- 1Department of Surgery and Anesthesiology, The Affiliated Jiangning Hospital of Nanjing Medical University, Nangjing, China
- 2Department of Anesthesiology, Huainan First People’s Hospital, The First Affiliated Hospital of Anhui University of Science and Technology, Huainan, China
Gastric cancer is a common and highly lethal malignancy of the digestive system, with surgical resection as the primary treatment approach. However, postoperative analgesia management remains a major clinical challenge. Postoperative pain not only affects recovery speed but may also lead to complications, thereby influencing prognosis. Recent research on postoperative pain following gastric cancer surgery has expanded, exploring various analgesic methods, including pharmacological therapy, neuraxial blocks, and non-pharmacological approaches, with growing emphasis on individualized analgesia protocols. Despite the proposal of multiple analgesic techniques, current research indicates that their effectiveness and safety are still inadequately assessed in clinical applications. This review aims to discuss the physiological mechanisms of postoperative pain following gastric cancer surgery, modern analgesic strategies, and related research, to provide a theoretical basis and clinical guidance for improving postoperative quality of life.
Introduction
Gastric cancer is a leading cause of cancer-related mortality worldwide, with surgical resection considered the primary treatment option. Postoperative pain is a common complication in gastric cancer patients, significantly affecting recovery and quality of life, highlighting the importance of research into effective postoperative analgesia strategies (1). Recent research on postoperative analgesia following gastric cancer surgery has made notable progress, particularly in pain management techniques and pharmacological (2). However, it is still required further exploration of pain mechanisms and the development of optimal practice protocols to improve patient outcomes.
Patient-controlled intravenous analgesia (PCIA) and patient-controlled epidural analgesia (PCEA) are two primary methods for postoperative analgesia following gastric cancer surgery (3, 4). It is reported that PCEA provides superior pain relief in older adult gastric cancer patients. Compared to PCIA, patients in the PCEA group showed significantly lower Visual Analog Scale (VAS) scores on postoperative days 1, 2, and 3, along with shorter hospital stays (5). Additionally, a study indicated that in patients undergoing laparoscopic gastrectomy, PCEA demonstrated non-inferiority in pain relief compared to PCIA, with similar postoperative recovery parameters (4). These studies suggest that selecting the appropriate analgesic method is crucial for optimizing postoperative pain management. In addition to traditional analgesia methods, ultrasound-guided neuraxial blocks have been introduced into postoperative pain management for gastric cancer surgery. For example, transversus abdominis plane block (TAPB) significantly reduces opioid consumption within 24 h postoperatively and improves pain scores, particularly during coughing (6). These results suggest that TAPB, as an emerging analgesic technique, holds promise in reducing postoperative pain and opioid requirements, thereby minimizing related side effects. Furthermore, studies have shown that erector spinae plane block (ESPB) (7), rectus sheath block (RSB) (8), quadratus lumborum block (QLB) (9), paravertebral block (PVB) (10), transcutaneous electrical nerve stimulation (TEAS) (11), and intraoral acupuncture therapy (12) can reduce opioid use during the perioperative period in gastric cancer surgery patients, enhancing analgesic efficacy. The application of enhanced recovery after surgery (ERAS) protocols following gastric cancer surgery has garnered significant attention. By optimizing preoperative, intraoperative, and postoperative management, ERAS protocols can significantly reduce postoperative hospital stays and complication rates (13). Systematic reviews and meta-analyses indicate that patients undergoing ERAS protocols experience better postoperative recovery, including shorter hospital stays and lower complication rates (14). These findings offer new insights into postoperative pain management following gastric cancer surgery, emphasizing the importance of a comprehensive approach.
In this review, we discussed the physiological mechanisms of postoperative pain following gastric cancer surgery, modern analgesic strategies, and related research, aiming to provide a theoretical basis and clinical guidance for improving postoperative quality of life.
Physiological mechanisms of postoperative pain after radical gastrectomy for gastric cancer
Impact of surgical trauma on pain
Gastric cancer surgery typically involves extensive tissue resection and reconstruction, which can cause significant pain responses (15). The incision cuts the nerve endings in the skin, subcutaneous tissue, muscles (such as the rectus abdominis), fascia and peritoneum, which is the most direct and superficial source of pain. Moreover, gastrectomy and reconstruction, surgical traction, abdominal adhesion and changes in digestive function can lead to damage, inflammation or ischemia of organs in the abdominal cavity, which is the deeper visceral pain (16). Furthermore, the sensation of the abdominal skin is primarily supplied by specific cutaneous nerves, mainly the intercostal and subcostal nerves. These nerves run through the area where the gastric cancer surgery incision is made, particularly the upper abdominal vertical or subcostal incision in open surgery. During the operation, cutting, pulling, and suturing tissues can inevitably cause varying degrees of damage to these cutaneous nerves. Cutaneous nerve injury and neuropathic pain are the most common causes of chronic postoperative pain (17). Additionally, tissue damage activates nociceptors during surgery, leading to the release of proinflammatory mediators such as prostaglandins and interleukins. These substances not only directly stimulate nerve endings but also trigger local inflammatory reactions, which further exacerbate pain perception (18). The intensity of postoperative pain is closely related to the type of surgery, postoperative complications, and the patient's psychological state (19). Therefore, understanding the impact of surgical trauma on pain is essential for developing effective postoperative analgesia protocols.
Role of the nervous system in pain
The nervous system plays a central role in both the perception and transmission of pain. After surgery, both peripheral and central nervous system reactions influence the intensity and duration of pain (20). Peripheral nociceptive neurons release neurotransmitters when injured or inflamed, activating spinal cord neurons that transmit pain signals to the brain (21). Furthermore, spinal cord neurons amplify pain signals during transmission, which increases pain sensitivity, a phenomenon known as “central sensitization” (22). This phenomenon is particularly evident after gastric cancer surgery, where trauma and inflammation alter nervous system function, making patients more sensitive to pain (23). It was shown that neurotransmitters and modulators in the nervous system, such as norepinephrine and serotonin, participate in pain regulation, influencing pain intensity and duration (24). Therefore, interventions targeting the nervous system, such as neuraxial blocks or pharmacological therapies, may help alleviate postoperative pain.
Relationship between inflammatory response and chronic pain
The postoperative inflammatory response is a key mechanism in the development of chronic pain. Following surgical trauma, local tissues release a significant amount of inflammatory mediators, activating immune cells and triggering an inflammatory response (25). Although this inflammatory response is a protective mechanism, if excessive or prolonged, it may lead to chronic pain (17). It is reported that levels of inflammatory markers are often significantly elevated in patients with chronic pain, indicating a close correlation between inflammation and pain (26). Additionally, the occurrence of chronic pain is linked to plastic changes in the nervous system. Persistent inflammatory stimulation may cause structural and functional changes in neurons, creating a “memory” of pain, leading to pain persistence even without obvious stimulation (27). Psychological factors, such as anxiety and depression, are also considered to play significant roles in the development of chronic pain (28). Chronic pain not only impacts patients' quality of life but may also delay postoperative recovery. Therefore, controlling the postoperative inflammatory response and providing early psychological interventions may be crucial strategies for preventing and managing chronic pain.
Placebo and contextual effects on postoperative analgesia
In recent years, placebo and nocebo effects have been extensively documented in different medical conditions, including pain. The placebo effect can play a prominent role in the cognitive strategies that can facilitate motor performance in sport and physical practice (29). Moreover, the invasiveness and ritual nature of the surgery may be explained through contextual effects and expectations (30). However, the study of Rossettini et al. (31) showed that there were no unique results of the occurrence and magnitude of placebo and nocebo effects in chronic pain patients, mainly due to the heterogeneity of pain.
Application of traditional analgesia methods
Nonsteroidal anti-inflammatory drugs (NSAIDs)
NSAIDs are commonly used for postoperative analgesia after gastric cancer surgery due to their effective analgesic and anti-inflammatory properties (32). NSAIDs alleviate pain and inflammation by inhibiting cyclooxygenase (COX), which reduces prostaglandin synthesis (33). After gastric cancer surgery, the use of NSAID can significantly reduce postoperative pain scores, decrease opioid dependence, and lower the risk of related side effects (34). However, NSAID use carries certain risks, especially in patients with gastrointestinal diseases or bleeding tendencies, as it may increase the risk of gastrointestinal bleeding and ulcers (35). It is shown that selective COX-2 inhibitors, such as meloxicam and celecoxib, offer significantly better gastrointestinal safety than traditional non-selective NSAIDs. However, their effects on cardiovascular and renal function require further investigation (36). Therefore, clinicians must weigh the analgesic benefits of NSAIDs against potential risks and select an appropriate dosing regimen.
Use of opioids
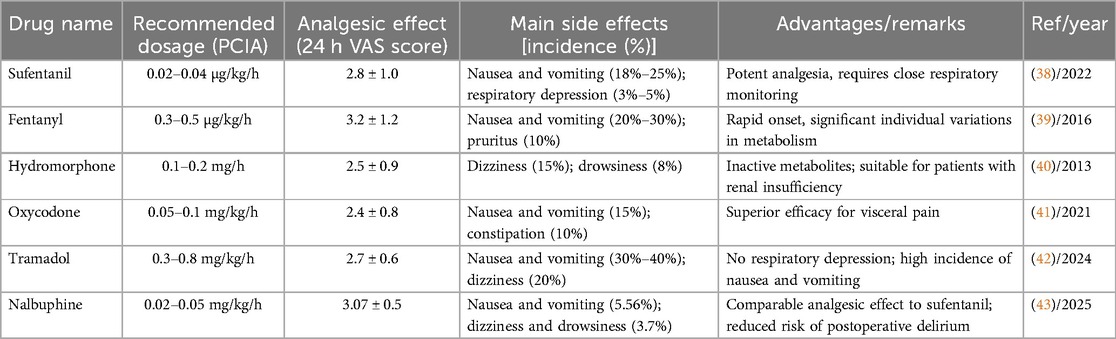
Opioids remain a crucial option for managing postoperative pain after gastric cancer surgery, particularly in patients with severe pain (37). Common opioid receptor agonists include sufentanil, fentanyl, hydromorphone, and oxycodone, while opioid receptor agonist-antagonists include nalbuphine, butorphanol, dezocine, and the weak opioid tramadol. Opioids reduce pain perception by binding to opioid receptors in the central nervous system. However, their use carries risks of side effects, including nausea, vomiting, respiratory depression, dizziness, somnolence, and addiction (38). Research in Table 1 indicates that sufentanil and oxycodone are better suited for controlling visceral pain after gastric cancer surgery due to their high lipophilicity and strong analgesic potency. Hydromorphone's metabolism is independent of renal function, making it suitable for patients with renal insufficiency. Newer drugs, such as nalbuphine, show potential in reducing delirium and respiratory depression (39–43). In recent years, concerns about opioid abuse have led to a trend in clinical practice toward adopting multimodal analgesia protocols to reduce opioid use, thereby minimizing the incidence of related adverse reactions (44). Therefore, the rational use of opioid analgesics, combined with other analgesic methods, is key to improving the effectiveness of postoperative pain management after gastric cancer surgery.
Strategies for combined use of analgesic drugs
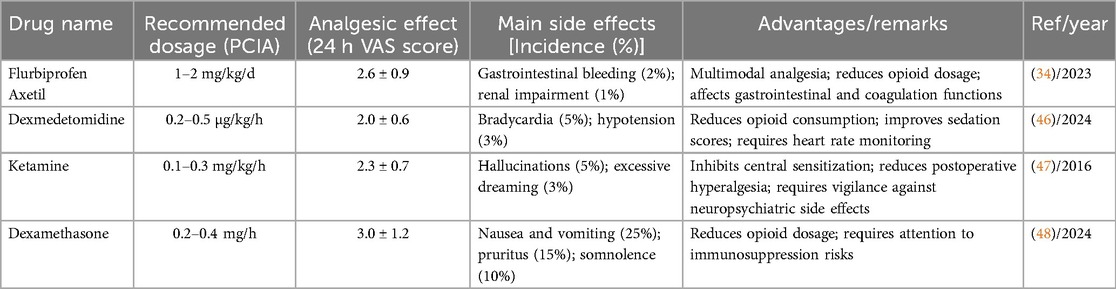
The combined use of analgesic drugs has shown favorable outcomes in managing pain following gastric cancer surgery (45). This strategy typically involves combining multiple analgesic drugs with different mechanisms of action, including NSAIDs, opioids, and adjuvant drugs (e.g., dexmedetomidine, ketamine, and glucocorticoids) to improve analgesic efficacy and minimize side effects. Research presented in Table 2 shows that combining PCIA with NSAIDs is economical and practical but requires assessment of gastrointestinal risks and is contraindicated in patients with coagulation disorders. By rationally selecting and combining different analgesic drugs, clinicians can develop individualized regimens tailored to each patient's specific conditions, optimizing analgesic efficacy while minimizing side effects. This strategy is increasingly recognized in modern pain management and is a crucial component of postoperative analgesia for gastric cancer.
Emerging analgesic techniques
Application of peripheral nerve blocks
Peripheral nerve blocks, particularly ultrasound-guided techniques, have gained increasing attention for postoperative analgesia in gastric cancer patients (49). Research in Table 3 shows that TAPB, ESPB, RSB, QLB, and PVB provide significant analgesic effects after gastric cancer surgery, reducing opioid consumption and postoperative pain scores within 24 h. TAPB, ESPB, and QLB offer advantages in laparoscopic surgery by reducing opioid dependence. Combined blocks (e.g., TAPB + ESPB) may improve visceral pain control, although further clinical evidence is needed to support this (6–10). These findings suggest that nerve block techniques may have opioid-sparing effects in postoperative analgesia, thereby reducing opioid-related adverse reactions.
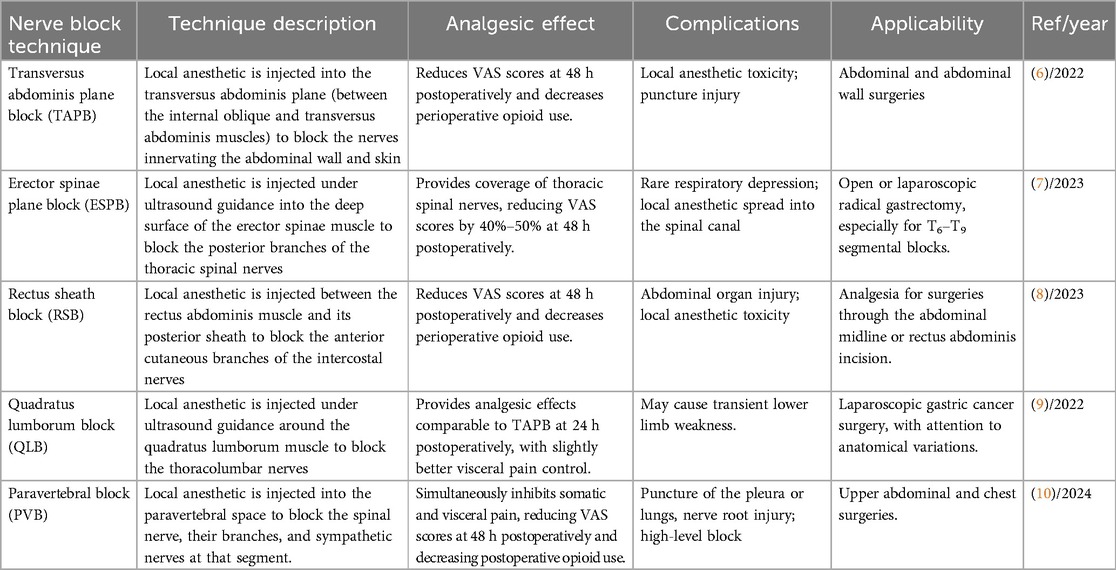
Table 3. Applications of different nerve block techniques in postoperative analgesia for gastric cancer patients.
Application of central analgesia techniques
Central analgesia techniques, such as PCEA, are crucial in managing postoperative pain following gastric cancer surgery. Studies have shown that PCEA provides superior analgesia and results in better postoperative recovery compared to PCIA (3, 4). A comparative study showed that patients undergoing laparoscopic gastrectomy who received epidural analgesia had significantly lower pain scores within 24 h postoperatively and shorter hospital stays than those in the PCIA group. However, the epidural group had higher risks of hypotension, urinary retention, and motor blockade, requiring hemodynamic monitoring during the perioperative period (4). Epidural analgesia also accelerates gastrointestinal function recovery, which is crucial for the postoperative rehabilitation of gastric cancer patients (50). Additionally, it is reported that a single intrathecal morphine injection for analgesia in patients after radical gastrectomy can alleviate postoperative pain. However, the duration of analgesia is significantly shorter, and its controllability is inferior to that of PCEA (51). These findings suggest that central analgesia techniques are valuable in improving postoperative pain management and facilitating recovery.
Impact of physical therapy on postoperative pain
Physical therapy has demonstrated positive effects in managing pain following gastric cancer surgery. Research indicates that multimodal physical therapy interventions, when implemented postoperatively, can significantly improve pain perception and functional recovery in patients. For example, studies using TEAS or acupuncture therapy as adjuvant therapy have shown that TEAS effectively reduces pain after gastric surgery, decreases perioperative opioid use, and improves digestive function (11, 52, 53). These results suggest that physical therapy, as part of a comprehensive treatment plan, can effectively alleviate postoperative pain and promote recovery, supporting its widespread application in pain management following gastric cancer surgery.
Impact of psychological factors on postoperative pain management
Psychological factors play a vital role in postoperative pain management (54). It is shown that patients' psychological states, especially anxiety and depression, significantly influence pain perception and the effectiveness of pain management (55). Anxiety and depression not only exacerbate patients' subjective pain experience but also increase the demand for analgesic medications, impacting postoperative recovery and quality of life (56). Therefore, interventions targeting psychological factors are essential in postoperative pain management.
Relationship between anxiety and pain perception
Anxiety is a significant psychological factor influencing postoperative pain perception (57). It is reported that anxiety influences pain perception by increasing sensitivity to pain and decreasing tolerance, with patients who report higher preoperative anxiety levels often experiencing more severe postoperative pain (24). Specifically, anxious patients may have stronger emotional responses to pain, intensifying their pain experience (58). Furthermore, anxiety increases pain expectancy, which in turn exacerbates pain perception (59). Therefore, assessing and managing preoperative anxiety is vital for enhancing postoperative pain management.
Effectiveness of psychological intervention measures
Psychological interventions have shown positive effects in improving postoperative pain management (60). Psychological interventions, such as cognitive-behavioral therapy (CBT) and relaxation training, can effectively reduce anxiety levels, thereby alleviating postoperative pain (61). For example, a study found that patients who received psychological interventions had significantly lower postoperative pain scores than those who did not, and reduced their dependence on medications, thereby lowering potential drug side effects (62). Additionally, psychological interventions improve patients' psychological states, enhance their coping abilities, and promote overall postoperative recovery outcomes (63). Therefore, incorporating psychological interventions into postoperative pain management can significantly improve patient satisfaction and quality of life.
Patient education and self-management
Patient education is equally essential in postoperative pain management (64). By providing knowledge and skills for pain management, patients can better understand and cope with postoperative pain, thereby improving their self-management abilities (65). Moreover, educating patients on how to use analgesic medications, recognize pain signals, and adopt effective self-management strategies can significantly reduce postoperative pain perception (66). Furthermore, patient education helps establish reasonable expectations and reduces fear of pain, thereby improving psychological well-being and postoperative recovery outcomes (64). In summary, integrating psychological interventions, patient education, and self-management strategies will enhance the overall effectiveness of postoperative pain management.
Application of alternative therapies
Alternative therapies, including traditional Chinese medicine, massage, and acupuncture, are gaining attention in the management of postoperative pain following gastric cancer surgery (67). These therapies target pain management through various mechanisms, effectively alleviating postoperative pain and promoting overall rehabilitation. For example, it is shown that traditional Chinese nursing interventions significantly reduce postoperative pain scores and improve patients' physiological and psychological states (68). Buccal acupuncture therapy, which stimulates various acupuncture points, can enhance the effectiveness of PCIA in patients undergoing laparoscopic radical gastrectomy for gastric cancer, reduce opioid use, and facilitate postoperative recovery (12). Additionally, alternative therapies generally have fewer side effects and are suitable for long-term use, offering patients more treatment options (69). With further research into their effectiveness and safety, alternative therapies are expected to play an increasingly significant role in pain management in the future.
Multimodal analgesia strategies
Multimodal analgesia is the established gold standard for postoperative pain management in gastric cancer patients. It aims to achieve synergistic analgesic effects while reducing the dosage and adverse effects of single-agent therapies, particularly opioids, through the combination of pharmacologically distinct agents and techniques (70, 71). The formulation of an analgesic regimen should account for surgical approach, patient-specific factors, and ERAS protocols. For example: PCEA combined with NSAID) and gabapentin is recommended in open gastrectomy (3), while TAPB or ESPB with a selective COX-2 inhibitor is advised in Laparoscopic gastrectomy (7, 8). For older adult or renally impaired patients, NSAIDs are contraindicated and regional nerve blocks are preferred (36). Moreover, Adjunctive ketamine may be utilized for opioid-tolerant patients (47). Within ERAS pathways, the key objectives include maintaining VAS scores <4 within 24 h postoperatively, minimizing opioid consumption, and discontinuing opioids within 48 h when feasible (72). The current consensus advocates a four-step analgesic protocol: priority regional anesthesia (PCEA/TAP/ESPB), Maximized non-opioid foundation (acetaminophen + COX-2 inhibitor), opioids as rescue therapy (preferably via PCIA) and dynamic reassessment at 72 h (regimen adjustment until VAS ≤4) (70, 71).
Future research directions and challenges
Exploration of individualized analgesic strategies
The exploration of individualized analgesic strategies is crucial in postoperative pain management following gastric cancer surgery. Research has shown that personalized analgesic regimens can significantly improve postoperative recovery and quality of life (73). By analyzing patients' physiological characteristics, psychological states, and pain perceptions, medical teams can create more tailored analgesic plans. Individualizing analgesic approaches based on these individual differences enhances analgesic effectiveness and reduces the risk of adverse drug reactions. For example, using biomarkers to predict the severity of postoperative pain can help anesthesiologists select the most appropriate analgesic medications and administration methods, achieving individualized pain management (74). Advances in genomics and pharmacogenomics allow researchers to predict patients' responses to specific analgesics, optimizing medication selection (75). Additionally, patients' personal pain goals (PPG) should be considered to ensure pain management plans align with their needs and expectations (76). Therefore, future research should focus on developing more precise assessment tools to tailor individualized analgesic strategies to patients' specific needs early in the postoperative period.
Importance of multidisciplinary collaboration in pain management
Multidisciplinary collaboration is crucial in pain management, especially in complex cases like those involving gastric cancer surgery patients. By integrating the expertise of surgeons, anesthesiologists, pain management specialists, and nursing teams, a more comprehensive pain management plan can be developed (77). For example, anesthesiologists can provide targeted analgesic techniques, while surgeons can adjust analgesic strategies based on surgery type and patient condition. Research shows that interdisciplinary collaboration improves pain control outcomes and reduces postoperative complications (78). Additionally, establishing effective communication mechanisms and collaboration platforms is key to enabling multidisciplinary cooperation (79). Future research should explore ways to optimize multidisciplinary team collaboration models to enhance pain management effectiveness following gastric cancer surgery and improve patients' quality of life.
Development of novel analgesic drugs
As understanding of pain mechanisms deepens, the development of novel analgesic drugs has become a key focus for improving postoperative pain management following gastric cancer surgery (80). Currently, traditional analgesics, such as opioids, present a challenge in balancing efficacy with side effects (81). Novel drugs, including neuropeptide-based medications and targeted therapies, are being extensively studied. These drugs target pain pathways through various mechanisms, offering more effective pain relief with fewer side effects (82). Additionally, nano-drug delivery systems offer new possibilities for analgesic drug development by enabling more precise drug release and minimizing side effects (83). Moreover, drug discovery methods that leverage artificial intelligence and big data technologies are accelerating the development of analgesic drugs (84). Future research should focus on the clinical application of these novel drugs, evaluating their effectiveness in pain management following gastric cancer surgery, and exploring their potential for personalized treatments to provide safer and more effective analgesic solutions for patients.
Limitations
There were several limitations in this review. First of all, this paper mainly reviewed the postoperative analgesic regimen for patients with radical gastrectomy, and did not conduct a specific meta-analysis. Secondly, perhaps some pictures could improve the quality of this review, but the tables in the manuscript have covered the main analgesic drugs and protocols currently being focused on and applied. Additionally, as the mainstream method of regional anesthesia techniques, peripheral nerve block were more extensive and effective than local infiltration anesthesia, which was not discussed in this review.
Conclusions
Existing studies suggest that traditional pharmacological treatments remain the primary approach for alleviating postoperative pain in gastric cancer patients. However, as understanding of pain mechanisms deepens, emerging techniques such as neural blockade, ultrasound-guided analgesia, and multimodal analgesic strategies are increasingly showing their importance. Despite the abundance of literature on different analgesic techniques and their effects, balancing the findings from various studies remains a challenge in practical applications. Therefore, future research should emphasize developing personalized pain management plans based on patients’ specific conditions. Integrating psychological interventions with pain management will enhance patients' quality of life and postoperative recovery outcomes, an area ripe for further exploration and validation. In summary, the prospects for research in postoperative pain management following gastric cancer surgery are broad. Future research should emphasize individualized and comprehensive management strategies to meet the needs of diverse patients, ultimately enhancing postoperative quality of life and satisfaction.
Author contributions
LL: Conceptualization, Writing – original draft, Writing – review & editing. YZ: Conceptualization, Investigation, Writing – original draft. HC: Methodology, Writing – review & editing. JZ: Writing – review & editing, Validation, Methodology, Supervision. MD: Validation, Writing – review & editing. QW: Resources, Validation, Writing – review & editing. JL: Methodology, Writing – review & editing, Supervision. WW: Supervision, Writing – original draft, Writing – review & editing, Validation, Investigation.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This review was supported by the Plan of Science and Technology Benefit to the People of Jiangning District in Nanjing (No. 2023078S).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
PCIA, patient-controlled intravenous analgesia; PCEA, patient-controlled epidural analgesia; VAS, visual analog scale; TAPB, transvs. abdominis plane block; ESPB, erector spinae plane block; RSB, rectus sheath block; QLB, quadratus lumborum block; PVB, paravertebral block; TEAS, transcutaneous electrical nerve stimulation; ERAS, enhanced recovery after surgery; NSAIDs, nonsteroidal anti-inflammatory drugs; COX, cyclooxygenase; CBT, cognitive-behavioral therapy; PPG, Patients' personal pain goals.
References
1. Shin S, Kim HI, Kim NY, Lee KY, Kim DW, Yoo YC. Effect of postoperative analgesia technique on the prognosis of gastric cancer: a retrospective analysis. Oncotarget. (2017) 8(61):104594–604. doi: 10.18632/oncotarget.21979
2. Xu L, Yao L, Qin J, Xu H. Efficacy of multimodal analgesia based on the concept of enhanced recovery after surgery in laparoscopic radical gastrectomy for gastric cancer. Pak J Med Sci. (2024) 40(10):2190–5. doi: 10.12669/pjms.40.10.10088
3. Hsu FK, Chang WK, Lin KJ, Chu TJ, Fang WL, Chang KY. Effect of epidural analgesia on cancer outcomes after gastric cancer resection: a single-centre cohort study in Taiwan. BMJ Open. (2022) 12(3):e053050. doi: 10.1136/bmjopen-2021-053050
4. Kikuchi S, Matsusaki T, Mitsuhashi T, Kuroda S, Kashima H, Takata N, et al. Epidural versus patient-controlled intravenous analgesia on pain relief and recovery after laparoscopic gastrectomy for gastric cancer: randomized clinical trial. BJS Open. (2024) 8(1):zrad161. doi: 10.1093/bjsopen/zrad161
5. Yu J, Zheng T, Yuan A, Wang W, Li Z, Cao S. The role of patient-controlled epidural analgesia in the short-term outcomes of laparoscopic-assisted gastrectomy in elderly gastric cancer patients. J Surg Res. (2025) 306:257–65. doi: 10.1016/j.jss.2024.11.008
6. Yoon S, Song GY, Lee J, Lee HJ, Kong SH, Kim WH, et al. Ultrasound-guided bilateral subcostal transversus abdominis plane block in gastric cancer patients undergoing laparoscopic gastrectomy: a randomised-controlled double-blinded study. Surg Endosc. (2022) 36(2):1044–52. doi: 10.1007/s00464-021-08370-9
7. Ridolfi M, Conti D, Antognozzi E, Garulli G, Monari F, Montomoli J, et al. Erector spinae plane block as part of an opiod-sparing anesthesia in enhanced recovery after surgery program in laparoscopic gastric surgery. Minerva Anestesiol. (2023) 89(1–2):108–9. doi: 10.23736/S0375-9393.22.16813-6
8. Shi DW, Zhou XD, Wang FJ, Wang J, Liu Y, Niu Y, et al. Opioid-sparing effect of multi-point incision-based rectus sheath block in laparoscopic-assisted radical gastrectomy: a randomized clinical trial. J Clin Med. (2023) 12(4):1414. doi: 10.3390/jcm12041414
9. Jiao R, Peng S, Wang L, Feng M, Li Y, Sun J, et al. Ultrasound-guided quadratus lumborum block combined with general anaesthesia or general anaesthesia alone for laparoscopic radical gastrectomy for gastric adenocarcinoma: a monocentric retrospective study. Int J Gen Med. (2022) 15:7739–50. doi: 10.2147/IJGM.S382757
10. Xiong YF, Wei BZ, Wang YF, Li XF, Liu C. Paravertebral block’s effect on analgesia and inflammation in advanced gastric cancer patients undergoing transarterial chemoembolization and microwave ablation. World J Gastrointest Surg. (2024) 16(1):196–204. doi: 10.4240/wjgs.v16.i1.196
11. Chang XL, Liu XM, An LX, Zheng JY, Zhang K. Effects of transcutaneous electrical acupoint stimulation (TEAS) on postoperative pain in patients undergoing gastric and esophageal ESD surgery: a study protocol for a prospective randomized controlled trial. BMC Complement Med Ther. (2023) 23(1):253. doi: 10.1186/s12906-023-04075-9
12. Zhu DX, Yang YL, Yang L, Zhao YY, Xie YY, Wang W, et al. Effects of buccal acupuncture on postoperative analgesia in elderly patients undergoing laparoscopic radical gastrectomy: a randomized controlled trial. Front Neurol. (2024) 15:1408360. doi: 10.3389/fneur.2024.1408360
13. Parakonthun T, Gonggetyai G, Nampoolsuksan C, Suwatthanarak T, Tawantanakorn T, Swangsri J, et al. Higher compliance with the enhanced recovery after surgery protocol improves postoperative recovery and 6-month mortality in upper gastrointestinal surgery. Surg Pract Sci. (2024) 19:100265. doi: 10.1016/j.sipas.2024.100265
14. Wang Y, Luo S, Wang S. Evaluation of enhanced recovery after surgery for gastric cancer patients undergoing gastrectomy: a systematic review and meta-analysis. Wideochir Inne Tech Maloinwazyjne. (2023) 18(4):551–64. doi: 10.5114/wiitm.2023.131723
15. Lee S, Suh YS, Berlth F, Kang SH, Park SH, Park YS, et al. Feasibility and safety of pure single-incision laparoscopic total and proximal gastrectomy for early gastric cancer: propensity score-matched comparison to multiport totally laparoscopic approach. Surg Endosc. (2023) 37(12):9665–75. doi: 10.1007/s00464-023-10490-3
16. Lu CP, Gao Y, Zhang ZH. Enhanced recovery after surgery continuity nursing in elderly gastric cancer patients. World J Gastrointest Surg. (2025) 17(5):103340. doi: 10.4240/wjgs.v17.i5.103340
17. Huang X, Du H, Aihemaiti M, Liu T, Chen N, Yu W, et al. Laparoscopic-assisted versus open D2 gastrectomy for advanced gastric cancer in highly selective patients: short-term surgical and chemotherapy outcomes of a prospective cohort study. Am J Clin Oncol. (2019) 42(5):459–65. doi: 10.1097/COC.0000000000000534
18. Marshall TJ, Watne LO, Sanders RD. Mechanisms of perioperative neuronal injury and the search for therapies. Br J Anaesth. (2025) 134(4):906–8. doi: 10.1016/j.bja.2024.12.032
19. Zhi X, Kuang X, Li J. The impact of perioperative events on cancer recurrence and metastasis in patients after radical gastrectomy: a review. Cancers. (2022) 14(14):3496. doi: 10.3390/cancers14143496
20. Ontario Health. Peripheral nerve stimulation for chronic neuropathic pain: a health technology assessment. Ont Health Technol Assess Ser. (2024) 24(10):1–131.
21. Ahmed U, Baloch M. Deafferentation in pain medicine: a narrative review of mechanisms and management. J Pain Palliat Care Pharmacother. (2024) 39(1):114–23. doi: 10.1080/15360288.2024.2432640
22. Imamura M, Filardi RM, Lacerda GJM, Pacheco-Barrios K, Shinzato G, Battistella LR, et al. The role of maladaptive plasticity in modulating pain pressure threshold post-spinal cord injury. Healthcare. (2025) 13(3):247. doi: 10.3390/healthcare13030247
23. Li CY, Wang YF, Luo LK, Yang XJ. Present situation of minimally invasive surgical treatment for early gastric cancer. World J Gastrointest Oncol. (2024) 16(4):1154–65. doi: 10.4251/wjgo.v16.i4.1154
24. Ohashi N, Uta D, Ohashi M, Baba H. Norepinephrine restores inhibitory tone of spinal lamina X circuitry, thus contributing to analgesia against inflammatory pain. Neuroscience. (2022) 490:224–35. doi: 10.1016/j.neuroscience.2022.03.023
25. Tassou A, Richebe P, Rivat C. Mechanisms of chronic postsurgical pain. Reg Anesth Pain Med. (2025) 50(2):77–85. doi: 10.1136/rapm-2024-105964
26. Segelcke D, Sondermann JR, Kappert C, Pradier B, Görlich D, Fobker M, et al. Blood proteomics and multimodal risk profiling of human volunteers after incision injury: a translational study for advancing personalized pain management after surgery. Pharmacol Res. (2025) 212:107580. doi: 10.1016/j.phrs.2025.107580
27. Beckers P, Charlier M, Richter LA, Braconnier P, Desmet N, Massie A, et al. Implication of system xc-in complete Freund’s adjuvant-induced peripheral inflammation and associated nociceptive sensitization. Neuropharmacology. (2025) 269:110340. doi: 10.1016/j.neuropharm.2025.110340
28. Tukanova KH, Chidambaram S, Guidozzi N, Hanna GB, McGregor AH, Markar SR. Physiotherapy regimens in esophagectomy and gastrectomy: a systematic review and meta-analysis. Ann Surg Oncol. (2022) 29(5):3148–67. doi: 10.1245/s10434-021-11122-7
29. Rossettini G, Emadi Andani M, Dalla Negra F, Testa M, Tinazzi M, Fiorio M. The placebo effect in the motor domain is differently modulated by the external and internal focus of attention. Sci Rep. (2018) 8(1):12296. doi: 10.1038/s41598-018-30228-9
30. Ezzatvar Y, Dueñas L, Balasch-Bernat M, Lluch-Girbés E, Rossettini G. Which portion of physiotherapy Treatments’ effect is not attributable to the specific effects in people with musculoskeletal pain? A meta-analysis of randomized placebo-controlled trials. J Orthop Sports Phys Ther. (2024) 54(6):391–9. doi: 10.2519/jospt.2024.12126
31. Rossettini G, Campaci F, Bialosky J, Huysmans E, Vase L, Carlino E. The biology of placebo and nocebo effects on experimental and chronic pain: state of the art. J Clin Med. (2023) 12(12):4113. doi: 10.3390/jcm12124113
32. Tołoczko-Iwaniuk N, Dziemiańczyk-Pakieła D, Nowaszewska BK, Celińska-Janowicz K, Miltyk W. Celecoxib in cancer therapy and prevention—review. Curr Drug Targets. (2019) 20(3):302–15. doi: 10.2174/1389450119666180803121737
33. Pawlędzio S, Ziemniak M, Wang X, Woźniak K, Malinska M. Understanding the selectivity of nonsteroidal anti-inflammatory drugs for cyclooxygenases using quantum crystallography and electrostatic interaction energy. IUCrJ. (2025) 12(Pt 2):208–22. doi: 10.1107/S2052252525000053
34. Kim SJ, Jeon CH, Lee HH, Song KY, Seo HS. Impact of postoperative NSAIDs (IV-PCA) use on short-term outcomes after laparoscopic gastrectomy for the patients of gastric cancer. Surg Endosc. (2023) 37(2):1123–31. doi: 10.1007/s00464-022-09600-4
35. Goyal R, Gupta S, Sharma P, Sharma M. Insights into prospects of novel NSAID prodrugs in the management of gastrointestinal toxicity: a perspective review. Recent Adv Inflamm Allergy Drug Discov. (2024) 18(1):2–10. doi: 10.2174/0127722708278736231205055035
36. Vaja R, Ferreira P, Portas L, Ahmetaj-Shala B, Cypaite N, Gashaw H, et al. Vascular and inflammatory biomarkers of cardiovascular events in non-steroidal anti-inflammatory drug users. Eur Heart J Open. (2024) 4(6):oeae088. doi: 10.1093/ehjopen/oeae088
37. Pu J, Wang N, Huang ZK, He XY, Yuan HB. Correlation between gene polymorphism and opioid efficacy in patients with gastric or intestinal cancer. Eur Rev Med Pharmacol Sci. (2019) 23(21):9393–410. doi: 10.26355/eurrev_201911_19432
38. Li J, Li S, Yu L, Wei J, Li S, Tan H. The effects of resistin gene polymorphism on pain thresholds and postoperative sufentanil consumption in gastric cancer patients. J Pain Res. (2022) 15:1995–2004. doi: 10.2147/JPR.S372845
39. Ding Z, Wang K, Wang B, Zhou N, Li H, Yan B. Efficacy and tolerability of oxycodone versus fentanyl for intravenous patient-controlled analgesia after gastrointestinal laparotomy: a prospective, randomized, double-blind study. Medicine. (2016) 95(39):e4943. doi: 10.1097/MD.0000000000004943
40. Ziemann-Gimmel P, Hensel P, Koppman J, Marema R. Multimodal analgesia reduces narcotic requirements and antiemetic rescue medication in laparoscopic Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis. (2013) 9(6):975–80. doi: 10.1016/j.soard.2013.02.003
41. Lao WL, Song QL, Jiang ZM, Chen WD, Zheng XH, Chen ZH. The effect of oxycodone on post-operative pain and inflammatory cytokine release in elderly patients undergoing laparoscopic gastrectomy. Front Med. (2021) 8:700025. doi: 10.3389/fmed.2021.700025
42. Sato T, Ono S, Sato T, Tanaka R, Kamo Y, Suzuki T. Safety and efficacy of combined injection of pure-μ-opioid agonist with tramadol as an opioid induction agent for opioid-naïve cancer patients. Palliat Med Rep. (2024) 5(1):340–9. doi: 10.1089/pmr.2023.0061
43. Qian JL, Wang J, Shen ZY, Xu BQ, Shen DP, Yang C. Effect of nalbuphine on analgesia and pain factors after gastric cancer resection. World J Gastrointest Surg. (2025) 17(1):99327. doi: 10.4240/wjgs.v17.i1.99327
44. Kang SH, Lee Y, Min SH, Park YS, Ahn SH, Park DJ, et al. Multimodal enhanced recovery after surgery (ERAS) program is the optimal perioperative care in patients undergoing totally laparoscopic distal gastrectomy for gastric cancer: a prospective, randomized, clinical trial. Ann Surg Oncol. (2018) 25(11):3231–8. doi: 10.1245/s10434-018-6625-0
45. Wang Y, Wang L, Chen H, Xu Y, Zheng X, Wang G. The effects of intra- and post-operative anaesthesia and analgesia choice on outcome after gastric cancer resection: a retrospective study. Oncotarget. (2017) 8(37):62658–65. doi: 10.18632/oncotarget.16724
46. Zhao GG, Lou C, Gao RL, Lei FX, Zhao J. Combined use of dexmedetomidine and nalbuphine in laparoscopic radical gastrectomy for gastric cancer. World J Gastrointest Oncol. (2024) 16(7):2952–9. doi: 10.4251/wjgo.v16.i7.2952
47. Lin L, Liu S, Chen Z, Lin S. Effect of ketamine combined with butorphanol on emergence agitation of postoperative patients with gastric cancer. Ther Clin Risk Manag. (2016) 12:713–7. doi: 10.2147/TCRM.S103060
48. Zeng H, Yin F, Fan L, Li C, Lin H, Liu F, et al. Combination of dexamethasone and dexmedetomidine as adjuvants of transversus abdominis plane block for postoperative analgesia in gastric cancer patients: a double-blinded randomized controlled trial. J Clin Anesth. (2024) 97:111543. doi: 10.1016/j.jclinane.2024.111543
49. Liu R, Qin H, Wang M, Li K, Zhao G. Transversus abdominis plane block with general anesthesia blunts the perioperative stress response in patients undergoing radical gastrectomy. BMC Anesthesiol. (2019) 19(1):205. doi: 10.1186/s12871-019-0861-0
50. Wang L, Li X, Chen H, Liang J, Wang Y. Effect of patient-controlled epidural analgesia versus patient-controlled intravenous analgesia on postoperative pain management and short-term outcomes after gastric cancer resection: a retrospective analysis of 3,042 consecutive patients between 2010 and 2015. J Pain Res. (2018) 11:1743–9. doi: 10.2147/JPR.S168892
51. Desjardins P, Ménassa M, Desbiens F, Gagné JP, Hogue JC, Poirier É. Effect of single-shot intrathecal morphine versus continuous epidural analgesia on length of stay after gastrectomy for cancer: a retrospective cohort study. Gastric Cancer. (2023) 26(4):648–52. doi: 10.1007/s10120-023-01386-1
52. Zhou X, Cao SG, Tan XJ, Liu XD, Li ZQ, Kong LX, et al. Effects of transcutaneous electrical acupoint stimulation (TEAS) on postoperative recovery in patients with gastric cancer: a randomized controlled trial. Cancer Manag Res. (2021) 13:1449–58. doi: 10.2147/CMAR.S292325
53. Wang Y, Wang L, Ni X, Jiang M, Zhao L. Effect of acupuncture therapy for postoperative gastrointestinal dysfunction in gastric and colorectal cancers: an umbrella review. Front Oncol. (2024) 14:1291524. doi: 10.3389/fonc.2024.1291524
54. Zhang M, Li R, Chen H, Zhou J, Zhang Y. Application and efficacy evaluation of an NBASS-APS pain management model in postoperative analgesia for gastric cancer patients. J BUON. (2018) 23(5):1426–31.30570869
55. Demir E, Doğan G, Kiraz M, Akdağli Ekici A, Kayir S, Ekici M, et al. Current trends in pain management: a bibliometric analysis for the 1980-to-2023 period. Medicine. (2025) 104(3):e41319. doi: 10.1097/MD.0000000000041319
56. St John IJ, Englund HM. Improving patient discharge education through daily educational bursts: a pilot study. J Nurses Prof Dev. (2020) 36(5):283–7. doi: 10.1097/NND.0000000000000627
57. Schreiber KL, Wilson JM, Chen YK. Recognizing pain phenotypes: biopsychosocial sources of variability in the transition to chronic postsurgical pain. Reg Anesth Pain Med. (2025) 50(2):86–92. doi: 10.1136/rapm-2024-105602
58. Ishida Y, Okada T, Kobayashi T, Funatsu K, Uchino H. Pain management of acute and chronic postoperative pain. Cureus. (2022) 14(4):e23999. doi: 10.7759/cureus.23999
59. Stamenkovic DM, Selvaraj S, Venkatraman S, Arshad A, Rancic NK, Dragojevic-Simic VM, et al. Anesthesia for patients with psychiatric illnesses: a narrative review with emphasis on preoperative assessment and postoperative recovery and pain. Minerva Anestesiol. (2020) 86(10):1089–102. doi: 10.23736/S0375-9393.20.14259-7
60. Darnall BD, Abshire L, Courtney RE, Davin S. Upskilling pain relief after surgery: a scoping review of perioperative behavioral intervention efficacy and practical considerations for implementation. Reg Anesth Pain Med. (2025) 50(2):93–101. doi: 10.1136/rapm-2024-105601
61. Grimmett C, Heneka N, Chambers S. Psychological interventions prior to cancer surgery: a review of reviews. Curr Anesthesiol Rep. (2022) 12(1):78–87. doi: 10.1007/s40140-021-00505-x
62. Orenius T, Silén E, Nuortimo A, Ristolainen L. Psychological interventions in preventing chronicity of sub-acute back pain: a systematic review. Scand J Pain. (2022) 22(2):211–7. doi: 10.1515/sjpain-2021-0063
63. Kneebone II, Munday I, Van Zanden BE, Thomas S, Newton-John T. Psychological interventions for post stroke pain: a systematic review. Neuropsychol Rehabil. (2023) 33(7):1304–24. doi: 10.1080/09602011.2022.2070506
64. Niyonkuru E, Iqbal MA, Zhang X, Ma P. Complementary approaches to postoperative pain management: a review of non-pharmacological interventions. Pain Ther. (2025) 14(1):121–44. doi: 10.1007/s40122-024-00688-1
65. Liu QR, Dai YC, Ji MH, Liu PM, Dong YY, Yang JJ. Risk factors for acute postsurgical pain: a narrative review. J Pain Res. (2024) 17:1793–804. doi: 10.2147/JPR.S462112
66. Small C, Laycock H. Acute postoperative pain management. Br J Surg. (2020) 107(2):e70–80. doi: 10.1002/bjs.11477
67. Wang XQ, Xiao L, Duan PB, Xu Q, Yang LH, Wang AQ, et al. The feasibility and efficacy of perioperative auricular acupuncture technique via intradermal needle buried for postoperative movement-evoked pain after open radical gastrectomy: a randomized controlled pilot trial. Explore. (2022) 18(1):36–43. doi: 10.1016/j.explore.2021.09.007
68. Hong Z, Yi J, Ming L, Huiqin L, Jing W, Chuanbing H. Evaluation index system of core competence of traditional Chinese medicine nurse specialists: a qualitative evidence synthesis. Nurse Educ Pract. (2025) 84:104290. doi: 10.1016/j.nepr.2025.104290
69. Angelini E, Josefsson C, Ögren C, Andréll P, Wolf A, Ringdal M. Patients' experiences of TENS as a postoperative pain relief method in the post-anesthesia care unit after laparoscopic cholecystectomy: a qualitative study. BMC Anesthesiol. (2025) 25(1):18. doi: 10.1186/s12871-024-02872-4
70. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. (2016) 17(2):131–57. doi: 10.1016/j.jpain.2015.12.008
71. Yamagata Y, Yoshikawa T, Yura M, Otsuki S, Morita S, Katai H, et al. Current status of the “enhanced recovery after surgery” program in gastric cancer surgery. Ann Gastroenterol Surg. (2019) 3(3):231–8. doi: 10.1002/ags3.12232
72. Hu Y, Hsu AW, Strong VE. Enhanced recovery after major gastrectomy for cancer. Ann Surg Oncol. (2021) 28(12):6947–54. doi: 10.1245/s10434-021-09906-y
73. Kinoshita J, Fushida S, Kaji M, Oyama K, Fujimoto D, Hirono Y, et al. A randomized controlled trial of postoperative intravenous acetaminophen plus thoracic epidural analgesia vs. thoracic epidural analgesia alone after gastrectomy for gastric cancer. Gastric Cancer. (2019) 22(2):392–402. doi: 10.1007/s10120-018-0863-5
74. Li J, Li S, Zhang T, Yu L, Wei J, Wu M, et al. Label-free serum proteomics for the identification of the putative biomarkers of postoperative pain in patients with gastric cancer. Mol Omics. (2023) 19(4):351–61. doi: 10.1039/D2MO00296E
75. Li S, Toneman MK, Diatchenko L, Parisien M, Vissers KCP, Ten Broek RPG, et al. Genome-wide association study on chronic postsurgical pain in the UK Biobank. Br J Anaesth. (2025) 134(3):783–92. doi: 10.1016/j.bja.2024.12.008
76. Ehrlich O, Lackowski A, Glover TL, Vallerand AH. Use of goals in cancer pain management: a systematic review. J Pain Symptom Manage. (2024) 68(3):e194–205. doi: 10.1016/j.jpainsymman.2024.05.026
77. Geum MJ, Ahn JH, Kim JS, Kim SH, Son ES, Hu YJ, et al. Interprofessional collaboration between a multidisciplinary palliative care team and the team pharmacist on pain management. Am J Hosp Palliat Care. (2019) 36(7):616–22. doi: 10.1177/1049909119829047
78. Ablin JN. Nociplastic pain: a critical paradigm for multidisciplinary recognition and management. J Clin Med. (2024) 13(19):5741. doi: 10.3390/jcm13195741
79. Hinneburg J, Zacher S, Berger-Höger B, Berger-Thürmel K, Kratzer V, Steckelberg A, et al. Enhancing transsectoral interdisciplinary patient-centered care for patients with rare cancers: protocol for a mixed methods process evaluation. JMIR Res Protoc. (2023) 12:e49731. doi: 10.2196/49731
80. Yang CT, Lai ZZ, Zheng ZH, Kang JM, Xian M, Wang RY, et al. A novel pH-controlled hydrogen sulfide donor protects gastric mucosa from aspirin-induced injury. J Cell Mol Med. (2017) 21(10):2441–51. doi: 10.1111/jcmm.13166
81. Robinson CL, D’Souza RS, Yazdi C, Diejomaoh EM, Schatman ME, Emerick T, et al. Reviewing the potential role of artificial intelligence in delivering personalized and interactive pain medicine education for chronic pain patients. J Pain Res. (2024) 17:923–9. doi: 10.2147/JPR.S439452
82. Tétreault P, Besserer-Offroy É, Brouillette RL, René A, Murza A, Fanelli R, et al. Pain relief devoid of opioid side effects following central action of a silylated neurotensin analog. Eur J Pharmacol. (2020) 882:173174. doi: 10.1016/j.ejphar.2020.173174
83. Sharma VK, Mamontov E, Tyagi M. Effects of NSAIDs on the nanoscopic dynamics of lipid membrane. Biochim Biophys Acta Biomembr. (2020) 1862(2):183100. doi: 10.1016/j.bbamem.2019.183100
Keywords: stomach neoplasms, analgesia, pain management, pain mechanisms, clinical studies as topic
Citation: Li L, Zhao Y, Chen H, Zhao J, Dai M, Wang Q, Lv J and Wang W (2025) Insights and progress on postoperative analgesia of radical gastrectomy for gastric cancer: a comprehensive review. Front. Pain Res. 6:1601220. doi: 10.3389/fpain.2025.1601220
Received: 3 April 2025; Accepted: 17 June 2025;
Published: 30 June 2025.
Edited by:
Caroline M. Speksnijder, University Medical Center Utrecht, NetherlandsReviewed by:
Giacomo Rossettini, University of Verona, ItalyEngin Ihsan Turan, Istanbul Kanuni Sultan Süleyman Eğitim ve Araştırma Hastanesi, Türkiye
Copyright: © 2025 Li, Zhao, Chen, Zhao, Dai, Wang, Lv and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Wang, d2FuZ3dlaTIwMjRAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Linghui Li
Linghui Li Yuanyuan Zhao
Yuanyuan Zhao Huan Chen1,†
Huan Chen1,† Qi Wang
Qi Wang Wei Wang
Wei Wang