- 1Department of Ecology and Conservation Biology, Texas A&M University, College Station, TX, United States
- 2Cook’s Branch Conservancy, Montgomery, TX, United States
Prescribed burning is a common forest management tool, and is expected to affect soil carbon (C) content and dynamics, yet data on this remain limited. Here we report the effect of prescribed burning frequency on net soil C balance in three loblolly-shortleaf pine stands that have undergone different fire regimes over the past 20 years: low-intensity ground fires applied (i) annually, (ii) intermittently (every 2–4 years), or (iii) not at all. Prior to the initiation of differential burn frequencies in 2001, all stands underwent minimal management. Differences in soil C pools and fluxes were attributed to burn frequency treatments. Frequent burns reduced fine root biomass and thus soil autotrophic respiration (Ra). Indirectly, lower fine root detritus production also resulted in reduced heterotrophic respiration (Rh). Fine root productivity and mortality, however, were similar across burn frequencies, resulting in faster fine root turnover with burning. Conversely, the no-burn stand had the highest fine root biomass (BFR) and the highest Ra: BFR ratio (although statistically non-significant), suggesting higher investment in the maintenance of fine roots. Combined with the highest total belowground C flux, and highest soil CO2 efflux, especially from Ra, but also from Rh, the results suggest greater metabolic activity belowground in the no-burn than burned treatments, possibly due to greater mycorrhizal colonization. As a result of these mutually offsetting responses, the net soil C balance did not significantly differ by burn frequency, ranging from −71 ± 123 to −167 ± 104 g C m−2 year−1.
1 Introduction
Forest soils represent a major global carbon (C) reservoir, with organic C stocks in the top 1 meter estimated at approximately 383 petagrams (Pg; 1 Pg = 1015 g), exceeding the C stored in live biomass (363 Pg) (Pan et al., 2011). While detecting a change in this large and spatially variable pool is challenging, it is now recognized that the soil C content varies on the scale of years to decades (Chen et al., 2015; Liu et al., 2025), and is responsive to management activities (Ameray et al., 2021; Jandl et al., 2007; Mayer et al., 2020). Of these, prescribed burning that is used widely in the southern United States (Fox et al., 2007; Waldrop and Goodrick, 2012) may also have an unaccounted impact on soil C. Prescribed burning is used primarily for reducing wildfire risk and facilitating habitat management and ecological restoration (Calkin et al., 2015; Kalies and Kent, 2016). Improved quantitative understanding of this management approach is critical for balancing the safety climate mitigation, economic, and soil health benefits of managed forest ecosystems (Kaarakka et al., 2021; Vargas et al., 2013).
Long-term applications of frequent, low-intensity prescribed burns (typically with fire return intervals of 1–4 years) are common and intended to maintain fuel loads at manageable levels and reduce wildfire risk (Davis et al., 2024). The effect of such management regimes on mineral soil C content has been mixed. While many studies have reported insignificant effects [(e.g., Binkley et al., 1992; Coates et al., 2018; Hatten et al., 2008; Matosziuk et al., 2019; Oliver et al., 2015), others have noted slight increases in soil C (Godwin et al., 2017)]. Changes in soil C storage are closely associated with alterations in C inputs and outputs to the soil environment, with fires contributing to both immediate and long-lasting effects (Frouz, 2024). Soil heterotrophic respiration, the primary soil C output, can increase due to physical soil disturbances and an increase in fire-driven dead organic matter (e.g., aboveground litterfall and fine root mortality). Alternatively, it may decrease by a reduction in substrate supply (e.g., root exudates) due to dead or damaged live aboveground biomass. Most studies to date have shown litter decomposition is generally unaffected by frequent burns in southern US pine communities (Ficken and Wright, 2017; Liechty and Reinke, 2020), however, the effect may become more pronounced when fires become more severe, especially in conjunction with other disturbances, such as drought after autumn fires (Hatten et al., 2008). It has also been suggested that pyrogenic C may facilitate long-term C sequestration (Matosziuk et al., 2019; Pellegrini et al., 2021), but evidence for it remains controversial (Fontúrbel et al., 2021).
Repeated prescribed fires that reduce litter layer, modify understory cover and taxonomic composition, and dry the soil are expected to affect C allocation patterns within ecosystems, such as changes in total belowground carbon flux, invertebrates, and both root symbiont and free-living saprotrophic microbial communities. These phenomena can result directly from fires, but also indirectly due to fire-driven changes in forest stand structure and competition over time. Previous studies have reported that fire-driven changes in the plant community and detritus inputs associated with varying fire frequencies can induce shifts in the soil microbial community and mycorrhizal colonization (Fox et al., 2024; Hart et al., 2005; Oliver et al., 2015). In particular, frequent prescribed fires (e.g., 1–2 year intervals) have been shown to significantly affect mycorrhizal symbioses in the southern pine ecosystems (Fox et al., 2024; Hart et al., 2005). Given the complex mechanisms involved, the net effect of fires on soil C balance remains uncertain.
Soil C processing remains a key uncertainty in the ecosystem C cycle and land surface models (Friedlingstein et al., 1999; Lawrence et al., 2019; Wieder et al., 2018). Despite major conceptual (Kuzyakov, 2010; Lehmann et al., 2020; Schmidt et al., 2011) and methodological advances (Cotrufo et al., 2013; Soldatova et al., 2024; Sulman et al., 2014) in recent decades, uncertainties have persisted because of the complexity of the belowground trophic, competitive, and symbiotic relationships. Many conceptually recognized pools and fluxes, such as root exudates and production and turnover of mycorrhizal fungal mycelium, are difficult to quantify experimentally (Frey, 2019). For example, although mycorrhizal fungi play crucial roles in temperate forest ecosystems, accounting for 27–34% of the net primary productivity (NPP) in temperate mixed coniferous-deciduous forests (Allen and Kitajima, 2014) and 4–35% across temperate forest stands dominated by coniferous and deciduous broadleaf trees (Ouimette et al., 2020), quantitative in-situ studies of their production, turnover, and respiration remain few (Ekblad et al., 2013). This knowledge gap has hindered the accurate modeling of ecosystem C dynamics (Chapin et al., 2009).
In this study, we aim to quantify the effect of prescribed burn frequency on soil carbon balance (ΔSOC = C inputs – C outputs) and its components (i.e., aboveground litterfall production, fine root mortality, and soil heterotrophic CO2 efflux), estimate mycorrhizal fungal production, and critically evaluate uncertainties and main assumptions. We hypothesized that frequent prescribed burning would decrease total belowground carbon flux (H1a) and net soil C balance (H1b) by removing some of the aboveground detritus inputs. We also hypothesized that frequent burning would reduce fine root biomass (H2a) or shift them deeper in the soil (H2b), and potentially accelerate their turnover (H2c).
2 Materials and methods
2.1 Study site
The study was conducted in three adjacent shortleaf pine-loblolly pine stands at Cook’s Branch Conservancy (CBC) in Montgomery County, TX, located in a humid subtropical climate. The average annual precipitation was 1,331 mm (2001–2023), with an average air temperature of 28.6°C in July and 11.2°C in January, as recorded at the Conroe weather station located approximately 28 km from the study site (30.5333 N, 95.7833 W, Elevation: 60 m) (Western Regional Climate Center, 2024). The terrain is flat, with slope angles ranging from 0 to 5%.
The property is divided into about 80 burn management units, ranging in size from 100 to 300 acres, that have been managed with a consistent fire regime since 2001, with prescribed burn intervals occurring either annually or every 2 to 4 years. Additionally, the study included a 290-acre stand that has been excluded from fire management practices since 2001. Prior to 2001, the entire property had received minimal active management (and no comprehensive records), since natural recovery after a harvest and agricultural use about a century earlier.
From among the management units, three stands differing solely in their prescribed fire interval, but similar in mean stand age, vegetation composition, and soils, were chosen for this study. They are labeled as annual burn (AB), intermittent burn (IB; fire interval of 2–4 years for the past 20 years), and fire-excluded or no-burn (NB; no fires since 2001) stands. The soils were classified as moderately well-drained loamy fine sands, specifically, with Conroe loamy fine sand in the AB and IB stands, and Conroe and Lilbert loamy fine sand in the NB stand, respectively (Soil Survey Staff, n.d.). For the purposes of the current study, these soil classes are identical – the texture of both soil series is identical down to 65 cm, and their drainage characteristics are very similar. The possible existence of a clay layer at 80–200 cm was not verified (a characteristic of the Conroe series, but not of the Lilbert series).
All measurements were carried out on four replicate plots per stand. Each plot was 0.18 acres (730 m2, 15.25 m radius) in size, selected so as to include 15–50 live trees. The prescribed burns in the burn stands (AB and IB) were consistently low intensity, primarily consuming surface fuels and ground-level vegetation, with minimal impact on mature overstory trees. These burns were typically conducted during the fall to spring period, with ignition timing determined by site-specific fuel loads and short-term weather conditions, including air temperature, relative humidity, and wind speed in the days preceding and during the burn events. The most recent prescribed burns took place in January 2019 and September 2020 in the AB stand, and in December 2017 in the IB stand.
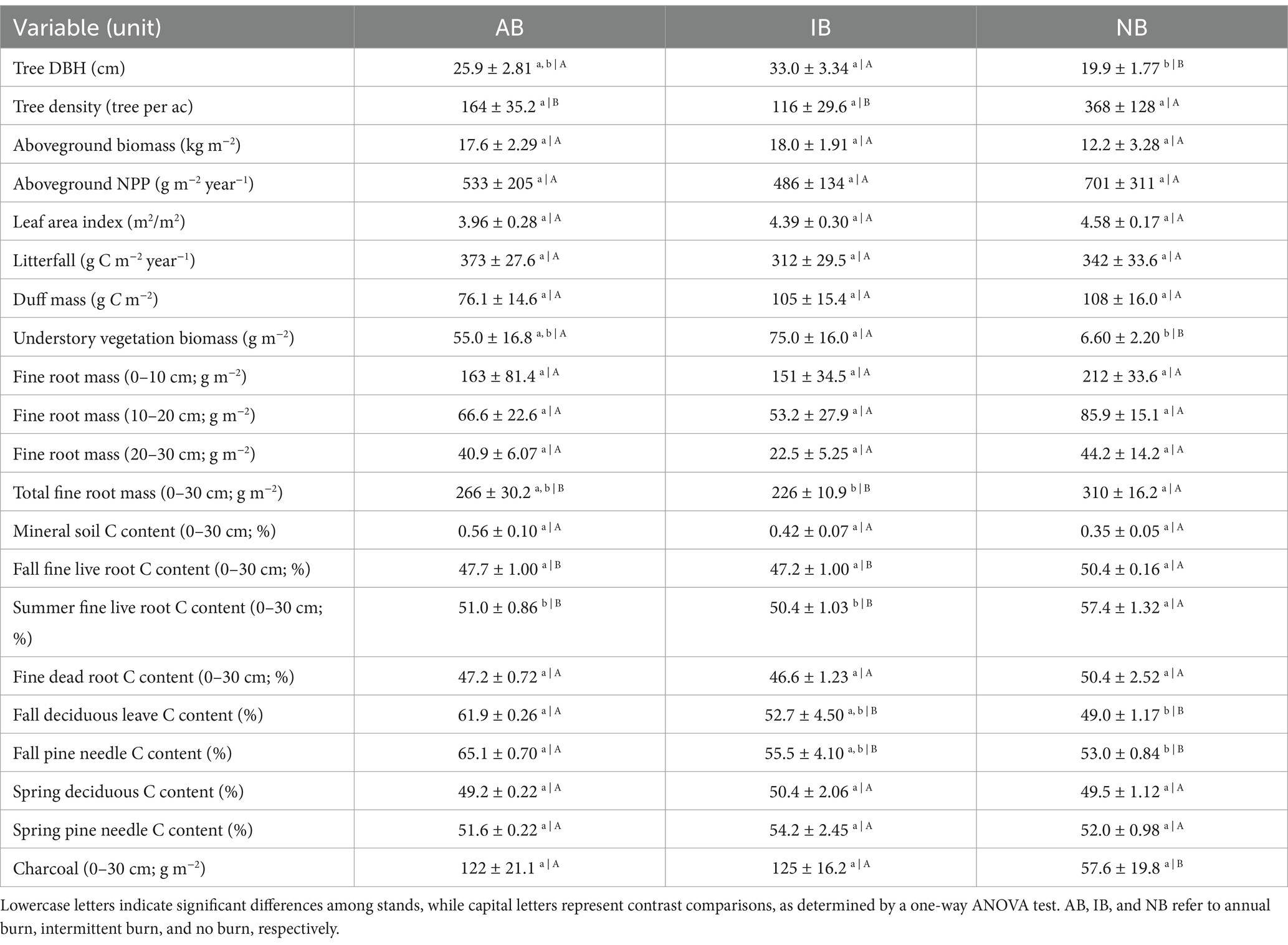
The overstory vegetation within the study site is dominated by loblolly pine (Pinus taeda) and shortleaf pine (Pinus echinata), with some water oak (Quercus nigra), American elm (Ulmus americana), and yaupon (Ilex vomitoria; in the NB stand). The understory vegetation consists of American beautyberry (Callicarpa americana), Cherokee sedge (Carex cherokeensis), hemlock rosettegrass (Dichanthelium portoricense), and wood oats (Chasmanthium sessiliflorum) (Keith, 2012). The mean canopy height was 14.5 m, and the mean leaf area index was 4.0–4.6 m2 m−2. Additional stand characteristics are listed in Table 1.
2.2 Soil carbon balance
Soil C balance can be calculated as:
where , , , , , and are the detritus production rates of foliage, fine roots, snags, mycorrhizal fungi, branches, and coarse roots. T is lateral transport, E is exudation, L is leaching, is heterotrophic CO2 production, and d is disturbance (i.e., fire). The variables in parentheses were considered negligible and are discussed below. In forest ecosystems on flat terrain and the absence of disturbances, over 90% of annual C inputs occur via litterfall and fine root mortality, and over 99% of C losses occur via heterotrophic activity (Noormets et al., 2015). However, given that our active study sites are managed by prescribed burning, losses through disturbance can also be significant. Fire-driven C loss from surface detritus was estimated to be 62% of the duff layer in the AB stand (Clark et al., 2020), and incorporated in the ∆SOC calculation. IB and NB stands did not experience fire during the study period, and their d = 0. Coarse woody debris (DCW), coarse root detritus (DCR), and branchfall (DBF) were assumed to be insignificant on the annual scale. The decomposition of coarse woody materials (DBF and DCR) is about an order of magnitude slower than that of fine aboveground litter and fine roots (King et al., 1997), and C from these tissues may take years to enter into soil C processing. Furthermore, branchfall and treefall are sporadic and exhibit high spatial variability, often occurring during strong wind events, none of which occurred during the study period. Therefore, we omitted DCW, DCR, and DBF from ΔSOC calculations. While some leaching (L) of dissolved organic carbon (DOC) is possible, most published reports suggest it is small in comparison to other components of the ∆SOC. For example, Wang et al. (2019) reported that 0.04% of the total DOC leached out from the soil profile, and Kindler et al. (2011) reported a range from 2.8 g C m−2 yr.−1 in a sandy pine stand in the Netherlands to 16 g C m−2 year−1 in loamy sand in a beech forest in Denmark. Therefore, L was assumed to be negligible in the ΔSOC calculations but will be acknowledged as a potential source of error when discussing treatment differences in the Discussion. Similarly, lateral transport (T) was assumed to be negligible due to the predominantly flat terrain of the study sites, with slopes generally < 2%. However, the order of magnitude of these fluxes is considered when interpreting the significance of treatment differences in the Discussion. The effect of fire was primarily in the removal of the understory and ground vegetation, whereas no change was detected in the soil charcoal content, even immediately after the 2020 prescribed burn in the AB stand (Supplementary Figure S3). Contributions by root and fungal exudates were not measured in this study. Finally, while detritus production of mycorrhizal fungi (e.g., extramatrical hyphal turnover) could contribute significantly to soil C inputs, the magnitude of this flux remains highly variable and challenging to quantify. Therefore, Dfungi was not included in ∆SOC calculations. However, its potential role in C cycling was acknowledged and discussed in Section 4.3.
2.3 Field measurements
2.3.1 Stand measurement
Diameter at breast height (DBH, 1.35 m), tree heights, and species at each study plot were recorded for all trees greater than 7 cm in diameter in March 2020 and 2021. The aboveground vegetation biomass of each plot was estimated based on these records using allometric equations developed for the southern (Cieszewski et al., 2021; Priest et al., 2015; Tiller et al., 2017) or the contiguous US (Jenkins et al., 2003) depending on the availability of species-specific equations. Aboveground NPP of each stand was estimated by tracking the aboveground biomass increments of individual surviving trees and the ingrowth of new trees between 2020 and 2021 (Clark et al., 2001). The Leaf Area Index (LAI) was measured in August 2020 and April 2021 using a plant canopy analyzer (LAI-2000, LI-COR Biosciences, Lincoln, NE, USA).
2.3.2 Soil CO2 efflux measurement
Soil CO2 efflux (μmol CO2 m−2 s−1) was measured using an infrared gas analyzer (LI 8100, LI-COR Biosciences) in conjunction with a 20 cm diameter survey chamber (LI 8100–103, LI-COR Biosciences). During measurements, the chamber was placed on top of polyvinyl chloride (PVC) collars. Each plot (4 plots per stand) contained five control collars (20 cm interior diameter and 5 cm height) used to measure total soil CO2 efflux (SR) and five root exclusion collars (20 cm interior diameter and 30 cm height) used to estimate heterotrophic soil CO2 efflux (Rh). The five pairs of collars were placed in a circular arrangement, spaced 75 degrees apart. Within each pair, the collars were positioned 1–1.5 meters apart to prevent disturbance from the root exclusion collar. Root exclusion collars were inserted 20–25 cm (as deep as they would go) into the ground to isolate the Rh contribution to SR. Before the installation of root exclusion collars, initial differences in surface CO2 fluxes between adjacent control and root exclusion collars were measured and accounted for in subsequent monthly measurements to identify the proportion of Rh in SR. Both control and root exclusion collars were initially installed in October 2020 and reinstalled in April 2021 to maintain accurate Rh measurements. All soil CO2 efflux measurements were taken between 8:00 and 17:00. To maintain bare soil in the respiration collar, all aboveground live vegetation within collars was clipped before measurements.
During soil respiration measurements, soil temperature at a 5 cm depth was recorded using a digital temperature probe, and soil moisture content (m3/m3) at a 5 cm depth was measured using a soil moisture probe (HydroSense II, Campbell Scientific, Logan UT, USA) adjacent to each control PVC collar.
Monthly SR rates were estimated by scaling up the mean fluxes of the plot-averaged measured rate (μmol CO2 m−2 s−1) (Giardina and Ryan, 2002). Due to an extended rainy period, no field measurements were available for the NB stand in May 2021. Hence, the monthly SR values for that month were estimated using linear interpolation between the preceding and subsequent months.
Temporal changes in flux ratios between root exclusion and control collars were used to determine when pure Rh values were observed. Stabilized ratios of Rh and SR (hereafter referred to as “Rh: SR ratio”) for dormant and growing seasons were established based on the decay curves of this ratio within each study plot. Monthly Rh was then estimated by multiplying the monthly SR by the Rh: SR ratios. The dormant season Rh: SR ratio was used to estimate monthly Rh between November 2020 and April 2021, whereas the growing season Rh: SR ratio was used for the remaining months. Monthly autotrophic soil CO2 efflux (Ra), which consists of root and mycorrhizal respiration, was derived by subtracting Rh from SR.
2.3.3 Soil sampling and detritus input collection
For fine root productivity and mortality estimates, soil samples were collected at monthly intervals between September 2020 and August 2021 using a soil core (5.2 cm diameter with 33 cm length) from five locations within each study plot. For the vertical root profile, soil samples were also collected in 10 cm increments down to 30 cm from one location within each study plot in the spring of 2021. All soil samples were stored in plastic bags and transported to the Texas A&M University College Station campus, where they were frozen at −20°C until processing. The soil samples were washed with deionized water using a 1-mm sieve to remove soil particles. Roots were then sorted based on diameter into fine roots (< 2 mm diameter) and coarse roots (> 2 mm diameter), and further categorized as dead and live roots based on color and texture (Vogt and Persson, 1991). Charcoal particles caught in the sieve were also extracted. After oven-drying at 68°C and reaching a stabilized weight, the dry weight of each root category and charcoal particles was recorded.
Fine root productivity (NPPFR) and mortality (DFR) were calculated using a decision matrix (Assefa et al., 2017; Brunner et al., 2012; Gower et al., 1992; McClaugherty et al., 1982; Yuan and Chen, 2013), maximum-minimum (McClaugherty et al., 1982), and compartment flow methods (Santantonio and Grace, 1987). The decision matrix method provided the greatest consistency of productivity and mortality estimates in time and among plots and was therefore chosen as the preferred method. To reduce potential outlier effects further, we report here the average of four different variants of the decision matrix method, which are detailed in Supplementary Table S2. These methods rely on plot-averaged differences in live fine and dead fine root biomass (ΔLF and ΔDF, respectively) across sampling intervals. We excluded the maximum-minimum method due to its tendency to underestimate root production (Brunner et al., 2012), which was also observed in our study. Similarly, the compartment flow method was not used as it calculates mortality based on constant decomposition rates and derives production estimates from calculated mortality, which we found less appropriate for our study. Turnover rates of fine roots were estimated by dividing fine root productivity (NPPFR) by the averaged fine root biomass (BFR) over a sampling year (McClaugherty et al., 1982) (i.e., Turnover rates = NPPFR/BFR). Coarse root biomass was estimated allometrically as the ratio of root compartment biomass to total aboveground biomass (Chojnacky et al., 2014).
Aboveground fine litterfall detritus (i.e., pine needles, deciduous leaves, small twigs, and reproductive tissue) was collected monthly between September 2020 and August 2021 at three locations within each study plot using litter traps with a surface area of 0.86 m2 at a height of 0.5 m. Duff layer, consisting of litter accumulated on the ground surface, was sampled in July–August 2020 at the same three locations using a 0.11 m2 quadrat. Understory vegetation, composed of live plants below the canopy, was sampled in July 2023 at three locations near litter traps at each study plot with a 0.25 m2 quadrat. All collected samples were oven-dried at 68°C until a constant mass.
The C content of soil, live and dead fine roots, as well as fine litter (including pine needles and deciduous leaves), were measured at each stand twice a year. The samples were oven-dried at 68°C, ground with a mixer mill (MM 400, Retsch, Newtown, PA, USA), and analyzed using an elemental analyzer (FlashSmart NC Soil, Thermo Fisher Scientific, Waltham, MA, USA) to determine tissue and soil C content.
2.4 Total belowground carbon flux and fungal production
Annual mycorrhizal fungal production was estimated similarly to Ouimette et al. (2020) (Equation 2), combining two established mass balance models of total belowground carbon flux (TBCF). The first model calculates TBCF as the sum of the production of fine roots (NPPFR), coarse roots (NPPCR), mycorrhizal fungi (NPPfungi), autotrophic respiration (Ra), and exudation (E) [Equation 2a; Chapin et al., 2009]. Even though both NPPfungi and E are unknown, E has been reported to be about an order of magnitude smaller than NPPfungi [E generally less than 25 g C m−2 year−1 (Phillips et al., 2011; Yin et al., 2014) versus NPPfungi up to 200 g C m−2 year−1 (Allen and Kitajima, 2014; Deng et al., 2023)]. The second model considers TBCF as comprising four primary components: soil respiration (SR), aboveground litterfall (DL), and the production of coarse roots (NPPCR) and the changes in soil C (ΔSOC) [Equation 2b; Davidson et al., 2002; Nadelhoffer et al., 1998]. As mentioned before, leaching (L) is considered negligible. This equation was used to calculate TBCF for all stands. Thus, we estimate the latter as the mass balance residual:
rearranged from Chapin et al. (2009):
and TBCF estimated independently as per Davidson et al. (2002) and Nadelhoffer et al. (1998):
2.5 Data analysis
The annual soil C balance and TBCF were calculated from plot average pools and fluxes, and standard errors of stands in the following sections were derived from variabilities between study plots (n = 4).
The temperature response of SR and its components in each stand was assessed by an exponential Q10 function [Equation 3: Van't Hoff (1898)] using monthly measurements of soil temperature (T) and SR.
where is an SR at a given soil temperature at a depth of 5 cm, and is reference respiration at a reference temperature , and is temperature sensitivity. and were derived by minimizing the residual sum of squares through nonlinear least squares analysis using the “nls_table” function in the “forestmangr” package in R (Braga et al., 2023). The reference temperature was selected close to the annual mean soil temperature at all stands, which was 20.3°C in AB, 21.4°C in IB, and 19.3°C in NB stands.
The annual flux of each component was calculated by summing the monthly means for each plot, except for NPPCR, which was derived from sequential annual stand measurements. Stand differences in monthly soil temperature and moisture were determined with a linear mixed-effects model for repeated measures using the R package “lme4” (Bates et al., 2015). Prior to analysis, variables were subjected to transformation using the Box-Cox method to meet the criteria of normality, as necessary. In the nested-mixed model, months of the year were treated as fixed effects, while study plots were treated as random effects. Where significant differences were found, the Tukey test was used for post-hoc analysis. The comparisons between (i) burned (AB + IB) versus unburned (NB) stands and (ii) AB versus (IB + NB) stands were also investigated using the contrast function in the “emmeans” package in R (Lenth, 2024).
One-way ANOVA analysis (the aov function in the “stats” package in R) was used to assess significant differences in annual values among stands, as well as to conduct contrast comparisons. The level of significance was determined at p-value < 0.05. All the analyses were conducted in R (version 4.3.3) (R Core Team, 2024) and implemented in RStudio (version 2023.12.1) (Posit team, 2024).
3 Results
3.1 Stand characteristics
The three study stands differed in standing biomass quantity and distribution. The NB stand had the greatest overstory tree density, but the lowest tree diameter, tree biomass, and ground cover biomass (Table 1). The summer LAI and duff mass were slightly lower in the AB stand than in the IB and NB stands (Table 1). Fine root biomass was significantly lower in the burned stands compared to the NB stand (p = 0.04, Table 1). Most of the fine root biomass (62.7%) was concentrated in the top 10 cm of soil, with 24.5% in the 10–20 cm layer and 12.8% in the 20–30 cm layer. No significant differences in fine root biomass distribution were observed across stands at any depth, contrary to the expectation of a downward shift of roots in the soil profile in response to frequent burning (H2b). The monthly average charcoal particle mass was significantly higher in the burned stands than in the NB stand (p = 0.01), but no differences were detected between AB and IB stands (Table 1) or before and after the fire (Supplementary Figure S3). The understory vegetation biomass was significantly greater in burned stands (AB and IB) than in the NB stand (p < 0.01, Table 1), attributable to the dense yaupon mid-story in the NB stand. The slightly lower LAI in the AB stand leads to earlier cooling of the soil in the fall and earlier warming in the spring than in the other stands, accompanied by higher soil moisture in the AB stand in the late fall and slower water use in the spring. The NB stand had lower soil temperatures than the burned stands (p = 0.002, Figure 1).

Figure 1. Temporal changes in (a) soil temperature and (b) soil moisture at a depth of 5 cm in AB (red), IB (green), and NB (blue) stands. The error bars indicate the standard deviation of four study plots. A nested mixed effect model, with stand, month of the year, and their interaction as fixed effects and study plot as a random effect, was used for analysis. Post-hoc analysis was conducted to investigate statistical differences among stands, with results displayed as letters (monthly) and in tables (overall). Soil moisture measurements were not taken at the NB stand in May 2021 due to extended rainy periods and in August 2021 due to instrument issues; thus, these months were excluded from the analysis.
3.2 Soil carbon inputs
The mean standing fine root biomass over the sampling year was greater in the NB stand (310 ± 16.2 g m−2) than in the burned stands (266 ± 30.2 in AB and 226 ± 10.9 g m−2 in IB, p = 0.04; Table 1). Live fine roots constituted approximately 52–57% of the total fine root biomass across stands, while dead fine roots comprised 43–48%. Live fine root biomass showed a weak seasonal pattern, with the highest values in early summer 2021, while no seasonality was detected in dead fine root biomass. Across all stands, the majority of fine root biomass was concentrated in the top 10 cm of soil (62.7%), and the differences in root biomass between the topsoil and depths of 10–20, and 20–30 cm were significant in the IB and NB stands (both p < 0.01; Table 1).
The annual fine root mortality rates were 211 ± 40.2, 184 ± 38.4, and 238 ± 32.4 g C m−2 year−1 (mean ± SE) in AB, IB, and NB stands, respectively. The annual fine root productivity was 205 ± 18.3, 212 ± 15.6, and 234 ± 31.4 g C m−2 year−1 in AB, IB, and NB stands, respectively. No significant differences were observed in either productivity or mortality between the stands. Turnover rates of fine roots were 1.57 ± 0.11, 1.87 ± 0.12, and 1.50 ± 0.14 in AB, IB, and NB stands, respectively.
3.3 Soil CO2 emission
The annual SR was 1,138 ± 66.3, 1,047 ± 65.6, and 1,455 ± 64.9 g C m−2 year−1 (mean ± SE) in the AB, IB, and NB stands, respectively. The annual SR values in the burned stands were significantly lower than the NB stand (by 21.9% in AB and by 28.0% in IB; p < 0.05). The Q10 values of SR and Ra were significantly greater in the NB stand compared to the burned stands (p < 0.01 and p = 0.03, respectively; Figure 2), whereas the Q10 values of Rh were similar among stands. Reference respirations of SR and Ra were also greater in the NB than in the burned stands (p = 0.02 and p = 0.08, respectively).
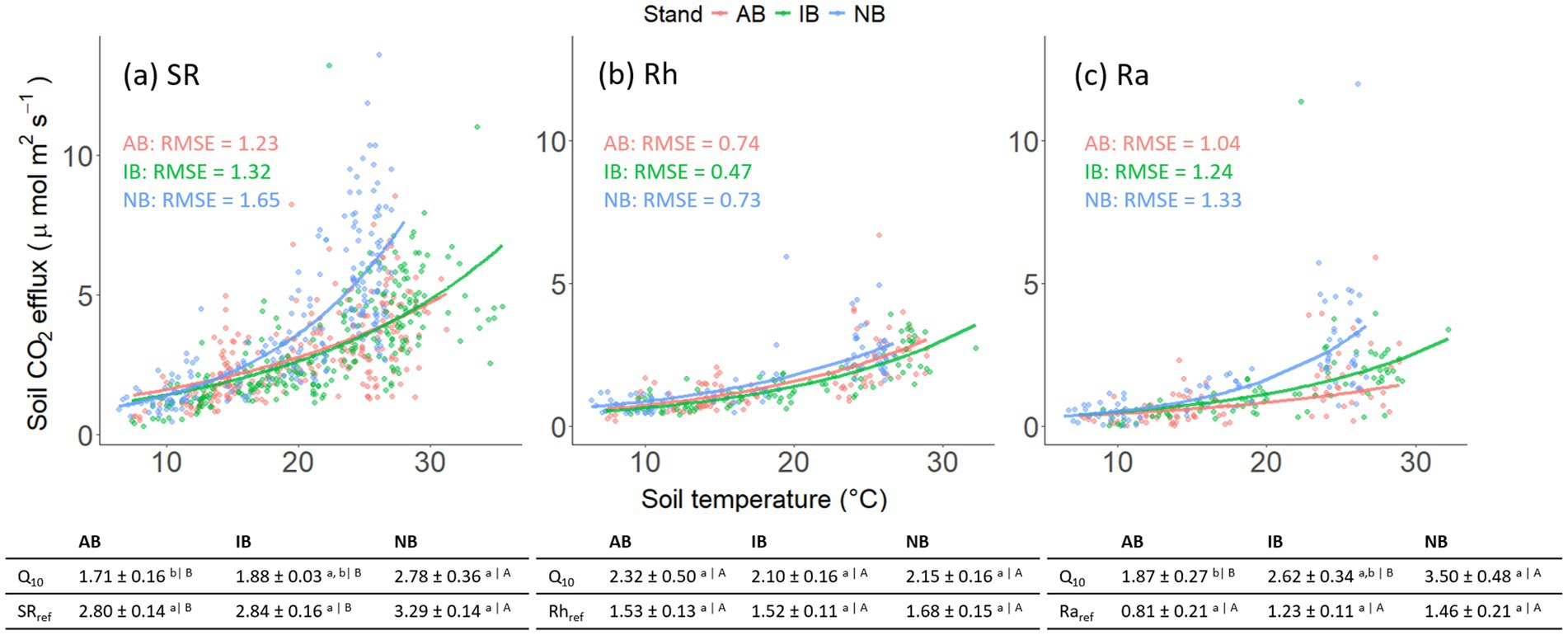
Figure 2. Temperature responses of (a) soil CO2 efflux (SR), (b) heterotrophic soil CO2 efflux (Rh), and (c) autotrophic soil CO2 efflux (Ra) in the AB (red), IB (green), and NB (blue) stands, respectively. The regression curves indicate the exponential relationships (Equation 3) for each stand. The obtained Q10 values and reference respirations (mean ± SE) were presented in the tables along with post-hoc one-way ANOVA results. Note that the data points in Rh and Ra were collected during periods when Rh: SR ratios had stabilized.
Soil CO2 efflux from root exclusion collars stabilized in month 4 during the dormant season and in month 5 during the growing season based on the flux decay curves (Supplementary Figure S2). The heterotrophic fraction increased with annual fire frequency, with AB being higher than IB and NB stands (Supplementary Figure S2), but this change was due to lower Ra rather than higher Rh. The Rh: SR ratio was also higher in the dormant season than in the growing season (0.62 ± 0.03 vs. 0.51 ± 0.03, p = 0.02). The annual Rh was 663 ± 54.0, 567 ± 68.1, and 747 ± 61.4 g C m−2 year−1 in AB, IB, and NB, respectively. The annual Ra was 474 ± 80.0, 480 ± 28.8, and 708 ± 108 g C m−2 year−1 in AB, IB, and NB, respectively, with the NB stand having greater annual Ra than burned sites (p = 0.03).
3.4 Soil carbon balance and total belowground carbon flux
The annual soil C balance (ΔSOC), calculated with Equation 1, was −126 ± 31.4, −71.0 ± 122, and −167 ± 104 g C m−2 year−1 in the AB, IB, and NB stands, respectively (Figure 3). There were no significant differences in ΔSOC among stands. The C loss of duff during the prescribed burn in the AB stand was estimated as 47.2 ± 9.03 g C m−2 year−1.
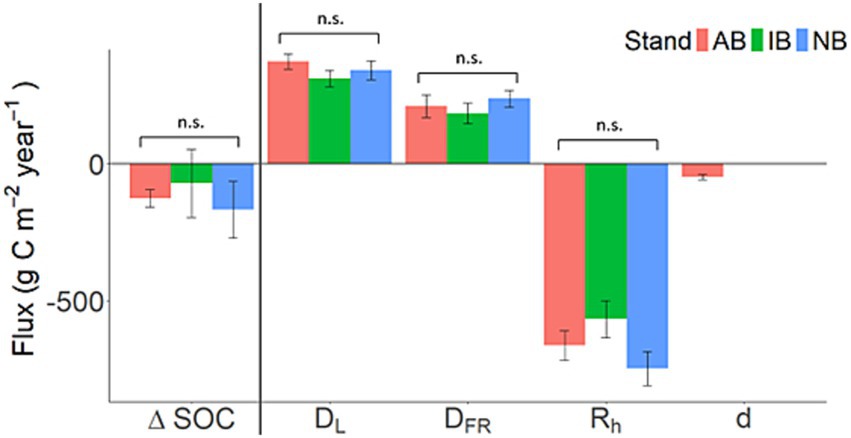
Figure 3. Net annual soil C balance (ΔSOC) and its component fluxes - DL: aboveground litterfall; DFR: fine root mortality; Rh: heterotrophic respiration; d: disturbance (g C m−2 year−1). The error bars show the standard error for the four study plots. Soil C inputs were displayed as positive, while C losses as negative. “n.s.” denotes non-significant differences based on one-way ANOVA.
The C content in live fine roots was significantly greater during peak biomass in June 2021 than during the low biomass in September 2020 (p = 0.001). Live fine roots in the NB stand had higher C content than those in the burned stands for both collections (p = 0.01 and p < 0.001, Table 1). The primary components in fine litterfall were pine needles (AB: 41.5 ± 0.40%, IB: 66.7 ± 4.34%, NB: 39.5 ± 6.5%) and broadleaf leaves (AB: 33.9 ± 0.72%, IB: 8.49 ± 4.33%, NB: 41.4 ± 4.03%) across stands. The weighted C contents for these percentages were estimated to be 63.6 ± 0.43%, 54.9 ± 4.06%, and 51.0 ± 0.93% for the dormant season, while 50.5 ± 0.09%, 53.4 ± 2.35%, and 50.7 ± 1.05% for the growing season in the AB, IB, and NB stands, respectively.
The TBCF, calculated using Equation 2b, was significantly higher in the NB stand at 1018 ± 88.2 g C m−2 year−1, in contrast to the AB (698 ± 64.1 g C m−2 year−1, p = 0.02) and IB stands (713 ± 36.2 g C m−2 year−1, p = 0.02), respectively (Figure 4). Across stands, TBCF was predominantly allocated to Ra, accounting for 68 to 69%, followed by NPPFR (30% in burned stands vs. 23% in NB; p = 0.02), and NPPCR (7%). NPPCR was estimated as 54.0 ± 20.4, 49.2 ± 13.5, and 71.8 ± 32.0 g C m−2 year−1, while NPPfungi was estimated at −41.6 ± 25.1, −27.9 ± 24.3, and 3.98 ± 51.7 g C m−2 year−1 for the AB, IB, and NB stands, respectively. No significant differences were found in either NPPCR or NPPfungi among stands. The negative value of NPPfungi may be used as an estimate of the cumulative error in the ΔSOC components.
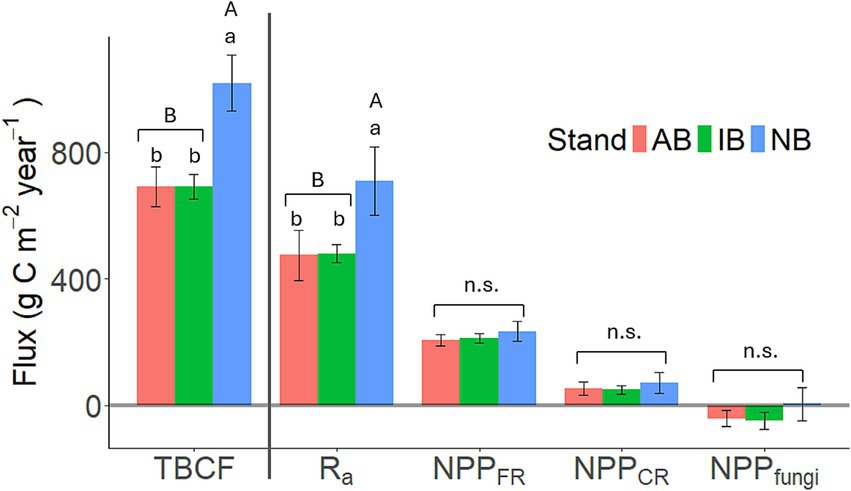
Figure 4. Total belowground carbon flux (TBCF; g C m−2 year−1) and its component fluxes (Ra: autotrophic respiration; NPPFR: fine root productivity; NPPCR: coarse root productivity; NPPfungi: mycorrhizal fungal productivity) (g C m−2 year−1). The error bars represent the standard error for the four study plots. Lowercase letters indicate significant differences among stands, while capital letters denote differences between burned (AB + IB) and unburned stands, as determined by a one-way ANOVA. “n.s.” indicates non-significant differences.
4 Discussion
4.1 Limits of inference
The current study is an unreplicated comparison of three individual burn units on similar soil, identical climates, and similar site histories. Prior to the initiation of the different burn frequencies in 2001, the stands were in similar condition, following decades of fire exclusion. The findings are interpreted as direct or indirect consequences of different burn frequencies implemented for 20 years. Extrapolation of current findings to other sites should be done with caution and with consideration to specific vegetation, climate, edaphic, and biotic interactions in mind.
4.2 Assumptions and uncertainties
In this study, we assumed that the contributions of DCW, DBF, DCR, T, E, and L to the ΔSOC calculations were negligible. Kindler et al. (2011) reported annual DOC leaching losses (L) in European forests averaging 8.3 ± 4.9 g C m−2 year−1, with a range from 2.8 ± 0.9 g C m−2 year−1 in a sandy pine forest to 16.2 ± 0.4 g C m−2 year−1 in a beech forest on loamy sand. Others have estimated that in the US Midwest root and fungal exudation (E) may be up to 24 g C m−2 year−1 (Phillips et al., 2011; Yin et al., 2014). While omitting these fluxes in Equation 1 may potentially underestimate ΔSOC and TBCF by as much as 40 g C m−2 year−1, this would not alter the results significantly.
Second, the AB stand was the only stand affected by fire during the study period, while the disturbance loss (d) was zero in the IB and NB stands. If the loss of duff and ground cover during a prescribed fire was proportionally identical to the estimated loss at the AB stand, the average annualized d in the IB stand would have been 21.8 ± 3.17 g C m−2 year−1, and ΔSOC -92.8 ± 126 g C m−2 year−1. This would not change the relative ranking of ΔSOC or the statistical significance difference among the stands. It is also possible that the fuel consumption rate may have differed from this estimate.
Third, the NPPCR is estimated with allometric equations, whereby coarse root biomass is scaled proportionally to tree diameter (Chojnacky et al., 2014). While these relationships are species- and region-specific, they are constant for all three treatments in the current study. As explained later, we concluded that belowground C allocation was affected by the different burn frequencies. Yet, we were unable to assess the extent to which this could have affected the allometric relationships. Errors in NPPCR could affect the mass balance closure (Equation 2) and the value of NPPfungi.
Given that Rh is the primary contributor to ΔSOC and Ra of TBCF, respectively (Figures 3, 4), the annual SR estimation holds the greatest potential for introducing errors or biases in these balances, as well as NPPfungi. One possible source of error in SR estimation could arise from the upscaling from point measurements to the annual scale. To address this, we compared SR estimates derived from monthly observations with those derived from daily SR accumulation, utilizing the Q10 equation based on half-hourly soil temperature measurements in the IB stand. These two estimates came to within 1% of one another (1,047 and 1,053 g C m−2 year−1). Moreover, the SR and its components observed in this study are consistent with previous studies in southern pine communities with repeated prescribed burns (Godwin et al., 2017). Hence, we conclude that the estimates of SR, Rh, and Ra are reasonable.
Uncertainties associated with the estimation of fine root production and mortality, coupled with potentially high mycorrhizal fungal turnover rates, could affect the estimation of NPPfungi. Often, Rh significantly exceeds total detritus production (DFR + DL) (Noormets et al., 2015), with the difference usually attributed to fungal production or root and fungal exudates (Ouimette et al., 2020). It has been estimated that about 25% of fine root production may be in fungal sheaths (Ouimette et al., 2013), which, if omitted, could result in an error of up to 59 g C m−2 year−1 in the current study. Moreover, the turnover of mycorrhizal necromass also contributes to detritus inputs. However, the carbon use efficiency of the mycorrhizal, free-living saprotrophic fungi and bacteria may vary significantly (Soares and Rousk, 2019; Wang and Kuzyakov, 2023). Extramatrical hyphal turnover for ectomycorrhizal fungi has been estimated at 13 times per year (95% CI: 10.5–19.5) in a loblolly pine stand in North Carolina (Ekblad et al., 2016) and 10 ± 3 times per year in a longleaf pine stand in Georgia (Hendricks et al., 2016). Although these turnover rates may not directly reflect the dynamics of bulk mycorrhizal structures, it is possible that NPPfungi derived from monthly sequential soil cores is underestimated. This relatively rapid turnover may also be underestimated in Rh measurements obtained using root exclusion collars, as these collars may suppress Rh if high levels of root exudates were used to sustain microbial activity.
Finally, we should note that earlier studies may also have relied on incorrect assumptions, which may have affected the NPPfungi estimates. For example, the assumption of no net change in soil C by Ouimette et al. (2020) may or may not have been the reason for their mass balance and isotopic estimates of NPPfungi along a 10-site gradient. In the current study, neglecting changes in mineral soil C would have resulted in NPPfungi of 84–171 g C m−2 year−1 instead of the −27.9 - 4.0 g C m−2 year−1. That said, the stand differences in NPPfungi looked similar in both calculations (not shown).
4.3 Detritus production and soil CO2 efflux
The main soil C input was the aboveground litterfall (59–65%), whereas fine root mortality contributed 35–41%. The duff layer biomass (as well as depth) was 35% lower in AB than in IB and NB (Table 1). There was no significant difference between the IB and NB stands, suggesting that the duff layer recovered within the 2-4-year burn interval at the IB stand. Earlier reports have found that similar low-intensity fires did not alter DL (Espinosa et al., 2018), but that over time, fire exclusion manifested in greater duff layer biomass compared to frequently burned stands (Godwin et al., 2017).
As hypothesized in H2a, fine root biomass was significantly greater in the NB stand than in the burned stands (p = 0.04, Table 1), however, fine root productivity and mortality were similar among the stands. This resulted in higher fine root turnover in the burned stands (1.57 in AB and 1.87 in IB) than in the NB stand (1.50), though these differences were not statistically significant, consistent with H2c. Both fine root productivity (NPPFR = 205–234 g C m−2 year−1) and mortality (DFR = 184–238 g C m−2 year−1) were similar to the range commonly reported for pine forests using the sequential coring method (NPPFR: 242–862 g m−2 year−1; DFR: 199–862 g m−2 year−1) (Han et al., 2016; Makkonen and Helmisaari, 1999; Persson, 1980; Santantonio and Grace, 1987; Yuan and Chen, 2013).
The SR in the NB stand was consistently greater than that in the burned stands, in line with earlier studies on shortleaf-loblolly plantations (Godwin et al., 2017), and primarily due to the difference in Ra. While the absolute magnitude of Rh was greater than Ra (with annual averaged heterotrophic fraction of 0.61, 0.55, and 0.54 in AB, IB, and NB, respectively), the treatment differences were more pronounced in Ra. Additionally, the Q10 value of Ra was significantly greater in the NB stand compared to the burned stands (p < 0.05), with the Q10 of Ra exceeding that of Rh in the NB stand (3.50 vs. 2.15; p = 0.04). Ra was also higher in NB than the burned stands in proportion to BFR (3.83, 4.27, and 4.51 in AB, IB, and NB, respectively), even though the differences were not statistically significant. The combination of greater standing BFR, lower turnover, and higher respiratory cost of fine roots in NB than the burned stands suggests that the roots may carry some additional value, perhaps through higher mycorrhizal colonization, even though visual inspection during root sorting did not reveal differences among treatments. Given that Ra is tightly coupled to C availability from photosynthesis (Fenn et al., 2010; Kuzyakov and Gavrichkova, 2010), the stands also likely differ in the C allocation patterns. The higher Q10 of Ra at NB than the burned stands, as well as a positive correlation between TBCF and Q10 of Ra (r = 0.69, p = 0.01; data not shown), support this interpretation, as higher temperatures correlate with radiative input that also drives photosynthesis. Even though the difference between the temperature responses of AB and IB is small (Figure 2c), it is still apparent that the gradient of Q10, and thus potentially belowground allocation, decreases progressively with burn frequency. Interestingly, the consistent Q10 values of Rh contrast with the findings of Butler et al. (2019), who reported an increase in Q10 in frequent fire stands due to an increased level of law-quality recalcitrant organic matter. Our study did not observe a detectable charcoal pulse even after the fire in the AB stand (Supplementary Figure S3), aligning with findings from ponderosa pine forests in Oregon subjected to repeated spring-prescribed burns (Matosziuk et al., 2019). The absence of pyrogenic C changes in the mineral soil post-fire suggests that dormant season-prescribed burns in our sites may be too low in intensity to promote its accumulation.
The omission of fungal detritus from the soil C balance estimate is due to uncertainty associated with its magnitude. While some studies report mycorrhizal mycelial turnover of 7–10 yr.−1 (Hagenbo et al., 2021), others have noted that turnover declines drastically with age, down to 1 yr.−1 (Hagenbo et al., 2017). Given that the trees in this study are estimated to be 70–100 years old, the turnover is expected at the lower end of this range. On the other hand, the regular prescribed burning at AB and IB stands may increase the turnover. It is also unclear whether the fungal communities in these stands had the same species composition. While some earlier studies have reported minimal effects of low-intensity prescribed burns on decomposition (i.e., Rh) in pine communities in the southern US (Ficken and Wright, 2017; Liechty and Reinke, 2020), there are also reports suggesting that repeated low-intensity burns can alter fungal community composition (Fox et al., 2024; Oliver et al., 2015). In the current study, we did not measure fungal taxonomic composition, and even the NPPfungi estimates are mass balance residuals, making functional interpretation challenging. Nevertheless, variations in fungal composition among stands in our study site are suggested by the differing relationships between NPPfungi and the SR components.
4.4 Annual soil carbon balance and total belowground carbon flux
Our initial hypothesis of greater annual soil C loss in the burned stands than in the unburned stand was proven false (H1b; Figure 3). All sites had a similar and negative ΔSOC, losing 126 ± 31.4, 71.0 ± 123, and 167 ± 104 g C m−2 year−1 (AB, IB, and NB stands, respectively). However, even though ΔSOC did not differ significantly among the three stands, C processing clearly responded to the frequency of prescribed burning. The NB stand had greater NPPCR and TBCF than the AB and IB stands (H1a). NPPfungi was also numerically higher, although the large variances of this flux kept the stand differences from manifesting as statistically significant (Figure 4). Obviously, NPPfungi cannot be negative, but given the large magnitude of other fluxes, and the small sample size (n = 4) for resolving the differences, we postulate that it is still notable (and significant) that several independent lines of evidence consistently point to the likely greater presence of mycorrhizal fungi in the NB than burned treatments. More direct measurements in the future will be used to test this hypothesis.
At the same time, both Ra and Rh were also higher in NB than the burned stands (Figures 3, 4), while the difference between the AB and IB stands was minimal. Although fine root mortality did not significantly differ between stands, the Ra was lower at AB and IB than NB (Figure 4), correlating with BFR (Table 1) and NPPfungi. Similar to earlier reports (Giardina et al., 2014; Kuzyakov et al., 2019), higher TBCF correlated with higher Ra (r = 0.79, p < 0.001). The Ra estimates in the burned stands (474 and 480 g C m−2 year−1 in AB and IB stands, respectively) were consistent with prior studies in southern pine ecosystems (Kim et al., 2025; Maier et al., 2004). Somewhat unexpectedly, our results showed that the change in belowground activity came from both greater autotrophic as well as heterotrophic processes in the NB stand than the burned stands, with the between-stand differences on the annual scale being about 2-fold greater in Ra than Rh. While it is only logical that TBCF also supports the decomposer community, the correlations of Ra and Rh with NPPFR, NPPfungi, DL, and DFR were weak throughout (not shown).
In conclusion, prescribed burning appears to trigger a suite of related changes in belowground C dynamics, collectively reducing both belowground autotrophic and heterotrophic activity. Whether this cascade of changes starts with lower belowground C allocation by plants or with the mortality of fungi resulting in lower belowground C sink, and more disposable fine roots, is yet to be elucidated. Most of the C allocated belowground was immediately used to support the entire complexity of the belowground ecosystem, of which the current study only quantified root biomass. We did not find the hypothesized differential soil C balance, but we did observe that 20 years of fire exclusion had manifested in greater fine root biomass, lower fine root turnover, slightly greater respiratory investment per unit of fine roots, and, seemingly, greater fungal colonization. The latter, in particular, should be further investigated using techniques such as DNA metabarcoding or stable isotope labeling to better characterize shifts in symbiotic associations. The current study indicates that fire management decisions influence not only surface conditions, but also fundamental processes of belowground C allocation and dynamics, and have thus implications for climate mitigation and soil health.
Data availability statement
'The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://doi.org/10.15482/USDA.ADC/28235093.v1, https://github.com/moekaono/soil_C_balance_CBC.
Author contributions
MO: Conceptualization, Investigation, Software, Writing – original draft, Formal analysis, Data curation, Visualization, Methodology. AN: Conceptualization, Supervision, Project administration, Funding acquisition, Resources, Methodology, Writing – review & editing, Investigation. SM: Project administration, Conceptualization, Writing – review & editing, Resources.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This project was supported by The Cynthia and George Mitchell Foundation and Texas A&M AgriLife. MO was supported by the ITO Foundation and McMillan-Ward Fellowship.
Acknowledgments
We thank Seth Massery, Tyler Smith, Connor Kenny, and Evan DuPont for assistance with fieldwork and sample processing, and Kathy Hutson for logistical support at the site. We also thank Joe Hamrick, Kevin Mundorff, Eric Keith, and Ross Carrie from Raven Environmental Services for sharing fire history and vegetation data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1602557/full#supplementary-material
References
Allen, M. F., and Kitajima, K. (2014). Net primary production of ectomycorrhizas in a California forest. Fungal Ecol. 10, 81–90. doi: 10.1016/j.funeco.2014.01.007
Ameray, A., Bergeron, Y., Valeria, O., Montoro Girona, M., and Cavard, X. (2021). Forest carbon management: a review of Silvicultural practices and management strategies across boreal, temperate and tropical forests. Curr. For. Rep. 7, 245–266. doi: 10.1007/s40725-021-00151-w
Assefa, D., Rewald, B., Sandén, H., and Godbold, D. L. (2017). Fine root dynamics in Afromontane Forest and adjacent land uses in the northwest Ethiopian highlands. Forests 8:249. doi: 10.3390/f8070249
Bates, D., Mächler, M., Bolker, B., and Walker, S. (2015). Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67, 1–48. doi: 10.18637/jss.v067.i01
Binkley, D., Richter, D., David, M. B., and Caldwell, B. (1992). Soil chemistry in a loblolly/longleaf pine Forest with interval burning. Ecol. Appl. 2, 157–164. doi: 10.2307/1941772
Braga, S. R., Oliveira, M. L. R. D., and Gorgens, E. B. (2023). Forestmangr: Forest mensuration and management. In (Version 0.9.6) Available online at: https://CRAN.R-project.org/package=forestmangr
Brunner, I., Bakker, M. R., Björk, R. G., Hirano, Y., Lukac, M., Aranda, X., et al. (2012). Fine-root turnover rates of European forests revisited: an analysis of data from sequential coring and ingrowth cores. Plant Soil 362, 357–372. doi: 10.1007/s11104-012-1313-5
Butler, O. M., Lewis, T., Rashti, M. R., and Chen, C. R. (2019). Energetic efficiency and temperature sensitivity of soil heterotrophic respiration vary with decadal-scale fire history in a wet sclerophyll forest. Soil Biol. Biochem. 134, 62–71. doi: 10.1016/j.soilbio.2019.03.022
Calkin, D. E., Thompson, M. P., and Finney, M. A. (2015). Negative consequences of positive feedbacks in US wildfire management. For. Ecosyst. 2, 1–10. doi: 10.1186/s40663-015-0033-8
Chapin, F. S., McFarland, J., David McGuire, A., Euskirchen, E. S., Ruess, R. W., and Kielland, K. (2009). The changing global carbon cycle: linking plant–soil carbon dynamics to global consequences. J. Ecol. 97, 840–850. doi: 10.1111/j.1365-2745.2009.01529.x
Chen, L., Smith, P., and Yang, Y. (2015). How has soil carbon stock changed over recent decades? Glob. Chang. Biol. 21, 3197–3199. doi: 10.1111/gcb.12992
Chojnacky, D. C., Heath, L. S., and Jenkins, J. C. (2014). Updated generalized biomass equations for north American tree species. Forestry 87, 129–151. doi: 10.1093/forestry/cpt053
Cieszewski, C. J., Zasada, M., Lowe, R. C., and Liu, S. (2021). Estimating biomass and carbon storage by Georgia Forest types and species groups using the FIA data diameters, basal areas, site indices, and Total Heights. Forests 12:141. doi: 10.3390/f12020141
Clark, D. A., Brown, S., Kicklighter, D. W., Chambers, J. Q., Thomlinson, J. R., and Ni, J. (2001). Measuring net primary production in forests: concepts and Field methods. Ecol. Appl. 11, 356–370. doi: 10.2307/3060894
Clark, K. L., Heilman, W. E., Skowronski, N. S., Gallagher, M. R., Mueller, E., Hadden, R. M., et al. (2020). Fire behavior, fuel consumption, and turbulence and energy exchange during prescribed fires in pitch pine forests. Atmos. 11:242. doi: 10.3390/atmos11030242
Coates, T. A., Hagan, D. L., Aust, W. M., Johnson, A., Keen, J. C., Chow, A. T., et al. (2018). Mineral soil chemical properties as influenced by long-term use of prescribed fire with differing frequencies in a southeastern coastal plain pine Forest. Forests 9:739. doi: 10.3390/f9120739
Cotrufo, M. F., Wallenstein, M. D., Boot, C. M., Denef, K., and Paul, E. (2013). The microbial efficiency-matrix stabilization (MEMS) framework integrates plant litter decomposition with soil organic matter stabilization: do labile plant inputs form stable soil organic matter? Glob. Chang. Biol. 19, 988–995. doi: 10.1111/gcb.12113
Davidson, E. A., Savage, K., Bolstad, P., Clark, D. A., Curtis, P. S., Ellsworth, D. S., et al. (2002). Belowground carbon allocation in forests estimated from litterfall and IRGA-based soil respiration measurements. Agric. For. Meteorol. 113, 39–51. doi: 10.1016/S0168-1923(02)00101-6
Davis, K. T., Peeler, J., Fargione, J., Haugo, R. D., Metlen, K. L., Robles, M. D., et al. (2024). Tamm review: a meta-analysis of thinning, prescribed fire, and wildfire effects on subsequent wildfire severity in conifer dominated forests of the Western US. For. Ecol. Manag. 561:121885. doi: 10.1016/j.foreco.2024.121885
Deng, M., Hu, S., Guo, L., Jiang, L., Huang, Y., Schmid, B., et al. (2023). Tree mycorrhizal association types control biodiversity-productivity relationship in a subtropical forest. Sci. Adv. 9:eadd4468. doi: 10.1126/sciadv.add4468
Ekblad, A., Mikusinska, A., Ågren, G. I., Menichetti, L., Wallander, H., Vilgalys, R., et al. (2016). Production and turnover of ectomycorrhizal extramatrical mycelial biomass and necromass under elevated CO2 and nitrogen fertilization. New Phytol. 211, 874–885. doi: 10.1111/nph.13961
Ekblad, A., Wallander, H., Godbold, D. L., Cruz, C., Johnson, D., Baldrian, P., et al. (2013). The production and turnover of extramatrical mycelium of ectomycorrhizal fungi in forest soils: role in carbon cycling. Plant Soil 366, 1–27. doi: 10.1007/s11104-013-1630-3
Espinosa, J., Madrigal, J., De La Cruz, A. C., Guijarro, M., Jimenez, E., and Hernando, C. (2018). Short-term effects of prescribed burning on litterfall biomass in mixed stands of Pinus nigra and Pinus pinaster and pure stands of Pinus nigra in the Cuenca Mountains (central-eastern Spain). Sci. Total Environ. 618, 941–951. doi: 10.1016/j.scitotenv.2017.08.291
Fenn, K. M., Malhi, Y., and Morecroft, M. D. (2010). Soil CO2 efflux in a temperate deciduous forest: environmental drivers and component contributions. Soil Biol. Biochem. 42, 1685–1693. doi: 10.1016/j.soilbio.2010.05.028
Ficken, C. D., and Wright, J. P. (2017). Effects of fire frequency on litter decomposition as mediated by changes to litter chemistry and soil environmental conditions. PLoS One 12:e0186292. doi: 10.1371/journal.pone.0186292
Fontúrbel, T., Carrera, N., Vega, J. A., and Fernández, C. (2021). The effect of repeated prescribed burning on soil properties: a review. Forests 12:767. doi: 10.3390/f12060767
Fox, T. R., Jokela, E. J., and Allen, H. L. (2007). The development of pine plantation Silviculture in the southern United States. J. For. 105, 337–347. doi: 10.1093/jof/105.7.337
Fox, S., Taylor, M. K., Callaham, M., and Jumpponen, A. (2024). Fire-excluded and frequently burned longleaf pine forests have contrasting soil microbial communities. For. Ecol. Manag. 551:121519. doi: 10.1016/j.foreco.2023.121519
Frey, S. D. (2019). Mycorrhizal fungi as mediators of soil organic matter dynamics. Annu. Rev. Ecol. Evol. Syst. 50, 237–259. doi: 10.1146/annurev-ecolsys-110617-062331
Friedlingstein, P., Joel, G., Field, C. B., and Fung, I. Y. (1999). Toward an allocation scheme for global terrestrial carbon models. Glob. Chang. Biol. 5, 755–770. doi: 10.1046/j.1365-2486.1999.00269.x
Frouz, J. (2024). Plant-soil feedback across spatiotemporal scales from immediate effects to legacy. Soil Biol. Biochem. 189:109289. doi: 10.1016/j.soilbio.2023.109289
Giardina, C. P., Litton, C. M., Crow, S. E., and Asner, G. P. (2014). Warming-related increases in soil CO2 efflux are explained by increased below-ground carbon flux. Nat. Clim. Chang. 4, 822–827. doi: 10.1038/nclimate2322
Giardina, C. P., and Ryan, M. G. (2002). Total belowground carbon allocation in a fast-growing Eucalyptus plantation estimated using a carbon balance approach. Ecosystems 5, 487–499. doi: 10.1007/s10021-002-0130-8
Godwin, D., Kobziar, L., and Robertson, K. (2017). Effects of fire frequency and soil temperature on soil CO2 efflux rates in old-Field pine-grassland forests. Forests 8:274. doi: 10.3390/f8080274
Gower, S. T., Vogt, K. A., and Grier, C. C. (1992). Carbon dynamics of Rocky Mountain Douglas-fir: influence of water and nutrient availability. Ecol. Monogr. 62, 43–65. doi: 10.2307/2937170
Hagenbo, A., Clemmensen, K. E., Finlay, R. D., Kyaschenko, J., Lindahl, B. D., Fransson, P., et al. (2017). Changes in turnover rather than production regulate biomass of ectomycorrhizal fungal mycelium across a Pinus sylvestris chronosequence. New Phytol. 214, 424–431. doi: 10.1111/nph.14379
Hagenbo, A., Piñuela, Y., Castaño, C., Martínez, J., de-Miguel, S., Alday, J., et al. (2021). Production and turnover of mycorrhizal soil mycelium relate to variation in drought conditions in Mediterraneanpinus pinaster, pinus sylvestrisandQuercus ilexforests. New Phytol. 230, 1609–1622. doi: 10.1111/nph.17012
Han, S. H., Yun, S., Lee, J., Kim, S., Chang, H., and Son, Y. (2016). Estimating the production and mortality of fine roots using minirhizotrons in a Pinus densiflora forest in Gwangneung, Korea. J. For. Res. 27, 1029–1035. doi: 10.1007/s11676-016-0221-6
Hart, S. C., Classen, A. T., and Wright, R. J. (2005). Long-term interval burning alters fine root and mycorrhizal dynamics in a ponderosa pine forest. J. Appl. Ecol. 42, 752–761. doi: 10.1111/j.1365-2664.2005.01055.x
Hatten, J. A., Zabowski, D., Ogden, A., and Thies, W. (2008). Soil organic matter in a ponderosa pine forest with varying seasons and intervals of prescribed burn. For. Ecol. Manag. 255, 2555–2565. doi: 10.1016/j.foreco.2008.01.016
Hendricks, J. J., Mitchell, R. J., Kuehn, K. A., and Pecot, S. D. (2016). Ectomycorrhizal fungal mycelia turnover in a longleaf pine forest. New Phytol. 209, 1693–1704. doi: 10.1111/nph.13729
Jandl, R., Lindner, M., Vesterdal, L., Bauwens, B., Baritz, R., Hagedorn, F., et al. (2007). How strongly can forest management influence soil carbon sequestration? Geoderma 137, 253–268. doi: 10.1016/j.geoderma.2006.09.003
Jenkins, J. C., Chojnacky, D. C., Heath, L. S., and Birdsey, R. A. (2003). National-scale biomass estimators for United States tree species. For. Sci. 49, 12–35. doi: 10.1093/forestscience/49.1.12
Kaarakka, L., Cornett, M., Domke, G., Ontl, T., and Dee, L. E. (2021). Improved forest management as a natural climate solution: a review. Ecol. Solut. Evid. 2:e12090. doi: 10.1002/2688-8319.12090
Kalies, E. L., and Kent, L. L. Y. (2016). Tamm review: are fuel treatments effective at achieving ecological and social objectives? A systematic review. For. Ecol. Manag. 375, 84–95. doi: 10.1016/j.foreco.2016.05.021
Keith, E. L. (2012). “Baseline establishment of thirty FIRE monitoring handbook (FMH)” in Vegetation plots at cook’s branch conservancy in montgomery county (Texas).
Kim, D., Baniya, B., Ono, M., and Noormets, A. (2025). Is drought the primary driver of the seasonality of carbon allocation in a subtropical pine forest? ESS Open Archive. doi: 10.22541/essoar.174775973.32248154/v1
Kindler, R., Siemens, J. A. N., Kaiser, K., Walmsley, D. C., Bernhofer, C., Buchmann, N., et al. (2011). Dissolved carbon leaching from soil is a crucial component of the net ecosystem carbon balance. Glob. Chang. Biol. 17, 1167–1185. doi: 10.1111/j.1365-2486.2010.02282.x
King, J. S., Allen, H. L., Dougherty, P., and Strain, B. R. (1997). Decomposition of roots in loblolly pine: effects of nutrient and water availability and root size class on mass loss and nutrient dynamics. Plant Soil 195, 171–184. doi: 10.1023/A:1004248232450
Kuzyakov, Y. (2010). Priming effects: interactions between living and dead organic matter. Soil Biol. Biochem. 42, 1363–1371. doi: 10.1016/j.soilbio.2010.04.003
Kuzyakov, Y., and Gavrichkova, O. (2010). REVIEW: time lag between photosynthesis and carbon dioxide efflux from soil: a review of mechanisms and controls. Glob. Chang. Biol. 16, 3386–3406. doi: 10.1111/j.1365-2486.2010.02179.x
Kuzyakov, Y., Horwath, W. R., Dorodnikov, M., and Blagodatskaya, E. (2019). Review and synthesis of the effects of elevated atmospheric CO2 on soil processes: no changes in pools, but increased fluxes and accelerated cycles. Soil Biol. Biochem. 128, 66–78. doi: 10.1016/j.soilbio.2018.10.005
Lawrence, D. M., Fisher, R. A., Koven, C. D., Oleson, K. W., Swenson, S. C., Bonan, G., et al. (2019). The community land model version 5: description of new features, benchmarking, and impact of forcing uncertainty. J. Adv. Model. Earth Syst. 11, 4245–4287. doi: 10.1029/2018MS001583
Lehmann, J., Hansel, C. M., Kaiser, C., Kleber, M., Maher, K., Manzoni, S., et al. (2020). Persistence of soil organic carbon caused by functional complexity. Nat. Geosci. 13, 529–534. doi: 10.1038/s41561-020-0612-3
Lenth, R. V. (2024). Emmeans: estimated marginal means, aka least-squares means. In (Version 1.10.0). Available online at: https://CRAN.R-project.org/package=emmeans
Liechty, H. O., and Reinke, M. (2020). The influence of repeated prescribed fire on decomposition and nutrient release in uneven-aged loblolly–shortleaf pine stands. Fire Ecol. 16:6. doi: 10.1186/s42408-019-0064-6
Liu, M., Zheng, S., Pendall, E., Smith, P., Liu, J., Li, J., et al. (2025). Unprotected carbon dominates decadal soil carbon increase. Nat. Commun. 16:2008. doi: 10.1038/s41467-025-57354-z
Maier, C. A., Albaugh, T. J., Lee Allen, H., and Dougherty, P. M. (2004). Respiratory carbon use and carbon storage in mid-rotation loblolly pine (Pinus taeda L.) plantations: the effect of site resources on the stand carbon balance. Glob. Chang. Biol. 10, 1335–1350. doi: 10.1111/j.1529-8817.2003.00809.x
Makkonen, K., and Helmisaari, H.-S. (1999). Assessing fine-root biomass and production in a scots pine stand-comparison of soil core and root ingrowth core methods. Plant Soil 210, 43–50. doi: 10.1023/A:1004629212604
Matosziuk, L. M., Alleau, Y., Kerns, B. K., Bailey, J., Johnson, M. G., and Hatten, J. A. (2019). Effects of season and interval of prescribed burns on pyrogenic carbon in ponderosa pine stands in the southern Blue Mountains, Oregon, USA. Geoderma 348, 1–11. doi: 10.1016/j.geoderma.2019.04.009
Mayer, M., Prescott, C. E., Abaker, W. E. A., Augusto, L., Cécillon, L., Ferreira, G. W. D., et al. (2020). Tamm review: influence of forest management activities on soil organic carbon stocks: a knowledge synthesis. For. Ecol. Manag. 466:118127. doi: 10.1016/j.foreco.2020.118127
McClaugherty, A. C., Aber, D. J., and Melillo, M. J. (1982). The role of fine roots in the organic matter and nitrogen budgets of two forested ecosystems. Ecology 63, 1481–1490. doi: 10.2307/1938874
Nadelhoffer, K. J., Raich, J. W., and Aber, J. (1998). A global trend in belowground carbon allocation: comment. Ecology 79, 1822–1825. doi: 10.1890/0012-9658(1998)079[1822:AGTIBC]2.0.CO;2
Noormets, A., Epron, D., Domec, J. C., McNulty, S. G., Fox, T., Sun, G., et al. (2015). Effects of forest management on productivity and carbon sequestration: a review and hypothesis. For. Ecol. Manag. 355, 124–140. doi: 10.1016/j.foreco.2015.05.019
Oliver, A. K., Callaham, M. A., and Jumpponen, A. (2015). Soil fungal communities respond compositionally to recurring frequent prescribed burning in a managed southeastern US forest ecosystem. For. Ecol. Manag. 345, 1–9. doi: 10.1016/j.foreco.2015.02.020
Ouimette, A., Guo, D., Hobbie, E., and Gu, J. (2013). Insights into root growth, function, and mycorrhizal abundance from chemical and isotopic data across root orders. Plant Soil 367, 313–326. doi: 10.1007/s11104-012-1464-4
Ouimette, A. P., Ollinger, S. V., Lepine, L. C., Stephens, R. B., Rowe, R. J., Vadeboncoeur, M. A., et al. (2020). Accounting for carbon flux to mycorrhizal Fungi may resolve discrepancies in Forest carbon budgets. Ecosystems 23, 715–729. doi: 10.1007/s10021-019-00440-3
Pan, Y., Birdsey, R. A., Fang, J., Houghton, R., Kauppi, P. E., Kurz, W. A., et al. (2011). A large and persistent carbon sink in the world’s forests. Science 333, 988–993. doi: 10.1126/science.1201609
Pellegrini, A. F. A., Caprio, A. C., Georgiou, K., Finnegan, C., Hobbie, S. E., Hatten, J. A., et al. (2021). Low-intensity frequent fires in coniferous forests transform soil organic matter in ways that may offset ecosystem carbon losses. Glob. Chang. Biol. 27, 3810–3823. doi: 10.1111/gcb.15648
Persson, H. (1980). Fine-root production, mortality and decomposition in Forest ecosystems. Vegetatio 41, 101–109. doi: 10.1007/BF00121422
Phillips, R. P., Finzi, A. C., and Bernhardt, E. S. (2011). Enhanced root exudation induces microbial feedbacks to N cycling in a pine forest under long-term CO2 fumigation. Ecol. Lett. 14, 187–194. doi: 10.1111/j.1461-0248.2010.01570.x
Posit team. (2024). RStudio: Integrated development environment for R. In (Version 2023.12.1.402) Posit Software, PBC. Available online at: http://www.posit.co/ (Accessed January 1, 2024).
Priest, J., Stovall, J., Coble, D., Oswald, B., and Williams, H. (2015). Loblolly pine growth patterns on reclaimed Mineland: Allometry, biomass, and volume. Forests 6, 3547–3581. doi: 10.3390/f6103547
R Core Team. (2024). R: A Language and Environment for Statistical Computing. In R Foundation for Statistical Computing. Available online at: https://www.R-project.org/ (Accessed January 1, 2024).
Santantonio, D., and Grace, J. C. (1987). Estimating fine-root production and turnover from biomass and decomposition data: a compartment–flow model. Can. J. For. Res. 17, 900–908. doi: 10.1139/x87-141
Schmidt, M. W. I., Torn, M. S., Abiven, S., Dittmar, T., Guggenberger, G., Janssens, I. A., et al. (2011). Persistence of soil organic matter as an ecosystem property. Nature 478, 49–56. doi: 10.1038/nature10386
Soares, M., and Rousk, J. (2019). Microbial growth and carbon use efficiency in soil: links to fungal-bacterial dominance, SOC-quality and stoichiometry. Soil Biol. Biochem. 131, 195–205. doi: 10.1016/j.soilbio.2019.01.010
Soil Survey Staff. Web soil survey. Natural Resources Conservation Service, United States Department of Agriculture. Available online at: http://websoilsurvey.nrcs.usda.gov/ (Accessed February 18, 2020).
Soldatova, E., Krasilnikov, S., and Kuzyakov, Y. (2024). Soil organic matter turnover: global implications from δ13C and δ15N signatures. Sci. Total Environ. 912:169423. doi: 10.1016/j.scitotenv.2023.169423
Sulman, B. N., Phillips, R. P., Oishi, A. C., Shevliakova, E., and Pacala, S. W. (2014). Microbe-driven turnover offsets mineral-mediated storage of soil carbon under elevated CO2. Nat. Clim. Chang. 4, 1099–1102. doi: 10.1038/nclimate2436
Tiller, M. B., Oswald, B. P., Frantzen, A. S., Conway, W. C., and Hung, I. (2017). Biomass estimations of invasives yaupon, Chinese privet and Chinese tallow in East Texas hardwood and pine ecosystems. Forest Res. 6:208. doi: 10.4172/21689776.1000208
Van't Hoff, J. H. (1898). “Lectures on theoretical and physical chemistry. Part I. Chemical dynamics (translated by R. A. Lehfeldt),” London: Edward Arnold. pp. 224–229.
Vargas, R., Paz, F., and de Jong, B. (2013). Quantification of forest degradation and belowground carbon dynamics: ongoing challenges for monitoring, reporting and verification activities for REDD+. Carbon Manag. 4, 579–582. doi: 10.4155/cmt.13.63
Vogt, K., and Persson, H. (1991). “Measuring growth and development of roots,” in Techniques and approaches in forest tree ecophysiology. eds. J. P. Lassoie and T. M. Hinckley (Boca Raton, Ann Arbor, Boston: CRC Press), 477–501.
Waldrop, T. A., and Goodrick, S. L. (2012). Introduction to prescribed fires in southern ecosystems. Science update SRS-054. Asheville, NC: US Department of Agriculture Forest Service, Southern Research Station. Available online at: https://www.srs.fs.usda.gov/pubs/su/su_srs054.pdf#page=8.15
Wang, C., and Kuzyakov, Y. (2023). Energy use efficiency of soil microorganisms: driven by carbon recycling and reduction. Glob. Chang. Biol. 29, 6170–6187. doi: 10.1111/gcb.16925
Wang, M., Tian, Q., Liao, C., Zhao, R., Wang, D., Wu, Y., et al. (2019). The fate of litter-derived dissolved organic carbon in forest soils: results from an incubation experiment. Biogeochemistry 144, 133–147. doi: 10.1007/s10533-019-00576-3
Western Regional Climate Center. (2024). Available online at: https://wrcc.dri.edu/cgi-bin/rawMAIN.pl?sdTCON (Accessed September 2, 2024).
Wieder, W. R., Hartman, M. D., Sulman, B. N., Wang, Y.-P., Koven, C. D., and Bonan, G. B. (2018). Carbon cycle confidence and uncertainty: exploring variation among soil biogeochemical models. Glob. Chang. Biol. 24, 1563–1579. doi: 10.1111/gcb.13979
Yin, H., Wheeler, E., and Phillips, R. P. (2014). Root-induced changes in nutrient cycling in forests depend on exudation rates. Soil Biol. Biochem. 78, 213–221. doi: 10.1016/j.soilbio.2014.07.022
Keywords: soil carbon balance, soil respiration, total belowground carbon flux, prescribed fire frequency, Pinus taeda, Pinus echinata
Citation: Ono M, Noormets A and Mitchell S (2025) The effect of the frequency of prescribed burning on annual soil carbon balance in a loblolly-shortleaf pine forest in East Texas. Front. For. Glob. Change. 8:1602557. doi: 10.3389/ffgc.2025.1602557
Edited by:
Anteneh Tesfaye Tekleyohannes, Ethiopian Environment and Forest Research Institute, EthiopiaReviewed by:
Anastasia Christopoulou, National and Kapodistrian University of Athens, GreeceMenassie Yimer, Ethiopian Forestry Development, Ethiopia
Copyright © 2025 Ono, Noormets and Mitchell. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Moeka Ono, bW9ub0B0YW11LmVkdQ==; Asko Noormets, bm9vcm1ldHNAdGFtdS5lZHU=
 Moeka Ono
Moeka Ono Asko Noormets
Asko Noormets Sarah Mitchell2
Sarah Mitchell2