- 1Department of Health, Medicine and Caring Sciences, and Pain and Rehabilitation Center, Linköping University, Linköping, Sweden
- 2Department of Activity and Health in Linköping, Linköping University, Linköping, Sweden
- 3Department of Health, Medicine and Caring Sciences, Linköping University, Linköping, Sweden
- 4Department of Health, Medicine and Caring Sciences, Unit of Physiotherapy, Linköping University, Linköping, Sweden
- 5Occupational and Environmental Medicine Centre, Department of Health, Medicine and Caring Sciences, Unit of Clinical Medicine, Linköping University, Linköping, Sweden
- 6Rehabilitation Research Laboratory 2rLab, Department of Business Economics, Health and Social Care, University of Applied Sciences and Arts of Southern Switzerland, Manno, Switzerland
- 7Primary Health Care Center Valla, Linköping, Sweden
Background: Previous studies have demonstrated an independent association between pain and frailty, but knowledge about this association with different pain characteristics is limited.
Objective: This study was embedded in a prospective, pragmatic, matched-control multicenter trial at 19 primary care practices in south-eastern Sweden (ClinicalTrials.gov 170608, ID: NCT03180606), aiming to investigate the association between frailty and pain characteristics among older people (75+) at high risk of hospitalization.
Methods: High risk of hospitalization was identified using case-finding algorithm including 32 diagnostic codes of morbidities and healthcare use. Frailty was assessed by a nurse-physician team using Clinical Frailty Scale (N = 389). Data on pain aspects, physical and ADL functioning were collected in the self-reported questionnaires.
Results: One in three (n = 133, 34%) was classified as frail. About 36% (n = 142) reported frequent pain (from several times per week to constantly). Slightly over 40% reported pain lasting longer than 3 months (n = 163, 41.9%) and/or having regional or widespread pain (n = 165, 42.4%). In comparison to non-frail peers, frail participants reported higher pain intensity, more ADL-dependency, less physical activity, and more anxiety/depression (p < 0.01). In logistic regression analysis, pain frequency [Odds Ratio (OR) 1.8, 95% confidence interval (CI): 1.2–2.8] was associated with frailty. However, the models with ADL-staircase score (OR: 1.4, 95% CI: 1.2–1.6) had a higher explanatory power (Nagelkerke R2: 0.39) in predicting frailty than those without this aspect (R2: 0.10 and 0.13).
Conclusion: In older people at high risk of hospitalization, pain frequency seemed to be related to frailty, whilst ADL dependency demonstrated a stronger association.
1 Introduction
The aging population increases rapidly around the world due to longer life expectancy, medical development, and improvement of health care service. Most older people is healthy, but multimorbidity (including pain conditions) and frailty increase with age. Frailty is characterized by an age-related decline in function in various organ systems, leading to increased vulnerability to external stressors (1–3). This vulnerability leads to a reduction in a frail person's functional ability and a high risk of hospitalization (4, 5). In a previous interview study with a group of frail older adults, we found that many of them adapted to their health problems and functional decline but kept a strong desire to live independently. They expressed fear of pain and suffering as well as losing independence in the end-of-life (6).
The International Association for the Study of Pain (IASP) defines pain as “An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage” (7). Pain is a personal experience and pain characteristics are often measured such as frequency, duration, intensity, localization and spreading (8). Chronic pain, referring to pain persisting or recurring at least three months, is viewed as a disease of its own defined by the biopsychological model of pain (9). It has a multidimensional aspect and interacts with biological, psychological, and social factors reciprocally (9). It is well-known that chronic pain is associated with a higher degree of mortality, morbidity, and healthcare consumption. The sufferings of chronic pain are also common in the old age, as many older people with chronic pain report disability, lower life satisfaction, and poorer health status in general (10).
Previous research demonstrated an independent association between pain and frailty, after accounting for comorbidities and psychosocial factors (11). However, knowledge about this association with pain characteristics is rather limited, and functional aspects have yet to be considered together with pain characteristics. The current study aimed to investigate the association between frailty and pain characteristics in older adults with a high risk of hospitalization. We also explored this relationship with consideration of physical functioning and psychological well-being.
2 Methods
2.1 Study population from an interventional study trial
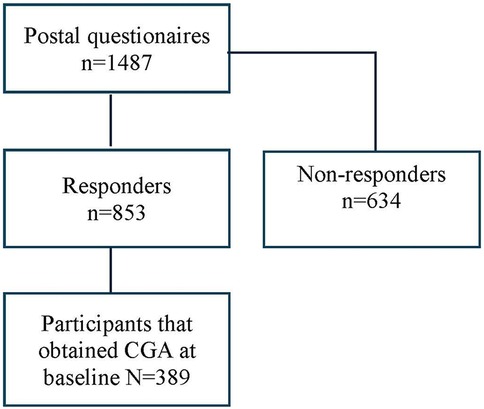
This study was embedded in a prospective, pragmatic, matched-control multicenter trial at 19 primary care practices in south-eastern Sweden (12). In March 2017, we identified 1,604 individuals aged 75 years older with a high risk of hospitalization using case-finding algorithm including 32 diagnostic codes of morbidities related to unplanned admission to hospital (13). The trial included Intervention Group and Control Group at baseline, 1-year and 2-year follow-ups (Trial registration: ClinicalTrials.gov 170608, ID: NCT03180606) (14). All participants were invited to complete the postal questionnaires covering pain aspects and other health conditions on up to three occasions. The proactive healthcare in Intervention Group, led by patients' responsible general practitioner (GP), consists of a health assessment using the standardized evaluation form—the Primary Care Assessment Tool for Elderly (PASTEL) (15), to identify the health-related problems in need of medical treatment, rehabilitation, or prevention. PASTEL is constructed for the project and is based on the holistic approach of Comprehensive Geriatric Assessment (CGA) (15). PASTEL combines the diagnostic and therapeutic purposes, covering medical, functional, psychological, and social domains. An overview of patients' medical history including an evaluation of current medication use as well as physical measures were included in the clinical assessment. The GPs and nurses were introduced to the CGA tool, Frailty assessment with the Clinical Frailty Scale and the process of intervention prior to the study. For Intervention Group, this individual pragmatic assessment led to further rehabilitation and prevention such as home-based exercise program, prescription of assistance technology (e.g., walker, adapted toilet, bath/shower technology) and the extent to facilitating assistance (i.e., transportation service, community assistance, and personal alarm) in everyday life when needed. CGA was not included in usual care for Control Group. A postal questionnaire was delivered to all participants at three times during the study period for both groups. The current study population was restricted to all participants who received Comprehensive Geriatric Assessment (CGA) at baseline year (N = 389) and answered the first postal questionnaire (Figure 1). The study was approved by the regional ethical review board in Linköping (Dnr: 2016/347-31). All participants filled in an informal consent.
2.2 Measures
2.2.1 Socio-demographic factors
The information on age and sex of one person were from Statistics Sweden (SCB). Highest education level (no education, less than nine years of compulsory school or similar, nine years compulsory school or similar, secondary school, or university/college), living situation (living alone, with cohabitant or with children) and marital status (married/co-habited and unmarried/widow) were asked in the postal questionnaire.
2.2.2 Pain characteristics: frequency, duration, intensity and extent
Pain frequency was measured by letting patients assess how often they experience pain, with the scale ranging from 1 to 5: 1 = “never”; 2 = “occasionally”; 3 = “once per day”; 4 = “several times per day”; and 5 = “constantly”.
Pain duration was measured by asking “How long have you been suffering from pain?”. The answer was given by the number of months. The answer was also recorded as chronic pain (lasting longer than 3 months) and no chronic pain.
Pain intensity was measured by a visual analog scale reflecting pain over the preceding seven days (VAS Pain–7d). Scores are made by marking a 100 mm line, with 0 mm meaning no pain and 100 mm = worst imaginable pain.
Pain extent was used to identify pain localization and spreading, quantified through pain drawings. Participants provided information about their pain location by coloring two body charts attached in the postal questionnaire. One displayed a frontal and the other a ventral view of the body. All drawings were further digitalized and processed by calculating the total number of pixels in each drawing (1,024 × 78 pixel) (16, 17). Furthermore, using the manikin, local pain was generated when it was marked on just one section (or two sections when sections were equally marked on the front and back of e.g., hip, knee, shoulder, or arm). Widespread pain was pain marked in at least two sections in two contralateral limbs and the axial skeleton and marked equally on the front and on the back of the body. Regional pain was defined as pain shaded on the manikin that did not meet the criteria for the other two categories (18, 19).
2.2.3 Frailty
The process of measuring frailty was divided into three processes (12, 20). First, an interview with multiple-choice questions and self-rating health was conducted by a nurse using the PASTEL tool (15). The second part consisted of a physical examination, a review of medical records and an evaluation of the patient's thoughts about current and future healthcare needs. A third step was that the medical team (GP together with the nurse) made an estimation of frailty using Clinical Frailty Scale (CFS) (21–23). CFS has a scoring system from 1 (very fit) to 9 (terminally ill) and it is complimented by a visual chart to assist in the classification of frailty. Higher scores in CFS indicate an increased risk of mortality and morbidity. The original CFS has high reliability and validity (21, 23). Based on the skewed distribution of the number of participants assessed over the different frailty scores (from 1 to 9), CFS was also categorized to non-frail category (scoring 0–4) and frail category (scoring 5–9) (22, 23).
2.2.4 Anxiety/depression
EQ-5D-3L is a generic measurement for health-related quality of life and consists of five dimensions: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression (24). Each dimension can be answered with the three following levels: no problems (level 1), some problems (level 2), and extreme problems (level 3). In this study, we used the dimension of anxiety/depression to measure the perceived anxiety/depression.
2.2.5 Physical functioning
In the postal questionnaire, the participants were asked about their daily physical activity (e.g., walking or bicycling to the store, going out with dogs, gardening, shoveling snow or some other activity) and regular physical exercise (exercise/sport/open-air activity on a week basis, but not included in the everyday life activities above) during the past 6 months. A combination of the answers to both questions generated the physical activity level 1–4: 1 = inactivity, 2 = low activity, 3 = moderate activity, and 4 = high activity (25, 26). Good internal consistency (Cronbach's alpha of 0.79) was demonstrated in the present study.
Activity of Daily Living (ADL) was measured by the extended version of KatźADL index (ADL-staircase) (27). ADL-staircase covers both Personal ADL (PADL) and Instrumental ADL (IADL), containing the following 10 activities: feeding, cleaning, bathing, toilet visits, dressing, shopping, transportation, feeding, continence, and transfer. Each activity is graded by three levels (1 = independent, 2 = partially dependent, and 3 = dependent) and the total score is summarized into a scale from 10 to 30, where higher scores indicate greater dependency. Good construct validity has been reported previously (28). Good internal consistency (Cronbach's alpha of 0.86) was demonstrated in the present study.
2.3 Statistical analysis
Statistical analyses were conducted in IBM SPSS statistics (version 29.0. NY, USA). Data are reported as mean with standard deviations (SD), median with interquartile ranges (IQR) and as numbers with percentages (%) where appropriate. χ2-test for comparison between different categorical variables and Mann–Whitney U test for non-normally distributed variables were performed. A p-value under 0.05 was regarded as statistically significant. Effect size (ES) calculation for within-group analysis were computed using a calculator when appropriate (Phi-value for the Pearson χ2-test and rank-biserial correlation r for Mann–Whitney U test). Small ES is considered when Phi or r = 0.10–0.29, medium ES when Phi or r = 0.30–0.49, and large ES when phi or r = 0.50 and higher values (29). Correlation analysis between CFS grading and variables of interest was performed using Spearman's Rho correlation test.
To explore the odds of frailty (CFS scoring ≥5), binary logistic regression analysis (Forward, likelihood ratio) was performed to explore the odds of frailty. In comparison to Backward selections, we chose an exploratory approach due to lack of previous evidence. Results were displayed as odds ratio (OR) and 95% confidence interval (CI 95%). Firstly, univariate analysis was performed first for each predictor. A lax criterion with p-value <0.25 was used to select candidate variables due to limited prior studies in this field (30). Then, three multivariate regression models were performed by selecting (Forward, likelihood) pain characteristics (Model 1), psychological aspect (Model 2), and physical functioning (Model 3). Sociodemographic factors were selected as confounding factors to be entered in the models based on prior knowledge (31–34). We further examined collinearity among the categorical variables using the phi (Φ, Φ ≥ 0.30 indicating high correlation) (35). Due to the collinearity between marital status and living situation, we selected marital status as it showed significance regarding frailty status (Table 1). The Hosmer and Lemeshow test was used to examine the goodness of fit (p > 0.05). Logistic regression analyses use listwise deletion to handle the missing cases. To examine if missing data can lead to biased results, we also performed a sensitivity analysis where we used multiple imputation to handle the missing data as a comparison to the results based on “real completed cases” (see Supplementary Material).
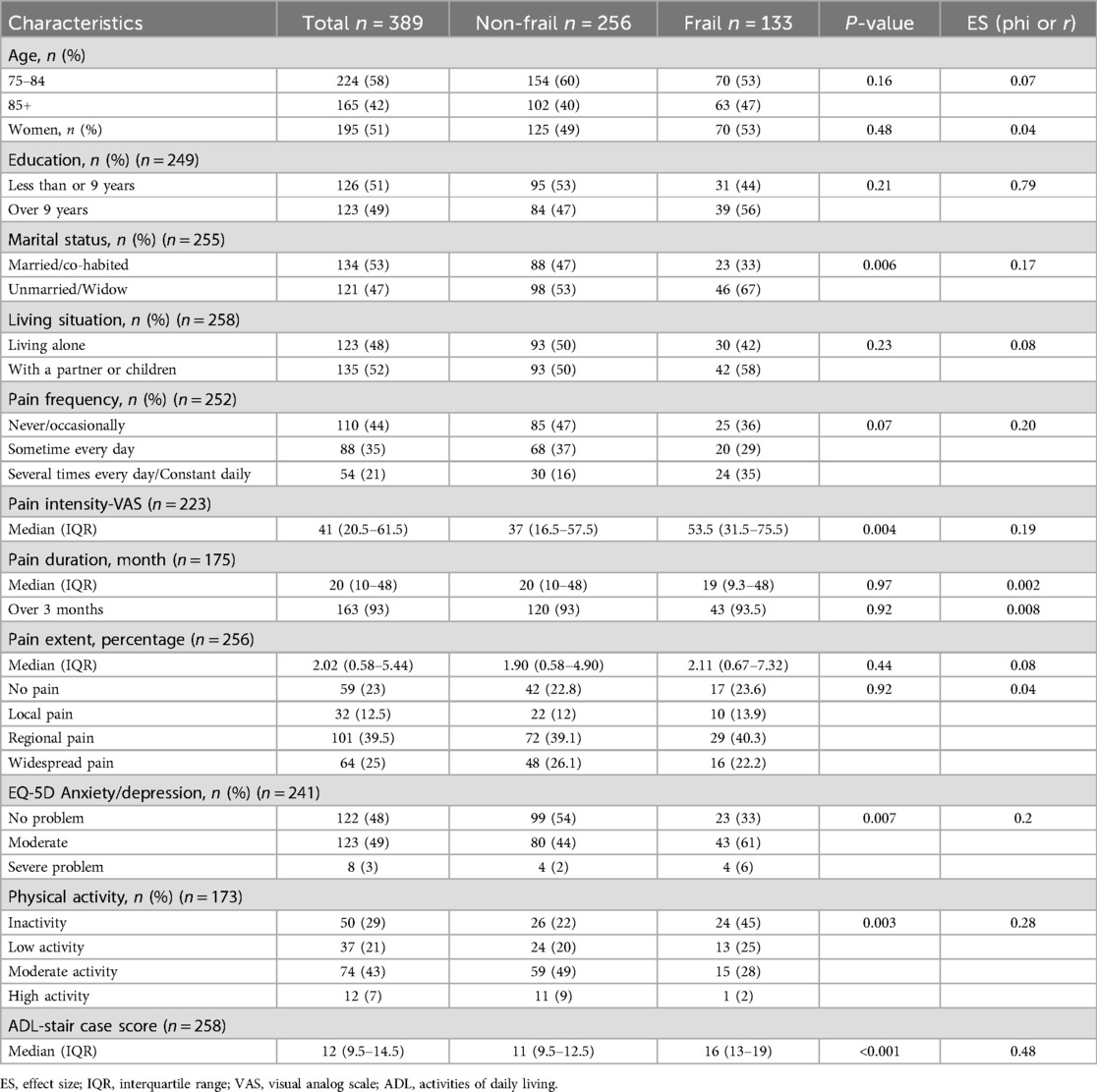
Table 1. Comparison of different factors between individuals with and without frailty. Results are reported as number of participants (%) unless otherwise stated.
3 Results
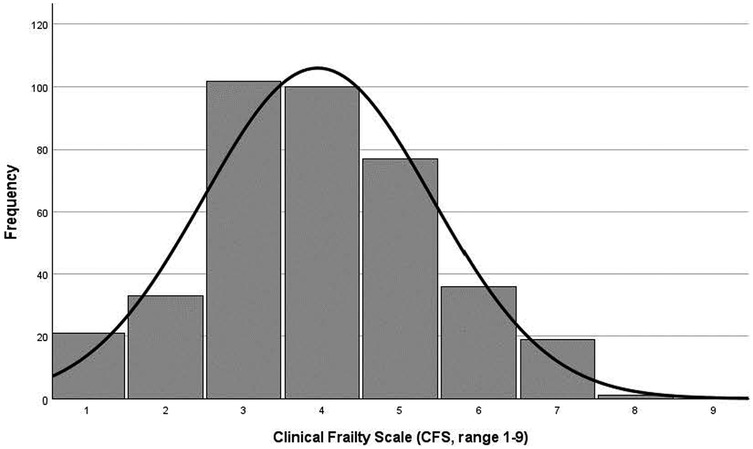
Mean age was 83.45 ± 5.09 years and 51% were female in this study population (Table 1). Mean CFS score was 3.95 ± 1.46, and approximately every other participant was classified as less vital (102, 26.2%) or vulnerable (100, 25.7%, Figure 2). Over one-third was detected as having frailty (CFS ≥ 5, 133, 34%), with almost equal distribution in both sexes (49% of men and 51% of women, respectively).
As shown in Table 1, approximately half of the participants completed high school or higher education, and no significant difference was found between Frailty Group (FG) and Non-Frailty Group (NFG). More participants in FG (67%) were unmarried or widowed than NFG participants (53%, p = 0.006, small ES). The likelihood of living alone or living with others (partner and/or children) was similar regardless of frailty status (approximately 50%, p > 0.05).
With non-responded participants (missing values) included, more than one in three (142, 36.5%) reported frequent pain (sometime every day, several times every day, and constantly daily), slightly over 40% had pain lasting longer than 3 months (163, 41.9%), and regional or widespread pain (165, 42.4%). It was extremely common to report chronic pain among the valid responses (163, 93%). Significant difference between the groups was found in pain intensity, where it was higher in FG (Median 53.5, IQR 31.5–75.5) than that in NFG (Median 37, IQR 16.5–57.5, p = 0.004, small ES).
FG participants were more likely than NFG peers to report severe anxiety/depression (p = 0.007, small ES). Regarding physical functioning, significantly lower physical activity level was showed in FG compared to NFG (p = 0.003, small ES). Higher scores of ADL-staircase were found in FG (Medan 16, IQR 13–19) compared to NFG (Median 11, IQR 9.5–12.5, p < 0.001, medium ES), indicating more ADL-dependency.
CFS grading was significantly correlated to most pain characteristics, psychological aspect and physical functioning (rho: 0.14–0.59, Table 2). Pain intensity showed significant correlations to all variables of interest. Pain frequency, EQ-5D anxiety/depression and score of ADL-staircase were correlated to all other variables except pain duration. Pain duration was only correlated to two other pain aspects (pain intensity and pain extent, p < 0.05).
Univariate logistic analysis showed all pain characteristics except pain duration were significantly associated to frailty (Table 3). In multivariate logistic regression analysis, pain frequency (OR: 1.79, 95% CI: 1.15–2.77) was significantly associated with frailty (Table 3, Model 1). The association decreased to a marginal insignificance (p = 0.052) when anxiety/depression was tested into the model (Table 3, Model 2). When the score of ADL-staircase was considered in the model fitting, neither pain frequency nor anxiety/depression remained to show significant associations (Table 3, Model 3). This model with ADL-staircase score (OR: 1.39, 95% CI: 1.22–1.58) had a higher explanatory power (Nagelkerke R2: 0.39) in predicting frailty than those without this aspect (R2: 0.10 and 0.13). The final model 3 was also examined by including one pain variable (frequency, intensity, extent and duration) at a time (Logistic regression, Enter). None of the pain characteristics showed significant association with frailty (see Supplementary Material).
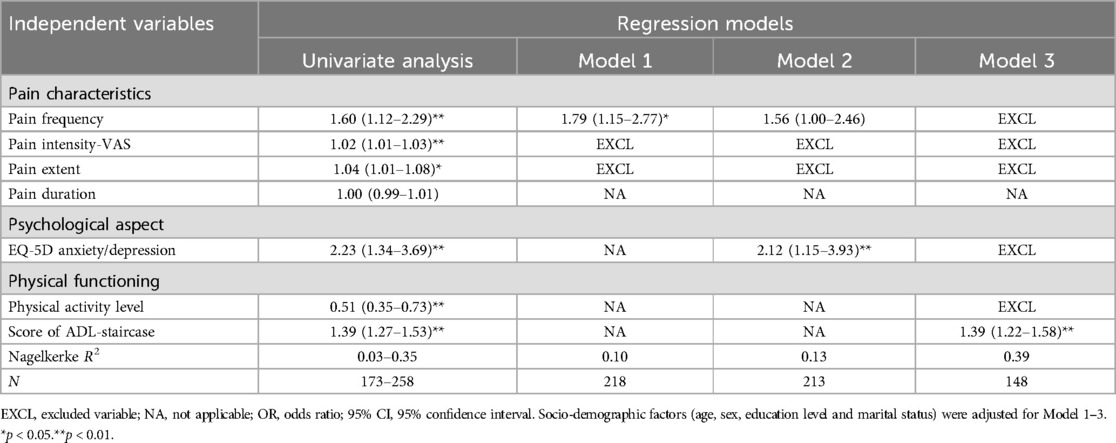
Table 3. Result of multivariate logistic regression (forward LR) model. OR (95% CI). Frailty (CFS score ≥ 5) was the dependent variable of interest.
4 Discussion
The main finding of this study is that pain characteristics (frequency, intensity, extent, and duration) were not associated with the severity of frailty in an aging population with high risk of future hospitalization. Once pain frequency was included in the logistic regression model after accounting for psychological aspect and physical functioning, the other pain characteristics did not contribute additional information. In comparison, ADL dependency had a stronger association with frailty than pain characteristics and psychological aspect (anxiety/depression).
It is hypothesized that the mechanism on pain-frailty relationship is that pain, whether chronic, severe, or widespread, may infringe on different physiological systems leading to decreased ability to perform physical activities (36). Regarding pain as an impact on physical function, researchers found pain contributed to a limitation in movement, fatigue, and lower nutritional intake (37). The experience of pain potentially creates a state of vulnerability to stressors, which may explain why individuals with pain are more prone to develop frailty and experience worsening frailty (36, 38). Pain, particularly chronic pain, has also been linked to a higher incidence of anxiety and depression (38). This in turn is more likely to lead to different consequences such as a sedentary lifestyle, malnutrition, and weight loss, which are known prerequisites for frailty development (39).
To our knowledge, few studies have investigated different pain characteristics and the associations to frailty in aging populations. According to a systematic review on the relationship between chronic pain and frailty among community-dwelling older adults, previous studies mostly focused on investigating how chronic pain influences frailty, but not considering pain intensity, frequency, and extent (11). Another study concluded that chronic pain, rather than pain intensity is positively associated with the grading of frailty in older hospitalized patients (40). This reinforces the idea that the main determinant of frailty is chronicity of pain. Moreover, the study found that widespread pain (vs. localized pain) predicted a higher grading of frailty. Comparatively, pain extent measured by pain drawings was not significantly associated with frailty in our study, when pain frequency was considered in the regression analysis. A clear difference from the above-mentioned studies is that our study population can be classified as a vulnerable group. For example, we noted an extremely high prevalence of chronic pain among the valid responses (93%), which is much higher than community-dwelled populations.
Without accounting for physical functioning, psychological problem (anxiety/depression) was a significant predictor to frailty. The results showed that the odds of being frail was 2.12 times higher for each level increase in anxiety or depression measured by EQ-5D (Table 3, Model 2). This finding was in agreement with literature (41). The significant effect, however, disappeared after the score of ADL-staircase was considered in the analysis (Table 3, Model 3). To be noted, perceived anxiety or depression should not be regarded equally as clinical anxiety or depressive symptoms that usually included clinical instruments in measurement. We speculate that the self-perceived psychological well-being might have a less impact on frailty than that was measured by clinical instruments (41).
In terms of ADL-dependency, we found that ADL-staircase score was a significant predictor for frailty. This finding can also be confirmed by earlier studies (42, 43). As individuals experienced difficulties in performing ADLs, they became more susceptible to physical and cognitive decline, which could ultimately result in frailty (42). Managing ADLs is essential for maintaining independence and quality of life, and impairment can lead to a loss of functionality and an increased risk of adverse health outcomes. To date, ADL assessment is included in frailty screening for assessing physical function and for identifying individuals who are at risk of functional decline. A systematic literature search assessing ADL in frailty instruments found it unclear whether disability in ADL should be considered an outcome of frailty, a characteristic of frailty, or a predictor of frailty (43). This inconclusiveness reflects the heterogeneity of the way frailty and ADL disability are defined and the subsequent different approaches toward the interrelationship between frailty and ADL disability. That study highlighted the need for a more comprehensive and standardized definition of the specific ADLs to be included in the frailty instruments.
From a pain rehabilitation perspective, it is reasonable to address rehabilitation interventions on improving, delaying or preventing deterioration of functional status and ADL ability, which also matches one of the main goals of proactive healthcare. Our clinical practice may shift from merely prescribing pain medications to rehabilitation approaches. It is well acknowledged that multiple pharmacologic agents might not always be of help (44, 45). Non-pharmacological approaches, such as referrals to the rehabilitation team (physiotherapist, occupational therapists, dieticians, etc.) are supposed to be included in the team-based interventions for pain management among vulnerable older people. With this knowledge, health professionals may also challenge themselves in motivating and supporting vulnerable older people in pain rehabilitation and everyday life, and in enhancing independence at home (46). More studies on the effectiveness of different rehabilitation interventions in older adults at risk of frailty and functional decline are also needed.
4.1 Strengths and limitations
To our knowledge, this is the first study to investigate the association between different pain characteristics and frailty among older people with a risk of future hospitalization. As the population also was part of a pragmatic intervention study in primary care, it is a highly relevant group to study from a clinical perspective. The instruments used to measure frailty and pain extent are well validated (17, 21, 24, 28).
Some limitations need to be considered. Firstly, the data collected in this study are embedded in a larger trial and the questionnaires were not specifically related to the aim of this study, which could have contributed to a high number of nonresponses to pain-related questions. Individuals might be more likely to complete questions related to their current health state and skip others they thought were irrelevant. For example, a minority of the participants (n = 36, 9.3%) did not answer any question of pain aspects. It is difficult for us to distinguish no pain complaint from missing values (left the question blank). A second limitation is response bias, due to a minority of the responses being provided by close relatives or caregivers (proxy). Some items (e.g., pain extent measured by pain drawing), could have excluded older people with impaired cognitive capacity if they could not get help from proxy. Thirdly, the cross-sectional study design limits the interpretation of causal-effect relationship. We only analyzed the relationship between different pain characteristics and frailty at one time point. Our results cannot draw any conclusion on factors associated with onset and/or development of frailty (36).
4.2 Conclusion
In older adults (75+) with a high risk for future hospitalization, pain frequency seemed to be related to frailty (CFS ≥ 5). When psychological aspect and physical functioning were considered, ADL dependency showed a stronger association with frailty than pain frequency. The study findings suggest that pain frequency and ADL dependency should be recognized as pertinent health issues to be addressed in pain rehabilitation to identify frailty in vulnerable older adults. Enhancing ADL functioning can be a realistic goal in pain rehabilitation as this aspect showed a strong association with frailty.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethical review board in Linköping. The studies were conducted in accordance with the local legislation and institutional requirements all participants gave written informed consent for participation in this study. For a small group, the consent was provided by a legal guardian/next of kin.
Author contributions
H-JD: Conceptualization, Data curation, Formal analysis, Funding acquisition, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Formal analysis, Writing – original draft, Writing – review & editing. MJ: Funding acquisition, Investigation, Project administration, Validation, Writing – review & editing. AP: Formal Analysis, Funding acquisition, Project administration, Resources, Validation, Visualization, Writing – review & editing. MB: Methodology, Validation, Writing – review & editing. MN: Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The research was funded by the County Council of Östergötland and Linköping University from the strategic research fund for ‘Health Care and Welfare’ [Grant number 2016186–14].
Acknowledgments
We acknowledge and thank the respondents.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1576691/full#supplementary-material
References
1. Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K. Frailty in elderly people. Lancet. (2013) 381(9868):752–62. doi: 10.1016/S0140-6736(12)62167-9
2. Hoogendijk EO, Afilalo J, Ensrud KE, Kowal P, Onder G, Fried LP. Frailty: implications for clinical practice and public health. Lancet. (2019) 394(10206):1365–75. doi: 10.1016/S0140-6736(19)31786-6
3. Rodriguez-Mañas L, Fried LP. Frailty in the clinical scenario. Lancet. (2015) 385(9968):e7–9. doi: 10.1016/S0140-6736(14)61595-6
4. Ye L, van Grieken A, Alhambra-Borrás T, Zhou S, Clough G, Markaki A, et al. Interplay of physical, psychological, and social frailty among community-dwelling older adults in five European countries: a longitudinal study. J Urban Health. (2024) 101(4):730–9. doi: 10.1007/s11524-024-00831-5
5. Boucher EL, Gan JM, Rothwell PM, Shepperd S, Pendlebury ST. Prevalence and outcomes of frailty in unplanned hospital admissions: a systematic review and meta-analysis of hospital-wide and general (internal) medicine cohorts. eClinicalMedicine. (2023) 59:101947. doi: 10.1016/j.eclinm.2023.101947
6. Olaison A, Cedersund E, Marcusson J, Nord M, Sverker A. ‘Do you have a future when you are 93?’ frail older person’s perceptions about the future and end of life—a qualitative interview study in primary care. Scand J Prim Health Care. (2022) 40(4):417–25. doi: 10.1080/02813432.2022.2139348
7. Raja SN, Carr DB, Cohen M, Finnerup NB, Flor H, Gibson S, et al. The revised international association for the study of pain definition of pain: concepts, challenges, and compromises. Pain. (2020) 161(9):1976–82. doi: 10.1097/j.pain.0000000000001939
8. Fillingim RB, Loeser JD, Baron R, Edwards RR. Assessment of chronic pain: domains, methods, and mechanisms. J Pain. (2016) 17(9 Suppl):T10–20. doi: 10.1016/j.jpain.2015.08.010
9. Cohen SP, Vase L, Hooten WM. Chronic pain: an update on burden, best practices, and new advances. Lancet. (2021) 397(10289):2082–97. doi: 10.1016/S0140-6736(21)00393-7
10. Karadag Arli S, Bakan AB, Varol E, Aslan G. Investigation of pain and life satisfaction in older adults. Geriatr Gerontol Int. (2018) 18(1):5–11. doi: 10.1111/ggi.13125
11. Otones Reyes P, Garcia Perea E, Pedraz Marcos A. Chronic pain and frailty in community-dwelling older adults: a systematic review. Pain Manag Nurs. (2019) 20(4):309–15. doi: 10.1016/j.pmn.2019.01.003
12. Marcusson J, Nord M, Johansson MM, Alwin J, Levin LA, Dannapfel P, et al. Proactive healthcare for frail elderly persons: study protocol for a prospective controlled primary care intervention in Sweden. BMJ Open. (2019) 9(5):e027847. doi: 10.1136/bmjopen-2018-027847
13. Marcusson J, Nord M, Dong HJ, Lyth J. Clinically useful prediction of hospital admissions in an older population. BMC Geriatr. (2020) 20(1):95. doi: 10.1186/s12877-020-1475-6
14. Dong HJ, Peolsson A, Johansson MM. Effects of proactive healthcare on pain, physical and activities of daily living functioning in vulnerable older adults with chronic pain: a pragmatic clinical trial with one- and two-year follow-up. Eur Geriatr Med. (2024) 15(3):709–18. doi: 10.1007/s41999-024-00952-9
15. Nord M, Östgren CJ, Marcusson J, Johansson M. Staff experiences of a new tool for comprehensive geriatric assessment in primary care (PASTEL): a focus group study. Scand J Prim Health Care. (2020) 38(2):132–45. doi: 10.1080/02813432.2020.1755786
16. Johansson MM, Barbero M, Peolsson A, Falla D, Cescon C, Folli A, et al. Pain characteristics and quality of life in older people at high risk of future hospitalization. Int J Environ Res Public Health. (2021) 18(3):958. doi: 10.3390/ijerph18030958
17. Barbero M, Moresi F, Leoni D, Gatti R, Egloff M, Falla D. Test–retest reliability of pain extent and pain location using a novel method for pain drawing analysis. Eur J Pain. (2015) 19(8):1129–38. doi: 10.1002/ejp.636
18. Grimby-Ekman AG, Bjork J, Larsson B. Comorbidities, intensity, frequency and duration of pain, daily functioning and health care seeking in local, regional, and widespread pain—a descriptive population-based survey (SwePain). BMC Musculoskelet Disord. (2015) 16:165. doi: 10.1186/s12891-015-0631-1
19. Margolis RB, Tait RC, Krause SJ. A rating system for use with patient pain drawings. Pain. (1986) 24(1):57–65. doi: 10.1016/0304-3959(86)90026-6
20. Nord M. Proactive Primary Care for Older Adults at High Risk of Hospital Admission. Linköping: Linköping University (2022).
21. Mendiratta P, Schoo C, Latif R. Clinical frailty scale. In: StatPearls. Treasure Island, FL: StatPearls Publishing (2022). https://www.ncbi.nlm.nih.gov/books/NBK559009/
22. Ekerstad N, Cederholm T, Boström AM, De Geer L, Ekdahl A, Guidetti S, et al. Clinical frailty scale—a proxy estimate of biological age. Lakartidningen. (2022) 119:22040. https://lakartidningen.se/klinik-och-vetenskap-1/artiklar-1/klinisk-oversikt/2022/11/clinical-frailty-scale-skorhet-ar-ett-satt-att-skatta-biologisk-alder/36345801
23. Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, et al. A global clinical measure of fitness and frailty in elderly people. Cmaj. (2005) 173(5):489–95. doi: 10.1503/cmaj.050051
24. Szende A, Janssen B, Cabases J. Self-Reported Population Health: An International Perspective Based on EQ-5D. Dordrecht, NL: Springer (2014).
25. Peolsson A, Almkvist C, Dahlberg C, Lindqvist S, Pettersson S. Age- and sex-specific reference values of a test of neck muscle endurance. J Manipulative Physiol Ther. (2007) 30(3):171–7. doi: 10.1016/j.jmpt.2007.01.008
26. Leijon ME, Stark-Ekman D, Nilsen P, Ekberg K, Walter L, Stahle A, et al. Is there a demand for physical activity interventions provided by the health care sector? Findings from a population survey. BMC Public Health. (2010) 10:34. doi: 10.1186/1471-2458-10-34
27. Sonn U, Asberg KH. Assessment of activities of daily living in the elderly. A study of a population of 76-year-olds in Gothenburg, Sweden. J Rehabil Med. (1991) 23(4):193–202. doi: 10.2340/165020011991193202
28. Sonn U. Longitudinal studies of dependence in daily life activities among elderly persons. Scand J Rehabil Med Suppl. (1996) 34:1–35.8701230
29. Kim HY. Statistical notes for clinical researchers: chi-squared test and fisher’s exact test. Restor Dent Endod. (2017) 42(2):152–5. doi: 10.5395/rde.2017.42.2.152
31. Fulop T, Larbi A, Witkowski JM, McElhaney J, Loeb M, Mitnitski A, et al. Aging, frailty and age-related diseases. Biogerontology. (2010) 11(5):547–63. doi: 10.1007/s10522-010-9287-2
32. Brigola AG, Alexandre TDS, Inouye K, Yassuda MS, Pavarini SCI, Mioshi E. Limited formal education is strongly associated with lower cognitive status, functional disability and frailty status in older adults. Dement Neuropsychol. (2019) 13(2):216–24. doi: 10.1590/1980-57642018dn13-020011
33. Zhang Q, Guo H, Gu H, Zhao X. Gender-associated factors for frailty and their impact on hospitalization and mortality among community-dwelling older adults: a cross-sectional population-based study. PeerJ. (2018) 6:e4326. doi: 10.7717/peerj.4326
34. Kojima G, Walters K, Iliffe S, Taniguchi Y, Tamiya N. Marital status and risk of physical frailty: a systematic review and meta-analysis. J Am Med Dir Assoc. (2020) 21(3):322–30. doi: 10.1016/j.jamda.2019.09.017
35. Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates and Proportions. 3rd edn. Hoboken, NJ: Wiley-Interscience (2003).
36. Wade KF, Marshall A, Vanhoutte B, Wu FCW, O’Neill TW, Lee DM. Does pain predict frailty in older men and women? Findings from the english longitudinal study of ageing (ELSA). The Journals of Gerontology: Series A. (2016) 72(3):403–9. doi: 10.1093/gerona/glw226
37. Coelho T, Paúl C, Gobbens RJJ, Fernandes L. Multidimensional frailty and pain in community dwelling elderly. Pain Med. (2017) 18(4):693–701. doi: 10.1111/pme.12746
38. Lin T, Zhao Y, Xia X, Ge N, Yue J. Association between frailty and chronic pain among older adults: a systematic review and meta-analysis. Eur Geriatr Med. (2020) 11(6):945–59. doi: 10.1007/s41999-020-00382-3
39. Zhao W, Zhang Y, Liu X, Yue J, Hou L, Xia X, et al. Comorbid depressive and anxiety symptoms and frailty among older adults: findings from the west China health and aging trend study. J Affect Disord. (2020) 277:970–6. doi: 10.1016/j.jad.2020.08.070
40. Ardoino I, Franchi C, Nobili A, Mannucci PM, Corli O. Pain and frailty in hospitalized older adults. Pain Ther. (2020) 9(2):727–40. doi: 10.1007/s40122-020-00202-3
41. Soysal P, Veronese N, Thompson T, Kahl KG, Fernandes BS, Prina AM, et al. Relationship between depression and frailty in older adults: a systematic review and meta-analysis. Ageing Res Rev. (2017) 36:78–87. doi: 10.1016/j.arr.2017.03.005
42. Cruz DTD, Vieira MT, Bastos RR, Leite ICG. Factors associated with frailty in a community-dwelling population of older adults. Rev Saude Publica. (2017) 51:106. doi: 10.11606/S1518-8787.2017051007098
43. Costenoble A, Knoop V, Vermeiren S, Vella RA, Debain A, Rossi G, et al. A comprehensive overview of activities of daily living in existing frailty instruments: a systematic literature search. Gerontologist. (2019) 61(3):e12–22. doi: 10.1093/geront/gnz147
44. Deng LX, Patel K, Miaskowski C, Maravilla I, Schear S, Garrigues S, et al. Prevalence and characteristics of moderate to severe pain among hospitalized older adults. J Am Geriatr Soc. (2018) 66(9):1744–51. doi: 10.1111/jgs.15459
45. Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuropsychopharmacol Biol Psychiatry. (2019) 93:284–90. doi: 10.1016/j.pnpbp.2019.04.006
Keywords: chronic pain, ADL, physical functioning, frailty, aging
Citation: Dong H-J, Yang J, Johansson MM, Peolsson A, Barbero M and Nord M (2025) Association between frailty and pain in older people at high risk of future hospitalization. Front. Pain Res. 6:1576691. doi: 10.3389/fpain.2025.1576691
Received: 14 February 2025; Accepted: 7 April 2025;
Published: 28 April 2025.
Edited by:
Kelly Marie Naugle, Indiana University-Purdue University Indianapolis, United StatesReviewed by:
Marina Kohlsdorf, University of Brasilia, BrazilValentin Max Vetter, Charité University Medicine Berlin, Germany
Copyright: © 2025 Dong, Yang, Johansson, Peolsson, Barbero and Nord. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Huan-Ji Dong, aHVhbmppLmRvbmdAbGl1LnNl
†ORCID:
Huan-Ji Dong
orcid.org/0000-0001-7051-1234
Maria M. Johansson
orcid.org/0000-0003-4166-7269
Anneli Peolsson
orcid.org/0000-0002-6075-4432
Marco Barbero
orcid.org/0000-0001-8579-0686
Magnus Nord
orcid.org/0000-0002-3257-2981
 Huan-Ji Dong
Huan-Ji Dong Joakim Yang1
Joakim Yang1 Marco Barbero
Marco Barbero