- 1Department of Anesthesiology, Toyama University Hospital, Toyama, Toyama, Japan
- 2Department of Anesthesiology and Intensive Care Unit, Gunma University Hospital, Maebashi, Gunma, Japan
Introduction: Sleep disturbances have been shown to exacerbate pain sensitivity and prolong recovery from pain. However, conventional animal models of sleep disturbance, which involve physical disruptions, such as water or forced movement, might not fully represent modern human sleep disorders.
Methods: We utilized a novel sleep disorder model, the perpetual avoidance of water on a wheel (PAWW) model, which induces spontaneous activity, chronic stress, and abnormal sleep–wake cycles in mice. We investigated the effects of a kappa opioid receptor (KOR) antagonist, nor-binaltorphimine (nor-BNI), on pain recovery in a postoperative pain model in mice in a state of disordered sleep. After 1 week of acclimation, the mice were housed in PAWW or regular cages for 2 weeks. Sleep conditions were evaluated using electroencephalogram and electromyogram recordings, and postoperative pain recovery following plantar incision was assessed using von Frey tests. We also examined the effects of nor-BNI on pain recovery.
Results: The evaluation showed that PAWW housing significantly increased activity during the light phase, disrupted sleep patterns, and delayed postoperative pain recovery. The administration of nor-BNI alleviated the delayed pain recovery.
Discussion: These findings suggest that sleep disorders, such as those modeled by PAWW, could delay postoperative pain recovery, and that KOR antagonists might provide therapeutic benefits in the management of delayed recovery of postoperative pain induced by sleep disorders.
1 Introduction
Clinical studies have reported that sleep disorders in perioperative patients exacerbate postoperative pain (1–3). However, well-evidenced treatments for this issue have not been established. While basic research is essential for developing novel treatments grounded in scientific mechanisms, traditional animal models of sleep disturbance used in such studies often fail to replicate the sleep disorders experienced by humans in modern society. The conventional animal models rely on physical stimuli or acute strong stress, such as single platform-on-water, forced movement, mechanical stimulation, and fear-conditioning, to interrupt sleep (4–6), which does not accurately mimic the more subtle sleep disorders associated with modern stressors.
To address these limitations, we utilized the perpetual avoidance of water on a wheel (PAWW) model. In this model, mice are housed in a running wheel set above shallow water, promoting chronic voluntary movement and disrupting their sleep–wake cycle without acute physical stress (7). Although the PAWW model induces chronic stress, its primary feature is disruption of the normal sleep–wake cycle via increased spontaneous activity. In this study, we focused on the sleep impairment aspect of the PAWW model as it pertains to postoperative pain recovery. While we acknowledge that stress and sleep disturbances are closely interrelated, our experimental design aimed to investigate the effects of stress-induced sleep disruption on pain recovery, rather than measuring stress responses.
Pain and sleep are interlinked through common brain regions such as the frontal cortex, hypothalamus, and brainstem (8, 9), and via shared neurotransmitters, including opioids (10–13), monoamines (14–17), and orexin (18, 19). Dysregulation of these pathways contributes to both sleep disturbances and pain sensitization. Accordingly, pharmacologic targets such as antidepressants (20, 21), orexin receptor antagonists (22), kappa opioid receptor (KOR) antagonists (8, 9), and melatonin receptor agonists (23) are promising candidates for addressing both conditions.
Recent evidence has highlighted the role of dynorphin/KOR signaling in the anterior cingulate cortex and hypothalamus is implicated in sleep fragmentation and emotional dysregulation. Systemic KOR antagonism restores normal sleep in chronic pain models without altering baseline sleep in sham animals (8, 9). Moreover KOR signaling has also been reported to induce mechanical hypersensitivity in rodent pain models (24, 25). Although the underlying mechanisms of these effects remain unclear, they might involve interactions between the anterior cingulate cortex, hypothalamic orexin neurons, and ascending monoaminergic systems in the brain stem (14, 15, 26).
In the present study,we investigated the effect of nor-binaltorphimine (nor-BNI), a selective and long-acting KOR antagonist, on postoperative pain recovery in mice experiencing sleep disturbance induced by PAWW housing.
2 Materials and methods
2.1 Animals
This study was carried out according to the principles of the Act on Welfare and Management of Animals and the Guidelines for Proper Conduct of Animal Experiments of the Science Council of Japan, and followed the Guiding Principles for the Care and Use of Laboratory Animals at the University of Toyama. The experiments involved 8-week-old male C57BL/6J mice (Japan SLC), maintained at 22–26°C with a 12 h light–dark cycle. Zeitgeber time (ZT) 0 and ZT 12 represent the light onset (07:00) and offset times (19:00), respectively. Food and water were provided ad libitum. Every effort was made to minimize the number and suffering of the animals used in the experiments.
2.2 Study design
Animals were randomly assigned to either the PAWW or control group before the start of the experiments, with allocation being independent of the baseline number of wheel rotations. There was no significant difference in baseline wheel rotations between the PAWW and control groups, confirming the equivalence of locomotor activity prior to experimental manipulation.
Experiment 1. After a 1-week acclimation period in regular wheel cages, the number of wheel rotations was counted for 24 h with an automatic counter. Mice were then either placed in PAWW cages or kept in regular cages for 2 weeks, based on their group allocation. Two weeks after the start of PAWW housing, the number of wheel rotations was recorded again for 24 h (Figure 1A).
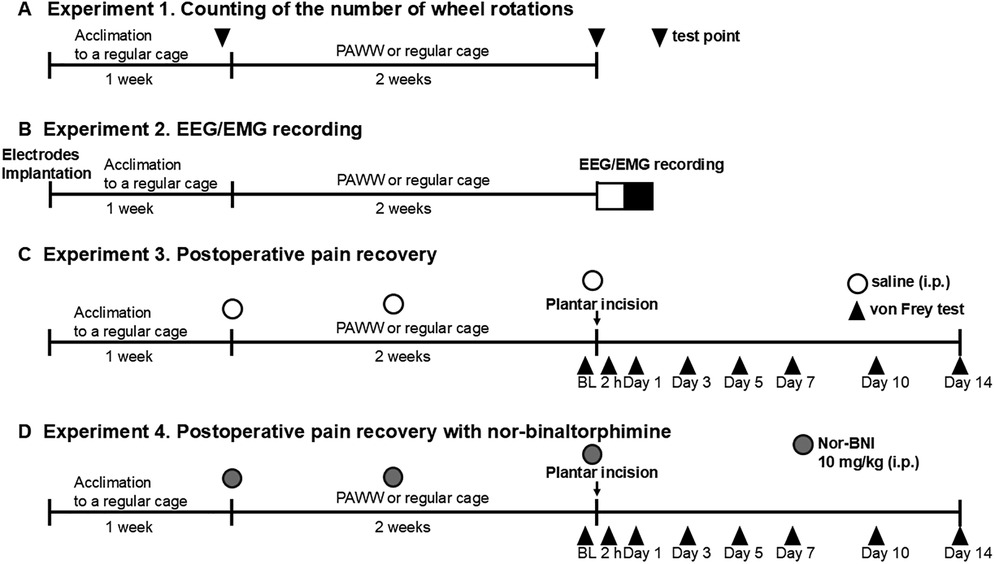
Figure 1. Experimental timeline. The timeline of each experiment, including the duration of housing in PAWW or regular cages and the timing of each treatment and measurement, is presented. The number of wheel rotations was counted after 1 week of acclimation in regular cages (baseline) and 2 weeks of housing in either PAWW cages or regular cages in experiment 1 (A) EEGs/EMGs were recorded after 1 week of acclimation in regular cages and 2 weeks of housing in either PAWW cages or regular cages in experiment 2 (B) Mice were subjected to von Frey tests at certain test points before and after the plantar incision surgery in experiment 3 (C) In experiment 4 (D), they were treated with nor-BNI in addition to the same protocol as in experiment 3. BL, baseline; nor-BNI, nor-binaltorphimine; PAWW, perpetual avoidance of water on a wheel; EEG, electroencephalogram; EMG, electromyogram.
Experiment 2. Mice underwent surgical EEG and EMG electrode implantation and were then placed in the regular wheel cages for the 1-week acclimation period. After acclimation, they were placed in PAWW cages or retained in the regular cages for 2 weeks. Thereafter, electroencephalograms (EEG) and electromyograms (EMG) were recorded for 24 h, as described below (Figure 1B).
Experiment 3. A plantar incision was performed as described below, after the mice were kept in PAWW cages or regular cages on the same schedule as in the previous two experiments. Von Frey testing was performed 2 h before surgery, and on days 1, 3, 5, 7, 10, and 14 post-surgery, to evaluate the course of postoperative pain recovery. Additionally, saline, which was also used as the vehicle for nor-BNI, was administered intraperitoneally before and one week after the start of PAWW rearing, as well as before baseline von Frey testing prior to the plantar incision surgery (a total of three doses), to maintain uniformity in experimental conditions and handling as in the next experiment, allowing for comparisons (Figure 1C).
Experiment 4. Mice breeding, plantar incision surgery, and von Frey tests were performed at the same schedule as in Experiment 3 in mice kept in both PAWW cages and regular cages. Additionally, 10 mg/kg of the long-acting kappa opioid receptor antagonist, nor-BNI (27), was administered intraperitoneally before and 1 week after the start of PAWW rearing, and before baseline von Frey testing prior to the plantar incision surgery (a total of three doses) to all the animals (Figure 1D).
2.3 Sleep disorder model
All the mice were individually housed in regular cages (length 225 mm × width 335 mm × height 210 mm), which are standard plastic cages equipped with a running wheel (diameter 140 mm × width 60 mm) and wood chip bedding, allowing them to sleep normally (RWC-15; Melquest, Toyama, Japan) for one week before exposure to stress. On the first day of exposure to stress, mice that were randomly allocated to the PAWW group were placed in the PAWW cage (SW-15-SD; Melquest, Toyama, Japan). The PAWW cage is a plastic cage (length 160 mm × width 180 mm × height 230 mm) that contains a centrally positioned, freely rotating running wheel (diameter 140 mm × width 60 mm), but with the mice being confined within the wheel. A water bottle and food dispenser were positioned within easy reach of the mice. The bottom of the PAWW cage is filled with water to a shallow depth, and no bedding is provided. However, the lower part of the wheel does not submerge in the water. Although the mice do not come in touch with the water, they tend to avoid the lowest position of the wheel. This aversion promotes sustained voluntary movement, leading to chronic sleep–wake cycle disturbances when housed in the PAWW cage for several weeks (7). The mouse group that continued to be kept in the regular wheel cage (RWC-15) without being transferred to a PAWW cage was designated as the control group. The cages were placed under conventional light on ventilated shelves, and the water in the PAWW cages was changed every day to keep the water clean. The number of wheel rotations of each mouse was recorded for 24 h with an automatic counter, then averaged for each 12 h light–dark phase.
2.4 EEG/EMG recording and sleep analysis
We recorded EEGs and EMGs to evaluate the sleep condition for 24 h, as previously described (12, 13). Briefly, mice were mounted in a stereotaxic head holder, and EEG and EMG electrodes were implanted for polysomnographic recordings (Pinnacle Technology, USA) under 3% isoflurane anesthesia before acclimation to the regular cage. Two stainless steel EEG recording screws were positioned 1 mm anterior to the bregma or lambda, both 1.5 mm lateral to the midline. EMG activity was monitored using Teflon-coated steel wires placed bilaterally into both trapezius muscles. The electrodes and the surgical site were covered with acrylic resin. The collected data were analyzed using appropriate software (SLEEPSIGN Kissei Comtec, Japan). Vigilance in every 10 s epoch was automatically classified into three stages, arousal, rapid eye movement (REM), and non-REM sleep; according to the standard criteria: (1) arousal was defined by a high EMG amplitude and low EEG amplitude; (2) REM sleep was defined by a low EMG amplitude, low EEG amplitude, and high θ wave activity; and (3) non-REM sleep was defined by a low EMG amplitude, high EEG amplitude, and high δ wave activity (16, 17). The defined sleep–wake stages were visually examined, and corrected if necessary.
2.5 Postoperative pain model
As a postoperative pain model, a 0.5 cm longitudinal incision was made through the mouse's plantar skin, fascia, and muscle under general anesthesia with 3% isoflurane (28). The incision site was sutured using 4-0 silk.
2.6 Mechanical allodynia assessment
Mechanical allodynia was assessed using von Frey filaments at different points before and after the plantar incision surgery. Mice were placed in chambers with wire mesh floors and allowed to habituate for 30 min. Von Frey filaments (range: 0.04–4.0 g) were alternately applied to the ipsilateral and contralateral hind paws until the filament buckled. The paw withdrawal threshold was determined using the up-down method, as previously described (29). Data were analyzed using UpDown Reader software (30). After completing the assessment, the mice were euthanized in accordance with institutional ethical guidelines depending on the requirements of the specific experimental protocol.
2.7 Preparation of nor-BNI
Nor-BNI (Abcam, CAS Number: 113158-34-2) was dissolved in a saline vehicle to a final concentration of 10 mg/ml. The solution was prepared before each experiment and stored at 4°C to maintain stability.
2.8 Statistical analysis
All data are expressed as the mean ± standard error of the mean (SEM). One-way ANOVA was used to analyze the number of wheel rotations. Repeated-measures two-way ANOVA was employed to analyze the percentage of sleep and the percentage of REM sleep, as these variables were measured in the same animals at multiple time points. Similarly, repeated-measures two-way ANOVA was applied to the von Frey test results. For all analyses, post hoc comparisons were performed using the Bonferroni multiple comparisons test. Statistical significance was set at P < 0.05. All data were tested for normality using the Kolmogorov–Smirnov test, with P < 0.05 considered indicative of deviation from a normal distribution. These statistical analyses were performed using Prism version 9.2 (GraphPad Software, La Jolla, CA, USA). Effect sizes were calculated from preliminary experiments, and the required sample size was estimated using G*Power 3.1 to achieve an actual power of >0.8. The effect sizes for comparisons in each experiment were estimated as follows: 1.8 for wheel rotations, 3.3 for sleep quantity determined by EEG, and 1.9 for the von Frey test in the postoperative pain model.
3 Results
The results of the various experimental conditions adopted to assess the effects of sleep deprivation, pain and nor-BNI treatment on postoperative pain recovery are shown below. All data met the assumption of normality (P > 0.05), as assessed by the Kolmogorov–Smirnov test.
In experiment 1 (Figure 1A), during the light phase, one-way ANOVA revealed a significant group effect [F(2,28) = 19.60, P < 0.00001, η² = 0.58]. post hoc Bonferroni tests showed that the number of wheel rotations in the PAWW cage group (n = 9) was significantly higher (720 ± 135.7 rotations) than that in the regular cage group (n = 7, 11 ± 17.1 rotations; P < 0.001) (Figure 2A). Additionally, the PAWW cage group exhibited a significant increase in light phase rotations compared to baseline (192 ± 39.7 rotations; P < 0.001) (Figure 2A). During the dark phase, one-way ANOVA revealed a significant group effect [F(2,28) = 7.37, P < 0.01, η² = 0.35]. post hoc Bonferroni tests showed that the number of wheel rotations in the PAWW cage group (10,611 ± 1,801 rotations) was significantly lower compared to the regular cage group (16,564 ± 1,141 rotations; P < 0.05) (Figure 2B). Similarly, the number of dark phase rotations in the PAWW cage group also showed a significant decrease compared to baseline (17,403 ± 1,058 rotations; P < 0.01) (Figure 2B).
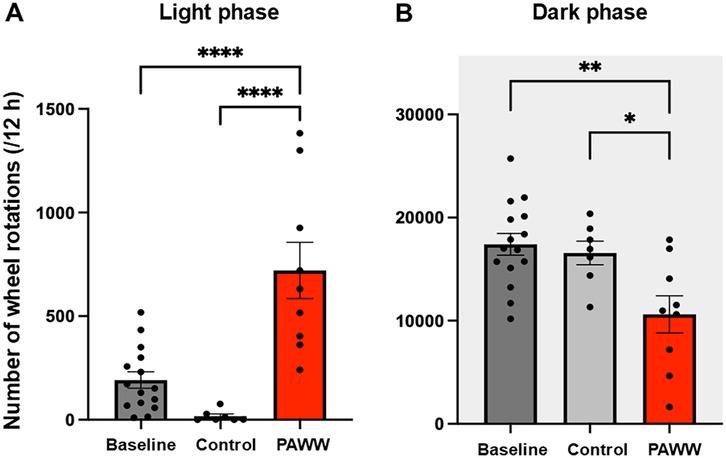
Figure 2. The number of wheel rotations of the mice in the PAWW and regular cages. (A) The number of wheel rotations recorded during the light phase (7:00–19:00). (B) The number of wheel rotations recorded during the dark phase (19:00–7:00). The measurements were taken during a 1-week acclimation period in regular cages (baseline, n = 15) and a 2-week housing period in either PAWW cages (n = 8) or regular cages (n = 7), and their average values were calculated. The results are presented as bar graphs. All data are expressed as the mean ± standard error of the mean. One-way ANOVA was used for statistical tests, and Bonferroni's multiple comparison test was performed as a post hoc test. Values were considered statistically significant at *P < 0.05, **P < 0.01, and ****P < 0.0001. PAWW, perpetual avoidance of water on a wheel.
In experiment 2 (Figure 1B), in the sleep percentage evaluation by EEG/EMG, a repeated-measures two-way ANOVA revealed a significant effect of the within-group comparisons [F(1, 6) = 237.81, P < 0.0001, η² = 0.86], and the effect of the between-group comparisons was also significant [F(1, 6) = 14.11, P < 0.01, η² = 0.080], but the interaction between phase and group was not significant [F(1, 6) = 1.36, P = 0.29, η² = 0.0049]. post hoc Bonferroni tests revealed that the PAWW cage group (n = 4) exhibited a reduction in total sleep time (60.9 ± 1.4%) during the light phase, compared to the regular cage group (n = 4, 75.8 ± 5.2%; P < 0.01) (Figure 3A). There was no significant difference between the PAWW cage group (24.8 ± 1.7%) and the regular cage group (33.8 ± 1.0%) in total sleep time during the dark phase.
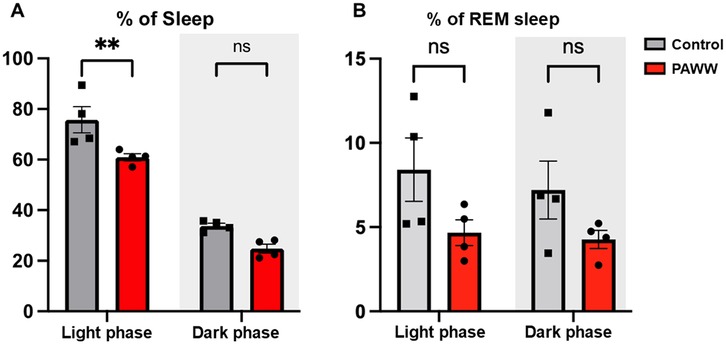
Figure 3. The percentage of sleep time of the mice in the PAWW and regular cages. The percentage of sleep time (A) and the percentage of REM sleep during sleep time (B) were calculated from EEG/EMG waves during the light phase (7:00–19:00) and dark phase (19:00–7:00) after 1 week of habituation and 2 weeks of housing in either PAWW cages (n = 4) or regular cages (n = 4). The results are presented as bar graphs. All data are expressed as the mean ± standard error of the mean. Repeated measures two-way ANOVA was used for statistical tests, and Bonferroni's multiple comparison test was performed as a post hoc test. The results were considered statistically significant at **P < 0.01. PAWW, perpetual avoidance of water on a wheel.
In the evaluation of the REM sleep percentage, a repeated-measures two-way ANOVA revealed that there were no significant effects of either phase [F(1, 6) = 2.00, P = 0.21, η² = 0.019] or group [F(1, 6) = 3.31, P = 0.12, η² = 0.33]. The interaction between phase and group was also not significant [F(1, 6) = 0.52, P = 0.50, η² = 0.0049]. post hoc Bonferroni tests revealed that the PAWW cage group also showed a lower percentage of REM sleep during the light phase (4.7 ± 0.8%) compared to the regular cage group (8.9 ± 2.1%), although this difference was not statistically significant (Figure 3B). During the dark phase, the percentage of REM sleep was 4.3 ± 0.5% in the PAWW cage group and 7.7 ± 1.9% in the regular cage group, with no significant difference between them.
In Experiment 3 (Figure 1C), following the plantar incision, pain behavior was assessed using the von Frey test. A repeated-measures two-way ANOVA revealed there were significant effects of groups [F(3, 28) = 55.64, p < 0.0001, η² = 0.35], and days [F(7, 196) = 12.90, p < 0.0001, η² = 0.15]. A significant interaction effect between days and groups was also observed [F(21, 196) = 6.80, p < 0.0001, η² = 0.20]. post hoc Bonferroni tests revealed that in the regular cage group (n = 8), the pain threshold in the ipsilateral hind paw decreased until post-surgery day 1, followed by recovery starting on day 3. In contrast, the PAWW cage group (n = 8) exhibited a prolonged decrease in pain threshold, with no statistically significant difference between the ipsilateral and contralateral hind paws two weeks after surgery (Figure 4A, Supplementary Table S1A).
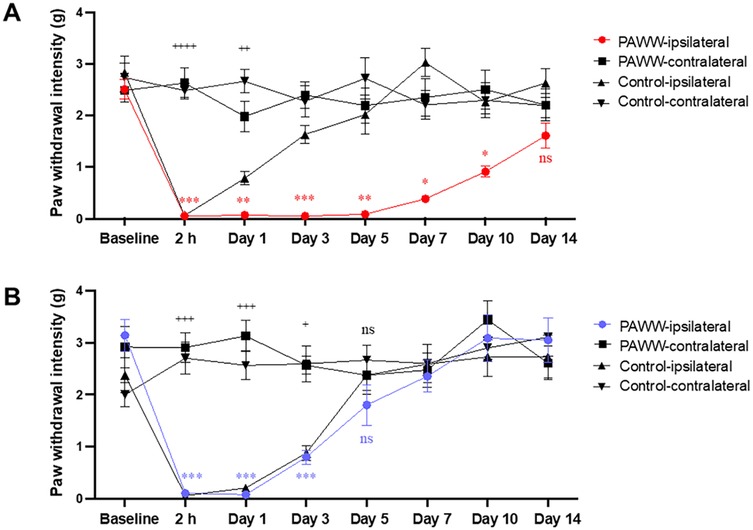
Figure 4. Effects of nor-BNI on the time course of mechanical pain threshold in the perioperative period. Paw withdrawal intensity was calculated using the up–down method of von Frey tests at specific test points before and after plantar incision surgery in mice housed in either PAWW cages (n = 8) or regular cages (n = 8) (A) Paw withdrawal intensity was also evaluated following nor-BNI treatment, administered as required, to mice in either PAWW cages (n = 8) or regular cages (n = 8) (B) All data are expressed as the mean ± standard error of the mean. *P < 0.05, **P < 0.01, and ***P < 0.001 for comparisons between PAWW-ipsilateral and PAWW-contralateral, and +P < 0.05, ++P < 0.01, +++P < 0.001, and ++++P < 0.0001 for comparisons between control-ipsilateral and control-contralateral, as assessed by repeated measures two-way ANOVA with Bonferroni's multiple comparison test as a post hoc test. nor-BNI, nor-binaltorphimine; PAWW, perpetual avoidance of water on a wheel.
In Experiment 4, nor-BNI was administered at three time points, followed by von Frey tests to evaluate the course of postoperative pain recovery (Figure 1D). A repeated-measures two-way ANOVA revealed there were significant effects of groups [F(3, 28) = 17.22, p < 0.0001, η² = 0.14], and days [F(7, 196) = 18.41, p < 0.0001, η² = 0.21]. A significant interaction effect between days and groups was also observed [F(21, 196) = 6.95, p < 0.0001, η² = 0.24]. post hoc Bonferroni tests revealed that in the regular cage group (n = 8), the pain threshold in the ipsilateral hind paw decreased until post-surgery day 3, followed by recovery starting on day 5. Similarly, the PAWW cage group (n = 8) exhibited a decrease in pain threshold up to day 3, with recovery beginning on day 5 (Figure 4B, Supplementary Table S1B).
4 Discussion
Our findings demonstrate that housing mice in a PAWW cage induces sleep disturbances and delays recovery from postoperative pain following a plantar incision. Notably, nor-BNI, a KOR antagonist, alleviated the delayed recovery of the mechanical pain threshold.
In our study, PAWW housing significantly increased spontaneous activity during the light phase and decreased activity during the dark phase, disrupting circadian locomotor rhythms consistent with previous reports (7). EEG recordings confirmed a significant reduction in total sleep time during the light phase, with a decreasing trend also observed during the dark phase. The mismatch observed during the dark phase, where physical activity is reduced but sleep also tends to decrease, is not merely a sign of hyperactivity, but rather reflects a broader dysregulation of sleep homeostasis. Similar patterns have been reported in human studies, in which chronic stress leads to reductions in both sleep and physical activity (31).
Compared to traditional sleep deprivation models, PAWW offers a more physiological approach to mimicking chronic sleep disturbance (Table 1). The single platform-on-water model and bar rotation method effectively induce sleep deprivation, but cause excessive acute stress and severe physical impairment, limiting their feasibility in long-term studies (4–6, 32–34). The social defeat model induces fragmented sleep and decreased pain thresholds, but has low reproducibility for sleep disturbances and primarily relies on short-term acute stress exposure (35, 36). The PAWW model, in contrast, induces chronic sleep-wake cycle disruptions without excessive acute stress or severe physical distress. It effectively mimics the circadian rhythm dysregulation seen in human sleep disorders, with characteristic alterations in corticosterone secretion rhythms and weight loss despite increased appetite (7).
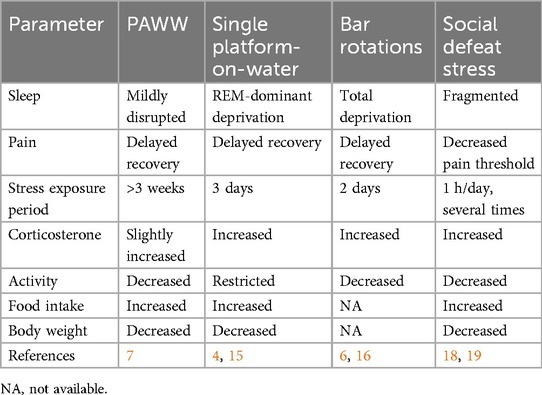
Table 1. Comparison of sleep, pain, and other stress responses in various animal models of sleep disturbance.
Poor sleep quality is a well-established risk factor for hyperalgesia and spontaneous pain (37–39). Several mechanisms may underlie this relationship, including opioid system dysfunction (12, 13), altered orexin signaling (18, 19), monoaminergic imbalance (serotonin, noradrenaline, dopamine) (16, 17), and immune and endocrine systems (40). Sleep disruption might delay tissue recovery, since sleep-wake cycles are essential regulators of immune function and tissue repair (41). In our study, PAWW-induced sleep disturbances prolonged postoperative pain recovery, further supporting this link. However, since sleep and stress are interdependent, their relative contributions remain uncertain. While measuring corticosterone levels could have provided insights into stress involvement, we opted not to include this measurement in this study, in order to minimize additional handling stress that could have confounded the results. Future research should include stress biomarkers (e.g., corticosterone, pro-inflammatory cytokines) to clarify the individual contributions of sleep disruption and stress-induced hyperalgesia. Exploring dual-targeted interventions may offer new therapeutic insights.
These findings may have clinical relevance for perioperative patients, who are highly susceptible to stress and sleep disturbances. Addressing sleep disturbances could be key to improving postoperative recovery. However, currently, only a few well-evidenced interventions have addressed sleep disorders in this context (42). Mice and humans share a 24 h circadian rhythm, and their sleep influences the nervous and endocrine systems similarly in both species (43). However, key differences exist: mice are nocturnal, have shorter sleep cycles (∼10–15 min between NREM and REM sleep), and exhibit polyphasic sleep patterns. These differences highlight the need for further clinical validation when translating findings from rodent models to human patients. Nonetheless, our results strongly suggest that targeting sleep disturbances might be a promising approach to improving postoperative pain recovery.
Nor-BNI is unlikely to have direct and immediate analgesic effects, since no improvement in pain thresholds was observed between 2 and 24 h post-administration. Previous studies also report a delayed onset of nor-BNI's effects (24, 44), likely due to indirect modulation of stress, sleep architecture, and pain processing (24, 25, 44, 45). Nor-BNI may also enhance wound healing by improving sleep and immune responses, both of which are closely linked to pain recovery processes (41). Although nor-BNI improved postoperative pain recovery, its underlying mechanisms remain uncertain. Given prior evidence that KOR antagonists normalize sleep in chronic pain models (8), further EEG analyses post-nor-BNI are needed to clarify whether sleep restoration mediates its effects. While we focused on KOR antagonism, it remains possible that nor-BNI's effects on pain recovery involve interactions with other neurotransmitter systems, including serotonin and dopamine (46, 47), both of which are essential for pain modulation. Tricyclic antidepressants, which enhance monoaminergic transmission, are widely used for chronic pain treatment. Thus, nor-BNI's effects may involve downstream interactions with monoaminergic systems. Future studies should quantify changes in these neurotransmitters following nor-BNI treatment and assess the therapeutic potential of combining KOR antagonists with monoamine modulators.
In addition to central mechanisms, peripheral KOR signaling may also modulate pain. KORs in peripheral sensory neurons have been implicated in nociceptive processing (48). While peripheral KOR agonists suppress inflammation and hypersensitivity, they may also contribute to opioid tolerance and hyperalgesia. Our study did not evaluate local KOR expression or inflammatory markers. Further investigation could elucidate the peripheral contributions of KOR antagonism. Because nor-BNI was administered intraperitoneally, it may have affected both central and peripheral KORs. Clarifying the site-specific contributions will require future studies employing localized drug delivery (e.g., intracerebral injection) or region-specific genetic approaches such as conditional knockouts.
While testing a KOR agonist could have provided further insights, our primary aim was to investigate the efficacy of KOR blockade. Nor-BNI was selected due to its long-lasting action, ensuring sustained receptor inhibition. Previous literature has reported that KOR activation can induce mechanical hypersensitivity (24, 25), and KOR agonists can disrupt sleep (10), suggesting that KOR agonists may exacerbate pain recovery. These findings support the rationale for targeting KOR antagonism in our experimental context. We acknowledge this as an important future direction, and we need to explore the differential effects of KOR agonists vs. antagonists on sleep-related pain outcomes in subsequent studies. Further investigations are required to determine whether the observed effects are specific to KOR blockade or involve additional neurotransmitter systems. Future studies incorporating KOR agonists, monoamine modulators, and glutamate antagonists may clarify the complex mechanisms underlying sleep-related pain enhancement.
The limitations of this study include the short duration of observation for postoperative pain recovery and sleep disturbances. While we demonstrated that sleep disruption delays pain recovery, we could not assess long-term consequences, such as chronic pain development, due to the limited observation period. Future studies with extended monitoring periods are needed to evaluate potential long-term effects. Additionally, although we observed a trend toward REM sleep reduction in the PAWW group, this difference did not reach statistical significance. This suggests that a larger sample size or more precise EEG analysis methods might be necessary for better assessment of REM sleep alterations and their potential impact on pain recovery. Furthermore, while nor-BNI improved postoperative pain recovery, the underlying mechanisms remain unclear. Future studies should include EEG assessments following nor-BNI treatment, along with investigations into other potential mechanisms, such as monoaminergic or neuroimmune interactions, to elucidate the precise role of KOR antagonism in postoperative pain recovery. Additionally, our study utilized only male mice to minimize variability related to the estrous cycle and to maintain consistency with prior PAWW model studies. Future research is warranted to determine whether similar effects are observed in female mice, which may provide further insight into sex-specific mechanisms in sleep–pain interactions and KOR signaling.
In conclusion, our study demonstrates that the PAWW model, which mimics sleep disorders in modern society, delays recovery from postoperative pain. Importantly, our findings suggest that KOR antagonism could be a promising option to prevent or treat prolonged pain induced by sleep disorders in perioperative patients. This potential application of KOR antagonists in perioperative care is the key takeaway of our research.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The animal study was approved by Committee of Ethics in Animal Experiments of the University of Toyama. The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
HI: Conceptualization, Methodology, Project administration, Writing – original draft. MK: Writing – review & editing, Conceptualization, Methodology. MY: Conceptualization, Writing – review & editing. SS: Conceptualization, Writing – review & editing. TT: Supervision, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Acknowledgments
This research was supported by a Grant-in-Aid for Early-Career Scientists (No. 21K16534) from the Japan Society for the Promotion of Science. We would like to thank Forte Science Communications for English language editing of this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1516935/full#supplementary-material
References
1. Bjurström MF, Irwin MR, Bodelsson M, Smith MT, Mattsson-Carlgren N. Preoperative sleep quality and adverse pain outcomes after total hip arthroplasty. Eur J Pain. (2021) 25:1482–92. doi: 10.1002/ejp.1761
2. Wang J-P, Lu S-F, Guo L-N, Ren C-G, Zhang Z-W. Poor preoperative sleep quality is a risk factor for severe postoperative pain after breast cancer surgery: a prospective cohort study. Medicine (Baltimore). (2019) 98:e17708. doi: 10.1097/MD.0000000000017708
3. Purcell KF, Scarcella N, Chun D, Holland C, Stauffer TP, Bolognesi M, et al. Treating sleep disorders after total hip and total knee arthroplasty. Orthop Clin North Am. (2023) 54:397–405. doi: 10.1016/j.ocl.2023.05.008
4. Kim SH, Park JY, Shin HE, Lee Sb, Ryu DW, Kim TW, et al. The influence of rapid eye movement sleep deprivation on nociceptive transmission and the duration of facial allodynia in rats: a behavioral and Fos immunohistochemical study. J Headache Pain. (2019) 20:21. doi: 10.1186/s10194-019-0977-0
5. Feng P, Ma Y. Instrumental REM sleep deprivation in neonates leads to adult depression-like behaviors in rats. Sleep. (2003) 26:990–6. doi: 10.1093/sleep/26.8.990
6. Huang Y, Xu R, Liu Q, Zhang X, Mao Y, Yang Y, et al. Glucose competition between endothelial cells in the blood-spinal cord barrier and infiltrating regulatory T cells is linked to sleep restriction-induced hyperalgesia. BMC Med. (2024) 22:189. doi: 10.1186/s12916-024-03413-z
7. Miyazaki K, Itoh N, Ohyama S, Kadota K, Oishi K. Continuous exposure to a novel stressor based on water aversion induces abnormal circadian locomotor rhythms and sleep-wake cycles in mice. PLoS One. (2013) 8:e55452. doi: 10.1371/journal.pone.0055452
8. Haack M, Simpson N, Sethna N, Kaur S, Mullington J. Sleep deficiency and chronic pain: potential underlying mechanisms and clinical implications. Neuropsychopharmacology. (2020) 45:205–16. doi: 10.1038/s41386-019-0439-z
9. Li MT, Robinson CL, Ruan QZ, Surapaneni S, Southerland W. The influence of sleep disturbance on chronic pain. Curr Pain Headache Rep. (2022) 26:795–804. doi: 10.1007/s11916-022-01074-2
10. Ito H, Navratilova E, Vagnerova B, Watanabe M, Kopruszinski C, Moreira de Souza LH, et al. Chronic pain recruits hypothalamic dynorphin/kappa opioid receptor signalling to promote wakefulness and vigilance. Brain. (2023) 146:1186–99. doi: 10.1093/brain/awac153
11. Lillo Vizin RC, Ito H, Kopruszinski CM, Ikegami M, Ikegami D, Yue X, et al. Cortical kappa opioid receptors integrate negative affect and sleep disturbance. Transl Psychiatry. (2024) 14:417. doi: 10.1038/s41398-024-03123-3
12. Ukponmwan OE, Rupreht J, Dzoljic MR. REM sleep deprivation decreases the antinociceptive property of enkephalinase-inhibition, morphine and cold-water-swim. Gen Pharmacol. (1984) 15:255–8. doi: 10.1016/0306-3623(84)90170-8
13. Nascimento DC, Andersen ML, Hipólide DC, Nobrega JN, Tufik S. Pain hypersensitivity induced by paradoxical sleep deprivation is not due to altered binding to brain mu-opioid receptors. Behav Brain Res. (2007) 178:216–20. doi: 10.1016/j.bbr.2006.12.016
14. Ito H, Yanase M, Yamashita A, Kitabatake C, Hamada A, Suhara Y, et al. Analysis of sleep disorders under pain using an optogenetic tool: possible involvement of the activation of dorsal raphe nucleus-serotonergic neurons. Mol Brain. (2013) 6:2–4. doi: 10.1186/1756-6606-6-59
15. Koh K, Hamada A, Hamada Y, Yanase M, Sakaki M, Someya K, et al. Possible involvement of activated locus coeruleus-noradrenergic neurons in pain-related sleep disorders. Neurosci Lett. (2015) 589:200–6. doi: 10.1016/j.neulet.2014.12.002
16. Millan MJ. Descending control of pain. Prog Neurobiol. (2002) 66:355–474. doi: 10.1016/s0301-0082(02)00009-6
17. Davies SK, Ang JE, Revell VL, Holmes B, Mann A, Robertson FP, et al. Effect of sleep deprivation on the human metabolome. Proc Natl Acad Sci. (2014) 111:10761–6. doi: 10.1073/pnas.1402663111
18. Yamamoto T, Nozaki-Taguchi N, Chiba T. Analgesic effect of intrathecally administered orexin-A in the rat formalin test and in the rat hot plate test. Br J Pharmacol. (2002) 137:170–6. doi: 10.1038/sj.bjp.0704851
19. Toyama S, Shimoyama N, Shimoyama M. The analgesic effect of orexin-A in a murine model of chemotherapy-induced neuropathic pain. Neuropeptides. (2017) 61:95–100. doi: 10.1016/j.npep.2016.12.007
20. Ito H, Takemura Y, Aoki Y, Hattori M, Horikawa H, Yamazaki M. Analysis of the effects of a tricyclic antidepressant on secondary sleep disturbance induced by chronic pain in a preclinical model. PLoS One. (2020) 15:1–12. doi: 10.1371/journal.pone.0243325
21. Ito H, Tsuneki H, Sasaoka T, Toyooka N, Matsuo M, Yamazaki M. Suvorexant and mirtazapine improve chronic pain-related changes in parameters of sleep and voluntary physical performance in mice with sciatic nerve ligation. PLoS One. (2022) 17:1–14. doi: 10.1371/journal.pone.0264386
22. Roehrs T, Withrow D, Koshorek G, Verkler J, Bazan L, Roth T. Sleep and pain in humans with fibromyalgia and comorbid insomnia: double-blind, crossover study of suvorexant 20 mg versus placebo. J Clin Sleep Med. (2020) 16:415–21. doi: 10.5664/jcsm.8220
23. Palmer ACS, Souza A, dos Santos VS, Cavalheiro JAC, Schuh F, Zucatto AE, et al. The effects of melatonin on the descending pain inhibitory system and neural plasticity markers in breast cancer patients receiving chemotherapy: randomized, double-blinded, placebo-controlled trial. Front Pharmacol. (2019) 10:1382. doi: 10.3389/fphar.2019.01382
24. Nation KM, De Felice M, Hernandez PI, Dodick DW, Neugebauer V, Navratilova E, et al. Lateralized kappa opioid receptor signaling from the amygdala central nucleus promotes stress-induced functional pain. Pain. (2018) 159:919–28. doi: 10.1097/j.pain.0000000000001167
25. Navratilova E, Ji G, Phelps C, Qu C, Hein M, Yakhnitsa V, et al. Kappa opioid signaling in the central nucleus of the amygdala promotes disinhibition and aversiveness of chronic neuropathic pain. Pain. (2019) 160:824–32. doi: 10.1097/j.pain.0000000000001458
26. Ferrari LL, Park D, Zhu L, Palmer MR, Broadhurst RY, Arrigoni E. Regulation of lateral hypothalamic orexin activity by local GABAergic neurons. J Neurosci. (2018) 38:1588–99. doi: 10.1523/JNEUROSCI.1925-17.2017
27. Kishioka S, Kiguchi N, Kobayashi Y, Yamamoto C, Saika F, Wakida N, et al. Pharmacokinetic evidence for the long-lasting effect of nor-binaltorphimine, a potent kappa opioid receptor antagonist, in mice. Neurosci Lett. (2013) 552:98–102. doi: 10.1016/j.neulet.2013.07.040
28. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. (1996) 64:493–502. doi: 10.1016/0304-3959(95)01441-1
29. Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. (1994) 53:55–63. doi: 10.1016/0165-0270(94)90144-9
30. Gonzalez-Cano R, Boivin B, Bullock D, Cornelissen L, Andrews N, Costigan M. Up-down reader: an open source program for efficiently processing 50% von frey thresholds. Front Pharmacol. (2018) 9:433. doi: 10.3389/fphar.2018.00433
31. Stanton R, To QG, Khalesi S, Williams SL, Alley SJ, Thwaite TL, et al. Depression, anxiety and stress during COVID-19: associations with changes in physical activity, sleep, tobacco and alcohol use in Australian adults. Int J Environ Res Public Health. (2020) 17(11):4065. doi: 10.3390/ijerph17114065
32. Lopez-Rodriguez F, Kim J, Poland RE. Total sleep deprivation decreases immobility in the forced-swim test. Neuropsychopharmacology. (2004) 29:1105–11. doi: 10.1038/sj.npp.1300406
33. Dumaine JE, Ashley NT. Acute sleep fragmentation induces tissue-specific changes in cytokine gene expression and increases serum corticosterone concentration. Am J Physiol—Regul Integr Comp Physiol. (2015) 308:R1062–9. doi: 10.1152/ajpregu.00049.2015
34. Pandey A, Kar SK. Rapid eye movement sleep deprivation of rat generates ROS in the hepatocytes and makes them more susceptible to oxidative stress. Sleep Sci. (2018) 11:245–53. doi: 10.5935/1984-0063.20180039
35. La Porta C, Plum T, Palme R, Mack M, Tappe-Theodor A. Repeated social defeat stress differently affects arthritis-associated hypersensitivity in male and female mice. Brain Behav Immun. (2024) 119:572–96. doi: 10.1016/j.bbi.2024.04.025
36. Kinn AM, Grønli J, Fiske E, Kuipers S, Ursin R, Murison R, et al. A double exposure to social defeat induces sub-chronic effects on sleep and open field behaviour in rats. Physiol Behav. (2008) 95:553–61. doi: 10.1016/j.physbeh.2008.07.031
37. Finan PH, Goodin BR, Smith MT. The association of sleep and pain: an update and a path forward. J Pain. (2013) 14:1539–52. doi: 10.1016/j.jpain.2013.08.007
38. Afolalu EF, Ramlee F, Tang NKY. Effects of sleep changes on pain-related health outcomes in the general population: a systematic review of longitudinal studies with exploratory meta-analysis. Sleep Med Rev. (2018) 39:82–97. doi: 10.1016/j.smrv.2017.08.001
39. Simpson NS, Scott-Sutherland J, Gautam S, Sethna N, Haack M. Chronic exposure to insufficient sleep alters processes of pain habituation and sensitization. Pain. (2018) 159:33–40. doi: 10.1097/j.pain.0000000000001053
40. Goodin BR, Smith MT, Quinn NB, King CD, McGuire L. Poor sleep quality and exaggerated salivary cortisol reactivity to the cold pressor task predict greater acute pain severity in a non-clinical sample. Biol Psychol. (2012) 91:36–41. doi: 10.1016/j.biopsycho.2012.02.020
41. Besedovsky L, Lange T, Born J. Sleep and immune function. Pflugers Arch Eur J Physiol. (2012) 463:121–37. doi: 10.1007/s00424-011-1044-0
42. Chouchou F, Khoury S, Chauny JM, Denis R, Lavigne GJ. Postoperative sleep disruptions: a potential catalyst of acute pain? Sleep Med Rev. (2014) 18:273–82. doi: 10.1016/j.smrv.2013.07.002
43. Vyazovskiy VV, Delogu A. NREM and REM sleep: complementary roles in recovery after wakefulness. Neuroscientist. (2014) 20:203–19. doi: 10.1177/1073858413518152
44. Kopruszinski CM, Navratilova E, Swiokla J, Dodick DW, Chessell IP, Porreca F. A novel, injury-free rodent model of vulnerability for assessment of acute and preventive therapies reveals temporal contributions of CGRP-receptor activation in migraine-like pain. Cephalalgia. (2021) 41:305–17. doi: 10.1177/0333102420959794
45. Xie JY, De Felice M, Kopruszinski CM, Eyde N, LaVigne J, Remeniuk B, et al. Kappa opioid receptor antagonists: a possible new class of therapeutics for migraine prevention. Cephalalgia. (2017) 37:780–94. doi: 10.1177/0333102417702120
46. Pirino BE, Spodnick MB, Gargiulo AT, Curtis GR, Barson JR, Karkhanis AN. Kappa-opioid receptor-dependent changes in dopamine and anxiety-like or approach-avoidance behavior occur differentially across the nucleus accumbens shell rostro-caudal axis. Neuropharmacology. (2020) 181:108341. doi: 10.1016/j.neuropharm.2020.108341
47. West AM, Holleran KM, Jones SR. Kappa opioid receptors reduce serotonin uptake and escitalopram efficacy in the mouse substantia nigra pars reticulata. Int J Mol Sci. (2023) 24:2080. doi: 10.3390/ijms24032080
Keywords: postoperative pain, pain recovery, sleep disorder, kappa opioid receptor, norbinaltorphimine, dynorphin
Citation: Ito H, Kawakami M, Yoshida M, Sugimoto S and Takazawa T (2025) Effects of a kappa opioid receptor antagonist on delayed postoperative pain recovery in a novel mouse sleep disorder model. Front. Pain Res. 6:1516935. doi: 10.3389/fpain.2025.1516935
Received: 25 October 2024; Accepted: 19 June 2025;
Published: 4 July 2025.
Edited by:
Chi-Kun Tong, Columbia University, United StatesReviewed by:
Ana Margarida Ferreira Cunha, University of Minho, PortugalParamita Basu, University of Pittsburgh, United States
Copyright: © 2025 Ito, Kawakami, Yoshida, Sugimoto and Takazawa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tomonori Takazawa, dGFrYXphd3RAbWVkLnUtdG95YW1hLmFjLmpw
 Hisakatsu Ito
Hisakatsu Ito Masaaki Kawakami
Masaaki Kawakami Masashi Yoshida
Masashi Yoshida Sadamu Sugimoto
Sadamu Sugimoto Tomonori Takazawa
Tomonori Takazawa