- 1State Key Laboratory of Plateau Ecology and Agriculture, Qinghai University, Xining, China
- 2Key Laboratory for Water Quality and Conservation of the Pearl River Delta, Ministry of Education; School of Environmental Science and Engineering, Guangzhou University, Guangzhou, China
- 3Qinghai Geological Survey, Xining, China
- 4Key Laboratory of Green and High-end Utilization of Salt Lake Resources, Qinghai Institute of Salt Lakes, Chinese Academy of Sciences, Xining, China
This research investigates the distinctions between surface and deep brines in the Salt Lake region of the Qaidam Basin, with an emphasis on their physicochemical properties, organic matter content, heavy metal concentrations, organic pollutants, and microbial community structures. Both surface and deep brine samples were subjected to analysis for total and dissolved organic carbon, heavy metals (specifically Mn, Pb, and Cd), and pollutants, including phthalate esters (PAEs), halogenated compounds, and sulfides. The microbial communities were characterized through high-throughput sequencing, and redundancy analysis (RDA) coupled with correlation heatmaps was employed to evaluate the relationships between pollutants and microbial communities. The findings revealed that surface brines contained higher levels of organic matter, whereas deep brines exhibited significantly elevated concentrations of heavy metals and pollutants. The microbial community composition also varied, with Proteobacteria being predominant in deep brines and Firmicutes in surface brines, along with notable shifts at the genus level. Statistical analyses identified pollutants, particularly Pb, Cd, PAEs, halogenated compounds, and sulfides, as major determinants of microbial community variation. The findings indicate that the accumulation of pollutants in deep brines significantly impacts microbial community structures and ecological functions. Integrating microbial response data into environmental risk assessments is crucial for the sustainable development of deep brine resources in the Qaidam Basin.
1 Introduction
Human activities are significantly impacting the ecological stability of natural hypersaline environments, particularly in arid and semi-arid regions. The intensification of salt mining, petroleum extraction, and industrial brine discharge has led to the increased introduction of various pollutants, both organic and inorganic, into surface brine systems (Corsellis et al., 2016; Sayed et al., 2021). These pollutants tend to accumulate in closed or semi-closed water bodies (Rojo-Nieto et al., 2011; Wang et al., 2016), disrupting indigenous microbial communities and impairing critical ecological functions such as biogeochemical cycling and pollutant degradation (Bai et al., 2017). While pollution in freshwater and marine systems has garnered considerable attention in recent years (Huang et al., 2025; Mossotto et al., 2025), the mechanisms by which microorganisms in hypersaline ecosystems respond to pollution remain largely underexplored.
Hypersaline environments, characterized by extreme salinity, elevated osmotic pressure, and limited nutrient availability, support uniquely adapted halophilic microbial communities, comprising a diverse array of bacteria and archaea (Menéndez-Serra et al., 2020; Santini et al., 2022). These microorganisms are integral to ecosystem stability, exhibiting distinctive metabolic capabilities, that significantly contribute to the biogeochemical cycling of carbon, nitrogen, sulfur, and other essential elements (Wang and Bao, 2022; Feng et al., 2023). However, the introduction of exogenous stressors including not only organic compounds but also heavy metals such as Mn, Cd, Co and Cu can profoundly disrupt these microbial networks. Heavy metals are known to exert toxic effects on cellular processes, inhibit microbial growth and enzymatic activity, and induce alterations in community structure and functional potential (Lichter et al., 2006; Cristiano et al., 2021). Their accumulation in brine environments, particularly in regions lacking historical baseline data, complicates ecological risk assessments.
The Qaidam Basin, located in the northern Qinghai-Tibet Plateau, exemplifies a typical arid inland basin characterized by extensive distributions of salt lakes and deep brine systems (Zheng et al., 2016). This area serves as an exemplary natural setting for examining the effects of environmental pollution on hypersaline ecosystems. Its unique geological and climatic conditions give rise to two contrasting brine environments: geochemically stable deep gravel-layer brine (Bai et al., 2024; Fan et al., 2024), which remains isolated from surface influence, and surface brines that are directly impacted by anthropogenic disturbances. Due to high evaporation and poor hydrological exchange, surface brines are particularly susceptible to the accumulation of both organic and inorganic pollutants, including heavy metals and persistent organic compounds (Minella et al., 2013). Recent surveys have revealed that organic contaminants originating from industrial activities and transportation have permeated both surface and, to a lesser extent, deep brine systems (Besser and Hamed, 2019; Wang et al., 2023), thereby providing a natural “deep–surface” comparative framework for studying pollution and microbial ecological responses.
While previous research has investigated microbial diversity in salt lakes (Oren et al., 2009; Tazi et al., 2014), comprehensive comparisons of microbial community structures between surface and deep brines, particularly concerning environmental pollution, remain scarce. It remains uncertain whether deep brines preserve their indigenous microbial assemblages or have been altered by anthropogenic pollutants. Moreover, the impact of various pollutants—especially heavy metals and organic compounds—on the composition and diversity of microbial communities in hypersaline environments is not well understood. Understanding these relationships is essential for evaluating ecological risks and predicting microbial responses in extreme environments under escalating environmental pressures.
In this study, we conducted an investigation on three representative brine samples from the Qaidam Basin, comprising two deep gravel-layer brines and one surface brine. Employing 16S rRNA high-throughput sequencing, gas chromatography-mass spectrometry (GC-MS) for organic pollutant profiling, and inductively coupled plasma mass spectrometry (ICP-MS) for trace metal analysis, our objectives were to: 1) compare the microbial community composition between surface and deep brines; 2) analyze the distribution patterns of heavy metals and organic pollutants across different brine environments; and 3) explore potential microbial responses to complex pollution stress. The outcomes of this study are anticipated to provide significant insights into pollutant–microbe interactions within deep and surface hypersaline systems, thereby contributing to future environmental monitoring and pollution risk assessments in extreme saline environments.
2 Materials and methods
2.1 Hydrogeological conditions
The study area is situated in the northwestern region of the Qaidam Basin. The predominant stratigraphic units exposed in this region are the Upper Pleistocene deposits of the Quaternary System. The lithological composition primarily consists of gray to gray-white gravel and sand, with an approximate thickness of 3 m. The groundwater within this study area is part of the Mahai Basin, a subsidiary basin of the Qaidam Basin. The groundwater in the Mahai Basin is primarily sourced from atmospheric precipitation and the melting of ice and snow in the mid to high-altitude mountainous regions of the northern and eastern Qilian Mountains. It is subsequently depleted through lake water discharge and surface evaporation. The groundwater dynamics are influenced and regulated by a multitude of factors, including geological structures, landforms, lithofacies, climate, and hydrological conditions. The hilly regions serve as natural barriers to groundwater flow, while the extensive unconsolidated deposits in the piedmont alluvial fans and lacustrine plains to the east and north provide substantial capacity for groundwater storage.
The deep brine examined in this research is characterized as fracture pore water within clastic rock formations. The aquifer’s lithology comprises Neogene and Paleogene sandstone, silt-fine sandstone, as well as Cretaceous and Jurassic sandstone. This highly mineralized brine frequently coexists with oil and natural gas deposits. The burial depth of the water-bearing rock formations typically ranges from 300 to 2018 m. The chemical composition of the deep brine in the study area is predominantly of the chloride type, exhibiting moderate mineralization.
2.2 Sampling locations
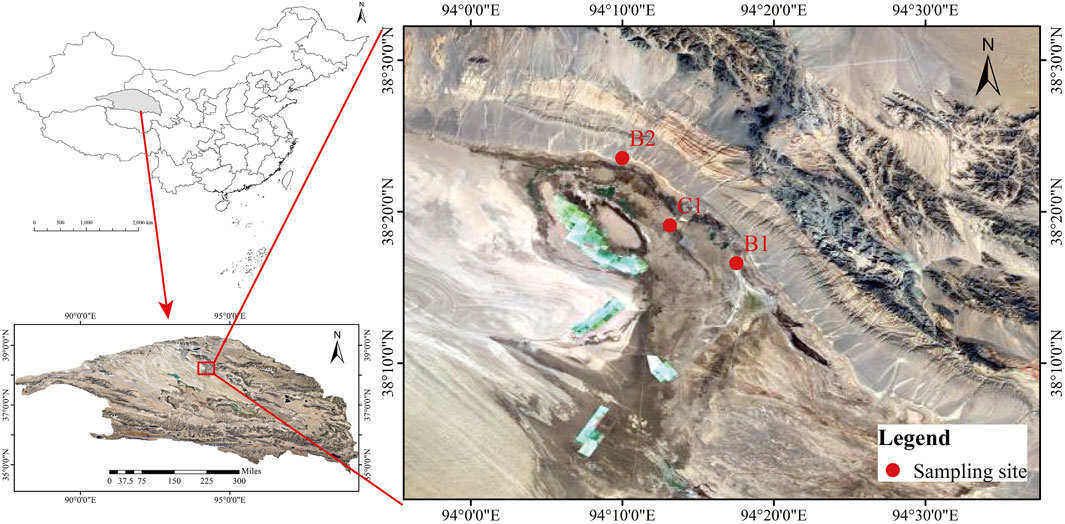
This study selected two deep brine samples and one surface brine sample as research subjects, based on variations in water types and spatial distribution. These samples are designated as B1, B2, and C1 (Figure 1). B1 and B2 are deep brine samples situated within the same tectonic unit in the western region of the Qaidam Basin, north of Dezong Mahai Lake. In contrast, C1 is a surface brine sample collected from the Mahai Mining Area. The sampling depths of B1 and B2 are 1092.45 m and 1122.32 m, respectively, and the horizontal distance is approximately 16 km. Despite their similar burial depths and essentially identical tectonic backgrounds, the distinct geographical locations of B1 and B2 reflect differences in spatial distribution within the deep brine system, thereby facilitating the understanding of lateral variation characteristics in this system. C1 is situated between B1 and B2 and primarily derives from DeZong Mahai Lake, representing a typical surface brine environment. The origins of the deep brines in B1 and B2 are preliminarily attributed to mountain-front leaching, which percolates underground to form these deposits.
2.3 Sample collection, transportation, and storage
In August 2024, water sampling was conducted at three locations: B1 (deep brine), B2 (deep brine), and C1 (surface brine). Five samples were collected at each sampling point, with each sample being 1L, totaling 15 samples. At each location, three samples were collected using sterile water sampling bags for 16sRNA detection, and two samples were collected using PVC bottles (rinsed three times with the water sample before collection) for heavy metal detection and GC-MS. The samples were stored at 4 °C, transported to the laboratory, and immediately subjected to microbial testing and routine ion analysis.
2.4 Heavy metal analysis
Samples were digested with HClO4, HNO3, HF, and HCl, diluted with dilute HCl, and analyzed by ICP-OES (Agilent 5110, Malaysia). For elevated Bi/Hg/Mo/Ag/W concentrations, appropriate dilutions were prepared prior to ICP-MS analysis. After correcting for spectral interferences, final results were obtained. All samples were measured in triplicate, with precision (relative deviation, RD) and accuracy (relative error, RE) controlled to <10%.
2.5 Physicochemical analysis of brines
All samples were diluted to salinity <2 g/L prior to analysis. Carbonate/bicarbonate, chloride, and magnesium were quantified by volumetric titration, while potassium, sodium, calcium, and sulfate were measured using ICP-OES (Agilent 5900, United States). Triplicate measurements yielded relative standard deviations (RSD) < 5% for all analytes.
2.6 GC-TOF-MS analysis
5 mL sample and 10 μL internal standard were added into a 20 mL Agilent headspace bottle, then placed on the sampling tray and analyzed by gas chromatograph coupled with a time-of-flight mass spectrometer (GC-TOF-MS).
GC-TOF-MS analysis was performed using an Agilent 7890B gas chromatograph coupled with a time-of-flight mass spectrometry system (Pegasus BT, Leco). A DB-WAX (30 m × 0.25 mm × 0.25 um)capillary column was used for separation. Helium was used as the carrier gas at a constant flow rate of 1.0 mL/min. The temperature of injection was set to 245 °C. The source temperature was 220 °C. The initial temperature was kept at 40 °C for 3 min, then raised to 105 °C at a rate of 6 °C min−1, then raised to 180 °C at a rate of 4 °C min−1, then raised to 245 °C at a rate of 10 °C min−1 and kept for 5 min at 300 °C. The energy was 70 eV in electron impact mode. The mass spectrometry data were acquired in full-scan mode with the m/z range of 35–450 at a rate of 15 spectra per second. To assess the stability and reproducibility of the instrumental analysis, quality control (QC) samples were prepared by pooling aliquots from all individual samples and were analyzed alongside the experimental samples.
2.7 16s RNA sequencing
Firstly, the samples from the three sterile water sampling bags were filtered using a vacuum pump with a 0.22-micron organic filter membrane. After filtration, the filter membrane was placed into a cryotube and stored at −80 °C, then sent for analysis. This part of the process was completed by Zhongke New Life Co., Ltd. Then, Total genome DNA from samples were extracted using Mag-bind soil DNA kit (Omega), and tests the purity and concentration of DNA. For microbial analysis, each sample was subjected to six technical replicates to ensure the accuracy and reliability of the results. According to the selection of sequencing region, the selected V3-V4 variable region was amplified by PCR using specific primers with Barcode and high-fidelity DNA polymerase. PCR products were detected by 2% agarose gel electrophoresis, and the target fragments were cut and recovered by Quant-iT PicoGreen dsDNA Assay Kit. Referring to the preliminary quantitative results of electrophoresis, the PCR amplification recovered products were detected and quantified with the Microplate reader (BioTek, FLx800) fluorescence quantitative system, and the corresponding proportions were mixed according to the sequencing requirements of each sample. The library was constructed using TruSeq Nano DNA LT Library Prep Kit from Illumina. The constructed library is inspected by Agilent Bioanalyzer 2100 and Promega Quanti Fluor. After the library is qualified, it is sequenced.
2.8 Data analysis
Raw sequencing data were in FASTQ format. Paired-end reads were then preprocessed using cutadapt software to detect and cut off the adapter. After trimming, paired-end reads were filtering low quality sequences, denoised, merged and detect and cut off the chimera reads using DADA2 with the default parameters of QIIME2. At last, the software output the representative reads and the ASV abundance table. The representative read of each ASV was selected using QIIME 2 package. Representative sequence reads were taxonomically annotated using the SILVA database (version 138, 16S/18S rDNA) through the classify-sklearn classifier with default parameters. Alpha and beta diversity indices were calculated using QIIME2. Alpha diversity assesses microbial richness and diversity within individual samples, while beta diversity compares microbial community composition between samples.
Due to the limited environmental monitoring infrastructure and lack of reliable baseline pollution data in the study area, the screening criteria for organic pollutants detected by GC-MS were established with reference to the U.S. EPA’s Integrated Risk Information System (IRIS) database and the National Institute of Standards and Technology (NIST). The correlations between dominant microorganisms and hydrochemical factors were analyzed using SPSS software (IBM SPSS Statistics 23) and represented the results through a correlation heat map. The Mantel test and redundancy analysis (RDA) were performed using R software (v 4.5.0) to determine the response relationships between microbial community diversity and hydrochemical factors.
3 Results
3.1 Physicochemical properties of brine samples
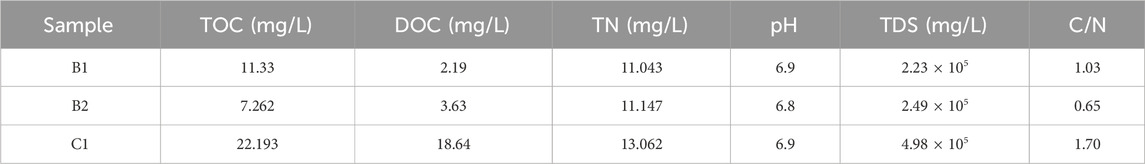
In order to gain insight into the variability of organic matter and its sources in surface and deep brines, this study examined the characteristics of the distribution of Total Organic Carbon (TOC), Dissolved Organic Carbon (DOC), Total Nitrogen (TN), pH, and Total dissolved solids (TDS) of different samples (Table 1).
The results revealed that although B1 and B2 were collected at similar depths and within the same structural unit, they exhibited substantial differences in organic matter content. Specifically, B1 contained significantly higher organic matter (11.33%) than B2 (7.26%), whereas B2 demonstrated elevated dissolved organic carbon levels (3.63%) compared to B1 (2.19%). As shown in the table, the pH values of both surface and deep brines exhibit minimal variation, maintaining a neutral range of 6.8–6.9. According to the groundwater quality standard (GB/T 14848-2017), deep brine should be classified as a Class V water body and should not be used as a source of domestic drinking water. And TDS levels exceed the standard, possibly due to hydrogeological influences. Based on TDS classification, both surface and deep brine are categorized as brine. The TDS in surface water exceed those in deep brine, a phenomenon closely linked to evaporation processes occurring within the mining area.
Additionally, the organic matter content in surface brine is two to three times higher than that in deep brine, while the total nitrogen content remains relatively consistent between the two. The surface brine has the highest DOC concentration of 18.64 mg/L, which is 6–9 times higher than that of deep brine.
3.2 Pollutant distribution characteristics
3.2.1 Heavy metal pollutant distribution characteristics
According to the groundwater quality standard (GB/T14848-2017), analysis of Figures 2a–c reveals that both surface and deep brines are contaminated with heavy metals to varying extents. Currently, three primary heavy metal pollutants have been identified: Mn, Cd and Pb. Notably, while the concentrations of most heavy metals in deep brines exhibit minimal variation, the Mn concentration in sample B2 (average 9.70 mg/L) is significantly higher than in sample B1 (average 3.00 mg/L). Furthermore, deep brine generally exhibits higher concentrations of heavy metals compared to surface brine. Specifically, Mn concentrations in surface brine range from 0.48 to 0.52 mg/L, whereas in deep brine, they range from 2.84 to 10.10 mg/L. The Mn concentration in deep brine significantly exceeds the background value, reaching levels 2 to 7 times higher. Additionally, the concentrations of Cd (0.02–0.04 mg/L) and Pb (0.12–0.18 mg/L) in deep brine are slightly elevated compared to those in surface brine (0.01–0.03 mg/L for Cd and 0.01 mg/L for Pb).

Figure 2. Contents of organic pollutants and heavy metal pollutants in deep brine and surface brine. (a): Mn content, (b): Cd content, (c): Pb content, (d): PAHs content, (e): PAEs content, (f): Halogenated compounds content, (g): S-containing organics compounds content.
3.2.2 Volatile organic pollutant distribution characteristics
Utilizing the databases from the National Institute of Standards and Technology (NIST) and U.S. EPA Integrated Risk Information System (IRIS), we classified anthropogenic contaminants in both deep and surface brines into four categories: polycyclic aromatic hydrocarbons (PAHs), phthalate esters (PAEs), halogenated hydrocarbons, and sulfur-containing compounds. In deep brines, we identified five PAH species, compared to four in surface brines, three PAEs, compared to four, four halogenated hydrocarbons, compared to seven, and one sulfur-containing compound, compared to three (Supplementary Table S1). Although surface brines exhibited a greater diversity of organic pollutants, with a total of 15 species compared to 13 in deep brines, the concentrations of contaminants were consistently higher in deep brines (Figures 2d–g).
3.3 Microbial diversity and community structure
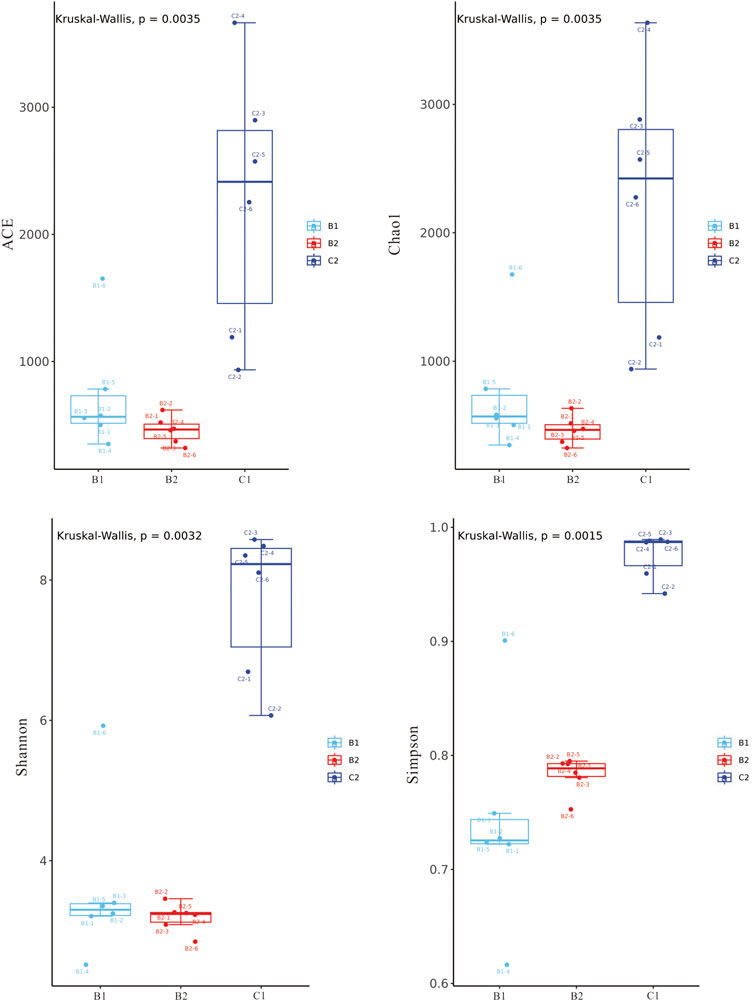
The Alpha diversity index values of the microbial communities in surface and deep brines are summarized in Supplementary Table S2. The coverage values of each sample were greater than 99%, indicating that most microorganisms could be explained by the applied sequencing depth. The ACE and Chao1 indexes used to estimate the total number of microbial species ranged from 319.32 to 1651.56 and 324.25 to 1675.12 in deep brines, and from 933.92 to 3665.13 and 938.73 to 2882.30 in surface brine, respectively. The Shannon and Simpson indexes, used to estimate microbial diversity in samples, ranged from 2.52 to 5.92 and 0.62 to 0.96 in deep brines, and from 6.69 to 8.58 and 0.94 to 0.99 in surface brine (Figure 3).
The composition of microbial communities exhibited significant differences between surface and deep brines. At the phylum level, four phyla were predominant (>1%) in deep brines (Figure 4a). Among these dominant phyla, Proteobacteria and Halanaerobiaeota collectively constituted over 80%. In surface brines, four phyla were also dominant, with Proteobacteria and Firmicutes account for 74% (Figure 4a). To further elucidate the differences in microbial communities between surface and deep brines, the microbial composition was analyzed at the genus level (Figure 4b). At this level, it was observed that common genera varied considerably among samples, including those from deep brines. In sample B1, four genera were dominant (>1%), namely, including Halovibrio, f_Halomonadaceae|g_uncultured, Halanaerobaculum and Halanaerobacter, with Halovibrio and Halanaerobaculum together comprising 73% of the total. In sample B2, three genera were dominant (>1%), including f_Halomonadaceae|g_uncultured, Halanaerobium and Paraliobacillus, with f_Halomonadaceae|g_uncultured and Halanaerobium together accounting for 77% of the total. In sample C1, only two genera were dominant (>1%), namely, f_uncultured|g_uncultured and Thiohalorhabdus, and these two dominant genera together account for 74%.

Figure 4. Distribution of microbial community structure in brine: (a,b) present the composition of the brine microbial community at the phylum and genus levels respectively, (c): shows the Venn diagram of the OTU sample distribution, (d): shows the PCoA analysis of the brine sample.
The Venn diagram presented in Figure 4c delineates the distribution of microorganisms across various samples. In the deep brine environment, sample B1 exhibits 1,172 unique Operational Taxonomic Units (OTUs), while sample B2 contains 441 unique OTUs. The surface brine sample harbors the highest number of unique OTUs, totaling of 7,738. Among the three samples, B1 and B2 share 115 OTUs, B2 and C1 share 88 OTUs, B1 and C1 share 444 OTUs, and all three samples collectively share 462 OTUs. These findings indicate significant differences in the structural composition of microbial communities between surface and deep brine environments. As depicted in the Principal Coordinate Analysis (PCoA) correlation analysis in Figure 4d, the principal components PC1 and PC2 account for 76.64% and 10.57% of the variance in microbial community composition, respectively. The PCoA results reveal that the microbial community structures within deep brine samples are similar and form distinct clusters, yet they differ markedly from those in surface brine samples.
Figure 5 illustrates the microbial taxa with LDA scores exceeding 2, indicating significant intergroup differences, alongside their phylogenetic clades. The blue nodes denote surface brine, which contains 311 significantly differentiated species primarily from the phyla Firmicutes, Actinobacteriota, and Bacteroidota. In contrast, the green and red nodes represent deep brine layers B2 and B1, which harbor 31 and 16 distinct microbial taxa, respectively, predominantly from the phyla Proteobacteria and Halanaerobiaeota. The results demonstrate substantially higher abundance of differential microbial communities in surface brine compared to the deep layers (311 vs. 31/16), likely due to the more open environmental conditions of the surface brine. Further investigation is necessary to explore potential correlations with environmental factors.

Figure 5. The potential biomarker was defined by LEfSe. (a) shows a histogram of linear discriminant analysis (LDA) scores for features rich in differences between groups. The log LDA score threshold for discriminant features is set to 2.0, (b) shows a cladistic plot representing significant differences in classification between deep and surface brine formations.
3.4 Relationship among microbial community structure, pollutants and environmental factors
Through a detailed analysis of the correlations among pollutants, environmental factors, and microbial communities, a correlation heatmap was constructed (Figure 6), elucidating the intricate interactions between water chemistry, pollutants, and microbial composition. The findings indicated that, with the exception of PAHs and Mn, all other pollutants exerted a significant influence on the five dominant microbial groups in the brine. Although most water chemistry parameters did not exhibit strong correlations with PAHs and Mn, TC and TOC were notable exceptions, showing certain degrees of association. Specifically, Pb showed strong positive correlations with TN, TOC, TC, sulfate (SO42-), magnesium (Mg2+), TDS, and DOC. Cd was strongly correlated with TDS and potassium (K+). PAEs exhibited significant correlations with DOC, TDS, K+, Mg2+, SO42-, TC, and TOC. Halogenated compounds were closely related to DOC, TDS, K+, Mg2+, SO42-, TC, TOC, and TN. Sulfides showed strong correlations with DOC, Ca2+, Mg2+, SO42-, TC, TOC, and TN. Overall, the majority of pollutants were strongly associated with organic matter in the brine. Furthermore, significant correlations were identified among water chemistry parameters, as well as between water chemistry and pollutants. Consequently, further research is warranted to investigate the interactions among microbial community structure, water chemistry, and pollutants. This will enhance our understanding of the effects of pollutants on microbial composition and ecological functions.

Figure 6. The correlation heat map depicting the relationships between different hydrochemical factors, as well as between hydrochemical factors and dominant phylum in deep brines and surface brine of the study area (All variables were normalized using Z-score transformation to enable direct comparison across different units and magnitudes).
This study conducted Redundancy Analysis (RDA) and Principal Component Analysis (PCA) to evaluate the impact of pollutants and hydrochemical factors on the microbial community structure in both deep and surface brine environments. The RDA findings demonstrated that the first two axes, RDA1 and RDA2, accounted for 70.23% and 25.41% of the variation in the native microbial community structure within high-salinity water bodies affected by organic and heavy metal pollution (Figure 7). Collectively, the influence of 20 variables explained 95.64% of the observed variation in microbial community composition. Notably, both organic and heavy metal pollutants significantly influenced the variation in microbial communities in deep brine. Importantly, both organic and heavy metal pollutants were found to significantly affect the variation in microbial communities in deep brine. Specifically, the presence of Cd, Pb, PAEs, halogenated compounds, and sulfur-containing organics exhibited strong correlations with the distribution of dominant deep-brine genera such as Halanaerobaculum, Halanaerobacter, and Halovibrio, as indicated by the RDA analysis. These genera exhibited distinct directional associations with the previously mentioned pollutants along the primary RDA axis (RDA1, 70.23%), indicating that fluctuations in pollutant concentrations are intricately connected to the structuring of microbial communities in hypersaline subsurface environments.
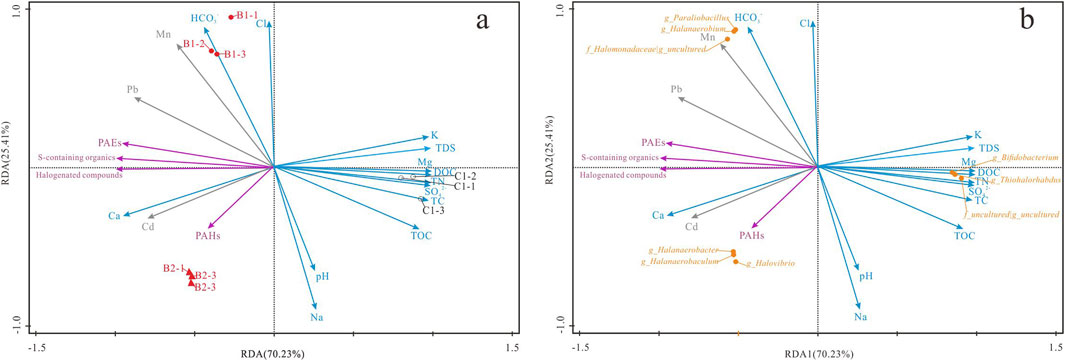
Figure 7. The response relationship between indigenous microflora and hydrochemical factors in deep brines and surface brine. (a) displays the RDA diagram, highlighting the correlation between samples and hydrochemical factors, (b) show cases the RDA diagram, revealing the correlation between dominant genera and hydrochemical factors in groundwater.
4 Discussion
4.1 Composition and sources of organic matter in brine
Dissolved organic matter (DOM) is prevalent in high-salinity waters (Xu and Guo, 2017) and plays a crucial role in influencing nutrient cycling (Mopper et al., 1991), pH buffering capacity (Carter et al., 2012), and the solubility, toxicity, and bioavailability of metals and organic pollutants (Worms et al., 2019). In this study, we found that the TDS, TC, TOC, and DOC in surface brine were elevated compared to those in deep brine, although the total nitrogen content did not exhibit a substantial difference. Previous research has demonstrated that increasing salinity in lakes correlates with an increase in TDS, and the average molecular weight of DOM in brine tends to be larger with a higher degree of oxidation (Xu et al., 2020). Moreover, surface brine is more exposed to external influences compared to deep brine, potentially leading to greater impacts from external organic matter, such as atmospheric precipitation. Based on the analysis of the carbon-to-nitrogen (C/N) ratio, both surface and deep brines demonstrated C/N values below 2. This ratio indicates that the organic matter in these environments predominantly originates from algae (Khan et al., 2015), with a higher concentration of algae present in surface water relative to deep brine. Studies have demonstrated that microbial communities in brine, especially halophilic bacteria, are capable of efficiently utilizing organic nitrogen (Wang and Cui, 2024) and consuming organic carbon (Bonfá et al., 2013; Bonaglia et al., 2020), thereby significantly reducing the C/N ratio. This efficient utilization of organic nitrogen by microbes generally leads to an imbalance between organic carbon and nitrogen, further decreasing the C/N ratio.
Despite B1 and B2 being sampled from comparable depths within the same tectonic unit, their organic matter compositions exhibit notable differences. Specifically, B1 is characterized by a higher total organic carbon (TOC) content of 11.33%, whereas B2 contains a greater proportion of dissolved organic carbon (DOC) at 3.63%. This pattern implies that the organic matter in B2 may have undergone more extensive microbial degradation, leading to an increased presence of soluble organic fractions (Servais et al., 2020), while B1 retains a larger amount of particulate or recalcitrant organic components. This divergence is likely attributable to localized microenvironmental variations between the two boreholes. For example, increased ionic strength could lead to organic matter salting-out effects, thereby promoting the formation of colloids and particulate matter (Tóth et al., 2005). These findings underscore the existence of significant spatial heterogeneity in organic carbon composition and reactivity, even within the same deep brine system. Conversely, while microbial activity predominantly influences brine, external pollutants, notably anthropogenic contamination, may also directly contribute to organic nitrogen inputs (Valiente et al., 2022), potentially accounting for the exceptionally low C/N ratio observed. Thus, although microbial activity remains the principal factor, the impact of external organic nitrogen should be taken into account to offer a more comprehensive understanding of the variations in C/N ratios within brine.
4.2 Mechanisms and accumulation of heavy metals
In both surface and deep brines, we identified three heavy metals with significantly elevated concentrations: Mn, Cd, and Pb. Manganese (Mn) is recognized for its potential to induce a range of neurotoxic symptoms in clinical contexts, with chronic or acute exposure potentially resulting in severe outcomes, including cognitive and psychiatric symptoms, Parkinson’s disease, motor dysfunction, and other neurodegenerative disorders (Das et al., 2015). Mn is widely distributed in geological formations, particularly with in aquifer sediments, and can leach into groundwater, thereby constituting a common chemical component (Wu et al., 2019). In natural environments, the predominant mechanism facilitating the release and migration of Mn from aquifer sediments to groundwater is its redox dissolution (Moon et al., 2020). Although Mn in groundwater primarily originates from natural sources (Zhai et al., 2019), research has also indicated that its concentration may be influenced by anthropogenic activities (Li et al., 2020). Previous research has demonstrated a positive correlation between Mn concentrations in groundwater and organic matter content (Liu et al., 2020). Additionally, the presence of organic pollutants has been shown to facilitate the release of Mn from sediments into the water (Liu et al., 2019; Zhai et al., 2021). Contrary to these findings, the present study observed that despite lower organic matter content in deep brines compared to surface brines, Mn concentrations were significantly elevated, exceeding typical industrial heavy metal pollution levels in groundwater (0.663 mg/L) as reported by Adeyemi and Ojekunle (2021). Furthermore, our data indicate substantially higher Mn concentrations in site B2 relative to site B1, which may be associated with increased levels of DOC. This suggests that DOC could potentially enhance Mn mobility through microbially-mediated and chemical reduction processes (Jiann et al., 2013).
Simultaneously, we propose the hypothesis that sediments within deep aquifers may exhibit elevated concentrations of Mn. Additionally, the relatively isolated geographical environment of deep brines may contribute to the accumulation of heavy metals to a certain degree (Stamatis et al., 2019). Pb and Cd, recognized as prevalent heavy metal contaminants, are ubiquitously found in soils (Zhou et al., 2019), rivers (Nasrabadi, 2015), and lakes (Şener et al., 2023), thereby posing substantial threats to both environmental integrity and human health. Studies have shown that Pb and Cd in soils not only affect plant growth but also presents potential health risks to animals and humans through bioaccumulation in the food chain (Chrysochoou and Dermatas, 2015). In rivers and lakes, Pb and Cd pollution predominantly arises from anthropogenic activities (Niu et al., 2021), including wastewater discharge and fertilizer application (Fei et al., 2020). In recent years, Pb and Cd contamination has also been found in high salinity environments, such as salt springs (Rezaei et al., 2019), salt lakes (Baati et al., 2022), and sediments (Rezaei et al., 2021), with primary sources attributed to industrial wastewater from factories, oilfields, and the deposition of industrial particles. This study found that the concentrations of Pb and Cd in deep brines were comparable to those found in freshwater lakes with values of 0.111 mg/L and 0.135 mg/L, respectively (Muneer et al., 2022). However, these concentrations were significantly elevated compared to those in surface brines, thereby reinforcing the hypothesis that closed environments facilitate the accumulation of heavy metals.
4.3 Types and possible sources of organic pollutants
In this study, organic pollutants characterized by their persistence and bioaccumulative properties, were identified in both surface and deep brine samples. Four major classes were detected: PAHs, PAEs, halogenated compounds, and S-containing organics. PAHs, which consist of multiple fused aromatic rings, are well-documented for their carcinogenic potential and environmental persistence (McCarrick et al., 2019). Research indicates shown that low-molecular-weight PAHs exhibit greater water solubility, whereas high-molecular-weight PAHs are more prone to accumulation in suspended solids and sediments (Rabodonirina et al., 2015). In lakes and rivers, PAHs predominantly originate from the combustion of fossil fuels or biomass (e.g., shrubs or forests) (Wei et al., 2015), as well as from petroleum contamination (Bandowe et al., 2014). Conversely, in saline lakes, atmospheric deposition regarded as the principal source of PAHs (Idowu et al., 2020). The present study found that the PAH concentrations in surface and deep brines were comparable, measuring 0.26% and 0.20%, respectively. In light of the absence of vegetation and oil fields within the study area, it is inferred that atmospheric deposition constitutes the primary source of PAHs in surface brine. This observation further substantiates the hypothesis that deep brine originates from the infiltration of snowmelt into subsurface strata, resulting in the formation of highly mineralized brine under closed conditions (Hongpu et al., 2022). PAEs, extensively utilized as plasticizers, are frequently detected in aquatic environments and sediments. Elevated concentrations of PAEs have been reported in lakes and rivers situated near industrial zones (Gong et al., 2024). In our study, the presence of PAEs in brine is likely attributable to wastewater discharge from mining activities. The elevated levels of PAEs detected in deep brine suggest their accumulation within the confined subsurface environment over time. Halogenated hydrocarbons in saline lakes predominantly originate from biological halogenation processes, which are intensified by elevated sodium chloride concentrations (Timms, 2009).
Previous research has indicated that concentrations of halogenated hydrocarbons are elevated in neutral to alkaline saline lakes compared to acidic ones (Ruecker et al., 2015). Additionally, halophilic microorganisms and enzyme-mediated reactions are pivotal in the formation of halogens within hypersaline environments (Rhew et al., 2000). In this study, deep brine exhibited a significantly higher content of halogenated compounds (1.48%) than surface brine (0.19%), despite comparable NaCl concentrations. This suggests that microbial communities associated with halogenation processes may be considerably more abundant in deep brine. S-containing organic compounds have garnered significant attention due to their implications in atmospheric chemistry and global climate regulation (Andreae and Crutzen, 1997). In freshwater lakes, sulfide formation is generally linked to microbial activity (Hu et al., 2007) and the metabolism of sulfur-containing amino acids (Caron and Kramer, 1994). Conversely, in saline lakes, sulfide formation is more closely related to microbial degradation and sulfate reduction processes (Fritz and Bachofen, 2000; Torfstein et al., 2005).
Furthermore, salinity alterations resulting from anthropogenic activities may influence microbial community functions, thereby impacting sulfide production (Mr et al., 2017). In our study area, the deep brine, although currently undeveloped, exhibited a higher concentration of S-containing organic compounds compared to the surface brine. This suggests a greater prevalence of sulfate-reducing bacteria in the deep brine. Microbial community analysis corroborated this finding, revealing that the relative abundance of Proteobacteria in deep brine was more than three times greater than in surface brine, while the abundance of Halanaerobiaeota was over 200 times higher. Previous research has identified Proteobacteria as major sulfate-reducing species in high-salinity environments (Santos et al., 2020). Additionally, Boidi et al. (2022) reported that sulfate-reducing functional groups within the microbial mats of Laguna Negra’s high-salinity environments are predominantly composed of Halanaerobiaeota, underscoring the significant role of sulfate reduction in deep brine.
4.4 Differences in microbial community structure and the effects of pollutants on microorganisms
As shown in Figure 4A, the microbial community in deep brine was predominantly composed of the phylum Proteobacteria, whereas Firmicutes emerged as the most abundant phylum in surface brine. Within the Proteobacteria, both α-Proteobacteria and γ-Proteobacteria are extensively acknowledged as pivotal microbial groups involved in the degradation of organic pollutants (Moxley and Schmidt, 2012; Cui et al., 2014; Moghadam et al., 2014). Firmicutes, frequently associated with petroleum degradation, flourish in eutrophic environments and plays an important role in carbon and nitrogen cycling (Hellal et al., 2021; Fang et al., 2023). In addition to Proteobacteria, the deep brine also exhibited relatively high proportions of Firmicutes (6.84%–15.65%) and Halanacrobiacota (12.95%–26.35%). Conversely, surface brine demonstrated higher relative abundances of Actinobacteriota (13.06%) and Bacteroidota (10.26%). These dominant microbial phyla have been documented in hypersaline environments and are known for their capabilities in degrading organic pollutants and heavy metals (Ye et al., 2016; Alvarez et al., 2017; Liu et al., 2023).
At the genus level (Figure 4B), several taxa exhibited high relative abundances within the brine samples, including f_Halomonadaceae|g_uncultured, Halovibrio, Halanaerobaculum, Halanaerobium, Bifidobacterium, Paraliobacillus, and Thiohalorhabdus. Notably, substantial differences were observed among the microbial communities at the genus level across samples B1, B2, and C1. Specifically, Halovibrio was predominant in B1, f_Halomonadaceae|g_uncultured was the most prevalent in B2, while Bifidobacterium was the dominant genus in C1. These observations indicate significant differences in the indigenous microbial composition between deep and surface brine, potentially attributable to variations in hydrogeological conditions, physicochemical properties, pollutant concentrations, and organic matter composition. Importantly, despite the high salinity conditions, the majority of the dominant genera identified possess known capabilities for degrading heavy metals and organic contaminants (Ottoson, 2009; Mohammadipanah et al., 2015; Cui et al., 2019; Boltyanskaya et al., 2023). Figure 4C illustrates the unique and shared OTUs between deep brine and surface brine samples. The deep brine contained the highest number of unique OTUs, totaling 7,738, indicating significantly greater microbial diversity compared to surface brine.
Additionally, PCoA demonstrated a distinct separation between the microbial communities of surface and deep brine, with a high degree of similarity observed between samples B1 and B2. Remarkably, RDA indicated that pollutants exerted a more pronounced influence on the microbial communities in deep brine compared to those in surface brine. The structure of local microbial communities was significantly shaped by most organic pollutants and heavy metals. Interestingly, PAHs exhibited a relatively minor impact on the microbial community, potentially due to their limited bioavailability in brine systems (Lindgren et al., 2014). Nonetheless, considering that the ecological effects of PAHs can vary substantially across different environmental contexts (Picariello et al., 2020), it is crucial to further investigate the conditions under which PAHs might influence microbial community dynamics in brine systems. In contrast to the long-exposed surface brine, deep brine exists in a more isolated environment. However, under high-salinity conditions, organic pollutants and heavy metals tend to accumulate and persist more readily in deep brine, exerting stronger impacts on subterranean microbial ecosystems. Consequently, greater emphasis must be placed on these factors when evaluating the future exploitation and utilization of deep brine resources.
5 Conclusion
This study conducted a systematic comparison of the surface and deep brines from the Qaidam Basin, focusing on their physicochemical properties, organic matter composition, distributions of heavy metals and organic pollutants, and microbial community structures. The results revealed that surface brine exhibited significantly higher contents of TC, TOC, and DOC, whereas deep brine showed elevated levels of Mn, Pb, and Cd. This suggests that closed subsurface environments are conducive to the accumulation of heavy metals. Furthermore, the low C/N ratios in both brine types imply a predominantly microbial origin of organic matter, potentially supplemented by exogenous organic nitrogen.
Among the pollutants identified, PAHs, PAEs, halogenated compounds, and S-containing organics were more concentrated in deep brines. This enrichment suggests that their sources are likely related to atmospheric deposition, biogenic halogenation, industrial activities, and wastewater discharge from mining operations. Microbial community analysis showed that Proteobacteria predominated in deep brines, whereas Firmicutes were more prevalent in surface brines. Variations at the genus level, OTU distributions, and PCoA results confirmed significant microbial divergence between the two environments. Furthermore, RDA and correlation heatmaps indicated that pollutants such as Cd, Pb, PAEs, halogenated compounds, and sulfides exerted substantial influences on microbial community structures, with more pronounced pollutant-driven effects observed in deep brines.
In summary, the enclosed and hypersaline environments of deep brines not only promote the accumulation and preservation of heavy metals and organic pollutants but also substantially influence the structuring of microbial communities. These findings highlight that microbial responses not only mirror the ecological stress induced by pollutants but also play a pivotal role in shaping ecological risks. Consequently, these factors should be meticulously considered in the development of future strategies for deep brine resource exploitation and pollution management.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding authors.
Author contributions
XL: Resources, Funding acquisition, Conceptualization, Validation, Project administration, Visualization, Writing – review and editing, Methodology, Formal Analysis, Writing – original draft. QW: Software, Writing – original draft. ZW: Formal Analysis, Writing – original draft. YM: Writing – original draft, Resources, Data curation. ZS: Writing – original draft, Investigation. DQ: Project administration, Writing – original draft, Writing – review and editing, Supervision, Validation. ZM: Writing – original draft, Project administration, Validation, Conceptualization, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Key Research and Development and Transformation Program Project of the Qinghai Provincial Department of Science and Technology (2024-QY-207).
Acknowledgments
The authors are thankful to the support of Qinghai University for the use of laboratory facilities.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1646864/full#supplementary-material
References
Adeyemi, A. A., and Ojekunle, Z. O. (2021). Concentrations and health risk assessment of industrial heavy metals pollution in groundwater in Ogun state, Nigeria. Sci. Afr. 11, e00666. doi:10.1016/j.sciaf.2020.e00666
Alvarez, A., Saez, J. M., Davila Costa, J. S., Colin, V. L., Fuentes, M. S., Cuozzo, S. A., et al. (2017). Actinobacteria: current research and perspectives for bioremediation of pesticides and heavy metals. Chemosphere 166, 41–62. doi:10.1016/j.chemosphere.2016.09.070
Andreae, M. O., and Crutzen, P. J. (1997). Atmospheric Aerosols: biogeochemical sources and role in atmospheric chemistry. Science 276, 1052–1058. doi:10.1126/science.276.5315.1052
Baati, H., Bahloul, M., Amdouni, R., and Azri, C. (2022). Behavior assessment of moderately halophilic bacteria in brines highly enriched with heavy metals: Sfax solar saltern (Tunisia), A case study. Geomicrobiol. J. 39, 341–351. doi:10.1080/01490451.2021.2008548
Bai, Y., Huo, Y., Liao, K., and Qu, J. (2017). Influence of microbial community diversity and function on pollutant removal in ecological wastewater treatment. Appl. Microbiol. Biotechnol. 101, 7293–7302. doi:10.1007/s00253-017-8464-5
Bai, H., Pan, T., Han, G., Fan, Q., Miao, Q., and Bu, H. (2024). Hydrochemical characteristics and genesis of sand–gravel brine deposits in the Mahai Basin of the northern Qinghai–Tibetan plateau. Water 16, 3562. doi:10.3390/w16243562
Bandowe, B. A. M., Lueso, M. G., and Wilcke, W. (2014). Oxygenated polycyclic aromatic hydrocarbons and azaarenes in urban soils: a comparison of a tropical city (Bangkok) with two temperate cities (Bratislava and Gothenburg). Chemosphere 107, 407–414. doi:10.1016/j.chemosphere.2014.01.017
Besser, H., and Hamed, Y. (2019). Causes and risk evaluation of oil and brine contamination in the Lower Cretaceous Continental Intercalaire aquifer in the Kebili region of southern Tunisia using chemical fingerprinting techniques. Environ. Pollut. 253, 412–423. doi:10.1016/j.envpol.2019.07.020
Boidi, F. J., Mlewski, E. C., Fernández, G. C., Flores, M. R., Gérard, E., Farías, M. E., et al. (2022). Community Vertical composition of the Laguna Negra hypersaline microbial mat, Puna region (Argentinean Andes). Biology 11, 831. doi:10.3390/biology11060831
Boltyanskaya, Y., Zhilina, T., Grouzdev, D., Detkova, E., Pimenov, N., and Kevbrin, V. (2023). Halanaerobium polyolivorans sp. nov.—a Novel halophilic Alkalitolerant bacterium capable of Polyol degradation: Physiological properties and genomic insights. Microorganisms 11, 2325. doi:10.3390/microorganisms11092325
Bonaglia, S., Broman, E., Brindefalk, B., Hedlund, E., Hjorth, T., Rolff, C., et al. (2020). Activated carbon stimulates microbial diversity and PAH biodegradation under anaerobic conditions in oil-polluted sediments. Chemosphere 248, 126023. doi:10.1016/j.chemosphere.2020.126023
Bonfá, M. R. L., Grossman, M. J., Piubeli, F., Mellado, E., and Durrant, L. R. (2013). Phenol degradation by halophilic bacteria isolated from hypersaline environments. Biodegradation 24, 699–709. doi:10.1007/s10532-012-9617-y
Caron, F., and Kramer, J. R. (1994). Formation of volatile sulfides in freshwater environments. Sci. Total Environ. 153, 177–194. doi:10.1016/0048-9697(94)90197-X
Carter, H. T., Tipping, E., Koprivnjak, J.-F., Miller, M. P., Cookson, B., and Hamilton-Taylor, J. (2012). Freshwater DOM quantity and quality from a two-component model of UV absorbance. Water Res. 46, 4532–4542. doi:10.1016/j.watres.2012.05.021
Chrysochoou, M., and Dermatas, D. (2015). Editorial. J. Hazard Mater 281, 1. doi:10.1016/j.jhazmat.2014.10.006
Corsellis, Y. Y., Krasovec, M. M., Sylvi, L. L., Cuny, P. P., and Militon, C. C. (2016). Oil removal and effects of spilled oil on active microbial communities in close to salt-saturation brines. Extremophiles 20, 235–250. doi:10.1007/s00792-016-0818-x
Cristiano, W., Giacoma, C., Carere, M., and Mancini, L. (2021). Chemical pollution as a driver of biodiversity loss and potential deterioration of ecosystem services in Eastern Africa: a critical review. South Afr. J. Sci. 117. doi:10.17159/sajs.2021/9541
Cui, Z., Xu, G., Gao, W., Li, Q., Yang, B., Yang, G., et al. (2014). Isolation and characterization of Cycloclasticus strains from Yellow Sea sediments and biodegradation of pyrene and fluoranthene by their syntrophic association with Marinobacter strains. Int. Biodeterior. and Biodegrad. 91, 45–51. doi:10.1016/j.ibiod.2014.03.005
Cui, Y.-X., Biswal, B. K., Guo, G., Deng, Y.-F., Huang, H., Chen, G.-H., et al. (2019). Biological nitrogen removal from wastewater using sulphur-driven autotrophic denitrification. Appl. Microbiol. Biotechnol. 103, 6023–6039. doi:10.1007/s00253-019-09935-4
Das, A. P., Ghosh, S., Mohanty, S., and Sukla, L. B. (2015). “Advances in Manganese pollution and its bioremediation,” in Environmental microbial Biotechnology. Editors L. B. Sukla, N. Pradhan, S. Panda, and B. K. Mishra (Cham: Springer International Publishing), 313–328. doi:10.1007/978-3-319-19018-1_16
Fan, Q., Han, G., Chen, T., Pang, T., Bai, H., Liu, J., et al. (2024). Inheritance recharge of subsurface brine constrains on formation of K-bearing sand-gravel brine in the alluvial fan zone of mountain-basin system on the Qinghai-Tibetan Plateau. J. Hydrology 645, 132029. doi:10.1016/j.jhydrol.2024.132029
Fang, T., Wang, T., Zhao, M., Bai, L., Deng, Y., and Ruan, W. (2023). Food waste digestate composting enhancement by sodium polyacrylate addition: effects on nitrogen transformation processes and bacterial community dynamics. J. Environ. Manag. 325, 116531. doi:10.1016/j.jenvman.2022.116531
Fei, X., Lou, Z., Xiao, R., Ren, Z., and Lv, X. (2020). Contamination assessment and source apportionment of heavy metals in agricultural soil through the synthesis of PMF and GeogDetector models. Sci. Total Environ. 747, 141293. doi:10.1016/j.scitotenv.2020.141293
Feng, L., Zhang, Z., Yang, G., Wu, G., Yang, Q., and Chen, Q. (2023). Microbial communities and sediment nitrogen cycle in a coastal eutrophic lake with salinity and nutrients shifted by seawater intrusion. Environ. Res. 225, 115590. doi:10.1016/j.envres.2023.115590
Fritz, M., and Bachofen, R. (2000). Volatile organic sulfur compounds in a Meromictic Alpine lake. Acta hydrochimica hydrobiologica 28, 185–192. doi:10.1002/1521-401x(20004)28:4<185::aid-aheh185>3.0.co;2-v
Gong, X., Xiong, L., Xing, J., Deng, Y., Qihui, S., Sun, J., et al. (2024). Implications on freshwater lake-river ecosystem protection suggested by organic micropollutant (OMP) priority list. J. Hazard Mater 461, 132580. doi:10.1016/j.jhazmat.2023.132580
Hellal, J., Joulian, C., Urien, C., Ferreira, S., Denonfoux, J., Hermon, L., et al. (2021). Chlorinated ethene biodegradation and associated bacterial taxa in multi-polluted groundwater: insights from biomolecular markers and stable isotope analysis. Sci. Total Environ. 763, 142950. doi:10.1016/j.scitotenv.2020.142950
Hongpu, L., Xianhua, H., Mianping, Z., Fu, F., Xixi, L., and Shuli, W. (2022). Metallogenic model and prospecting direction of Pleistocene gravel brine Potassium deposit in western Qaidam Basin. J. Lake Sci. 34, 1–13. doi:10.18307/2022.0327
Hu, H., Mylon, S. E., and Benoit, G. (2007). Volatile organic sulfur compounds in a stratified lake. Chemosphere 67, 911–919. doi:10.1016/j.chemosphere.2006.11.012
Huang, Y., Li, F., Wang, C., Teng, Y., Sun, R., Tang, J., et al. (2025). Predictive framework for species Sensitivity distribution Curves of emerging contaminants: a comparative study in marine and freshwater environments. Environ. Sci. and Technol. 59, 8016–8026. doi:10.1021/acs.est.4c12654
Idowu, O., Carbery, M., O’Connor, W., and Thavamani, P. (2020). Speciation and source apportionment of polycyclic aromatic compounds (PACs) in sediments of the largest salt water lake of Australia. Chemosphere 246, 125779. doi:10.1016/j.chemosphere.2019.125779
Jiann, K.-T., Santschi, P. H., and Presley, B. J. (2013). Relationships between geochemical parameters (pH, DOC, SPM, EDTA concentrations) and trace metal (Cd, Co, Cu, Fe, Mn, Ni, Pb, Zn) concentrations in river waters of Texas (USA). Aquat. Geochem 19, 173–193. doi:10.1007/s10498-013-9187-6
Khan, N. S., Vane, C. H., Horton, B. P., Hillier, C., Riding, J. B., and Kendrick, C. P. (2015). The application of δ13C, TOC and C/N geochemistry to reconstruct Holocene relative sea levels and paleoenvironments in the Thames Estuary, UK. J. Quat. Sci. 30, 417–433. doi:10.1002/jqs.2784
Li, Q., Zhang, H., Guo, S., Fu, K., Liao, L., Xu, Y., et al. (2020). Groundwater pollution source apportionment using principal component analysis in a multiple land-use area in southwestern China. Environ. Sci. Pollut. Res. 27, 9000–9011. doi:10.1007/s11356-019-06126-6
Lichter, J., Caron, H., Pasakarnis, T. S., Rodgers, S. L., Squiers, T. S., and Todd, C. S. (2006). The ecological Collapse and partial Recovery of a freshwater Tidal ecosystem. nena 13, 153–178. doi:10.1656/1092-6194(2006)13[153:tecapr]2.0.co;2
Lindgren, J. F., Hassellöv, I.-M., and Dahllöf, I. (2014). PAH effects on meio- and microbial benthic communities strongly depend on bioavailability. Aquat. Toxicol. 146, 230–238. doi:10.1016/j.aquatox.2013.11.013
Liu, S., Liu, H., Wang, Z., Cui, Y., Chen, R., Peng, Z., et al. (2019). Benzene promotes microbial Fe(III) reduction and flavins secretion. Geochimica Cosmochimica Acta 264, 92–104. doi:10.1016/j.gca.2019.08.013
Liu, W., Wang, Y., Li, J., Qian, K., and Xie, X. (2020). Indices of the dual roles of OM as electron donor and complexing compound involved in as and Fe mobilization in aquifer systems of the Datong Basin. Environ. Pollut. 262, 114305. doi:10.1016/j.envpol.2020.114305
Liu, Y.-H., Mohamad, O. A. A., Gao, L., Xie, Y.-G., Abdugheni, R., Huang, Y., et al. (2023). Sediment prokaryotic microbial community and potential biogeochemical cycle from saline lakes shaped by habitat. Microbiol. Res. 270, 127342. doi:10.1016/j.micres.2023.127342
McCarrick, S., Cunha, V., Zapletal, O., Vondráček, J., and Dreij, K. (2019). In vitro and in vivo genotoxicity of oxygenated polycyclic aromatic hydrocarbons. Environ. Pollut. 246, 678–687. doi:10.1016/j.envpol.2018.12.092
Menéndez-Serra, M., Ontiveros, V. J., Triadó-Margarit, X., Alonso, D., and Casamayor, E. O. (2020). Dynamics and ecological distributions of the Archaea microbiome from inland saline lakes (Monegros Desert, Spain). FEMS Microbiol. Ecol. 96, fiaa019. doi:10.1093/femsec/fiaa019
Minella, M., Maurino, V., Minero, C., and Vione, D. (2013). Modelling photochemical transformation of emerging organic pollutants in surface waters: effect of water level fluctuations following outflow or evaporation, relevant to arid and semi-arid environments. Int. J. Environ. Anal. Chem. 93, 1698–1717. doi:10.1080/03067319.2013.803284
Moghadam, S., Mohsen, E., Gholamhossein, A., Khazaei, N., and Karbasi, N. (2014). Statistical Optimization of Crude oil biodegradation by Marinobacter sp. isolated from Qeshm Island, Iran. Iran. J. Biotechnol. 12, 35–41. doi:10.5812/ijb.15392
Mohammadipanah, F., Hamedi, J., and Dehhaghi, M. (2015). “Halophilic bacteria: Potentials and applications in Biotechnology,” in Halophiles: Biodiversity and Sustainable exploitation. Editors D. K. Maheshwari, and M. Saraf (Cham: Springer International Publishing), 277–321. doi:10.1007/978-3-319-14595-2_11
Moon, J.-W., Paradis, C. J., Joyner, D. C., Von Netzer, F., Majumder, E. L., Dixon, E. R., et al. (2020). Characterization of subsurface media from locations up- and down-gradient of a uranium-contaminated aquifer. Chemosphere 255, 126951. doi:10.1016/j.chemosphere.2020.126951
Mopper, K., Zhou, X., Kieber, R. J., Kieber, D. J., Sikorski, R. J., and Jones, R. D. (1991). Photochemical degradation of dissolved organic carbon and its impact on the oceanic carbon cycle. Nature 353, 60–62. doi:10.1038/353060a0
Mossotto, C., Anselmi, S., Trevisan, S., Provenza, F., Maganza, A., Gabetti, A., et al. (2025). Assessing the toxicity of gadolinium in freshwater and marine ecosystems: effects across trophic levels. Environ. Toxicol. Pharmacol. 115, 104673. doi:10.1016/j.etap.2025.104673
Moxley, K., and Schmidt, S. (2012). Isolation of a phenol-utilizing marine bacterium from Durban Harbour (South Africa) and its preliminary characterization as Marinobacter sp. KM2. Water Sci. Technol. 65, 932–939. doi:10.2166/wst.2012.940
Mr, L., C, A., N, F., G, S., J, A., E, A., et al. (2017). Microbialite response to an anthropogenic salinity gradient in Great Salt Lake, Utah. Geobiology 15, 131–145. doi:10.1111/gbi.12201
Muneer, J., AlObaid, A., Ullah, R., Rehman, K. U., and Erinle, K. O. (2022). Appraisal of toxic metals in water, bottom sediments and fish of fresh water lake. J. King Saud Univ. - Sci. 34, 101685. doi:10.1016/j.jksus.2021.101685
Nasrabadi, T. (2015). An indexapproach tometallic pollution in riverwaters. Int. J. Environ. Res. 9, 385–394.
Niu, Y., Chen, F., Li, Y., and Ren, B. (2021). Trends and sources of heavy metal pollution in global river and lake sediments from 1970 to 2018. Rev. Environ. Contam. Toxicol. 257, 1–35. doi:10.1007/398_2020_59
Oren, A., Baxter, B., and Weimer, B. (2009). Microbial communities in salt lakes: phylogenetic diversity, metabolic diversity, and in situ activities. Nat. Resour. Environ. Issues 15. Available online at: https://digitalcommons.usu.edu/nrei/vol15/iss1/51.
Ottoson, J. R. (2009). Bifidobacterial survival in surface water and implications for microbial source tracking. Can. J. Microbiol. 55, 642–647. doi:10.1139/W09-007
Picariello, E., Baldantoni, D., and De Nicola, F. (2020). Acute effects of PAH contamination on microbial community of different forest soils. Environ. Pollut. 262, 114378. doi:10.1016/j.envpol.2020.114378
Rabodonirina, S., Net, S., Ouddane, B., Merhaby, D., Dumoulin, D., Popescu, T., et al. (2015). Distribution of persistent organic pollutants (PAHs, Me-PAHs, PCBs) in dissolved, particulate and sedimentary phases in freshwater systems. Environ. Pollut. 206, 38–48. doi:10.1016/j.envpol.2015.06.023
Rezaei, M., Zarasvandi, A., Heidari, M., and Azhdari, A. (2019). Geochemistry of heavy metals in brine springs of Khouzestan province: Tracing of pollution potential of water resources and Persian Gulf. J. Mar. Sci. Technol. 18, 75–87. doi:10.22113/jmst.2019.166210.2249
Rezaei, M., Zarasvandi, A., Azdari, A., Mousavi, S. S., Heidari, M., and Azizi, N. (2021). Assessment of geological source and geochemical dispersion of heavy metals in the sediments of brine springs in Khouzestan Province. Adv. Appl. Geol. 11, 349–364. doi:10.22055/aag.2020.35770.2182
Rhew, R. C., Miller, B. R., and Weiss, R. F. (2000). Natural methyl bromide and methyl chloride emissions from coastal salt marshes. Nature 403, 292–295. doi:10.1038/35002043
Rojo-Nieto, E., Garrido-Pérez, C., Anfuso-Melfi, G., Lopez-Aguayo, F., Sales-Marquez, D., and Perales-Vargas-Machuca, J. A. (2011). The zoning of semi-enclosed bodies of water according to the sediment pollution: the Bay of Algeciras as a case example. Estuaries Coasts 34, 1129–1139. doi:10.1007/s12237-011-9389-3
Ruecker, A., Weigold, P., Behrens, S., Jochmann, M., Barajas, X. L. O., and Kappler, A. (2015). Halogenated hydrocarbon formation in a moderately acidic salt lake in Western Australia – role of abiotic and biotic processes. Environ. Chem. 12, 406–414. doi:10.1071/EN14202
Santini, T. C., Gramenz, L., Southam, G., and Zammit, C. (2022). Microbial community structure is most strongly associated with geographical distance and pH in Salt Lake sediments. Front. Microbiol. 13, 920056. doi:10.3389/fmicb.2022.920056
Santos, J. C. dos, Lopes, D. R. G., Da Silva, J. D., De Oliveira, M. D., Dias, R. S., Lima, H. S., et al. (2020). Diversity of sulfate-reducing prokaryotes in petroleum production water and oil samples. Int. Biodeterior. and Biodegrad. 151, 104966. doi:10.1016/j.ibiod.2020.104966
Sayed, K., Baloo, L., and Sharma, N. K. (2021). Bioremediation of total petroleum hydrocarbons (TPH) by Bioaugmentation and Biostimulation in water with Floating oil Spill Containment Booms as Bioreactor basin. Int. J. Environ. Res. Public Health 18, 2226. doi:10.3390/ijerph18052226
Şener, E., Şener, Ş., and Bulut, C. (2023). Assessment of heavy metal pollution and quality in lake water and sediment by various index methods and GIS: a case study in Beyşehir Lake, Turkey. Mar. Pollut. Bull. 192, 115101. doi:10.1016/j.marpolbul.2023.115101
Servais, S., Kominoski, J. S., Coronado-Molina, C., Bauman, L., Davis, S. E., Gaiser, E. E., et al. (2020). Effects of Saltwater Pulses on soil microbial enzymes and organic matter Breakdown in freshwater and Brackish coastal Wetlands. Estuaries Coasts 43, 814–830. doi:10.1007/s12237-020-00708-1
Stamatis, N., Kamidis, N., Pigada, P., Sylaios, G., and Koutrakis, E. (2019). Quality Indicators and possible ecological risks of heavy metals in the sediments of three semi-closed east Mediterranean Gulfs. Toxics 7, 30. doi:10.3390/toxics7020030
Tazi, L., Breakwell, D. P., Harker, A. R., and Crandall, K. A. (2014). Life in extreme environments: microbial diversity in Great Salt Lake, Utah. Extremophiles 18, 525–535. doi:10.1007/s00792-014-0637-x
Timms, B. (2009). Study of the saline lakes of the Esperance Hinterland, Western Australia, with special reference to the roles of acidity and episodicity. Nat. Resour. Environ. Issues 15. Available online at: https://digitalcommons.usu.edu/nrei/vol15/iss1/44.
Torfstein, A., Gavrieli, I., and Stein, M. (2005). The sources and evolution of sulfur in the hypersaline Lake Lisan (paleo-Dead Sea). Earth Planet. Sci. Lett. 236, 61–77. doi:10.1016/j.epsl.2005.04.026
Tóth, J., Kardos-Fodor, A., and Halász-Péterfi, S. (2005). The formation of fine particles by salting-out precipitation. Chem. Eng. Process. Process Intensif. 44, 193–200. doi:10.1016/j.cep.2004.02.013
Valiente, N., Jirsa, F., and Gómez-Alday, J. J. (2022). Saline lakes as barriers against pollution: a multidisciplinary overview. Limnetica 41, 281–303. doi:10.23818/limn.41.17
Wang, Y., and Bao, G. (2022). Diversity of prokaryotic microorganisms in alkaline saline soil of the Qarhan Salt Lake area in the Qinghai–Tibet plateau. Sci. Rep. 12, 3365. doi:10.1038/s41598-022-07311-3
Wang, L., and Cui, Y.-W. (2024). Mutualistic symbiosis of fungi and nitrogen-fixing bacteria in halophilic aerobic granular sludge treating nitrogen-deficient hypersaline organic wastewater. Bioresour. Technol. 394, 130183. doi:10.1016/j.biortech.2023.130183
Wang, L., Lim, C. K., Dang, H., Hanson, T. E., and Klotz, M. G. (2016). D1FHS, the type Strain of the Ammonia-Oxidizing bacterium Nitrosococcus wardiae spec. Nov.: enrichment, isolation, phylogenetic, and growth Physiological characterization. Front. Microbiol. 7, 512. doi:10.3389/fmicb.2016.00512
Wang, X., Ren, L., Long, T., Geng, C., and Tian, X. (2023). Migration and remediation of organic liquid pollutants in porous soils and sedimentary rocks: a review. Environ. Chem. Lett. 21, 479–496. doi:10.1007/s10311-022-01506-w
Wei, C., Han, Y., Bandowe, B. A. M., Cao, J., Huang, R.-J., Ni, H., et al. (2015). Occurrence, gas/particle partitioning and carcinogenic risk of polycyclic aromatic hydrocarbons and their oxygen and nitrogen containing derivatives in Xi’an, central China. Sci. Total Environ. 505, 814–822. doi:10.1016/j.scitotenv.2014.10.054
Worms, I. A. M., Chmiel, H. E., Traber, J., Tofield-Pasche, N., and Slaveykova, V. I. (2019). Dissolved organic matter and associated trace metal dynamics from river to lake, under ice-Covered and ice-Free conditions. Environ. Sci. Technol. 53, 14134–14143. doi:10.1021/acs.est.9b02184
Wu, B., Amelung, W., Xing, Y., Bol, R., and Berns, A. E. (2019). Iron cycling and isotope fractionation in terrestrial ecosystems. Earth-Science Rev. 190, 323–352. doi:10.1016/j.earscirev.2018.12.012
Xu, H., and Guo, L. (2017). Molecular size-dependent abundance and composition of dissolved organic matter in river, lake and sea waters. Water Res. 117, 115–126. doi:10.1016/j.watres.2017.04.006
Xu, W., Gao, Q., He, C., Shi, Q., Hou, Z.-Q., and Zhao, H.-Z. (2020). Using ESI FT-ICR MS to characterize dissolved organic matter in salt lakes with different salinity. Environ. Sci. Technol. 54, 12929–12937. doi:10.1021/acs.est.0c01681
Ye, T., Cai, H., Liu, X., and Jiang, H.-L. (2016). Dominance of Oscillospira and Bacteroides in the bacterial community associated with the degradation of high-concentration dimethyl sulfide under iron-reducing condition. Ann. Microbiol. 66, 1199–1206. doi:10.1007/s13213-016-1207-5
Zhai, Y., Zheng, F., Zhao, X., Xia, X., and Teng, Y. (2019). Identification of hydrochemical genesis and screening of typical groundwater pollutants impacting human health: a case study in Northeast China. Environ. Pollut. 252, 1202–1215. doi:10.1016/j.envpol.2019.05.158
Zhai, Y., Han, Y., Xia, X., Li, X., Lu, H., Teng, Y., et al. (2021). Anthropogenic organic pollutants in groundwater increase releases of Fe and Mn from aquifer sediments: impacts of pollution degree, mineral content, and pH. Water 13, 1920. doi:10.3390/w13141920
Zheng, M., Zhang, Y., Liu, X., Qi, W., Kong, F., Nie, Z., et al. (2016). Progress and Prospects of Salt Lake research in China. Acta Geol. Sin. - Engl. Ed. 90, 1195–1235. doi:10.1111/1755-6724.12767
Keywords: deep brine, heavy metal pollution, organic pollutants, microbial community structure, extreme environment
Citation: Lu X, Wang Q, Wang Z, Ma Y, Shi Z, Qi D and Ma Z (2025) Pollutant distribution characteristics and microbial response mechanisms in surface and deep brines of the Qaidam Basin. Front. Environ. Sci. 13:1646864. doi: 10.3389/fenvs.2025.1646864
Received: 14 June 2025; Accepted: 18 August 2025;
Published: 10 September 2025.
Edited by:
Yaoguang Guo, Shanghai Polytechnic University, ChinaCopyright © 2025 Lu, Wang, Wang, Ma, Shi, Qi and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Delin Qi, ZGVsaW5xaUAxMjYuY29t; Zhe Ma, bWF6aGVAaXNsLmFjLmNu
 Xiaohang Lu
Xiaohang Lu Qiugui Wang
Qiugui Wang Zhendong Wang3
Zhendong Wang3 Delin Qi
Delin Qi Zhe Ma
Zhe Ma