- 1Department of Anesthesiology & Pain Medicine, University of Washington, Seattle, WA, United States
- 2Center for Child Health, Behavior & Development, Seattle Children’s Research Institute, Seattle, WA, United States
- 3Inserm U987, APHP, UVSQ, Paris-Saclay University, Ambroise Pare Hospital, Boulogne-Billancourt, France
- 4Department of Surgery and Institute of Medical Science, University of Toronto, Toronto, ON, Canada
- 5Krembil Brain Institute, University Health Network, Toronto, ON, Canada
- 6Department of Anesthesiology, Weill Cornell Medicine, New York, NY, United States
- 7Pain Outcomes Lab, Department of Anesthesiology, Translational Neuroscience (Formerly Pharmacology), and Public Health Sciences, Wake Forest University School of Medicine, Winston-Salem, NC, United States
- 8Department of Psychology, York University, Toronto, ON, Canada
- 9Nuffield Department of Clinical Neuroscience, University of Oxford, Oxford, United Kingdom
- 10Nuffield Department of Anaesthesia, Oxford University Hospitals NHS Foundation Trust, Oxford, United Kingdom
- 11Department of Neuroscience and Center for Advanced Pain Studies, University of Texas at Dallas, Dallas, TX, United States
- 12Department of Anesthesiology, Perioperative Care, and Pain Medicine, NYU Grossman School of Medicine, New York, NY, United States
- 13Department of Population Health—Division of Medical Ethics, NYU Grossman School of Medicine, New York, NY, United States
- 14Department of Anesthesiology, Pharmacology & Therapeutics, The University of British Columbia, Vancouver, BC, Canada
- 15Department of Anesthesia, St. Paul’s Hospital/Providence Health Care, Vancouver, BC, Canada
- 16Department of Cardiovascular Sciences, Catholic University Leuven, Leuven, Belgium
- 17Department of Anesthesiology, University Hospitals Leuven, Leuven, Belgium
- 18Department of Academic Anaesthesia, Sheffield Teaching Hospitals NHS Foundation Trust, Sheffield, United Kingdom
- 19Centre for Applied Health and Social Care Research (CARe), Sheffield Hallam University, Sheffield, United Kingdom
- 20Department of Anesthesiology & Pharmacology, University of California, San Diego, CA, United States
- 21Laboratory of Clinical Neurophysiology, Department of Neurology, Rambam Medical Center, Technion Faculty of Medicine, Haifa, Israel
1 Introduction
We are a group of journal editors1 dedicated to advancing discoveries and innovations in basic, translational, and clinical research across anesthesiology and pain-related disciplines, which play a crucial role in reducing the burden of pain, improving health, enhancing perioperative outcomes, and optimizing healthcare delivery. Across scientific disciplines, concerns have been raised about research quality and trustworthiness (1, 2). While these challenges are not unique to pain and anesthesiology research, we recognize this as a judicious opportunity to raise awareness and collaborate across our journals to align and strengthen initiatives to enhance research integrity, trust, and impact across our field.
In a 2005 landmark paper, John Ioannidis concluded with the dramatic and troubling assertion that “most published research findings are false,” stimulating a large focus in the biomedical research community on understanding issues of integrity, reproducibility, and replication that continues to be relevant to this day (3). Indeed, there are many instances in which authors, institutions, funders, publishers and journals have failed to embody the core values that produce trustworthy science. The trustworthiness of research is affected by both intentional actions (e.g., fabrication and falsification of data, lack of rigor, image manipulation) and unintentional actions (e.g., inadequate oversight, awareness and understanding of both technical and scientific issues). Most concerning are instances of research misconduct including fabrication, falsification, or plagiarism sometimes revealed by failure to replicate or reproduce results, duplication of publications, a rise in the number of retractions (4, 5), and calls for larger numbers of papers to be retracted (e.g., 2). In support of Ioannidis's disquiet, some reviews (e.g., 6, 7) report low replication rates of positive findings in the social and life sciences across clinical trials, epidemiological research, and molecular studies.
In anesthesiology specifically, low agreement has been found between randomized clinical trials (RCTs) and meta-analytic findings for clinical pain interventions, where positive findings in meta-analyses were often not confirmed by subsequent large RCTs. For example, using individual patient data from RCTs published in Anaesthesia, Carlisle (8) demonstrated that almost half of the databases had false data as detected from the duplication of figures, tables, and other data from published work; the duplication of data in the rows and columns of spreadsheets; impossible values; and incorrect data analytic strategies and calculations.
Reproducibility, clinical validity, and utility in pain and anesthesiology research are often compromised by non-representative samples (e.g., limited representation on characteristics such as race, ethnicity, age, sex/gender, or socioeconomic status that do not match population-level data of those most affected by pain) (9–11), reliance on surrogate outcomes with limited clinical relevance, underutilization of common data elements and core outcome sets, underpowered studies prone to false-negative results, and flawed statistical analysis plans that generate misleading conclusions (12).
To ensure integrity of the literature, retraction of articles may be necessary due to such issues as major errors, data fabrication, plagiarism, or unethical research practices. Authors are encouraged to identify errors in their own work and may request a corrigendum to correct the literature. However, when ethical issues are brought to a journal's attention, they have a duty to investigate, and when there is conclusive evidence, to impose a retraction to alert readers that the findings and conclusions cannot be relied upon (13). Retractions, when reported, can have a widespread impact due to the interconnectedness of studies attributed to the same authors (14). In the field of anesthesiology, the Retraction Watch Leaderboard (15) indicates four of the top ten authors are anesthesiologists, and two of these individuals occupy the top two positions (https://retractionwatch.com/the-retraction-watch-leaderboard/. Systematic reviews have summarized characteristics of retracted publications for research misconduct in pain (e.g., 16) and anesthesiology research (e.g., 17). Concerns regarding retractions in all scientific fields are particularly noteworthy because they undermine trust in science, can have a lasting impact on conclusions made about treatments and, ultimately, impact clinical practice. In one study by O'Connell et al. (18), a set of 8 untrustworthy trials (i.e., identified due to concerns including data anomalies and implausible results), in spinal pain was determined to substantially impact the results of subsequent recommendations made in systematic reviews and international clinical practice guidelines in management of spinal pain.
Meta-research studies regarding open science practices highlight critical remaining gaps across many fields in reproducible research practices, open access data, and availability of protocols (e.g., 1). In 2018, Lee et al. (19) examined open science efforts in the pain field including preregistration of trials, sharing code, data, reproducible workflows, and the use of reporting guidelines. Among ten pain journals, a low level of engagement with open and transparent research policies was identified at that time. Cashin et al. (20) also reviewed the policies of ten leading pain journals and determined that there were few journal policies adhering to transparency standards for review and publication. These observations have fueled many recent efforts and initiatives in open science including in pain and anesthesiology research.
Open and transparent research practices as embodied in the “open science” movement provide a more complete and accurate report of the research conducted and what was found, and share important aspects of the research process (e.g., availability of study materials, data and code) (21). Trust and transparency are interwoven because when research is conducted and reported openly and transparently it increases confidence in the findings by enabling verification, replication, and critical appraisal.
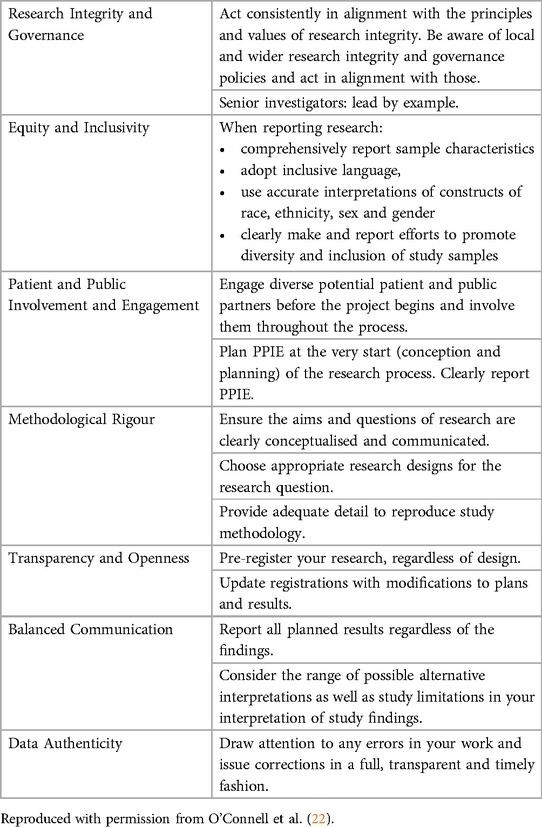
For pain science to advance with groundbreaking discoveries and translation into clinical impact, it is important to produce high-quality, trustworthy research. Building on their earlier recommendations, O'Connell and colleagues (22) recently presented a comprehensive framework for building trustworthy pain research called ENhancing TRUSTworthiness in Pain Evidence (ENTRUST-PE). The ENTRUST-PE framework conceptualizes the construct “Trustworthiness” of research to be supported by seven core values [see Figure 1 reproduced from (22)]:
1. Governance and Integrity (e.g., follow principles of research integrity and comply with regulatory guidelines, disclose conflicts of interest);
2. Equity, Diversity, and Inclusivity (e.g., plan strategies to maximize inclusivity at the preparation and initiation of the research);
3. Patient and Public Involvement and Engagement (e.g., embed partnership with people with lived experience throughout the research process);
4. Methodological Rigor (e.g., value, conduct, and promote high-quality methodologically rigorous research including in clinical studies with a focus on patient-centered outcomes, adequate power, and an appropriate analysis plan);
5. Transparency and Openness (e.g., adopt open research practices that include sharing of data, materials, and code);
6. Balanced Communication (e.g., report results accurately and comprehensively irrespective of the finding); and
7. Data Authenticity (e.g., commit to timely correction or removal of errors in the published literature).
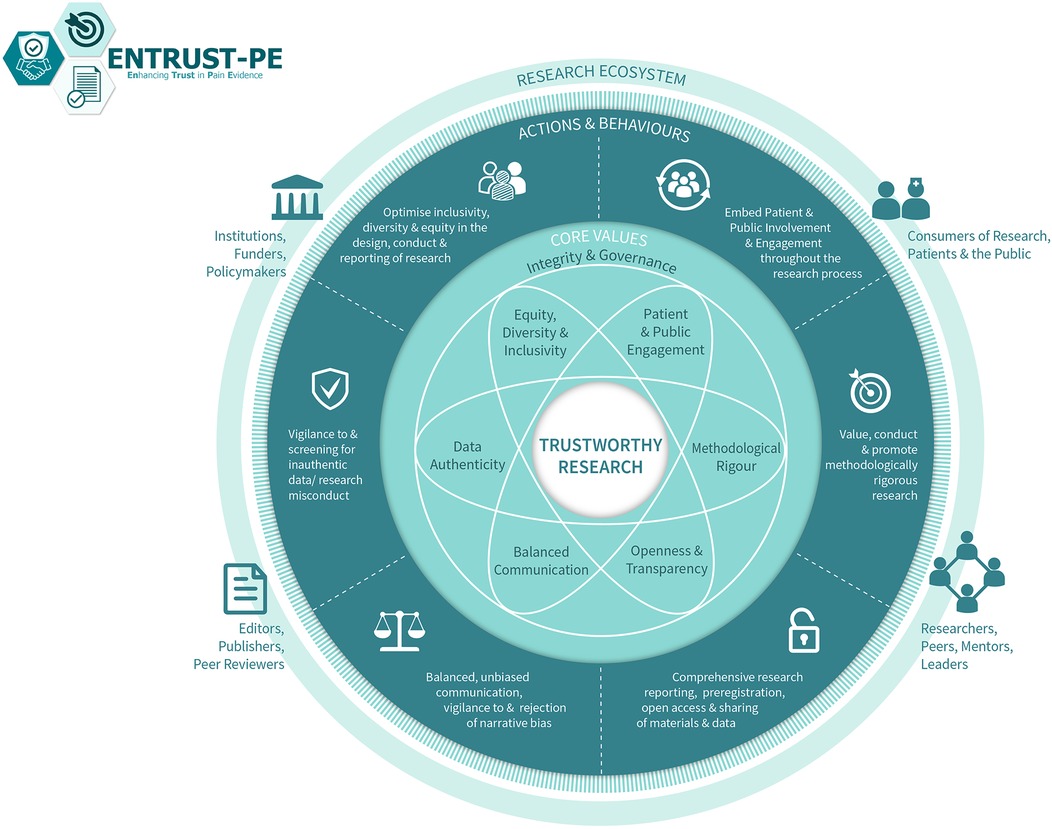
Figure 1. The ENhancing TRUSTworthiness in pain evidence framework (ENTRUST-PE). Reproduced with permission from O’Connell et al. (22).
Recommendations were made for short-term as well as more extended-term actions and behaviors for several different stakeholder groups (e.g., researchers, institutions, publishers, funders, policymakers and regulators, peer reviewers) to support trustworthy research within each of the core values of ENTRUST. These recommendations are intended to guide the development of a strategy for enhancing trustworthy research, rather than serving as a mandated policy.
From the perspective of engagement with our journals, here we focus on recommendations for researchers and editors/publishers.
2 Guidance for researchers who produce, review, and consume research
We strongly recommend that researchers thoroughly review the proposed framework, which we as editors endorse, and explore the full suite of resources available through the ENTRUST-PE network project. These can be accessed at https://entrust-pe.org and on the Open Science Framework (https://osf.io/cua7g/?view_only=ec1d9e6b1d774dbca9306ff5ae4dec67). The initiative is designed to support researchers to understand how to conduct and report science in a manner that enhances the transparency and trustworthiness of their work. By following these recommendations, researchers can provide the highest quality of research and facilitate confidence in pain science. Moreover, peer reviewers and consumers of research can be alert to potential issues of methodological rigor, transparency, lack of equity and inclusivity, and markers of potential data inauthenticity or research misconduct that play a critical role in raising concerns to editors and publishers when these are identified. Recently, both the International Association for the Study of Pain (IASP) (23) and the European Pain Federation (EFIC) (24) endorsed the ENTRUST-PE initiative, recognizing that trustworthy research not only benefits investigators and healthcare professionals but also serves patients and the public by promoting science that produces more effective pain management strategies.
For a concise, actionable summary, we reproduce the guidance provided by O'Connell et al. (22), which outlines practical suggestions researchers can implement immediately to align with the core values of the ENTRUST-PE framework (see Table 1).
3 Journal initiatives
As editors of journals in the fields of pain science and anesthesiology, we wish to amplify the ENTRUST-PE framework (22) and support efforts to promote, teach, and enforce principles and values underpinning high quality and trustworthy research. Here, we highlight four areas where we collectively aspire to take a leadership role in enhancing the trustworthiness of research in the journals we serve.
3.1 Evaluate journal policies on transparency and openness to inform potential improvements
As highlighted in several prior reviews (19, 20), journals can use existing tools to conduct self-assessments of their policies and procedures. Tools have been developed to facilitate transparency including the Transparency and Openness Evaluation Tool (20) and the Centre for Open Science (COS) Transparency Factor (25). As a first step, pain and anesthesiology journals can sign on to COS as signatories (if they are not already) to express support of transparency and openness principles. In addition, the Transparency and Openness Factor metric provides information on where opportunities exist for improvement, which can contribute to decision making and policy development by Editors and Publishers to improve transparency and openness. For example, this can guide changes to journals (26) along such areas as research pre-registration where appropriate, reporting guidelines, open data analytic codes and materials, transparent reporting of authorship contributions, and defining the role of the corresponding author as the point of contact for accountability and transparency.
We plan to undertake an updated and coordinated self-assessment process across our 15 journals using the procedures outlined by Cashin et al. (20). This will provide a critical update on current engagement efforts with transparency standards across a larger number of pain and anesthesiology journals. Such an assessment will provide the journals with a list of potential areas for improvement to guide their efforts.
3.2 Gain access to automated tools to improve transparency and trustworthiness, while fostering innovation in new methodologies
Innovations are needed to support a range of automated processes to enhance transparency and integrity. At present, multiple checks of transparency and trustworthiness are conducted manually by reviewers and editorial teams. Journals can carry out protocols in the work flow prior to the initiation of peer review around many indicators for quality, trustworthiness, and ethics concerns such as possible image manipulation, internal inconsistencies in referral to figures and tables, text plagiarism, adherence to reporting checklists, registration of systematic reviews, identifying discrepancies between research registrations (e.g., ClinicalTrials.gov) and reporting of clinical trial outcomes, and the inclusion of relevant animal and human review board approvals, to name a few. One example of checking for random sampling in RCTs is the method suggested by Carlisle and colleagues, but (a) this is labor-intensive and (b) does not apply where recruitment has not been entirely random (27, 28). Although there are automated processes to check for duplicate text, there are none yet to assist with these data integrity checks, and this requires dedicated staff effort. In this regard, several publishers/journals have introduced advanced technology (i.e., artificial intelligence) to detect duplicate manuscript submissions across all their respective journal platforms. Others have initiated “flag alerts” for authorships that include individuals who have been associated with multiple manuscript retractions. Additional automated processes are needed to help authors, reviewers, and editors to standardize more thorough yet efficient approaches to enhance transparency of reporting and enhance trustworthiness of published work.
Several approaches can be used to identify areas for improvement in this area. For example, we can engage in robust discussions with our publishers to emphasize the importance of automated tools, checks, and alerts, and advocate for their implementation in our journals. In addition, we can continue to advocate for adequate staffing to enable the critical checks needed for pre-review of submissions by the journal, which requires explicit formal training of a stable journal staff. While using advanced technology and providing journal staff entails a heightened responsibility of the publisher with possible financial consequences, it increases our confidence in the integrity of the research and builds trust in our science. We can also provide guidance and when possible, share resources (e.g., “how to guidance”) with our authors to enhance their own knowledge of tools to increase trustworthy science. For example, some reference management software (e.g., Zotero [Corporation for Digital Scholarship, Vienna, VA, USA], EndNote™ [Clarivate, London, UK]) have capabilities to check references for retractions (29).
3.3 Create a platform for collaboration among editors of leading pain and anesthesiology journals
This editorial highlights a significant collaboration among editors of leading pain and anesthesiology journals, which can serve as a foundation for continued engagement. We suggest holding online annual meetings and developing other platforms for information exchange for this group to discuss emerging trends, ethical concerns, and resource sharing. This may also serve as a forum for discussing general or specific integrity concerns and addressing the removal of inauthentic data from the literature, while ensuring confidentiality and privacy are upheld. We also recognize that there are barriers to engaging in transparency and integrity standards and anticipate initiating dialogue to better understand these barriers and how journals can support authors without increasing burden.
3.4 Offer educational opportunities and resources to professional societies, forums, journal reviewers, and early-career professionals
Journals can be an important resource to guide and teach researchers and consumers about transparency and integrity standards, and we see several opportunities to make an impact. For example, one opportunity to introduce standards for trustworthiness is through the system adopted by several of our journals for manuscript review mentorship and editorial fellowship that provides tutorials, training, and experience reviewing or managing manuscripts. Moreover, we can leverage our partnerships with the professional societies that are associated with many of our journals to offer training and instruction on transparency and integrity. This could include professional development programs for reviewers, as well as early-career faculty (e.g., North American Pain School), and offerings developed by groups such as the International Association for the Study of Pain's Early Career Network, (https://www.iasp-pain.org/early-career-network/), and by setting expectations for presenting and sharing research at scientific meetings (e.g., checking for retractions of any published studies discussed in presentations). Our journals can help disseminate information on tools targeting researchers directly (30) that can be made available to authors in a toolkit to assist them in pursuing values of openness and integrity. For example, statistical assessment tools to assess the accuracy of reported findings may be implemented by running simple, automated error checks, such as using the StatCheck tool (31). It should be stressed that increasing the education provided enhances quality, reliability, and integrity.
4 Conclusions
Ultimately, as a community of scientists and clinicians in pain and anesthesiology, we must recognize that trust is a dynamic and multifaceted concept. It requires ongoing effort to maintain, once lost is hard to regain, and it is built through consistent actions and open communication. Resources are available through the ENTRUST-PE framework that can guide actions and values to promote trust and integrity. These principles apply to all scientific fields beyond those that are pain-related and we encourage other specialties to harmonize such efforts. As editors, we will work together to advance the trustworthiness of research through upholding rigorous standards, ethical conduct, and open dialogue. By doing so, we can strengthen the foundation of trust in research and ensure that anesthesia and pain science continue to optimally inform care for people undergoing anesthesia or living with pain.
Author contributions
TMP: Conceptualization, Supervision, Writing – original draft. DB: Conceptualization, Writing – review & editing. KD: Conceptualization, Writing – review & editing. HH: Conceptualization, Writing – review & editing. RH: Conceptualization, Writing – review & editing. JK: Conceptualization, Writing – review & editing. JP: Conceptualization, Writing – review & editing. TJP: Conceptualization, Writing – review & editing. MS: Conceptualization, Writing – review & editing. SS: Conceptualization, Writing – review & editing. DT: Conceptualization, Writing – review & editing. MV: Conceptualization, Writing – review & editing. MW: Conceptualization, Writing – review & editing. TY: Conceptualization, Writing – review & editing. DY: Conceptualization, Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
None of the authors have disclosures directly related to this work. TMP receives research grant funding from NIH, provides consultation to TriveniBio, and serves as Editor-in-Chief, The Journal of Pain. DB serves as Editor-in-Chief, European Journal of Pain. KDD serves as Editor-in-Chief, PAIN. HCH receives research grant funding from NIH and serves as Editor-in-Chief, British Journal of Anaesthesia. RWH has received cooperative research grants from NIH and Nevro, Inc., provides expert/consulting to State Farm, and serves as Editor-in-Chief, Pain Medicine. JK is the Research Director of ManagingLife and serves as Editor-in-Chief, Canadian Journal of Pain. JJP is Editor-in-Chief, Anesthesia & Analgesia. TJP is a co-founder of and holds equity in 4E Therapeutics, NuvoNuro, PARMedics, Nerveli, and Doloromics, has received research grants from AbbVie, Eli Lilly, Grunenthal, Evommune, GSK, l\Hoba Therapeutics, and The National Institutes of Health, and serves as Editor-in-Chief, Neurobiology of Pain. MVdV has received honoraria for lectures and/or consultancy from CSL Behring, CSL Vifor, BBraun, Werfen, Viatris, CAF-DCF and Aquettant, and serves as Editor-in-Chief, European Journal of Anesthesiology. MS is Senior Medical Advisor, APURANO Pharma, and serves as Editor-in-Chief, Journal of Pain Research. SKWS holds the Dr. Jean Templeton Hugill Chair in Anesthesia, supported by the Dr. Jean Templeton Hugill Endowment for Anesthesia Memorial Fund (Faculty of Medicine, The University of British Columbia, Vancouver, BC, Canada) and gratefully acknowledges the Department of Anesthesia, St. Paul's Hospital/Providence Health Care (Vancouver, BC, Canada) for ongoing support. SKWS serves as Editor-in-Chief, Canadian Journal of Anesthesia. DCT holds the John and Emma Bonica Endowed Chair in Anesthesiology & Pain Research at the University of Washington, has received research grants from NIH and NIOSH, and has provided consultation to Vertex. DCT is also Associate Director of Analgesic, Anesthetic, and Addiction Clinical Trials Translations, Innovations, Opportunities, and Networks & Pediatric Anesthesia Safety Initiative (ACTTION/PASI). DCT serves as Editor-in-Chief, Clinical Journal of Pain. MDW serves as Editor-in-Chief, Anaesthesia. TLY is co-founder of Raft Pharmaceuticals, serves on the Scientific Advisory Board for Navega Therapeutics, and serves as the Editor-in-Chief for Frontiers in Pain Research. DY is a partner in a current Horizons grant, holds equity in BrainsGate and Theranica, and receives honorariums from Dr Reddy and from IASP. DY serves as Editor-in-Chief, PAIN Reports.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Footnote
1. ^Drs. Matthew Wiles, Editor-in-Chief, Anaesthesia; Jaideep Pandit, Editor-in-Chief, Anesthesia & Analgesia; Hugh Hemmings, Editor-in-Chief, British Journal of Anaesthesia; Stephan K, W. Schwarz, Editor-in-Chief, Canadian Journal of Anesthesia/Journal canadien d’anesthésie; Joel Katz, Editor-in-Chief, Canadian Journal of Pain; Dennis Turk, Editor-in-Chief, Clinical Journal of Pain, Marc Van de Velde, Editor-in-Chief, European Journal of Anaesthesiology; Didier Bouhassira, Editor-in-Chief, European Journal of Pain; Tony Yaksh, Editor-in-Chief, Frontiers in Pain Research; Michael Schatman, Editor-in-Chief, Journal of Pain Research; Theodore Price, Editor-in-Chief, Neurobiology of Pain; Karen Davis, Editor-in-Chief, PAIN; Robert Hurley, Editor-in-Chief, Pain Medicine; David Yarnitsky, Editor-in-Chief, PAIN Reports; and Tonya Palermo, Editor-in-Chief, The Journal of Pain.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
This article is being published almost simultaneously in all journals listed to reach as many readers as possible.
Anaesthesia; Anesthesia & Analgesia; British Journal of Anaesthesia; Canadian Journal of Anesthesia/Journal canadien d'anesthésie; Canadian Journal of Pain; Clinical Journal of Pain, European Journal of Anaesthesiology; European Journal of Pain; Frontiers in Pain Research; Journal of Pain Research; Neurobiology of Pain; PAIN; Pain Medicine; PAIN Reports; The Journal of Pain.
References
1. Wallach JD, Boyack KW, Ioannidis JPA. Reproducible research practices, transparency, and open access data in the biomedical literature, 2015–2017. PLoS Biol. (2018) 16(11):e2006930. doi: 10.1371/journal.pbio.2006930
2. Ioannidis JPA. What meta-research has taught us about research and changes to research practices. J Econ Surv. (2024):1–12. doi: 10.1111/joes.12666
3. Ioannidis JPA. Why most published research findings are false. PLoS Med. (2005) 2(8):e124. doi: 10.1371/journal.pmed.0020124
4. Hemmings HC, Shafer SL. Further retractions of articles by Joachim Boldt. Br J Anaesth. (2020) 125(3):409–11. doi: 10.1016/j.bja.2020.02.024
5. Audisio K, Soletti GJ, Cancelli G, Olaria RP, Rahouma M, Gaudino M, et al. Systematic review of retracted articles in critical care medicine. Br J Anaesth. (2022) 128(4):e292–4. doi: 10.1016/j.bja.2022.01.021
6. Open Science Collaboration. Estimating the reproducibility of psychological science. Science. (2015) 349(6251):aac4716. doi: 10.1126/science.aac4716
7. Camerer CF, Dreber A, Holzmeister F, Ho TH, Huber J, Johannesson M, et al. Evaluating the replicability of social science experiments in nature and science between 2010 and 2015. Nat Hum Behav. (2018) 2(9):637–44. doi: 10.1038/s41562-018-0399-z
8. Carlisle JB. False individual patient data and zombie randomised controlled trials submitted to Anaesthesia. Anaesthesia. (2021) 76(4):472–9. doi: 10.1111/anae.15263
9. Palermo TM, Davis KD, Bouhassira D, Hurley RW, Katz JD, Keefe FJ, et al. Promoting inclusion, diversity, and equity in pain science. J Pain. (2023) 24(2):187–91. doi: 10.1016/j.jpain.2022.11.005
10. Davis KD. The value of equity, diversity, and inclusion principles and sex/gender considerations in pain research. Pain. (2025) 166(4):713. doi: 10.1097/j.pain.0000000000003569
11. Milam AJ, Pandit JJ. The anesthesia & analgesia strategy for the “people and health advocacy” section: economic, academic, and healthcare dividends of diversity, equity, and inclusion. Anesth Analg. (2025) 140(5):1093–8. doi: 10.1213/ANE.0000000000007468
12. Moore A, Fisher E, Eccleston C. Flawed, futile, and fabricated-features that limit confidence in clinical research in pain and anaesthesia: a narrative review. Br J Anaesth. (2023) 130(3):287–95. doi: 10.1016/j.bja.2022.09.030
13. Retraction guidelines. COPE: Committee on publication ethics. (2019). Available online at: https://publicationethics.org/guidance/guideline/retraction-guidelines cm (Accessed April 9, 2025).
14. Shafer SL. Editor’s note: notices of retraction. Anesth Analg. (2011) 112(5):1246–7. doi: 10.1213/ANE.0b013e31821a8542
15. The Retraction Watch Leaderboard. Retraction Watch. Available online at: https://retractionwatch.com/the-retraction-watch-leaderboard/ (Accessed February 21, 2025)
16. Ferraro MC, Moore RA, De C, Williams AC, Fisher E, Stewart G, et al. Characteristics of retracted publications related to pain research: a systematic review. Pain. (2023) 164(11):2397–404. doi: 10.1097/j.pain.0000000000002947
17. Nair S, Yean C, Yoo J, Leff J, Delphin E, Adams DC. Reasons for article retraction in anesthesiology: a comprehensive analysis. Can J Anaesth. (2020) 67(1):57–63. doi: 10.1007/s12630-019-01508-3
18. O’Connell N, Moore RA, Stewart G, Fisher E, Hearn L, Eccleston C, et al. Trials we cannot trust: investigating their impact on systematic reviews and clinical guidelines in spinal pain. J Pain. (2023) 24(12):2103–30. doi: 10.1016/j.jpain.2023.07.003
19. Lee H, Lamb SE, Bagg MK, Toomey E, Cashin AG, Moseley GL. Reproducible and replicable pain research: a critical review. Pain. (2018) 159(9):1683–9. doi: 10.1097/j.pain.0000000000001254
20. Cashin AG, Bagg MK, Richards GC, Toomey E, McAuley JH, Lee H. Limited engagement with transparent and open science standards in the policies of pain journals: a cross-sectional evaluation. BMJ Evid-Based Med. (2021) 26(6):313–9. doi: 10.1136/bmjebm-2019-111296
21. Okonya O, Rorah D, Tritz D, Umberham B, Wiley M, Vassar M. Analysis of practices to promote reproducibility and transparency in anaesthesiology research. Br J Anaesth. (2020) 125(5):835–42. doi: 10.1016/j.bja.2020.03.035
22. O’Connell NE, Belton J, Crombez G, Eccleston C, Fisher E, Ferraro MC, et al. Enhancing the trustworthiness of pain research: a call to action. J Pain. (2025) 28:104736. doi: 10.1016/j.jpain.2024.104736
23. IASP Formally Endorses the ENhancing TRUST in Pain Evidence (ENTRUST-PE) Framework. International Association for the Study of Pain (IASP). Available online at: https://www.iasp-pain.org/publications/iasp-news/iasp-formally-endorses-the-enhancing-trust-in-pain-evidence-entrust-pe-framework/ (Accessed April 6, 2025).
24. EFIC Endorses ENTRUST-PE: Advancing Trustworthiness in Pain Research. European Pain Federation. (March 4, 2025). Available online at: https://europeanpainfederation.eu/news/efic-endorses-entrust-pe-advancing-trustworthiness-in-pain-research/ (Accessed April 6, 2025)
25. Nosek BA, Alter G, Banks GC, Borsboom D, Bowman SD, Breckler SJ, et al. Promoting an open research culture. Science. (2015) 348(6242):1422–5. doi: 10.1126/science.aab2374
26. Cashin AG, Fisher E, Soliman N, Palermo TM. Promoting openness and transparency to advance pain science: new initiatives at the journal of pain. J Pain. (2024) 25(8):104604. doi: 10.1016/j.jpain.2024.104604
27. Pandit JJ. On statistical methods to test if sampling in trials is genuinely random. Anaesthesia. (2012) 67(5):456–62. doi: 10.1111/j.1365-2044.2012.07114.x
28. Carlisle JB, Dexter F, Pandit JJ, Shafer SL, Yentis SM. Calculating the probability of random sampling for continuous variables in submitted or published randomised controlled trials. Anaesthesia. (2015) 70(7):848–58. doi: 10.1111/anae.13126
29. Ferraro MC, Soliman N, Fisher E, Cashin AG. Reducing the spread of retracted pain research. J Pain. (2025) 27:104747. doi: 10.1016/j.jpain.2024.104747
30. Korbmacher M, Azevedo F, Pennington CR, Hartmann H, Pownall M, Schmidt K, et al. The replication crisis has led to positive structural, procedural, and community changes. Commun Psychol. (2023) 1(1):1–13. doi: 10.1038/s44271-023-00003-2
Keywords: clinical trial, pain, anesthesiology, data reproducibility, data authenticity, research integrity, open science
Citation: Palermo TM, Bouhassira D, Davis KD, Hemmings HC Jr, Hurley RW, Katz J, Pandit JJ, Price TJ, Schatman ME, Schwarz SKW, Turk DC, Van de Velde M, Wiles MD, Yaksh TL and Yarnitsky D (2025) Editorial commitment to trust and integrity in science: implications for pain and anesthesiology research. Front. Pain Res. 6:1653869. doi: 10.3389/fpain.2025.1653869
Received: 25 June 2025; Accepted: 24 July 2025;
Published: 12 August 2025.
Edited by:
Roi Treister, University of Haifa, IsraelReviewed by:
Carina Crucho, New University of Lisboa, PortugalCopyright: © 2025 Palermo, Bouhassira, Davis, Hemmings, Hurley, Katz, Pandit, Price, Schatman, Schwarz, Turk, Van de Velde, Wiles, Yaksh and Yarnitsky. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tonya M. Palermo, dG9ueWEucGFsZXJtb0BzZWF0dGxlY2hpbGRyZW5zLm9yZw==
 Tonya M. Palermo
Tonya M. Palermo Didier Bouhassira3
Didier Bouhassira3 Hugh C. Hemmings Jr
Hugh C. Hemmings Jr Joel Katz
Joel Katz Jaideep J. Pandit
Jaideep J. Pandit Theodore J. Price
Theodore J. Price Tony L. Yaksh
Tony L. Yaksh