- 1Tris Pharma, Monmouth Junction, NJ, United States
- 2Avalon Health Economics, Morristown, NJ | Miami, FL, United States
- 3Albany Medical College, Albany, NY, United States
The prevalence of acute pain has grown substantially over the past two decades, due primarily to more surgeries, an aging population, and the rapid growth in the prevalence of metabolic disease. Although opioids are often the only effective treatment for many types of acute pain, especially severe acute pain, their use, even over a short period of time, comes with substantial risks of dependence, misuse, and diversion. Moreover, a large fraction of the patients currently suffering from opioid use disorder and those dying from opioid overdoses had their first exposure as pain patients. Conversely, refraining from using opioids in cases where other treatment options are ineffective creates a different set of risks. This potential undertreatment of acute pain, especially severe acute pain, increases the risk of acute pain transitioning to chronic pain. The use of opioids to treat acute pain and the ineffective treatment of acute pain have important implications for population health and health care costs.
1 Introduction
Managing acute pain in the United States remains a critical clinical and public health challenge. Since 1998, the prevalence of pain among U.S. adults has increased by 25% (1). Pain is reported in nearly 80% of emergency department (ED) visits in the U.S., with a median reported pain intensity score of 8 out of 10 on numeric rating scales (2). The prevalence of moderate to severe acute pain continues to rise, often stemming from post-operative procedures but also associated with the rising prevalence of metabolic disorders and an aging population. The rising prevalence of pain has imposed a significant burden on both patients and healthcare systems. Moreover, individuals with poorly treated acute pain are at greater risk for developing chronic pain, which further widens the treatment gap. Although opioids are an effective treatment for acute pain, especially severe acute pain, providers and patients have, understandably, approached opioid-based treatments with trepidation, and clinical practice guidelines have not completely resolved these apprehensions in the wake of the opioid crisis in the U.S. Consequently, the aftermath of the opioid crisis has resulted in a greater risk of the undertreatment of acute pain.
Acute pain is defined by the International Association for the Study of Pain (IASP) as pain that “happens suddenly, starts out sharp or intense, and serves as a warning sign of disease or threat to the body. It is caused by injury, surgery, illness, trauma, or painful medical procedures and generally lasts from a few minutes to less than six months.” (3). The IASP definition further notes that “acute pain usually disappears whenever the underlying cause is treated or healed.” Examples of acute pain include pain associated with surgery, musculoskeletal injuries (e.g., broken bones, sprains, and strains), dental procedures, soft tissue injuries (e.g., cuts, puncture wounds, burns), blunt force trauma, labor and childbirth, and headaches (4). Subacute pain, as labeled by the U.S. Centers for Disease Control and Prevention (CDC), is generally defined as pain lasting one to three months. However, the literature focused on subacute pain does not consistently apply this threshold, with some sources classifying acute pain as episodes of six weeks or less in duration, and subacute with episodes of seven to twelve weeks. While the duration of subacute pain may in some cases overlap with that of acute pain, it is generally recognized as a separate clinical stage, whereas chronic pain generally extends beyond the three-month mark (5, 6).
Aside from different durations of pain there are different pain conditions, e.g., neuropathic pain, visceral pain, inflammatory pain, etc. that may respond differently to treatment as a result of their underlying mechanisms and characteristics (7). For example, neuropathic pain is usually characterized as damage to the nerve, often leading to burning, aching, or stabbing sensations and can either be acute or chronic. In contrast, inflammatory pain is typically caused by inflammation in tissues like joints or muscles, and tends to be relatively more localized, examples of which include musculoskeletal pain, trauma-related pain, dental pain, and post-surgical pain (8). Understanding these causal pathways is critical to improving patient outcomes, preventing misuse of prescription pain medications, and reducing the undertreatment of pain. In turn, better treatment of pain has the potential to also reduce the total costs of treatment. The focus of this paper is to identify and further explore the causes of the treatment gap in acute pain, highlighting the economic and clinical impacts of undertreatment. In the sections that follow, we discuss these dynamics, focusing on the challenges associated with the use of opioids in first-line treatment of acute pain.
2 Demand
The demand for pain treatment in the U.S. and globally has been increasing, primarily for three reasons: (1) an increase in the total number and rate of surgical procedures; (2) an increase in the proportion of the population over the age of 65, along with a lengthening of life expectancy; and (3) an increase in the prevalence of metabolic diseases, such as obesity and diabetes.
There has been an increase in the overall volume of surgical procedures. Apart from a slight dip during the COVID-19 pandemic, rates of surgeries in the U.S. have been steadily increasing for decades (9). This is especially the case for orthopedic procedures, where in the two-decade period from 2000 to 2019, the estimated annual volume of total hip arthroplasty (THA) and total knee arthroplasty (TKA) increased by 177% and 156%, respectively (10). In addition to increased demand for pain management associated with surgical volume, concomitant trends toward shorter lengths of hospital stays and migration of care to outpatient settings has further magnified demand for pain treatment (11, 12).
In addition, the proportion of the U.S. population that is aged 65 and over has grown steadily over the past several decades, and is expected to more than double by 2040 (13). Increased aging of the population, increased life expectancy, and improved survival rates from cancer and other chronic diseases has added further demands on the health system to develop more effective means of controlling acute and chronic pain for a growing number of individuals who are living longer with acute and chronic health problems (14, 15). Among the most worrisome trends in the prevalence of chronic health problems has been the steep growth in prevalence of metabolic diseases, such as obesity and diabetes, both of which are associated with increased prevalence of acute pain. Beyond the impact on acute pain, research has also shown that metabolic disorders, specifically those involving abnormal glucose metabolism, have been identified as significant risk factors for the development and persistence of chronic pain (16, 17). For example, Mäntyselkä et al. observed that diabetes mellitus and elevated plasma glucose were associated with daily chronic pain, increasing the risk of chronic pain in adults by almost 2.5 times (17).
From 2000 to 2018, obesity prevalence increased an unprecedented 39%, and rates of severe obesity increased an alarming 96% over the same time period (18). The connection between obesity and pain is both direct and indirect. The direct effect has been observed in studies of reported pain by individuals with varying degrees of obesity. For example, Hitt et al. found that Class II obese respondents (i.e., those with BMI of 35 to 39.9) were 1.9 times more likely to report severe pain, and Class III obese individuals were 2.3 times more likely to report severe pain (19). Although the causal pathways between obesity and chronic pain remains unclear, as a result of complex and bidirectional mechanisms, evidence has shown that individuals with obesity can have up to 45% higher risk of chronic pain compared to those of normal weight (20). This may be due in part to the fact that obesity can promote insulin resistance and accelerate the progression of peripheral neuropathy (21). The mechanisms associated with obesity also impact the overall burden and undertreatment of acute pain. Individuals with obesity demonstrated more than double the odds of intra-procedural acute pain compared to those without obesity (22). Additionally, pain management remains a challenge within this population, as the use of opioids, particularly among those with obstructive sleep apnea, is associated with increased risk of opioid-induced central sleep apnea and respiratory depression, which can result in higher mortality (23). These safety concerns often limit pain management, potentially further contributing to the undertreatment of acute pain.
The second pathway linking obesity and demand for pain treatment is indirect, as obesity is causative of a variety of serious health conditions that increase the need for medical care services, including pain management. Generally, these conditions include, but are not limited to, coronary artery disease, heart failure, cardiac arrhythmia, stroke, insulin resistance, diabetes mellitus, hypertension, sleep apnea, arthritis, musculoskeletal conditions, and certain types of cancer (24–27). Diabetes, in particular, is projected to affect 10.9% of the global population by 2045, an increase from 9.3% in 2019, with significant implications for pain management (28). Diabetes complications, such as neuropathy and poor wound healing, contribute to both acute and chronic pain, complicating treatment (29). In addition, surgical interventions related to diabetes, such as amputations, often result in severe pain, further driving the demand for pain management interventions. Growth in metabolic diseases has largely overlapped with the aging of the population, the cumulative effect of which has been a marked rise in the demand for pain management (30); since 1998, pain prevalence in U.S. adults has increased more than 25% (1).
3 Treatment
There are several treatments and approaches aimed at the management of acute pain. These approaches are generally categorized as non-pharmacologic and pharmacologic treatments. Non-pharmacological treatments include, for example, physical therapy, biofeedback, cognitive behavioral therapy, and massage therapy. While these types of treatments have been shown to be effective in some instances, their efficacy is generally limited to mild to moderate pain and by lower rates of adherence and barriers to access (31). Pharmacologic treatments are typically partitioned into opioid vs. non-opioid drugs such as acetaminophen and non-steroidal anti-inflammatory drugs (NSAIDs). In practice, optimal pain management frequently relies on a multimodal approach that combines these therapies. For procedures such as total knee arthroplasty, peripheral nerve blocks are commonly used to manage postoperative pain; however, protocols like those at Brigham and Women's Hospital (BWH) indicate that these blocks are typically administered either as a one-time dose or as a continuous infusion for only a brief period following surgery, often discontinued by the morning of the first postoperative day (32). Therefore, while opioids are generally reserved for moderate to severe pain, they are often relied upon for longer-term pain management following a procedure, as there have been no analgesics superior or as effective as opioids.
3.1 Serious adverse events
There is understandable trepidation regarding the use of opioids for pain. The serious adverse events (SAEs) and adverse drug reactions (ADRs) associated with opioids have been broadly discussed in the literature, increasingly in recent years due to the opioid crisis and the revised CPGs regarding acute pain. As current pain treatment guidelines generally acknowledge, despite their efficacy, opioids are associated with several important SAEs, notably physical dependence, euphoria, risk of overdose, and respiratory depression, which together can lead to significant negative outcomes. In addition, opioids are also associated with substantial humanistic and economic burden, mainly because they are linked to greater risk of misuse and diversion, both of which have the potential to magnify the risks associated with dependence and overdose.
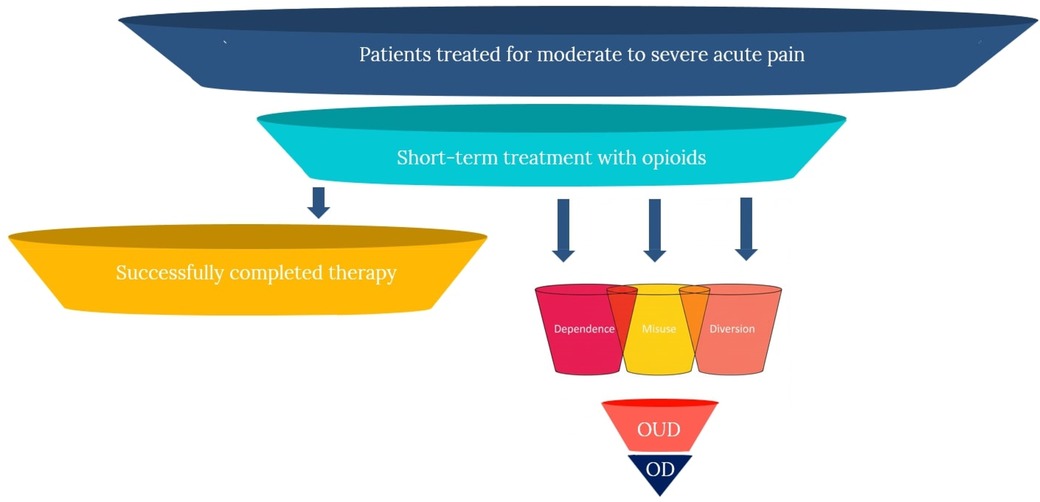
As such, opioids are also associated with what economists refer to as “negative externalities,” where the negative effects of consumption extend beyond proximal clinical effects (i.e., SAEs and ADRs), and have the potential of generating substantial “secondary” harms in the community. Accordingly, in the case of opioids, it is more appropriate to consider the tradeoffs and limitations more broadly, as inclusive of these primary and secondary effects, resulting in the following five domains of negative effects: (1) risk of dependence; (2) risk of misuse; (3) risk of progression to use disorders; (4) risk of overdose; and (5) risk of diversion and secondary harms. The figure below illustrates the potential patient pathways following short-term opioid treatment for acute pain, demonstrating both successful resolution and progression to various harms. These effects are portrayed in Figure 1 below.
3.2 Dependence
The risk of physical dependence following treatment with opioids is an important clinical consideration and has also been a source of trepidation among patients considering opioid-based treatment approaches (33, 34). Opioid dependence develops in 11%–13% of patients initially prescribed opioids (35, 36). Schroeder et al., for example, in a matched case control study of opioid-naive adolescents and young adults, found that index opioid prescriptions were associated with a 6.8% absolute risk increase in persistent opioid use after dental procedures, which are usually associated with relatively short duration of pain (37). Moreover, opioid dependence can occur over a relatively short period of time, with some studies showing dependence occurring as soon as five days after an opioid prescription is obtained (38), and in some cases even after the patient no longer reports experiencing pain symptoms (39). This suggests that opioid use may continue beyond the need for pain relief, but instead due to emerging dependence.
In a large study of opioid-naive patients (surgical and non-surgical) all of the surgical procedures were associated with an increased risk of chronic opioid use, with odds ratios ranging from 1.28 for cesarean delivery to 5.10 for TKA (40). In another large study of opioid-naive patients undergoing short-stay surgery, Alam et al. found that patients receiving an opioid prescription within 7 days post-surgery were 44% more likely to become long-term opioid users within one year, compared to controls (41). Similar patterns have been observed among hospital discharges more generally; for example, Calcaterra et al. found that opioid receipt within 72 h of discharge was associated with increased likelihood of chronic opioid use and greater subsequent opioid refills one year post-discharge, compared to controls (42). Discontinuing therapy or reducing dosage, even when tapered per FDA approved prescribing information and medical guidelines, has been shown to be challenging and has a relatively high likelihood of failure (43, 44). Often tapering results in opioid use lasting significantly longer than necessary to alleviate symptoms (44), and tapering has also been shown to increase the probability of ED visits and hospitalizations (45).
3.3 Misuse
Data from the 2021 National Survey on Drug Use and Health (NSDUH) showed that, of the 68.5 million (27.0%) U.S. civilian adults who used prescription opioids, 8.3 million (12.1%) misused them (46). However, studies of misuse have shown that rates can vary substantially depending on population, methods, and timeframe, with some estimates of the prevalence of misuse as high as 29% (47). Apart from the aforementioned elevated risk of dependence and addiction, opioids are particularly prone to misuse because they cause euphoria, often even at doses needed for analgesia (48). This is the driving factor contributing to opioid misuse; often patients receive an initial prescription, experience feelings of euphoria, and then are induced to subsequently misuse, in many cases after pain has subsided.
Studies have shown that the experience of euphoria plays a significant role in driving the desire to continue using opioids beyond the point of medical need (49, 50). In a study of emergency department patients treated for pain with opioids, 25% reported experiencing pain relief in combination with sensations of being “high” and feelings of euphoria (51). In another study of ED patients with acute pain, patients randomized to opioids were significantly more likely to report that the medications were associated with feelings of “blissfulness.” (50) This euphoric sensation can trigger drug-seeking behavior, leading patients to misuse the medication in an attempt to recreate the euphoric feeling (52, 53). In turn, opioid seeking behavior can result in individuals cycling among euphoria, withdrawal, and craving, a process that is associated with increasing rates of misuse (54, 55) and, ultimately, poor treatment outcomes (56, 57). These effects have been shown to be dose dependent. For example, in a large study of commercially insured patients, Brat et al. found that total duration of opioid use was a strong predictor of misuse, with each refill and additional week of opioid use associated with an adjusted increase in the rate of misuse of 44% (58).
The risk profile for opioids also includes factors that transcend the traditional concepts of SAEs and ADRs. One of the most important of these differences is the propensity for prescription opioids to be “diverted” for non-medical use. Several studies have closely examined the sources of misused opioids. One of the most comprehensive of these studies is by Park and Wu, who analyzed data from the NSDUH (59). Across all age and sex categories, the authors found that 36.6% of individuals who reported opioid misuse during the past year obtained the opioids directly from the health system. The largest fraction (40.3%) obtained opioids unlawfully from friends and family members, and another 23.1% obtained them unlawfully from a variety of other sources, including drug dealers. These results are broadly consistent with other studies. For example, Schepis et al., in two different studies, found very consistent results within similar age groups (60, 61).
3.4 Progression to use disorder
The cycle of acute pain, opioid dependence, addiction, misuse, and poorly managed pain leaves individuals at substantially higher risk for progression to substance use disorders. The term “opioid use disorder” (OUD), which is listed in the American Psychiatric Association DSM-5, generally refers to repeated or consistent misuse of opioids, and includes more clinically serious forms of abuse or addiction (62). According to estimates based on the 2021 NSDUH, among US adults with past-year prescription opioid use, 4.8 million (7.0%) had prescription opioid use disorder (46). Similar to estimates of rates of misuse, rates of OUD vary depending on population, methods, and timeframe, with some estimates of OUD as high as 12% (47). Prescription opioids can in some cases serve as a “gateway” to non-medical use and progression to OUD (63). For example, Butler et al. found that among ED patients reporting nonmedical opioid use or heroin use, 59% indicated initial exposure via prescription opioids (64). A large study of dental patients conducted by Schroeder et al. found that 5.8% of opioid-exposed individuals experienced one or more subsequent health care encounters associated with an opioid abuse-related diagnosis, compared with 0.4% in the control group (37). These effects have also been shown to be dose dependent. In a large study of adolescents and young adults with opioid prescription claims in a large commercial claims database, Chua et al. found that each increase in daily opioid dosage category was associated with higher overdose risk [adjusted odds ratio (AOR), 1.18; 95% CI, 1.05–1.31] (65). In some cases prescription opioids can also act as a gateway to abuse of other more potent forms of opioids, including heroin and synthetic fentanyl, as well as progression to other forms of SUD (66, 67).
3.5 Overdose
While most definitions of overdose associated it with “excessive use,” more recent definitions have focused instead on physiology; for example, “a condition or physical state produced by the ingestion, injection, inhalation of or exposure to a deleterious agent.” (68). Individuals who use opioids are at increased risk for drug overdose (69, 70). Opioid overdose can include a mix of severe reactions, including hypoxia, respiratory distress, peripheral vasodilatation and hypotension, nausea and vomiting, psychiatric distress (e.g., anxiety, agitation, and dysphoria), and seizures (71).
The risk of overdose is particularly high among individuals who misuse opioids, for several reasons. First, those who misuse may take higher doses due to euphoria-seeking behavior (72). Second, misuse often progresses to non-oral administration as a means of obtaining a more rapid onset of effects (73), thereby giving the body less time to adapt to the respiratory depressant effects and increasing the risk of overdose. Opioid overdose is typically accompanied by respiratory depression, which is characterized by life-threatening changes in breathing frequency and oxygen saturation (74, 75). This risk is substantially greater in patients who are concomitantly prescribed other drugs that have the potential to inhibit respiratory function, such as benzodiazepines, gabapentin/pregabalin, first generation antihistamines, and tranquilizers (76). These drugs are often prescribed alongside opioids (77, 78). For example, individuals experiencing acute or chronic pain are more likely to also experience anxiety, and are therefore more likely to be prescribed benzodiazepines (79). Similarly, individuals experiencing acute or chronic pain are also more likely to be prescribed other pain medications, such as gabapentin (80, 81). First-generation antihistamines are also often used alongside opioids, typically to alleviate opioid side effects, such as pruritis and nausea (82).
These co-prescribed treatments have been shown to affect respiratory function additively and synergistically with opioids (77, 78, 83). For example, in 2016, the FDA issued its strongest warning against prescribing benzodiazepines alongside opioids due to evidence of increased risk of overdose and mortality (84). Similar concerns have been raised regarding opioid use concurrently with gabapentin and pregabalin (85–87). In addition, alcohol can potentiate opioid-induced respiratory depression (88). The risk of overdose is significantly greater in patients with underlying respiratory conditions, such as sleep apnea or asthma, and in patients with renal or hepatic impairment, as those conditions can significantly increase exposure to opioids (89–91). For example, Boitor et al. found that patients diagnosed with renal failure were more than two times more likely to experience opioid-induced respiratory depression compared to those without diagnosed renal failure (83). Often, renal and hepatic impairment is undiagnosed or can be acute due to infection or adverse effects of other drugs, thereby increasing risk of opioid-induced respiratory depression. Moreover, tolerance to opioid effects can develop rapidly, causing the need to increase dose levels; however, tolerance to the respiratory effects develops more slowly (92). Thus, as dosage increases, individuals with these comorbid conditions are at substantially increased risk of overdose.
In addition to co-prescribing with other drugs that can suppress respiratory function, opioid use disorder is also associated with “polysubstance use” (PSU), which is generally defined as the misuse of more than one drug at the same time or over a relatively short period (93). In 2019, among all deaths attributable to some form of drug overdose, nearly 50% involved observable PSU (93). Similarly, most opioid deaths involve PSU (94). For example, Morley et al. analyzed data from the Global Drug Survey and found that misuse of illicit drugs was associated with a four-fold greater likelihood of opioid misuse (95). Consequently, the risk of overdose and respiratory depression increases significantly when opioid use or misuse is accompanied by PSU, which can include non-medical use of the aforementioned co-prescribed drugs (especially benzodiazepines and tranquilizers), alcohol, cocaine, and other illicit drugs. The added risk associated with PSU is evident in much of the data on opioid mortality (96).
4 Treatment gaps
Substantial gaps in acute pain treatment persist with the rise in prevalence of acute pain, despite the availability of effective treatments, (30). For example, in a study of patients at a tertiary care hospital, about 50% of patients reported that their pain had not been relieved (97). Another study found that only 60% of ED patients received pain medications, but 74% continued to report moderate to severe pain upon discharge (98). Similarly, a large national survey found that more than 80% of patients experienced acute post-surgical pain within two weeks of discharge, and most of those patients indicated that their pain was moderate to severe (99).
4.1 Opioids
In many cases, moderate to severe acute pain can only effectively be treated with opioids (100–102). As mentioned previously, no other analgesics have demonstrated superior or even similar efficacy to opioids for the treatment of nociceptive pain. However, despite their clinical effectiveness, the use of opioids is, understandably, associated with considerable apprehension, for many of the reasons discussed in the previous section. Apprehension regarding opioid use to treat moderate to severe acute pain is generally attributable to four factors: (1) uncertainty on the part of providers to prescribe opioids, due to challenges in assessing risk-benefit tradeoffs and perceptions regarding opioid prescribing guidelines; (2) uncertainty on the part of patients, who also experience challenges understanding risk-benefit tradeoffs; (3) increased prevalence of state-level policies, which have either substantially restricted access to opioids or generated uncertainty regarding appropriate use and access to opioids; and (4) underappreciation of the long-term consequences of ineffectively treating acute pain. However, given the relatively recent prominence of opioid information in the medical literature and mainstream media, especially regarding the crisis of addiction and overdose, the ascension in the importance of these factors is not particularly surprising. More importantly, these factors have direct medical consequences, because each one of the factors has the potential to impede access to effective management of acute pain.
4.2 Providers
Uncertainty and reluctance on the part of providers to prescribe opioids has been a substantial impediment to the management of acute and chronic pain (30). Provider reluctance and uncertainty regarding opioids intensified during the opioid crisis, with some providers expressing general concerns regarding dependence, misuse, addiction, and diversion (103–106). In a recent comprehensive review of the literature on physician attitudes toward opioid prescribing, Bell et al. identified five common themes regarding opioid prescribing challenges: (1) uncertainty stemming from the subjectivity of pain; (2) concern regarding patient SAEs and community effects, such as diversion; (3) previous negative experiences, such as negative professional feedback and patient feedback; (4) uncertainty and confusion regarding interpretation and implementation of CPGs; and (5) conflicting institutional policies and burdensome administrative requirements (107). More generally, another contributing factor leading to gaps in acute pain management has been lack of consistency on the part of providers in following acute pain CPGs. For example, a recent systematic review identified 20 pain management quality measures, but only three of which focused specifically on postoperative pain management, leading the authors to conclude that “these results indicate a lack of measures to guide and promote quality postoperative pain management.” (108). Again, given the high level of attention garnered by the crisis of addiction and overdose, these reactions on the part of providers have been understandable. Perhaps more importantly, there exist no readily available solutions to provider reluctance and apprehension.
4.3 Patients
Many of the same factors affecting provider prescribing have also impacted patients (102). Fear of longer-term dependence and addiction are generally the main concerns expressed by patients (33). In addition, for some patients there is a perceived stigma associated with opioid use, and for these patients, opioid use can trigger feelings of shame, which can be exacerbated by uncertainty regarding risk of dependence and addition and discomfort in communicating concerns to their providers (109). Even patients who have been previously treated with opioids share some of these concerns; for example, Vargas-Schaffer et al. found that, in a sample of longer term opioid users, 40% maintained a “negative attitude” toward opioids, compared to 32% and 22% with positive and neutral attitudes, respectively (110). Again, reluctance on the part of patients is not unexpected given that many of their providers share the same reluctance.
4.4 Guidelines and policy
In addition to provider and patient factors, state and federal policy has impacted the use of opioids for moderate to severe acute pain. Prior to 2016, clinical practice guidelines (CPGs) for the treatment of acute pain generally considered opioids as first-line treatment, primarily due to better efficacy relative to alternative treatments (102, 111, 112). In 2016, the U.S. Centers for Disease Control and Prevention (CDC) issued opioid prescribing guidelines in response to increasing rates of opioid dependency and overdose, with the main thrust of the guidelines aimed at encouraging greater caution in prescribing of opioids for acute and chronic pain (113). However, the CDC guidelines were by most accounts “over interpreted,” resulting in the exacerbation of many of the problems that prompted opioid prescribing initially; that is, the management of the escalating prevalence of acute and chronic pain (114). Specifically, the CDC guidelines resulted in an increased propensity for providers to be overly rigid regarding dosage, duration, tapering, and discontinuation, and to misapply the guidelines to populations outside of the guideline scope (e.g., the misapplication of chronic pain recommendations to acute pain management) (115). Moreover, following the original 2016 CDC guidelines, one study found that only two thirds of opioid prescribing physicians were even aware of the existence of the guideline (116). While no current study directly links the introduction of recent CDC opioid prescribing guidelines to increased rates of chronic postsurgical pain, there is strong and consistent evidence in the literature that inadequate management of acute pain, especially during the postoperative period, significantly increases the risk of chronic pain (117–121). For example, Zhang et al. specifically examined how mechanisms across multiple domains such as epigenetic changes, including DNA methylation and histone modifications that alter the expression of pain-related genes, can contribute to the development of chronic pain, emphasizing that the timing of intervention is crucial for disrupting this progression and preventing pain chronification (121).
Some believed that the CDC guidelines were either unclear or advocated for excessive levels of caution, prompting the CDC to release revised guidelines in 2022 (122). However, the main objective of the 2022 revisions was largely the same, encouraging providers to exercise extreme caution regarding opioid utilization, and consider opioid treatments only in cases where non-opioid treatments have failed or where the likelihood of SAEs precluded non-opioid options. Although much of the focus of the 2016 CDC opioid guidelines was on the overuse of opioids in the treatment of chronic pain, the guidelines also addressed acute pain by recommending limiting opioid use for acute pain to 3–7 days (113). Professional medical societies generally followed the CDC guidance in issuing their own acute pain treatment CPGs (123–127).
Following the CDC guidelines, many states in the U.S. began to implement their own restrictions and recommendations. As of 2021, 29 states had some kind of limitation on the duration of opioid prescriptions, with 15 states imposing moderate restrictions, such as limiting initial prescriptions to 7 days. Three states restricted prescriptions to a 30-day supply, and 10 states had more stringent limits, including restrictions based on dosage. States like West Virginia, Kentucky, and Florida have implemented some of the more restrictive measures (128). Overall, these policies have been shown to have resulted in substantial reductions in opioid prescribing (129). Moreover, at the federal level, Congress recently introduced H.R. 5172, the “Non-Opioids Prevent Addiction in the Nation Act,” which is conventionally known as the “NO PAIN Act.” The Act would essentially increase financial incentives, via reimbursement mechanisms, to prescribe non-opioid treatments. In addition, heightened scrutiny by the U.S. Drug Enforcement Administration (DEA) regarding opioid manufacturing, shipments and utilization (130). The burden of acute pain may be exacerbated with the focus of these guidelines and policies shifting from the treatment of pain to the mitigation of treatment side effects, in light of the current treatment options.
4.5 Pain progression
The consequences of insufficient treatment of acute pain extend beyond short-term patient comfort. A particularly important issue that leads to significant patient clinical and economic burden is the progression of poorly treated acute pain to chronic pain, sometimes referred to the “chronification” of acute pain (117, 118, 131–136). There is evidence of this process occurring, with the proportion of post-surgical patients developing chronic pain (i.e., chronic post-surgical pain, or CPSP) in the range of 10%–40%, depending on the type of surgery (137, 138). Hip arthroplasty, for example, has been associated with a 27% risk of CPSP, and knee arthroplasty associated with a 13%−44% risk of CPSP (138). In addition to chronic post-surgical pain, there is also evidence of chronification of acute pain following ED visits. For example, in a study of ED patients, those who departed the ED with a high pain score (e.g., 7/10 or higher) were significantly more likely to develop chronic pain within 90 days (139). In addition, another long-term negative outcome of ineffective pain treatment and resulting chronification is that it has been shown to increase the likelihood of dementia in the elderly (140–142).
4.6 Economic impact
Despite the increase in the prevalence of moderate to severe acute pain, opioid prescriptions have fallen dramatically over the past decade, particularly after the release of the CDC guidelines and the implementation of state policies restricting opioid use (143). After a period of steadily increasing rates from the late 1990s until around 2012, opioid prescription rates began to steadily fall and continued to fall through 2020, from about 250 million prescriptions in 2012 to less than 150 million prescriptions in 2020 (144). Due to apprehensions regarding opioids, over that time period, opioids have been largely replaced by less effective non-opioid options.
The high prevalence of pain, the undertreatment of acute and chronic pain, and the progression of undertreated pain is associated with significant economic burden. The attributable costs of pain, including pain treatment, have been found to vary considerably depending on causal pathways and type and intensity of treatment. Some broad estimates were developed by Gaskin and Richard, who estimated the total costs of pain (acute and chronic) in the U.S. using data from the Medical Expenditure Panel Survey (MEPS), finding that the attributable “excess” healthcare costs of pain summed to nearly $436 billion (in 2024 dollars) (145).1 The impact of pain on work productivity, as measured by absenteeism, presenteeism, and depressed wages, added another $487 billion, bringing the total attributable cost of pain to $923 billion (in 2024 dollars). Their analysis also found that moderate pain doubled healthcare costs, whereas severe pain tripled these costs. Notably, these costs, which were based on 2010 data, are likely an underestimate of total attributable pain costs in more recent years, mainly due to the increasing prevalence of acute pain and the rapid growth of interventional pain techniques, the utilization of which increased more than two-fold from 2000 to 2013. (146) Private insurers bear the largest proportion of healthcare costs attributable to pain (43%) (147).
Moreover, in addition to these costs are the many indirect expenses tied to the use of opioids for pain management, including the long-term impact of opioid use disorder, misuse, and overdose. In 2017 alone, the total economic burden of OUD and opioid-related fatal overdoses in the U.S. was approximately $1.02 trillion, including $92 billion from lost productivity and $23 billion from criminal justice-related expenditures (148). As previously discussed, many physicians have historically opted to treat pain with opioids; however, the substantial healthcare and societal costs associated with opioid use disorder and overdoses indicate that it may be time to adopt alternative, non-opioid approaches to pain management. The rising indirect and societal costs mentioned above has partially played a role in shifts in opioid prescribing practices.
5 Discussion
The challenge of managing acute pain with opioids is a difficult conundrum, as both prescribing and not prescribing opioids present significant risks. On one hand, opioid use for acute pain poses significant risks for adverse effects, such as dependence, misuse, OUD, overdose, and increased healthcare utilization and costs. Patients who are prescribed opioids postoperatively or for acute pain often experience higher rates of persistent opioid use, which in turn leads to more ED visits, hospitalizations, and the need for treatment. Moreover, opioids prescribed to individuals with pain are frequently diverted to others who misuse them. While opioids are effective at managing severe to moderate pain, the risk of associated adverse events and potential for misuse make it a challenging option.
On the other hand, withholding or restricting prescribing opioids due to these risks can lead to undertreatment of acute pain, which itself has serious consequences. The undertreatment of acute pain can result in the chronification of pain. Patients who do not receive appropriate pain management are more likely to suffer from poor recovery, worse outcomes, and long-term disability, resulting in diminished quality of life. The fear of OUD and the apprehension surrounding opioid use have led to more conservative prescribing practices by physicians and patients often refusing treatment, leaving some patients undertreated. This situation highlights the need for innovative pain management alternatives that are as effective as opioids but without the associated risks of dependence, misuse, progression to use disorders, and overdose. The healthcare system also faces systematic barriers, such as practice guidelines and state and federal regulations that restrict the prescription of opioids even for patients for whom the risk-benefit tradeoff favor prescribing. Consequently, these restrictions, intended to curb misuse and address the ongoing opioid crisis, often result in the undertreatment of patients.
6 Conclusions
The current approach to managing acute pain continues to be a difficult balancing act. The use of opioids, while often necessary to effectively manage acute pain, carries significant risks, including dependence, misuse, OUD, and overdose. On the other hand, not treating pain adequately can lead to worse short-term outcomes, the chronification of acute pain, and poor long-term outcomes. As a result, there is compelling demand for new and innovative pain management options that offer the efficacy of opioids without the associated risks. When such options are available, it is important that guidelines are reformed in order to appropriately treat pain while also minimizing harm and preventing opioid misuse, especially as we continue to address the opioid crisis.
Author contributions
JH: Project administration, Supervision, Writing – review & editing. JS: Conceptualization, Methodology, Project administration, Writing – original draft, Writing – review & editing. MD: Writing – review & editing. DF: Writing – review & editing. CA: Project administration, Writing – review & editing. EO: Visualization, Writing – review & editing. JP: Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was supported by Tris Pharma.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Footnote
1. ^Data reported by authors in 2010 dollars, trended forward to 2024 using the U.S. Consumer Price Index (“CPI”).
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Nahin RL, Sayer B, Stussman BJ, Feinberg TM. Eighteen-year trends in the prevalence of, and health care use for, noncancer pain in the United States: data from the medical expenditure panel survey. J Pain. (2019) 20(7):796–809. doi: 10.1016/j.jpain.2019.01.003
2. Kent M, Tighe P, Belfer I, Brennan TJ, Bruehl S, Brummett C, et al. The ACTTION-American pain society-American academy of pain medicine pain taxonomy (AAAPT): a multidimensional approach to classifying acute pain conditions. J Pain. (2017) 18(5):479. doi: 10.1016/j.jpain.2017.02.421
3. IASP. Acute Pain. Washington, D.C.: International Association for the Study of Pain (2021). Available online at: https://www.iasp-pain.org/resources/topics/acute-pain/
4. NINDS. Pain. Washington, D.C.: National Institute of Neurological Disorders and Stroke (2024). Available online at: https://www.ninds.nih.gov/health-information/disorders/pain
5. CDC. Opioid Therapy and Different Types of Pain. Atlanta, GA: U.S. Centers for Disease Control and Prevention (2024). Available online at: https://www.cdc.gov/overdose-prevention/manage-treat-pain/index.html
6. Chanda ML, Alvin MD, Schnitzer TJ, Apkarian AV. Pain characteristic differences between subacute and chronic back pain. J Pain. (2011) 12(7):792–800. doi: 10.1016/j.jpain.2011.01.008
7. Chou R, Wagner J, Ahmed AY, Blazina I, Brodt E, Buckley DI, et al. Treatments for Acute Pain: A Systematic Review. Rockville (MD): U.S. Agency for Healthcare Research and Quality (AHRQ) (2020).
8. Bennett GJ. Can we distinguish between inflammatory and neuropathic pain? Pain Res Manag. (2006) 11:11A–5A. doi: 10.1155/2006/237251
9. Mattingly AS, Rose L, Eddington HS, Trickey AW, Cullen MR, Morris AM, et al. Trends in US surgical procedures and health care system response to policies curtailing elective surgical operations during the COVID-19 pandemic. JAMA Netw Open. (2021) 4(12):e2138038. doi: 10.1001/jamanetworkopen.2021.38038
10. Shichman I, Roof M, Askew N, Nherera L, Rozell JC, Seyler TM, et al. Projections and epidemiology of primary hip and knee arthroplasty in medicare patients to 2040–2060. JB JS Open Access. (2023) 8(1):e22.00112. doi: 10.2106/JBJS.OA.22.00112
11. Charipova K, Gress KL, Urits I, Viswanath O, Kaye AD. Management of patients with chronic pain in ambulatory surgery centers. Cureus. (2020) 12(9):e10408. doi: 10.7759/cureus.10408
12. Park R, Mohiuddin M, Arellano R, Pogatzki-Zahn E, Klar G, Gilron I. Prevalence of postoperative pain after hospital discharge: systematic review and meta-analysis. Pain Rep. (2023) 8(3):e1075. doi: 10.1097/PR9.0000000000001075
14. Jairam V, Yang DX, Verma V, Yu JB, Park HS. National patterns in prescription opioid use and misuse among cancer survivors in the United States. JAMA Netw Open. (2020) 3(8):e2013605. doi: 10.1001/jamanetworkopen.2020.13605
15. Zajacova A, Grol-Prokopczyk H, Zimmer Z. Pain trends among American adults, 2002–2018: patterns, disparities, and correlates. Demography. (2021) 58(2):711–38. doi: 10.1215/00703370-8977691
16. Ma L, Wang Y, Zhao Y, Sun M, Zhu T, Zhou C. The relationship between abnormal glucose metabolism and chronic pain. Cell Biosci. (2025) 15(1):86. doi: 10.1186/s13578-025-01430-w
17. Mäntyselkä P, Miettola J, Niskanen L, Kumpusalo E. Chronic pain, impaired glucose tolerance and diabetes: a community-based study. Pain. (2008) 137(1):34–40. doi: 10.1016/j.pain.2007.08.007
18. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of Obesity and Severe Obesity Among Adults: United States, 2017–2018 (NCHS Data Brief, No 360). Hyattsville, MD: National Center for Health Statistics (2020).
19. Hitt HC, McMillen RC, Thornton-Neaves T, Koch K, Cosby AG. Comorbidity of obesity and pain in a general population: results from the Southern pain prevalence study. J Pain. (2007) 8(5):430–6. doi: 10.1016/j.jpain.2006.12.003
20. Zhong Y, Tian K, Zhu Y, Li Y. Chronic pain and obesity in community-dwelling adults: findings from the national health and nutrition examination survey. J Pain Res. (2024) 17:3115–25. doi: 10.2147/JPR.S470855
21. Bonomo R, Kramer S, Aubert VM. Obesity-associated neuropathy: recent preclinical studies and proposed mechanisms. Antioxid Redox Signal. (2022) 37(7–9):597–612. doi: 10.1089/ars.2021.0278
22. Dong P, Wang H, Yan F, Zhang Z. Risk of acute pain in obese patients undergoing atrial fibrillation ablation. J Pain Res. (2025) 18:2549–57. doi: 10.2147/JPR.S517820
23. Cooney MF. Optimizing acute pain management in the obese patient: treatment and monitoring considerations. J Perianesth Nurs. (2016) 31(3):269–76. doi: 10.1016/j.jopan.2015.12.008
24. Afshin A, Forouzanfar MH, Reitsma MB, Sur P, Estep K, Lee A, et al. Health effects of overweight and obesity in 195 countries over 25 years. N Engl J Med. (2017) 377(1):13–27. doi: 10.1056/NEJMoa1614362
25. Pi-Sunyer FX. Comorbidities of overweight and obesity: current evidence and research issues. Med Sci Sports Exerc. (1999) 31(11 Suppl):S602–8. doi: 10.1097/00005768-199911001-00019
26. Rea TD, Heckbert SR, Kaplan RC, Psaty BM, Smith NL, Lemaitre RN, et al. Body mass index and the risk of recurrent coronary events following acute myocardial infarction. Am J Cardiol. (2001) 88(5):467–72. doi: 10.1016/S0002-9149(01)01720-9
27. Sung H, Siegel RL, Torre LA, Pearson-Stuttard J, Islami F, Fedewa SA, et al. Global patterns in excess body weight and the associated cancer burden. CA Cancer J Clin. (2019) 69(2):88–112. doi: 10.3322/caac.21499
28. Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas. Diabetes Res Clin Pract. (2019) 157:107843. doi: 10.1016/j.diabres.2019.107843
29. Sudore RL, Karter AJ, Huang ES, Moffet HH, Laiteerapong N, Schenker Y, et al. Symptom burden of adults with type 2 diabetes across the disease course: diabetes & aging study. J Gen Intern Med. (2012) 27:1674–81. doi: 10.1007/s11606-012-2132-3
30. Sinatra R. Causes and consequences of inadequate management of acute pain. Pain Med. (2010) 11(12):1859–71. doi: 10.1111/j.1526-4637.2010.00983.x
31. Bushnell MC, Frangos E, Madian N. Non-pharmacological treatment of pain: grand challenge and future opportunities. Front Pain Res (Lausanne). (2021) 2:696783. doi: 10.3389/fpain.2021.696783
32. Ghazinouri R, Rubin A. Total Knee Arthroplasty Protocol. Boston, MA: The Brigham and Women’s Hospital, Inc., Department of Rehabilitation Services (2012).
33. Bulls HW, Hamm M, Wasilko R, Cameron FA, Belin S, Goodin BR, et al. “I refused to get addicted to opioids”: exploring attitudes about opioid use disorder in patients with advanced cancer pain and their support people. J Pain. (2023) 24(6):1030–8. doi: 10.1016/j.jpain.2023.01.015
34. Lewis ET, Combs A, Trafton JA. Reasons for under-use of prescribed opioid medications by patients in pain. Pain Med. (2010) 11(6):861–71. doi: 10.1111/j.1526-4637.2010.00868.x
35. Fishbain DA, Cole B, Lewis J, Rosomoff HL, Rosomoff RS. What percentage of chronic nonmalignant pain patients exposed to chronic opioid analgesic therapy develop abuse/addiction and/or aberrant drug-related behaviors? A structured evidence-based review. Pain Med. (2008) 9(4):444–59. doi: 10.1111/j.1526-4637.2007.00370.x
36. Meisel ZF, Lupulescu-Mann N, Charlesworth CJ, Kim H, Sun BC. Conversion to persistent or high-risk opioid use after a new prescription from the emergency department: evidence from Washington medicaid beneficiaries. Ann Emerg Med. (2019) 74(5):611–21. doi: 10.1016/j.annemergmed.2019.04.007
37. Schroeder AR, Dehghan M, Newman TB, Bentley JP, Park KT. Association of opioid prescriptions from dental clinicians for US adolescents and young adults with subsequent opioid use and abuse. JAMA Intern Med. (2019) 179(2):145–52. doi: 10.1001/jamainternmed.2018.5419
38. Shah A, Hayes CJ, Martin BC. Characteristics of initial prescription episodes and likelihood of long-term opioid use—United States, 2006–2015. MMWR Morb Mortal Wkly Rep. (2017) 66(10):265–9. doi: 10.15585/mmwr.mm6610a1
39. Neuman MD, Bateman BT, Wunsch H. Inappropriate opioid prescription after surgery. Lancet. (2019) 393(10180):1547–57. doi: 10.1016/S0140-6736(19)30428-3
40. Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. (2016) 176(9):1286–93. doi: 10.1001/jamainternmed.2016.3298
41. Alam A, Gomes T, Zheng H, Mamdani MM, Juurlink DN, Bell CM. Long-term analgesic use after low-risk surgery: a retrospective cohort study. Arch Intern Med. (2012) 172(5):425–30. doi: 10.1001/archinternmed.2011.1827
42. Calcaterra SL, Yamashita TE, Min SJ, Keniston A, Frank JW, Binswanger IA. Opioid prescribing at hospital discharge contributes to chronic opioid use. J Gen Intern Med. (2016) 31(5):478–85. doi: 10.1007/s11606-015-3539-4
43. Henry SG, Paterniti DA, Feng B, Iosif AM, Kravitz RL, Weinberg G, et al. Patients’ experience with opioid tapering: a conceptual model with recommendations for clinicians. J Pain. (2019) 20(2):181–91. doi: 10.1016/j.jpain.2018.09.001
44. Davis MP, Digwood G, Mehta Z, McPherson ML. Tapering opioids: a comprehensive qualitative review. Ann Palliat Med. (2020) 9(2):586–610. doi: 10.21037/apm.2019.12.10
45. Magnan EM, Tancredi DJ, Xing G, Agnoli A, Jerant A, Fenton JJ. Association between opioid tapering and subsequent health care use, medication adherence, and chronic condition control. JAMA Netw Open. (2023) 6(2):e2255101. doi: 10.1001/jamanetworkopen.2022.55101
46. Han B, Jones CM, Einstein EB, Dowell D, Compton WM. Prescription opioid use disorder among adults reporting prescription opioid use with or without misuse in the United States. J Clin Psychiatry. (2024) 85(3):24m15258. doi: 10.4088/JCP.24m15258
47. Vowles KE, McEntee ML, Julnes PS, Frohe T, Ney JP, van der Goes DN. Rates of opioid misuse, abuse, and addiction in chronic pain: a systematic review and data synthesis. Pain. (2015) 156(4):569–76. doi: 10.1097/01.j.pain.0000460357.01998.f1
48. Savage SR, Kirsh KL, Passik SD. Challenges in using opioids to treat pain in persons with substance use disorders. Addict Sci Clin Pract. (2008) 4(2):4–25. doi: 10.1151/ascp08424
49. Abril Ochoa L, Naeem F, White DJ, Bijur PE, Friedman BW. Opioid-induced Euphoria among emergency department patients with acute severe pain: an analysis of data from a randomized trial. Acad Emerg Med. (2020) 27(11):1100–5. doi: 10.1111/acem.13946
50. Sapkota A, Takematsu M, Adewunmi V, Gupta C, Williams AR, Friedman BW. Oxycodone induced euphoria in ED patients with acute musculoskeletal pain. A secondary analysis of data from a randomized trial. Am J Emerg Med. (2022) 53:240–4. doi: 10.1016/j.ajem.2022.01.016
51. Caplan M, Friedman BW, Siebert J, Takematsu M, Adewunmi V, Gupta C, et al. Use of clinical phenotypes to characterize emergency department patients administered intravenous opioids for acute pain. Clin Exp Emerg Med. (2023) 10(3):327–32. doi: 10.15441/ceem.23.018
52. Remillard D, Kaye AD, McAnally H. Oxycodone’s unparalleled addictive potential: is it time for a moratorium? Curr Pain Headache Rep. (2019) 23(2):15. doi: 10.1007/s11916-019-0751-7
53. Hansen GR. The drug-seeking patient in the emergency room. Emerg Med Clin North Am. (2005) 23(2):349–65. doi: 10.1016/j.emc.2004.12.006
54. Kakko J, Alho H, Baldacchino A, Molina R, Nava FA, Shaya G. Craving in opioid use disorder: from neurobiology to clinical practice. Front Psychiatry. (2019) 10:592. doi: 10.3389/fpsyt.2019.00592
55. Vest N, Reynolds CJ, Tragesser SL. Impulsivity and risk for prescription opioid misuse in a chronic pain patient sample. Addict Behav. (2016) 60:184–90. doi: 10.1016/j.addbeh.2016.04.015
56. Ellis MS, Kasper Z, Cicero T. Assessment of chronic pain management in the treatment of opioid use disorder: gaps in care and implications for treatment outcomes. J Pain. (2021) 22(4):432–9. doi: 10.1016/j.jpain.2020.10.005
57. Morgan MM, Christie MJ. Analysis of opioid efficacy, tolerance, addiction and dependence from cell culture to human. Br J Pharmacol. (2011) 164(4):1322–34. doi: 10.1111/j.1476-5381.2011.01335.x
58. Brat GA, Agniel D, Beam A, Yorkgitis B, Bicket M, Homer M, et al. Postsurgical prescriptions for opioid naive patients and association with overdose and misuse: retrospective cohort study. Br Med J. (2018) 360:j5790. doi: 10.1136/bmj.j5790
59. Park JY, Wu LT. Sources of misused prescription opioids and their association with prescription opioid use disorder in the United States: sex and age differences. Subst Use Misuse. (2020) 55(6):928–36. doi: 10.1080/10826084.2020.1713818
60. Schepis TS, McCabe SE, Teter CJ. Sources of opioid medication for misuse in older adults: results from a nationally representative survey. Pain. (2018) 159(8):1543–9. doi: 10.1097/j.pain.0000000000001241
61. Schepis TS, Wilens TE, McCabe SE. Prescription drug misuse: sources of controlled medications in adolescents. J Am Acad Child Adolesc Psychiatry. (2019) 58(7):670–80.e4. doi: 10.1016/j.jaac.2018.09.438
62. Dydyk AM, Jain NK, Gupta M. Opioid Use Disorder. StatPearls. Treasure Island (FL): StatPearls Publishing LLC. (2021).
63. Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription-opioid use and heroin use. N Engl J Med. (2016) 374(2):154–63. doi: 10.1056/NEJMra1508490
64. Butler MM, Ancona RM, Beauchamp GA, Yamin CK, Winstanley EL, Hart KW, et al. Emergency department prescription opioids as an initial exposure preceding addiction. Ann Emerg Med. (2016) 68(2):202–8. doi: 10.1016/j.annemergmed.2015.11.033
65. Chua KP, Brummett CM, Conti RM, Bohnert A. Association of opioid prescribing patterns with prescription opioid overdose in adolescents and young adults. JAMA Pediatr. (2020) 174(2):141–8. doi: 10.1001/jamapediatrics.2019.4878
66. Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers—United States, 2002–2004 and 2008–2010. Drug Alcohol Depend. (2013) 132(1–2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007
67. Lankenau SE, Teti M, Silva K, Jackson Bloom J, Harocopos A, Treese M. Initiation into prescription opioid misuse amongst young injection drug users. Int J Drug Policy. (2012) 23(1):37–44. doi: 10.1016/j.drugpo.2011.05.014
68. Chen C-Y, Chen WJ. Chapter 19—overdose. In: Miller PM, editor. Principles of Addiction. San Diego: Academic Press (2013). p. 187–92.
69. Lyons RM, Yule AM, Schiff D, Bagley SM, Wilens TE. Risk factors for drug overdose in young people: a systematic review of the literature. J Child Adolesc Psychopharmacol. (2019) 29(7):487–97. doi: 10.1089/cap.2019.0013
70. Webster LR. Risk factors for opioid-use disorder and overdose. Anesth Analg. (2017) 125(5):1741–8.29049118
71. Schiller E, Goyal A, Mechanic O. Opioid Overdose. Treasure Island (FL): StatPearls Publishing (2023).
72. Coyle DT, Pratt CY, Ocran-Appiah J, Secora A, Kornegay C, Staffa J. Opioid analgesic dose and the risk of misuse, overdose, and death: a narrative review. Pharmacoepidemiol Drug Saf. (2018) 27(5):464–72. doi: 10.1002/pds.4366
73. Green JL, Bucher Bartelson B, Le Lait MC, Roland CL, Masters ET, Mardekian J, et al. Medical outcomes associated with prescription opioid abuse via oral and non-oral routes of administration. Drug Alcohol Depend. (2017) 175:140–5. doi: 10.1016/j.drugalcdep.2017.01.039
74. Bateman JT, Saunders SE, Levitt ES. Understanding and countering opioid-induced respiratory depression. Br J Pharmacol. (2023) 180(7):813–28. doi: 10.1111/bph.15580
75. Boom M, Niesters M, Sarton E, Aarts L, Smith TW, Dahan A. Non-analgesic effects of opioids: opioid-induced respiratory depression. Curr Pharm Des. (2012) 18(37):5994–6004. doi: 10.2174/138161212803582469
76. Gerlach LB, Olfson M, Kales HC, Maust DT. Opioids and other central nervous system-active polypharmacy in older adults in the United States. J Am Geriatr Soc. (2017) 65(9):2052–6. doi: 10.1111/jgs.14930
77. Rhee TG. Coprescribing of benzodiazepines and opioids in older adults: rates, correlates, and national trends. J Gerontol A Biol Sci Med Sci. (2019) 74(12):1910–5. doi: 10.1093/gerona/gly283
78. Santo L, Rui P, Ashman JJ. Physician office visits at which benzodiazepines were prescribed: findings from 2014 to 2016 national ambulatory medical care survey. Natl Health Stat Report. (2020) 31(137):1–16.
79. Rogers AH, Zvolensky MJ, Ditre JW, Buckner JD, Asmundson GJG. Association of opioid misuse with anxiety and depression: a systematic review of the literature. Clin Psychol Rev. (2021) 84:101978. doi: 10.1016/j.cpr.2021.101978
80. Bongiovanni T, Gan S, Finlayson E, Ross JS, Harrison JD, Boscardin WJ, et al. Trends in the use of gabapentinoids and opioids in the postoperative period among older adults. JAMA Netw Open. (2023) 6(6):e2318626. doi: 10.1001/jamanetworkopen.2023.18626
81. Chen C, Lo-Ciganic WH, Winterstein AG, Tighe P, Wei YJ. Concurrent use of prescription opioids and gabapentinoids in older adults. Am J Prev Med. (2022) 62(4):519–28. doi: 10.1016/j.amepre.2021.08.024
82. Dinwiddie AT, Tanz LJ, Bitting J. Notes from the field: antihistamine positivity and involvement in drug overdose deaths—44 jurisdictions, United States, 2019–2020. MMWR Morb Mortal Wkly Rep. (2022) 71(41):1308–10. doi: 10.15585/mmwr.mm7141a4
83. Boitor M, Ballard A, Emed J, Le May S, Gélinas C. Risk factors for severe opioid-induced respiratory depression in hospitalized adults: a case-control study. Can J Pain. (2020) 4(1):103–10. doi: 10.1080/24740527.2020.1714431
84. FDA. FDA Warns About Serious Risks and Death When Combining Opioid Pain or Cough Medicines with Benzodiazepines; Requires its Strongest Warning. Washington, D.C.: U.S. Food and Drug Administration (2016).
85. Abrahamsson T, Berge J, Öjehagen A, Håkansson A. Benzodiazepine, z-drug and pregabalin prescriptions and mortality among patients in opioid maintenance treatment-A nation-wide register-based open cohort study. Drug Alcohol Depend. (2017) 174:58–64. doi: 10.1016/j.drugalcdep.2017.01.013
86. Humpert SR, Reveles KR, Bhakta K, Torrez SB, Evoy KE. Association of gabapentinoids with opioid-related overdose in the inpatient setting: a single center retrospective case-control study. Hosp Pharm. (2024) 59(2):188–97. doi: 10.1177/00185787231206522
87. Olopoenia A, Camelo-Castillo W, Qato DM, Adekoya A, Palumbo F, Sera L, et al. Adverse outcomes associated with concurrent gabapentin, opioid, and benzodiazepine utilization: a nested case-control study. Lancet Reg Health Am. (2022) 13:100302. doi: 10.1016/j.lana.2022.100302
88. Witkiewitz K, Vowles KE. Alcohol and opioid use, co-use, and chronic pain in the context of the opioid epidemic: a critical review. Alcohol Clin Exp Res. (2018) 42(3):478–88. doi: 10.1111/acer.13594
89. Langstengel J, Yaggi HK. Sleep deficiency and opioid use disorder: trajectory, mechanisms, and interventions. Sleep Med Clin. (2024) 19(4):625–38. doi: 10.1016/j.jsmc.2024.07.009
90. Odoma VA, Pitliya A, AlEdani E, Bhangu J, Javed K, Manshahia PK, et al. Opioid prescription in patients with chronic kidney disease: a systematic review of comparing safety and efficacy of opioid use in chronic kidney disease patients. Cureus. (2023) 15(9):e45485. doi: 10.7759/cureus.45485
91. Serinelli S, Arunkumar P, Stoppacher R, Wang D, Gitto L. Relationship between opiates and asthma in the determination of death. J Forensic Leg Med. (2020) 74:102030. doi: 10.1016/j.jflm.2020.102030
92. Algera MH, Olofsen E, Moss L, Dobbins RL, Niesters M, van Velzen M, et al. Tolerance to opioid-induced respiratory depression in chronic high-dose opioid users: a model-based comparison with opioid-naïve individuals. Clin Pharmacol Ther. (2021) 109(3):637–45. doi: 10.1002/cpt.2027
93. CDC. Polysubstance Use Facts. Washington, D.C.: U.S. Centers for Disease Control and Prevention (2022).
94. Cicero TJ, Ellis MS, Kasper ZA. Polysubstance use: a broader understanding of substance use during the opioid crisis. Am J Public Health. (2020) 110(2):244–50. doi: 10.2105/AJPH.2019.305412
95. Morley KI, Ferris JA, Winstock AR, Lynskey MT. Polysubstance use and misuse or abuse of prescription opioid analgesics: a multi-level analysis of international data. Pain. (2017) 158(6):1138–44. doi: 10.1097/j.pain.0000000000000892
96. O'Brien P, Henke RM, Schaefer MB, Lin J, Creedon TB. Adverse events among adult medicaid enrollees with opioid use disorder and co-occurring substance use disorders. Drug Alcohol Depend. (2021) 221:108555. doi: 10.1016/j.drugalcdep.2021.108555
97. Guru V, Dubinsky I. The patient vs. caregiver perception of acute pain in the emergency department. J Emerg Med. (2000) 18(1):7–12. doi: 10.1016/S0736-4679(99)00153-5
98. Todd KH, Ducharme J, Choiniere M, Crandall CS, Fosnocht DE, Homel P, et al. Pain in the emergency department: results of the pain and emergency medicine initiative (PEMI) multicenter study. J Pain. (2007) 8(6):460–6. doi: 10.1016/j.jpain.2006.12.005
99. Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. (2003) 97(2):534–40. doi: 10.1213/01.ANE.0000068822.10113.9E
100. Chou R, Wagner J, Ahmed A, Blazina I, Brodt E, Buckley D, et al. Treatments for Acute Pain: A Systematic Review AHRQ Publication No. 20(21)-EHC006. Rockville, MD: U.S. Agency for Healthcare Research and Quality (2022).
101. Sachs CJ. Oral analgesics for acute nonspecific pain. Am Fam Physician. (2005) 71(5):913–8. doi: 10.1017/cbo9780511576706.015
102. NASEM. In: Bonnie RJ, Ford MA, Phillips JK, editors. Pain Management and the Opioid Epidemic: Balancing Societal and Indidivual Benefits and Risks of Prescription Opioid Use. Washington, D.C.: National Academies Press (2017).
103. Hooten WM, Bruce BK. Beliefs and attitudes about prescribing opioids among healthcare providers seeking continuing medical education. J Opioid Manag. (2011) 7(6):417–24. doi: 10.5055/jom.2011.0082
104. Spitz A, Moore AA, Papaleontiou M, Granieri E, Turner BJ, Reid MC. Primary care providers’ perspective on prescribing opioids to older adults with chronic non-cancer pain: a qualitative study. BMC Geriatr. (2011) 11:35. doi: 10.1186/1471-2318-11-35
105. Wolfert MZ, Gilson AM, Dahl JL, Cleary JF. Opioid analgesics for pain control: wisconsin physicians’ knowledge, beliefs, attitudes, and prescribing practices. Pain Med. (2010) 11(3):425–34. doi: 10.1111/j.1526-4637.2009.00761.x
106. Jamison RN, Sheehan KA, Scanlan E, Matthews M, Ross EL. Beliefs and attitudes about opioid prescribing and chronic pain management: survey of primary care providers. J Opioid Manag. (2014) 10(6):375–82. doi: 10.5055/jom.2014.0234
107. Bell LV, Fitzgerald SF, Flusk D, Poulin PA, Rash JA. Healthcare provider knowledge, beliefs, and attitudes regarding opioids for chronic non-cancer pain in North America prior to the emergence of COVID-19: a systematic review of qualitative research. Can J Pain. (2023) 7(1):2156331. doi: 10.1080/24740527.2022.2156331
108. Joseph JM, Gori D, Curtin C, Hah J, Ho VT, Asch SM, et al. Gaps in standardized postoperative pain management quality measures: a systematic review. Surgery. (2022) 171(2):453–8. doi: 10.1016/j.surg.2021.08.004
109. Harsanyi H, Cuthbert C, Schulte F. The stigma surrounding opioid use as a barrier to cancer-pain management: an overview of experiences with fear, shame, and poorly controlled pain in the context of advanced cancer. Curr Oncol. (2023) 30(6):5835–48. doi: 10.3390/curroncol30060437
110. Vargas-Schaffer G, Cogan J. Attitudes toward opioids and risk of misuse/abuse in patients with chronic noncancer pain receiving long-term opioid therapy. Pain Med. (2018) 19(2):319–27. doi: 10.1093/pm/pnw338
111. Chou R, Gordon DB, de Leon-Casasola OA, Rosenberg JM, Bickler S, Brennan T, et al. Management of postoperative pain: a clinical practice guideline from the American pain society, the American society of regional anesthesia and pain medicine, and the American society of Anesthesiologists’ committee on regional anesthesia, executive committee, and administrative council. J Pain. (2016) 17(2):131–57. doi: 10.1016/j.jpain.2015.12.008
112. Daoust R, Paquet J, Marquis M, Williamson D, Fontaine G, Chauny JM, et al. Efficacy of prescribed opioids for acute pain after being discharged from the emergency department: a systematic review and meta-analysis. Acad Emerg Med. (2023) 30(12):1253–63. doi: 10.1111/acem.14790
113. Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain–United States, 2016. JAMA. (2016) 315(15):1624–45. doi: 10.1001/jama.2016.1464
114. Kroenke K, Alford DP, Argoff C, Canlas B, Covington E, Frank JW, et al. Challenges with implementing the centers for disease control and prevention opioid guideline: a consensus panel report. Pain Med. (2019) 20(4):724–35. doi: 10.1093/pm/pny307
115. Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. (2019) 380(24):2285–7. doi: 10.1056/NEJMp1904190
116. Ebbert JO, Philpot LM, Clements CM, Lovely JK, Nicholson WT, Jenkins SM, et al. Attitudes, beliefs, practices, and concerns among clinicians prescribing opioids in a large academic institution. Pain Med. (2018) 19(9):1790–8. doi: 10.1093/pm/pnx140
117. Borsook D, Youssef AM, Simons L, Elman I, Eccleston C. When pain gets stuck: the evolution of pain chronification and treatment resistance. Pain. (2018) 159(12):2421–36. doi: 10.1097/j.pain.0000000000001401
118. Pozek JP, Beausang D, Baratta JL, Viscusi ER. The acute to chronic pain transition: can chronic pain be prevented? Med Clin North Am. (2016) 100(1):17–30. doi: 10.1016/j.mcna.2015.08.005
119. Althaus A, Arránz Becker O, Moser KH, Lux EA, Weber F, Neugebauer E, et al. Postoperative pain trajectories and pain chronification-an empirical typology of pain patients. Pain Med. (2018) 19(12):2536–45. doi: 10.1093/pm/pny099
120. Martuliak I, Golubnitschaja O, Chvala L, Kapalla M, Ferencik M, Bubeliny M, et al. Pain chronification risk assessment: advanced phenotyping and scoring for prediction and treatments tailored to individualized patient profile. EPMA J. (2024) 15(4):739–50. doi: 10.1007/s13167-024-00383-3
121. Zhang S, Ning Y, Yang Y, Mu G, Yang Y, Ren C, et al. Decoding pain chronification: mechanisms of the acute-to-chronic transition. Front Mol Neurosci. (2025) 18:1596367. doi: 10.3389/fnmol.2025.1596367
122. Dowell D, Ragan KR, Jones CM, Baldwin GT, Chou R. CDC clinical practice guideline for prescribing opioids for pain—United States, 2022. MMWR Recomm Rep. (2022) 71(3):1–95. doi: 10.15585/mmwr.rr7103a1
123. Amaechi O, Huffman MM, Featherstone K. Pharmacologic therapy for acute pain. Am Fam Physician. (2021) 104(1):63–72.34264611
124. Bohnert ASB, Guy GP Jr, Losby JL. Opioid prescribing in the United States before and after the centers for disease control and prevention’s 2016 opioid guideline. Ann Intern Med. (2018) 169(6):367–75. doi: 10.7326/m18-1243
125. Carrasco-Labra A, Polk DE, Urquhart O, Aghaloo T, Claytor JW Jr, Dhar V, et al. Evidence-based clinical practice guideline for the pharmacologic management of acute dental pain in adolescents, adults, and older adults: a report from the American dental association science and research institute, the university of Pittsburgh, and the university of Pennsylvania. J Am Dent Assoc. (2024) 155(2):102–17.e9. doi: 10.1016/j.adaj.2023.10.009
126. Guy GP, Zhang K, Bohm MK, Losby J, Lewis B, Young R, et al. Vital signs: changes in opioid prescribing in the United States, 2006–2015. MMWR Morb Mortal Wkly Rep. (2017) 66(26):697–704. doi: 10.15585/mmwr.mm6626a4
127. Hsu JR, Mir H, Wally MK, Seymour RB. Clinical practice guidelines for pain management in acute musculoskeletal injury. J Orthop Trauma. (2019) 33(5):e158–e82. doi: 10.1097/BOT.0000000000001430
129. Stein BD, Sheng F, Taylor EA, Dick AW, Sorbero M, Pacula RL. The effect of state policies on rates of high-risk prescribing of an initial opioid analgesic. Drug Alcohol Depend. (2022) 231:109232. doi: 10.1016/j.drugalcdep.2021.109232
130. CBO. The Opioid Crisis and Recent Federal Policy Responses. Washington, DC: U.S. Congressional Budget Office (2022).
131. Morlion B, Coluzzi F, Aldington D, Kocot-Kepska M, Pergolizzi J, Mangas AC, et al. Pain chronification: what should a non-pain medicine specialist know? Curr Med Res Opin. (2018) 34(7):1169–78. doi: 10.1080/03007995.2018.1449738
132. Pak DJ, Yong RJ, Kaye AD, Urman RD. Chronification of pain: mechanisms, current understanding, and clinical implications. Curr Pain Headache Rep. (2018) 22(2):9. doi: 10.1007/s11916-018-0666-8
133. Pergolizzi JV Jr, Raffa RB, Taylor R Jr. Treating acute pain in light of the chronification of pain. Pain Manag Nurs. (2014) 15(1):380–90. doi: 10.1016/j.pmn.2012.07.004
134. Fregoso G, Wang A, Tseng K, Wang J. Transition from acute to chronic pain: evaluating risk for chronic postsurgical pain. Pain Physician. (2019) 22(5):479–88. doi: 10.36076/ppj/2019.22.479
135. Kehlet H, Jensen TS, Woolf CJ. Persistent postsurgical pain: risk factors and prevention. Lancet. (2006) 367(9522):1618–25. doi: 10.1016/s0140-6736(06)68700-x
136. Luo D, Fan Z, Yin W. Chronic post-surgical pain after total knee arthroplasty: a narrative review. Perioper Med (Lond). (2024) 13(1):108. doi: 10.1186/s13741-024-00466-9
137. Bruce J, Quinlan J. Chronic post surgical pain. Rev Pain. (2011) 5(3):23–9. doi: 10.1177/204946371100500306
138. Schug SA, Bruce J. Risk stratification for the development of chronic postsurgical pain. Pain Rep. (2017) 2(6):e627. doi: 10.1097/pr9.0000000000000627
139. Ten Doesschate SFH, Kuijper TM, Koopman S, Mol S, Colen-Kroon L, Brown VV. Pain severity at emergency department discharge as a predictor for chronification of pain. Pain Rep. (2022) 7(6):e1048. doi: 10.1097/PR9.0000000000001048
140. El-Tallawy SN, Ahmed RS, Shabi SM, Al-Zabidi FZ, Zaidi ARZ, Varrassi G, et al. The challenges of pain assessment in geriatric patients with dementia: a review. Cureus. (2023) 15(11):e49639. doi: 10.7759/cureus.49639
141. Herr K. Pain assessment strategies in older patients. J Pain. (2011) 12(3 Suppl 1):S3–s13. doi: 10.1016/j.jpain.2010.11.011
142. Katz B. The science and art of pain management in older persons: case study and discussion. Pain Med. (2012) 13(Suppl 2):S72–8. doi: 10.1111/j.1526-4637.2011.01315.x
143. Salvatore PP, Guy GP, Mikosz CA. Changes in opioid dispensing by medical specialties after the release of the 2016 CDC guideline for prescribing opioids for chronic pain. Pain Med. (2022) 23(11):1908–14. doi: 10.1093/pm/pnac068
144. CDC. Opioid Precribing Data, 2006-2020. Based on IQVIA Xponent Data, 2006–2020; Reported by U.S. Washington, DC: Centers for Disease Control and Prevention (2022).
145. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. (2012) 13(8):715–24. doi: 10.1016/j.jpain.2012.03.009
146. Manchikanti L, Pampati V, Falco FJ, Hirsch JA. An updated assessment of utilization of interventional pain management techniques in the medicare population: 2000–2013. Pain Physician. (2015) 18(2):E115–27. doi: 10.36076/ppj/2015.18.E115
147. IOM. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. U.S. Washington, DC: National Institutes for Health, Institute of Medicine (IOM) (2011).
Keywords: acute pain, opioid use disorder (OUD), pain management, undertreatment, chronification of pain, healthcare costs
Citation: Hackworth JC, Schneider JE, Do Valle M, Fam D, Argoff C, Offidani E and Potenziano J (2025) The burden of acute pain in the U.S. in the wake of the opioid crisis. Front. Pain Res. 6:1642035. doi: 10.3389/fpain.2025.1642035
Received: 5 June 2025; Accepted: 12 September 2025;
Published: 7 October 2025.
Edited by:
Xin Zhang, Wuxi People’s Hospital Affiliated to Nanjing Medical University, ChinaReviewed by:
Zhi-Yong Tan, Hebei University, ChinaMatthew Halma, Frontline COVID-19 Critical Care Alliance, United States
Copyright: © 2025 Hackworth, Schneider, Do Valle, Fam, Argoff, Offidani and Potenziano. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maggie Do Valle, bWFnZ2llLmRvdmFsbGVAYXZhbG9uZWNvbi5jb20=
 James C. Hackworth1
James C. Hackworth1 Maggie Do Valle
Maggie Do Valle David Fam
David Fam Emanuela Offidani
Emanuela Offidani