- 1Yunnan Key Laboratory for Plateau Mountain Ecology and Restoration of Degraded Environments & School of Ecology and Environmental Sciences, Yunnan University, Kunming, Yunnan, China
- 2Central Yunnan Field Scientific Station for Restoration of Ecological Function & Yunnan International Joint Research Center of Plateau Lake Ecological Restoration and Watershed Management, Yunnan Think Tank for Ecological Civilization Construction, Yunnan University, Kunming, China
- 3Southwestern United Graduate School & Institute of International Rivers and Eco-security, Yunnan University, Kunming, China
- 4The Ecological and Environmental Monitoring Station of DEEY in Kunming, China
- 5School of Agronomy and Life Science, Kunming University, Kunming, China
- 6School of Chemistry, Biology and Environmental Science, Yuxi Normal University, Yuxi, China
Soil degradation caused by activities such as mining is a severe global environmental issue that drastically disrupts ecosystems. Utilizing plants and their root secretions for ecological remediation is a crucial pathway to restoring these damaged lands. The factors and mechanisms by which plant exudates in mining wastelands drive successional processes in mining areas remain largely unknown. To investigate the plant community succession over time during natural remediation and elucidate how their rhizosphere processes influence the speciation and bioavailability of soil heavy metals. The space-time substitution sampling method was used to continuously monitor the research areas at six different restoration stages of the abandoned Huize Pb–Zn mine site. Additionally, the effects of restoring plants on the total contents and speciation of soil heavy metals (Pb, Cd, Mn, Zn, Cu, Fe) were compared. The results showed that there were 31 dominant plants from 17 families, a shift from herbs to shrubs to evergreen trees. Specifically, the rhizosphere soil exhibited a significantly higher clay content compared to the non-rhizosphere soil. Furthermore, β-glucosidase played a crucial dual role: it contributed to the reduction of total heavy metal content while simultaneously enhancing metal bioavailability. Artemisia argyi Levl. et Van, Populus davidiana Dode, and Pteris vittata L. exhibit different distribution strategies for heavy metals. Artemisia argyi Levl. Populus davidiana Dode primarily transfers and accumulates heavy metals in its leaves, demonstrating its potential for phytoremediation via phytoextraction. In contrast, P. vittata predominantly sequesters heavy metals in its roots, characterizing it as a typical root accumulator or stabilizing plant. Collectively, this research reveals that natural plant succession, driven by key rhizosphere processes like enzyme activity, is an effective driver of ecological restoration in mining wastelands. The distinct heavy metal allocation strategies observed among the dominant species not only elucidate the mechanisms behind this remediation but also provide a scientific basis for selecting and combining plants for targeted phytoremediation.
1 Introduction
The exploitation of mineral resources has seriously damaged the soil structure (Yu and Zahidi, 2023), not only compromising ecosystem functions and biodiversity but also exacerbating soil erosion and groundwater contamination (Boldy et al., 2021). The soil in the mining area is impoverished, and the content of heavy metals and other toxic and harmful elements remains high (Zhu et al., 2024). Therefore, a central scientific challenge in the ecological restoration of mining areas lies in mitigating the bioavailability and toxicity of harmful elements while concurrently improving soil fertility under these adverse conditions (Li et al., 2024). Physical remediation in ecological remediation of mining sites is costly, usually impractical, inefficient in treatment, and not applicable to large-scale contaminated soils (Khan and Jones, 2009). Chemical remediation impoverishes the physical properties of the soil (Fernández-Caliani et al., 2021).
In contrast, phytoremediation has emerged as a cost-effective, ecologically harmonious, and widely applicable alternative (David et al., 2021). In barren and contaminated environments, the rhizosphere process represents a central mechanism for successful phytoremediation, wherein root exudates perform an essential function (Chen et al., 2016). These exudates, comprising low-molecular-weight organic acids and other compounds, directly and indirectly influence heavy metal dynamics by altering soil pH, enhancing mineral weathering, and forming complexes with metals. Consequently, they modify the speciation, bioavailability, and ultimate fate of heavy metals (Lu et al., 2022). For instance, organic acids can mobilize bound metals, facilitating plant uptake or leaching, while simultaneously shaping unique microbial communities that contribute to detoxification and soil health improvement (Beauchemin et al., 2019).
The composition and concentration of root exudates vary among plant species, plant development stage, and soil environmental factors (Ash et al., 2016). Low-molecular-weight organic acids are active components of root exudates that mobilize heavy metals in the soil and facilitate their migration or transport (Najafi and Jalali, 2015; Hinsinger et al., 2009). Root exudates affect the geochemical stability of mine tailings by changing their mineral composition (Beauchemin et al., 2019). Plant root exudates have high nitrogen and carbon contents, providing nutrients and energy to microbes, thereby stimulating microbial diversity (Lu et al., 2022; Tian et al., 2008). Root exudates also improve soil physicochemical properties, and roots secrete a large variety of organic and inorganic substances into the soil, which alter the soil physicochemical properties and affect the speciation and total contents of heavy metals in soil (Hinsinger et al., 2009). Root exudates may also affect the uptake of heavy metals by influencing the kinetics of chemical reactions in the soil environment (Wang and Mulligan, 2012). The efficacy of phytoremediation largely depends on the ability of pioneer or dominant plants to improve soil fertility and mitigate toxicity via root exudates. Thus, it is essential to study the enrichment, migration, and transformation characteristics of heavy metals based on the dominant plants in abandoned mining areas, and to explore the effect of restoring vegetation on polluted soils. In the process of vegetation restoration, with the increase of restoration time and the evolution of vegetation types, the content and speciation of heavy metals in soil may change (Zhao et al., 2024). For example, some heavy metals may be absorbed by plants and enriched in plants, thereby reducing the content of heavy metals in soil (Ali et al., 2013). Other heavy metals may undergo speciated transformation due to the influence of the plant rhizosphere and reduce their bioavailability (Bolan et al., 2014).
Many studies focus on the construction and development path of aboveground vegetation in abandoned metal mining areas (Li et al., 2022). Existing studies tend to focus on the role of inter-root secretion of one or a few species of plants on specific heavy metals (e.g., cadmium, nickel, arsenic, etc.) (Bi et al., 2022). However, there is a wide range of heavy metals in actual abandoned metal mining areas and complex pollution by multiple heavy metals may exist at the same time (Geetha et al., 2023). Most of the studies are short-term potting or greenhouse experiments, lacking long-term field trial data, making it difficult to assess the long-term effectiveness of vegetation restoration and the sustained impact on soil fertility (Pérez-Hernández et al., 2021). This limitation is compounded by the fact that different plant species secrete distinct substances and exhibit varied remediation efficiency (Yang K. et al., 2022). Existing studies often focus on a single plant, lacking comprehensive screening and optimization of multiple species, particularly their adaptability under varying soil conditions. Therefore, the applicability of existing studies in dealing with complex heavy metal pollution situations is limited. The ability of vegetation in different succession stages to absorb, enrich and transform heavy metals in soil may be different. Therefore, it is of great significance to study the variation of soil heavy metals in different succession stages for understanding the effect of vegetation restoration on soil heavy metals.
Wang et al. (2015) reported the soil heavy metal contents throughout most of China according to specific evaluation criteria. Metal ores are mainly distributed in central, southwestern, and eastern China, particularly in Yunnan Province (Wang et al., 2015). Yunnan Province is located in a vast area of mineral resources, especially nonferrous metals, in Southwest China. In 2011, there were 142 mineral deposits in Yunnan Province, accounting for 83% of the country’s discovered reserves (Shi, 2013). Located in Yunnan Province, the Huize Pb–Zn mine is known for its long-term, large-scale mining and smelting, and has a long history tracing back to the Western Han Dynasty. However, this project has generated many mining waste areas, which seriously endanger the local environment (Li et al., 2016). The area’s complex multi-metal contamination, combined with ongoing natural recovery processes, makes it an ideal natural laboratory.
This study systematically investigated the level of soil heavy metal pollution across different stages of natural restoration at the abandoned Huize Pb–Zn mine. We moved beyond single-species and short-term approaches by exploring the absorption and transfer of heavy metals by multiple dominant plants within a natural succession, linking these processes to underlying soil physicochemical properties. We hypothesized that (1) plants would significantly alter the rhizospheric environment during the recovery process, (2) rhizospheric exudates would significantly affect the speciation and total contents of heavy metals in soil, and (3) rhizospheric exudates would change the vertical distribution of heavy metals by affecting their speciation and total contents. The main objectives were to describe the distribution characteristics of heavy metals at different vegetation succession stages and to identify the crucial linkages between soil heavy metal dynamics and the process of vegetation restoration, thereby providing a mechanistic basis for guided ecological recovery.
2 Materials and methods
2.1 Site description, plant survey and sample collection
The study area was in the mining village of Kuang Town, Huize County (103°40′–103°76′E, 26°30′–26°65′N) (Zhou et al., 2018), located in Qujing, northeastern Yunnan Central Plateau. The study area has a more than 100-year history of Zn smelting, and long-term soil smelting without environmental protection measures has led to serious Pb, Zn, cadmium (Cd), and copper (Cu) pollution in the soil (Qi et al., 2016). This mining area has not been artificially restored, and clusters of multiple patches of plant restoration have formed naturally on abandoned land after decades of abandonment following open-pit mining (Wang et al., 2025).
We set up plots representing six vegetation succession stages (S1–S6) in the core area of the mine (Figure 1; Table 1) using the “space instead of time” method. Four botanical surveys were conducted at the sample site from October 2021 to October 2022 (October and December) using the minimum quadrat area method to determine species composition and dominant plants at each successional stage. Nine quadrats were set up for each succession stage, yielding a total of 54 quadrats. The specific quadrats included 1 m × 1 m plots in secondary bare land, 2 m × 2 m plots in sparse grassland, 5 m × 5 m plots in tall grassland and scrub-grassland, 10 m × 10 m plots in savanna and 10 m × 10 m broadleaf-coniferous forest.
Bulk (Non-rhizosphere) soil was sampled from five randomly placed 1 m × 1 m quadrats per successional stage (S1-S6). After removal of surface litter, soil samples from depths of 0–10 cm topsoil,10–20 cm, 20–30 cm in each quadrat were collected using the five-point sampling method (i.e., from the four corners and centre of a square), resulting in a total of 90 samples. Each sample was then mixed, passed through a 2-mm plastic sieve, and divided into two parts. These portions were stored in sterile bags at 4 °C and transported to the laboratory. Based on the vegetation survey, the dominant plant species at each succession stage were identified. The selected species were as follows: Picris divaricata Vaniot in S2, Artemisia argyi Levl. et Van in S3, Pteris vittata L. in S4, Populus davidiana Dode P. davidiana Dode in S5, and both Pinus yunnanensis and Cotoneaster in S6. The rhizosphere soil, defined as the soil tightly adhering to the root surface (within approximately 2 mm), was collected by gently brushing the roots with a sterile soft-bristled brush (Brown et al., 2020; Ling et al., 2021). For each dominant species at a given stage, soil from several plant individuals was pooled to create a homogenized composite sample, thus forming one representative sample per species per stage. All samples were immediately placed in sterile 50-mL centrifuge tubes, maintained at 4 °C, and transported to the laboratory within 24 h for subsequent microbial and physicochemical analysis.
2.2 Physicochemical properties and heavy metal determination
The soil aggregate was separated by mixing 20 g of air-dried soil with a 1.85 g cm−3 density sodium iodide solution, followed by shaking at 120 rpm for 10 min to separate the light fraction (<1.85 g cm−3). The supernatant (free light fraction) was washed with 0.01 M calcium chloride and deionized water, and dried at 60 °C for 48 h. The particles were wet-sieved at 53 μm and divided by grain size into sand, silt, and clay fractions; the silt and clay fractions were separated by centrifugation (Cotrufo et al., 2015). The sieved soil was finely ground with a ball mill (MM 400; Retsch, Haan, Germany) and analyzed for soil total carbon (TC) and total nitrogen (TN) using a CN elemental analyzer (Elementar Analysensysteme GmbH, Frankfurt, Germany) (Nivelle et al., 2016). A kt-10 magnetic susceptibility meter (ZHONGMEDILIAN, China) was used to measure soil magnetic susceptibility (De Mello et al., 2023). After the air-dried soil was passed through a 100-mesh nylon sieve plate, the pH of a 5:1 water–soil suspension was measured with a pH meter (Crison, BA, Spain). The mixture was vigorously stirred for 2 min, allowed to stand for 30 min, and then measured with a pH meter (Ciarkowska, 2017). Humic acid (HA) was isolated following the International Humus Society method (www.ihss.gatech.edu). Soil cation exchange was determined by cobalt trichloride hexaaminochloride extraction spectrophotometry (HJ 889–2017). Soil bulk density (BD) was determined using the ring knife method; soil samples collected with a ring knife were oven-dried at 30 °C for 19–20 h, and the dry weight was determined; the BD (g cm−3) was calculated as the ratio of the dry weight of the soil core to the internal volume of the metal core (BD = dry weight/100) (Pan et al., 2022).
The Technical Standards for Geological Survey of China Geological Survey (DD 2005-03) were used to analyze the different forms of heavy metals in the soil samples. For the morphological analysis, the solution was subjected to fractional and ultrasonic extraction. After extraction, the samples were subjected to inductively coupled plasma mass spectrometry (ICP-MS) for analysis and determination. ICP-MS determined the heavy metals in different vegetative tissues after digestion.
The soil β-D-glucosidase content was tested with a nitrophenol-based colourimetric assay. Soil sucrase was determined following the dinitrosalicylic acid colourimetric method. Soil polyphenol oxidase was determined using the o-Benzenetriol colourimetric method. Soil dehydrogenase was determined using the iodonitrotetrazolium chloride method. Soil phosphatase was determined following a colourimetric method with sodium benzene phosphate. Soil protease was determined using the ninhydrin colourimetric method. All index analyses were performed in triplicate, and the values were calibrated using a blank control. These activities were measured following the methods described by Dick (2011).
2.3 Quality assurance and statistical analyses
Rigorous quality assurance and control protocols were implemented to ensure the accuracy and reliability of all soil physicochemical data. For the ICP-MS analysis of heavy metals, quality control measures included the routine analysis of method blanks and duplicate samples with every batch of 10 samples to monitor contamination and assess precision. Additionally, a mid-point calibration standard was analyzed after every 20 samples to verify instrumental stability, with recoveries required to be within 85%–115%. The entire procedure was validated using certified reference materials. For the soil enzyme activity assays, substrate and reagent blanks were included, and approximately 20% of the samples were analyzed in duplicate to ensure reproducibility, maintaining a relative standard deviation of less than 10%. The experimental data are presented as the mean ± standard deviation of three replicates. Significant differences in soil physicochemical properties, enzyme activities, microbial alpha diversity, and Species richness between rhizosphere and bulk soils across all successional stages were assessed by analysis of variance (ANOVA) followed by Duncan’s test at p < 0.05. The results of these comparisons were visualized using column charts generated with Microsoft Excel. The relationships between environmental factors (soil magnetic susceptibility, total carbon, total nitrogen, pH, β-glucosidase, dehydrogenase, and protease) and heavy metals (total and available concentrations) were assessed using Pearson correlation analysis. The correlation matrix was visualized with Origin 2023 software. The microbial community structure (beta-diversity) was analyzed using principal coordinate analysis (PCoA) based on the Bray-Curtis distance, and the significant differences in community composition between rhizosphere and bulk soils were tested using analysis of similarities (ANOSIM). Structural equation model (SEM) was applied to quantify the direct effects and indirect pathways by which rhizosphere secretions and soil properties influence the concentrations of total and available heavy metals. The lavaan package in the R language was used for both calculations and graphical representation.
3 Results
3.1 Vegetation succession gradient constructs divergent soil environments
After four consecutive vegetation surveys, about 80 plant species (Supplementary Table S1) from 39 families (Supplementary Table S1) and 31 dominant species (Supplementary Table S1) from 17 families (Asteraceae, Fabaceae, Poaceae, Berberaceae, Commelinaceae, Clusiaceae, Rosaceae, Liliaceae, Plantaginaceae, Cyperaceae, Pinaceae, Pteridacea, Salicaceae, Anchovyaceae, Masanaceae, Oleaceae, and Cupressaceae) were collected. The main changes in the plant ecotypes during the natural succession of the abandoned mining area changed from annual herbs (Asteraceae and Fabaceae) to perennial herbs (Rosaceae and Asteraceae), and then to deciduous shrubs (Clusiaceae) and finally evergreen and deciduous trees (Pinaceae and Salicaceae). With the progression of the successional continuum, the bulk soil exhibited a decreasing trend in sand content, while the clay content and magnetic susceptibility (MS) correspondingly increased (Figures 2a,d). The total concentrations of Pb, Cu, and Mn in the bulk soil exhibited a unimodal pattern along the successional chronosequence, with their highest levels occurring during stage 4 (S4) (Figures 3a,c,e).
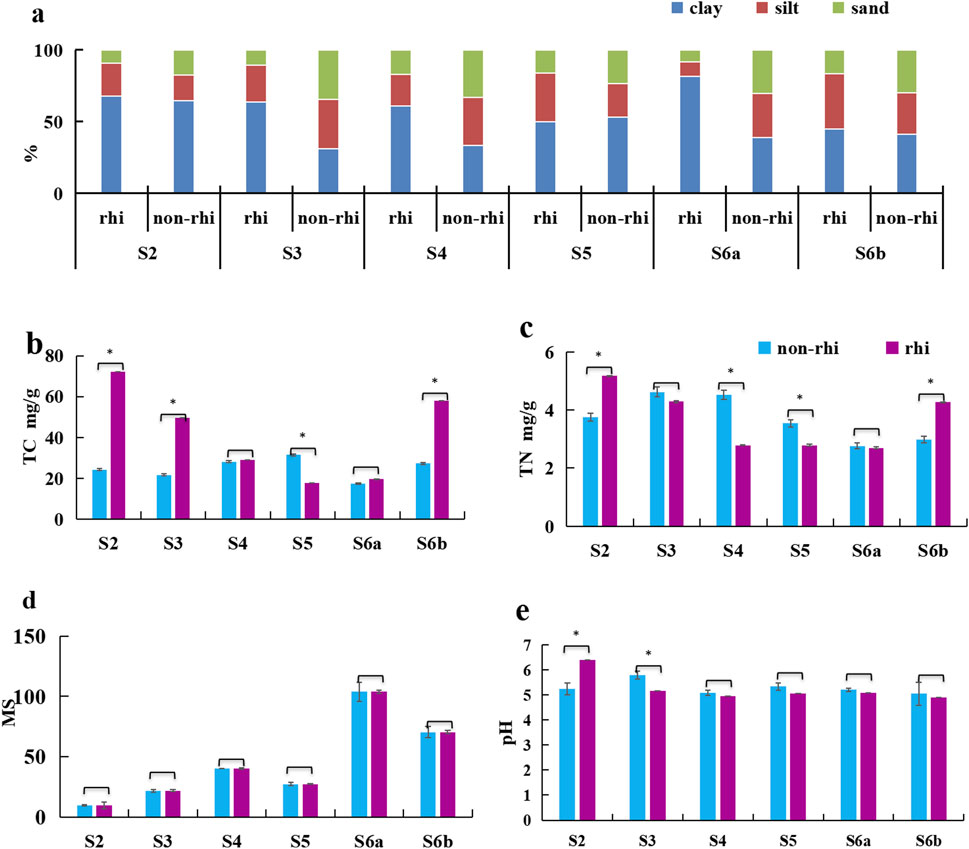
Figure 2. Differences in the soil physicochemical properties of rhizospheric (rhi) and non-rhizospheric (non-rhi) soils: (a) soil texture, (b) total carbon, (c) total nitrogen, (d) magnetic susceptibility, and (e) soil pH. * indicates a significant difference, P < 0.05.
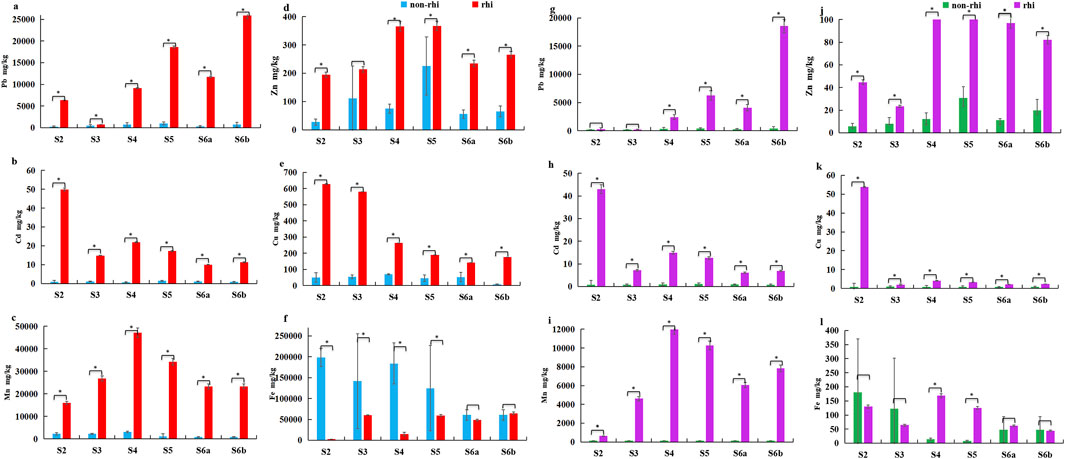
Figure 3. Distributions of heavy metals in rhizospheric (rhi) and non-rhizospheric (non-rhi) soils at different vegetation succession stages: total contents of (a) lead (Pb), (b) cadmium (Cd), (c) manganese (Mn), (d) zinc (Zn), (e) copper (Cu), and (f) iron (Fe); contents of available (g) Pb, (h) Cd, (i) Mn, (j) Zn, (k) Cu, and (l) Fe. * indicates a significant difference, P < 0.05.
3.2 Rhizosphere-induced enrichment of heavy metals and shifts in soil properties
The clay content was significantly higher in rhizospheric than non-rhizospheric soil (P < 0.05) (Figure 2a). During the early and late succession stages, the TC content was considerably higher in rhizospheric than non-rhizospheric soil (P < 0.05) (Figure 2b). TN and magnetic susceptibility did not differ between rhizospheric and non-rhizospheric soils (Figures 2c,d).
The total contents of heavy metals, such as Pb, Zn, Cd, Cu, and manganese (Mn), were significantly higher in rhizospheric than non-rhizospheric soil (P < 0.05) (Figure 3); however, the iron (Fe) content was significantly higher in non-rhizospheric than rhizospheric soil (P < 0.05) (Figure 3f). The contents of available Pb, Zn, Cd, Cu, and Mn were significantly higher in rhizospheric than non-rhizospheric soil (P < 0.05) (Figure 3). The heavy metal contents in the rhizospheric soil of the dominant plants differed markedly, and the total and available Pb and Zn were higher in the rhizospheric soil of woody plants than in the rhizospheric soil of herbaceous plants (P < 0.05) (Figure 3).
Over the course of succession, HA contents first decreased and then increased in non-rhizospheric soil. The HA content was higher, and the fulvic acid content lower, in rhizospheric than non-rhizospheric soil during the early succession stages. Still, the opposite was true during late succession stages. β-Glucosidase activity was significantly higher in the rhizospheric soil of herbaceous plants than woody plants (P < 0.05) (Figure 4b). The activities of β-glucosidase and dehydrogenase were significantly higher in the rhizospheric soil of Picris divaricata Vaniot than those in the rhizospheric soil of the other successional stages (P < 0.05) (Figures 4b,c). Significant differences in soil dehydrogenase, acid phosphatase, and protease were detected in the rhizosphere and non-rhizosphere (P < 0.05) (Figure 4). No significant differences in the activities of sucrase, polyphenol oxidase, catalase, or cellulase were detected in the rhizospheric soils of the dominant plants (Figure 4).

Figure 4. Distributions of rhizospheric (rhi) and non-rhizospheric (non-rhi) soil exudates: (a) humic acid, (b) soil β-glucosidase, (c) soil dehydrogenases, (d) soil acid protease, (e) fulvic acid, (f) soil polyphenol oxidase, (g) soil acid phosphatase, and (h) soil sucrase. * indicates a significant difference, P < 0.05.
At the S4, S5, S6a, and S6b stages, the Shannon index of rhizosphere soil was significantly higher than that of non-rhizosphere soil, indicating greater microbial species richness and evenness in the rhizosphere soil during these successional stages (Supplementary Figure S1a). Although no significant difference was observed at the S3 stage, the rhizosphere soil index was slightly lower than that of non-rhizosphere soil (Supplementary Figure S1a). Across all successional stages, the Chao1 index values of rhizosphere and non-rhizosphere soil were comparable, suggesting that the rhizosphere soil has a limited effect on enhancing microbial species richness (Supplementary Figure S1b). The distribution of samples from different plants and growth stages was visualized along the first two principal components (PC1 and PC2), which explained 13.92% and 11.44% of the total variation, respectively (Supplementary Figure S2). The rhizosphere soil microbial community structure is not random but is driven by both the plant species and the successional stage (Supplementary Figure S2).
3.3 Rhizosphere effects and plant accumulation of heavy metals
The TN content in non-rhizospheric soil was significantly negatively correlated with the contents of available heavy metals and positively correlated with soil β-glucosidase and acid phosphatase activities (P < 0.05) (Figure 5). The total and available heavy metal contents in rhizospheric soil were closely correlated with environmental factors; the total contents of heavy metals in rhizospheric soil were significantly negatively correlated with rhizospheric exudates (P < 0.05), whereas the contents of available heavy metals were significantly positively correlated with rhizospheric exudates (P < 0.05) (Figure 5).

Figure 5. Correlations between environmental factors and heavy metals in (a) non-rhizospheric soil and (b) rhizospheric soil. Abbreviations: soil magnetic susceptibility (MC), total carbon (TC), total nitrogen (TN), soil pH (pH), total heavy metals (MT), available heavy metals (MA), soil β-glucosidase activity (SβG), dehydrogenase activity (Sd), protease activity (Sap). *Indicates a significant difference, P < 0.05.
The capacity for heavy metal accumulation varies significantly among different plant species. As shown in Supplementary Table S1, the concentrations of Zn and Pb in Populus davidiana Dode were considerably higher than those in other plants. Moreover, the accumulation of heavy metals differs across various organs within the same species. In Artemisia argyi Levl. et Vant and P. davidiana Dode, the concentrations of Zn and Pb were higher in leaves than in roots.
In contrast, heavy metals in Pteris vittata L. were predominantly accumulated in the roots. The translocation factor (TF) of Zn and Cd in leaves of A. argyi Levl. et Vant and P. davidiana Dode exceeded 1, indicating a strong ability to transport Cd from roots to leaves. Conversely, the TF value for Pb in leaves of P. vittata L. was less than 1, suggesting a limited capacity for Pb translocation from roots to leaves.
3.4 Direct and indirect effects of vegetation on heavy metals
Changes in environmental factors during soil restoration can directly or indirectly affect the total heavy metal contents in soil. Root exudates had a greater effect on the total and available heavy metal contents in rhizospheric soil than in non-rhizospheric soil (Figure 6). Soil in the rhizosphere, the β-glucosidase (β = −0.419, P = 0.00), dehydrogenase (β = −0.310, P = 0.00), and protease (β = −0.508, P = 0.005) activities directly negatively affected the total heavy metal contents. Moreover, Soil β-glucosidase had a significant positive effect on the contents of available heavy metals (β = 0.446, P = 0.00). Indirectly, β-glucosidase acted on the total contents and speciation of heavy metals through the environmental factors TC and pH (Figure 6). MT in bulk soil is mainly regulated by pH (negatively) and TN and TC (positively), and enzyme activities (SβG, Sd) indirectly affect MT through mediating TN and TC (Figure 6a). MA in bulk soil is mainly regulated by TN (negatively) and TC (positively), and enzyme activities (SβG, Sd) indirectly affect MA through mediating TN and TC (Figure 6b). The rhizosphere soil MT is significantly affected by pH (negatively) and TN (positively). Protease (Sap) indirectly enhances MT by regulating pH, indicating that root exudates (such as organic acids) may change the rhizosphere pH, thereby affecting the forms of heavy metals (Figure 6c). The rhizosphere soil MA is significantly affected by TN (positively) and TC (positively), and the effect of TC is much stronger than that in the non-rhizosphere. This indicates that root exudates (such as organic matter) increase rhizosphere TC, thereby promoting the release of available heavy metals (Figure 6d).
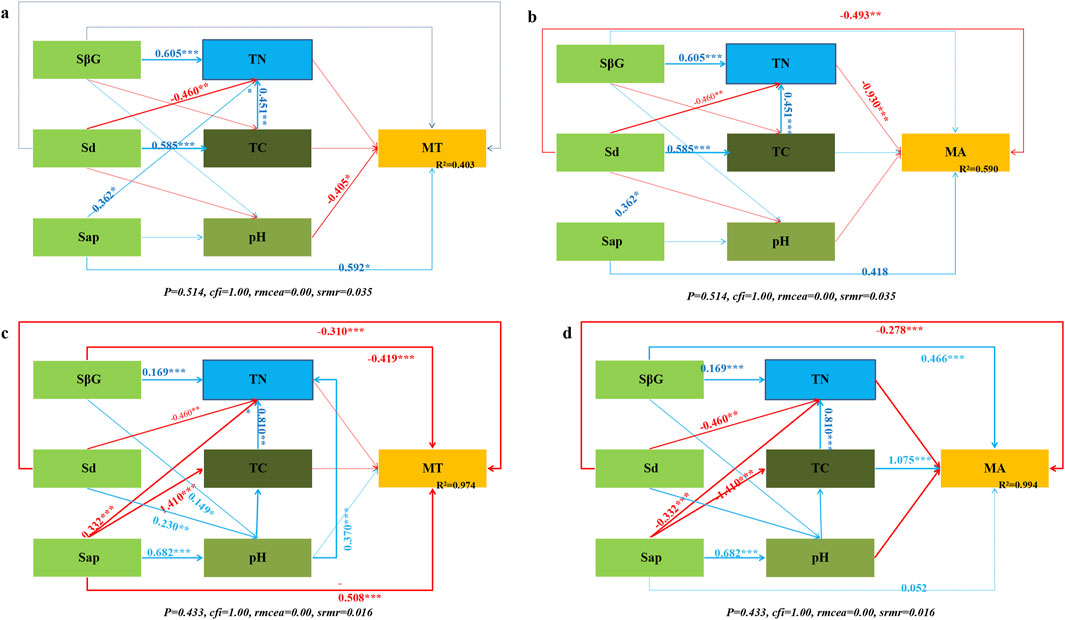
Figure 6. Structural equations of the effects of environmental factors on the total and available heavy metal contents: (a) total heavy metal contents in non-rhizospheric soil, (b) contents of available heavy metals in non-rhizospheric soil, (c) total heavy metal contents in rhizospheric soil, (d) contents of available heavy metals in rhizospheric soil. Abbreviations: soil magnetic susceptibility (MC), total carbon (TC), total nitrogen (TN), soil pH (pH), total heavy metals (MT), available heavy metals (MA), soil β-glucosidase activity (SβG), dehydrogenase activity (Sd), and protease activity (Sap). * indicates a significant difference, P < 0.05.
4 Discussion
4.1 Improvements in soil environmental conditions with natural succession
Plant community succession fundamentally alters soil properties and biological activities, constructing a divergent soil environment. Heavy metal contamination decreased, and pH, organic matter (OM), TC, TN, and soil enzyme activities of β-glucosidase and protease in soil increased with increasing age of mine restoration (Khan et al., 2024). This study found that the contents of carbon and nitrogen in the rhizosphere soil of herbaceous plants are significantly higher than those in non-rhizosphere soil. Herbaceous species are the main plants to appear in early succession stages, and their ecological functions are crucial for soil carbon and nitrogen accumulation (Yuan et al., 2023). Initially, a few herbaceous seeds rapidly germinate and survive in polluted and barren areas (Wu et al., 2021; Zhu et al., 2018; Karaca et al., 2017; Yang et al., 2013). Their rapid growth supports rapid carbon fixation, which effectively increases the soil carbon content; the establishment of herbaceous plant communities effectively increases soil C content during mine restoration (Zhang et al., 2023). As vegetation cover is produced, Plants input organic matter into the soil through root exudates (such as sugars, amino acids) and litter (leaves, root residues). After being decomposed by microorganisms, these are converted into soil organic matter (SOM), which becomes the core source of the soil carbon pool (Yang X. F. et al., 2022). In addition, herbaceous plants (especially legumes) convert atmospheric nitrogen into an available form through symbiotic nitrogen fixation (in cooperation with rhizobia), significantly increasing the total nitrogen (TN) content of the soil. This forms a synergistic effect of “carbon sequestration - nitrogen input” and accelerates the restoration of soil fertility in mining areas. The root exudates form organic acids, which help decompose coarse to fine particles, such as clay. Root exudates also release mucilage and organic polysaccharides, which facilitate the formation of aggregates and the accumulation of organic carbon in soil (Badri and Vivanco, 2009). Plant roots secrete organic substances as they grow, and these substances can react with minerals in the soil to facilitate the agglomeration and stabilization of soil particles. In addition, the physical effect of roots and their activity may also lead to changes in the physical structure of the soil, such as the rearrangement and compaction of soil particles. Consistent with the findings of this study, the clay content was significantly higher in rhizospheric than non-rhizospheric soil, which may prevent the loss of fine soil particles (Gu et al., 2018; Wan et al., 2023). In summary, the physical effect of roots on soil improvement, root exudates, and the input of organic matter from dead roots and litter collectively affect soil properties and stabilize the soil structure (Qu et al., 2016; Wan et al., 2023).
Biodiversity relies on feedback within the ecosystem and complementary activities among species, which accumulate over time (Jiao et al., 2022). Both the total amount and the available content of heavy metals in rhizosphere soil are significantly higher than those in non-rhizosphere soil, and this phenomenon is closely related to the unique microenvironment of the rhizosphere. Firstly, root exudates (such as organic acids, polysaccharides, etc.) can not only dissolve heavy metals in the soil through acidification (Jiao et al., 2022; Castellanos-Barliza and León-Peláez, 2023). The acidic groups contained in it can also form soluble complexes with heavy metal ions, thereby acting as carriers and promoting the migration and enrichment of heavy metals to the rhizosphere (Wu et al., 2024). Improved soil properties are an essential factor affecting the migration of heavy metals, and the enrichment of heavy metals adhered to particles increases with decreasing soil particle size (Onyatta and Huang, 2006). The rhizosphere soil aggregate structure, formed by the promotion of root activity, exhibits a stronger adsorption and retention capacity for heavy metals attached to fine particles, thereby increasing the total amount of heavy metals in the rhizosphere (Wu et al., 2024). Soil organic matter promotes the transformation of heavy metals into an organically bound state (Li et al., 2019). Increased organic matter contents further improve the heavy metal adsorption capacity of soil.
The rhizosphere environment plays a central role in improving the soil ecosystem during vegetation succession, which is clearly evidenced by the significant changes in the microbial community. β-glucosidase hydrolyses non-reducing sugars, such as cellobiose and other water-soluble cellulose dextrins, to release glucose, which serves as the primary carbon source for microorganisms. In the middle and late stages of succession (S4, S5, S6), the Shannon index of microorganisms in the rhizosphere soil is significantly higher than that in the non-rhizosphere soil, indicating that the species richness and evenness of the rhizosphere microbial community have been improved considerably with the successional process. It reveals that the rhizosphere environment selectively enriches specific microbial groups, thereby optimizing the composition and structure of the community (Chen et al., 2024). Principal Coordinate Analysis (PCoA) further confirmed that there is a significant separation in the microbial community structure between rhizosphere and non-rhizosphere soils. This positive succession of microbial communities is closely linked to the improvement of the rhizosphere environment. During the succession process, plants, through root exudates (such as organic acids, mucilage, and polysaccharides), not only directly provide carbon sources for specific microorganisms but also create a more favourable microenvironment for microbial survival by improving soil aggregate structure and regulating pH values (Yang et al., 2025). Therefore, the optimization of rhizosphere microbial community structure and the enhancement of their functions are key biological indicators for the successful colonization of vegetation and the restoration of soil ecosystem functions driven by succession. Together, they promote the practical cycling of nutrients and the reconstruction of soil health (Duan et al., 2021).
4.2 Rhizosphere exudates drive heavy metal transformation and migration
Plants enhance soil enzyme activity and activate the metal ion forms in the rhizosphere, thereby improving the vertical migration capacity of metal ions and reducing soil heavy metal pollution. The physicochemical properties and enzyme activities of rhizospheric soils are essential for phytoremediation of heavy metal-contaminated soils (Lin et al., 2021). The structural equation model (SEM) of this study reveals that the increase in β-glucosidase (SβG) activity has a significant positive direct effect on the available forms of heavy metals in the rhizosphere soil. This indicates that β-glucosidase promotes the mineralization of soil organic matter (Tripathy et al., 2014). On the one hand, it releases the immobilized heavy metals; on the other hand, the produced soluble organic matter may form mobile complexes with heavy metals (Mishra et al., 2017; Yao et al., 2022). These combined effects increase the bioavailability of heavy metals, thereby facilitating the absorption by plants and their transfer to the aboveground parts, and ultimately reducing the total amount of heavy metals in the soil. Soil protease (Sap) has been proven to be another key factor affecting the total amount of heavy metals in the rhizosphere. SEM path analysis shows that protease indirectly exerts an adverse effect on the total amount of heavy metals by significantly reducing the rhizosphere pH (Yang et al., 2017). The mechanism may be that the protease catalyzes the hydrolysis of proteins to produce products such as amino acids. These substances can not only serve as a nitrogen source for microorganisms to promote their metabolism and acid production, but also may act as chelating agents to change the solid-liquid distribution balance of heavy metals (Ma et al., 2016), thereby promoting the vertical migration of heavy metals or their leaching into the lower soil layers. Soil enzymes, including β-glucosidase, urease, protease, and phosphatase, have essential roles in nutrient use and cycling (Xu et al., 2019). Their activities affect the contents of available nutrients in the soil and, in turn, plant growth, which reflects ecological health (Xian et al., 2015).
Different dominant plants exhibit significant differences in their strategies for the migration and transformation of heavy metals, which directly affects the remediation effect. Hyperaccumulator plants like Pteris vittata exhibit a root sequestration strategy for Pb (TF < 1) (Yao et al., 2020). It indicates that lead is strongly immobilized in the root system and rarely translocates to the aboveground parts. Root sequestration can reduce the metal burden in the aboveground parts of plants, decreasing secondary pollution where metals return to the soil through plant residues. Meanwhile, organic acids and viscous polysaccharides secreted by roots can form metal complexes in the rhizosphere, further reducing the bioavailability of soluble Pb. However, plants such as Artemisia argyi and Populus davidiana show strong aboveground translocation capacity for Zn and Cd (TF > 1) (Zhang et al., 2024; Qiao et al., 2023; Yan et al., 2020). This type of plant transports heavy metals from the root zone to the aboveground tissues through rapid root-stem-leaf translocation, thereby removing them from the surface soil. Woody plants can promote the migration of heavy metals to deeper soil due to their deep root system characteristics, which is one of the essential reasons for the reduction of heavy metal content in surface soil in the later stages of succession (Pulford and Watson, 2003). Woody plants such as Populus simonii have well-developed root systems that can penetrate soil layers more than 200 cm to absorb water (Zirdie et al., 2020). While deep roots absorb soil moisture, they also simultaneously absorb and transport metal ions downward through the xylem, causing heavy metals in the surface soil to migrate to deeper layers gradually. Vertical distribution studies show that heavy metals are prone to downward migration in acidic or weakly acidic soils, and the phenomenon of deep accumulation is particularly pronounced under long-term leaching conditions (Ge et al., 2024). Therefore, deep-rooted woody plants such as Populus davidiana can significantly reduce the heavy metal concentration in surface soil through the root ‘suction-transport-deposition’ mechanism during the later stages of succession, providing a safer substrate for subsequent vegetation restoration (Li et al., 2009). The emergence and succession of functional diversity in this plant are the core biological mechanisms by which rhizosphere effects are fully exerted and soil heavy metal pollution is effectively reduced during vegetation restoration.
5 Conclusion
This study demonstrates that the ecological succession from herbaceous to woody plants constructs a functionally differentiated soil microenvironment, characterized by the enrichment of clay and organic carbon, alongside the optimization of microbial diversity and community structure. Our findings reveal the pivotal role of rhizosphere enzyme activities, wherein β-glucosidase significantly enhances the bioavailability of heavy metals by activating them in the soil, thereby improving plant extraction efficiency. Furthermore, the research identifies distinct, complementary strategies among plant species: Artemisia argyi and Populus davidiana act as efficient phytoextractors for Zn and Cd, demonstrating a pronounced ability to transfer these metals to their aboveground tissues. In contrast, P. vittata functions as a stabilizer plant, exhibiting a strong capacity for root sequestration of lead. Additionally, deep-rooted woody plants facilitate the vertical migration of heavy metals, effectively reducing the contaminant load in the surface soil. Collectively, these findings indicate that the natural succession process inherently forms a synergistic and highly effective system for heavy metal remediation. The outcomes of this research provide valuable practical insights for guiding species selection and the construction of functional plant communities in the ecological restoration of mining areas. Future studies should be expanded to include an assessment of the socio-economic feasibility of applying this restoration model to other mining regions, thereby promoting more cost-effective and sustainable environmental remediation strategies based on nature-based solutions.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
X-YY: Conceptualization, Investigation, Methodology, Data curation, Software, Visualization, Writing – original draft, Writing – review and editing. TL: Conceptualization, Supervision, Writing – review and editing. X-NW: Investigation, Data curation, Writing – review and editing. C-EL: Project administration, Supervision, Writing – review and editing. S-YL: Investigation, Data curation, Writing – review and editing. S-XP: Supervision, Writing – review and editing. S-CW: Investigation, Writing – review and editing. L-QZ: Supervision, Writing – review and editing. C-QD: Conceptualization, Resources, Supervision, Funding acquisition, Project administration, Writing – review and editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was supported by the National Natural Science Foundation of China (32371707), China Yunnan Provincial R&D Programs (202405AF140014, 202302AO370015), the Graduate Research Innovation Project of Yunnan University (KC-22221078), Special Basic Cooperative Programs of Yunnan Provincial Undergraduate Universities’ Association (202101BA070001-154). China Postdoctoral Science Foundation (2025M772752); Caiyun Postdoctoral Innovation Project of Yunnan Province, China.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2025.1698742/full#supplementary-material
References
Ali, H., Khan, E., and Sajad, M. A. (2013). Phytoremediation of heavy metals-Concepts and applications. Chemosphere 91 (7), 869–881. doi:10.1016/j.chemosphere.2013.01.075
Ash, C., Tejnecky, V., Boruvka, L., and Drábek, O. (2016). Different low-molecular-mass organic acids specifically control leaching of arsenic and lead from contaminated soil. J. Contam. Hydrology 187, 18–30. doi:10.1016/j.jconhyd.2016.01.009
Badri, D. V., and Vivanco, J. M. (2009). Regulation and function of root exudates. Plant Cell Environ. 32 (6), 666–681. doi:10.1111/j.1365-3040.2009.01926.x
Beauchemin, S., Langley, S., and MacKinnon, T. (2019). Geochemical properties of 40-year old forested pyrrhotite tailings and impact of organic acids on metal cycling. Appl. Geochem. 110, 104437. doi:10.1016/j.apgeochem.2019.104437
Bi, B. Y., Yuan, Y., Zhang, H., Wu, Z. H., Wang, Y., and Han, F. P. (2022). Rhizospheric soil metabolites mediated microbial community changes of Pinus sylvestris var. mongolica across stand ages in the Mu us desert. Appl. Soil Ecol. 169, 10422. doi:10.1016/j.apsoil.2021.104222
Bolan, N., Kunhikrishnan, A., Thangarajan, R., Kumpiene, J., Park, J., Makino, T., et al. (2014). Remediation of heavy metal(loid)s contaminated soils - to mobilize or to immobilize? J. Hazard. Materals 266, 141–166. doi:10.1016/j.jhazmat.2013.12.018
Boldy, R., Santini, T., Annandale, M., Erskine, P. D., and Sonter, L. J. (2021). Understanding the impacts of mining on ecosystem services through a systematic review. Extr. Industries Soc. 8, 457–466. doi:10.1016/j.exis.2020.12.005
Brown, S. P., Grillo, M. A., Podowski, J. C., and Heath, K. D. (2020). Soil origin and plant genotype structure distinct microbiome compartments in the model legume Medicago truncatula. Microbiome 8 (1), 139. doi:10.1186/s40168-020-00915-9
Castellanos-Barliza, J., and León-Peláez, J. D. (2023). Soil recovery in a chronosequence of revegetated coal mine spoils in Colombian drylands: a view from the assessment of physical-chemical and biological properties. Geoderma Reg. 33, e00652. doi:10.1016/j.geodrs.2023.e00652
Chen, Z. J., Tian, Y. H., Zhang, Y., Song, B. R., Li, H. C., and Chen, Z. H. (2016). Effects of root organic exudates on rhizosphere microbes and nutrient removal in the constructed wetlands. Ecol. Eng. 92, 243–250. doi:10.1016/j.ecoleng.2016.04.001
Chen, M. F., Acharya, S. M., Yee, M. O., Cabugao, K. G. M., and Chakraborty, R. (2024). Developing stable, simplified, functional consortia from brachypodium rhizosphere for microbial application in sustainable agriculture. Front. Microbiol. 15, 1401794. doi:10.3389/fmicb.2024.1401794
Ciarkowska, K. (2017). Organic matter transformation and porosity development in non-reclaimed mining soils of different ages and vegetation covers: a field study of soils of the zinc and lead ore area in SE Poland. J. Soils Sediments 17 (8), 2066–2079. doi:10.1007/s11368-017-1678-4
Cotrufo, M. F., Soong, J. L., Horton, A. J., Campbell, E. E., Haddix, M. L., Wall, D. H., et al. (2015). Formation of soil organic matter via biochemical and physical pathways of litter mass loss. Nat. Geosci. 8 (10), 776–779. doi:10.1038/ngeo2520
David, O. A., Akin-Fajiye, M., Akomolafe, G. F., Akanmu, A. O., and Ogunlowo, I. I. (2021). Post-mine succession and short term effects of coal mining in a Guinea savanna ecosystem. Acta Oecol. 112, 103766. doi:10.1016/j.actao.2021.103766
De Mello, D. C., Veloso, G. V., Moquedace, C. M., Oliveira, I. D., De Oliveira, F. S., Gomes, L. C., et al. (2023). Radiometric and magnetic susceptibility characterization of soil profiles: geophysical data and their relationship with antarctic periglacial processes, pedogenesis, and lithology. Catena 232, 107427. doi:10.1016/j.catena.2023.107427
Duan, R. Y., Lin, Y. X., Zhang, J. N., Huang, M. Y., Du, Y. H., Yang, L., et al. (2021). Changes in diversity and composition of rhizosphere bacterial community during natural restoration stages in antimony mine. Peerj 9, e12302. doi:10.7717/peerj.12302
Fernández-Caliani, J. C., Giraldez, M. I., Waken, W. H., Del Río, Z. M. ., and Córdoba, F. (2021). Soil quality changes in an Iberian pyrite mine site 15 years after land reclamation. Catena 206, 105538. doi:10.1016/j.catena.2021.105538
Ge, L., Fang, F. M., Zhou, H., Yao, Y. R., Tan, H. R., Wang, F., et al. (2024). Vertical distribution characteristics and migration patterns of heavy metals in different soil types of caizi Lake wetlan. Environ. Chem. 43 (03), 933–941. doi:10.7524/j.issn.0254-6108.2022081803
Geetha, N., Sunilkumar, C. R., Bhavya, G., Nandini, B., Abhijith, P., Satapute, P., et al. (2023). Warhorses in soil bioremediation: seed biopriming with PGPF secretome to phytostimulate crop health under heavy metal stress. Environ. Res. 216, 114498. doi:10.1016/j.envres.2022.114498
Gu, C. J., Mu, X. M., Gao, P. ., Zhao, G. J., Sun, W. Y., Tatarko, J., et al. (2018). Influence of vegetation restoration on soil physical properties in the loess Plateau, China. J. Soils Sediments 19 (2), 716–728. doi:10.1007/s11368-018-2083-3
Hinsinger, P., Bengough, A. G., Vetterlein, D., and Young, I. M. (2009). Rhizosphere: biophysics, biogeochemistry and ecological relevance. Plant Soil 321 (1-2), 117–152. doi:10.1007/s11104-008-9885-9
Jiao, S., Lu, Y. H., and Wei, G. H. (2022). Soil multitrophic network complexity enhances the link between biodiversity and multifunctionality in agricultural systems. Glob. Change Biol. 28 (1), 140–153. doi:10.1111/gcb.15917
Karaca, A., Karaca, A., Kocacinar, F., and Cuci, Y. (2017). The effects of seed treatment with melatonin on germination and emergence performance of pepper seeds under chilling stress. J. Agric. Sci. 23 (2), 167–176. doi:10.15832/TBD.58349
Khan, M. I. R., and Jones, D. L. (2009). Effect of composts, lime and diammonium phosphate on the phytoavailability of heavy metals in a copper mine tailing soil. Pedosphere 19 (5), 631–641. doi:10.1016/S1002-0160(09)60158-2
Khan, S. R., Singh, P. C., Schmettow, M., Singh, S. K., and Rastogi, N. (2024). Exploring the influence of ground-dwelling ant bioturbation activity on physico-chemical, biological properties and heavy metal pollution in coal mine spoil. Pedobiologia 104, 150960. doi:10.1016/j.pedobi.2024.150960
Li, S. X., Tang, B., Chang, W. J., Liu, W. X., Ma, F. J., and Gu, Q. B. (2009). Vertical distribution characteristics of heavy metals in soils at typical nonferrous metal smelting sites in Hunan Province and identification of key influencing factors. Res. Environ. Sci. 38 (09), 2001–2009. doi:10.13198/j.issn.1001-6929.2025.06.20
Li, Z. L., Zhou, J., Mao, J. R., Santosh, M., Yu, M. G., Li, Y. Q., et al. (2016). Zircon U-Pb geochronology and geochemical characteristics of the mafic intrusions in northwestern Guizhou province, and their significances to the lead-zinc mineralization. Acta Geol. Sin. 90 (05), 933–949. doi:10.1016/j.lithos.2013.07.014
Li, B. C., Wang, J. C., Yao, L. R., Meng, Y. X., Ma, X. L., Si, E. J., et al. (2019). Halophyte Halogeton glomeratus, a promising candidate for phytoremediation of heavy metal-contaminated saline soils. Plant Soil 442 (1-2), 323–331. doi:10.1007/s11104-019-04152-4
Li, T., Wu, M. H., Duan, C. Q., Li, S. Y., and Liu, C. E. (2022). The effect of different restoration approaches on vegetation development in metal mines. Sci. Total Environ. 806 (3), 150626. doi:10.1016/j.scitotenv.2021.150626
Li, C. H., Ji, Y. K., Ma, N., Zhang, J., Zhang, H., Ji, C. J., et al. (2024). Positive effects of vegetation restoration on the soil properties of post-mining land. Plant Soil 497, 93–103. doi:10.1007/s11104-022-05864-w
Lin, H., Liu, C. J., Li, B., and Dong, Y. B. (2021). Trifolium repens L. regulated phytoremediation of heavy metal contaminated soil by promoting soil enzyme activities and beneficial rhizosphere associated microorganisms. J. Hazard. Mater. 402, 123829. doi:10.1016/j.jhazmat.2020.123829
Ling, N., Wang, T., and Kuzyakov, Y. (2021). Rhizosphere bacteriome structure and functions. Nat. Commun. 12 (1), 836. doi:10.1038/s41467-022-28448-9
Lu, Z. X., Wang, P., Ou, H. B. ., Wei, S. X., Wu, L. C., Jiang, Y., et al. (2022). Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in southwest China. For. Ecol. Manag. 508, 120002. doi:10.1016/j.foreco.2021.120002
Ma, Y., Oliveira, R. S., Freitas, H., and Zhang, C. (2016). Biochemical and molecular mechanisms of plant-microbe-metal interactions: relevance for phytoremediation. Front. plant Sci. 7, 918. doi:10.3389/fpls.2016.00918
Mishra, J., Singh, R., and Arora, N. K. (2017). Alleviation of heavy metal stress in plants and remediation of soil by rhizosphere microorganisms. Front. Microbiol. 8, 1706. doi:10.3389/fmicb.2017.01706
Najafi, S., and Jalali, M. (2015). Effects of organic acids on cadmium and copper sorption and desorption by two calcareous soils. Environ. Monit. Assess. 187 (9), 585. doi:10.1007/s10661-015-4804-z
Nivelle, E., Verzeaux, J., Habbib, H., Kuzyakov, Y., Decocq, G., Roger, D., et al. (2016). Functional response of soil microbial communities to tillage, cover crops and nitrogen fertilization. Appl. Soil Ecol. 108, 147–155. doi:10.1016/j.apsoil.2016.08.004
Onyatta, J. O., and Huang, P. M. (2006). Distribution of applied cadmium in different size fractions of soils after incubation. Biol. Fertil. Soils 42 (5), 432–436. doi:10.1007/s00374-006-0087-4
Pan, L. M., Chen, Y. Y., Xu, Y., Li, J., and Lu, H. Z. (2022). A model for soil moisture content prediction based on the change in ultrasonic velocity and bulk density of tillage soil under alternating drying and wetting conditions. Measurement 189, 110504. doi:10.1016/j.measurement.2021.110504
Pérez-Hernández, H., Fernández-Luqueño, F., Huerta-Lwanga, E., Mendoza-Vega, J., Alvarez-Solís, J. D., and Pérez-Moreno, A. Y. (2021). A field experiment regarding the behavior of endogenous earthworms exposed to iron and titanium engineered nanoparticles in a natural forest soil. Int. J. Environ. Res. 15 (5), 849–858. doi:10.1007/s41742-021-00360-y
Pulford, I. D., and Watson, C. (2003). Phytoremediation of heavy metal-contaminated land by trees - a review. Environ. Interational 29 (4), 529–540. doi:10.1016/S0160-4120(02)00152-6
Qi, J. Y., Zhang, H. L., Li, X. P., Lu, J., and Zhang, G. S. (2016). Concentrations, spatial distribution, and risk assessment of soil heavy metals in a Zn-Pb mine district in southern China. Environ. Monit. Assess. 188 (7), 413. doi:10.1007/s10661-016-5406-0
Qiao, K., Shan, Q. H., Zhang, H. Z., Lv, F. L., and Zhou, A. M. (2023). Populus euphratica plant cadmium tolerance PePCR3 improves cadmium tolerance. Tree Physiol. 43 (11), 1950–1963. doi:10.1093/treephys/tpad103
Qu, L. Y., Huang, Y. Y., Ma, K. M., Zhang, Y. X., and Biere, A. (2016). Effects of plant cover on properties of rhizosphere and inter-plant soil in a semiarid valley, SW China. Soil Biol. Biochem. 94, 1–9. doi:10.1016/j.soilbio.2015.11.004
Shi, X. P. (2013). China's small coal mine policy in the 2000s: a case study of trusteeship and consolidation. Resour. Policy 38 (4), 598–604. doi:10.1016/j.resourpol.2013.09.009
Tian, Y., Haibara, K., Toda, H., Ding, F. J., Liu, Y. H., and Choi, D. S. (2008). Microbial biomass and activity along a natural pH gradient in forest soils in a karst region of the upper yangtze river, China. J. For. Res. 13 (4), 205–214. doi:10.1007/s10310-008-0073-9
Tripathy, S., Bhattacharyya, P., Mohapatra, R., Som, A., and Chowdhury, D. (2014). Influence of different fractions of heavy metals on microbial ecophysiological indicators and enzyme activities in century old municipal solid waste amended soil. Ecol. Eng. 70, 25–34. doi:10.1016/j.ecoleng.2014.04.013
Wan, R. P., Luo, D. Y., Liu, J. Y., Zhang, Y., Xiang, Y. Q., Yan, W., et al. (2023). Superior improvement on soil quality by pennisetum sinese vegetation restoration in the dry-hot valley region, SW China. Sci. Total Environ. 878, 163185. doi:10.1016/j.scitotenv.2023.163185
Wang, S. L., and Mulligan, C. N. (2012). Effects of three low-molecular-weight organic acids (LMWOAs) and pH on the mobilization of arsenic and heavy metals (cu, Pb, and zn) from mine tailings. Environ. Geochem. Health 35 (1), 111–118. doi:10.1007/s10653-012-9461-3
Wang, S. P., Wang, Y. H., Zhang, R. J., Wang, W. T., Xu, D. Q., Guo, J., et al. (2015). Historical levels of heavy metals reconstructed from sedimentary record in the hejiang river, located in a typical mining region of southern China. Sci. Total Environ. 532, 645–654. doi:10.1016/j.scitotenv.2015.06.035
Wang, S. C., Li, T., Yuan, X. Q., Yu, J., Luan, Z. F., Guo, Z. L., et al. (2025). Biotic and abiotic drivers of soil carbon, nitrogen and phosphorus and metal dynamic changes during spontaneous restoration of Pb–Zn mining wastelands. J. Hazard. Mater. 490, 137818. doi:10.1016/j.jhazmat.2025.137818
Wu, B. H., Peng, H., Sheng, M. P., Luo, H. Y., Wang, X. T., Zhang, R., et al. (2021). Evaluation of phytoremediation potential of native dominant plants and spatial distribution of heavy metals in abandoned mining area in southwest China. Ecotoxicol. Environ. Saf. 220, 112368. doi:10.1016/j.ecoenv.2021.112368
Wu, B. H., Li, X., Lin, S. K., Jiao, R. F., Yang, X., Shi, A. A., et al. (2024). Miscanthus sp. root exudate alters rhizosphere microbial community to drive soil aggregation for heavy metal immobilization. Sci. Total Environ. 949, 175009. doi:10.1016/j.scitotenv.2024.175009
Xian, Y., Wang, M. E., and Chen, W. P. (2015). Quantitative assessment on soil enzyme activities of heavy metal contaminated soils with various soil properties. Chemosphere 139, 604–608. doi:10.1016/j.chemosphere.2014.12.060
Xu, L. X., Han, Y. S., Yi, M. ., Yi, H. L., Guo, E. H., and Zhang, A. Y. (2019). Shift of millet rhizosphere bacterial community during the maturation of parent soil revealed by 16S rDNA high-throughput sequencing. Appl. Soil Ecol. 135, 157–165. doi:10.1016/j.apsoil.2018.12.004
Yan, A., Wang, Y. M., Tan, S. N., Yusof, M. L. M., Ghosh, S., and Chen, Z. (2020). Phytoremediation: a promising approach for revegetation of heavy metal-polluted land. Front. Plant Sci. 11, 359. doi:10.3389/fpls.2020.00359
Yang, S. X., Liang, S. C., Yi, L. B., Xu, B. B., Cao, J. B., Guo, Y. F., et al. (2013). Heavy metal accumulation and phytostabilization potential of dominant plant species growing on manganese mine tailings. Front. Environ. Sci. Eng. 8 (3), 394–404. doi:10.1007/s11783-013-0602-4
Yang, Y. R., Dong, M., Cao, Y. P., Wang, J. L., Tang, M., and Ban, Y. (2017). Comparisons of soil properties, enzyme activities and microbial communities in heavy metal contaminated bulk and rhizosphere soils of Robinia pseudoacacia L. in the northern foot of qinling Mountain. Forests 8 (11), 430. doi:10.3390/f8110430
Yang, K., Zhu, J. J., Zhang, W. W., Zhang, Q., Lu, D. L., Zhang, Y. K., et al. (2022). Litter decomposition and nutrient release from monospecific and mixed litters: comparisons of litter quality, fauna and decomposition site effects. J. Ecol. 110 (7), 1673–1686. doi:10.1111/1365-2745.13902
Yang, X. F., He, Y. H., Liu, B., Guo, H., Xue, L., Duan, Y. W., et al. (2022). Alfalfa's response to atrazine stress and its secreted atrazine metabolites. Ecotoxicol. Environ. Saf. 241, 113780. doi:10.1016/j.ecoenv.2022.113780
Yang, H., Pang, Y., Yang, Y., Wang, D. X., and Wang, Y. C. (2025). Habitat heterogeneity of nitrogen and phosphorus cycling functional genes in rhizosphere microorganisms of Pinus tabuliformis in qinling Mountains, China. Microorganisms 13 (6), 1275. doi:10.3390/microorganisms13061275
Yao, Y., Wang, X., Yang, X., Wang, L., and Xu, H. (2020). Root sequestration and translocation of phytoremediation of Pb by the hyperaccumulator Pteris vittata L. Int. J. Phytoremediation 22 (6), 579–586. doi:10.1080/15226514.2019.1696744
Yao, W. B., Huang, L., Yang, Z. H., and Zhao, F. P. (2022). Effects of organic acids on heavy metal release or immobilization in contaminated soil. Trans. nonferrous Metals Soc. China 32 (4), 1277–1289. doi:10.1016/S1003-6326(22)65873-4
Yu, H. X., and Zahidi, I. (2023). Spatial and temporal variation of vegetation cover in the main mining area of qibaoshan town, China: potential impacts from mining damage, solid waste discharge and land reclamation. Sci. Total Environ. 859, 160392. doi:10.1016/j.scitotenv.2022.160392
Yuan, X. Q., Guo, Z. L., Wang, S. C., Zhao, L. Q., Yuan, M. X., Gao, Y. H., et al. (2023). Drivers and mechanisms of spontaneous plant community succession in abandoned Pb-Zn mining areas in Yunnan, China. Sci. Total Environ. 904, 166871. doi:10.1016/j.scitotenv.2023.166871
Zhang, Z., Wang, K., Li, G., Xie, X., Chang, X., and Zheng, J. (2023). Beyond shrub dieback: understory plant diversity, soil water and soil carbon storage were improved in a semi-arid region. For. Ecol. Manag. 545, 121267. doi:10.1016/j.foreco.2023.121267
Zhang, Z., Zhong, J. S., Guo, X. Z., Xu, C., Huang, D. Y., Liu, J., et al. (2024). Differential effects of pH on cadmium accumulation in Artemisia argyi growing in low and moderately cadmium-contaminated paddy soils. Chem. Biol. Tehnologies Agric. 11 (1), 158. doi:10.1186/s40538-024-00690-x
Zhao, D. Y., Bol, R., Wang, J. P., Jin, J. Y., Wang, Y. H., Wang, T. X., et al. (2024). Soil heavy metal pollution promotes extracellular enzyme production by mediating microbial community structure during vegetation restoration of metallic tailing reservoir. Sci. Total Environ. 948, 174783. doi:10.1016/j.scitotenv.2024.174783
Zhou, J. X., Xiang, Z. Z., Zhou, M. F., Feng, Y. X., Luo, K., Huang, Z. L., et al. (2018). The giant upper yangtze Pb–Zn province in SW China: reviews, new advances and a new genetic model. J. Asian Earth Sci. 154, 280–315. doi:10.1016/j.jseaes.2017.12.032
Zhu, G. X., Xiao, H. Y., Guo, Q. J., Song, B., Zheng, G. D., Zhang, Z. Y., et al. (2018). Heavy metal contents and enrichment characteristics of dominant plants in wasteland of the downstream of a lead-zinc mining area in Guangxi, southwest China. Ecotoxicol. Environ. Saf. 151, 266–271. doi:10.1016/j.ecoenv.2018.01.011
Zhu, Y., An, Y. F., Li, X. Y., Cheng, L., and Lv, S. J. (2024). Geochemical characteristics and health risks of heavy metals in agricultural soils and crops from a coal mining area in Anhui province, China. Environ. Res. 241, 117670. doi:10.1016/j.envres.2023.117670
Keywords: heavy metal migration, phytoremediation, succession dynamics, plant exudates, rhizosphere processes
Citation: Yu X, Li T, Wu X, Liu C, Li S, Peng S, Wang S, Zhao L and Duan C (2025) Exudates of dominant plants regulate rhizospheric soil total and available heavy metals and facilitates natural restoration succession in an abandoned metal mining area. Front. Environ. Sci. 13:1698742. doi: 10.3389/fenvs.2025.1698742
Received: 04 September 2025; Accepted: 27 October 2025;
Published: 19 November 2025.
Edited by:
Haibo Zhang, Zhejiang Agriculture and Forestry University, ChinaReviewed by:
Leonce Dusengemungu, Copperbelt University, ZambiaLijuan Sun, Shanghai Academy of Agricultural Sciences, China
Copyright © 2025 Yu, Li, Wu, Liu, Li, Peng, Wang, Zhao and Duan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Changqun Duan, Y2hxZHVhbkB5bnUuZWR1LmNu
†These authors have contributed equally to this work
 Xiaoya Yu
Xiaoya Yu Ting Li
Ting Li Xiaoni Wu5
Xiaoni Wu5 Chang’e Liu
Chang’e Liu Changqun Duan
Changqun Duan

