- 1School of Zhongbei, Nanjing Normal University, Zhenjiang, China
- 2School of Environment, Nanjing Normal University, Nanjing, China
Purpose: Biochar application is considered a promising strategy for mitigating agricultural phosphorus (P) shortages. However, it remains uncertain whether biochar enhances soil phosphorus availability for crops beyond functioning as an external phosphorus source. Additionally, the role of phosphorus-solubilizing bacteria (PSB) in phosphorus transformation during biochar amendment is poorly understood, including whether PSB exerts a net positive or negative effect.
Methods: Four different soils were incubated with or without phosphorus-depleted biochar (PDB), followed by Hedley phosphorus fractionation and leaching column experiments. Illumina MiSeq high-throughput sequencing targeting the phoD gene was used to analyze PSB diversity and community structure. Soil phosphatase activity and related properties were quantified.
Results: Hedley fractionation revealed that PDB application reduced labile phosphorus while increasing HCl-extractable and residual phosphorus across all soils. Leaching experiments confirmed that PDB reduced inorganic and total phosphorus leaching in all soil types. Acid phosphatase activity was inhibited by PDB in upland soils (a peach orchard, a tea plantation, and a vegetable field). phoD-based sequencing indicated an increased relative abundance of dominant PSB genera in upland soils but a decreased abundance in paddy soils under PDB treatment. PDB did not significantly alter PSB diversity.
Conclusion: Phosphorus-depleted biochar reduces potentially available phosphorus fractions and mitigates phosphorus leaching, supporting aquatic ecosystem protection. However, it does not enhance short-term soil phosphorus fertility in agricultural systems. Both the mechanisms underlying PSB abundance shifts after biochar application and the ways in which plants respond to the altered P fractions require further investigation.
1 Introduction
Soil phosphorus (P) depletion poses a growing global threat (Alewell et al., 2020). An estimated 29%–32% of the world’s cropland exhibits soil phosphorus deficits, despite intensive fertilizer inputs (Lun et al., 2018; MacDonald et al., 2011). This deficiency primarily stems from the formation of stable soil-bound phosphorus, as explained by the precipitation–particulate and adsorption–penetration theories (Barrow, 2020). Consequently, global phosphorus-use efficiency averages only 46%, with Eastern Asia recording the lowest value (23%) (Lun et al., 2018). Enhancing soil phosphorus utilization efficiency, particularly by converting bound phosphorus to plant-available forms rather than increasing application rates, is therefore a critical strategy for sustainable agriculture.
Biochar soil amendments can modulate plant-available phosphorus pools, especially the inorganic and soluble fractions (Hossain et al., 2020; Zhang et al., 2025). This dynamic carries significant implications for both crop productivity and aquatic ecosystem protection. The prevailing literature suggests that biochar typically increases surface soil available phosphorus by 45%–260% (Gao et al., 2019; Joseph et al., 2021). However, contradictory findings exist (Li et al., 2020), rendering biochar’s net impact contentious. Crucially, agricultural constraints more commonly involve scarcity of available phosphorus than insufficiency of total phosphorus. Biochar enriched with inherent phosphorus functionally resembles direct fertilizer application. The core agronomic value of biochar is its ability to mobilize recalcitrant native soil phosphorus to improve utilization efficiency. This function, however, requires that the confounding effects of biochar-borne phosphorus be minimized. Variations in biochar feedstock induce substantial heterogeneity in phosphorus content (Tesfaye et al., 2021), while soil type and biochar–soil interactions further modulate outcomes (Gul and Whalen, 2016).
Phosphate-solubilizing bacteria (PSB) mineralize insoluble phosphorus into plant-accessible forms and may critically mediate phosphorus bioavailability under biochar amendment (Bai et al., 2024; Fan et al., 2025). For instance, rice straw biochar stimulated Bacillus mucilaginosus phosphorus solubilization, increasing dissolved phosphorus by 43% versus controls (Lu et al., 2023). PSB exhibits high community diversity across soils (Kour et al., 2021). However, a fundamental question remains unresolved: does biochar amendment enhance or inhibit PSB-mediated phosphorus transformation?
To address this knowledge gap, we conducted an incubation study using four distinct soils amended with phosphorus-depleted biochar (PDB). Through integrated Hedley phosphorus fractionation, leaching experiments, and alkaline phosphatase gene phoD-targeted Illumina MiSeq sequencing, we aimed to a) eliminate the confounding effects of biochar-derived phosphorus, b) track transformations among soil phosphorus pools, c) quantify PDB-induced shifts in PSB abundance and diversity, and d) determine cross-soil variability in these responses.
2 Materials and methods
2.1 Preparation of soils and biochar
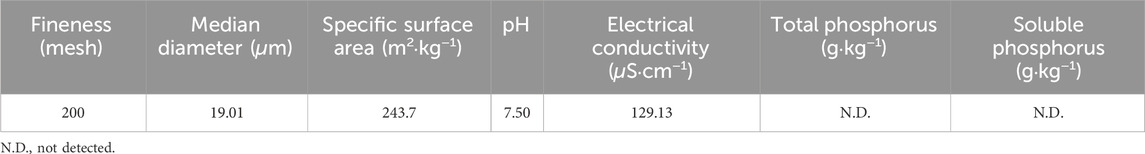
The sampling sites were located in Danyang City, southern Jiangsu Province, China, under a humid subtropical monsoon climate (mean annual temperature: 16.5 °C; precipitation: 1,043 mm). Surface soils (0–20 cm depth) were collected in February 2023 from four different field sites: a vegetable field (VF), a tea garden (TG), a rice paddy (RP), and a peach garden (PG). VF, TG, and PG represented upland soils, with VF and TG having a cultivation history of >10 years and PG approximately 5 years. RP represented flooded paddy soil and was sampled post-harvest. For each soil site, three replicate samples were collected randomly. Soils were air-dried, ground, sieved (<2 mm), and stored at 4 °C. Soil pH values were 6.2 (VF), 5.5 (TG), 5.3 (RP), and 5.3 (PG); additional properties (coordinates and phosphorus contents) are provided in Supplementary Table S1. PDB derived from coconut shells (Zhengzhou Niute Agricultural Technology Co., Ltd., Henan, China) was used, exhibiting no detectable phosphorus content (Table 1).
2.2 Soil incubation and column leaching experiment
PDB was thoroughly mixed into each soil at 5% (w/w), with unamended soils serving as controls. Treatments included four PDB-amended groups (VF + PDB, PG + PDB, TG + PDB, and RP + PDB) and four unamended controls (VF, PG, TG, and RP), each with three replicates. Subsamples from each treatment were allocated to incubation and leaching experiments. The incubation proceeded for 30 days at 20 °C in the dark. For leaching assays, 24 plexiglass columns (internal diameter: 80 mm; height: 500 mm) were prepared with a 5-cm base layer of quartz sand, overlain by 20 cm of soil (1.00 kg). Deionized water (200 mL per event) was applied in five events to simulate rainfall. The next event was initiated only after the leachate from the previous event had completely stopped flowing. The soil was maintained at saturated water content during each leaching event. Inorganic phosphorus (Pi) and total phosphorus (TP) concentrations in leachates were quantified.
2.3 Analysis of Hedley phosphorus fractions and soil properties
After incubation (as described in Section 2.2), the soil was thoroughly homogenized and quartered to obtain a representative subsample for subsequent extraction. Soil phosphorus fractions were quantified using a modified Hedley sequential extraction procedure (Yang et al., 2024). Soluble P, NaHCO3-P, NaOH-P, hydrochloric acid-extractable P (HCl-P), and residual P were extracted with deionized water, 0.5 mol·L−1 NaHCO3, 0.1 mol·L−1 NaOH, 1.0 mol·L−1 HCl, and mixed acid (H2SO4 and HClO4), respectively. The phosphorus concentrations in all extracts were determined by molybdenum antimony colorimetry using a UV-1800 spectrophotometer (Shanghai Mapada Instruments Co. Ltd., China). Soil electrical conductivity (EC) was measured in 1:5 (w/v) soil–water suspensions, while pH was determined electrometrically in 1:2.5 (w/v) suspensions (DDS-11A/PHS-3C, INASE Scientific Instruments Co. Ltd., Shanghai, China). TP was quantified after strong acid digestion using the same colorimetric method.
2.4 High-throughput sequencing of phosphate-solubilizing bacteria
Soil DNA was extracted from the incubated samples using a FastDNA™ SPIN Kit for Soil (MP Biomedicals LLC, Santa Ana, CA, United States). The phoD gene was amplified with primers phoD-733F/phoD-1083R on a GeneAmp® 9700 Thermal Cycler (Life Technologies, Foster City, CA, United States). Amplicons were sequenced on an Illumina MiSeq PE300 platform (Majorbio, Shanghai, China). The sequencing process is described by Qin et al. (2018). These sequences were deposited in GenBank under the accession numbers SRX30327776–SRX30327799. Using UPARSE (ver. 7.1, http://drive5.com/uparse/) at 97% similarity, the sequences were clustered into operational taxonomic units (OTUs) with chimera removal. Taxonomic annotation was performed using the RDP classifier. Alpha diversity was assessed using the Chao1 (richness), Shannon/Simpson (diversity), and coverage indices. The community composition analysis focused on the top eight phyla and genera by relative abundance. Sequence data will be deposited upon manuscript acceptance.
2.5 Analysis of soil acid phosphatase activity
Soil acid phosphatase (ACP) activity was determined using disodium p-nitrobenzene phosphate as the substrate. For each assay, 1 g of air-dried soil was incubated with 0.2 mL toluene (to inhibit microbial activity), followed by sequential addition of 4 mL hydrochloric acid buffer (pH 6.5) and 1 mL disodium p-nitrobenzene phosphate solution. After 1 h of incubation at 37 °C, ACP activity was quantified by measuring p-nitrophenol release at 420 nm using a UV-1800 spectrophotometer (Shanghai Mapada Instruments Co. Ltd., China).
2.6 Statistical analysis
One- and two-way ANOVA (Duncan method) and stepwise regression analysis were performed using IBM SPSS Statistics 18 (IBM, Chicago, IL, United States). Pearson correlation and stepwise regression analyses were also conducted to evaluate the relationships between PSB diversity indices and environmental factors. Redundancy analysis (RDA) was conducted using Canoco 5 software (Microcomputer Power, Ithaca, NY, United States) to examine the associations between environmental factors and PSB community composition. Figures were generated using Origin 8.0 (OriginLab Corporation, Northampton, MA, United States), and the final graphical composition (layout, color editing, and figure assembly) was completed in Adobe Illustrator CS6 (Adobe Systems, San Diego, CA, United States).
3 Results
3.1 Effects of PDB on soil properties and phosphatase activity
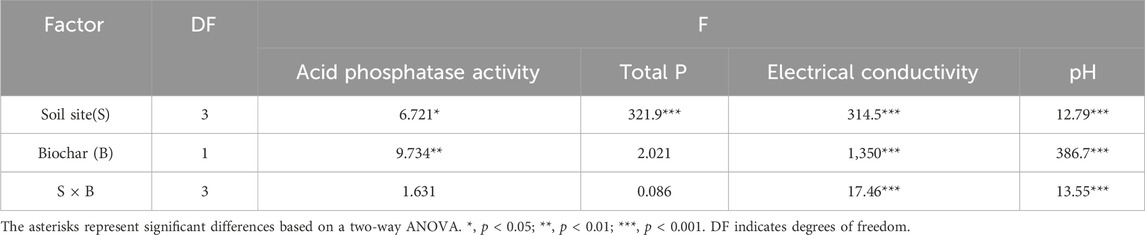
A two-way ANOVA demonstrated significant main effects of soil types and PDB amendment on all soil properties (Table 2), with Figure 1 revealing distinct variations across the four different soils. In non-amended soils, TP was highest in VF (2.32 g·kg−1), significantly exceeding the other treatments, whereas PG showed the lowest TP (0.48 g·kg−1); the addition of PDB did not significantly alter the TP content. Soil pH in unamended VF (6.21) was significantly higher than in the other untreated soils (5.25–5.46), but PDB amendment increased pH significantly (p < 0.05) across all soils tested (VF: 7.12, +0.91 increase; TG: 6.56, +1.10; PG: 7.31, +1.99; RP: 7.21, +1.93). EC exhibited the following pattern: VF (0.214 mS·cm−1) > RP (0.0615 mS·cm−1) > PG (0.0325 mS·cm−1) ≈ TG (0.0319 mS·cm−1), and the addition of PDB significantly elevated EC values by 0.121–0.1868 mS·cm−1. Crucially, the PDB application significantly reduced acid phosphatase activity (F = 9.734; p < 0.01), with VF displaying the highest baseline activity (significantly greater than that of RP/PG); after amendment, VF + PDB and TG + PDB showed significant decreases relative to their non-PDB controls. PDB did not affect acid phosphatase activity in RP soil.
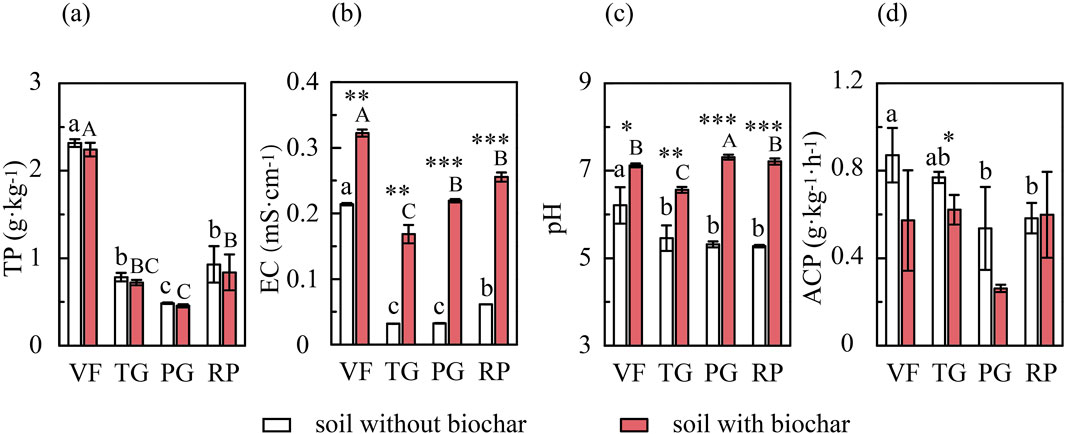
Figure 1. Effects of PDB on some soil properties in different field sites. (a) Soil total P content; (b) Soil electrical conductivity; (c) Soil pH value; (d) Soil acid phosphatase activity. VF, vegetable field; TG, tea garden; PG, peach garden; RP, rice paddy. Bars represent mean values; error bars indicate standard deviations (n = 3). Lowercase letters denote significant differences (p < 0.05) among the four tested soils without PDB addition (one-way ANOVA and Duncan’s test), whereas uppercase letters denote significant differences (p < 0.05) among the four different soils with PDB addition. Asterisks indicate significant differences between corresponding soils with (+PDB) and without (−PDB) biochar addition, determined using the independent samples t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001).
3.2 Effects of PDB on soil phosphorus fractions
P fraction changes induced by PDB amendment are shown in Figure 2. A consistent decrease in soluble P (H2O-P) occurred across all four soils (a 6.6%–35.6% reduction), whereas the HCl-P and residual P fractions increased by 5.7%–107.3% and 20.5%–62.5%, respectively. Residual P dominated in PG soils, regardless of PDB treatment, while NaOH-P and residual P prevailed in TG and RP soils. The effects of PDB on the NaOH-P and NaHCO3-P fractions were soil-specific: NaOH-P decreased in VF (by 76.0 mg·kg−1), TG (by 25.3 mg·kg−1), and RP (by 9.6 mg·kg−1) soils but increased in PG (by 24.3 mg·kg−1) soils following PDB addition. Conversely, the NaHCO3-P increased in TG (by 21.5 mg·kg−1), PG (by 5.9 mg·kg−1), and RP (by 7.1 mg·kg−1) soils, yet decreased in VF (by 58.5 mg·kg−1) soils with PDB amendment.
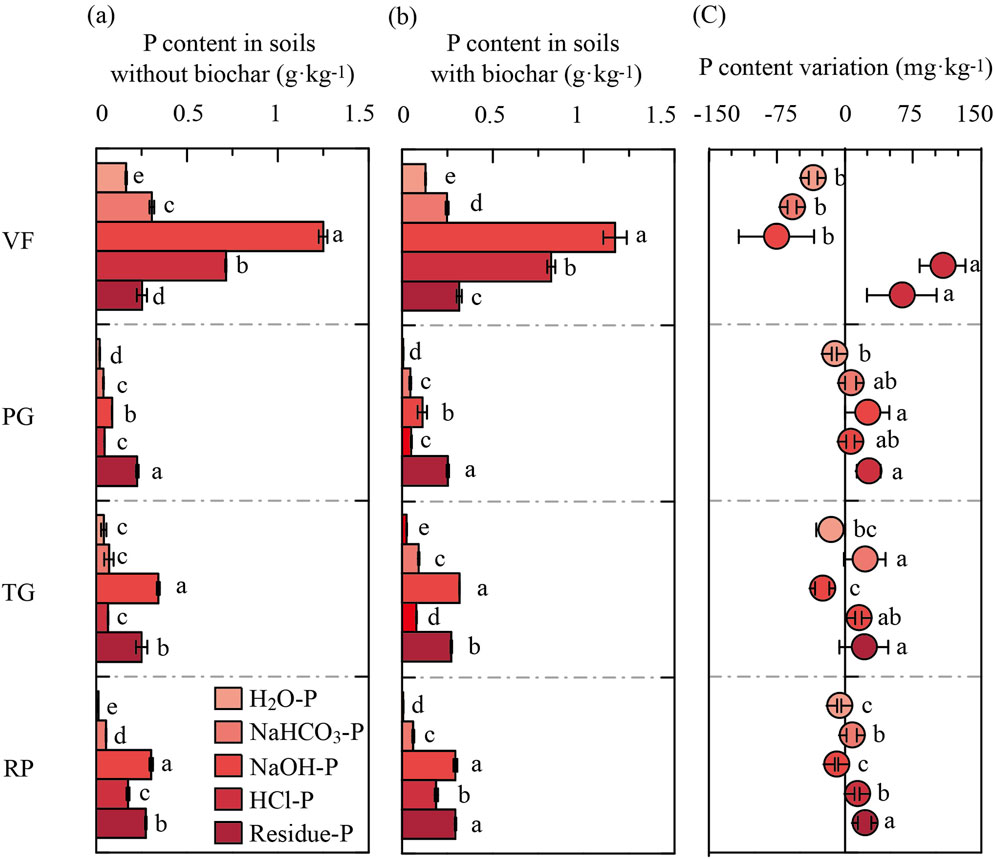
Figure 2. Effects of PDB application on soil phosphorus (P) fractions in different soil sites. VF, vegetable field; TG, tea garden; PG, peach garden; RP, rice paddy. (a) P content in soil P fractions without PDB addition. (b) P content in soil P fractions after PDB addition. (c) Change in P content of each fraction following PDB application relative to non-amended soils. Lowercase letters denote significant differences (p < 0.05) among P fractions within the same treatment. Error bars represent standard deviations (n = 3).
3.3 Effects of PDB on soil phosphorus leaching
Phosphorus leaching responses to PDB amendment across the four tested soils are shown in Figure 3. PDB addition substantially reduced the cumulative losses of TP and Pi in VF soils by 83.7% and 82.7%, respectively. Similarly, TP and Pi losses in TG, PG, and RP soils decreased by 32.95%–61.38% and 1.04%–22.39%, respectively. Notably, without PDB amendment, VF soils exhibited the highest cumulative TP and Pi losses after five rainfall leaching events, significantly exceeding all other soils.
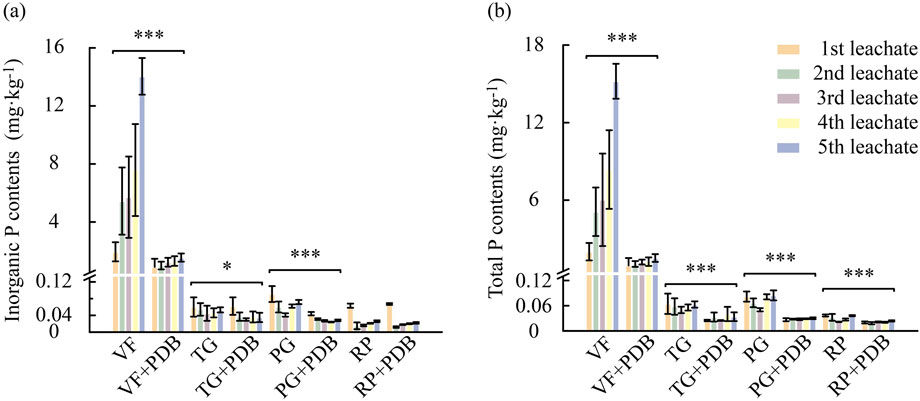
Figure 3. Effects of PDB application on leachate inorganic phosphorus (a) and total phosphorus (b) content during five sequential leaching events. Asterisks denote significant differences between the + PDB and −PDB treatments, as determined using an independent samples t-test (*, p < 0.05; **, p < 0.01; ***, p < 0.001). Error bars represent standard deviations (n = 3).
3.4 Effects of PDB on the diversity of soil PSB
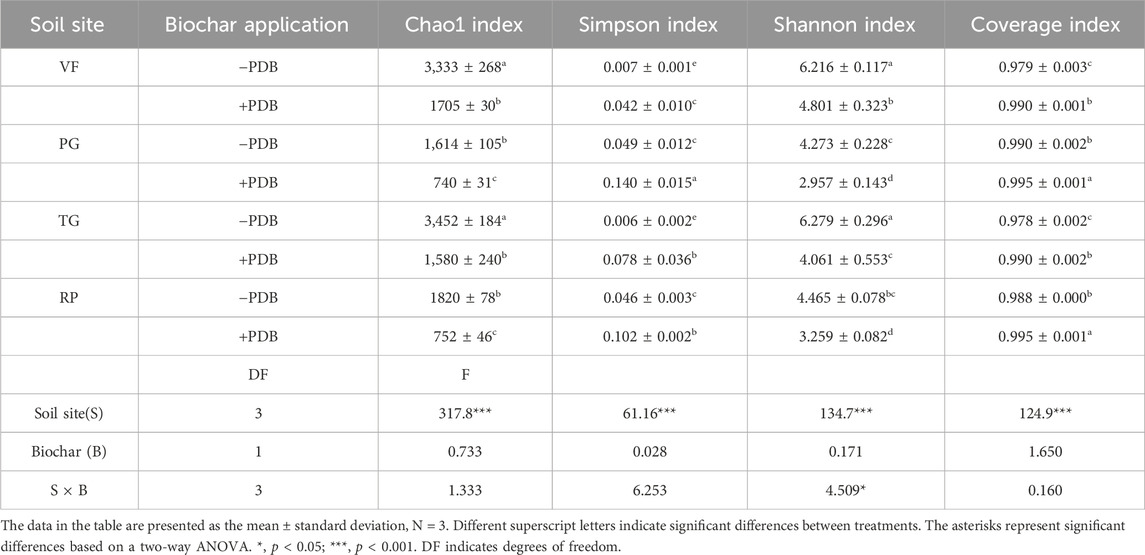
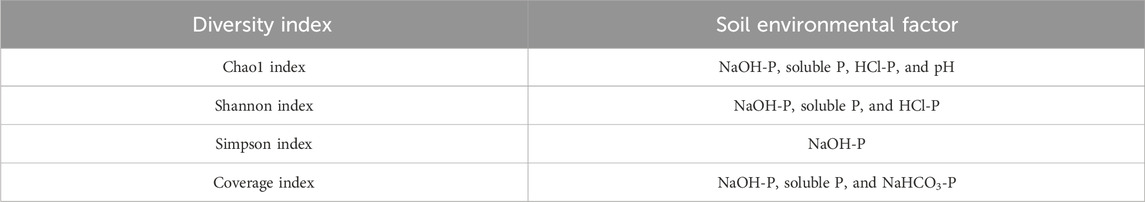
The PSB diversity indices (Chao1, Shannon, Simpson, and coverage) presented in Table 3 exhibited significant variation across the soils tested (p < 0.001), but PDB amendment did not significantly alter these metrics. Stepwise regression identified NaOH-P content as the primary predictor of α-diversity (Table 4), explaining its critical role in structuring PSB communities.
3.5 Effects of PDB on the relative abundance of soil PSB
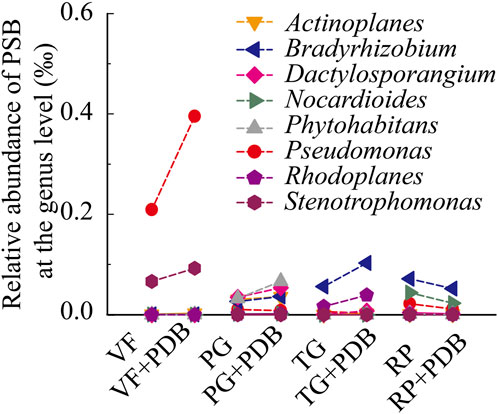
The PDB amendment increased the relative abundance of dominant genera in upland soils (VF, TG, and PG; Figure 4). According to the relative abundance data, Pseudomonas and Stenotrophomonas were the dominant genera in VF soils; Bradyrhizobium prevailed in TG; and PG was characterized by Bradyrhizobium, Dactylosporangium, Phytohabitans, and Actinoplanes. In contrast, RP soils—dominated by Bradyrhizobium and Nocardioides—exhibited decreased relative abundances of these genera post-PDB. Redundancy analysis (RDA) at the genus level (Figure 5) indicated that RDA1 (axis 1) and RDA2 (axis 2) collectively explained 50.79% of PSB community variation. NaOH-P, TP, NaHCO3-P, and ACP explained 27.6%, 14.9%, 9.5%, and 6.5% of the constrained variance in bacterial community composition, respectively. NaOH-P and TP contents were positively correlated with the abundance of Pseudomonas and Stenotrophomonas but negatively correlated with the abundance of Bradyrhizobium, Dactylosporangium, Phytohabitans, Actinoplanes, and Rhodoplanes.
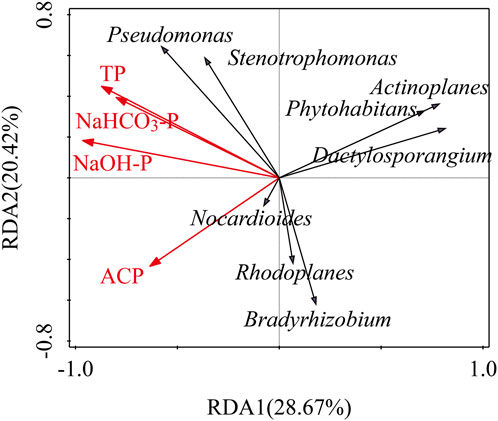
Figure 5. Redundancy analysis (RDA) illustrating the relationships between PSB community structure at the genus level and environmental factors across sample sites. Red arrows: environmental factors; black arrows: bacterial taxa abundance. Acute angles between arrows indicate positive correlations, whereas obtuse angles denote negative correlations.
4 Discussion
4.1 PDB reduces phosphorus bioavailability across different soils
Although conventional biochar typically enhances soil bioavailable phosphorus (Gao et al., 2019; Joseph et al., 2021), this study is the first to demonstrate that PDB—excluding interference from inherent phosphorus—consistently diminished potentially available P fractions in all tested soils. This finding is corroborated by our leaching experiments showing reduced soluble phosphorus loss. Our results suggest that available phosphorus reduction primarily occurs through the conversion of H2O-P into stable forms, specifically HCl-P and residual phosphorus (Figure 2). The underlying mechanisms might involve PDB-mediated transformation through the following processes: (1) precipitation with surface cations (Mg/Ca in alkaline soils; Fe/Al in acidic soils), which forms insoluble crystals (Chen et al., 2018; Dai et al., 2020) and (2) physical adsorption via porous structures and a high surface area, which limits phosphorus dissolution (Zhu et al., 2005). Crucially, phosphorus-rich biochar increases potentially available P fractions because (i) its exogenous phosphorus directly supplements plant-available pools (Liu et al., 2018) and (ii) pyrolysis-derived phosphorus compounds occupy adsorption sites, increasing the surface’s negative charge and inhibiting soil phosphorus adsorption (Zhao et al., 2017). This dual mechanism resolves the apparent contradiction in the literature regarding biochar’s phosphorus effects. It is important to note that this was a short-term incubation study, and the findings do not incorporate the potential influences of soil–plant interactions.
4.2 PDB suppresses soil phosphatase activity
ACP mediates organic phosphorus mineralization in acidic soils, a critical process in phosphorus-deficient ecosystems (Eivazi and Tabatabai, 1977; Nannipieri et al., 2011). Although phosphorus-rich biochar inconsistently affects ACP (positive: Masto et al., 2013; Lu et al., 2023; negative; Jin et al., 2016; Song et al., 2020; neutral; Yang et al., 2016; Pokharel et al., 2020), PDB consistently inhibited ACP activity in our study, which could limit the potentially plant-available P-pool in soils. We attribute this to the following: (1) direct enzyme adsorption: PDB’s porous surface adsorbs ACP, inducing conformational changes that reduce activity by >90% (Foster et al., 2018); and (2) microbial mediation: altered electron shuttling and solute transformation impair phosphatase-producing microbes (Yuan et al., 2017). Although we did not observe a significant correlation between pH and ACP, which could be attributed to the constrained sample size, it is necessary to expand the study to confirm these findings in the future.
4.3 PDB alone does not alter PSB α-diversity
Although biochar can enhance bacterial α-diversity by creating distinct microenvironments (e.g., black soil under soybean; Yao et al., 2017), the PDB amendment in our study showed no significant effect on PSB diversity indices (Table 3). This discrepancy may stem from soil-specific factors, particularly moisture and oxygen gradients, which override the influence of biochar on microenvironments. Crucially, stepwise regression identified the NaOH-P content as the primary predictor of α-diversity (Table 4). NaOH-P originates from Al/Fe-bound phosphorus and organic phosphorus in humic/fulvic acids (Qin et al., 2019). Given that NaOH-P acts as a pivotal sink/source of labile-to-stable phosphorus (Zheng et al., 2002) and correlates with dissolved oxygen dynamics (Zhang et al., 2018), we propose that PDB indirectly modulates PSB diversity through oxygen-mediated regulation of NaOH-P fractions, such as Al- or Fe-bound P, along with humic and fulvic acid P, rather than direct microbial habitat alteration.
4.4 Land-use patterns mediate PDB effects on PSB community composition
PDB amendment differentially altered PSB genus-level abundances, with dominant genera increasing in upland soils (VF, TG, and PG) but decreasing in paddy soils (RP) (Figure 4). This divergence underscores a possible effect of land use, such as water management, rather than cropping patterns, as the primary control. For example, Bradyrhizobium (a multifunctional genus involved in nitrogen fixation, denitrification, and phosphorus solubilization; Mirriam et al., 2023) exhibited opposing responses to biochar across studies: decreased (He et al., 2021; Yao et al., 2017) versus increased at low application rates (Li et al., 2022). We attribute this to PDB’s oxygen-modulating role: in flooded paddy soils, biochar improves porosity and aeration, alleviating hypoxia to favor Bradyrhizobium growth. RDA analysis indicated NaOH-P (explaining 27.6% of genus variance; Figure 5) as the key driver, consistent with its oxygen-dependent solubilization dynamics (Zhang et al., 2018). This mechanistically links PDB-induced oxygen shifts to NaOH-P bioavailability and ultimately PSB assemblage. Additionally, a negative correlation between soil total phosphorus and the relative abundance of Bradyrhizobium has been documented (Griebsch et al., 2020). However, the specific reasons for this correlation are not yet understood. We acknowledge that other soil properties not measured in this study may also have contributed to these changes.
5 Conclusion
In summary, this study demonstrates that biochar application, irrespective of its phosphorus content, alters soil phosphorus fractions, inhibits soil acid phosphatase activity, and reduces soil phosphorus loss. These findings indicate that biochar holds potential for maintaining soil nutrient status and mitigating non-point source agricultural pollution. Notably, the relative abundance of phosphate-solubilizing bacteria varied significantly across different soils. In addition, the elucidation of specific land-use types is an important field for future research. Further investigation is necessary to elucidate the interactions among biochar, phosphate-solubilizing bacteria, and soil management practices concerning soil phosphorus transformation.
Data availability statement
The authors acknowledge that the data presented in this study must be deposited and made publicly available in an acceptable repository prior to publication. Frontiers cannot accept a manuscript that does not adhere to our open data policies.
Author contributions
HQ: Writing – original draft, Writing – review and editing. XH: Formal analysis, Methodology, Writing – original draft. JC: Data curation, Methodology, Software, Writing – original draft. YZ: Data curation, Investigation, Methodology, Writing – original draft. SW: Data curation, Methodology, Software, Writing – original draft. SL: Methodology, Writing – original draft. YX: Resources, Writing – original draft. XY: Methodology, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Natural Science Research of the Jiangsu Higher Education Institutions of China (grant number: 20KJD610003).
Acknowledgments
The authors thank Mr. Pengfei Qin and Ms. Yan Tang for their assistance with sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1663371/full#supplementary-material
References
Alewell, C., Ringeval, B., Ballabio, C., Robinson, D. A., Panagos, P., and Borrelli, P. (2020). Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 11, 4546. doi:10.1038/s41467-020-18326-7
Bai, K., Wang, W., Zhang, J., Yao, P., Cai, C., Xie, Z., et al. (2024). Effects of phosphorus-solubilizing bacteria and biochar application on phosphorus availability and tomato growth under phosphorus stress. BMC Biol. 22, 211. doi:10.1186/s12915-024-02011-y
Barrow, N. J. (2020). Comparing two theories about the nature of soil phosphate. Eur. J. Soil Sci. 72, 679–685. doi:10.1111/ejss.13027
Chen, Q., Qin, J., Sun, P., Cheng, Z., and Shen, G. (2018). Cow dung-derived engineered biochar for reclaiming phosphate from aqueous solution and its validation as slow-release fertilizer in soil crop system. J. Clean. Prod. 172, 2009–2018. doi:10.1016/j.jclepro.2017.11.224
Dai, Y., Wang, W., Lu, L., Yan, L., and Yu, D. (2020). Utilization of biochar for the removal of nitrogen and phosphorus. J. Clean. Prod. 257, 120573. doi:10.1016/j.jclepro.2020.120573
Eivazi, F., and Tabatabai, M. (1977). Phosphatases in soils. Soil Biol. Biochem. 9 (3), 167–172. doi:10.1016/0038-0717(77)90070-0
Fan, B., Zhao, L., Yang, F., Zhao, C., and Li, Z. (2025). Biochar promotes phosphorus solubilization by reconstructing soil organic acid and microorganism networks. Agronomy 15, 1163. doi:10.3390/agronomy15051163
Foster, E., Fogle, E., and Cotrufo, M. (2018). Sorption to biochar impacts β-glucosidase and phosphatase enzyme activities. Agriculture 8, 158. doi:10.3390/agriculture8100158
Gao, S., DeLuca, T. H., and Cleveland, C. C. (2019). Biochar additions alter phosphorus and nitrogen availability in agricultural ecosystems: a meta-analysis. Sci. Total Environ. 654, 463–472. doi:10.1016/j.scitotenv.2018.11.124
Griebsch, A., Matschiavelli, N., Lewandowska, S., and Schmidtke, K. (2020). Presence of bradyrhizobium sp. under continental conditions in central europe. Agriculture 10 (10), 446. doi:10.3390/agriculture10100446
Gul, S., and Whalen, J. K. (2016). Biochemical cycling of nitrogen and phosphorus in biochar-amended soils. Soil Biol. Biochem. 103, 1–15. doi:10.1016/j.soilbio.2016.08.001
He, X., Augusto, L., Goll, D. S., Ringeval, B., Wang, Y., Helfenstein, J., et al. (2021). Global patterns and drivers of soil total phosphorus concentration. Earth Syst. Sci. Data 13, 5831–5846. doi:10.5194/essd-13-5831-2021
Hossain, M. Z., Bahar, M. M., Sarkar, B., Donne, S. W., Ok, Y. S., Palansooriya, K. N., et al. (2020). Biochar and its importance on nutrient dynamics in soil and plant. Biochar 2, 379–420. doi:10.1007/s42773-020-00065-z
Jin, Y., Liang, X., He, M., Liu, Y., Tian, G., and Shi, J. (2016). Manure biochar influence upon soil properties, phosphorus distribution and phosphatase activities: a microcosm incubation study. Chemosphere 142, 128–135. doi:10.1016/j.chemosphere.2015.07.015
Joseph, S., Cowie, A. L., Van Zwieten, L., Bolan, N., Budai, A., Buss, W., et al. (2021). How biochar works, and when it doesn't: a review of mechanisms controlling soil and plant responses to biochar. GCB Bioenergy 13 (11), 1731–1764. doi:10.1111/gcbb.12885
Kour, D., Rana, K. L., Kaur, T., Yadav, N., Yadav, A. N., Kumar, M., et al. (2021). Biodiversity, current developments and potential biotechnological applications of phosphorus-solubilizing and -mobilizing microbes: a review. Pedosphere 31, 43–75. doi:10.1016/s1002-0160(20)60057-1
Li, H., Li, Y., Xu, Y., and Lu, X. (2020). Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 244, 125471. doi:10.1016/j.chemosphere.2019.125471
Li, X., Romanya, J., Li, N., Xiang, Y., Yang, J., and Han, X. (2022). Biochar fertilization effects on soil bacterial community and soil phosphorus forms depends on the application rate. Sci. Total Environ. 843, 157022. doi:10.1016/j.scitotenv.2022.157022
Liu, L., Tan, Z., Gong, H., and Huang, Q. (2018). Migration and transformation mechanisms of nutrient elements (N, P, K) within biochar in straw–biochar–soil–plant systems: a review. ACS Sustain. Chem. Eng. 7 (1), 22–32. doi:10.1021/acssuschemeng.8b04253
Lu, J., Liu, S., Chen, W., and Meng, J. (2023). Study on the mechanism of biochar affecting the effectiveness of phosphate solubilizing bacteria. World J. Microbiol. Biotechnol. 39, 87. doi:10.1007/s11274-023-03533-3
Lun, F., Liu, J., Ciais, P., Nesme, T., Chang, J., Wang, R., et al. (2018). Global and regional phosphorus budgets in agricultural systems and their implications for phosphorus-use efficiency. Earth Syst. Sci. Data 10, 1–18. doi:10.5194/essd-10-1-2018
MacDonald, G. K., Bennett, E. M., Potter, P. A., and Ramankutty, N. (2011). Agronomic phosphorus imbalances across the world's croplands. Proc. Natl. Acad. Sci. U. S. A. 108, 3086–3091. doi:10.1073/pnas.1010808108
Masto, R. E., Kumar, S., Rout, T. K., Sarkar, P., George, J., and Ram, L. C. (2013). Biochar from water hyacinth (Eichornia crassipes) and its impact on soil biological activity. Catena 111, 64–71. doi:10.1016/j.catena.2013.06.025
Mirriam, A., Mugwe, J., Nasar, J., Kisaka, O., Ranjan, S., and Gitari, H. (2023). Role of phosphorus and inoculation with bradyrhizobium in enhancing soybean production. Adv. Agric. 2023 (3), 1–14. doi:10.1155/2023/3231623
Nannipieri, P., Giagnoni, L., Landi, L., and Renella, G. (2011). Role of phosphatase enzymes in soil. In: Phosphorus in action: biological processes in soil phosphorus cycling. Berlin, Heidelberg: Springer. p. 215–243.
Pokharel, P., Ma, Z., and Chang, S. X. (2020). Biochar increases soil microbial biomass with changes in extra- and intracellular enzyme activities: a global meta-analysis. Biochar 2, 65–79. doi:10.1007/s42773-020-00039-1
Qin, H., Han, C., Jin, Z., Wu, L., Deng, H., Zhu, G., et al. (2018). Vertical distribution and community composition of anammox bacteria in sediments of a eutrophic shallow lake. J. Appl. Microbiol. 125 (1), 121–132. doi:10.1111/jam.13758
Qin, G., Wu, J., Zheng, X., Zhou, R., and Wei, Z. (2019). Phosphorus forms and associated properties along an urban–rural gradient in Southern China. Water 11, 2504. doi:10.3390/w11122504
Song, X., Razavi, B. S., Ludwig, B., Zamanian, K., Zang, H., Kuzyakov, Y., et al. (2020). Combined biochar and nitrogen application stimulates enzyme activity and root plasticity. Sci. Total Environ. 735, 139393. doi:10.1016/j.scitotenv.2020.139393
Tesfaye, F., Liu, X., Zheng, J., Cheng, K., Bian, R., Zhang, X., et al. (2021). Could biochar amendment be a tool to improve soil availability and plant uptake of phosphorus? A meta-analysis of published experiments. Environ. Sci. Pollut. Res. Int. 28, 34108–34120. doi:10.1007/s11356-021-14119-7
Yang, X., Liu, J., McGrouther, K., Huang, H., Lu, K., Guo, X., et al. (2016). Effect of biochar on the extractability of heavy metals (Cd, Cu, Pb, and Zn) and enzyme activity in soil. Environ. Sci. Pollut. R. 23, 974–984. doi:10.1007/s11356-015-4233-0
Yang, Y., Zhang, J., Chang, X., Chen, L., Liu, Y., Xu, Q., et al. (2024). Green manure incorporation enhanced soil labile phosphorus and fruit tree growth. Front. Plant Sci. 15, 1356224. doi:10.3389/fpls.2024.1356224
Yao, Q., Liu, J., Yu, Z., Li, Y., Jin, J., Liu, X., et al. (2017). Changes of bacterial community compositions after three years of biochar application in a black soil of northeast China. Appl. Soil. Ecol. 113, 11–21. doi:10.1016/j.apsoil.2017.01.007
Yuan, Y., Bolan, N., Prévoteau, A., Vithanage, M., Biswas, J. K., Ok, Y. S., et al. (2017). Applications of biochar in redox-mediated reactions. Bioresour. Technol. 246, 271–281. doi:10.1016/j.biortech.2017.06.154
Zhang, Z., Hu, H., Wan, C., Peng, J., Xu, F., and Shi, F. (2018). Lateral and longitudinal variation in phosphorus fractions in surface sediment and adjacent riparian soil in the Three Gorges Reservoir, China. Environ. Sci. Pollut. Res. Int. 25, 31262–31271. doi:10.1007/s11356-018-3087-7
Zhang, L., Chang, L., Liu, H., de Jesús Puy Alquiza, M., and Li, Y. (2025). Biochar application to soils can regulate soil phosphorus availability: a review. Biochar 7, 13. doi:10.1007/s42773-024-00415-1
Zhao, S., Wang, B., Gao, Q., Gao, Y., and Liu, S. (2017). Adsorption of phosphorus by different biochars. Spectrosc. Lett. 50 (2), 73–80. doi:10.1080/00387010.2017.1287091
Zheng, Z., Simard, R. R., Lafond, J., and Parent, L. E. (2002). Pathways of soil phosphorus transformations after 8 years of cultivation under contrasting cropping practices. Soil Sci. Soc. Am. J. 66, 999–1007. doi:10.2136/sssaj2002.0999
Keywords: biochar amendment, phosphorus loss, phosphorus fractionation, phosphatase activity, phosphorus-solubilizing microorganisms
Citation: Qin H, Huan X, Cui J, Zhou Y, Wang S, Liu S, Xing Y and Yu X (2025) Effects of biochar on phosphorus fraction, phosphatase activity, and phosphorus-solubilizing bacterial abundance: a phosphorus-depleted biochar design. Front. Environ. Sci. 13:1663371. doi: 10.3389/fenvs.2025.1663371
Received: 10 July 2025; Accepted: 16 September 2025;
Published: 01 October 2025.
Edited by:
Oliver Matthias Wiche, Zittau/Görlitz University of Applied Sciences, GermanyReviewed by:
Diolina Moura Silva, Federal University of Espirito Santo, BrazilAbubakar Dar, The Islamia University of Bahawalpur, Pakistan
Manfred Sager, Bioforschung Austria, Austria
Copyright © 2025 Qin, Huan, Cui, Zhou, Wang, Liu, Xing and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongyi Qin, cWluaG9uZ3lpaUAxNjMuY29t
 Hongyi Qin
Hongyi Qin Xinyu Huan2
Xinyu Huan2