- 1Brain Health Institute, Westport, CT, United States
- 2Department of Neurology, University of Toledo, Toledo, OH, United States
- 3Department of Research, Hartford HealthCare, Hartford, CT, United States
- 4Lumiio Inc., Calgary, AB, Canada
- 5Department of Clinical Neurosciences, University of Calgary, Calgary, AB, Canada
- 6University Otolaryngology, Providence, RI, United States
Background: Rhinosinusitis (RS) is a leading reason for antibiotic prescriptions but treatment satisfaction is low. Misdiagnosis may contribute to poor outcomes, as migraine—often underrecognized—can mimic RS symptoms, with studies showing overlap between RS and migraine diagnoses. Our aims were to explore the demographics and clinical features of facial pain or pressure (FPP), its relationship with migraine and RS, and distinguish symptoms between these overlapping conditions.
Methods: The HEADS Registry, a web-based survey, targets adults with head and/or neck symptoms. Participants who answered “yes” (FPP+) or “no” (FPP−) to experiencing recurrent facial or sinus pain/pressure were included in this analysis. The ID Migraine screening tool was used to classify participants as ID Migraine+ or ID Migraine−. Demographics, symptoms, disability, history of allergies, sinusitis, and antibiotic use were compared between 1) FPP+ and FPP− groups, 2) FPP+/ ID Migraine+ and FPP+/ID Migraine−, and 3) FPP+/ID Migraine− and FPP−/ID Migraine+ subgroups. Continuous variables were compared using independent samples t-test or Mann–Whitney U, and categorical variables were compared using chi-square or Fisher's exact test.
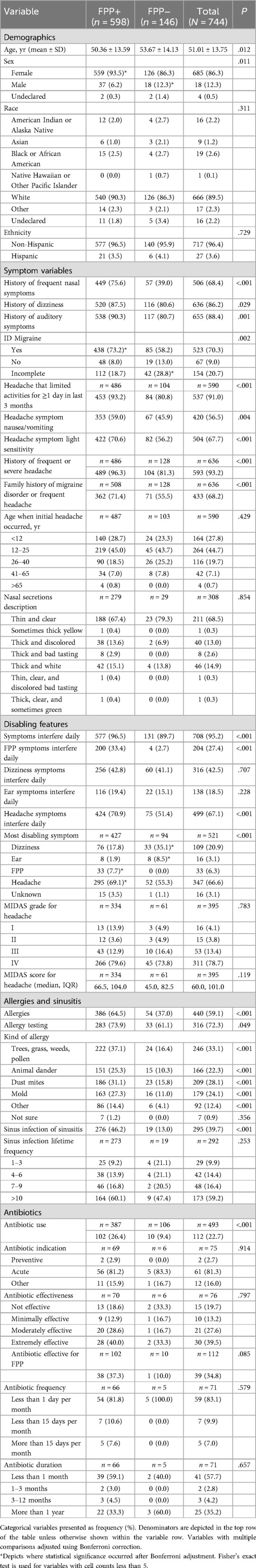
Results: The FPP+ group (n = 598) was younger, more often female, and reported higher rates of nasal, vestibular, and otologic symptoms compared to the FPP− group (n = 146). They also had more severe headaches, migraine-associated symptoms, and higher ID Migraine screening rates. The FPP+ group reported greater daily symptom interference, and more allergies, sinus infections, and antibiotic use. Those who screened positive for migraine (FPP+/ID Migraine+, n = 438) had more severe symptoms, greater disability, and more frequent forehead/eye pain. FPP+/ID Migraine− (n = 48) participants were more likely to report nasal symptoms, allergies, and sinus infections, while FPP−/ID Migraine+ (n = 85) participants reported more disabling headaches.
Conclusion: In this exploratory analysis, FPP was strongly associated with headache, including migraine, as well as allergies, rhinosinusitis, and antibiotic use. The low reported effectiveness of antibiotics suggests potential misdiagnosis. Findings that migraine, plus autonomic, vestibular, otologic symptoms are associated with FPP, highlight the need to expand the differential diagnosis beyond infectious causes. These insights, along with ongoing registry improvements, will support efforts to refine diagnostic accuracy and optimize treatment strategies for neurologic, otologic, and rhinologic conditions.
Introduction
Each year in the United States, nearly one in 10 adults is diagnosed with rhinosinusitis (RS), the acute form of which is characterized by either purulent nasal discharge accompanied by nasal obstruction, facial pain-pressure-fullness, or both (1). The majority of those diagnosed with RS report severe symptoms and substantial adverse impact on daily functioning, and it is among the most common diagnoses for which antibiotics are prescribed (2, 3). Despite the high per annum costs of RS ($22 billion, direct and indirect) (4), treatment effectiveness and satisfaction is low (5–9). This could be related to the appropriateness of the prescribed antibiotic for the underlying infection, but these findings also raise the possibility that a subset of persons not responding to RS treatment are misdiagnosed. One common condition, characterized by symptoms that may overlap with those of RS, is migraine. Migraine is poorly recognized by patients and also widely underdiagnosed and misdiagnosed by both general practitioners and specialists (10). According to a recent study, 60% of people diagnosed with migraine reported one or more rhinologic symptoms and 20% met the formal criteria for rhinosinusitis during migraine attacks (11). In a 2018 study of 1,458 random patients presenting to 14 different otolaryngology clinics, those who screened positively for migraine 89.1% reported facial pressure (12). Notably, clinical studies have also shown that the majority of people diagnosed with RS met diagnostic criteria for migraine. In addition, studies highlight symptom improvement in this population with migraine-specific treatments, as opposed to antibiotics and/or surgery (13–15).
Although it not unusual for common conditions like RS and migraine to co-occur, we hypothesize that in a subset of persons experiencing recurrent episodes of facial/sinus pain or pressure (FPP), there may be an underlying migraine-like neurogenic etiology distinct from the infectious etiology of rhinosinusitis. With the ultimate goal of improving diagnostic accuracy and management for migraine and RS in persons presenting with FPP, the initial aim of this exploratory study was to examine and describe the demographic and clinical characteristics associated with FPP, its relationship with migraine, defined by symptomatology, and relationship with RS history and treatment effectiveness. Our second aim was to examine differences in demographics and clinical characteristics between subgroups, namely (1) FPP with migraine vs. FPP without migraine, and (2) FPP without migraine vs. migraine without FPP, in order to identify symptoms at the intersection of these two overlapping conditions, and those that distinguish them. With an eye toward clinical applicability of our findings, we chose to study a cohort based on a self-reported symptom, facial/sinus pain or pressure, rather than on the diagnosis of a condition that presumes a specific temporal course [e.g., persistent idiopathic facial pain (16), anatomic origin [e.g., sinus headache, orofacial pain (17)] or pathophysiology (e.g., infectious, allergic, neurogenic)]. In this study migraine status was determined using a validated screening tool with a high positive predictive value (18). We utilized the recently established Headache, Ear, Auditory, Dizzy and Sinus (HEADS) registry, which contains information on demographics, clinical characteristics, comorbid conditions, and response to treatment. The long-term goals of the HEADS Registry are to refine clinical diagnostic and treatment guidelines for neurologic, otologic and rhinologic conditions, improve outcomes and advance medical education.
Materials and methods
The HEADS Registry is a prospective, patient-reported set of questions (available in English and Spanish) deployed through a web-based portal. The registry is operated by the Association of Migraine Disorders (AMD) and registry data collection is IRB-approved [Advarra IRB, HEADS Registry (Pro0070553)].
Participants
From July 2023 to September 2024, participants in the HEADS registry were recruited through a variety of means, including their medical professionals, AMD contact channels, social media, and partnership with nonprofit organizations, the Coalition for Headache and Migraine Patients (CHAMP) and Vestibular Disorders Association (VeDA). Recruitment into the registry was based on participants having head and/or neck symptoms, including headache, rhinosinusitis (chronic or recurring), and dizziness. Other criteria for registry participation included residence in the United States, aged 18 years or older, ability to understand English or Spanish, and signed the informed consent. This study included a subset of the registry responding either “yes” [FPP positive (FPP+)] or “no” [FPP negative (FPP−)] to the question, “Have you repeatedly experienced pain or pressure anywhere across your face or in your sinuses?”.
Registry questionnaire
The HEADS registry questionnaire was developed from a trial survey completed by 1,631 participants, then refined for clarity and completeness by a team of otolaryngologists, neurologists, physical therapists, psychologists, and patient advocates. There are 12 sections of the survey consisting of 50 head and neck symptom-related questions (see Supplementary Material), however, for this analysis we focused on questions relevant to our specific aims. Branching logic was used to limit question burden where possible. All questions were optional. To identify individuals with high likelihood of migraine, the validated, self-reported ID Migraine screening tool was utilized. ID Migraine consists of three questions about headaches occurring during the prior three months involving (1) limitation of activities for at least one day in the past 3 months, (2) nausea or vomiting, and (3) light sensitivity. A positive screen is a “yes” answer to two or more of the questions and indicates a positive predictive value of migraine of 93% (18). Another validated tool, Migraine Disability Assessment (MIDAS), was used to determine headache-related disability (19).
Statistical analysis methods
Data were assessed for normality and to ensure assumptions were met for the statistical tests conducted. Patient demographics, clinical symptoms, disabling features, allergy history and testing, sinusitis history, and related antibiotic use were compared based on FPP status, i.e., FPP+ group vs. FPP− group. We conducted subgroup analyses to further explore if there were differences between participants who screened positive for migraine using the three-item ID Migraine tool (ID Migraine+) vs. participants who screened negative (ID Migraine−) within the FPP+ group. We also compared the subgroup with FPP but no migraine (FPP+/ID Migraine−) to the subgroup with migraine but no FPP (FPP−/ID Migraine+) to explore differences when isolating FPP+ patients and ID Migraine+ patients. The subgroup analyses excluded participants who did not respond to the ID Migraine questions. Continuous variables were compared between groups using an independent samples t-test or Mann–Whitney U test depending on distribution of data, and presented as mean ± standard deviation or median, interquartile range, respectively. Categorical variables were compared using chi-square test or Fisher's exact test and presented as frequency (%). Bonferroni correction was used to adjust for multiple comparisons when appropriate. Statistical analysis was performed using Statistical Package for the Social Sciences, Version 29.0 (IBM Corp., Armonk, NY, USA) and P < .05 was established as the level of statistical significance.
Results
Study enrollment and population demographics
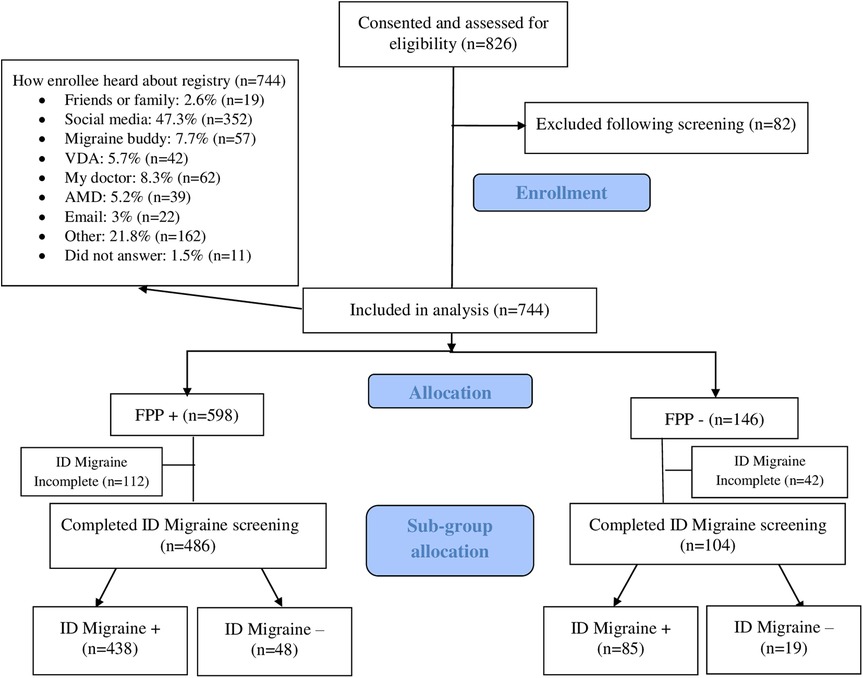
Over the 14 months of our study, a total of 826 participants with head and/or neck symptoms consented to participate in the HEADS registry (Figure 1, flow chart). After exclusion following screening, 744 participants were included, nearly half coming to the study through social media (Figure 1, flow chart). Of the 744 participants, 598 (80.4%) were FPP+, while 146 (19.6%) were FPP−. In the FPP+ group, 486 participants completed the ID Migraine screen, with 438 (90.1%) stratified to the ID Migraine+ subgroup, and 48 (9.9%) to the ID Migraine− subgroup. In the FPP− group, 104 participants completed the ID Migraine screen, with 85 (81.7%) stratified to the ID Migraine+ subgroup.
Demographics, symptom variables, disabling features, allergies and sinusitis in FPP+ and FPP− groups
Demographics
Participants in the FPP+ group were younger in years (50.36 ± 13.59 vs. 53.67 ± 14.13, P = .012), and more likely to be female (93.5% vs. 86.3%, P = .011) than those in the FPP− group. There were no significant differences in race or ethnicity between groups and participants (FPP+ vs. FPP−) were predominantly White (90.3% vs. 86.3%) and non-Hispanic (96.5% vs. 96.4%; Table 1).
Symptom variables
Participants in the FPP+ group reported a greater rate of frequent nasal symptoms (e.g., runny nose, or nasal congestion) than the FPP− group (75.6% vs. 39.0%, P < .001) but no significant differences in nature of nasal secretions (Table 1). History of vestibular (e.g., dizziness, feeling off balance or having a spinning sensation) and otologic symptoms (e.g., ringing in ears, ear pressure, ear pain, sensation of blocked ears, or difficulty hearing) were also more common in FPP+ participants. A history of frequent or severe headache was common in the total sample (93.2%), but prevalence was higher in the FPP+ group (96.3% vs. 81.3%, P < .001). Participants in the FPP+ group also reported more migraine-associated symptoms with headache, including nausea/vomiting (59.0% vs. 45.9%, P = .004), sensitivity to light (70.6% vs. 56.2%, P < .001), and limitation of activities (93.2% vs. 80.8%, P < .001). The proportion with a positive ID Migraine screen was higher in the FPP+ group (73.2% vs. 58.2%, P = .002). In addition, FPP+ participants were more likely to have a family history of migraine or frequent headache (71.4% vs. 55.5%, P < .001).
Disabling features
The FPP+ group reported greater rates of daily interference for any symptom in general (96.5% vs. 89.7%, P < .001), and specifically those of FPP (33.4% vs. 2.7%, P < .001) and headache (70.9% vs. 51.4%, P < .001) (Table 1). Participants in the FPP+ group more commonly reported headache as their most disabling symptom (69.1% vs. 55.3%, P < .05 after Bonferroni adjustment), whereas a greater proportion of FPP− participants reported vestibular (35.1% vs. 17.8%, P < .05 after Bonferroni adjustment) and/or otologic symptoms (8.5% vs. 1.9%, P < .05 after Bonferroni adjustment) as their most disabling symptoms. There were no statistically significant differences between groups for MIDAS score or grade.
Allergies and sinusitis
The FPP+ participants reported greater rates of allergies (64.5% vs. 37.0%, P < .001), allergy testing (73.9% vs. 61.1%, P = .049), sinus infection or sinusitis (46.2% vs. 13.0%, P < .001), and antibiotic use for FPP symptoms (26.4 vs. 9.4%, P < .001) than FPP− participants (Table 1). There were, however, no significant differences between groups for lifetime frequency of sinus infection, nor for antibiotic indication, effectiveness, frequency, or duration of antibiotic use.
Demographics, symptoms, disabling features, allergies and sinusitis in FPP+ group's ID Migraine+ and ID Migraine− subgroups
Results from the sub-group analysis examining demographics, symptoms, disabling features, and allergies and sinusitis between ID Migraine+ (n = 438) vs. ID Migraine− (n = 48) among FPP+ participants (n = 486) are shown in Table 2.
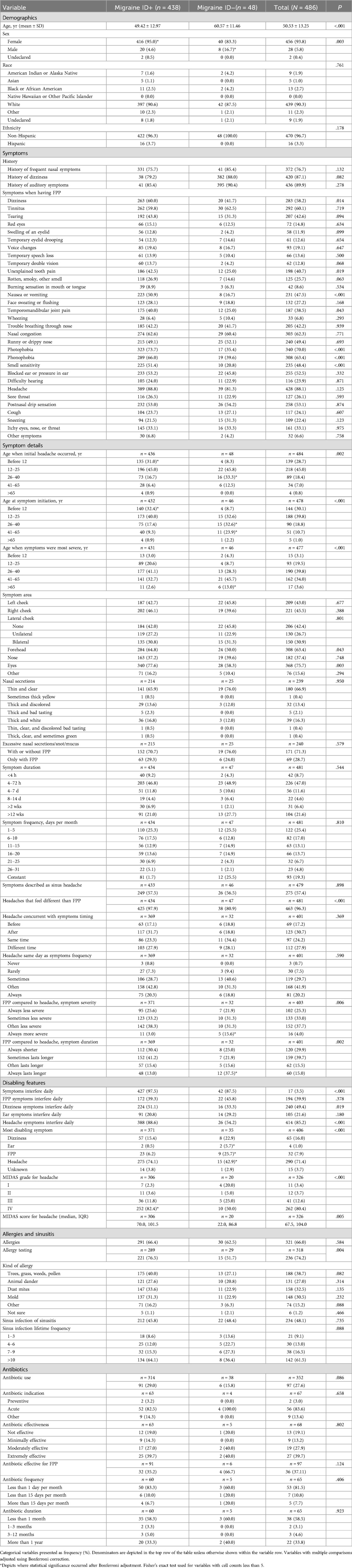
Table 2. Patient demographics and symptom characteristics between patients who are FPP+ and migraine ID+ vs. patients who are FPP+ and Migraine ID−.
Demographics
Participants in the ID Migraine+ subgroup were younger in years (49.42 ± 12.97 vs. 60.57 ± 11.46, P < .001), and more likely to be female (95.0% vs. 83.3%, P = .003) than the ID Migraine− subgroup. There were no differences in race and ethnicity between subgroups.
Symptom variables
History of frequent nasal symptoms, vestibular symptoms and otologic symptoms exceeded 75% in each of the FPP+ ID Migraine subgroups, and there were no significant differences. Participants were asked to select all that apply from a list of 30 symptoms involving the face, head, sinuses, nose, eyes, ears, mouth, throat, and respiratory tract in response to the question, “When you have facial/sinus pain or pressure, what are the symptoms that you experience?”. Headache was the most commonly reported symptom during FPP symptoms, but there were no significant differences between ID Migraine+ and ID Migraine− subgroups for this variable (88.8% vs. 81.3%, P = .125). A number of migraine-associated symptoms were, however, more frequently reported by the ID Migraine+ participants, including photophobia, phonophobia, osmophobia, nausea or vomiting, and dizziness. Temporomandibular joint pain and unexplained tooth pain were also more common in the ID Migraine+ subgroup. There were no significant differences between ID Migraine+ subgroups for any other symptoms, including those commonly described in persons with allergies and rhinosinusitis, although the larger proportion of eye tearing, eyelid swelling, and malodorous (rotten, smoky) smell in the ID Migraine+ subgroup approached significance and may warrant continued observation as registry recruitment increases.
Symptom details
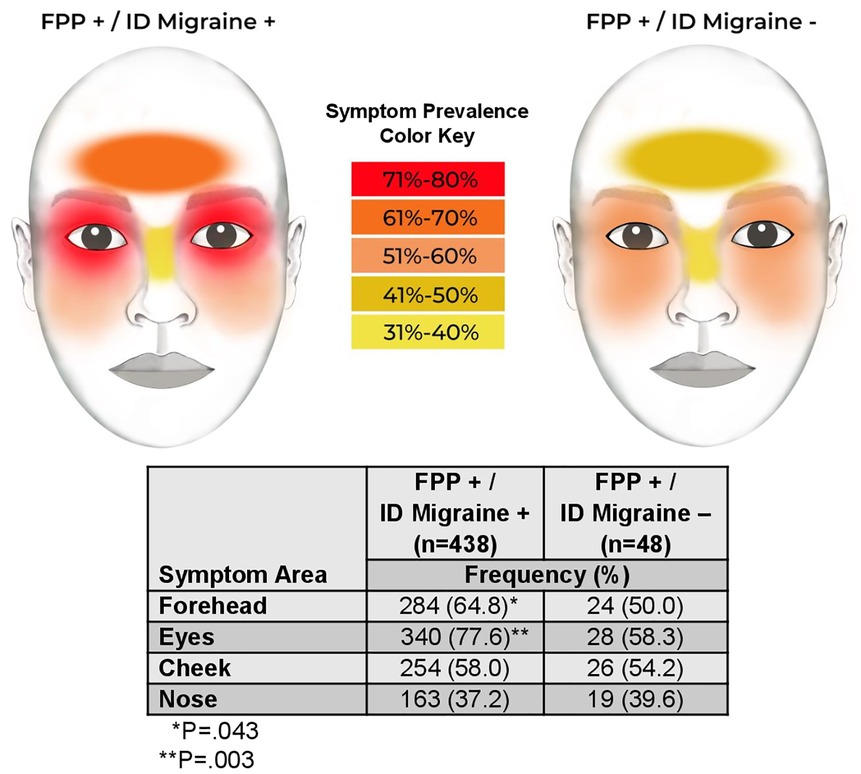
Both headache and FPP symptoms were more likely to start before age 12 years in the ID Migraine+ subgroup, and at a later age in the ID Migraine-subgroup. Furthermore, ID Migraine+ participants were more likely to report their most severe symptoms at an earlier age, whereas they occurred after age 65 years in the ID Migraine− participants. Pain or pressure location was also different between subgroups, occurring more frequently over the forehead and eyes in the ID Migraine+ participants (Figure 2). There were no differences in the nature of nasal secretions between subgroups or whether excessive secretions occurred only during FPP symptoms or at other times as well. In addition, FPP symptom duration and frequency did not differ based on ID Migraine status. In response to questions addressing the interface of FPP symptoms and headache symptoms, a greater proportion of ID Migraine+ participants reported that their FPP symptoms felt different than their headache symptoms compared to ID Migraine− participants (97.9% vs. 80.9%, P < .001). There were, however, no differences between the subgroups in the temporal relationship between headache and FPP symptoms. The ID Migraine− subgroup was significantly more likely to describe FPP symptoms as “always more severe” (15.6% vs 3.0%, P < .05 after Bonferroni adjustment) and “always lasting longer” (37.5% vs. 13.0%, P < .05 after Bonferroni adjustment) than their headache.
Disabling features
The ID Migraine+ subgroup reported greater rates of daily interference for any symptom in general (97.5% vs. 87.5%, P < .001), and specifically for headache (88.6% vs. 54.2%, P < .001) and dizziness (51.1% vs. 33.3%, P = .019) (Table 2). Participants in the ID Migraine+ subgroup most frequently reported headache as their most disabling symptom (74.1% vs. 42.9%, P < .05 after Bonferroni adjustment) followed by FPP (25.7% vs. 6.2%, P < .05 after Bonferroni adjustment), whereas a greater proportion of ID Migraine− participants reported otologic symptoms (5.7% vs. 0.5%, P < .05 after Bonferroni adjustment) as their most disabling symptom. Compared to ID Migraine− participants, a greater proportion of ID Migraine+ participants had a MIDAS grade of IV and higher median MIDAS score.
Allergies and sinusitis
The proportion of persons with allergies in the FPP+ group was 66% overall and did not vary significantly between ID Migraine subgroups, although the allergy testing rating was greater in the ID Migraine+ subgroup (76.5% vs. 51.7%, P = .004). There was no difference in the proportions with sinus infection or sinusitis (45.8% vs. 48.4%, P = .735). There were also no significant differences between groups for lifetime frequency of sinus infection, nor for antibiotic use, indication, effectiveness, frequency, or use duration.
Headache and associated symptoms in FPP+ group's ID Migraine+ and ID Migraine− subgroups
To further explore differences between FPP+ ID Migraine subgroups for occurrence of headache in addition to photophobia, phonophobia, and nausea or vomiting, we examined composite variables. In the ID Migraine+ subgroup (n = 438), 302 (68.9%) participants had headache and photophobia. In the ID Migraine− subgroup (n = 48), 16 (33.3%) participants had headache and photophobia. The group difference of 35.6% was statistically significant (P < .001). Of those who had headache in the ID Migraine+ subgroup (n = 389), 302 (77.6%) had photophobia. Of those who had headache in the ID Migraine− subgroup (n = 39), 16 (41.0%) had photophobia. The subgroup difference of 36.6% was statistically significant (P < .001).
In the ID Migraine+ subgroup, 273 (62.3%) participants had headache and phonophobia. In the ID Migraine− subgroup, 19 (39.6%) participants had headache and phonophobia. The subgroup difference of 22.7% was statistically significant (P = .002). Of those who had headache in the ID Migraine+ subgroup, 273 (70.2%) had phonophobia. Of those who had headache in the ID Migraine− subgroup, 19 (48.7%) had phonophobia. The subgroup difference of 21.5% was statistically significant (P = .006).
In the ID Migraine+ subgroup, 213 (48.6%) participants had headache and nausea or vomiting. In the ID Migraine− subgroup, 8 (16.7%) participants had headache and nausea or vomiting. The subgroup difference of 31.9% was statistically significant (P < .001). Of those who had headache in the ID Migraine+ subgroup, 213 (54.8%) had nausea or vomiting. Of those who had headache in the ID Migraine− subgroup, 8 (20.5%) had nausea or vomiting. The subgroup difference of 34.3% was statistically significant (P < .001).
Demographics, symptoms, disabling features, allergies and sinusitis in FPP+ group's ID Migraine− and FPP− group's ID Migraine+ subgroups
Results from the subgroup analysis examining demographics, symptom variables, disabling features, and allergies and sinusitis between FPP+ participants without migraine (n = 48) and ID Migraine+ participants without FPP (n = 85) are in Table 3.
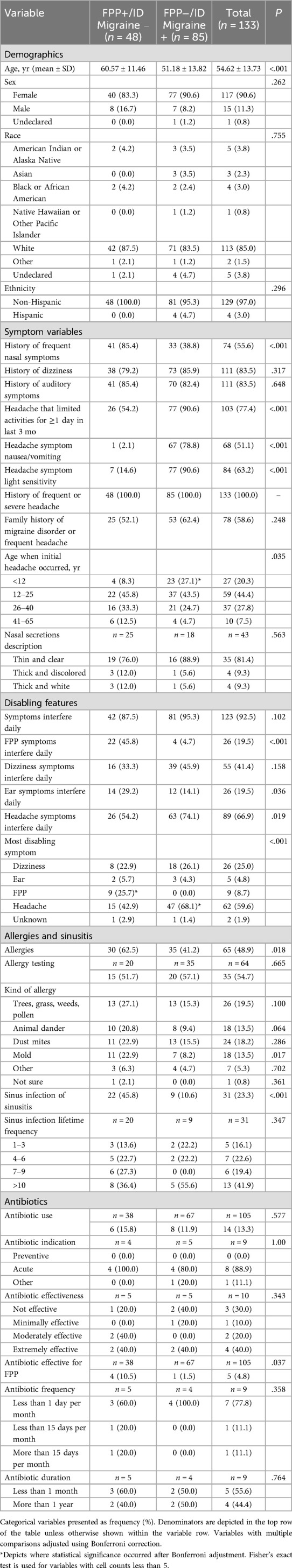
Table 3. Patient demographic and symptom characteristics: FPP+/ID Migraine− (isolated FPP) vs. FPP−/ID Migraine+ (Isolated Migraine).
Demographics
Participants in the FPP+/ID Migraine− subgroup were older, but there were no significant differences in sex, race, or ethnicity compared to the FPP−/ID Migraine+ subgroup.
Symptoms
In the FPP (FPP+/ID Migraine−) and migraine (FPP−/ID Migraine+) subgroups, 100% of participants reported history of frequent or severe headache. The proportion with initial headache below the age of 12 years was higher in the FPP−/ID Migraine+ subgroup. History of frequent nasal symptoms was more than twice as common in the FPP+/ID Migraine− subgroup (85.4% vs. 38.8%, P < .001), whereas the proportions reporting vestibular and otologic symptoms were elevated and similar in both subgroups. Headache severe enough to restrict activity or associated with nausea/vomiting or light sensitivity was higher in the FPP−/ID Migraine+ subgroup.
Disabling features
There were differences between the subgroups for the most disabling symptoms (P < .001), with a higher proportion reporting headache in the FPP−/ID Migraine+ subgroup (68.1% vs. 42.9%), and a higher proportion reporting FPP symptoms in the FPP+/ID Migraine− subgroup (25.7% vs. 0.0%).
Allergies and sinusitis
Participants in the FPP+/ID Migraine− subgroup were also more likely to report allergies (62.5% vs. 41.2%, P = .018), a history of sinus infection (45.8% vs. 10.6%, P < .001), and antibiotic effectiveness for FPP symptoms (10.5% vs. 1.5%, P = .037).
Discussion
The premise on which this report is founded is that people with facial pain report that they consider this a symptom characteristically distinct from their headache. Among the FPP+ registrants, 97.9% (425/434) of migraine ID+ reported feeling that their headaches were different from their FPP. In contrast, 80.9% (38/47) who were Migraine ID- reported that same distinction. Our first aim, to investigate and describe the demographic and clinical characteristics of FPP that might implicate either migraine or RS as the underlying etiology, revealed a number of noteworthy findings. First and foremost, in this population the relationship between FPP and headache, and in particular migraine, was robust and multifaceted. Migraine diagnosis, based on a validated screening tool utilizing headache-associated restriction of activity, photophobia, and nausea and vomiting, was significantly more common in participants with FPP than those without. Our findings of an FPP demographic profile reminiscent of migraine (i.e., younger, more likely female), a higher proportion with family history of migraine or severe headache, and headache as the most common reason for disability may be related to the outsized proportion of migraine in this population, but it does not exclude the potential of shared hormonal and genetic mechanisms contributing to the pathophysiology of both migraine and FPP. The FPP group had a higher proportion of nasal symptoms commonly described with RS, as well as vestibular and otologic symptoms, which are decidedly less common in RS (20–22). These symptoms, however, may also be caused by the non-infectious inflammatory activation of autonomic and vestibular pathways that occurs in migraine (23–25), so are not precise in differentiating the conditions.
Responses to direct questions about alternative diagnoses, revealed that both allergies and history of sinusitis were more common in the FPP cohort, implicating a role of immune/inflammatory pathways in the connection between FPP and these disorders. We also identified an association of allergies and RS with FPP in a small-sample comparison of an FPP subgroup without migraine to a migraine subgroup without FPP. A recent Mendelian randomization study failed to demonstrate a causal relationship of allergic rhinitis and migraine, or the reverse (26). In light of this, findings from American Migraine Prevalence and Prevention study (27) showing that allergic rhinitis (coupled with a nonallergic rhinitis) was associated with higher migraine frequency and disability suggest that the epidemiological overlap may be related to shared mechanisms, a theory that requires further research (28). With regards to RS, our finding that antibiotics were more frequently prescribed in the FPP group, but were not effective, raises concern for misdiagnosis, which both delays proper treatment and contributes to the unnecessary use of antibiotics (29). Our findings of positive association of migraine and FPP coupled with the prominent overlap of symptoms between migraine and RS, should prompt early consideration of migraine and migraine screening, particularly when other causes of FPP have been ruled out by diagnostic evaluation with endoscopy and CT.
Our second aim, to examine demographics and clinical symptoms in persons with both FPP and migraine (compared to FFP without migraine), and in those where each condition occurs in isolation (FPP vs. migraine), revealed that in both subgroup comparisons those with migraine were younger, more likely to report early onset headache, and to describe headache as the predominant disabling symptom. Calling into question the role of migraine in the etiology of autonomic, vestibular and otologic symptoms, are our subgroup findings that (1) there were no significant differences in the prevalence of these three symptom constellations between the FPP subgroups with and without migraine, (2) there were no significant differences in the prevalence of vestibular and otologic symptoms in the FPP vs. migraine subgroup comparison, and (3) the prevalence of nasal/autonomic symptom in the isolated FPP subgroup was twice that of the isolated migraine subgroup. The etiology of these symptoms in migraine and FPP together and in isolation deserves further study in a population less enriched by headache. Neurogenic mechanisms may, however, have accounted for increased prevalence of facial pain over the forehead and eyes in persons from the FPP with migraine subgroup compared to the FPP subgroup without migraine, potentially by a migraine-related effect on different special sensory and autonomic neural networks. Our findings also raise the question of whether FPP and related symptoms may be neurogenically mediated as a variant of migraine without headache or migraine-defining associated symptoms.
Limitations and future directions
There are a number of limitations in this exploratory study. Self-administered and self-reported surveys in general have the potential for recall bias, social desirability bias, and misinterpretation of questions. We aimed to minimize these biases through a number of measures: (1) limiting recall to 1 year; (2) including a “contact us” page on the registry site where participants can contact registry staff to ask questions; and (3) standardizing questions by using consistent terminology, sentence structure, and 6th grade level writing for easy comprehension.
There was also potential selection bias, with nearly half of participants coming to the registry from social media related to migraine. This likely accounted for the high prevalence of headache, and specifically migraine in our sample, and contributed to the finding that headache was the most common and most disabling symptom described by members in both groups (FPP+ and FPP−) and subgroups (ID Migraine+ and ID Migraine−, FPP+/ID Migraine− and FPP−/ID Migraine+). We will incorporate broader recruitment strategies beyond migraine groups to include individuals with head and neck symptoms without migraine. We also acknowledge that the sample sizes, especially for subgroup analyses, are small and likely not adequately powered to detect differences for many of the variables being compared.
Our preliminary findings indicate a lack of diversity in our sample with smaller proportions of men, Black, and Hispanic persons than found in the overall US population. We aim to ensure representation of the US population, and therefore the registry questionnaires are available in English and Spanish, however, additional recruitment strategies are necessary to engage with diverse communities by expanding the registry to include underserved healthcare settings (e.g., community health care centers and First Nations/indigenous communities).
Another limitation is missing data for some variables due to the fact that all questions have up to this point been optional, resulting in smaller sample sizes for some variables. To address this issue, we will require responses for all survey questions for future enrollees. In addition, a question on the throbbing or pulsating quality of headache pain, part of the International Classification of Headache Disorders 3rd edition criteria for migraine (16), was not included in the original questionnaire but will be included in the future version. Finally, given the cross-sectional nature of our preliminary findings, the relationship of any observed symptom or comorbidity to migraine pathophysiology remains speculative. A more robust sample with multiple data time points will also allow for more comprehensive statistical methods including multivariate analysis such as mixed effects modeling and regression analysis.
Further exploration of the relationship between FPP, rhinosinusitis and migraine will benefit from additional registry recruitment, including persons with rhinosinusitis, head, and neck symptoms, but no history of migraine, in addition to collection and documentation of objective clinical data, including neuroimaging, sinus computed tomography and endoscopy, as well as response to therapies prescribed for migraine, allergies and rhinosinusitis. Utilization of a more comprehensive, validated tool for diagnosis of migraine, its subsets and associated symptoms, as well as collection of a wide range of symptoms (e.g., autonomic, vestibular, and otologic) may be useful in capturing migraine's full spectrum of presentation. Such data will help fill a significant knowledge gap in the literature (30), which stems from the diagnostic difficulties regarding the etiology of facial pain and pressure symptoms that may include nasal congestion and rhinorrhea in the absence of any radiologic evidence of sinus infection or inflammation.
Conclusion
This exploratory study of facial pain or pressure in a population from the HEADS Registry demonstrates an association of FPP with headache, including migraine, as well as with allergies, rhinosinusitis and related antibiotic use. The low rate of antibiotic effectiveness suggests misdiagnosis in at least a subset of those treated. Our finding of an association of FPP with autonomic, vestibular and otologic symptoms suggests neurogenic underpinnings, as distinct from an infectious etiology, but clinical characteristics may not be sufficient to make an accurate diagnosis. Uncovering these complexities and expanding the differential diagnosis to include conditions of neurological etiology, may lead to more accurate diagnostic and effective therapeutic approaches. This study has also revealed some of the limitations of the recently established HEADS registry, prompting addition and revision of questions, improvement in recruitment practices, and documentation of diagnostic studies, treatments, and outcomes. These preliminary findings and proceeding registry modifications will be key steps for the aim of ultimately using the HEADS Registry to improve outcomes for persons with neurologic, otologic and rhinologic conditions.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Advarra IRB, HEADS Registry (Pro0070553). The studies were conducted in accordance with the local legislation and institutional requirements. The participants provided their written informed consent to participate in this study.
Author contributions
DK: Conceptualization, Methodology, Writing – review & editing. GT: Conceptualization, Methodology, Writing – review & editing. GP: Conceptualization, Formal analysis, Methodology, Writing – review & editing. VH: Data curation, Writing – review & editing. FG: Conceptualization, Supervision, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. The study was supported by the Association of Migraine Disorders (AMD).
Acknowledgments
We would like to thank all individuals who participate in the registry, as well as the Coalition for Headache and Migraine Patients (CHAMP) and Vestibular Disorders Association (VeDA) for their support in recruitment. We would also like to thank Lumiio Inc. for their support in developing, launching, and operating the HEADS registry. We also thank Leigh Serth for assisting with manuscript formatting and reference preparation; Ma'ayan Epstein for reference preparation; Liam Laidlaw for assistance with data acquisition; Monica Mallimpalli, PhD for reviewing the final manuscript and providing feedback; and Elaine Alibrandi for creating facial images for Figure 2.
Conflict of interest
DEK served on advisory boards, consulted, and/or been a speaker or contributing author for AbbVie, Pfizer, Eli Lilly, Cefaly technology, the American Headache Society and JOGO health. GET is a consultant for Lundbeck and owns common stock in Johnson & Johnson. GAP is a consultant for the Association of Migraine Disorders and Cefaly Technology. VLH has nothing to disclose. FAG is the President of the Association of Migraine Disorders and consultant for Pfizer and Aerin Medical.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1625442/full#supplementary-material
References
1. Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, Brook I, Ashok Kumar K, Kramper M, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. (2015) 152(2 Suppl):S1–39.25832968
2. Hirsch AG, Stewart WF, Sundaresan AS, Young AJ, Kennedy TL, Scott Greene J, et al. Nasal and sinus symptoms and chronic rhinosinusitis in a population-based sample. Allergy. (2017) 72(2):274–81. doi: 10.1111/all.13042
3. Palmer JN, Messina JC, Biletch R, Grosel K, Mahmoud RA. A cross-sectional, population-based survey of U.S. Adults with symptoms of chronic rhinosinusitis. Allergy Asthma Proc. (2019) 40(1):48–56. doi: 10.2500/aap.2019.40.4182
4. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. (2015) 125(7):1547–56. doi: 10.1002/lary.25180
5. Donnelly JP, Baddley JW, Wang HE. Antibiotic utilization for acute respiratory tract infections in U.S. Emergency departments. Antimicrob Agents Chemother. (2014) 58(3):1451–7. doi: 10.1128/AAC.02039-13
6. Sng WJ, Wang DY. Efficacy and side effects of antibiotics in the treatment of acute rhinosinusitis: a systematic review. Rhinology. (2015) 53(1):3–9. doi: 10.4193/Rhino13.225
7. Smith SS, Evans CT, Tan BK, Chandra RK, Smith SB, Kern RC. National burden of antibiotic use for adult rhinosinusitis. J Allergy Clin Immunol. (2013) 132(5):1230–2. doi: 10.1016/j.jaci.2013.07.009
8. Lemiengre MB, van Driel ML, Merenstein D, Liira H, Mäkelä M, Sutter D, et al. Antibiotics for acute rhinosinusitis in adults. Cochrane Database Syst Rev. (2018) 9(9):CD006089. doi: 10.1002/14651858.CD006089
9. Barshak MB, Durand ML. The role of infection and antibiotics in chronic rhinosinusitis. Laryngoscope Investig Otolaryngol. (2017) 2(1):36–42. doi: 10.1002/lio2.61
10. Viana M, Khaliq F, Zecca C, Figuerola MDL, Sances G, Di Piero V, et al. Poor patient awareness and frequent misdiagnosis of migraine: findings from a large transcontinental cohort. Eur J Neurol. (2020) 27(3):536–41. doi: 10.1111/ene.14098
11. Straburzynski M, Nowaczewska M, Czapinska-Ciepiela EK, Gryglas-Dworak A, Budrewicz S, Waliszewska-Prosół M. Sinonasal symptoms in migraine without aura: results from the cross-sectional “Migraine in Poland” study. Front Neurol. (2023) 14:1321261. doi: 10.3389/fneur.2023.1321261
12. Schulz KA, Esmati E, Godley FA, Hill CL, Monfared A, Teixido M, et al. Patterns of migraine disease in otolaryngology: a CHEER network study. Otolaryngol Head Neck Surg. (2018) 159(1):42–50. doi: 10.1177/0194599818764387
13. Kari E, DelGaudio JM. Treatment of Sinus headache as migraine: the diagnostic utility of triptans. Laryngoscope. (2008) 118(12):2235–9. doi: 10.1097/MLG.0b013e318182f81d
14. Al-Hashel JY, Ahmed SF, Alroughani R, Goadsby PJ. Migraine misdiagnosis as a sinusitis, a delay that can last for many years. J Headache Pain. (2013) 14(1):97. doi: 10.1186/1129-2377-14-97
15. Lal D, Rounds A, Dodick DW. Comprehensive management of patients presenting to the otolaryngologist for sinus pressure, pain, or headache. Laryngoscope. (2015) 125(2):303–10. doi: 10.1002/lary.24926
16. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia. (2018) 38(1):1–211. doi: 10.1177/0333102417738202
17. International classification of orofacial pain, 1st edition (ICOP). Cephalalgia. (2020) 40(2):129–221. doi: 10.1177/0333102419893823
18. Lipton RB, Dodick D, Sadovsky R, Kolodner K, Endicott J, Hettiarachchi J, et al. A self-administered screener for migraine in primary care: the ID migraine validation study. Neurology. (2003) 61(3):375–82. doi: 10.1212/01.WNL.0000078940.53438.83
19. Stewart WF, Lipton RB, Dowson AJ, Sawyer J. Development and testing of the migraine disability assessment (MIDAS) questionnaire to assess headache-related disability. Neurology. (2001) 56(6 Suppl 1):S20–28. doi: 10.1212/WNL.56.suppl_1.S20
20. Yao A, Wilson JA, Ball SL. Autonomic nervous system dysfunction and sinonasal symptoms. Allergy Rhinol (Providence). (2018) 9:2152656718764233. doi: 10.1177/2152656718764233
21. Brody-Camp S, Risey JA, McCoul ED. Vestibular characteristics of patients with chronic rhinosinusitis. OTO Open. (2018) 2(4):2473974X18804993. doi: 10.1177/2473974X18804993
22. Muskat G. Induced rhinogenic vertigo, dizziness and tinnitus; is there a real pathophysiologic connection or not? Rom J Rhinol. (2017) 7(28):205–6.
23. Villar-Martinez MD, Goadsby PJ. Vestibular migraine: an update. Curr Opin Neurol. (2024) 37(3):252–63. doi: 10.1097/WCO.0000000000001257
24. Benjamin T, Gillard D, Abouzari M, Djalilian HR, Sharon JD. Vestibular and auditory manifestations of migraine. Curr Opin Neurol. (2022) 35(1):84–9. doi: 10.1097/WCO.0000000000001024
25. Noseda R. Cerebro-Cerebellar networks in migraine symptoms and headache. Front Pain Res. (2022) 3:940923. doi: 10.3389/fpain.2022.940923
26. Lv H, Liu K, Xie Y, Wang Y, Chen S, Liu P, et al. No causal association between allergic rhinitis and migraine: a Mendelian randomization study. Eur J Med Res. (2024) 29(1):78. doi: 10.1186/s40001-024-01682-1
27. Martin VT, Fanning KM, Serrano D, Buse DC, Reed ML, Bernstein JA, et al. Chronic rhinitis and its association with headache frequency and disability in persons with migraine: results of the American migraine prevalence and prevention (AMPP) study. Cephalalgia. (2014) 34(5):336–48. doi: 10.1177/0333102413512031
28. Ferretti A, Gatto M, Velardi M, Di Nardo G, Foiadelli T, Terrin G, et al. Migraine, allergy, and histamine: is there a link? J Clin Med. (2023) 12(10):3566. doi: 10.3390/jcm12103566
29. Kim JR, Park TJ, Agapova M, Blumenfeld A, Smith JH, Shah D, et al. Healthcare resource use and costs associated with the misdiagnosis of migraine. Headache. (2025) 65(1):35–44. doi: 10.1111/head.14822
Keywords: facial pain, migraine, rhinosinusitis, allergies, antibiotics, facial pressure, nasal congestion, rhinorrhea
Citation: Kuruvilla DE, Tietjen GE, Panza GA, Hodgkinson VL and Godley III FA (2025) Facial/sinus pain or pressure and migraine: exploratory findings from the HEADS registry. Front. Pain Res. 6:1625442. doi: 10.3389/fpain.2025.1625442
Received: 8 May 2025; Accepted: 24 July 2025;
Published: 21 August 2025.
Edited by:
James Russell Couch, University of Oklahoma Health Sciences Center, United StatesReviewed by:
Marta Waliszewska-Prosół, Wroclaw Medical University, PolandAnand Kumar, Banaras Hindu University, India
Copyright: © 2025 Kuruvilla, Tietjen, Panza, Hodgkinson and Godley, III. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Deena E. Kuruvilla, RGVlbmFLdXJ1dmlsbGFAZ21haWwuY29t
 Deena E. Kuruvilla
Deena E. Kuruvilla Gretchen E. Tietjen
Gretchen E. Tietjen Gregory A. Panza
Gregory A. Panza Victoria L. Hodgkinson
Victoria L. Hodgkinson Frederick A. Godley III
Frederick A. Godley III