genes of the sediment samples using Illumina MiSeq high-throughput sequencing technology to study the spatial and temporal variations of the bacterial community in the reservoir sediments. The results showed that the sediment was rich in bacterial diversity: a total of 63 phyla, 192 classes, 459 orders, 761 families, and 1,485 genera of the bacterial domain were detected in the sediment samples from Zhangjiayan Reservoir, with Proteobacteria and Actinobacteria as the dominant phyla. The bacterial communities exhibited significantly distinct clustering patterns between spring/summer and autumn/winter seasons (P < 0.05), with both diversity indices and taxonomic abundance being markedly higher during spring and summer compared to autumn and winter periods. In terms of community structure, the spring and summer samples showed more concentrated clustering characteristics, whereas the fall and winter samples showed a significantly dispersed distribution pattern. DO and SRP in the overlying water, as well as pH, phosphorus, ALP and TOC of the sediments were the key influencing factors for the differences in bacterial community structure. The relative abundance of the dominant genera norank_f_Anaerolineaceae and Dechloromonas was significantly and positively correlated with SRP content (P < 0.01). The relative abundance of hgcI_clade was highly significantly negatively correlated with SRP content (P < 0.01). The average clustering coefficient and modularity coefficient of the co-occurrence network of bacterial communities in sediments were at a high level, characterized by significant seasonal variations, and significantly positively correlated with dissolved reactive phosphorus (SRP) concentration (P < 0.01). These results enhance our understanding of bacterial community structure, composition, distribution, and their roles in phosphorus cycling in Southwest China’s sub-deep lake reservoirs, providing valuable insights for eutrophication treatment and aquatic ecosystem protection.
1 Introduction
Eutrophication refers to the process and phenomenon of excessive nitrogen, phosphorus and other nutrients in the water body, causing the aquatic ecosystem to change from an anaerobic state to a higher eutrophic state (Smith, 2003). Eutrophication of lakes and reservoirs can lead to ecological and environmental problems such as excessive algal blooms, receding submerged plants, deterioration of water quality, destruction of water resource values, etc., which can seriously affect the safety of drinking water (Dunck et al., 2015).
The presence of phosphorus and its chemical behavior play a key role in aquatic ecosystems, not only because it directly affects the primary productivity of water bodies, but also because it influences the ecological distribution of aquatic organisms. For this reason, phosphorus is often recognized as the limiting nutrient in the aquatic environment. Although water also contains important nutrients such as nitrogen and carbon, the regulation of these elements is far more complex. This is primarily because their interactions with the atmosphere, along with the atmospheric nitrogen fixation carried out by certain cyanobacteria, are difficult to assess—a complexity that makes the release of phosphorus a matter of particular interest (Daniel et al., 1999). Sources of phosphorus in lakes and reservoirs can be categorized as exogenous and endogenous according to the route of entry. Research points out (Sas and Augustin, 1989), release of nutrients in water sediments is one of the important sources of phosphorus in water, that is, endogenous pollution. After the control of exogenous pollution, endogenous pollution may still cause algal outbreaks, so that the water body continues to be in a state of eutrophication for many years.
The release of nutrients from water sediments is a physical, chemical and biological process, in which the release of phosphorus is mainly related to the degree of degradation of microorganisms on the surface of its sediments (Li et al., 2006), and are affected by various environmental factors, mainly including dissolved oxygen in water, temperature, PH, redox potential, and disturbance of the water body (hydrodynamic conditions) (Sun, 2000). The study of the morphology and migration transformation of phosphorus in water is the key to the prevention and control of eutrophication (Morton et al., 2005). Among them, microbial activity has a significant impact on the migration and transformation of phosphorus, and sediment plays an important role in the material cycle as the main pollution source and the key area of microbial aggregation in lake water (Jin et al., 2004). It was pointed out that microbial activities would have a greater impact on sediment redox potential, pH and phosphorus transport, and that aerobic and anaerobic metabolism of microorganisms would have a buffering effect on seasonal changes (Zhan et al., 2007; Lazzaretti-Ulmer and Hanselmann, 1999) Microbial activity at the sediment-overlying water interface is critical for the biogeochemical cycling of phosphorus during eutrophication processes (Cesare et al., 2020).
Some studies have shown that changes in bacteria directly affect nutrient cycling, while environmental factors such as phosphorus salt concentration can in turn affect bacterial structure (Pan et al., 2020). Kuang et al. (2023). explored the abundance, diversity, and metabolic pathways of microorganisms in Baiyangdian, a shallow eutrophic lake sediment, and revealed the patterns and mechanisms of carbon, nitrogen, phosphorus, and sulfur cycling under lake eutrophication. Several studies have focused on the diversity, composition and changes of bacteria in lake reservoirs, but they have mainly focused on plateau deep-water lakes (Zhu et al., 2012; Guo et al., 2016)、shallow lake (Wan, 2017; Huang et al., 2016)and the northern lakesand northern lakes (Zhang et al., 2017; Wang et al., 2009; Wang et al., 2018a). In contrast, there are few studies on bacteria in sub-deep drinking water sources in southwest China. Sub-deep lakes, classified by their water depth ranging between 10 and 30 m (Zheng et al., 2021), typically exhibit moderate nutrient levels. This intermediate trophic status supports relatively balanced aquatic ecosystems while potentially allowing for limited algal proliferation. Due to the moderate characteristics of water depth and nutrient levels, sub deep lakes and reservoirs typically have abundant biodiversity, which can support the survival of various aquatic plants, fish, and microorganisms. Compared to shallow lakes and reservoirs, sub deep lakes and reservoirs may exhibit significant temperature stratification, which can sometimes affect the oxygen distribution and biological activity of the water body. During the thermal stratification period, the water body can be vertically divided into a mixed layer, a thermocline layer, and a stagnant layer from top to bottom (Jones et al., 2008; Guan, 2020).The vertical distribution of dissolved oxygen is significantly affected by thermal stratification, and a layered structure similar to that of water thermal stratification can also appear vertically. From the surface to the bottom of the water, there are mixed layers, oxygen thermocline layers, and stagnant oxygen layers (Liu, 2019). When thermal stratification has a significant impact on the structure of dissolved oxygen stratification, anoxic zones may appear in the water body, which can lead to the death of aquatic animals and phytoplankton due to hypoxia, thereby posing a threat to the aquatic ecological environment (González-Piana et al., 2018) Dissolved oxygen regulates nutrient cycling and energy transfer by affecting the respiration and metabolic processes of microorganisms. The stratification in spring and summer can lead to bottom water hypoxia, which is the main driving factor for sediment redox conditions and phosphorus release. A study by Li Yong et al. (Li et al., 2022) on Sancha Lake, a sub-deep reservoir in Southwest China, has demonstrated that the structure and diversity of microbial communities in this reservoir exert a significant impact on phosphorus release. This finding provides important empirical evidence for understanding the association between microorganisms and nutrient cycling in sub-deep aquatic ecosystems, and also highlights the necessity of conducting research on microbial communities and phosphorus migration/transformation mechanisms in similar water bodies (such as Zhangjiayan Reservoir).
Therefore, exploring the spatial and temporal distribution characteristics of bacteria in the sediments of Zhangjiayan Reservoir and their response mechanisms to environmental factors, and clarifying the interaction mechanisms between the main driving factors and key species in the phosphorus cycle are of great theoretical significance and practical value for the development of precise pollution control and ecological restoration strategies.
For many years, phosphorus has been the limiting factor for eutrophication in Zhangjiayan Reservoir (Yu et al., 2024). In recent years, the water quality of Zhangjiayan Reservoir has reached the standard of Ⅲ class of surface water except for total phosphorus, but the water body has been eutrophication, seasonal cyanobacteria phenomenon, and there is a risk of further aggravation of eutrophication (Yu et al., 2024). Therefore, it is of great significance to investigate the spatial and temporal characteristics of the sediment bacterial community in Zhangjiayan Reservoir sediments, the mechanism of response to environmental factors, and the impacts of the structural composition of the bacterial community and the dominant genera of the bacteria on the release of phosphorus from the sediments in order to formulate the targeted strategies for pollution management and ecological remediation. It is of great significance for the development of targeted pollution management and ecological remediation strategies.
Based on the above research background, the following hypotheses are proposed.
1. Zhangjiayan sediments are rich in microbial diversity, and their microbial structural composition has obvious seasonal changes in response to environmental factors.
2. The structural composition of the bacterial community in Zhangjiayan Reservoir sediments may play an important role in phosphorus cycling. The relative abundance of dominant bacterial genera was highly correlated with the SRP concentration of the overlying water. The average clustering coefficients and modularity coefficients of the microbial co-occurrence network were both significantly correlated with SRP concentrations.
2 Materials and methods
2.1 Sampling locations and sample collection
Zhangjiayan Reservoir (E30˚24′06″∼E30˚28′25″,N104˚14′35″ ∼N104˚19′48″) islocated in the eastern new district of Sichuan Province, which belongs to the basin of Jiangxi River in the upper reaches of the Yangtze River. The area of the reservoir is 862,400 m2, with an average water depth of 16.5 m and the deepest water depth of 28.5 m, which is a typical sub-deep-type lake in southwest China. The water source of Zhangjiayan Reservoir comes from natural rainfall (including Zhangjiayan River) and the confluence of the main stream of Minjiang River on the western Sichuan Plateau via Dongfeng Canal. The agricultural irrigation and drainage period is from March to July every year. Influenced by the rainy season and agricultural irrigation and drainage, the flow rate of the water body in and out of the reservoir area is relatively fast during the spring and summer seasons. The catchment area of Zhangjiayan Reservoir is forested land and some agricultural land, with no industrial pollution sources, no livestock and poultry breeding, and exogenous domestic pollutants have been effectively controlled. Zhangjiayan Reservoir is an important source of drinking water, providing life-sustaining water for more than 300,000 people downstream of Jianyang City.
Based on the characteristics of Zhangjiayan Reservoir sediments and eutrophication status, five sampling sites were set up in the whole reservoir area (Figure 1). L1 is located near the incoming water area at the confluence of Dongfeng Canal and the river, with a water depth of 5 m. L2 is situated at the tail of the reservoir, where the water depth is relatively shallow at 14 m and there are many bends. L3 lies in the center of the reservoir, featuring a wide water area and a relatively deep water depth of 18 m. L4 is near the outlet, with a wide water area, relatively strong water flow, a shallow bottom sludge layer, and a water depth of 16 m. L5 is located at the dam, where the water flow is relatively weak, the bottom sludge layer is deep, and the water depth is 21 m, which is relatively deep. Surface sediment samples (0∼5 cm) were collected at each sampling site in spring (April), summer (July), fall (November) and winter (January) of 2022–2023 using HL-CN grab-type Peterson dredge. Three sediment samples were collected at each site and sealed in polyethylene bags. Once collected, the samples were quickly refrigerated and transported to the laboratory. Some of the sediment samples were stored at 4 °C for analysis of physical and chemical parameters within 24 h, while others were stored at −80 °C for DNA extraction. Meanwhile, some of the sediment samples were refrigerated for 12 h, vacuum freeze-dried for 48 h, ground and passed through a 200-mesh sieve, and then sealed and stored at −20 °C, which will be used to determine the content of various phosphorus forms. In addition, each sampling point in Zhangjiayan Reservoir used an airtight water sampler to collect surface overlying water about 5 cm above the mud-water interface, and three water samples were collected from each sampling point, and the samples were preserved and transported according to the “Specification for Monitoring of the Water Environment SL219-98”. The physical and chemical analysis of the collected samples should be completed within 24 h after the samples are refrigerated with ice packs and stored in 4 °C cold storage.
2.2 Methods for analyzing physical and chemical factors in the overlying water and sediments of Zhangjiyan Reservoir
The physical and chemical indicators of the overlying water of Zhangjiyan Reservoir were determined according to the standards set by the Ministry of Ecology and Environment of the People’s Republic of China and the Methods of Water and Wastewater Monitoring and Analysis (Fourth Edition) (Table 1). The determination of each phosphorus was carried out as follows: firstly, potassium persulfate was directly applied to the overlying water samples, and then TP was measured by the malachite green-phosphomolybdenum heteropolyacid spectrophotometric method. Soluble reactive phosphorus (SRP) was determined without further treatment by potassium persulfate digestion and ammonium molybdate spectrophotometry on a 0.45 μm membrane filter.
Physical and chemical factors of the sediments in Zhangjiayan Reservoir were measured as shown in Table 2. The pH value of the sediments was measured by mixing the sediments with distilled water in the ratio of 1:5 using the glass electrode method. In addition, ALP activity was determined based on the Anupama method (Thengodkar and Sivakami, 2010). The specific experimental procedure is as follows: Approximately 0.5 g of fresh sediment sample was placed into a sterilized reaction tube, followed by the addition of 10 mL of 0.5 mol/L Tris-HCl buffer (pH 8.6). After thorough mixing, the mixture was sonicated for 45 s. Subsequently, 1 mL of 10 mmol/L p-NPP (p-nitrophenyl phosphate) solution was added as a substrate, and the mixture was stirred evenly and incubated in a 37 °C water bath for 1 h. The reaction was terminated by adding 2 mL of 1 mol/L NaOH solution. The sample was then centrifuged at 5,000 rpm for 10 min under refrigeration, and the supernatant was collected. The absorbance of the supernatant was measured using a spectrophotometer at a wavelength of 410 nm, and the ALP activity per gram of dry-weight sediment was calculated according to the standard curve of PNP, expressed as micromoles of p-NPP produced per hour per gram of sediment. Additionally, each sample was subjected to three replicate experiments to ensure data accuracy.
In this study, phosphorus was extracted by the SMT method recommended by the European Committee for Standardization (ECSB) (Ye et al., 2023) was used to classify and extract phosphorus in the sediment of Zhangjiayan Reservoir. The specific steps are as follows: For 200 mg of freeze-dried sediment samples, total phosphorus (TP) is determined by spectrophotometry after extraction with 1 mol/L HCl following ignition; inorganic phosphorus (IP) is extracted with 1 mol/L HCl, and organic phosphorus (OP) is extracted with 1 mol/L HCl from the residual sediment after ignition; iron-aluminum bound phosphorus (NaOH-P) is determined after extraction with 1 mol/L NaOH and subsequent treatment, while calcium bound phosphorus (HCl-P) is extracted and determined with 1 mol/L HCl from the residual sediment after washing; all phosphorus contents are measured using the malachite green-phosphomolybdate heteropoly acid spectrophotometric method, with three parallel determinations for each sample.
2.3 DNA extraction, PCR amplification, and high-throughput sequencing
Given the high water content of the sediments, after centrifugation, DNA extraction was carried out using the FastDNA® Spin Kitfor Soil (MP Biomedicals, U.S.) kit, which was performed in strict accordance with the operating instructions. DNA integrity was checked by 1.0% agarose gel electrophoresis, and after extraction, DNA concentration was quantified using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). To ensure the reproducibility of the experiments and the accuracy of the data, all DNA samples were stored at −80 °C and three replicates of each sample were performed.
Bacterial primers 338F (5′-ACTCCTACGGGGAGGCAGCA-3′) and 806R (5′-GGACTACHVGGGGTWTCTAAT-3′) were used for PCR amplification and high-throughput sequencing (Liu et al., 2016).
The reaction mixture for PCR amplification (total volume 25 μL) consisted of 4 µL of 5× FastPfu Buffer, 2 µL of 2.5 mM dNTPs, 0.8 µL of upstream primer (5 µM), 0.8 µL of downstream primer (5 µM), 0.4 µL of FastPfu DNA polymerase, 0.2 µL of BSA, 10 ng of genomic DNA, and deionized water (ddH2O) to 20 µL.
The amplification program was set to 30 cycles, each cycle consisting of denaturation at 95 °C for 30 s, annealing at 50 °C for 30 s, and extension at 72 °C for 30 s. At the end of the amplification program, the sample was extended at 72 °C for an additional 10 min and finally stored at 4 °C. Detection of the products was accomplished by 2% agarose gel electrophoresis, and the amplified fragments were purified and recovered using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). The experiment was repeated three times for each sample to ensure the accuracy of the results.
High-throughput sequencing was performed by Shanghai Meiji Biomedical Technology Co. All PCR products were quantified by Quantus™ Fluorometer and sequencing libraries were constructed using Illumina’s NEXTFLEX Rapid DNA-Seq Kit. The quality-tested libraries were then bipartite sequenced on the Illumina Miseq PE300 platform and the raw sequencing data were uploaded to the NCBI database.
3 Data analysis
3.1 Data processing
During data processing, the DADA2 plug-in from the Qiime2 software package was used for quality control and noise reduction (Bolyen et al., 2019), the generated data were defined as ASVs (amplicon sequence variants). In addition, chloroplast and mitochondrial sequences were removed from all samples to enhance the accuracy of data analysis. To minimize the effect of sequencing depth on Alpha diversity and Beta diversity analysis, the number of sequences was uniformly adjusted to 20,000 for all samples to ensure an average sequence coverage (Goods coverage) of 99.09% for each sample.
3.2 Sequence processing and statistical analysis
Based on the Sliva 16S rRNA gene database (v138http://greengenes.secondgenome.com), the ASVs were analyzed by species taxonomy using the Blast classifier in Qiime2 (http://www.mothur.org/wiki/Calculators) (Quast et al., 2012), mothur was used to calculate the alpha diversity index chao, shannon index, etc., and R software was used to make abundance distribution maps of the taxonomic units with higher abundance in the samples. The Kruskal–Wallis rank sum test was used to analyze the between-group differences in Alpha diversity. The similarity of bacterial structures among samples was examined using Principal CoordinatesAnalysis (PCoA) method analysis (Li et al., 2017). Kruskal–Wallis multiple tests were used to test hypotheses about species among bacteria from multiple sample groups and to identify bacterial taxa with significant differences in abundance among different groups (Ye et al., 2017). After screening the environmental factors by the VIF method, Canonical Correlation Analysis (CCA) was used to investigate the effects of the environmental factors on the structure of the bacterial community (Chen et al., 2017). In nature, microorganisms tend to form complex networks of interactions (such as cooperation and competition) (Faust and Raes, 2012), ecological co-occurrence networks are structural models that characterize the interrelationships between species at the community level, describing the patterns of species interactions and the basic structure of communities through the frequency of occurrence and abundance of species in the community (Wan et al., 2022), which is now widely used (Huber P et al., 2020; Ouyang T. et al., 2022). The topological characteristic parameters of the ecological coexistence network (such as modularity, centrality, network nodes and edges) can reflect the stability of bacteria and reveal the function and characteristics of the community (Cardona et al., 2016) and this mutualistic relationship can be significantly affected by external environmental changes (Zhang et al., 2021) The co-occurrence network at genus level was visualized using Gephi software (Bastian et al., 2009), and in order to reduce the complexity of the network, the bacterial genera with the top 50 relative abundances and occurring in ≥50% of the samples were retained. Spearman’s correlation coefficient R ≥ 0.6, significance P < 0.05. Some parameters of the network (number of nodes and edges, average path length, clustering coefficients, and modularity) were computed using the igraph package in the R environment. The role of individual nodes is illustrated by the intra-module connectivity Zi (the extent to which a node is connected to other nodes in its own module) and inter-module connectivity Pi (the extent to which a node is connected to different modules) (Guimera and Amaral, 2005). Node attributes can be classified into four types based on their topological characteristics, including: Module hubs (module centroids, nodes with high connectivity within a module, Zi > 2.5 and Pi < 0.62), Connectors (connected nodes, nodes with high connectivity between two modules, Zi < 2.5 and Pi > 0.62), Network hubs (network centroids, nodes with high connectivity between two modules, Zi < 2.5 and Pi > 0.62), and Network hubs (network centroids, nodes with high connectivity between two modules, Zi < 2.5 and Pi > 0.62). hubs (network centers, nodes with high connectivity in the whole network, Zi > 2.5 and Pi > 0.62), and peripherals (peripheral nodes, nodes that do not have high connectivity both within and between modules, Zi < 2.5 and Pi < 0.62) (Deng et al., 2012). In addition to peripheral nodes, the other three types of nodes are usually regarded as critical nodes and are the focus of functional and structural analysis of network modules.
Statistical analysis of the data was done using Oringin2021 and SPSS22.0.
4 Results and analysis
4.1 Physical and chemical properties of overlying water
The averaged results of the physicochemical indicators of the overlying water body of Zhangjiayan Reservoir for the four seasons of spring (April), summer (July), autumn (November), and winter (January) from 2022 to 2023 are shown in Table 3. The water temperature ranges from 9.97 °C to 28.35 °C, with the highest values recorded in summer and lower levels during spring and autumn, while winter temperatures are the lowest. The inflow area and reservoir tail exhibit higher temperatures, whereas the dam section shows the coldest conditions. These temperature fluctuations reveal seasonal variations and differences across sampling points, which may be related to stratification phenomena in lake reservoirs and water body circulation patterns. The DO values ranged from 5.31 to 10.42 mg/L, and the DO content of the water body is usually low in the summer season, which is mainly attributable to the metabolism of aquatic organisms is enhanced, and the oxygen consumption for organic matter decomposition increases. On the contrary, in winter, the dissolved oxygen in the water body is relatively high due to a substantial decrease in the demand for oxygen as a result of the weakening of biological activities. Dissolved oxygen levels in spring and fall, on the other hand, are intermediate between summer and winter, withspring levels usually slightly higher than those in fall. This phenomenon may be attributed to the fact that the plankton, which grows vigorously in summer, begins to decay in the fall and consumes a large amount of oxygen during thedecomposition of its detritus and remains.

Table 3. Physical and chemical indicators of overlying water of Zhangjiayan Reservoir in different seasons.
TN values ranged from 0.44 to 0.99 mg/L, higher in the summer than in the spring, and lower in the fall and winter. TN was lower in the inflow area and the tail of the reservoir, and higher in the dam and the core of the reservoir. The average concentration of TN was 0.76 mg/L, which indicated a certain degree of nitrogen contamination of the lake and the reservoir. The CODCr values ranged from 5.42 to 15.36 mg/L, with the highest values in the summer, the second highest in the spring, and the lowest in the fall and winter. CODCr values were lowest at the tail of the reservoir and highest at the dam. TP values ranged from 0.165g to 0.566 mg/L, with an average value of 0.031 mg/L for the whole lake, the highest in summer, lower in spring and fall, and the lowest in winter. TP was higher in the dam and the center of the reservoir, lower at the tail of the reservoir and the inlet area. The seasonal variation pattern of SRP was consistent with that of TP, with the gradual rise from the inlet area to the center of the reservoir, the slight decrease in the outlet, and the highest at the dam. SRP is the highest at the dam.
4.2 Physical and chemical properties of sediments
The physicochemical indicators of sediment in Zhangjiayan Reservoir in spring, summer, fall and winter from 2022 to 2023 are shown in Table 4, and the point changes of sediment physicochemical indicators are shown in Figure 2.
The pH value of sediment in Zhangjiayan Reservoir ranges from 6.44 to 8.29, with the highest in winter, the second highest in fall, and the lowest in spring and summer, and there is a significant difference between spring, summer, and fall/winter (P < 0.05). As can be seen in Figure 3, pH was highest in the incoming water area and lowest at the dam. The average TOC content of Zhangjiayan sediment at each sampling point varied from 8.46 to 13.14 mg/g, with a lake-wide average of 10.31 mg/g. As can be seen in Figure 3, TOC content was lower in the inflow area and the tail end of the reservoir, and was highest at the dam, with significant differences between spring, summer, and fall/winter (P < 0.05).

Figure 3. Changes in physical and chemical indicator points in overlying water of Zhangjiayan reservoir.
The TP content at each sampling point ranged from 0.213 to 0.322 mg/g, with an average of 0.260 mg/g. The TP content basically showed a high level in autumn and winter and a low level in spring and summer, i.e., winter > autumn > spring > summer, and there was a significant difference between spring, summer, and autumn and winter (P < 0.05). This is related to the high demand for phosphorus D by the vigorous growth of water body plankton in spring and summer, and the excessive decomposition of sediment phosphorus in the absence of water body phosphorus. As can be seen from Figure 3, TP was highest in the center of the reservoir and at the dam, and lowest in the incoming water area.
In the sediment of Zhangjiayan Reservoir, the content variation range of OP at each sampling point is 0.034–0.105 mg/g, with an average of 0.071 mg/g, and the seasonal variation was the highest in summer, the second highest in spring, the lowest in fall and winter, and the difference between spring, summer, and autumn/winter was significant; there was not much difference in the distribution of the points; the IP content was 0.1160.267 mg/g, with an average of 0.190 mg/g, and the seasonal variation was the high in fall/winter, the low in spring/winter, and the difference between spring, summer, and fall/winter was significant; the distribution of points did not differ much. The IP content was 0.116–0.267 mg/g, average 0.190 mg/g, seasonal variation was high in autumn and winter, low in spring and summer, significant difference between spring and summer and autumn and winter, higher content in the center of the reservoir and dam, lower content in the inflow area and the tail end of the reservoir, and 0.0800.118 mg/g, average 0.198 mg/g, seasonal variation was high in winter, followed by fall, and low in spring and summer, there was no significant difference between seasons, and the content was gradually increased from the inflow area to the dam, and 0.0460.149 mg/g, average 0.093 mg/g, no significant difference between seasons. NaOH-P content was 0.0460.149 mg/g, with an average of 0.093 mg/g, high in spring and summer, low in fall and winter, with significant differences between spring and summer and fall and winter, and the content was lower in the inflow area and the end of the reservoir and higher at the dam. (Except for HCl-P, there were significant differences between spring-summer and fall-winter for all indicators.)
4.3 Bacterial diversity and abundance
Illumina Miseq high-throughput sequencing of the V3∼V4 highly variable region of the 16S rRNA gene in sediment DNA, after quality control, sequence splicing and chimera removal, a total of 1,087,666 valid sequences were obtained from 20 samples, with an average length of 416.64 bp. The number of sequences in the samples ranged from 31,381 to 101,869, with an average of 54,383. The number of sequences in the samples ranged from 31,381 to 101,869, with an average of 54,383. The coverage of all the samples exceeded 0.9, which indicated that the sequencing depth obtained could adequately cover the majority of microbial species, and was sufficient to reveal the diversity among different bacteria. A total of 70,149 ASVs were identified in the validated sequences, and a comparison of ASVs from sediment samples from the four seasons, as shown in the Venn diagram (Figure 4), showed that the total number of ASVs in the four seasons was 26,147. The number of ASVs unique to each season was as follows: 11,586 in the spring, 9,996 in thesummer, 1,329 in the fall, and 772 in the winter. The number of ASVs was in the order of the highest in the spring, followed by the summer, lower in the fall, and lowest in the winter.
Using the statistical data of bacterial ASVs isolated from sediments, the abundance and diversity of bacteria were analyzed, and the abundance indices (Ace and Chao) and diversity indices (Shannon and Simpson) were calculated. The specific data are shown in Table 5. The bacterial diversity in the sediments of Zhangjiayan Reservoir shows high richness. Specifically, the Ace index ranges from 189.39 to 4417.65, the Chao index ranges from 88.76 to 5,576.85, the Shannon index ranges from 1.98 to 7.65, and the Simpson index ranges from 0.0033 to 0.0419. Further diversity analysis reveals a clearer pattern: both the bacterial abundance indices and diversity indices show a consistent trend of being significantly higher in spring and summer than in autumn and winter, and the order according to the numerical values is spring > summer > autumn > winter. Statistical analysis shows that the ACE, Chao, Shannon and Simpson indices in autumn are significantly different from those in spring and summer (P < 0.05) but not significantly different from those in winter. Similarly, these indices in winter are significantly different from those in spring and summer (P < 0.05), but there is no significant difference between the indices in spring and summer. This indicates that spring and summer are the “active periods” for bacterial diversity in the sediments of Zhangjiayan Reservoir, while the community structure tends to be simplified in autumn and winter.
4.4 Taxonomic composition and seasonal changes of bacterial communities
A total of 63 phyla, 192 orders, 459 orders, 761 families, and 1,485 genera of the bacterial domain were detected in the sediment samples from Zhangjiayan Reservoir. The statistics of the number of bacterial taxa at each taxonomic level in each sampling site during the four seasons are shown in Table 5.
4.4.1 Phylum level
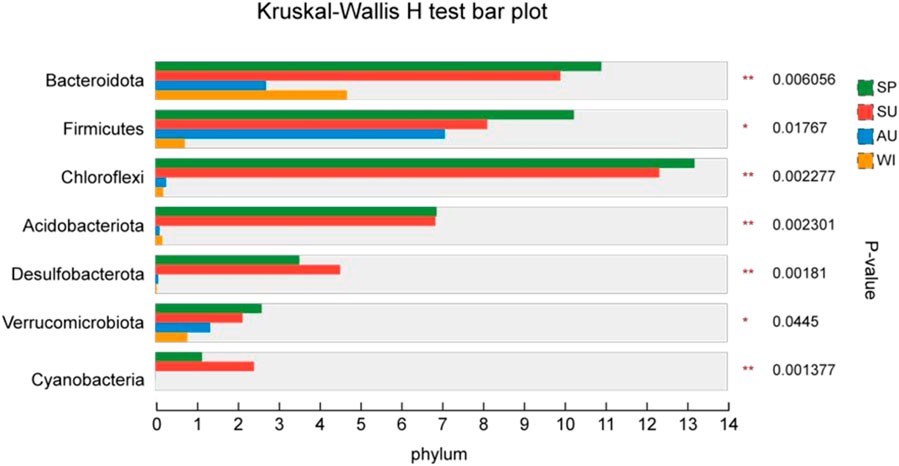
The distribution and relative abundance of the top 15 bacterial taxa in thesediment of Zhangjiayan Reservoir in each sampling site in all four seasons at the phylum level are shown in Figure 5. The microbial groups with higher abundance (average abundance >1%) were ranked as follows: Proteobacteria (relative abundance10.82%–87.45%,average abundance 33.11%)、Actinobacteriota (6.88%–73.68%,28.61%)、Bacteroidota (0.89%–14.12%,7.05%)、Firmicutes (0.06%–17.18%,6.68%)、Chloroflex (0–31.44,6.46%)、Acidobacteriota (0–9.06%,3.44%)、Desulfobacterota (0–7.37%,2.01%)、Verrucomicrobiota (0.21%–4.03%,1.72%)、Cyanobacteria (0.29%–4.29%,1.16%)。Proteobacteria and Actinobacteria dominated the sediment bacteria in Zhangjiayan Reservoir.

Figure 5. Distribution and relative abundance of the top 15 bacterial taxa in the four seasons of Zhangjiayan sediments at the gate level.
The most abundant microbial populations were present in all four sampling seasons, but there were large differences in abundance. According to the Wilcoxon test (Figure 6), the mean abundance of Bacteroidota, Firmicutes, Chloroflex Acidobacteriota, Desulfobacterota, Verrucomicrobiota, and Cyanobacteria varied significantly (P < 0.05) between seasons. The mean abundance of Bacteroidota was significantly different between seasons (P < 0.05), with Bacteroidota being most abundant in spring, second most abundant in summer, and less abundant in fall and winter, and Firmicutes and Verrucomicrobiota decreasing from spring to winter. Chloroflex, Acidobacteriota, Desulfobacterota had significantly higher relative abundance in spring and summer than in fall and winter (P < 0.05). Cyanobacteria were most abundant in summer, second most abundant in spring, and less abundant in fall and winter.
4.4.2 Genus level
At the genus level, the distribution and relative abundance of abundant microbial populations in Zhangjiayan Reservoir at each sampling site during the four seasons are shown in Figure 7. Of the 1,716 genera detected in Zhangjiayan Reservoir, six genera with relative abundance >1% were hgcI_clade (relative abundance 0.12%–56.53%, average abundance 15.43%), CL500-29_marine_group (0.07%–30.84%, 8.45%), Acinetobacter (0.04%–78.72%, 10.86%), norank_f_Anaerolineaceae (0%–6.77%, 1.72%), Dechloromonas (0.01%–5.66%, 1.33%), norank_f_Bacteroidetes_vadinHA17 (0%–3.34%, 1.09%). We can find that a large number of unidentified bacteria such as in norank_f_Anaerolineaceae, norank_f_Bacteroidetes_vadinHA17 are included in the classification at the genus level, and even their relative abundance is not small, which makes it difficult to analyze the community composition of bacteria. However, it also means that the Zhangjiayan Reservoir sediments are rich in microbial diversity, which contains unknown bacterial taxa that are valuable resources for us to explore.

Figure 7. Distribution and relative abundance of the top 15 bacterial taxa at genus level in four seasons of Zhangjiayan sediments.
At the genus level, Wilcoxon tests were done for genera with relative abundance >1%, and the results are shown in Figure 8. hgcI_clade, CL500-29_marine_group, Acinetobacter, norank_f_Anaerolineaceae, and Dechloromonas, The mean abundance of norank_f_Bacteroidetes_vadinHA17 differed significantly between the four seasons (P < 0.05). hgcI_clade and Dechloromonas were most abundant in the winter, second in the fall, and less abundant in the spring and summer. CL500-29_marine_group is most abundant in the fall, second most abundant in the winter, and almost less abundant in the spring and summer. Acinetobacter decreases from spring to winter. norank_f_Anaerolineaceae is most abundant in summer, second most abundant in spring, and less abundant in fall and winter. Dechloromonas is most abundant in winter, second most abundant in fall, and less abundant in spring and summernorank_f_Anaerolineaceae is most abundant in summer, second most abundant in spring, and less abundant in fall. Bacteroidetes_vadinHA17 is most abundant in the spring, second most abundant in the summer, and less abundant in the fall and winter.
Zhangjiayan Reservoir sediment bacteria show distinct seasonal patterns across taxonomic levels. At the phylum level, Proteobacteria and Actinobacteriota dominate, with seven major phyla (e.g., Bacteroidota, Firmicutes) exhibiting significant seasonal abundance shifts (P < 0.05), mostly peaking in spring/summer. At the genus level, top taxa like Acinetobacter and norank_f_Anaerolineaceae also display clear seasonal trends, contrasting with winter-dominant groups such as hgcI_clade.
4.5 Comparative analysis of bacterial community composition and structure
Bray-Curtis based PCoA analysis was used to evaluate the similarities and differences in the bacterial composition of Zhangjiayan Reservoir sediments, and the results are shown in Figure 9. It can be seen that spring and summer bacteria were mainly concentrated in quadrants I and IV, while fall and winter samples were distributed in quadrants II and III, showing a clear spatial separation between the two seasonal groups. This distribution pattern points to a significant difference in bacterial structure between spring and summer and fall and winter seasons. During the spring and summer seasons, the bacterial structures tended to cluster at different sampling sites, suggesting that the effects of environmental type or spatial heterogeneity on bacteria were relatively limited during these seasons. On the contrary, in the fall and winter seasons, the bacteria at the sampling sites showed greater dispersion as the environmental conditions changed, suggesting that changes in environmental type had a greater impact on bacterial structure.

Figure 9. PCoA analysis of bacterial community structure in Zhangjiayan Reservoir based on genus level.
This clear separation between seasonal groups, combined with the tight clustering of samples within each season, strongly suggests that temporal dynamics are the predominant driver of sediment bacterial community structure in this reservoir, outweighing the effects of spatial heterogeneity among the sampled sites.
4.6 Correlation between bacterial communities and environmental physical and chemical factors
After the environmental factors were preferentially selected by the VIF method, the bacterial community structure of Zhangjiayan Reservoir sediments was investigated by CCA analysis at the genus level, and it was found that these structures were significantly influenced by the physicochemical properties of the sediments (Figure 10). Key environmental influences included overlying water DO, SRP, sediment pH, TP, IP, OP, and ALP, which together explained 41.12% of the bacterial community distribution at the genus level, with the two major axes contributing 28.78% and 12.34%. Although the environmental conditions in different seasons had different effects on the composition of the bacterial community, the SRP in the overlying water, the pH of the sediment and the phosphorus content were closely related to the seasonal variations, and these factors were the main environmental factors contributing to the changes in the bacterial structure.
It was found that most of the major bacteria (mean abundance >1%) in the sediments showed significant correlations (P < 0.01 or P < 0.05) with overlying water DO, SRP and sediment pH, TOC, TP, NaOH-P, OP, ALP (see Table 6). The seasonal variation of bacterial communities in the sediments of Zhangjiayan Reservoir was mainly influenced by the key influencing factors of the water body and sediments.

Table 6. Correlation analysis of relative abundance (mean abundance >1%) of sediment bacteria at the genus level with physical and chemical factors.
To further identify the bacterial taxa critical to eutrophication in Zhangjiayan Reservoir, linear regression analyses were conducted between major bacterial genera in sediments (average abundance >1%) and SRP in the overlying water (Figure 11). The results revealed a clear differentiation pattern of phosphorus-cycling functional groups: the relative abundances of norank_f_Anaerolineaceae and Dechloromonas showed a highly significant positive correlation with SRP content (P < 0.01), while hgcI_clade exhibited a highly significant negative correlation (P < 0.01).

Figure 11. Correlation between relative abundance and SRP content of major genera (average abundance >1%).
This pattern suggests that these three bacterial groups play distinct roles in phosphorus exchange at the sediment-overlying water interface: the former two may contribute to SRP accumulation by decomposing organic phosphorus or promoting phosphorus release, while the latter may reduce SRP in water through absorption and fixation. This functional complementarity between “releasers and absorbers” makes them core microbial taxa regulating phosphorus cycling and eutrophication processes in the reservoir.
4.7 Microbial co-occurrence network
This study constructed a microbial co-occurrence network for four seasons of sediment from Zhangjiayan Reservoir (Figure 12) and performed corresponding ZiPi analysis to identify key species in the microbial association network. In the networks of all four seasons, three module hubs and nineteen connectors were identified. In spring, Streptococcus, Gaella, Aurantimicrobium, and Glutamicibacter were identified as four connectors. In summer, Bacillus was identified as one module hub, along with Limnohabitans, Gaella, norank_f_Sporichthaceae, and seven other connectors. In autumn, BD1-7_clade was identified as one module hub, along with norank_f_Sporichthaceae, Ro-mboutsia, and Planococcus, totaling three connectors. In winter, Polynucleobacter was identified as one module hub, along with Arenimonas, Flavobacterium, Pseudorhodobacter, and Methylotenera, totaling four connectors. Gaella was identified as a connector in both spring and summer, while norank_f_Sporichthaceae was identified as a connector in both summer and autumn. From the perspective of seasonal variation trend, the clustering coefficient reaches a peak in summer, gradually decreases in autumn and winter but remains relatively stable, and is lowest in spring. The modular coefficient also shows the same seasonal variation.

Figure 12. Microbial correlation network diagram. (a) Spring microbial co-occurrence network. (b) Summer microbial co-occurrence network. (c) Autumn Microbial Co-occurrence Network. (d) Winter microbial co-occurrence network. The default display of species information in the co-occurrence network graph is for species with absolute values of correlation coefficient data greater than or equal to 0.6, P < 0.05; the size of the nodes in the graph indicates the size of the species abundance, and genera from the same phylum are uniformly colored; the connecting line in red indicates positive correlation, and the connecting line in green indicates negative correlation.
Linear regression analysis (Figure 13) showed that both modularity coefficients and clustering coefficients in the microbial network topology coefficients were significantly and positively correlated (P < 0.01) with SRP content in the water column.

Figure 13. Microbial network topology coefficients versus SRP of overlying water. Results calculated based on the average value of three groups of parallel samples (12 samples in total).
5 Discussion
5.1 Bacterial composition and diversity
Proteobacteria (average abundance 33.11%) and Actinobacteriota (28.61%) dominated the sediments of Zhangjiayan Reservoir. Peng et al., (2015) studied the bacterial structure of sediments from Donghu Lake in Wuhan and found that Proteobacteria and Actinobacteria were the main phyla in the sediments. Meng et al. (2024) took Yuehai Lake, Rhinoceros Lake and Mingcui Lake as the research objects to explore the community structure of lake sediment bacteria and their influencing factors, in which the most dominant phyla all contained Proteobacteria phylum. Chen et al. (2024) In the study of bacterial communities in lake sediments on the Qinghai-Tibet Plateau, Proteobacteria and Actinobacteria were also found to be dominant bacterial groups. It can be seen that the composition of the dominant bacterial taxa in lake reservoirs is similar at different spatial scales, and the phylums Proteobacteria and Actinobacteria may be the dominant bacterial groups in freshwater ecosystem sediments.
At the genus level, hgcI_clade (15.43%) was the genus with the highest relative abundance in the sediments of Zhangjiayan Reservoir. Keshri (Keshri et al., 2018) Similar findings were found in studies of temperate freshwater lakes in France. Wang Huan et al. also concluded that hgcI_clade was the dominant bacterial species in the reservoir in their study on the structural characteristics of the bacterial community in Biliuhe Reservoir, Dalian City (Wang et al., 2018a). Bacteria of the genus hgcI_clade were generally dominant in freshwater lake and reservoir sediments under different geographic regions and environmental conditions, suggesting that they play a key role in freshwater ecosystems worldwide. The microbial taxa in Zhangjiayan Reservoir had different levels of bacterial diversity in spring and summer compared with fall and winter, and the bacterial diversity was significantly higher in spring and summer than in fall and winter (P < 0.05). Peng et al. (2015) studying the bacterial community structure of East Lake and South Lake in Wuhan similarly found that microbial abundance and diversity indices were higher in spring and summer than in fall and winter. Unlike the results of this study, Zhang et al. (Zhang L et al., 2019) in studying the seasonal variation of bacteria in Chaohu Lake sediments, it was found that the diversity was higher in summer and winter than in fall and spring. The microbial diversity in Zhangjiayan Reservoir’s sediments shows seasonal variations with higher levels during spring and summer compared to autumn and winter. This phenomenon is closely related to the reservoir’s unique hydrological characteristics as a “sub-deep lake,” particularly its seasonal fluctuations in thermocline dynamics and disturbance intensity. As a sub-deep water body, Zhangjiayan Reservoir exhibits disturbance intensity that lies between shallow and deep lakes: In shallow lakes without stable thermoclines, disturbances can uniformly affect the entire water column; whereas in deep lakes with stable thermoclines, disturbances primarily impact only the surface layer (Woolway and Merchant, 2019). The thermohaline layer in Zhangjiayan Reservoir exists seasonally and is relatively unstable, which may become a variable regulating microbial habitat.
The results of PCoA analysis indicated (Figure 11) that the bacterial structure of Zhangjiayan Reservoir sediments showed seasonal variations throughout the four seasons, and in particular, the bacterial structure in the spring and summer seasons yielded a significant difference from that in the fall and winter seasons (P < 0.05). Similarly Li et al. (Li et al., 2019) analyzed the bacterial community structure in the sediments of Sancha Lake, a sub-deep lake in Southwest China, and also found that they showed obvious seasonal variations, with a significant difference between spring and non-spring There were significant differences in bacterial structure.
This phenomenon may occur when water temperature crosses the critical threshold for thermocline formation, potentially inducing qualitative changes in microbial metabolic activity and interspecific competition, thereby triggering seasonal shifts in community structure. As a sub-deep reservoir, Zhangjiayan Reservoir exhibits a more pronounced “warm season - cold season” dichotomous differentiation pattern in its microbial communities. This pattern is closely associated with the significant annual temperature variation (reaching 18 °C) characteristic of southwestern China’s climate. The marked seasonal temperature differences regulate thermocline stability by disrupting stratification during warm seasons (enhancing habitat heterogeneity) while maintaining stable stratification during cold seasons (compressing microhabitat variations), ultimately shaping this distinct seasonal differentiation pattern.
5.2 Response mechanism of environmental factors
It has been found that the activities of phosphorus solubilizing microorganisms are closely related to environmental factors (Gu et al., 2025). For example, pH, organic matter content, and dissolved oxygen affect the phosphorus dissolving capacity of PSB.
These environmental factors, through their regulation of the physicochemical properties of lake and reservoir waters, can significantly affect the dynamic changes in the structure of bacterial communities, and can reveal the response mechanism of bacterial communities to environmental changes.
The correlation analysis of the dominant bacterial genera with environmental factors in the sediment of Zhangjiayan Reservoir (Figure 10) and the Pearson correlation analysis (Table 6) showed that the main dominant bacterial taxa in the sediment of Zhangjiayan Reservoir had significant correlations (P < 0.01 or 0.05) with the environmental factors pH, TOC, TP, DO, NaOH-P, OP, SRP and ALP. Yuan et al. (Niu et al., 2015) found that TN, TP and transparency were the main factors affecting bacteria in Lake Dongting. Huang et al. (Huang et al., 2016) found that bacteria in Poyang Lake were strongly influenced by TN and TP. Gao Hui-Qin et al. found that the main influencing factors of sediment bacteria in five lakes, namely, Jiulongkou, Dazhong Lake, Wu Turban Dang, Centipede Lake, and Desheng Lake, were more or less the same, which were TOC and TP (Gao et al., 2011). The conclusion that the lake is mainly affected by the concentration of nutrients is similar to that of Dongting Lake, Poyang Lake and Taihu Lake. The eutrophication caused by environmental factors such as phosphorus leads to the influence and shaping of bacterial structure in the sediments of Zhangjiayan Reservoir. In addition, numerous studies have confirmed that pH, DO, TOC, and phosphorus are key factors influencing the structure of bacterial communities within sediments. Winters et al. (Winters et al., 2014)found that the structure of bacterial communities in Laurentian Great Lakes sediments was primarily influenced by phosphate, nitrate, and ammonium salts. Seymour et al. (Seymour et al., 2007) showed that the structure of bacterial communities in mangrove estuarine sediments is strongly correlated with DO. The significant correlation between the bacterial structure of Zhangjiayan Reservoir sediments and environmental factors, especially the close correlation with phosphorus and related physicochemical parameters, together reveal the potential mechanism by which environmental factors influence the phosphorus cycling process in the reservoir by regulating the dominant bacterial communities. The study by Balaji Prasath et al. demonstrated that the spatiotemporal distribution characteristics and adsorption-release behaviors of phosphorus in shallow marine sediments of Sansha Bay, Fujian Province were closely correlated with bacterial community structure, confirming phosphorus as a key environmental factor regulating sediment bacterial communities (Barathan et al., 2021).These studies collectively demonstrate that the core driving factors influencing sediment bacterial community structure vary across different lake ecosystems due to differences in hydrological characteristics and trophic status: Dongting Lake exhibits the combined effects of TN, TP, and water transparency; Poyang Lake is primarily regulated by TN and TP; while Zhangjiayan Reservoir shows synergistic influences of pH, DO, TOC, and various phosphorus forms. However, the common patterns across ecosystems remain significant—the fundamental regulatory roles of DO, pH, and phosphorus persist throughout. As a key driver of water eutrophication, phosphorus plays an irreplaceable core role in shaping bacterial community structure in sediments of phosphorus-limited lakes, further confirming its critical position in microbial community assembly within freshwater ecosystems.
The composition of bacterial communities in the sediments of Zhangjiayan Reservoir exhibits significant differentiation due to dynamic changes in seasonal environmental factors. Among these, the seasonal differences in the relative abundance of dominant community taxa, the structural proportion of functional flora, and species richness are particularly prominent. As a key stress environmental factor, the spatiotemporal dynamics of sediment phosphorus, through microhabitat shaping and adaptive selection of microbial communities, serve as the core mechanism driving community differentiation. This study found that bacterial communities in Zhangjiayan Reservoir show a pattern of aggregation in spring and summer and dispersion in autumn and winter (Figure 9), which is consistent with the research results of Newton, Ryan J and others (Newton and McMahon, 2011). During spring and summer, rising temperatures enhance vertical water mixing, leading to more homogeneous distribution of sedimentary phosphorus. Concurrently, algal germination (spring) and vigorous growth (summer) increase phosphorus demand. Under these spatiotemporally stable phosphorus-limited conditions, microbial communities undergo a “homogenizing selection” effect: phosphorus-metabolizing functional groups (e.g., polyphosphate-accumulating organisms and phosphate-solubilizing bacteria) adapted to moderate-high phosphorus environments proliferate widely due to consistent resource availability. This results in convergent phosphorus utilization strategies across sampling sites, manifesting as clustered community composition with relatively stable dominant populations. In autumn and winter, water temperature decreases and disturbances weaken. Meanwhile, algal sedimentation is unevenly distributed due to differences in water depth and uneven eutrophication levels, further exacerbating this heterogeneity. This leads to the formation of high-phosphorus microzones in local areas due to phosphorus retention, while other areas maintain a low-phosphorus state due to restricted diffusion. Such differential phosphorus stress drives the microbial community toward “adaptive differentiation”: phosphorus-stress-tolerant degrading bacteria become the dominant taxa in high-phosphorus microzones, whereas oligotrophic bacteria with efficient phosphorus utilization are enriched in low-phosphorus areas. The microbial communities at different sampling sites show a discrete distribution due to the differentiation of phosphorus adaptation strategies, and the structure of dominant bacterial communities undergoes significant reorganization along the phosphorus content gradient.
Correlation analysis between dominant bacterial genera and environmental factors in Zhangjiayan Reservoir sediments (Figure 10) reveals that dominant genera exhibit seasonal distribution patterns due to their differential adaptation to overlying water DO and SRP, as well as sediment pH, TOC, and phosphorus fractions. During spring and summer, favorable DO levels and phosphorus availability support the aggregation of specific bacterial genera, while autumn and winter fluctuations in TOC and phosphorus fractions drive community restructuring. This mechanism, whereby environmental factors shape the ecological niches of dominant genera to regulate seasonal community succession, highlights the core regulatory role of phosphorus and other physicochemical factors in structuring freshwater sediment microbial communities. Collectively, these findings demonstrate the potential mechanisms through which environmental factors influence reservoir phosphorus cycling processes by regulating dominant bacterial populations.
5.3 Role of bacteria in phosphorus cycling
The migration and transformation of phosphorus in sediments are jointly driven by biological and abiotic mechanisms. The biological phosphorus-solubilizing mechanism is mainly achieved through the metabolic activities of functional microbial communities. The phosphorus-solubilizing mechanisms of phosphorus-solubilizing bacteria (PSB) are divided into inorganic phosphorus-solubilizing mechanisms and organic phosphorus-solubilizing mechanisms. Among them, the mechanisms of PSB solubilizing inorganic phosphorus mainly include: organic acid solubilization mechanism, hydrogen proton exchange, chelation theory, and redox theory. The main mechanism of PSB solubilizing organic phosphorus is the phosphatase theory. In the process of solubilizing inorganic phosphorus, phosphorus-solubilizing bacteria mainly dissociate H+ through organic acid production to lower the pH. Meanwhile, the acidic environment not only promotes the combination of H+ and PO43- and the chelation of metal ions by organic acid ions to release phosphorus, but also may reduce DO due to microbial respiratory consumption, forming a synergistic cycle between pH, DO, and inorganic phosphorus solubilization (Khan et al., 2014). The solubilization of organic phosphorus is primarily driven by hydrolytic enzymes such as phosphatases secreted by organophosphorus-solubilizing bacteria (OPB). Among these enzymes, alkaline phosphatase (ALP) is distributed both intracellularly and extracellularly, and its synthesis is influenced by environmental phosphorus content. pH regulates the suitable operating range and activity of ALP (which exhibits strong activity in neutral or alkaline environments), thereby further controlling the solubilized forms of organic phosphorus substrates and the efficiency of hydrolytic transformation. A mutually associated regulatory relationship is formed between the two (Singh and Reddy, 2011). In addition, existing studies have shown that the phosphorus content in overlying water is closely related to the speciation and content of phosphorus in sediments (Xie et al., 2022). When the content of soluble reactive phosphorus (SRP) in water is at a high level, it usually indicates that the water is in an oxidizing environment. This oxidizing environment can exert an inhibitory effect on water productivity, thereby slowing down the mineralization rate of organic matter in sediments and significantly reducing the release flux of sediment phosphorus to the overlying water. In contrast, when the SRP content in water is low, under the synergistic action of multiple factors such as DO, pH value, and microbial communities, phosphorus in sediments will be released into the water body. It supplements the water phosphorus pool in the form of SRP, thereby reducing the phosphorus content in sediments (Song et al., 2024). A complex feedback mechanism is formed between the transformation of phosphorus forms and environmental factors. The enrichment of soluble reactive phosphorus (SRP) may trigger a chain reaction of “algal bloom - decay - hypoxia” (Deng et al., 2023), while the hydrolysis of OP and the transformation of iron-bound phosphorus (Fe-P) can fine-tune the pH value of the system (Kim et al., 2003). These interactions collectively shape the succession trajectory of microbial communities (Hu et al., 2024).
This dynamic association between phosphorus cycling and microbial communities can be more intuitively verified in specific aquatic ecosystems. During the phosphorus cycling process in the sediments of Zhangjiayan Reservoir, the seasonal changes in alkaline phosphatase (ALP) activity, pH, and DO exhibit significant synergistic regulatory characteristics. Under low-temperature conditions in autumn and winter, the high DO and weakly alkaline environment of the water body collectively promote ALP activity to reach its peak, driving efficient mineralization of organic phosphorus to compensate for the deficiency of SRP in the overlying water. During the massive algal bloom period in spring and summer, the lower dissolved oxygen levels and slightly acidic environment inhibit alkaline phosphatase activity, while promoting the release of inorganic phosphorus through the secretion of organic acids. Regression analysis between horizontal dominant bacterial groups in reservoir sediments and SRPs in overlying water revealed highly significant correlations (P < 0.01) between relative abundance of hgcI_clade, Dechloromonas, and norank_f_Anaerolineaceae families and SRP concentrations. Notably, Dechloromonas exhibited a significant negative correlation with SRP levels (P < 0.01), while hgcI_clade and norank_f_Anaerolineaceae showed positive correlations (P < 0.01). These findings indicate that Dechloromonas species typically demonstrate higher abundance in enhanced biological phosphorus removal (EBPR) systems (Petriglieri et al., 2021), Furthermore, hgcI_clade and norank_f_Anaerolineaceae may be involved in the phosphorus-solubilizing process (Li et al., 2022). These bacterial communities may play a key role in phosphorus transport and transformation at the sediment-water interface. Therefore, the bacterial communities in the genera hgcI_clade, Dechloromonas and norank_f_Anaerolineaceae play important roles in the transport and transformation of endogenous phosphorus in sediments and their impact on eutrophication of water bodies. From the perspective of microbial functional mechanisms, the realization of this key role is closely related to the metabolic characteristics of bacterial communities. Existing studies have shown that functional flora within bacterial communities can participate in the migration and transformation of phosphorus at the sediment-water interface through synergistic effects such as the metabolic trait of “phosphorus accumulation and release”, providing electron donors for symbiotic flora, and secreting organic acids and functional enzymes (He et al., 2018; Sun et al., 2025; Liao et al., 2025; Van Mourik et al., 2008). It is hypothesized that the functional flora in the sediments of this study also possess such ecological functions. Meanwhile, the dynamic changes in SRP concentration in the overlying water can reversely regulate the metabolic activities of sediment microorganisms, thereby forming a dynamically balanced system of phosphorus cycling. In addition, abiotic factors can also affect the transformation and release of phosphorus forms through pH, redox potential, temperature, and iron-aluminum oxides. However, their intensity of action and regulatory flexibility are relatively weaker by comparison (Gächter and Müller, 2003). Future research needs to further explore the interactions between abiotic and biotic factors to more comprehensively reveal the driving mechanisms of sediment phosphorus cycling.
The results of the microbial co-occurrence network showed that there were different key species in the sediment microorganisms of Zhangjiayan Reservoir in different seasons (Figure 12). This may be caused by changes in environmental factors such as water temperature, DO, and SRP content due to changes in temperature. Microbial co-occurrence networks are not static structures; the differences in seasonal keystone species essentially reflect the community’s adaptive responses to seasonal fluctuations in environmental factors. In the Zhangjiayan Reservoir ecosystem, seasonal changes significantly affect the phosphorus cycling process in sediments. During the period of rising water temperatures in summer, algal residues accumulate in sediments, and the abundance of microbial taxa responsible for organic matter degradation in sediments increases accordingly. These microorganisms produce large amounts of organic acids during the degradation of organic matter, resulting in a weakly acidic pH in the reservoir during spring and summer. This, on one hand, directly promotes the dissolution of inorganic phosphorus, and on the other hand, inhibits the activity of alkaline phosphatase (ALP). The chemical phosphorus-solubilizing effect in this acidic environment continuously releases soluble reactive phosphorus (SRP), providing a sufficient phosphorus source for the vigorous growth of algae. In contrast, when entering autumn and winter, as temperatures decrease and algal activity weakens, the sediment environment undergoes transformation: the pH rises to neutral-alkaline, and dissolved oxygen levels increase. This environmental change prompts cold-tolerant microbial communities to adjust their metabolic strategies, significantly enhancing ALP activity to maintain the supply of SRP in the water body through efficient mineralization of organic phosphorus in sediments. Notably, even under low-temperature conditions in winter, microorganisms can maintain basic phosphorus-solubilizing functions through community structure reorganization (such as the increased relative abundance of Actinobacteria in the reservoir in this study) and optimization of metabolic pathways (such as enhanced intracellular ALP synthesis). This adaptive response constitutes a dynamic balance mechanism for phosphorus cycling at the sediment-water interface of the reservoir, which not only meets the phosphorus demands of the ecosystem in different seasons but also avoids excessive accumulation or deficiency of phosphorus.
The seasonal succession of microbial co-occurrence networks is essentially a dynamic adaptation strategy of the community to environmental changes. By regulating the abundance and activity of key functional taxa, differentiated phosphorus cycling patterns are established. This adaptive mechanism is closely related to the structural characteristics of microbial networks.
Linear regression analysis showed that the modularity coefficient and clustering coefficient of the microbial network were significantly and positively correlated (P < 0.01) with SRP content (Figure 13). The average clustering coefficients and modularity coefficients of the sediment microbial co-occurrence network were at a high level, which were overall larger than the corresponding values in most similar studies, showing strong aggregation characteristics and high modularity within the bacteria. When the clustering coefficient >0.5, the water body showed more obvious eutrophication (Zhang et al., 2022), and higher clustering coefficients indicate the existence of close interactions among microorganisms (Yang et al., 2019). Modularity describes the resistance of the system to the environment, and higher modularity coefficients indicate that the network is more resistant to interference (Manirakiza et al., 2024). Modularity coefficient >0.4 indicates that the network has a distinct modular structure (Barberan et al., 2012). Previous studies have shown that high clustering coefficients and modularity coefficients characterizing bacterial communities may be useful in enhancing interspecies interactions and improving network stability by facilitating phosphorus transport and transformation processes at the sediment-overlying water interface (Yang et al., 2019; Chen et al., 2023; Lu et al., 2025)The phosphorus content in the sediments of Zhangjiayan Reservoir may affect the bacterial community structure and promote the formation of co-occurrence networks with high clustering coefficients (0.539–0.643) and high modularity coefficients (0.457–0.624). These network characteristics are significantly positively correlated with the SRP concentration in the overlying water (P < 0.01), indicating that under phosphorus-stressed environments, bacteria adapt to environmental pressures by enhancing the tightness of interspecific interactions and improving network stability. Meanwhile, this optimized network structure may play an important regulatory role in the phosphorus cycling process at the sediment-water interface.
Our current 16S rRNA-based analysis provided initial insights into sediment microbial communities and their putative roles in phosphorus solubilization. Due to the lack of empirical vertical profile data for water temperature and dissolved oxygen, the discussion on thermal stratification and its impacts on bottom-water chemistry and phosphorus cycling in this study relies on theoretical inference based on the typical characteristics of sub-deep reservoirs. To advance this work, an integrated multi-omics approach is proposed: (1) metagenomics to characterize the spatial-temporal dynamics of phosphorus-solubilizing functional genes and their environmental regulation thresholds, and (2) combined metatranscriptomic and metabolomic analyses to validate functional gene expression and track key metabolic pathways during phosphorus solubilization. (3) In the future, we will also conduct targeted analyses and studies to supplement the empirical vertical profile data of water temperature and dissolved oxygen, and further integrate these new data with the multi-omics results to refine the understanding of thermal stratification effects on phosphorus cycling.
6 Conclusion
This study systematically analyzed the bacterial communities in Zhangjiayan Reservoir sediments using MiSeq high-throughput sequencing, identifying an exceptionally high diversity comprising 63 phyla, 1,485 genera, and 26,147 ASVs, with Proteobacteria and Actinobacteria as the dominant phyla. PCoA revealed significantly distinct clusters between spring/summer and autumn/winter communities, showing higher diversity and abundance in warmer seasons. Key environmental drivers included overlying water DO and SRP, along with sediment pH, phosphorus fractions, ALP, and TOC. Co-occurrence network analysis demonstrated high average clustering and modularity coefficients, both significantly positively correlated with SRP content (p < 0.01). Dominant bacterial taxa also showed significant SRP correlations (p < 0.01). These findings demonstrate that: (1) sediment bacterial communities exhibit pronounced seasonal dynamics in response to environmental changes, and (2) their modular network structure’s strong SRP coupling suggests crucial ecological roles in reservoir phosphorus cycling.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
YL: Conceptualization, Methodology, Writing – review and editing. YL: Data curation, Investigation, Visualization, Writing – original draft. XY: Investigation, Software, Writing – original draft. SG: Formal Analysis, Visualization, Writing – original draft. JD: Formal Analysis, Visualization, Writing – original draft. JW: Investigation, Visualization, Writing – original draft.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Barathan, B. P., Lin, Z. R., Su, Y. P., She, C. X., Lin, H., Zhang, C. W., et al. (2021). Adsorption-release characteristics of phosphorus and the community of phosphorus accumulating organisms of sediments in a shallow lake. J. Sustain. 13, 11501. doi:10.3390/su132011501
Barberan, A., Bates, S. T., Casamayor, E. O., and Fierer, N. F. (2012). Using network analysis to explore co-occurrence patterns in soil microbial communities. J. ISME J. 6, 343–351. doi:10.1038/ismej.2011.119
Bastian, M., Heymann, S., and Jacomy, M. (2009). Gephi: an open source software for exploring and manipulating networks. C. Proc. Int. AAAI Conf. Web Soc. Media 3, 361–362. doi:10.1609/icwsm.v3i1.13937
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable andextensible microbiome data science using QIIME 2. J. Nat. Biotechnol. 37, 852–857. doi:10.1038/s41587-019-0209-9
Cardona, C., Weisenhorn, P., Henry, C., and Gilbert, J. A. (2016). Network-based metabolic analysis and microbial community modeling. J. Curr. Opin. Microbiol. 31, 124–131. doi:10.1016/j.mib.2016.03.008
Cesare, A., Pjevac, P., Eckert, E., Curkov, N., Miko Sparica, M., Corno, G., et al. (2020). The role of metal contamination in shaping microbial communities in heavily polluted marine sediments. J. Environ. Pollut. 26, 114823. doi:10.1016/j.envpol.2020.114823
Chen, X., Hou, F., Wu, Y., and Cheng, Y. (2017). Bacterial and fungal community structures in loess plateau grasslands with different grazing intensities. J. Front. Microbiol. 8, 606. doi:10.3389/fmicb.2017.00606
Chen, X., Han, X., Wang, X., Guo, Z., Yan, J., Lu, X., et al. (2023). Inversion tillage with straw incorporation affects the patterns of soil microbial co occurrence and multi-nutrient cycling in a Hapli-Udic Cambisol. J. J. Integr. Agric. 22, 1546–1559. doi:10.1016/j.jia.2022.12.011
Chen, X. T., Du, C., Wang, J., and Shen, J. (2024). Salinity gradient distribution patterns and driving factors of lake sediment microbial communities on the Tibetan Plateau. J. Lake Sci. 37, 238–254. doi:10.18307/2025.0137
Daniel, T. C., Sharpley, A. N., and Lemunyon, J. L. (1999). Agricultural phosphorus and eutrophication: a symposium Overview. J. Environ. Qual. 27, 251–257. doi:10.2134/jeq1998.00472425002700020002x
Deng, Y., Jiang, Y. H., Yang, Y., He, Z., Luo, F., and Zhou, J. (2012). Molecular ecological network analyses. J. BMC Bioinforma. 13, 113. doi:10.1186/1471-2105-13-113
Deng, Y., Yan, Y., Wu, Y., Liu, G., Ma, J., Xu, X., et al. (2023). Response of aquatic plant decomposition to invasive algal organic matter mediated by the co-metabolism effect in eutrophic lakes. J. J. Environ. Manag. 329, 117037. doi:10.1016/j.jenvman.2022.117037
Dunck, B., Limafernandes, E., Cassio, F., Cunha, A., Rodrigues, L., and Pascoal, C. (2015). Responses of primary production, leaf litter decomposition and associated communities to stream eutrophication. J. Environ. Pollut. 202, 32–40. doi:10.1016/j.envpol.2015.03.014
Faust, K., and Raes, J. (2012). Microbial interactions, from networks to models. J. Nat. Rev. Microbiol. 10, 538–550. doi:10.1038/nrmicro2832
Gächter, R., and Müller, B. (2003). Why the phosphorus retention of lakes does not necessarily depend on the oxygen supply to their sediment surface. J. Limnol. Oceanogr. 48, 929–933. doi:10.4319/lo.2003.48.2.0929
Gao, H. Q., Liu, L., and Fang, Z. J. (2011). Physicochemical properties and microbial diversity of surface sediments of lakes in summer. J. J. Hohai Univ. Nat. Sci. Ed. 39, 361–366. doi:10.1016/S1671-2927(11)60313-1
González-Piana, M., Piccardo, A., Ferrer, C., Brena, B., Pirez, M., Fabian, D., et al. (2018). Effects of wind mixing in a stratified water column on toxic cyanobacteria and Microcystin-LR distribution in a subtropical reservoir. J. Bull. Environ. Contam. Toxicol. 101, 611–616. doi:10.1007/s00128-018-2446-x
Gu, X. L., Qiao, Q. C., Yu, Y. C., Li, X., Long, X. E., and Yan, S. R. (2025). Study on the key drivers of microbial distribution in water and sediment of an artificial lake in the early stage. J. J. Environ. Eng. Technol. 15, 130–140. doi:10.12153/j.issn.1674-991X.20240100
Guan, Z. (2020). Formation mechanism and regulation of minimum dissolved oxygen in thermocline of typical reservoirs in Lancang River. Wuhan: D. Hubei University of Technology.
Guimera, R., and Amaral, L. A. N. (2005). Functional cartography of complex metabolic networks. J. . Nat. 33, 895–900. doi:10.1038/nature03288
Guo, X. F., Li, Z. Y., Dong, M. H., Zhou, B., Yang, L. Y., and Li, S. L. (2016). Spatial distribution of yeasts in Fuxian Lake, a Yunnan plateau lake, and its relationship with environmental factors. J. Lake Sci. 28, 358–369. doi:10.18307/2016.0216
He, Q., Song, Q., Zhang, S., Zhang, W., and Wang, H. (2018). Simultaneous nitrification, denitrification and phosphorus removal in an aerobic granular sequencing batch reactor with mixed carbon sources: reactor performance, extracellular polymeric substances and microbial successions. J. Chem. Eng. J. 331, 841–849. doi:10.1016/j.cej.2017.09.060
Hu, D., Li, S., and Liu, G. (2024). Advances in research on the impact of non-biological environmental factors on microbial community succession. J. J. Beihang Univ. 50, 2677–2687. doi:10.13700/j.bh.1001-5965.2022.0736
Huang, X., Hu, B., Wang, P., Chen, X., and Xu, B. (2016). Microbial diversity in lake-river ecotone of Poyang Lake, China. J. Environ. Earth Sci. 75, 965–967. doi:10.1007/s12665-016-5473-0
Huber, P., Metz, S., Unrein, F., Mayora, G., Sarmento, H., and Devercelli, M. (2020). Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. J. ISME J. 14, 2951–2966. doi:10.1038/s41396-020-0723-2
Jin, X. C., Wang, S. G., and Jiang, X. (2004). A preliminary study on the three-phase structural model of lake water-sediment interface. J. Environ. Sci. Res. 17, 1–5. doi:10.3321/j.issn:1001-6929.2004.z1.001
Jones, I. D., Winfield, I. J., and Carse, F. (2008). Assessment of long-term changes in habitat availability for Arctic charr (Salvelinus alpinus) in a temperate lake using oxygen profiles and hydroacoustic surveys. J. Freshw. Biol. 53, 393–402. doi:10.1111/j.1365-2427.2007.01902.x
Keshri, J., Pradeep Ram, A., and Sime Ngando, T. (2018). Distinctive patterns in the taxonomical Resolution of bacterioplankton in the sediment and Pore waters of contrasted freshwater lakes. J. Microb. Ecol. 75, 662–673. doi:10.1007/s00248-017-1074-z
Khan, M. S., Zaidi, A., and Ahmad, E. (2014). Mechanism of phosphate solubilization and physiological functions of phosphate-solubilizing microorganisms. M. Berlin: Springer, 31–62.
Kim, L. H., Choi, E., and Stenstrom, M. K. (2003). Sediment characteristics, phosphorus types and phosphorus release rates between river and lake sediments. J. Chemosphere. 50, 53–61. doi:10.1016/s0045-6535(02)00310-7
Kuang, B., Xiao, R., Hu, Y., Wang, Y., Zhang, L., Wei, Z., et al. (2023). Metagenomics reveals biogeochemical processes carried out by sediment microbial communities in a shallow eutrophic freshwater lake. J. Front. Microbiol. 11 (13)–1112669. doi:10.3389/fmicb.2022.1112669
Lazzaretti-Ulmer, M. A., and Hanselmann, K. W. (1999). Seasonal variati-on of the microbially regulated buffering capacity at sediment-water interfaces i-n a freshwater lake. J. Aquat. Sci. 61, 59–74. doi:10.1007/s000270050052
Li, Y. (2023). The spatio-temporal distribution of alkaline phosphatase activity and phoD gene abundance and diversity in the sediment of Sancha Lake. J. Sci. Rep. 13, 1–14. doi:10.1038/s41598-023-29983-1
Li, W. X., Feng, H. Y., Yang, Z. F., and Ruan, X. H. (2006). Eutrophication of water bodies and nutrient release from water body sediments. J. Geol. Bull. 25, 602–608. doi:10.3969/j.issn.1671-2552.2006.05.012
Li, T., Long, M., Li, H., Gatesoupe, F.-J., Zhang, X., Zhang, Q., et al. (2017). Multi-Omics analysis reveals a correlation between the host phylogeny, gut microbiota and metabolite profiles in cyprinid fishes. J. Front. Microbiol. 8, 454–11. doi:10.3389/fmicb.2017.00454
Li, Y., Zhang, J., Zhang, J., Xu, W., and Mou, Z. (2019). Microbial community structure in the sediments and its relation to environmental factors in eutrophicated Sancha Lake. J. Int. J. Environ. Res. Public Health 31, 1931. doi:10.3390/ijerph16111931
Li, Y., Yu, X., Zheng, J., Gong, Z., and Xu, W. (2022). Diversity and phosphate solubilizing characteristics of Cultivable organophosphorus-Mineralizing bacteria in the sediments of Sancha Lake.Int. J. Environ. Res. Public Health. 19, 2320–2320. doi:10.3390/ijerph19042320
Liao, M., Liu, G., He, Q., Liu, W., Qiu, Y., Tian, Y., et al. (2025). In-situ remediation of water and sediment in aquaculture system using novel ecological floating bed coupled with microbial electrochemical system. J. Bioresour. Technol. 432, 132660. doi:10.1016/j.biortech.2025.132660
Liu, C., Zhao, D., Ma, W., Guo, Y., Wang, A., Wang, Q., et al. (2016). Denitrifying sulfide removal process on high-salinity wastewaters in the presence of Halomonas sp. J. Appl. Microbiol. Biotechnol. 100, 1421–1426. doi:10.1007/s00253-015-7039-6
Liu, C., Liu, X., Zhou, H., Wang, S., and Li, B. (2019). Temporal and spatial evolution characteristics and driving factors of reservoir anoxic zone. J. J. Hydraulic Eng. 50, 1479–1490.
Lu, L., Tang, N., Zhu, Z., Wang, R., Gao, X., Yan, M., et al. (2025). Unraveling the interaction of dissolved organic matter and microorganisms with internal phosphorus cycling in the floodplain lake ecosystem. J. Environ. Res. 270, 120966. doi:10.1016/j.envres.2025.120966
Manirakiza, B., Zhang, S., Addo, F. G., Yu, M., and Alklaf, S. A. (2024). Interactions between water quality and microbes in epiphytic biofilm and superficial sediment of lake in trophic agriculture area. J. Sci. Total Environ. 912, 169321. doi:10.1016/j.scitotenv.2023.169321
Meng, J. J., Liu, S. Y., Qiu, X. C., and Zhou, R. J. (2024). Microbial community structure of typical lake sediments in Yinchuan City and its response to heavy metals. J. Environ. Sci. 45, 2727–2740. doi:10.13227/j.hjkx.202305247
Morton, S. C., Glindemann, D., Wang, X., Niu, X., and Edwards, M. (2005). Analysis of reduced phosphorus in samples of environmental interest. J. Environ. Sci. and Technol. 39, 4369–4376. doi:10.1021/es0401038
Newton, R. J., and McMahon, K. D. (2011). Seasonal differences in bacterial community composition following nutrient additions in a eutrophic lake. J. Environ. Microbiol. 13, 887–899. doi:10.1111/j.1462-2920.2010.02387.x
Niu, Y., Yu, H., and Jiang, X. (2015). Within-lake heterogeneity of environmental factors structuring bacterial community composition in Lake Dongting, China. J. World J. Microbiol. Biotechnol. 31, 1683–1689. doi:10.1007/s11274-015-1917-z
Ouyang, T., Yang, S. Q., Zhao, L., Ji, L. L., Shi, J.-Q., and Wu, Z. X. (2022). Temporal heterogeneity of bacterial communities and their responses to Raphidiopsis raciborskii blooms. J. Microbiol. Res. 262, 127098. doi:10.1016/j.micres.2022.127098
Pan, F. X., Lai, X. S., Li, X., Zhao, Y. Q., Wang, S. Z., and Wang, H. (2020). Characterization of nitrogen removal effect of different wetland plants and functional diversity of inter-root soil bacteria. J. Environ. Sci. Res. 33, 1497–1503. doi:10.13198/j.issn.1001-6929.2019.10.12
Peng, L., Zhao, J. W., Zhang, Y., Hua, Y. M., Zhu, D. W., and Liu, G. L. (2015). Seasonal changes of microbial diversity in urban eutrophic lake sediments. J. J. Appl. Environ. Biol. 21, 1012–1018. doi:10.3724/SP.J.1145.2015.05011
Petriglieri, F., Singleton, C., Peces, M., Petersen, J. F., Nierychlo, M., and Nielsen, P. H. (2021). “Candidatus Dechloromonas phosphoritropha” and “Ca. D. phosphorivorans”, novel polyphosphate accumulating organisms abundant in wastewater treatment systems. Nov. polyphosphate accumulating Org. Abund. wastewater Treat. Syst. J. ISME J. 15, 3605–3614. doi:10.1038/s41396-021-01029-2
Quast, C., Pruesse, E., Yilmaz, P., Gerken, J., Schweer, T., Yarza, P., et al. (2012). The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596. doi:10.1093/nar/gks1219
Sas, H., and Augustin, St (1989). Lake restoration by reduction of nutrient loading—expectations, experiences, extrapolation. Academic Verlag Richarz.
Seymour, J. R., Seuront, L., and Mitchell, J. G. (2007). Microscale gradients of planktonic microbial communities above the sediment surface in a mangrove estuary. J. Estuar. Coast. Shelf Sci. 73, 651–666. doi:10.1016/j.ecss.2007.03.004
Singh, H., and Reddy, M. S. (2011). Effect of inoculation with phosphate solubilizing fungus on growth and nutrient uptake of wheat and maize plants fertilized with rock phosphate in alkaline soils. J. Eur. J. Soil Biol. 47, 30–34. doi:10.1016/j.ejsobi.2010.10.005
Smith, V. H. (2003). Eutrophication of freshwater and coastal marine ecosystems, a global problem. J. Environ. Sci. Pollut. Res. Int. 10, 126–139. doi:10.1065/espr2002.12.142
Song , L., Gao, S., Fan, X., Lu, W., Feng, M., and Wang, J (2024). Influence of taihu meiliang bay algal bloom decomposition on phosphorus migration and transformation in sediments. J. Acta Scientiae Circumstantiae 44 (11), 184–192. doi:10.13671/j.hjkxxb.2024.0253
Sun, Y. M. (2000). Adsorption and release of nutrient salts in Chaohu Lake sediment Hefei University of technology.
Sun, T., Zhu, L., Huang, T., Tao, P., Bao, Y., Wang, B., et al. (2025). Seasonal distribution patterns of P-cycling-related microbes and its association with internal phosphorus release in the eutrophic Lake Chaohu, China. J. Environ. Sci. 154, 226–237. doi:10.1016/j.jes.2024.07.028
Thengodkar, R. R. M., and Sivakami, S. (2010). Degradation of chlorpyrifos by an alkaline phosphatase from the cyanobacterium Spirulina platensis. J. Biodegrad. 21, 637–644. doi:10.1007/s10532-010-9331-6
Van Mourik, A., Bleumink-Pluym, N. M. C., van Dijk, L., van Putten, J. P. M., and Wösten, M. M. S. M. (2008). Functional analysis of a Campylobacter jejuni alkaline phosphatase secreted via the Tat export machinery. J. Microbiol. 154, 584–592. doi:10.1099/mic.0.2007/012120-0
Wan, Y., Bai, Y., He, J., Zhang, Y., Li, R., and Ruan, X. (2017). Temporal and spatial variations of aquatic environmental characteristics and sediment bacterial community in five regions of Lake Taihu. J. Aquat. Ecol. 51, 343–358. doi:10.1007/s10452-017-9621-8
Wan, L. L., Chen, Z. F., Guo, J., Tong, L. H., Ren, L. J., Han, B. P., et al. (2022). Principles of biological co-occurrence network and its application in freshwater ecosystem assessment. J. Lake Sci. 341765-1787. doi:10.18307/2022.0601
Wang, X. D., Zhai, Z. H., Zhao, S., Dai, Y. H., Wang, S. J., Zhao, S. Y., et al. (2009). Bacterial community diversity in Miyun Reservoir in different seasons. J. J. Ecol. 29, 3919–3927. doi:10.3321/j.issn:1000-0933.2009.07.055
Wang, H., Li, Y., and Zhao, W. (2018a). Spatial and temporal distribution characteristics of bacterial populations in three large water source reservoirs in Liaoning Province. J. J. Microbiol. 38, 75–83. doi:10.3969/j.issn.1005-7021.2018.03.013
Wang, H., Zhao, W., Xie, Z. G., Wei, J., and Jiang, Y. (2018b). Characterization of microbial community structure and its key drivers in Biliuhe Reservoir. J. Environ. Sci. 39, 3660–3669. doi:10.13227/j.hjkx.201711022
Winters, A. D., Marsh, T. L., Brenden, T. O., and Faisal, M. (2014). Molecular characterization of bacterial communities associated with sediments in the Laurentian Great Lakes. J. J. Gt. Lakes. Res. 409, 640–645. doi:10.1016/j.jglr.2014.04.008
Woolway, R. I., and Merchant, C. J. (2019). Worldwide alteration of lake mixing regimes in response to climate change. J. Nat. Geosci. 12, 271–276. doi:10.1038/s41561-019-0322-x
Xie, F., Luo, K., and Zhu, Y. (2024). Occurrence forms of phosphorus in sediments of nan yi lake and their impact on overlying water. J. Environ. Sci. 42 (11), 5318–5327. doi:10.0000/j.zghjkx.1000-6923.20224218509
Yang, Y. Z., Gao, Y. C., Huang, X. N., Ni, P., Wu, Y. N., Deng, Y., et al. (2019). Adaptive shifts of bacterioplankton communities in response to nitrogen enrichment in a highly polluted river. J. Environ. Pollut. 245, 290–299. doi:10.1016/j.envpol.2018.11.002
Ye, J., Joseph, S. D., Ji, M., Nielsen, S., Mitchell, D. R. G., Donne, S., et al. (2017). Chemolithotrophic processes in the bacterial communities on the surface of mineral-enriched biochars. J. ISME J. 11, 1087–1101. doi:10.1038/ismej.2016.187
Ye, G. K., E, S. Z., Chen, Z. Y., Yuan, J. H., Lu, G. B., Zhang, P., et al. (2023). Progress in the study of phosphorus in soil and its classification. J. Chin. Agron. Bull. 39, 96–102. doi:10.11924/j.issn.1000-6850.casb2022-0041
Yu, X., Li, Y., Wu, Y., Gao, H., Liu, W., Liu, H., et al. (2024). Seasonal changes of prokaryotic microbial community structure in Zhangjiayan reservoir and its response to environmental factors. J. Sci. Rep. 14, 5513. doi:10.1038/s41598-024-55702-5
Zhan, Z., Yang, L. Y., Song, W., Xiao, Y. G., and Lin, B. G. (2007). The influence of microorganisms on the total phosphorus of sediments from Lake Taihu. J. Henan Sci. 25, 839–841. doi:10.3969/j.issn.1004-3918.2007.05.043
Zhang, Y. J., Li, K., Zhu, H. R., and Zhang, H. X. (2017). Characterization of seasonal changes in bacterial structure in Beihai Lake. J. Environ. Sci. 38, 3319–3329. doi:10.13227/j.hjkx.201612253
Zhang, L., Cheng, Y., Gao, G., and Jiang, J. (2019). Spatial-temporal variation of bacterial communities in sediments in Lake Chaohu, a large, shallow eutrophic Lake in China. Int. J. Environ. Res. Public Health 16, 3966. doi:10.3390/ijerph16203966
Zhang, H., Zong, R., He, H., Liu, K., Yan, M., Miao, Y., et al. (2021). Biogeographic distribution patterns of algal community in different urban lakes in China: insights into the dynamics and co-existence. J. J. Environ. Sci. 100, 216–227. doi:10.1016/j.jes.2020.07.024
Zhang, H., Yang, L., Li, Y., Wang, C., Zhang, W., Wang, L., et al. (2022). Pollution gradients shape the co-occurrence networks and interactions of sedimentary bacterial communities in Taihu Lake, a shallow eutrophic lake. J. J. Environ. Manag. 305, 114380. doi:10.1016/j.jenvman.2021.114380
Zheng, Y., Liu, S., Liu, H., Ma, C., Jiang, R., and Yan, B. (2021). Seasonal stratification characteristics of water bodies in Yashen-type reservoirs. J. J. Yunnan Agric. Univ. Nat. Sci. 36, 359–370. doi:10.12101/j.issn.1004-390X(n).201907059
Keywords: Zhangjiayan reservoir, sediment, high-throughput sequencing, bacterialcommunity, environmental factors, eutrophication
Citation: Li Y, Li Y, Yu X, Gong S, Du J and Wang J (2025) The temporal and spatial variation characteristics of bacterial communities in Zhangjiayan reservoir sediments and their response mechanisms to environmental factors. Front. Environ. Sci. 13:1640325. doi: 10.3389/fenvs.2025.1640325
Received: 04 June 2025; Accepted: 12 September 2025;
Published: 13 October 2025.
Edited by:
Min Zhang, China Institute of Water Resources and Hydropower Research, ChinaReviewed by:
Xianfu Zhao, Ministry of Water Resources & Chinese Academy of Sciences, ChinaZhao Feng Guo, Chinese Academy of Sciences (CAS), China
Copyright © 2025 Li, Li, Yu, Gong, Du and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yong Li, bGl5b25nQHN3anR1LmVkdS5jbg==
 Yong Li
Yong Li Yajie Li1
Yajie Li1