Abstract
Ponderosa pine (Pinus ponderosa) is one of the most valuable American pines growing naturally in the western and Pacific states of Arizona and California. Based on previously published research, its ecological valence makes this species suitable for introduction worldwide, including Europe. In Central Europe, climate change—the primary cause of significant dieback of native tree species, such as Norway spruce and Scots pine—has increased the need to explore new methods to ensure forest stand sustainability. Introducing previously overlooked tree species, such as ponderosa pine could help address this challenge. We reviewed 229 research sources to analyze P. ponderosa’s potential for utilization in new areas. The existing research from its native distribution range indicates ecological plasticity and strong resistance to drought and climatic extremes. Production parameters were evaluated in young European forest stands with a stand volume of 430 m3⋅ha–1 at the age of 45, pointing toward a promising use in the forestry sector. In European forestry, ponderosa pine’s importance could grow due to its adaptability to warm and dry climates and tolerance of diverse soil conditions. Moreover, the extraordinary quality and texture of the wood, as well as ponderosa’s biodiversity and ornamental functions, make the species destined to become part of future landscapes and forest ecosystems of Central Europe under changed climatic conditions. However, we also see challenges and scientific gaps associated with the management of ponderosa pine and its introduction to mixtures with native tree species without prior verification and silviculture recommendations.
1 Introduction
Global climate change represents a significant challenge for many sectors—and forestry has been one of the most threatened over the last few decades. Currently, forest ecosystems are facing an increasing rate of biotic and abiotic disturbances, be it increased mortality of native Central European tree species, primarily Norway spruce (Picea abies [L.] Karst.; Jandl, 2020; Knoke et al., 2021; Romeiro et al., 2022), or mass dieback of Scots pine (Pinus sylvestris L.; Rigling et al., 2018; Lemaire et al., 2022). Climate change may also disturb the wood supply chain by reducing the amount of high-quality wood for industry due to fluctuations caused by high wildfire occurrences or insect pest outbreaks (Rodríguez Silva et al., 2012; Vakula et al., 2015).
One option for afforestation in areas where forests have been disturbed, is to find alternatives to native tree species that no longer thrive under current climatic conditions. Moreover, the adaptation of forest ecosystems should be based on what the expected climate will be like in the upcoming decades. Therefore, the increase of non-native tree species proportions, such as those from North America or Asia (Brus et al., 2019) could be a part of a successful solution in Central European forests. According to Forest Europe (2015), non-native species occupy approximately 4.5% of European forest area (approximately 9.5 million ha). Many of them are critical for ensuring wood production in particular countries, e.g., Pinus nigra (J. F. Arnold), Pinus contorta (Douglas ex Loudon), or Pinus radiata (D. Don). However, significant differences remain in our understanding of the ecological aspects, as well as the quality and quantity of wood production. In particular, there is a lack of comprehensive knowledge about the potential of specific introduced tree species, especially regarding their resistance to extreme climatic conditions under ongoing climate change. For example, the potential of P. nigra in Europe has already been described by Vacek et al. (2023). The prospective management and use of lodgepole pine (P. contorta) in Europe was extensively described by Novotný et al. (2018).
Pinus ponderosa (Douglas ex C. Lawson) is another introduced pine species with considerable potential that has not yet been systematically evaluated in European conditions. This species is one of the most commercially used trees in North America, where it covers millions of hectares and provides high-quality wood (Oliver et al., 1990). Its ecological valence and economic value are why scientists, foresters, and the timber industry are paying more attention (Farjon and Styles, 1997). This growing interest is supported by the favorable mechanical and technological properties of the wood, such as good workability and sufficient strength and durability, making it a potential alternative to native conifers in Europe such as Scots pine (Kretschmann, 2010; Ross and Forest Products Laboratory, 2010). Geographically, P. ponderosa is native to areas from Canada to Central America (Price et al., 1998). It is also widespread in Western Europe and the Czechia (Pokorný, 1963), where it has been tested predominately in hilly areas. It is often planted in parks and woodlands as an attractive landscape feature (Hieke, 1994). However, planting it in standard forest stands is rare. The species prefers well-drained soils and a continental to subcontinental climate, and it is generally tolerant to drought and poor soil conditions (Farjon, 2010, 2021), which adds to its silvicultural appeal. Compared to regions in the Southern Hemisphere (Dezzotti et al., 2009; Mathias et al., 2023) the introduction of P. ponderosa in Europe is not expected to pose significant risks related to invasiveness or the spread of associated pests.
Systematic reviews describing P. ponderosa’s growth and production potential have only been performed in the area where it naturally grows (Krannitz and Duralia, 2004; Callaham, 2013). They are lacking for newly established stands in Europe with different soil and climatic conditions, which can be the main drivers for the successful growth of introduced tree species in non-native areas. Therefore, the objective of this literature review is to evaluate in detail the opportunities and risks of P. ponderosa cultivation in European forestry, based on the comparison of research results from native and non-native areas. The specific aims are to provide a detailed overview of (i) species description, (ii) taxonomy, (iii) distribution, (iv) ecology, (v) silviculture and production potential, (vi) wood properties, (vii) diseases and threats, and (viii) adaptation to climate change.
2 Materials and methods
This manuscript was prepared using information from research databases Scopus, Web of Science, and Google Scholar. The search covered a wide range of definitions and keywords related to P. ponderosa: P. ponderosa, taxonomy, cultivation, silviculture, growth, productivity, harvesting, use, and wood properties in both native and introduced distribution areas, i.e., European research and review articles, books, and conference proceedings were searched for in the study. Other sources, such as technical reports or dissertations, were also evaluated. A total of 229 references were included in this article – 137 research articles, 12 review articles, 28 books, 15 conference proceedings, and 37 other sources. In the past, P. ponderosa topics were rare, but with the increasing interest in this species since the mid-20th century, it was deemed unnecessary to establish a search window. The most recent manuscript was found in 2025, while the oldest was published in 1931. The selected manuscripts are from various countries worldwide, but most articles are from the United States.
3 Description of the species
Pinus ponderosa is not only one of the most important pine species in the U.S. in terms of forestry and timber industry, but also a symbol of the western part of North America. For example, P. ponderosa is the state tree of Montana (Kral, 1993). P. ponderosa is an evergreen monoecious tree species. It grows to a height of 50 m (83 m maximum) and a diameter of 120 cm (265 cm maximum) in its native distribution range. Taylor (2022) reports that the tallest P. ponderosa (P. ponderosa subsp. benthamiana) from the Sierra Nevada—Stanislaus National Forest—reaches a height of 83.66 m and is likely to be the tallest pine in the world.1 In Europe, the tallest ponderosa pine reaches a height of 48.3 m with a diameter at breast height (dbh) of 161 cm in the United Kingdom, followed by a tree of 47.3 m in height with a dbh of 109 cm in Belgium.2 The trunk is usually straight and sturdy. Branches are thick, usually 3–6 branches on a hinge, set to the trunk at an angle of 51°–56° from the top of the young trees. The bark on mature trees is vertically fissured, dark brown or dark gray to black. On old trees, it is already light yellow to yellowish brown, thick with broad stripes separated by shallow fissures. The tree forms a massive root system with a deep main root (taproot) (Oliver and Ryker, 1990; Callaham, 2013).
This pine species is characterized by three needles in the fascicle (Figure 1), but P. ponderosa subsp. scopulorum and P. ponderosa subsp. readiana often have only two. The needles are finely serrated and pointed, finely lined on all faces with vents. They are 13–25 cm long, yellow-green to blue-green, lighter and shorter at higher elevations and in xeric conditions. The needles rarely turn gray but are more likely to at higher altitudes and with the onset of winter. The needles are flexible and in bundles of three; they remain green for an average of 1.4–7.6 years, longer at higher altitudes (Callaham, 2013). The needles are enclosed at the base by sheaths of braces, which reach 10%–15% of their length in the first year (Helmers, 1943; Conkle and Critchfield, 1988; Callaham, 2013). P. ponderosa needles are toxic for pregnant cows, especially in the last trimester of pregnancy (Panter et al., 1990; James, 1999) due to labdane resin acid, ICA (Gardner and Panter, 1994).
FIGURE 1

Tree habitus, branch with cones, needles, and scales with the seed of Pinus ponderosa (Douglas ex C. Lawson).
On the oldest bundles, they leave a thick, dark brown to black sheath 4–8 mm long. The vegetative buds are covered with loosely appressed ovoid, sharp scales that are conspicuously lined with short brown hairlike projections. The scales are dark reddish-brown to light gray-brown, covered with resin in summer and occasionally in winter as well. Vegetative shoots are green or brownish-green and sticky, elongating once a year.
The production of cones in P. ponderosa typically starts at approximately 20 years old and can continue until the tree reaches a maximum age of 350 years. However, the superior seeds are produced by trees that fall within the 60–160-year-old range (Bonner and Karrfalt, 2008). The trees can live over 600 years (Youngblood et al., 2004) as OldList gives the oldest known P. ponderosa from measurements by Stan Kitchen from a drier climate in the Wah Wah Mountains. Using the cross-dating method, he counted 929 annual rings and estimated the final age at 941 years.3
The number of seeds in a cone varies from 50 to 70, depending on many abiotic and biotic factors, such as high temperature, precipitation, or insect pests, which can also affect seed growth and mortality (Krannitz and Duralia, 2004). Male cones borne in clusters of 5–30 are dark red or reddish-brown to reddish-purple, long-cylindrical, and 20–90 mm long. Male cones in the first year are 5–25 mm long, semi-double at the peduncle, initially red, then turning greenish-brown to gray, with short, appressed scales. Cones are either single or in clusters of usually 3–5, sessile, oblong-ovate-conical, 8–10–15 cm long, and 3–5 cm wide, and deciduous the following year after opening. The shield is flat-angled with a straight keel, while the umbilicus is conspicuously spiny (Conkle and Critchfield, 1988; Callaham, 2013).
The spines are usually erect, symmetrical, and straight to slightly curved at maturity. The color of the cones during the second summer ranges from apple green to reddish brown to dark purple. The cones are sessile, deciduous during the first winter after maturity, with a very short pedicel and a few basal scales that usually remain temporarily on the branch after the cone has fallen. The scales of the seed cones (134–159) are slightly concave, usually rounded at the apex. The apophysis is yellow to brown, shiny, and slightly raised along the transverse keel. The adaxial surface of the cones is slightly concave, dull yellow to reddish brown. The abaxial surface is dark brown, purple, or blackish (Farjon, 2005, 2021).
Pinus ponderosa produces seeds periodically, usually after mast years, and cone production is limited to a few or no cones (McDonald, 1992; Shepperd et al., 2006; Mooney et al., 2011). Generally, mast years occur from 3 to 8 years (Roeser, 1941; Fowells and Schubert, 1956; Larson and Schubert, 1970; Dahms, 1975). Seeds are dark brown or mottled, about two-thirds as wide as long, 4–5 mm × 6–8 mm in diameter, and are not translucent. Seed wings are 3.3–4.3 times the length of the seed, 16–25 mm long, thin, semi-transparent, light brown, sometimes dark brown, broadly striped in the middle or slightly below the middle, with a rounded apex (Pokorný, 1963; Pilát, 1964; Murphy, 1994; Farjon, 2001, 2010; Callaham, 2013). Individual morphological characteristics of P. ponderosa partially vary between subspecies (Callaham, 2013).
4 Taxonomy
Pinus ponderosa (Douglas ex C. Lawson), known as yellow western pine, was discovered by David Douglas in 1826 near Spokane, WA (Little, 1979), and was described by Lawson in 1836. The taxonomy of P. ponderosa has not been resolved due to differing opinions (Weidman, 1939; Wells, 1964a; Read, 1980; Kitzmiller, 1990; Lauria, 1991, 1996a; 1996b; Rehfeldt, 1993, 1999; Cregg, 1994; Farjon and Styles, 1997; Willyard et al., 2009, 2017, Willyard et al., 2021a; Callaham, 2013; Potter et al., 2013; López-Reyes et al., 2015). Provenance studies of P. ponderosa began in 1913 and have been described by numerous authors (Wells, 1964b; Read, 1980, 1984; Rehfeldt, 1986, 1990, 1991, 1993, 1999; Conkle and Critchfield, 1988). There have been a series of provenance studies to determine patterns of genetic variation in a broad north-south range extending from the Canadian border to nearly Mexico. Smith (1977), after studying xylem monoterpenes from 68 sites, divided P. ponderosa into five regional types with four transition zones. Critchfield (1984) demonstrated the close identity of Pinus washoensis H. Mason and Stockwell with Weidman’s “North Plateau ponderosa pine.”
-
Pinus ponderosa Dougl. ex C. Lawson var. ponderosa is a typical heavy pine, ranging south from British Columbia, Idaho, Washington, Oregon, and northeastern California.
-
Rocky Mountain ponderosa pine, P. ponderosa var. scopulorum Engelm. (1880), E. Murray (1982), elevated the subspecies in Montana and Wyoming, east of the Continental Divide and extending south through Utah, Colorado, and Nebraska into Arizona, New Mexico, Oklahoma, and West Texas to Mexico.
-
Arizona pine, P. ponderosa var. arizonica (Engelm.) Shaw, growing in extreme southwestern New Mexico, southeastern Arizona, and adjacent northern Mexico.
Based on morphological data and climatic parameters, Callaham (2013) concluded that there are five subspecies of P. ponderosa in the western U.S. states and adjacent Canada (Figure 2):
FIGURE 2
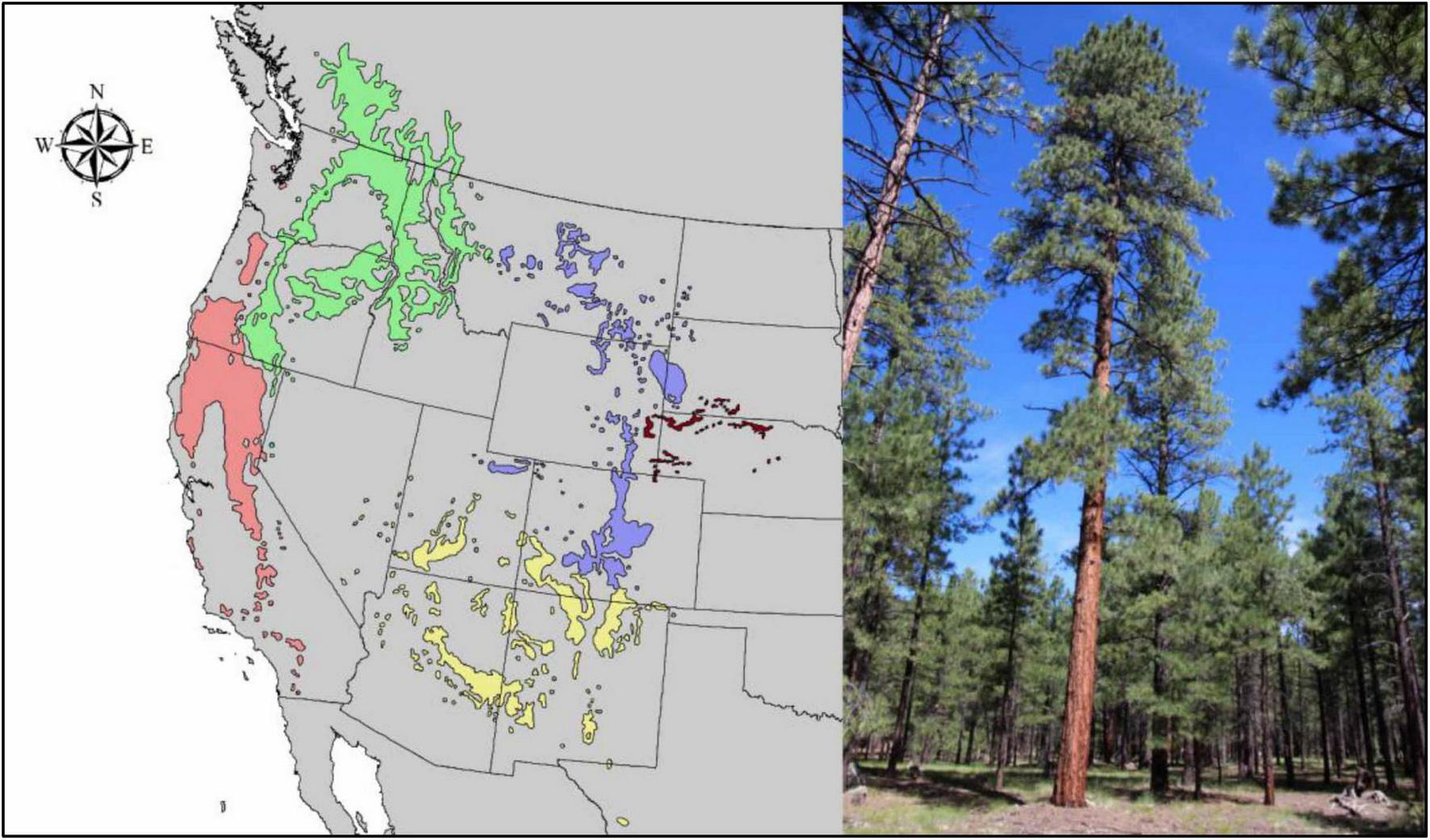
Distribution map of ponderosa pine according to Callaham (2013) with illustrated areas of the five main subspecies: Pinus ponderosa subsp. P. ponderosa (green), P. ponderosa subsp. P. critchfieldiana (red), P. ponderosa subsp. P. scopulorum (blue), P. ponderosa subsp. P. readiana (brown), and P. ponderosa subsp. P. brachyptera (yellow).
-
Pinus ponderosa subsp. P. ponderosa, Columbia ponderosa pine,
-
Pinus ponderosa subsp. critchfieldiana Callaham, subsp. nov., Pacific ponderosa pine,
-
Pinus ponderosa subsp. scopulorum (Engelm. and S. Watson) E. Murray, Rocky Mountain ponderosa pine,
-
Pinus ponderosa subsp. readiana Callaham, subsp. nov., central High Plains ponderosa pine,
-
Pinus ponderosa subsp. brachyptera (Engelm. and Wislizenus) Callaham, comb. nov., Southwestern ponderosa pine.
These subspecies of P. ponderosa closely match the geographic races proposed by Weidman (1939), the ecotypes proposed by Wells (1964b) and Haller (1965), the regions and zones proposed by Smith (1977), and the geographic clusters proposed by Read (1980, 1984).
However, according to Willyard et al. (2021a), based on nuclear genetics, there are only three subspecies of P. ponderosa:
-
Pinus ponderosa subsp. P. ponderosa Douglas ex Lawson 1836,
-
Pinus ponderosa subsp. P. benthamiana (Hartw.) Silba 2009,
-
Pinus ponderosa subsp. P. washoensis (Mason & Stockwell) E. Murray 1982.
Mitochondrial studies suggest complexity within each subspecies, with mitochondrial haplotypes varying significantly across the species range (Potter et al., 2013; Willyard et al., 2021b). Molecular clock analyses (Lascoux et al., 2004; Potter et al., 2013) suggest that the separation of P. ponderosa and P. scopulorum occurred more than 250,000 years ago and that the separation may have persisted from the onset of the Glacial/Interglacial cycles at the Pliocene/Pleistocene boundary or even earlier, with the separation occurring when tectonism created the basin and range structure of central Nevada in what was formerly a montane plateau (Potter et al., 2013). In addition to taxonomy, recent research using state-of-the-art genetic methods has focused on the issue of advancing global climate change, both from an ecosystem perspective and from the perspective of management and its adaptation to ongoing processes (Skov et al., 2005; Xiaoyan et al., 2013; Rehfeldt et al., 2014; Jolly et al., 2015; McDowell and Allen, 2015; Mcdowell et al., 2016; Singleton et al., 2019; Willyard et al., 2021a).
5 Distribution
Geographically, P. ponderosa’s native range extends from Canada to Central America (Price et al., 1998). It is naturally distributed in western North America, from British Columbia to California and northern Mexico in the south and east to northwestern Nebraska, covering millions of hectares. The natural distribution of this species includes British Columbia, Arizona, California, Colorado, Idaho, Northeastern Mexico, Northwestern Mexico, Montana, Nebraska, Nevada, New Mexico, North Dakota, Oklahoma, Oregon, South Dakota, Texas, Utah, Washington, and Wyoming (Baker and Korstian, 1931; Meyer, 1938; Little, 1979; Thompson et al., 1999; Kolb et al., 2019).
Individual subspecies vary in their natural distribution:
-
Pinus ponderosa subsp. P. ponderosa, Columbia ponderosa pine—southward from southern British Columbia, east of the Cascade Range in Washington, Oregon, and northeastern California, and eastward to the Continental Divide.
-
Pinus ponderosa subsp. P. critchfieldiana Callaham, subsp. nov., Pacific ponderosa pine—from Puget Sound, Washington, southward to Southern California.
-
Pinus ponderosa subsp. P. scopulorum (Engelm. in S. Watson) E. Murray, Rocky Mountain ponderosa pine—from the Continental Divide in Montana eastward to the Black Hills of South Dakota and southward into Utah and Colorado.
-
Pinus ponderosa subsp. P. readiana Callaham, subsp. nov., Central High Plains ponderosa pine—restricted to Nebraska and adjacent areas in South Dakota, Wyoming, and Colorado.
-
Pinus ponderosa subsp. P. brachyptera (Engelm. in Wislizenus) Callaham, comb. nov., Southwestern ponderosa pine—in Arizona, New Mexico, Oklahoma, and southwestern Texas, and, in all likelihood, extending into Mexico (Callaham, 2013).
The current distribution of P. ponderosa forests in its natural range is likely the result of its rapid expansion during the Holocene (Betancourt, 1986; Anderson, 1989).
In Europe, P. ponderosa was successfully introduced two centuries ago (Hermann, 1987). For example, in the Czechia, P. ponderosa was first noticed in 1827 (Pokorný, 1963) and first planted in 1845 in the Sychrov chateau park (Hieke, 1994). At the same time, ponderosa was also introduced in Great Britain, but recently, it has been used primarily as an ornamental plant and is rarely found in forest stands (Streets, 1961; Parrantt, 2020). On the other hand, it has been experimentally planted in forest stands in the Czechia (Poleno, 1985; Kaňák, 2004). Besides these two countries, P. ponderosa was introduced to many other European countries, e.g., Austria, France, Germany, Greece, Hungary, Ireland, Italy, Poland, Romania, Spain, and Sweden—in parks, arboretums, and forests (Tutin et al., 1968; Landry, 1978; Krauze-Baranowska et al., 2002; Dobignard and Chatelain, 2010; Stace, 2019). However, the total forest cover of this species has not yet been measured in Europe. P. ponderosa has also been introduced to other continents, such as Australia and Oceania, e.g., to South Australia and New Zealand (Weston, 1957; Burdon et al., 1991; Orchard et al., 1998), Asia, e.g., China (Gong et al., 1997), and South America, e.g., to Argentina (Bachmann, 1972; Broquen et al., 1998; Gyenge et al., 2012).
From the perspective of introduction into Europe and phytosanitary considerations, P. ponderosa is known to be susceptible to several pests and pathogens in its native range (McHugh et al., 2003; Fettig et al., 2007). However, these organisms are currently absent or not established in Europe, which minimizes the immediate risk of pathogen transfer associated with P. ponderosa introduction (Haffey et al., 2018). Ecologically, P. ponderosa thrives in environments differing from most native European pine species (Oliver et al., 1990; Caudullo et al., 2016), which may reduce competition and phytopathological impacts. Overall, while P. ponderosa does not currently pose significant phytosanitary threats in Europe, the potential for future pest or disease introduction via this species warrants ongoing surveillance and risk assessment.
6 Habitat and ecology
Pinus ponderosa has a wide ecological spectrum of environmental conditions where it can grow, which could be one reason for its successful introduction into new areas. It is a light-tolerant tree species that grows in forests and woodlands in predominately arid habitats (Farjon, 2001, 2010). It occurs from the lowlands to the mountains, reaching up to 3,200 m above sea level. Muir (1894) found it grows well on moist moraines, gravelly lake basins, arctic ridges, and arid lava covers. In the lower elevations of the mountainous west, these forests are adjacent to grasslands, pine-juniper woodlands, or chaparral (scrub). Their ecotone varies in width (Milo and Moir, 1986). In the higher elevations, P. ponderosa stands are usually next to mixed, mostly coniferous forests (Eyre, 1980).
Regarding habitat and vegetation, there are two types of P. ponderosa forests—xerophilous and mesophilous. In xerophilous (drier) forests, P. ponderosa occurs as a climax tree in predominantly monospecific stands and regenerates during the mid to late stages of its life cycle (Dick-Peddie, 1993; Moir and Fletcher, 1996; Moir et al., 1997). In mesophilous (wetter) forests, P. ponderosa is part of mixed conifer stands. Its regeneration occurs only during the early to mid part of the developmental cycle, although older trees may persist until the end of their life cycle (Johnson, 1993, 1994; Moir et al., 1997).
The average annual air temperatures in xerophilous forests range between 6°C and 9°C and precipitation between 480 and 600 mm, while in mesophilous forests, they are between 4°C and 7°C and 660–700 mm. In xerophilous forests, the driest periods are in May and June, and the understory vegetation, primarily grasses, is severely desiccated and flammable (Swetnam, 1990; Swetnam and Betancourt, 1990; Swetnam and Baisan, 1996). In mesic forests, drought does not usually occur during the growing season (Eyre, 1980; Johnson, 1994).
It grows on a wide range of soils, from stony and sandy on acidic rock to limestone and basalt. Soil types include podzols, rankers, rangelands, lithosols, cambisols, and volcanic cinder soils; loamy and sandy soils predominate (Brady and Weil, 1999; Hyndman, 1985; Kaye and Hart, 1998; Abella and Covington, 2006).
The xerophilous forests are dominated by pure stands of P. ponderosa, sometimes with small admixtures of oaks, junipers, and other pine species (Quercus grisea, Quercus arizonica, Quercus emoryi, Juniperus spp., Pinus edulis, Pinus discolor, Pinus californiarum). Mixed or intermixed woody species form the middle or bottom layer below P. ponderosa (Marshall, 1957; Fischer and Bradley, 1987; Bradley et al., 1992). In mesophilous forests, they usually form mixed stands. In the southern part of the range, it grows together with Abies concolor and Pinus lambertiana, while in the northern part, predominately with Pseudotsuga menziesii var. glauca. Locally, Picea pungens, Pinus strobiformis, and Populus tremuloides are also intermixed or interspersed (Jones, 1974; Bradley et al., 1992; Dick-Peddie, 1993; Johnson, 1993, 1994). The mixing of woody species varies among subregions. In California, Calocedrus decurrens, P. menziesii, A. concolor, and P. lambertiana are common. In Oregon, P. contorta, A. concolor, and P. menziesii grow, while Larix occidentalis and Picea engelmannii occur sporadically. In Washington and Idaho, P. menziesii and A. concolor are sometimes found, and in Montana, it is P. menziesii and L. occidentalis (Block and Finch, 1997).
The structure of P. ponderosa stands was initially open parkland, dominated by large, old trees, mostly of similar age, that are relatively fire tolerant (Weaver, 1943; Covington and Moore, 1994; Moore et al., 2004; Brown et al., 2015). Fires recorded in the old-growth tree rings have occurred every 8–15 years on average (Brown et al., 2015).
7 Silviculture and production
7.1 Reforestation and silviculture under wildfire threat
The cultivation of P. ponderosa has received considerable attention in western North America, as it is the most economically important tree on the continent (Fiedler and Arno, 2015). In California alone, approximately 280,000 ha of both private and National Forest lands are covered by such plantations (Zhang et al., 2019). Indeed, P. ponderosa is one of the best examples of the excellent adaptation of conifers to wildfire. Mature trees are not threatened by low-intensity fires due to their dense, fire-resistant boles. P. ponderosa forests, which are predominantly or co-dominantly composed of P. ponderosa, have long relied on wildfire as a crucial ecological disturbance (Korb et al., 2019). Historically, ponderosa stands were frequently dominated by low to moderate-severity surface fires, or mixed-severity fires that involved both moderate and high-severity crown fires (Brown and Cook, 2006; Iniguez et al., 2009; Scholl and Taylor, 2010; Sherriff et al., 2014). Moreover, as climate change continues, the incidence of fires worldwide increases. Wildfires play a critical role in P. ponderosa forests, both ecologically and economically (Swetnam and Baisan, 1996; Laughlin et al., 2004; Ouzts et al., 2015) due to their distribution in high fire-risk areas. Research indicates that the regeneration of P. ponderosa in post-fire areas is slow and not very effective, particularly during the first decade following a fire (Roccaforte et al., 2012; Ritchie and Knapp, 2014). Presumedly, the natural regeneration of ponderosa forests may even take a few decades (Bonnet et al., 2005; Haire and McGarigal, 2010), primarily because of large areas for regeneration with limited access to seed sources (Haire and McGarigal, 2010; Puhlick et al., 2012). Ponderosa seeds are relatively large and, therefore, do not disperse over long distances. They have a short lifespan (Howard, 2003). This phenomenon presents a danger to the forests in the Southwestern United States, as it may lead to their conversion into non-forested ecosystems (Savage and Mast, 2005; Dore et al., 2012; Roccaforte et al., 2012; Savage et al., 2013). This risk is becoming increasingly relevant due to the rising number of large-scale wildfires associated with climate change. Regeneration of ponderosa pine is severely limited beyond 150 m from a seed source, further hindering natural recovery (Haffey et al., 2018). On the other hand, with sufficient numbers of surviving mature seed trees, post-fire conditions—bare ground and open space—enable a successful natural regeneration of P. ponderosa (Howard, 2003; Oliver and Ryker, 1990).
The second, more promising option for reestablishing ponderosa forests is artificial regeneration (planting) (Schubert, 1970, 1974). However, Vickers et al. (2018) reported a lower survival rate of seedlings planted in West Texas in two periods—22%–34% after fall planting and 9%–25% after late-summer planting. The main reasons for the high seedling mortality were the occurrence of pocket gophers and drought. In Arizona and New Mexico, Ouzts et al. (2015) noticed the high variability of survival rates between research plots 5–8 years after artificial reforestation. The survival rate varied from 0% to 60%. Many authors offered options to improve the survival rate of ponderosa seedlings through different silvicultural methods and treatments, e.g., planting season (Vickers et al., 2018), irregular cluster planting designs (Vickers et al., 2018; North et al., 2019), selection of drought-adapted seed sources (Kolb et al., 2016), among others (Engeman et al., 1999; Pinto et al., 2011; Tricker et al., 2013; North et al., 2019).
However, competition from pioneer shrubs, such as Arctostaphylos and Ceanothus spp., may affect the seeds, seedling germination, and growth (McDonald and Fiddler, 2010; Zhang et al., 2013a). Therefore, it is essential to implement effective weeding control measures to enhance the survival rate of P. ponderosa. Reducing grass and shrub competition with herbicides can improve young trees’ growth (Powers and Reynolds, 1999; Moore et al., 2023). Fertilization can be employed to increase the effect of weed control (Moore et al., 2023). However, fertilizing without initially removing competing plants may even be counterproductive in supporting weed growth (Zhang et al., 2022).
A great deal of attention has focused on stand management, particularly thinning intensity, which might reduce the negative impact of wildfire and improve the health status of managed forest stands (Covington et al., 1997; Waltz et al., 2014; Kalies and Yocom Kent, 2016). Many authors point out the need for tree reduction during the first thinning (Schubert, 1971; Ffolliott et al., 2000). Consequently, the stand ring base and volume will also change. Moreover, the natural regeneration was significantly reduced after the first thinning. This short-term management of density reduction may improve light conditions and influence the subsequent growth within the next 10 years after the thinning (Malchus and Ffolliott, 1999; Ffolliott et al., 2000). In xerophilous stands, natural regeneration was also severely reduced to minimize fire hazard (Gottfried and DeBano, 1990). These thinning operations produce a large volume of predominately small-diameter trees and woody by-products, such as wood chips (Hampton et al., 2008; Lowell et al., 2008). Contrastingly, managed dense stands of P. ponderosa are considerably less adapted to fire (Weatherspoon et al., 1992; Skinner and Chang, 1996). When fire does occur, it is likely to be highly destructive and destroy large areas of the stand. Since the mid-1970s, some forest managers have attempted to reintroduce low-intensity fire into this ecosystem, but their efforts have often been thwarted by the high cost of monitoring these fires (Swetnam and Baisan, 1996; Gutsell and Johnson, 1996; Laughlin et al., 2004; Waltz et al., 2014; Ouzts et al., 2015).
7.2 Production and productivity
In the early 1970s, an emphasis on timber production changed to a more holistic view of the natural resource management of P. ponderosa. In North America, where the species is native, production parameters vary greatly depending on altitude, habitat, or silvicultural interventions. For example, tree densities in old-growth P. ponderosa forests in Eastern Oregon and Northern California ranged from 58 to 1,853 trees⋅ha–1 and basal area from 18.9 to 30.9 m2⋅ha–1 (Youngblood et al., 2004). Research focusing on different types of treatment in Arizona showed that the control (unthinned) plots reached an average number of trees 3,160 per ha and a basal area of 47 m2⋅ha–1, while the heavily thinned stands had 70 trees, i.e., 7 m2⋅ha–1 (McDowell et al., 2006). The high production potential of this pine species was proven by a study by Zhang et al. (2013b) from Oregon and California, where periodic annual increment reached a respectable 18.7 m3⋅ha–1⋅yr–1.
Less information comes from non-native areas. In the Czechia, Podrázský et al. (2020) found above-average growth characteristics of P. ponderosa compared to seven other native and exotic pine species. In comparison, P. ponderosa reached the highest stand volume of 430 m3⋅ha–1 at age 45 years, while the lowest yield was calculated for Pinus strobus—112 m3⋅ha–1. High production potential (223 m3⋅ha–1) was observed in Pinus jeffreyi in the same study area. In a forest reclamation site on a former coal mine, P. ponderosa reached a 2% lower stand volume when compared to the average of the 12 tree species tested, including native and non-native ones (Vacek et al., 2021a). In another study from Czechia, the stand volume of P. ponderosa was 335 m3⋅ha–1 at the age of 50 years, which is 12% less than the native P. sylvestris but 53% more than, for example, P. contorta on the same site. Generally, P. ponderosa grows more slowly in the early years and can eventually overtake P. sylvestris in growth parameters (Pokorný, 1963). Production of P. ponderosa according to selected studies is summarized in Table 1.
TABLE 1
| Study | Country | Altitude (m a.s.l.) |
Age (year) | DBH (cm) | Height (m) | Basal area (m2⋅ha–1) | Volume (m3⋅ha–1) | Density (trees⋅ha–1) | Climate classification |
| North America—native | |||||||||
| Youngblood et al., 2004 | Oregon California |
900–2,000 | To 618 | 58.8–61.0 | 25.6–30.9 | 18.9–30.9 | 58–1,853 | Csb | |
| Hagmann et al., 2013 | Oregon | 1,270–2,300 | Multi | 8–14 | 38–72 | Csb | |||
| Vaughan et al., 2019 | Arizona | 2,266 | 100 | 28.5–51.0 | 10.9–39.2 | 57–601 | Csb | ||
| McDowell et al., 2006 | Arizona | 2,266 | 87 | 13.4–47.0 | 11.1–19.5 | 7–45 | 70–3,160 | Csb | |
| Robertson and Bowser, 1999 | Colorado | 214 | 25.8 | 241 | Bsk | ||||
| Negrón et al., 2008 | South Dakota and Wyoming | 1,814–1,827 | 21.1–22.8 | 22.0–26.3 | 583–630 | Dfb | |||
| Zhang et al., 2013b | Oregon California |
1,240–1,520 | 20–65 | 9.9–18.0 | 35.4–55.1 | 2,200–5,260 | Dsb, Csb | ||
| Europe—introduced | |||||||||
| Forest Data Bank in Poland | Poland | 300 | 88 | 40 | 21 | Cfb | |||
| Insinna et al., 2007 | Germany | 14 | 112 | 50.2 | 28.8 | Cfb | |||
| Podrázský et al., 2020 | Czechia | 300–345 | 35 | 37.0 | 18.2 | 49.4 | 430 | 460 | Cfb |
| Vacek et al., 2021a | Czechia | 440 | 48 | 15.1 | 10.6 | 35.6 | 183 | 2,000 | Cfb |
| Zeidler et al., 2024 | Czechia | 430 | 50 | 23.6 | 13.7 | 335 | Cfb | ||
| Australia and Oceania—introduced | |||||||||
| Burdon et al., 1991 | New Zealand | 300 | 28 | 34.6 | 16.0 | Cfb | |||
| 375 | 29 | 29.3 | 14.4 | Cfb | |||||
| 500 | 28–29 | 22.5 | 9.5 | Cfb | |||||
Overview of available publications related to Pinus ponderosa (Douglas ex C. Lawson) production parameters.
DBH, diameter at breast height; MAI, mean annual increment; Köppen climate type: Csb, warm-summer Mediterranean; Bsk, cold semi-arid; Dfb, warm-summer humid continental; Dsb, warm-summer Mediterranean continental; Cfb, oceanic.
8 Importance and use
8.1 Wood quality and utilization
Pinus ponderosa is a softwood (conifer) tree species. On the cross-section of the stem, its wood is divided into heartwood and sapwood. The heartwood is indicated by a light reddish-brown color, while the sapwood is from pale yellow to almost white (Ross and Forest Products Laboratory, 2010). Sapwood covers a relatively significant area of the cross-section (Domec and Gartner, 2003; Ross and Forest Products Laboratory, 2010). Domec and Gartner (2003) found that ponderosa sapwood covers 29-54-116 rings from young, mature, and old-growth trees at the trunk base. The juvenile wood may cover up to 30 rings in conifers and is characterized by somewhat poor wood properties and small sizes of anatomical elements (Panshin and Zeeuw, 1980). In pines, the wood properties exhibit an increase in their intensity from the pith to the bark (Zeidler et al., 2024). P. ponderosa sawtimber from the outer stem is known to be relatively lightweight and to have poor wood properties (Ross and Forest Products Laboratory, 2010).
The wood typically has straight grain and moderate shrinkage (Zeidler et al., 2024) showed that volumetric shrinkage for P. ponderosa was 14.4% for samples collected in the Czechia, while its native area showed 3.9% for radial, 6.2% for tangential, and 9.7% for volumetric shrinkage (Ross and Forest Products Laboratory, 2010). The wood density of P. ponderosa in the native area of its distribution varied between studies, ranging from 380 to 464 kg⋅m–3 (Tsoumis, 1991; Alden, 1997; Vaughan et al., 2019, 2021). Similar results were obtained in Central Europe by Zeidler et al. (2024) who reported an average density of P. ponderosa growth on post-mining sites of approximately 426 kg⋅m–3. In New Zealand, Ledgard and Belton (1985) observed slightly lower wood density: 361 kg⋅m–3. The modulus of rupture of P. ponderosa wood also varied from a very low value of approximately 34 MPa (Mackes et al., 2005) to about 64 MPa (Alden, 1997), or even 65 MPa (Ross and Forest Products Laboratory, 2010), which corresponds to a modulus of elasticity ranging from 5.633 to 8.900 MPa (Alden, 1997; Mackes et al., 2005; Ross and Forest Products Laboratory, 2010). In the case of compression parallel to the grain, the average value for ponderosa is 36.7 MPa, and over four times lower compression perpendicular to the grain (Ross and Forest Products Laboratory, 2010). In Europe, Zeidler et al. (2024) noticed a lower average value for compression—26.2 MPa—which varied from 17.7 to 37.7 MPa.
Due to its high quality and large distribution in its native range, P. ponderosa wood is the most commercially important and versatile in western North America (Little, 1971, 1979; Safford, 2013; Western Wood Products Association, 2018). In the past, P. ponderosa wood was widely used for log cabin construction, lumber production, as firewood in mines, and to power steam locomotives (Meyer, 1938). Current uses include structural lumber, furniture lumber, miscellaneous cabinetry, firewood, log cabin construction (Western Wood Products Association, 2018), and, to a lesser extent, for pole production, mine timber, posts, or railroad crossties. The highest-quality wood is reserved for molding, blinds, doors, or even sashes. Low-grade wood is popular for making boxes and crates. Knotty P. ponderosa is often employed for interior woodwork. Its use is similar to Scots pine in Europe. The primary regions known to produce P. ponderosa are located in Oregon, Washington, and California. In addition, Idaho and Montana are considered other significant producing states (Ross and Forest Products Laboratory, 2010). In Europe, the potential of P. ponderosa is not yet recognized due to its sparse distribution. However, available studies have shown that compared to other pine species in Europe, it is not on par with Scots pine in similar habitats (Zeidler et al., 2024).
8.2 Other uses
The Nez Perce Indians harvested the inner bark to feed their horses in early spring when the forage was still buried deep under the snow. Cambium was scraped for food (often only in times of famine) by the Klamath, Sanpoil, Spokane, Colville, Okanogan, and Thompson-Nicola tribal nations. Seeds were also gathered for food. Most everyone ate the seeds, usually roasted, sometimes ground into flour. The pitch or sap was used by various tribes, including the Cheyenne, Flathead, Okanogan, Colville, Paiute, and Thompson-Nicola, for a wide variety of purposes, such as an ointment for sores, cuts, and earaches; to reduce inflammation of the eyes; to treat back pain or rheumatism. Decoctions of green needles have also been used for similar medicinal purposes. Many tribes used pitch as glue or putty. The Diegueño tribe made baskets from the needles, and the Karok and Maidu tribes made them from finer roots. The wood was used for 6,000 years in Indian dwellings to make hollowed-out canoes, and, of course, as fuel (Peattie, 1991; Schoenherr, 2017).
In Europe, P. ponderosa is also used for forest reclamation and soil improvement (Vacek et al., 2021a). For example, in the reclamation of former landfills, faster soil formation, low bulk density, higher porosity, pH, and nutrient levels were observed in P. ponderosa stands (Spasić et al., 2024). Cones are commonly used in handicrafts and decorations. This pine species is rarely used as Christmas trees due to its long needles; Scots pine is preferred instead (Johnson et al., 1997). P. ponderosa also has important scenic, recreational, and aesthetic values due to large tree sizes and low overstory density (Pokorný, 1963; Brown, 1984).
9 Risks and diseases
A serious threat to P. ponderosa is advancing global climate change, particularly in the Southwestern United States, where tree mortality has increased due to more frequent long-term droughts, warmer temperatures, and associated fire and insect attacks (Westerling et al., 2006; van Mantgem et al., 2009; Meddens et al., 2012; Xiaoyan et al., 2013; Allen et al., 2015; Cohen et al., 2016). In western North America, several species of Dendroctonus, particularly Dendroctonus ponderosae, Dendroctonus valens, and Dendroctonus brevicomis, are locally damaging in P. ponderosa stands, but are vital parts of the ecosystem. Their periodic outbreaks could potentially cause widespread mortality in mature forests over large areas, particularly in post-fire stands (McHugh and Kolb, 2003; McHugh et al., 2003; Fettig et al., 2007; Negrón et al., 2016). In California, they have caused significant mortality in these stands (Oliver, 1997). There is also a higher incidence of mistletoe (Arceuthobium spp.) in naturally weakened stands (Barrett and Roth, 1985; Filip, 2005; Abella and Denton, 2009).
Ponderosa seeds and cones can also be attacked by several insects, such as cone beetle (Conophthorus ponderosae), cone moths (Dioryctria spp. or Eucosma spp.), cone weevils (Conotrachelus neomexicanus), or cone bugs (Leptoglossus occidentalis), seed worms (Cydia piperana), and parasitic wasps or chalcids (Megastigmus albifrons) (Keefover-Ring and Linhart, 2010). These insects are only found on the P. ponderosa trees from the Colorado Front Range and display a tendency to limit their movements to only one or a few nearby trees (Bodenham and Stevens, 1981).
Major root diseases of P. ponderosa include Armillaria ostoyae, Heterobasidion annosum, and Leptographium wageneri var. ponderosum, which cause stunting to loss of growth, breakage or windthrow, and mortality. These root diseases are spread by contact with roots, aerial spores, or insect vectors, depending on the species of the root disease fungus. The trunk partially decays, mainly due to infection by Phellinus pini, Fomitopsis officinalis, and Phaeolus schweinitzii, but the post-decay is slowed by the highly resinous nature of the wood (Filip, 2001, 2005). The trunk disease is caused by Endocronartium harknessii, Cronartium comandrae, Cronartium coleosporioides, and Cronartium ribicola, which cause reduced tree growth and even death. Seedlings, young plants, and mature trees can also be affected (Allen, 1996; Filip, 2005).
Pine tree diseases are caused by Mycosphaerella pini, Lophodermella concolor, Lophodermella morbida, and Lophodermium pinastri. These fungi regularly cause needle discoloration at 2–3 years old when environmental conditions are favorable for infection development. Recently, P. ponderosa has suffered from dieback caused by the microscopic fungus Sphaeropsis sapinea (Filip, 2005). Quail, squirrels, and many other wildlife species consume the seeds of P. ponderosa (Little, 1979). Introduced to New Zealand in 1865, P. ponderosa is considered an invasive tree species because of its negative impact on native communities (Burdon et al., 1991).
In general, the introduction of non-native species carries risks that may lead to their rapid spread and the displacement of native species from their natural ecosystems. Ponderosa pine does not have the status of an invasive species in most of the world, with the exception of some regions in the Southern Hemisphere, such as New Zealand (McAlpine and Howell, 2024; Mathias et al., 2023). The same phenomenon of treating ponderosa pine as an invasive species also occurs in Argentina, Australia, and Chile (Richardson and Rejmánek, 2004; Iglesias et al., 2022). In northwestern Patagonia, P. ponderosa has demonstrated invasive tendencies, with natural regeneration expanding beyond plantation borders at an average rate of up to 10 m per year, especially under disturbed conditions. Although factors such as dense ground vegetation, poor soils, and lack of mycorrhizal associations can hinder its spread, its potential to establish and persist in novel habitats has been clearly documented (Dezzotti et al., 2009). So far, no such evidence of spontaneous invasive spread has been reported from European countries.
10 Adaptation to climate change
The ability of P. ponderosa to adapt to different climatic conditions is the subject of anxiety and uncertainty, as well as prospects and expectations. In its natural range in the Southwestern United States, threatening climatic conditions can trigger damage to populations (Kolb et al., 2019; Davis et al., 2019). Foresters are particularly concerned about reduced growth rates due to warmer temperatures and decreased water availability, which can hinder tree growth and increase susceptibility to pests and diseases. Weakened trees are more vulnerable to insect and pathogen attacks. Climate change can also alter fire regimes—and changes in fire patterns disrupt the natural regeneration cycle of P. ponderosa. These situations raise the question about range shifts. As conditions become less suitable, P. ponderosa populations may migrate to higher elevations or latitudes. To mitigate these impacts, scientists and forest managers are investigating strategies such as assisted migration and drought-resistant genotypes (Dixit et al., 2020) including the increased adaptation to extreme environmental conditions by testing the survival or growth of seedlings with different phenotypes, which was conducted in Arizona (Dixit et al., 2021), but has not yet been evaluated under European conditions.
Pinus ponderosa is significantly more sensitive to changes in precipitation than temperature, making it challenging to predict its response to climate change, given the uncertainty surrounding future precipitation patterns (Kusnierczyk and Ettl, 2002). In regions such as central and southern Europe, where projected climate change is expected to involve greater increases in temperature than shifts in precipitation, this species may represent a suitable candidate due to its relative tolerance to rising temperatures (Carvalho et al., 2021; Ghazi et al., 2023). One of the options for adapting to climate change can be silvicultural practices (Vacek et al., 2023). From a management perspective, P. ponderosa trees in low-density plantations can withstand severe drought without substantial impact on their water relations, particularly in larger trees. In contrast, trees in high-density stands experience more pronounced drought effects on water relations, stem growth, and leaf area, likely resulting in lower growth resilience and prolonged drought impacts (Gyenge et al., 2012).
In contrast, in its non-native range of distribution, there are hopes for P. ponderosa to support or partially substitute local declining populations of native species, e.g., Norway spruce or Scots pine in Central Europe (Toth et al., 2020; Netherer et al., 2024), which originally had a temperate climate, but is now affected by climate change. In a wider introduction, it may be possible to rely in part on the research results reviewed so far. However, research on P. ponderosa from Europe is scarce. Podrázský et al. (2020) evaluated and confirmed the high production potential and growth stability of P. ponderosa compared to other tree species, including introduced ones. Similar observations were made by Insinna et al. (2006), who confirmed 60% higher productivity of P. ponderosa in comparison with Scots pine in dry growing conditions. High production is associated with biomass production and carbon sequestration, which plays an essential role in mitigating ongoing global climate change (Insinna et al., 2006; Berndes et al., 2016; Vacek et al., 2023). More recent tree-ring studies (Kerhoulas et al., 2017; McCullough et al., 2017; Fuchs et al., 2019; Fletcher et al., 2019; Arco Molina et al., 2024) indicate a decrease in radial growth due to ongoing climate change, but at the same time, they also show the adaptive capacity of P. ponderosa to cope with a drier climate. Based on the current knowledge, P. ponderosa looks promising in Central Europe, especially from the point of production value and, possibly, higher tolerance to climate extremes, particularly higher temperatures. The tolerance is determined by the adaptation to the climatic conditions in its original homeland, including the southern parts of North America.
When considering assisted migration and climate change adaptation, it is crucial to carefully evaluate and understand the ecological risks associated with species introductions (Probert et al., 2020). This includes assessing potential invasiveness, impacts on native biodiversity, and long-term ecosystem effects. Effective management involves ongoing monitoring and risk mitigation strategies to prevent unintended negative consequences (Meyerson and Mooney, 2007; Simberloff et al., 2013). A precautionary approach is necessary to balance the benefits of assisted migration with the protection of native ecosystems (Vitt et al., 2010).
Despite its potential, the limited research has focused primarily on incorporating P. ponderosa into mixtures of native species in newly planted forest stands. As described earlier, mixed forest stands are more resilient to climatic extremes, e.g., when precipitation and temperature have a greater effect on the growth of monospecific rather than mixed stands (Vacek et al., 2021b). There is a considerable scientific gap that needs to be addressed in future research on all introduced tree species, including P. ponderosa. The favorable production parameters, resistance, and ecology of P. ponderosa should provide new motivation for further verification of the production characteristics and other factors of existing stands, and particularly the motivation to establish new research-based forest stands with varying degrees of the admixture of this introduced tree species among native ones.
11 Conclusion
Native to North America, P. ponderosa exhibits a robust potential for cultivation in various climatic conditions throughout Europe due to its high adaptability. Previously published studies have shown that its ability to thrive in stressful environments, including extreme climate conditions, such as drought and poor soil stands, makes it a promising species in the context of ongoing climate change. Its resilience to drought periods and capacity to grow in diverse soil types suggest that it could be a suitable option for European reforestation projects, particularly in areas threatened by desertification or for forest reclamation. Low-density stands should be preferred in areas prone to severe drought, as this promotes growth resistance and stability of water conditions compared to high-density stands. However, P. ponderosa cultivation should be supplemented by a careful evaluation of its ecological impacts, including potential effects on native ecosystems, where we see limitations in studies already conducted, which focused on wood production characteristics. Monitoring its growth and adaptability under different conditions while assessing the risk of invasive behavior will be crucial. The potential for commercial forestry use of P. ponderosa in Europe is significant, especially given its high-quality timber, relatively fast growth, and positive effect on soil conditions. However, there have been no previous studies on silvicultural recommendations for mixtures with native tree species, highlighting another critical scientific gap. Therefore, the suitability of the species and its provenance for specific European regions requires further investigation, and more research is needed on its long-term impact on local biodiversity. If properly managed and implemented, ponderosa pine could contribute positively to commercial forestry and forest restoration efforts.
Statements
Author contributions
KT: Data curation, Formal analysis, Methodology, Writing – original draft. ZV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JCu: Conceptualization, Supervision, Validation, Writing – review & editing. SV: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. VB: Writing – original draft. AZ: Writing – original draft. VT: Validation, Writing – original draft. JG: Writing – original draft. JČe: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was funded by the Czech University of Life Sciences Prague, Faculty of Forestry and Wood Sciences (Excellent Team 2025) and by the Ministry of Agriculture of the Czech Republic (No. QK22020045).
Acknowledgments
We would like to thank Jitka Šišáková, an expert in the field, and Richard Lee Manore, a native speaker, for proofreading the article. We also want to thank Josef Macek for the graphic design of the figures.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Abella S. Covington W. (2006). Forest ecosystems of an Arizona Pinus ponderosa landscape: Multifactor classification and implications for ecological restoration.J. Biogeogr.331368–1383. 10.1111/j.1365-2699.2006.01513.x
2
Abella S. Denton C. (2009). Spatial variation in reference conditions: Historical tree density and pattern on a Pinus ponderosa landscape.Can. J. For. Res.392391–2403. 10.1139/X09-146
3
Alden H. (1997). Softwoods of North America.Madison: US Department of Agriculture, Forest Service, Forest Products Laboratory.
4
Allen C. (1996). Fire effects in southwestern forests: Proceedings of the second La Mesa fire symposium.Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station.
5
Allen C. Breshears D. McDowell N. (2015). On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the Anthropocene.Ecosphere6:art129. 10.1890/ES15-00203.1
6
Anderson R. (1989). “Development of the southwestern ponderosa pine forests: What do we really know?,” in Multiresource Management of Ponderosa Pine Forests, edsTecleA.CovingtonW. W.HamreR. H. (Fort Collins, CO: U.S. Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station), 15–22.
7
Arco Molina J. Altman J. Rai S. Korznikov K. Pejcho V. Dvorsky M. et al (2024). Climate drivers of Pinus ponderosa tree development on volcanic tephra deposits in the Southwestern USA: Insights from radial increment and wood density variations.Dendrochronologia88:126242. 10.1016/j.dendro.2024.126242
8
Bachmann B. (1972). Afforestation in the andean foothills of Patagonia, Argentina.Argentina Rev. For. Argentina16166–169.
9
Baker F. Korstian C. (1931). Suitability of brush lands in the intermountain region for the growth of natural or planted western yellow pine forests.Washington, DC: US Department of Agriculture.
10
Barrett J. Roth L. (1985). Response of dwarf mistletoe-infested ponderosa pine to thinning: 1. Sapling growth. Pacific Northwest research station.Washington, DC: Forest Service, US Department of Agriculture, 10.2737/pnw-rp-330
11
Berndes G Abt B Asikainen A Cowie A Dale V Egnell G et al . (2016). Forest biomass, carbon neutrality and climate change mitigation.From Sci. Policy31–27. 10.36333/fs03
12
Betancourt J. (1986). “Paleoecology of pinyon-juniper woodlands: summary. In: RL Everett, compiler,” in Proceedings-pinyon-juniper conference, (Ogden: U.S. Department of Agriculture, Forest Service, Intermountain Research Station), 129–139.
13
Block W. Finch D. (1997). Songbird ecology in southwestern ponderosa pine forests: a literature review.Fort Collins, CO: US Dep Agric.
14
Bodenham J. Stevens R. (1981). Insects associated with second-year ponderosa pine cones, larimer and boulder counties, Colorado.Southwest Nat.26375–378. 10.2307/3671079
15
Bonner F. Karrfalt R. (2008). The woody plant seed manual.Washington, DC: U.S. Department of Agriculture, Forest Service.
16
Bonnet V. Schoettle A. Shepperd W. (2005). Postfire environmental conditions influence the spatial pattern of regeneration for Pinus ponderosa.Can. J. For. Res.3537–47. 10.1139/x04-157
17
Bradley A. Noste N. V. Fischer W. (1992). Fire ecology of forests and woodlands in Utah.Ogden: US Department of Agriculture, Forest Service, Intermountain Research Station.
18
Brady N. Weil R. (1999). The nature and properties of soils.Prentice Hall, Inc: Upper Saddle River, 881.
19
Broquen P. Girardin J. Falbo G. Álvarez O. (1998). Prediction models of site index in Pinus ponderosa Dougl. from soil features, west andinopatagonia, 37°-41° S.República Argentina. Revista Bosque1971–79. 10.4206/bosque.1998.v19n1-08
20
Brown P. Cook B. (2006). Early settlement forest structure in Black Hills ponderosa pine forests.For. Ecol. Manage223284–290. 10.1016/J.FORECO.2005.11.008
21
Brown P. Battaglia M. Fornwalt P. Gannon B. Huckaby L. Julian C. et al (2015). Historical. (1860). forest structure in ponderosa pine forests of the northern Front Range, Colorado.Can. J. For. Res.451462–1473. 10.1139/cjfr-2014-0387
22
Brown T. (1984). Modeling forest scenic beauty: concepts and application to ponderosa pine.Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest.
23
Brus R. Pötzelsberger E. Lapin K. Brundu G. Orazio C. Straigyte L. (2019). Extent, distribution and origin of non-native forest tree species in Europe.Scand. J. For. Res.34533–544. 10.1080/02827581.2019.1676464
24
Burdon R. Miller J. Knowles F. (1991). Introduced forest trees in New Zealand: recognition, role, and seed source. 10. Ponderosa and Jeffrey pines: Pinus ponderosa P. Lawson et Lawson, Pinus jeffreyi Grev. et Balf.Rotorua: Forest Research Institute, Ministry of Forestry.
25
Callaham R. (2013). Pinus ponderosa: a taxonomic review with five subspecies in the United States. Albany, CA: U.S. Department of Agriculture, Forest Service, Pacific Southwest Research Station.
26
Carvalho D. Cardoso Pereira S. Rocha A. (2021). Future surface temperatures over Europe according to CMIP6 climate projections: An analysis with original and bias-corrected data.Clim Change167:10. 10.1007/s10584-021-03159-0
27
Caudullo G. De Rigo D. Mauri A. Houston Durrant T. San-Miguel-Ayanz J. (2016). European atlas of forest tree species. Publications Office of the European Union. 10.2760/776635
28
Cohen W. Yang Z. Stehman S. V. Schroeder T. Bell D. Masek J. et al (2016). Forest disturbance across the conterminous United States from 1985–2012: The emerging dominance of forest decline.For Ecol Manage360242–252. 10.1016/j.foreco.2015.10.042
29
Conkle M. Critchfield W. (1988). Genetic variation and hybridization of ponderosa pine.Ponderosa Pine Species Manag.27:43.
30
Covington W. Moore M. (1994). Postsettlement changes in natural fire regimes and forest structure.J. Sustainable For.2153–181. 10.1300/J091v02n01_07
31
Covington W. Fule P. Moore M. (1997). Restoring ecosystem health in ponderosa pine forests of the Southwest.J For9523–29. 10.1093/jof/95.4.23
32
Cregg B. (1994). Carbon allocation, gas exchange, and needle morphology of Pinus ponderosa genotypes known to differ in growth and survival under imposed drought.Tree Physiol.14883–898. 10.1093/treephys/14.7-8-9.883
33
Critchfield W. B. (1984). Crossability and relationships of Washoe pine.Madroño31, 144–170.
34
Dahms W. (1975). Seed production of central Oregon ponderosa and lodgepole pines.Portland: Pacific Northwest Forest and Range Experiment Station, Forest Service.
35
Davis K. Dobrowski S. Higuera P. (2019). Wildfires and climate change push low-elevation forests across a critical climate threshold for tree regeneration.Proc. Natl. Acad. Sci.1166193–6198. 10.1073/pnas.1815107116
36
Dezzotti A. Sbrancia R. Mortoro A. Monte C. (2009). Invasión biológica de Pinus ponderosa y Pinus contorta: Estudio de caso de una plantación en la Patagonia noroccidental.Invest. Agraria Sistemas Recursos Forestales18181–191. 10.5424/fs/2009182-01061
37
Dick-Peddie W. (1993). New Mexico vegetation: past, present, and future.Albuquerque: University New Mexico Press.
38
Dixit A. Kolb T. Burney O. (2020). Provenance geographical and climatic characteristics influence budburst phenology of southwestern ponderosa pine seedlings.Forests11:1067. 10.3390/f11101067
39
Dixit A Kolb T. E Burney O Mock K Grady K. (2021). Provenance variation in early survival, growth, and carbon isotope discrimination of southwestern ponderosa pine growing in three common gardens across an elevational gradient.Forests12:1561. 10.3390/f12111561
40
Dobignard A. Chatelain C. (2010). Index synonymique de la flore d’Afrique du Nord Volume 1: Pteridophyta, Gymnospermae, Monocotyledonae.Ville Genève Éditions Conservatoire Jardin Botaniques Genève1:455.
41
Domec J. Gartner B. (2003). Relationship between growth rates and xylem hydraulic characteristics in young, mature and old-growth ponderosa pine trees.Plant Cell Environ26471–483. 10.1046/j.1365-3040.2003.00978.x
42
Dore S Montes-Helu M Hart S. C Hungate B. A Koch G. W Moon J. B et al . (2012). Recovery of ponderosa pine ecosystem carbon and water fluxes from thinning and stand-replacing fire.Glob. Chang. Biol.183171–3185. 10.1111/j.1365-2486.2012.02775.x
43
Engeman R. M Anthony R. M Barnes V. G. Jr. Krupa H. W Evans J. (1999). Evaluations of plastic mesh tubes for protecting conifer seedlings from pocket gophers in three Western states.Western J. Appl. For.1486–90. 10.1093/wjaf/14.2.86
44
Eyre F. (1980). Forest cover types of the United States and Canada.Washington, DC: Society of American Foresters.
45
Farjon A. (2001). World checklist and bibliography of conifers, 2nd Edn. London Borough of Richmond upon Thames: The Royal Botanic Gardens, Kew.
46
Farjon A. (2005). Pines: drawings and descriptions of the genus Pinus.Leiden: Brill Academic Publishers.
47
Farjon A. (2010). A handbook of the world’s conifers: revised and updated edition.Leiden: Brill Academic Publishers.
48
Farjon A. (2021). Pines: drawings and descriptions of the genus Pinus.Leiden: Brill Academic Publishers.
49
Farjon A. Styles B. (1997). Pinus. (Pinaceae). flora neotropical monograph 75.New York, NY: The New York Botanical Gardens.
50
Fettig C. J Klepzig K. D Billings R. F Munson A. S Nebeker T. E Negrón J. F et al . (2007). The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States.For. Ecol. Manage23824–53. 10.1016/j.foreco.2006.10.011
51
Ffolliott P. Baker M. Gottfried G. (2000). Heavy thinning of ponderosa pine stands: An Arizona case study. (Research Paper/RMRS–RP–22).Department Agric. For. Serv. Rocky Mountain Res. Station22:6. 10.2737/RMRS-RP-22
52
Fiedler C. Arno S. (2015). Ponderosa: People, Fire, and the West’s Most Iconic Tree.Missoula: Mountain press Publishing Company.
53
Filip G. (2001). Managing tree wounding and stem decay in Oregon forests.Corvallis: Oregon State University Extension Service, 12.
54
Filip G. (2005). Diseases as agents of disturbance in ponderosa pine.USDA Forest Service Gen Tech Rep PSW-GTR198227–232.
55
Fischer W. Bradley A. (1987). Fire ecology of western Montana forest habitat types.Ogden: U.S. Department of Agriculture, Forest Service, Intermountain Research Station.
56
Fletcher T Touchan R Lepley K Rouini N Bloye R Tremarelli T. S et al . (2019). Two reconstructions of august–july precipitation for central Northern Arizona from tree rings.Tree Ring Res75116–126. 10.3959/1536-1098-75.2.116
57
Forest Europe,. (2015). “State of Europe’s forests 2015,” in Proceedings of the Ministerial Conference on the Protection of Forests in Europe.Spain: Forests Europe, 314.
58
Fowells H. Schubert G. (1956). Seed crops of forest trees in the pine region of California.Washington, DC: US Department of Agriculture.
59
Fuchs L. Stevens L. Fulé P. (2019). Dendrochronological assessment of springs effects on ponderosa pine growth, Arizona, USA.For. Ecol. Manage.43589–96. 10.1016/j.foreco.2018.12.049
60
Gardner D. Panter K. (1994). Ammodendrine and related piperidine alkaloid levels in the blood plasma of cattle, sheep and goats fed Lupinus formosus.J. Nat. Toxins3, 107–116.
61
Ghazi B. Przybylak R. Pospieszyńska A. (2023). Projection of climate change impacts on extreme temperature and precipitation in Central Poland.Sci. Rep.13:18772.
62
Gong Z. Wu W. Ci Z. (1997). Studies on introduction of Pinus ponderosa.J. Northeast For. Univer.259–12.
63
Gottfried G. DeBano L. (1990). “Streamflow and water quality responses to preharvest prescribed burning in an undisturbed ponderosa pine watershed,” in Effects of fire management on southwestern natural resources. RMRS-GTR-191, ed.KrammesJ. (Fort Collins, CO: USDA Forest Service), 222–231.
64
Gutsell S. Johnson E. (1996). How fire scars are formed: Coupling a disturbance process to its ecological effect.Can. J. For. Res.26166–174. 10.1139/x26-020
65
Gyenge J. Fernández M. Varela S. (2012). Short- and long-term responses to seasonal drought in ponderosa pines growing at different plantation densities in Patagonia, South America.Trees Struct. Funct.261905–1917. 10.1007/s00468-012-0759-7
66
Haffey C. Sisk T. Allen C. Thode A. Margollis E. (2018). Limits to ponderosa pine regeneration following large high-severity forest fires in the United States Southwest.Fire Ecol.14143–163. 10.4996/fireecology.140114316
67
Hagmann R. Franklin J. Johnson K. (2013). Historical structure and composition of ponderosa pine and mixed-conifer forests in south-central Oregon.For. Ecol. Manage304492–504. 10.1016/J.FORECO.2013.04.005
68
Haire S. McGarigal K. (2010). Effects of landscape patterns of fire severity on regenerating ponderosa pine forests. (Pinus ponderosa). in New Mexico and Arizona, USA.Landsc. Ecol.251055–1069. 10.1007/s10980-010-9480-3
69
Haller J. (1965). Pinus washoensis in Oregon: taxonomic and evolutionary implications, 6th Edn. Hoboken, NJ: Wiley.
70
Hampton H. M Sesnie S. E Dickson B. G Rundall J. M Sisk T. D Snider G. B et al . (2008). Analysis of small-diameter wood supply in northern Arizona-Final report.ForestERA106311–316.
71
Helmers A. (1943). The ecological anatomy of ponderosa pine needles.Am. Midl. Nat.2955–71. 10.2307/2420979
72
Hermann R. (1987). North American tree species in Europe.J. For.8527–32. 10.1093/jof/85.12.27
73
Hieke K. (1994). Lexikon okrasných dřevin.Praha: Helma.
74
Howard J. L. (2003). Pinus ponderosa var. brachyptera, P. p. var. scopulorum. Fire effects information system. Missoula, MT: US Department of Agriculture, Forest Service, Rocky Mountain Research Station, Fire Sciences Laboratory.
75
Hyndman D. (1985). Petrology of igneous and metamorphic rocks.McGraw-Hill Book CO: New York.
76
Iglesias A. L. Nuñez M. A. Paritsis J. (2022). The potential effect of climate change on the establishment of invasive pines in Patagonia. Plant Ecol.223, 1207–1218. 10.1007/s11258-022-01268-z
77
Iniguez J. Swetnam T. Baisan C. (2009). Spatially and temporally variable fire regime on Rincon Peak.Arizona, USA. Fire Ecol.53–21. 10.4996/fireecology.0501003
78
Insinna P. Götz B. Aas G. Schill H. (2006). Comparative Investigations on the Growth of Pinus ponderosa Dougl. ex P. Laws. and Pinus sylvestris L. in NE-Germany.Archiv für Forstwesen und Landschaftsökologie40106–113.
79
Insinna P. Jalkanen R. Götz B. (2007). Climate impact on 100-year old foliage chronologies of Scots pine and Ponderosa pine in the northeast lowlands of Brandenburg.Germany: Silva Fennica.
80
James L. (1999). Teratological research at the USDA-ARS poisonous plant research laboratory.J. Nat. Toxins863–80.
81
Jandl R. (2020). Climate-induced challenges of Norway spruce in Northern Austria.Trees Forests People1:100008. 10.1016/j.tfp.2020.100008
82
Johnson J. Kreh R. Torbert J. (1997). Comparison of scotch pine christmas tree varieties in Virginia.Southern J. Appl. For.2157–63. 10.1093/sjaf/21.2.57
83
Johnson M. (1993). Changing conditions in Southwestern forests and implications on land stewardship.Albuquerque: U.S. Department of Agriculture, Forest Service.
84
Johnson M. (1994). Changes in southwestern forests: Stewardship implications.J. For.9216–19. 10.1093/jof/92.12.16
85
Jolly W. M Cochrane M. A Freeborn P. H Holden Z. A Brown T. J Williamson G. J et al . (2015). Climate-induced variations in global wildfire danger from 1979 to 2013.Nat. Commun.6:7537. 10.1038/ncomms8537
86
Jones J. (1974). Silviculture of southwestern mixed conifers and aspen: the status of our knowledge.Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest.
87
Kalies E. Yocom Kent L. (2016). Tamm Review: Are fuel treatments effective at achieving ecological and social objectives? A systematic review.For. Ecol. Manage37584–95. 10.1016/j.foreco.2016.05.021
88
Kaňák J. (2004). “Možnosti a úskalí introdukce některých druhů rodu Pinus. In: Perspektivy lesnické dendrologie a šlechtění lesních dřevin,” in In Proceedings of the Sborník z konference.Kostelec nad Černými lesy, 5.
89
Kaye J. Hart S. (1998). Ecological restoration alters nitrogen transformations in a ponderosa pine–bunchgrass ecosystem.Ecol. Appl.81052–1060. 10.1890/1051-07611998008[1052:ERANTI]2.0.CO;2
90
Keefover-Ring K. Linhart Y. B. (2010). Variable chemistry and herbivory of ponderosa pine cones.Int. J. Plant Sci.171293–302. 10.1086/650155
91
Kerhoulas L. Kolb T. Koch G. (2017). The influence of monsoon climate on latewood growth of southwestern ponderosa pine.Forests8:140. 10.3390/f8050140
92
Kitzmiller J. (1990). Managing genetic diversity in a tree improvement program.For. Ecol. Manage35131–149. 10.1016/0378-1127(90)90237-6
93
Knoke T Gosling E Thom D Chreptun C Rammig A Seidl R. (2021). Economic losses from natural disturbances in Norway spruce forests – A quantification using Monte-Carlo simulations.Ecol. Econ.185:107046. 10.1016/J.ECOLECON.2021.107046
94
Kolb T. Dixit A. Burney O. (2019). Challenges and opportunities for maintaining ponderosa pine forests in the southwestern United States. Tree Planters’.Notes62104–112.
95
Kolb T. Grady K. McEttrick M. Herrero A. (2016). Local-Scale drought adaptation of ponderosa pine seedlings at habitat ecotones.For. Sci.62641–651. 10.5849/forsci.16-049
96
Korb J. Fornwalt P. Stevens-Rumann C. (2019). What drives ponderosa pine regeneration following wildfire in the western United States?For. Ecol. Manage454:117663. 10.16/J.FORECO.2019.117663
97
Kral R. (1993). “Pinus. Flora of North America editorial committee,” in In: Flora of North America North of Mexico.Oxford University Press: New York, 372–398.
98
Krannitz P. Duralia T. (2004). Cone and seed production in Pinus ponderosa: A review.West. N. Am. Nat.64208–218.
99
Krauze-Baranowska M Mardarowicz M Wiwart M Pobłocka L Dynowska M. (2002). Antifungal activity of the essential oils from some species of the genus pinus.Z Naturforsch C J Biosci.57478–482. 10.1515/znc-2002-5-613
100
Kretschmann D. (2010). Mechanical properties of wood. Wood handbook: wood as an engineering material: chapter 5 Centennial ed General technical report FPL; GTR-190.Madison, WI: US Dept of Agriculture, Forest Service, Forest Products Laboratory, 51–546.
101
Kusnierczyk E. Ettl G. (2002). Growth response of ponderosa pine. (Pinus ponderosa). to climate in the eastern Cascade mountains, Washington, U.S.A.: Implications for climatic change1.Écoscience9544–551. 10.1080/11956860.2002.11682742
102
Landry P. (1978). Reflections on the taxonomic divisions and subdivisions of a genus: The example of the genus Pinus.Bull. Soc. Botanique France125507–519.
103
Larson M. Schubert G. (1970). Cone crops of ponderosa pine in central Arizona, including the influence of Abert squirrels.Fort Collins: Rocky Mountain Forest and Range Experiment Station.
104
Lascoux M. Palmé A. Cheddadi R. Latta R. (2004). Impact of Ice Ages on the genetic structure of trees and shrubs.Philos. Trans. R. Soc. Lond. B Biol. Sci.359197–207. 10.1098/rstb.2003.1390
105
Laughlin D. C Bakker J. D Stoddard M. T Daniels M. L Springer J. D Gildar C. N et al . (2004). Toward reference conditions: Wildfire effects on flora in an old-growth ponderosa pine forest.For. Ecol. Manage199137–152. 10.1016/j.foreco.2004.05.034
106
Lauria F. (1991). Taxonomy, systematics, and phylogeny of Pinus, subsection Ponderosae Loudon. (Pinaceae).Alternative concepts. Linz. Biol. Beitr.23129–202.
107
Lauria F. (1996a). The identity of Pinus ponderosa Douglas ex C. Lawson. (Pinaceae).Linz. Biol. Beitr.28999–1052.
108
Lauria F. (1996b). Typification of Pinus benthamiana Hartw.(Pinaceae), a taxon deserving renewed botanical examination.Ann. Nat. Hist. Mus. Wien Ser. B Bot. Zool.98, 427–446.
109
Ledgard N. Belton M. (1985). Exotic trees in the Canterbury high country.N. Z. J. For. Sci.15298–323.
110
Lemaire J. Vennetier M. Prévosto B. Cailleret M. (2022). Interactive effects of abiotic factors and biotic agents on Scots pine dieback: A multivariate modeling approach in southeast France.For. Ecol. Manage526:120543. 10.1016/j.foreco.2022.120543
111
Little E. (1971). Atlas of United States trees. Vol. 1. Conifers and important hardwoods.Washington, DC: U.S. Dept. of Agriculture, Forest Service.
112
Little E. (1979). Checklist of United States trees. (native and naturalized).Washington, D.C: Forest Service, US Department of Agriculture.
113
López-Reyes A. de la Rosa J. P. Ortiz E. Gernandt D. S. (2015). Morphological, molecular, and ecological divergence in Pinus douglasiana and P. maximinoi.Syst. Bot.40658–670. 10.1600/036364415X689384
114
Lowell E. C Becker D. R Rummer R Larson D Wadleigh L. (2008). An integrated approach to evaluating the economic costs of wildfire hazard reduction through wood utilization opportunities in the Southwestern United States.Forest Sci.54273–283. 10.1093/forestscience/54.3.273
115
Mackes K. Shepperd W. Jennings C. (2005). Evaluating the bending properties of clear wood specimens produced from small-diameter ponderosa pine trees.For. Prod. J.55:72.
116
Malchus B. Ffolliott P. (1999). “Interdisciplinary land use along the Mogollon Rim,” in History of watershed research in the Central Arizona Highlands, ed.BakerM. <suffix>Jr.</suffix> (Fort Collins: U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station), 27–34.
117
Marshall J. (1957). Birds of pine-oak woodland in southern Arizona and adjacent Mexico. Pacific Coast Avifauna 32.Cooper Ornithol. Soc. Berkeley CAL321–125.
118
Mathias S. van Galen L. Jarvie S. Larcombe M. (2023). Range reshuffling: Climate change, invasive species, and the case of Nothofagus forests in Aotearoa New Zealand.Divers. Distrib.291402–1419. 10.1111/ddi.13767
119
McAlpine K. G. Howell C. J. (2024). List of environmental weeds in New Zealand 2024. Science for Conservation, 340.
120
McCullough I. Davis F. Williams A. (2017). A range of possibilities: Assessing geographic variation in climate sensitivity of ponderosa pine using tree rings.For. Ecol. Manage402223–233. 10.1016/j.foreco.2017.07.025
121
McDonald P. (1992). Estimating seed crops of conifer and hardwood species.Can. J. For. Res.22832–838. 10.1139/x92-112
122
McDonald P. Fiddler G. (2010). Twenty-five years of managing vegetation in conifer plantations in northern and central California: results, application, principles, and challenges. Gen Tech Rep PSW-GTR-231.Albany, CA: US Department of Agriculture, Forest Service, Pacific Southwest Research Station.
123
McDowell N. G Adams H. D Bailey J. D Hess M Kolb T. E. (2006). Homeostatic maintenance of ponderosa pine gas exchange in response to stand density changes.Ecol. Appl.161164–1182. 10.1890/1051-07612006016[1164:HMOPPG]2.0.CO;2
124
McDowell N. Allen C. (2015). Darcy’s law predicts widespread forest mortality under climate warming.Nat. Clim. Chang.5669–672. 10.1038/nclimate2641
125
McDowell N. G Williams A. P Xu C Pockman W. T Dickman L. T Sevanto S et al . (2016). Multi-scale predictions of massive conifer mortality due to chronic temperature rise.Nat. Clim. Chang.6295–300. 10.1038/NCLIMATE3143
126
McHugh C. Kolb T. (2003). Ponderosa pine mortality following fire in northern Arizona.Int. J. Wildl and Fire127–22. 10.1071/WF02054
127
McHugh C. Kolb T. Wilson J. (2003). Bark beetle attacks on ponderosa pine following fire in Northern Arizona.Environ. Entomol.32510–522. 10.1603/0046-225X-32.3.510
128
Meddens A. Hicke J. Ferguson C. (2012). Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States.Ecol. Appl.221876–1891. 10.1890/11-1785.1
129
Meyer W. (1938). Yield of even-aged stands of ponderosa pine.Washington, DC: Technical Bulletin of the United States Department of Agriculture.
130
Meyerson L. Mooney H. (2007). Invasive alien species in an era of globalization.Front. Ecol. Environ.5:199–208. 10.1890/1540-929520075[199:IASIAE]2.0.CO;2
131
Milo L. Moir W. (1986). Forest and woodland habitat types. (plant associations). of Southern New Mexico and Central Arizona. (north of the Mogollon Rim), Ed. 2 Edn. Masthead NE: Department of Agriculture Forest Service, Southwestern Region 2.
132
Moir W. Fletcher R. (1996). Forest diversity in New Mexico.New Mexico J. Sci.36232–254.
133
Moir W. Geils B. Benoit M. Scurlock Dan . (1997). “Ecology of southwestern ponderosa pine forests,” in Songbird ecology in southwestern ponderosa pine forests: a literature review, edsBlockW. M.FinchD. M. (Fort Collins: Deptartment of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station), 3–27.
134
Mooney K. Linhart Y. Snyder M. (2011). Masting in ponderosa pine: Comparisons of pollen and seed over space and time.Oecologia165651–661. 10.1007/s00442-010-1742-x
135
Moore J. Fan Z. Shaw T. (2023). Silvicultural treatments affect growth and foliar nutrients in a young ponderosa Pine Stand.For. Sci.69435–442. 10.1093/forsci/fxad010
136
Moore M Huffman D Fulé P Covington W Crouse E. (2004). Comparison of historical and contemporary forest structure and composition on permanent plots in Southwestern Ponderosa pine forests.For. Sci.50162–176. 10.1093/forestscience/50.2.162
137
Muir J. (1894). The mountains of California.New York: The Century, CO.
138
Murphy A. (1994). Graced by pines: the ponderosa pine in the American west.Missoula, MT: Mountain press Publishing Company.
139
Negrón J. Allen K. Cook B. Withrow J. (2008). Susceptibility of ponderosa pine, Pinus ponderosa. (Dougl. ex Laws.), to mountain pine beetle, Dendroctonus ponderosae Hopkins, attack in uneven-aged stands in the Black Hills of South Dakota and Wyoming USA.For. Ecol. Manage254327–334. 10.1016/J.FORECO.2007.08.018
140
Negrón J. F McMillin J Sieg C. H Fowler J. F Allen K. K Wadleigh L. L et al . (2016). Variables associated with the occurrence of Ips beetles, red turpentine beetle and wood borers in live and dead ponderosa pines with post-fire injury.Agric. For. Entomol.18313–326. 10.1111/afe.12163
141
Netherer S Lehmanski L Bachlehner A Rosner S Savi T Schmidt A et al . (2024). Drought increases Norway spruce susceptibility to the Eurasian spruce bark beetle and its associated fungi.New Phytol.2421000–1017. 10.1111/nph.19635
142
North M. P Stevens J. T Greene D. F Coppoletta M Knapp E. E Latimer A. M et al . (2019). Tamm Review: Reforestation for resilience in dry western U.S. forests.For. Ecol. Manage432209–224. 10.1016/j.foreco.2018.09.007
143
Novotný P. Fulín M. Čáp J. Dostál J. (2018). Lodgepole pine. (Pinus contorta Douglas ex Loudon). from the perspective of its possible utilization in conditions of changing Central European climate. Conifers.London: IntechOpen.
144
Oliver W. Ryker R. (1990). Pinus ponderosa Dougl. ex Laws. ponderosa pine. Silvics of north America, edsBurnsR. M.HonkalaB. H. (Washington, DC: US Department of Agriculture, Forest Service), 413.
145
Oliver W. Ryker R. Burns R. Honkala B. (1990). “Ponderosa pine,” in Silvics of North America. Agriculture Handbook, (Washington, DC: U.S. Department of Agriculture, Forest Servicepp), 413–424.
146
Oliver W. W. (1997). Twenty five-year growth and mortality of planted ponderosa pine repeatedly thinned to different stand densities in northern California. West. J. Appl. For.12, 122–130. 10.1093/wjaf/12.4.122
147
Orchard A. McCarthy P. Patrick M. (1998). Flora of Australia 48.Canberra: Australian Government Publishing Service.
148
Ouzts J. Kolb T. Huffman D. Sánchez Meador A. (2015). Post-fire ponderosa pine regeneration with and without planting in Arizona and New Mexico.For. Ecol. Manage354281–290. 10.1016/J.FORECO.2015.06.001
149
Panshin A. Zeeuw C. (1980). Textbook of Wood Technology.New York: McGraw-Hill Book Company.
150
Panter K. E James L. F Molyneux R. J Short R. E Sisson D. V. (1990). Premature bovine parturition induced by ponderosa pine: Effects of pine needles, bark and branch tips.Cornell Vet.80329–338. 10.1021/jf00039a031
151
Parrantt M. (2020). Pinus ponderosa Douglas ex Lawson & C.Lawson in BSBI Online Plant Atlas 2020. Available online at: https://plantatlas2020.org/atlas/2cd4p9h.2he(accessed December 7, 2024).
152
Peattie D. (1991). A Natural History of Trees of Eastern and Central North America.Boston, MA: Houghton Mifflin.
153
Pilát A. (1964). Jehličnaté stromy a keře našich zahrad a parků.Praha: Československá akademie věd.
154
Pinto J. Dumroese R. Davis A. Landis T. (2011). Conducting seedling stocktype trials: A new approach to an old question.J. For.109293–299. 10.1093/jof/109.5.293
155
Podrázský V. Vacek Z. Vacek S. Vítámvás J. Gallo J. Prokůpková A. et al (2020). Production potential and structural variability of pine stands in the Czech Republic: Scots pine. (Pinus sylvestris L.). vs. introduced pines-case study and problem review.J. For. Sci.66197–207. 10.17221/42/2020-JFS
156
Pokorný J. (1963). Jehličnany lesů a parků.Prague: SZN.
157
Poleno Z. (1985). Příměstské lesy.Prague: SZN - Státní zemědělské nakladatelství.
158
Potter K. Hipkins V. Mahalovich M. Means R. (2013). Mitochondrial DNA haplotype distribution patterns in Pinus ponderosa. (Pinaceae): Range-wide evolutionary history and implications for conservation.Am. J. Bot.1001562–1579. 10.3732/ajb.1300039
159
Powers R. Reynolds P. (1999). Ten-year responses of ponderosa pine plantations to repeated vegetation and nutrient control along an environmental gradient.Can. J. For. Res.291027–1038. 10.1139/x99-104
160
Price R. Liston A. Strauss H. (1998). “Phylogeny and systematics of Pinus,” in Ecology and Biogeography of Pinus, ed.RichardsonD. (Cambridge: Cambridge University Press), 49–68.
161
Probert A. Ward D. Beggs J. Lin S. Stanley M. (2020). Conceptual risk framework: Integrating ecological risk of introduced species with recipient ecosystems.Bioscience7071–79. 10.1093/biosci/biz131
162
Puhlick J. Laughlin D. Moore M. (2012). Factors influencing ponderosa pine regeneration in the southwestern USA.For. Ecol. Manage26410–19. 10.1016/J.FORECO.2011.10.002
163
Read R. (1980). Genetic variation in seedling progeny of ponderosa pine provenances.For. Sci.26:a0001. 10.1093/forestscience/26.s2.a0001
164
Read R. (1984). Ten-year performance of ponderosa pine provenances in the Great Plains of North America.Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest.
165
Rehfeldt G. (1986). Adaptive Variation in Pinus Ponderosa from Intermountain Regions: Middle Columbia River System. II.Ogden: US Department of Agriculture, Forest Service, Intermountain Forest and Range.
166
Rehfeldt G. (1990). Genetic differentiation among populations of pinus ponderosa from the Upper Colorado River Basin.Botanical Gazette151125–137. 10.1086/337812
167
Rehfeldt G. (1991). A model of genetic variation for Pinus ponderosa in the Inland Northwest. (USA): Applications in gene resource management.Canadian Journal of Forest Research211491–1500.
168
Rehfeldt G. (1993). Genetic variation in the Ponderosae of the Southwest.Am. J. Bot.80330–343. 10.1002/j.1537-2197.1993.tb13807.x
169
Rehfeldt G. (1999). Systematics and genetic structure of Ponderosae taxa. (Pinaceae). inhabiting the mountain islands of the Southwest.Am. J. Bot.86741–752. 10.2307/2656584
170
Rehfeldt G. E Jaquish B. C López-Upton J Sáenz-Romero C St Clair J. B Leites L. P et al . (2014). Comparative genetic responses to climate for the varieties of Pinus ponderosa and Pseudotsuga menziesii: Realized climate niches.For. Ecol. Manage.324126–137. 10.1016/j.foreco.2014.02.035
171
Richardson D. M. Rejmánek M. (2004). Conifers as invasive aliens: A global survey and predictive framework. Divers. Distrib.10, 321–331.
172
Rigling A Moser B Feichtinger L Gärtner H Giuggiola A Hug C et al . (2018). 20 years of Scots pine dieback in Valais. (Switzerland): A retrospect and new results.Schweizerische Zeitschrift für Forstwesen169242–250.
173
Ritchie M. Knapp E. (2014). Establishment of a long-term fire salvage study in an interior ponderosa pine forest.J For112395–400. 10.5849/jof.13-093
174
Robertson P. Bowser Y. (1999). Coarse woody debris in mature Pinus ponderosa stands in Colorado.J. Torrey Bot. Society13255–267. 10.2307/2997280
175
Roccaforte J. Fulé P. Chancellor W. Laughlin D. (2012). Woody debris and tree regeneration dynamics following severe wildfires in Arizona ponderosa pine forests.Can. J. For. Res.42593–604. 10.1139/x2012-010
176
Rodríguez Silva F. Ramón Molina J. González-Cabán A. Machuca M. ÁH. (2012). Economic vulnerability of timber resources to forest fires.J Environ Manage10016–21. 10.1016/J.JENVMAN.2011.12.026
177
Roeser J. (1941). Some aspects of flower and cone production in ponderosa pine.J For39534–536. 10.1093/jof/39.6.534
178
Romeiro J. M. N Eid T Antón-Fernández C Kangas A Trømborg E. (2022). Natural disturbances risks in European Boreal and Temperate forests and their links to climate change – A review of modelling approaches.For. Ecol. Manage509:120071. 10.1016/J.FORECO.2022.120071
179
Ross R. Forest Products Laboratory. (2010). Wood handbook: wood as an engineering material.Madison, WI: Department of Agriculture, Forest Service, Forest Products Laboratory, 10.2737/fpl-gtr-190
180
Safford H. (2013). Natural Range of Variation. (NRV). for yellow pine and mixed conifer forests in the bioregional assessment area, including the Sierra Nevada, southern Cascades, and Modoc and Inyo National Forests.Vallejo, CA: US Forest Service Research and Development.
181
Savage M. Mast J. (2005). How resilient are southwestern ponderosa pine forests after crown fires?Can. J. For. Res.35967–977. 10.1139/x05-028
182
Savage M. Mast J. Feddema J. (2013). Double whammy: High-severity fire and drought in ponderosa pine forests of the Southwest.Can. J. For. Res.43570–583. 10.1139/cjfr-2012-0404
183
Schoenherr A. (2017). A natural history of California.California: University of California Press.
184
Scholl A. Taylor A. (2010). Fire regimes, forest change, and self-organization in an old-growth mixed-conifer forest, Yosemite National Park, USA.Ecol. Appl.20362–380. 10.1890/08-2324.1
185
Schubert G. (1970). Artificial reforestation practices for the Southwest.Washington, DC: US Department of Agriculture, Forest Service.
186
Schubert G. (1971). Growth response of even-aged ponderosa pine related to stand density levels in Arizona.J For69857–860. 10.1093/jof/69.12.857
187
Schubert G. (1974). Silviculture of southwestern ponderosa pine: the status of our knowledge.Fort Collins, CO: Rocky Mountain Forest and Range Experiment Station, Forest Service.
188
Shepperd W. Edminster C. Mata S. (2006). Long-Term Seedfall, Establishment, Survival, and Growth of Natural and Planted Ponderosa Pine in the Colorado Front Range.West. J. Appl. For.2119–26. 10.1093/wjaf/21.1.19
189
Sherriff R. L Platt R. V Veblen T. T Schoennagel T. L Gartner M. H. (2014). Historical, observed, and modeled wildfire severity in montane forests of the colorado front range.PLoS One9:e0106971. 10.1371/journal.pone.0106971
190
Simberloff D Martin J.-L Genovesi P Maris V Wardle D. A Aronson J et al . (2013). Impacts of biological invasions: What’s what and the way forward.Trends Ecol Evol2858–66. 10.1016/j.tree.2012.07.013
191
Singleton M. Thode A. Sánchez Meador A. Iniguez J. (2019). Increasing trends in high-severity fire in the southwestern USA from 1984 to 2015.For Ecol Manage433709–719. 10.1016/j.foreco.2018.11.039
192
Skinner C. Chang C. (1996). Fire regimes, past and present. In sierra nevada ecosystem project: final report to congress, Vol. II. Davis: Centers for Water and Wildland Resources, University of California, 1041–1069.
193
Skov K. Kolb T. Wallin K. (2005). Difference in Radial Growth Response to Restoration Thinning and Burning Treatments Between Young and Old Ponderosa Pine in Arizona.West. J. Appl. For.2036–43. 10.1093/wjaf/20.1.36
194
Smith R. (1977). Monoterpenes of ponderosa pine zylem resin in Western United States.Washington, DC: Department of Agriculture, Forest Service.
195
Spasić M Vacek O Vejvodová K Tejnecký V Vokurková P Križová P et al . (2024). Which trees form the best soil? Reclaimed mine soil properties under 22 tree species: 50 years later—assessment of physical and chemical properties.Eur. J. For. Res.143561–579. 10.1007/s10342-023-01637-x
196
Stace C. (2019). New Flora of the British Isles, edition 4 Edn. Stowmarket: C&M Floristics.
197
Streets R. (1961). Exotic forest trees in the British Commonwealth.Oxford: Clarendon Press.
198
Swetnam T. (1990). “Fire history and climate in the southwestern United States,” in Proceedings of the symposium on effects of fire management of southwestern United States natural resources. Gen. Tech. Rep. RM-GTR-191, (Fort Collins, CO: US Department of Agriculture, Forest Service, Rocky Mountain Forest and Range Experiment Station). 10.1126/science.249.4972.1017
199
Swetnam T. Baisan C. (1996). Historical fire regime patterns in the southwestern United States since AD 1700. United States Department of Agriculture Forest Service General Technical Report RM 11–32.Washington, DC: US Forest Service.
200
Swetnam T. Betancourt J. (1990). Fire-southern oscillation relations in the southwestern United States.Science2491017–1020.
201
Taylor M. (2022). World’s tallest pine - 268.29 ft. Ponderosa. (272.3’ 2022). plus oregon ponderosa AF points champion. Available online at: https://www.mdvaden.com/pine_tallest.shtml(accessed February 28, 2025).
202
Thompson R. Anderson K. Bartlein P. (1999). Atlas of relations between climatic parameters and distributions of important trees and shrubs in North America.Reston, VI: US Department of the Interior, US Geological Survey.
203
Toth D. Maitah M. Maitah K. Jarolínová V. (2020). The impacts of calamity logging on the development of spruce wood prices in Czech forestry.Forests11:283. 10.3390/f11030283
204
Tricker P. J Rodríguez López C. M Hadley P Wagstaff C Wilkinson M. J. (2013). Pre-conditioning the epigenetic response to high vapor pressure deficit increases the drought tolerance of Arabidopsis thaliana.Plant. Signal. Behav.8:e25974. 10.4161/psb.25974
205
Tsoumis G. (1991). Science and technology of wood. (structur, properties, utilization).New York: Van Nostrand Reinhold.
206
Tutin T. G Heywood V. H Burges N. A Moore D. M Valentine D. H Walters S et al . (1968). Flora Europaea.Cambridge: Cambridge University Press.
207
Vacek Z Cukor J Vacek S Linda R Prokůpková A Podrázský V et al . (2021a). Production potential, biodiversity and soil properties of forest reclamations: Opportunities or risk of introduced coniferous tree species under climate change?Eur. J. For. Res.1401243–1266. 10.1007/s10342-021-01392-x
208
Vacek Z Prokůpková A Vacek S Bulušek D Šimůnek V Hájek V et al . (2021b). Mixed vs. monospecific mountain forests in response to climate change: Structural and growth perspectives of Norway spruce and European beech.For. Ecol. Manage488:119019. 10.1016/j.foreco.2021.119019
209
Vacek Z. Vacek S. Cukor J. (2023). European forests under global climate change: Review of tree growth processes, crises and management strategies.J. Environ. Manage332:117353. 10.1016/J.JENVMAN.2023.117353
210
Vakula J Zúbrik M Galko J Gubka A Kunca A Nikolov C et al . (2015). Influence of selected factors on bark beetle outbreak dynamics in the Western Carpathians.For. J.61149–156. 10.1515/forj-2015-0023
211
van Mantgem P. J Stephenson N. L Byrne J. C Daniels L. D Franklin J. F Fuleì P. Z. et al (2009). Widespread increase of tree mortality rates in the Western United States.Science323521–524. 10.1126/science.1165000
212
Vaughan D Auty D Dahlen J Sánchez Meador A. J Mackes K. H. (2021). Modelling variation in wood stiffness of Pinus ponderosa using static bending and acoustic measurements.For. Int. J. For. Res.94232–243. 10.1093/forestry/cpaa030
213
Vaughan D Auty D Kolb T. E Sánchez Meador A. J Mackes K. H Dahlen J et al . (2019). Climate has a larger effect than stand basal area on wood density in Pinus ponderosa var. scopulorum in the southwestern USA.Ann. For. Sci.76:85. 10.1007/s13595-019-0869-0
214
Vickers L. Houser J. Rooni J. Guldin J. (2018). “Restoring ponderosa pine in the Davis Mountains of west Texas: impacts of planting practices on seedling survival,” in Proceedings of the Silvicultural Research Conference, (Washington, DC: United States Department of Agriculture), 82.
215
Vitt P Havens K Kramer A. T Sollenberger D Yates E. (2010). Assisted migration of plants: Changes in latitudes, changes in attitudes.Biol. Conserv.14318–27. 10.1016/j.biocon.2009.08.015
216
Waltz A. E. M Stoddard M. T Kalies E. L Springer J. D Huffman D. W Sánchez Meador A. (2014). Effectiveness of fuel reduction treatments: Assessing metrics of forest resiliency and wildfire severity after the Wallow Fire.AZ. For. Ecol. Manage33443–52. 10.1016/j.foreco.2014.08.026
217
Weatherspoon C. Husari S. van Wagtendonk J. (1992). “Fire and fuels management in relation to owl habitat in forests of the Sierra Nevada and southern California,” in The California spotted owl: a technical assessment of its current status, edsVernerJ.McKelveyK. S.NoonB. R. (Vallejo, CA: Pacific Southwest Research Station, Forest Service, U.S. Department of Agriculture), 247–260.
218
Weaver H. (1943). Fire As An Ecological and Silvicultural Factor in the Ponderosa-Pine Region of the Pacific Slope.J. For.417–15. 10.1093/jof/41.1.7
219
Weidman R. (1939). Evidences of racial influence in a 25-year test of ponderosa pine.J. Agric. Res.59855–887.
220
Wells O. (1964a). Geographic variation in ponderosa pine. II. Correlations between progeny performance and characteristics of the native habitat. Silvae Genet. 13, 89–103.
221
Wells O. (1964b). Geographic variation in ponderosa pine. I. The ecotypes and their distribution. Silvae Genet. 13, 126–164.
222
Westerling A. Hidalgo H. Cayan D. Swetnam T. (2006). Warming and Earlier Spring Increase Western U.S. Forest Wildfire Activity.Science313940–943. 10.1126/science.1128834
223
Western Wood Products Association (2018). Ponderosa pine. Available online at: www.wwpa.org/western-lumber/species/ponderosa-pine.(accessed December 17, 2024)
224
Weston G. (1957). “Exotic forest trees in New Zealand,” in Paper prepared for the seventh British Commonwealth Forestry Conference, Australia and New Zealand, (New Zealand Forests Service Bul), 104.
225
Willyard A. Cronn R. Liston A. (2009). Reticulate evolution and incomplete lineage sorting among the ponderosa pines.Mol Phylogenet Evol52498–511. 10.1016/j.ympev.2009.02.011
226
Willyard A Gernandt D. S Cooper B Douglas C Finch K Karemera H et al . (2021a). Phylogenomics in the hard pines. (Pinus subsection Ponderosae; Pinaceae). confirms paraphyly in Pinus ponderosa, and places Pinus jeffreyi with the California big cone pines.Syst. Bot.46538–561. 10.1600/036364421X16312067913435
227
Willyard A. Gernandt D. López-Reyes A. Potter K. (2021b). Mitochondrial phylogeography of the ponderosa pines: Widespread gene capture, interspecific sharing, and two unique lineages.Tree Genet. Genomes17:47. 10.1007/s11295-021-01529-4
228
Willyard A Gernandt D. S Potter K Hipkins V Marquardt P Mahalovich M. F et al . (2017). Pinus ponderosa: A checkered past obscured four species.Am. J. Bot.104161–181. 10.3732/ajb.1600336
229
Xiaoyan J Rauscher S. A Ringler T. D Lawrence D. M Williams A. P Allen C. D et al . (2013). Projected future changes in vegetation in western North America in the 21st century.J. Clim.263671–3687. 10.1175/JCLI-D-12-00430.1
230
Youngblood A. Max T. Coe K. (2004). Stand structure in eastside old-growth ponderosa pine forests of Oregon and northern California.For. Ecol. Manage199191–217. 10.1016/J.FORECO.2004.05.056
231
Zeidler A Borůvka V Tomczak K Vacek Z Cukor J Vacek S et al . (2024). The potential of non-native pines for timber production—A case study from afforested post-mining sites.Forests15:1388. 10.3390/f15081388
232
Zhang J. Finley K. Knapp E. (2019). Resilience of a ponderosa pine plantation to a backfiring operation during a mid-summer wildfire.Int. J. Wildland Fire28981–992. 10.1071/WF19033
233
Zhang J Finley K. A Young D. H Fiddler G. O Looney C. (2022). Growth response of ponderosa pine to intensive cultural treatments varies with site quality and plantation age.For. Sci.68212–225. 10.1093/forsci/fxab065
234
Zhang J. Powers R. Oliver W. Young D. (2013a). Response of ponderosa pine plantations to competing vegetation control in Northern California, USA: A meta-analysis.For. Int. J. For. Res.863–11. 10.1093/forestry/cps054
235
Zhang J. Ritchie M. Maguire D. Oliver W. (2013b). Thinning ponderosa pine. (Pinus ponderosa). stands reduces mortality while maintaining stand productivity.Can. J. For. Res.43311–320. 10.1139/cjfr-2012-0411
Summary
Keywords
silviculture, production, ecological valence, introduced tree species, climate change, ponderosa pine
Citation
Tomczak K, Vacek Z, Cukor J, Vacek S, Bažant V, Zeidler A, Trojan V, Gallo J and Černý J (2025) Unlocking Pinus ponderosa (Douglas ex C. Lawson) potential: a comprehensive review of results from native and introduced areas. Front. For. Glob. Change 8:1585253. doi: 10.3389/ffgc.2025.1585253
Received
28 February 2025
Accepted
21 July 2025
Published
19 August 2025
Volume
8 - 2025
Edited by
David Ellison, University of Bern, Switzerland
Reviewed by
Alan Feest, University of Bristol, United Kingdom
Silvio Daniele Oggioni, University of Milan, Italy
Updates
Copyright
© 2025 Tomczak, Vacek, Cukor, Vacek, Bažant, Zeidler, Trojan, Gallo and Černý.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jan Cukor, cukor@fld.czu.cz
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.