- 1School of Nuclear Science and Technology, Southwest University of Science and Technology, Mianyang, China
- 2National Co-Innovation Center for Nuclear Waste Disposal and Environmental Safety, Southwest University of Science and Technology, Mianyang, China
- 3State Key Laboratory of Featured Metal Materials and Life-cycle Safety for Composite Structures, School of Resources, Environment and Materials, Guangxi University, Nanning, China
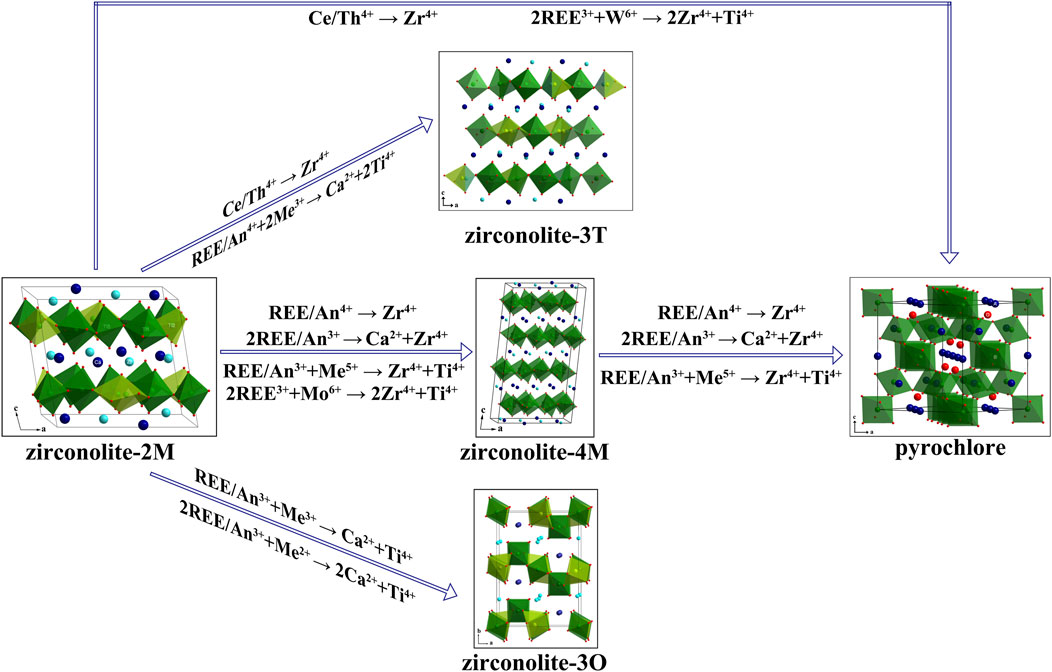
Zirconolite waste forms are advanced ceramic materials for the immobilisation of HLW, particularly actinides. This paper presents a systematic review of seven common substitution mechanisms of nuclear wastes in zirconolite, categorized into two types: charge-compensated substitution and direct substitution. For those substitution modes, three primary phase evolution pathways were identified: i zirconolite-2M to zirconolite-4M and/or to pyrochlore; ii zirconolite-2M to zirconolite-3O; iii zirconolite-2M to zirconolite-3T. The formation of zirconolite-3T or zirconolite-4M as intermediate phases is typically influenced by substitution behaviour, preparation conditions, or fabrication methods. Additionally, the substitution mechanisms of actinides (or REE analogue) in zirconolite were systematically investigated, corresponding to the seven substitution designs. Notably, the preferential occupation of An/REE in Ca sites was verified at simultaneous direct substitution in both Ca and Zr sites. Whilst extensive studies has explored An/REE substitution mechanisms in zirconolite and identified phase evolution pathways to zirconolite-3O, -3T, -4M, and pyrochlore, the substitution behaviour, radiation stability and chemical durability of these defect-fluorite derivatives warrant further systematic investigation.
1 Introduction
Nuclear power will assume an increasingly significant role in the future global energy structure owing to its high energy density, environmental friendliness and sustainability. It also plays a vital role in mitigating greenhouse gases, particularly in achieving China’s carbon peaking and carbon neutrality goals. However, their unique challenge of radioactive waste severely constrains nuclear energy development. The radioactive wastes from spent nuclear fuel contain fission product gases (e.g., Xe, I), metallic fission products (e.g., Mo, Tc, Pb), oxides-forming fission products (e.g., Zr, Ba, Cs, Nb, lanthanides), and transuranic elements (e.g., Pu, Np, Am, Cm) (Ewing, 2015). Radioactive wastes are typically classified as low-level waste (LLW), intermediate-level waste (ILW), and high-level waste (HLW) according to radionuclide inventory, decay heat, and half-life. Robust disposal solutions have been developed and implemented for LLW and short-lived ILW (Hua and Deng, 2006; Corkhill and Hyatt, 2018; IAEA, 2022). HLW has attracted significant concern due to its long-term radioactivity, high decay heat, chemical and biological toxicity (Slovic et al., 1991; Ewing, 1999; Corkhill and Hyatt, 2018). Should HLW not be safely disposed, resultant environmental and public health impacts could trigger a crisis in public trust, further constraining the development of nuclear energy.
For long-term nuclear waste disposal, numerous options, including outer space, rock melting, sea disposal, ice sheets, were historically considered. Currently, deep geological disposal is accepted by most countries as the definitive solution of radioactive wastes, particularly HLW containing actinides. This preference stems from its multi-barrier disposal systems which could prevent the radionuclides from reaching humans and the environment (Corkhill and Hyatt, 2018; Ewing and Park, 2021). Prior to the deep geological disposal, HLW and long-lived ILW require immobilization in glass, ceramic (or SYNROC) or glass-ceramic matrices (Donald et al., 1997; Ewing, 1999; Weber et al., 2009; Corkhill and Hyatt, 2018). Glass waste forms could accommodate approximately 40 different elements, ∼35 wt.% HLW loadings, and have been industrially implemented in several countries (Donald et al., 1997; Weber et al., 2009; Sengupta, 2012; Corkhill and Hyatt, 2018). Vitrification technologies include the induction-heated metallic Melter, cold-crucible Melter, and joule-heated ceramic Melter, but the disadvantages of glass waste forms limited the application in the immobilisation of HLW, for instance, low solubility for special and important radionuclides (e.g., actinides and Tc), thermodynamic metastability, and problematic phase segregation (e.g., yellow phase formation) (Donald et al., 1997; Vance, 2007; Yudintsev et al., 2007; Weber et al., 2009; Ojovan et al., 2021). Ceramic waste forms can immobilise radionuclides within crystalline lattices, offering superior actinides (An) solubility, chemical durability, and radiation tolerance (Donald et al., 1997; Ewing, 1999; Vance, 2007; Yudintsev et al., 2007; Weber et al., 2009; Gregg and Vance, 2017; Orlova and Ojovan, 2019). Ceramic matrices have been regarded as second-generation waste forms, including zirconolite, pyrochlore, zircon, garnet, hollandite, and monazite, but they lack industrial implementation (Donald et al., 1997; Ewing et al., 2004; Vance, 2007; Weber et al., 2009; Wang and Liang, 2012; Stefanovsky and Yudintsev, 2016; Gregg and Vance, 2017; Vance et al., 2017; Fuentes et al., 2018; McMaster et al., 2018; Finkeldei et al., 2021; Lumpkin and Aughterson, 2021; Yudintsev et al., 2023). Glass-ceramic waste forms are designed to merge the advantages of both ceramic and glass to immobilise HLW, incorporate specific waste streams, and facilitate industrial-scale production with existing vitrification infrastructure (Rawlings et al., 2006; Vance, 2007; Yudintsev et al., 2007; Weber et al., 2009; Vance et al., 2012). As a pragmatic compromise, glass-ceramics have been attractive in recent decades. Sphene-based, zirconolite-based and other glass ceramics were devised and studied widely, but the optimization of fabrication process, immobilisation mechanism and performance characteristics require further research (Lee et al., 2006; Rawlings et al., 2006; Weber et al., 2009; Vance et al., 2012; Corkhill and Hyatt, 2018; Ojovan et al., 2021). Consequently, ceramics represent the most promising matrices for the immobilisation of HLW, especially for actinides. Recent advances in high-entropy ceramics offer novel approaches for multi-radionuclide solidification (Wang et al., 2024; Wang et al., 2025). The oxides ceramic systems, as zirconolite, pyrochlore, garnet, perovskite, have emerged as leading candidates for actinides immobilisation.
Zirconolite, as one of fluorite-derived structures, was first proposed as a main constituent of SYNROC for the immobilisation of HLW towards the end of the 1970s (Ringwood et al., 1979). Since then, the research interest in zirconolite waste forms has grown, and several review articles concerning zirconolite crystal chemistry and stability have emerged. In the 1990s, Matzke et al. reviewed the immobilisation of several actinides and their rare-earth element analogues in zirconolite (Matzke et al., 1990). However, the solid-state behaviour of actinides in zirconolite remained poorly understood. Gieré et al. subsequently summarised 24 possible substitution types and nine typical chemical compositions in natural zirconolite, based on a statistical study of ∼300 chemical analyses (Gieré et al., 1998). More recently, Blackburn et al. reviewed the crystal chemistry and aqueous durability of zirconolite, focusing on the incorporation of Ce/Pu/U in the Ca2+ site with charge compensators and the Zr4+ site, noting typical elemental release rates from zirconolite of less than 10–5 g m–2 d–1 (Blackburn et al., 2021a). The solid solution limits of neutron absorbing additives (Gd, Hf, Sm, In, Cd, B) and Pu in zirconolite were also reviewed (Blackburn et al., 2023). Based on considerations of cost and neutronics, they proposed that Gd and Hf are the most promising oxides for the candidate Pu-zirconolite phase. Yudintsev et al. have provided a detailed analysis of zirconolite-structured phases for the immobilisation of REE-actinide-rich wastes, identifying cold pressing and sintering (CPS), pressure-assisted sintering techniques, or melt crystallization as the most feasible industrial-scale processing routes (Yudintsev et al., 2023). Therefore, this article discusses the substitution mechanism within synthetic zirconolite as a potential matrix for rare-earth elements (REE) and actinides fractions of HLW. It aims to provide a comprehensive literature review on the design and application of REE and actinide solidification from the perspective of substitution mechanism in zirconolite.
2 The substitution mechanism of REE/An in zirconolite
2.1 Theoretical substitution
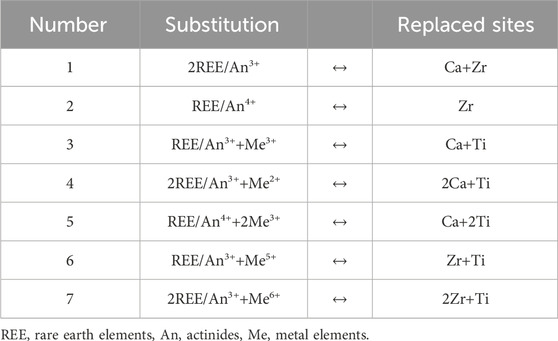
The ideal chemical formula of zirconolite is CaZrTi2O7 (or ABC2O7), space group C2/c (No.15). There are three-type cations, with their coordination environments being CaO8, ZrO7, TiO6 and TiO5 (Rossell, 1980; Whittle et al., 2012). Owing to its three distinct cation sites and the principle of isomorphism, zirconolite exhibits highly flexible element substitution behaviour. Gieré et al. proposed 24 potential substitutions types in zirconolite, but only a few theoretically possible substitutions are significant for the immobilisation of nuclear wastes (Gieré et al., 1998). Over the latest 30 years, numerous studies have been published on the incorporation of REE/An into zirconolite, with the most theoretically important substitutions were listed in Table 1 (Gieré et al., 1998; Weber et al., 2009; Blackburn et al., 2021a; Yudintsev et al., 2023). These substitutions could be classified into two typical scenarios: direct substitution and co-substitution with charge compensators such as Al3+, Fe2+/3+, Mn2+, Cr3+, Nb5+ and other metal (Me) elements.
2.2 No charge compensator
Generally, REE/An ions may occupy Ca, Zr, and Ti sites in zirconolite through direct substitution, either individually or via simultaneous multi-cation replacement. The most significant substitutions are types 1# and 2#, which require no charge compensation. Extensive research exists on CaZrTi2O7-REE2Ti2O7 (REE = Ce, Nd, Gd, Sm, Dy, Ho, Y) systems (Jafar et al., 2014a; Jafar et al., 2014b; Jafar et al., 2016; Meng et al., 2016; Zhang et al., 2016b; Zhang et al., 2017c; Yin et al., 2018; Zhang et al., 2018; Zhang et al., 2019; Blackburn et al., 2022; Ji et al., 2024). The dominant crystal phase evolution pathway has been identified from zirconolite-2M to zirconolite-4M, then to pyrochlore. However, high dopant concentrations of Nd and Ce yield primary multiphase assemblages rather than pyrochlore (Jafar et al., 2014b; Zhang et al., 2020). This behaviour can be attributed to the larger ionic radii of Nd3+ and Ce3+, which exceed the stable RA/RB cationic radii ratio required for ordered pyrochlore formation (Ewing et al., 2004). The possible preferential substitution of Ln3+ (Ln = Ce, Gd, Nd, Sm, Dy, Ho) in Ca site of zirconolite-2M structure were determined by XANES, Rietveld refinement and other method. However, this preference was not observed in Ca1-xZr1-xY2xTi2O7 system due to smaller ionic radii of Y3+ (Jafar et al., 2016). For pyrochlore (A2B2O7), all the REE only occupy the A site (Blackburn et al., 2022; Ji et al., 2024). In addition, Analogous zirconolite-to-pyrochlore transformations occur in CaHfTi2O7-(Gd/Ce)2Ti2O7 systems (Perera et al., 2002; Zhang et al., 2020).
The direct substitution of An4+ and Ce4+ at Zr sites of zirconolite has been extensively studied owing to favourable charge balance and comparable ionic radii. In CaZr1-xMxTi2O7 (M = Ce, U, Pu, Np) systems, phase evolution typically progresses from zirconolite-2M to zirconolite-4M to pyrochlore (Vance et al., 1994b; Begg et al., 1997; Vance et al., 2002; Clark et al., 2017). However, CaZr1-xThxTi2O7 and select SHS sintered CaZr1-xCexTi2O7 systems exhibited direct transformation of zirconolite-2M to pyrochlore, bypassing the zirconolite-4M phase (Zhang et al., 2015; Blackburn et al., 2021b). Moreover, Wen et al. reported the phase evolution of zirconolite-2M to zirconolite-3T in CaZr1-xCexTi2O7 (x = 0–0.5) samples synthesised via solid-state reaction using CaF2, Ti, TiO2, ZrO2, CeO2 and Fe2O3 (Wen et al., 2015). This pathway was observed in CaZr1-xThxTi2O7 (x = 0–0.5) samples sintered under reducing conditions (Blackburn et al., 2021b). Interestingly, graphite-die hot pressing of CaZr0.8Pu4+0.2Ti2O7 resulted in: i complete reduction of Pu4+ to Pu3+, ii Pu3+ substitution at Ca sites of zirconolite, and iii ∼50% perovskite phase formation (Begg et al., 1997). Multiple studies reported perovskite emergence in CaZr1-xCexTi2O7 systems at elevated Ce concentrations (Zhang et al., 2015; Zhang et al., 2017a; Blackburn et al., 2020a; Kong et al., 2021). The substitution of U4+ in Zr sites of zirconolite was determined by Sharivastava et al. using Rietveld refinement (Shrivastava et al., 2005; Shrivastava et al., 2006). Subramani et al. discovered that U4+/6+ incorporated into Zr sites of zirconolite for CaZr1-xUxTi2O7+x (0 ≤ x ≤ 0.4) (Subramani et al., 2020). Contrastingly, Zhang et al. identified predominant substitution of Ce at Ca sites rather than Zr sites in CaZr1-xCexTi2O7 (x = 0–0.5) samples when MoO3 and Ti served as oxidant and reductant, respectively (Zhang et al., 2016a). It can be attributed to the reduction of Ce4+ to Ce3+. In addition, Gatehouse et al. reported zirconolite formation at x = 0.85–1.3 in CaZrxTi3-xO7 system, indicating mutual Zr/Ti site occupancy (Gatehouse et al., 1981). Nevertheless, there are no articles on the substitution of REE/An at Ti sites. It could be caused by the smaller ionic radii and lower coordination number of Ti relative to Zr, Ca, and REE/An.
2.3 Low-valency ions as charge compensators
Trivalent actinides preferentially occupy the Ca sites in zirconolite. Change compensation could be achieved through coupled substitutions at Ti sites using low-valency ions. The low-valency ions are defined as those with oxidation states below +4 (i.e., lower than Ti4+), such as Mg2+, Fe2+/3+, Al3+, which are used in industry. In 1994, Vance et al. conducted pioneering work incorporating Nd3+, Ce3+/4+, U4+, Pu3+, Np3+ into zirconolite with Al3+ as charge compensator (Vance et al., 1994a; Vance et al., 1994b). They found the phase transformation of monoclinic to orthorhombic zirconolite in Ca1-xNd/CexZrTi2-xAlxO7 samples (Vance et al., 1994b). Begg et al. found that the reducing conditions induced the formation of perovskite (∼10%) in Ca0.8Pu3+0.2HfTi1.8Al0.2O7 and Ca0.8Pu4+0.2HfTi1.6Al0.4O7 samples (Begg et al., 1997). Stefanovsky et al. documented the possible phase evolution of zirconolite-2M to higher symmetry structure (zirconolite-3T/6T at x = 0.4–1.0, zirconolite-3Oat x→1.0) in Ca1-xGdxZrTi2-xAlxO7 (0 ≤ x ≤ 1) (Stefanovsky et al., 2002). Zhang et al. synthesised Nd-Al co-doped zirconolite with CuO oxidant, confirming the substitution of Nd at Ca sites and Al at Ti sites (Zhang et al., 2017b). Ji and co-worker had been systematically investigated the phase evolution and substitution mechanism of Ca1-xLnxZrTi2-xAlxO7, Ca1-xLnxZrTi2-xFexO7 and Ca1-xLnxZrTi2-x(Al, Fe)xO7 (Ln = La, Nd, Gd, Ho, Yb; x = 0–1) solid solutions (Ji et al., 2018; Ma et al., 2018; Ji et al., 2020). They found that: i phase evolution of zirconolite-2M to zirconolite-3O, ii Ln completely incorporated into zirconolite (2M/3O) except La, iii the substitution of Ln in Ca sites and Al/Fe in Ti sites. A detail research was published on substitution behaviour of Nd co-doped zirconolite with low charge compensator (Mg2+) (Zhou et al., 2022). The results indicated: i phase transformation from zirconolite-2M to zirconolite-3O, ii the substitution of Nd in CaO8 sites and Mg in TiO5 sites for zirconolite-2M, and iii the substitution of Nd in CaO8 site and Mg in TiO4+TiO5 sites for zirconolite-3O. Furthermore, Zhang et al. reported the phase transformation from zirconolite-2M to zirconolite-3T in Ca1-xSmxZrTi2-xAlxO7 compounds when CuO was used as oxidant (Zhang et al., 2019). The phase evolution of zirconolite-2M to zirconolite-3T were observed in Ce4+ co-doped zirconolite with two or three trivalent metal cations as charge compensator (Ai et al., 2024a; Ai et al., 2024b). Moreover, the same pathway (zirconolite-2M to zirconolite-3T) was found in Ca1-xPuxZrTi2-xFe2xO7 (x = 0.1–0.7) (Gilbert et al., 2010). The zirconolite-2M could accommodate ∼35 at% Ce at Ca sites with Cr3+ as charge compensator in Ca1-xCexZrTi2-xCr2xO7 samples (Blackburn et al., 2020b). For Ca1-xCexZrTi2-xCrxFexO7, zirconolite-2M could be formed at x ≤ 0.25 (Chen et al., 2023). In addition, Hayashizak et al. found that Ce-Al co-doped could be to prevent perovskite formation than that without charge compensator (Hayashizaki et al., 2025). Wei et al. incorporated Pr (as surrogate of Np) into Ca site with Al3+ as charge compensator (Wei et al., 2021). The zirconolite-2M phase could be more than 93 wt.%.
2.4 High-valency ions as charge compensator
High-valency ions are defined as elements with oxidation states exceeding +4 (i.e., higher than Ti4+) in zirconolite waste forms. The high-valency ions (as Nb5+, Mo6+) have been found in spent nuclear fuel and natural zirconolite, so that it is possible to immobilize actinides when employed these high-valency ions as charge compensator (Gieré et al., 1998; Ewing, 2015; Chukanov et al., 2018). Ji et al. systematically investigated the high-valency ion (Nb5+) substitution in CaZr1-xNdxTi2-xNbxO7 (x = 0.05–1) systems, observing the fluorite-derived phase evolution: zirconolite-2M to zirconolite-4M and then to pyrochlore, with zirconolite-3T appearing as a minor intermediate phase between zirconolite-2M and zirconolite-4M. (Ji et al., 2021). Nd3+ preferentially occupied Zr sites of ziconolite-2M alongside Nb5+ in Ti1 sites. For pyrochlore (A2B2O7) structure, the substitution mechanism comprised: i Ca2++Nd3++Zr4+ at A sites, ii Ti4++Nb5+ at B sites. Song et al. reported identical phase evolution in CaZr1-xSmxTi2-xNbxO7 (x = 0.1–1.0) solid solutions though without zirconolite-3T intermediate phase (Song et al., 2025). Huangfu et al. conducted detailed analysis of CaZr1-2xREE2xTi2-xMo6+xO7 (REE = Nd, Sm, Gd, Ho, Yb) samples, noting: i the phase evolution of zirconolite-2M to zirconolite-4M, ii ∼25 at.% solubility limit in zirconolite (2M and 4M), iii phase separation into “yellow phase” (CaMoO4) at x > 0.25, coexisting with pyrochlore (Huangfu et al., 2024). In zirconolite-2M structure, Mo6+ ion preferentially occupies TiO5 site. REE (e.g., Sm, Ho) could mainly replace the 8f Zr sites with a considerable amount substitution in 8f Ca sites. In addition, the phase evolution was studied in details for W6+ co-doped CaZr1-2xLn2xTi2-xW6+xO7 (Ln = Nd, Sm, Gd, Ho, Yb) (Huang et al., 2024). In this condition, zirconolite-2M directly transformed to pyrochlore with secondary “yellow phase”.
3 Conclusion and outlook
The seven most important substitution mechanisms for actinides (or their REE surrogates) in zirconolite were systematically discussed and summarised. Figure 1 summarises the phase evolution pathways for these substitution mechanisms, which comprise two classes: charge-compensated substitution and direct substitution. For co-substitution with low-valency charge compensators, the phase evolution pathway may progress from zirconolite-2M to zirconolite-3O. Zirconolite-2M would transform to zirconolite-4M and subsequently to pyrochlore for direct substitution and high-valency ion (Nb5+) substitution modes. For higher-valency ions (Me6+), phase evolutions from zirconolite-2M to zirconolite-4M, or directly from zirconolite-2M to pyrochlore, were identified. These are accompanied by the appearance of the hazardous “yellow phase” at high dopant concentrations. Moreover, phase evolution of zirconolite-2M to zirconolite-3T would be observed in substitution modes 5# and partially 2#, under reducing conditions or others special situation. Furthermore, substitution mechanisms, ionic radii, preparation conditions, and processing parameters, amongst other factors, significantly influence phase evolution and the formation of intermediate phases. For model 1#, An/REE preferentially occupy the Ca sites of zirconolite. The preferential or main occupation of An/REE in zirconolite-2M corresponds to the substitution mechanisms detailed in Table 1. Although numerous studies have investigated substitution mechanisms with promising results, other performance indicators of zirconolite under different substitution mechanisms and concentrations, such as irradiation resistance, chemical durability, and mechanical performance, require further investigation. In addition, the substitution mechanisms discussed above are predicated on the zirconolite-2M structure. However, as evidenced by the preceding analysis, substitution induces phase evolution to zirconolite-3O, zirconolite-3T, zirconolite-4M, and pyrochlore. To evaluate the long-term performance of zirconolite waste forms in deep geological disposal, a comprehensive investigation is required into the substitution behaviour, irradiation stability, chemical durability, and other critical properties of these defect-fluorite derived structural ceramics. Finally, this review provides a valuable reference for the design and application of zirconolite in nuclear waste disposal, aerospace materials, and advance functional ceramics. We recommend that future research should prioritize evaluating the long-term performance of multiphase ceramic assemblages resulting from phase evolution, particularly those with defect-fluorite derived structures.
Author contributions
SJ: Project administration, Funding acquisition, Formal Analysis, Investigation, Writing – original draft, Writing – review and editing, Conceptualization. CZ: Formal Analysis, Writing – original draft, Data curation, Investigation. XJ: Investigation, Formal Analysis, Writing – original draft, Data curation. CG: Formal Analysis, Data curation, Writing – original draft. YZ: Formal Analysis, Writing – review and editing, Supervision. XC: Formal Analysis, Supervision, Writing – review and editing. TD: Resources, Project administration, Writing – review and editing, Investigation, Funding acquisition. TJ: Formal Analysis, Writing – review and editing, Data curation. CL: Funding acquisition, Conceptualization, Resources, Project administration, Supervision, Writing – review and editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (U2167221, 12105235, 52361002), Guangxi Science and Technology Major Program (No. AA23073019) and Guangxi Science and Technology Base and Talent Special Project (No. GKAD23026259). The Center for Instrumental Analysis of Guangxi University is acknowledged for providing research facilities and resources for the experiments.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ai, Q., Sun, S. K., Hao, P. W., Chen, Y. B., Zhao, Y., Tan, S. H., et al. (2024a). Synergistic immobilisation of CeO2 as a surrogate for PuO2 and metallic FeCrAl alloy via zirconolite wasteform. Ceram. Int. 50 (8), 12924–12933. doi:10.1016/j.ceramint.2024.01.199
Ai, Q., Sun, S. K., Hao, P. W., Chen, Y. B., Zhao, Y., Tan, S. H., et al. (2024b). Co-immobilisation of a PuO2 surrogate and contaminated stainless steel within a zirconolite matrix. J. Am. Ceram. Soc. 107 (9), 5773–5785. doi:10.1111/jace.19901
Begg, B. D., Vance, E. R., Day, R. A., Hambley, M., and Conradson, S. D. (1997). Plutonium and neptunium incorporation in zirconolite. Mater. Res. Soc. Symposium - Proc. 465, 325–332. doi:10.1557/proc-465-325
Blackburn, L. R., Sun, S., Gardner, L. J., Maddrell, E. R., Stennett, M. C., and Hyatt, N. C. (2020a). A systematic investigation of the phase assemblage and microstructure of the zirconolite CaZr1-xCexTi2O7 system. J. Nucl. Mater. 535, 152137. doi:10.1016/j.jnucmat.2020.152137
Blackburn, L. R., Sun, S. K., Lawson, S. M., Gardner, L. J., Ding, H., Corkhill, C. L., et al. (2020b). Synthesis and characterisation of Ca1-xCexZrTi2-2xCr2xO7: analogue zirconolite wasteform for the immobilisation of stockpiled UK plutonium. J. Eur. Ceram. Soc. 40 (15), 5909–5919. doi:10.1016/j.jeurceramsoc.2020.05.066
Blackburn, L. R., Bailey, D. J., Sun, S. K., Gardner, L. J., Stennett, M. C., Corkhill, C. L., et al. (2021a). Review of zirconolite crystal chemistry and aqueous durability. Adv. Appl. Ceram. 120 (2), 69–83. doi:10.1080/17436753.2021.1877596
Blackburn, L. R., Sun, S. K., Gardner, L. J., Maddrell, E. R., Stennett, M. C., Corkhill, C. L., et al. (2021b). Synthesis, structure, and characterization of the thorium zirconolite CaZr1-xThxTi2O7 system. J. Am. Ceram. Soc. 104 (7), 2937–2951. doi:10.1111/jace.17627
Blackburn, L. R., Townsend, L. T., Lawson, S. M., Mason, A. R., Stennett, M. C., Sun, S. K., et al. (2022). Phase evolution in the CaZrTi2O7-Dy2Ti2O7 system: a potential host phase for minor actinide immobilisation. Inorg. Chem. 61 (15), 5744–5756. doi:10.1021/acs.inorgchem.1c03816
Blackburn, L. R., Corkhill, C. L., and Hyatt, N. C. (2023). A review of zirconolite solid solution regimes for plutonium and candidate neutron absorbing additives. Ceramics 6 (3), 1330–1347. doi:10.3390/ceramics6030082
Chen, Y. B., Wu, J. Y., Huang, K. H., Sun, S. K., Ai, Q., Bao, W. C., et al. (2023). Influence of the dual charge compensator on solid solution of the air-sintered Ca1-xCexZrTi2-2xFexCrxO7 zirconolite. Ceram. Int. 49 (19), 31517–31523. doi:10.1016/j.ceramint.2023.07.102
Chukanov, N. V., Zubkova, N. V., Britvin, S. N., Pekov, I. V., Vigasina, M. F., Schäfer, C., et al. (2018). Nöggerathite-(Ce), (Ce,Ca)2Zr2(Nb,Ti)(Ti,Nb)2Fe2+O14, a new zirconolite-related mineral from the eifel volcanic region, Germany. Minerals 8 (10), 449. doi:10.3390/min8100449
Clark, B. M., Sundaram, S. K., and Misture, S. T. (2017). Polymorphic transitions in cerium-substituted zirconolite (CaZrTi2O7). Sci. Rep. 7 (1), 5920–10. doi:10.1038/s41598-017-06407-5
Corkhill, C., and Hyatt, N. (2018). Nuclear waste management. IOP Publishing, 1–18. doi:10.1088/978-0-7503-1638-5
Donald, I. W., Metcalfe, B. L., and Taylor, R. N. J. (1997). The immobilization of high level radioactive wastes using ceramics and glasses. J. Mater. Sci. 32 (22), 5851–5887. doi:10.1023/a:1018646507438
Ewing, R. C. (1999). Nuclear waste forms for actinides. Proc. Natl. Acad. Sci. 96 (7), 3432–3439. doi:10.1073/pnas.96.7.3432
Ewing, R. C. (2015). Long-term storage of spent nuclear fuel. Nat. Mater. 14 (3), 252–257. doi:10.1038/nmat4226
Ewing, R. C., and Park, S. (2021). in Encyclopedia of nuclear energy. Editor E. Greenspan (Oxford: Elsevier), 588–602.
Ewing, R. C., Weber, W. J., and Lian, J. (2004). Nuclear waste disposal-pyrochlore (A2B2O7): nuclear waste form for the immobilisation of plutonium and “minor” actinides. J. Appl. Phys. 95 (11 I), 5949–5971. doi:10.1063/1.1707213
Finkeldei, S. C., Chang, S., Ionescu, M., Oldfield, D., Davis, J., Lumpkin, G. R., et al. (2021). Insight into disorder, stress and strain of radiation damaged pyrochlores: a possible mechanism for the appearance of defect fluorite. Front. Chem. 9 (November), 706736–20. doi:10.3389/fchem.2021.706736
Fuentes, A. F., Montemayor, S. M., Maczka, M., Lang, M., Ewing, R. C., and Amador, U. (2018). A critical review of existing criteria for the prediction of pyrochlore formation and stability. Inorg. Chem. 57 (19), 12093–12105. doi:10.1021/acs.inorgchem.8b01665
Gatehouse, B. M., Grey, I. E., Hill, R. J., and Rossell, H. J. (1981). Zirconolite, CaZrxTi3-xO7; structure refinements for near-end-member compositions with x = 0.85 and 1.30. Acta Crystallogr. Sect. B 37 (2), 306–312. doi:10.1107/s0567740881002914
Gieré, R., Williams, C. T., and Lumpkin, G. R. (1998). Chemical characteristics of natural zirconolite. Schweiz. Mineral. Petrogr. Mittl. 78 (3), 433–459. doi:10.5169/seals-59299
Gilbert, M. R., Selfslag, C., Walter, M., Stennett, M. C., Somers, J., Hyatt, N. C., et al. (2010). Synthesis and characterisation of Pu-doped zirconolites –(Ca1−xPux)Zr(Ti2-2xFe2x)O7. IOP Conf. Ser. Mater. Sci. Eng. 9, 012007. doi:10.1088/1757-899x/9/1/012007
Gregg, D. J., and Vance, E. R. (2017). Synroc tailored waste forms for actinide immobilization. Radiochim. Acta 105 (11), 907–925. doi:10.1515/ract-2016-2604
Hayashizaki, K., Hirooka, S., Yamada, T., Sunaoshi, T., Murakami, T., and Saito, K. (2025). Reduction and phase transformation of Ce-Doped zirconolites. Ceram. (Basel). 8, 24. doi:10.3390/ceramics8010024
Hua, B., and Deng, B. (2006). Radioactive wastes. Water Environ. Res. 78 (10), 1856–1882. doi:10.2175/106143006x119431
Huang, Y., Yang, T., Wang, K., Liao, C. Z., Huangfu, Z., Fang, S., et al. (2024). Effects of high valency and polarizability ion (W6+) on the phase evolution of CaZr1–2xLn2xTi2–xWxO7 zirconolite-based solid solution (ln = Nd, Sm, Gd, Ho, yb). Ceram. Int. 50 (21PB), 42986–42998. doi:10.1016/j.ceramint.2024.08.145
Huangfu, Z., Yang, T., Ma, S., Wang, K., Shih, K., Yang, W., et al. (2024). High valency of charge compensator (Mo6+) to substitute Ti site in REE doped zirconolite (REE=Nd, Sm, Gd, Ho and yb): solid solubility, phase evolution and structural analysis. Ceram. Int. 50 (15), 26351–26360. doi:10.1016/j.ceramint.2024.04.271
Iaea (2022). Status and trends in spent fuel and radioactive waste management. Vienna: International Atomic Energy Agency.
Jafar, M., Sengupta, P., Achary, S. N., and Tyagi, A. K. (2014a). Phase evolution and microstructural studies in CaZrTi2O7 (zirconolite)–Sm2Ti2O7 (pyrochlore) system. J. Eur. Ceram. Soc. 34 (16), 4373–4381. doi:10.1016/j.jeurceramsoc.2014.07.001
Jafar, M., Sengupta, P., Achary, S. N., Tyagi, A. K., and Vance, L. (2014b). Phase evolution and microstructural studies in CaZrTi2O7–Nd2Ti2O7 system. J. Am. Ceram. Soc. 97 (2), 609–616. doi:10.1111/jace.12664
Jafar, M., Achary, S. N., Salke, N. P., Sahu, A. K., Rao, R., and Tyagi, A. K. (2016). X-ray diffraction and raman spectroscopic investigations on CaZrTi2O7-Y2Ti2O7 system: delineation of phase fields consisting of potential ceramic host materials. J. Nucl. Mater. 475, 192–199. doi:10.1016/j.jnucmat.2016.04.016
Ji, S., Li, Y., Ma, S., Liu, C., Shih, K., and Liao, C.-Z. (2018). Synergistic effects of Ln and Fe Co-Doping on phase evolution of Ca1-xLnxZrTi2-xFexO7 (ln = La, Nd, Gd, Ho, yb) ceramics. J. Nucl. Mater. 511, 428–437. doi:10.1016/j.jnucmat.2018.09.043
Ji, S., Su, M., Liao, C., Ma, S., Wang, Z., Shih, K., et al. (2020). Synchrotron x-ray spectroscopy investigation of the Ca1−xLnxZrTi2−x(Al, Fe)xO7 zirconolite ceramics (ln = La, Nd, Gd, Ho, yb). J. Am. Ceram. Soc. 103 (2), 1463–1475. doi:10.1111/jace.16832
Ji, S., Liao, C.-Z., Chen, S., Zhang, K., Shih, K., Chang, C.-K., et al. (2021). Higher valency ion substitution causing different fluorite-derived structures in CaZr1-xNdxTi2-xNbxO7 (0.05 ≤ x ≤ 1) solid solution. Ceram. Int. 47 (2), 2694–2704. doi:10.1016/j.ceramint.2020.09.119
Ji, X., Song, Y., Wang, J., Ding, Y., Ji, S., and Duan, T. (2024). Phase evolution, solubility and substitution behaviour of CaZrTi2O7–Ho2Ti2O7 ceramics for potential nuclear waste immobilisation. Ceram. Int. 50 (24), 55342–55350. doi:10.1016/j.ceramint.2024.10.392
Kong, L., Karatchevtseva, I., Zhang, Y., and Wei, T. (2021). The incorporation of Nd or Ce in CaZrTi2O7 zirconolite: ceramic versus glass-ceramic. J. Nucl. Mater. 543, 152583. doi:10.1016/j.jnucmat.2020.152583
Lee, W. E., Ojovan, M. I., Stennett, M. C., and Hyatt, N. C. (2006). Immobilisation of radioactive waste in glasses, glass composite materials and ceramics. Adv. Appl. Ceram. 105 (1), 3–12. doi:10.1179/174367606x81669
Lumpkin, G. R., and Aughterson, R. D. (2021). Perspectives on pyrochlores, defect fluorites, and related compounds: building blocks for chemical diversity and functionality. Front. Chem. 9 (November), 778140–12. doi:10.3389/fchem.2021.778140
Ma, S., Ji, S., Liao, C., Liu, C., Shih, K., and He, W. (2018). Effects of ionic radius on phase evolution in Ln-Al co-doped Ca1-xLnxZrTi2-xAlxO7 (ln = La, Nd, Gd, Ho, yb) solid solutions. Ceram. Int. 44 (13), 15124–15132. doi:10.1016/j.ceramint.2018.05.149
Matzke, H. J. J., Ray, I. L. F., Seatonberry, B. W., Thiele, H., Trisoglio, C., Walker, C. T., et al. (1990). Incorporation of transuranic elements in titanate nuclear waste ceramics. J. Am. Ceram. Soc. 73 (2), 370–378. doi:10.1111/j.1151-2916.1990.tb06520.x
Mcmaster, S. A., Ram, R., Faris, N., and Pownceby, M. I. (2018). Radionuclide disposal using the pyrochlore supergroup of minerals as a host matrix—A review. J. Hazard. Mater. 360 (August), 257–269. doi:10.1016/j.jhazmat.2018.08.037
Meng, C., Ding, X., Li, W., Zhao, J., and Yang, H. (2016). Phase structure evolution and chemical durability studies of Ce-doped zirconolite–pyrochlore synroc for radioactive waste storage. J. Mater. Sci. 51 (11), 5207–5215. doi:10.1007/s10853-016-9822-x
Ojovan, M. I., Petrov, V. A., and Yudintsev, S. V. (2021). Glass crystalline materials as advanced nuclear wasteforms. Sustain. Switz. 13 (8), 4117. doi:10.3390/su13084117
Orlova, A. I., and Ojovan, M. I. (2019). Ceramic mineral waste-forms for nuclear waste immobilization. Materials 12 (16), 2638. doi:10.3390/ma12162638
Perera, D. S., Stewart, M. W. A., Li, H., Day, R. A., and Vance, E. R. (2002). Tentative phase relationships in the system CaHfTi2O7-Gd2Ti2O7 with up to 15 mol% additions of Al2TiO5 and MgTi2O5. J. Am. Ceram. Soc. 85 (12), 2919–2924. doi:10.1111/j.1151-2916.2002.tb00556.x
Rawlings, R. D., Wu, J. P., and Boccaccini, A. R. (2006). Glass-ceramics: their production from wastes-A review. J. Mater. Sci. 41 (3), 733–761. doi:10.1007/s10853-006-6554-3
Ringwood, A. E., Kesson, S. E., Ware, N. G., Hibberson, W., and Major, A. (1979). Immobilisation of high level nuclear reactor wastes in SYNROC. Nature 278 (5701), 219–223. doi:10.1038/278219a0
Sengupta, P. (2012). A review on immobilization of phosphate containing high level nuclear wastes within glass matrix – present status and future challenges. J. Hazard. Mater. 235-236, 17–28. doi:10.1016/j.jhazmat.2012.07.039
Shrivastava, O. P., Kumar, N., and Sharma, I. B. (2005). Synthesis, characterization and structural refinement of polycrystalline uranium substituted zirconolite. J. Mater. Sci. 40 (11), 2945–2950. doi:10.1007/s10853-005-2431-8
Shrivastava, O. P., Kumar, N., and Sharma, I. B. (2006). Synthesis, characterization and structural refinement of polycrystalline uranium substituted zirconolite CaZr0.9U0.1Ti2O7. Radiochim. Acta 94 (6-7), 339–342. doi:10.1524/ract.2006.94.6.339
Slovic, P., Flynn, J. H., and Layman, M. (1991). Perceived risk, trust, and the politics of nuclear waste. Science 254 (5038), 1603–1607. doi:10.1126/science.254.5038.1603
Song, Y., Ji, X., Tang, K., Zhang, Y., Xu, T., Ji, S., et al. (2025). The structural evolution and chemical durability of the CaZr1-xSmxTi2-xNbxO7 defect fluorite-derived ceramics. J. Am. Ceram. Soc. n/a (n/a), e20693. doi:10.1111/jace.20693
Stefanovsky, S. V., and Yudintsev, S. V. (2016). Titanates, zirconates, aluminates and ferrites as waste forms for actinide immobilization. Russ. Chem. Rev. 85 (9), 962–994. doi:10.1070/rcr4606
Stefanovsky, S. V., Troole, A. Y., Lapina, M. I., Nikonov, B. S., Sivstov, A. V., and Yudinstev, S. V. (2002). XRD, SEM and TEM study of the Gd-Doped zirconolites. Mater. Res. Soc. Symposia Proc. 713, JJ11.14–15. doi:10.1557/proc-713-jj11.14
Subramani, T., Baker, J., Xu, H., and Navrotsky, A. (2020). Synthesis, characterization, and enthalpies of formation of uranium substituted zirconolites. ACS Earth Space Chem. 4 (10), 1878–1887. doi:10.1021/acsearthspacechem.0c00182
Vance, E. (2007). Development of ceramic waste forms for high-level nuclear waste over the last 30 years. MRS Online Proc. Libr. 985 (1), 401. doi:10.1557/proc-985-0985-nn04-01
Vance, E. R., Angel, P. J., Begg, B. D., and Day, R. A. (1994a). Zirconolite-rich titanate ceramics for high-level actinide wastes. Mater. Res. Soc. Symposium Proc. 333, 293–298. doi:10.1557/proc-333-293
Vance, E. R., Ball, C. J., Day, R. A., Smith, K. L., Blackford, M. G., Begg, B. D., et al. (1994b). Actinide and rare Earth incorporation into zirconolite. J. Alloys Compd. 213-214 (C), 406–409. doi:10.1016/0925-8388(94)90945-8
Vance, E. R., Lumpkin, G. R., Carter, M. L., Cassidy, D. J., Ball, C. J., Day, R. A., et al. (2002). Incorporation of uranium in zirconolite (CaZrTi2O7). J. Am. Ceram. Soc. 85 (7), 1853–1859. doi:10.1111/j.1151-2916.2002.tb00364.x
Vance, E. R., Stewart, M. W. A., and Moricca, S. (2012). Advanced ceramics and glass-ceramics for immobilisation of ILW and HLW. Mater. Res. Soc. Symposium Proc. 1475, 163–172. doi:10.1557/opl.2012.571
Vance, E. R., Chavara, D. T., and Gregg, D. J. (2017). Synroc development—Past and present applications. MRS Energy and Sustain. 4, 8. doi:10.1557/mre.2017.9
Wang, L., and Liang, T. (2012). Ceramics for high level radioactive waste solidification. J. Adv. Ceram. 1 (3), 194–203. doi:10.1007/s40145-012-0019-8
Wang, Z., Zhou, L., Liu, C., and Li, Y. (2024). The irradiation resistance and mechanical properties of the high-entropy zirconate pyrochlore (La0.2Nd0.2Sm0.2Eu0.2Gd0.2)2Zr2O7. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 549, 165285. doi:10.1016/j.nimb.2024.165285
Wang, H., Hu, H., Wang, Z., Zhou, L., Liu, C., Song, Y., et al. (2025). A reevaluation of radiation resistance in high-entropy pyrochlores: the role of entropy. J. Eur. Ceram. Soc. 45 (12), 117462. doi:10.1016/j.jeurceramsoc.2025.117462
Weber, W. J., Navrotsky, A., Stefanovsky, S., Vance, E. R., and Vernaz, E. (2009). Materials science of high-level immobilisation. MRS Bull. 34 (01), 46–52. doi:10.1557/mrs2009.12
Wei, Z. J., Bao, W., Sun, S. K., Blackburn, L. R., Tan, S. H., Gardner, L. J., et al. (2021). Synthesis of zirconolite-2M ceramics for immobilisation of neptunium. Ceram. Int. 47 (1), 1047–1052. doi:10.1016/j.ceramint.2020.08.220
Wen, G., Zhang, K., Yin, D., and Zhang, H. (2015). Solid-state reaction synthesis and aqueous durability of Ce-doped zirconolite-rich ceramics. J. Nucl. Mater. 466, 113–119. doi:10.1016/j.jnucmat.2015.07.047
Whittle, K. R., Hyatt, N. C., Smith, K. L., Margiolaki, I., Berry, F. J., Knight, K. S., et al. (2012). Combined neutron and X-ray diffraction determination of disorder in doped zirconolite-2M. Am. Mineralogist 97 (2-3), 291–298. doi:10.2138/am.2012.3848
Yin, D., Zhang, K., Peng, L., He, Z., Liu, Y., Zhang, H., et al. (2018). Solid-state reaction synthesis and chemical stability studies in Nd-doped zirconolite-rich ceramics. J. Rare Earths 36 (5), 492–498. doi:10.1016/j.jre.2017.10.005
Yudintsev, S. V., Stefanovsky, S. V., and Ewing, R. C. (2007). in Structural chemistry of inorganic actinide compounds. Editors S. V. Krivovichev, P. C. Burns, and I. G. Tananaev (Elsevier), 457–490.
Yudintsev, S. V., Nickolsky, M. S., Ojovan, M. I., Stefanovsky, O. I., Malkovsky, V. I., Ulanova, A. S., et al. (2023). Zirconolite matrices for the immobilization of REE–actinide wastes. Ceramics 6 (3), 1573–1622. doi:10.3390/ceramics6030098
Zhang, K., Wen, G., Zhang, H., and Teng, Y. (2015). Self-propagating high-temperature synthesis of CeO2 incorporated zirconolite-rich waste forms and the aqueous durability. J. Eur. Ceram. Soc. 35 (11), 3085–3093. doi:10.1016/j.jeurceramsoc.2015.04.025
Zhang, K., Wen, G., Yin, D., and Zhang, H. (2016a). SHS/QP synthesis and characterization of Ce-Bearing zirconolite-rich waste forms using MoO3 as the oxidant. J. Am. Ceram. Soc. 99 (6), 1894–1901. doi:10.1111/jace.14143
Zhang, K., Yin, D., Peng, L., and Zhang, H. (2016b). Self-propagating synthesis and characterization of Ca1−xZr1−xNd2xTi2O7 using CuO as the oxidant. Ceram. Int. 42 (9), 11387–11392. doi:10.1016/j.ceramint.2016.04.066
Zhang, K., Yin, D., Peng, L., and Wu, J. (2017a). Self-propagating synthesis and CeO2 immobilisation of zirconolite-rich composites using CuO as the oxidant. Ceram. Int. 43 (1), 1415–1423. doi:10.1016/j.ceramint.2016.10.103
Zhang, K., Yin, D., Peng, L., Wu, J., and Xue, J. (2017b). Combustion synthesis and characterization of Nd-Al codoped zirconolite-rich waste forms using CuO as the oxidant. Ceram. Int. 43 (9), 7094–7098. doi:10.1016/j.ceramint.2017.02.140
Zhang, K., Yin, D., Peng, L., Wu, J., and Xue, J. (2017c). Self-propagating synthesis of Nd2O3-incorporated zirconolite/Mo composites and their aqueous durability. J. Nucl. Mater. 491, 177–182. doi:10.1016/j.jnucmat.2017.04.044
Zhang, Y. B., Wang, J., Wang, J. X., Huang, Y., Luo, P., Liang, X. F., et al. (2018). Phase evolution, microstructure and chemical stability of Ca1-xZr1-xGd2xTi2O7 (0.0 ≤ x ≤ 1.0) system for immobilizing nuclear waste. Ceram. Int. 44 (12), 13572–13579. doi:10.1016/j.ceramint.2018.04.191
Zhang, K., Yin, D., He, Z., Xue, J., Zhao, W., and Zhang, H. (2019). Combustion synthesis and characterizations of Sm2O3 doped zirconolite-rich waste forms with CuO as oxidant. J. Rare Earths 37 (12), 1359–1365. doi:10.1016/j.jre.2018.11.020
Zhang, Z., Spiers, K. M., Vance, E. R., and Davis, J. (2020). Partitioning of Ce, as a simulant for Pu, in a multiphase ceramic nuclear waste form. J. Am. Ceram. Soc. 103 (10), 5515–5524. doi:10.1111/jace.17277
Zhou, Y., Liao, C., Leung, K. M., Ma, S., Chan, T. S., and Shih, K. (2022). Low charge compensator (Mg2+) causing a new REE-end 3O structure (REE=Rare Earth element) and a different phase transformation in Nd3+ Co-doped zirconolite: investigation by X-ray structural analysis. Ceram. Int. 48 (13), 18596–18604. doi:10.1016/j.ceramint.2022.03.131
Keywords: zirconolite, substitution mechanism, nuclear waste form, phase evolution, actinides
Citation: Ji S, Zhao C, Ji X, Gu C, Zhang Y, Chen X, Duan T, Ju T and Liao C (2025) Substitution mechanisms of zirconolite for nuclear waste form: a mini review. Front. Environ. Sci. 13:1656188. doi: 10.3389/fenvs.2025.1656188
Received: 29 June 2025; Accepted: 02 September 2025;
Published: 15 September 2025.
Edited by:
Nsikak U. Benson, Topfaith University, NigeriaReviewed by:
Lewis Blackburn, The University of Sheffield, United KingdomCopyright © 2025 Ji, Zhao, Ji, Gu, Zhang, Chen, Duan, Ju and Liao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiyin Ji, amlzaHlAc3d1c3QuZWR1LmNu; Changzhong Liao, bGlhb2N6MjlAY29ubmVjdC5oa3UuaGs=
 Shiyin Ji
Shiyin Ji Chuanhang Zhao1
Chuanhang Zhao1 Changzhong Liao
Changzhong Liao