- 1Department of Anesthesiology, Perioperative Care and Pain Medicine, New York University Grossman School of Medicine, New York, NY, United States
- 2Interdisciplinary Pain Research Program, New York University Grossman School of Medicine, New York, NY, United States
- 3Department of Psychiatry, New York University Grossman School of Medicine, New York, NY, United States
- 4Department of Neuroscience & Physiology, Neuroscience Institute, New York University Grossman School of Medicine, New York, NY, United States
- 5Department of Biomedical Engineering, New York University Tandon School of Engineering, New York, NY, United States
Introduction: This study aims to investigate the interregional functional connectivity in chronic back pain patients with widespread hyperalgesia, patients with localized back pain, and pain-free controls using stimulus-evoked high-density EEG recordings.
Methods: We conducted high-density EEG recordings to compare the functional connectivity and betweenness centrality between these groups.
Results: Compared with controls, chronic pain patients showed altered functional connectivity between regions that process cognitive information and regions that process sensory or affective information. Widespread hyperalgesia, however, is further differentiated from localized pain by decreased inter-hemispheric connectivity of sensory and affective areas and increased intra-hemispheric connectivity between sensory and cognitive cortices. Graph-theoretic analysis showed that whereas chronic pain is associated with decreased centrality of prefrontal, orbitofrontal, and cingulate areas, widespread hyperalgesia is distinguished by increased centrality of prefrontal and insular areas.
Discussion: Together, our results show that although widespread hyperalgesia shares certain features with localized pain, it is further characterized by distinct cortical mechanisms.
Introduction
Widespread hyperalgesia, heightened pain sensitivity outside the primary area of disease (1–4), is prevalent in pain conditions like osteoarthritis, rheumatoid arthritis, and low back pain (5–8). It correlates with higher pain intensity and daily activity interference, worsening overall health (9). Despite its prevalence and associated morbidity, its mechanisms remain poorly studied.
Imaging studies and serum or plasma markers often don't correlate with widespread hyperalgesia symptoms (10, 11). Given the non-anatomical pain distribution and hypersensitivity, the central nervous system (CNS) is believed to play a key role, with widespread pain or widespread hyperalgesia likely involving nociplastic mechanisms (12–25). Key regions in aversive and cognitive processing of pain include the anterior cingulate cortex (ACC), insular cortex (IC), prefrontal cortex (PFC) and medial orbitofrontal cortex (mOFC) (14, 17, 26–45). The ACC, a hub for aversive processing (26–34, 46, 47), has been shown in animal and human studies to causally regulate generalized aversive response to nociceptive inputs, suggesting its involvement in the mechanisms underlying widespread hyperalgesia (12, 15, 26, 48). The IC is another critical region for aversive processing implicated in fibromyalgia and other rheumatological disorders that show nociplastic features (49–51). Both the ACC and the anterior IC have been shown to process bottom-up aversive experiences (52, 53), while the PFC and mOFC are known to provide top-down cognitive regulation of pain (17, 45, 54–56).
Despite ongoing research, two key questions about widespread pain and hyperalgesia remain unanswered. First, what circuit mechanisms cause disordered nociceptive processing in unrelated, disease-free anatomical regions characterizing widespread hyperalgesia? Second, does widespread hyperalgesia represent a quantitative increase in the strength of maladaptive plasticity in nociceptive circuits already existing in chronic localized pain, or is it caused by distinct circuit changes not seen in localized pain?
Although widespread hyperalgesia may occur in both animals and humans, animal models of widespread hyperalgesia may not accurately reflect clinical conditions. Therefore, human neuroimaging pain studies offer valuable insight and neural biomarkers. While resting-state brain activity provides characterization of functional connectivity (FC), it often lacks specificity due to unknown influences from other cognitive processes. To fully understand the mechanisms of widespread hyperalgesia, it is important to study neural activity specifically during active period of nociceptive processing. Thus, we conducted noxious stimulus-evoked EEG recordings in chronic low back pain patients with widespread hyperalgesia, localized low back pain, and pain-free controls. Our findings indicate that while chronic pain patients exhibit numerous alterations in FC in response to noxious stimuli, widespread hyperalgesia differs from localized pain by distinctly reduced inter-hemispheric connectivity within sensory areas and affective areas. Such inter-hemispheric synchronization deterioration is coupled with an increase in intra-hemispheric connectivity between sensory and non-sensory cortical regions. Furthermore, whereas chronic pain is associated with decreased centrality of the prefrontal, orbitofrontal and cingulate cortical areas, widespread hyperalgesia is characterized by increased centrality of prefrontal and insular cortical areas. Our results indicate that, although widespread hyperalgesia shares certain common features with chronic localized pain at the cortical level, it is also characterized by a distinct set of circuit mechanisms.
Methods
Study participants
This study was approved by the New York University Grossman School of Medicine Institutional Review Board (8/22/2019, #i19-01088) and conducted in accordance with the latest version of the Declaration of Helsinki. Written informed consent was obtained from all participants.
Inclusion criteria for chronic back pain patients were diagnosis of chronic low back pain lasting longer than 6 months with a baseline average back pain intensity >4 on a 0–10 numerical rating scale; age between 18 and 75 years; and American Society of Anesthesiologists (ASA) physical status 1–3. Exclusion criteria included acute lumbosacral radiculopathy with sensory or motor symptoms, systemic signs or symptoms, cognitive impairment (by history) or clinical signs of altered mental status; history of schizophrenia; daily benzodiazepine use; and pregnancy.
Assessment of pain, function, and mood
Prior to EEG recordings, participants underwent a comprehensive assessment of pain, function, and mood based on recommendations from the National Institutes of Health (NIH) Task force on research standards for chronic low back pain (57). PROMIS numeric rating scale—pain intensity, PROMIS pain interference 4a, PROMIS anxiety 8a, PROMIS depression 8a, and PROMIS sleep disturbance assessed symptoms over the preceding week. The McGill Pain Questionnaire short form was used to assess the multidimensional component of pain. PROMIS physical function 4a assessed physical function.
EEG recordings and mechanical stimulation
Brain activity was recorded using high-density electroencephalography (EEG), equipped with two integrated bipolar leads for vertical electrooculogram (EOG; 64-channel Quik-Cap Neo Net, Compumedics Neuroscan, Charlotte, NC, USA) with the ground electrode positioned on the left cheek. The EEG cap was interfaced with a 64-channel Neuroscan SynAmps 2/RT and Nuevo Amplifier (Compumedics Neuroscan, Charlotte, NC, USA). Each recording session began with two 5-minute baseline recordings (5 minutes with eyes closed, followed by 5 minutes with eyes open) prior to the administration of mechanical stimuli. Participants were blindfolded during the EEG recordings and asked to stay relaxed and in a wakeful state during the behavioral tasks. Weighted mechanical pinprick stimulators (MRC System GmbH, Heidelberg, Germany) exerting forces of 32 mN and 256 mN were used to apply mechanical stimuli both to the lower back and the dorsum of the right hand. 10–20 trials per force were applied at each site with stimulations delivered in random order with an interstimulus interval of approximately 10 s. Participants were asked to rate each stimulus on a 0–10 numeric rating scale, with 0 indicating no pain and 10 indicating high pain. All data were captured using the Curry 8 software (Compumedics Neuroscan, Charlotte, NC, USA) with a sampling rate of 1,000 Hz.
Pain phenotyping
A threshold for hyperalgesic response to 32 mN stimulation to the hand, a site typically not affected by back pain, was defined as 2 standard deviations above the mean pain rating in control participants. Chronic low back pain participants reporting pain scores below this threshold with 32 mN stimuli to the hand were defined as having chronic localized pain. Chronic low back pain participants reporting pain scores above this threshold with 32 mN stimuli to the hand were defined as having widespread hyperalgesia.
EEG preprocessing
MNE-Python (version 1.6.1) was utilized for preprocessing (58). First, raw signals were down-sampled to a rate of 400 Hz and a band-pass filter between 1 and 100 Hz was applied. A band-stop filter with 3 Hz width was applied at 60 Hz to eliminate electrical line noise. Noisy EEG channels were identified and subsequently interpolated using PyPREP (59). Criteria for noisy channel detection included low signal-to-noise ratio (SNR), lack of correlation with other channels, low or high relative deviations, presence of high-frequency noise, and poor prediction by other channels based on the random sample consensus approach.
All signals were re-referenced to the average reference. An independent component analysis (ICA) based on the fast ICA algorithm was conducted on the EEG data within the −2.5–2.5 s peri-stimulus time windows. This process utilized a number of independent components (ICs) equivalent to half the number of EEG channels (60). ICs that represented artifacts originating from eye movements, recorded in the EOG electrode, were removed from the EEG data.
The cleaned data were analyzed using functions in MNE-Python, in addition to custom-written Python code. Data were segmented into epochs ranging from −2.5 to 2.5 s in peri-stimulus time. Noisy epochs were identified using the AutoReject package based on Bayesian optimization and were automatically marked for rejection (61). Automatically rejected epochs accurately matched trials marked in the recording notes as containing movement.
To highlight changes in oscillatory activity, epochs were z-scored relative to their pre-stimulus baselines. Z-scored epochs were achieved by subtracting the mean of the baseline period (−2.5–0.0 s) from each epoch (−2.5–2.5 s), followed by division by the standard deviation of the baseline. This procedure ensures a common scale for all epochs, facilitating more accurate comparisons and analyses.
Source model
To project sensor-space time series to source space, the Minimum Norm Estimate (MNE) was employed, using its implementation in MNE-Python (62). The surface-based, three-shell boundary element model used for anatomical reconstruction was derived from “fsaverage”—a template brain MRI constructed from 40 brain MRI scans (63–65). Loose-orientation was set to 0.2 for inverse solution computation to allow source space dipoles freedom of rotation without deviating extensively from an orientation perpendicular to the cortex. The regions of interest (ROIs) selected for source-localization were the dorsal and rostral anterior cingulate cortices (dACC, rACC), the dorsolateral prefrontal cortex (dlPFC), the medial orbitofrontal cortex (mOFC), the primary somatosensory cortex (S1), and the insular cortex (IC). Both left and right hemisphere regions were considered for connectivity analysis, resulting in a total of 12 regions.
Source-space frequency-domain representations
To secure a robust stimulus response from each participant, source-space z-scored epochs were averaged. Next, frequency-domain representations were estimated from the averaged source-space z-scored epochs using a batch of multitapers with digital prolate spheroidal sequence (DPSS) windows, one for each of the canonical frequency bands— theta (4–8 Hz), alpha (8–13 Hz), beta (13–30 Hz), low-gamma (30–58.5 Hz) and high-gamma (61.5–100 Hz).
Functional connectivity (FC) analysis
Connectivity analyses of EEG data were performed using a phase-based approach (66). Phase-based connectivity measures rely on temporal synchronization of brain activity, and thus are more strongly affected by contextual factors. In this study, functional connectivity was investigated using the debiased weighted phase lag index-square estimator (dwPLI) (67). dwPLI is a well-established and highly sensitive phase-based connectivity, a modification of the weighted phase lag index (wPLI), offering a more robust measure against noise and volume conduction effects. It quantifies the asymmetry of phase differences and is debiased to minimize the influence of random phase relationships. The values range from 0 to 1, with 0 indicating either no interaction or symmetric phase differences, and values approaching 1 indicating strong asymmetric phase coupling. Thus, dwPLI is resistant to volume conduction without the risk of reduced sensitivity, as real synchrony at zero phase lag is also discarded. If the wPLI exceeds the phase lag index (PLI), the dwPLI will be negatively biased for small sample sizes, resulting in values below 0.
Centrality analysis
To better understand the network topology of chronic pain, we analyzed the betweenness centrality of the source space ROIs. Node betweenness centrality, a concept borrowed from graph theory, is the number of shortest paths between pairs of other nodes that pass through a given node. Nodes with high betweenness centrality serve as a bridge between many pairs of other nodes. In our context, each ROI is a node in the pain processing network, and the connections between them, based on dwPLI, are the edges. Regions in the brain with high betweenness centrality act as hubs in the network.
Weighted betweenness centrality was computed using betweenness_wei.m from Brain Connectivity Toolbox (15, 68). Because centrality must be estimated from a connection-length matrix, the inverse of each connectivity matrix was taken prior to centrality estimation. The resulting centrality vector (BC, 1xN) is normalized to the range [0,1] as BC[(N-1)x(N-2)], where N is the number of nodes in the network.
Functional grouping
To reduce the degree of freedom and improve the detection statistics in the presence of multiple comparisons, individual cortical regions were grouped into sensory (S1), affective (ACC and IC) and cognitive areas (mOFC and dlPFC), for both left and right hemispheres. To achieve this, three reduction techniques were tested: mean, median, and maximum. For either FC or centrality measure, the mean, median, and maximum value of the regions within each group were computed, resulting in a connectivity matrix (or centrality vector) with N = 6, for the three groups in both hemispheres. Maximum value was selected as the reduction technique for functional grouping.
Statistical analysis
Behavioral data
Pain numeric ratings were analyzed using IBM SPSS Statistical Software (Version 28, IBM, New York, United States) and GraphPad Prism (Version 9.4.1, GraphPad Software, Boston, United States). Results were expressed as mean ± standard deviation (SD), standard error of mean (SEM) or median [interquartile range] for continuous variables. Results were expressed as frequency and percentage for categorical variables. An unpaired t-test was used to compare mean pain scores of chronic low back pain patients with localized pain vs. widespread pain. P < 0.05 was considered significant.
Analysis of FC and graph-theoretic measures
FC measures were compared using the non-parametric Mann–Whitney U-test to account for non-Gaussian distributions in the EEG data of each participant. Results were expressed as mean ± SEM for continuous variables. P < 0.05 was considered significant.
Results
A subset of chronic pain patients demonstrates widespread hyperalgesia
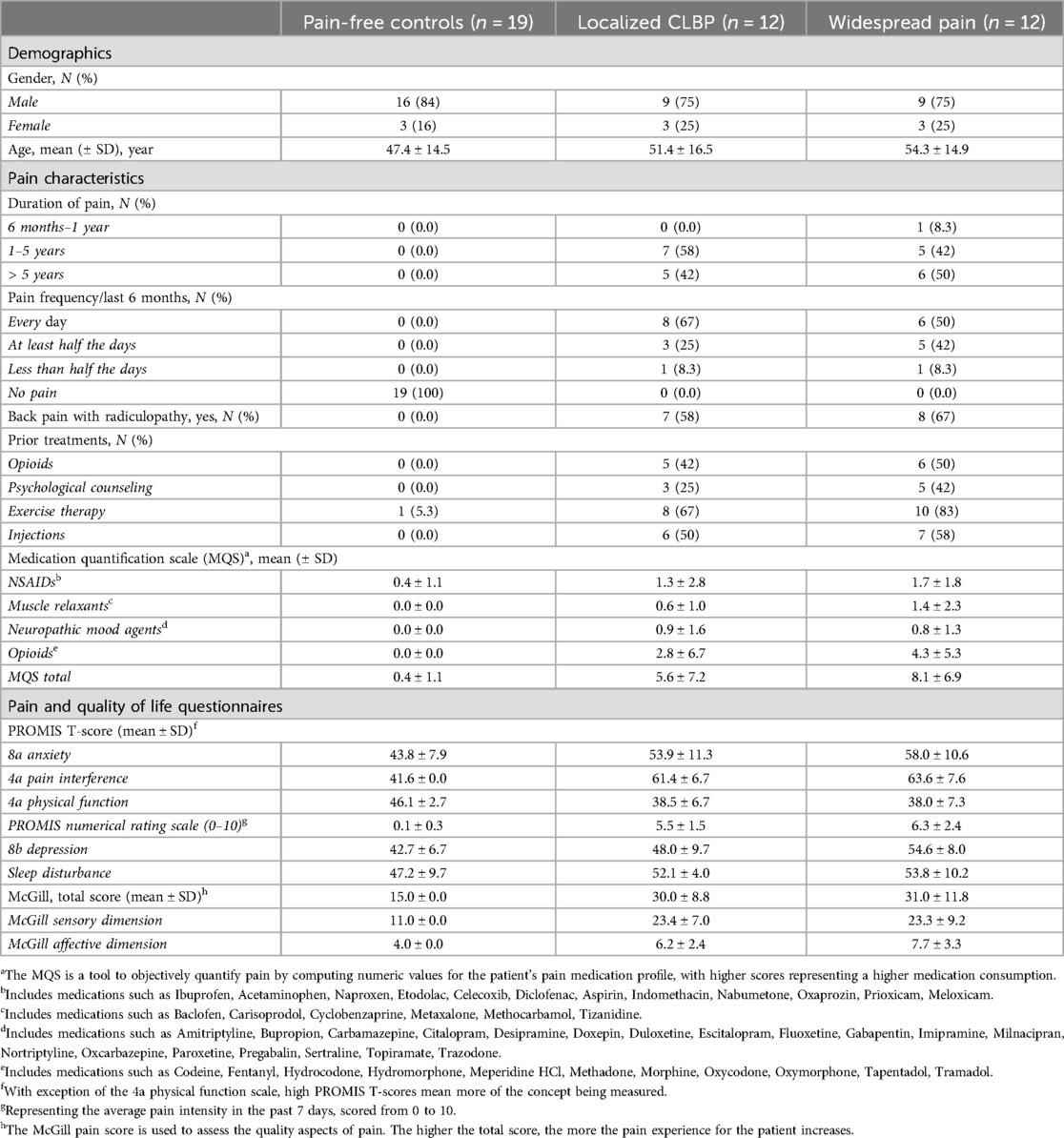
We used a high-density (64-channel) EEG cap with two integrated bipolar leads for horizontal and vertical electrooculogram (EOG; 64-channel Quik-Cap Neo Net, Compumedics Neuroscan, Charlotte, NC, USA) to measure brain activity before, during and after the application of noxious stimuli to the dorsum of the right hand of all participants (n = 43; 24 with chronic low back pain and 19 pain-free control participants; Table 1). None of the participants had chronic pain in the right hand, thus allowing us to evaluate how the presence of chronic pain alters normal cortical nociceptive response or hyperalgesic response. Two calibrated mechanical stimulations were used to provide acute noxious inputs (32 mN and 256 mN). Whereas the 32 mN mechanical stimulation did not trigger pain in control subjects, the 256 mN mechanical stimulation did (17). Next, we separated chronic low back pain patients with widespread hyperalgesia (n = 12) from patients with chronic localized low back pain (n = 12), using hyperalgesic response to the 32 mN stimulation to the hand as a criterion (Figure 1, Table 1; see Materials and Methods). Patients with widespread hyperalgesia reported higher pain scores for both the 32 mN stimulus and the 256 mN stimulus to the dorsum of their right hand (Figure 1B).
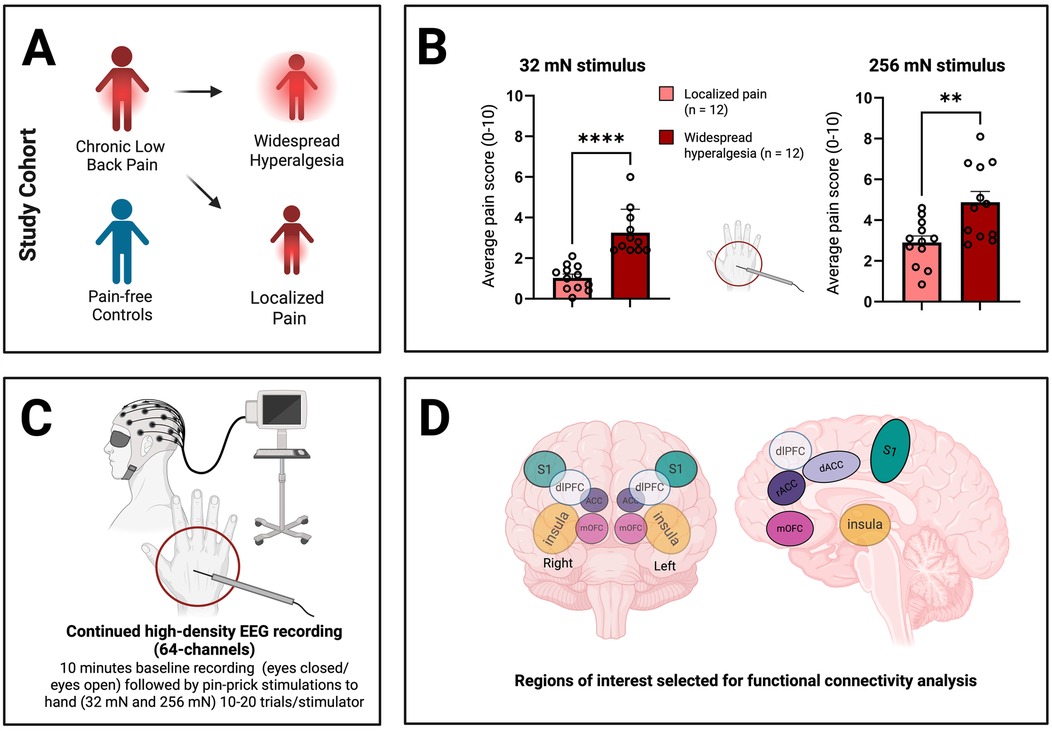
Figure 1. Characterization of patients with widespread hyperalgesia using high-density electroencephalography (EEG) recordings. (A) Patients experiencing chronic low back pain, further characterized into those with only localized pain and those with widespread hyperalgesia (see Materials and Methods), as well as pain-free controls underwent EEG recordings. (B) Patients with widespread hyperalgesia show increased sensitivity to both 32 mN stimulus and 256 mN stimulus to the dorsum of their hand. Unpaired t-test, p ≤ 0.05 (*). p ≤ 0.01 (**), p ≤ 0.001 (***) and p ≤ 0.0001 (****). (C) Resting-state EEG was collected, including 5-minute intervals with eyes open and 5-minutes with eyes closed. Additionally, EEG recordings were performed during the application of pinprick stimuli (32 mN vs. 256 mN) to the dorsum of the right hand. (D) EEG data was analyzed and source localization was performed to isolate cortical areas known to play prominent roles in pain processing (regions of interest, ROIs). These include the primary somatosensory cortex (S1), insula (IC), anterior cingulate cortex (ACC; including rostral ACC or rACC and dorsal ACC or dACC), medial orbitofrontal cortex (mOFC), and dorsolateral prefrontal cortex (dlPFC). Figure creation was assisted by biorender.com.
Chronic pain is associated with changes in cortico-cortical connectivity in response to a noxious stimulus
We conducted stimulus-evoked EEG recordings in all patients (Figure 1C). After source localization, we examined interregional FC among cortical regions using a phase-coupling method known as debiased weighted phase lag index (dwPLI) (67, 69) (Figure 1D). Pain has sensory, affective and cognitive dimensions, and different cortical regions have primary roles in each of these dimensions. We focused our inquiry on cortical areas known to process these different dimensions of pain: primary somatosensory cortex (S1), ACC, IC, mOFC and dorsolateral PFC (dlPFC) (Figure 1D). Further, to understand how distinct groups of cortices interact at the level of sensory, affective and cognitive dimensions, we adopted a functional grouping strategy in FC analysis in the frequency domain where oscillations at different frequency bands are associated with distinct mechanisms in pain processing. This grouping strategy also enhances the power of our analysis. Thus, we grouped individual cortical regions into sensory (S1), affective (ACC and IC) and cognitive areas (mOFC and dlPFC), then performed FC analysis at the group level. We examined how FC was altered in chronic pain patients in response to a noxious stimulus (256 mN). Here, we found that in response to a noxious stimulus, chronic pain is associated with increased FC between cognitive areas and the sensory cortex that is contralateral to the stimulus, but decreased FC between cognitive areas and the ipsilateral sensory cortex in the beta frequency (13–30 Hz), as well as increased FC between cognitive and affective cortices in the high-gamma frequency (61.5–100 Hz) (Figure 2). In contrast, FC at other frequency bands failed to reach statistical significance.
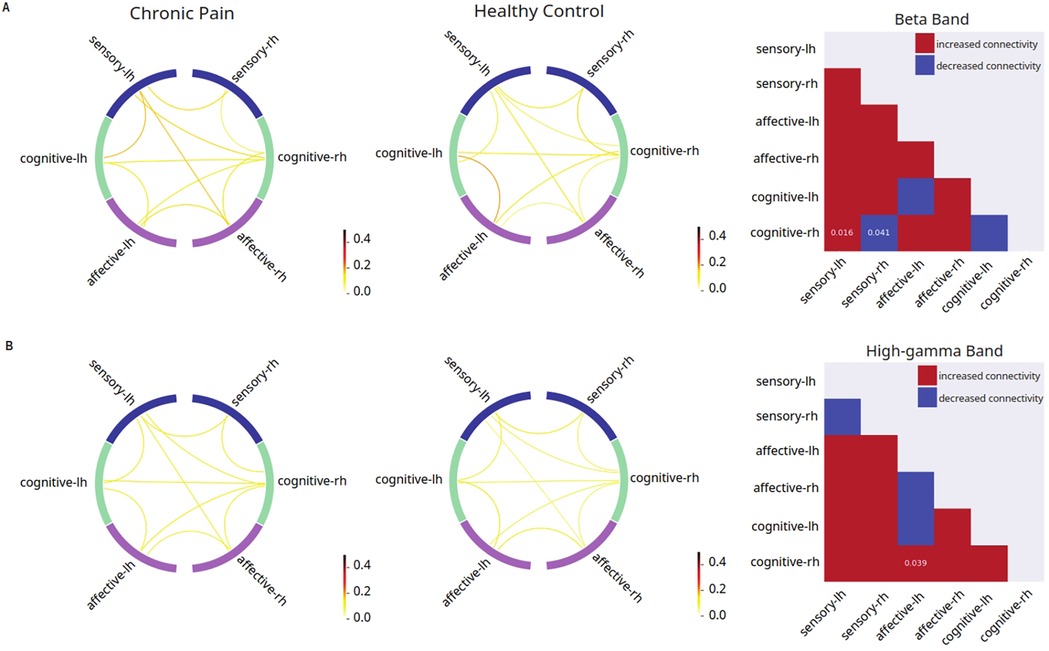
Figure 2. Disrupted mean connectivity across functionally grouped cortical networks are found in chronic pain patients. Left columns: FC between grouped cortical networks in patients with chronic low back pain (n = 24); middle columns: FC between grouped cortical networks in pain-free controls (n = 19); right columns: matrix comparing mean FC in patients with chronic low back pain vs. pain-free controls (red indicates higher mean connectivity in chronic low back pain patients as compared to controls, blue indicates lower mean connectivity in chronic low back pain patients as compared to controls, p-values displayed on matrix indicate statistically significant differences between the two mean connectivities). (A) Noxious mechanical stimulation with 256 mN resulted in increased debiased weighted phase lag index (dwPLI) between cognitive-processing cortical areas and the sensory cortex that is contralateral to the stimulus (p = 0.016), but decreased FC between cognitive areas and the ipsilateral sensory cortex (p = 0.041) in the beta band of the chronic low back pain group as compared to pain-free controls. (B) Noxious stimulation resulted in increased dwPLI connectivity between right hemisphere cognitive processing regions and left hemisphere affective processing regions in the high-gamma band (p = 0.0388) of the chronic low back pain group as compared to pain-free controls.
A unique pattern of grouped FC distinguishes widespread hyperalgesia from localized pain
Next, we asked whether widespread pain has its own distinct mechanistic features not found in patients who experience only localized pain (Figure 3). Thus, we conducted grouped FC analysis for widespread hyperalgesia vs. localized pain. We found that, compared with localized pain, widespread hyperalgesia is associated with a number of changes in FC in response to the noxious (256 mN) stimulus. These changes include decreased inter-hemispheric FC between the sensory areas and between affective areas in the alpha frequency (8–13 Hz), and increased FC between the cognitive areas and the sensory cortex ipsilateral to the stimulus in the beta frequency (Figure 3). These results indicate that widespread hyperalgesia has its own mechanistic features compared with localized pain.
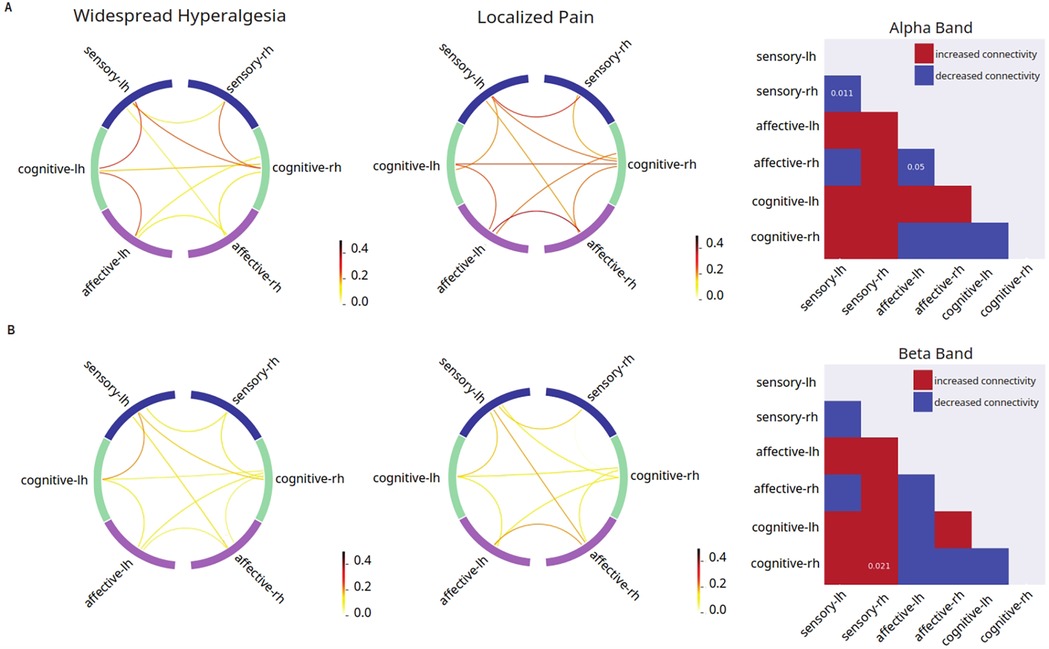
Figure 3. Unique features in mean connectivity across functionally grouped cortical networks in patients with widespread hyperalgesia. Left columns: FC between grouped cortical networks in patients with widespread hyperalgesia (n = 12); middle columns: FC between grouped cortical networks in patients with localized pain (n = 12); right columns: matrix comparing mean FC in patients with widespread hyperalgesia vs. patients with localized pain (red indicates higher mean connectivity in widespread hyperalgesia patients as compared to localized pain patients, blue indicates lower mean connectivity in widespread hyperalgesia patients as compared to localized pain patients, p-values displayed on matrix indicate statistically significant differences between the two mean connectivities). (A) Noxious mechanical stimulation with 256 mN resulted in decreased inter-hemispheric dwPLI connectivity between the sensory cortices and affective cortices (p = 0.011, p = 0.0496) in the alpha band of the widespread hyperalgesia group as compared to the chronic localized pain group. (B) Noxious stimulation resulted in increased dwPLI connectivity between the cognitive processing regions and the sensory cortex that is ipsilateral to stimulus in the beta band (p = 0.021) of the widespread hyperalgesia group as compared to the chronic localized pain group.
Patients with widespread hyperalgesia display distinct changes in nodal centrality in the cortical functional network
To further understand the role of each individual cortical area in driving the overall functional structure in response to noxious inputs, we used an independent graph-theoretic approach to calculate the betweenness centrality, which characterizes how important a cortical area is in organizing a network response to a nociceptive input. We denoted each of the cortical areas (S1, ACC, IC, mOFC, or dlPFC) as a node in pain processing. Our results reveal that chronic pain patients, compared with control subjects, showed decreased centrality of the right dlPFC in the theta frequency (4–8 Hz) (Figure 4A), decreased centrality of the left mOFC but increased centrality of the right dorsal ACC in the beta frequency (Figure 4B), and decreased centrality of the left rostral ACC in the high-gamma frequency (Figure 4C). In contrast, when we compared patients with widespread hyperalgesia with patients with localized back pain, a distinct set of nodal centrality emerged. Here, we found increased centrality in the right dlPFC in the alpha frequency (Figure 5A), increased centrality of the right IC in the beta frequency (Figure 5B), and decreased centrality in the right rostral ACC in both the beta and low-gamma (30–58.5 Hz) frequencies (Figures 5B,C) in the widespread hyperalgesia cohort. These results suggest that different cortical mechanisms may be responsible for widespread hyperalgesia as compared with chronic localized pain.
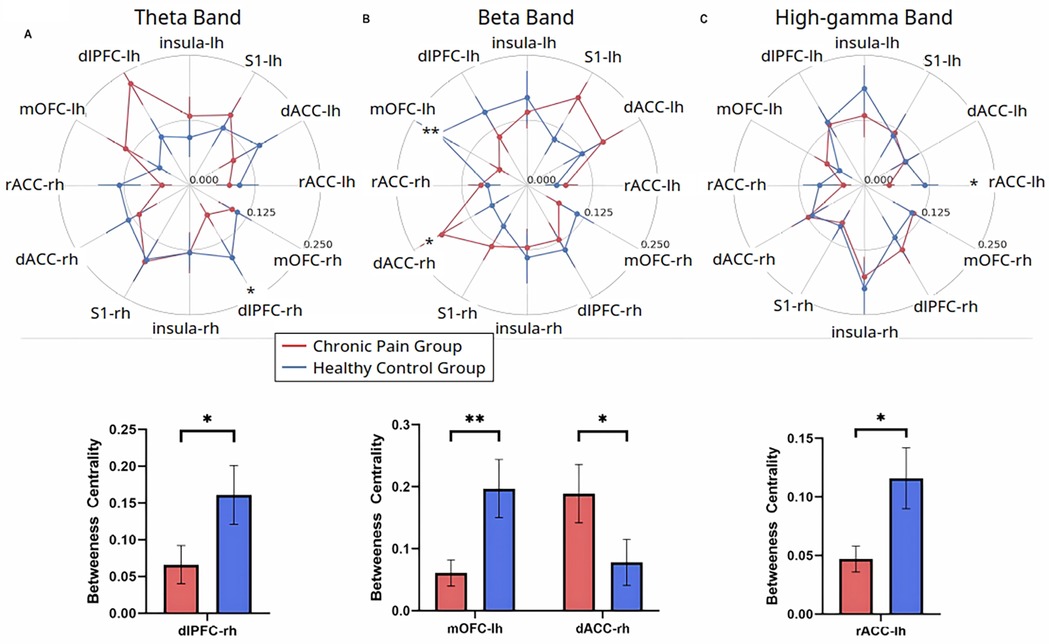
Figure 4. Chronic pain is characterized by changes in node centrality in theta, beta, and high-gamma frequency oscillations. (A) Noxious mechanical stimulation with 256 mN resulted in decreased centrality of the right hemisphere dorsolateral PFC in the theta band (p = 0.0302), (B) Noxious stimulation decreased centrality of the left hemisphere medial OFC (p = 0.0095) and increased centrality of the right hemisphere dorsal ACC (p = 0.0269) in the beta band. (C) Noxious stimulation decreased centrality of the left hemisphere rostral ACC in the high-gamma band (p = 0.0441) in the chronic low back pain group (n = 24) as compared to pain-free controls (n = 19). Data are shown as mean +/− SEM.

Figure 5. Select features in node centrality further characterize widespread hyperalgesia from localized pain. (A) Noxious mechanical stimulation with 256 mN resulted in increased centrality of the right hemisphere dorsolateral PFC in the theta band (p = 0.016). (B) Noxious stimulation decreased centrality of the right hemisphere rostral ACC (p = 0.0275) and increased centrality of the right hemisphere insular cortex (p = 0.0483) in the beta band. (C) Noxious stimulation decreased centrality of the right hemisphere rostral ACC in the low-gamma band (p = 0.0024) in patients with widespread hyperalgesia (n = 12) as compared to patients with localized pain (n = 12). Data are shown as mean +/− SEM.
Discussion
EEG connectomes offer a powerful tool for studying brain connectivity and advancing our understanding of brain function and dysfunction in both healthy and pathological conditions. In this study, we examined interregional FC among cortical circuits in response to a noxious stimulus in chronic pain patients and pain-free controls. We found that chronic pain patients demonstrated a large number of changes in FC between cognitive-, affective-, and sensory-processing regions in a frequency-dependent manner. More importantly, widespread hyperalgesia, commonly found among chronic pain patients, is further distinguished from chronic localized pain by a specific set of FC changes, indicating unique pathogenetic features.
Study designs to investigate nociceptive processing in patients with widespread hyperalgesia
Two prominent questions in chronic pain research are (1) what are the circuit mechanisms that give rise to disordered nociceptive processing in unrelated, disease-free areas in the body in chronic pain, and (2) is widespread hyperalgesia a progressively worse condition of chronic localized pain, or is it caused by a distinct set of circuit changes that are not found with localized pain? Several features of our study design enable us to specifically address these two key questions. First, in contrast to prior studies in neuroimaging, which primarily investigate resting-state changes, we focused our inquiry on stimulus-evoked neurophysiological changes. Taking advantage of the temporal precision of EEG recordings, we are able to analyze brain circuits specifically associated with nociceptive processing by performing FC analysis after EEG source localization. Another key feature of our study design is the application of noxious stimuli to a disease-free site, enabling us to investigate generalized, widespread hypersensitivity rather than localized hypersensitivity. Furthermore, comparing patients with chronic localized pain to patients with widespread hyperalgesia, we are able to isolate selective mechanistic features of widespread hyperalgesia.
Functional connectivity associated with nociceptive processing in chronic pain patients
A key finding in our study is that, in response to a noxious stimulus, there was a large range of changes in FC across multiple cortical areas. This is not surprising, given that nociceptive inputs are processed in a distributive manner throughout the neocortex. The convergence of the nociceptive information carried by distributive circuits is critical for the overall experience of pain, and these circuits may be synchronized by neural oscillations recruited locally that then propagate across long-range projections (70).
Prior fMRI and EEG studies have primarily focused on resting-state FC. In these studies, several featured networks have emerged as highly relevant, including the default mode network (DMN) and salience network (SN) (53, 71). The DMN comprises many of the prefrontal cortical regions and has been implicated in widespread hyperalgesia and pain in fibromyalgia and other rheumatological disorders (49–51). The SN, comprised of the IC and ACC, has also been shown to process the bottom-up sensory stimulus-driven aversive experience, which includes pain (52, 53). Mood disturbances associated with fibromyalgia and other chronic pain conditions have been correlated with changes in the DMN and SN, as well as in their functional connection to the IC and rostral ACC (72, 73).
In contrast to prior studies of resting-state changes in the brain, our study focused on nociceptive processing, specifically in response to a noxious stimulus at a disease-free site, to uncover potential disruptions in brain circuits for endogenous nociceptive processing. Several key regions comprising the DMN and SN, nevertheless, featured prominently in our analysis. For example, we found altered nodal centrality of ACC as well as dlPFC. In addition, we found increased FC between cognitive areas and the sensory cortex that is contralateral to the stimulus, but decreased FC between cognitive areas and the ipsilateral sensory cortex. Our results, in the context of the prior work on resting-state FC, indicate that altered FC at baseline can also translate into disorderly nociceptive processing. These results are further compatible with data from animal models of chronic pain (12, 15, 23).
Cortico-cortical FC can vary from individual to individual, and thus we conducted a functional grouping analysis by combining different cortical areas into groups that process the sensory information (S1), affective information (ACC and IC), or cognitive information (dlPFC and mOFC) of pain. With this analysis framework, we find that chronic pain patients, including those with localized pain and those with widespread hyperalgesia, display a very different FC pattern compared with control subjects. Specifically, chronic pain is associated with increased connectivity between cognitive areas and the sensory cortex that is contralateral to the stimulus, as well as between cognitive and affective cortices. These changes are most prominent in the beta and high-gamma frequencies. Faster frequency (e.g., gamma) oscillations are typically confined to a small neuronal space and are known to involve bottom-up sensory processing, whereas slower frequency (theta, alpha and beta) oscillations are recruited from larger brain networks sometimes associated with persistent brain states such as the state of chronic pain (74–76). Both beta and gamma oscillations in frontal and prefrontal cortical areas have been shown to be positively correlated with ongoing pain (25), whereas gamma oscillations in sensory and prefrontal cortices are known to process sensory and aversive signals, respectively, and have been shown to correlate with evoked stimulus intensity (74, 77–84). Thus, our findings showing alterations in FC in these frequencies indicate enhanced nociceptive processing in response to a noxious stimulus in chronic pain patients.
Unique mechanistic features distinguish widespread hyperalgesia from chronic localized pain
A key finding in our study is that, in contrast to patients who have chronic localized back pain, patients with widespread hyperalgesia showed additional changes throughout the cortex. At the network level, we found that widespread hyperalgesia is distinguished from localized pain by decreased inter-hemispheric FC between the sensory areas and between affective processing areas in the alpha frequency, and at the same time increased FC between the cognitive processing areas and the sensory area ipsilateral to the stimulus in the beta frequency in response to a noxious stimulus.
Inter-hemispheric FC plays an important role in integrating cognitive, affective and sensory circuits, and is in fact one of the salient and stable features of intrinsic brain function (85). Decreased inter-hemispheric FC has been shown in fMRI studies to have a strong correlation with a number of neuropsychiatric disorders, notably major depression and autism (85–88). There is also emerging evidence from resting-state fMRI studies for disordered inter-hemispheric FC in certain chronic pain conditions (89). Since we did not observe this deficit in inter-hemispheric FC of sensory areas when we compared chronic pain patients with controls, this selective disruption in FC indicates that widespread hyperalgesia is not simply a progression from chronic localized pain but has distinct network features that may be responsible for widespread hypersensitivity.
In addition, our graph-theoretic analysis demonstrated that widespread hyperalgesia is further characterized by increased centrality of the IC. This was not seen among chronic pain patients as a whole. Given the important role of the IC in processing the aversive component of pain (35), this result is not surprising, especially in the context that patients with widespread hyperalgesia are also more likely to experience heightened aversive or affective components of pain and have higher comorbidity with mood disorders (9, 90).
Overall, our results indicate that while some maladaptive cortical circuit disruptions are found in both widespread hyperalgesia and localized pain groups, other cortical mechanisms underlying widespread hyperalgesia may be distinct features not found with localized pain. These results thus shed important light on the mechanisms of widespread or nociplastic pain.
There are several limitations of our study that present opportunities for future studies. First, due to our focus on some of the well-known cortical pain-processing nodes, we did not study FC involving all brain areas critical for pain regulation. For example, subcortical regions which are known to be involved in pain processing include hippocampus, amygdala and nucleus accumbens. Future studies need to examine the roles of these regions in nociceptive functional connectivity, using techniques such as fMRI or intracranial recordings. Secondly, as we grouped distinct cortical regions into networks (sensory, affective and cognitive), there is the possibility that we have reduced the multi-functionality of certain cortical regions. For example, while the IC is known to regulate affective component of pain, studies have shown that it also has a role in sensory processing (91, 92). Likewise, PFC has roles in both cognitive and to a lesser degree affective processing of nociceptive inputs. Thirdly, neuroplasticity is known to play a key role in widespread pain, where both glial cells and neurons can interact to cause persistent synaptic changes in both the brain and spinal cord (6, 93), and future studies are needed to identify the relationship between these cellular mechanisms and the long-range FC identified in the present study. Lastly, more male than female patients enrolled in our study, and thus studies are needed to further characterize sex differences in widespread pain (94).
Our present results indicate that chronic pain causes disruptions in functional connectivity in response to nociceptive inputs. More importantly, whereas chronic pain in general is associated with decreased centrality of prefrontal, orbitofrontal, and cingulate areas, widespread hyperalgesia is further distinguished by increased centrality of prefrontal and insular areas. Therefore, although widespread hyperalgesia shares some features with chronic localized pain, it is also characterized by distinct cortical mechanisms. Insights into these mechanistic differences can enhance our theoretical understanding of chronic pain. At the same time, understanding how disrupted cortical connectivity contributes to widespread hyperalgesia can open new avenues for targeted interventions from a translational point of view.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by New York University Grossman School of Medicine Institutional Review Board (8/22/2019, #i19-01088) and conducted in accordance with the latest version of the Declaration of Helsinki. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
GK: Data curation, Formal analysis, Investigation, Methodology, Writing – original draft, Writing – review & editing. MR: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Writing – original draft, Writing – review & editing. KE: Data curation, Formal analysis, Investigation, Writing – review & editing. QZ: Data curation, Formal analysis, Investigation, Methodology, Supervision, Writing – review & editing. DO: Conceptualization, Data curation, Investigation, Methodology, Project administration, Writing – review & editing. CG: Conceptualization, Investigation, Methodology, Project administration, Supervision, Writing – review & editing. LV: Conceptualization, Investigation, Methodology, Project administration, Writing – review & editing. LD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Supervision, Visualization, Writing – original draft, Writing – review & editing. ZC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Supervision, Validation, Writing – review & editing. JW: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The authors acknowledge funding from the Interdisciplinary Pain Research Program; Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU Grossman School of Medicine. Any opinions, findings, and conclusions or recommendations expressed in this article are solely those of the authors and do not necessarily reflect the views of the funding agencies.
Conflict of interest
JW is a cofounder of Pallas Technologies, Inc., and ZSC is a scientific advisor of Pallas Technologies, Inc. JW and ZSC are inventors of a pending US patent application of pain treatment technology.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Heisler AC, Song J, Dunlop DD, Wohlfahrt A, Bingham CO 3rd, Bolster MB, et al. Association of pain centralization and patient-reported pain in active rheumatoid arthritis. Arthritis Care Res (Hoboken). (2020) 72:1122–9. doi: 10.1002/acr.23994
2. Lee YC, Bingham CO 3rd, Edwards RR, Marder W, Phillips K, Bolster MB, et al. Association between pain sensitization and disease activity in patients with rheumatoid arthritis: a cross-sectional study. Arthritis Care Res (Hoboken). (2018) 70:197–204. doi: 10.1002/acr.23266
3. Neville SJ, Clauw AD, Moser SE, Urquhart AG, Clauw DJ, Brummett CM, et al. Association between the 2011 fibromyalgia survey criteria and multisite pain sensitivity in knee osteoarthritis. Clin J Pain. (2018) 34:909–17. doi: 10.1097/AJP.0000000000000619
4. Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken). (2011) 63:320–7. doi: 10.1002/acr.20373
5. Nijs J, George SZ, Clauw DJ, Fernández-de-las-Peñas C, Kosek E, Ickmans K, et al. Central sensitisation in chronic pain conditions: latest discoveries and their potential for precision medicine. Lancet Rheumatol. (2021) 3:e383–92. doi: 10.1016/S2665-9913(21)00032-1
6. Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Hauser W. Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet. (2021) 397:2098–110. doi: 10.1016/S0140-6736(21)00392-5
7. Murphy AE, Minhas D, Clauw DJ, Lee YC. Identifying and managing nociplastic pain in individuals with rheumatic diseases: a narrative review. Arthritis Care Res (Hoboken). (2023) 75:2215–22. doi: 10.1002/acr.25104
8. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. (2009) 10:895–926. doi: 10.1016/j.jpain.2009.06.012
9. Schaefer C, Mann R, Masters ET, Cappelleri JC, Daniel SR, Zlateva G, et al. The comparative burden of chronic widespread pain and fibromyalgia in the United States. Pain Pract. (2016) 16:565–79. doi: 10.1111/papr.12302
10. Finan PH, Buenaver LF, Bounds SC, Hussain S, Park RJ, Haque UJ, et al. Discordance between pain and radiographic severity in knee osteoarthritis: findings from quantitative sensory testing of central sensitization. Arthritis Rheum. (2013) 65:363–72. doi: 10.1002/art.34646
11. Lee YC, Cui J, Lu B, Frits ML, Iannaccone CK, Shadick NA, et al. Pain persists in DAS28 rheumatoid arthritis remission but not in ACR/EULAR remission: a longitudinal observational study. Arthritis Res Ther. (2011) 13:R83. doi: 10.1186/ar3353
12. Zhang Q, Manders T, Tong AP, Yang R, Garg A, Martinez E, et al. Chronic pain induces generalized enhancement of aversion. eLife. (2017) 6:e25302. doi: 10.7554/eLife.25302
13. Singh A, Patel D, Li A, Hu L, Zhang Q, Liu Y, et al. Mapping cortical integration of sensory and affective pain pathways. Curr Biol. (2020) 30:1703–1715.e5. doi: 10.1016/j.cub.2020.02.091
14. Dale J, Zhou H, Zhang Q, Martinez E, Hu S, Liu K, et al. Scaling up cortical control inhibits pain. Cell Rep. (2018) 23:1301–13. doi: 10.1016/j.celrep.2018.03.139
15. Li A, Liu Y, Zhang Q, Friesner I, Jee HJ, Chen ZS, et al. Disrupted population coding in the prefrontal cortex underlies pain aversion. Cell Rep. (2021) 37:109978. doi: 10.1016/j.celrep.2021.109978
16. Guo X, Zhang Q, Singh A, Wang J, Chen ZS. Granger causality analysis of rat cortical functional connectivity in pain. J Neural Eng. (2020) 17:016050. doi: 10.1088/1741-2552/ab6cba
17. Kenefati G, Rockholt MM, Ok D, McCartin M, Zhang Q, Sun G, et al. Changes in alpha, theta, and gamma oscillations in distinct cortical areas are associated with altered acute pain responses in chronic low back pain patients. Front Neurosci. (2023) 17:1278183. doi: 10.3389/fnins.2023.1278183
18. Sun G, Wen Z, Ok D, Doan L, Wang J, Chen ZS. Detecting acute pain signals from human EEG. J Neurosci Methods. (2021) 347:108964. doi: 10.1016/j.jneumeth.2020.108964
19. Stegemann A, Liu S, Retana Romero OA, Oswald MJ, Han Y, Beretta CA, et al. Prefrontal engrams of long-term fear memory perpetuate pain perception. Nat Neurosci. (2023) 26:820–9. doi: 10.1038/s41593-023-01291-x
20. Tan LL, Oswald MJ, Heinl C, Retana Romero OA, Kaushalya SK, Monyer H, et al. Gamma oscillations in somatosensory cortex recruit prefrontal and descending serotonergic pathways in aversion and nociception. Nat Commun. (2019) 10:983. doi: 10.1038/s41467-019-08873-z
21. Tan LL, Pelzer P, Heinl C, Tang W, Gangadharan V, Flor H, et al. A pathway from midcingulate cortex to posterior insula gates nociceptive hypersensitivity. Nat Neurosci. (2017) 20:1591–601. doi: 10.1038/nn.4645
22. Alonso-Matielo H, Zhang Z, Gambeta E, Huang J, Chen L, de Melo GO, et al. Inhibitory insula-ACC projections modulate affective but not sensory aspects of neuropathic pain. Mol Brain. (2023) 16:64. doi: 10.1186/s13041-023-01052-8
23. Zhang Z, Gadotti VM, Chen L, Souza IA, Stemkowski PL, Zamponi GW. Role of prelimbic GABAergic circuits in sensory and emotional aspects of neuropathic pain. Cell Rep. (2015) 12:752–9. doi: 10.1016/j.celrep.2015.07.001
24. Ta Dinh S, Nickel MM, Tiemann L, May ES, Heitmann H, Hohn VD, et al. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. Pain. (2019) 160:2751–65. doi: 10.1097/j.pain.0000000000001666
25. May ES, Nickel MM, Ta Dinh S, Tiemann L, Heitmann H, Voth I, et al. Prefrontal gamma oscillations reflect ongoing pain intensity in chronic back pain patients. Hum Brain Mapp. (2019) 40:293–305. doi: 10.1002/hbm.24373
26. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. (1997) 277:968–71. doi: 10.1126/science.277.5328.968
27. Foltz EL, White LE. The role of rostral cingulumotomy in “pain” relief. Int J Neurol. (1968) 6:353–73.5759640
28. Talbot JD, Villemure JG, Bushnell MC, Duncan GH. Evaluation of pain perception after anterior capsulotomy: a case report. Somatosens Mot Res. (1995) 12:115–26. doi: 10.3109/08990229509101503
29. Koyama T, Kato K, Mikami A. During pain-avoidance neurons activated in the macaque anterior cingulate and caudate. Neurosci Lett. (2000) 283:17–20. doi: 10.1016/S0304-3940(00)00894-6
30. Koyama T, Kato K, Tanaka YZ, Mikami A. Anterior cingulate activity during pain-avoidance and reward tasks in monkeys. Neurosci Res. (2001) 39:421–30. doi: 10.1016/S0168-0102(01)00197-3
31. Qu C, King T, Okun A, Lai J, Fields HL, Porreca F. Lesion of the rostral anterior cingulate cortex eliminates the aversiveness of spontaneous neuropathic pain following partial or complete axotomy. Pain. (2011) 152:1641–8. doi: 10.1016/j.pain.2011.03.002
32. Johansen JP, Fields HL, Manning BH. The affective component of pain in rodents: direct evidence for a contribution of the anterior cingulate cortex. Proc Natl Acad Sci U S A. (2011) 98:8077–82. doi: 10.1073/pnas.141218998
33. LaGraize SC, Borzan J, Peng YB, Fuchs PN. Selective regulation of pain affect following activation of the opioid anterior cingulate cortex system. Exp Neurol. (2006) 197:22–30. doi: 10.1016/j.expneurol.2005.05.008
34. Lubar JF. Effect of medial cortical lesions on the avoidance behavior of the cat. J Comp Physiol Psychol. (1964) 58:38–46. doi: 10.1037/h0041014
35. Segerdahl AR, Mezue M, Okell TW, Farrar JT, Tracey I. The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci. (2015) 18:499–500. doi: 10.1038/nn.3969
36. Talbot JD, Marrett S, Evans AC, Meyer E, Bushnell MC, Duncan GH. Multiple representations of pain in human cerebral cortex. Science. (1991) 251:1355–8. doi: 10.1126/science.2003220
37. Martinez E, Lin HH, Zhou H, Dale J, Liu K, Wang J. Corticostriatal regulation of acute pain. Front Cell Neurosci. (2017) 11:146. doi: 10.3389/fncel.2017.00146
38. Lee M, Manders TR, Eberle SE, Su C, D'Amour J, Yang R, et al. Activation of corticostriatal circuitry relieves chronic neuropathic pain. J Neurosci. (2015) 35:5247–59. doi: 10.1523/JNEUROSCI.3494-14.2015
39. Wang G-Q, Cen C, Li C, Cao S, Wang N, Zhou Z, et al. Deactivation of excitatory neurons in the prelimbic cortex via Cdk5 promotes pain sensation and anxiety. Nat Commun. (2015) 6:1–16. doi: 10.1038/ncomms8660
40. Cheriyan J, Sheets PL. Altered excitability and local connectivity of mPFC-PAG neurons in a mouse model of neuropathic pain. J Neurosci. (2018) 38:4829–39. doi: 10.1523/JNEUROSCI.2731-17.2018
41. Jones AF, Sheets PL. Sex-specific disruption of distinct mPFC inhibitory neurons in spared-nerve injury model of neuropathic pain. Cell Rep. (2020) 31:107729. doi: 10.1016/j.celrep.2020.107729
42. Zhang Q, Hu S, Talay R, Xiao Z, Rosenberg D, Liu Y, et al. A prototype closed-loop brain-machine interface for the study and treatment of pain. Nat Biomed Eng. (2021) 10:1038. doi: 10.1038/s41551-021-00736-7
43. Sun G, Zeng F, McCartin M, Zhang Q, Xu H, Liu Y, et al. Closed-loop stimulation using a multiregion brain-machine interface has analgesic effects in rodents. Sci Transl Med. (2022) 14:eabm5868. doi: 10.1126/scitranslmed.abm5868
44. Hardy SG. Analgesia elicited by prefrontal stimulation. Brain Res. (1985) 339:281–4. doi: 10.1016/0006-8993(85)90093-9
45. Shirvalkar P, Prosky J, Chin G, Ahmadipour P, Sani OG, Desai M, et al. First-in-human prediction of chronic pain state using intracranial neural biomarkers. Nat Neurosci. (2023) 26:1090–9. doi: 10.1038/s41593-023-01338-z
46. Turnbull IM. Bilateral cingulumotomy combined with thalamotomy or mesencephalic tractotomy for pain. Surg Gynecol Obstet. (1972) 134:958–62.4113363
47. Craig AD, Reiman EM, Evans A, Bushnell MC. Functional imaging of an illusion of pain. Nature. (1996) 384:258–60. doi: 10.1038/384258a0
48. Zhou H, Zhang Q, Martinez E, Dale J, Hu S, Zhang E, et al. Ketamine reduces aversion in rodent pain models by suppressing hyperactivity of the anterior cingulate cortex. Nat Commun. (2018) 9:3751. doi: 10.1038/s41467-018-06295-x
49. Basu N, Kaplan CM, Ichesco E, Larkin T, Harris RE, Murray A, et al. Neurobiologic features of fibromyalgia are also present among rheumatoid arthritis patients. Arthritis Rheumatol. (2018) 70:1000–7. doi: 10.1002/art.40451
50. Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. (2010) 62:2545–55. doi: 10.1002/art.27497
51. Napadow V, Kim J, Clauw DJ, Harris RE. Decreased intrinsic brain connectivity is associated with reduced clinical pain in fibromyalgia. Arthritis Rheum. (2012) 64:2398–403. doi: 10.1002/art.34412
52. Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, et al. Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci. (2007) 27:2349–56. doi: 10.1523/JNEUROSCI.5587-06.2007
53. Hemington KS, Wu Q, Kucyi A, Inman RD, Davis KD. Abnormal cross-network functional connectivity in chronic pain and its association with clinical symptoms. Brain Struct Funct. (2016) 221:4203–19. doi: 10.1007/s00429-015-1161-1
54. Petrovic P, Kalso E, Petersson KM, Ingvar M. Placebo and opioid analgesia- imaging a shared neuronal network. Science. (2002) 295:1737–40. doi: 10.1126/science.1067176
55. Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. (2005) 6:691–702. doi: 10.1038/nrn1747
56. Rockholt MM, Kenefati G, Doan LV, Chen ZS, Wang J. In search of a composite biomarker for chronic pain by way of EEG and machine learning: where do we currently stand? Front Neurosci. (2023) 17:1186418. doi: 10.3389/fnins.2023.1186418
57. Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. National institutes of health task force on research standards for chronic low back p, report of the national institutes of health task force on research standards for chronic low back pain. J Manipulative Physiol Ther. (2014) 37:449–67. doi: 10.1016/j.jmpt.2014.07.006
58. Gramfort A, Luessi M, Larson E, Engemann DA, Strohmeier D, Brodbeck C, et al. MEG and EEG data analysis with MNE-python. Front Neurosci. (2013) 7:267. doi: 10.3389/fnins.2013.00267
59. Bigdely-Shamlo N, Mullen T, Kothe C, Su KM, Robbins KA. The PREP pipeline: standardized preprocessing for large-scale EEG analysis. Front Neuroinform. (2015) 9:16. doi: 10.3389/fninf.2015.00016
60. Hyvarinen A. Fast and robust fixed-point algorithms for independent component analysis. IEEE Trans Neural Netw. (1999) 10:626–34. doi: 10.1109/72.761722
61. Jas M, Engemann DA, Bekhti Y, Raimondo F, Gramfort A. Autoreject: automated artifact rejection for MEG and EEG data. Neuroimage. (2017) 159:417–29. doi: 10.1016/j.neuroimage.2017.06.030
62. Dale AM, Liu AK, Fischl BR, Buckner RL, Belliveau JW, Lewine JD, et al. Dynamic statistical parametric mapping: combining fMRI and MEG for high-resolution imaging of cortical activity. Neuron. (2000) 26:55–67. doi: 10.1016/S0896-6273(00)81138-1
63. Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Hum Brain Mapp. (1999) 8:272–84. doi: 10.1002/(SICI)1097-0193(1999)8:4%3C272::AID-HBM10%3E3.0.CO;2-4
64. Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, et al. Automatically parcellating the human cerebral cortex. Cereb Cortex. (2004) 14:11–22. doi: 10.1093/cercor/bhg087
65. Destrieux C, Fischl B, Dale A, Halgren E. Automatic parcellation of human cortical gyri and sulci using standard anatomical nomenclature. Neuroimage. (2010) 53:1–15. doi: 10.1016/j.neuroimage.2010.06.010
66. Mostame P, Sadaghiani S. Phase- and amplitude-coupling are tied by an intrinsic spatial organization but show divergent stimulus-related changes. Neuroimage. (2020) 219:117051. doi: 10.1016/j.neuroimage.2020.117051
67. Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CM. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. (2011) 55:1548–65. doi: 10.1016/j.neuroimage.2011.01.055
68. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. (2010) 52:1059–69. doi: 10.1016/j.neuroimage.2009.10.003
69. Stam CJ, Nolte G, Daffertshofer A. Phase lag index: assessment of functional connectivity from multi channel EEG and MEG with diminished bias from common sources. Hum Brain Mapp. (2007) 28:1178–93. doi: 10.1002/hbm.20346
70. Buzsaki G, Draguhn A. Neuronal oscillations in cortical networks. Science. (2004) 304:1926–9. doi: 10.1126/science.1099745
71. Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp. (2009) 30:2731–45. doi: 10.1002/hbm.20705
72. Loggia ML, Kim J, Gollub RL, Vangel MG, Kirsch I, Kong J, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. (2013) 154:24–33. doi: 10.1016/j.pain.2012.07.029
73. Kucyi A, Davis KD. The dynamic pain connectome. Trends Neurosci. (2015) 38:86–95. doi: 10.1016/j.tins.2014.11.006
74. Ploner M, May ES. Electroencephalography and magnetoencephalography in pain research-current state and future perspectives. Pain. (2018) 159:206–11. doi: 10.1097/j.pain.0000000000001087
75. Heitmann H, Gil Avila C, Nickel MM, Ta Dinh S, May ES, Tiemann L, et al. Longitudinal resting-state electroencephalography in patients with chronic pain undergoing interdisciplinary multimodal pain therapy. Pain. (2022) 163:e997–e1005. doi: 10.1097/j.pain.0000000000002565
76. Kisler LB, Kim JA, Hemington KS, Rogachov A, Cheng JC, Bosma RL, et al. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin. (2020) 26:102241. doi: 10.1016/j.nicl.2020.102241
77. Zhang ZG, Hu L, Hung YS, Mouraux A, Iannetti GD. Gamma-band oscillations in the primary somatosensory cortex–a direct and obligatory correlate of subjective pain intensity. J Neurosci. (2012) 32:7429–38. doi: 10.1523/JNEUROSCI.5877-11.2012
78. Gross J, Schnitzler A, Timmermann L, Ploner M. Gamma oscillations in human primary somatosensory cortex reflect pain perception. PLoS Biol. (2007) 5:e133. doi: 10.1371/journal.pbio.0050133
79. Baroni A, Severini G, Straudi S, Buja S, Borsato S, Basaglia N. Hyperalgesia and central sensitization in subjects with chronic orofacial pain: analysis of pain thresholds and EEG biomarkers. Front Neurosci. (2020) 14:552650. doi: 10.3389/fnins.2020.552650
80. Zhou R, Wang J, Qi W, Liu F-Y, Yi M, Guo H, et al. Elevated resting state gamma oscillatory activities in electroencephalogram of patients with post-herpetic neuralgia. Front Neurosci. (2018) 12:750. doi: 10.3389/fnins.2018.00750
81. Michels L, Moazami-Goudarzi M, Jeanmonod D. Correlations between EEG and clinical outcome in chronic neuropathic pain: surgical effects and treatment resistance. Brain Imaging Behav. (2011) 5:329–48. doi: 10.1007/s11682-011-9135-2
82. Vanneste S, De Ridder D. Chronic pain as a brain imbalance between pain input and pain suppression. Brain Commun. (2021) 3:fcab014. doi: 10.1093/braincomms/fcab014
83. Schulz E, May ES, Postorino M, Tiemann L, Nickel MM, Witkovsky V, et al. Prefrontal gamma oscillations encode tonic pain in humans. Cereb Cortex. (2015) 25:4407–14. doi: 10.1093/cercor/bhv043
84. Mouraux A, Iannetti GD. The search for pain biomarkers in the human brain. Brain. (2018) 141:3290–307. doi: 10.1093/brain/awy281
85. Gee DG, Biswal BB, Kelly C, Stark DE, Margulies DS, Shehzad Z, et al. Low frequency fluctuations reveal integrated and segregated processing among the cerebral hemispheres. Neuroimage. (2011) 54:517–27. doi: 10.1016/j.neuroimage.2010.05.073
86. Guo W, Liu F, Dai Y, Jiang M, Zhang J, Yu L, et al. Decreased interhemispheric resting-state functional connectivity in first-episode, drug-naive major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. (2013) 41:24–9. doi: 10.1016/j.pnpbp.2012.11.003
87. Hermesdorf M, Sundermann B, Feder S, Schwindt W, Minnerup J, Arolt V, et al. Major depressive disorder: findings of reduced homotopic connectivity and investigation of underlying structural mechanisms. Hum Brain Mapp. (2016) 37:1209–17. doi: 10.1002/hbm.23097
88. Jiang X, Shen Y, Yao J, Zhang L, Xu L, Feng R, et al. Connectome analysis of functional and structural hemispheric brain networks in major depressive disorder. Transl Psychiatry. (2019) 9:136. doi: 10.1038/s41398-019-0467-9
89. Sevel LS, Letzen JE, Staud R, Robinson ME. Interhemispheric dorsolateral prefrontal Cortex connectivity is associated with individual differences in pain sensitivity in healthy controls. Brain Connect. (2016) 6:357–64. doi: 10.1089/brain.2015.0405
90. Heisler AC, Song J, Muhammad LN, Wohlfahrt A, Marder W, Bolster MB, et al. Association of dysregulated central pain processing and response to disease-modifying antirheumatic drug therapy in rheumatoid arthritis. Arthritis Rheumatol. (2020) 72:2017–24. doi: 10.1002/art.41440
91. Liu CC, Moosa S, Quigg M, Elias WJ. Anterior insula stimulation increases pain threshold in humans: a pilot study. J Neurosurg. (2021) 135:1487–92. doi: 10.3171/2020.10.JNS203323
92. Liberati G, Algoet M, Santos SF, Ribeiro-Vaz JG, Raftopoulos C, Mouraux A. Tonic thermonociceptive stimulation selectively modulates ongoing neural oscillations in the human posterior insula: evidence from intracerebral EEG. Neuroimage. (2019) 188:70–83. doi: 10.1016/j.neuroimage.2018.11.059
93. Ji RR, Nackley A, Huh Y, Terrando N, Maixner W. Neuroinflammation and central sensitization in chronic and widespread pain. Anesthesiology. (2018) 129(2):343–66. doi: 10.1097/ALN.0000000000002130
Keywords: chronic pain, chronic low back pain, pain mechanisms, pain phenotyping, hyperalgesia, functional connectivity, EEG
Citation: Kenefati G, Rockholt MM, Eisert K, Zhang Q, Ok D, Gharibo CG, Voiculescu LD, Doan LV, Chen ZS and Wang J (2025) Disruptions in cortical circuit connectivity distinguish widespread hyperalgesia from localized pain. Front. Pain Res. 6:1548500. doi: 10.3389/fpain.2025.1548500
Received: 19 December 2024; Accepted: 12 May 2025;
Published: 20 June 2025.
Edited by:
Serge Marchand, Université de Sherbrooke, CanadaReviewed by:
Mayank Gautam, University of Pennsylvania, United StatesIvan Jonassen Rimstad, Oslo University Hospital, Norway
Copyright: © 2025 Kenefati, Rockholt, Eisert, Zhang, Ok, Gharibo, Voiculescu, Doan, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jing Wang, amluZy53YW5nMkBueXVsYW5nb25lLm9yZw==
†These authors have contributed equally to this work
 George Kenefati
George Kenefati Mika M. Rockholt
Mika M. Rockholt Katherine Eisert1
Katherine Eisert1 Qiaosheng Zhang
Qiaosheng Zhang Lisa V. Doan
Lisa V. Doan Zhe Sage Chen
Zhe Sage Chen Jing Wang
Jing Wang