Abstract
Fine root decomposition is a key process influencing the element cycling of forest ecosystems and is sensitive to increasing atmospheric nitrogen (N) deposition. However, the specific relationship between soil chemical characteristics and the nutrient release patterns of fine root decomposition under increasing N deposition is not fully understood. We conducted a decomposition experiment with three N addition levels using lower order (order 1–3) and higher order (order 4–5) of Castanopsis platyacantha fine root and observed its decomposition rates and nutrient dynamics from March 2016 to April 2018 in a subtropical forest of China. Soil pH, carbon fractions, and N fractions were measured and served as explanatory variables to explore the relationship with root decomposition rates through stepwise linear regression. After 2 years of decomposition, high N addition had a larger mass remaining than the control treatment for the lower-order root. Soil nitrate greatly explained the root decomposition rates among N treatments (R2 = 0.712 for lower-order roots and R2 = 0.520 for higher-order roots). Nitrogen addition did not affect the remaining root N, P, K, Ca, Mg, and Mn. The remaining ratio of C/P and C/Mn in lower-order roots and C/Mn in higher-order roots was significantly increased in high N addition. Our results indicated that among different N treatments, soil nitrate content significantly affected rates of fine root decomposition in subtropical forests in China. However, N addition did not significantly affect the amount of nutrients released during root decomposition except for significantly changing the release rate of P in lower-order roots and Mn in both lower- and higher-order roots. In the future, the impact of N deposition on Mn cycling and its further effect on C storage in forests deserves attention.
1 Introduction
Litter decomposition is a fundamental biogeochemical process and plays an imperative role in soil organic matter storage and nutrient cycling in forests (Yang et al., 2021; Awasthi et al., 2022; Lalnunzira and Tripathi, 2018). Any disturbance that affects the litter decomposition can have pervasive effects on ecosystem functioning (Manral et al., 2020). Numerous studies have focused on the role of leaf litter decomposition, but the contribution of root litter decomposition to soil C and nutrient status remains poorly understood due to associated methodological problems and time constraints (See et al., 2019; Minerovic et al., 2018). In forests, fine roots contribute 48% of the annual litter inputs that represent a substantial C and nutrient pool (Freschet et al., 2013), and the average residence time of root-derived C was estimated to be 2.4 times as long as shoot-derived C (Rasse et al., 2005). The decomposition of fine roots is influenced by root chemical attributes, such as acid-unhydrolyzable components and nitrogen content, as well as decomposition-related environmental factors, including soil pH, soil nutrient availability, and temperature. (He et al., 2025; Lalnunzira and Tripathi, 2018; Silver and Miya, 2001).
The great acceleration of fossil fuel use and fertilizer consumption since the industrial revolution has greatly increased the concentration of reactive nitrogen (N), including NH4+ and NO3−, in the atmosphere, thereby enhancing N deposition to terrestrial ecosystems (Steffen et al., 2015; Galloway et al., 2008). The rate of terrestrial N deposition in China increased from 13 kg N ha−1 a−1 in 1961 to 21 kg N ha−1 a−1 in 2010 (Liu et al., 2013). Although the rate of increase slowed down after 2010, it is still high (Yu et al., 2019). As one of the important global changes, N deposition significantly affects the functioning of different forest ecosystems, including litter decomposition (Huang et al., 2025; Singh and Tripathi, 2000). The response of fine root decomposition to N deposition has been revealed in some forest ecosystems (e.g., Fu et al., 2022; Tu et al., 2015; Jiang and Li, 2018; Gang et al., 2019). Most of those studies reported that N addition inhibited fine root decomposition rates and usually attributed to increased acid-unhydrolyzable matter remaining and decreased cellulolytic and ligninolytic enzyme activity (e.g., Kou et al., 2018; Sun et al., 2016a; Sun et al., 2016b; Xia et al., 2017, 2018). Litter decomposition is a microbe-dominated process (Yang et al., 2021); thus, decreased root decomposition rate under N addition implies decreased soil microbes’ activities under N addition (Silver and Miya, 2001). In fact, comprehensive meta-analysis revealed that N addition decreased soil microbial biomass and heterotrophic respiration in the forest ecosystems (Treseder, 2008; Zhou et al., 2014). The reason behind this is that N addition alters the soil environment, such as soil pH and/or soil C and N availability, because the soil environment drives soil microbes’ changes and determines microbial community and biomass (Philippot et al., 2024; Xun et al., 2015). However, based on our knowledge, the potential relationship between soil chemical characters and fine root decomposition rates is seldom explored, especially under increasing N deposition.
Litter nutrient release pattern varies with nutrient types and exhibits the different immobilizing patterns that pose different impacts on forest ecosystem functions (Lalnunzira and Tripathi, 2018; Pandey et al., 2007). Nutrient release amount and rates during litter decomposition are critical for productivity and functions in forest ecosystems, which usually have a tightly coupled cycling among multiple nutrients, and the amount of annual nutrient cycling between soil and plant is much larger than annual nutrient net input or loss (Zechmeister-Boltenstern et al., 2015). Except for N and phosphorus (P), other nutrient dynamics during root decomposition seldom get attention, especially under N addition. Although the N and P limitations in the forest ecosystems are more extensive globally, other nutrients such as potassium (K), calcium (Ca), and magnesium (Mg) all play an irreplaceable role in plant growth and metabolism and even became a limited nutrient in some ecosystems (Peng et al., 2020; Zhang et al., 2020). Whether decreased fine root decomposition rates under N addition affect multiple nutrient release patterns needs to be verified in the field. Some soluble nutrients can easily be released from litter by physical leaching, and the remaining nutrient requires microorganism breakdown of complex chemical structures initially (Peng et al., 2017). Thus, the decrease of acid-unhydrolyzable matter decomposition due to N addition will potentially impede the normal release of nutrients. Tu et al. (2015) observed that N addition significantly decreased the release of N, P, K, Ca, and Mg of Pleioblastus amarus fine root after 2 years of decomposition.
Except for the absolute nutrient release amount, which is usually characterized by nutrient remaining, the response of relative multiple nutrient release rates under N addition is also important, which can be characterized by the remaining ratio between carbon and nutrient. The stoichiometry of plants and microorganisms is generally maintained within certain limits (Zechmeister-Boltenstern et al., 2015). Thus, the change of nutrient relative cycling rates may enhance some nutrient limitations.
The Rainy Zone of Western China is a large and complex ecotone located on the western edge of the Sichuan Basin. Here, warm, moist air from the basin is readily brought to supersaturation, generating substantial precipitation in this region (Peng et al., 2020). The mean annual total atmospheric N deposition flux during 2017–2019 was 24.3 kg N ha−1 year−1, even though dry deposition was underestimated, and was significantly higher than the critical load (10–15 kg N ha−1 year−1) (Zhang et al., 2020). In this area, we conducted a field N addition experiment in a subtropical forest which has received monthly N additions since 2013, aiming to test the response of multiple ecosystem processes to increasing N deposition. In this study, the lower-order (order 1–3) and higher-order (order 4–5) fine roots of Castanopsis platyacantha were incubated in the field for 2 years by the litterbag method under three N addition treatments. We aim to explore (1) the potential relationship between fine root decomposition rates and soil chemical characters, including soil pH, soil C, and N fractions under N addition, and (2) the response of multiple nutrient release to N addition in subtropical forests.
2 Materials and methods
2.1 Site description
The study site is located at Mujianggan (29°32′35″N, 103°15′41″E; 1,600 m a.s.l.) in Wawushan National Forest Park in Sichuan Province, China (Supplementary Figure S1). This area is located in the center of the Rainy Zone of Western China and has a humid subtropical, highland, monsoon-influenced climate with an average annual temperature of 10 − 14°C, annual precipitation of 2,323 mm, and annual average relative humidity of 85–90%. This forest was logged in 1956 and then restored to a secondary evergreen broad-leaved forest dominated by Castanopsis platyacantha and Schima sinensis. The mean diameter at the breast height for C. platyacantha and S. sinensis stands was 23.8 cm and 25.4 cm in 2012, respectively. The soil is classified as Lithic Dystrudepts according to USDA soil taxonomy, and the average soil depth is more than 1 m. The root biomass of the 0–10 cm soil horizon was 3.5 kg m−3, and specific soil properties were reported by Peng et al. (2017).
2.2 Experimental design
A monthly NH4NO3 addition experiment was conducted from April 2013 to April 2018. Three N addition rates were set: control (CK, background N deposition level+0 kg N ha−1 a−1), low N (LN, background N deposition level+50 kg N ha−1 a−1), and high N (HN, background N deposition level+150 kg N ha−1 a−1). The LN treatment simulated N deposition scenarios for 2050, when some world regions, especially the large areas of South and East Asia, are projected to receive >50 kg N ha−1 year−1 (Galloway et al., 2008). The HN treatment was to simulate N saturation scenarios. Nine plots (20 m × 20 m) were designated at intervals of more than 20 m in October 2012 and were randomly assigned to three N treatments with three replicates each. Aqueous NH4NO3 was monthly applied to the soil surface in each plot using a sprayer. The control plots received an equivalent volume of water without NH4NO3.
2.3 Root collection, treatment, and root litterbag preparation
A similar forest near the experimental plots was selected. C. platyacantha roots in the 0–10 cm soil layer were collected by the soil block method in October 2015. Specifically, soil blocks were dug around the C. platyacantha tree stem. Fine roots were carefully collected by hand. The soil was brushed off and cleaned with deionized water in the laboratory. Dead C. platyacantha roots were discarded based on color (the epidermis of live C. platyacantha roots is red) and texture. Root orders were classified according to Pregitzer et al. (2002), with root samples divided into two groups: lower-order roots (order 1–3), which exhibit strong absorption capacity, and higher-order roots (order 4–5), characterized by enhanced transport capacity (McCormack et al., 2015). All root samples were air-dried at room temperature for 1 month. The moisture content of air-dried root samples was measured gravimetrically after oven-drying at 65°C for 48 h, to convert the air-dried weight to oven-dry weight. Then, 2.70 g (oven-dry weight) of lower-order or higher-order root samples were placed in a 10 cm × 10 cm nylon bag (pore size 120 um).
2.4 Root litterbag field incubation, harvest, and measurement
Three points were chosen in each plot for the litterbag. Seven lower-order and higher-order root litterbags were inserted into the 0–10 cm soil at an angle of 45o at each point in March 2016 (after 3 years of N additions treatment). Totally, each plot buried 21 lower-order root litterbags and 21 higher-order root litterbags. Litterbags were collected in May, August, and November 2016, March, July, and December 2017, and April 2018. In each collection, one lower-order and one higher-order root litterbags were collected from each point in all plots. Collected litterbags were air-dried, after which the roots were cleaned of adhering material. The root samples were dried at 65°C for 48 h and weighed. Finally, they were ground for chemical analysis.
The element concentrations of initial (undecomposed) and decomposed root samples were measured. Carbon concentration (g kg−1) was measured by the dry combustion method (Nelson and Sommers, 1982). Nitrogen concentration (g kg−1) was determined by the Kjeldahl method using an automatic distillation unit (VELP, Milano, Italy). Phosphorus (P) concentration (g kg−1) was determined using the ammonium molybdate-stannous chloride colorimetric method (Nelson and Sommers, 1982). Samples (0.3 g) were digested with perchloric acid–nitric acid (HClO4 − HNO3); then, the concentrations (g kg−1) of potassium (K), calcium (Ca), magnesium (Mg), and manganese (Mn) were analyzed using an atomic absorption spectrophotometer (TAS − 986, PGENERAL, Beijing, China). The percentage of ash in root samples was measured at 550°C for 6 h in a muffle furnace. For the initial samples, the acid-unhydrolyzable and acid-hydrolysable matters (g kg−1) were determined using an acid detergent fiber method described by Ryan et al. (1990). The initial root samples’ chemical properties are shown in Supplementary Table S1.
2.5 Soil sampling and measurement
In August 2018, 0–10 cm of soil was collected using a soil auger at each plot. After removing the visible roots, part of the fresh soil was used for soil ammonium (NH4+), nitrate (NO3−), and extractable dissolved organic carbon (EDOC) measurement immediately after being sieved through a 2-mm mesh. The remaining soil was air-dried at room temperature thoroughly; then, soil samples were sieved through a 2-mm mesh for pH measurement and sieved through a 0.25-mm mesh for soil total organic carbon (TOC), total nitrogen (TN), and readily oxidizable carbon (ROC) measurement.
Soil pH (soil/water: 1:2.5) was measured using a glass electrode. The soil NH4+ and NO3− contents were measured by the colorimetric method using a 2 M KCl soil extraction solution. Soil EDOC was analyzed by a total CN analyzer (Shimadzu model TOC-VcPH+TNM-1, Kyoto, Japan) after extraction using a 0.5-M K2SO4 solution. Soil total organic carbon (TOC) was measured by the dichromate digestion method. Soil total nitrogen (TN) was determined using the Kjeldahl method. Soil ROC was measured using the KMnO4 oxidation method (Blair et al., 1995).
2.6 Data analysis
The ash-free mass was used for all calculations and analyses. The remaining mass and nutrients (N, P, K, Ca, Mg, and Mn) for each harvest time were calculated and expressed as a proportion of the corresponding value in initial root samples. The relationship between the mass remaining and decomposition time was described using a single-exponent decomposition model, y = ae–kt, where y is the remaining mass at time t (year) and k (year−1) is the decomposition constant (Olson, 1963). To explore the effect of N addition on the nutrient relative release rate, the remaining ratio between C and each nutrient was calculated using the final harvested root sample data.
The normal distribution (Shapiro–Wilk’s test) and equality of variance (Levene’s test) were tested for each parameter before further analysis, and a logarithmic transformation was applied when these assumptions were not met. Plot mean values were used in analyses unless otherwise stated. To determine the effect of N additions on root mass and nutrients remaining during decomposition, repeated measures analyses of variance (ANOVAs) were performed with N addition as main effects and time as the within-subject factor. One-way ANOVA with Tukey’s honestly significant test was used to analyze the effect of N treatment on mass and nutrient remaining at each sampling time. One-way ANOVA with Tukey’s honestly significant test was used to analyze the effect of N treatment on k, and the ratio between C and nutrient after 2 years of decomposition. The multivariable effects of soil chemical characters and decomposition constant k were analyzed by stepwise linear regression. All analyses were conducted using SPSS 20.0 for Windows (IBM SPSS Inc., Chicago, USA). Significant effects were determined by α = 0.05. Values are expressed as mean ± standard error (SE).
3 Results
3.1 Mass remaining
After 2 years of decomposition, lower-order root mass remaining in HN treatments (69.0 ± 1.8%) was significantly larger than in CK (59.5 ± 2.1%) and LN (63.53 ± 1.1%) (Figure 1). Higher-order root mass remaining in CK, LN, and HN was 50.7 ± 0.3%, 50.7 ± 2.5%, and 56.5 ± 3.2%, respectively, after 2 years of decomposition (Figure 1). There was a significant single-exponent relationship between root mass remaining and decomposition time in all treatments (p < 0.01, 0.788 ≤ R2 ≤ 0.952, Table 1). For the lower-order roots, the k value was 0.24, 0.22, and 0.16 in CK, LN, and HN, respectively, with a significant difference between CK and HN. For higher-order roots, the k value was 0.33, 0.31, and 0.29 in CK, LN, and HN, respectively.
Figure 1

Percentages of mass remaining for lower-order and higher-order roots during 2 years of decomposition (mean ± SE, n = 3). * Denotes a significant difference among N treatments by one-way ANOVA with Tukey’s honestly significant test.
Table 1
| Root groups | Treatments | P | k (a−1) |
|---|---|---|---|
| Lower-order (orders 1–3) |
CK | <0.01 | 0.24 ± 0.01 a |
| LN | <0.01 | 0.22 ± 0.01 ab | |
| HN | <0.01 | 0.16 ± 0.02 c | |
| Higher-order (orders 4–5) |
CK | <0.01 | 0.33 ± 0.01 a |
| LN | <0.01 | 0.31 ± 0.01 a | |
| HN | <0.01 | 0.29 ± 0.02 a |
Decomposition constant k in the single-exponential regression equation of the mass remaining of C. platyacantha fine root.
Small letters are the results of one-way ANOVA with Tukey’s honestly significant test within each root group, and different small letters denote significant differences at the 0.05 level.
3.2 Soil chemical and decomposition constant k
Using soil pH, TOC, TN, NH4+, NO3−, EDOC, and ROC as explaining variables (detail data in Supplementary Table S2) and decomposition constant k as response variable, we performed stepwise linear regression. We got the best model for lower-order root, which was k = 0.319–0.004 × c[NO3−] (c[NO3−]: soil nitrate concentration; R2 = 0.712; p < 0.01), and for higher-order root, which was k = 0.376–0.002 × c[NO3−] (R2 = 0.520; p = 0.028) (Figure 2).
Figure 2
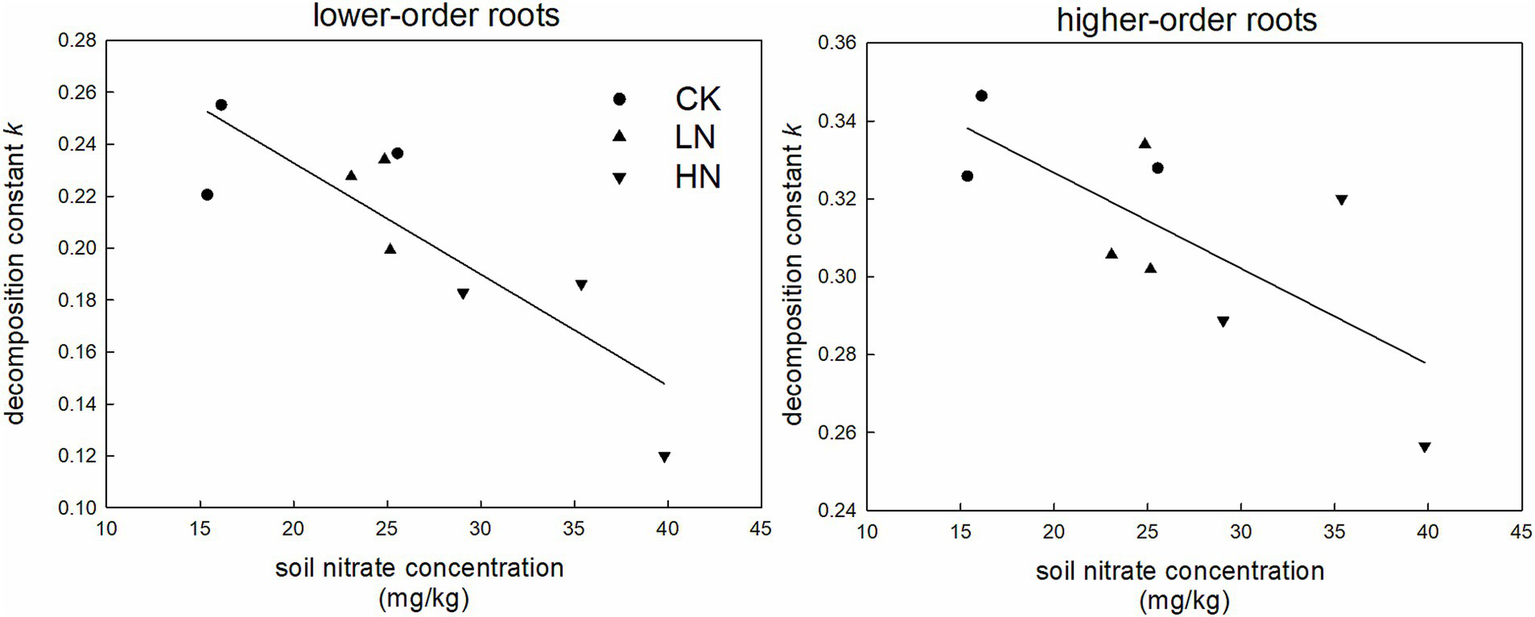
Linear relationship between soil nitrate concentration and decomposition constant k for lower-order and higher-order roots.
3.3 Nutrients remaining and ratio
Repeated measures ANOVA indicated that decomposition time significantly affects root N, P, K, Ca, Mg, or Mn remaining (p < 0.01), while N addition effect and the interactive effect were not significant (Table 2). After 2 years of decomposition, the remaining lower-order roots N, P, K, Ca, Mg, and Mn were 22.0 ± 0.8%, 78.7 ± 5.1%, 26.9 ± 7.2%, 30.0 ± 0.6%, 5.6 ± 1.5%, and 47.5 ± 8.6%, respectively, in the CK treatment (Figures 3, 4). One-way ANOVA results indicated that lower-order roots in CK had a significantly lower C/P and C/Mn ratio than in LN and HN after 2 years of decomposition (Figure 5). High-order roots had a significantly higher C/Mn ratio in HN than in CK and LN (Figure 5).
Table 2
| Remaining | Lower-order roots | Higher-order roots | ||||
|---|---|---|---|---|---|---|
| Time | Nitrogen treatment | Time × N interactive | Time | Nitrogen treatment | Time × N interactive | |
| N | <0.01 | 0.746 | 0.702 | <0.01 | 0.181 | 0.755 |
| P | <0.01 | 0.143 | 0.600 | <0.01 | 0.858 | 0.387 |
| K | <0.01 | 0.153 | 0.238 | <0.01 | 0.108 | 0.423 |
| Ca | <0.01 | 0.550 | 0.349 | <0.01 | 0.518 | 0.550 |
| Mg | <0.01 | 0.974 | 0.209 | <0.01 | 0.947 | 0.421 |
| Mn | <0.01 | 0.289 | 0.240 | <0.01 | 0.204 | 0.123 |
Repeated measures ANOVA of C. platyacantha lower-order and higher-order fine root nutrients remaining.
Figure 3
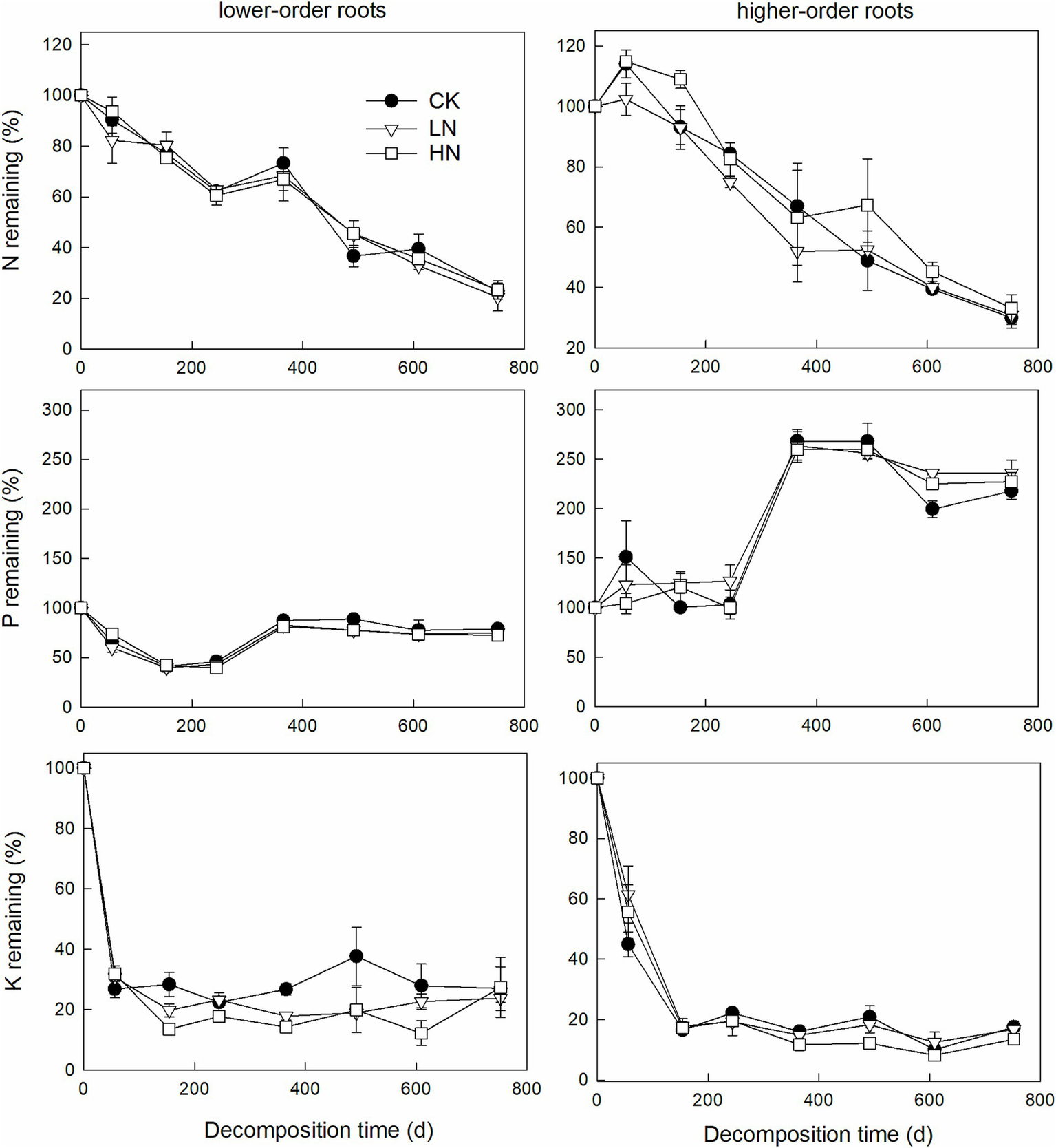
Root N, P, and K remaining dynamic for lower-order and higher-order roots during 2 years of decomposition (mean ± SE, n = 3).
Figure 4
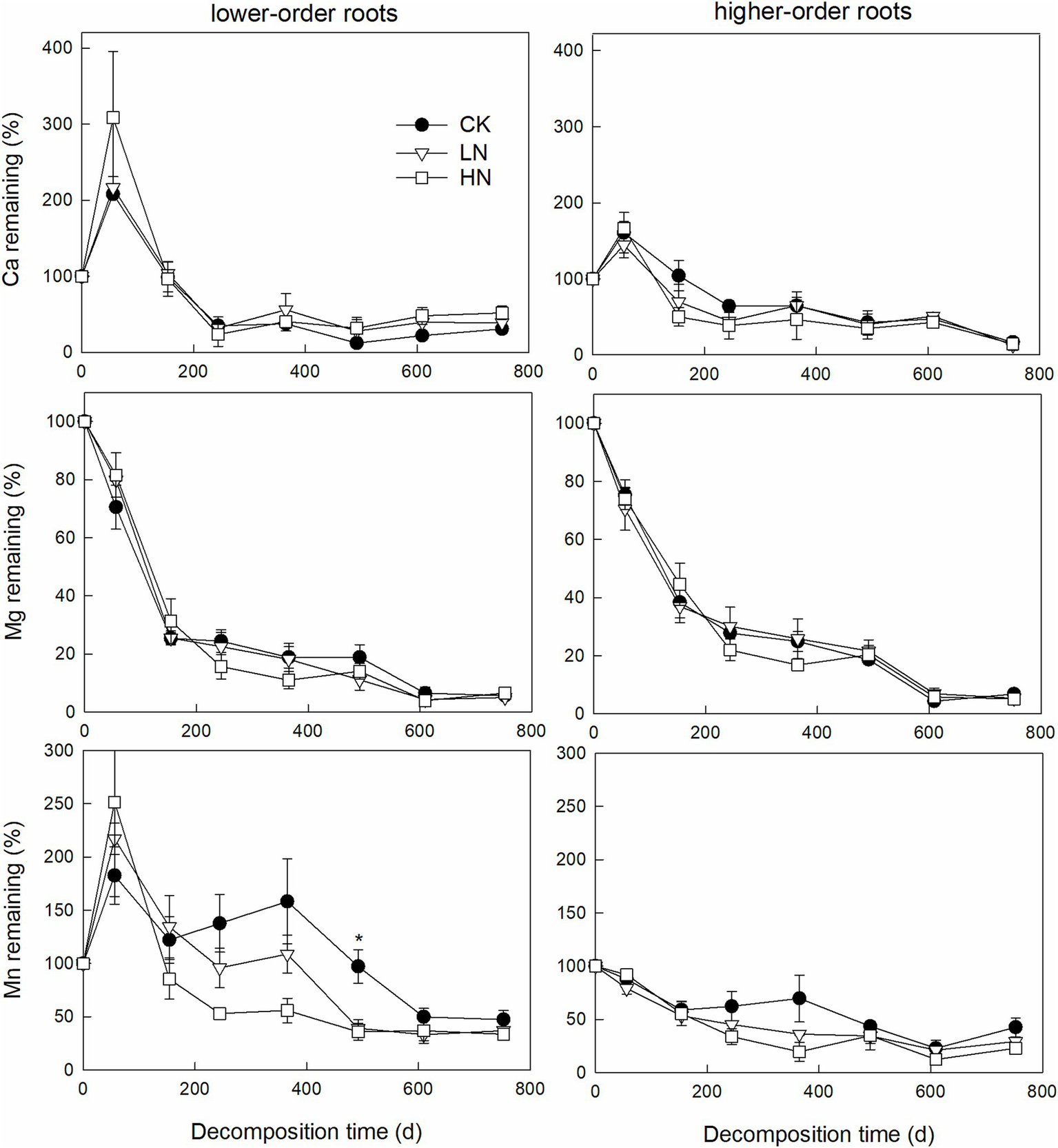
Root Ca, Mg, and Mn remaining dynamic for lower-order and higher-order roots during 2 years of decomposition (mean ± SE, n = 3). * Denotes a significant difference among N treatments by one-way ANOVA with Tukey’s honestly significant test.
Figure 5
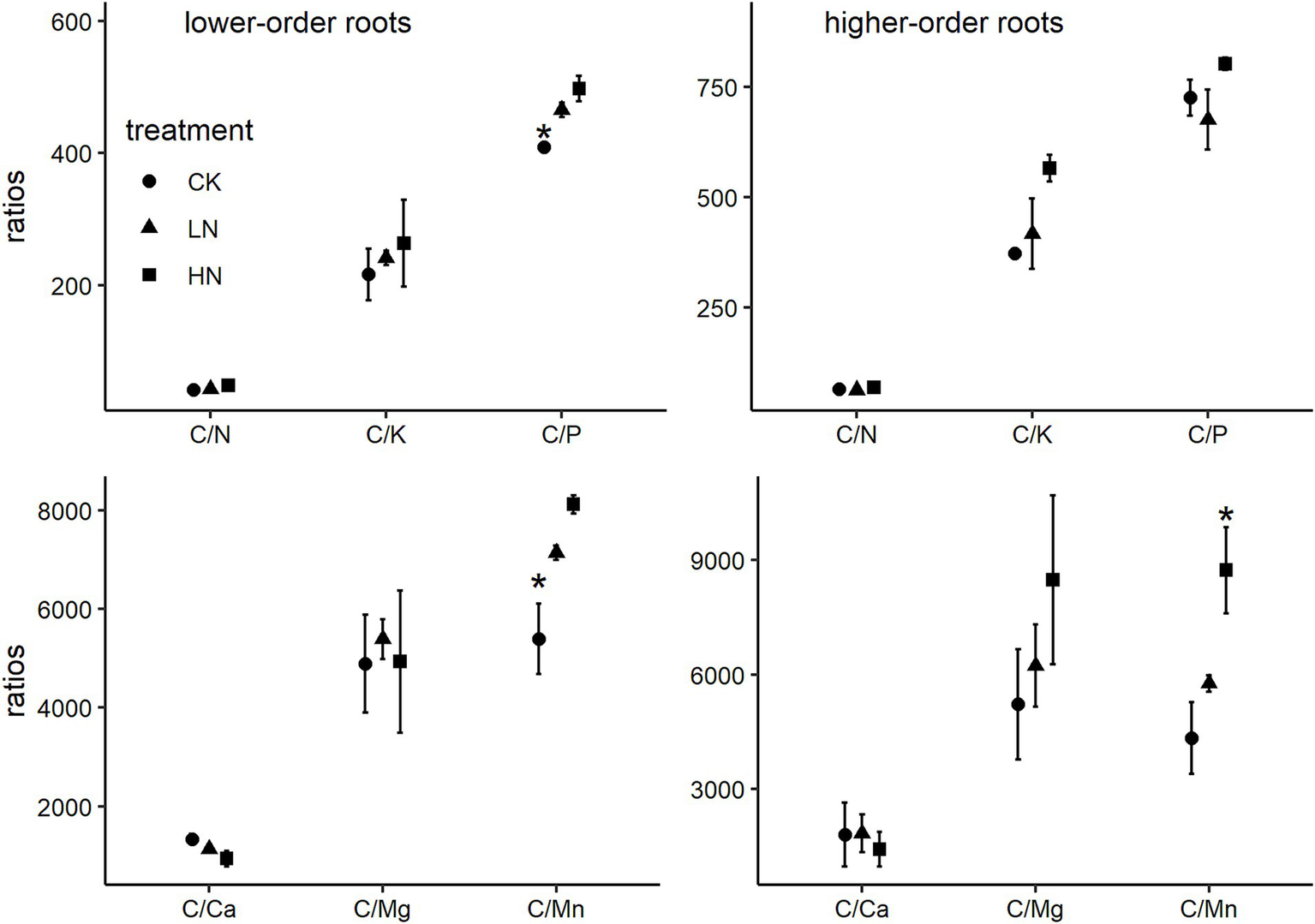
Ratio between root C and nutrient remaining after 2 years of decomposition (mean ± SE, n = 3). * Denotes a significant difference among N treatments by one-way ANOVA with Tukey’s honestly significant test.
4 Discussion
4.1 Relationship between soil chemical and decomposition constant k
After 2 years of decomposition, HN significantly decreased the lower-order root decomposition rate, as indicated by a larger mass remaining (Figure 1) and a lower decomposition constant k in HN than in CK (Table 1). Among different N treatments, soil nitrate content significantly affected rates of fine root decomposition (71.2% in lower-order root; 52.0% in higher-order root) in this subtropical forest, as supported by the result of stepwise linear regression. Soil NO3− concentration was the only variable accepted by the best regression model although seven soil chemical characters, including soil pH, TOC, TN, NH4+, NO3−, EDOC, and ROC, were offered as potential explanatory variables in stepwise linear regression of decomposition constant k. Furthermore, fine root decomposition constant k showed a significant negative relationship with soil NO3− (Figure 2). These results implied that N addition affected microbial-mediated root decomposition processes by affecting soil nitrate nitrogen content. Nitrogen is an essential nutrient for microbes, and increasing soil NO3− content can promote soil microbe growth, especially in N-limited ecosystems (Zhang et al., 2019). With the increase in N addition, soil NO3− is increased and HN is significantly higher than CK and LN in our research site (Supplementary Table S2). However, under chronic N addition and non-N limited forest ecosystem, soil acidification occurs with the increase of soil NO3− content (Huang et al., 2014; Wang Z. et al., 2023). In this subtropical forest, HN reduced soil pH by 0.23 compared with CK (Supplementary Table S2). Globally, Tian and Niu (2015) found that N addition average decreased soil pH by 0.26 through a meta-analysis. Soil pH has a strong linkage with microbial community structure (Ning et al., 2020), C metabolic process (Malik et al., 2018), and microbial gene abundance (Wan et al., 2021). Wang X. et al. (2023), who conducted a meta-analysis of a global dataset, found that soil pH regulated the responses of soil bacterial diversity and richness to N addition. N addition induced soil acidification, which could profoundly impact soil decomposer communities and their functions indicated by decreased enzyme activity during root decomposition under N addition (Kou et al., 2015). In our research site, although the community functions of soil microorganisms under different N treatments were not measured, HN and LN significantly decreased soil microbial biomass carbon by 37 and 52% compared with CK after 2 years of N addition (Peng et al., 2017). Thus, we deduced that the dual role of soil NO3− in characterizing N availability and soil acidification may make it become a good indicator to predict root decomposition rate under N addition in subtropical forest.
Soil NO3− content had a stronger effect on lower-order roots than higher-order roots according to the slope of the regression model (slope of lower-order root: −0.004; slope of higher-order root: −0.002; Figure 2), which indicated that N addition had a more pronounced effect on lower-order roots. This result was consistent with the findings of Kou et al. (2018), who observed that N addition inhibited lower-order root decomposition from the early decomposition stage and higher-order root was in the late stage in Pinus elliottii plantations. The different litter qualities were the main reason since they had a similar decomposition environment in the field (Kou et al., 2015). The lower-order and higher-order roots of C. platyacantha had similar C content, but the lower-order root had a significantly higher acid-unhydrolyzable matter content than the higher-order root (Supplementary Table S1). Acid-unhydrolyzable content usually limited the overall litter decomposition rates (Kou et al., 2015), explaining the faster decomposition rates of higher-order roots than lower-order roots in this forest (Table 1). Nitrogen addition mainly decreased fine root acid-unhydrolyzable matter decomposition (Bonner et al., 2019) and thus had a stronger inhibition on C. platyacantha lower-order roots.
4.2 Multiple nutrients release during fine root decomposition
After 2 years of decomposition, the C. platyacantha lower-order and higher-order roots remaining C/Mn from HN were increased, compared with CK. That indicated a relatively higher Mn release under N addition. Manganese is an essential cofactor for lignin-degrading enzymes (Berg et al., 2015), and lignin depolymerization is the rate-limiting step in root decomposition (Kou et al., 2018). Nitrogen addition accelerates the Mn release relative to C, which could potentially affect the subsequent root decomposition. In this forest, Peng et al. (2022) conducted a 5-year leaf litter decomposition experiment and found that N addition slows litter decomposition accompanied by accelerated Mn release. Chen et al. (2023) reported that root litter Mn concentration and remaining mass of Schima superba, Cleyera japonica, and Eurya loquaiana were negatively correlated during the whole decomposition period, and root Mn concentration greatly indicates root decomposition rates across soil layers, root orders, and species. Those results indicated that N addition accelerates the release of Mn from root litter, which is of great significance for decomposition processes in the forest ecosystem. Under N addition, the accelerated release and leaching of Mn lead to a reduction in Mn bioavailability (Whalen et al., 2018). Furthermore, Mn regulates the stabilizing and destabilizing of soil organic matter (Li et al., 2021). Therefore, the impact of N addition on the Mn cycle will profoundly influence soil C cycling. Promoting the combination of N and lignin to form more recalcitrant chemical matter was an important potential mechanism that N addition decreased root decomposition (Bonner et al., 2019; Rinkes et al., 2016). However, our result did not support that because the C/N in fine root litter did not show a significant difference among the three N addition treatments.
Nitrogen addition did not affect the absolute release amount for N, K, Ca, and Mg in this subtropical forest. Root N dynamics of C. platyacantha were not significantly affected by N addition, similar to Jiang and Li (2018), but different from Tu et al. (2015) and Kou et al. (2015). They found that N addition significantly increased the root N remaining. Gang et al. (2019) and Jing et al. (2019) showed that the effect of N addition on root N remaining depended on N addition rates and root order, respectively. Similar to N, the response of the remaining P to N addition is also variable. Our results and Jiang and Li (2018) indicated that N addition had no significant effect on root P remaining. However, Gang et al. (2019) and Jing et al. (2019) found that N addition could have a different effect on root P remaining, and it depends on root order. These different results seem to indicate that the impact of N addition on N and P release patterns during root decomposition is site-specific and may be affected by nutrient content, both litter itself and the soil. By the way, N addition inhibited litter decomposition mainly occurred in later decomposition stages (Bonner et al., 2019). Therefore, the duration of the root decomposition experiment will also affect the final nutrient release patterns under different N addition treatments.
Castanopsis platyacantha root rapidly releases K and Mg during initial root decomposition, similar to Fahey et al. (1988), indicating that physical leaching dominated K and Mg release, and no evidence indicates that N addition can disturb this process yet. Silver and Miya (2001) speculated that Ca plays an important role in root decomposition based on a positive relationship between decomposition and initial Ca concentration. Ca is an essential element for many fungi decomposers, and compared with forest litter, they have a higher Ca concentration (Cromack et al., 1977). In this forest, both C. platyacantha lower-order and higher-order roots showed Ca enrichment in the initial stage, but the remaining C/Ca in the root samples increased after 2 years of decomposition compared to the initial root samples. This implies that decomposers need a relatively higher Ca concentration in the initial decomposition stage; however, N addition does not affect Ca dynamics during root decomposition. However, our results indicated that root litter nutrient release pattern varies with nutrient types (Figures 3, 4), and more than 70% of root N, K, Ca, and Mg released after 2 years of decomposition indicated that root decomposition plays an important role in nutrient cycling in subtropical forests.
5 Conclusion
Among different nitrogen treatments, soil nitrate content significantly affected rates of root decomposition in subtropical forests in China. However, nitrogen addition did not significantly affect the amount of nutrients released during root decomposition except for significantly changing the release rate of P in lower-order roots and Mn in both lower- and higher-order roots. Under long-term N deposition, N management ecosystem should be optimized to maintain the coupling relationships among multiple elements.
Statements
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GC: Conceptualization, Methodology, Visualization, Writing – original draft, Writing – review & editing. YC: Conceptualization, Methodology, Writing – review & editing. XY: Conceptualization, Methodology, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by the Sichuan Academy of Agricultural Sciences 2024 Talent Introduction and Training Special Project (30114-03-24003).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1595417/full#supplementary-material
References
1
Awasthi P. Bargali K. Bargali S. S. Khatri K. (2022). Nutrient return through decomposing Coriaria nepalensis litter in degraded hills of Kumaun Himalaya, India. Front. For. Glob. Change5:1008939. doi: 10.3389/ffgc.2022.1008939
2
Berg B. Erhagen B. Johansson M. B. Nilsson M. Stendahl J. Trum F. et al . (2015). Manganese in the litter fall-forest floor continuum of boreal and temperate pine and spruce forest ecosystems – a review. For. Ecol. Manag.358, 248–260. doi: 10.1016/j.foreco.2015.09.021
3
Blair G. Lefroy R. Lisle L. (1995). Soil carbon fractions based on their degree of oxidation, and the development of a carbon management index for agricultural systems. Aust. J. Agric. Res.46, 1459–1466. doi: 10.1071/AR9951459
4
Bonner M. T. Castro D. Schneider A. N. Sundström G. Hurry V. Street N. R. et al . (2019). Why does nitrogen addition to forest soils inhibit decomposition?Soil Biol. Biochem.137:107570. doi: 10.1016/j.soilbio.2019.107570
5
Chen G. Sun Y. Chen Y. Ma W. Zhong Q. Li Y. et al . (2023). Manganese indicates root decomposition rates across soil layer, root order, and tree species: evidence from a subtropical forest. Soil Biol. Biochem.181:109023. doi: 10.1016/j.soilbio.2023.109023
6
Cromack K. Sollins P. Todd R. L. Crossley D. A. Fender W. M. Fogel R. et al . (1977). “Soil microorganism—arthropod interactions: Fungi as major calcium and sodium sources” In: Mattson, W. J. (eds.) The Role of Arthropods in Forest Ecosystems. Proceedings in Life Sciences. Berlin, Heidelberg: Springer. doi: 10.1007/978-3-642-88448-1_9
7
Fahey T. J. Hughes J. W. Pu M. Arthur M. A. (1988). Root decomposition and nutrient flux following whole-tree harvest of northern hardwood forest. For. Sci.34, 744–768. doi: 10.1093/forestscience/34.3.744
8
Freschet G. T. Cornwell W. K. Wardle D. A. Elumeeva T. G. Liu W. Jackson B. G. et al . (2013). Linking litter decomposition of above- and below-ground organs to plant-soil feedbacks worldwide. J. Ecol.101, 943–952. doi: 10.1111/1365-2745.12092
9
Fu X. Xu C. Geng Q. Ma X. Zhang H. Cai B. et al . (2022). Effects of nitrogen application on the decomposition of fine roots in temperate forests: a meta-analysis. Plant Soil472, 77–89. doi: 10.1007/s11104-021-05176-5
10
Galloway J. N. Townsend A. R. Erisman J. W. Bekunda M. Cai Z. Freney J. R. et al . (2008). Transformation of the nitrogen cycle: recent trends, questions, and potential solutions. Science320, 889–892. doi: 10.1126/science.1136674
11
Gang Q. Chang S. X. Lin G. Zhao Q. Mao B. Zeng D. H. (2019). Exogenous and endogenous nitrogen differentially affect the decomposition of fine roots of different diameter classes of Mongolian pine in semi-arid Northeast China. Plant Soil436, 109–122. doi: 10.1007/s11104-018-03910-0
12
He W. Mäkiranta P. Ojanen P. Korrensalo A. Laiho R. (2025). Dynamics of fine-root decomposition and its response to site nutrient regimes in boreal drained-peatland and mineral-soil forests. For. Ecol. Manag.582:122564. doi: 10.1016/j.foreco.2025.122564
13
Huang X. Li Y. Yu S. Cui Y. Guan F. Li Y. et al . (2025). Nitrogen deposition mitigates long-term phosphorus input-induced stimulative effects on soil respiration in a tropical forest. Geoderma453:117142. doi: 10.1016/j.geoderma.2024.117142
14
Huang J. Mo J. Zhang W. Lu X. (2014). Research on acidification in forest soil driven by atmospheric nitrogen deposition. Acta Ecol. Sin.34, 302–310. doi: 10.1016/j.chnaes.2014.10.002
15
Jiang L. Li S. (2018). Alterations of early-stage decomposition of leaves and absorptive roots by deposition of nitrogen and phosphorus have contrasting mechanisms. Soil Biol. Biochem.127, 213–222. doi: 10.1016/j.soilbio.2018.09.037
16
Jing H. Zhang P. Li J. Yao X. Liu G. Wang G. (2019). Effect of nitrogen addition on the decomposition and release of compounds from fine roots with different diameters: the importance of initial substrate chemistry. Plant Soil438, 281–296. doi: 10.1007/s11104-019-04017-w
17
Kou L. Chen W. Zhang X. Gao W. Yang H. Li D. et al . (2015). Differential responses of needle and branch order-based root decay to nitrogen addition: dominant effects of acid-unhydrolyzable residue and microbial enzymes. Plant Soil394, 315–327. doi: 10.1007/s11104-015-2517-2
18
Kou L. Jiang L. Fu X. Dai X. Wang H. Li S. (2018). Nitrogen deposition increases root production and turnover but slows root decomposition in Pinus elliottii plantations. New Phytol.218, 1450–1461. doi: 10.1111/nph.15066
19
Lalnunzira C. Tripathi S. K. (2018). Leaf and root production, decomposition and carbon and nitrogen fluxes during stand development in tropical moist forests, north-East India. Soil Res.56, 306–317. doi: 10.1071/SR16265
20
Li H. Santos F. Butler K. Herndon E. (2021). A critical review on the multiple roles of manganese in stabilizing and destabilizing soil organic matter. Environ. Sci. Technol.55, 12136–12152. doi: 10.1021/acs.est.1c00299
21
Liu X. Zhang Y. Han W. Tang A. Shen J. Cui Z. et al . (2013). Enhanced nitrogen deposition over China. Nature494, 459–462. doi: 10.1038/nature11917
22
Malik A. A. Puissant J. Buckeridge K. M. Goodall T. Jehmlich N. Chowdhury S. et al . (2018). Land use driven change in soil pH affects microbial carbon cycling processes. Nat. Commun.9:3591. doi: 10.1038/s41467-018-05980-1
23
Manral V. Bargali K. Bargali S. S. Shahi C. (2020). Changes in soil biochemical properties following replacement of Banj oak forest with Chir pine in central Himalaya, India. Ecol. Process.9:30. doi: 10.1186/s13717-020-00235-8
24
McCormack M. L. Dickie I. A. Eissenstat D. M. Fahey T. J. Fernandez C. W. Guo D. et al . (2015). Redefining fine roots improves understanding of below-ground contributions to terrestrial biosphere processes. New Phytol.207, 505–518. doi: 10.1111/nph.13363
25
Minerovic A. J. Valverde-Barrantes O. J. Blackwood C. B. (2018). Physical and microbial mechanisms of decomposition vary in importance among root orders and tree species with differing chemical and morphological traits. Soil Biol. Biochem.124, 142–149. doi: 10.1016/j.soilbio.2018.06.006
26
Nelson D. W. Sommers L. E. (1982). “Total carbon, organic carbon, and organic matter” In: Page, A. L., Miller, R. H., Keeney, D. R. (Eds.), Methods of soil analysis. Part 2, 2nd ed. Madison, WI: Agron. Monogr. 9, ASA and SSSA. 539–579. doi: 10.2136/sssabookser5.3.c34
27
Ning Q. Chen L. Jia Z. Zhang C. Ma D. Li F. et al . (2020). Multiple long-term observations reveal a strategy for soil pH-dependent fertilization and fungal communities in support of agricultural production. Agric. Ecosyst. Environ.293:106837. doi: 10.1016/j.agee.2020.106837
28
Olson J. S. (1963). Energy storage and the balance of producers and decomposers in ecological systems. Ecology44, 322–331. doi: 10.2307/1932179
29
Pandey R. R. Sharma G. Tripathi S. K. Singh A. K. (2007). Litterfall, litter decomposition and nutrient dynamics in a subtropical natural oak forest and managed plantation in northeastern India. For. Ecol. Manag.240, 96–104. doi: 10.1016/j.foreco.200
30
Peng Y. Chen G. Chen G. Li S. Peng T. Qiu X. et al . (2017). Soil biochemical responses to nitrogen addition in a secondary evergreen broad-leaved forest ecosystem. Sci. Rep.7:2783. doi: 10.1038/s41598-017-03044-w
31
Peng Y. Li Y. Song S. Chen Y. Chen G. Tu L. (2022). Nitrogen addition slows litter decomposition accompanied by accelerated manganese release: a five-year experiment in a subtropical evergreen broadleaf forest. Soil Biol. Biochem.165:108511. doi: 10.1016/j.soilbio.2021.108511
32
Peng Y. Song S. Li Z. Li S. Chen G. Hu H. et al . (2020). Influences of nitrogen addition and aboveground litter-input manipulations on soil respiration and biochemical properties in a subtropical forest. Soil Biol. Biochem.142:107694. doi: 10.1016/j.soilbio.2019.107694
33
Philippot L. Chenu C. Kappler A. Rillig M. C. Fierer N. (2024). The interplay between microbial communities and soil properties. Nat. Rev. Microbiol.22, 226–239. doi: 10.1038/s41579-023-00980-5
34
Pregitzer K. S. DeForest J. L. Burton A. J. Allen M. F. Ruess R. W. Hendrick R. L. (2002). Fine root architecture of nine north American trees. Ecol. Monogr.72, 293–309. doi: 10.2307/3100029
35
Rasse D. P. Rumpel C. Dignac M. F. (2005). Is soil carbon mostly root carbon? Mechanisms for a specific stabilisation. Plant Soil269, 341–356. doi: 10.1007/s11104-004-0907-y
36
Rinkes Z. L. Bertrand I. Amin B. A. Z. Grandy A. S. Wickings K. Weintraub M. N. (2016). Nitrogen alters microbial enzyme dynamics but not lignin chemistry during maize decomposition. Biogeochemistry128, 171–186. doi: 10.1007/s10533-016-0201-0
37
Ryan M. G. Melillo J. M. Ricca A. (1990). A comparison of methods for determining proximate carbon fractions of forest litter. Can. J. For. Res.20, 166–171. doi: 10.1139/x90-023
38
See C. R. McCormack M. L. Hobbie S. E. Flores-Moreno H. Silver W. L. Kennedy P. G. et al . (2019). Global patterns in fine root decomposition: climate, chemistry, mycorrhizal association and woodiness. Ecol. Lett.22, 946–953. doi: 10.1111/ele.13248
39
Silver W. L. Miya R. K. (2001). Global patterns in root decomposition: comparisons of climate and litter quality effects. Oecologia129, 407–419. doi: 10.1007/s004420100740
40
Singh K. P. Tripathi S. K. (2000). Impact of environmental nutrient loading on the structure and functioning of terrestrial ecosystems. Curr. Sci.79, 316–323.
41
Steffen W. Broadgate W. Deutsch L. Gaffney O. Ludwig C. (2015). The trajectory of the anthropocene: the great acceleration. Anthropocene Rev.2, 81–98. doi: 10.1177/2053019614564785
42
Sun T. Dong L. Wang Z. Lü X. Mao Z. (2016a). Effects of long-term nitrogen deposition on fine root decomposition and its extracellular enzyme activities in temperate forests. Soil Biol. Biochem.93, 50–59. doi: 10.1016/j.soilbio.2015.10.023
43
Sun T. Dong L. Zhang L. Wu Z. Wang Q. Li Y. et al . (2016b). Early stage fine-root decomposition and its relationship with root order and soil depth in a Larix gmelinii plantation. Forests7:234. doi: 10.3390/f7100234
44
Tian D. Niu S. (2015). A global analysis of soil acidification caused by nitrogen addition. Environ. Res. Lett.10:024019. doi: 10.1088/1748-9326/10/2/024019
45
Treseder K. K. (2008). Nitrogen additions and microbial biomass: a meta-analysis of ecosystem studies. Ecol. Lett.11, 1111–1120. doi: 10.1111/j.1461-0248.2008.01230.x
46
Tu L. Peng Y. Chen G. Hu H. Xiao Y. Hu T. et al . (2015). Direct and indirect effects of nitrogen additions on fine root decomposition in a subtropical bamboo forest. Plant Soil389, 273–288. doi: 10.1007/s11104-014-2353-9
47
Wan W. Hao X. Xing Y. Liu S. Zhang X. Li X. et al . (2021). Spatial differences in soil microbial diversity caused by pH-driven organic phosphorus mineralization. Land Degrad. Dev.32, 766–776. doi: 10.1002/ldr.3734
48
Wang X. Feng J. Ao G. Qin W. Han M. Shen Y. et al . (2023). Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness. Soil Biol. Biochem.179:108982. doi: 10.1016/j.soilbio.2023.108982
49
Wang Z. Tao T. Wang H. Chen J. Small G. E. Johnson D. et al . (2023). Forms of nitrogen inputs regulate the intensity of soil acidification. Glob. Chang. Biol.29, 4044–4055. doi: 10.1111/gcb.16746
50
Whalen E. D. Smith R. G. Grandy A. S. Frey S. D. (2018). Manganese limitation as a mechanism for reduced decomposition in soils under atmospheric nitrogen deposition. Soil Biol. Biochem.127, 252–263. doi: 10.1016/j.soilbio.2018.09.025
51
Xia M. Talhelm A. F. Pregitzer K. S. (2017). Chronic nitrogen deposition influences the chemical dynamics of leaf litter and fine roots during decomposition. Soil Biol. Biochem.112, 24–34. doi: 10.1016/j.soilbio.2017.04.011
52
Xia M. Talhelm A. F. Pregitzer K. S. (2018). Long-term simulated atmospheric nitrogen deposition alters leaf and fine root decomposition. Ecosystems21, 1–14. doi: 10.1007/s10021-017-0130-3
53
Xun W. Huang T. Zhao J. Ran W. Wang B. Shen Q. et al . (2015). Environmental conditions rather than microbial inoculum composition determine the bacterial composition, microbial biomass and enzymatic activity of reconstructed soil microbial communities. Soil Biol. Biochem.90, 10–18. doi: 10.1016/j.soilbio.2015.07.018
54
Yang X. Wang X. Xiao S. Liu Z. Zhou X. Du G. et al . (2021). Dominant plants affect litter decomposition mainly through modifications of the soil microbial community. Soil Biol. Biochem.161:108399. doi: 10.1016/j.soilbio.2021.108399
55
Yu G. Jia Y. He N. Zhu J. Chen Z. Wang Q. et al . (2019). Stabilization of atmospheric nitrogen deposition in China over the past decade. Nat. Geosci.12, 424–429. doi: 10.1038/s41561-019-0352-4
56
Zechmeister-Boltenstern S. Keiblinger K. M. Mooshammer M. Peñuelas J. Richter A. Sardans J. et al . (2015). The application of ecological stoichiometry to plant–microbial–soil organic matter transformations. Ecol. Monogr.85, 133–155. doi: 10.1890/14-0777.1
57
Zhang Y. Cao Y. Tang Y. Ying Q. Hopke P. K. Zeng Y. et al . (2020). Wet deposition of sulfur and nitrogen at Mt. Emei in the West China rain zone, southwestern China: status, inter-annual changes, and sources. Sci. Total Environ.713:136676. doi: 10.1016/j.scitotenv.2020.136676
58
Zhang J. Yang H. Wang J. (2019). Soil and climate determine differential responses of soil respiration to nitrogen and acid deposition along a forest transect. Eur. J. Soil Bio.93:103097. doi: 10.1016/j.ejsobi.2019.103097 others
59
Zhou L. Zhou X. Zhang B. Lu M. Luo Y. Liu L. et al . (2014). Different responses of soil respiration and its components to nitrogen addition among biomes: a meta-analysis. Glob. Chang. Biol.20, 2332–2343. doi: 10.1111/gcb.12490
Summary
Keywords
soil chemical, root decomposition, nitrogen addition, lower-order roots, higher-order roots, nutrient dynamics
Citation
Chen G, Chen Y and Yao X (2025) Soil nitrate drives fine root decomposition under nitrogen addition in a subtropical forest. Front. For. Glob. Change 8:1595417. doi: 10.3389/ffgc.2025.1595417
Received
18 March 2025
Accepted
07 May 2025
Published
30 May 2025
Volume
8 - 2025
Edited by
Xiankai Lu, Chinese Academy of Sciences (CAS), China
Reviewed by
Pengpeng Duan, Chinese Academy of Sciences (CAS), China
Jiahuan Guo, Hainan University, China
Updates
Copyright
© 2025 Chen, Chen and Yao.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xingzhu Yao, xingzhuyao@scsaas.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.