Abstract
Introduction:
Compared with natural restoration, the recovery of soil ecological function in plantations is relatively lagging. Exogenous phosphorus (P) input and arbuscular mycorrhizal fungi (AMF) inoculation could eliminate or alleviate nutrient limitation in soil for plants. However, the differences in AMF community structure associated with P addition and AMF inoculation and their effects on soil nutrients remain unclear. The rhizosphere is vital for plant productivity and soil ecosystem function.
Methods:
Based on in situ P addition (Ca(H2PO4)2 fertilizer) and AMF inoculation (Rhizophagus intraradices inoculant) treatments in plantations in typical karst areas in Hunan Province, China, stratified rhizosphere soil samples of 0–15 and 15–30 cm were collected to determine alkaliolytic nitrogen (AN), available phosphorus (AP), soil organic carbon (SOC) contents, and AMF community composition. The corresponding non-rhizosphere soil samples were collected with cutting rings to determine their physical properties.
Results:
After P addition, AP contents in both the 0–15 and 15–30 cm layers increased significantly. In addition, AN contents in 0–15 and 15–30 cm layers and SOC content in the 0–15 cm layer increased, with no significant changes. After AMF inoculation, AP content in the 15–30 cm layer and SOC content in the 0–15 cm layer increased, with no significant changes. P addition and AMF inoculation increased AMF colonization intensity significantly in the roots, in turn altering the relative abundance in the AMF community and increasing Chao1 richness, Pielou’s evenness, and Simpson’s and Shannon’s diversity indices. Compared with the control, the relationships between soil nutrients and other properties varied after P addition and AMF inoculation. The effects of AMF community relative abundance and diversity on soil nutrients changed after P addition and AMF inoculation, with explanations for soil nutrient variations of the former both decreasing and the latter both increasing.
Discussion:
The results clarify the responses of rhizosphere soil properties to exogenous P input and AMF inoculation in plantations in karst areas. This can facilitate soil ecological function restoration and sustainable recovery of fragile ecosystems.
1 Introduction
As fragile ecological environments, karst areas are subject to various stresses, such as rocky desertification, land degradation, and water scarcity (Jiang et al., 2014). One of the largest continuous karst areas globally, the southwest karst area in China, has relatively high landscape heterogeneity and high-intensity human disturbance (Zhang et al., 2017). Exposed rocks, as well as shallow and discontinuous soils, are common (Hu et al., 2018). The degree of rocky desertification in Hunan Province ranks fourth in the southwest karst area, including eight provinces or cities of China. However, its management began later, i.e., in 2008 (Wei et al., 2018). One of the most effective ecological restoration methods, afforestation, can alter soil physicochemical properties and microbial community structure while enhancing ecosystem services (Hu et al., 2018; Lu et al., 2022; Wang et al., 2018). Against a background of ecosystem protection enhancement, major ecosystem restoration projects have been implemented to control rocky desertification in China over the years, which decreased the rocky desertification area in karst areas from 129,600 km2 in 2005 to 67,390 km2 in 2021, with the recovery effect attributed to vegetation restoration being remarkable (Tong et al., 2018).
Vegetation restoration modes are of great importance for maintaining and improving karst ecosystems’ stability. Compared with natural enclosures, artificial vegetation restoration promotes rapid community succession by improving soil nutrients, enzyme activity, and microbial biomass more effectively (Lu et al., 2022). However, compared with secondary or primary forests, soil function and ecosystem stability in plantations are relatively poor; therefore, proper interventions are important for the sustainability of degraded karst ecosystems. Nitrogen (N) and phosphorus (P) in soil are essential macronutrients required for plant development and participate in almost all major biochemical and metabolic processes (Liu et al., 2024; Zheng et al., 2023). In karst areas, soil N and P are key factors limiting vegetation growth, with forests being particularly P-limited (Zhang et al., 2015).
Exogenous P input is one of the most direct solutions to P deficiency as it increases available P in soils. However, the low-use efficiency and adverse environmental effects of P fertilizers are the main factors limiting their widespread application (Etesami et al., 2021). Arbuscular mycorrhizal fungi (AMF) can form symbioses with more than 80% of plant species on earth (Tedersoo et al., 2020), in turn increasing plant resistance to abiotic stress, improving mineral uptake (particularly P), enhancing water relations, and providing protection against soil-borne pathogens (Smith and Read, 2008; Wang and Rengel, 2024).
The effects of AMF on P uptake are well established. AMF prevent the available P from re-precipitating before plants can access it. AMF drive up to 80% of the total P uptake in plants (Xu et al., 2018; Yang et al., 2022). Symbiosis and its effects vary with the soil nutrient status (Jia et al., 2023; Xu et al., 2018). To date, relatively few studies have addressed the differences in AMF communities caused by P addition and AMF inoculation, which affect soil nutrients. Most previous studies have focused on pot experiments, and relatively few studies related to AMF inoculation have been conducted in karst areas based on field experiments. Therefore, how P addition and AMF inoculation influence soil AMF community composition and their effects on soil nutrients should be explored.
The rhizosphere, a narrow zone of soil surrounded and influenced by plant roots, has high numbers of microorganisms and invertebrates and is one of the most dynamic interfaces in the natural environment (Philippot et al., 2013). Therefore, based on in situ field experiments, rhizosphere soil samples were collected from plantations in typical karst rocky desertification areas in Hunan Province, China. The objectives of the present study were (1) to investigate the responses of plantation rhizosphere soil nutrients to P addition and AMF inoculation and (2) to quantify the relative contributions of factors influencing nutrient variations based on changes in AMF community structure in a karst rocky desertification area. Clarifying the responses of soil properties to external nutrient input and internal key microbial addition can help to break through the bottleneck of lagging soil ecological function restoration and support the sustainable recovery of fragile terrestrial ecosystems.
2 Materials and methods
2.1 Study area
Shaoyang County (110°40′–110°59′E, 26°40′–27°06′N) is located in the southwest of central Hunan Province, in the upper reaches of the Zijiang River. It borders Shaodong and Qidong Counties to the east, Dongan and Xinning Counties to the south, Wugang City and Longhui County to the west, and Xinshao County and Shaoyang City to the north. It is located in the transitional zone from the southwest edge of the Hengshao Hilly Basin to the mountains. The study area is located in the typical karst rocky desertification area of Lijiaping (Figure 1) in Shaoyang County. It is a typical soluble, hill-depressed landform. The altitude of the sampling site was approximately 400–585 m. The site is characterized by a subtropical monsoon climate with a mean annual temperature of 16.9 °C and a mean annual precipitation of 1,399 mm from 2000 to 2021. The parent material is mainly carbonate rock, and the soil type in karst areas is Calcisols (IUSS Working Group WRB, 2022). Vegetation restoration was implemented after agricultural abandonment in 2010. Vegetation cover was relatively low, mainly including trees such as Platycladus orientalis (L.) Franco, Pinus elliottii Engelm, and Liquidambar formosana Hance, as well as shrubs and grasses such as Vitex negundo var. cannabifolia (Sieb. and Zucc.) Hand., Miscanthus floridulus (Lab.) Warb. ex Schum. et Lau, and Imperata cylindrica (L.) P. Beauv.
Figure 1
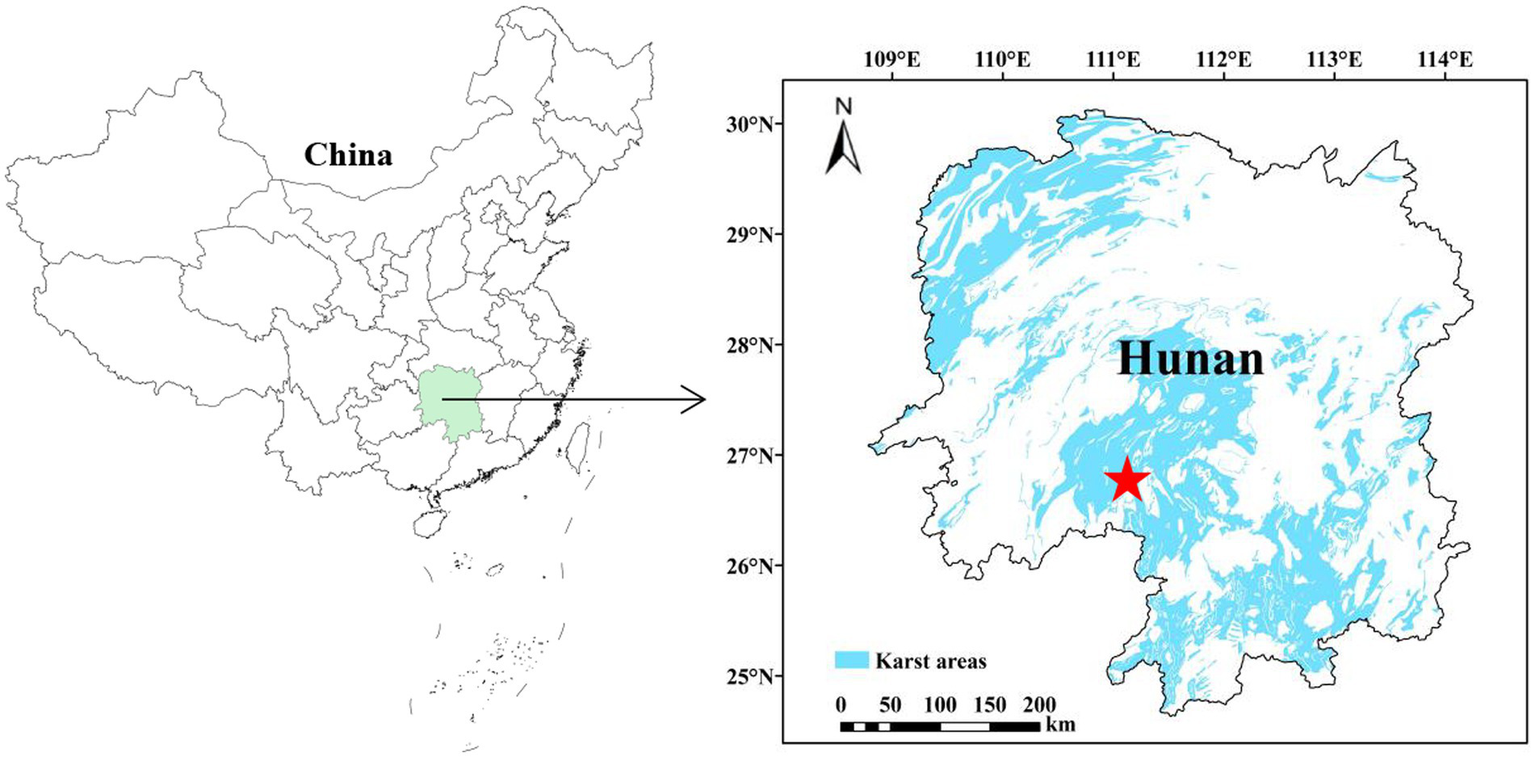
Location of the study area and soil sample collection.
2.2 Soil sampling
In March 2023, three sampling lines (repeats) were selected in a middle-aged (10–15 years) mixed plantation forest on a karst slope land, with an incline of approximately 35°. The mixed forest comprised Platycladus orientalis (L.) Franco, Pinus elliottii Engelm, and Liquidambar formosana Hance, and it was dominated by P. orientalis. The sampling line extended approximately 100 m from the top to the hillside of the slope. Samples were collected from the upper, middle, and lower slopes at approximately 50 m apart. Considering the horizontal extension of the roots, around the dominant trees (growing well and similarly), three 1 m × 1 m small sampling quadrats (three replicates) were selected randomly for P addition (+P), AMF inoculation (+AMF), and the control (CK) treatments in the upper, middle, and lower sampling plots. Plot sizes for the different line transects were set at 20 m × 20 m in the +P and +AMF treatments. After removing the litter, 100 g P fertilizer/m2 (approximately 20 g P/m2) and 60 g inoculant/m2 (approximately 3,000 spores/m2) were added to the soil. The litter was then returned to its original location. The P fertilizer used was Ca(H2PO4)2 with a purity of 98%, which was sprayed evenly onto the sampling quadrats after dissolving in pure water. The microbial inoculant was obtained from Guangxi Academy of Agricultural Sciences, Nanning, China, and the tested strain was Rhizophagus intraradices (Walker et al., 2021), which contained at least 50 spores/g. Three months later (June 2023), based on the soil adhering to roots after the shaking method (Yang et al., 2022), rhizosphere soil samples of the +P, +AMF, and CK groups were collected in stratified layers (0–15 and 15–30 cm), respectively. Cutting rings from the corresponding non-rhizosphere soils were also collected. A total of 54 rhizosphere soil samples and 54 cutting rings were collected. Part of the obtained rhizosphere soil samples, divided by a quarter method, were brought back to the lab and air-dried through a 2-mm screen for chemical analysis. Other small parts, wrapped in tin foil and placed in a sterilized cloth bag, were immediately placed in liquid nitrogen, then brought back to the lab for freeze-drying, grinding, and sub-packing, and eventually stored at −80 °C for microbial community composition analysis. Cutting rings of the soil were used to determine the soil’s physical properties.
2.3 Fine roots collection and determination of AMF colonization intensity
Considering the vertical extension of roots, along with soil sampling, fine roots of the dominant trees were collected in the 0–15 and 15–30 cm soil layers in different treatments (CK, +P, and + AMF) to determine AMF colonization intensity in the roots. The fine roots preserved in formaldehyde–acetic acid–ethanol fixative were cut into 1–2 cm segments, rinsed thoroughly with pure water, and then immersed in a 20% KOH solution for 2 h in a 60 °C water bath. Darker root samples were immersed in a 3% H2O2 solution for bleaching. They were then rinsed under running water for 5 min and acidified with 5% CH3COOH solution for 5 min. They were stained in a 60 °C water bath for 30 min using acetic ink. The samples were soaked in pure water for > 12 h for decolorization (Yang et al., 2010). A total of 20–30 root samples were selected randomly from each soil layer at different slope positions, and root colonization was observed under a microscope. The root segments were graded according to the degree of mycorrhizal colonization, and the number of root segments in the corresponding grade was recorded. The details were as follows:
N1, colonization rate > 90%; N2, colonization rate of 50–90%; N3, colonization rate of 10–50%; N4, colonization rate of 1–10%; N5, colonization rate < 1%; and N, total number of root segments.
Based on the weighted method of the root segment colonization rate proposed by Biermann and Linderman (1981), AMF colonization intensity (Equation 1) in the roots was calculated using the following formula:
2.4 Microbial composition and diversity analysis
Soil DNA was extracted from each soil sample (0.5 g) using a MagBeads FastDNA Kit for Soil (MP Biomedicals, Thomas Irvine, CA, USA). The extracted DNA was subjected to 0.8% agarose gel electrophoresis for molecular size determination, followed by quantification using a Nanodrop. A PCR instrument (Bio-Rad S1000; Bio-Rad Laboratories, Hercules, CA, USA) was used to amplify the AMF gene. The primers used were AMV4.5NF (5′-AAGCTCGTAGTTGAATTTCG-3′) and AMDGR (5′-CCCAACTATCCCTATTAATCAT-3′), with a length of approximately 280 bp (Xiao et al., 2019). The PCR reaction used NEB Q5 high-fidelity DNA polymerase, including components such as 5 × reaction buffer (5 μL), 5 × GC buffer (5 μL), dNTPs (2.5 mM; 2 μL), forward primer (10 μM; 1 μL), reverse primer (10 μM; 1 uL), DNA template (2 μL), ddH2O (8.75 μL), and Q5 high-fidelity DNA polymerase (0.25 μL). The PCR cycle started with a pre-denaturation at 98 °C for 3 min, followed by denaturation at 98 °C for 30 s, annealing at 52 °C for 30 s, and extension at 72 °C for 45 s. The cycle was then repeated 26 times. A final extension was then performed at 72 °C for 5 min, and the product was stored at 12 °C. Amplification results were subjected to 2% agarose gel electrophoresis, and the target fragments were recovered using an Axygen Gel Recovery Kit (Axygen Inc., Union City, CA, USA). The PCR products were quantified using a Quant-iT PicoGreen dsDNA Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) on a microplate reader (BioTek, FLx800, Agilent Technologies, Santa Clara, CA, USA), and the samples were mixed according to the required data volume for each sample. The amplified products were sequenced on the NovaSeq 6000 (Illumina Inc., San Diego, CA, USA) platform. In the present study, the diversity of AMF community structure was characterized by Chao1 richness, Pielou’s evenness, and Simpson’s and Shannon’s diversity indices (Simpson, 1949; Xu et al., 2018).
2.5 Analysis of soil physicochemical properties
Soil alkaliolytic nitrogen (AN) was determined using the alkaliolytic diffusion method. Available phosphorus (AP) was determined using the 0.5 mol/L NaHCO3 extraction method. Soil organic carbon (SOC) was measured by wet oxidation using the dichromate redox colorimetric method (Bao, 2000). Soil pH was determined using a digital pH meter at a water/soil ratio of 1:2.5. Soil cation exchange capacity (Ca2+) was determined using the ammonium acetate exchange method and measured by X-ray fluorescence spectrometry (ICP-OES Optima 7,000 DV, PerkinElmer, USA) (Liu et al., 2024). Physical properties, such as soil volumetric water content (VWC), bulk density (BD), capillary porosity (CP), and non-capillary porosity (NCP), were measured using the cutting ring method. Total porosity (TP) was the sum of CP and NCP. Soil aeration (SA) was defined as the difference between TP and VWC. Soil porosity ratio (SP) was defined as the ratio of TP/(1-TP).
2.6 Statistical analysis
Data were checked for normality and homogeneity of variance and, if necessary, were transformed. A one-way analysis of variance (ANOVA) with the least significant difference test at a p-value of < 0.05 was used to determine whether there was a significant difference between any two datasets. Pearson’s correlation analysis at a 95% confidence level was used to determine the relationships among soil properties. Redundancy analysis (RDA) was performed to explore the effects of other soil properties on soil nutrients and to determine their relative contributions. Considering the values of relative abundances of AMF community composition, only four genera, Paraglomus, Glomus, Ambispora, and Claroideoglomus (average values ≥ 1%), were counted in the correlation analysis and RDA. IBM SPSS Statistics 21.0 (IBM Corp., Armonk, NY, USA) and Origin 8.0 (Originlab Inc., Northampton, MA, USA) for Windows were used for statistical analysis and visualization, respectively.
3 Results
3.1 Responses of rhizosphere soil nutrients to P addition and AMF inoculation
In the study area, the average contents of AN, AP, and SOC in 0–15 and 15–30 cm layers were 105.1 mg/kg, 1.5 mg/kg, and 17.6 g/kg, respectively, and 104.6 mg/kg, 1.3 mg/kg, and 15.9 g/kg, respectively (Figure 2, CK).
Figure 2
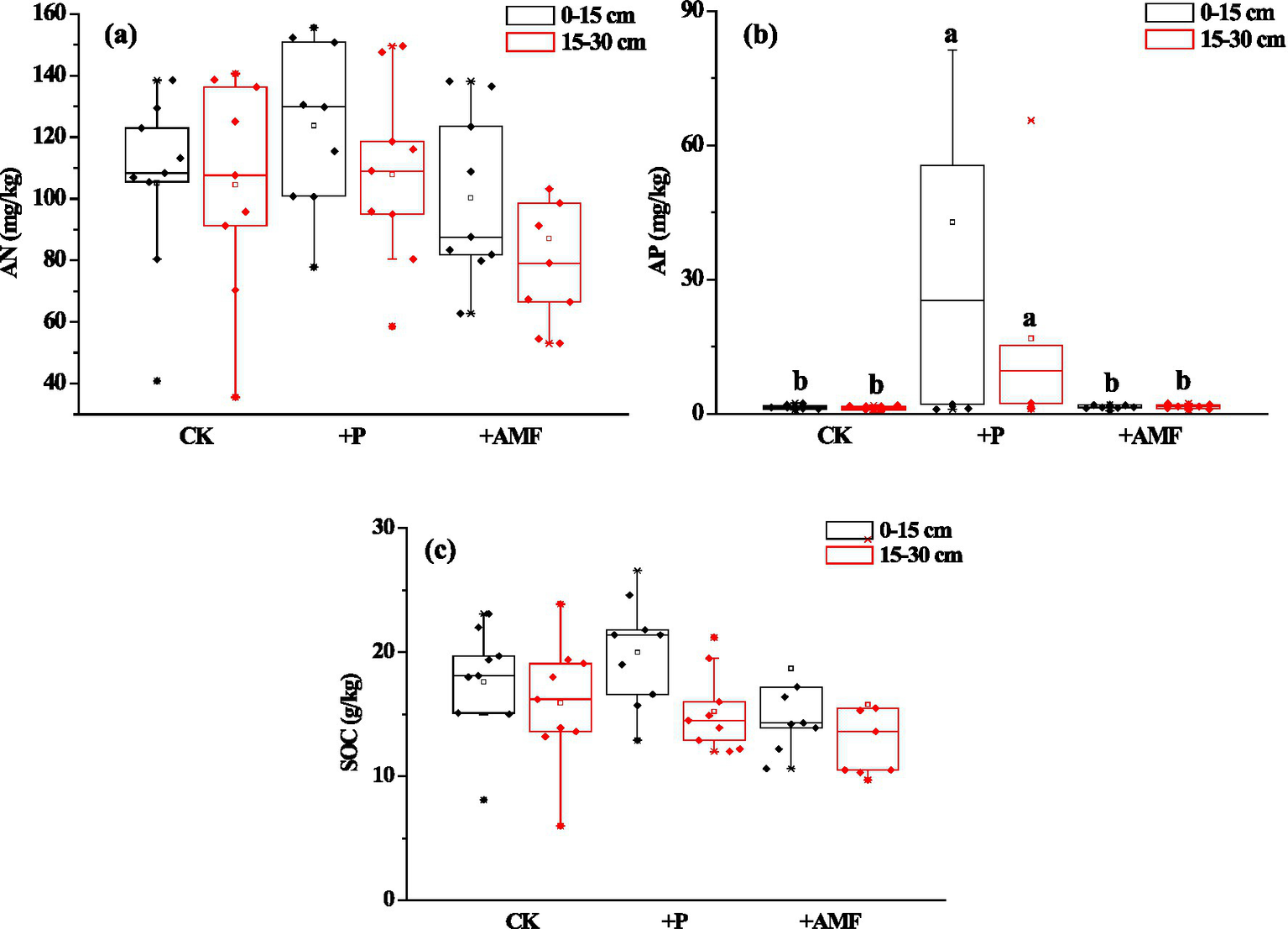
(a) AN, (b) AP, and (c) SOC contents of different soil layers in different treatments. Data plot is a box and the data overlap. N = 9. Different letters represent significant differences between treatments in the same soil layer at a level of p < 0.05. CK, the control; +P, P addition; +AMF, AMF inoculation.
Compared with the control, the average AN contents in the 0–15 and 15–30 cm layers both increased after P addition. In addition, they decreased after AMF inoculation, with all the differences being non-significant (p > 0.05) (Figure 2a). In addition, the changes in the 15–30 cm layer was larger than that in the 0–15 cm. After P addition, the average contents of AP in 0–15 and 15–30 cm layers both increased significantly (p < 0.05). In addition, after AMF inoculation, the average AP content in the 15–30 cm layer also increased, although the change was non-significant (Figure 2b). After P addition and AMF inoculation, the average SOC contents increased in the 0–15 cm soil layer but decreased in the 15–30 cm soil layer, with no significant changes (Figure 2c).
3.2 Changes in rhizosphere soil AMF community related to P addition and AMF inoculation
3.2.1 AMF colonization intensity in fine roots
In the 0–15 cm soil layer, AMF colonization intensities in fine roots after P addition and AMF inoculation were significantly higher than those in the control. A similar pattern was observed in the 15–30 cm soil layer (Figure 3). After AMF inoculation, AMF colonization intensity in the 0–15 cm layer was significantly higher than that in the 15–30 cm.
Figure 3
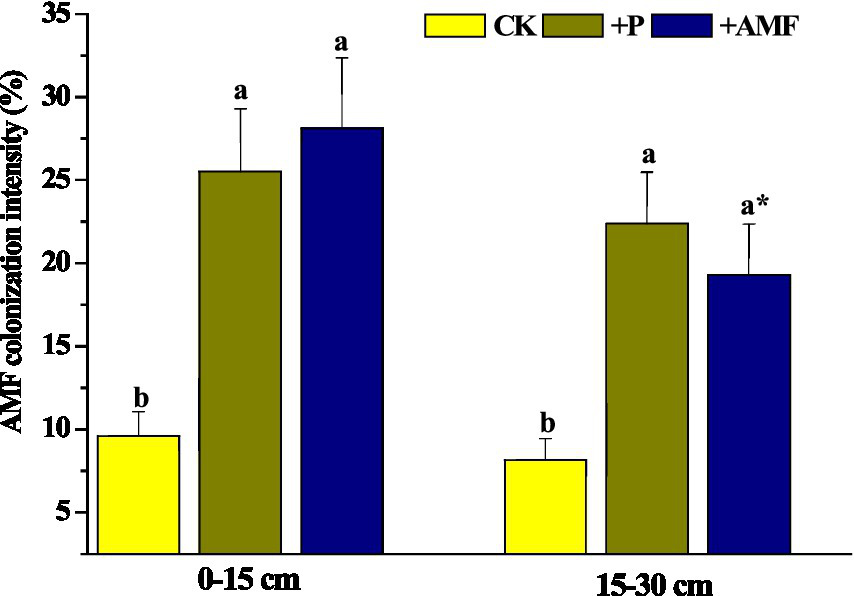
AMF colonization intensity of roots under different treatments. Different letters represent significant differences between treatments in the same soil layer at a level of p < 0.05. * Represents significant differences in the same treatment between the two soil layers at a level of p < 0.05. CK, the control; +P, P addition; +AMF, AMF inoculation.
3.2.2 Changes in the relative abundances of different genera in AMF community
Eight genera were identified in the AMF community in the study area, namely, Paraglomus, Glomus, Ambispora, Claroideoglomus, Archaeospora, Scutellospora, Diversispora, and Acaulospora, among which Paraglomus, Glomus, and Ambispora were the dominant genera (Figure 4).
Figure 4
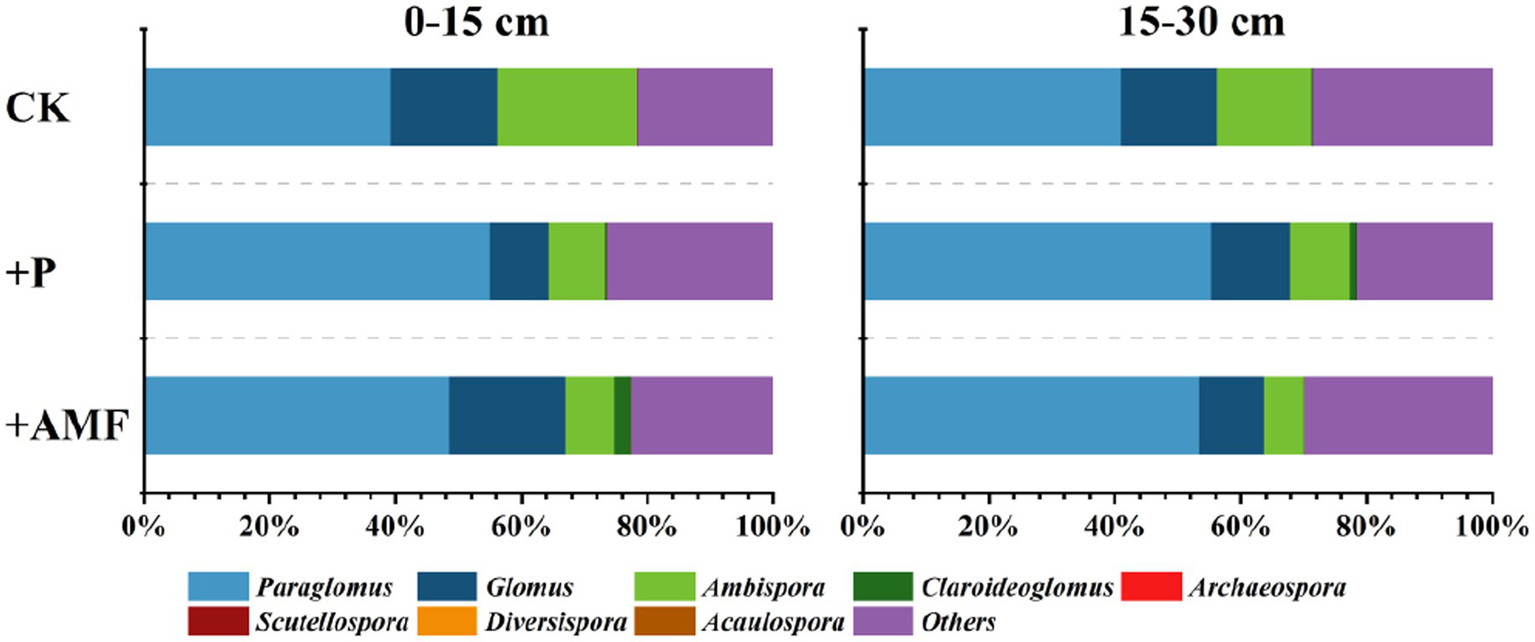
Changes in relative abundances of different genera in AMF community composition in different soil layers after P addition and AMF inoculation.
After P addition, the relative abundance of Paraglomus increased. Meanwhile, those of Glomus and Ambispora decreased at both the 0–15 and 15–30 cm depths. In contrast, after AMF inoculation, the relative abundance of Glomus increased in the 0–15 cm layer, whereas that in the 15–30 cm layer decreased. Similarly, the relative abundance of Paraglomus increased in the 0–15 and 15–30 cm soil layers after AMF inoculation.
3.2.3 Changes in AMF community diversity indices
Overall, compared with the control, all diversity indices increased at both the 0–15 and 15–30 cm depths (Figure 5). After P addition, the Chao1 richness index at 0–15 and 15–30 cm layers and Shannon diversity index at the 0–15 cm layer were significantly (p < 0.05) higher than those of the control. In addition, after AMF inoculation, the Chao1 richness index at the 0–15 and 15–30 cm layers, Pielou’s evenness, and Simpson’s and Shannon’s diversity indices in the 0–15 cm layer increased significantly (p < 0.05).
Figure 5
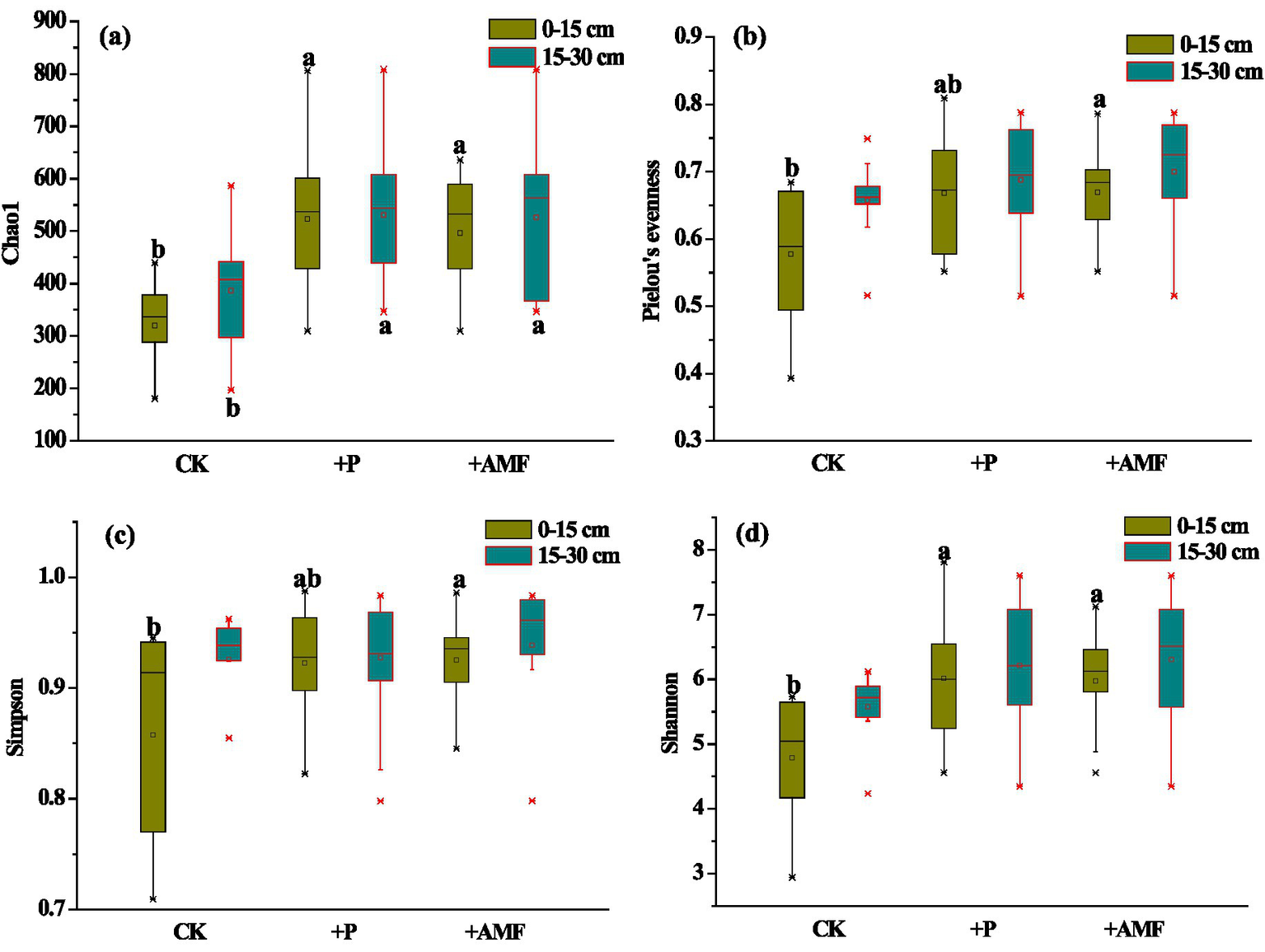
Changes in diversity indices: (a) Chao1 richness, (b) Pielou’s evenness, (c) Simpson, and (d) Shannon diversity index of AMF community in different soil layers after P addition and AMF inoculation. N = 9. Different letters represent significant differences between treatments in the same soil layer at a level of p < 0.05.
3.3 Factors influencing rhizosphere soil nutrients related to P addition and AMF inoculation
The pH, exchangeable Ca2+ content, physical properties such as VWC, BD, NCP, CP, TP, SA, and SP of non-rhizosphere soil (as shown in Table 1), and AMF community composition and structure such as diversity indexes and relative abundances of the four genera (average values ≥ 1%) of rhizosphere soil were explored in the present study.
Table 1
| Treatment/Index | pH | Ca2+ | VWC | BD | NCP | CP | TP | SA | SP |
|---|---|---|---|---|---|---|---|---|---|
| CK | 5.9 ± 0.5a | 2.3 ± 0.3b | 0.11 ± 0.01c | 1.12 ± 0.08a | 0.07 ± 0.01a | 0.38 ± 0.03a | 0.45 ± 0.03a | 0.34 ± 0.03a | 0.82 ± 0.10a |
| +P | 5.8 ± 0.3a | 2.4 ± 0.3b | 0.12 ± 0.01b | 1.12 ± 0.07a | 0.04 ± 0.01b | 0.37 ± 0.04a | 0.42 ± 0.04b | 0.30 ± 0.04b | 0.72 ± 0.13b |
| +AMF | 5.6 ± 0.5a | 2.7 ± 0.3a | 0.13 ± 0.02a | 1.12 ± 0.10a | 0.04 ± 0.01b | 0.38 ± 0.03a | 0.42 ± 0.03b | 0.29 ± 0.03b | 0.72 ± 0.10b |
Other chemical properties of rhizosphere soil and physical properties of non-rhizosphere soil in different treatments.
Data are mean ± SD; N = 18; CK, the control; +P, P addition; +AMF, AMF inoculation. Ca2+ (g/kg), content of exchangeable Ca2+; VWC, volumetric water content; BD (g/cm3), bulk density; NCP, non-capillary porosity; CP, capillary porosity; TP, total porosity; SA, soil aeration; SP, ratio of soil porosity. Different letters represent significant differences between treatments at a level of p < 0.05.
3.3.1 Relationships among soil properties
Compared with the control, the relationship between AN and AP changed after P addition and AMF inoculation, with no significant correlation (Figure 6). The relationships between pH, AMF community composition, such as the relative abundance of Claroideoglomus, and physical properties, such as CP, TP, and SA, changed significantly. In contrast, after AMF inoculation, the correlation coefficients between AMF community composition and some physical properties were weaker, but the directions also changed. The relationships between soil nutrients and other soil properties changed directly or indirectly after P addition and AMF inoculation.
Figure 6
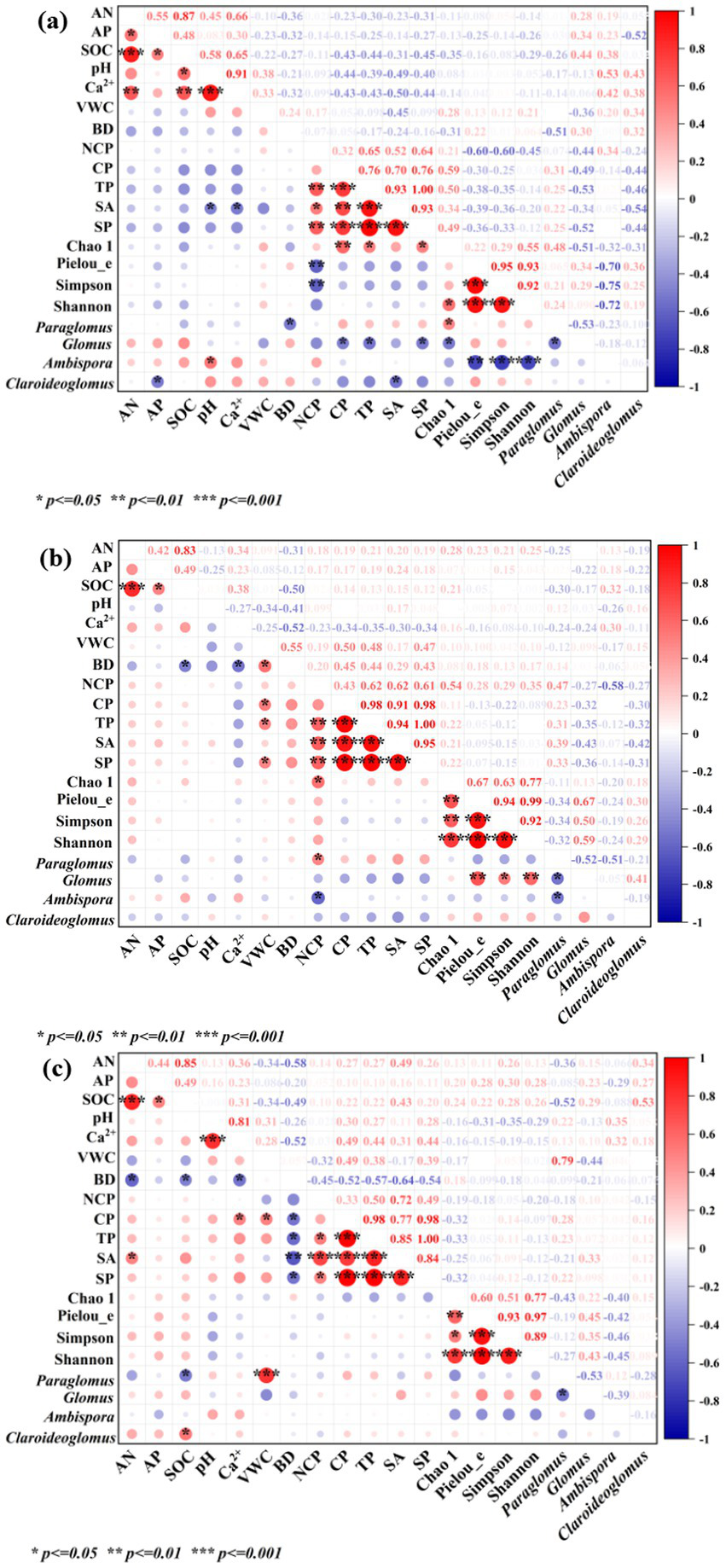
Relationships between soil nutrients and other soil properties in different treatments: (a) CK, (b) P addition, and (c) AMF inoculation. AN, alkaliolytic nitrogen; AP, available phosphorus; SOC, soil organic carbon; VWC, volumetric water content; BD, bulk density; NCP, non-capillary porosity; CP, capillary porosity; TP, total porosity; SA, soil aeration; SP, ratio of soil porosity; Paraglomus, Glomus, Ambispora, and Claroideoglomus were the relative abundance values.
3.3.2 Effects of influencing factors on soil nutrient variations
Compared with the control, the most prominent changes were the effects of the relative abundances and diversity indices of AMF community on soil nutrients after P addition and AMF inoculation (Figure 7). Similarly, the effect directions of Chao1 richness, Pielou’s evenness, and Simpson’s diversity indices on AN, AP, and SOC changed after P addition and AMF inoculation. In contrast, the effect of the relative abundance of Glomus on soil nutrients was negative after P addition. In addition, the effect directions of the relative abundances of Ambispora and Claroideoglomus both changed after AMF inoculation.
Figure 7
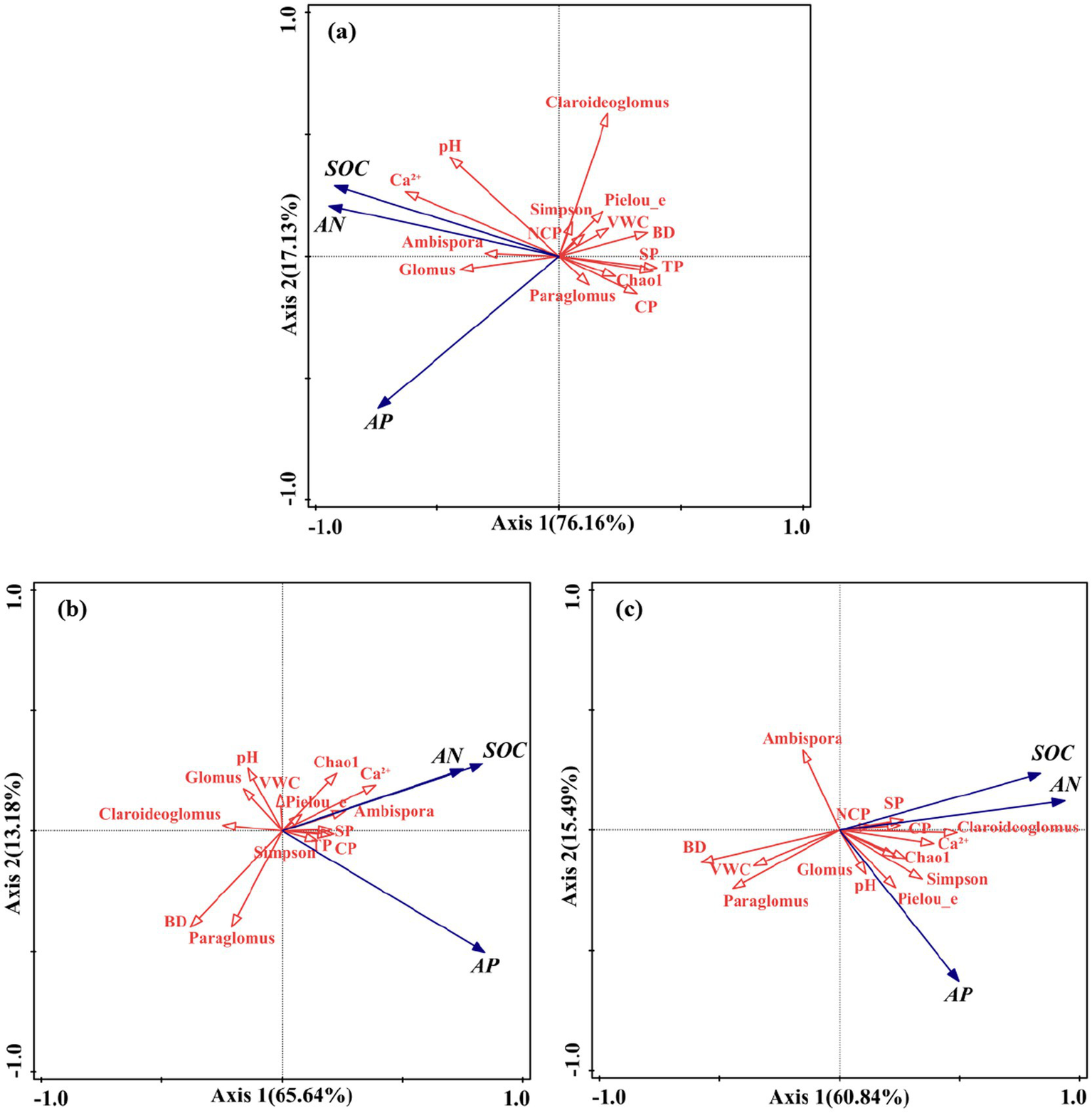
Redundancy analysis (RDA) of other soil properties influencing soil nutrients in different treatments: (a) CK, (b) P addition, and (c) AMF inoculation.
Compared with the control, the total variance in soil nutrients explained by other soil properties decreased after both P addition and AMF inoculation (Figure 8). The explanation of the relative abundances of AMF community composition decreased, while that of Chao1 richness, Pielou’s evenness, and Simpson’s diversity indices increased after P addition and AMF inoculation. Explanations of exchange Ca2+ for soil nutrient variations decreased obviously both after P addition and AMF inoculation, with values shifting from 31.5 to 2.0 and 3.5%, respectively.
Figure 8
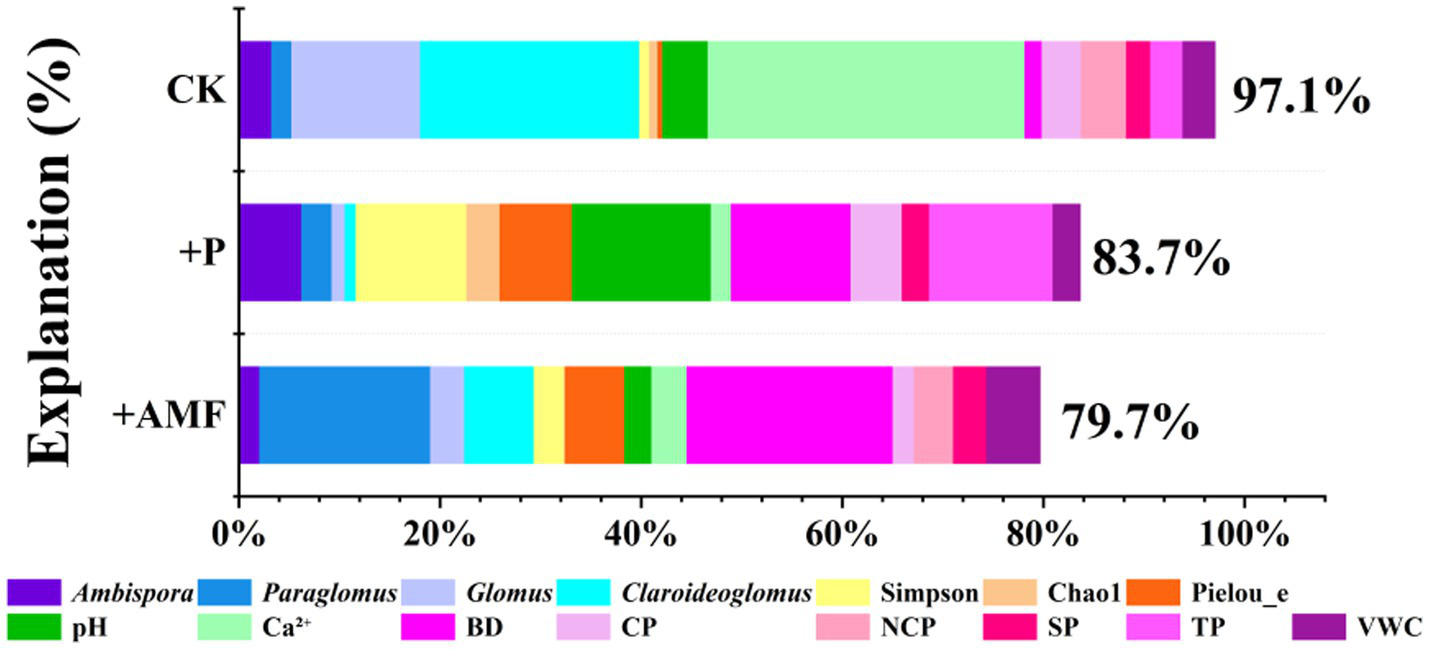
Explanations of other soil properties for soil nutrient variations under different treatments.
4 Discussion
4.1 Rhizosphere soil nutrients in karst areas
Compared to non-karst soils, the development of karst soils, that is, calcareous soils, is closely related to rock dissolution (Bonacci, 2009). Its dual surface and underground spatial structure, with shallow soil and low water storage capacity, make soil nutrients the key factors limiting vegetation growth (Jiang et al., 2014). Soil N and P have been demonstrated to be the key nutrients limiting vegetation growth in terrestrial ecosystems (Liu et al., 2024; Zheng et al., 2023). However, in karst ecosystems, soil N may no longer limit plants in later vegetation succession, such as secondary and primary forests, and it is only deficient during the initial stage of vegetation restoration (Song et al., 2019; Zhang et al., 2015). The rhizosphere is complex and dynamic, and understanding its ecology and development is key to enhancing plant productivity and ecosystem functioning (Philippot et al., 2013). Therefore, in the present study, rhizosphere soil nutrients such as AN and AP (Figures 2a,b, CK) were studied in plantations in karst areas. The results showed that the AN content was in the range of 35.6–140.6 mg/kg, whereas the AP content was in the range of 0.3–2.4 mg/kg. Compared to bulk soil, it is more vital to clarify the nutrient status of rhizosphere soil for vegetation restoration in vulnerable areas.
Rhizosphere soil AN, with average contents being 106.7 and 104.8 mg/kg in the 0–15 and 15–30 cm layers, respectively (Figure 2a, CK), was lower than those in other subtropical karst or non-karst forests (Lu et al., 2022; Zhang et al., 2014, 2015). This finding indicated that soil N was still the limiting nutrient in the study area. As an essential plant nutrient, P is required for C metabolism, N fixation, energy generation and transfer, enzyme activation, membrane formation, and key biological molecules (Schachtman et al., 1998). Soil P is an essential element limiting plant growth, approximately 30–65% of which is present in organic form and cannot be used directly by plants. In the present study, the average contents of rhizosphere soil AP were 1.5 and 1.3 mg/kg at the 0–15 and 15–30 cm layers, respectively (Figure 2b, CK), which ranked sixth (last) (<3 mg/kg) in the nutrient grading standard of the Second National Soil Census in China. Compared to other subtropical forests in karst and non-karst areas (Liu et al., 2023; Lu et al., 2022; Zhang et al., 2014, 2015), AP was extremely deficient. The SOC contents at the 0–15 and 15–30 cm layers were 17.6 and 15.9 g/kg in the present study, respectively (Figure 2c, CK), which ranked fourth (10–20 g/kg) in the nutrient grading standard of the Second National Soil Census in China. In karst areas, previous studies have indicated that SOC content is neither lower nor higher than that in some non-karst areas (Hu et al., 2018; Wang et al., 2018). The low SOC stock was attributed to the shallow soil layer.
4.2 Effect of P addition on soil nutrients
In calcareous soils, the strong adsorption and precipitation of P by calcium can result in P insufficiency (Hinsinger, 2001). Therefore, P availability is a major factor limiting vegetation recovery (Zhang et al., 2015). P fertilizers are generally recommended for the management of soil P deficiency. As shown in this study, the AP contents at the 0–15 and 15–30 cm layers increased significantly after P addition (Figure 2b). However, more than 80% of the P fertilizer applied to the soil is lost due to the adsorption and fixation processes (Vance et al., 2003) or is transformed into organic forms (Holford, 1997), which represent 40–80% of the total P in soil (Bünemann et al., 2010). Therefore, the availability of P added to plants is limited, comprising approximately 0.1% of the total P (Etesami et al., 2021). In the present study, more than 20 g P/m2 was added to the soil. However, the increases in AP content at the 0–15 and 15–30 cm layers were 41.3 and 15.5 mg/kg, respectively, with a low use efficiency.
Exogenous P input also affected soil C and N. As shown in the present study, contents of SOC at the 0–15 cm depth and AN at the 0–15 and 15–30 cm depths increased after P addition (Figures 2a,c). Most studies have demonstrated that the soil microbial community structure is sensitive to changes in soil properties caused by the fertilizer additions (Allison and Martiny, 2008; Liu et al., 2021; Tian et al., 2022; Xiao et al., 2019). For example, Xiao et al. (2019) observed that AMF diversity was sensitive to P addition, whereas AMF abundance was sensitive to N addition in karst grasslands. In the present study, soil AMF community composition and structure also changed after P addition, with prominent changes in the relative abundances of Paraglomus, Glomus, and Ambispora (Figure 4) and increases in diversity indices (Figure5). In calcareous soils, microbial community dynamics were reported to be significantly correlated with exchangeable Ca2+ and AP contents during vegetation degradation (Tang et al., 2019). Such changes have a significant influence on C and N bio-geochemistry. Therefore, in the present study, P addition increased AP content (Figure 2) directly and then influenced the AMF community directly or indirectly (Figure 4), ultimately affecting AN and SOC contents (Figures 2a,c).
4.3 Effect of AMF inoculation on soil nutrients
Arbuscular mycorrhizal fungi colonize the roots of host plants and promote plant growth owing to the improved nutrient uptake (Emmett et al., 2021; Farzaneh et al., 2011), especially in nutrient-deficient areas. AMF are widespread in subtropical forests. In karst areas, Glomus is the most likely dominant genus (Xiao et al., 2019). In the present study, Paraglomus, Glomus, and Ambispora were the dominant genera (Figure 4). AMF inoculation can directly affect the symbiosis with plants by increasing the colonization rate in roots and changing microbial activity, community composition, and biomass (Etesami et al., 2021; Xu et al., 2018). As demonstrated in the present study, compared with the control, AMF colonization intensity in roots increased significantly (Figure 3), and the relative abundances of different genera in the AMF community differed after inoculation (Figure 4). Furthermore, changes in the microbe-related inoculation can affect other soil properties, such as nutrients. In the present study, although not all changes in AN, AP, and SOC were significant after AMF inoculation, the contents of AP at the 15–30 cm layer and SOC at the 0–15 cm layer increased compared to the control (Figures 2b,c). The increase in AP content was consistent with previous studies, indicating that inoculation could reduce soil P consumption during vegetation growth by releasing more AP. However, the decrease in AN content at the 0–15 and 15–30 cm depths after AMF inoculation may be due to expanded mycorrhizal fungal hyphae, which can facilitate plants in absorbing more nutrients from the soil. Soil C is closely related to N; larger changes in AN content in the 15–30 cm layer may lead to a decrease in SOC content after AMF inoculation. Changes in SOC content indicate that AMF play a crucial role in below-ground C dynamics. They facilitate soil N and P uptake in host plants in exchange for C (Zheng et al., 2022).
5 Conclusion
In the study area, only the rhizosphere soil AP contents at the 0–15 and 15–30 cm layers increased significantly after P addition. In addition, AN and SOC contents in the 0–15 cm layer increased, with no significant changes. After AMF inoculation, only the contents of AP in the 15–30 cm layer and SOC in the 0–15 cm layer increased, with no significant changes. P addition and AMF inoculation increased AMF colonization intensity in the roots significantly. They altered the relative abundance of AMF community composition, which resulted in an increase in Chao1 richness, Pielou’s evenness, and Simpson’s and Shannon’s diversity indices. Chao1 richness index at the two soil layers and Shannon diversity index at the 0–15 cm layer increased significantly after P addition and AMF inoculation, whereas Pielou’s evenness and Simpson’s diversity indices in the 0–15 cm layer increased significantly only after AMF inoculation. Compared with the control, the relationships between soil nutrients and other soil properties varied after P addition and AMF inoculation. The effects of AMF relative abundance and diversity on soil nutrients changed after P addition and AMF inoculation, with the variation explained by relative abundance decreasing and the variation explained by diversity increasing. The results clarified the response characteristics of soil properties to external P input and internal AMF inoculation, which could provide a theoretical basis for the enhancement of soil ecological functions and support the sustainable recovery of karst vulnerable ecosystems.
Statements
Data availability statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author contributions
MW: Formal analysis, Funding acquisition, Software, Writing – original draft, Writing – review & editing. YH: Data curation, Formal analysis, Software, Writing – original draft. QL: Formal analysis, Software, Writing – original draft. XH: Formal analysis, Software, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by the National Natural Science Foundation of China [grant no. 42101316], the Natural Science Foundation of Hunan Province [grant no. 2022JJ40866], Outstanding Youth Project of Department of Education, Hunan Province [grant no. 20B613], and the Open Research Fund of Technology Innovation Center for Ecological Conservation and Restoration in Dongting Lake Basin, Ministry of Natural Resources [grant no. 2023007].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Gen AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1
Allison S. D. Martiny J. B. H. (2008). Resistance, resilience, and redundancy in microbial communities. PNAS105, 11512–11519. doi: 10.1073/pnas.0801925105
2
Bao S. D. (2000). Soil and agricultural chemistry analysis. 3rd Edn. Beijing: China Agriculture Publishing House.
3
Biermann B. Linderman R. G. (1981). Quantifying vesicular-arbuscular mycorrhizas: a proposed method towards standardization. New Phytol.87, 63–67. doi: 10.1111/j.1469-8137.1981.tb01690.x
4
Bonacci O. (2009). Karst landscape ecohydrology. In:Proceedings of International Symposium on Water Management and Hydraulic Engineering (Ohrid), p. 781–790.
5
Bünemann E. K. Oberson A. Frossard E. (2010). Phosphorus in action: Biological processes in soil phosphorus cycling. Berlin: Springer Science & Business Media.
6
Emmett B. Lévesque-Tremblay V. Harrison M. (2021). Conserved and reproducible bacterial communities associate with extraradical hyphae of arbuscular mycorrhizal fungi. ISME J.15, 2276–2288. doi: 10.1038/s41396-021-00920-2
7
Etesami H. Jeong B. R. Glick B. R. (2021). Contribution of arbuscular mycorrhizal fungi, phosphate-solubilizing bacteria, and silicon to P uptake by plant. Front. Plant Sci.12:699618. doi: 10.3389/fpls.2021.699618
8
Farzaneh M. Vierheilig H. Loessl A. Kaul H. P. (2011). Arbuscular mycorrhiza enhances nutrient uptake in chickpea. Plant Soil Environ.57, 465–470. doi: 10.17221/133/2011-PSE
9
Hinsinger P. (2001). Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil237, 173–195. doi: 10.1023/A:1013351617532
10
Holford I. C. R. (1997). Soil phosphorus: its measurement, and its uptake by plants. Soil Res.35, 227–240. doi: 10.1071/s96047
11
Hu P. L. Liu S. J. Ye Y. Y. Zhang W. Wang K. L. Su Y. R. (2018). Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev.29, 387–397. doi: 10.1002/ldr.2876
12
IUSS Working Group WRB (2022). World reference base for soil resources. International soil classification system for naming soils and creating legends for soil maps. 4th Edn. Vienna, Austria: International Union of Soil Sciences (IUSS).
13
Jia T. Zhang Y. Yao Y. S. Wang Y. Liang X. L. Zheng M. Y. et al . (2023). Effects of AMF inoculation on the eco-physiological characteristics of Imperata cylindrica under differing soil nitrogen conditions. Front. Plant Sci.14:1134995. doi: 10.3389/fpls.2023.1134995
14
Jiang Z. C. Lian Y. Q. Qin X. Q. (2014). Rocky desertification in Southwest China: impacts, causes, and restoration. Earth-Sci. Rev.132, 1–12. doi: 10.1016/j.earscirev.2014.01.005
15
Liu M. H. Shen Y. K. Li Q. Xiao W. F. Song X. Z. (2021). Arbuscular mycorrhizal fungal colonization and soil pH induced by nitrogen and phosphorus additions affects leaf C:N:P stoichiometry in Chinese fir (Cunninghamia lanceolata) forests. Plant soil461:421–440. doi: 10.1007/s11104-021-04831-1
16
Liu S. Zhang X. Y. Pan J. X. Ma Z. Q. (2023). Differences in available phosphorus in temperate and subtropical forest soils. Appl. Soil Ecol.187:104849. doi: 10.1016/j.apsoil.2023.104849
17
Liu L. J. Zhu Q. L. Yang L. Elrys A. S. Sun J. F. Ni K. et al . (2024). Afforestation increases soil inorganic N supply capacity and lowers plant N limitation in subtropical karst areas. Geoderma443:116848. doi: 10.1016/j.geoderma.2024.116848
18
Lu Z. X. Wang P. Ou H. B. Wei S. X. Wu L. C. Jiang Y. et al . (2022). Effects of different vegetation restoration on soil nutrients, enzyme activities, and microbial communities in degraded karst landscapes in Southwest China. For. Ecol. Manag.508:120002. doi: 10.1016/j.foreco.2021.120002
19
Philippot L. Raaijmakers J. M. Lemanceau P. van der Putten W. H. (2013). Going back to the roots: the microbial ecology of the rhizosphere. Nat. Rev. Microbiol.11, 789–799. doi: 10.1038/nrmicro3109
20
Schachtman D. P. Reid R. J. Ayling S. M. (1998). Phosphorus uptake by plants: from soil to cell. Plant Physiol.116, 447–453. doi: 10.1104/pp.116.2.447
21
Simpson E. H. (1949). Measurement of diversity. Nature163:688. doi: 10.1038/163688a0
22
Smith S. E. Read D. J. (2008). Mycorrhizal symbiosis. London: Academic Press.
23
Song M. He T. G. Chen H. Wang K. L. Li D. J. (2019). Dynamics of soil gross nitrogen transformations during post-agricultural succession in a subtropical karst region. Geoderma341, 1–9. doi: 10.1016/j.geoderma.2019.01.034
24
Tang J. Tang X. X. Qin Y. M. He Q. S. Yi Y. Ji Z. L. (2019). Karst rocky desertification progress: soil calcium as a possible driving force. Sci. Total Environ.649, 1250–1259. doi: 10.1016/j.scitotenv.2018.08.242
25
Tedersoo L. Bahram M. Zobel M. (2020). How mycorrhizal associations drive plant population and community biology. Science367:867. doi: 10.1126/science.aba1223
26
Tian J. H. Lu X. Chen Q. Q. Kuang X. Z. Liang C. Y. Deng L. S. et al . (2022). Phosphorus fertilization affects soybean rhizosphere phosphorus dynamics and the bacterial community in karst soils. Plant Soil475, 137–152. doi: 10.1007/s11104-020-04662-6
27
Tong X. W. Brandt M. Yue Y. M. Horion S. Wang K. L. Keersmaecker W. et al . (2018). Increased vegetation growth and carbon stock in China karst via ecological engineering. Nat. Sustain.1, 44–50. doi: 10.1038/s41893-017-0004-x
28
Vance C. P. Uhde-Stone C. Allan D. L. (2003). Phosphorus acquisition and use: critical adaptations by plants for securing a nonrenewable resource. New Phytol.157, 423–447. doi: 10.1046/j.1469-8137.2003.00695.x
29
Walker C. Schüßler A. Vincent B. Cranenbrouck S. Declerck S. (2021). Anchoring the species Rhizophagus intraradices (formerly Glomus intraradices). Fungal Syst. Evol.8, 179–201. doi: 10.3114/fuse.2021.08.14
30
Wang M. M. Chen H. S. Zhang W. Wang K. L. (2018). Soil nutrients and stoichiometric ratios as affected by land use and lithology at county scale in a karst area, Southwest China. Sci. Total Environ.619, 1299–1307. doi: 10.1016/j.scitotenv.2017.11.175
31
Wang F. Y. Rengel Z. (2024). Disentangling the contributions of arbuscular mycorrhizal fungi to soil multifunctionality. Pedosphere34, 269–278. doi: 10.1016/j.pedsph.2023.12.015
32
Wei X. C. Deng X. W. Xiang W. H. Lei P. F. Ou Y. S. Wen H. F. et al . (2018). Calcium content and high calcium adaptation of plants in karst areas of southwestern Hunan, China. Biogeosciences15, 2991–3002. doi: 10.5194/bg-15-2991-2018
33
Xiao D. Che R. X. Liu X. Tan Y. J. Yang R. Zhang W. et al . (2019). Arbuscular mycorrhizal fungi abundance was sensitive to nitrogen addition but diversity was sensitive to phosphorus addition in karst ecosystems. Biol. Fertil. Soils55, 457–469. doi: 10.1007/s00374-019-01362-x
34
Xu J. Liu S. J. Song S. R. Guo H. L. Tang J. J. Yong J. W. H. et al . (2018). Arbuscular mycorrhizal fungi influence decomposition and the associated soil microbial community under different soil phosphorus availability. Soil Biol. Biochem.120, 181–190. doi: 10.1016/j.soilbio.2018.02.010
35
Yang Y. N. Ba L. Bai X. N. Zhang L. Z. Wang D. L. (2010). An improved method to stain arbuscular mycorrhizal fungi in plant roots. Acta Ecol. Sin.30, 0774–0779. doi: 10.20103/j.stxb.2010.03.027
36
Yang Y. Zhang X. Y. Hartley I. P. Dungait J. A. J. Wen X. F. Li D. D. et al . (2022). Contrasting rhizosphere soil nutrient economy of plants associated with arbuscular mycorrhizal and ectomycorrhizal fungi in karst forests. Plant Soil470, 95–96. doi: 10.1007/s11104-021-05032-6
37
Zhang Z. H. Hu B. Q. Hu G. (2014). Spatial heterogeneity of soil chemical properties in a subtropical karst forest, Southwest China. Sci. World J.2014:473651. doi: 10.1155/2014/473651
38
Zhang C. H. Qi X. K. Wang K. L. Zhang M. Y. Yue Y. M. (2017). The application of geospatial techniques in monitoring karst vegetation recovery in Southwest China: a review. Progress Physical Geography Earth Environ.41, 450–477. doi: 10.1177/0309133317714246
39
Zhang W. Zhao J. Pan F. J. Li D. J. Chen H. S. Wang K. L. (2015). Changes in nitrogen and phosphorus limitation during secondary succession in a karst region in Southwest China. Plant Soil391, 77–91. doi: 10.1007/s11104-015-2406-8
40
Zheng X. An Z. F. Cao M. M. Wu F. Guan X. Chang S. X. et al . (2022). Arbuscular mycorrhizal hyphal respiration makes a large contribution to soil respiration in a subtropical forest under various N input rates. Sci. Total Environ.852:158309. doi: 10.1016/j.scitotenv.2022.158309
41
Zheng C. H. Wan L. Wang R. S. Wang G. Dong L. Yang T. et al . (2023). Effects of rock lithology and soil nutrients on nitrogen and phosphorus mobility in trees in non-karst and karst forests of Southwest China. For. Ecol. Manag.548:121392. doi: 10.1016/j.foreco.2023.121392
Summary
Keywords
AMF inoculation, karst ecosystem, plantation, P addition, soil nutrient
Citation
Wang M, Hu Y, Li Q and He X (2025) Responses of rhizosphere soil nutrients to phosphorus addition and arbuscular mycorrhizal fungi inoculation in karst plantations. Front. For. Glob. Change 8:1680540. doi: 10.3389/ffgc.2025.1680540
Received
06 August 2025
Accepted
06 October 2025
Published
23 October 2025
Volume
8 - 2025
Edited by
Mehrdad Zarafshar, Linnaeus University, Sweden
Reviewed by
Fayuan Wang, Qingdao University of Science and Technology, China
Shubhransu Nayak, Odisha Biodiversity Board, India
Miaomiao Zhang, Jinan University, China
Updates
Copyright
© 2025 Wang, Hu, Li and He.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Miaomiao Wang, miaomiaowang@csuft.edu.cn
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.