Abstract
Mixed plantations have emerged as a key afforestation strategy for enhancing the stability and sustainability of forest ecosystems. However, their effects on soil microbial diversity and ecosystem multifunctionality (EMF) remain inconsistent. Here, we compiled a dataset from 121 peer-reviewed publications and conducted a meta-analysis to evaluate the effects of different mixed plantation strategies on soil microbial diversity and EMF. The results showed that mixed plantations significantly increased bacterial and fungal Shannon indices by 1.43 and 7.4%, respectively, compared to monocultures. In addition, mixed plantations enhanced key ecosystem functions, including increased soil organic carbon, nutrient availability, and moisture retention, while reducing soil bulk density. Collectively, these improvements resulted in a 19.25% increase in the EMF index. Further analysis revealed significant positive correlations between both bacterial and fungal diversity and EMF, as well as significant associations between EMF and key soil physical properties. Moreover, the effects of mixed plantations on microbial diversity and ecosystem functions varied depending on the mixing strategy. Overall, our findings highlight the ecological benefits of mixed plantations for soil microbial diversity and EMF, supporting more effective and sustainable afforestation practices.
1 Introduction
Biodiversity loss disrupts ecosystem structure and functioning, posing a major threat to long-term ecological stability and human wellbeing (Jaureguiberry et al., 2022). Forest ecosystems cover approximately 31% of the Earth’s terrestrial surface and play a crucial role in maintaining global biodiversity and providing ecosystem services and functions, including carbon storage, nutrient cycling, water conservation, and climate regulation (FAO, 2024). In recent decades, with the growing demand for ecosystem services and the need to avoid the ecological crises caused by excessive logging of natural forests, the area of forest plantations now comprises approximately 7% of the global forest cover (Liu S. et al., 2018). Notably, China has established the world’s largest area of forest plantations through a series of major afforestation projects, representing more than 25% of the global planted forest area (Ma et al., 2025). China’s forest plantations possess several distinctive characteristics that set them apart globally. In terms of dominant species, they are predominantly composed of fast-growing timber trees such as Populus species (Populus tomentosa) and Cunninghamia lanceolata, which are managed for high-yield timber production (Sui et al., 2022). Beyond their economic value, these plantations are strategically established to fulfill critical ecological functions, most notably soil and water conservation, which is vital for rehabilitating degraded land across the country. Among various afforestation models, monoculture plantations have become the prevailing afforestation model owing to their high productivity and management simplicity (Li T. et al., 2022). However, long-term, large-scale monoculture plantations are known to cause a range of ecological problems, such as soil erosion, declines in soil quality, reduced biodiversity, and diminished ecosystem service capacity (Li et al., 2021; Li P. et al., 2022; Zhou et al., 2024). In contrast, mixed plantations are receiving increasing attention due to their potential to enhance biodiversity, improve ecosystem resilience, and maintain multiple ecosystem functions (Gamfeldt et al., 2013; Xie et al., 2018; Zhou et al., 2024).
Soil microbial communities, as a vital component of global biodiversity, play key roles in ecosystem functioning (Delgado-Baquerizo et al., 2016; Bastida et al., 2021). Studies have shown that mixed plantations can influence soil microbial diversity by altering soil moisture content, pH, and nutrient availability (An et al., 2019; Ding et al., 2022; Xu et al., 2022). Despite increasing research on how mixed plantations affect soil microbial communities, results remain inconsistent and no clear consensus has emerged. Some studies report that mixed plantations significantly enhance soil microbial diversity (Xu et al., 2022; Liu L. et al., 2023; Li N. et al., 2024), while others report either negative effects or no significant differences compared to monocultures (Xu et al., 2021; Pan et al., 2023; Yang et al., 2024). Variations in tree species composition, stand age, and planting density may contribute to the variable effects on soil microbial diversity (Zhang et al., 2023; Zhang et al., 2024).
Biodiversity has been widely recognized as a key driver of ecosystem multifunctionality (EMF) in numerous studies (Lefcheck et al., 2015; Mensah et al., 2020; Yan et al., 2023). Earlier research tended to focus on the relationship between plant diversity and EMF (Maestre et al., 2012; van der Plas et al., 2016; Gross et al., 2017). The relationship between soil microbial diversity and EMF has received growing attention with the rapid advancement of molecular biology (Shu et al., 2023; Sun et al., 2023; Zhang et al., 2025). Previous studies have found a significant positive correlation between soil microbial diversity and EMF (Delgado-Baquerizo et al., 2020). As research has become more nuanced, some studies have revealed that fungal diversity positively influences EMF (Duan et al., 2021), whereas bacterial diversity appears to have a less pronounced effect (Chen et al., 2023). These findings suggest that trade-offs may exist between different types of soil microbial diversity and their contributions to EMF (Sun et al., 2023).
Although mixed plantations are increasingly adopted in afforestation practices, the specific relationships between mixed plantations, soil microbial diversity, and EMF remain unclear. Previous field-based empirical studies have been largely confined to local or small spatial scales, limiting their applicability to broader regions. Moreover, existing meta-analyses on mixed plantations have primarily focused on either soil microbial diversity or specific ecosystem functions (Gong et al., 2021; Yan et al., 2021; Xiang et al., 2022), with few attempts to quantitatively integrate both aspects. To address these gaps, this study conducts a quantitative meta-analysis across China, a representative region for large-scale plantation development, to simultaneously evaluate the effects of mixed plantations on soil microbial diversity and EMF. This integrated approach provides new insights for optimizing mixed plantation management and promoting ecosystem sustainability.
Here, we compiled a comprehensive database comprising 332 paired observations from monoculture and mixed plantations for meta-analysis to evaluate soil microbial diversity and EMF. Based on our meta-analysis, we aimed to (1) quantify the impact of mixed plantations on soil microbial diversity; (2) evaluate their effects on various ecosystem functions and EMF; and (3) explore the relationships between microbial diversity and EMF in mixed plantation systems. For robust assessment of microbial alpha diversity, we employed the Shannon index as our primary metric, following established methodologies (He et al., 2013). Furthermore, we hypothesized that factors such as the presence or absence of nitrogen-fixing tree species, the age structure of mixed plantations, and species mixture type significantly influence soil microbial diversity and ecosystem functioning.
2 Materials and methods
2.1 Data collection
We conducted a comprehensive literature search of peer-reviewed journal articles published before 31 December 2024, using the Web of Science and China National Knowledge Infrastructure (CNKI) databases. The literature screening was performed following the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) (Moher et al., 2009; Figure 1). Various keyword combinations were used for the search, such as: (mixed forest or mixed plantation or plantation or different forest stand types) AND (soil microbial diversity or soil fungal diversity or soil bacteria diversity or soil microbial biomass or soil microbial community) AND (ecosystem multifunctionality or soil physical and chemical properties). Additionally, relevant peer-reviewed literature was further supplemented by cross-checking the reference lists of the included articles. The studies were subsequently screened according to the following criteria: (1) only studies conducted within China were included; (2) only field studies conducted in plantation forests with at least three replicates were included, excluding those conducted in natural forests; (3) to ensure comparability, each study had to include both mixed plantations (treatment; composed of two tree species) and monoculture plantations (control; consisting of one of these two species) at the same experimental site; (4) only studies focusing on the entire community of soil bacteria and fungi were included, excluding those that focused on specific microbial taxa; (5) data were extracted only from the 0 to 20 cm soil layer; (6) the diversity index of soil microbial community was calculated based on the high-throughput sequencing method (Wang et al., 2023); (7) if a publication included multiple experiments under different treatments, seasons or years, stand ages, or mixing proportions, each independent experiment was considered as one observation in the meta-analysis.
FIGURE 1
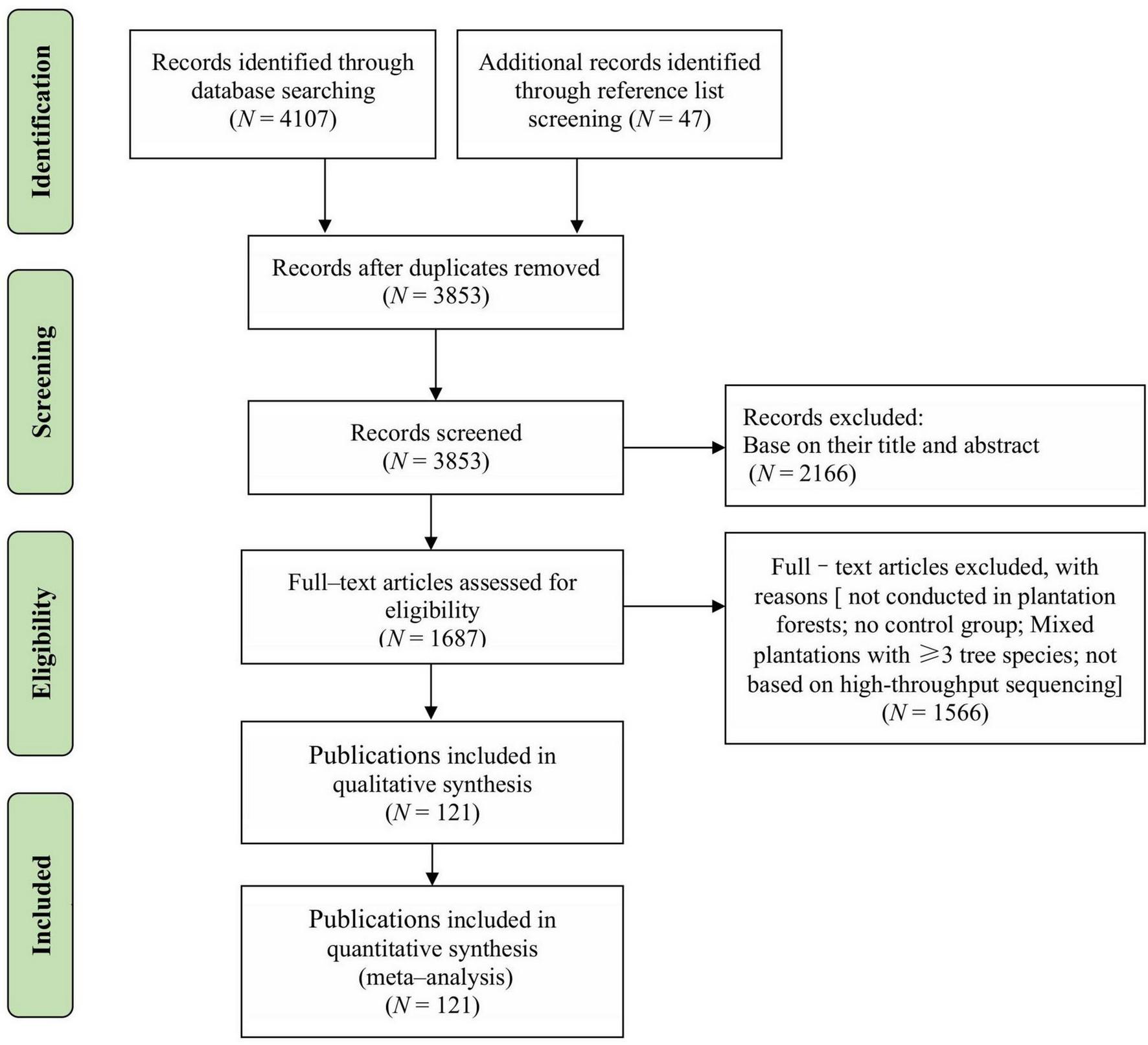
PRISMA diagram showing the process of locating publications included in this meta– analysis.
2.2 Data extraction
For each study included in this meta-analysis, we extracted key variables, including the mean, standard deviation (SD), and sample size for plant properties [litter biomass (LB), root biomass (RB)], soil physicochemical properties [bulk density (BD), soil moisture content (SM), soil pH (pH), soil organic carbon (SOC), total nitrogen (TN), total phosphorus (TP), available phosphorus (AP), and available potassium (AK), ammonium nitrogen (NH4–-N), nitrate nitrogen (NO3–-N), SOC/TN ratio (C/N)], microbial attributes [microbial biomass carbon (MBC), microbial biomass nitrogen (MBN), bacterial Shannon index, and fungal Shannon index, and enzyme activity urease activity (URE)].
All original data were extracted from the figures, tables, and supplementary material of the publications. Specifically, data presented in tables were directly extracted, whereas graphical data were digitized using the GetData Graph Digitizer (version 2.24). When only the median, range, or interquartile range was reported, we estimated the mean and standard deviation using the method proposed by Wan et al. (2014). If the original studies reported standard errors (SE) instead of SD, we converted SE to SD using the formula SD = SE* . Additionally, we collected site characteristics, including geographic location (latitude and longitude), elevation, mean annual temperature, mean annual precipitation, mixed plantation type, mixing ratio, and stand age. Stand age was defined as the number of years between forest establishment (or species introduction) and sampling. Based on these criteria, the databases used for the Meta-analysis comprise 332 observations at 104 sites from 121 peer-reviewed publications. The study sites included in this study are shown in Figure 2.
FIGURE 2
Distribution of all study sites included in this meta-analysis across China.
We categorized the collected data based on three mixing strategy classifications:
-
(1)
Nitrogen-fixing status (N-fixing status): classified based on the presence or absence of nitrogen-fixing tree species as nitrogen-fixing mixed plantations (NF) and non-nitrogen-fixing mixed plantations (NNF).
-
(2)
Age structure: classified as uneven-aged mixed plantations (UA) and even-aged mixed plantations (EA).
-
(3)
Species mixture: classified as coniferous–broadleaf mixed plantations (CB) and broadleaf–broadleaf mixed plantations (BB). Coniferous–coniferous mixed plantations were excluded from the classification due to an insufficient number of available studies (n < 3).
2.3 Assessment of ecosystem multifunctionality index
We selected 13 indicators related to ecosystem functioning from the database to assess EMF, including LB, RB, SOC, TN, TP, AP, AK, NO3–-N, NH4–-N, MBC, MBN, C/N, and URE. These indicators were selected because they have been recognized in previous studies as good descriptors of ecosystem functions and services (Yuan et al., 2021; Liu et al., 2025). To ensure consistency in directional change, the values of the C/N ratio were multiplied by –1, since higher values represent a negative effect on ecosystem functioning (Ma et al., 2021). The EMF index was calculated as the mean of effect sizes across the 13 selected indicators, following the averaging approach widely used in multifunctionality studies (Shu et al., 2024). This approach was adopted because it provides an intuitive and easily interpretable metric that gives balanced consideration to the overall performance of multiple ecosystem functions.
2.4 Meta-analysis
We used the natural log-transformed effect size (RR) to quantify the impact of mixed plantations on soil microbial diversity and individual ecosystem functions (Hedges et al., 1999), as shown in Eq. (1):
where Xt and Xc are the average values of the selected variables in the mixed plantation (treatment group) and monoculture plantation (control group), respectively.
The variance (v) of RR was calculated using Eq. (2):
where Nt and Nc represent the sample size of mixed plantation and monoculture plantation, respectively; SDt and SDc denote the standard deviations (SD) of the mixed plantation and monoculture plantation, respectively. If the SD was unavailable and could not be estimated, it was imputed as 10% of the corresponding mean, following Li X. et al. (2020).
To facilitate interpretation, the weighted effect sizes (lnRR) were back-transformed and presented as percentage changes using Eq. (3) (Wang et al., 2023):
We used the “metafor” package (version 4.6.0) in R to perform a meta-analysis with a random-effects model (Viechtbauer, 2010). This model was chosen to account for heterogeneity arising from differences in site conditions and experimental designs among the included studies, which cannot be addressed by a fixed-effects model. The “rma” function was employed to calculate the lnRR and the corresponding 95% confidence interval (CI). If the 95% CI did not overlap with zero, the effect of mixing on the given variable was considered significant (p < 0.05).
We used between-group heterogeneity tests (QB) to compare effect sizes across different mixing strategies. A significant QB value (PQB < 0.05) indicates significant differences in the weighted effect sizes between groups for a given variable.
To examine whether soil microbial diversity affected EMF, we used ordinary least squares regressions to analyze the relationships between soil bacterial and fungal diversity and EMF. In addition, we also analyzed the relationships between soil physical properties and EMF. For the regression model, we used the Shapiro-Wilk test to assess the normality of the residuals and the Breusch-Pagan test to examine homoscedasticity (Zhang et al., 2022). For both tests, p > 0.05 was considered to indicate that the assumptions of normality and homoscedasticity were not violated.
Given that some variables lacking reported SD were included in this meta-analysis, we conducted a sensitivity analysis to assess the possible bias introduced by this inclusion (Gao et al., 2021). Specifically, we calculated and compared the lnRR and 95% CIs using all available data and a subset excluding studies without SD. If the lnRR were similar and their 95% CIs overlapped, the inclusion of all data for that variable was deemed acceptable for our meta-analysis. Furthermore, we employed both Egger’s regression test and Fail-Safe N analysis to comprehensively assess potential publication bias in this meta-analysis (Egger et al., 1997; Rosenberg, 2005). If the Fail-Safe N exceeded 5n + 10 (where n is the sample size), the results were considered robust and free from publication bias. Sensitivity analysis indicated that the inclusion of data without SD did not change the direction and statistical significance of our results (Table 1), and no potential publication bias was detected (Table 2).
TABLE 1
| Variables | Data class | QT | n | lnRR (95%CI) |
|---|---|---|---|---|
| LB | Full data | 2253.4 | 49 | 0.2740 (0.1854, 0.3626) |
| Reduced data | 2175.3 | 46 | 0.2671 (0.1774, 0.3569) | |
| RB | Full data | 533.2 | 34 | 0.3415 (0.2529, 0.4301) |
| Reduced data | 473.7 | 28 | 0.3028 (0.2039, 0.4017) | |
| SOC | Full data | 7523.2 | 232 | 0.1822 (0.1495, 0.2150) |
| Reduced data | 7371.1 | 222 | 0.1899 (0.1570, 0.2228) | |
| TN | Full data | 4116.2 | 245 | 0.2120 (0.1802, 0.2438) |
| Reduced data | 3974.6 | 235 | 0.2101 (0.1779, 0.2422) | |
| TP | Full data | 12445.1 | 204 | 0.1351 (0.0913, 0.1788) |
| Reduced data | 11885.9 | 194 | 0.1379 (0.0967, 0.1792) | |
| AP | Full data | 13263.5 | 163 | 0.2059 (0.1450, 0.2669) |
| Reduced data | 12979.0 | 159 | 0.1896 (0.1302, 0.2491) | |
| AK | Full data | 19347.2 | 93 | 0.1323 (0.0658, 0.1988) |
| Reduced data | 19309.0 | 84 | 0.1305 (0.0578, 0.2031) | |
| NH4–-N | Full data | 1619.9 | 115 | 0.1179 (0.0634, 0.1725) |
| Reduced data | 1619.4 | 114 | 0.1191 (0.0640, 0.1741) | |
| NO3–-N | Full data | 14973.3 | 113 | 0.2235 (0.1112, 0.3358) |
| Reduced data | 14961.3 | 112 | 0.2259 (0.1126, 0.3392) | |
| C/N | Full data | 9964.6 | 90 | −0.0318 (−0.0750, 0.0115) |
| Reduced data | 9964.3 | 89 | −0.0309 (−0.0747, 0.0128) | |
| H bacteria | Full data | 1441.1 | 94 | 0.0142 (0.0052, 0.0233) |
| Reduced data | 1439.0 | 91 | 0.0147 (0.0056, 0.0238) | |
| H fungi | Full data | 155.8 | 56 | 0.0714 (0.0298, 0.1130) |
| Reduced data | 154.1 | 54 | 0.0764 (0.0334, 0.1194) |
Sensitivity analysis for the effects of mixed plantations on soil microbial diversity and ecosystem multifunctionality.
Full data includes all data from studies that directly provided standard deviation (SD) and those with SD obtained through calculation. Reduced data includes data only from studies that directly provided SD. QT, total heterogeneity; n, sample size; lnRR, weighted effect size; CI, confidence interval. The effect of mixed plantations is considered statistically significant if the 95% CI does not overlap with zero. LB, litter biomass; RB, root biomass; SOC, soil organic carbon; TN, total nitrogen; TP, total phosphorus; AP, available phosphorus; AK, available potassium; NH4–-N, ammonium nitrogen; -N, nitrate nitrogen; C/N, SOC/TN ratio; Hbacteria, bacterial Shannon index; Hfungi, fungal Shannon index.
TABLE 2
| Variables | n | Egger’s test | Exist of bias | Fail-safe number | Is the analysis robust | |
|---|---|---|---|---|---|---|
| z | p-value | |||||
| LB | 49 | 0.7667 | 0.4433 | No | 45821 | Yes |
| RB | 34 | 0.6111 | 0.5412 | No | 7299 | Yes |
| BD | 71 | −0.8986 | 0.3689 | No | 8799 | Yes |
| SM | 114 | −0.0263 | 0.9791 | No | 24276 | Yes |
| pH | 199 | −0.5416 | 0.5881 | No | 38103 | Yes |
| SOC | 232 | 0.3920 | 0.6950 | No | 260777 | Yes |
| TN | 245 | 0.6439 | 0.5197 | No | 239661 | Yes |
| TP | 204 | −0.1269 | 0.8990 | No | 134160 | Yes |
| AP | 163 | −0.6434 | 0.5200 | No | 195440 | Yes |
| AK | 93 | −0.7276 | 0.4669 | No | 88751 | Yes |
| NH4–-N | 115 | 1.1819 | 0.2372 | No | 9810 | Yes |
| NO3–-N | 113 | −2.5209 | 0.0117 | Yes | 62913 | Yes |
| C/N | 90 | 1.0708 | 0.2843 | No | 3633 | Yes |
| MBC | 83 | −1.7033 | 0.0885 | No | 62229 | Yes |
| MBN | 57 | −2.0445 | 0.0409 | Yes | 14740 | Yes |
| URE | 53 | 1.8059 | 0.0709 | No | 11672 | Yes |
| H bacteria | 94 | −0.5851 | 0.5585 | No | 5102 | Yes |
| H fungi | 56 | 1.1110 | 0.2666 | No | 648 | Yes |
Results of publication bias.
A p-value > 0.05 in Egger’s regression test indicates no evidence of publication bias. If the fail-safe number is greater than 5n + 10 (where n is the sample size), the results are considered robust regardless of any potential publication bias. LB, litter biomass; RB, root biomass; BD, bulk density; SM, soil moisture content; pH, soil pH; SOC, soil organic carbon; TN, soil total nitrogen; TP, total phosphorus; AP, available phosphorus; AK, available potassium; NH4–-N, ammonium nitrogen; NO3–-N, nitrate nitrogen; C/N, SOC/TN ratio; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; URE, urease activity; Hbacteria, bacterial Shannon index; Hfungi, fungal Shannon index.
All statistical analyses and visualizations were performed in R version 4.4.1 (R Core Team, 2024).
3 Results
3.1 Effects of mixed plantations on soil microbial diversity
The Shannon indices of bacterial and fungal communities were both significantly higher in mixed plantations than in monocultures, with increases of 1.43% (p < 0.01) and 7.4% (p < 0.001), respectively (Figure 3). Further analysis revealed that this increase was significant regardless of the age structure of the mixed plantations. Specifically, the Shannon indices of bacteria and fungi increased by 2.00 and 14.56%, respectively, in uneven-aged mixed plantations, and by 1.53 and 5.59% in even-aged mixed plantations (all p < 0.05) (Figure 3). While nitrogen-fixing mixed plantations did not significantly affect the bacterial Shannon index (p > 0.05) (Figure 3A), they significantly increased the fungal Shannon index by 7.14% (p < 0.05) (Figure 3B). In contrast, broadleaf–broadleaf mixed plantations significantly increased the bacterial Shannon index by 2.38% (p < 0.05) (Figure 3A), but had no significant effect on the fungal Shannon index (p > 0.05) (Figure 3B).
FIGURE 3
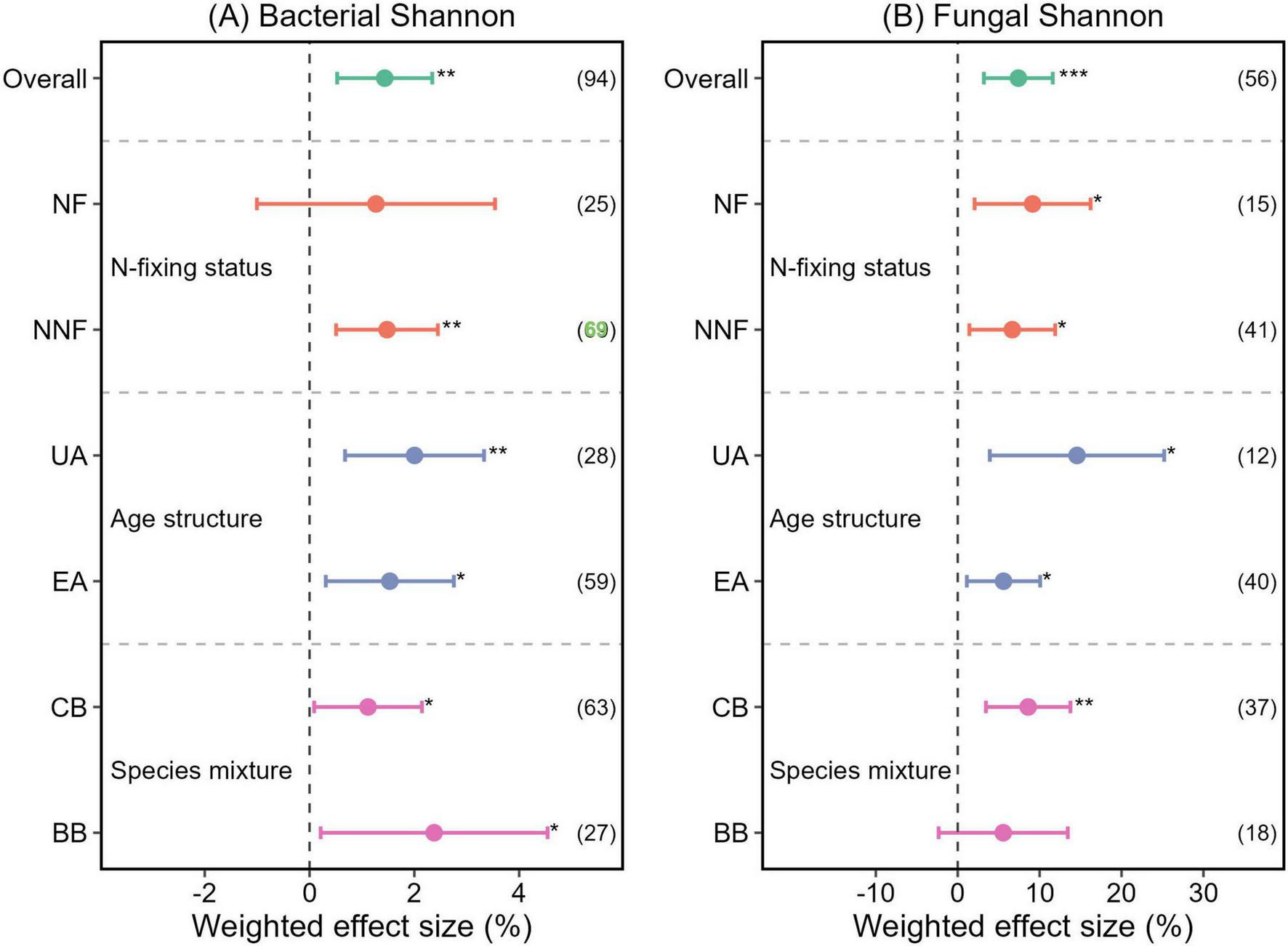
Weighted effect sizes (A) of soil bacterial Shannon index and (B) of soil fungal Shannon index in mixed plantations under different mixing strategies. Error bars represent 95% confidence intervals (CIs) of the weighted effect sizes. An effect is considered statistically significant if the 95% CI does not overlap with zero (p < 0.05). Sample sizes for each variable are shown in parentheses next to the corresponding labels. ***, **, and * denote p < 0.001, p < 0.01, and p < 0.05, respectively. NF, nitrogen-fixing mixed plantations; NNF, non-nitrogen-fixing mixed plantations; UA, uneven-aged mixed plantations; EA, even-aged mixed plantations; CB, coniferous-broadleaf mixed plantations; BB, broadleaf-broadleaf mixed plantations.
3.2 Effects of mixed plantations on ecosystem functions
Mixed plantations significantly increased plant biomass compared to monocultures, with LB and RB rising by 31.52 and 40.71%, respectively (p < 0.001) (Figure 4). Mixed plantations exhibited higher levels of SM, pH, SOC, TN, TP, AP, AK, NH4–-N, and NO3–-N concentrations, with varying magnitudes across indicators (Figure 4). Among all indicators, the NO3–-N concentration showed the largest increase, rising by 25.04% overall (p < 0.001) (Figure 4), and by 61.91% specifically in nitrogen-fixing mixed plantations (p < 0.001) (Figure 5A). In contrast, the BD level in mixed plantations decreased by 5.66% (p < 0.001) (Figure 4). Notably, the C/N ratio decreased significantly by 13.39%, but only in uneven-aged mixed plantations (p < 0.001) (Figure 5B).
FIGURE 4
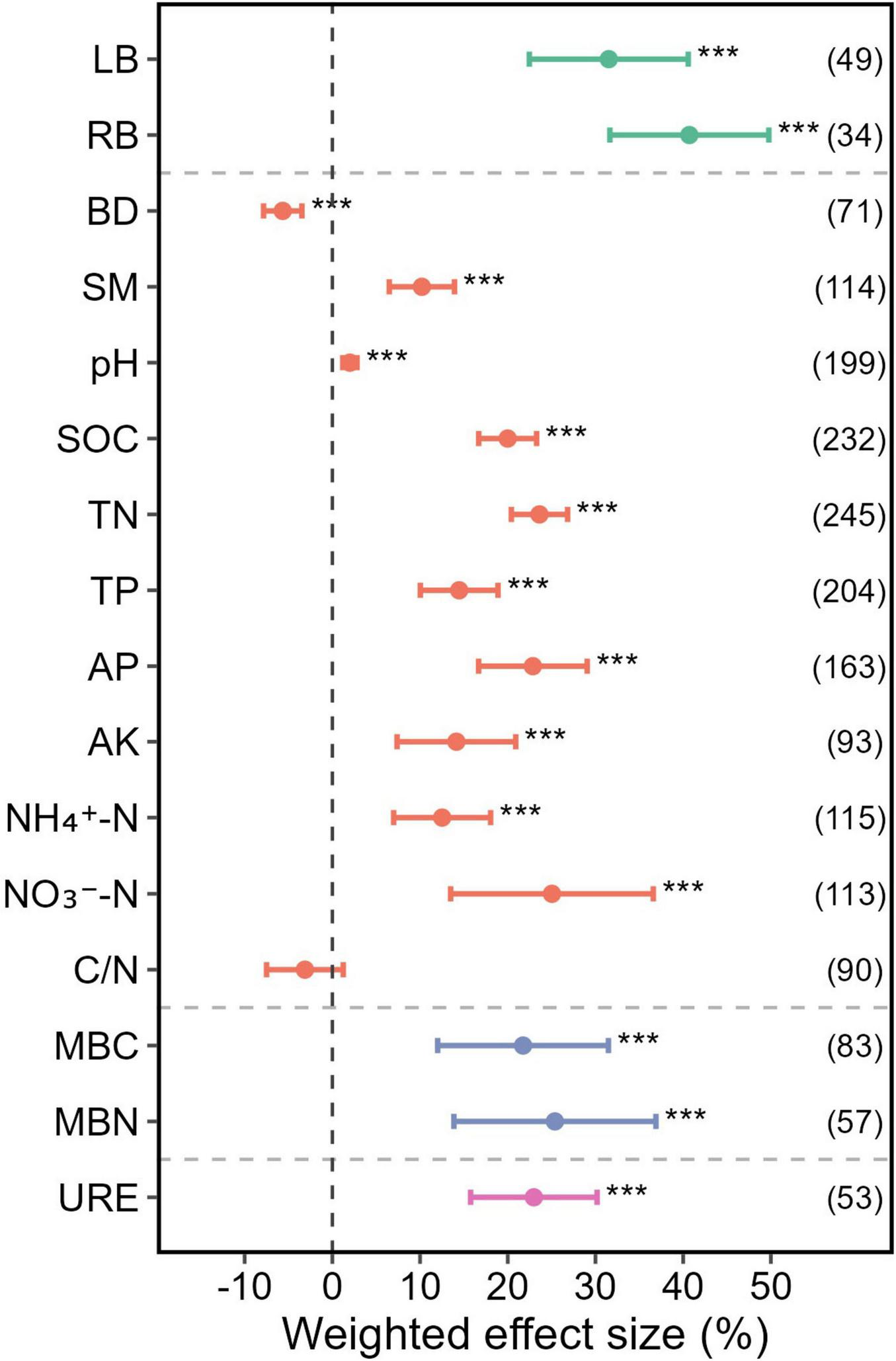
Weighted effect sizes of individual ecosystem functions (LB, RB, SOC, TN, TP, AP, AK, NH4–-N, NO3–-N, C/N, MBC, MBN, and URE) and soil properties, including physical (BD, SM) and chemical (pH) characteristics, in mixed plantations. Error bars represent 95% confidence intervals (CIs) of the weighted effect sizes. An effect is considered statistically significant if the 95% CI does not overlap with zero (p < 0.05). Sample sizes for each variable are shown in parentheses next to the corresponding labels. *** denote p < 0.001, respectively. LB, litter biomass; RB, root biomass; BD, bulk density; SM, soil moisture content; pH, soil pH; SOC, soil organic carbon; TN, soil total nitrogen; TP, soil total phosphorus; AP, soil available phosphorus; AK, soil available potassium; NH4–-N, soil ammonium nitrogen; NO3–-N, soil nitrate nitrogen; C/N, SOC/TN ratio; MBC, microbial biomass carbon; MBN, microbial biomass nitrogen; URE, urease activity.
FIGURE 5
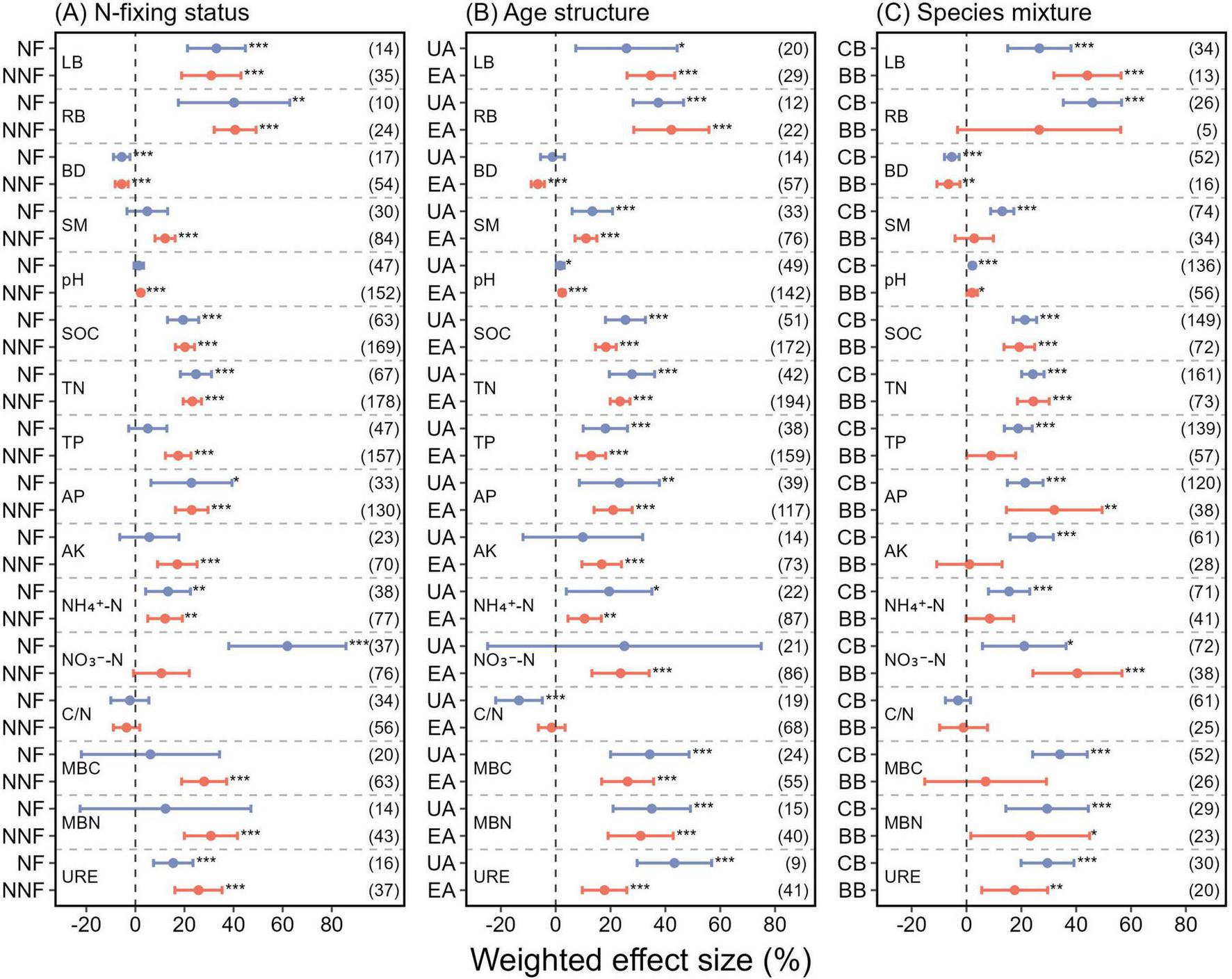
Effects of (A) N-fixing status (NF, nitrogen-fixing mixed plantations; NNF, non-nitrogen-fixing mixed plantations), (B) age structure (UA, uneven-aged mixed plantations; EA, even-aged mixed plantations), and (C) species mixture (CB, coniferous-broadleaf mixed plantations; BB, broadleaf-broadleaf mixed plantations) on multiple ecosystem functions and soil physical properties. Error bars represent 95% confidence intervals (CIs) of the weighted effect sizes. An effect is considered statistically significant if the 95% CI does not overlap with zero (p < 0.05). Sample sizes for each variable are shown in parentheses next to the corresponding labels. ***, **, and * denote p < 0.001, p < 0.01, and p < 0.05, respectively. See Figure 4 caption for abbreviation definitions.
Microbial biomass was significantly greater in mixed plantations, with MBC and MBN increasing by 21.74 and 25.38%, respectively, compared to monocultures (p < 0.001) (Figure 4).
Soil urease activity also increased significantly in mixed plantations, showing a 22.98% rise compared to monocultures (p < 0.001) (Figure 4). This stimulatory effect on urease activity was consistent across all examined mixing strategies (Figures 5A–C).
3.3 Effects of mixed plantations on EMF and its drivers
Compared to monoculture plantations, mixed plantations significantly increased the EMF index by 19.25% (p < 0.001) (Figure 6). This positive effect was consistent across all mixing strategies, although the magnitude of the increase did not differ significantly among them (p > 0.05) (Table 3). Linear regression analysis showed that the EMF index was positively correlated with both bacterial and fungal Shannon indices (p < 0.05) (Figure 7 and Table 4). Additionally, among soil physical properties, EMF was positively associated with SM and negatively associated with BD (both p < 0.001) (Figure 8 and Table 4).
FIGURE 6
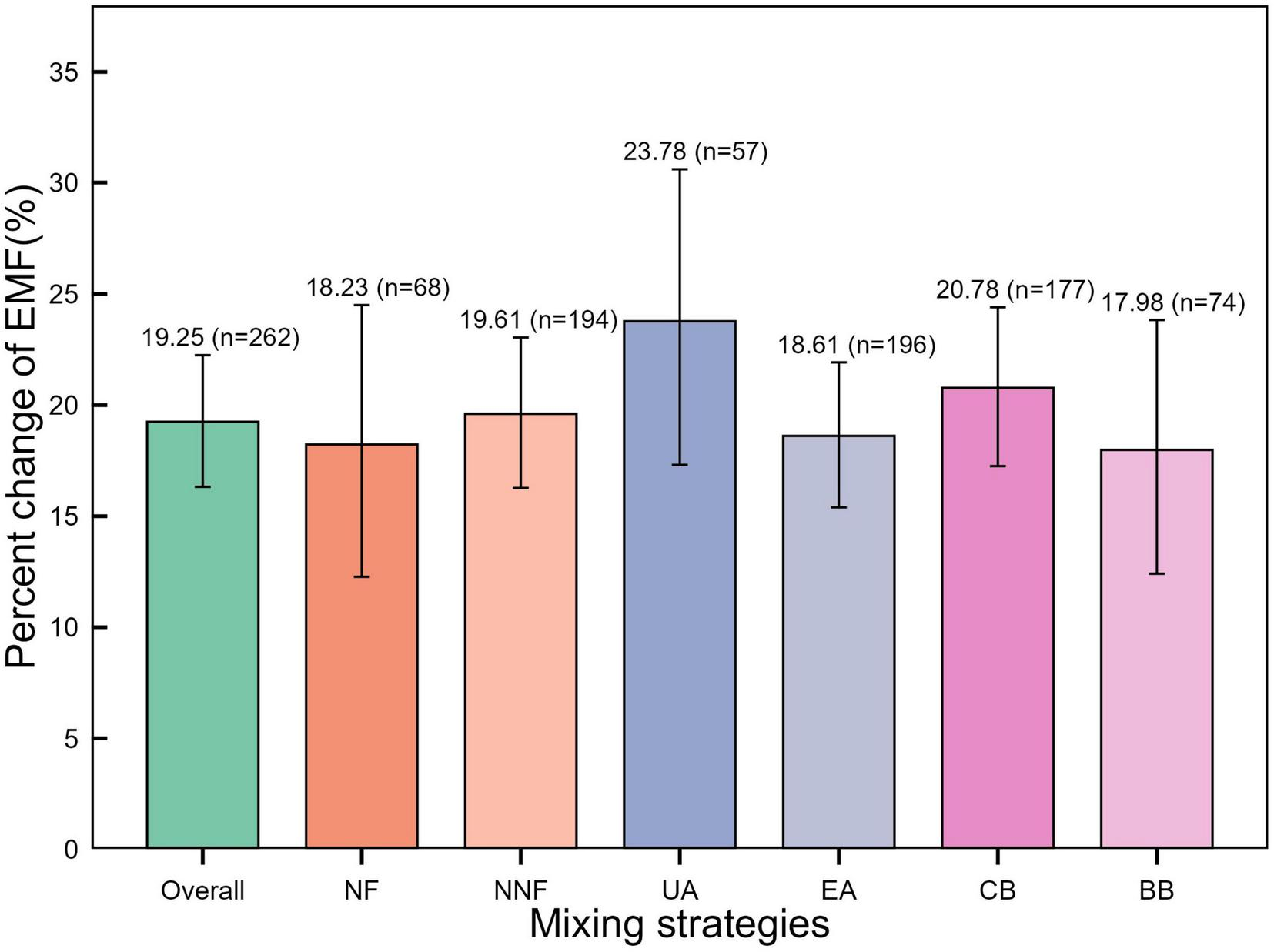
Percent change in ecosystem multifunctionality (EMF) across different mixing strategies in mixed plantations. NF, nitrogen-fixing mixed plantations; NNF, non-nitrogen-fixing mixed plantations; UA, uneven-aged mixed plantations; EA, even-aged mixed plantations; CB, coniferous-broadleaf mixed plantations; BB, broadleaf-broadleaf mixed plantations.
TABLE 3
| Variables | N-fixing status | Age structure | Species mixture | |||
|---|---|---|---|---|---|---|
| Qb | P | Qb | P | Qb | P | |
| LB | 0.04 | 0.843 | 0.61 | 0.433 | 1.76 | 0.185 |
| RB | 0.01 | 0.918 | 0.06 | 0.803 | 1.10 | 0.293 |
| BD | 0.02 | 0.883 | 3.43 | 0.064 | 0.24 | 0.623 |
| SM | 2.37 | 0.124 | 0.26 | 0.611 | 5.71 | < 0.05 |
| pH | 0.58 | 0.448 | 0.54 | 0.464 | 0.01 | 0.932 |
| SOC | 0.03 | 0.865 | 2.11 | 0.146 | 0.22 | 0.641 |
| TN | 0.11 | 0.739 | 0.62 | 0.433 | 0.001 | 0.973 |
| TP | 4.52 | <0.05 | 0.57 | 0.448 | 3.09 | 0.079 |
| AP | 0.0001 | 0.989 | 0.07 | 0.790 | 1.39 | 0.239 |
| AK | 1.72 | 0.189 | 0.38 | 0.538 | 8.27 | <0.01 |
| NH4+-N | 0.04 | 0.851 | 0.98 | 0.322 | 1.13 | 0.288 |
| NO3–-N | 11.33 | <0.001 | 0.39 | 0.535 | 1.58 | 0.208 |
| C/N | 0.08 | 0.778 | 6.39 | <0.05 | 0.18 | 0.672 |
| MBC | 2.74 | 0.098 | 0.43 | 0.512 | 4.35 | <0.05 |
| MBN | 1.15 | 0.284 | 0.04 | 0.835 | 0.10 | 0.755 |
| URE | 0.84 | 0.361 | 4.81 | <0.05 | 1.58 | 0.208 |
| H bacteria | 0.02 | 0.890 | 0.2 | 0.651 | 1.49 | 0.222 |
| H fungi | 0.30 | 0.581 | 2.33 | 0.127 | 0.48 | 0.490 |
| EMF | 0.16 | 0.690 | 2.02 | 0.156 | 0.68 | 0.409 |
Between-group heterogeneity (Qb and P-values) for each variable under different mixing strategies.
Qb values represent the between-group heterogeneity, with p < 0.05 indicating a statistically significant difference.
FIGURE 7
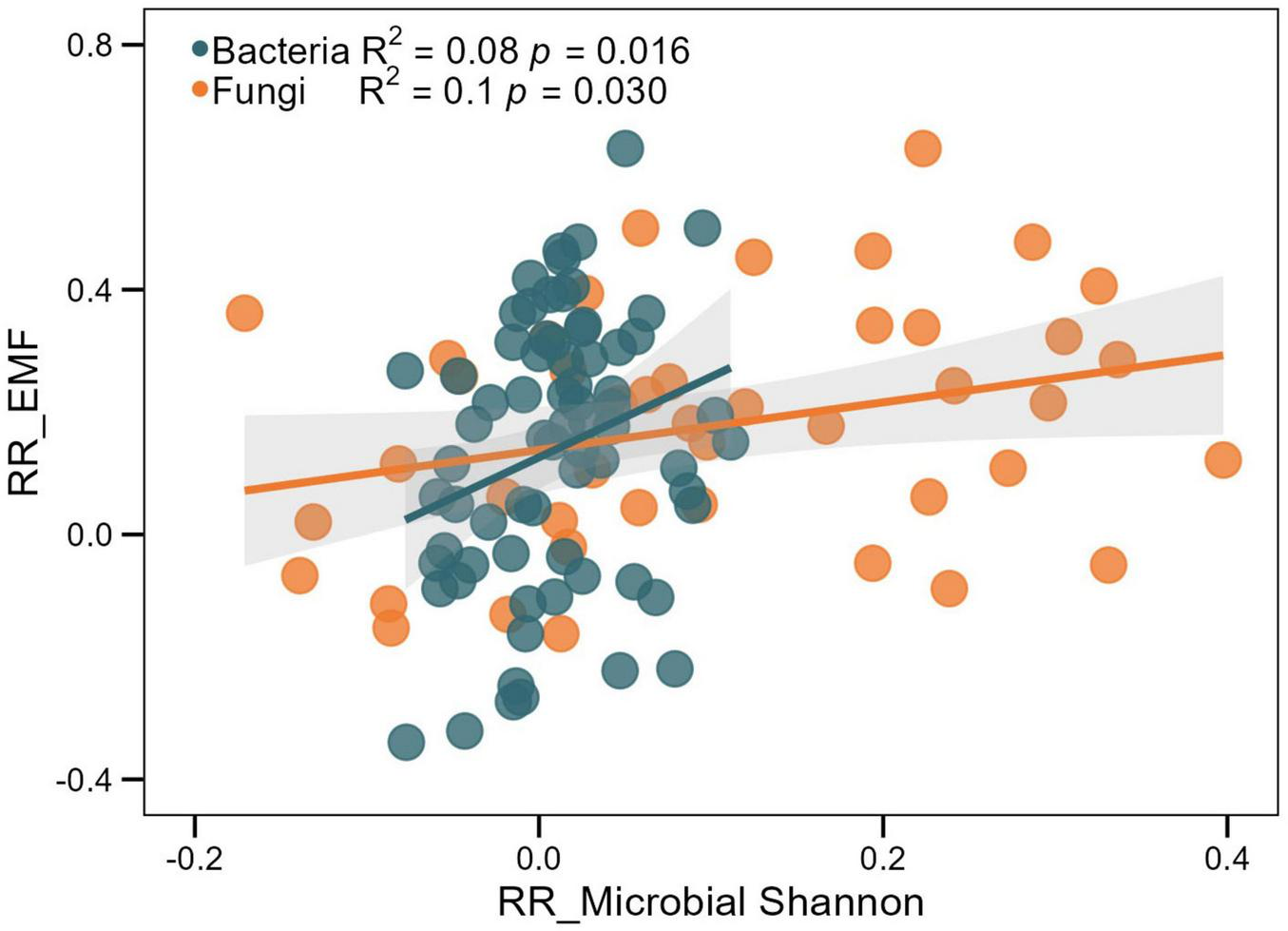
Relationships between the effect sizes (RR) of soil bacterial and fungal Shannon indices and the RR of ecosystem multifunctionality (EMF). Light gray areas indicate the 95% confidence intervals, with the central lines representing linear regressions.
TABLE 4
| Variables | n | Intercept | Slope | R 2 | P | Shapiro-Wilk | Breusch-Pagan | ||
|---|---|---|---|---|---|---|---|---|---|
| W | p | χ2 | p | ||||||
| H bacteria | 72 | 0.116 | 1.503 | 0.080 | 0.016 | 0.976 | 0.181 | 0.008 | 0.931 |
| H fungi | 45 | 0.134 | 0.415 | 0.105 | 0.030 | 0.982 | 0.704 | 0.194 | 0.660 |
| SM | 114 | 0.104 | 0.413 | 0.156 | < 0.001 | 0.995 | 0.940 | 0.103 | 0.748 |
| BD | 71 | 0.083 | −0.867 | 0.211 | < 0.001 | 0.975 | 0.163 | 0.316 | 0.574 |
Linear relationships between the effect sizes (RR) of soil microbial diversity and physical properties, and the RR of ecosystem multifunctionality (EMF).
“n” represents the sample size for each regression analysis. The Shapiro-Wilk and Breusch-Pagan tests were used to assess residual normality and homoscedasticity, respectively. For both tests, p > 0.05 indicates that model assumptions were met.
FIGURE 8
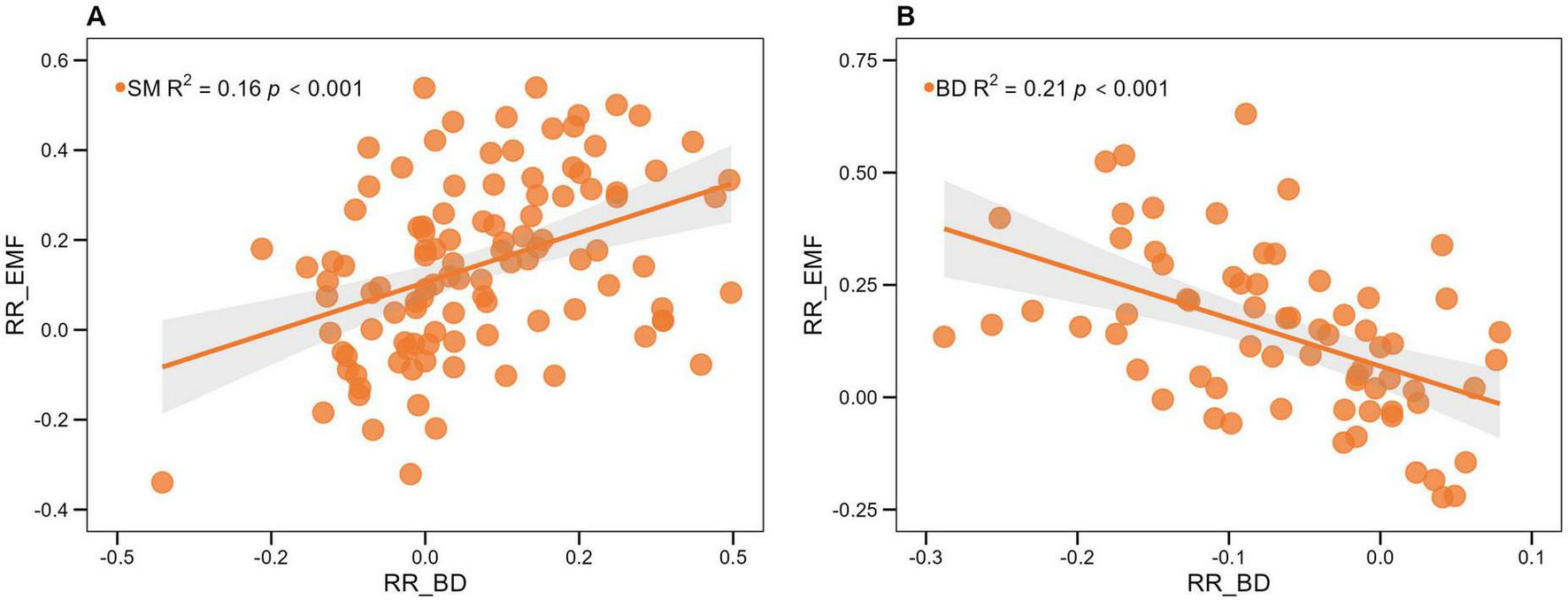
Relationships between the effect sizes (RR) of soil moisture content (SM, A) or soil bulk density (BD, B) and the RR of ecosystem multifunctionality (EMF). Light gray areas indicate the 95% confidence intervals, with the central lines representing linear regressions.
4 Discussion
4.1 Positive effects of mixed plantations on soil microbial diversity
Our meta-analysis showed that the effect sizes of mixed plantations on the Shannon index were positive (Figures 3a,b), suggesting an overall increase in soil microbial biodiversity. Previous studies have found that mixed plantations generally support higher microbial diversity than monocultures, mainly due to their more diverse tree species composition, which provides diverse organic inputs and fosters heterogeneous microhabitats (Li et al., 2015; Khlifa et al., 2017). Litter and root exudates from different tree species offer diverse nutritional substrates for microbes, while also improving the soil microenvironment and increasing the availability of soil nutrients, thereby supporting a more diverse microbial community (Li et al., 2015; Khlifa et al., 2017). Higher microbial diversity is widely recognized as an indicator of healthier soils, promoting more efficient nutrient cycling, organic matter decomposition, and resistance to pests and diseases (Zhu et al., 2021). Notably, microbial diversity is not determined solely by tree species composition; it is also shaped by multiple biotic and abiotic factors, such as pH, nutrient availability, and microbial interactions, which may influence the observed patterns (Ding et al., 2022; Wang Y. et al., 2022).
Moreover, our results revealed that different mixing strategies had divergent effects on bacterial and fungal diversity. Specifically, nitrogen-fixing mixed plantations significantly increased the fungal Shannon index, while bacterial diversity showed no significant response (Figure 3). This pattern suggests that fungal communities may be more responsive than bacterial communities to the presence of nitrogen-fixing species (Zhang et al., 2020; Liu et al., 2022). Introducing nitrogen-fixing species into monocultures may enhance plant nutrient-use efficiency, increasing the input of structurally complex plant residues and root exudates rich in macromolecular organic matter (Wu et al., 2025). Fungi, as primary decomposers of recalcitrant compounds such as lignin and cellulose, may benefit from these additional resources, potentially contributing to the observed increase in fungal diversity. While nitrogen-fixing species may contribute to higher fungal diversity, this effect likely operates together with other environmental and biological factors (Li N. et al., 2024). Moreover, bacterial diversity remained largely unaffected, possibly reflecting a lower sensitivity to tree species composition and a greater influence of abiotic factors such as soil depth and moisture (Xu Z. et al., 2023). In contrast, broadleaf–broadleaf mixed plantations increased the bacterial Shannon index only (Figure 3A), likely because faster decomposition of broadleaf litter accelerates nutrient turnover, providing more labile carbon and nutrients for bacterial communities (Wang et al., 2018; Zhuang et al., 2023). Collectively, these results suggest that bacterial and fungal communities exhibit distinct responses to different mixing strategies (Bai et al., 2025; Xiao et al., 2022).
4.2 Improved ecosystem functions in response to various mixing strategies
Our findings showed that mixed plantations significantly increased LB and RB compared to monocultures (Figure 4). This pattern may be driven by more efficient resource use and niche complementarity in mixed plantations. For instance, mixed plantations often develop stratified canopies that enable species to optimize light capture through vertical niche differentiation. This structural advantage persists over time, resulting in higher litter production in mixed plantations than in monocultures, thereby increasing litter biomass (Wu et al., 2019). Moreover, root niche partitioning and complementary resource use in mixed plantations likely enhance fine root biomass by facilitating more efficient use of belowground resources (Ma and Chen, 2016).
Plants primarily absorb nitrogen in the forms of ammonium (NH4–) and nitrate (NO3–) from the soil (Wang et al., 2005). Mixed plantations have been shown to enhance the concentrations of both NH4–-N and NO3–-N in the soil (Yu et al., 2015; Zhou et al., 2023). In our study, the concentrations of NH4–-N and NO3–-N were both significantly increased in mixed plantations (Figure 4). Notably, NO3–-N levels increased by 61.91% in nitrogen-fixing mixed plantations (Figure 5A), which was significantly higher than in non-nitrogen-fixing mixed plantations (Table 3). This finding aligns with previous studies, suggesting that nitrogen-fixing species enhance soil nitrogen availability (Hoogmoed et al., 2014). This increase is likely attributable to the ability of the associated tree species to fix nitrogen symbiotically, leading to greater nitrogen input into the soil (Mus et al., 2016). Furthermore, mixed plantations may promote nitrification by soil microbes, accelerating the conversion of NH4–-N to NO3–-N and further increasing NO3–-N levels (Liu C.-A et al., 2018). While this shift improves nitrogen availability and promotes plant growth, it may also increase the risk of nitrogen loss, an aspect requiring further investigation (Liang et al., 2020).
In addition, we found that only uneven-aged mixed plantations significantly reduced the C/N ratio (Figure 5B). The C/N ratio is a proxy for nitrogen mineralization potential, with lower values typically indicating higher rates of nutrient release (Liu and Lu, 2024). The decrease in the C/N ratio in uneven-aged mixed plantations may be associated with the timing of species introduction and the resulting age structure (Ling et al., 2022). As new species are introduced, the quantity and quality of surface litter increase, providing more substrates for soil microbes. This stimulates microbial activity, leading to faster decomposition and increased nitrogen mineralization and release, significantly increasing soil nitrogen content and thereby reducing the C/N ratio (Ling et al., 2022; Zhang et al., 2023).
Our study also found that mixed plantations reduced BD (Figure 4). Lower bulk density improves soil aeration, which facilitates root respiration and enhances root growth and nutrient uptake, consistent with the findings of Guo et al. (2023). The reduction in soil bulk density often stems from the complementary distribution of deep and shallow roots in mixed plantations, which increases soil porosity (Liu et al., 2021). Additionally, the greater litter input and faster decomposition in mixed plantations promote the formation of soil aggregates via humus accumulation, thereby improving soil pore structure (Qin et al., 2013). In contrast, monocultures, with limited root distribution and lower, slower-decomposing litter input, tend to have higher soil bulk density (Qin et al., 2013; Liu et al., 2021). Furthermore, mixed plantations significantly increased SM (Figure 4), possibly due to the thick litter and debris layer retaining moisture and reducing water loss through evaporation (Liu B. et al., 2023). Mixed plantations have been confirmed to be more effective than monocultures in enhancing soil water retention (Gong et al., 2024). Therefore, from the perspective of soil and water conservation, afforestation with mixed species is preferable to monoculture.
Changes in soil microbial biomass reflect shifts in microbial community structure and serve as key indicators of soil fertility and microbial metabolic activity (Zhang et al., 2014). Urease activity, closely linked to soil nitrogen availability, is also a crucial indicator of soil quality (Dong et al., 2023). In this study, mixed plantations led to marked increases in MBC, MBN, and urease activity (Figure 4). Their higher species diversity results in a broader range of organic inputs, providing diverse substrates that stimulate microbial activity (Singh et al., 2012). Furthermore, differences in species composition in mixed plantations lead to higher nitrogen content and lower C/N ratios in litter, accelerating decomposition and enhancing microbial activity (Peng et al., 2025), thus increasing MBC and MBN relative to monocultures. However, it is worth noting that while litter can enhance microbial biomass, it may also trigger the priming effect, accelerating the decomposition of native soil organic carbon and potentially resulting in a net loss or reduced stability of soil carbon stocks (Feng et al., 2022). The increase in urease activity may be due to the elevated levels of inorganic nitrogen in mixed plantations, which provide more substrates for urease and stimulate its activity (Luo et al., 2022). Other studies have suggested that enhanced enzyme activity is often the result of multiple interacting factors, including increased soil pH, improved aeration, and higher availability of nutrients (Guo et al., 2023).
4.3 Correlations between the EMF and soil microbial diversity and physical properties
Numerous studies have demonstrated that mixed plantations generally outperform monocultures across a range of ecosystem functions (Pretzsch et al., 2015; Gong et al., 2021; Dai et al., 2023). Building on these findings, our study adopted an integrative perspective by examining EMF, and showed that mixed plantations significantly enhanced overall ecosystem performance (Figure 6), consistent with previous findings (Xu H. et al., 2023). This result highlights the ecological advantage of mixed plantations in simultaneously promoting multiple ecosystem functions. However, other studies have suggested that differences in EMF between mixed and monoculture plantations may depend on the specific tree species used in afforestation (Li X. et al., 2024). Species composition influences interspecific interactions—such as complementarity or competition—which in turn significantly affect EMF levels. These underlying mechanisms merit further investigation.
Regression analyses indicated a significant correlation between EMF and both soil microbial diversity and physical properties. Soil microbial communities were key predictors of EMF and important indicators for forest management (Wang J. et al., 2022). Consistent with previous findings (Shi et al., 2021; Li et al., 2023), the EMF index showed significant positive correlations with both bacterial and fungal Shannon indices (Figure 7). Such positive relationships may arise from multifunctional niche complementarity and interspecific functional dissimilarity among microbial taxa (Luo et al., 2018). Soil microbial communities contribute directly to essential processes such as organic matter decomposition and nutrient mineralization, which support multiple ecosystem functions (Delgado-Baquerizo et al., 2016). Moreover, EMF was positively correlated with SM but negatively correlated with BD (Figures 8A,B), indicating that improved soil structure and moisture conditions can enhance EMF. High bulk density may reduce soil porosity and aeration, constraining root growth and microbial activity and ultimately decreasing EMF (Niu et al., 2024), whereas optimal soil moisture facilitates nutrient transport and plant growth, thereby enhancing multiple ecosystem functions (Li P. et al., 2022; Wang et al., 2024). Although these factors were significantly correlated with EMF, they explained only a small proportion of its variation (Table 4), suggesting that other unmeasured factors such as aboveground biodiversity, litter quality, and climatic conditions likely also contribute to EMF regulation.
4.4 Limitations and future directions
Our meta-analysis demonstrates that mixed plantations generally exert positive effects on soil microbial diversity and a range of ecosystem functions. In addition, it provides insights into the drivers of EMF. Nonetheless, several limitations should be acknowledged. The main limitations of this study include: (1) a focus on widely reported factors, which precluded the inclusion of other key variables like mixing proportion and stand age due to data scarcity; (2) a restricted geographic scope and limited sample size, which reduced the representation of diverse mixing ratios; and (3) the lack of consideration for microbial communities in deeper soil horizons. Consequently, future studies should aim to broaden geographic coverage, investigate deeper soil profiles, and examine a wider range of species compositions to advance our understanding of how mixed plantations shape microbial diversity and ecosystem processes.
5 Conclusion
This meta-analysis revealed that mixed plantations increased soil microbial diversity and enhanced EMF. Importantly, the positive correlation between microbial diversity and EMF underscores a potential central role of soil microbial communities in sustaining multiple ecosystem functions. Moreover, the significant relationships between EMF and soil physical properties underscore the importance of a well-structured soil environment in supporting ecosystem functioning. Although the study confirms the general benefits of mixed plantations, it also reveals that their effectiveness varies with N-fixing status, age structure, and species mixture type. In summary, mixed plantations offer significant ecological advantages over monocultures by enhancing soil microbial diversity and improving multiple ecosystem functions. Consequently, mixed plantations are a more effective afforestation strategy than monocultures in maintaining biodiversity and enhancing ecosystem multifunctionality.
Statements
Data availability statement
The original contributions presented in this study are included in this article/Supplementary material, further inquiries can be directed to the corresponding author.
Author contributions
KX: Conceptualization, Data curation, Formal analysis, Methodology, Software, Validation, Visualization, Writing – original draft. YX: Funding acquisition, Methodology, Project administration, Supervision, Writing – review & editing. XL: Conceptualization, Funding acquisition, Methodology, Supervision, Writing – review & editing.
Funding
The author(s) declare financial support was received for the research and/or publication of this article. This work was funded by the National Key R&D program of China (grant 2023YFE0112801).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/ffgc.2025.1694630/full#supplementary-material
References
1
An R. Ma F. Cui H. Qin G. Huang Y. Tian Q. (2019). Analysis of bacterial community structure and diversity characteristics of mixed forest of Robinia pseudoacacia and Ailanthus altissima and there pure forest in the Yellow River Delta.Acta Ecol. Sin.397960–7967. 10.5846/stxb201809041886
2
Bai Y. Zhou Y. Du J. Zhang X. Feng J. Feng J. (2025). Tree species influence microbiome-mediated nutrient sequestration in soil aggregates of subtropical plantations in China.Appl. Soil Ecol.209:106034. 10.1016/j.apsoil.2025.106034
3
Bastida F. Eldridge D. J. Garcia C. Kenny Png G. Bardgett R. D. Delgado-Baquerizo M. (2021). Soil microbial diversity-biomass relationships are driven by soil carbon content across global biomes.ISME J.152081–2091. 10.1038/s41396-021-00906-0
4
Chen C. Yin G. Hou L. Jiang Y. Sun D. Liang X. et al (2023). Reclamation of tidal flats to paddy soils reshuffles the soil microbiomes along a 53-year reclamation chronosequence: Evidence from assembly processes, co-occurrence patterns and multifunctionality.Environ. Int.179:108151. 10.1016/j.envint.2023.108151
5
Dai J. Li Y. Wang L. (2023). Mixed-species plantations alleviate deep soil water depletion and facilitate hydrological niche partitioning compared to pure plantations.For. Ecol. Manage.539:121017. 10.1016/j.foreco.2023.121017
6
Delgado-Baquerizo M. Maestre F. T. Reich P. B. Jeffries T. C. Gaitan J. J. Encinar D. et al (2016). Microbial diversity drives multifunctionality in terrestrial ecosystems.Nat. Commun.7:10541. 10.1038/ncomms10541
7
Delgado-Baquerizo M. Reich P. B. Trivedi C. Eldridge D. J. Abades S. Alfaro F. D. et al (2020). Multiple elements of soil biodiversity drive ecosystem functions across biomes.Nat. Ecol. Evol.4210–220. 10.1038/s41559-019-1084-y
8
Ding K. Zhang Y. Yrjälä K. Tong Z. Zhang J. (2022). The introduction of Phoebe bournei into Cunninghamia lanceolata monoculture plantations increased microbial network complexity and shifted keystone taxa.For. Ecol. Manage.509:120072. 10.1016/j.foreco.2022.120072
9
Dong Q. Wang H. Du X. Zou J. Cui X. Zhao H. (2023). Characteristics of soil urease activity in typical stand types in low mountainous area of Northeast China.Chin. J. Appl. Environ. Biol.29690–695. 10.19675/j.cnki.1006-687x.2022.03050
10
Duan B. B. Ren Y. Z. Zhang L. Q. Suzhou C. X. Chen G. Q. Cui P. et al (2021). Fungal diversity rather than bacterial diversity drives the ecosystem multifunctionality of vineyards in a semi-arid region.Int. J. Agric. Biol. Eng.14126–136. 10.25165/j.ijabe.20211406.5560
11
Egger M. Davey Smith G. Schneider M. Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test.BMJ315629–634. 10.1136/bmj.315.7109.629
12
FAO (2024). The State of the World’s Forests 2024: Forest-Sector Innovations Towards a More Sustainable Future.Rome: Food and Agriculture Organization of the United Nations.
13
Feng J. He K. Zhang Q. Han M. Zhu B. (2022). Changes in plant inputs alter soil carbon and microbial communities in forest ecosystems.Global Change Biol.283426–3440. 10.1111/gcb.16107
14
Gamfeldt L. Snäll T. Bagchi R. Jonsson M. Gustafsson L. Kjellander P. et al (2013). Higher levels of multiple ecosystem services are found in forests with more tree species.Nat. Commun.4:1340. 10.1038/ncomms2328
15
Gao D. Bai E. Yang Y. Zong S. Hagedorn F. (2021). A global meta-analysis on freeze-thaw effects on soil carbon and phosphorus cycling.Soil Biol. Biochem.159:108283. 10.1016/j.soilbio.2021.108283
16
Gong C. Tan Q. Liu G. Xu M. (2021). Impacts of species mixture on soil nitrogen stocks in the Loess Plateau of China.For. Ecol. Manage.491:119145. 10.1016/j.foreco.2021.119145
17
Gong C. Tan Q. Liu G. Xu M. (2024). Positive effects of mixed-species plantations on soil water storage across the Chinese Loess Plateau.For. Ecol. Manage.552:121571. 10.1016/j.foreco.2023.121571
18
Gross N. Bagousse-Pinguet Y. L. Liancourt P. Berdugo M. Gotelli N. J. Maestre F. T. (2017). Functional trait diversity maximizes ecosystem multifunctionality.Nat. Ecol. Evol.1:0132. 10.1038/s41559-017-0132
19
Guo J. Feng H. McNie P. Liu Q. Xu X. Pan C. et al (2023). Species mixing improves soil properties and enzymatic activities in Chinese fir plantations: A meta-analysis.CATENA220:106723. 10.1016/j.catena.2022.106723
20
He Y. Zhou B.-J. Deng G.-H. Jiang X.-T. Zhang H. Zhou H.-W. (2013). Comparison of microbial diversity determined with the same variable tag sequence extracted from two different PCR amplicons.BMC Microbiol.13:208. 10.1186/1471-2180-13-208
21
Hedges L. V. Gurevitch J. Curtis P. S. (1999). The meta-analysis of response ratios in experimental ecology.Ecology801150–1156. 10.2307/177062
22
Hoogmoed M. Cunningham S. C. Baker P. J. Beringer J. Cavagnaro T. R. (2014). Is there more soil carbon under nitrogen-fixing trees than under non-nitrogen-fixing trees in mixed-species restoration plantings?Agricult. Ecosyst. Environ.18880–84. 10.1016/j.agee.2014.02.013
23
Jaureguiberry P. Titeux N. Wiemers M. Bowler D. E. Coscieme L. Golden A. S. et al (2022). The direct drivers of recent global anthropogenic biodiversity loss.Sci. Adv.8:eabm9982. 10.1126/sciadv.abm9982
24
Khlifa R. Paquette A. Messier C. Reich P. B. Munson A. D. (2017). Do temperate tree species diversity and identity influence soil microbial community function and composition?Ecol. Evol.77965–7974. 10.1002/ece3.3313
25
Lefcheck J. S. Byrnes J. E. K. Isbell F. Gamfeldt L. Griffin J. N. Eisenhauer N. et al (2015). Biodiversity enhances ecosystem multifunctionality across trophic levels and habitats.Nat. Commun.6:6936. 10.1038/ncomms7936
26
Li J. Zhou X. Yan J. Li H. He J. (2015). Effects of regenerating vegetation on soil enzyme activity and microbial structure in reclaimed soils on a surface coal mine site.Appl. Soil Ecol.8756–62. 10.1016/j.apsoil.2014.11.010
27
Li N. Zhang Y. Qu Z. Liu B. Huang L. Ming A. et al (2024). Mixed and continuous cropping eucalyptus plantation facilitated soil carbon cycling and fungal community diversity after a 14-year field trail.Indust. Crops Prod.210:118157. 10.1016/j.indcrop.2024.118157
28
Li P. Yang Z. Yan P. Wu D. (2022). Quality evaluation of mixed plantations of Pinus massoniana and Castanopsis hystrix based on the soil erosion characteristics and soil physical and chemical properties.J. Central S. Univ. For. Technol.42104–116. 10.1016/j.foreco.2017.08.025
29
Li S. Huang X. Lang X. Shen J. Xu F. Su J. (2020). Cumulative effects of multiple biodiversity attributes and abiotic factors on ecosystem multifunctionality in the Jinsha River valley of southwestern China.For. Ecol. Manage.472:118281. 10.1016/j.foreco.2020.118281
30
Li S. Huang X. Tang R. Li J. Zhu B. Su J. (2023). Soil microbial diversity and network complexity sustain ecosystem multifunctionality following afforestation in a dry-hot valley savanna.CATENA231:107329. 10.1016/j.catena.2023.107329
31
Li T. Wu S. Wang L. Lv F. Lan Z. (2022). Progress in National and International Research on Mixed Forest.World For. Res.3542–48. 10.13348/j.cnki.sjlyyj.2022.0039.y
32
Li W. Q. Huang Y. X. Chen F. S. Liu Y. Q. Lin X. F. Zong Y. Y. et al (2021). Mixing with broad-leaved trees shapes the rhizosphere soil fungal communities of coniferous tree species in subtropical forests.For. Ecol. Manage.480:118664. 10.1016/j.foreco.2020.118664
33
Li X. Liu Y. Wu G. Lie Z. Sheng H. Aguila L. C. R. et al (2024). Mixed plantations do not necessarily provide higher ecosystem multifunctionality than monoculture plantations.Sci. Total Environ.914:170156. 10.1016/j.scitotenv.2024.170156
34
Li X. Wang T. Chang S. X. Jiang X. Song Y. (2020). Biochar increases soil microbial biomass but has variable effects on microbial diversity: a meta-analysis.Sci. Total Environ.749:141593. 10.1016/j.scitotenv.2020.141593
35
Liang K. Jiang Y. Qi J. Fuller K. Nyiraneza J. Meng F.-R. (2020). Characterizing the impacts of land use on nitrate load and water yield in an agricultural watershed in Atlantic Canada.Sci. Total Environ.729:138793. 10.1016/j.scitotenv.2020.138793
36
Ling G. Shen H. Fan R. Zhang N. Xia C. Hu W. (2022). Growth and stoichiometry characteristics of C, N and P in Soil under Mixed Plantation of Different Aged Cunninghamia lanceolata and Phoebe bournei.J. Zhejiang For. Sci. Technol.4214–20. 10.3969/j.issn.1001-3776.2022.06.003
37
Liu B. Zhen B. Pan Q. He Z. (2023). Study on soil remediation effect of open-pit mine dump under different reclamation modes.J. Anhui Agric. Sci.5153–57. 10.1016/j.catena.2024.108193
38
Liu C.-A. Nie Y. Zhang Y.-M. Tang J.-W. Siddique K. H. M. (2018). Introduction of a leguminous shrub to a rubber plantation changed the soil carbon and nitrogen fractions and ameliorated soil environments.Sci. Rep.8:17324. 10.1038/s41598-018-35762-0
39
Liu H. Lu X. (2024). Leaf–Soil C:N:P Stoichiometry and Homeostasis Characteristics of Plantations in the Yellow River Floodplain in Western Shandong, China.Forests15:1433. 10.3390/f15081433
40
Liu H. Liang Y. Liu J. Zhang Q. Liu Y. Zeng J. et al (2025). Long-term no-tillage enhanced soil multifunctionality and reduced microbial metabolic entropy.Appl. Soil Ecol.206:105876. 10.1016/j.apsoil.2025.105876
41
Liu J. Wei Y. Du H. Zhu W. Zhou Y. Yin Y. (2022). Effects of intercropping between morus alba and nitrogen fixing species on soil microbial community structure and diversity.Forests13:1345. 10.3390/f13091345
42
Liu L. He T. Zhu N. Peng Y. Gao X. Liu Z. et al (2023). Effects of afforestation patterns on soil nutrient and microbial community diversity in rocky desertification areas.Forests14:2370. 10.3390/f14122370
43
Liu S. Yang Y. Wang H. (2018). Development strategy and management countermeasures of planted forests in China: transforming from timber-centered single objective management towards multi-purpose management for enhancing quality and benefits of ecosystem services.Acta Ecol. Sin.381–10. 10.5846/stxb201712072201
44
Liu X. Su S. Li Y. Wang W. (2021). Study on the ecological benefits of a plantation mixed forest model in the Loess Plateau.Arid Zone Res.38380–391. 10.1016/j.foreco.2023.121571
45
Luo G. Rensing C. Chen H. Liu M. Wang M. Guo S. et al (2018). Deciphering the associations between soil microbial diversity and ecosystem multifunctionality driven by long-term fertilization management.Funct. Ecol.321103–1116. 10.1111/1365-2435.13039
46
Luo H. Liu W. Yang M. Ye S. Cheng F. (2022). Differences of soil physicochemical property, phenolic acid and enzyme activities in different Eucalyptus mixed plantations.J. Northwest A F Univ.50:76. 10.13207/j.cnki.jnwafu.2022.12.007
47
Ma L. Ma J. Yan P. Tian F. Peñuelas J. Rao M. P. et al (2025). Planted forests in China have higher drought risk than natural forests.Glob. Change Biol.31:e70055. 10.1111/gcb.70055
48
Ma X. Geng Q. Zhang H. Bian C. Chen H. Y. H. Jiang D. et al (2021). Global negative effects of nutrient enrichment on arbuscular mycorrhizal fungi, plant diversity and ecosystem multifunctionality.New Phytol.2292957–2969. 10.1111/nph.17077
49
Ma Z. Chen H. Y. H. (2016). Effects of species diversity on fine root productivity in diverse ecosystems: a global meta-analysis.Global Ecol. Biogeogr.251387–1396. 10.1111/geb.12488
50
Maestre F. T. Quero J. L. Gotelli N. J. Escudero A. Ochoa V. Delgado-Baquerizo M. et al (2012). Plant species richness and ecosystem multifunctionality in Global Drylands.Science335214–218. 10.1126/science.1215442
51
Mensah S. Salako K. V. Assogbadjo A. Glèlè Kakaï R. Sinsin B. Seifert T. (2020). Functional trait diversity is a stronger predictor of multifunctionality than dominance: Evidence from an Afromontane forest in South Africa.Ecol. Indicat.115:106415. 10.1016/j.ecolind.2020.106415
52
Moher D. Liberati A. Tetzlaff J. Altman D. G. The P. G. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.PLoS Med.6:e1000097. 10.1371/journal.pmed.1000097
53
Mus F. Crook M. B. Garcia K. Garcia Costas A. Geddes B. A. Kouri E. D. et al (2016). Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes.Appl. Environ. Microbiol.823698–3710. 10.1128/AEM.01055-16
54
Niu W. Ding J. Fu B. Zhao W. Han Y. Zhou A. et al (2024). Ecosystem multifunctionality is more related to the indirect effects than to the direct effects of human management in China’s drylands.J. Environ. Manage.368:122259. 10.1016/j.jenvman.2024.122259
55
Pan C. Sun C. Yu W. Guo J. Yu Y. Li X. (2023). Mixed planting enhances soil multi-nutrient cycling by homogenizing microbial communities across soil vertical scale.Land Degrad. Dev.341477–1490. 10.1002/ldr.4547
56
Peng S. Liu Y. Mao R. Liu Y. Fan Y. Zhou Y. et al (2025). Dynamic effects of Pinus massoniana replanted with broad-leaved of Schima superba on soil microbial biomass carbon and nitrogen.J. Guangxi Normal Univ.43150–160. 10.16088/j.issn.1001-6600.2024062801
57
Pretzsch H. del Rio M. Ammer C. Avdagic A. Barbeito I. Bielak K. et al (2015). Growth and yield of mixed versus pure stands of Scots pine (Pinus sylvestris L.) and European beech (Fagus sylvatica L.) analysed along a productivity gradient through Europe.Eur. J. For. Res.134927–947. 10.1007/s10342-015-0900-4
58
Qin J. Tang X. Yang X. (2013). Effects of soil physical and chemical properties on different forest types of Pinus massoniana.Ecol. Environ. Sci.22598–604. 10.16258/j.cnki.1674-5906.2013.04.012
59
R Core Team (2024). R: A Language and Environment for Statistical Computing.Vienna: R Foundation for Statistical Computing.
60
Rosenberg M. S. (2005). The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis.Evolution59464–468. 10.1111/j.0014-3820.2005.tb01004.x
61
Shi X. Wang J. Lucas-Borja M. E. Wang Z. Li X. Huang Z. (2021). Microbial diversity regulates ecosystem multifunctionality during natural secondary succession.J. Appl. Ecol.582833–2842. 10.1111/1365-2664.14015
62
Shu X. Liu W. Hu Y. Xia L. Fan K. Zhang Y. et al (2023). Ecosystem multifunctionality and soil microbial communities in response to ecological restoration in an alpine degraded grassland.Front. Plant Sci.14:1173962. 10.3389/fpls.2023.1173962
63
Shu X. Ye Q. Huang H. Xia L. Tang H. Liu X. et al (2024). Effects of grazing exclusion on soil microbial diversity and its functionality in grasslands: a meta-analysis.Front. Plant Sci.15:1366821. 10.3389/fpls.2024.1366821
64
Singh K. Singh B. Singh R. R. (2012). Changes in physico-chemical, microbial and enzymatic activities during restoration of degraded sodic land: Ecological suitability of mixed forest over monoculture plantation.CATENA9657–67. 10.1016/j.catena.2012.04.007
65
Sui J. Yu Q. Yang K. Yang J. Li C. Liu X. (2022). Effects of Bacillus subtilis T6-1 on the rhizosphere microbial community structure of continuous cropping poplar.Biology11:791. 10.3390/biology11050791
66
Sun K. Cai J. Liu X. Yang L. Li H. Wang G. et al (2023). Effects of nitrogen and phosphorus supply levels and ratios on soil microbial diversity-ecosystem multifunctionality relationships in a coastal nontidal wetland.Sci. Total Environ.874:162472. 10.1016/j.scitotenv.2023.162472
67
van der Plas F. Manning P. Allan E. Scherer-Lorenzen M. Verheyen K. Wirth C. et al (2016). Jack-of-all-trades effects drive biodiversity–ecosystem multifunctionality relationships in European forests.Nat. Commun.7:11109. 10.1038/ncomms11109
68
Viechtbauer W. (2010). Conducting meta-analyses in R with the metafor Package.J. Stat. Softw.361–48. 10.18637/jss.v036.i03
69
Wan X. Wang W. Liu J. Tong T. (2014). Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range.BMC Med. Res. Methodol.14:135. 10.1186/1471-2288-14-135
70
Wang B. Yang X. Zhu Y. Tu Y. Wang D. Indree T. (2024). Plant diversity mediates the response of ecosystem multifunctionality to climate factor in Eastern Eurasian Steppe.Glob. Ecol. Conserv.50:e02827. 10.1016/j.gecco.2024.e02827
71
Wang J. Shi X. Lucas-Borja M. E. Lam S. K. Wang Z. Huang Z. (2022). Plants, soil properties and microbes directly and positively drive ecosystem multifunctionality in a plantation chronosequence.Land Degrad. Dev.333049–3057. 10.1002/ldr.4371
72
Wang X. Feng J. Ao G. Qin W. Han M. Shen Y. et al (2023). Globally nitrogen addition alters soil microbial community structure, but has minor effects on soil microbial diversity and richness.Soil Biol. Biochem.179:108982. 10.1016/j.soilbio.2023.108982
73
Wang X. Guo Y. Zhao H. Meng F. Liu G. (2018). Decomposition characteristics and its nutrient dynamics of the leaf litter mixtures of Larix principis-rupprechtii and Betula platyphylla.For. Ecol. Sci.3329–36. 10.13466/j.cnki.lyzygl.2018.03.017
74
Wang Y. Ma L. Liu Z. Chen J. Song H. Wang J. et al (2022). Microbial interactions play an important role in regulating the effects of plant species on soil bacterial diversity.Front. Microbiol.13:984200. 10.3389/fmicb.2022.984200
75
Wang Y. Zou G. Fu H. Liu H. (2005). Development and advance of soil nitrogen mineralization.Chin. Agric. Sci. Bulle.21203–208. 10.3969/j.issn.1000-6850.2005.10.056
76
Wu W. Zhou X. Wen Y. Zhu H. You Y. Qin Z. et al (2019). Coniferous-broadleaf mixture increases soil microbial biomass and functions accompanied by improved stand biomass and litter production in subtropical China.Forests10:879. 10.3390/f10100879
77
Wu Y. Zhang S. Xie G. Shao Y. Shi S. Lin J. et al (2025). Response of leaf functional traits and rhizosphere microbial communities of Castanopsis hystrix in three subtropical plantations with leguminous or non-leguminous trees.Forests16:367. 10.3390/f16020367
78
Xiang Y. Li Y. Luo X. Liu Y. Huang P. Yao B. et al (2022). Mixed plantations enhance more soil organic carbon stocks than monocultures across China: implication for optimizing afforestation/reforestation strategies.Sci. Total Environ.821:153449. 10.1016/j.scitotenv.2022.153449
79
Xiao Y. Huang Z. Li Y. Zhang Y. Wang M. (2022). Soil microbial community structure and diversity of typical vegetation types in Chishui River Basin.Res. Soil Water Conserv.29275–283. 10.13869/j.cnki.rswc.2022.06.003
80
Xie H. Wang G. G. Yu M. (2018). Ecosystem multifunctionality is highly related to the shelterbelt structure and plant species diversity in mixed shelterbelts of eastern China.Glob. Ecol. Conserv.16:e00470. 10.1016/j.gecco.2018.e00470
81
Xu H. Wei X. Cheng X. (2023). Fungal diversity dominates the response of multifunctionality to the conversion of pure plantations into two-aged mixed plantations.Sci. Total Environ.866:161384. 10.1016/j.scitotenv.2022.161384
82
Xu Y. Li C. Zhu W. Wang Z. Wu L. Du A. (2022). Effects of enrichmemt planting with native tree species on bacterial community structure and potential impact on Eucalyptus plantations in southern China.J. For. Res.331349–1363. 10.1007/s11676-021-01433-6
83
Xu Y. Ren S. Liang Y. Du A. Li C. Wang Z. et al (2021). Soil nutrient supply and tree species drive changes in soil microbial communities during the transformation of a multi-generation Eucalyptus plantation.Appl. Soil Ecol.166:103991. 10.1016/j.apsoil.2021.103991
84
Xu Z. Hu Z. Jiao S. Bell S. M. Xu Q. Ma L. et al (2023). Depth-dependent effects of tree species identity on soil microbial community characteristics and multifunctionality.Sci. Total Environ.878:162972. 10.1016/j.scitotenv.2023.162972
85
Yan K. Dong Y. Gong Y. Zhu Q. Wang Y. (2021). Climatic and edaphic factors affecting soil bacterial community biodiversity in different forests of China.CATENA207:105675. 10.1016/j.catena.2021.105675
86
Yan P. Fernández-Martínez M. Van Meerbeek K. Yu G. Migliavacca M. He N. (2023). The essential role of biodiversity in the key axes of ecosystem function.Glob. Change Biol.294569–4585. 10.1111/gcb.16666
87
Yang X. Tian X. Wu Q. (2024). Soil bacterial community diversity of different forest types in southern subtropical Guangxi and their correlation to soil chemical properties.J. S. Agric.55954–963. 10.3969/j.issn.2095-1191.2024.04.005
88
Yu X. Liu X. Zhao Z. Liu J. Zhang S. (2015). Effect of monospecific and mixed sea-buckthorn (Hippophae rhamnoides) plantations on the structure and activity of soil microbial communities.PLoS One10:e0117505. 10.1371/journal.pone.0117505
89
Yuan Z. Q. Ali A. Loreau M. Ding F. Liu S. F. Sanaei A. et al (2021). Divergent above- and below-ground biodiversity pathways mediate disturbance impacts on temperate forest multifunctionality.Glob. Change Biol.272883–2894. 10.1111/gcb.15606
90
Zhang L. Li H. Wu C. Linghu G. Zhu H. Khamphilavong K. et al (2023). Far-reaching effects on soil properties and underground microbial ecosystem after the introduction of black locusts in forest.Front. Ecol. Evolut.11:1210498. 10.3389/fevo.2023.1210498
91
Zhang M. Wan Z. Gao W. Zhang Y. (2024). Effects of Chinese fir retention density on soil bacterial community structure in Chinese Fir and Betula luminifera mixed forests plantations in China.Forests15:2107. 10.3390/f15122107
92
Zhang R. Tian D. Chen H. Y. H. Seabloom E. W. Han G. Wang S. et al (2022). Biodiversity alleviates the decrease of grassland multifunctionality under grazing disturbance: a global meta-analysis.Global Ecol. Biogeogr.31155–167. 10.1111/geb.13408
93
Zhang Y. Hou L. Li Z. Zhao D. Song L. Shao G. et al (2020). Leguminous supplementation increases the resilience of soil microbial community and nutrients in Chinese fir plantations.Sci. Total Environ.703:134917. 10.1016/j.scitotenv.2019.134917
94
Zhang Y. Luo Z. Li L. Nian L. Li L. Niu Y. et al (2025). Nitrogen fertilization shapes soil microbial diversity and ecosystem multifunctionality by modulating soil nutrients.Microorganisms13:540. 10.3390/microorganisms13030540
95
Zhang Y. Ni J. Zhou C. Fan F. Xie D. (2014). Effects of configuration mode of crop-mulberry system in purple arid hillside field on SMBC and SMBN in the Three Gorges Reservoir.Chin. J. Eco-Agricult.22766–773. 10.3724/SP.J.1011.2014.40377
96
Zhou C. Gao Q. Tigabu M. Wang S. Cao S. Yu Y. (2024). Continuous planting of Chinese fir monocultures significantly influences dissolved organic matter content and microbial assembly processes.Sci. Total Environ.926:171943. 10.1016/j.scitotenv.2024.171943
97
Zhou J. Zhang J. Tian S.-T. Chen E. (2023). Soil carbon and nitrogen contents and aggregate composition of different forest types in Southeast Henan.J. Northwest For. Univ.3874–81. 10.3969/j.issn.1001-7461.2023.04.09
98
Zhu Y. Peng J. Wei Z. Shen Q. Fusuo Z. (2021). Linking the soil microbiome to soil health.Sci. Sin.511–11. 10.1360/SSV-2020-0320
99
Zhuang J. Liu Z. Huang Y. Zhou H. Xian J. Sun S. et al (2023). Community characteristics of soil bacteria and fungi in Robinia pseudoacacia plantations based on high-throughput sequencing.J. Central S. Univ. For. Technol.4392–100. 10.14067/j.cnki.1673-923x.2023.04.010
Summary
Keywords
afforestation strategies, mixed plantations, soil microbial diversity, ecosystem multifunctionality, meta-analysis
Citation
Xie K, Xu Y and Lu X (2025) Effects of mixed plantations on soil microbial diversity and ecosystem multifunctionality across China: a meta-analysis. Front. For. Glob. Change 8:1694630. doi: 10.3389/ffgc.2025.1694630
Received
28 August 2025
Accepted
29 October 2025
Published
19 November 2025
Volume
8 - 2025
Edited by
Shuai Ouyang, Central South University Forestry and Technology, China
Reviewed by
Junhong Zhang, Zhejiang Agriculture and Forestry University, China
Dongmei Fan, Zhejiang University, China
Updates
Copyright
© 2025 Xie, Xu and Lu.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xinghui Lu, luxinghui_0@163.comYue Xu, xuyue@caf.ac.cn
†These authors have contributed equally to this work
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.