- 1School of Life Sciences, Department of Earth and Environmental Sciences, The Chinese University of Hong Kong, Shatin, Hong Kong SAR, China
- 2State Key Laboratory of Marine Environmental Health, City University of Hong Kong, Hong Kong, Hong Kong SAR, China
- 3Department of Biology, University of North Carolina at Greensboro, Greensboro, NC, United States
- 4Division of Environmental Science and Engineering, Pohang University of Science and Technology, Pohang, Republic of Korea
- 5School of Fisheries, Aquaculture, and Aquatic Sciences, Auburn University, Auburn, AL, United States
Global biogeochemical cycling of trace elements is under influences of anthropogenic inputs and climate change, and their trophic transfers within natural food webs are not yet fully characterized. The present study investigated the trophodynamics of four trace elements (essential: copper and zinc, and non-essential: arsenic and cadmium) in a river food web and compared those with an upland forest food web in a semi-remote watershed in northern California, United States. We found empirical evidence of biomagnification for copper, zinc, and cadmium in the lower trophic levels of both aquatic and terrestrial food webs while we showed biodiminution for arsenic in food webs within the river, but not the forest as shown by calculating Trophic Magnification Factor (TMF), a direct and robust approach to explore the biomagnification efficiency of trace elements along the food web. There was a positive intercorrelation between copper, zinc, and cadmium among the food web components, indicating potential common mechanisms in controlling the trophic transfer of these three cationic trace elements in the natural food webs.
Introduction
Trace elements are natural components of the biosphere and widely exist in terrestrial and aquatic environments (Luoma, 1983). Rock weathering is one of the important processes controlling the availability of trace elements in natural ecosystems (Loureiro and Hepp, 2020). Therefore, living organisms can be exposed to a range of trace elements in both terrestrial and aquatic environments even without anthropogenic activities. However, it is well known that many anthropogenic activities have altered the natural biogeochemical cycles of different trace elements, through accelerating their emissions to the environment (Luoma, 1983). Also, climate change can pose many direct or indirect changes to metal cycling processes (Nedrich and Burton, 2017), and thus our environments are under multiple stresses from metal pollution. Not limited to the amount of trace element, but in what chemical form (i.e., speciation) is present, it strongly determines their bioavailability and the presence of trophic transfer in the natural environment (Szynkowska et al., 2018).
Most of the published work has focused on the metal pollution problems in contaminated ecosystems such as mining and industrial areas (Cain et al., 2004), but our understanding of trace element bioaccumulation and their trophic transfers in relatively pristine environments are relatively limited.
Previous research has shown that freshwater invertebrates such as Daphnia magna can take up aqueous metals through exoskeleton as well as dietary metals (e.g., from phytoplankton) through assimilation (Loureiro et al., 2018; Tsui and Wang, 2007). Once absorbed or assimilated, metals can be redistributed to the biologically inactive tissues such as the exoskeleton for regulation and excretion by ecdysis (Loureiro and Hepp, 2020; Rainbow, 2002). Excessive metals in the tissues can also be “detoxified” by binding with metallothionein as well as metal granules, which can be excreted from the biological tissues or stored in an inactive form indefinitely (Rainbow, 2002).
At the base of natural food webs, invertebrates are known to play an important role in transferring trace elements up the food webs (Tsui and Wang, 2007), and examining the base of food webs (e.g., from basal resources to invertebrates) would provide an unbiased assessment on trophic transfer of trace elements among habitat types because higher trophic level consumers (e.g., songbirds) are known to integrate food prey items over multiple sources and even a range of habitats, thus their body burden of trace elements may not reflect the “locally-derived” sources (Baxter et al., 2005).
Among different metals, the organic form of Hg, methylmercury (MeHg), is the only trace element consistently showing strong biomagnification among diverse food webs, including both aquatic and terrestrial ecosystems (Lavoie et al., 2013; Selin, 2009; Tsui et al., 2019). However, for many metals they have not been found to consistently biomagnify (Cardwell et al., 2013) but exceptions were reported before. For example, Croteau et al. (2005) reported biomagnification of cadmium (Cd) but not copper (Cu) in a freshwater food web consisting of epiphytic algae, invertebrates and fishes, leading to 15 times difference of Cd concentrations within two trophic levels. Their results on Cu would be similar to those of another study with Florida apple snails (Hoang et al., 2008). In other cases, strong biodiminution has been shown in food webs for elements such as arsenic (As) and lead (Pb) (Chen and Folt, 2000), showing empirical evidence of the food web regulation of toxic elements. However, somewhat more conflicting results have been observed among terrestrial food webs (Dar et al., 2019).
In view of the increasing anthropogenic pollution and global climate change, this study was aimed to provide a baseline characterization of food web trophic transfer of trace elements among freshwater and forest food webs in relatively pristine forested watersheds to allow any future comparison. Specifically, we examined the bioaccumulation and trophic transfer of four common trace elements, As, Cd, Cu and zinc (Zn) in freshwater and terrestrial food webs in a semi-remote forested watershed at northern California (United States of America) without any known local point sources. The study ecosystem has been previously assessed for biogeochemical Hg cycling and the Hg levels were similar to many other background ecosystems receiving mainly atmospheric deposition (Tsui et al., 2009; Tsui et al., 2019). Cu and Zn are essential micronutrients and are responsible for the metabolism of the organisms, whereas As and Cd are generally classified as non-essential elements with no recognized biological role in humans (Kaur et al., 2023). These four trace elements were selected since they could be grouped as essential vs. non-essential elements, cationic vs. oxoanionic speciation, we speculate that other heavy metals could indeed show similar behaviors, allowing us to determine whether it biomagnifies or biodiminishes along the food chain (Alloway, 2012; Peana et al., 2021). To our knowledge, it should be the first study to compare multiple trace elements across both aquatic and terrestrial food webs within the same pristine watershed, aiming to explore the trace element transfer and interaction between aquatic and adjacent terrestrial environment.
To understand better on the trophic transfer of these trace elements, stable nitrogen isotope (δ15N) was used to examine trophic relationships in food webs with the calculation of Trophic Magnification Factor as a common approach to evaluate the trophic transfer efficiency of trace element transfer (Lavoie et al., 2013; Pelletier et al., 2025; Tsui et al., 2019). Meanwhile, stable C isotope (δ13C) was used to track energy sources and differentiate feeding habits, explaining how the trace elements get into the food web and transfer among organisms within and between habitats (Wen et al., 2024).
Within a forest, not only nutrients such as fatty acids but also contaminants such as trace elements could be carried from aquatic habitats to terrestrial ecosystems when aquatic insect larvae emerge as adults (e.g., dragonfly and mayfly, etc.) and are consumed by riparian predators (e.g., bats, birds and spiders) (Moyo et al., 2017; Speir et al., 2014) or detritus flow with fallen terrestrial prey being eaten by aquatic predators. The interaction between these two processes remains poorly resolved. The nutritional status of organisms potentially influences the rate of contaminant uptake and accumulation (Kainz et al., 2008). This study also aims to investigate how these metal fluxes between aquatic and terrestrial systems operates.
Materials and methods
Study site, sample collection and processing
Our study sites were in the Angelo Coast Range Reserve (39.718239, −123.652512) in Branscomb, Mendocino County of northern California, United States (Tsui et al., 2009; Tsui et al., 2019). Briefly, invertebrate samples were mainly collected in three locations: i) a section of South Fork Eel River, a third order river, within the reserve with a drainage basin of 150 km2 and a canopy cover of ∼39%; ii) a riparian area within 5 m of the stream bank, and iii) an upland forest with mixed deciduous and coniferous stands.
In the river, basal resources including submerged leaf litter, filamentous alga (Cladophora glomerata), and nitrogen-fixing cyanobacterium (Nostoc pruniforme) were collected by personnel wearing non-powdered vinyl gloves. Biofilm on the substrates was scraped with acid-washed plastic brushes, followed by rinsing them with river water into an acid-cleaned Teflon bottle. Invertebrates were collected with clean stainless-steel forceps and nets, followed by transferring them into clean tubes individually while fish were collected with clean dip nets. In the riparian zone, spiders and emerging aquatic insects were collected by clean forceps and nets. In the upland forest far from any water sources (> 100 m), basal resources including leaf litter, surface soil (0–5 cm) and decomposing bark were collected while invertebrates were collected by clean forceps and nets and/or light traps, followed by transferring them into clean tubes or zip-lock bags individually. Samples were transported on dry ice back to the analytical laboratory, then immediately frozen at −20 °C, followed by lyophilization, and homogenization into fine powders using trace-metal clean approach (Tsui et al., 2012). All samples were classified according to their functional feeding groups with reference to previous literature (Ramírez and Gutiérrez-Fonseca, 2014).
Stable isotope analyses
Individual dry samples were weighed using a microbalance (± 0.1 mg) into a tin capsule (Costech Analytical Technologies Inc.). Samples wrapped in the tin capsules were shipped to the Arizona Climate and Ecosystems Isotope Laboratory (Northern Arizona University, Flagstaff, Arizona, United States of America) and analyzed by a Carlo Erba NC2100 Elemental Analyzer coupled with continuous-flow Thermo Scientific DELTA V Advantage Mass Spectrometer for stable carbon (δ13C) and nitrogen (δ15N) isotope ratios. The repeated analyses of a standard reference material (NIST 1547 Peach Leaves) (n = 20) gave an analytical uncertainty (2SD) of 0.12‰ for δ13C and 0.26‰ for δ15N.
Trace element determination
Individual dry samples were weighed (0.1 ± 0.01 g) into acid-cleaned Teflon digestion vessels (Savillex), and to which we added 4 mL of trace-metal grade nitric acid and 1 mL of reagent grade hydrogen peroxide (both obtained from Thermo Fisher Scientific). First, the digestion vessels were left loosely capped overnight at room temperature and on the following day, the vessels were completely sealed by a pair of wrenches, and the sample digestion was conducted at 80 °C overnight. After cooling, 1 mL of the acid digest was diluted 10 times by ultrapure water (18.2 MΩ/cm; Thermo Scientific Barnstead). Each batch of digestion includes reagent blank, duplicates of selected samples, and standard reference materials (SRMs: NIST-1515 Apple Leaves; TORT-3: Lobster hepatopancreas; DORM-4: Fish protein). All diluted samples were analyzed by PerkinElmer NexION 300 ICP-MS while here we only reported results for Cu, Zn, Cd, and As. The mean recoveries of trace elements from the three SRMs were as below: NIST-1515 Apple leaves (110% for Cu; 96% for Zn; 117% for Cd; not certified for As; n = 3); TORT-3 Lobster hepatopancreas (111% for Cu; 97% for Zn; 97% for Cd; 109% for As; n = 8); and DORM-4 Fish protein (122% for Cu; 99% for Zn; 104% for Cd; 97% for As; n = 7). Data were reported as µg/g on a dry mass basis and expressed as median ± mean absolute deviation (MAD).
Trophic magnification factor (TMF)
To quantitatively evaluate this trophic transfer process for all four elements, the trophic magnification factor (TMF) was used and was statistically derived (Fisk et al., 2001). It should be noted that TMF is essentially the same as the trophic magnification slope commonly used by other studies (e.g., Lavoie et al., 2013). In brief, TMF was estimated through the linear regression between δ15N and the log10-transformed elemental concentration (i.e., log10[M]). According to Boldrocchi et al. (2021), the equation of the linear regression could be written as: log10[M] = a + b * (δ15N), where a and b refer to the y-intercept and slope of the equation, respectively, and TMF = 10b. Biomagnification of trace elements in TMF > 1 and TMF < 1 respectively indicates biodiminution (Boldrocchi et al., 2021).
Statistical analysis
All data analyses were conducted using R Studio. Shapiro-Wilk tests were conducted to test the normality of all data before further analysis. Due to the violation of the normality test the non-parametric Wilcoxon/Kruskal-Wallis (Rank Sums) test and the Post hoc Dunn’s test were used to evaluate if any difference in metal concentration exists between habitats and different habitat groups. Also, non-parametric Spearman correlations were used to investigate the relationship between trace elements. Finally, a linear regression was used to evaluate trends in metal concentration and relative trophic position to derive TMF. All statistical tests were statistically significant if p is < 0.05. All units are expressed in μg g-1 for all concentration analysis.
Results & Discussion
Food web structures
In the aquatic–riparian ecosystems, the basal resources consist of benthic algae (e.g., algal biofilms, Cladophora and Nostoc), coarse particulate organic matter (CPOM), and macrophytes. These basal resources are consumed by primary consumers such as filter-feeding mussels and fly larvae, as well as scrapers including mayflies and shrimps. Secondary consumers include carnivorous invertebrates (Hemiptera, Odonata and Trichoptera) and insectivorous fishes (Cypriniformes and Salmoniformes). At the top of the aquatic food web, terrestrial riparian predators such as spiders (Araneae) feed on emerging aquatic insects, thereby linking the aquatic and riparian ecosystems.
In the adjacent forest system, basal resources are represented by leaf litter and detritus. These support detritivores (Blattodea, Polydesmida) and herbivores (Hemiptera, Orthoptera). These groups are in turn preyed upon by carnivorous invertebrates (i.e., Coleoptera and Lithobiomorpha), fed by upper predators like scorpions and spiders. The forest food web thus tends to be more detritus-based compared to the algal–detrital mixture comprising the base of the aquatic food web.
Figure 1 and Supplementary Figure S1 summarize the isotopic data of both δ13C and δ15N in the stream and riparian food webs and the forest food webs. It first appears that the stream and riparian food webs (from −29.7 to −13.2‰) have a much wider range of δ13C than their terrestrial counterparts (from −28.7 to −22.7‰). However, the high values of δ13C were mainly derived from a cyanobacterium (Nostoc) and biofilm in the stream, while the rest of the food webs have a slightly narrower range (Supplementary Figure S1, left). The large variation of δ13C in these basal resources (e.g., Cladophara, Nostoc) may be due to the difference in water velocity, and CO2 availability in the stream, as revealed by a previous study (Finlay et al., 1999). Therefore, the high bioavailability of CO2 favors the assimilation of C in Nostoc, however, it is speculated that the cyanobacterium has little contribution, if any, to the stream food web, as revealed by the disconnected energy flow (i.e., mismatched δ13C signals) to the higher trophic level animal consumers.
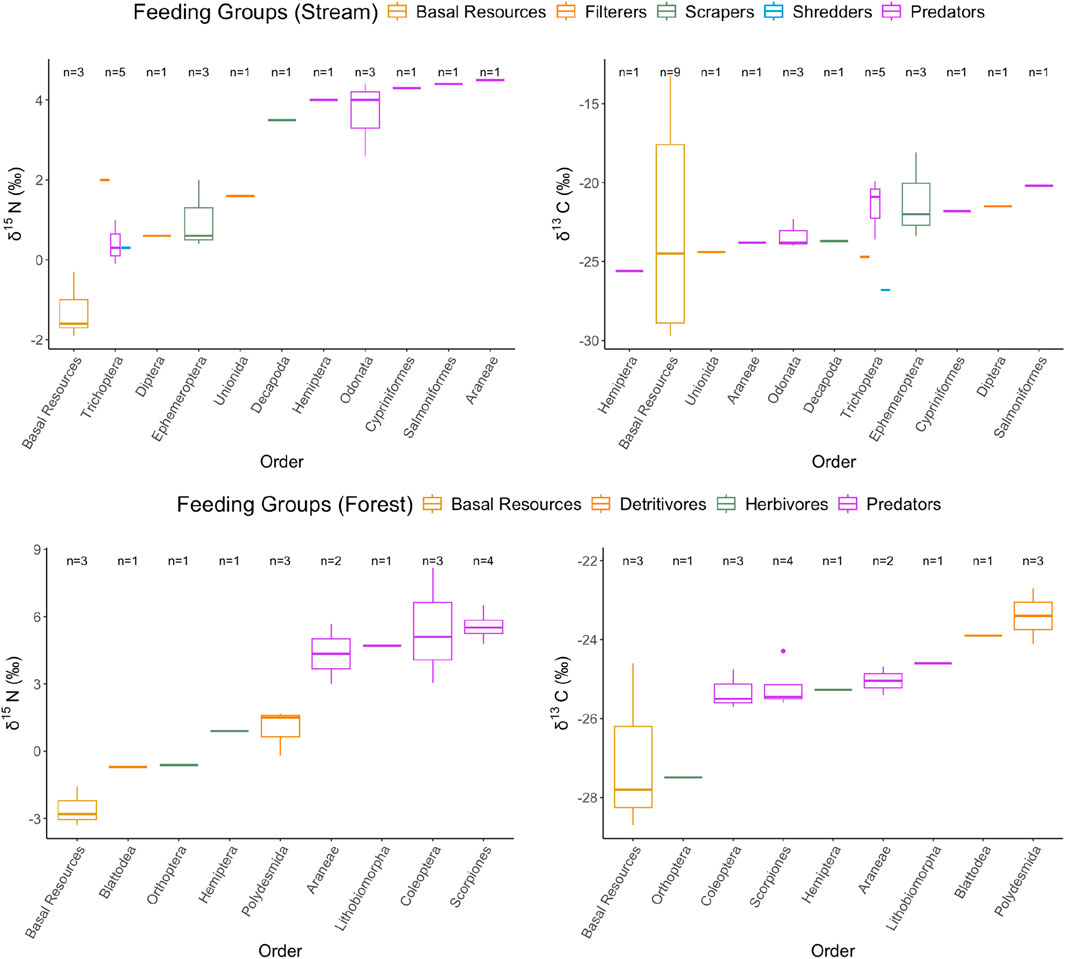
Figure 1. Boxplots of δ13C and δ15N value of all samples collected in the stream and terrestrial food web in the study, the order of samples in x-axis are arranged in ascending median value, functional feeding group are classified by colors.
Among the forest food webs, millipedes showed the highest value of δ13C (at −22.8‰) while (C3) leaf litter had the lowest value (at −28.8‰). Overall, it appears that all aquatic and terrestrial animal consumers had a narrower range of δ13C than the basal resources (litter and algae) in their respective habitats, with the terrestrial animal consumers having a much narrower range of δ13C than their aquatic counterparts (Supplementary Figure S1). For the forest food webs, it has been hypothesized that the microbial processing of leaf litter would shift the δ13C toward higher values in the terrestrial habitats and that would represent the ultimate energy sources to the terrestrial invertebrates (Hyodo, 2015).
For δ15N, all the basal resources in both habitats ranged from −3.3 to −0.5‰, and the animal consumers in general had a stepwise increase with an overall range from −1.1‰ to 7.6‰. Interestingly, while the “baseline” δ15N appeared to be similar for both forest and stream, the δ15N for the terrestrial invertebrate consumers is much higher than those of stream predatory invertebrates (max. ∼8.2‰) and stream fish (max. ∼4.5‰) with a much higher averaged trophic levels (TL) of predators in terrestrial environment (TLForest: 3.29 ± 0.45; TLStream: 2.26 ± 0.54). One explanation is that the lengthened food chain in the forest is due to the involvement of microbial decomposition of leaf litter and that may add additional trophic steps above the basal resources (Steffan et al., 2017), which if true can enhance the biomagnification of trace elements, if any.
The dual isotope plots (δ13C vs. δ15N, i.e., Supplementary Figure S1) revealed that the studied organisms are representing the typical food webs of both habitats. As expected, δ15N values increase progressively from basal resources through primary consumers to higher trophic consumers, reflecting the stepwise enrichment of the stable N isotope with each level of trophic transfer.
Trace element bioaccumulation
This study focused on two essential elements (Cu and Zn) and two non-essential elements (As and Cd) (Figure 2). For Cu, the lowest averaged concentrations were found in biofilms and autotrophs while the highest averaged concentration were not found in predators. Zn shows a similar trend to Cu. Like Cu and Zn, Cd was found to be averagely lowest in biofilm and autotrophs, however, only predators in forest environments exhibit the highest mean Cd level. Unlike the others, the lowest mean As levels were found in predators and detritivores in forest (which showed the highest averaged concentration in Cu and Zn).
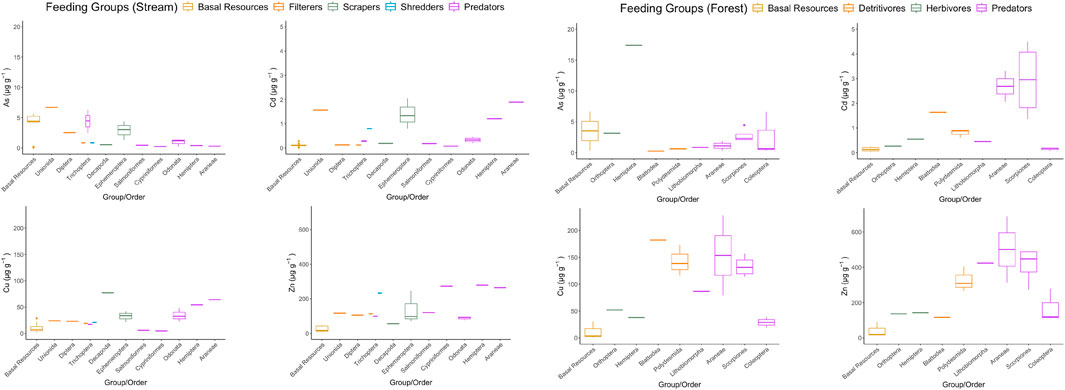
Figure 2. Boxplots of arsenic (As), cadmium (Cd), copper (Cu) and zinc (Zn) concentrations in the stream and riparian food webs (left) and the forest food webs (right) in this study. Note that the order of samples in the x-axis is arranged in ascending median value; functional feeding groups are classified by colors.
Comparing the food webs between habitats, significant differences existed for Cu, Zn and Cd (Kruskal-Wallis H test, p < 0.05). In general, trace element concentrations were higher in the terrestrial food webs. However, there was no significant difference in trace element concentrations between the basal resources in both habitats. When focusing on predators and invertebrates, significant variations were observed for Cu and Zn (Figure 2), for example, crayfish and fish in the aquatic environment.
Crayfish had higher Cu and Zn levels than expected (log Cu: 1.89, log Zn: 1.75) (Figure 2), which were also observed in another research (Balzani et al., 2021). Both are essential elements to the crustaceans, which are preferably accumulated in their body. For example, high level of Cu is associated with the needs of hemocyanin metabolism in their hemolymph, which is copper-dependent and crucial in transporting oxygen through the body and replacing hemoglobin (Dairain et al., 2019). It is a protein cofactor for a number of enzymes. Many enzymes, such as carbonic anhydrases and other biological processes, are required for metabolism, e.g., protein production requires Zn (Griboff et al., 2018), thus an enhanced level of Zn is observed in Decapoda.
Trace element concentrations on biofilms exceeds those measured in some aquatic vertebrates (e.g., log Cu: 1.41 in algal biofilms and 0.76 in Salmoniformes). Since biofilms colonize on both substrates and organismal surfaces, they may act as source from two ways, not only for filter feeders ingesting biofilm directly (internal assimilation), but also for organisms who could host biofilm growth on their body surface (external adsorption) (Barnes et al., 2024; Cetinić et al., 2021). Consequently, together with the isotopic data, the presence of biofilm associated metals could be potentially recognized as a pathway influencing metal dynamics especially at the base of the aquatic food webs, elevating the levels in some of the aquatic invertebrates (e.g., log Cu: 1.38 in Unionida). However, these metals are not internalized and may be loosely associated, making them prone to desorption to water and less available for trophic transfer which warrants further studies in testing this hypothesis.
Metallothionein naturally exists in organisms especially in vertebrates (Newman, 2009). Their functions are to regulate the uptake, accumulation, and excretion rates of these trace elements in biota (Griboff et al., 2018). This would explain a lower value of Cu was observed in fish (log Cu: 0.664 in Cypriniformes, log Cu: 0.763 in Salmoniformes) than in other aquatic invertebrates (e.g., log Cu: 1.89 in Decapoda) (Figure 2; Supplementary Table S2). Similar to Cu, Zn is essential for many biological functions, such as metabolism processes that the organisms would preferably be preserved in their organs for later excretion (Rainbow, 2007). This may be a possible explanation why Zn could be maintained at a high level in fish, while Cu is biodiluted in their body (log Cu: 0.664, log Zn: 2.44 in Cypriniformes) (Figure 2; Supplementary Table S2). Some shredders prevent feeding on leaf detritus that are contaminated (Schaller et al., 2011), therefore, their decomposition rate is weakened (Schultheis et al., 1997). The trace element is then accumulated in their body instead. It may be an explanation why the shredders, Lepidostomatidae, are rich in metal content with respect to their trophic levels (e.g., log Zn: 2.37) (Figure 2; Supplementary Table S1).
Meanwhile, a relatively low level of Cd was observed among vertebrates (e.g., log Cd: 1.12, −0.909 in Cypriniformes and Salmoniformes, respectively) (Figure 2; Supplementary Table S2). Cd exists in the stream and cannot be easily absorbed and preserved in muscle tissues. Meanwhile, it is accumulated in organs such as livers, and gills, instead of muscles (Griboff et al., 2018) that would be regulated more easily. At the same time, these elements can be absorbed by chitinous exoskeletons (Pereira et al., 2010) that may not be consumed directly by their predators. Therefore, Cd might be biodiluted at a higher trophic level (Campbell et al., 2005). A remarkably high value of trace elements was observed in millipedes, especially Cu (log Cu: 2.24) (Figure 2; Supplementary Table S3), which may be due to the great necessity of Cu to produce hemocyanin in millipedes (Jaenicke et al., 1999). Such a phenomenon may also be applied to other detritivores in the study such as termites.
Relationships between trace element and stable isotope values
Bioaccumulation indicates the adsorption of trace elements in organisms in the natural environment over time (Croteau et al., 2005). When it transfers to a higher trophic level along the food chain, biomagnification occurs. Trace elements vary in term of their chemical properties that their fate would be different when entering the food web. It depends on the strategies used by the organisms, biomagnification could occur if there are clear excretion strategies (Loureiro et al., 2018). The trophic magnification factor (TMF) was calculated to explore the potential of biomagnification of trace elements in the food web (Croteau et al., 2005). The range of TMFs varied from 0.769 (As) to 1.39 (Zn) in aquatic environments and from 0.982 (As) to 1.26 (Zn) in terrestrial environments. Statistically significant biomagnification (p < 0.05, TMF > 1) was evidenced for Cu, Zn, and Cd in both habitats (Figure 3). Regression model showing TMF > 1 with p < 0.05 was found for As in streams while no significant relationship (p > 0.05) was found for As in forest (Figure 3).
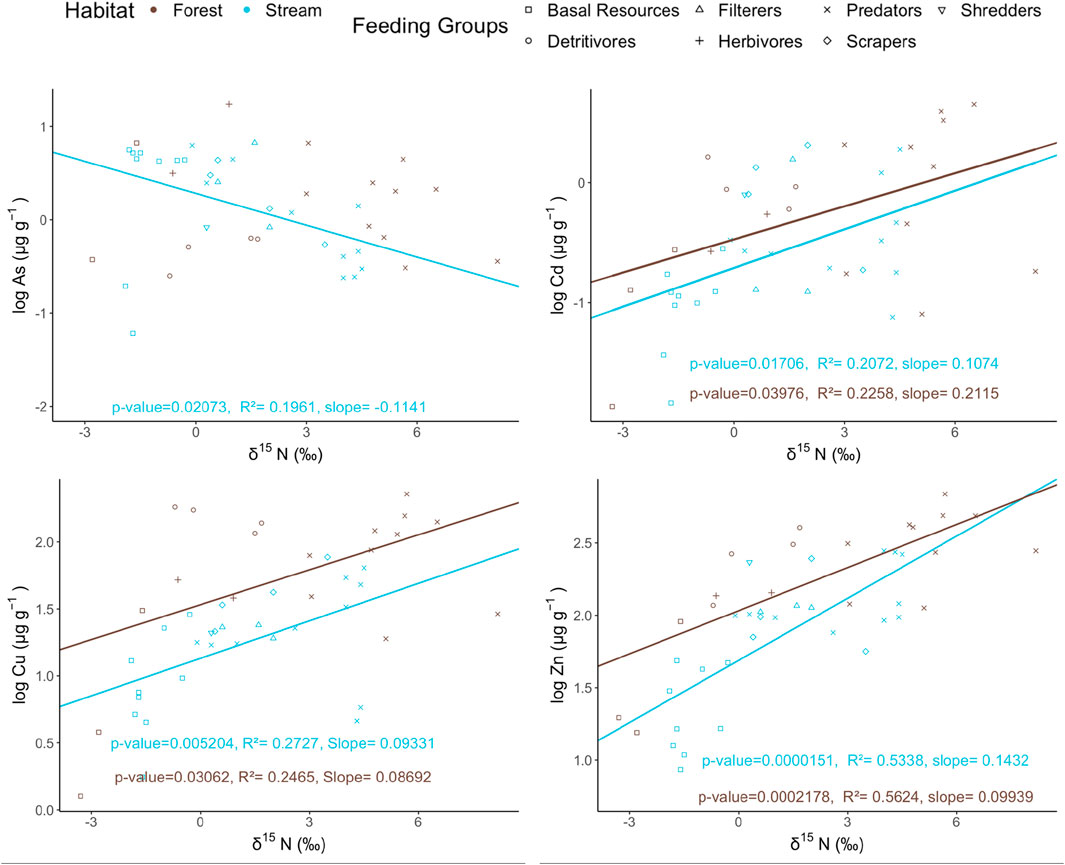
Figure 3. Comparison of the relationship between δ15N and the log-transformed trace element concentrations (μg g-1 dry weight) in both habitats, where blue and brown points represent the stream and forest ecosystems, respectively.
In general, biomagnification of MeHg has been observed across many different aquatic studies (Lavoie et al., 2013) and relatively limited in terrestrial studies (Tsui et al., 2019), which all showed a positive relationship between the concentration of MeHg and relative trophic position (i.e., TMF > 1). However, it is still a bone of contention in other trace elements, including the target metals in the study. Previous studies have shown that the TMF value in temperate stream and forest food webs would be ∼1.6 and ∼1.7 respectively (Lavoie et al., 2013; Tsui et al., 2019), which is showing higher biomagnifying effect of MeHg than other trace elements along the food webs. While some previous studies reported biomagnification of some trace elements (Campbell et al., 2005; Majer et al., 2014; Ward et al., 2012), direct comparison with our findings is not feasible because the species involved, the length of the food webs, and the environmental parameters between habitats were very different.
Our studied food webs mostly consisted of basal resources and invertebrates (which accounted for 96% of all samples). Basal resources such as biofilm and litter are important food sources, especially for the herbivorous invertebrates (Cain et al., 2011). In addition, the absence of essential regulatory and detoxification mechanisms in vertebrates or efficient accumulation of essential elements, such as Cu and Zn in invertebrates, contribute to the presence of biomagnification (Nfon et al., 2009).
Trophic transfer of trace elements is not solely influenced by concentration, but also by speciation, which controls their bioavailability and the extent of biological assimilation (Tessier and Turner, 1995). Cationic elements such as Cu, Zn, and Cd are more bioavailable through the way shared with essential nutrients, causing potential bioaccumulation and biomagnification (Szynkowska et al., 2018).
The opposite of biomagnification, biodiminution, was present for As in the aquatic food web only, which was similar to the previous findings in freshwater food webs (Chen and Folt, 2000). One possible explanation is related to the homeostatic control that the trace element concentration is maintained to a certain level (Nfon et al., 2009) regardless of the food source. It is more commonly found in organisms at higher trophic level, invertebrates. More details of past studies on trace element trophic transfer can be found in Supplementary Table S4.
In contrast to Cd, Cu and Zn, As largely occurs as oxyanions which are less efficiently and rapidly excreted by organisms, especially its organic form (Garbinski et al., 2019; Michopoulos, 2021), hence, it does not favor the occurrence of bioaccumulation and biomagnification (Campbell et al., 2005). In organisms, especially invertebrates, the absorbed As is preserved in organs like muscle and exoskeleton that reduce the possibility of trophic transfer as limited by the body growth (Nfon et al., 2009) or mobilize them in the form that cannot disrupt the essential biological function (Hopkin, 1989). When comparing the slopes of the regression lines between habitats, no significant variation was found among all trace elements, (ANCOVA, p > 0.05). Habitats difference does not influence the difference in TMF, the extent of biomagnification.
As exists mainly as inorganic oxyanions (arsenate AsO43- and arsenite AsO33-) in aquatic ecosystems, and thus As species are taken up less efficiently than cationic metals and are often rapidly metabolized into less toxic organic forms (e.g., arsenobetaine and arsenosugars) that are readily excreted, potentially leading to biodiminution in aquatic food webs (Garbinski et al., 2019; Kabata-Pendias, 2010). In upland forest soils, As (III) decreases on the forest floor by transforming it into the methylated forms and thus detoxifies the As content (Michopoulos, 2021), allowing higher As excretion, especially to those soil invertebrates and fungi, where a lower As concentration was observed in those detritivores (Figure 3). Further to that, an inconsistent trend between habitats suggested that As may not be efficiently retained in aquatic insects, limiting the contaminant transfer to the terrestrial ecosystems unlike cationic metals. Hence, As showed a contrasting trophic transfer between different environments, where As biodiminishes along the aquatic food web but showed insignificant relationship with trophic position in the forest food web.
Interelement relationships
From the median value (µg g-1) of the metal concentration in all samples, Zn showed the highest concentration in both habitats (Stream: 94.3 ± 46.5; Forest: 96.6 ± 47.8), followed by Cu (Stream: 20.0 ± 12.6; Forest: 20.9 ± 11.8), As (Stream: 2.0 ± 1.7; Forest: 2.5 ± 2.0) and the lowest were found for Cd (Stream: 0.2 ± 0.09; Forest: 0.2 ± 0.1). Zn and Cu are essential elements while the remaining two are non-essential. Therefore, it is obvious that the concentration of essential elements found in the samples should have a higher value as they are important components for metabolism. Zn significantly has a higher concentration than Cu, and Zn is considered to be much less toxic than Cu (Clements et al., 2013; Loureiro et al., 2018). Indeed, Cu could affect the decomposition and metabolic rates, especially in shredders (Loureiro et al., 2018) while Zn is more available in the environment (Rainbow, 2007).
To explore the relationship between trace elements, Spearman’s rank correlations tests were performed, indicating that a significant positive correlation exists among Zn, Cu, and Cd in both habitats (Figure 4; Supplementary Table S5). LogCu-LogCd had the highest coefficient (r = 0.718, 0.847 in stream and forest, respectively) while the value was the lowest in LogCu-LogZn (i.e., inter-essential-element) relationship (r = 0.441, 0.684 in stream and forest, respectively). Cu (Cu2+), Zn (Zn2+), and Cd (Cd2+) are all cationic trace metals. They could be mainly assimilated through similar transporters (e.g., calcium channels). This shared pathways may explain the positive intercorrelation and suggests that once they are bioavailable in ionic forms, organisms could accumulate them more efficiently (Marchetti, 2013). The non-essential metal Cd can mimic Zn and Cu, having the uptake and binding pathways similar to those of essential metals by competiting for transporters or metallothionein binding (Leavitt et al., 1979), potentially strengthening the interaction and metal transfer between habitats.
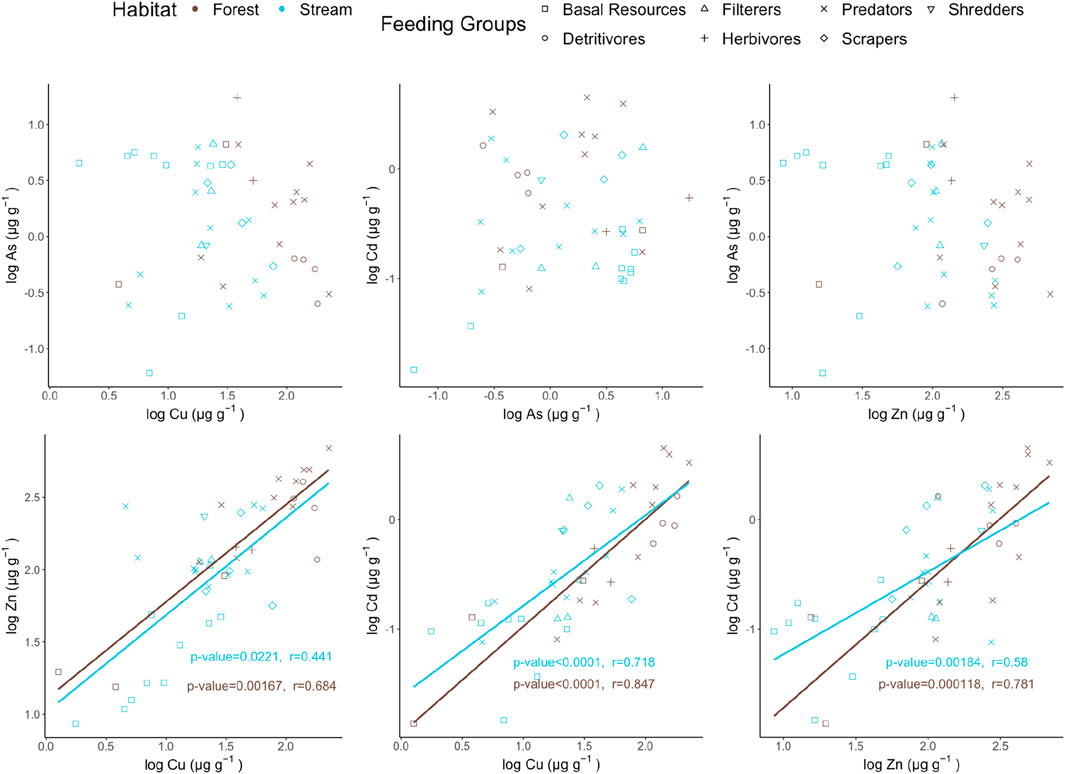
Figure 4. Correlation between trace elements by Spearman’s Rank Correlation Test, where blue and brown points represent the stream and forest ecosystems, respectively.
Metals interact in two ways, including antagonism and the stimulation of intake, which affects the amount available in the organisms (Luoma, 1983). Antagonism, the reduced effect (e.g., toxicity) due to the combination of two agents (e.g., metals) has been reported in previous studies (Olszowy-Tomczyk, 2020). Such a phenomenon has been found between Hg and Cd in crabs; Cu and Pb in phytoplankton (van Hattum et al., 1991). On the other hand, one metal bioaccumulation could be enhanced by the other metal through the production of binding sites, for example, metallothionein, as a result, both metals are more elevated (Rainbow, 2002). For example, a study on cephalopods by Bustamante, (1998) showed that metallothionein highly attracted Cu in regulating Zn.
Summary
Although it is generally assumed that trace element (except MeHg) biomagnification does not exist commonly in nature (Gray, 2002), our study shows that such a phenomenon may be more common than expected in natural food webs. From our analyses, Cd, Cu and Zn biomagnify in both freshwater and terrestrial food webs while only As biodiminishes in the freshwater ecosystem, with the latter being similar to the findings to a previous lake food web study (Chen and Folt, 2000) and showed a contrasting pattern in the terrestrial food webs. Our study adds to the growing number of investigations reporting biomagnification of Cu, Zn, and Cd in freshwater and terrestrial ecosystems (Croteau et al., 2005; Ikemoto et al., 2008).
Apart from species or individual-specific variation (i.e., metabolic rate, etc.), the element itself, for instance, the environmental bioavailability and speciation, as well as external input, such as the presence of biofilm or cross-habitat transfer, are possible drivers determining the trophic transfer of these pollutants in the natural environment, either biomagnification or biodiminution or neither nor them.
The extent of biomagnification or biodiminution could be influenced by a number of factors. These variations can be attributed to i) variations of metal bioavailability across environmental conditions (e.g., temperature, pH, etc.) and habitat types; ii) different physiological strategies among organisms to accumulate and regulate trace elements including such as storage and excretion (Rainbow, 2002); and iii) interspecific and intraspecific differences in physical and biological differences such as sex, body size, feeding preference, etc. (Augusto et al., 2022). Regarding the comparison between habitats, transfer of energy and nutrients can exist between aquatic and terrestrial habitats (Baxter et al., 2005), however, we are deemed to be of minor contribution for trace elements, especially As, which did show a consistent trend between habitats.
For future studies, we strongly suggest to 1) increase the sample size or inclusion of vertebrates, 2) explore the influence of environmental factors, and 3) examine the role of chemical speciation of different trace elements across habitats to make a more comprehensive analysis. This study establishes key baseline investigation on trophic transfer of trace elements in aquatic and forest food webs under an elevating global climate change and anthropogenic influence.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
The manuscript presents research on animals that do not require ethical approval for their study.
Author contributions
MC: Formal Analysis, Writing – original draft. MT: Project administration, Conceptualization, Supervision, Investigation, Resources, Funding acquisition, Writing – review and editing, Methodology. MMe: Methodology, Data curation, Writing – review and editing. SK: Data curation, Writing – review and editing. TH: Data curation, Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by a National Science Foundation award to MT.K. Tsui (DEB-1354811), and a competitive General Research Fund award from the Hong Kong Research Grants Council to MT.K. Tsui (14300724).
Acknowledgments
AcknowledgementsWe thank G. Rios-Sotelo for assisting sample collection in the field. We acknowledge the logistical support of Angelo Coast Range Reserve, University of California for this work.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1657891/full#supplementary-material
References
Alloway, B. J. (2012). Heavy metals in soils: trace metals and metalloids in soils and their bioavailability. 3rd ed. 2013, Vol. 22. doi:10.1007/978-94-007-4470-7Springer Nat.
Augusto, F. G., Graça, M. A., Martinelli, L. A., Caçador, I., and Arce-Funck, J. (2022). Do aquatic insects disperse metals from contaminated streams to land? Hydrobiologia 849, 1437–1451. doi:10.1007/s10750-021-04793-6
Balzani, P., Haubrock, P. J., Russo, F., Kouba, A., Haase, P., Veselý, L., et al. (2021). Combining metal and stable isotope analyses to disentangle contaminant transfer in a freshwater community dominated by alien species. Environ. Pollut. 268, 115781. doi:10.1016/j.envpol.2020.115781
Barnes, J., Balestra, B., Knee, K. L., Frederick, J. A., Landaverde, N., and Meiller, J. (2024). Freshwater algal biofilm assemblages are more effective than invertebrate assemblages at aggregating microplastics. Heliyon 10 (1), e23239. doi:10.1016/j.heliyon.2023.e23239
Baxter, C. V., Fausch, K. D., and Saunders, W. C. (2005). Tangled webs: reciprocal flows of invertebrate prey link streams and riparian zones. Freshw. Biol. 50, 201–220. doi:10.1111/j.1365-2427.2004.01328.x
Boldrocchi, G., Spanu, D., Mazzoni, M., Omar, M., Baneschi, I., Boschi, C., et al. (2021). Bioaccumulation and biomagnification in elasmobranchs: a concurrent assessment of trophic transfer of trace elements in 12 species from the Indian Ocean. Mar. Pollut. Bull. 172, 112853. doi:10.1016/j.marpolbul.2021.112853
Bustamante, P. (1998). “Bioaccumulation des éléments traces (métaux et terres rares) chez les mollusques céphalopodes et bivalves pectinidés,” in Implication de leur biodisponibilité pour le transfert vers les prédateurs. Ph.D. Thesis. Université de La Rochelle. France.
Cain, D. J., Luoma, S. N., and Wallace, W. G. (2004). Linking metal bioaccumulation of aquatic insects to their distribution patterns in a mining-impacted river. Environ. Toxicol. Chem. 23, 1463–1473. doi:10.1897/03-291
Cain, D., Croteau, M. N., and Luoma, S. (2011). Bioaccumulation dynamics and exposure routes of Cd and Cu among species of aquatic mayflies. Environ. Toxicol. Chem. 30, 2532–2541. doi:10.1002/etc.663
Campbell, L. M., Norstrom, R. J., Hobson, K. A., Muir, D. C., Backus, S., and Fisk, A. T. (2005). Mercury and other trace elements in a pelagic arctic marine food web (Northwater Polynya, Baffin Bay). Sci. Total Environ. 351, 247–263. doi:10.1016/j.scitotenv.2005.02.043
Cardwell, R. D., DeForest, D. K., Brix, K. V., and Adams, W. J. (2013). Do Cd, Cu, Ni, Pb, and Zn biomagnify in aquatic ecosystems? Rev. Environ. Contam. Toxicol. 226, 101–122. doi:10.1007/978-1-4614-6898-1_4
Cetinić, K. A., Previšić, A., and Rožman, M. (2021). Holo-and hemimetabolism of aquatic insects: implications for a differential cross-ecosystem flux of metals. Environ. Pollut. 277, 116798. doi:10.1016/j.envpol.2021.116798
Chen, C. Y., and Folt, C. L. (2000). Bioaccumulation and diminution of arsenic and lead in a freshwater food web. Environ. Sci. Technol. 34, 3878–3884. doi:10.1021/es991070c
Clements, W. H., Cadmus, P., and Brinkman, S. F. (2013). Responses of aquatic insects to Cu and Zn in stream microcosms: understanding differences between single species tests and field responses. Environ. Sci. Technol. 47, 7506–7513. doi:10.1021/es401255h
Croteau, M. N., Luoma, S. N., and Stewart, A. R. (2005). Trophic transfer of metals along freshwater food webs: evidence of cadmium biomagnification in nature. Limnol. Oceanogr. 50, 1511–1519. doi:10.4319/lo.2005.50.5.1511
Dairain, A., Legeay, A., Gonzalez, P., Baudrimont, M., Gourves, P. Y., and de Montaudouin, X. (2019). Seasonal influence of parasitism on contamination patterns of the mud shrimp Upogebia cf. pusilla in an area of low pollution. Sci. Total Environ. 692, 319–332. doi:10.1016/j.scitotenv.2019.07.258
Dar, M. I., Green, I. D., and Khan, F. A. (2019). Trace metal contamination: transfer and fate in food chains of terrestrial invertebrates. Food Webs 20, e00116. doi:10.1016/j.fooweb.2019.e00116
Finlay, J. C., Power, M. E., and Cabana, G. (1999). Effects of water velocity on algal carbon isotope ratios: implications for river food web studies. Limnol. Oceanography, 44 (5), 1198–1203.
Fisk, A. T., Hobson, K. A., and Norstrom, R. J. (2001). Influence of chemical and biological factors on trophic transfer of persistent organic pollutants in the Northwater Polynya marine food web. Environ. Sci. Technol. 35, 1700–1738. doi:10.1021/es010719m
Garbinski, L. D., Rosen, B. P., and Chen, J. (2019). Pathways of arsenic uptake and efflux. Environ. Int. 126, 585–597. doi:10.1016/j.envint.2019.02.058
Gray, J. S. (2002). Biomagnification in marine systems: the perspective of an ecologist. Mar. Pollut. Bull. 45, 46–52. doi:10.1016/s0025-326x(01)00323-x
Griboff, J., Horacek, M., Wunderlin, D. A., and Monferran, M. V. (2018). Bioaccumulation and trophic transfer of metals, as and Se through a freshwater food web affected by antrophic pollution in Córdoba, Argentina. Ecotoxicol. Environ. Saf. 148, 275–284. doi:10.1016/j.ecoenv.2017.10.028
Hoang, T. C., Rogevich, E. C., Rand, G. M., and Frakes, R. A. (2008). Copper uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa). Ecotoxicology 17, 605–615. doi:10.1007/s10646-008-0243-8
Hopkin, S. P. (1989). Ecophysiology of metals in terrestrial invertebrates. Elsevier Applied Science Publishers.
Hyodo, F. (2015). Use of stable carbon and nitrogen isotopes in insect trophic ecology. Entomol. Sci. 18 (3), 295–312.
Ikemoto, T., Tu, N. P. C., Okuda, N., Iwata, A., Omori, K., Tanabe, S., et al. (2008). Biomagnification of trace elements in the aquatic food web in the Mekong Delta, South Vietnam using stable carbon and nitrogen isotope analysis. Arch. Environ. Contam. Toxicol. 54, 504–515. doi:10.1007/s00244-007-9058-5
Jaenicke, E., Decker, H., Gebauer, W., Markl, J., and Burmester, T. (1999). Identification, structure, and properties of hemocyanins from diplopod Myriapoda. J. Biol. Chem. 274, 29071–29074. doi:10.1074/jbc.274.41.29071
Kabata-Pendias, A. (2010). Trace elements in soils and plants. 4th edition. Boca Raton: CRC Press. doi:10.1201/b10158
Kainz, M., Arts, M. T., and Mazumder, A. (2008). Essential versus potentially toxic dietary substances: a seasonal comparison of essential fatty acids and methyl mercury concentrations in the planktonic food web. Environ. Pollut. 155 (2), 262–270. doi:10.1016/j.envpol.2007.11.021
Kaur, H., Kaur, H., Kaur, H., and Srivastava, S. (2023). The beneficial roles of trace and ultratrace elements in plants. Plant Growth Regul. 100 (2), 219–236. doi:10.1007/s10725-022-00837-6
Lavoie, R. A., Jardine, T. D., Chumchal, M. M., Kidd, K. A., and Campbell, L. M. (2013). Biomagnification of mercury in aquatic food webs: a worldwide meta-analysis. Environ. Sci. Technol. 47, 13385–13394. doi:10.1021/es403103t
Leavitt, S. W., Dueser, R. D., and Goodell, H. G. (1979). Plant regulation of essential and non-essential heavy metals. J. Appl. Ecol. 16, 203–212. doi:10.2307/2402739
Loureiro, R. C., and Hepp, L. U. (2020). Stream contamination by trace elements: biota incorporation and phytoremediation. Acta Limnol. Bras. 32, e201. doi:10.1590/s2179-975x2219
Loureiro, R. C., Menegat, M. N., Restello, R. M., and Hepp, L. U. (2018). Incorporation of zinc and copper by insects of different functional feeding groups in agricultural streams. Environ. Sci. Pollut. Res. 25, 17402–17408. doi:10.1007/s11356-018-1971-9
Luoma, S. N. (1983). Bioavailability of trace metals to aquatic organisms - a review. Sci. Total Environ. 28, 1–22. doi:10.1016/s0048-9697(83)80004-7
Majer, A. P., Petti, M. A. V., Corbisier, T. N., Ribeiro, A. P., Theophilo, C. Y. S., de Lima Ferreira, P. A., et al. (2014). Bioaccumulation of potentially toxic trace elements in benthic organisms of Admiralty Bay (King George Island, Antarctica). Mar. Pollut. Bull. 79, 321–325. doi:10.1016/j.marpolbul.2013.12.015
Marchetti, C. (2013). Role of calcium channels in heavy metal toxicity. Int. Sch. Res. Notices 2013 (1), 1–9. doi:10.1155/2013/184360
Michopoulos, P. (2021). Arsenic in forests–a short review. Folia Oecol. 48 (1), 35–41. doi:10.2478/foecol-2021-0004
Moyo, S., Chari, L. D., Villet, M. H., and Richoux, N. B. (2017). Decoupled reciprocal subsidies of biomass and fatty acids in fluxes of invertebrates between a temperate river and the adjacent land. Aquat. Sci. 79, 689–703. doi:10.1007/s00027-017-0529-0
Nedrich, S. M., and Burton, G. A. (2017). Indirect effects of climate change on zinc cycling in sediments: the role of changing water levels. Environ. Toxicol. Chem. 36, 2456–2464. doi:10.1002/etc.3783
Nfon, E., Cousins, I. T., Järvinen, O., Mukherjee, A. B., Verta, M., and Broman, D. (2009). Trophodynamics of mercury and other trace elements in a pelagic food chain from the Baltic sea. Sci. Total Environ. 407, 6267–6274. doi:10.1016/j.scitotenv.2009.08.032
Peana, M., Pelucelli, A., Medici, S., Cappai, R., Nurchi, V. M., and Zoroddu, M. A. (2021). Metal toxicity and speciation: a review. Curr. Med. Chem. 28 (35), 7190–7208. doi:10.2174/0929867328666210324161205
Pelletier, A. R., Villamarin, F., Campos-Silva, J. V., Scabin, A. B., Doig, L. E., and Jardine, T. D. (2025). Trophic magnification rates of eighteen trace elements in freshwater food webs. Sci. Total Environ. 958, 178069. doi:10.1016/j.scitotenv.2024.178069
Olszowy-Tomczyk, M. (2020). Synergistic, antagonistic and additive antioxidant effects in the binary mixtures. Phytochem. Rev. 19 (1), 63–103.
Pereira, A. A., Van Hattum, B., De Boer, J., Van Bodegom, P. M., Rezende, C. E., and Salomons, W. (2010). Trace elements and carbon and nitrogen stable isotopes in organisms from a tropical coastal lagoon. Arch. Environ. Contam. Toxicol. 59, 464–477. doi:10.1007/s00244-010-9489-2
Rainbow, P. S. (2002). Trace metal concentrations in aquatic invertebrates: why and so what? Environ. Pollut. 120, 497–507. doi:10.1016/s0269-7491(02)00238-5
Rainbow, P. S. (2007). Trace metal bioaccumulation: models, metabolic availability and toxicity. Environ. Int. 33, 576–582. doi:10.1016/j.envint.2006.05.007
Ramírez, A., and Gutiérrez-Fonseca, P. E. (2014). Functional feeding groups of aquatic insect families in Latin America: a critical analysis and review of existing literature. Rev. Biol. Trop. 62, 155–167. doi:10.15517/rbt.v62i0.15785
Schaller, J., Brackhage, C., Mkandawire, M., and Dudel, E. G. (2011). Metal/metalloid accumulation/remobilization during aquatic litter decomposition in freshwater: a review. Sci. Total Environ. 409, 4891–4898. doi:10.1016/j.scitotenv.2011.08.006
Schultheis, A. S., Sanchez, M., and Hendricks, A. C. (1997). Structural and functional responses of stream insects to copper pollution. Hydrobiologia 346, 85–93. doi:10.1023/a:1002909914774
Selin, N. E. (2009). Global biogeochemical cycling of mercury: a review. Annu. Rev. Environ. Resour. 34, 43–63. doi:10.1146/annurev.environ.051308.084314
Speir, S. L., Chumchal, M. M., Drenner, R. W., Cocke, W. G., Lewis, M. E., and Whitt, H. J. (2014). Methyl mercury and stable isotopes of nitrogen reveal that a terrestrial spider has a diet of emergent aquatic insects. Environ. Toxicol. Chem. 33 (11), 2506–2509. doi:10.1002/etc.2700
Steffan, S. A., Chikaraishi, Y., Dharampal, P. S., Pauli, J. N., Guédot, C., and Ohkouchi, N. (2017). Unpacking brown food-webs: animal trophic identity reflects rampant microbivory. Ecol. Evol. 7 (10), 3532–3541.
Szynkowska, M. I., Pawlaczyk, A., and Maćkiewicz, E. (2018). Bioaccumulation and biomagnification of trace elements in the environment. Recent Adv. Trace Elem., 251–276. doi:10.1002/9781119133780.ch13
A. Tessier, and D. R. Turner (1995). Metal speciation and bioavailability in aquatic systems (Chichester: Wiley), 3, 103.
Tsui, M. T. K., and Wang, W. X. (2007). Biokinetics and tolerance development of toxic metals in Daphnia magna. Environ. Toxicol. Chem. 26, 1023–1032. doi:10.1897/06-430r.1
Tsui, M. T. K., Finlay, J. C., and Nater, E. A. (2009). Mercury bioaccumulation in a stream network. Environ. Sci. Technol. 43, 7016–7022. doi:10.1021/es901525w
Tsui, M. T. K., Blum, J. D., Kwon, S. Y., Finlay, J. C., Balogh, S. J., and Nollet, Y. H. (2012). Sources and transfers of methylmercury in adjacent river and forest food webs. Environ. Sci. Technol. 46, 10957–10964. doi:10.1021/es3019836
Tsui, M. T. K., Liu, S., Brasso, R. L., Blum, J. D., Kwon, S. Y., Ulus, Y., et al. (2019). Controls of methylmercury bioaccumulation in forest floor food webs. Environ. Sci. Technol. 53, 2434–2440. doi:10.1021/acs.est.8b06053
van Hattum, B., Timmermans, K. R., and Govers, H. A. (1991). Abiotic and biotic factors influencing in situ trace metal levels in macroinvertebrates in freshwater ecosystems. Environ. Toxicol. Chem. 10 (2), 275–292.
Ward, D. M., Mayes, B., Sturup, S., Folt, C. L., and Chen, C. Y. (2012). Assessing element-specific patterns of bioaccumulation across new England lakes. Sci. Total Environ. 421, 230–237. doi:10.1016/j.scitotenv.2012.01.058
Keywords: biomagnification, trophic transfer, trace elements, food webs, river, forest
Citation: Cheng ML-H, Tsui MT-K, Monteverde MR, Kwon SY and Hoang TC (2025) Trophic transfer of trace elements within and between aquatic and terrestrial food webs in a forested watershed. Front. Environ. Sci. 13:1657891. doi: 10.3389/fenvs.2025.1657891
Received: 02 July 2025; Accepted: 13 October 2025;
Published: 10 November 2025.
Edited by:
Jiang Helong, Chinese Academy of Sciences (CAS), ChinaReviewed by:
Nathalie Gassama, Université de Tours, FranceXingchun Li, Northeast Forestry University, China
Copyright © 2025 Cheng, Tsui, Monteverde, Kwon and Hoang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Martin Tsz-Ki Tsui, bXRrdHN1aUBjdWhrLmVkdS5oaw==
 Matthew Long-Hei Cheng
Matthew Long-Hei Cheng Martin Tsz-Ki Tsui
Martin Tsz-Ki Tsui Matthew R. Monteverde3
Matthew R. Monteverde3 Sae Yun Kwon
Sae Yun Kwon Tham C. Hoang
Tham C. Hoang