- 1Department of Emergency Medicine, Augusta University, Augusta GA, United States
- 2Harmonic Scientific LLC, Lewes, DE, United States
- 3Kaizo Clinical Research Institute, Landover, MD, United States
- 4Department of Health Policy and Administration, Penn State College, University Park, PA, United States
- 5Department of Psychology, Georgia State University, Atlanta, GA, United States
- 6Department of Statistics and Analytical Sciences, Kennesaw State University, Kennesaw, GA, United States
Background: Low back pain (LBP) is the most common reason for outpatient opioid prescribing: a quarter of patients receive prescriptions, leading to opioid use disorder (OUD) in 5%. Guideline-recommended multimodal interventions often face implementation barriers, and effective modalities (e.g., electrical stimulation) lack coverage. A multimodal mechanical stimulation (M-Stim) device for LBP has demonstrated safety and efficacy in pain reduction, but its impact on opioid use has not yet been determined.
Methods: As part of an NIH-funded double-blind study to reduce pain and opioid use, patients with moderate-to-severe LBP presenting to two suburban chiropractic centers were randomized to receive either the M-Stim device or a transcutaneous electrical nerve stimulation (TENS) unit for 30 min daily, in addition to other therapies. Analgesic use was reported daily for 28 days, with new prescribing followed weekly for 3 months. The primary outcome was prescribing in the opioid-naïve subjects. Secondary endpoints included risk factors for prolonged use in the opioid-naïve subjects, milligram morphine equivalents (MME) for opioid users between the first and last 2 weeks, and prescribing compared with national rates.
Results: After informed consent, 159 eligible patients were randomized to M-Stim (87) or TENS (72) (mean age 42.6 years, 54% female, BMI 30.9, NRS 5.5) between 23 June 2022 and 31 December 2023. Zero opioid-naïve M-Stim participants (n = 43) received prescriptions (0% vs. 8.6%, Fisher's exact p = 0.086), and those taking opioids used significantly fewer MME [7.5 (SD 3.54) vs. 498.5 MME (SD 474.9), p < 0.0001] for fewer of reported days [M-Stim 2/47 (4.2%)] compared with TENS [n = 36, 38/102 (37%), RR 0.11 (95% CI 0.28–0.44), p = 0.0018]. M-Stim significantly reduced MME in opioid users [−44.6% (32.33 MME), p = 0.02], use days for those with BMI ≥30 [−3 (99% CI −5.73 to −0.26), p = 0.032], and prescribing compared with national rates [9.8% vs. 25%, −63%, RR 0.32 (95% CI 0.16–0.66), p = 0.002] while TENS did not.
Conclusions: Among chiropractic patients with moderate-to-severe LBP, added use of a multimodal M-Stim device in the opioid-naïve subjects significantly reduced factors associated with OUD compared with TENS and reduced use days for those with BMI ≥30. This novel device is a potential alternative to prescribing opioids as first line for LBP management.
Clinical Trial Registration: https://clinicaltrials.gov/study/NCT04491175, identifier NCT04491175.
Introduction
Low back pain (LBP) affects 80% of Americans during their lifetimes (1), 60% each year, and is estimated to impact 57 million people in North America by 2050. Although opioid prescribing has declined in response to the national emergency, LBP remains the most common reason for ambulatory opioid prescribing, with 25%–32.5% of moderate-to-severe pain patients receiving prescriptions (2–4). As with other acute pain conditions, 5% of those prescribed opioids develop prolonged use (5, 6). In an opioid-naïve population, dose, duration, and use beyond 7 days are risk factors for progression to opioid use disorder (OUD) (6, 7). Among chronic opioid users, fear of pain is the primary barrier to reducing use (8). To prevent OUD and improve LBP outcomes, better pain relief alternatives are critically needed (9–11).
While clinical guidelines recommend non-pharmacologic approaches first (12, 13), delivery of effective alternatives at point of care is problematic. Physical therapy (14), spinal manipulation (15), fascial treatments, or acupuncture require both coverage and rapid referral (3). Approaches combining multiple patient-controlled modalities are most effective (16), but non-drug interventions are not part of standard medical curricula, and explaining multimodal options to patients is time-consuming. Moreover, evidence-based non-pharmacologic LBP modalities, such as thermal treatments, yoga, transcutaneous electrical stimulation (TENS) (17, 18), and high-frequency vibratory mechanical stimulation (M-Stim) (19–24), are excluded from ambulatory coverage: Medicare explicitly denies payment for “personal comfort” pain relief interventions [section 1862(a) of the Social Security Act] (6). Thus, time, familiarity, and cost considerations contribute to physicians prescribing opioids over multimodal options (13, 16, 25).
To bridge this gap, the National Institute on Drug Abuse (NIDA) funded the development of a novel non-invasive multimodal heat, pressure, and harmonic multifrequency vibration device as an opioid alternative. In a Phase 1 pilot study (26), this M-Stim device reduced LBP by 57% after 20 min. The present double-blind active-controlled trial tested the hypothesis that moderate-to-severe LBP patients would be less likely to seek out and use opioids with a multimodal pain relief device than an established single-modality active control. The primary outcome was reduced prescribing in the opioid-naïve subjects compared with TENS over 3 months. Secondary endpoints included the reduction of risk factors associated with prolonged use in opioid-naïve subjects, including milligram morphine equivalents (MME) and days of use. Because of the potential for reduced prescribing due to dispensing any device, additional outcomes included prescribing compared with historical national rates and change in MME for opioid users tracked daily between the first and last 2 weeks.
Methods
Device description
Non-specific mechanical low back pain has no definitive radiologic findings (27) and is variably attributed to dysfunction of the paraspinal muscles, neuromotor instability, and fascial inflammation (28), with central pain sensitization and psychologic components (29, 30). The M-Stim device delivers mechanical force to the thoracolumbar field through a 6″ × 8″ thermoconductive metal plate held by a 54″ compressive neoprene belt (26). The plate was first described by Lundeberg (31), who reported that a single 100 Hz or 200 Hz motor on a 6″ × 8″ flat plate reduced low back pain more effectively than TENS. The lower frequency has since been shown to improve neuromotor reflexes and proprioception (32), while the 200 Hz Lundeberg found more effective activates spinal gating to reduce sharp pain transmission (33). To concentrate the vibratory mechanical force, curves on the M-Stim device amplify pressure on the paraspinal muscles. Three motors in harmonic frequencies (50 Hz, 100 Hz, and 200 Hz) with amplitude 0.03 m/s2–0.1 m/s2 interact in eight possible therapeutic cycles programmed to deliver stochastic force in frequencies associated with myriad tissue effects [e.g., oxytocin release (34), reduction of fatty changes (35), decreased inflammation and disc degeneration (36), and vasodilation (37)]. To activate force-gated Piezo1 and Piezo2 ion channels mediating these processes at variable tissue depths (38–41), constructive interference between frequencies enhances the amplitude for deeper mechanical energy penetration. Five intensity settings increase both amplitude and frequency (Figure 1).
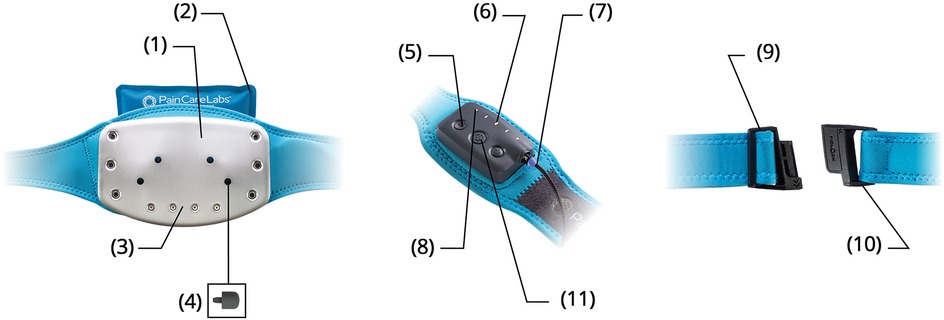
Figure 1. Contoured temperature plate (1). Natural clay ice/heat pack (2). Multi-vibration motor array (3). Trigger point acupressure nubs (4). Five intensity settings (5). LED cycle and intensity display (6). Magnetic charging cable (7). Eight therapy cycles (8). Custom-fit waistband (9). Slide-N-Lock magnetic buckle (10). Haptic touch control panel (11).
Fascial tissue gliding is enhanced with heat (42), while cryotherapy decreases inflammatory cytokine production (43). Thus, thermal heat and cold packs can be placed behind the plate. Pressure enhances fascial glide and forces extracellular liquid into gliding hyaluronic complexes, so the holes in the plate allow for four different locations of a 1.5 cm silicone acupressure nub.
To reinforce the device's benefit as a part of reducing central sensitization (25), the 200 Hz frequency reduces nociceptor firing on contact (44–46), while heat can be comforting or reduce spasm. The device is easy to apply to reduce catastrophizing and helplessness, while the multiple settings and options are intended to improve self-efficacy (feeling empowered to choose), all of which are associated with reduced opioid intake (47).
Study design
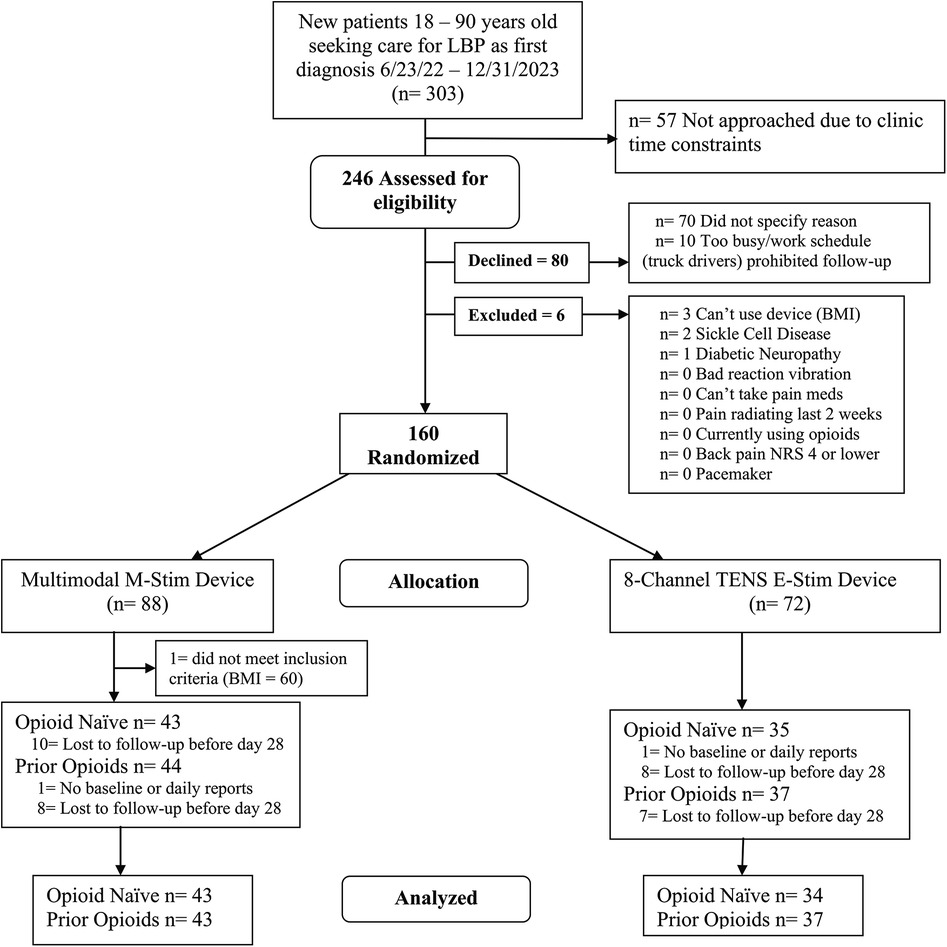
This randomized double-blind active-controlled trial was designed to evaluate the efficacy of multimodal M-Stim compared with TENS to reduce opioid prescribing and pain in patients with moderate-to-severe LBP [≥4 on a 0–10 numeric rating scale (NRS)]. Between June 2022 and December 2023, 160 adults aged between 20 and 75 years were recruited at two chiropractic and motor vehicle collision (MVC) physical therapy referral clinics in Maryland and Virginia, with follow-up completed in July 2024. The sites had no access to practitioners who could prescribe opioids. Enrollment was stratified by chronicity for pain outcomes [chronic (cLBP) ≥3 months (n = 100) or acute (aLBP) <3 months (n = 60)] with opioid use tracked daily for 28 days and then weekly for 3 months. The exclusion criteria included radicular pain, sickle cell disease, sensitivity to cold or vibration, a pacemaker, skin lesions in the low back, or inability to apply the devices as directed (Supplementary Material S1). The Kaizo Clinical Research Institutional Review Board approved the trials, which were registered with ClinicalTrials.gov NCT04491175 and monitored for severe adverse events.
Interventions
Participants were randomized to add 30 min daily use of a prescription eight-channel electrical stimulation TENS unit (LG Smart TENS, LGMedSupply, Cherry Hill, NJ, USA; $125) or multimodal M-Stim (DuoTherm, Harmonic Scientific LLC, Lewes, DE, USA; reimbursement not yet determined) to any other treatments. A text and email prompted participants to record analgesic use, pain, and device use daily for 28 days, with prescribing outcomes followed weekly for 3 months.
The TENS unit delivers electrical stimulation via four electrodes with eight use channels, adjustable intensity, and duration. A systematic review reported TENS' immediate reduction of NRS pain intensity for both acute and chronic LBP at a standardized mean difference (SMD) of −0·96 (95% CI −1.14 to −0.78) (17), while a review of vibration for cLBP found reduced pain intensity SMD = −0.71 (95% CI −1.02 to −0.39) (23).
Procedure, randomization, and assessment
Prior to treatment, clinic intake staff assessed eligibility and completed informed consent for the study “to evaluate the effect of an electric or mechanical stimulation device on opioid use and pain relief.” All patients provided written informed consent.
After consent, a Qualtrics link on the data intake tablet randomly generated a study ID and coded device assignment with no blocking or additional stratification. Baseline pain intensity was recorded prior to learning the device assignment. After participants received their device, they watched the appropriate training video (Supplementary Material S1) for DuoTherm or LG-TENS and then initiated a 30 min use while completing registration entry. Registration data include the NIH Minimum Data set for low back pain studies (48), including the PROMIS Pain Intensity (4a), Physical Function (4a), Pain Interference (8a), Depression, Sullivan Pain Catastrophizing scale, and an inventory of 13 prior treatment options including cannabis and gabapentin.
Prior opioid use at registration was assessed with the questions “What short-acting/long-acting medications have you taken for your pain in the past? (Choose all that apply)” with a comprehensive list of opioid formulations and doses (DOSE tool, Supplementary Material S2). Daily and weekly opioid diary prompts used the short-/long-acting formulation questions, adding dose per pill (34), number of pills, and source. Sources presented in order were “prescribed to me for this event,” “prescribed to me for another event,” “given to me by a family member,” “given to me by a friend,” “given to me by an acquaintance or stranger,” or “purchased from someone without a prescription.” Clinic staff documented treatments received after enrollment.
Outcomes
The primary outcome was reduction in opioid prescribing to the opioid-naïve subjects. Opioid use status was considered “naïve” with no endorsement of any listed opioids and endorsement of “I have not taken any of these short-acting/long-acting medications in the past” at registration; otherwise participants were considered “prior users.” Receiving a prescription was a binary self-reported outcome where any opioid recorded as “prescribed to me for this event” was considered a new prescription. Secondary outcomes associated with a higher risk of subsequent OUD included self-reporting of milligrams of morphine equivalents, days of use, use in the past 7 days, and receiving a prescription 7 days after presentation (6). Quantitative MME were calculated from the reported opioid DOSE diaries using the health and human services conversion tables (3, 4, 49, 50).
To explore opioid use for chronic users, we established a baseline of use for the first 14 days and compared it to use during the second 14 days using MME as an indicator of pain reduction. Additional outcomes included prescribing rates compared with a national rate of 25%. ALBP pain relief and cLBP disability study outcomes are presented elsewhere.
Blinding assessment
Participants knew the study was intended to evaluate opioid use, but were blinded to whether the electrical or mechanical stimulation device powered the study hypothesis. The protocol statistician (KS) and study coordinator (JE-S) knew device assignments and had access to data, but did not conduct analysis. The PI (AB) and treating chiropractor (AH) made no outcome judgments and were blinded to allocation and all data during enrollment, with the PI accessing data only after study completion and data lock. The analyzing statisticians (JW, OT) were blinded to device assignment until the completion of analysis. The success of participant blinding was tested at 3 months with prompts, “What was the study trying to find out?”, “Select if you received…control or treatment”, and “How confident are you?”
Sample size calculation
At the time of study conception, no non-invasive opioid-reducing device research was available to estimate the effect size for a power analysis to prevent opioid prescribing. A retrospective spinal stimulation study of 59 cLBP chronic opioid users reported a 28% MME reduction after implantation (effect size 0.60) against standard care (51). Using this effect size for a two-sided significance of 0.05, 23 participants per group would detect a 30% prescribing reduction for our primary opioid outcome, similar to the 28% MME reduction. We planned to recruit 60 opioid-naïve subjects for prescribing outcomes anticipating a 10% attrition. Our primary pain outcome of treating disability powered the cLBP recruitment of 100 patients. However, if 60% of cLBP patients had chronic or ongoing opioid use (7), it would replicate the number of opioid users (59) in the spinal stimulation study.
Analysis
Opioid prescribing differences were calculated with percentages and relative risks for binary outcomes, using Fisher’s exact for cells with zero. Opioid use days included summary statistics (means, standard deviations) and Chi-squared tests. Paired t-tests were used to compare the change between the first and the last 14 days of MME for opioid users. As the number and missing data patterns of the diary entries and opioid use days did not statistically differ between intervention groups, we did not impute missing opioid use (missing data Supplementary Material S4) but instead calculated a linear mixed model (LMM) for the first month with daily reporting for all participants with at least one opiod diary input as a baseline (Figure 2). For demographic and pain history factors that differed between groups, we planned a regression analysis to assess any interaction of characteristics with opioid use. The two-tailed significance level was set at 0.05%, and 95% confidence intervals were reported. Data analysis was performed using StataNow/SE 18.5 and MedCalc Software Ltd. (https://www.medcalc.org/calc/fisher.php; Version 23.2.1; accessed 3 April 2025).
Results
Participants
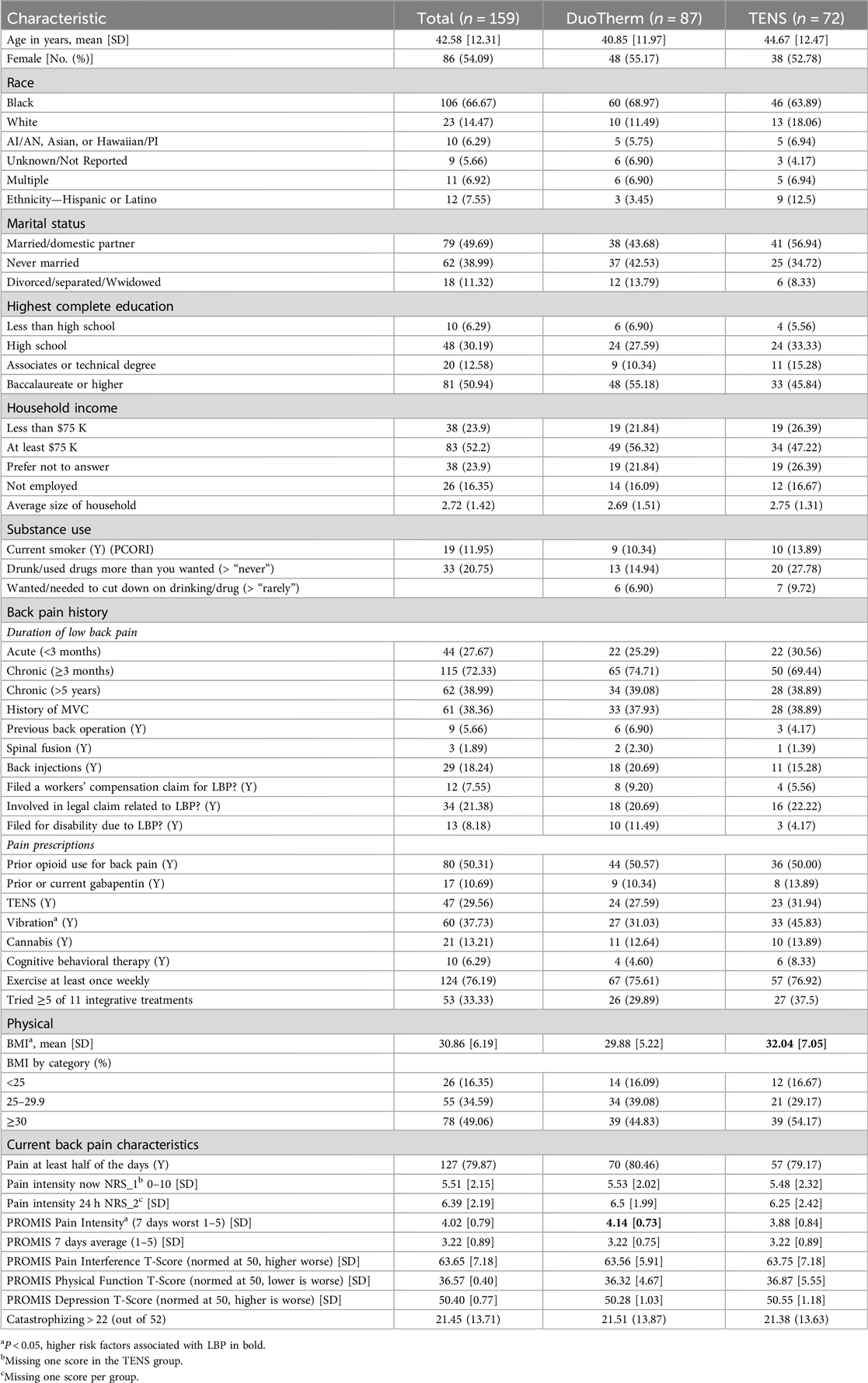
We enrolled 160 participants, of whom 159 were eligible (M-Stim = 87, TENS = 72) (Figure 2). One enrolled M-Stim patient (BMI = 60) was unable to apply the device. The majority were female (54.7%), non-Hispanic (94.3%), and Black or Multiple race (72.9%). The average age was 41.1 years (SD = 12.2), BMI 30.9%, and 31% had a household income of <$75,000. The average initial pain intensity was 5.46 on the 0–10 NRS, with a PROMIS Pain Interference T-score of 63.65 (high moderate severity). Based on registration reporting, 78 (49%) denied prior or current opioid use for back pain (“opioid-naïve”), while 81 (51%) acknowledged prior or current use (“prior user”). Demographic and pain history did not differ significantly by intervention group for 51 of the 54 data categories. The TENS group had a higher average BMI [32.04 (7.05) vs. 29.88 (5.22), p = 0.028], whereas the M-Stim group reported baseline PROMIS “worst pain intensity during the past 7 days” at 0.26 higher on a 1–5 scale and were less likely to endorse using vibration for prior pain management (Table 1). One participant per group filled no diary entries; 68 (78.2%) M-Stim and 60 (77.8%) TENS users reported through at least 1 month. Diary entry frequency did not differ by intervention, duration, or opioid use status. There were no reported device-related or significant adverse events.
Opioid prescribing and use in the opioid-naïve
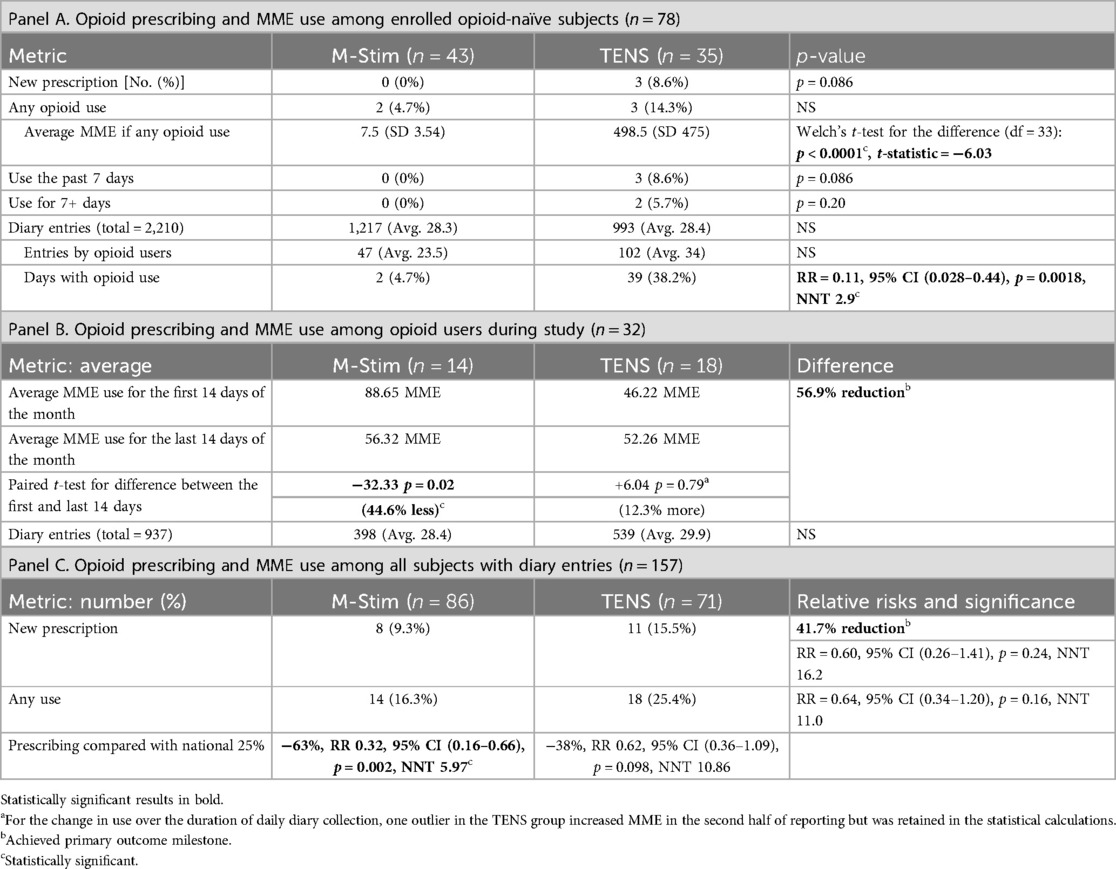
In the opioid-naïve cohort, zero M-Stim users (n = 43) received prescriptions compared with 3 of 36 using TENS (0% vs. 8.6%, Fisher's exact p = 0.086) (Table 2). For those who did take opioids, the M-Stim group averaged significantly reduced MME [7.5 (SD 3.54) vs. 498.5 MME (SD 474.9), p < 0.0001] with a lower risk of taking opioids on any daily entry: 2/47 (4.2%) using M-Stim compared with 38/102 (37%) entries by TENS users [RR 0.11 (95% CI 0.28–0.44, p = 0.0018, NNT 2.9]. All participants who took opioids in the TENS group used opioids after 7 days (p = 0.086); two TENS users (5.5%) had the additional OUD risk factor of opioid use for 7 or more days (p = 0.20). The two M-Stim participants each took one opioid on 1 day prior to day 7, obtained from sources other than a new prescription (Table 2).
Opioid prescribing and use overall
M-Stim users were 41.7% less likely to receive new prescriptions compared with TENS [7 of 86 (8.14%) vs. 11 of 71 (15.4%), RR 0.51, 95% CI 0.2099–1.256, p = 0.1441], which was not statistically significant (Table 2). Compared to national 25% rates, dispensing an M-Stim device significantly reduced prescribing (RR 0.3220, 95% CI 0.1570–0.6604, p = 0.002, NNT 5.971), while TENS devices did not (RR 0.6272, 95% CI 0.3608–1.094, p = 0.0982, NNT 10.858) (Table 2). Among 32 opioid users, M-Stim significantly reduced opioid use by 44.6% (32.33 MME, p = 0.02) while TENS users experienced a 12.3% increase (+6.04 MME, p = 0.79), but this was primarily due to one outlier (Figure 3).
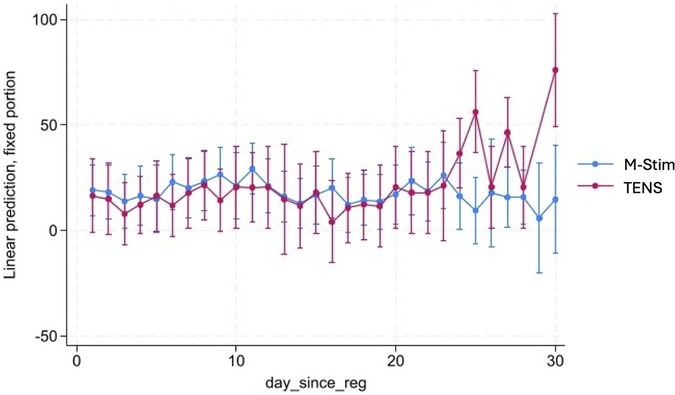
Figure 3. Linear mixed-effects model of MME change by opioid users. To evaluate opioid use patterns in the first month of intervention, we conducted a linear mixed-effects model (LMM-REML) examining daily milligram morphine equivalents (MME) for anyone with at least one positive MME value over 28 days, including the 30-day month diary timepoint reporting for the previous two days. Fixed effects included time (day since registration), condition [M-Stim (Condition 1) vs. TENS (Condition 2)], and their interaction (condition x time). A random intercept was induced for each participant to account for within-subject variability. The RE parameters were statistically significant, 106.58, 95%CI [61.56, 184.52]—suggesting substantial individual variation. There was also substantial variation that was not explained by the model. An LR test was statistically significant (P < 0.001)—indicating better fit than a single-level model. The effect of condition 1 versus condition 2 was not statistically significant.
Sources of opioid use
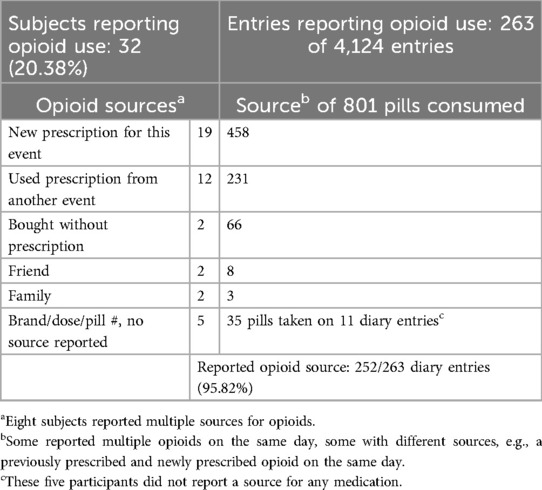
Of 4,124 diary entries, 801 opioid pills were reported among 263 diary entries, with 766 (95.6%) listing a source. In each intervention group, seven participants used opioids from sources other than new prescriptions (Table 3). Moreover, 28.8% of opioids reported were from prior prescribing rather than new prescriptions.
Blinding assessment and impact of BMI
The most common initial response in both groups was to answer the study purpose question without endorsing an allocation, which we considered “I don’t know.” Blinding guesses were made by 56 in the M-Stim group (8, control; 29, don't know; 19, treatment) and 49 using TENS (12, control; 26, don't know; 11, treatment). Using Bang's blinding index, M-Stim BBI = −0.032 (95% CI −0.54 to −0.06), and TENS BBI = −0.51 (95% CI −0.71 to −0.24), both significantly against their allocation groups. Using the James index, M-Stim = 0.54, and TENS = 0.71, where values >0.5 indicate adequate blinding.
Because BMI was significantly higher in the TENS group, opioid use and days of use were evaluated including BMI in a logistic regression model. While adding BMI did not significantly change results for MME, the opioid-naïve subjects with a BMI ≥30 had 3.0 fewer days of opioid use in the M-Stim group compared with the TENS group (99% CI −5.73 to −0.26, p = 0.032). For those with BMI ≥30, the percent of days using opioids vs. all days was 9.65% lower in the M-Stim group compared with the TENS group (99% CI −19.5% to −0.79%, p = 0.033).
Discussion
In the opioid-naïve subjects, the use of an M-Stim device significantly reduced risk factors associated with OUD for those who initiated opioids, including MME and days of use. Overall, opioid users reduced MME over the first 28 days significantly more using M-Stim than TENS, and M-Stim significantly reduced opioid use days for those with BMI ≥30. M-Stim users were prescribed opioids (8.14%) significantly less than the national moderate-to-severe LBP prescribing rate of 25%, but the 41.7% reduction compared with TENS was not statistically significant.
Low back pain is the leading cause of disability worldwide (52) and contributes $200–$600 billion in annual US healthcare costs (53–56). Risk factors for aLBP transitioning to cLBP include female sex (3), pain interfering with daily activities (48), low self-efficacy (helplessness), fear of pain (catastrophizing) (57), motor vehicle collision etiology (10), and receiving an opioid prescription (9, 58, 59). While LBP opioid prescribing fell after the declaration of the national crisis, LBP remains the most common reason for outpatient opioid prescribing in the USA (2, 60).
While current guidelines call for multimodal interventions instead of opioids (61, 62), alternative coverage and opioid familiarity inhibit physician recommendations (18, 63, 64). Immediate referrals work in other countries, but in the USA, the Patient-Centered Outcomes Research Institute (PCORI) declared that “implementation in practice has failed.” PCORI undertook a prospective trial in 76 clinics to reduce the 40% progression of aLBP to cLBP (3). After stratifying risk and providing services at the time of acute care presentation, this too failed, with 40% of moderate-to-severe aLBP still progressing to cLBP. Opioid prescribing was unchanged at 25%. Rather than referrals, effective devices are logical opioid-sparing interventions to dispense at presentation, balancing the physician's desire to act with the patient's impatience for relief.
The concept behind the DuoTherm device was initially to address pain with an evidence-based all-in-one neuromodulatory and thermal/pressure construct for easy prescribing at point of care. The device incorporated opioid reduction literature by reducing fear and increasing self-efficacy—factors associated with reduced chronic pain and OUD (25, 65, 66)—through multiple options and ease of use. Combining effective therapies of heat, cold, and pressure has synergistic effects on fascia, a common source of LBP (42). More recent discoveries, however, support the interplay of stochastic vibrations for vasodilation and mechanical force ion channel activation to reduce and address the muscle dysfunction associated with cLBP (67). The 200 Hz neuromodulatory frequency acutely blocks nociception via adenosine presynaptic inhibition in the dorsal horn, but is likely an additive rather than primary effect (33, 68). The range of frequencies applied across the entire thoracolumbar area address physiologic derangements from hypoperfusion to fatty changes (23), potentially providing pain relief by reversing newly understood mechanical causes of cLBP (30, 34, 69, 70). As such, M-Stim’s reduction of prescribing and opioid use may reflect processes other than reducing nociception.
LBP and opioid use are reciprocally linked to increased costs and disability: LBP increases the risk of OUD after surgery (71), and half of those on chronic opioids have LBP (7). The nature of opioid metabolism is implicated in the development of chronic pain. Opioids act on mu-opioid receptors (MORs), leading to antinociception through dopamine rewards. The receptors bring morphine into the cell and then return to the surface (72). After 3 days of opioid use, the receptors are more likely to be digested instead of returning; also around that time, increasing protein kinase C inhibits pain relief from opioids that are internalized (73, 74). In the face of decreased nociception, plastic processes in the brain increase pain perception to compensate for opioid-induced reduced sensitivity to physical risk (73). TENS acts in part via naloxone-reversible endogenous opioids, while vibration does not (31). If the receptors lose potency for exogenous opioids, reduced endogenous TENS efficacy might be expected after 3 days as well.
While no M-Stim opioid-naïve subjects were prescribed opioids, the rate for TENS users was only 8.7%, and not statistically significant. This may be clinically significant, however. The lead author of an international multidisciplinary team convened to address growing LBP prevalence (48) reported that one in 20 opioid-naïve patients prescribed opioids developed OUD (5), as did Hayden et al. (6) This OUD risk reporting is agnostic of pain etiology, whether for iatrogenic adult pain (75) or adolescent wisdom tooth removal (6.4%) (76). The current theory of a “reward deficient” genetic predisposition (77, 78) suggests that while reducing chronic opioid use is important, preventing initial prescribing is the critical goal. While not followed past typical time frames for prolonged use, 5% of our opioid-naïve TENS group had prolonged duration, late prescribing, and high MME risk factors for ongoing OUD. Using 2018 data, the mean value of averting one case of opioid misuse was estimated at $2.2 million, $325,125, and $244,030 in US societal, taxpayer, and healthcare costs, respectively (79). With 65 million LBP episodes yearly in the USA (52), if 25% of the 16.25 million seeking LBP relief receive opioids, the cost of 5% (203,125) becoming new prolonged opioid users is substantial.
To assess opioid use for outpatient low back pain, studies use prescribing (25%) or filled prescriptions (24.4%) (3, 6) as the accepted proxy, with a recent study confirming that all of the 94.2% filling prescriptions used them (80). Our study suggests this metric may underreport opioid consumption. Indeed, only 458 pills of 801 reported were “prescribed to me for this event,” implying that 42.8% of opioids would have been missed by validating against pharmacy records or prescription databases. While friends or family sources typically resulted in few use days, the two subjects who “bought without a prescription,” likely from an extra-legal source, averaged 10 days. Unused opioids from previous events were the second most common source after new prescriptions. As 90% of US households report having unused opioids (81), the prescription-only analyses are likely to underreport.
While there was no sham or placebo arm, the overall trend toward the superiority of M-Stim compared with TENS may be more relevant in light of TENS' established efficacy for pain intensity. In contrast, studies show subjects assume vibration works due to distraction (82). Participants using TENS did not appear to perceive their assignment as inferior; the James index of 0.71 and a Bang index of −0.51 suggest effective blinding leaning toward a more positive expectancy bias for TENS than M-Stim. That said, our average subject was obese. M-Stim reduced opioid use days significantly more in obese subjects, but increased adiposity may have confounded TENS' pain relief.
Future research should study TENS for opioid reduction against a sham or standard care, as it too may reduce unnecessary prescribing at a lower cost. As TENS and M-Stim work via different mechanisms, future devices could combine endogenous opioid TENS relief with M-Stim spinal gating, potentially reducing progression to cLBP. Finally, larger studies with chronic opioid users could assess if a multimodal M-Stim device is superior to an implanted stimulator, saving money and reducing morbidity.
Limitations
As the first investigation of a single non-invasive device to replace opioids acutely and reduce chronic pain, the ability to accurately power all outcomes was limited by a lack of consensus for meaningful reduction of exposure risk. While the primary outcome of prescribing in the opioid-naïve subjects did not reach statistical significance, opioid consumption metrics in this group and use day metrics for those with BMI ≥30 were statistically significant.
The decision to eliminate a potential bias from on-site prescribing required capturing real-world opioid use behaviors. The data collection algorithm was unverifiable through public records and may have over- or undercounted prescribing. Within-patient reporting, however, was highly consistent, and over 95% of entries reported a source. One M-Stim participant was counted as “new prescription” by protocol, but 30/32 of the subject's diaries before and after reported “to me for another event.” If misattributed, M-Stim would have significantly reduced prescribing compared with TENS. The fact that one subject could change significance underscores the need for more subjects and also suggests the DOSE tool should add verification if entries differ from previous trends.
Our choice of a chiropractic office could also have biased enrollment toward opioid-avoidant participants. One study of Vermont LBP health claims found that 22% of chiropractic and 35% of osteopathic patients filled opioid prescriptions over a year. In their cohort, however, the chiropractic users were significantly younger with less disability (83), and the osteopathic patients described resembled our population, of whom 51% had taken or were taking opioids for LBP.
The chiropractic environment may encourage acceptance of non-pharmaceutical and physical treatments. The FDA defines intractable pain as having tried three or more interventions without relief. By this definition, all but two participants had intractable pain, with over a quarter in each arm trying five or more non-pharmacologic interventions. Other environments may be less enthusiastic about applying a device rather than receiving a prescription.
We anticipated regression modeling in the case of unequal groups (e.g., BMI) and realized that providing any intervention for pain could reduce opioid prescribing. Hence, the comparison of prescribing against a national model in addition to against the control was added. We did not apply a Bonferroni adjustment, as the comparison was theoretically grounded in the construct of comparing two similar pain relief modalities (electricity vs. vibration) against a new device with additional opioid-sparing psychosocial considerations (multimodal options). Most importantly, the results favoring M-Stim to reduce opioid use were consistent across multiple analyses, only lacking significance due to being underpowered against a known pain relief device (84).
Conclusion
Among chiropractic patients with moderate-to-severe LBP, added use of a multimodal M-Stim device in the opioid-naïve subjects significantly reduced factors associated with OUD compared with TENS and reduced use days for those with BMI ≥30. Our results affirm guidelines recommending non-pharmacologic, multimodal LBP treatments as first line to mitigate opioid over-prescribing and signal a potential new approach for cLBP opioid reduction. M-Stim's multimodal approach combines therapies for local, neuromodulatory, and central pain, embodying best practices to reduce psychosocial factors linked to seeking opioids for pain. If verified in other environments, incorporating multimodal devices into routine care could reduce new opioid dependence and reduce excess prescribing. Finally, future studies should collect data on other opioid sources beyond point-of-care prescribing to get a full picture of opioid use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Kaizo Clinical Research Institute, Kaizo Health. The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
AB: Writing – original draft, Writing – review & editing. JE-S: Writing – review & editing. JW: Writing – review & editing. KS: Writing – review & editing. LC: Writing – review & editing. ML: Conceptualization, Writing – original draft.
Funding
The authors declare that financial support was received for the research and/or publication of this article. This study was funded by National Institutes of Health grants R44DA049631 (AB) and R44DA058952 (AB) as part of the Help End Addiction Long Term (HEAL) Program. The funders had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Acknowledgments
The authors are grateful to Bruce Davidson, MD, MPH, Clinical Professor, Washington State University Floyd College of Medicine, for the thoughtful review of the manuscript and to our Pain and Opioid Adjudication Committee members: Benjamin P. Van Dyke, PhD, Assistant Professor of Psychology Young Harris College; Traci Speed, MD, Assistant Professor, Psychiatry and Behavioral Sciences, Johns Hopkins University School of Medicine; and Anna Woodbury, MD, MSCR, C.Ac, Vice Chair of Research, Department of Anesthesiology and Pain Medicine, Emory University School of Medicine. We are particularly grateful to Jay Greenstein D.C., Allen Huffman D.C., and Michael Chase of Kaizo Health for their support.
In Memoriam
We acknowledge Louise Lawson, PhD, for her passion and statistical expertise supporting this research years before devices as opioid alternatives were potentially fundable. Lawson passed away on 16 April 2020.
Conflict of interest
AB is employed by Harmonic Scientific LLC. AB reports grants from the National Institutes of Health during the conduct of the study and is the inventor and owner of the DuoTherm technology. She receives no corporate fees or royalties but is on the scientific advisory board of Bright Therapeutics. During the time conducting the study, she received honoraria or speaking travel stipends from the Society for Pediatric Sedation, Voices for Non-opioid Choices, PROSA European Pediatric Sedation and Anesthesia Conference, the European Pediatric Anesthesia Society, and the Australia Child Life Association.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The authors declare that Generative AI was used in the creation of this manuscript. Generative AI was used to calculate the blinding statistics, but was not otherwise used in the writing of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1612572/full#supplementary-material
References
1. Clark S, Horton R. Low back pain: a major global challenge. Lancet. (2018) 391(10137):2302. doi: 10.1016/S0140-6736(18)30725-6
2. Leventhal EL, Nathanson LA, Landry AM. Variations in opioid prescribing behavior by physician training. West J Emerg Med. (2019) 20(3):428–32. doi: 10.5811/westjem.2019.3.39311
3. Delitto A, Patterson CG, Stevans JM, Brennan GP, Wegener ST, Morrisette DC, et al. PCORI Final Research Reports. Comparing Ways to Treat Low Back Pain and Prevent Chronic Pain and Disability—the TARGET Trial. Washington (DC): Patient-Centered Outcomes Research Institute (PCORI) (2021). Copyright © 2021. University of Pittsburgh. All Rights Reserved.
4. Walkerly A, Neugebauer RE, Misko B, Shively D, Singh S, Chahda B, et al. Prevalence, predictors and trends of opioid prescribing for lower back pain in United States emergency departments. J Clin Pharm Ther. (2021) 46(3):698–704. doi: 10.1111/jcpt.13324
5. Deyo RA, Hallvik SE, Hildebran C, Marino M, Dexter E, Irvine JM, et al. Association between initial opioid prescribing patterns and subsequent long-term use among opioid-naïve patients: a statewide retrospective cohort study. J Gen Intern Med. (2017) 32(1):21–7. doi: 10.1007/s11606-016-3810-3
6. Hayden JA, Ellis J, Asbridge M, Ogilvie R, Merdad R, Grant DAG, et al. Prolonged opioid use among opioid-naive individuals after prescription for nonspecific low back pain in the emergency department. Pain. (2021) 162(3):740–8. doi: 10.1097/j.pain.0000000000002075
7. Deyo RA, Von Korff M, Duhrkoop D. Opioids for low back pain. BMJ. (2015) 350:g6380. doi: 10.1136/bmj.g6380
8. Stumbo SP, Yarborough BJ, McCarty D, Weisner C, Green CA. Patient-reported pathways to opioid use disorders and pain-related barriers to treatment engagement. J Subst Abuse Treat. (2017) 73:47–54. doi: 10.1016/j.jsat.2016.11.003
9. Ibrahim AR, Elgamal ME, Moursi MO, Shraim BA, Shraim MA, Shraim M, et al. The association between early opioids prescribing and the length of disability in acute lower back pain: a systematic review and narrative synthesis. Int J Environ Res Public Health. (2022) 19(19):12114. doi: 10.3390/ijerph191912114
10. Nolet PS, Emary PC, Kristman VL, Murnaghan K, Zeegers MP, Freeman MD. Exposure to a motor vehicle collision and the risk of future back pain: a systematic review and meta-analysis. Accid Anal Prev. (2020) 142:105546. doi: 10.1016/j.aap.2020.105546
11. Menezes Costa LD, Maher CG, Hancock MJ, McAuley JH, Herbert RD, Costa LO. The prognosis of acute and persistent low-back pain: a meta-analysis. Can Med Assoc J. (2012) 184(11):E613–24. doi: 10.1503/cmaj.111271
12. Qaseem A, Wilt TJ, McLean RM, Forciea MA, Denberg TD, Barry MJ, et al. Noninvasive treatments for acute, subacute, and chronic low back pain: a clinical practice guideline from the American College of Physicians. Ann Intern Med. (2017) 166(7):514–30. doi: 10.7326/M16-2367
13. Dietz N, Sharma M, Adams S, Alhourani A, Ugiliweneza B, Wang D, et al. Enhanced recovery after surgery (ERAS) for spine surgery: a systematic review. World Neurosurg. (2019) 130:415–26. doi: 10.1016/j.wneu.2019.06.181
14. Sharif S, Jazaib Ali MY, Kirazlı Y, Vlok I, Zygourakis C, Zileli M. Acute back pain: the role of medication, physical medicine and rehabilitation: WFNS spine committee recommendations. World Neurosurg X. (2024) 23:100273. doi: 10.1016/j.wnsx.2024.100273
15. Paige NM, Miake-Lye IM, Booth MS, Beroes JM, Mardian AS, Dougherty P, et al. Association of spinal manipulative therapy with clinical benefit and harm for acute low back pain. JAMA, J Am Med Assoc. (2017) 317(14):1451–60. doi: 10.1001/jama.2017.3086
16. Schütze R, Rees C, Smith A, Slater H, Campbell JM, O’Sullivan P. How can we best reduce pain catastrophizing in adults with chronic noncancer pain? A systematic review and meta-analysis. J Pain. (2018) 19(3):233–56. doi: 10.1016/j.jpain.2017.09.010
17. Johnson MI, Paley CA, Jones G, Mulvey MR, Wittkopf PG. Efficacy and safety of transcutaneous electrical nerve stimulation (TENS) for acute and chronic pain in adults: a systematic review and meta-analysis of 381 studies (the meta-TENS study). BMJ Open. (2022) 12(2):e051073. doi: 10.1136/bmjopen-2021-051073
18. Gibson W, Wand BM, Meads C, Catley MJ, O'Connell NE. Transcutaneous electrical nerve stimulation (TENS) for chronic pain—an overview of Cochrane reviews. Cochrane Database Syst Rev. (2019) 4(4):Cd011890. doi: 10.1002/14651858.CD011890.pub3
19. Dehghan M, Farahbod F. The efficacy of thermotherapy and cryotherapy on pain relief in patients with acute low back pain, a clinical trial study. J Clin Diagn Res. (2014) 8(9):Lc01. doi: 10.7860/JCDR/2014/7404.4818
20. French SD, Cameron M, Walker BF, Reggars JW, Esterman AJ. A Cochrane review of superficial heat or cold for low back pain. Spine. (2006) 31(9):998–1006. doi: 10.1097/01.brs.0000214881.10814.64
21. Takamoto K, Bito I, Urakawa S, Sakai S, Kigawa M, Ono T, et al. Effects of compression at myofascial trigger points in patients with acute low back pain: a randomized controlled trial. Eur J Pain. (2015) 19(8):1186–96. doi: 10.1002/ejp.694
22. Lundeberg TC. Vibratory stimulation for the alleviation of chronic pain. Acta Physiol Scand Suppl. (1983) 523:1–51.6609524
23. Li Q, Liu P, Wang Z, Li X. Vibration therapy to improve pain and function in patients with chronic low back pain: a systematic review and meta-analysis. J Orthop Surg Res. (2023) 18(1):727. doi: 10.1186/s13018-023-04217-2
24. Nadler SF, Steiner DJ, Erasala GN, Hengehold DA, Abeln SB, Weingand KW. Continuous low-level heatwrap therapy for treating acute nonspecific low back pain. Arch Phys Med Rehabil. (2003) 84(3):329–34. doi: 10.1053/apmr.2003.50102
25. Darnall BD, Colloca L. International review of neurobiology. Int Rev Neurobiol. (2018) 139:129–57. doi: 10.1016/bs.irn.2018.07.022
26. Baxter AL, Thrasher A, Etnoyer-Slaski JL, Cohen LL. Multimodal mechanical stimulation reduces acute and chronic low back pain: pilot data from a HEAL phase 1 study. Front Pain Res. (2023) 4:1114633. doi: 10.3389/fpain.2023.1114633
27. Chou R, Deyo RA, Jarvik JG. Appropriate use of lumbar imaging for evaluation of low back pain. Radiol Clin North Am. (2012) 50(4):569–85. doi: 10.1016/j.rcl.2012.04.005
28. Stecco A, Bonaldi L, Fontanella CG, Stecco C, Pirri C. The effect of mechanical stress on hyaluronan fragments’ inflammatory cascade: clinical implications. Life. (2023) 13(12):2277. doi: 10.3390/life13122277
29. Langevin HM, Fox JR, Koptiuch C, Badger GJ, Greenan-Naumann AC, Bouffard NA, et al. Reduced thoracolumbar fascia shear strain in human chronic low back pain. BMC Musculoskelet Disord. (2011) 12:203. doi: 10.1186/1471-2474-12-203
30. Wilke J, Schleip R, Klingler W, Stecco C. The lumbodorsal fascia as a potential source of low back pain: a narrative review. BioMed Res Int. (2017) 2017:1. doi: 10.1155/2017/5349620
31. Lundeberg T. Naloxone does not reverse the pain-reducing effect of vibratory stimulation. Acta Anaesthesiol Scand. (1985) 29(2):212–6. doi: 10.1111/j.1399-6576.1985.tb02188.x
32. Fattorini L, Rodio A, Filippi GM, Pettorossi VE. Effectiveness of focal muscle vibration in the recovery of neuromotor hypofunction: a systematic review. J Funct Morphol Kinesiol. (2023) 8(3):103. doi: 10.3390/jfmk8030103
33. Salter MW, Henry JL. Evidence that adenosine mediates the depression of spinal dorsal horn neurons induced by peripheral vibration in the cat. Neuroscience. (1987) 22(2):631–50. doi: 10.1016/0306-4522(87)90359-9
34. Casale R, Hansson P. The analgesic effect of localized vibration: a systematic review. Part 1: the neurophysiological basis. Eur J Phys Rehabil Med. (2022) 58(2):306–15. doi: 10.23736/S1973-9087.22.07415-9
35. Rubin CT, Capilla E, Luu YK, Busa B, Crawford H, Nolan DJ, et al. Adipogenesis is inhibited by brief, daily exposure to high-frequency, extremely low-magnitude mechanical signals. Proc Natl Acad Sci U S A. (2007) 104(45):17879–84. doi: 10.1073/pnas.0708467104
36. Holguin N, Uzer G, Chiang FP, Rubin C, Judex S. Brief daily exposure to low-intensity vibration mitigates the degradation of the intervertebral disc in a frequency-specific manner. J Appl Physiol. (2011) 111(6):1846–53. doi: 10.1152/japplphysiol.00846.2011
37. Syabariyah S, Nurachmah E, Widjojo BD, Prasetyo S, Sanada H, Irianto , et al. The effect of vibration on the acceleration of wound healing of diabetic neuropathic foot ulcer: a prospective experimental study on human patients. Healthcare. (2023) 11(2):191. doi: 10.3390/healthcare11020191
38. Lei L, Wen Z, Cao M, Zhang H, Ling SK, Fu BS, et al. The emerging role of Piezo1 in the musculoskeletal system and disease. Theranostics. (2024) 14(10):3963–83. doi: 10.7150/thno.96959
39. Sonkodi B. Delayed-onset muscle soreness begins with a transient neural switch. Int J Mol Sci. (2025) 26(5):2319. doi: 10.3390/ijms26052319
40. Thammanichanon P, Kaewpitak A, Binlateh T, Leethanakul C. Interval vibration reduces orthodontic pain via a mechanism involving down-regulation of TRPV1 and CGRP. In Vivo. (2020) 34(5):2389–99. doi: 10.21873/invivo.12052
41. Mirzoev TM. Mechanotransduction for muscle protein synthesis via mechanically activated Ion channels. Life. (2023) 13(2):341–55. doi: 10.3390/life13020341
42. Stecco A, Gesi M, Stecco C, Stern R. Fascial components of the myofascial pain syndrome. Curr Pain Headache Rep. (2013) 17(8):352. doi: 10.1007/s11916-013-0352-9
43. Kunkle BF, Kothandaraman V, Goodloe JB, Curry EJ, Friedman RJ, Li X, et al. Orthopaedic application of cryotherapy. JBJS Rev. (2021) 9(1):e20.00016. doi: 10.2106/JBJS.RVW.20.00016
44. Salter MW, Henry JL. Physiological characteristics of responses of wide dynamic range spinal neurones to cutaneously applied vibration in the cat. Brain Res. (1990) 507(1):69–84. doi: 10.1016/0006-8993(90)90524-F
45. Zhao L, Qi P, Wang X, Su X, Liao L. Local analgesia for the relief of pain in children undergoing venipuncture and intravenous cannulation: a systematic review and network meta-analysis. BMC Anesthesiol. (2025) 25(1):115. doi: 10.1186/s12871-025-02991-6
46. Su HC, Hsieh CW, Lai NM, Chou PY, Lin PH, Chen KH. Using vibrating and cold device for pain relieves in children: a systematic review and meta-analysis of randomized controlled trials. J Pediatr Nurs. (2021) 61:23–33. doi: 10.1016/j.pedn.2021.02.027
47. Sullivan MD, Turner JA, DiLodovico C, D'Appollonio A, Stephens K, Chan YF. Prescription opioid taper support for outpatients with chronic pain: a randomized controlled trial. J Pain. (2017) 18(3):308–18. doi: 10.1016/j.jpain.2016.11.003
48. Deyo RA, Dworkin SF, Amtmann D, Andersson G, Borenstein D, Carragee E, et al. Report of the NIH task force on research standards for chronic low back pain. Phys Ther. (2015) 95(2):e1–e18. doi: 10.2522/ptj.2015.95.2.e1
49. (CMS) CfMMS. Opioid Oral Morphine Milligram Equivalent (MME) Conversion Factors Table for Prescription Drug Coverage (2020). Available at: https://www.hhs.gov/guidance/document/opioid-oral-morphine-milligram-equivalent-mme-conversion-factors-0 (Accessed May 30, 2024).
50. Jauregui JE, Nutt RJ, Margolis AM. Frequency of opioid prescribing for acute low back pain in a rural emergency department. Adv Emerg Nurs J. (2020) 42(3):210–4. doi: 10.1097/TME.0000000000000310
51. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 Khz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res. (2018) 11:2929–41. doi: 10.2147/JPR.S188795
52. Ferreira ML, de Luca K, Haile LM, Steinmetz JD, Culbreth GT, Cross M, et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. (2023) 5(6):e316–e29. doi: 10.1016/S2665-9913(23)00098-X
53. Casiano VE, Sarwan G, Dydyk AM, Varacallo M. Back Pain. StatPearls. Treasure Island (FL): StatPearls Publishing (2024).
54. Dieleman JL, Cao J, Chapin A, Chen C, Li Z, Liu A, et al. US health care spending by payer and health condition, 1996–2016. JAMA, J Am Med Assoc. (2020) 323(9):863–84. doi: 10.1001/jama.2020.0734
55. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. March 2015. Pain Physician. (2015) 18(2):E115–E27. doi: 10.36076/ppj/2015.18.E115
56. Gaskin D, Richard P. The Economic Costs of Pain in the United States. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. Washington (DC): National Academies Press (2011). p. 1–16.
57. Alhowimel AS, Alotaibi MA, Alenazi AM, Alqahtani BA, Alshehri MA, Alamam D, et al. Psychosocial predictors of pain and disability outcomes in people with chronic low back pain treated conservatively by guideline-based intervention: a systematic review. J Multidiscip Healthcare. (2021) 14:3549–59. doi: 10.2147/JMDH.S343494
58. Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. (2017) 125(5):1741–8. doi: 10.1213/ANE.0000000000002496
59. Webster BS, Verma SK, Gatchel RJ. Relationship between early opioid prescribing for acute occupational low back pain and disability duration, medical costs, subsequent surgery and late opioid use. Spine. (2007) 32(19):2127–32. doi: 10.1097/BRS.0b013e318145a731
60. Werthman AM, Jolley BD, Rivera A, Rusli MA. Emergency department management of low back pain: a comparative review of guidelines and practices. Cureus. (2024) 16(2):e53712. doi: 10.7759/cureus.53712
61. Skelly AC, Chou R, Dettori JR, Turner JA, Friedly JL, Rundell SD, et al. AHRQ Comparative Effectiveness Reviews. Noninvasive Nonpharmacological Treatment for Chronic Pain: A Systematic Review. Rockville (MD): Agency for Healthcare Research and Quality (US) (2018).
62. Foster NE, Anema JR, Cherkin D, Chou R, Cohen SP, Gross DP, et al. Prevention and treatment of low back pain: evidence, challenges, and promising directions. Lancet. (2018) 391(10137):2368–83. doi: 10.1016/S0140-6736(18)30489-6
63. Wu LC, Weng PW, Chen CH, Huang YY, Tsuang YH, Chiang CJ. Literature review and meta-analysis of transcutaneous electrical nerve stimulation in treating chronic back pain. Reg Anesth Pain Med. (2018) 43(4):425–33. doi: 10.1097/AAP.0000000000000740
64. Wolfe D, Rosenstein B, Fortin M. The effect of EMS, IFC, and TENS on patient-reported outcome measures for chronic low back pain: a systematic review and meta-analysis. Front Pain Res. (2024) 5:1346694. doi: 10.3389/fpain.2024.1346694
65. Börsbo B, Gerdle B, Peolsson M. Impact of the interaction between self-efficacy, symptoms and catastrophising on disability, quality of life and health in with chronic pain patients. Disabil Rehabil. (2010) 32(17):1387–96. doi: 10.3109/09638280903419269
66. Meints SM, Mawla I, Napadow V, Kong J, Gerber J, Chan ST, et al. The relationship between catastrophizing and altered pain sensitivity in patients with chronic low-back pain. Pain. (2019) 160(4):833–43. doi: 10.1097/j.pain.0000000000001461
67. Seyedhoseinpoor T, Taghipour M, Dadgoo M, Sanjari MA, Takamjani IE, Kazemnejad A, et al. Alteration of lumbar muscle morphology and composition in relation to low back pain: a systematic review and meta-analysis. Spine J. (2022) 22(4):660–76. doi: 10.1016/j.spinee.2021.10.018
68. Salter MW, De Koninck Y, Henry JL. Physiological roles for adenosine and ATP in synaptic transmission in the spinal dorsal horn. Prog Neurobiol. (1993) 41(2):125–56. doi: 10.1016/0301-0082(93)90006-E
69. Boucher JA, Abboud J, Nougarou F, Normand MC, Descarreaux M. The effects of vibration and muscle fatigue on trunk sensorimotor control in low back pain patients. PLoS One. (2015) 10(8):e0135838. doi: 10.1371/journal.pone.0135838
70. De Koninck Y, Henry JL. Peripheral vibration causes an adenosine-mediated postsynaptic inhibitory potential in dorsal horn neurons of the cat spinal cord. Neuroscience. (1992) 50(2):435–43. doi: 10.1016/0306-4522(92)90435-5
71. Lawal OD, Gold J, Murthy A, Ruchi R, Bavry E, Hume AL, et al. Rate and risk factors associated with prolonged opioid use after surgery. JAMA Network Open. (2020) 3(6):e207367. doi: 10.1001/jamanetworkopen.2020.7367
72. von Zastrow M, Sorkin A. Mechanisms for regulating and organizing receptor signaling by endocytosis. Annu Rev Biochem. (2021) 90:709–37. doi: 10.1146/annurev-biochem-081820-092427
73. Williams JT, Ingram SL, Henderson G, Chavkin C, von Zastrow M, Schulz S, et al. Regulation of µ-opioid receptors: desensitization, phosphorylation, internalization, and tolerance. Pharmacol Rev. (2013) 65(1):223–54. doi: 10.1124/pr.112.005942
74. Li J, Inoue A, Manglik A, von Zastrow M. Role of the G protein-coupled receptor kinase 2/3 N terminus in discriminating the endocytic effects of opioid agonist drugs. Mol Pharmacol. (2025) 107(1):100003. doi: 10.1124/molpharm.124.000951
75. Higgins C, Smith BH, Matthews K. Incidence of iatrogenic opioid dependence or abuse in patients with pain who were exposed to opioid analgesic therapy: a systematic review and meta-analysis. Br J Anaesth. (2018) 120(6):1335–44. doi: 10.1016/j.bja.2018.03.009
76. Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med. (2019) 179(2):145–52. doi: 10.1001/jamainternmed.2018.5419
77. von Zastrow M. Opioid receptor regulation. NeuroMol Med. (2004) 5(1):051–8. doi: 10.1385/NMM:5:1:051
78. Bieber CM, Fernandez K, Borsook D, Brennan MJ, Butler SF, Jamison RN, et al. Retrospective accounts of initial subjective effects of opioids in patients treated for pain who do or do not develop opioid addiction: a pilot case-control study. Exp Clin Psychopharmacol. (2008) 16(5):429–34. doi: 10.1037/1064-1297.16.5.429
79. Murphy SM. The cost of opioid use disorder and the value of aversion. Drug Alcohol Depend. (2020) 217:108382. doi: 10.1016/j.drugalcdep.2020.108382
80. Daoust R, Paquet J, Émond M, Iseppon M, Williamson D, Yan JW, et al. Opioid prescribing requirements to minimize unused medications after an emergency department visit for acute pain: a prospective cohort study. Can Med Assoc J. (2024) 196(25):E866–e74. doi: 10.1503/cmaj.231640
81. Sundararajan K, Ajrawat P, Canizares M, Power JD, Perruccio AV, Sarro A, et al. The potential for diversion of prescribed opioids among orthopaedic patients: results of an anonymous patient survey. PLoS One. (2021) 16(8):e0256741. doi: 10.1371/journal.pone.0256741
82. Harper DE, Hollins M. Is touch gating due to sensory or cognitive interference? Pain. (2012) 153(5):1082–90. doi: 10.1016/j.pain.2012.02.011
83. Whedon JM, Toler AWJ, Goehl JM, Kazal LA. Association between utilization of chiropractic services for treatment of low-back pain and use of prescription opioids. J Altern Complementary Med. (2018) 24(6):552–6. doi: 10.1089/acm.2017.0131
Keywords: opioid, M-Stim, DuoTherm, TENS, low back pain, multifidus, focal vibration
Citation: Baxter AL, Etnoyer-Slaski JL, Williams JAR, Swartout K, Cohen LL and Lawson ML (2025) Preventing opioid prescribing for low back pain using multimodal mechanical stimulation vs. TENS: a randomized-controlled trial. Front. Pain Res. 6:1612572. doi: 10.3389/fpain.2025.1612572
Received: 15 April 2025; Accepted: 16 June 2025;
Published: 10 July 2025.
Edited by:
Hewei Wang, Fudan University, ChinaReviewed by:
Liyi Chen, Newcastle University, United KingdomLiyu Fang, Zhejiang Chinese Medical University, China
Feixiang Huo, Jining Medical University, China
Copyright: © 2025 Baxter, Etnoyer-Slaski, Williams, Swartout, Cohen and Lawson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Amy L. Baxter, YWJheHRlckBBdWd1c3RhLmVkdQ==
†Deceased
 Amy L. Baxter
Amy L. Baxter Jena L. Etnoyer-Slaski3
Jena L. Etnoyer-Slaski3 Jessica Allia Rice Williams
Jessica Allia Rice Williams