- 1Department of Anesthesiology, Critical Care and Pain Medicine, Boston Children’s Hospital, Boston, MA, United States
- 2Department of Anaesthesia, Harvard Medical School, Boston, MA, United States
- 3Department of Pediatrics, Université de Montréal, Montreal, QC, Canada
- 4The Manton Center for Orphan Disease Research, Boston Children’s Hospital, Boston, MA, United States
- 5Division of Genetics and Genomics, Boston Children’s Hospital, Boston, MA, United States
- 6Department of Pediatrics, Harvard Medical School, Boston, MA, United States
Introduction: Snijders Blok-Campeau Syndrome (SNIBCPS) is a neurodevelopmental disorder characterized by intellectual disability, developmental delays, speech impairment, hypotonia, and distinctive facial features. Little is known about pain perception in children with cognitive impairments, such as patients with SNIBCPS. Although it has been noted that some individuals with SNIBCPS have decreased pain sensation and response to painful stimuli, these reports are anecdotal. Therefore, the objective was to better understand this syndrome and the affected individual's perception and response to pain through proxy-reported observational assessments.
Methods: Fifteen caregivers of individuals with a diagnosis of SNIBCPS participated in this mixed-methods anonymous survey study between July and September 2024. The survey questionnaires included the Pediatric Pain Profile, a Pain Sensory Questionnaire, the Non-Communicative Children's Pain Checklist-Revised, and the Individualized Numerical Rating Scale.
Results: Almost a quarter of our respondents reported insensitivity in the affected individual to hard impacts or pressure. Our findings highlight early and past painful experiences in individuals with SNIBCPS who have a range of behaviors to express their pain.
Discussion: Our findings bring awareness about the proper examination of individuals with SNIBCPS. Despite the small sample size, our findings suggest that pain and injuries may go unreported in individuals with SNIBCPS, and individualized parental observational scales may be beneficial for their healthcare providers and their caregivers.
Introduction
Snijders Blok-Campeau Syndrome (SNIBCPS, OMIM# 618205) is a rare neurodevelopmental disorder caused by mutations in the Chromodomain Helicase DNA Binding Protein 3 (CHD3) gene (1). The CHD3 gene is located on chromosome 17p13.1, and encodes a protein that is part of a chromatin remodeling complex called NuRD and plays an important role in regulating gene expression during early brain development. Mutations in CHD3 disrupt this process, leading to dysregulation of gene expression during critical stages of brain development.
SNIBCPS is characterized by intellectual disability, developmental delays, speech impairment, hypotonia, and distinctive facial features (1, 2). The degree of intellectual disability ranges from mild to severe, with most cases being moderate to severe (1–3). Other associated features may include seizures, autism spectrum disorder, behavioral problems, macrocephaly or more rarely microcephaly, and congenital malformations. The disorder follows an autosomal dominant inheritance pattern, meaning a single mutated copy of the CHD3 gene is sufficient to cause the condition. Various types of mutations in the CHD3 gene have been identified, including nonsense, missense, frameshift, and splice site mutations (1–7). Since there is no specific treatment for CHD3-related intellectual disability, management is primarily supportive and involves early intervention with speech, physical, and occupational therapies, as well as educational support and management of associated medical issues.
There is limited research about pain perception in children with cognitive impairments, such as patients with SNIBCPS. Importantly, children with severe cognitive impairments may express pain differently due to difficulties with communication, and are unable to provide self-reported pain intensities (8, 9). This has led to the assumption that children with cognitive impairments have decreased pain sensitivity (10). However, studies have shown in individuals with cognitive impairment, such as those with autism spectrum disorder, conflicting results, with one study reporting no difference in sensory function when compared to matched healthy controls (11, 12). Although it has been noted that four individuals with SNIBCPS have decreased pain sensation and response to painful stimuli, these reports are anecdotal (1, 2). Moreover, the absence of pain expression does not represent the absence of pain perception. Pain that is not managed properly could significantly decrease quality of life (13). Therefore, observational assessments of pain are used to better understand how patients with cognitive impairments, such as SNIBCPS, perceive pain. Nevertheless, empathy and compassion need to be prioritized in this population to foster a safe environment where the individual can express discomfort through non-verbal responses (14).
To our current knowledge, there are limited data on the pain perception of individuals with SNIBCPS. Therefore, the objective was to better understand this syndrome and their perception and response to pain through proxy-reported observational assessments. Our aims were to (1) determine the pain history and (2) determine the expression of pain of individuals with SNIBCPS. We hypothesized that individuals with SNIBCPS may experience additional sources of pain compared to typically developing children, and display different behaviors during a painful situation compared to a non-painful situation.
Materials and methods
Study approval, participants, and experimental design
Ethics approval was obtained from the Research Ethics Board of our Institution (IRB# P00045527). Caregivers (parents/legal guardians) of individuals with a diagnosis of SNIBCPS above the age of 18 years old were offered to participate in this mixed-methods survey study. Through a collaboration with the CHD3 Foundation (https://www.chd3.org/), targeted emails were sent once a month to their members between July and September 2024 to inform potential families to participate anonymously in this study. Because this was an anonymous survey, no protected health information was collected, and consent was provided by the completion of the survey. No incentive or compensation was offered to participants who completed the survey.
Outcome measures
The primary outcome variables included the questionnaires below. Secondary outcome measures include demographic data such as the current age, gender, and race of the respondent, as well as the current age, age of diagnosis, gender, and race of the child.
• The Pediatric Pain Profile (PPP) is an observational tool developed to assess and monitor pain in children with severe cognitive impairments and unable to verbally express their pain (8). The PPP is a validated 20 item behavior rating scale designed to interpret behaviors or signs of pain (15, 16). Each item is rated on a four-point Likert scale from “not at all” to “a great deal”. Caregivers were asked to assess the pain profile of the children's behaviour when they are “on a good day” and when they experience their “most troublesome pain” if applicable. Scores range from 0 to 60 in which scores of 14 or more are generally associated with moderate or severe pain. The pain history of the children was also collected to know how the child has coped with pain and injury in the past.
• The Pain Sensory questionnaire (PSQ) is an observational tool developed to understand what triggers a child's pain and what does not. The questionnaire has previously been used in a cohort of caregivers of individuals with Christianson syndrome (17). The first section of the questionnaire asks whether the child is insensitive or sensitive when faced different sensations (cold, heat, light touch, pressure, hard impact, gusts of air, smooth surface, rough surface). If the child was sensitive to a specific sensation, a follow-up question was asked to determine whether the child has an aversive reaction.
• The Non-Communicative Children's Pain Checklist—Revised (NCCPC-R) is an observational tool designed to describe the behaviors of a child with cognitive impairment or disabilities over a period of 2 h in a home setting (18). The NCCPC-R is a validated 30-item behavior frequency rating scale divided into seven sub-scales (vocal, social, facial, activity, body and limbs, physiological, and eating/sleeping) designed to describe the child's behavior when in pain. Each item is rated on a five-point Likert scale (0 = not at all, 1 = just a little, 2 = fairly often, 3 = very often, NA = not applicable). The caregivers were asked to fill out the NCCPC-R to describe the child's reactions or lack of reaction to different painful situations if applicable. The total scores range from 0 to 90 in which a score of 7 or more indicated that the child is experiencing pain.
• The Individualized Numeric Rating Scale (INRS) is a 0–10 numerical rating scale that includes space for caregivers to insert typical pain responses for a nonverbal individual with cognitive impairment (19, 20). Building upon the NRS in which numbers ranging from 0 to 10 are placed at equidistant points on a line where 0 equals no pain and 10 equals the worst pain imaginable), caregivers were asked to populate patient pain behaviors on the vertical line that corresponds to pain intensity for their child.
Sample size and statistical analysis
Given the rarity of this syndrome, there was no sample size calculated. However, with approximately 170 known cases, according the CHD3 Foundation's website, and approximately 3%–5% of those emailed are expected to digitally consent and respond to at least one survey question, a desired sample of at least 5 was envisioned.
Initial descriptive analyses of all demographic variables was conducted by calculating the means or medians and standard deviations or ranges for the continuous outcomes, and cross-tabulations for the categorical measures. For the first aim, summative qualitative content analysis was conducted to summarize the pain history of all children of the respondents, and how their child has coped with pain and injury/illness in the past. Summative qualitative content analysis which consists of interpreting, classifying, and comparing the comments to determine themes and patterns (21). One of the authors (DDO) independently read the transcripts multiple times to identify categories of similar comments. For the secondary aim, descriptive analyses of all questionnaires was conducted. Moreover, summative qualitative content analysis of the responses from the INRS was conducted to determine any themes used for caregivers descriptions of pain intensities.
Results
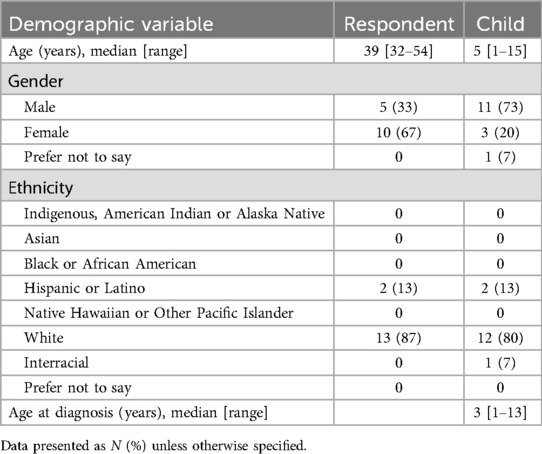
The survey was viewed 35 times and was started by 30 people. Among those, 25 respondents completed the demographics questionnaire, 15 respondents completed the first questionnaire (PPP), and six respondents completed the final questionnaire (INRS) leading to a 20% completion rate. The demographics of the 15 respondents/caregivers who completed the first questionnaire is summarized in Table 1. Five (33%) of the children were toddlers (1–3 years), five (33%) were of pre-school age (3–6 years), three (20%) were school-age (7–12 years), and two (13%) were adolescents (13–18 years).
Pain history of individuals with SNIBCPS
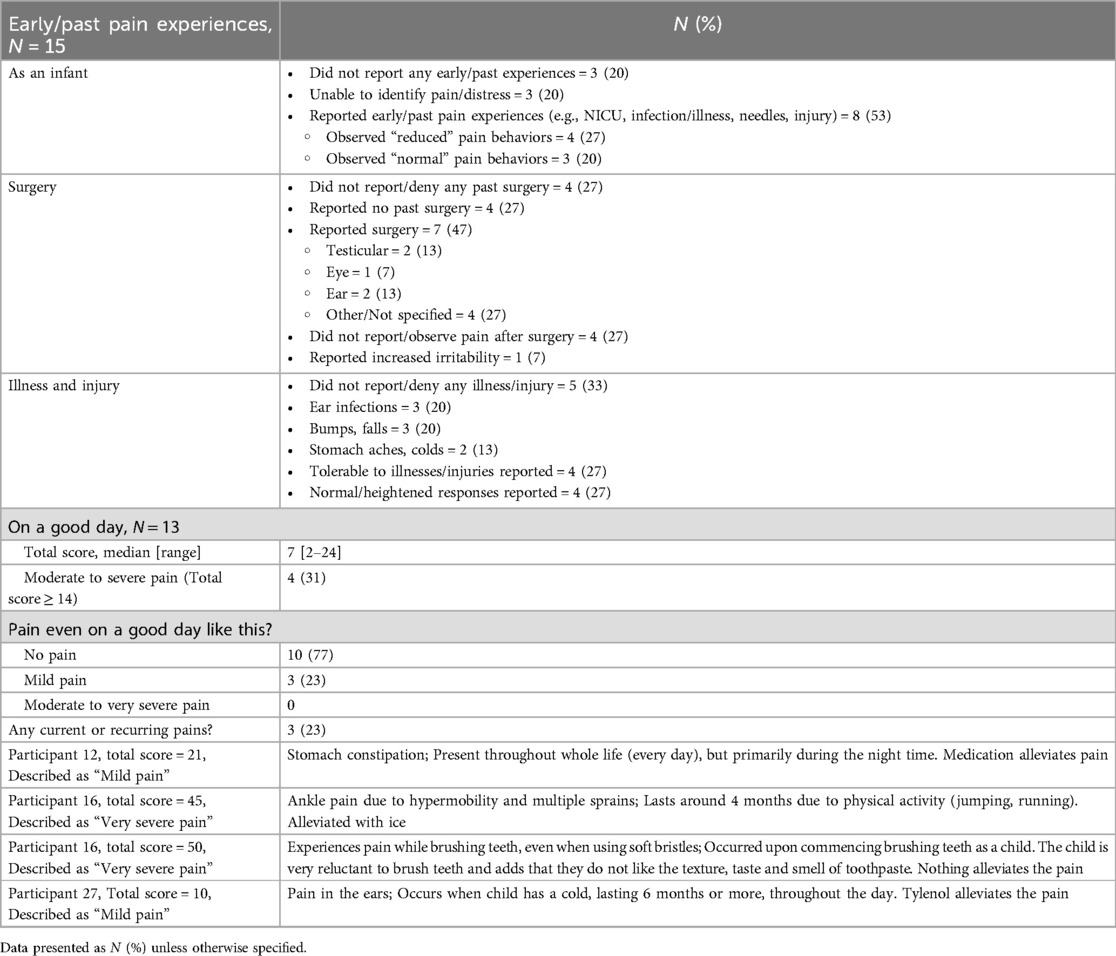
Fifteen respondents completed the pain history section of the Pediatric Pain Profile, while only thirteen (87%) completed the baseline assessments (Table 2). Only eight respondents (53%) reported early/past pain experiences from their child, such as being an inpatient in the neonatal intensive care unit, infections, illnesses, needles and injuries. However, only three of those respondents (38%) reported observing “normal” pain behaviors, such as crying or seeking comfort. Only 7 respondents (47%) reported past surgical experience in their child. Four of those respondents (57%) did not report or observe pain behaviors after surgery from their child. Eight respondents (53%) reported illnesses and injuries, such as ear infections, bumps, falls, and colds. However, there was an even split by the respondents in reporting tolerable behavior, or a normal or heightened response to the illness or injury.
From the baseline assessments of the respondents, even on a good day, three (23%) respondents reported mild pain in their child. Three respondents reported recurring pain in their child, with two of those respondents reporting their child is experiencing “mild pain”, while the other respondent reported their child was experiencing “very severe pain”.
Pain expression of individuals with SNIBCPS
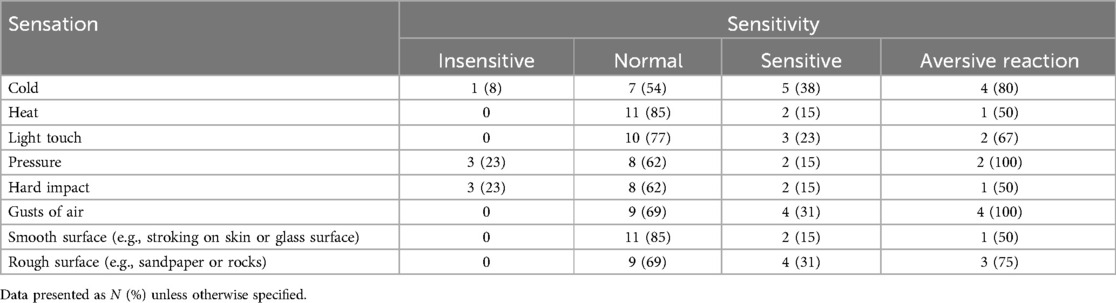
Thirteen respondents completed the Pain Sensory Questionnaire (Table 3). Up to three respondents (23%) reported their child to be insensitive to specific sensations, particularly pertaining to deep pressure and hard impact. On the other hand, two to five of the respondents (15%–38%) reported their children being sensitive to most sensations, with at least 50% of these respondents reporting that these sensations would trigger an aversive reaction in their children. For example, four of the respondents (31%) reported their children being sensitive to innocuous stimuli such as gusts of air, with all of these respondents reporting an aversive reaction from their children. Our findings highlight a wide range of sensitivity responses to noxious and innocuous stimuli.
Nine respondents completed the Non-Communicative Children's Pain Checklist—Revised (Table 4). There were nine painful situations that were reported that the children have not experienced. The situations in which a majority (>50%) of the respondents reported their child has been in included: tripping on stairs (n = 6, 67%), stubbing toes (n = 6, 67%), hitting into sharp furniture corners (n = 5, 56%), and infections (n = 6, 67%). When experiencing these situations, 67%–100% (n = 4–6) of those respondents reported scores indicating that the child is experiencing pain (total score ≥7) that needs to be addressed. When compiling all the responses reported by the respondents for all painful situations experienced, at least 33% of the respondents reported observing very often vocal, social, facial, and eating/sleeping behaviors (Figure 1).
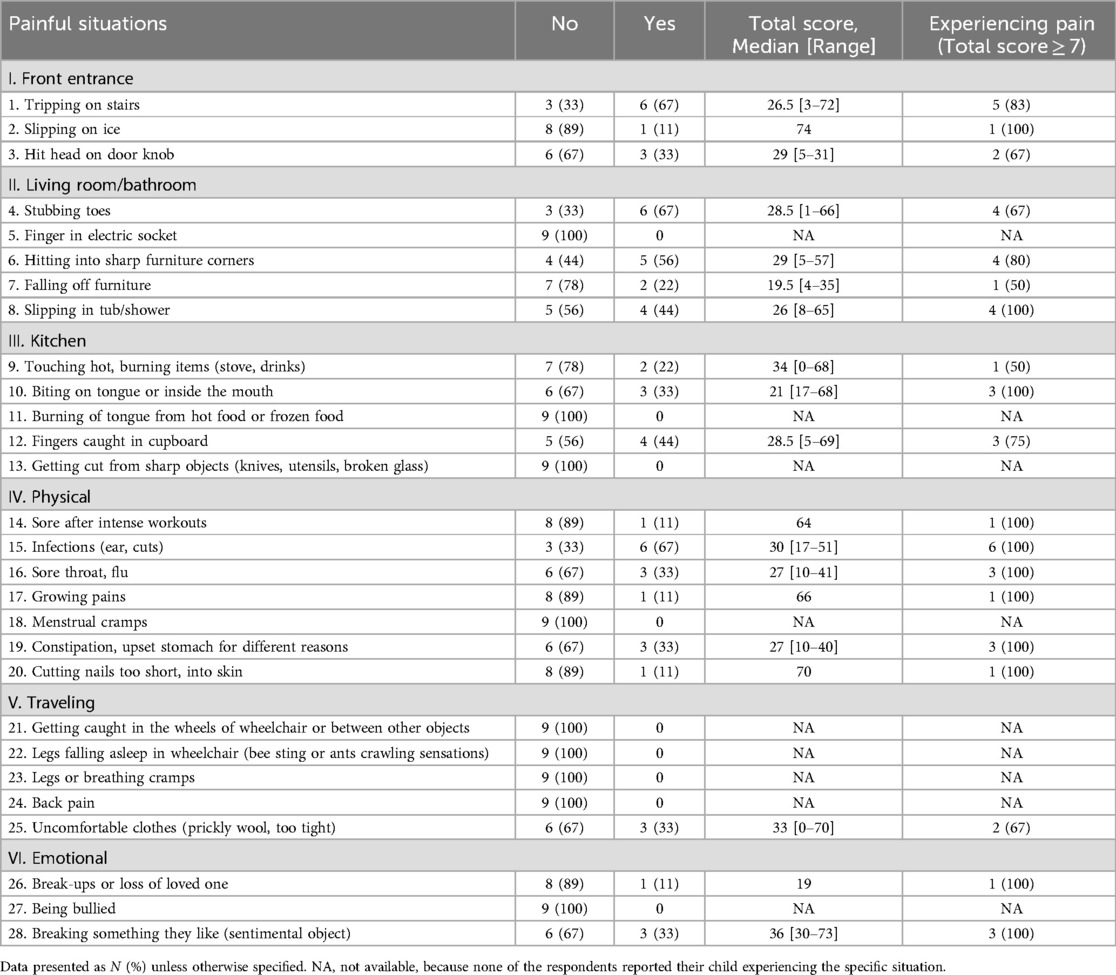
Table 4. Responses for the non-communicative children's pain checklist—revised for different painful situations, N = 9.
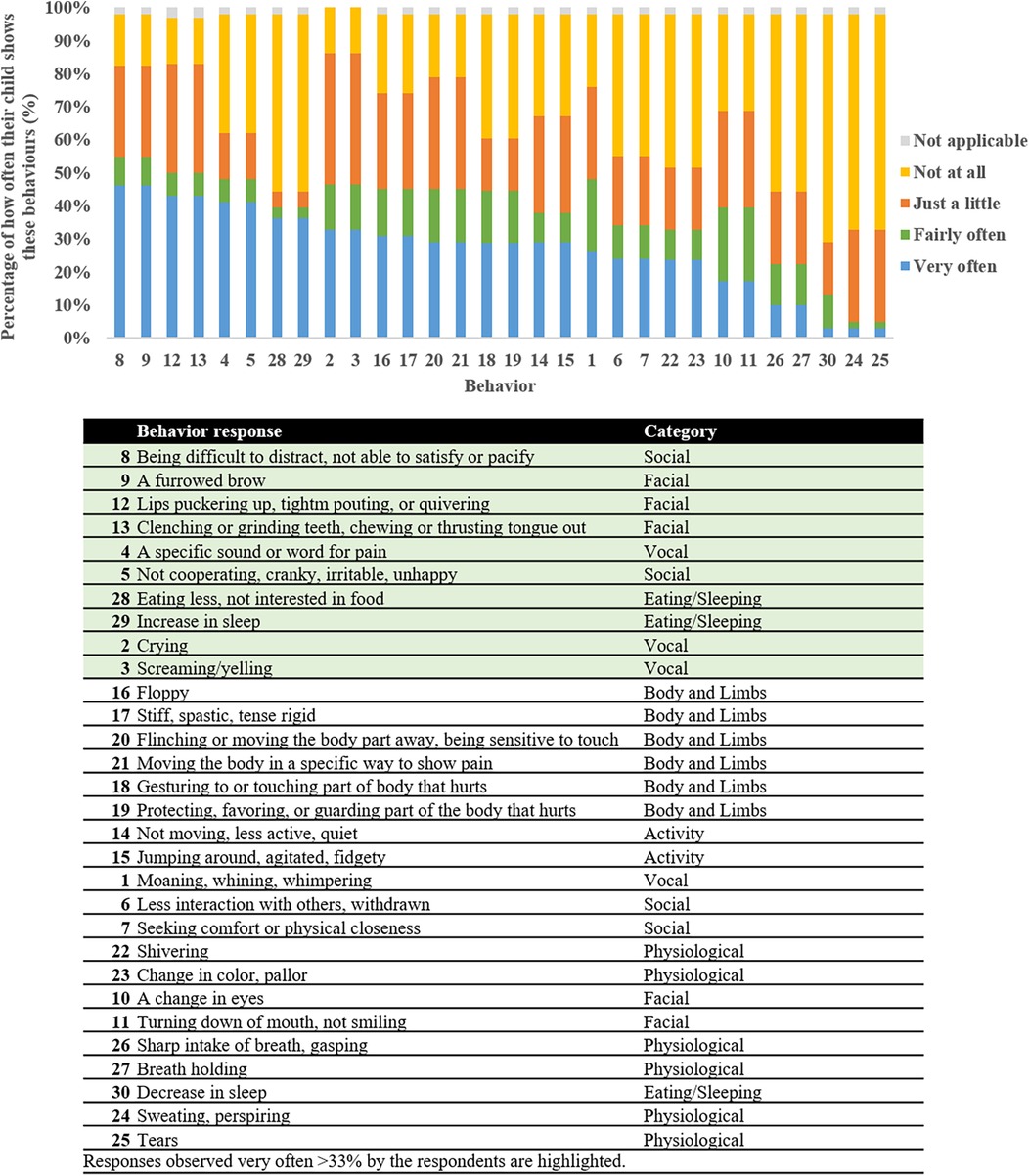
Figure 1. Rate types for each item of the non-communicative Children's pain checklist—revised by the respondents for all painful situations experienced ordered according to frequency of item occurrence. The highlighted responses represent behaviors reported by at least 33% of the respondents.
Six respondents completed the Individualized Numeric Rating (Figure 2A). Upon summative qualitative content analysis (Figure 2B), respondents reported their child to be happy, and present normal behavior during no or mild pain. However, frowning and a change in normal behavior may be observed by the respondents upon mild to moderate pain. During moderate to severe pain, respondents report observing vocal behavior representing pain, inconsolability, and self-harming behavior.
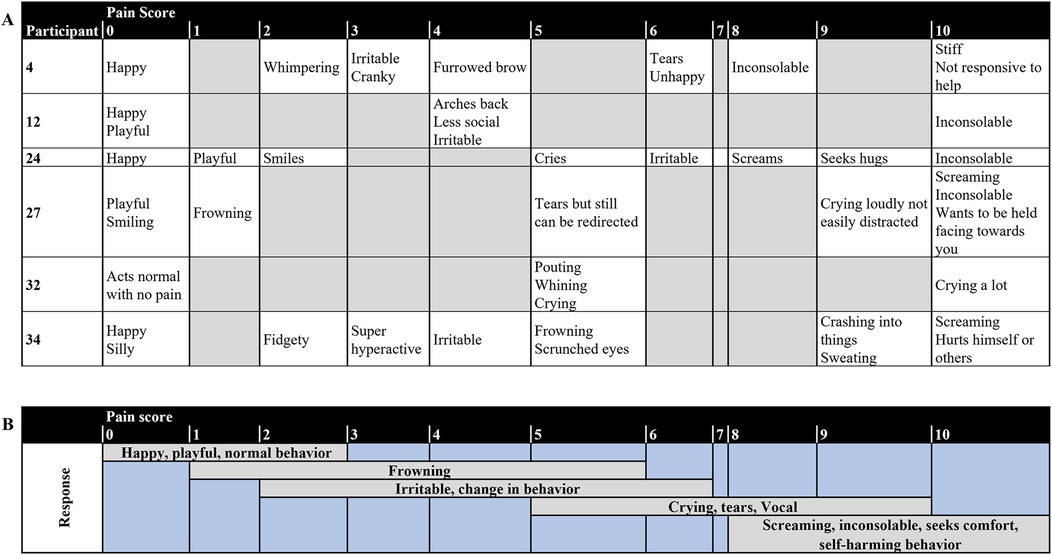
Figure 2. (A) Individual and (B) summary of responses from the Individualized numeric rating scale of the respondents (N = 6).
Discussion
This is the first study to investigate the experience and perception of pain of individuals with Snijders Blok-Campeau Syndrome. We report in 15 individuals with a diagnosis of SNIBCPS, that their caregivers report early and past painful experiences, and have a range of behaviors to express their pain.
At least 50% of the respondents reported past pain experiences as an infant, surgical experiences, illnesses and injuries in their child. However, when a painful experience was reported, respondents' observation of normal or reduced pain responses were nearly 50% split. One caregiver reported recurrent/current pain of their child as very severe for hypermobility and brushing teeth. Studies have shown that untreated or undertreated pain at infancy can lead to increased pain sensitivity (22, 23) and neurodevelopmental and socioeconomic problems (13, 24), and poor pain management or chronic pain in childhood can have consequential effects into adulthood (13, 25–28). Therefore, our findings highlights the need for proper assessment and treatment of pain in children with intellectual disability or developmental delay, especially when “reduced” pain responses are observed. The absence of pain responses in this population may not necessarily mean they feel less pain.
Our findings from the Pain Sensory Questionnaire highlight that although individuals with SNIBCPS may have cognitive impairments, a majority of them are observed to display normal or heightened reactions to diverse sensations. Coursimault et al. reported on a case diagnosed with severe intellectual disability, but associated with a frank happy demeanor, highlighting hypersociability may constitute a suggestive feature of CHD3 mutations (29). Reduced sensitivity may only be observed in a minority of individuals with SNIBCPS. Nevertheless, this is an important observation to note for caregivers, especially when respondents noted reduced sensitivity to noxious stimuli (e.g., pressure and hard impact), as unreported, undertreated or untreated pain could lead to decreased quality of life (13). Future directions may involve using quantitative sensory testing in this population to determine their somatosensory function and its association with the caregivers' observations. Although quantitative sensory testing primarily relies on the self-report of the subject regarding their sensation of mechanical or thermal stimuli, advances have been made investigating modified quantitative sensory testing protocols for individuals with intellectual or developmental disability (30, 31). Gunderson et al. recently investigated the feasibility of a modified quantitative sensory testing protocol in children with intellectual and developmental disabilities and reported that the modified approach was able to measure tactile reactivity for children with complex communication needs (32). Moreover, due to the rarity of SNIBCPS, proxy-administered quantitative sensory testing with a mobile tool-kit may be a direction to explore (33). A proxy-administered modified quantitative sensory testing approach may be useful to understand the sensory function of individuals with SNIBCPS in relation to developmental and behavioral responses to pain.
Regarding how individuals express pain during painful situations, respondents of our survey report primarily very often observing vocal, social, facial, and eating/sleeping behaviors. These responses were also noted in the individualized numerical rating scales from the respondents. Data from the previously published cohorts of individuals have suggested that most CHD3 pathogenic mutations were associated with delayed milestones in speech and language, with expressive language being more affected than receptive language (1–3). Moreover, CHD3 protein interacts with FOXP2, which is known to be implicated in language problems in Childhood Apraxia of Speech (34–36). The findings from the INRS highlight the need for proper assessment of individuals with SNIBCPS, especially since vocal expressions were assigned to moderate-to-severe pain scores. The social and eating/sleeping behavioral responses are important to note in this population as they are indirect behaviors related to pain. Findings from the INRS showed that a change in behavior was primarily assigned to mild pain, with inconsolability and self-harming behaviors assigned to severe pain. The INRS has been shown to have good reliability between parents, bedside nurse and research nurse in nonverbal children with intellectual disability (20). Therefore INRS may be a beneficial tool for clinicians, especially in acute or inpatient settings, or when atypical behaviors are reported (37). Healthcare providers for individuals with SNIBCPS should support their families/caregivers to develop their sense of knowledge and skills and to gain confidence in pain assessment (38). Another tool that can be beneficial is the revised and individualized Face Legs Activity Cry and Consolability (FLACC) behavioral pain assessment tool which has been validated in children with cognitive impairment (39). These tools can be used by caregivers to advocate for any under- or untreated pain.
Managing pain in patients with SNIBCPS requires healthcare providers to acquire the knowledge, empathy and compassion to identify and provide proper care. First and foremost, showing empathy and compassion is important to create a safe environment for the patient to feel empowered to express any discomfort non-verbally. Second, a comprehensive assessment of past pain experiences and behaviors observed from the patient's caregiver is important understand the patient's response to painful situations. Finally, an INRS or revised and individualized FLACC scale can be created in collaboration with the patient's caregivers for healthcare providers to effectively identify pain and provide personalized care.
Certain limitations should be considered when interpreting the results of this study. First, this was an anonymous survey with a small sample size with a majority of the participants with SNIBCPS being male and White. According to previous findings (3, 5), a majority of the variants in individuals with SNIBCPS that were inherited were maternally inherited. There was thus a female predominance observed among heterozygote parents, potentially indicating a female protective effect for CHD3 variation. Recent findings have observed no sex bias in the affected probands, or in the severity of the intellectual disability in the novo or inherited cases (3, 5). Nevertheless, our findings cannot be generalized to all individuals with SNIBCPS. With about 170 known cases, future studies with qualitative interviews with families may provide more insight on their experience and response of pain in other demographic groups. Second, many pain-related factors were not included in the data collection of this study, such as comorbidities, medication use, degree of intellectual disability, etc. A comprehensive biopsychosocial pain assessment is therefore warranted in this population. Third, although the parents/caregivers are considered “experts” regarding the pain expression of individuals with SNIBCPS, they can not fully understand their personal pain experience. Pain, as defined by the International Association for the Study of Pain, is “an unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (40). Although there has been work to identify pain biomarkers, there is still headway to bring these biomarkers into point-of care (41, 42). Lastly, the genetic variants involved for each individual was not collected, therefore, it is difficult to determine whether there is a genotype-phenotype relationship regarding the anecdotal decreased pain sensitivity observed in a subset of individuals with SNIBCPS. A registry of individuals with SNIBCPS is currently under development. Nevertheless, a follow-up study using similar methods including genetic information is warranted once the registry is operating.
In conclusion, our study brings awareness about the proper examination of individuals with Snijders Blok-Campeau Syndrome. Despite the small sample size, our findings suggest that pain and injuries may go unreported in individuals with SNIBCPS, and individualized parental observational scales may be beneficial for their healthcare providers. Future studies investigating the relationship between the genotype and the phenotype of this population, especially those with suspected hyposensitivity, is warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by IRB Office, Boston Children's Hospital. The studies were conducted in accordance with the local legislation and institutional requirements. The ethics committee/institutional review board waived the requirement of written informed consent for participation from the participants or the participants' legal guardians/next of kin because this was an anonymous survey, no protected health information was collected, and consent was provided by the completion of the survey.
Author contributions
DO: Conceptualization, Data curation, Formal analysis, Methodology, Project administration, Visualization, Writing – original draft, Writing – review & editing. PC: Writing – review & editing. CBe: Conceptualization, Writing – review & editing. CBr: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This manuscript was supported by the Sara Page Mayo Endowment for Pediatric Pain Research.
Acknowledgments
We would like to acknowledge Dr. Catherine E. Ferland, PhD, on their guidance and acceptance in using the Pain Sensory and Painful Situations questionnaire, and Dr. Jean C. Solodiuk, RN, PhD, on their guidance on using and analyzing the Individualized Numeric Rating Scale. We would also like to acknowledge the CHD3 Foundation on their collaboration and engagement in sharing the study with their members.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Snijders Blok L, Rousseau J, Twist J, Ehresmann S, Takaku M, Venselaar H, et al. CHD3 helicase domain mutations cause a neurodevelopmental syndrome with macrocephaly and impaired speech and language. Nat Commun. (2018) 9(1):4619. doi: 10.1038/s41467-018-06014-6
2. Drivas TG, Li D, Nair D, Alaimo JT, Alders M, Altmüller J, et al. A second cohort of CHD3 patients expands the molecular mechanisms known to cause Snijders Blok-Campeau syndrome. Eur J Hum Genet. (2020) 28(10):1422–31. doi: 10.1038/s41431-020-0654-4
3. van der Spek J, den Hoed J, Snijders Blok L, Dingemans AJM, Schijven D, Nellaker C, et al. Inherited variants in CHD3 show variable expressivity in Snijders Blok-Campeau syndrome. Genet Med. (2022) 24(6):1283–96. doi: 10.1016/j.gim.2022.02.014
4. Eising E, Carrion-Castillo A, Vino A, Strand EA, Jakielski KJ, Scerri TS, et al. A set of regulatory genes co-expressed in embryonic human brain is implicated in disrupted speech development. Mol Psychiatry. (2019) 24(7):1065–78. doi: 10.1038/s41380-018-0020-x
5. Pascual P, Tenorio-Castano J, Mignot C, Afenjar A, Arias P, Gallego-Zazo N, et al. Snijders Blok-Campeau syndrome: description of 20 additional individuals with variants in CHD3 and literature review. Genes (Basel). (2023) 14(9):1664. doi: 10.3390/genes14091664
6. Mizukami M, Ishikawa A, Miyazaki S, Tsuzuki A, Saito S, Niihori T, et al. A de novo CHD3 variant in a child with intellectual disability, autism, joint laxity, and dysmorphisms. Brain Dev. (2021) 43(4):563–5. doi: 10.1016/j.braindev.2020.12.004
7. LeBreton L, Allain EP, Parscan RC, Crapoulet N, Almaghraby A, Ben Amor M. A novel CHD3 variant in a patient with central precocious puberty: expanded phenotype of Snijders Blok-Campeau syndrome? Am J Med Genet A. (2023) 191(4):1065–9. doi: 10.1002/ajmg.a.63096
8. Breau LM, Burkitt C. Assessing pain in children with intellectual disabilities. Pain Res Manag. (2009) 14(2):116–20. doi: 10.1155/2009/642352
9. Barney CC, Andersen RD, Defrin R, Genik LM, McGuire BE, Symons FJ. Challenges in pain assessment and management among individuals with intellectual and developmental disabilities. Pain Rep. (2020) 5(4):e821. doi: 10.1097/PR9.0000000000000822
10. Genik LM, McMurtry CM, Breau LM. Caring for children with intellectual disabilities part 1: experience with the population, pain-related beliefs, and care decisions. Res Dev Disabil. (2017) 62:197–208. doi: 10.1016/j.ridd.2017.01.020
11. Fründt O, Grashorn W, Schöttle D, Peiker I, David N, Engel AK, et al. Quantitative sensory testing in adults with autism spectrum disorders. J Autism Dev Disord. (2017) 47(4):1183–92. doi: 10.1007/s10803-017-3041-4
12. Vaughan S, McGlone F, Poole H, Moore DJ. A quantitative sensory testing approach to pain in autism spectrum disorders. J Autism Dev Disord. (2020) 50(5):1607–20. doi: 10.1007/s10803-019-03918-0
13. Victoria NC, Murphy AZ. Exposure to early life pain: long term consequences and contributing mechanisms. Curr Opin Behav Sci. (2016) 7:61–8. doi: 10.1016/j.cobeha.2015.11.015
14. Miller EL, Okour A. Pain management among the cognitively impaired. Pain Manag Nurs. (2024) 25(2):101–3. doi: 10.1016/j.pmn.2024.03.015
15. Hunt A, Goldman A, Seers K, Crichton N, Mastroyannopoulou K, Moffat V, et al. Clinical validation of the paediatric pain profile. Dev Med Child Neurol. (2004) 46(1):9–18. doi: 10.1111/j.1469-8749.2004.tb00428.x
16. Hunt A, Wisbeach A, Seers K, Goldman A, Crichton N, Perry L, et al. Development of the paediatric pain profile: role of video analysis and saliva cortisol in validating a tool to assess pain in children with severe neurological disability. J Pain Symptom Manage. (2007) 33(3):276–89. doi: 10.1016/j.jpainsymman.2006.08.011
17. Premachandran S, Ocay DD, Beaulieu C, Balduzzi J, Ye DL, Davidova A, et al. Pain experience of children with Christianson syndrome. Pain. (2025) 166(7):1610–21. doi: 10.1097/j.pain.0000000000003522
18. Breau LM, McGrath PJ, Camfield CS, Finley GA. Psychometric properties of the non-communicating children’s pain checklist-revised. Pain. (2002) 99(1–2):349–57. doi: 10.1016/S0304-3959(02)00179-3
19. Solodiuk J, Curley MA. Pain assessment in nonverbal children with severe cognitive impairments: the individualized numeric rating scale (INRS). J Pediatr Nurs. (2003) 18(4):295–9. doi: 10.1016/S0882-5963(03)00090-3
20. Solodiuk JC, Scott-Sutherland J, Meyers M, Myette B, Shusterman C, Karian VE, et al. Validation of the individualized numeric rating scale (INRS): a pain assessment tool for nonverbal children with intellectual disability. Pain. (2010) 150(2):231–6. doi: 10.1016/j.pain.2010.03.016
21. Hsieh H-F, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. (2005) 15(9):1277–88. doi: 10.1177/1049732305276687
22. Goffaux P, Lafrenaye S, Morin M, Patural H, Demers G, Marchand S. Preterm births: can neonatal pain alter the development of endogenous gating systems? Eur J Pain. (2008) 12(7):945–51. doi: 10.1016/j.ejpain.2008.01.003
23. Valeri BO, Ranger M, Chau CM, Cepeda IL, Synnes A, Linhares MB, et al. Neonatal invasive procedures predict pain intensity at school age in children born very preterm. Clin J Pain. (2016) 32(12):1086–93. doi: 10.1097/AJP.0000000000000353
24. Grunau RE, Whitfield MF, Petrie-Thomas J, Synnes AR, Cepeda IL, Keidar A, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. (2009) 143(1–2):138–46. doi: 10.1016/j.pain.2009.02.014
25. Taddio A, Katz J, Ilersich AL, Koren G. Effect of neonatal circumcision on pain response during subsequent routine vaccination. Lancet. (1997) 349(9052):599–603. doi: 10.1016/S0140-6736(96)10316-0
26. Taddio A, Ipp M, Thivakaran S, Jamal A, Parikh C, Smart S, et al. Survey of the prevalence of immunization non-compliance due to needle fears in children and adults. Vaccine. (2012) 30(32):4807–12. doi: 10.1016/j.vaccine.2012.05.011
27. Hestbaek L, Leboeuf-Yde C, Kyvik KO, Manniche C. The course of low back pain from adolescence to adulthood: eight-year follow-up of 9600 twins. Spine (Phila Pa 1976). (2006) 31(4):468–72. doi: 10.1097/01.brs.0000199958.04073.d9
28. Brattberg G. Do pain problems in young school children persist into early adulthood? A 13-year follow-up. Eur J Pain. (2004) 8(3):187–99. doi: 10.1016/j.ejpain.2003.08.001
29. Coursimault J, Lecoquierre F, Saugier-Veber P, Drouin-Garraud V, Lechevallier J, Boland A, et al. Hypersociability associated with developmental delay, macrocephaly and facial dysmorphism points to CHD3 mutations. Eur J Med Genet. (2021) 64(4):104166. doi: 10.1016/j.ejmg.2021.104166
30. Barney CC, Tervo R, Wilcox GL, Symons FJ. A case-controlled investigation of tactile reactivity in young children with and without global developmental delay. Am J Intellect Dev Disabil. (2017) 122(5):409–21. doi: 10.1352/1944-7558-122.5.409
31. Cornelissen L, Donado C, Yu TW, Berde CB. Modified sensory testing in non-verbal patients receiving novel intrathecal therapies for neurological disorders. Front Neurol. (2022) 13:664710. doi: 10.3389/fneur.2022.664710
32. Gunderson J, Worthley E, Byiers B, Merbler A, Huebner A, Hofschulte D, et al. Modifying quantitative sensory testing to investigate tactile sensory function and behavioral reactivity in children with intellectual and developmental disabilities: establishing feasibility and testing sex, autism, and self-injury effects. J Neurodev Disord. (2025) 17(1):15. doi: 10.1186/s11689-025-09603-x
33. Ocay DD, Lobo K, Kim A, Halpin M, Berde CB. Development and validation of a home quantitative sensory testing tool-kit to assess changes in sensory and pain processing: a study in healthy young adults. Pain. (2025) 166(1):52–66. doi: 10.1097/j.pain.0000000000003320
34. Morgan A, Fisher SE, Scheffer I, Hildebrand M. FOXP2-related speech and language disorder. In: Adam MP, Feldman J, Mirzaa GM, Pagon RA, Wallace SE, Amemiya A, editors. GeneReviews® (online). Seattle, WA: University of Washington (2016). [updated 2023]. Available online at: https://www.ncbi.nlm.nih.gov/books/NBK368474/ (Accessed December 05, 2024).
35. Lai CS, Fisher SE, Hurst JA, Vargha-Khadem F, Monaco AP. A forkhead-domain gene is mutated in a severe speech and language disorder. Nature. (2001) 413(6855):519–23. doi: 10.1038/35097076
36. Graham SA, Fisher SE. Understanding language from a genomic perspective. Annu Rev Genet. (2015) 49:131–60. doi: 10.1146/annurev-genet-120213-092236
37. Hauer J, Houtrow AJ, Section on Hospice and Palliative Medicine, Council on Children with Disabilities. Pain assessment and treatment in children with significant impairment of the central nervous system. Pediatrics. (2017) 139(6):e20171002. doi: 10.1542/peds.2017-1002
38. Carter B, Arnott J, Simons J, Bray L. Developing a sense of knowing and acquiring the skills to manage pain in children with profound cognitive impairments: mothers’ perspectives. Pain Res Manag. (2017) 2017:2514920. doi: 10.1155/2017/2514920
39. Malviya S, Voepel-Lewis T, Burke C, Merkel S, Tait AR. The revised FLACC observational pain tool: improved reliability and validity for pain assessment in children with cognitive impairment. Paediatr Anaesth. (2006) 16(3):258–65. doi: 10.1111/j.1460-9592.2005.01773.x
40. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161(9):1976–82. doi: 10.1097/j.pain.0000000000001939
41. Niculescu AB, Le-Niculescu H, Levey DF, Roseberry K, Soe KC, Rogers J, et al. Towards precision medicine for pain: diagnostic biomarkers and repurposed drugs. Mol Psychiatry. (2019) 24(4):501–22. doi: 10.1038/s41380-018-0345-5
Keywords: pain, experience, perception, Snijders Blok-Campeau syndrome, CHD3
Citation: Ocay DD, Campeau PM, Berde CB and Brownstein CA (2025) Pain experience and perception in individuals with Snijders Blok-Campeau syndrome. Front. Pain Res. 6:1540422. doi: 10.3389/fpain.2025.1540422
Received: 5 December 2024; Accepted: 27 July 2025;
Published: 13 August 2025.
Edited by:
Tonia C. Onyeka, University of Nigeria, NigeriaReviewed by:
Caridad Velazquez Cardona, Grey’s Hospital, South AfricaDmytro Dmytriiev, National Pirogov Memorial Medical University, Ukraine
Bernie Carter, Edge Hill University, United Kingdom
Kareem Omran, King's College London, United Kingdom
Copyright: © 2025 Ocay, Campeau, Berde and Brownstein. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Don Daniel Ocay, ZG9uZGFuaWVsLm9jYXlAY2hpbGRyZW5zLmhhcnZhcmQuZWR1
 Don Daniel Ocay
Don Daniel Ocay Philippe M. Campeau
Philippe M. Campeau Charles B. Berde
Charles B. Berde Catherine A. Brownstein4,5,6
Catherine A. Brownstein4,5,6