- 1Jiangxi Key Laboratory for Intelligent Monitoring and Integrated Restoration of Watershed Ecosystem, Jiangxi University of Water Resources and Electric Power, Nanchang, China
- 2Jiangxi Key Laboratory of Watershed Soil and Water Conservation, Jiangxi Academy of Water Science and Engineering, Nanchang, China
- 3Xinhua Bureau of Agriculture and Rural Affairs, Loudi, China
Iron and calcium salts are two commonly used inactivators of sediment phosphorus (P). However, there is limited research on the effectiveness of their combined use. In this study, two enclosures (each 600 m2) were constructed in a eutrophic sub-lake within Poyang Lake (the largest freshwater lake in China), to examine the inhibitory effect of iron-calcium combined treatment (Fe&Ca treatment) on sediment P release. Subsequently, a sediment incubation experiment was conducted to investigate the impact of Fe treatment alone, Ca treatment alone, and Fe&Ca treatment on sediment P release fluxes and P fractions. The enclosure experiment demonstrated that the Fe&Ca treatment resulted in a decrease of 0.65 times in non-apatite inorganic P (NAIP) content in sediment, and an increase of 2.87 times in apatite P (AP) content. The sediment P release in the treated enclosure was significantly reduced by up to 90% under alkaline conditions compared to the control enclosure. In the sediment incubation experiment, Fe&Ca treatment effectively restrained P release and enhanced sediment AP content while reduced NAIP content. The transformation from NAIP to AP was likely primarily driven by Ca treatment, while Fe&Ca treatment stabilized water pH and consequently inhibited the release of sediment P and nitrogen. This study is the first to validate this transformation pathway and its inhibitory effect on P release. These findings emphasize the effectiveness of iron and calcium combination for minimizing the risk of P release from sediments, thereby offering a promising approach for in situ P control in lakes.
1 Introduction
Phosphorus (P) is an essential limiting factor that plays a key role in controlling eutrophication in lakes. The sources of P in water include external inputs, internal releases from sediments, and a small amount of atmospheric deposition (Lynam et al., 2023; Yao et al., 2023). As an important reservoir of P in lakes, sediments can act as both “sinks”, continuously absorbing and accumulating P from the overlying water, and “sources” that release P into the overlying water (Chen et al., 2025; Xing et al., 2025). Many studies have indicated that utilizing P precipitants for in situ adsorption and immobilization of P in water and sediments represents a cost-effective and efficient approach for eutrophic lake management (Lürling et al., 2016; McCorquodale-Bauer and Cicek, 2022).
Iron (Fe) and calcium (Ca) salts are two commonly used P precipitants, and their effectiveness has been substantiated through numerous field applications (Wang and Jiang, 2016; Ou et al., 2023). For example, Li et al. (2020) investigated the effect of ferric chloride (FeCl3) on sediment P in Dianchi Lake (China), and the results showed that FeCl3 could effectively inhibit the release of P from the sediments and reduce the total P (TP) concentration of the overlying water by about 87%. Dittrich et al. (2011) added calcium hydroxide (Ca(OH)2) in eutrophic Lake Luzin (Germany), and the results showed that Ca(OH)2 increased the P sedimentation rate in the water body and significantly reduced the sediment P release flux. However, the use of Fe and Ca has some limitations. When Fe ions enter the water, it hydrolyzes and thus reduces the pH of the water, which may adversely affect aquatic organisms (Wang and Jiang, 2016; Immers et al., 2015; Smolders et al., 2001). When the water pH increases (pH > 8), the effectiveness of Fe treatment decreases because the hydroxyl group can replace the adsorbed P (Kelly Vargas and Qi, 2019). In addition, under low-redox (anaerobic/anoxic) conditions, the iron-bound P is easily released, which significantly reduces the effectiveness of the Fe treatment (Wang and Jiang, 2016; Wang et al., 2021). Ca(OH)2 treatment may lead to an increase in the pH of the water body, which may adversely affect the growth of aquatic organisms. When the water pH decreases (pH < 5.5), P immobilized by Ca compounds can be easily released, resulting in a decrease in the efficacy of Ca treatment (Dittrich et al., 2011). In addition, precipitates generated by Ca treatment may cover the surface of plants and inhibit photosynthesis (Wang and Jiang, 2016).
In some field applications, Fe and Ca were combined to solve the problem of excessive pH changes in the water. For example, Waajen et al. (2016) used FeCl3 as a flocculant and P remover in the restoration of the eutrophic lake De Kuil (the Netherlands) and used Ca(OH)2 as a buffer for the water pH. Qiu et al. (2018) investigated phosphorus removal and recovery from wastewater, resulting in the development of a novel Fe(II)-Ca synergistic process utilizing a ferrous-calcium salt complex. This innovative process reduced phosphorus removal costs by 30.39% compared to traditional methods. Waajen et al. (2016) utilized Fe and Ca for the flocculation of lake pollutants and pH buffering, while Qiu et al. (2018) mainly focused on the combined treatment of industrial wastewater with Fe and Ca. However, there is almost no research on the combined mechanism of Fe and Ca on the release of nutrients from lake sediments.
Recently, our team conducted an enclosure experiment for ecological restoration in a severely eutrophic lake, employing combined in situ remediation methods and achieving satisfactory results (Li et al., 2024). The restoration methods encompassed the addition of Fe- and Ca-loaded red soil to effectively remove excessive P from the water and suppress sediment P release. FeCl3 and Ca(OH)2 were identified as the key components responsible for successful P removal. To further investigate the synergistic mechanism of Fe and Ca in P removal, this study further analyzed the sediment P fractions in both treated and untreated enclosures. Furthermore, a sediment incubation experiment was performed using sediment collected outside the enclosures to explore different inhibitory effects on sediment P release when Fe and Ca were added separately or simultaneously. It was hypothesized that: 1) FeCl3 and Ca(OH)2 exert different effects on sediment P inhibition; 2) the combined usage of FeCl3 and Ca(OH)2 exhibits a synergistic impact on sediment P inhibition; and 3) the potential synergistic mechanism of Fe and Ca may be related to the transformation of different forms of P and pH regulation in sediments.
2 Materials and methods
2.1 Study area and sediment collection
The study area was located at the inlet of Lake Nanhu (Figure 1), on the west edge of Poyang Lake, The largest freshwater lake in China. The inlet of Lake Nanhu has a long history of poultry farming, and it is currently the tail water discharge area of a sewage treatment plant, with heavy internal and external pollution and high N and P contents in the sediments. The annual TP and TN concentrations in the overlying water of the inlet of Lake Nanhu were ranged from 0.63 to 2.03 mg/L, with an average value of 1.25 mg/L, and ranged from 1.31 to 4.65 mg/L, with an average value of 2.77 mg/L, respectively (Li et al., 2024), which were characteristic of highly eutrophic lakes in China.
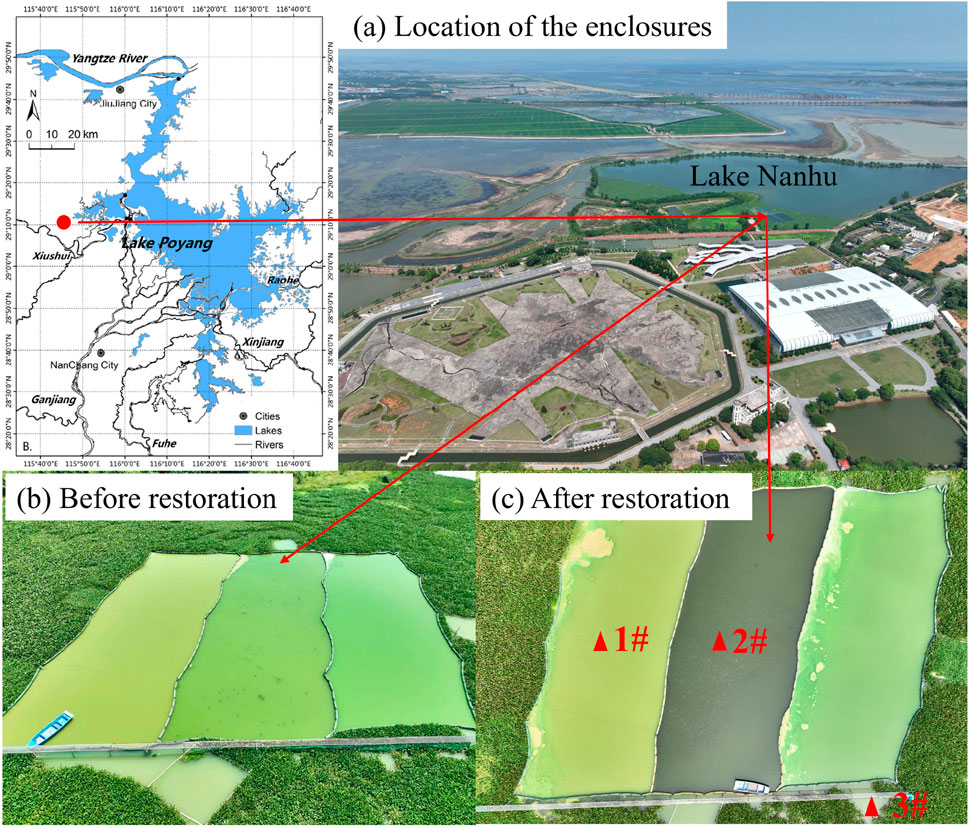
Figure 1. Location of the study area (solid red dot) and sediment sampling sites (solid red triangle). (a) Location of the enclosures. (b) Before restoration. (c) After restoration.
In September 2022, six 600 m2 impervious canvas enclosures (40 m in length, 15 m in width) were installed to study the inhibition effect of different treatments on sediment nutrients. The average water depth of the enclosures was 1.5 m. In 2023, an ecological restoration project was carried out in the enclosure 2#, and the enclosure 1# was served as the control enclosure without any treatment. The treatments in enclosure 2# involved the addition of 540 g/m2 of flocculant and 8 kg/m2 of adsorbent, following by submersed macrophytes planting (See Li et al. (2024) for details). The primary active component of the flocculant is FeCl3, while the main active component of the adsorbent is Ca(OH)2. To further study the effects of the above treatments on the sediment P fractions, surface sediments (0–10 cm) were collected in the center of enclosures 1# and 2# (Section 2.2). The 0–10 cm layer is the most active zone for phosphorus exchange. While deeper sediments may contribute, their influence is limited (Yin et al., 2022). Although submerged macrophytes were planted in the enclosure 2#, the results of a previous controlled experiment we conducted (Li et al., 2024) indicated that the influence of submerged macrophytes on sediment nutrients in the short term could be disregarded (data not published). Therefore, the observed changes in sediment P release characteristics were primarily attributed to the chemical treatments rather than biological uptake.
Furthermore, a sediment incubation experiment was performed using surface sediment collected outside the enclosures (3#) to explore different inhibitory effects on sediment P release when Fe and Ca were added separately or simultaneously (Section 2.3). The collected sediments were taken back to the laboratory and thoroughly mixed to remove impurities such as plant and animal debris and stones. A portion of the sediment was subjected to freeze-drying and pulverized into a powdered form for the purpose of determining its phosphorus fraction.
2.2 Sediment P release characteristics of enclosures 1# and 2#
Sediment P release characteristics were performed under different pH conditions (Table 1). The procedure included the following steps: 1) 1 g of dry sediments was added into flasks and 150 mL 0.02 mol/L KCl solution was added to maintain certain ion concentration; 2) 1 mol/L of HCl or NaOH solutions were added to the flasks according to the amount added in Table 1 to adjust the pH; 3) place the flasks on a thermostatic oscillator (at 25 °C) and shake them for 4 h, then let them stand for 24 h; 4) filter the extract and determine the soluble P content.
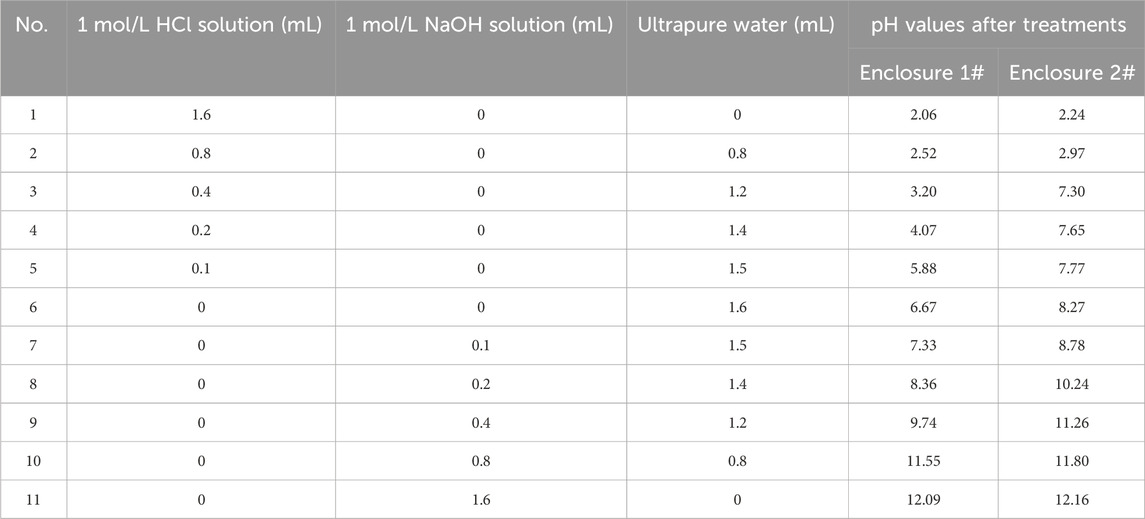
Table 1. Treatment of sediments collected from enclosures 1# and 2# and pH of the sediment extracts after treatments.
2.3 Design of the sediment incubation experiment
The collected sediments from site 3# were added to 12 cylindrical glass containers (diameter: 16.2 cm and height: 24.8 cm), each of which received 1.0 ± 0.05 kg of sediments. To determine the initial release rate of sediment nutrients, 3 L of ultrapure water was added to the sediment surface as overlying water and left for 3 days. Ultrapure water was used to eliminate interference from external ions. While it may slightly enhance release compared to lake water, it allows for better control and reproducibility. Three days later, four treatments were set up for the sediments (Figure 2). CK treatment was the control group without any treatment for the sediment. In the Fe treatment (labeled as Fe), FeCl3 solution was added to the overlying water of the sediments, with Fe3+ concentration being 90 mg/L. In the Ca treatment (labeled as Ca), Ca(OH)2 solution was added to the overlying water of the sediments, with Ca2+ concentration being 310 mg/L. In the Fe&Ca treatment (labeled as Fe&Ca), FeCl3 and Ca(OH)2 were added at the same time, with the Fe3+ concentration of 90 mg/L and the Ca2+ concentration of 310 mg/L. The amounts of Fe3+ and Ca2+ added were calculated based on the NAIP and AP contents in the sediment, aiming to achieve a 2:1 M ratio for Fe: P and a 5:1 M ratio for Ca: P, respectively, according to previous studies (Qiu et al., 2015; Li et al., 2020). These ratios are commonly applied in field restoration projects and ensure effective P immobilization. Three replicates were set up for each treatment.
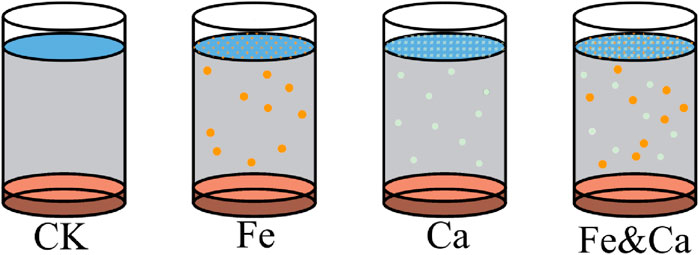
Figure 2. Different treatment groups for the two sediments. Each treatment had three replicate units. CK: sediment control group without treatment; Fe: sediment treated with ferric salt (FeCl3); Ca: sediment treated with calcium salt (Ca(OH)2); Fe&Ca: sediment treated with combined ferric and calcium salts.
The average water temperature was maintained at 26 °C ± 2 °C during the experiment. The overlying water was collected on days 0, 3, 6, 13, 20, 27, and 34 to determine the physicochemical indexes, and the overlying water in the containers was replenished with ultrapure water to the original water level after each sampling. At the end of the experiment, the overlying water was slowly poured off, and the sediments were mixed evenly, freeze-dried, and ground through a 100-mesh sieve to determine the contents of P, and N in the sediments.
2.4 Parameter measurement
TP and TN concentrations in the water and sediment were determined using standard methods (Huang et al., 1999). Five P fractions, namely, non-apatite inorganic P (NAIP, bound to Al, Fe, and Mn oxyhydrates), apatite P (AP, bound to Ca), organic P (OP), inorganic P (IP), and Total P (TP), in the sediments were determined using the Standards, Measurements and Testing Programme method (Pardo et al., 2004; Rydin, 2000).
2.5 Data analysis
The sediment nutrient release flux R (mg·m−2·d−1) was calculated as Equation 1 (Yin et al., 2022):
where V is the volume of the overlying water in the sediment column (L); Vj−1 is the volume of the j−1-th sampling from the column (L); Cn, C0, and Cj−1 are the concentration of a nutrient salt in the overlying water at the nth, the 0th (i.e., initial), and the j−1-th sampling, respectively (mg·L−1); Ca is the concentration of nutrient salts in the replenishment water (mg·L−1); S is the area of the sediment–water interface (m2); and t is the incubation time (d). The flux model assumes static conditions, which may simplify real hydrodynamic processes. Nevertheless, it provides a standardized measure for comparing treatments under controlled settings.
In this study, Origin 2022 (OriginLab Corporation, Northampton, MA, United States) was used for data processing and graphing, IBM SPSS Statistics 27 (IBM Corporation, Armonk, NY, United States) was employed for correlation analysis, and one-way analysis of variance (ANOVA) followed by Tukey’s test was applied to analyze the differences in the effects of the four treatments on P and N release from the sediments. All data were tested for normality and homogeneity before performing comparison. Data were logarithmic transformed to obtain normality and/or homogeneity if they did not meet the basic assumptions. The threshold for significance differences among groups was at the level of p < 0.05.
3 Results
3.1 Sediment P fractions of enclosures 1# and 2#
The TP content of the sediment in enclosure 1# was measured at 5.08 mg/g, while enclosure 2# exhibited a reduction of 30% compared to that observed in enclosure 1# (Figure 3a). The main component responsible for this reduction was IP, which accounted for more than 80% of TP. The NAIP and AP contents in the sediment of Enclosure 1# were measured at 4.33 mg/g and 0.36 mg/g, respectively. In comparison to Enclosure 1#, the NAIP content in the sediment from Enclosure 2# exhibited a significant decrease of 65.3%, while the AP content showed a substantial increase of 287.4% (Figure 3b).
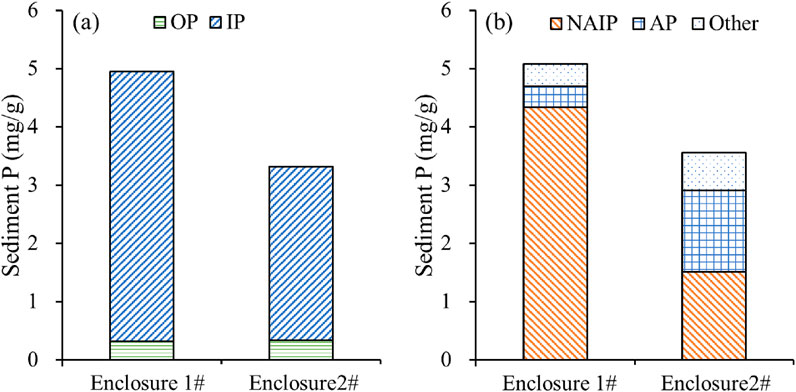
Figure 3. Sediment P fractions of enclosures 1# and 2#. (a) OP and IP; (b) NAIP and AP. “Other” refers to P fractions other than NAIP and AP, includes residual organic phosphorus and other non-extractable phosphorus forms not classified as NAIP or AP.
3.2 Sediment P release of enclosures 1# and 2#
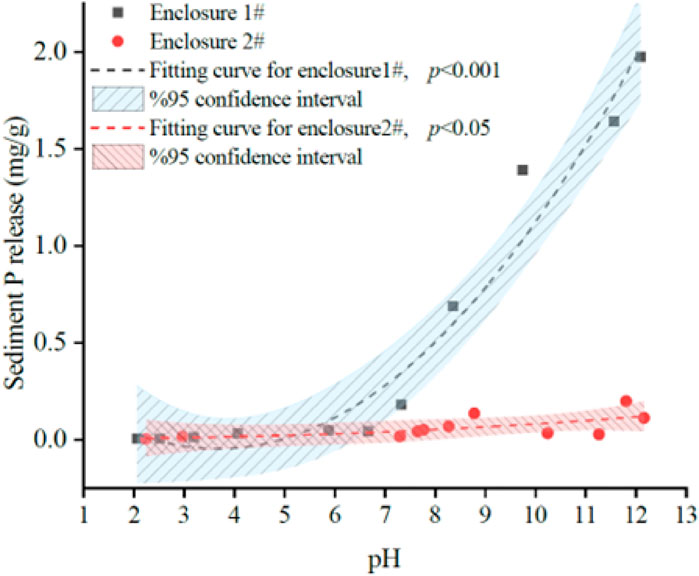
The sediment in enclosure 1# exhibited a slightly acidic with a pH of 6.67, whereas the sediment in enclosure 2# demonstrated an alkaline character with a pH of 8.27 (Table 1). The acid buffering capacity of the sediment in enclosure 2# was found to be superior to that in enclosure 1#, as evidenced by a smaller pH variation of the overlying water when a certain amount (≤0.4 mL) of 1 mol/L HCl was added.
Under acidic conditions, the P release of both sediments was minimal and exhibited a negative correlation with increasing acidity. With the increase in alkalinity, there was a significant enhancement in P release from sediments within enclosure 1#, reaching a maximum of 1.98 mg/g. In contrast, the P release trend within enclosure 2# was less pronounced and relatively slow, with a maximum release of only 0.20 mg/g, 5.7% of that observed in enclosure 1# (Figure 4).
3.3 Sediment P fractions in the sediment incubation experiment
The TP content of the CK treatment was 3.76 ± 0.13 mg/g. There was no significant difference between the Fe and CK treatments (p > 0.05). The sediment TP contents in the Ca and Fe&Ca treatments were significantly lower than the sediment TP content of the CK treatment (p < 0.05). The main component contributing to this decline was IP, which accounted for approximately 90% of TP (Figure 5a).
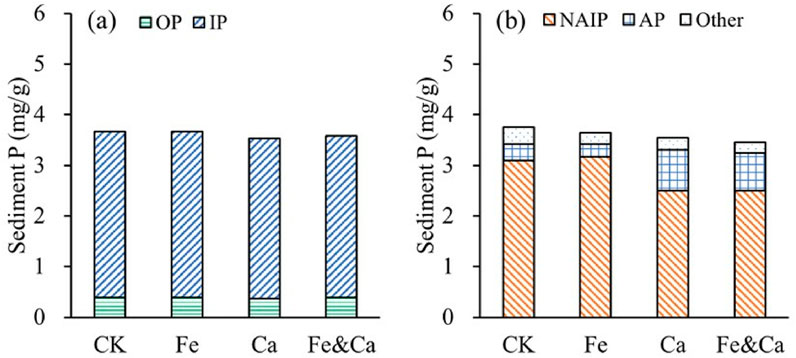
Figure 5. Sediment P fractions in the sediment incubation experiment. (a) OP and IP; (b) NAIP and AP. “Other” refers to P fractions other than NAIP and AP, includes residual organic phosphorus and other non-extractable phosphorus forms not classified as NAIP or AP.
The AP content in the CK treatment was 0.33 ± 0.03 mg/g, and the AP content in the sediments of the Fe treatment was significantly lower than that in the CK treatment (p < 0.05). The AP contents in the sediments of the Ca and Fe&Ca treatments were significantly higher than that in the CK treatment (2.48 and 2.30 times that in the CK treatment, respectively) (p < 0.05). The NAIP content in the sediment of the CK treatment was 3.10 ± 0.06 mg/g, and there was no significant difference between the Fe and CK treatments (p > 0.05). The NAIP contents in the sediments of the Ca and Fe&Ca treatments were significantly lower than those in the Fe and CK treatments (p < 0.05). Compared to CK and Fe treatments, the Ca and Fe&Ca treatments resulted in a 20% reduction in NAIP content and a 170% increase in AP content (Figure 5b).
3.4 P, N concentration in the overlying water and the P, N fluxes
The P content in the overlying water of CK treatment increased slowly with time, and the sediment P release flux was 2.78 mg·m−2·d−1 (Figure 6). In the Ca treatment, the P concentration in the overlying water decreased for a short time and then increased sharply. At the end of the experiment, the sediment P release flux was 5.54 mg·m−2·d−1, 2 times that of the CK treatment. In the Fe and Fe&Ca treatments, the P content in the overlying water was very low, and the sediment P release fluxes were −0.14 mg·m−2·d−1 and 0.37 mg·m−2·d−1, respectively, which were significantly lower than those of the CK and Ca treatments (p < 0.05).
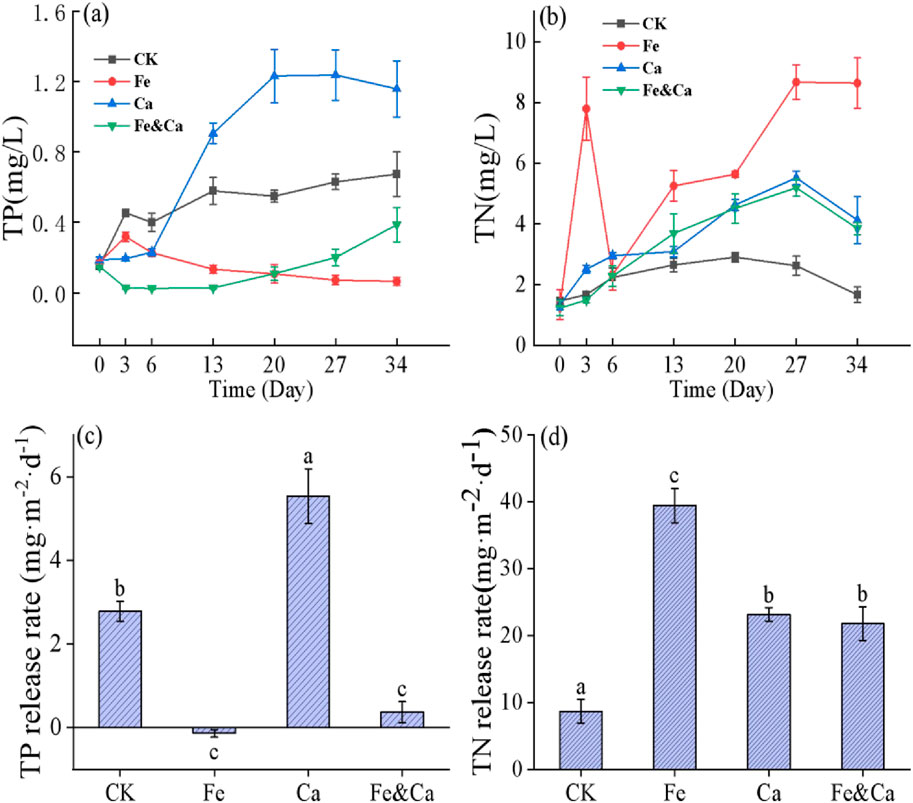
Figure 6. TP, TN concentrations in the overlying water (a, b) and the TP, TN release rates in the sediment (c, d) in different treatments. The error bars represent the standard deviation of averaged values. Different lowercase letters on the bars indicate significant differences at the p < 0.05 level among the treatments.
The N content in the overlying water of CK treatment varied little with time, and the sediment N release flux was 8.69 mg·m−2·d−1 (Figure 6). The sediment N release fluxes in the Fe, Ca, and Fe&Ca treatments were significantly higher than those of the CK treatment (4.54, 2.66, and 2.51 times those in the CK treatment, respectively). The Fe treatment exhibited the highest sediment N flux, while the Fe&Ca treatment showed a 46.2% decrease compared to the Fe treatment.
4 Discussion
The enclosure experiment demonstrated that the combined treatment of Fe and Ca shifted the sediment from weakly acidic to alkaline, resulting in a 2.87-fold increase in AP content and a 0.65-fold decrease in NAIP content, thereby enhancing the stability of sedimentary P. The present study further revealed that the combined treatment of Fe and Ca resulted in a remarkable 90% reduction in sediment P release under alkaline conditions. In water bodies characterized by intense photosynthesis, such as those affected by harmful algal blooms or excessive growth of submerged macrophytes, the pH level of water can even rise to 10 (Zamparas and Zacharias, 2014; Li et al., 2017). Hence, the combined application of Fe and Ca demonstrates great suitability for the ecological restoration project in eutrophic water, especially those with severe algal blooms. Fe and Ca are both elements that are abundant in the earth’s crust, with low costs and high ecological safety. While Fe and Ca are generally considered safe at applied doses, high concentrations may affect benthic organisms and submerged macrophytes. Large-scale application is feasible but requires site-specific optimization and further ecological risk assessment.
In the sediment incubation experiment, the Fe treatment significantly decreased the P content in the overlying water, and this reduction was sustained over time, indicating the relative stability of sediment-bound P and its limited release. Previous studies demonstrated that when the water was in an anoxic state, Fe3+ was reduced to Fe2+, which led to a secondary release of Fe-bound P from the sediments (Wang et al., 2024; Münch et al., 2024). Moreover, Fe-bound P is sensitive to water pH, and when the water pH increases, Fe-bound P is easily re-released from the sediments (Ping et al., 2023). The low release flux of sediment P observed in the Fe treatment in this study can be attributed to the high redox potential of the water, which hindered the reduction of Fe3+, and the sustained lower pH level after Fe treatment, which prevented the mobilization of Fe-bound P from sediments. However, the Fe treatment also resulted in a significant reduction of Ca-bound P in sediment, which may be due to the reduction of the water pH and the partial release of Ca-bound P because of Fe treatment (Andersson et al., 2016).
Studies have shown that when water pH is 10–12, P mainly exists in the form of H2PO4−, which easily forms insoluble hydroxyapatite precipitates with Ca, thus achieving the purpose of P removal (Li et al., 2023; Zhu et al., 2023). The present study also demonstrated that the application of Ca treatment led to a rapid reduction in the P content of the overlying water. However, over time, there was a gradual increase observed in the P content of the overlying water. This could potentially be attributed to the fact that Ca treatment led to an elevation in water pH and facilitated the release of NAIP from sediments. However, Ca treatment can facilitate the conversion of a fraction of NAIP into AP, thereby enhancing the sedimentary stability of phosphorus. Further studies should include measurements of microbial activity and organic matter decomposition to better understand these dynamics.
Qiu et al. (2015) demonstrated that the combined use of Fe and Ca could generate Fe-Ca complexes, which were effective in removing P from wastewater. The present study further demonstrated that the Fe&Ca treatment effectively suppressed sediment P release. The flux of sediment P release in the Fe&Ca treatment was comparable to that of the Fe treatment, but significantly lower than that of the Ca treatment. Moreover, the Fe&Ca treatment resulted in a significantly higher sediment AP content and a significantly lower NAIP content compared to the Fe treatment. The pH buffering capacity is primarily attributed to Ca(OH)2, which maintains alkaline conditions favorable for AP formation. FeCl3 hydrolysis mildly acidifies the water, but this is counteracted by Ca(OH)2. The synergistic pH stabilization can be represented as: Fe3+ + 3H2O → Fe(OH)3 + 3H+; Ca(OH)2 → Ca2+ + 2OH−. The OH− from Ca(OH)2 neutralizes H+ from Fe hydrolysis, thus stabilizing pH.
The increased nitrogen release in Fe, Ca, and Fe&Ca treatments may be due to several factors: (1) Fe addition could inhibit nitrification and promote dissimilatory nitrate reduction, leading to ammonium accumulation; (2) high pH from Ca(OH)2 may enhance organic nitrogen mineralization; (3) chemical treatments might disrupt sediment structure, releasing previously immobilized nitrogen. Although nitrogen release increased temporarily, the significant reduction in phosphorus release—the primary limiting nutrient in eutrophic lakes—likely outweighs this effect. These changes highlight the need to consider nitrogen dynamics when applying P immobilization agents, as unintended nitrogen release could offset eutrophication control efforts. Further studies should monitor long-term nitrogen dynamics and include nitrogen (e.g., NH4+, NO3−) speciation analysis to assess ecological trade-offs.
In conclusion, this study substantiated the hypotheses through both enclosure application and sediment incubation experiments, demonstrating distinct inhibitory effects of Fe treatment and Ca treatment on sediment P release. Furthermore, a synergistic effect was observed when both treatments were combined. However, further investigation (both incubation and field experiments) is required to fully understand the stability of the synergistic effect exhibited by the combined iron-calcium treatment across various environmental conditions, as well as to assess its long-term (>2 months or even >1 year) ecological implications.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
WL: Conceptualization, Formal Analysis, Funding acquisition, Writing – original draft, Writing – review and editing. WS: Data curation, Investigation, Methodology, Writing – review and editing. SL: Data curation, Formal Analysis, Methodology, Writing – original draft. TD: Data curation, Methodology, Resources, Writing – review and editing. JZ: Conceptualization, Funding acquisition, Supervision, Writing – review and editing. WR: Formal Analysis, Investigation, Validation, Writing – review and editing. YC: Conceptualization, Investigation, Resources, Writing – review and editing. YZ: Data curation, Investigation, Writing – review and editing. LL: Conceptualization, Funding acquisition, Project administration, Resources, Validation, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by the National Natural Science Foundation of China (52260026), the Science and Technology Project of Jiangxi Provincial Department of Water Resources (202325ZDKT09; 202426ZDKT30) and the Research Startup Fund for Introduced Talents at Jiangxi University of Water Resources and Electric Power (0101-01000576).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Andersson, K. O., Tighe, M. K., Guppy, C. N., Milham, P. J., and McLaren, T. I. (2016). The release of phosphorus in alkaline vertic soils as influenced by pH and by anion and cation sinks. Geoderma 264, 17–27. doi:10.1016/j.geoderma.2015.10.001
Chen, Y., Li, D., Liu, S., Zhang, Y., Yan, X., Song, X., et al. (2025). Long-term effects of dead algal deposition on sediment surfaces: behavior of endogenous phosphorus release in sediments. Water Res. 268, 122742. doi:10.1016/j.watres.2024.122742
Dittrich, M., Gabriel, O., Rutzen, C., and Koschel, R. (2011). Lake restoration by hypolimnetic Ca (OH)2 treatment: impact on phosphorus sedimentation and release from sediment. Sci. Total Environ. 409 (8), 1504–1515. doi:10.1016/j.scitotenv.2011.01.006
Huang, X. F., Chen, W. M., and Cai, Q. M. (1999). Survey, observation and analysis of Lake ecology. Standard methods for observation and analysis in Chinese ecosystem research network, series V.
Immers, A. K., Bakker, E. S., Van Donk, E., Ter Heerdt, G. N. J., Geurte, J. J. M., and Declerck, S. A. J. (2015). Fighting internal phosphorus loading: an evaluation of the large-scale application of gradual Fe-addition to a shallow peat lake. Ecol. Eng. 83, 78–89. doi:10.1016/j.ecoleng.2015.05.034
Kelly Vargas, K. G., and Qi, Z. (2019). P immobilizing materials for lake internal loading control: a review towards future developments. Crit. Rev. Environ. Sci. Technol. 49 (6), 518–552. doi:10.1080/10643389.2018.1551300
Li, W., Zhong, J., Yuan, G., Fu, H., Fan, H., Ni, L., et al. (2017). Stoichiometric characteristics of four submersed macrophytes in three plateau lakes with contrasting trophic statuses. Ecol. Eng. 99, 265–270. doi:10.1016/j.ecoleng.2016.11.059
Li, S. J., Lin, Z. G., Liu, M., Jiang, F. Z., Chen, J., Yang, X. J., et al. (2020). Effect of ferric chloride on phosphorus immobilization and speciation in dianchi Lake sediments. Ecotoxicol. Environ. Saf. 197, 110637. doi:10.1016/j.ecoenv.2020.110637
Li, S., Arnscheidt, J., Cassidy, R., Douglas, R. W., McGrogan, H. J., and Jordan, P. (2023). The spatial and temporal dynamics of sediment phosphorus attenuation and release in impacted stream catchments. Water Res. 245, 120663. doi:10.1016/j.watres.2023.120663
Li, W., Dai, T., Liu, J., Zhong, J., Wu, K., Gao, G., et al. (2024). Ferric-and calcium-loaded red soil assist colonization of submerged macrophyte for the in-situ remediation of eutrophic shallow lake: from mesocosm experiment to field enclosure application. Sci. Total Environ. 924, 171730. doi:10.1016/j.scitotenv.2024.171730
Lürling, M., Mackay, E., Reitzel, K., and Spears, B. M. (2016). Editorial–A critical perspective on geo-engineering for eutrophication management in lakes. Water Res. 97, 1–10. doi:10.1016/j.watres.2016.03.035
Lynam, M. M., Oriol, L., Mann, T., Dvonch, J. T., Barres, J. A., Gratz, L., et al. (2023). Atmospheric dry and wet deposition of total phosphorus to the great lakes. Atmos. Environ. 313, 120049. doi:10.1016/j.atmosenv.2023.120049
McCorquodale-Bauer, K., and Cicek, N. (2022). Zebra mussel shells as an alternative mineral resource for lime production as a phosphorus precipitant. Environ. Technol. 43 (10), 1446–1457. doi:10.1080/09593330.2020.1836029
Münch, M. A., van Kaam, R., As, K., Peiffer, S., Heerdt, G. T., Slomp, C. P., et al. (2024). Impact of iron addition on phosphorus dynamics in sediments of a shallow peat lake 10 years after treatment. Water Res. 248, 120844. doi:10.1016/j.watres.2023.120844
Ou, W. J., Lan, X., Guo, J., Cai, A., Liu, P., Liu, N., et al. (2023). Preparation of iron/calcium-modified biochar for phosphate removal from industrial wastewater. J. Clean. Prod. 383, 135468. doi:10.1016/j.jclepro.2022.135468
Pardo, P., Rauret, G., and López-Sánchez, J. F. (2004). Shortened screening method for phosphorus fractionation in sediments: a complementary approach to the standards, measurements and testing harmonised protocol. Anal. Chim. Acta 508 (2), 201–206. doi:10.1016/j.aca.2003.11.005
Ping, Q., Zhang, B., Zhang, Z., Lu, K., and Li, Y. (2023). Speciation analysis and formation mechanism of iron-phosphorus compounds during chemical phosphorus removal process. Chemosphere 310, 136852. doi:10.1016/j.chemosphere.2022.136852
Qiu, L., Zheng, P., Zhang, M., Yu, X. Q., and Ghulam, A. (2015). Phosphorus removal using ferric–calcium complex as precipitant: parameters optimization and phosphorus-recycling potential. Chem. Eng. J. 268, 230–235. doi:10.1016/j.cej.2014.12.107
Qiu, L., Zhang, M., Yu, X., and Zheng, P. (2018). A novel Fe (II)-Ca synergistic phosphorus removal process: process optimization and phosphorus recovery. Environ. Sci. Pollut. Res. 25, 1543–1550. doi:10.1007/s11356-017-0183-z
Rydin, E. (2000). Potentially mobile phosphorus in Lake Erken sediment. Water Res. 34 (7), 2037–2042. doi:10.1016/S0043-1354(99)00375-9
Smolders, A. J. P., Lamers, L. P. M., Moonen, M., Zwaga, K., and Roelofs, J. G. M. (2001). Controlling phosphate release from phosphate-enriched sediments by adding various iron compounds. Biogeochemistry 54, 219–228. doi:10.1023/A:1010660401527
Waajen, G., van Oosterhout, F., Douglas, G., and Lürling, M. (2016). Management of eutrophication in Lake De Kuil (The Netherlands) using combined flocculant–Lanthanum modified bentonite treatment. Water Res. 97, 83–95. doi:10.1016/j.watres.2015.11.034
Wang, C., and Jiang, H. L. (2016). Chemicals used for in situ immobilization to reduce the internal phosphorus loading from lake sediments for eutrophication control. Crit. Rev. Environ. Sci. Technol. 46 (10), 947–997. doi:10.1080/10643389.2016.1200330
Wang, Q., Liao, Z., Yao, D., Yang, Z., Wu, Y., and Tang, C. (2021). Phosphorus immobilization in water and sediment using iron-based materials: a review. Sci. Total Environ. 767, 144246. doi:10.1016/j.scitotenv.2020.144246
Wang, S. X., Huang, Y. X., Wu, Q. F., Yao, W., Lu, Y. Y., Huang, B. C., et al. (2024). A review of the application of iron oxides for phosphorus removal and recovery from wastewater. Crit. Rev. Environ. Sci. Technol. 54 (5), 405–423. doi:10.1080/10643389.2023.2242227
Xing, J., Zhou, Y., He, D., Guo, F., Li, P., Zhang, Y., et al. (2025). Sediment amplifies organic matter cycling and nutrients feedback in eutrophic lake zones. Water Res. 286, 124164. doi:10.1016/j.watres.2025.124164
Yao, X. L., Ding, R. N., Zhou, Y. Q., Wang, Z. W., Liu, Y., Fu, D. F., et al. (2023). How internal nutrient loading forms in shallow lakes: insights from benthic organic matter mineralization. Water Res. 245, 120544. doi:10.1016/j.watres.2023.120544
Yin, H., Zhang, M., Yin, P., and Li, J. Y. (2022). Characterization of internal phosphorus loading in the sediment of a large eutrophic lake (Lake Taihu, China). Water Res. 225, 119125. doi:10.1016/j.watres.2022.119125
Zamparas, M., and Zacharias, I. (2014). Restoration of eutrophic freshwater by managing internal nutrient loads. A review. Sci. Total Environ. 496, 551–562. doi:10.1016/j.scitotenv.2014.07.076
Keywords: eutrophication, restoration, sediment, apatite phosphorus, passivator
Citation: Li W, Sheng W, Liu S, Dai T, Zhong J, Ren W, Chen Y, Zhong Y and Luo L (2025) Transformation of non-apatite inorganic phosphorus to apatite phosphorus: a novel pathway for sediment phosphorus immobilization via iron-calcium synergistic treatment. Front. Environ. Sci. 13:1696023. doi: 10.3389/fenvs.2025.1696023
Received: 31 August 2025; Accepted: 22 September 2025;
Published: 01 October 2025.
Edited by:
Jun Hou, Hohai University, ChinaReviewed by:
Xiubao Chen, Chinese Academy of Fishery Sciences, ChinaJiangqi Qu, Beijing Research Center for Information Technology in Agriculture, China
Copyright © 2025 Li, Sheng, Liu, Dai, Zhong, Ren, Chen, Zhong and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liancong Luo, MjAyNDk5NDk3NUBuaXQuZWR1LmNu
†ORCID: Wei Li, orcid.org/0000-0002-1256-4423
 Wei Li
Wei Li Weijing Sheng1
Weijing Sheng1