- 1School of Population Health, Curtin University, Perth, WA, Australia
- 2Curtin Health Innovation Research Institute, Curtin University, Perth, WA, Australia
- 3Curtin Medical School, Curtin University, Perth, WA, Australia
Endometriosis is a complex and potentially debilitating condition that has major impact on quality of life. There is emerging evidence that biological compounds found in garlic (Allium sativum) may be effective for attenuating endometrial pain. Suggested mechanisms for efficacy include modulation of inflammation and potent antioxidant effects. Aged-garlic-extract (AGE) is a centuries old process describing ethanolic extracts of garlic bulbs for 12–20 months. The AGE formulation realised contains a complex array of stabilised biologics with significant immunomodulatory effects relevant to inflammatory conditions. This perspective article puts forward a hypothesis that AGE should be considered as a prophylactic to manage endometrial pain.
Introduction
Endometriosis is a chronic systemic disease that is pathologically characterised by uterine tissue proliferating outside of the uterine cavity (1, 2). The extra-uterine growth commonly occurs on the pelvic peritoneum and can also grow on ovaries, in the rectovaginal septum and in rare cases, exist in the pericardium, pleura, or brain (1). Endometriosis is suggested to be estrogen-dependent, however the physiological mechanisms for this association are not fully understood (1).
The American Society for Reproductive Medicine classification for endometriosis is a globally adopted scoring system where points are allocated regarding lesion characteristics and grouped into stages I (minimal) to IV (severe) (3). Stage I generally includes few superficial implants, whereas stage IV includes multiple large, deep implants and dense adhesions (3). However, a higher visually appointed severity score does not correlate with pain or symptoms experienced per se, which often results in delayed diagnosis (3, 4).
It is estimated that endometriosis affects 170 million people globally and ∼10% of women of reproductive age (1, 5–7). Symptoms include chronic pelvic pain, dysmenorrhea, dyspareunia, resulting in psychological effects and impaired social function (4, 8). Depression and anxiety due to chronic pain and stress surrounding fertility may amplify the burden of disease (9). Presently, there are limited treatments available to reduce endometrial progression, or attenuate endometrial pain, with significant impact on quality of life (4).
Aetiology and pathology
The prevailing paradigm for the aetiology of endometriosis is the theory of retrograde menstruation (1). It is proposed that uterine cells attach to distant sites from the peritoneal cavity due to the backflow of menstrual blood and cells (10). Women with endometriosis have an abnormally higher number of progenitor stem cells than women without the condition during menses (11), consistent with causality. Other pathways for disease progression include immune dysfunction, characterised by dysregulation of recruited leukocytes with limited capacity to clear fragments, increasing the propensity for lesion formation (1). Endometriotic lesions located outside the pelvis, in women without a uterus and indeed in men, indicate triggers other than retrograde menstruation (2). Coelomic metaplasia of mesothelial cells may also induce endometriosis (2).
The onset and progression of endometriosis is complex and multifactorial. Evidence suggests that either a single factor, or a combination of factors such as inflammation, oxidative stress, tumour promoting-genes, or endocrine modulation cell proliferation, microvascular tone, and angiogenesis, may be highly relevant (2, 12).
Garcia-Gomez et al. describes that inflammation is central to the pathophysiology of endometriosis and the symptoms of pain and infertility (13). However, there is complexity in delineating how immune cell function exacerbates, or attenuates endometrial disease progression. Macrophage migration inhibitory factor and monocyte chemoattractant protein-1 attracts monocytes/macrophages into endometriosis lesions (14). Inflammatory cytokines, prostaglandins and metalloproteinases are increased in endometriosis and may promote ectopic adhesion (12). Inflammatory mediators trigger epithelial-mesenchymal transition resulting in a cyclical inflammatory loop as a consequence of increased synthesis of vascular endothelial growth factor (VEGF) and angiogenesis (12, 15). Neutrophils have the ability to synthesise and secrete VEGF (16). The accumulation of these leukocytes in endometriosis has been proposed to be crucial for early disease progression due to the pro-angiogenic and inflammatory modulation effects (17, 18). Increased integrin secretion allows for ectopic implantation and concomitant hyperestrogenism can amplify lesion growth and development (12). Inadequate immunosurveillance may also exacerbate endometrial tissue proliferation. There is reduced cytotoxicity of natural killer cells in endometriosis patients which may be attributed to poor clearance of ectopic endometriotic cells (19). The dysregulation of the aforementioned pathways resulting in inflammation, invasion and metastasis could be considered as targets for endometriosis treatment (refer to Figure 1).
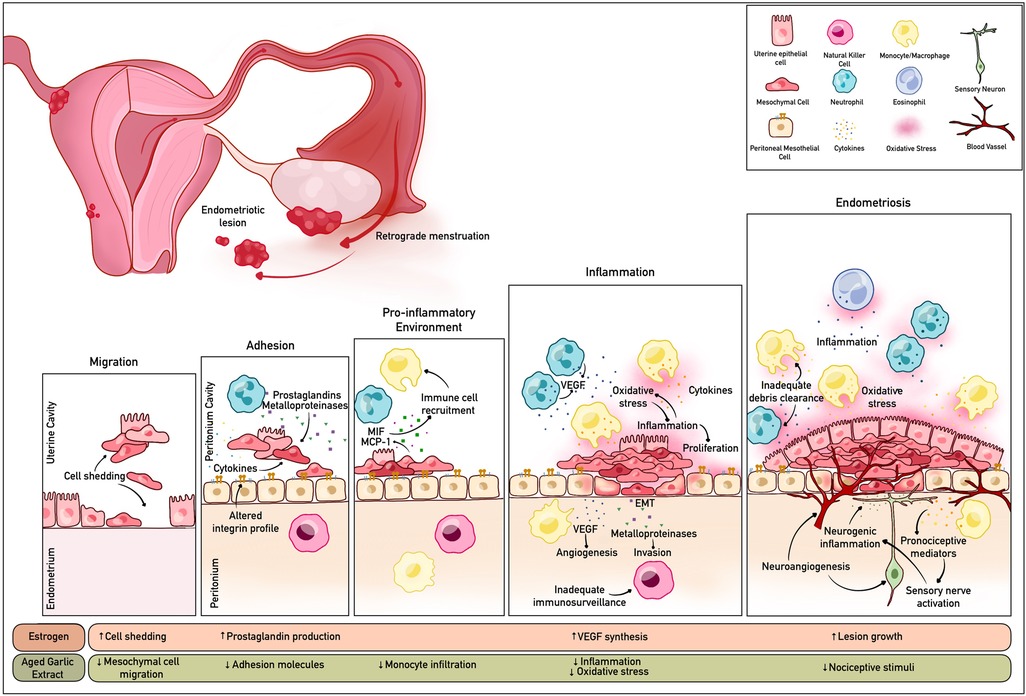
Figure 1. Schematic summary of the cellular disruption pathways, inflammation and oxidative stress associated with endometriosis. The bioactive compounds in AGE may modulate these mechanisms by targeting various pathways. Created with BioRender.com.
Endometrial pain treatment
The pain mechanisms associated with endometriosis are suggested to be positively associated with inflammation and angiogenesis resulting in neurogenic inflammation and cross-organ sensitization (20). Treatments for endometriosis are presently limited. Contemporary opportunities include surgical intervention, chronic use of nonsteroidal anti-inflammatory drugs, oral contraceptives, or endocrine modulation (gonadotrophin-releasing hormone agonists) (21). Surgical intervention can provide transient pain relief, however, recurrence within 5 years occurs in up to 50% of patients (22). Pharmacological interventions have limited efficacy and may have significant off-target effects including; osteoporosis, lipid profile changes and metrorrhagia (21).
In recent years nutraceuticals have gained increased attention as an alternative for endometriosis related pain relief. Bioactive compounds naturally occurring in plants have emerging evidence for anti-endometriotic effects, including quercetin, curcumin, resveratrol and naringenin (21). It has been suggested that garlic has anti-inflammatory and antioxidant effects that may be relevant in the context of endometriosis. A recent study by Amirsalari et al. found that consumption of 400 mg of garlic powder results in a significant reduction of pain symptoms in women with endometriosis (23). They suggest that garlic can reduce oxidative stress, prostaglandin production, limit proliferation of endometrial cells, and improve estrogen elimination (23). While this is the first human trial known using garlic as a nutraceutical for endometriosis, the results are consistent with a substantial body of literature describing the anti-inflammatory and antinociceptive effects of garlic.
Kim et al. found that in an in vitro model of activated endometrial stromal cells, vascular adhesion molecule-1 and intercellular adhesion molecule-1 expression was reduced in response to 500 ml hexane-extract of aged black garlic (HEABG) (24). This study reported that HEABG resulted in a reduction in the proliferation of endometrial stromal cells that were activated by tumour necrosis factor (TNF)-α (24). The biological elements of garlic may also be important in attenuating the damaging effects of oxidative stress associated with inflammation. Infertile women with endometriosis were found to have lower levels of peritoneal fluid and serum antioxidant markers (total antioxidant capacity, catalase enzyme and superoxide dismutase enzyme) content compared to infertile women without endometriosis (25).
Garlic is rich in N-acetylcysteine (NAC) which has been reported to have anti-proliferative effects in endometriomas. With 3 months of NAC supplementation, taken orally, mean diameter of lesions was reduced by 1.5 mm whereas those without treatment had a significant increase in lesion size diameter of 6.6 mm (26). These effects were attributed to the downregulatory effects of NAC on inflammatory pathways and inhibiting cell migration (26).
In humans, antinociceptive effects of garlic have been indicated in patients with knee osteoarthritis which has similar inflammatory sequelae to endometriosis with increased inflammatory factors and associated TNF-α (27–29). Similar findings have been reported in rheumatoid arthritis, which is subject to increased oxidative stress, and peripheral arterial occlusive disease (30, 31). It has been suggested that pain reduction may be directly attributable to the antioxidant effect of garlic (32). In animal studies there have been a number of induced models of pain demonstrating an attenuation with the provision of garlic derivatives (33). In murine models with central and peripheral induced pain, there was increased antinociception through the provision of garlic shoot extract (34).
AGE is processed by chopping raw garlic cloves and storing them in 15%–20% ethanol for up to 20 months (35). The main organosulphur compounds in AGE are water-soluble S-allyl-cysteine (SAC), allin, ɣ-glutamylcysteines, ɣ-glutamyl-S-allylcystine, and S-allylmercaptocysteine (SAMC) (32). The SAC and SAMC concentrations in AGE are estimated to be >60-fold more abundant per unit weight than in raw garlic due to the ageing process (36–38), enabling a pharmacological dose to be tested more readily clinically. These specific compounds have exceedingly potent immunomodulatory and antioxidant properties that may be relevant to endometriosis progression and pain management. Phenolic compounds in AGE such as allicin also contribute to antioxidant activity (36).
Conclusion
Endometriosis can result in a high burden of disability and is costly to treat. Prophylactive therapies that delay progression and/or decrease endometrial pain are a global health priority. A body of literature suggests that Allium sativum and particularly, ethanolic extracts of the garlic bulb containing enriched stabilised biologicals may be effective in attenuating endometrial progression and pain burden.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author/s.
Author contributions
ER was responsible for literature review, manuscript writing. VL, RT, DK and CJ were responsible for the conceptualization, literature review and manuscript preparation. AS was responsible for image construction. JM led the conceptualization, literature review and manuscript preparation. All authors contributed to the article and approved the submitted version.
Funding
The study will be funded by MSWA and supported by the Australian National Health and Medical Research Council (NHMRC).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Giudice LC, Kao LC. Endometriosis. Lancet. (2004) 364(9447):1789–99. doi: 10.1016/S0140-6736(04)17403-5
2. Taylor HS, Kotlyar AM, Flores VA. Endometriosis is a chronic systemic disease: clinical challenges and novel innovations. Lancet. (2021) 397(10276):839–52. doi: 10.1016/S0140-6736(21)00389-5
3. Lee SY, Koo YJ, Lee DH. Classification of endometriosis. Yeungnam Univ J Med. (2021) 38(1):10–8. doi: 10.12701/yujm.2020.00444
4. Gao X, Yeh YC, Outley J, Simon J, Botteman M, Spalding J. Health-related quality of life burden of women with endometriosis: a literature review. Curr Med Res Opin. (2006) 22(9):1787–97. doi: 10.1185/030079906X121084
5. Louis GM B, Hediger ML, Peterson CM, Croughan M, Sundaram R, Stanford J, et al. Incidence of endometriosis by study population and diagnostic method: the ENDO study. Fertil Steril. (2011) 96(2):360–5. doi: 10.1016/j.fertnstert.2011.05.087
6. Eskenazi B, Warner ML. Epidemiology of endometriosis. Obstet Gynecol Clin North Am. (1997) 24(2):235–58. doi: 10.1016/S0889-8545(05)70302-8
7. Adamson GD, Kennedy S, Hummelshoj L. Creating solutions in endometriosis: global collaboration through the world endometriosis research foundation. J Endometr. (2010) 2(1):3–6. doi: 10.1177/228402651000200102
8. Ballard K, Seaman H, De Vries C, Wright J. Can symptomatology help in the diagnosis of endometriosis? Findings from a national case–control study—part 1. BJOG. (2008) 115(11):1382–91. doi: 10.1111/j.1471-0528.2008.01878.x
9. Chen LC, Hsu JW, Huang KL, Bai YM, Su TP, Li CT, et al. Risk of developing major depression and anxiety disorders among women with endometriosis: a longitudinal follow-up study. J Affect Disord. (2016) 190:282–5. doi: 10.1016/j.jad.2015.10.030
10. Sampson JA. Metastatic or embolic endometriosis, due to the menstrual dissemination of endometrial tissue into the venous circulation. Am J Pathol. (1927) 3(2):93–110.43.19969738
11. Hapangama DK, Drury J, Da Silva L, Al-Lamee H, Earp A, Valentijn AJ, et al. Abnormally located SSEA1+/SOX9+ endometrial epithelial cells with a basalis-like phenotype in the eutopic functionalis layer may play a role in the pathogenesis of endometriosis. Hum Reprod. (2019) 34(1):56–68. doi: 10.1093/humrep/dey336
12. Kapoor R, Stratopoulou CA, Dolmans M-M. Pathogenesis of endometriosis: new insights into prospective therapies. Int J Mol Sci. (2021) 22(21):11700. doi: 10.3390/ijms222111700
13. García-Gómez E, Vázquez-Martínez ER, Reyes-Mayoral C, Cruz-Orozco OP, Camacho-Arroyo I, Cerbón M. Regulation of inflammation pathways and inflammasome by sex steroid hormones in endometriosis. Front Endocrinol. (2020) 10:4–10. doi: 10.3389/fendo.2019.00935
14. Akoum A, Kong J, Metz C, Beaumont M-C. Spontaneous and stimulated secretion of monocyte chemotactic protein-1 and macrophage migration inhibitory factor by peritoneal macrophages in women with and without endometriosis. Fertil Steril. (2002) 77(5):989–94. doi: 10.1016/S0015-0282(02)03082-0
15. Delbandi AA, Mahmoudi M, Shervin A, Heidari S, Kolahdouz-Mohammadi R, Zarnani AH. Evaluation of apoptosis and angiogenesis in ectopic and eutopic stromal cells of patients with endometriosis compared to non-endometriotic controls. BMC Womens Health. (2020) 20(1):3. doi: 10.1186/s12905-019-0865-4
16. Na Y-J, Yang S-H, Baek D-W, Lee D-H, Kim K-H, Choi Y-M, et al. Effects of peritoneal fluid from endometriosis patients on the release of vascular endothelial growth factor by neutrophils and monocytes. Hum Reprod. (2006) 21(7):1846–55. doi: 10.1093/humrep/del077
17. Takamura M, Koga K, Izumi G, Urata Y, Nagai M, Hasegawa A, et al. Neutrophil depletion reduces endometriotic lesion formation in mice. Am J Reprod Immunol. (2016) 76(3):193–8. doi: 10.1111/aji.12540
18. Symons LK, Miller JE, Tyryshkin K, Monsanto SP, Marks RM, Lingegowda H, et al. Neutrophil recruitment and function in endometriosis patients and a syngeneic murine model. FASEB J. (2020) 34(1):1558–75. doi: 10.1096/fj.201902272R
19. Jeung I, Cheon K, Kim M-R. Decreased cytotoxicity of peripheral and peritoneal natural killer cell in endometriosis. BioMed Res Int. (2016) 2016:2916070. doi: 10.1155/2016/2916070
20. Maddern J, Grundy L, Castro J, Brierley SM. Pain in endometriosis. Front Cell Neurosci. (2020) 14:4–9. doi: 10.3389/fncel.2020.590823
21. Gołąbek A, Kowalska K, Olejnik A. Polyphenols as a diet therapy concept for endometriosis—current opinion and future perspectives. Nutrients. (2021) 13(4):1347. doi: 10.3390/nu13041347
22. Guo S-W. Recurrence of endometriosis and its control. Hum Reprod Update. (2009) 15(4):441–61. doi: 10.1093/humupd/dmp007
23. Amirsalari S, Behboodi Moghadam Z, Taghizadeh Z, Jafar Abadi MN, Sabaghzadeh Irani P, Goodarzi S, et al. The effect of garlic tablets on the endometriosis-related pains: a randomized placebo-controlled clinical trial. Evid Based Complementary Altern Med. (2021) 2021:5547058. doi: 10.1155/2021/5547058
24. Kim K-H, Park JK, Choi Y-W, Kim Y-H, Lee EN, Lee J-R, et al. Hexane extract of aged black garlic reduces cell proliferation and attenuates the expression of ICAM-1 and VCAM-1 in TNF-α-activated human endometrial stromal cells. Int J Mol Med. (2013) 32(1):67–78. doi: 10.3892/ijmm.2013.1362
25. Osman HG, El-Refaey AAA, Aziz AAFA, El-Sokkary AMA, El-Saeed RA. Leptin and antioxidant profile in infertile women with endometriosis. J Endometr. (2010) 2(3):135–43. doi: 10.1177/228402651000200304
26. Porpora MG, Brunelli R, Costa G, Imperiale L, Krasnowska EK, Lundeberg T, et al. A promise in the treatment of endometriosis: an observational cohort study on ovarian endometrioma reduction by N-acetylcysteine. Evid Based Complementary Altern Med. (2013) 2013:240702. doi: 10.1155/2013/240702
27. Hussein NA, Sharara GM. Poster 9: effect of combined garlic therapy and comprehensive rehabilitation program versus comprehensive rehabilitation program alone on control of clinical manifestations and quality of life of knee osteoarthritis patients. Arch Phys Med Rehabil. (2007) 88(9):E12. doi: 10.1016/j.apmr.2007.06.584
28. Dehghani S, Alipoor E, Salimzadeh A, Yaseri M, Hosseini M, Feinle-Bisset C, et al. The effect of a garlic supplement on the pro-inflammatory adipocytokines, resistin and tumor necrosis factor-alpha, and on pain severity, in overweight or obese women with knee osteoarthritis. Phytomedicine. (2018) 48:70–5. doi: 10.1016/j.phymed.2018.04.060
29. Hosseinzadeh-Attar MJ, Alipoor E, Dehghani S, Salimzadeh A. Increased efficacy of a garlic supplement on knee osteoarthritis symptoms in patients with obesity. J Herb Med. (2020) 24:100392. doi: 10.1016/j.hermed.2020.100392
30. Kiesewetter H, Jung F, Jung EM, Blume J, Mrowietz C, Birk A, et al. Effects of garlic coated tablets in peripheral arterial occlusive disease. Clin Investig. (1993) 71(5):383–6. doi: 10.1007/BF00186628
31. Moosavian SP, Paknahad Z, Habibagahi Z. A randomized, double-blind, placebo-controlled clinical trial, evaluating the garlic supplement effects on some serum biomarkers of oxidative stress, and quality of life in women with rheumatoid arthritis. Int J Clin Pract. (2020) 74(7):e13498. doi: 10.1111/ijcp.13498
32. Hernández-Cruz EY, Silva-Islas CA, Maldonado PD, Pedraza-Chaverri J, Carballo-Villalobos AI. Antinociceptive effect of garlic, garlic preparations and derivative compounds. Eur J Pain. (2022) 26(5):947–64. doi: 10.1002/ejp.1935
33. Farjana F, Sakib N, Hasan M, Das P, Hossain A, Rahmatullah M. Antinociceptive activity studies with methanol extract of onion, garlic and ginger in mice. Adv Nat Appl Sci. (2014) 8:169–74.
34. Dange S, Mathew J, Datta A, Tilak A, Jadhav M. Evaluation of the analgesic efficacy of garlic shoots extract in experimental pain models in mice. Int J Basic Clin Pharmacol. (2016) 5:2393–6. doi: 10.18203/2319-2003.ijbcp20164094
35. Lawson LD. Garlic: a review of its medicinal effects and indicated active compounds. Phytomedicines of Europe. ACS symposium series. 691: American Chemical Society (1998). p. 176–209.
36. Borek C. Antioxidant health effects of aged garlic extract. J Nutr. (2001) 131(3):1010S–5S. doi: 10.1093/jn/131.3.1010S
37. Yudhistira B, Punthi F, Lin JA, Sulaimana AS, Chang CK, Hsieh CW. S-Allyl cysteine in garlic (Allium sativum): formation, biofunction, and resistance to food processing for value-added product development. Compr Rev Food Sci Food Saf. (2022) 21(3):2665–87. doi: 10.1111/1541-4337.12937
Keywords: endometriosis, pain, nutraceuticals, prophylactic, aged garlic extract
Citation: Redwood E, Lam V, Takechi R, Kerr DA, Jackaman C, Sharif A and Mamo JCL (2022) Aged garlic extract as a potential prophylactic to reduce the progression of endometriosis and associated pain burden. Front. Pain Res. 3:1057830. doi: 10.3389/fpain.2022.1057830
Received: 30 September 2022; Accepted: 31 October 2022;
Published: 17 November 2022.
Edited by:
Luke Grundy, Flinders University, AustraliaReviewed by:
Paramita Basu, University of Pittsburgh, United States© 2022 Redwood, Lam, Takechi, Kerr, Jackaman, Sharif and Mamo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: John Mamo ai5tYW1vQGN1cnRpbi5lZHUuYXU=
Specialty Section: This article was submitted to Abdominal and Pelvic Pain, a section of the journal Frontiers in Pain Research
 Emily Redwood
Emily Redwood Virginie Lam
Virginie Lam Ryusuke Takechi
Ryusuke Takechi Deborah Anne Kerr
Deborah Anne Kerr Connie Jackaman
Connie Jackaman Arazu Sharif2,3
Arazu Sharif2,3 John Charles Louis Mamo
John Charles Louis Mamo