- 1School of Basic and Applied Sciences, Maharaja Agrasen University, Baddi, India
- 2Department of Computer Science and Engineering, School of Computing, DIT University, Dehradun, Uttarakhand, India
- 3P. G. Department of Chemistry, Khalsa College, Amritsar, Punjab, India
- 4Cluster of Applied Sciences, UPES, Dehradun, Uttarakhand, India
- 5School of Environment and Disaster Management, Ramakrishna Mission Vivekananda Educational and Research Institute, Kolkata, West Bengal, India
- 6Swedish Meteorological and Hydrological Institute, Norrköping, Sweden
- 7Department of Physical Geography and Ecosystem Science, Lund University, Lund, Sweden
- 8National Centre for Medium Range Weather Forecasting (NCMRWF), Ministry of Earth Sciences, Noida, India
Surface water pollution consists of various pollutants like heavy metals (Cu, Cr, Pb, and Ni), metalloids (Se, As, and Ag), and organic pollutants (dyes, antibiotics, pharmaceuticals, pesticides, and phenolic compounds). The escalating issue of water pollution requires innovative solutions for mitigation and climate resilience. Although various advanced techniques are being developed for removing organic pollutants from surface water, nanoparticles (NPs) for the treatment of toxic dyes, antibiotics, and other organic pollutants are not yet efficiently used. In recent years, ZnO NPs have been studied for photocatalytic degradation of dyes and other organic pollutants. In the present review, efforts have been made to understand the role of ZnO NP in the area of treating contaminated surface water due to dyes and organic pollutants, aligning with the United Nations’ Sustainable Development Goals for clean water and sanitation. The review starts with the bibliographical analysis of the last 10 years from 2015 to June 2025, retrieved from the SCOPUS database. Based on the bibliographic analysis, an increasing trend has been noted in scholarly interest and publications related to ZnO NPs in the last decade. The review offers a detailed understanding of the role of ZnO NPs in removing organic pollutants and dyes from surface wastewater. The mechanisms of degradation, adsorption, and photocatalysis are discussed alongside environmental consequences due to the use of ZnO NPs. Additionally, challenges and future directions for enhancing the environmental performance of ZnO-based nanomaterials are explored. In conclusion, ZnO NPs play an important role in surface water purification; however, further research is needed to fully understand their long-term environmental impacts.
1 Introduction
Rapid industrial and population growth raises the demand for water, putting immense pressure on freshwater resources, resulting in poor-quality and deteriorated water resources (Sharma et al., 2024). Access to clean water has become a major challenge and global concern today. Fresh and clean water is both a resource and a foundation for agriculture, economic stability, disease-free life, community, and public health. Among various pollutants, pharmaceuticals, pesticides, synthetic dyes, and industrial solvents represent a significant threat due to their persistence, toxicity, and resistance to conventional treatment methods. Synthetic dyes, widely used in the textiles, leather, and plastic industries, contribute to the discoloration and oxygen depletion of aquatic ecosystems, thereby affecting photosynthetic activity and biodiversity. These synthetic dyes are often resistant to degradation, intensely colored, and toxic even at low concentrations (Alegbe and Uthman, 2024; Islam et al., 2023). They can obstruct sunlight penetration, disrupt photosynthesis in aquatic plants, and interfere with oxygen levels in the water.
Industrial chemicals such as phenols and surfactants further exacerbate these impacts by introducing carcinogenic and endocrine-disruption effects (Puri et al., 2023). These issues pose serious long-term risks to both environmental and human health. For this purpose, researchers, scientists, and engineers are working together to develop sustainable purification technologies.
One of the sustainable solutions is ensuring the removal of organic pollutants and purification of wastewater through photocatalytic processes. Organic pollutants like dyes, antibiotics, and pesticides are highly toxic, nonbiodegradable, and persistent pollutants and are not completely removed from wastewater by simple treatment processes. Thus, new technologies like solar photodegradation and photocatalysis with the help of nanoparticles (NPs) are being explored on a larger scale due to their ability to eliminate organic pollutants from wastewater and increase the reclamation and reuse of water resources for a longer time. Industries like textiles and power generation consume large quantities of water in their production and operations and release large quantities of effluent (Azanaw et al., 2022; Ip et al., 2009).
Over the past 30 years, India has emerged as a major manufacturer of dyes, supplying not only the textile industries but also sectors like printing, plastics, paints, rubber, paper, food, leather, cosmetics, and pharmaceuticals (Purkait et al., 2005). The effluent discharge from these industries has strong colors, high or low pH, and high chemical oxygen demand (COD). The wastewater is also highly polluted with heavy metals, unconsumed dyes, chemicals, and surfactants that can be toxic, mutagenic, and/or carcinogenic, impacting human health, aquatic life, and deteriorating ecosystems (Berradi et al., 2019; Hachem et al., 2001; Fosso-Kankeu et al., 2015; López Cisneros et al., 2002). The release of such effluents into the nearby water resources is currently one of the major concerns globally for both economic and ecological environments.
Many traditional treatment methods, like coagulation, flocculation, filtration, sedimentation, activated carbon adsorption, and/or chlorination, have been applied in the analysis of the effect of alterations and LA on antioxidant systems and the removal of organic pollutants, but these processes are not able to control microorganisms and other inorganic compounds present in surface or ground water sources. Thus, novel water treatment processes like advanced oxidation processes and UV disinfection techniques are being further developed to treat surface or ground water. In addition, the photocatalysis process is also explored by researchers by adopting a number of NPs, including zinc oxide (ZnO), on a larger scale due to its specific application and tendency to remove persistent organic pollutants from the wastewaters. NPs have unique properties, such as high surface area, reactivity, and photocatalytic activity, making them ideal for water purification applications (Awasthi et al., 2013; Kaur et al., 2024; Singh et al., 2020; Taghipour et al., 2019). These properties enable NPs to effectively remove contaminants, including organic pollutants, heavy metals, etc., from water, providing a promising solution for addressing global water pollution challenges.
Depending upon the requirements, different types of NPs with distinct characteristics are used for purification. Nanomaterials such as ZnO, graphene-based composites, titanium dioxide (TiO2), iron oxide nanoparticles (Fe3O4), and silver nanoparticles (Ag NPs) are used for water purification. In general, if the goal is cost effective, scalable UV-driven degradation of organic pollutants, ZnO NPs (especially doped or immobilized forms) generally provide better performance than other types of NPs. For visible light or sensitive environments, TiO2 hybrids are preferable despite higher costs.
Due to the rise in water pollution from dyes and chemicals, and the fast progress in using ZnO NPs for treatment, there is a need for an overview of the use of ZnO NPs as a water purifier. This review brings together the latest research, compares different approaches, and points out what still needs to be done for safer, cleaner water solutions. In the present review, the main objective of this current research is to evaluate the importance of ZnO NPs as photocatalysts to treat the organic pollutants in wastewater. The review starts with the bibliographic analysis of ZnO NPs for the last 10 years. After that, the techniques of ZnO NPs for the purification of water are discussed, followed by their mechanisms. Different modification methods for the treatment of wastewater, including pure, doped, immobilized, and biosynthesized forms of ZnO NPs, are discussed, providing researchers with different fabrication and perception toward the photocatalysis activity of ZnO when integrated into water treatment systems targeting different organic pollutants.
2 Bibliometric analysis for computing key research trends and geographic distributions
The flow diagram in Figure 1 illustrates the systematic process of selecting relevant journal papers for bibliographic analysis. A total of 535 records were initially identified through SCOPUS database searches using the keywords “ZnO nanoparticles, Dye, and Organic pollutants” on 5 August 2025. The results were screened by applying a publication year filter from 2015 to 2025, and 509 records remained. Subsequently, conference papers, book chapters, and retracted articles were excluded, narrowing the selection to 475 journal articles. Further screening was done by removing non-English language papers, resulting in a final set of 473 English-language journal articles. These 473 papers were considered relevant for bibliographic analysis and are presented in Figure 2. A significant increase in research is observed, as shown in Figure 2A. The average cumulative growth was 254% with respect to the year 2015. More articles may be published in the second 6 months of 2025, which will increase the average cumulative growth.
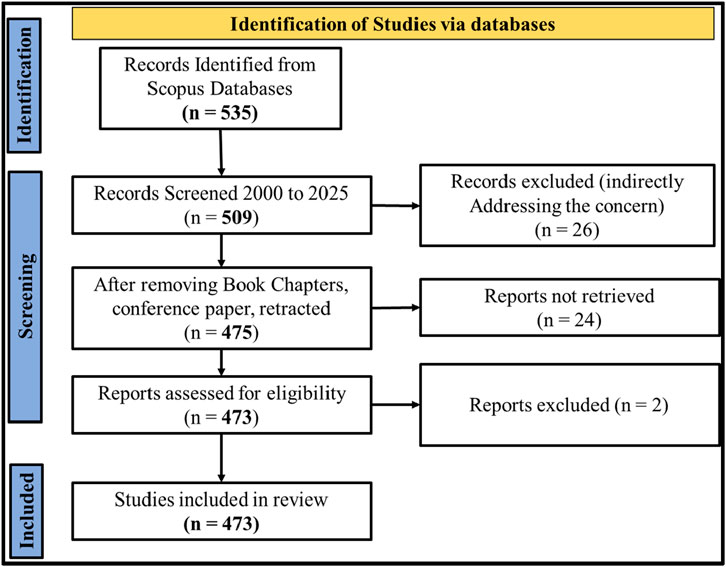
Figure 1. Flow diagram showing the systematic procedure to select relevant articles for bibliographic analysis.
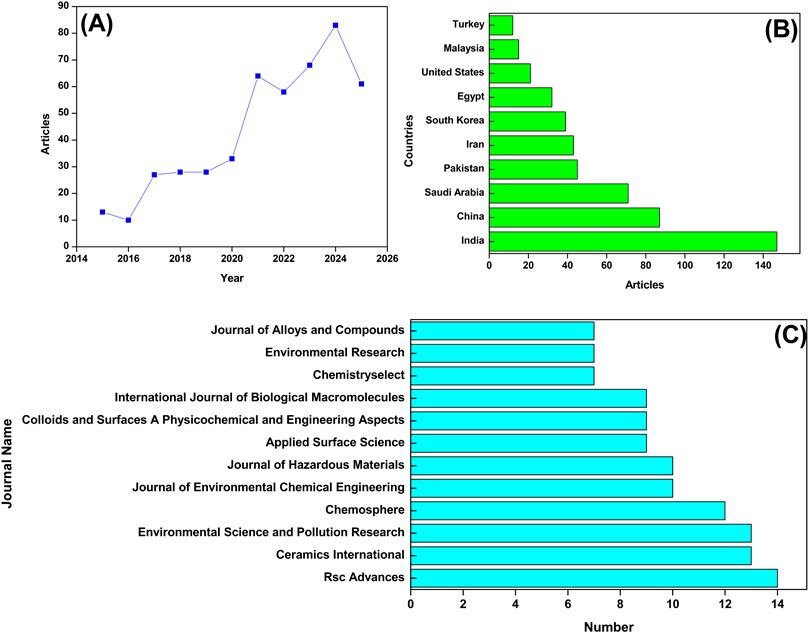
Figure 2. Bibliometric analysis focuses on the research trends in the area of ZnO NPs for the treatment of water contaminated with dye and organic pollutants. (A) Article trends between January 2015 and June 2025, (B) Articles from the top 10 countries, and (C) Top 10 journals.
Geographic contributions are shown in Figure 2B, indicating that the research in this field shows strong global participation, with major contributions from India, China, Saudi Arabia, Pakistan, Iran, South Korea, and several others, clearly reflecting a shared international effort and growing interest in tackling these important issues. The top 5 key publishing journals included RSC Advances, Ceramics International, Environmental Science and Pollution Research, Chemosphere, and Journal of Environmental Chemical Engineering (Figure 2C). These publications clearly indicate the growing significance, high impact, and global recognition of research in environmental sustainability and material science that highlights their interdisciplinary nature.
The bibliographic analysis revealed that researchers are showing increasing interest in the use of ZnO NPs for the treatment of wastewater, particularly in removing dyes and organic pollutants, highlighting their promising role in sustainable water purification technologies. Studies from various countries and research groups highlight the promising potential of ZnO NPs in environmental remediation, particularly due to their photocatalytic properties and versatility in different forms such as pure, doped, immobilized, and biosynthesized NPs. This expanding interest underscores the importance of understanding the underlying processes by which ZnO NPs interact with and break down harmful organic compounds. Hence, in the next section, the mechanism of ZnO NP degradation and how it works to make water treatment more effective are discussed.
3 Different techniques for the degradation of organic pollutants
Conventional physical and chemical techniques like physical separation, oxidation, coagulation, and adsorption have been used extensively to remove a variety of dyes from industrial wastewaters (Chowdhury and Viraraghavan, 2009). However, these processes cannot effectively treat a broad range of wastewater containing dyes, and they are very expensive treatments (Teixeira Tarley and Zezzi Arruda, 2004). Adsorption and oxidation are the two most convenient technologies for the removal of dyes from effluents. Oxidation methods are still a better technique as this process eliminates organic carbons. Photodegradation techniques adopted by industries for the treatment of dyes or organic contamination from water have been extensively explored because of their advantages of speedy oxidation of organic pollutants and less or no formation of secondary polycyclic products (Sobana et al., 2006). Physical methods like flocculation, coagulation, reverse osmosis, and adsorption are sometimes unable to biodegrade the dyes; rather, they only change the phase of the contaminants from one to another or remove suspended particles and some aggregates of dyes. However, the removal of soluble or permanent dyes from water is not achieved with high efficiency (Akyol et al., 2004; Ledakowicz et al., 2001). Chemical treatments with ozone or chlorine produce better results, but these methods releases mutagenic and carcinogenic by-products and are not economically feasible (Robinson et al., 2001; Kunkler et al., 2005) Several other methods of chemical treatment and filtration methods like ultrafiltration, microfiltration, nano-filtration, and reverse osmosis have been developed and are more often used in other industries, but they have high operational costs and release toxic contaminants to nearby waterbodies and in ecosystems. These methods, compared to the advanced oxidation or nanomaterials (e.g., ZnO NPs), have a lower removal efficiency but are used due to simplicity, particularly in large-scale municipal settings (Bal and Thakur, 2022). Hence, conventional methods are helpful for pre-treatment or primary removal but always require support with advanced processes such as photocatalysis or others to meet the maximum degradation of dye pollutants present in surface waters. For this purpose, many economically effective and better processes have been developed over the past decade. Advanced oxidation processes, which contain rational amounts of hydroxyl radical production, have been developed as a popular technique that affects water treatment (Glaze et al., 1987).
Photocatalysis breaks down organic pollutants, like dyes, into harmless components through a mineralization process (Almehizia et al., 2022; Lachheb et al., 2002; Tanaka et al., 2000). The removal of organic pollutants through photocatalytic technologies is considered the most important and cleanest chemical technique, due to their environmental friendliness.
Semiconductor photocatalysts have sparked considerable interest in providing solutions to the water pollution problem. This technology can efficiently degrade organic pollutants that have stable organic structures, as such pollutants are difficult to decompose. Process involves the use of SnO2, ZnO, and TiO2, which are semiconductors and are inexpensive, non-toxic, and largely available as photo catalysts. The effectiveness of semiconductors as photocatalysts depends on how well the wavelengths in which the emission wavelengths are used match the stimuli of gang splitting in the semiconductor. Heterogeneous photocatalytic oxidation processes are the target methods of nanotechnology and are currently under investigation (Ahmad et al., 2015; Meng et al., 2014; Meng and Ugaz, 2015). In recent decades, the use of ZnO as a photocatalyst for the decomposition of dyes in wastewater has been a more effective catalyst than other semiconductors (Daneshvar et al., 2004; Gouvêa et al., 2000; Lizama et al., 2002). Because of the similar energy bandgap of 3.37 eV for ZnO compared to TiO2, its photocatalytic behavior is well matched with that of TiO2(Chun and Yizhong, 1999). Many studies have confirmed that ZnO has better efficiency than photocatalyst TiO2, which dismantles several dyes, even in aqueous solutions (Sharma et al., 2017; Daneshvar et al., 2004). ZnO is an inexpensive catalyst. These nano-catalysts have been developed as important therapeutic tools in many environmental applications (Danwittayakul et al., 2015). Some of the transition metals, such as Cu2+, Mn2+, and Fe2+, have also been added to ZnO catalysts, indicating improved reactions in photocatalytic decomposition.
4 Mechanisms of how ZnO NPs degrade organic pollutants
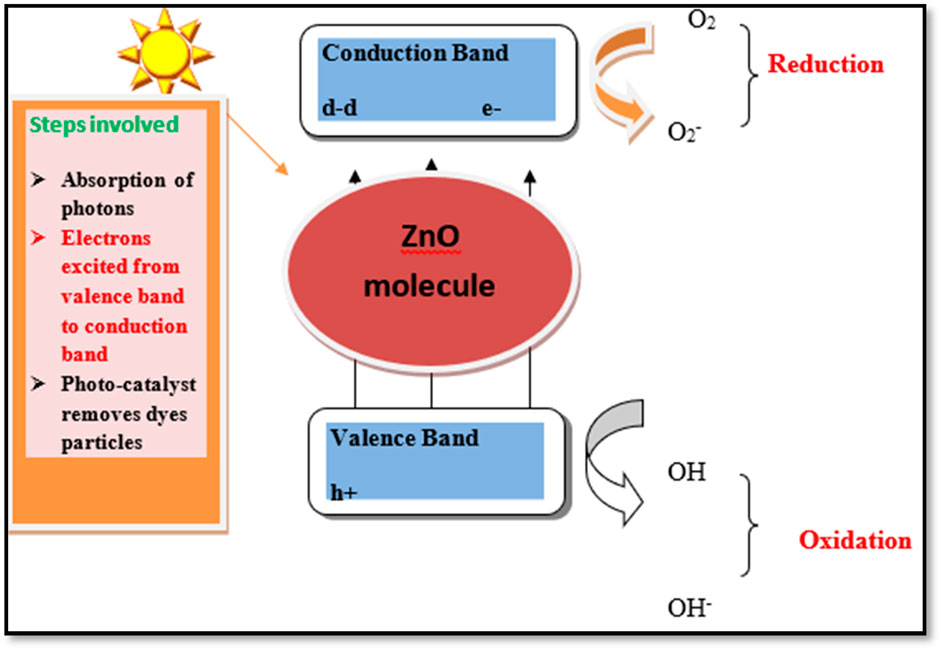
Photocatalysis is the mechanism that accelerates photoreaction in the presence of sunlight and a catalyst. The induction of light or absorption of photons excites the electron (e−) from the valence band to the conduction band of the semiconductor catalyst, creating a hole in the valence band. Thus, the radicals or electrons produced by the photo-generated catalyst remove the dye radicals and undergo chemical reduction to produce a superoxide radical anion (O2−). This type of reaction can be stimulated by the absorption of light with sufficient energy (Abou Zeid and Leprince-Wang, 2024; Sharma et al., 2016). At room temperature, ZnO has a significantly large direct band gap width of 3.37 eV, a large-scale proposed binding energy of 60 MEV, and deep purple/boundary-ultraviolet absorption (Figure 3). The various steps involved during the degradation of organic pollutants are (Herrmann, 1999) as follows:
a. Absorption of photons: Photons are induced in ZnO by solar light with photonic energy equal to hv, which is equal to or greater than the excitation energy. As a result, electrons from the lower energy-filled valence band are shifted and excited to a higher energy conduction band. The electron–hole pairs are produced due to the photo-induced process. The electron–hole pair migrates to the surface of ZnO and is involved in different oxidation and reduction reactions.
b. Reactive species generation: The hydrogen reacts with water molecules and hydroxide ions to produce hydroxyl radicals, and the electron reacts with oxygen to produce superoxide radical anions, and then finally, hydrogen peroxide (H2O2). H2O2 reacts with the superoxide radicals and forms hydroxyl radicals (OH• and OH−).
c. Degradation of pollutants: These hydroxyl radicals are strong oxidizing agents that react with organic pollutants that are adsorbed on the surface of ZnO and produce intermediate compounds that further change into harmless green compounds like water (H2O) and carbon dioxide (CO2) molecules (Rajamanickam and Shanthi, 2016).
ZnO, a highly versatile nanomaterial, can easily be manufactured in various structures such as nanorods, nanotubes, nanoplates, nanopellets, nanowires, and nanoflowers (Figure 4) (Kamal et al., 2023; Lee et al., 2023). This versatility is one of the reasons that these NP play an important role in the purification of wastewater. The degradation of rhodamine B dye, methylene blue, malachite green, and clofibrate acid in wastewater is from 80% to 98% by using photocatalytic ZnO nanomaterials (Al-Baghdadi et al., 2022; Bhapkar et al., 2023; Dodoo-Arhin et al., 2021; Mulmi et al., 2022; Zailan et al., 2020). Researchers are exploring the potential of various forms of ZnO nanostructures, like pure, doped, immobilized, and biosynthesized, along with different structures such as nanorods, nanotubes, nanoplates, nanopellets, nanowires, and nanoflowers (Kamal et al., 2023; Lee et al., 2023).
5 Degradation of dyes and organic pollutants from surface wastewater using different types of ZnO NPs
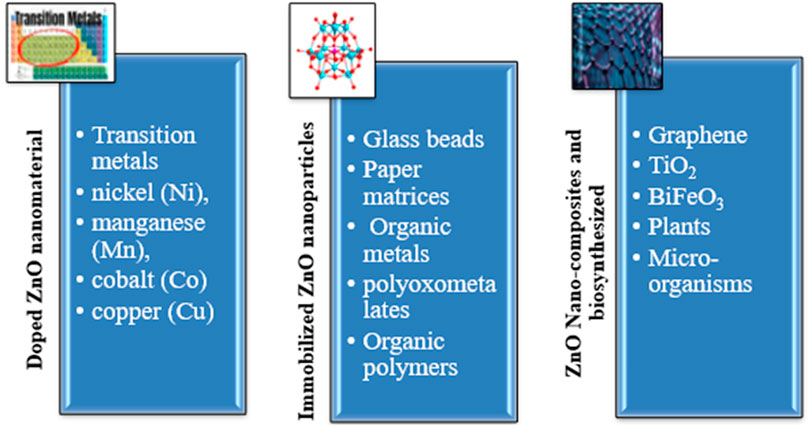
Recent advancements in the use of ZnO nanomaterials as photocatalysts have many advanced structures, including its pure form, doping, an immobilized form, and biosynthesized (Figure 5).
5.1 Pure ZnO NPs
Pure ZnO NPs are nanomaterials composed of zinc and oxygen atoms only. These pure ZnO NPs carry a wide band gap (approximately 3.2–3.3 eV) and provide a large surface area-to-volume ratio because of their nanoscale size. These nanomaterials have powerful photocatalytic properties, making them applicable for the degradation of organic pollutants in wastewater and other environmental remediation techniques by breaking complex organic polluting compounds into simpler non-toxic materials like water and carbon dioxide. In addition, pure ZnO NPs are cost-effective, easy to synthesize from plants and microorganisms, and biocompatible.
The photocatalytic degradation of organic pollutants like methyl orange dye was carried out by ZnO nanomaterial with a size of 200–250 nm under UV radiation. Using hydrothermal parameters, 200–250 nm sized ZnO NPs can be reused for several photocatalytic processes (Bazrafshan et al., 2019). Crystal violet dye can be removed from wastewater by ZnO NPs synthesized by decomposition of zinc acetate NPs (Franco et al., 2019). The Reactive Black 5 (RB5) azo dye is removed by ZnO nanoparticles under UV and Photo Fenton (FeSO4.7H2O/UV/H2O2) using heterogeneous advanced oxidation processes (Hassaan et al., 2019). Recently, researchers have worked on the degradation of rhodamine B dye, methylene blue, malachite green, and clofibrate acid in industrial wastewater using pure ZnO nanomaterials and found the highest degradation efficiency of 80%–98% in 45 min–120 min (Al-Baghdadi et al., 2022; Bhapkar et al., 2023; Dodoo-Arhin et al., 2021; Mulmi et al., 2022; Zailan et al., 2020).
5.2 Doped ZnO NPs
Doped NPs are generated by adding various transition metals like nickel (Ni), manganese (Mn), cobalt (Co), and copper (Cu) to undoped or pure ZnO nanomaterials that alter their morphological and electronic characteristics, increasing the ability of ZnO nanomaterials to degrade organic pollutants (Figure 6). For example, Ni-doped ZnO nanomaterials, Mn-doped ZnO NPs, and Co-doped nanomaterials have been shown to increase surface area, crystallite size, and band gap, making ZnO nanomaterials more effective in degrading organic dyes for environmental remediation. Moreover, the doped ZnO NPs enhance biocompatibility and antimicrobial efficacy. For example, Co-doped ZnO NPs and Ag-doped ZnO nanomaterials have shown increased antibacterial activity against Escherichia coli and Vibrio cholera pathogens present in wastewater. Ag-doped ZnO NPs under UV radiation degrade Direct Blue 15 dye and methylene blue up to 75% under ultraviolet and solar irradiation, and Cu-doped ZnO NPs degrade the dye with an efficiency of 70% (Ebrahimi et al., 2019a; Rodrigues et al., 2020).
The enhanced photocatalytic activity of doped ZnO is due to the transfer of photo-generated electrons from the conduction band of ZnO to the Ag Fermi level, which in turn, controls electron–hole recombination and also increases the surface plasmon resonance of transition metals for efficiently degrading under UV and solar irradiation (Abdel Messih et al., 2019; Abdel-Aziz et al., 2020). Doped ZnO nanorods have also been examined for the detection of different molecules of dyes like rhodamine 6G and pesticides like methyl parathion, so that water pollution can be better controlled (AlSalhi et al., 2023; Huo et al., 2021). Rates of 80% decomposition of GRL dyes, 86% degradation of methylene blue dye, and 70% of methyl orange and Direct Blue 15 dye were observed in ZnO/Fe2O3 (0.5 g and 1 g FeCl3) nanocomposites (Dhir, 2020; Ebrahimi et al., 2019b; Hashim et al., 2019; Prabakaran and Pillay, 2019). Methylene blue dye and BG dye can also be degraded by gold-doped ZnO NPs and Mn-doped ZnO, respectively, through photocatalytic degradation up to 90% (AlSalhi et al., 2023; Bekru et al., 2023; Bembibre et al., 2022; Ebrahimi et al., 2019a; Nguyen et al., 2022; Nithya et al., 2020; Selvaraj et al., 2022; Shkir et al., 2022). A 95% removal rate of methyl orange (40 mg/L) from wastewater within 45 min was achieved by using Cu-Pd doped ZnO nanocomposites with Cu-Pd 0.5 wt. percent (Sun et al., 2023). Photocatalytic degradation of Reactive Black 5 (RB5) and cefazolin up to 90% has been reported within 120 min (Ouriques Brasileiro et al., 2023).
5.3 Immobilized ZnO NPs
Immobilized ZnO NPs fixed onto solid surfaces or within matrices that enhance their performance, stability, and reusability in managing organic pollutants in wastewater are a promising advancement. Immobilization helps separate compounds, prevents nanoparticle aggregation, and reduces toxicity in water. Pure ZnO NPs immobilized on many substrates, like glass beads and paper matrices, demonstrated significant antimicrobial activity against pathogens like E. coli and showed effective photocatalytic degradation of certain contaminants like acetaminophen when exposed to visible light. For example, azo dyes are very difficult to remove from wastewater. Researchers studied a UV advanced oxidation process in which ZnO acts as a catalyst and is immobilized on a stone surface, where it then helps to degrade acid red 18 dye (Mahdizadeh et al., 2020). The immobilized zinc oxide performs three times better than pure ZnO NPs under UV light (Durmus et al., 2019). The degradation of methyl orange under UV radiation was 100% within 150 min, and the degradation of Congo red was 90% within 40 min, whereas pollutants like aniline and formaldehyde were degraded by 95% and 79%, respectively, using ZnO-Pb (Mano et al., 2020; Ranjithkumar et al., 2023; Wang et al., 2019).
5.4 Biosynthesized ZnO NPs
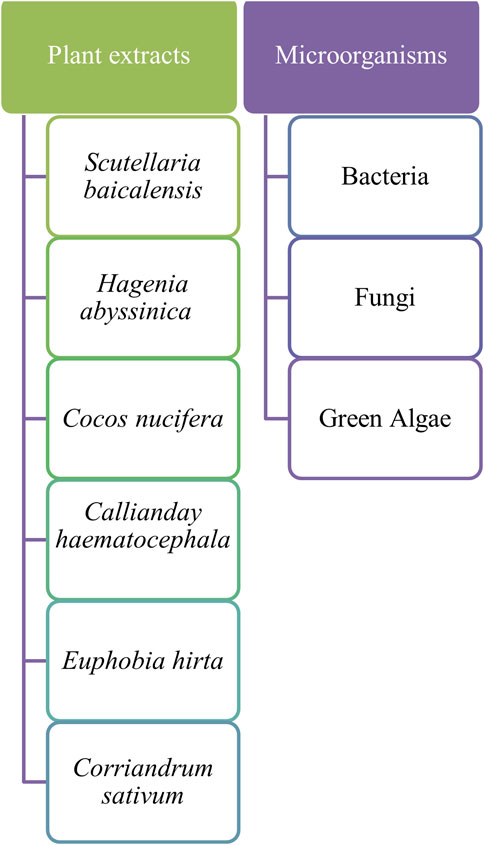
Biosynthesized ZnO NPs possess antibacterial activity against pathogens, are biocompatible, sustainable, and non-toxic, and work even at low concentrations, making them suitable for the remediation of organic pollutants in wastewater, agriculture, and photocatalytic degradation of environmental pollutants. These nanomaterials are also a cost effective, eco-friendly, and greener method for treating wastewater. A variety of plant extracts and microorganisms are reported to act as reducing agents and help in synthesizing ZnO NPs. ZnO NPs from microorganisms like bacteria, fungi, and green algae have the potential to degrade pathogens like Aspergillus niger, Bacillus subtilis, Escherichia coli, and Staphylococcus aureus (Figure 7). Plant extracts contain many phytochemicals like flavonoids, terpenoids, alkaloids, and polyphenols that function as reducing and stabilizing agents during nanoparticle synthesis. During the process of synthesizing ZnO NPs from plant extracts, a zinc precursor like zinc acetate is added to the plant extract of Cocos nucifera and a root extract of Scutellaria baicalensis. Zn2+ ions are reduced to ZnO NPs due to the action of aromatic hydroxyl groups of photochemicals (Anjum et al., 2017). Many organic pollutants and dyes like methyl orange and azo dyes discharged from industries like leather, paper, and textiles are degraded by biosynthesized ZnO by using a leaf extract of Hagenia abyssinica and Calliandra haematocephala, with an efficiency of 85% within 120–270 min (Vinayagam et al., 2020; Zewde and Geremew, 2022). Biosynthesized ZnO nanomaterials prepared from the extract of Corriandrum sativum, Viscum album, Synadenium grantii, and Trigonella foenum-graecum plants photodegrade organic pollutants like methylene blue, indigo carmine, rhodamine B, tetracycline, Congo red, and biphenyl A in good amounts up to 90% in a short period of time (Alshehri and Malik, 2019; Basit et al., 2023; Rafique et al., 2022; Sayadi et al., 2022).
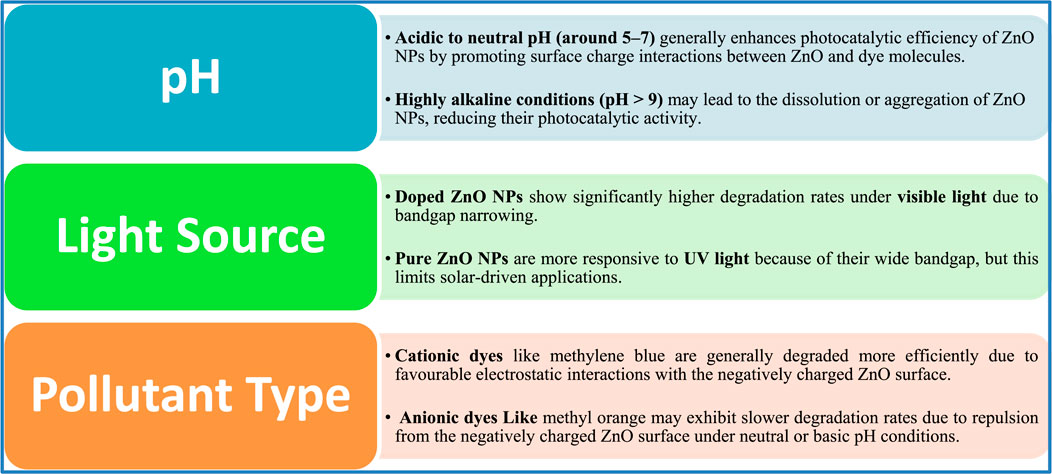
The photocatalytic performance of ZnO NPs is significantly influenced by parameters such as pH, light source, and pollutant type (Figure 8). Under acidic to neutral pH conditions (approximately 5–7), ZnO NPs exhibit increased degradation efficiency due to improved surface charge interactions with dye molecules (Mohamadpour and Amani, 2024). However, in highly alkaline environments (pH > 9), ZnO may aggregate or partially dissolve, which reduces its catalytic activity. Regarding the light source, pure ZnO performs optimally under UV light due to its wide bandgap (∼3.2 eV), while doping ZnO NPs, such as Ag-ZnO or N-ZnO, demonstrates superior efficiency under visible light owing to bandgap narrowing (Zhang et al., 2022). The type of pollutant also plays a crucial role; cationic dyes like methylene blue generally undergo faster degradation due to favorable electrostatic attraction to the ZnO surface. In contrast, anionic dyes such as methyl orange may degrade more slowly, particularly under neutral or alkaline conditions, because of repulsion from the negatively charged ZnO surface (Halim et al., 2025). These interdependent factors highlight the need to optimize conditions for maximum efficiency in real-world wastewater treatment scenarios.
6 Environmental consequences and regulatory considerations due to the use of ZnO NPs
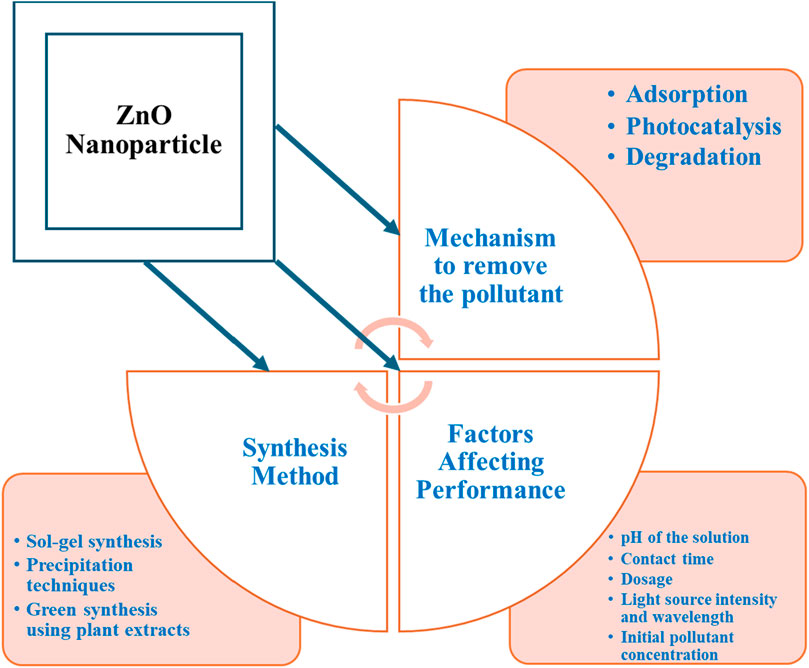
While zinc oxide NPs are considered relatively safe, there is still a risk of their causing secondary pollution or being released into the environment. ZnO NPs exhibit promising potential for wastewater treatment through mechanisms like adsorption and photocatalysis (Figure 9), where electrostatic interactions and surface complexation enable adsorption, and reactive oxygen species generated under UV or visible light degrade complex organic molecules into smaller, less harmful compounds or mineralize them into CO2 and H2O. The synthesis method, including sol–gel, hydrothermal, or precipitation techniques, influences particle size, morphology, and surface area, impacting treatment efficiency (Figure 9). Factors like pH, contact time, nanoparticle dosage, light intensity, and initial pollutant concentration also affect performance (Figure 9). While ZnO NPs show great potential for pollutant removal, their release into the environment raises concerns about toxicity to aquatic life, persistence, and bioaccumulation, highlighting the need for careful consideration of their environmental implications and sustainable use.
Although ZnO nanoparticles are widely researched and used as effective for degrading different types of pollutants in wastewater, their uncontrolled release into an aquatic system can cause serious environmental and ecological risks. When these particles are released in water bodies, they interact with the flora and fauna of the system, like microorganisms, phytoplankton, algae, and small aquatic animals, leading to oxidative stress, cell or membrane damage, or the death of cells or tissues due to the generation of reactive oxygen species. In addition, their small size enables them to penetrate deep into membranes, disrupting normal physiological functions. Over time, if not properly recovered, these ZnO NPs have the potential to accumulate in the aquatic food chain and food web, raising deep concerns about long-term toxicity and bioaccumulation in organisms, including fish, amphibians, and reptiles. In comparison, the traditional techniques like coagulation and flocculation are safer for the environment, ecosystems, flora, and fauna but are less effective due to their capacity to remove only suspended solids and create chemical sludge without thoroughly degrading complex organic pollutants and dyes.
To assess the environmental sustainability of ZnO NPs from manufacturing to disposal, it is important to assess their life cycle. Conventional synthesis, like sol-gel, precipitation, and hydrothermal methods, demands extensive energy and requires high temperatures, in addition to producing chemical waste that must be disposed of. Whereas green synthesis utilizes low-energy footprints via plant extracts or microorganisms, is less toxic, and creates minimal waste, making it more environmentally friendly. During the end-of-life stage, ZnO NPs, when dissolved or agglomerated in an aquatic environment, can break down, clump together, or change their form, which may affect their toxicity or mobility. Risks of ecotoxicological effects to ecosystems may occur through the accumulation of these particles in sediments or due to their uptake by aquatic organisms when particles persist or build up in the environment. To mitigate these risks, it is essential to develop and implement proper waste management strategies, such as capturing or recycling these particles. However, these approaches are still being researched, and standardized protocols are needed.
Hence, there is a need for a proper framework for engineered nanomaterials to ensure safe production, usage, and disposal. The regulatory framework in Europe (Registration, Evaluation, Authorization and Restriction of Chemicals (REACH)) and the Environmental Protection Agency (EPA) under the Toxic Substances Control Act (TSCA) in the United States provide some safe handling measures and mandates of reporting production volume, use patterns, etc. (Chávez-Hernández et al., 2024). The European Union’s REACH framework mandates detailed registration and safety assessments for nanoforms, focusing on toxicity, fate, and exposure. In the United States, the EPA regulates nanoscale materials under TSCA, requiring pre-manufacture notices and risk evaluations. However, existing frameworks often treat nanomaterials like conventional chemicals, not considering nano-specific risks such as size-dependent toxicity, environmental persistence, and bioavailability. There is a need to propose and develop nano-specific regulations based on the physical characteristics of NPs, toxicity test standards, and environmental release guidelines, especially for those that are directly discharged into aquatic systems.
7 Recovery and reusability
The advantages of using ZnO nanomaterials technology to mitigate water pollution are low cost, greater sustainability, high efficiency, non-polluting, and eco-friendliness. ZnO nanomaterials can be produced in greater amounts, reused many times, and synthesized easily by adopting methods like sol–gel, precipitation, and hydrothermal methods through different plants and microorganisms. The reusability and recovery of ZnO-based arrangements are important for environmental and economic sustainability. There are several methods used to reuse and recover ZnO NPs, like coating onto silica, glass beads, or ceramics, or by the incorporation of NP with Fe3O4, etc., for magnetic separation after wastewater treatment. Membrane integration is a method in which NPs embedded into nanocomposite membranes permit both photocatalysis and filtration, reducing the loss of nanomaterials. There are certain limitations to these technologies, such as specific temperature, pH, and types of pollutants present in wastewater. Future research may include the study of various factors that affect the photocatalytic activity of ZnO NPs. It will be important to explore more of the potential of ZnO NPs for large-scale mitigation of pollutants present in wastewater. Studies are also underway to test the toxicity of ZnO NPs for humans and the environment.
8 Challenges and limitations
Despite the promising photocatalytic potential for removing organic pollutants and dyes from surface wastewater, several limitations and challenges pose practical concerns and hinder the large-scale application of ZnO NPs. One major limitation is the reduced efficiency of ZnO NPs under visible light due to the wide bandgap, which restricts photocatalytic activation mostly to the ultraviolet range. Furthermore, sometimes the release of free ZnO NPs into nearby aquatic systems causes concerns about nanotoxicity, bioaccumulation in aquatic plants and animals, and ecological disruption. The rate of recovery of ZnO NPs remains inconsistent or sometimes becomes inefficient due to particle aggregation, less activity over cycles, and a lack of advanced immobilization techniques that also add complexity and cost. Other challenges and limitations are the difference between laboratory studies and real wastewater research. Laboratory studies focus on single or simple pollutants, while real surface wastewater contains complex mixtures of dyes, heavy metals, organic pollutants, and pharmaceuticals, requiring more comprehensive assessments. These challenges must be addressed properly for this advanced technology to be reliable and environmentally safe.
Photocatalytic NPs like ZnO offer high efficiency in degrading organic pollutants, especially under UV or visible light. However, this performance comes with environmental trade-offs. Free nanoparticles may leach into aquatic ecosystems, potentially causing toxicity to non-target organisms such as algae, fish, and beneficial microbes. Additionally, smaller particle sizes increase reactivity but also increase mobility and bioavailability, raising ecological concerns. While doping and surface modifications improve degradation efficiency, they may also alter toxicity profiles, making risk assessment more complex. Immobilized or composite-based systems reduce release risks but may compromise photocatalytic activity or regeneration potential. Thus, balancing performance with environmental safety remains a critical research challenge.
9 Future scope
There is a good scope to work in the area of ZnO-based technologies that focus on several areas that enhance their performance and sustainability. A major focus in the future should be on enhancing the visible light photocatalytic efficiency of ZnO nanoparticles due to their wide bandgap. Future technologies should aim for at least a three-fold increase in visible light absorption, targeting more than 85% degradation of certain organic pollutants and dyes within 60–90 min. Improvement of solar utilization is the other area where development of visible light-activated ZnO through doping with elements like nitrogen, silver, or iron is important, and one of the important future scopes in the area of treating surface water with ZnONPs. Improvement in charge separation and photocatalytic efficiency is a field that requires research, in which hybrid and composite materials will be tested and created with other semiconductor materials. To reduce the environmental impact and increase biocompatibility, work on the green synthesis approaches for extraction is also an important perspective. In future development, the biosynthesis of ZnO NPs using microorganisms or plant extracts should be standardized, with a standard level and benchmark for the size of NPs and zeta potential to ensure uniformity and consistency across batches.
Beyond laboratory studies, more pilot studies should be conducted for the industrial-level implementation of ZnO NPs, where more than 1000 L/day wastewater should be treated across varied seasons to reveal the challenges of complexity and operational costs. The integration of regulatory guidelines and environmental safety protocols is very important. Related to this, a comprehensive life cycle assessment that covers sourcing of raw material and nanoparticle synthesis, usage, recovery, reuse, and disposal should also be standardized in future studies for large-scale adoption and commercialization and to ensure the sustainability of ZnO NPs.
To tackle the environmental consequences, especially due to the emission of secondary pollutants directly into the environment, researchers are working on ways to recover and reuse these particles, such as using magnetic separation or immobilization on surfaces. Thorough sustainability evaluation studies are necessary to evaluate the environmental effects of ZnO NPs. Additionally, smart delivery systems that activate under specific conditions can improve selectivity and reduce environmental effects. Working on these areas can make NPs more sustainable and can lead to more efficient, environmentally friendly, and targeted solutions for various challenges.
10 Conclusion
In conclusion, ZnO NPs have emerged as a promising solution for mitigating surface water pollution, offering a sustainable strategy for climate resilience. Their photocatalytic properties enable effective degradation of organic pollutants, providing a novel approach for water remediation. This article reviewed the latest developments of ZnO NPs based removal of organic pollutants. Removal of various dyes and antibiotics has been summarized using ZnO NPs prepared by different methods. ZnO NPs can be used pure, doped with other components, immobilized, and biosynthesized for the purification of water. ZnO NPs offer a versatile approach to modify their structural, functional, and optical properties and enhance their suitability for large-scale water treatment and a wide range of other applications, environmental remediation, biomedical uses, and gas sensing. Ongoing research continues to address the research gaps and explore the new structures and complete potential of ZnO NPs in many other technological domains to develop as a sustainable, non-toxic, and efficient solution for mitigating water pollutants.
ZnO NPs represent a promising method for the removal of organic pollutants and dyes from surface wastewater. Continued research and innovation in synthesis methods, application strategies, and environmental safety assessments are essential to fully realize their potential in sustainable water treatment technologies. Using the influence of nanotechnology to work toward a more sustainable future is important, and the chances to provide clean water for generations to come will be bright.
Author contributions
NK: Investigation, Writing – original draft, Visualization, Formal Analysis. MB: Supervision, Methodology, Conceptualization, Validation, Writing – review and editing. PK: Validation, Supervision, Conceptualization, Writing – review and editing. KK: Formal Analysis, Writing – original draft, Visualization, Investigation. AA: Supervision, Writing – review and editing, Conceptualization, Validation, Project administration, Funding acquisition. SN: Writing – review and editing, Conceptualization, Validation. SD: Writing – review and editing, Validation, Conceptualization. AL: Funding acquisition, Writing – review and editing, Resources, Project administration.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abdel Messih, M. F., Ahmed, M. A., Soltan, A., and Anis, S. S. (2019). Synthesis and characterization of novel Ag/ZnO nanoparticles for photocatalytic degradation of methylene blue under UV and solar irradiation. J. Phys. Chem. Solids 135, 109086. doi:10.1016/j.jpcs.2019.109086
Abdel-Aziz, R., Ahmed, M. A., and Abdel-Messih, M. F. (2020). A novel UV and visible light driven photocatalyst AgIO4/ZnO nanoparticles with highly enhanced photocatalytic performance for removal of rhodamine B and indigo carmine dyes. J. Photochem. Photobiol. A Chem. 389, 112245. doi:10.1016/j.jphotochem.2019.112245
Abou Zeid, S., and Leprince-Wang, Y. (2024). Advancements in ZnO-based photocatalysts for water treatment: a comprehensive review. Crystals 14 (7), 611. doi:10.3390/cryst14070611
Ahmad, A., Mohd-Setapar, S. H., Chuong, C. S., Khatoon, A., Wani, W. A., Kumar, R., et al. (2015). Recent advances in new generation dye removal technologies: novel search for approaches to reprocess wastewater. RSC Adv. 5 (39), 30801–30818. doi:10.1039/C4RA16959J
Akyol, A., Yatmaz, H. C., and Bayramoglu, M. (2004). Photocatalytic decolorization of Remazol Red RR in aqueous ZnO suspensions. Appl. Catal. B Environ. 54 (1), 19–24. doi:10.1016/j.apcatb.2004.05.021
Al-Baghdadi, S. B., Abdullah, H. I., and Al-Amiery, A. A. (2022). Synthesis of ZnO nanoparticles for photodegradation of clofibrate acid as organic pollutant. Int. J. Health Sci., 3124–3131. doi:10.53730/ijhs.v6nS4.10000
Alegbe, E. O., and Uthman, T. O. (2024). A review of history, properties, classification, applications and challenges of natural and synthetic dyes. Heliyon 10 (13), e33646. doi:10.1016/j.heliyon.2024.e33646
Almehizia, A. A., Al-Omar, M. A., Naglah, A. M., Bhat, M. A., and Al-Shakliah, N. S. (2022). Facile synthesis and characterization of ZnO nanoparticles for studying their biological activities and photocatalytic degradation properties toward methylene blue dye. Alexandria Eng. J. 61 (3), 2386–2395. doi:10.1016/j.aej.2021.06.102
AlSalhi, M. S., Devanesan, S., Asemi, N., and Ahamed, A. (2023). Concurrent fabrication of ZnO–ZnFe2O4 hybrid nanocomposite for enhancing photocatalytic degradation of organic pollutants and its bacterial inactivation. Chemosphere 318, 137928. doi:10.1016/j.chemosphere.2023.137928
Alshehri, A. A., and Malik, M. A. (2019). Biogenic fabrication of ZnO nanoparticles using Trigonella foenum-graecum (Fenugreek) for proficient photocatalytic degradation of methylene blue under UV irradiation. J. Mater. Sci. Mater. Electron. 30 (17), 16156–16173. doi:10.1007/s10854-019-01985-8
Anjum, M., Kumar, R., and Barakat, M. A. (2017). Visible light driven photocatalytic degradation of organic pollutants in wastewater and real sludge using ZnO–ZnS/Ag 2 O–Ag 2 S nanocomposite. J. Taiwan Inst. Chem. Eng. 77, 227–235. doi:10.1016/j.jtice.2017.05.007
Awasthi, A., Wu, B. S., Liu, C. N., Chen, C. W., Uang, S. N., and Tsai, C. J. (2013). The effect of nanoparticle morphology on the measurement accuracy of mobility particle sizers. Mapan 28, 205–215. doi:10.1007/s12647-013-0068-7
Azanaw, A., Birlie, B., Teshome, B., and Jemberie, M. (2022). Textile effluent treatment methods and eco-friendly resolution of textile wastewater. Case Stud. Chem. Environ. Eng. 6, 100230. doi:10.1016/j.cscee.2022.100230
Bal, G., and Thakur, A. (2022). Distinct approaches of removal of dyes from wastewater: a review. Mater. Today Proc. 50, 1575–1579. doi:10.1016/j.matpr.2021.09.119
Basit, R. A., Abbasi, Z., Hafeez, M., Ahmad, P., Khan, J., Khandaker, M. U., et al. (2023). Successive photocatalytic degradation of methylene blue by ZnO, CuO and ZnO/CuO synthesized from coriandrum sativum plant extract via green synthesis technique. Crystals 13 (2), 281. doi:10.3390/cryst13020281
Bazrafshan, E., Al-Musawi, T. J., Silva, M. F., Panahi, A. H., Havangi, M., and Mostafapur, F. K. (2019). Photocatalytic degradation of catechol using ZnO nanoparticles as catalyst: optimizing the experimental parameters using the Box-Behnken statistical methodology and kinetic studies. Microchem. J. 147, 643–653. doi:10.1016/j.microc.2019.03.078
Bekru, A. G., Tufa, L. T., Zelekew, O. A., Gwak, J., Lee, J., and Sabir, F. K. (2023). Microwave-Assisted synthesis of rGO-ZnO/CuO nanocomposites for photocatalytic degradation of organic pollutants. Crystals 13 (1), 133. doi:10.3390/cryst13010133
Bembibre, A., Benamara, M., Hjiri, M., Gómez, E., Alamri, H. R., Dhahri, R., et al. (2022). Visible-light driven sonophotocatalytic removal of tetracycline using Ca-doped ZnO nanoparticles. Chem. Eng. J. 427, 132006. doi:10.1016/j.cej.2021.132006
Berradi, M., Hsissou, R., Khudhair, M., Assouag, M., Cherkaoui, O., El Bachiri, A., et al. (2019). Textile finishing dyes and their impact on aquatic environs. Heliyon 5 (11), e02711. doi:10.1016/j.heliyon.2019.e02711
Bhapkar, A., Prasad, R., Jaspal, D., Shirolkar, M., Gheisari, Kh., and Bhame, S. (2023). Visible light driven photocatalytic degradation of methylene blue by ZnO nanostructures synthesized by glycine nitrate auto combustion route. Inorg. Chem. Commun. 148, 110311. doi:10.1016/j.inoche.2022.110311
Chávez-Hernández, J. A., Velarde-Salcedo, A. J., Navarro-Tovar, G., and Gonzalez, C. (2024). Safe nanomaterials: from their use, application, and disposal to regulations. Nanoscale Adv. 6 (6), 1583–1610. doi:10.1039/D3NA01097J
Chowdhury, P., and Viraraghavan, T. (2009). Sonochemical degradation of chlorinated organic compounds, phenolic compounds and organic dyes – a review. Sci. Total Environ. 407 (8), 2474–2492. doi:10.1016/j.scitotenv.2008.12.031
Chun, H., and Yizhong, W. (1999). Decolorization and biodegradability of photocatalytic treated azo dyes and wool textile wastewater. Chemosphere 39 (12), 2107–2115. doi:10.1016/S0045-6535(99)00118-6
Daneshvar, N., Salari, D., and Khataee, A. R. (2004). Photocatalytic degradation of azo dye acid red 14 in water on ZnO as an alternative catalyst to TiO2. J. Photochem. Photobiol. A Chem. 162 (2–3), 317–322. doi:10.1016/S1010-6030(03)00378-2
Danwittayakul, S., Jaisai, M., and Dutta, J. (2015). Efficient solar photocatalytic degradation of textile wastewater using ZnO/ZTO composites. Appl. Catal. B Environ. 163, 1–8. doi:10.1016/j.apcatb.2014.07.042
Dhir, R. (2020). Photocatalytic degradation of methyl orange dye under UV irradiation in the presence of synthesized PVP capped pure and gadolinium doped ZnO nanoparticles. Chem. Phys. Lett. 746, 137302. doi:10.1016/j.cplett.2020.137302
Dodoo-Arhin, D., Asiedu, T., Agyei-Tuffour, B., Nyankson, E., Obada, D., and Mwabora, J. M. (2021). Photocatalytic degradation of Rhodamine dyes using zinc oxide nanoparticles. Mater. Today Proc. 38, 809–815. doi:10.1016/j.matpr.2020.04.597
Durmus, Z., Kurt, Z. B., and Durmus, A. (2019). A. Synthesis and Characterization of Graphene Oxide/Zinc Oxide (GO/ZnO) Nanocomposite and its Utilization for Photocatalytic Degradation of Basic Fuchsin Dye. Chemistry Select, 4, 271. doi:10.1002/slct.201803635
Ebrahimi, R., Hossienzadeh, K., Maleki, A., Ghanbari, R., Rezaee, R., Safari, M., et al. (2019a). Effects of doping zinc oxide nanoparticles with transition metals (Ag, Cu, Mn) on photocatalytic degradation of Direct Blue 15 dye under UV and visible light irradiation. J. Environ. Health Sci. Eng. 17 (1), 479–492. doi:10.1007/s40201-019-00366-x
Ebrahimi, R., Maleki, A., Zandsalimi, Y., Ghanbari, R., Shahmoradi, B., Rezaee, R., et al. (2019b). Photocatalytic degradation of organic dyes using WO3-doped ZnO nanoparticles fixed on a glass surface in aqueous solution. J. Industrial Eng. Chem. 73, 297–305. doi:10.1016/j.jiec.2019.01.041
Fosso-Kankeu, E., Mittal, H., Mishra, S. B., and Mishra, A. K. (2015). Gum ghatti and acrylic acid based biodegradable hydrogels for the effective adsorption of cationic dyes. J. Industrial Eng. Chem. 22, 171–178. doi:10.1016/j.jiec.2014.07.007
Franco, P., Sacco, O., De Marco, I., and Vaiano, V. (2019). Zinc oxide nanoparticles obtained by supercritical antisolvent precipitation for the photocatalytic degradation of crystal violet dye. Catalysts 9 (4), 346. doi:10.3390/catal9040346
Glaze, W. H., Kang, J.-W., and Chapin, D. H. (1987). The chemistry of water treatment processes involving ozone, hydrogen peroxide and ultraviolet radiation. Ozone Sci. and Eng. 9 (4), 335–352. doi:10.1080/01919518708552148
Gouvêa, C. A. K., Wypych, F., Moraes, S. G., Durán, N., Nagata, N., and Peralta-Zamora, P. (2000). Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 40 (4), 433–440. doi:10.1016/S0045-6535(99)00313-6
Hachem, C., Bocquillon, F., Zahraa, O., and Bouchy, M. (2001). Decolourization of textile industry wastewater by the photocatalytic degradation process. Dyes Pigments 49 (2), 117–125. doi:10.1016/S0143-7208(01)00014-6
Halim, O. M. A., Mustapha, N. H., Fudzi, S. N. M., Azhar, R., Zanal, N. I. N., Nazua, N. F., et al. (2025). A review on modified ZnO for the effective degradation of methylene blue and rhodamine B. Results Surfaces Interfaces 18, 100408. doi:10.1016/j.rsurfi.2024.100408
Hashim, F. S., Alkaim, A. F., Mahdi, S. M., and Omran Alkhayatt, A. H. (2019). Photocatalytic degradation of GRL dye from aqueous solutions in the presence of ZnO/Fe2O3 nanocomposites. Compos. Commun. 16, 111–116. doi:10.1016/j.coco.2019.09.008
Hassaan, M., El Katory, M., Ali, R., and El Nemr, A. (2019). Photocatalytic degradation of reactive black 5 using Photo-Fenton and ZnO nanoparticles under UV irradiation. Egypt. J. Chem. 0 (0), 0. doi:10.21608/ejchem.2019.15799.1955
Herrmann, J.-M. (1999). Heterogeneous photocatalysis: fundamentals and applications to the removal of various types of aqueous pollutants. Catal. Today 53 (1), 115–129. doi:10.1016/S0920-5861(99)00107-8
Huo, D., Chen, B., Li, M., Meng, G., Lei, Y., and Zhu, C. (2021). Template-assisted fabrication of Ag-nanoparticles@ZnO-nanorods array as recyclable 3D surface enhanced Raman scattering substrate for rapid detection of trace pesticides. Nanotechnology 32 (14), 145302. doi:10.1088/1361-6528/abc50e
Ip, A. W. M., Barford, J. P., and McKay, G. (2009). Reactive Black dye adsorption/desorption onto different adsorbents: effect of salt, surface chemistry, pore size and surface area. J. Colloid Interface Sci. 337 (1), 32–38. doi:10.1016/j.jcis.2009.05.015
Islam, T., Repon, M. R., Islam, T., Sarwar, Z., and Rahman, M. M. (2023). Impact of textile dyes on health and ecosystem: a review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 30 (4), 9207–9242. doi:10.1007/s11356-022-24398-3
Kamal, A., Saba, M., Kamal, A., Batool, M., Asif, M., Al-Mohaimeed, A. M., et al. (2023). Bioinspired green synthesis of bimetallic iron and zinc oxide nanoparticles using mushroom extract and use against Aspergillus niger; the most devastating fungi of the green world. Catalysts 13 (2), 400. doi:10.3390/catal13020400
Kaur, S., Garg, T., Joshi, A., Awasthi, A., Kumar, V., and Kumar, A. (2024). Potential effects of metal oxide nanoparticles on leguminous plants: practical implications and future perspectives. Sci. Hortic. 331, 113146. doi:10.1016/j.scienta.2024.113146
Kunkler, I. H., Fielding, R. G., Brebnei, J., Prescott, R., Maclean, J. R., Cairns, J., et al. (2005). A comprehensive approach for evaluating telemedicine-delivered multidisciplinary breast cancer meetings in southern Scotland. J. Telemedicine Telecare 11 (1_Suppl. l), 71–73. doi:10.1258/1357633054461804
Lachheb, H., Puzenat, E., Houas, A., Ksibi, M., Elaloui, E., Guillard, C., et al. (2002). Photocatalytic degradation of various types of dyes (alizarin S, crocein orange G, methyl red, Congo red, methylene blue) in water by UV-irradiated titania. Appl. Catal. B Environ. 39 (1), 75–90. doi:10.1016/S0926-3373(02)00078-4
Ledakowicz, S., Solecka, M., and Zylla, R. (2001). Biodegradation, decolourisation and detoxification of textile wastewater enhanced by advanced oxidation processes. J. Biotechnol. 89 (2–3), 175–184. doi:10.1016/S0168-1656(01)00296-6
Lee, D.-E., Kim, M.-K., Danish, M., and Jo, W.-K. (2023). State-of-the-art review on photocatalysis for efficient wastewater treatment: attractive approach in photocatalyst design and parameters affecting the photocatalytic degradation. Catal. Commun. 183, 106764. doi:10.1016/j.catcom.2023.106764
Lizama, C., Freer, J., Baeza, J., and Mansilla, H. D. (2002). Optimized photodegradation of reactive blue 19 on TiO2 and ZnO suspensions. Catal. Today 76 (2–4), 235–246. doi:10.1016/S0920-5861(02)00222-5
López Cisneros, R., Gutarra Espinoza, A., and Litter, M. I. (2002). Photodegradation of an azo dye of the textile industry. Chemosphere 48 (4), 393–399. doi:10.1016/S0045-6535(02)00117-0
Mahdizadeh, H., Nasiri, A., Gharaghani, M. A., and Yazdanpanah, G. (2020). Hybrid UV/COP advanced oxidation process using ZnO as a catalyst immobilized on a stone surface for degradation of acid red 18 dye. MethodsX 7, 101118. doi:10.1016/j.mex.2020.101118
Mano, G., Harinee, S., Sridhar, S., Ashok, M., and Viswanathan, A. (2020). Microwave assisted synthesis of ZnO-PbS heterojuction for degradation of organic pollutants under visible light. Sci. Rep. 10 (1), 2224. doi:10.1038/s41598-020-59066-4
Meng, F., and Ugaz, V. M. (2015). Instantaneous physico-chemical analysis of suspension-based nanomaterials. Sci. Rep. 5 (1), 9896. doi:10.1038/srep09896
Meng, F., King, M. D., Hassan, Y. A., and Ugaz, V. M. (2014). Localized fluorescent complexation enables rapid monitoring of airborne nanoparticles. Environ. Sci. Nano 1 (4), 358. doi:10.1039/C4EN00017J
Mohamadpour, F., and Amani, A. M. (2024). Photocatalytic systems: reactions, mechanism, and applications. RSC Adv. 14 (29), 20609–20645. doi:10.1039/D4RA03259D
Mulmi, D. D., Bhattarai, R., Thapa, R. B., Koju, R., and Nakarmi, M. L. (2022). Enhanced photocatalytic degradation of organic pollutants by lysozyme-mediated zinc oxide nanoparticles. Bull. Mater. Sci. 45 (2), 75. doi:10.1007/s12034-022-02657-w
Nguyen, L. T. T., Vo, D.-V. N., Nguyen, L. T. H., Duong, A. T. T., Nguyen, H. Q., Chu, N. M., et al. (2022). Synthesis, characterization, and application of ZnFe nanoparticles for photocatalytic degradation of Rhodamine B under visible-light illumination. Environ. Technol. and Innovation 25, 102130. doi:10.1016/j.eti.2021.102130
Nithya, R., Ragupathy, S., Sakthi, D., Arun, V., and Kannadasan, N. (2020). A study on Mn doped ZnO loaded on CSAC for the photocatalytic degradation of brilliant green dye. Chem. Phys. Lett. 755, 137769. doi:10.1016/j.cplett.2020.137769
Ouriques Brasileiro, I. L., Madeira, V. S., Lopes-Moriyama, A. L., and Rodrigues de Almeida Ramalho, M. L. (2023). Addition of g-C3N4 to ZnO and ZnFe2O4 to improve photocatalytic degradation of emerging organic pollutants. Ceram. Int. 49 (3), 4449–4459. doi:10.1016/j.ceramint.2022.09.331
Prabakaran, E., and Pillay, K. (2019). Synthesis of N-doped ZnO nanoparticles with cabbage morphology as a catalyst for the efficient photocatalytic degradation of methylene blue under UV and visible light. RSC Adv. 9 (13), 7509–7535. doi:10.1039/C8RA09962F
Puri, M., Gandhi, K., and Suresh Kumar, M. (2023). A global overview of endocrine disrupting chemicals in the environment: occurrence, effects, and treatment methods. Int. J. Environ. Sci. Technol. 20 (11), 12875–12902. doi:10.1007/s13762-022-04636-4
Purkait, M. K., DasGupta, S., and De, S. (2005). Adsorption of eosin dye on activated carbon and its surfactant based desorption. J. Environ. Manag. 76 (2), 135–142. doi:10.1016/j.jenvman.2005.01.012
Rafique, M., Tahir, R., Gillani, S. S. A., Tahir, M. B., Shakil, M., Iqbal, T., et al. (2022). Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem. 102 (1), 23–38. doi:10.1080/03067319.2020.1715379
Rajamanickam, D., and Shanthi, M. (2016). Photocatalytic degradation of an organic pollutant by zinc oxide – solar process. Arabian J. Chem. 9, S1858–S1868. doi:10.1016/j.arabjc.2012.05.006
Ranjithkumar, R., Van Nguyen, C., Wong, L. S., Thiruvengadam Nandagopal, J. G., Djearamane, S., Palanisamy, G., et al. (2023). Chitosan functionalized bismuth oxychloride/zinc oxide nanocomposite for enhanced photocatalytic degradation of Congo red. Int. J. Biol. Macromol. 225, 103–111. doi:10.1016/j.ijbiomac.2022.11.302
Robinson, T., McMullan, G., Marchant, R., and Nigam, P. (2001). Remediation of dyes in textile effluent: a critical review on current treatment technologies with a proposed alternative. Bioresour. Technol. 77 (3), 247–255. doi:10.1016/S0960-8524(00)00080-8
Rodrigues, J., Hatami, T., Rosa, J. M., Tambourgi, E. B., and Mei, L. H. I. (2020). Photocatalytic degradation using ZnO for the treatment of RB 19 and RB 21 dyes in industrial effluents and mathematical modeling of the process. Chem. Eng. Res. Des. 153, 294–305. doi:10.1016/j.cherd.2019.10.021
Sayadi, M. H., Ghollasimood, S., Ahmadpour, N., and Homaeigohar, S. (2022). Biosynthesis of the ZnO/SnO2 nanoparticles and characterization of their photocatalytic potential for removal of organic water pollutants. J. Photochem. Photobiol. A Chem. 425, 113662. doi:10.1016/j.jphotochem.2021.113662
Selvaraj, S., Palanivel, B., Patrick, S., Krishna Mohan, M., Navaneethan, M., Ponnusamy, S., et al. (2022). Effect of Sr doping in ZnO microspheres for solar light-driven photodegradation of organic pollutants. J. Mater. Sci. Mater. Electron. 33 (11), 8777–8788. doi:10.1007/s10854-021-06875-6
Sharma, A., Varshney, M., Chae, K. H., Shin, H. J., and Won, S. O. (2016). Investigation on the local electronic/atomic structure properties using XANES/EXAFS and photocatalyst application of Zr1− xCuxO2 (0≤ x≤ 0.2). Curr. Appl. Phys. 16 (10), 1326–1333. doi:10.1016/j.cap.2016.07.008
Sharma, A., Varshney, M., Shin, H. J., Lee, B. H., Chae, K. H., and Won, S. O. (2017). Effect of Cu insertion on structural, local electronic/atomic structure and photocatalyst properties of TiO2, ZnO and Ni (OH) 2 nanostructures: XANES-EXAFS study. Mater. Chem. Phys. 191, 129–144. doi:10.1016/j.matchemphys.2017.01.008
Sharma, M., Rawat, S., Kumar, D., Awasthi, A., Sarkar, A., Sidola, A., et al. (2024). The state of the Yamuna River: a detailed review of water quality assessment across the entire course in India. Appl. Water Sci. 14 (8), 175. doi:10.1007/s13201-024-02227-x
Shkir, M., Palanivel, B., Khan, A., Kumar, M., Chang, J.-H., Mani, A., et al. (2022). Enhanced photocatalytic activities of facile auto-combustion synthesized ZnO nanoparticles for wastewater treatment: an impact of Ni doping. Chemosphere 291, 132687. doi:10.1016/j.chemosphere.2021.132687
Singh, K., Kaur, M., Chauhan, I., Awasthi, A., Kumar, M., Thakur, A., et al. (2020). BN/NiO nanocomposites: structural, defect chemistry and electrical properties in hydrogen gas atmosphere. Ceram. Int. 46 (16), 26233–26237. doi:10.1016/j.ceramint.2020.07.084
Sobana, N., Muruganadham, M., and Swaminathan, M. (2006). Nano-Ag particles doped TiO2 for efficient photodegradation of Direct azo dyes. J. Mol. Catal. A Chem. 258 (1–2), 124–132. doi:10.1016/j.molcata.2006.05.013
Sun, H., Lee, S.-Y., and Park, S.-J. (2023). Bimetallic CuPd alloy nanoparticles decorated ZnO nanosheets with enhanced photocatalytic degradation of methyl orange dye. J. Colloid Interface Sci. 629, 87–96. doi:10.1016/j.jcis.2022.09.054
Taghipour, S., Hosseini, S. M., and Ataie-Ashtiani, B. (2019). Engineering nanomaterials for water and wastewater treatment: review of classifications, properties and applications. New J. Chem. 43 (21), 7902–7927. doi:10.1039/C9NJ00157C
Tanaka, K., Padermpole, K., and Hisanaga, T. (2000). Photocatalytic degradation of commercial azo dyes. Water Res. 34 (1), 327–333. doi:10.1016/S0043-1354(99)00093-7
Teixeira Tarley, C. R., and Zezzi Arruda, M. A. (2004). Biosorption of heavy metals using rice milling by-products. Characterisation and application for removal of metals from aqueous effluents. Chemosphere 54 (7), 987–995. doi:10.1016/j.chemosphere.2003.09.001
Vinayagam, R., Selvaraj, R., Arivalagan, P., and Varadavenkatesan, T. (2020). Synthesis, characterization and photocatalytic dye degradation capability of Calliandra haematocephala-mediated zinc oxide nanoflowers. J. Photochem. Photobiol. B Biol. 203, 111760. doi:10.1016/j.jphotobiol.2019.111760
Wang, L., Li, Z., Chen, J., Huang, Y., Zhang, H., and Qiu, H. (2019). Enhanced photocatalytic degradation of methyl orange by porous graphene/ZnO nanocomposite. Environ. Pollut. 249, 801–811. doi:10.1016/j.envpol.2019.03.071
Zailan, S. N., Bouaissi, A., Mahmed, N., and Abdullah, M. M. A. B. (2020). Influence of ZnO nanoparticles on mechanical properties and photocatalytic activity of self-cleaning ZnO-based geopolymer paste. J. Inorg. Organomet. Polym. Mater. 30 (6), 2007–2016. doi:10.1007/s10904-019-01399-3
Zewde, D., and Geremew, B. (2022). Biosynthesis of ZnO nanoparticles using Hagenia abyssinica leaf extracts; their photocatalytic and antibacterial activities. Environ. Pollut. Bioavailab. 34 (1), 224–235. doi:10.1080/26395940.2022.2081261
Keywords: ZnO nanoparticles, organic pollutants, dyes, wastewater purification, doped NPs, biosynthesized NPs
Citation: Kaur N, Bansal M, Kaur P, Kaur K, Awasthi A, Nippani SK, Das S and Lodh A (2025) Mitigating dye and organic pollutant-driven surface water pollution using ZnO nanoparticles: a sustainable strategy for climate resilience. Front. Environ. Sci. 13:1656031. doi: 10.3389/fenvs.2025.1656031
Received: 30 June 2025; Accepted: 09 September 2025;
Published: 01 October 2025.
Edited by:
Sandip Pal, Texas Tech University, United StatesReviewed by:
Suresh Babu Naidu krishna, Durban University of Technology, South AfricaPriyanka Mahajan, Panjab University, India
Abhishek Awasthi, Bastar University, India
Copyright © 2025 Kaur, Bansal, Kaur, Kaur, Awasthi, Nippani, Das and Lodh. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Abhishek Lodh, YWJoaXNoZWsubG9kaEBzbWhpLnNl, YWJoaXNoZWsuc21oaS5sb2RoQGdtYWlsLmNvbQ==, YWJoaXNoZWsubG9kaEBuYXRla28ubHUuc2U=; Amit Awasthi, YXdhc3RoaXRpZXRAZ21haWwuY29t; Mamta Bansal, bWFtdGFAbWF1LmVkdS5pbg==
†Present address: Abhishek Lodh, National Centre for Medium Range Weather Forecasting (NCMRWF), Ministry of Earth Sciences, Noida, India
 Navneet Kaur
Navneet Kaur Mamta Bansal
Mamta Bansal Prabhjot Kaur2
Prabhjot Kaur2 Amit Awasthi
Amit Awasthi Sumanta Das
Sumanta Das Abhishek Lodh
Abhishek Lodh