- 1Department of Anesthesiology and Perioperative Medicine, Division of Pain Medicine, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 2Department of Hematology and Oncology, University Hospitals Cleveland Medical Center, Seidman Cancer Center, Cleveland, OH, United States
- 3Case Western Reserve University, School of Medicine, Cleveland, OH, United States
- 4Department of Hospice and Palliative Medicine, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
- 5Department of Neurological Surgery, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
Chemotherapy-induced peripheral neuropathy (CIPN) presents a growing medical and financial burden on patients and the healthcare system alike. This has been treated with conservative and interventional care limited by efficacy, side effects, and lack of coverage. As such, there is an unmet treatment need for effective non-invasive or minimally invasive therapies for the treatment of CIPN. Scrambler therapy (ST) is a peripheral, non-invasive neuromodulation technique, which uses transcutaneous electrical stimulation to modulate pain signals. ST has shown mixed results in clinical trials; while some patients report symptom relief, more robust evidence is required before it can be widely recommended. This review article outlines the burden of CIPN and the current state of treatment, including pharmacological and interventional therapies. The emerging data on ST and its role in treating CIPN is highlighted, including a review of published observational and randomized controlled trials. We also discuss the gaps and challenges ahead in establishing this therapy as a standard of care.
Introduction
Chemotherapy-induced peripheral neuropathy (CIPN) is a dose-limiting side effect of several cancer chemotherapeutic agents that profoundly impacts quality of life and survivorship (1). Symptoms may arise from a single high-dose exposure or from cumulative effects of chemotherapy, often necessitating dose adjustments or early treatment discontinuation, which can worsen oncologic outcomes (2). The incidence depends on the drug, dose, combinations, duration, pre-existing diseases such as diabetes or chronic kidney disease, and individual susceptibility. Higher incidence rates have been seen with platinum-based compounds, vinca alkaloids, taxanes, and proteasome inhibitors with platinum agents being most neurotoxic (3). The incidence of CIPN is approximately 50%–90%. More than 80% of cancer survivors develop acute CIPN, which decreases patient tolerance to treatment (4). The incidence of CIPN approaches nearly 100% for some agents at higher doses, although differences in the definition, evaluation and reporting can lead to large variability in the reported occurrence (5). The prevalence of CIPN ranges from 19% to more than 85% (6).
CIPN primarily affects the peripheral nervous system, leading to sensory, motor, and autonomic dysfunction. The pathology differs based on the type of chemotherapy used. Platinum-based agents cause axonopathy, with preferential damage to large, myelinated sensory fibers in the dorsal root ganglia (5). This leads to stocking-glove sensory loss and neuropathic pain. Taxanes lead to microtubule dysfunction, impairing axonal transport, leading to distal sensory loss and pain (7). Bortezomib, a proteasome inhibitor, induces mitochondrial and endoplasmic reticulum stress, resulting in painful, distal sensory neuropathy (8).
In addition to the detrimental effects on pain and function, there is a significant economic burden associated with the development of CIPN. Patients with CIPN have significantly higher utilization of healthcare resources, including an increased chance of hospitalization, increased visits to the emergency department or outpatient clinic, and higher medication utilization (9). One database study of privately insured administrative claims records found that patients with CIPN on average had $17,344 (USD) higher healthcare costs during a 12-month period compared to patients without CIPN (10). In addition to higher direct costs related to treatment, indirect costs are also higher due to patient and caregiver work loss and disability costs (11). For example, pain, paresthesias and weakness in hands and/or feet secondary may lead to impaired dexterity, trouble feeling or manipulating objects, and difficulties ambulating—all of these factors may negatively impact a patient's ability to return to work or perform work-related tasks (12, 13).
The current state of management for chemotherapy-induced peripheral neuropathy
The standard of care for managing CIPN encompasses both preventive strategies and therapeutic interventions. No pharmacologic agents have demonstrated consistent efficacy in preventing CIPN. The American Society of Clinical Oncology (ASCO) advises against the use of several agents for CIPN prevention due to insufficient evidence, including acetyl-L-carnitine, amitriptyline, gabapentin/pregabalin, and calcium-magnesium (14).
ASCO advises clinicians to evaluate and discuss with patients the potential benefits of delaying, reducing, or discontinuing chemotherapy or switching to alternative agents, when patients experience severe CIPN (15). ASCO recommends duloxetine as the primary pharmacologic treatment for established CIPN. Clinical trials have shown that duloxetine can reduce neuropathic pain associated with CIPN (14). As a serotonin-norepinephrine reuptake inhibitor, duloxetine enhances the availability of the key neurotransmitters involved in activating the descending pain-modulating pathway (16). It may also reduce inflammation and nerve injury by inhibiting the activation of p38 and NF-kB (17). While tricyclic antidepressants and anticonvulsants are used for neuropathic pain, their efficacy in treating CIPN remains unproven. ASCO does not recommend these agents for CIPN treatment due to limited supporting evidence (14).
Several complementary and alternative medicines have been tested for treating CIPN. Exercise, acupuncture, mindfulness practices, yoga, meditation, and touch therapies like acupressure, reflexology, and massage, have been found to reduce CIPN symptoms and improve quality of life (4). Engaging in regular physical activity including stretching, walking, resistance training, and balance exercises, may help alleviate CIPN symptoms and improve functional outcomes (18). In addition, some nutrients and herbal medicines have shown potential therapeutic effects in patients with neuropathic pain (19).
The use of intrathecal pain therapies has a storied history in the treatment of refractory cancer pain (20), and both morphine and ziconotide are FDA-approved agents. The pivotal studies which formed the basis for FDA-approval reported on the treatment of refractory cancer pain, with most patients having mixed neuropathic and nociceptive pain (21, 22). It has been suggested that cancer patients with a life expectancy of 3–6 months or greater are appropriate candidates; however, its use has generally fallen out of favor due to changing practice patterns, as well as the need for frequent pump refills and potential device-related complications. Furthermore, there is no literature on the use of intrathecal therapy in the treatment of CIPN specifically, with most studies and current practice being focused on nociceptive or mixed pain.
There is significant enthusiasm for the use of spinal cord stimulation (SCS) in the treatment of CIPN. Preclinical studies have been supportive of its use in attenuating pain, such as demonstrating the inhibition of gait impairment and paclitaxel-induced mechanical and cold hypersensitivity in rats (23, 24). There are numerous case reports and case series supporting the use of SCS and dorsal root ganglion stimulation in the treatment of CIPN and other painful polyneuropathies (25, 26); however, its use is currently considered off-label. This is in contrast to the treatment of painful diabetic neuropathy, which is supported by level I evidence and is FDA-approved (27). Altogether, this suggests that SCS is a viable and potentially effective treatment option for CIPN, although more robust data are needed.
CIPN is both an acute and chronic complication of chemotherapy, with many patients developing lasting and debilitating pain (28). The current standard of care for treatment relies on a multimodal approach primarily consisting of medications, although side effect profiles, tolerance and dependency remain significant challenges. Although implantable neuromodulation devices have demonstrated efficacy, there is no robust data for either intrathecal therapy or SCS, and furthermore patients may not want to undergo a surgical procedure for pain control. As such, there is an unmet treatment need for non-invasive or minimally invasive therapies for the treatment of CIPN.
Scrambler therapy: mechanisms and indications
Scrambler therapy (ST), an electro-analgesia therapy, is a non-invasive treatment for chronic neuropathic and cancer-related pain (Figure 1). ST uses an algorithm to generate painless stimulation programs, which are then transmitted to the central nervous system transcutaneously (29, 30). The principle behind ST lies in its ability to disrupt the transmission of pain signals by replacing them with artificial pain-free signals (31). The mechanism of action of ST is not fully understood, but several theories have been proposed. The inventor of ST believes it works by stimulating C fiber surface receptors, which are responsible for transmitting sensory information, including pain, to the brain (29, 30). By delivering electrical stimulation through these receptors, ST may be able to “override” the pain signals, effectively blocking their transmission and reducing the patient's perception of pain. Another theory suggests that ST induces neuroplastic changes in the brain, essentially retraining the nervous system to interpret the signals from the affected area as non-painful through a series of repeated treatments (32). It is believed that ST alters pain perception at the brain level to relieve pain (33).
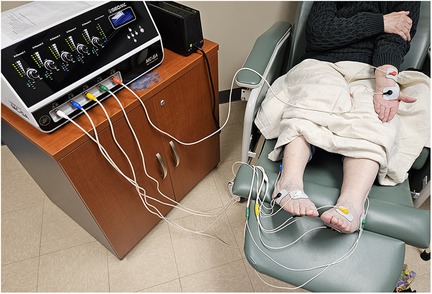
Figure 1. Example of the hardware and patient setup for scrambler therapy. Electrodes are applied to the painful areas to be treated.
ST is typically administered in a series of daily sessions, lasting approximately 30–60 min. Electrodes are placed on the skin at the pain site and along corresponding dermatomes. A low-intensity electrical current is carefully calibrated by the operator to ensure appropriate non-painful stimulation. ST has demonstrated effectiveness in alleviating various types of neuropathic pain, including post-herpetic neuralgia, diabetic neuropathy, and complex regional pain syndrome (34). Furthermore, ST has been explored as a potential treatment for cancer-related pain, specifically CIPN (29, 30).
The indications for ST continue to evolve, but it is generally considered an option for patients with chronic neuropathic and cancer-related pain. Appropriate patient selection is critical for successful outcomes, as individuals with certain underlying conditions or psychological factors may be less likely to respond to the treatment. ST does seem very promising for the treatment of difficult types and patterns of pain, although it is not a cure for chronic pain. Some patients may experience a recurrence of pain after a period, requiring additional treatment sessions to maintain the benefits (35).
Theoretically, there is risk of seizure with lead placement across the head, especially in patients with pre-existing seizure disorders. There may be risk of arrhythmia if placed across the heart, particularly if patients have a history of arrhythmia. But no such cases of seizures or arrhythmia have been reported in the literature. Leads should not be placed across or near implanted stimulation devices such as pacemakers, defibrillators, and SCSs. If necessary, the provider should consider placing these devices in surgical mode and arranging appropriate device management as needed should leads need to be placed in close proximity. ST initiation should be carefully set to avoid painful overstimulation, especially in allodynic or hypersensitive patients. A meta-analysis reported rare cases of contact dermatitis and minor ecchymosis at lead placement sites (47). ST safety has not been evaluated in patients who are pregnant or nursing.
The role of scrambler therapy in chemotherapy-induced peripheral neuropathy
ST is being studied and utilized as a means of reducing CIPN symptoms in the extremities, such as burning pain, dysesthesias, numbness, tingling, and motor dysfunction. By reducing these symptoms, patients can ideally lead more normal lives with less pain, improved sleep, and better function of their extremities. ST is primarily performed on patients who have completed chemotherapy but continue to have CIPN symptoms for months-to-years following treatment. While protocols vary, most institutions utilizing this therapy for CIPN offer 10 treatment sessions over the course of 2 weeks and will subsequently monitor for efficacy and duration of relief (36). If symptoms do improve significantly, but recur later, additional treatments may be considered at the discretion of the provider.
ST represents a novel, non-invasive, low-risk treatment option for those with lasting neuropathic symptoms after completion of their cancer treatment. Despite FDA-approval in 2009, Medicare and most commercial payers do not reimburse ST. Most patients pay a cash price per session or series of sessions. There are a small, but growing number of pain centers in the United States that offer ST. Many patients self-refer while others are recommended by an increasing number of specialists, particularly oncologists managing chemotherapy. Having more effective treatment options may allow some patients to maintain their chemotherapeutic regimen with less concern for debilitating neuropathic pain afterwards. Currently there is no research evaluating the neuroprotective effect on patients who are actively undergoing chemotherapy, but this could be a direction for future study.
Current evidence for scrambler therapy
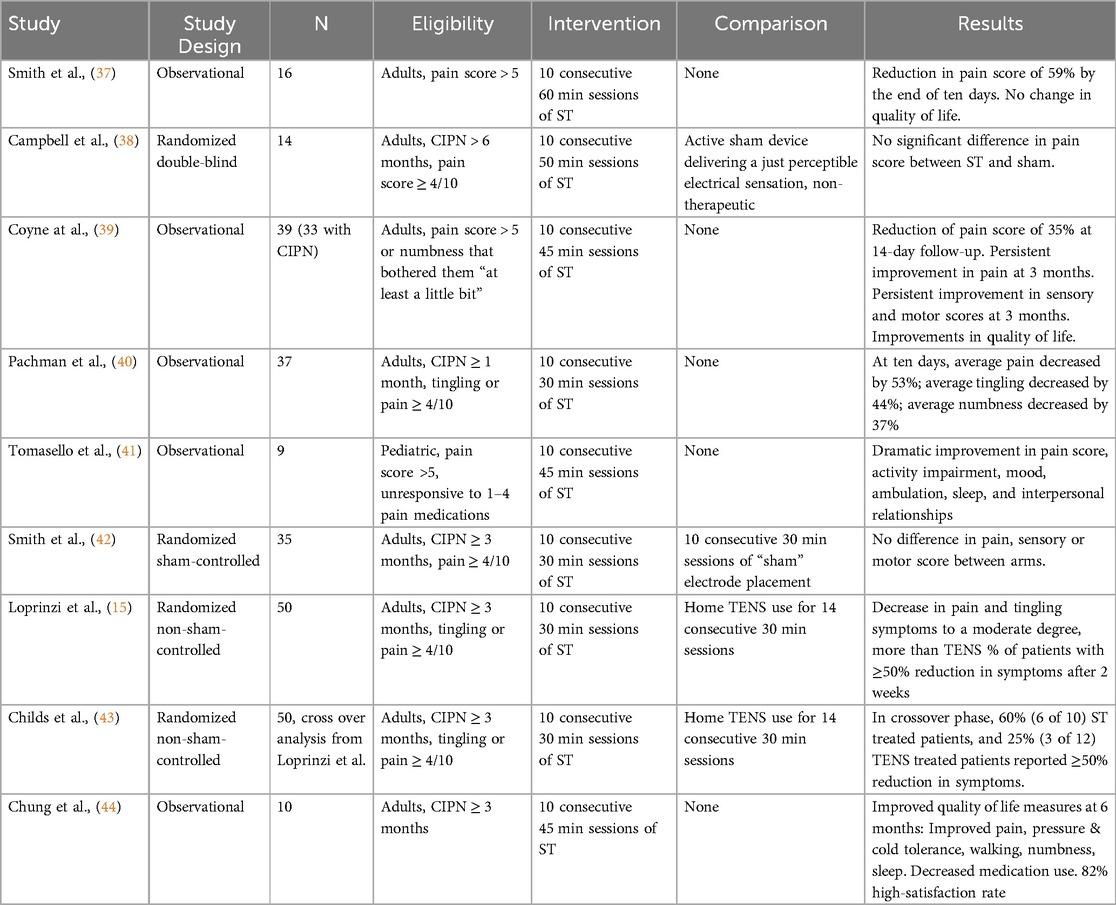
The role of ST in treating CIPN has been investigated in a limited number of pilot and randomized controlled trials, which are summarized in Table 1.
The first published trial evaluating the use of ST for the treatment of CIPN was published in 2010 (37). In this pilot study, 16 patients with CIPN received 10 daily one-hour ST treatments (37). There was a reduction in pain score by 59% by the end of treatment course. Despite these significant findings, most patients reported that their pain returned to pre-treatment levels 1 or 2 months after the end of therapy. There was also no change in quality-of-life metrics.
Two additional studies were published in 2013. Campbell et al. reported the first randomized double blind trial comparing ST to a novel active sham device designed to deliver a just perceptible electrical sensation (38). 14 patients were randomized to receive one of the two treatments for 10 daily 50 min sessions. There was no significant difference in the primary endpoint of change in pain between the treatment arms. Conversely, Coyne et al. found improvement in pain score at 14 days, 1 month, 2 month and 3 months of follow-up in an observational study (39). They also noted improvements in the sensory and motor components of CIPN, as well as improvement in general activity, mood, sleep and enjoyment of life.
In another study, 37 patients with CIPN received consecutive daily 30 min ST sessions for 10 days, after which their average pain score decreased by 52%. These patients' tingling score reduced by 44% and numbness score by 37%. Patients also reported improved quality of life over the course of treatment and at ten weeks follow-up (40).
Another pilot study randomized patients to ST or sham placement of electrodes. There was no significant difference in pain between the ST group and the sham group at 10, 28, 60 or 90 days, with small non-significant improvement in pain in both arms. Both arms demonstrated improvement in sensory and motor scores, suggesting the sham electrode placement may have had some therapeutic effect (42).
Loprinzi et al. compared ST to trans-electrical nerve stimulation (TENS) with 50 patients randomized to receive either two weeks of ST or two weeks of TENS therapy (15). A greater percentage of patients of receiving ST reported at least 50% reduction in pain scores and tingling scores compared to patients who received TENS (56% vs. 28% and 48% vs. 24%, respectively). More patients receiving ST were more likely to recommend the treatment they received than patients receiving TENS. This cohort of patients was further evaluated in a crossover analysis, where 22 of the 50 patients proceeded to the crossover phase. Patients were observed for 8 weeks then were allowed to cross over for an additional 10 weeks (2 weeks of treatment and 8 weeks of observation). The same trend was noted during the cross over phase of this trial, with 60% of the ST patients reaching ≥50% reduction in symptoms compared to only 25% of the TENS treated patients (43).
A pilot study showed ST may have a meaningful impact on quality-of-life measures impaired by CIPN. Patients were followed for 6 months post-ST. In addition to decreased pain, they displayed improved pressure and cold tolerance and improved ambulation. Patients exhibited decreased numbness and tingling and improved sleep. 82% of patients expressed high satisfaction and all denied adverse events (44).
Little is known about ST in pediatric patients. A study on 9 adolescents between the ages of 12 and 17 showed promise in treating pediatric CIPN. ST resulted in dramatic improvements in pain score, activity impairment, mood, ambulation, sleep, and interpersonal relationships (41).
There are many recurrent limitations across these trials, including low enrollment, short follow-up, and lack of a control arm. These studies have demonstrated mixed results, emphasizing the need for further research to understand the role of ST in the management of CIPN.
The future of scrambler therapy
CIPN can only be expected to become more prevalent as the population ages, cancer rates increase, and more patients receive chemotherapy. Patients with CIPN require solutions beyond medications with high side effect profiles. There are ongoing studies worldwide investigating wearable therapies, cryotherapy, and novel medications. ST has proven potential and is likely to play a growing role in the future of CIPN treatment. Compared to many medications, it does not carry a significant systemic side effect profile. Compared to implantable therapies such as SCS or intrathecal therapy, ST carries less potential risk as a transcutaneous treatment as well as lower overall cost. Collaboration with relevant specialists will be key to improving access to this therapy. Oncologists, neurologists, pain specialists, and physiotherapists should familiarize themselves with ST to provide the right resources for patients.
A challenge of ST therapy is the lack of standardization. Studies comparing session length such as 30 or 60 min may provide more uniform care and results. Likewise, investigations could clarify if less or more than 10 sessions are necessary for a clinically significant result. These results will contribute to a more standardized, replicable therapy (45). One of the inconveniences of ST is the time commitment required. Identifying the most effective number and length of sessions may expand access of this therapy. Future studies may show less and/or shorter sessions are required for clinically meaningful relief, but that evidence does not exist at this time. Successful, large-scale randomized control trials may help achieve Medicare and commercial payer coverage for ST. Currently, there is no evidence suggesting males or females are more responsive to ST. Further study in the pediatric population is necessary as well.
ST has potential in treating certain aspects of CIPN. Studies could further investigate its potential efficacy in treating numbness compared to pain compared to motor symptoms. Studies could be tailored towards specific chemotherapeutic agents, which could better prognosticate ST efficacy (46). ST should be evaluated for its role in treating CIPN during chemotherapy for acute pain relief, or potentially as a prophylactic therapy for the prevention of CIPN.
The time investment on behalf of the patient and possible need for repeat therapy leave questions on dosing and durability that remain unanswered. Nevertheless, ST is associated with minimal side effects and invasiveness, and its promising efficacy makes it an attractive and growing therapy, even if it is unlikely to be standalone. As studies continue, ST will likely serve as an adjunct or part of a multi-modal approach to treating CIPN. These nuances will have to be tailored to the individual patient as more data continues to emerge.
Conclusion
ST offers a low-risk option for patients who have already known significant risk from their cancer diagnosis through chemotherapy and resultant CIPN. Many patients are tired of expensive medications with variable efficacy and high side effect profiles. Others are not interested in more invasive procedures after the ordeal of cancer treatment. ST is non-invasive and shows significant promise in treating CIPN. As studies continue, treatments should become more standardized, as should our ability to better prognosticate success and durability.
Author contributions
HA: Project administration, Writing – original draft, Writing – review & editing. HV: Writing – original draft. AG: Writing – original draft. BM: Writing – original draft. MG: Writing – original draft. SR: Writing – review & editing. MS: Conceptualization, Funding acquisition, Project administration, Resources, Supervision, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Publication was supported by the George R. and Constance P. Lincoln Chair in Brain Health, which is an endowed chair for Michael D. Staudt.
Conflict of interest
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper. Staudt is a consultant for Boston Scientific, Curonix, Medtronic, NeuroOne, and Nevro, outside the submitted work. Aboumerhi is a consultant for SPR Therapeutics, outside the submitted work. Vucetic is a consultant for Abbott, Boston Scientific, Medtronic, Saluda, SPR Therapeutics and Stryker, outside the submitted work.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Mols F, Beijers T, Vreugdenhil G, van de Poll-Franse L. Chemotherapy-induced peripheral neuropathy and its association with quality of life: a systematic review. Support Care Cancer. (2014) 22(8):2261–9. doi: 10.1007/s00520-014-2255-7
2. Zajaczkowska R, Kocot-Kepska M, Leppert W, Wrzosek A, Mika J, Wordliczek J. Mechanisms of chemotherapy-induced peripheral neuropathy. Int J Mol Sci. (2019) 20(6):1451. doi: 10.3390/ijms20061451
3. Burgess J, Ferdousi M, Gosal D, Boon C, Matsumoto K, Marshall A, et al. Chemotherapy-induced peripheral neuropathy: epidemiology, pathomechanisms and treatment. Oncol Ther. (2021) 9(2):385–450. doi: 10.1007/s40487-021-00168-y
4. Gu J, Zhang H, Hu M, Liu L, Chen C, Wang J, et al. Complementary and alternative medicine in relation to chemotherapy-induced peripheral neuropathy: a narrative review. Explore (NY). (2024) 20(2):181–7. doi: 10.1016/j.explore.2023.08.010
5. Starobova H, Vetter I. Pathophysiology of chemotherapy-induced peripheral neuropathy. Front Mol Neurosci. (2017) 10:174. doi: 10.3389/fnmol.2017.00174
6. Mattar M, Umutoni F, Hassan MA, Wamburu MW, Turner R, Patton JS, et al. Chemotherapy-induced peripheral neuropathy: a recent update on pathophysiology and treatment. Life (Basel). (2024) 14(8):991. doi: 10.3390/life14080991
7. Sisignano M, Baron R, Scholich K, Geisslinger G. Mechanism-based treatment for chemotherapy-induced peripheral neuropathic pain. Nat Rev Neurol. (2014) 10(12):694–707. doi: 10.1038/nrneurol.2014.211
8. Cavaletti G, Marmiroli P. Chemotherapy-induced peripheral neurotoxicity. Nat Rev Neurol. (2010) 6(12):657–66. doi: 10.1038/nrneurol.2010.160
9. Song X, Wilson KL, Kagan J, Panjabi S. Cost of peripheral neuropathy in patients receiving treatment for multiple myeloma: a US administrative claims analysis. Ther Adv Hematol. (2019) 10:2040620719839025. doi: 10.1177/2040620719839025
10. Pike CT, Birnbaum HG, Muehlenbein CE, Pohl GM, Natale RB. Healthcare costs and workloss burden of patients with chemotherapy-associated peripheral neuropathy in breast, ovarian, head and neck, and nonsmall cell lung cancer. Chemother Res Pract. (2012) 2012:913848. doi: 10.1155/2012/913848
11. Calhoun EA, Chang CH, Welshman EE, Fishman DA, Lurain JR, Bennett CL. Evaluating the total costs of chemotherapy-induced toxicity: results from a pilot study with ovarian cancer patients. Oncologist. (2001) 6(5):441–5. doi: 10.1634/theoncologist.6-5-441
12. Zanville NR, Nudelman KN, Smith DJ, Von Ah D, McDonald BC, Champion VL, et al. Evaluating the impact of chemotherapy-induced peripheral neuropathy symptoms (CIPN-sx) on perceived ability to work in breast cancer survivors during the first year post-treatment. Support Care Cancer. (2016) 24(11):4779–89. doi: 10.1007/s00520-016-3329-5
13. Manfuku M, Inoue J, Yamanaka N, Kanamori H, Sumiyoshi K, Osumi M. Effects of taxane-induced peripheral neuropathy on hand dexterity impairment: evaluation of quantitative and subjective assessments. Support Care Cancer. (2024) 32(5):304. doi: 10.1007/s00520-024-08504-4
14. Loprinzi CL, Lacchetti C, Bleeker J, Cavaletti G, Chauhan C, Hertz DL, et al. Prevention and management of chemotherapy-induced peripheral neuropathy in survivors of adult cancers: ASCO guideline update. J Clin Oncol. (2020b) 38(28):3325–48. doi: 10.1200/JCO.20.01399
15. Loprinzi C, Le-Rademacher JG, Majithia N, McMurray RP, O'Neill CR, Bendel MA, et al. Scrambler therapy for chemotherapy neuropathy: a randomized phase II pilot trial. Support Care Cancer. (2020) 28(3):1183–97. doi: 10.1007/s00520-019-04881-3
16. Schuessler B. What do we know about duloxetine’s mode of action? evidence from animals to humans. BJOG. (2006) 113(Suppl 1):5–9. doi: 10.1111/j.1471-0528.2006.00877.x
17. Meng J, Zhang Q, Yang C, Xiao L, Xue Z, Zhu J. Duloxetine, a balanced serotonin-norepinephrine reuptake inhibitor, improves painful chemotherapy-induced peripheral neuropathy by inhibiting activation of p38 MAPK and NF-kappaB. Front Pharmacol. (2019) 10:365. doi: 10.3389/fphar.2019.00365
18. Tanay MAL, Armes J, Moss-Morris R, Rafferty AM, Robert G. A systematic review of behavioural and exercise interventions for the prevention and management of chemotherapy-induced peripheral neuropathy symptoms. J Cancer Surviv. (2023) 17(1):254–77. doi: 10.1007/s11764-021-00997-w
19. Klafke N, Bossert J, Kroger B, Neuberger P, Heyder U, Layer M, et al. Prevention and treatment of chemotherapy-induced peripheral neuropathy (CIPN) with non-pharmacological interventions: clinical recommendations from a systematic scoping review and an expert consensus process. Med Sci (Basel). (2023) 11(1):15. doi: 10.3390/medsci11010015
20. Chalil A, Staudt MD, Harland TA, Leimer EM, Bhullar R, Argoff CE. A safety review of approved intrathecal analgesics for chronic pain management. Expert Opin Drug Saf. (2021) 20(4):439–51. doi: 10.1080/14740338.2021.1889513
21. Smith TJ, Staats PS, Deer T, Stearns LJ, Rauck RL, Boortz-Marx RL, et al. Randomized clinical trial of an implantable drug delivery system compared with comprehensive medical management for refractory cancer pain: impact on pain, drug-related toxicity, and survival. J Clin Oncol. (2002) 20(19):4040–9. doi: 10.1200/JCO.2002.02.118
22. Staats PS, Yearwood T, Charapata SG, Presley RW, Wallace MS, Byas-Smith M, et al. Intrathecal ziconotide in the treatment of refractory pain in patients with cancer or AIDS: a randomized controlled trial. JAMA. (2004) 291(1):63–70. doi: 10.1001/jama.291.1.63
23. Sivanesan E, Stephens KE, Huang Q, Chen Z, Ford NC, Duan W, et al. Spinal cord stimulation prevents paclitaxel-induced mechanical and cold hypersensitivity and modulates spinal gene expression in rats. Pain Rep. (2019) 4(5):e785. doi: 10.1097/PR9.0000000000000785
24. Bakare AO, Stephens K, Sanchez KR, Liu V, Zheng L, Goel V, et al. Spinal cord stimulation attenuates paclitaxel-induced gait impairment and mechanical hypersensitivity via peripheral neuroprotective mechanisms in tumor-bearing rats. Reg Anesth Pain Med. (2024). doi: 10.1136/rapm-2024-105433
25. D’Souza RS, Her YF, Jin MY, Morsi M, Abd-Elsayed A. Neuromodulation therapy for chemotherapy-induced peripheral neuropathy: a systematic review. Biomedicines. (2022) 10(8):1909. doi: 10.3390/biomedicines10081909
26. Canos-Verdecho A, Bermejo A, Castel B, Izquierdo R, Robledo R, Gallach E, et al. Effects of spinal cord stimulation in patients with small fiber and associated comorbidities from neuropathy after multiple etiologies. J Clin Med. (2025) 14(2):652. doi: 10.3390/jcm14020652
27. Petersen EA, Stauss TG, Scowcroft JA, Brooks ES, White JL, Sills SM, et al. Effect of high-frequency (10-kHz) spinal cord stimulation in patients with painful diabetic neuropathy: a randomized clinical trial. JAMA Neurol. (2021) 78(6):687–98. doi: 10.1001/jamaneurol.2021.0538
28. Seretny M, Currie GL, Sena ES, Ramnarine S, Grant R, MacLeod MR, et al. Incidence, prevalence, and predictors of chemotherapy-induced peripheral neuropathy: a systematic review and meta-analysis. Pain. (2014) 155(12):2461–70. doi: 10.1016/j.pain.2014.09.020
29. Marineo G. Inside the scrambler therapy, a noninvasive treatment of chronic neuropathic and cancer pain: from the gate control theory to the active principle of information. Integr Cancer Ther. (2019) 18:1534735419845143. doi: 10.1177/1534735419845143
30. Medina R, Ho A, Reddy R, Chen J, Castellanos J. Narrative review of current neuromodulation modalities for spinal cord injury. Front Pain Res (Lausanne). (2023) 4:1143405. doi: 10.3389/fpain.2023.1143405
31. Zhang TC, Janik JJ, Grill WM. Mechanisms and models of spinal cord stimulation for the treatment of neuropathic pain. Brain Res. (2014) 1569:19–31. doi: 10.1016/j.brainres.2014.04.039
32. Majithia N, Smith TJ, Coyne PJ, Abdi S, Pachman DR, Lachance D, et al. Scrambler therapy for the management of chronic pain. Support Care Cancer. (2016) 24(6):2807–14. doi: 10.1007/s00520-016-3177-3
33. Congedi S, Spadini S, Di Pede C, Ometto M, Franceschi T, De Tommasi V, et al. Use of scrambler therapy in acute paediatric pain: a case report and review of the literature. Case Rep Pediatr. (2016) 2016:2628919. doi: 10.1155/2016/2628919
34. Lesenskyj AM, Maxwell CR, Brown S, Cruciani RA. A review of scrambler therapy for chronic neuropathic pain. J Pain Relief. (2016) 5(5):260. doi: 10.4172/2167-0846.1000260
35. Kashyap K, Vig S, Bhan S, Bhatnagar S. Scrambler therapy—a novel treatment approach for chronic postoperative pain. J Anaesthesiol Clin Pharmacol. (2020) 36(2):276–8. doi: 10.4103/joacp.JOACP_11_19
36. Marineo G, Iorno V, Gandini C, Moschini V, Smith TJ. Scrambler therapy may relieve chronic neuropathic pain more effectively than guideline-based drug management: results of a pilot, randomized, controlled trial. J Pain Symptom Manage. (2012) 43(1):87–95. doi: 10.1016/j.jpainsymman.2011.03.015
37. Smith TJ, Coyne PJ, Parker GL, Dodson P, Ramakrishnan V. Pilot trial of a patient-specific cutaneous electrostimulation device [MC5-A Calmare(R)] for chemotherapy-induced peripheral neuropathy. J Pain Symptom Manage. (2010) 40(6):883–91. doi: 10.1016/j.jpainsymman.2010.03.022
38. Campbell TC, Nimunkar AJ, Retseck J, Eickhoff JC, Backonja M, Cleary JF, et al. A randomized, double-blind study of “Scrambler” therapy versus sham for painful chemotherapy-induced peripheral neuropathy (CIPN). J Clin Oncol. (2013) 31(15):9635–9635. doi: 10.1200/jco.2013.31.15_suppl.9635
39. Coyne PJ, Wan W, Dodson P, Swainey C, Smith TJ. A trial of Scrambler therapy in the treatment of cancer pain syndromes and chronic chemotherapy-induced peripheral neuropathy. J Pain Palliat Care Pharmacother. (2013) 27(4):359–64. doi: 10.3109/15360288.2013.847519
40. Pachman DR, Weisbrod BL, Seisler DK, Barton DL, Fee-Schroeder KC, Smith TJ, et al. Pilot evaluation of Scrambler therapy for the treatment of chemotherapy-induced peripheral neuropathy. Support Care Cancer. (2015) 23(4):943–51. doi: 10.1007/s00520-014-2424-8
41. Tomasello C, Pinto RM, Mennini C, Conicella E, Stoppa F, Raucci U. Scrambler therapy efficacy and safety for neuropathic pain correlated with chemotherapy-induced peripheral neuropathy in adolescents: a preliminary study. Pediatr Blood Cancer. (2018) 65(7):e27064. doi: 10.1002/pbc.27064
42. Smith TJ, Razzak AR, Blackford AL, Ensminger J, Saiki C, Longo-Schoberlein D, et al. A pilot randomized sham-controlled trial of MC5-A scrambler therapy in the treatment of chronic chemotherapy-induced peripheral neuropathy (CIPN). J Palliat Care. (2020) 35(1):53–8. doi: 10.1177/0825859719827589
43. Childs DS, Le-Rademacher JG, McMurray R, Bendel M, O'Neill C, Smith TJ, et al. Randomized trial of scrambler therapy for chemotherapy-induced peripheral neuropathy: crossover analysis. J Pain Symptom Manage. (2021) 61(6):1247–53. doi: 10.1016/j.jpainsymman.2020.11.025
44. Chung M, Chen TH, Wang XS, Kim KH, Abdi S. The impact of scrambler therapy on pain and quality of life for chemotherapy-induced peripheral neuropathy: a pilot study. Pain Pract. (2024) 24(5):749–59. doi: 10.1111/papr.13355
45. Ricci M, Fabbri L, Pirotti S, Ruffilli N, Foca F, Maltoni M. Scrambler therapy: what’s new after 15 years? The results from 219 patients treated for chronic pain. Medicine (Baltimore). (2019) 98(2):e13895. doi: 10.1097/MD.0000000000013895
46. Jones KF, Wechsler S, Zulewski D, Wood L. Pharmacological and nonpharmacological management of chemotherapy-induced peripheral neuropathy: a scoping review of randomized controlled trials. J Palliat Med. (2022) 25(6):964–95. doi: 10.1089/jpm.2021.0512
Keywords: cancer, chemotherapy-induced peripheral neuropathy, chronic pain, neuropathic pain, neuropathy, review, scrambler therapy
Citation: Aboumerhi H, Vucetic H, Gruenzel A, Moftakhar B, Gupta M, Rao SK and Staudt MD (2025) The treatment of chemotherapy-induced peripheral neuropathy: a review of current management options and a potential role for scrambler therapy. Front. Pain Res. 6:1607102. doi: 10.3389/fpain.2025.1607102
Received: 7 April 2025; Accepted: 23 June 2025;
Published: 7 July 2025.
Edited by:
Anand Singh, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Panjamurthy Kuppusamy, University of Maryland, United StatesAjeena Ramanujan, University of Texas MD Anderson Cancer Center, United States
Copyright: © 2025 Aboumerhi, Vucetic, Gruenzel, Moftakhar, Gupta, Rao and Staudt. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael D. Staudt, TWljaGFlbC5TdGF1ZHRAVUhob3NwaXRhbHMub3Jn
 Hassan Aboumerhi
Hassan Aboumerhi Henry Vucetic
Henry Vucetic Andrew Gruenzel1
Andrew Gruenzel1 Michael D. Staudt
Michael D. Staudt