- 1RISE-Health, School of Health Sciences, University of Aveiro, Aveiro, Portugal
- 2RISE-Health, Department of Education and Psychology, University of Aveiro, Aveiro, Portugal
- 3RISE-Health, University of Aveiro, Aveiro, Portugal
- 4Department of Medical Sciences, Institute of Electronics and Informatics Engineering of Aveiro, University of Aveiro, Aveiro, Portugal
Introduction: Musculoskeletal pain is highly prevalent among older adults and a leading cause of disability. Digital health promises to deliver timely and quality care, but existing reviews fail to be specific for older adults, focus on a single type of technology or a single body site, and do not provide an integrated overview of the effectiveness of current digital interventions. This systematic review with meta-analysis (Prospero ID: CRD42024549668) aimed to assess the effectiveness of digital interventions for pain management in reducing pain intensity and self-reported disability in older adults with musculoskeletal pain.
Methods: We searched PubMed, Web of Science, Scopus, and Academic Search Complete from inception to April 2025; extracted data on participants, interventions, and primary (pain intensity and self-reported disability) and secondary outcomes (performance, pain-related psychological variables, and adverse events).
Results: Thirty-six RCTs were included (n = 4,041). Compared to other active interventions, older adults who received digital pain management reported lower pain intensity (SMD = −0.23, 95%CI = −0.37;−0.09) and lower self-reported disability (SMD = −0.22, 95%CI = −0.39;−0.04) at post-intervention. The effect was maintained at 6 months for pain intensity (SMD = −0.20; 95%CI = −0.38;−0.03), but not for disability (SMD = 0.13, 95%CI = −0.38;0.63). The certainty of evidence was low or very low, and heterogeneity was low to substantial. Most studies included domains judged as high risk of bias.
Discussion: The evidence is very uncertain on the effect of digital interventions on pain intensity and disability. They may decrease pain intensity and disability similarly to other interventions, but more research is needed to investigate the effect of digital interventions and identify key aspects that maximise the intervention.
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/view/CRD42024549668, PROSPERO CRD42024549668.
Introduction
Musculoskeletal pain affects more than 60% of older adults (1), with the low back, the hip, and the knee being the most common painful body sites (2). Most older adults report pain that is often or always present, is of at least moderate intensity, and located in three or more body sites (3). Furthermore, musculoskeletal pain is associated with decreased functioning, assessed through self-reported or performance-based measures (3). Pain also negatively impacts psychological well-being, being associated with decreased self-efficacy and increased anxiety (4), fear of movement (5) or catastrophizing, which, in turn, might also negatively impact older adultś ability to be physically active (6), further threatening a healthy and active aging.
Pain is one of the main reasons for healthcare use (7–9), with higher pain intensity and disability being drivers of care seeking (10, 11), burdening the healthcare system (2). Nevertheless, there are inequalities in access to adequate pain treatment, particularly non-pharmacological pain treatment (7, 8), that relate to high costs, remote healthcare centers, and difficulty accessing transportation (12).
Digital health offers the possibility to overcome current barriers to provide quality and timely non-pharmacological pain management interventions to larger numbers of individuals at lower costs (13). Digital health is a broad term that includes all tools and services using information and communication technology to support healthcare (14), including those accessed, for example, by a computer or a mobile phone. Also, digital health allows interaction with the health professional, which can be either synchronous, allowing real-time face-to-face interaction between the patient and the healthcare professional, or asynchronous, allowing the review of the patient's performance or data after the intervention (15). The diversity of digital means and interaction models enables the digital intervention's adjustment to the individual needs, characteristics, and preferences, and facilitates personalization (16), while having the potential to impact its use and effectiveness.
Existing systematic reviews suggest that some types of technology might be effective in reducing pain and improving functioning (17, 18), but fail to be specific for older adults (17), focus on a single type of technology, or are limited to one body site (17, 18) not providing an integrated overview of the effectiveness of digital pain interventions for older adults. Therefore, the primary aim of this systematic review was to assess the effectiveness of digital interventions delivered at a distance for pain management in reducing pain intensity and self-reported disability in older adults with musculoskeletal pain. The secondary aim was to explore the effectiveness of digital interventions targeting pain to improve performance-based measures (e.g., Timed Up and Go, gait velocity, grip strength) and pain-related psychological variables (self-efficacy, fear of movement, catastrophizing, and anxiety) in older adults with musculoskeletal pain. Adverse events were also characterised.
Methods
This systematic review followed PRISMA guidelines (19) and was registered in PROSPERO (registration number: CRD42024549668).
Search strategy
Pubmed, Web of Science, Scopus, and Academic Search Complete were searched from inception to the 20th of April 2024 and updated on the 14th of April 2025, using the search terms available in the Supplementary Material 1 - Search strategy. Search results were exported to CADIMA (https://www.cadima.info), and duplicates were identified and deleted. Reference lists of selected studies were checked for further relevant studies.
Selection of studies
Two authors (combined pairs of two of the four authors) independently screened the titles and abstracts, and the full texts. Discrepancies were resolved by consensus in a meeting with the four authors. At the full-text level, reasons for exclusion were documented. Reference screening was conducted with the support of CADIMA software.
Eligibility criteria
We included studies with older adults with acute (including post-surgical) or chronic musculoskeletal pain aged 60 and over (mean age of at least 60 years old). Musculoskeletal pain was defined as nociceptive pain that arises as part of a disease process directly affecting bone(s), joint(s), muscle(s), or related soft tissue(s) (20). Conditions usually considered as being musculoskeletal but for which the causes are incompletely understood (primary musculoskeletal pain), such as nonspecific back pain or fibromyalgia and chronic widespread pain, are classified in the CID-11 as primary pain (21) were also included.
Interventions included any pain management asynchronous or synchronous digital intervention delivered at a distance from the clinical center/hospital and constituting the main component of the intervention. This was defined as an intervention delivered via any web-based or online platforms, mobile applications, or virtual reality, accounting for at least 75% of the total intervention. The percentage of the intervention delivered digitally was calculated by dividing the total duration or total number of sessions of the intervention administered digitally by the total duration or total number of sessions of the intervention, respectively, multiplied by 100. Additionally, the participant and the health professional were in separate settings (e.g., the participant at home and the professional in a clinical environment). Studies employing digital interventions delivered by health professionals to participants in clinical settings were excluded as this was not considered to be delivered at a distance, and the potential for contact with healthcare professionals was very high, potentially affecting the effect of the intervention. Also, studies that used digital tools solely for data collection or self-monitoring (e.g., number of steps collected from a wearable sensor or mobile application) were excluded.
Comparisons included usual care, no treatment, waiting-list, a placebo (a digital intervention with limited features), or any non-digital pain management intervention. Studies using an active digital intervention in both arms were excluded, as these studies would not provide data on the beneficial effect of digital interventions.
Primary outcomes were pain intensity and self-reported disability measured using any validated instrument. Disability is usually characterized by both self-reported measures, which assess the individuals' perception of their capability to perform a range of tasks, and performance-based measures that capture how well an individual can perform a task and usually involve the completion or timing of strength, balance, or mobility tasks by an assessor (3, 22). Therefore, performance measures were also included as secondary outcomes. Additional secondary outcomes were: pain-related psychological factors (catastrophizing, fear of movement, self-efficacy, and anxiety) and adverse events. We collected outcome data immediately after treatment (baseline), 6-month follow-up (6 months), and 12-month follow-up.
The type of studies included were randomized controlled trials, as randomized controlled trials are one of the highest-quality trial designs for establishing effectiveness. We excluded other study designs, conference abstracts, dissertations, and papers that were not peer-reviewed.
Data extraction
A customized Excel form for data extraction was tested in three studies to ensure completeness of headings, clear and consistent coding, and response options, and to train researchers (23). The following data was extracted: authors and year of publication, participants' characteristics (age, sex, clinical condition), outcomes, general characteristics of the intervention (type, duration, frequency), characteristics of the digital intervention (delivered synchronously or asynchronously, type of technology used), personalization features of the digital intervention, adverse events and results. Personalization features were characterized using a previously used approach (24) that identified four possible personalization strategies: (i) goal setting (it involves defining goals considering the patients capabilities and preferences); (ii) adjusting the plan (it involves adjusting the intervention based on the capabilities of the participants and feedback throughout the intervention); (iii) using data-driven approaches (it involves gathering data on participants' health status and integrating those data into personalized interventions); and (iv) motivating behavioural changes (including text messages, reminders, and prompts).
Risk of bias
We used the Cochrane Risk of Bias tool (Rob-2) to judge the risk of bias (25). The domains covered by the tool (randomisation process, deviations from the intended interventions, missing outcome data, outcome measurement, selection of the reported outcome, and overall bias) were rated as “low,” “some concerns,” or “high” risk of bias. It was administered independently by at least two of the four authors, and by type of outcome (i.e., a separate Rob-2 was filled in for self-reported outcomes and clinical tests/performance measures administered by the clinician/assessor). Discrepancies were resolved by discussion among the four authors till a consensus was reached.
Grading of evidence
The certainty of evidence was assessed using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach and rated as high, moderate, low, or very low (26). Certainty in the meta-analysis results was downgraded for serious study limitations (one level if 25% of participants were from studies classified as high risk and two levels if the percentage was 50% or higher), inconsistency (downgrade one level if heterogeneity was high; I2≥ 75%), imprecision (downgraded one level if there were fewer than 400 participants in each arm), and publication bias (downgraded one level if there was evidence of publication bias assessed through visual inspection of funnel plots and Egger's test). Indirectness was not used to downgrade evidence as participants, comparisons, and outcomes were all directly relevant.
Summary of evidence
Meta-analysis
Comparisons or outcomes were performed at post-intervention and follow-up (when possible). Meta-analyses were conducted using SPSS (IBM, version 28). When data were not available or suitable (e.g., means not reported) for a meta-analysis, we contacted the corresponding author, requesting the necessary data. When needed data was not directly available, but it was possible to compute from available metrics (e.g., standard errors or confidence intervals to calculate SD), conversion was carried out as specified in the Cochrane Handbook (27). When lower scores of different instruments meant different things, the mean values from one set of studies were multiplied by −1 (27). All meta-analyses were conducted with random-effects models because of heterogeneity in study design and outcome measures across trials. We reported standardized mean differences (SMD) and respective 95% confidence intervals. SMD was interpreted as small (0.2), medium (0.5), and large (0.8). Heterogeneity was assessed using I2, interpreted as low heterogeneity (0%–40%), moderate heterogeneity (30%–60%), substantial heterogeneity (50%–90%), and considerable heterogeneity (75%–100%) (27). The findings of the meta-analysis were conveyed using the statements suggested by Santesso et al. (28), crossing the effect size and the level of certainty of evidence. Results are presented in graphics and a summary table of the effect sizes for individual studies.
Considering the moderate heterogeneity of the main meta-analyses, we explored subgroup analysis for type of patients (i.e., patients with chronic conditions and patients with post-surgery conditions) and sensitivity analysis isolating studies with asynchronous administration of the digital intervention and no personalization/one personalization strategy.
Lower pain intensity, lower self-perceived functioning scores, and lower performance scores represent better outcomes. When more than one instrument was used to assess the same outcome of interest, we prioritize the outcome reported more consistently across studies.
Results
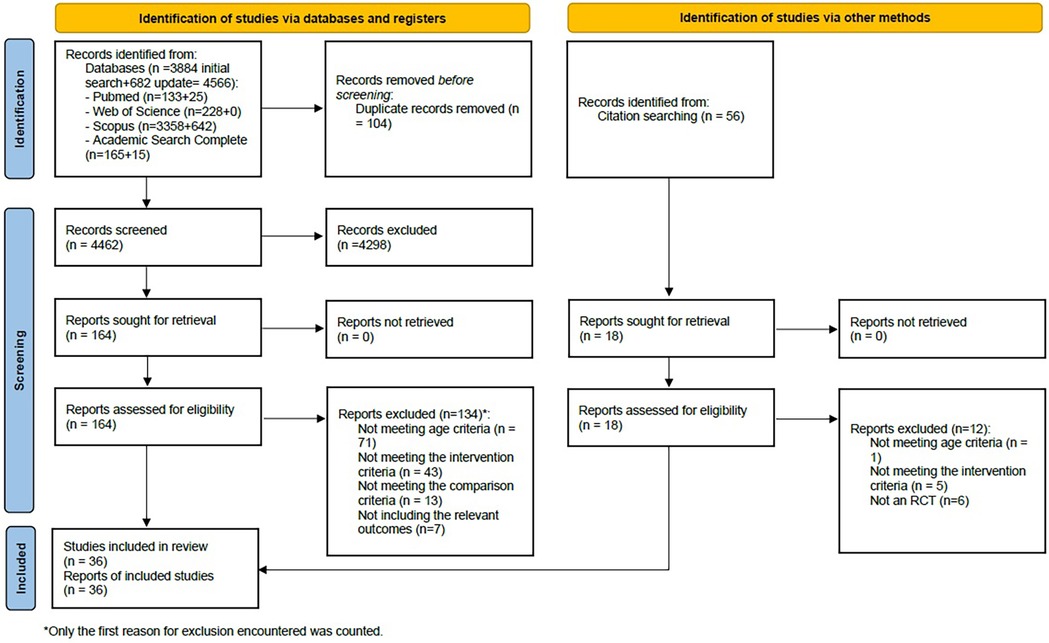
The search yielded 4,566 records, and after the removal of duplicates, 4,462 unique references were screened, of which 181 full texts were read (164 resulting from database searches and 17 resulting from citation searching). A total of 36 articles, reflecting 36 studies, met the inclusion criteria and were included in this systematic review. The PRISMA flowchart (Figure 1) presents the numbers throughout the selection phases and reasons for exclusion. Corresponding authors of eight articles were contacted, requesting additional information, but only one replied.
Study characteristics
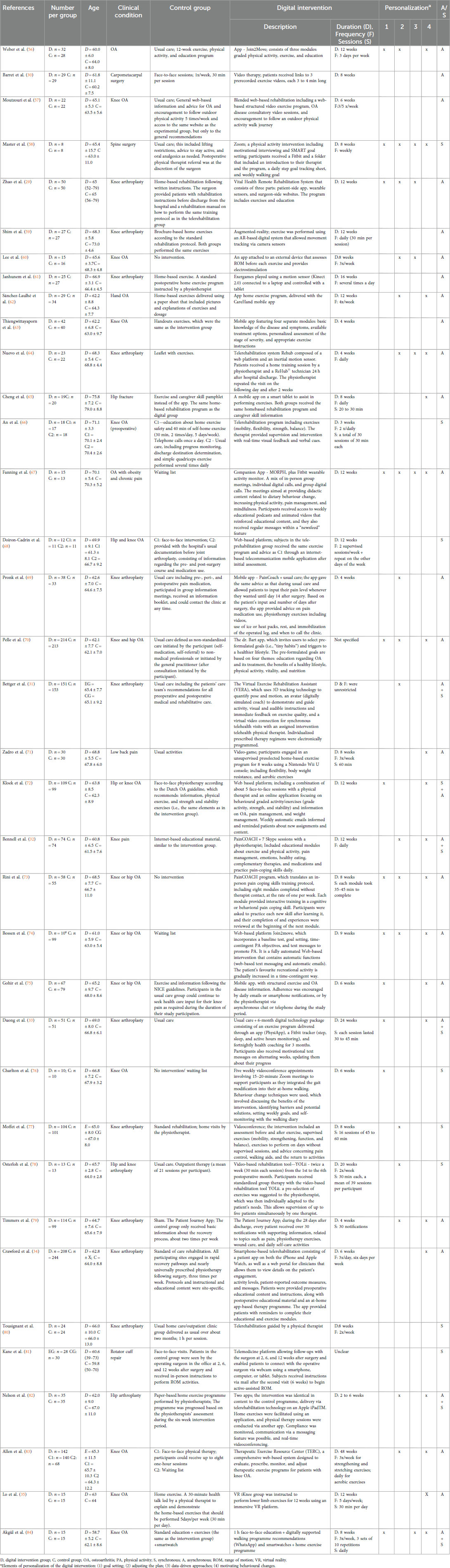
A total of 36 manuscripts were included that assessed at least one of the main variables of interest (pain intensity or functioning). Included studies represented 4,041 participants, of which 2,270 were females (56.2%). Nineteen studies involved patients with chronic conditions (knee or hip osteoarthritis: n = 17; hand osteoarthritis: n = 1; low back pain: n = 1), while 17 involved patients with acute conditions (hip fracture: n = 1) or submitted to surgical procedures (knee or hip arthroplasty: n = 13; rotator cuff repair: n = 1; carpometacarpal arthroplasty: n = 1; spine surgery: n = 1). The general characteristics of the studies are presented in Table 1.
The digital intervention was delivered synchronously, via teleconference software, in 8 (22%) studies, and asynchronously, via mobile apps, web-based platforms, video, exergames, or virtual reality, with or without external sensors for data collection, in 22 (61%) studies. In addition, 6 (17%) studies combined a synchronous and an asynchronous component.
The digital intervention was compared against other interventions in 31 (86%) studies, defined as usual care (n = 14), face-to-face intervention (n = 3), home care (n = 5), a brochure with information/exercises (n = 4), and general online/app-delivered information (n = 2). Also, of the 31 studies, two studies included two comparison groups receiving a combination of usual care, home exercises, or face-to-face care, and another study included one active comparison arm (usual care) and a waiting list control. Most of the studies using a comparator defined as “usual care”, “face-to-face intervention”, and “home care” involved some form of education and exercise. The remaining 5 (14%) of the 36 included studies used a no-intervention/wait-list group as a control.
Regarding personalization strategies, 9 (25%) studies did not include any, 9 (25%) included only 1, and an additional 9 (25%) studies included two. Only 9 (25%) studies used 3 or 4 personalization strategies.
The duration of the digital intervention varied (range: 3 weeks–48 weeks), with 24 (67%) studies reporting 8 or more weeks, 10 (28%) reporting 6 or fewer weeks, and 2 (5%) were unclear.
Of 36 included studies, 32 reported on pain intensity, 34 reported on self-reported disability, and 22 reported on a performance measure. The most commonly used instruments to assess pain intensity were the numeric pain rating scale or the visual analogue scale (n = 19, 59%). The most commonly used instruments for self-reported disability were the Western Ontario and McMaster Universities Arthritis Index (WOMAC) or WOMAC function subscale (n = 13, 38%), and the Hip or Knee Disability and Osteoarthritis Outcome Score (HOOS or KOOS; n = 12, 35%). Performance was assessed using mainly the Timed Up and Go test (n = 12; 55%).
Adverse events
Studies that reported on adverse events n = 21 (58%) reported either no adverse/serious events or a similar rate of events between both groups (n = 14, 67%). Of these, 7 studies reported more concrete data: a 2% rate of adverse events in each group (29), 1 person in the digital group intervention developed a complex regional pain syndrome (30), a rate of falls of 19.4% in the group receiving the digital intervention against 14.6% in the control group and a mean(±sd) of rehospitalizations in 12 weeks of 0.1 ± 0.3 in the experimental group and of 0.2 ± 0.5 in the control group (31), that more participants in the intervention group (n = 22) than the control group (n = 3) reported minor, mainly unrelated to the intervention, adverse events (15 and 3 events, respectively) (32), adverse events related to skin irritation due to the bandages used to affix tracking sensors (n = 3 out of 51 participants) (33), emergency department visits within 90 days were lower in the digital group intervention when compared to the control group [n (%): 16 (8.2) vs. 5 (2.5), p < 0.013] (34) and in a study using virtual reality (35), 33% of participants reported cybersickness.
Risk of bias
For subjective measurements (pain intensity and self-reported disability), the overall risk of bias was judged as high, due to bias in domain 4 (measurement of the outcome) introduced by lack of blinding. Among the remaining domains, bias was considered low in 26 studies (72%) for domain 1 (randomization process), 21 (58%) for domain 2 (deviations from intended interventions), 86% for domain 3 (missing outcome data) and 5 (14%) for domain 5 (selection of the reported results). For performance measurements and the 22 studies that reported on them, the overall risk of bias was judged as some concerns for 7 studies (32%) and high risk for 15 (68%) studies. Low risk of bias was found for 14 (64%) studies in domain 1, 10 (45%) in domain 2, 19 (86%) in domain 3, 12 (55%) in domain 4, and 3 (14%) in domain 5. The risk of bias for pain intensity and self-reported disability (patient-reported outcomes) is presented in Figure 2. The risk of bias for performance-based measures is presented in Supplementary Material 2.
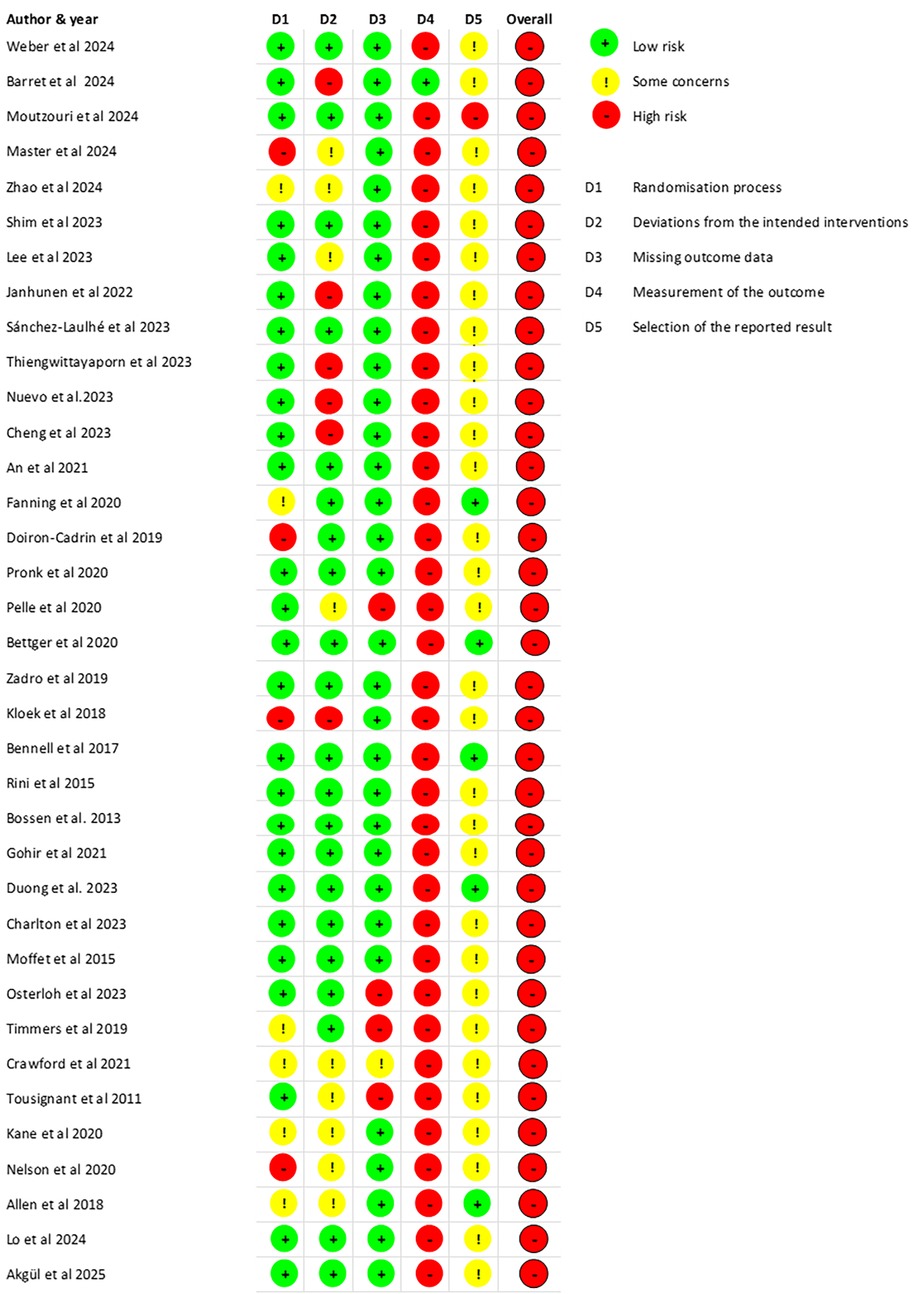
Figure 2. Risk of bias for all studies (pain intensity and self-reported disability—patient-reported outcome measures).
Meta-analysis on the effects of the digital intervention
Of the 32 studies that reported pain intensity, 29 provided data that could be included in the meta-analysis (23 compared a digital intervention against another active intervention, and 6 against no intervention). Of the 33 studies that reported self-reported disability, 28 provided data that could be included in the meta-analysis (24 compared a digital intervention against another active intervention, and 4 against no intervention). GRADE tables for all meta-analyses performed are presented in Supplementary Material 3. Additionally, sample size, mean, and standard deviation at post-intervention and follow-up for studies included in the meta-analysis are presented in Supplementary Material 4.
Effect of the digital interventions when compared against other forms of treatment on pain intensity and self-reported disability
There was low certainty evidence of a small beneficial effect of digital interventions in reducing pain intensity at post-treatment (SMD = −0.23, 95%CI = −0.37 to −0.09, I2 57%, k = 23; Figure 3), and very low certainty of evidence that this beneficial effect was maintained at 6 months follow-up (SMD = −0.20; 95%CI = −0.38 to −0.03; I2 = 0%; k = 3). No meta-analysis was possible at 12-month follow-up (k = 2).
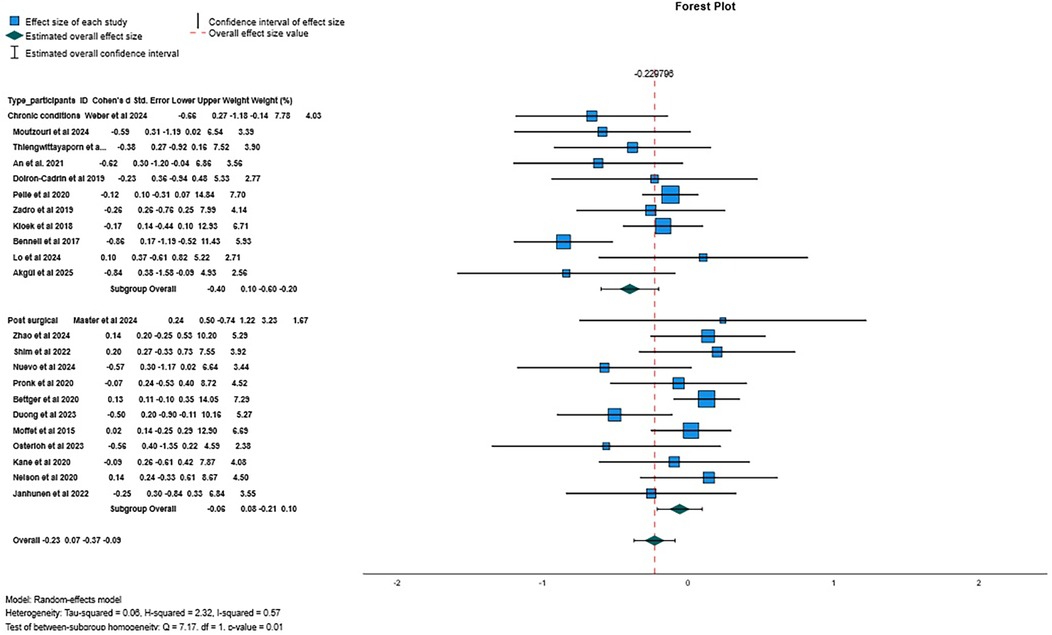
Figure 3. Forest plot for pain intensity when comparing digital interventions against other interventions.
There was low certainty evidence of a small beneficial effect of digital interventions in reducing disability at post-treatment (SMD = −0.22, 95%CI = −0.39 to −0.04; I2 78%, k = 24; Figure 4), and very low certainty of evidence that this effect was not maintained at 6-month follow-up (SMD = 0.13, 95%CI = −0.38 to 0.63; I2 70%, k = 3), neither at 12-month follow-up (SMD = −0.06, 95%CI = −0.23 to 0.11; I2 = 9%, k = 4). Additional flowcharts are presented in Supplementary Material 5. A qualitative description of the results of studies not included in the meta-analyses is presented in Supplementary Material 6.
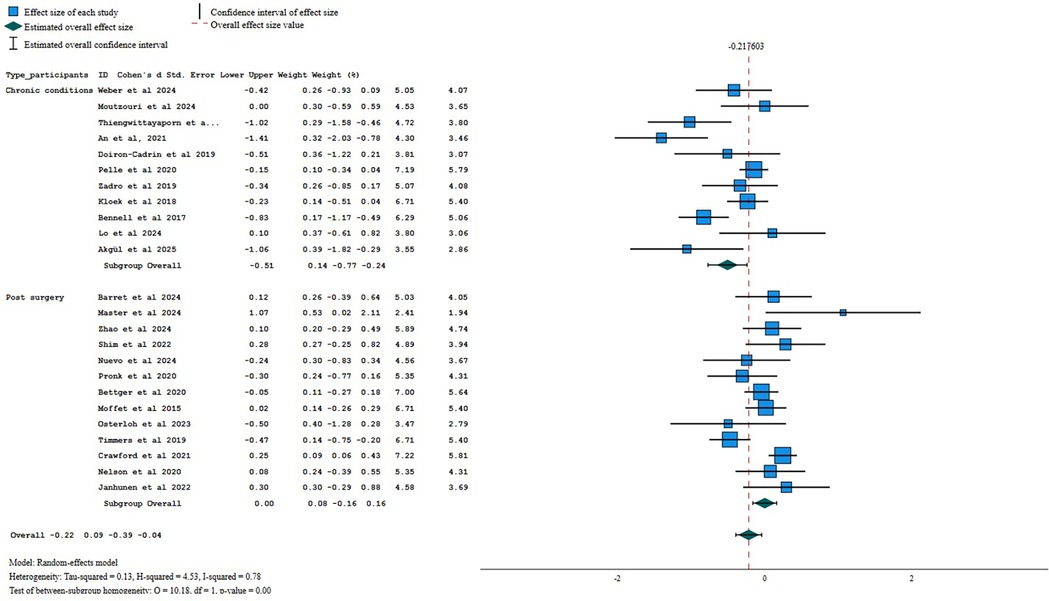
Figure 4. Forest plot for self-reported disability when comparing digital interventions against other interventions.
Effect of the digital interventions when compared to no intervention
Meta-analysis included only studies with chronic conditions
There was very low certainty of evidence of a small beneficial effect of digital interventions in reducing pain intensity at post-treatment (SMD = −0.24, 95%CI = −0.40 to −0.08, I2 0%, k = 6).
There was very low certainty of evidence of no between-group differences at post-treatment for self-reported disability (SMD = −0.09, 95%CI = −0.30 to 0.12, I2 0%, k = 4).
No meta-analysis was possible at follow-up due to the small number of studies with the same time-point (k < 3).
Effect of the digital interventions on performance (secondary outcome)
Of the 22 studies that reported performance, 18 provided data for the meta-analysis (15 compared a digital intervention against another active intervention and 3 against no intervention).
When compared against other interventions, there was very low certainty evidence of a small beneficial effect of digital interventions improving performance at post-treatment (SMD = −0.26, 95%CI = −0.44 to −0.08; I2 65%, k = 15; Figure 5). No meta-analysis was possible at 6- or 12-month follow-up (k < 3 for both follow-up time points).
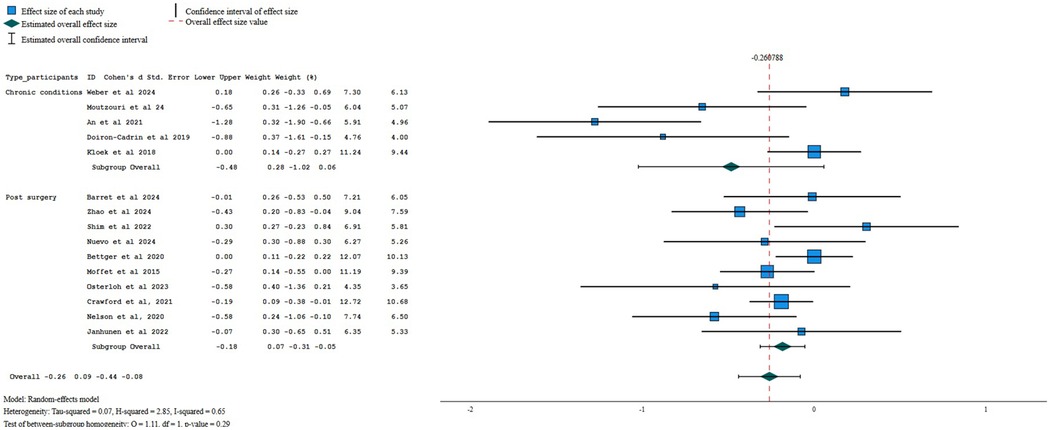
Figure 5. Forest plot for performance when comparing digital interventions against other interventions.
When compared against no interventions, there was very low certainty of evidence of a small beneficial effect of digital interventions in improving performance at post-treatment for older adults with chronic painful conditions (SMD = −0.49, 95%CI = −0.95 to −0.03, I2 0%, k = 3).
Effect of the digital interventions on psychological variables (secondary outcome)
Six studies assessed at least one psychological variable (self-efficacy, fear of movement, catastrophizing, or anxiety). Meta-analysis was possible only for self-efficacy at post-intervention, when the comparison group was other interventions, and there was very low certainty of evidence of no between-group differences (SMD = 0.39; 95%CI −0.19 to 0.98, I2 87%; k = 3).
Subgroup and sensitivity analysis for the comparison of digital interventions against other interventions
Given the variability in patients' characteristics, particularly post-operative individuals and patients with chronic conditions, and the moderate heterogeneity identified, we conducted a subgroup analysis based on the type of patients and a sensitivity analysis grouping only studies using one or no personalization strategies, and studies using asynchronous digital interventions. These analyses were only possible when the comparisons were other interventions, due to the small number of studies that used no intervention as a comparison.
Sub-group analysis by type of patients revealed low certainty of evidence of a small beneficial effect of digital interventions when compared to other interventions for pain intensity in participants with chronic conditions (−0.40, 95% CI −0.60 to −0.20; p = 0.00; I2 53%, k = 11) but not for patients who underwent a surgery (−0.06, 95% CI −0.21 to 0.10; p = 0.00, I2 30%; k = 12). Sensitivity analysis, for pain intensity showed a small beneficial effect of asynchronous digital interventions (−0.18, 95% CI −0.34 to −0.03, p = 0.02, I2 18%, k = 11; low certainty of evidence). When aggregating only the studies using one or no personalization strategies, no between-group difference was found (SMD = −0.14, 95% CI −0.31 to 0.02, p = 0.20; I2 = 2%; k = 7; low certainty of evidence).
For self-reported disability, sub-group analysis by type of patients revealed low certainty of evidence of a medium beneficial effect of digital interventions when compared to other interventions for patients with chronic conditions (−0.51, 95% CI −0.77 to −0.24; I2 74%, k = 11), but no difference between interventions for post-operative patients (SMD = 0.00; 95% CI −0.16 to 0.16; I2 = 54%; k = 13). Sensitivity analysis, using only the asynchronous administration of digital interventions, showed no between-group differences (−0.13, 95%=−0.31 to 0.04, I2 65%, k = 15; low certainty of evidence). When aggregating only the studies using one or no personalization strategies, no between-group differences were found (SMD = −0.18, 95%CI −0.43 to 0.07; I2 = 79%; k = 14; low certainty of evidence).
Sub-group analysis by type of patients revealed very low certainty evidence of a small beneficial effect of digital interventions when compared to other interventions for performance in postoperative patients (−0.18, 95% CI −0.31 to −0.05; I2 20%, k = 10) but no difference between interventions for patients with chronic conditions (SMD = −0.48; 95% CI −1.02 to 0.06; I2 82%; k = 5). Sensitivity analysis, using only the asynchronous administration of digital interventions, showed no between-group differences (−0.13, 95%=−0.27 to 0.01, I2 14%, k = 9; low certainty of evidence). When aggregating only the studies using one or no personalization strategies, a small beneficial effect was found for performance (SMD = −0.30, 95%CI −0.57 to −0.04; I2 = 76%; k = 10; very low certainty of evidence).
Subgroup analysis did not apply when the comparison was no intervention (all studies in the same type of patients), and sensitivity analysis was not possible (k < 3).
Discussion
This review is a comprehensive evaluation of the effectiveness of digital interventions for older adults with pain. Our results suggest that digital interventions may reduce pain intensity and pain disability slightly at post-intervention and compared to other interventions for older adults with painful chronic conditions, but not for older adults with post-surgical/acute conditions. The evidence is very uncertain on the effect of digital interventions at follow-up on pain intensity and disability. The evidence also suggests that digital interventions may improve performance compared to other interventions, but only for older adults with post-surgical conditions. The evidence is very uncertain and scarce about the effect of digital interventions on self-efficacy. This review provides a broad overview of the effectiveness of digital interventions delivered at a distance for older adults, adding to previous reviews that focus on a single body region, clinical condition or intervention (for example, exercise), do not limit studies to those conducted in older adults or do not clarify whether the digital intervention administered at a distance corresponds to the main component of the intervention (36, 37).
Sub-group analysis by type of patients and sensitivity analysis for studies using fewer personalization strategies and delivering the intervention asynchronously showed a decrease in heterogeneity of varying degrees for pain intensity. For self-reported disability and performance, a decrease in heterogeneity was found only in the analyses by type of patients and asynchronous interventions. These findings suggest that type of patients, number of personalization strategies and mode of administration (synchronous vs. asynchronous) partially explain the variability across studies, but its impact on variability depends on the outcome. Furthermore, the sensitivity analysis also suggests that the mode of administration of the digital intervention (synchronous or asynchronous) might impact its effectiveness, as when analysing only the trials with an asynchronous administration of the intervention, the between-group differences for pain disability and performance were no longer present. Similarly, when analysing the trials with no or one personalization strategy, the between-group differences were no longer present for pain intensity and pain disability. Caution should be taken when interpreting the sensitivity analysis, as a few of them included a low number of studies. Nevertheless, these results highlight the importance of reporting the mode of administration (synchronous vs. asynchronous) and the number of personalization strategies in future trials. These factors should also be considered when aggregating data from different trials.
It is unclear why the type of subjects, mode of administration, and interaction strategies impact the effectiveness of digital interventions. Conceivably, there is more room for improvement in patients submitted to a recent surgery, which, adding to the general positive expectations of patients (38), might contribute to improvements that are less dependent on the specific characteristics of the intervention. Older adults value the received support, the ability to establish a continuous care relationship, and human communication when using digital services (39, 40), which might be perceived as less present in asynchronous interventions. Older adults prefer synchronous communication over asynchronous communication, which is believed to improve communication and comprehension (41). The interaction with the physical therapist, even if asynchronous, but frequent (daily), was described as important for support and encouragement. Older adults might have difficulties with technology (41), which can be mitigated by synchronous intervention as the clinician can help solve any issues. Recent studies also showed that a synchronous intervention decreased anxiety and pain intensity in a few body regions (not all) to a greater extent than an asynchronous digital intervention in adults with fibromyalgia (42) and no differences between synchronous and asynchronous digital delivery of exercises for adults with neck pain (42). Different strategies can be used to promote engagement in asynchronous interventions, including providing the possibility of contacting the healthcare professional, using interactive content, building a sense of community with peers through discussion forums or collaborative activities, and providing feedback by healthcare professionals. Whether the relevance of the mode of administration differs across clinical conditions and age ranges requires further investigation.
Personalization, more likely in synchronous and interactive interventions, is also valued by older adults (39). Self-monitoring, self-motivation, goal setting, and personalized feedback are a few of the strategies identified as key for a successful digital intervention (43) and for motivating individuals to behaviour change (44), such as adhering to the digital intervention or performing the recommended exercise. A previous review on the effectiveness of digital health interventions for older adults with cancer also concluded that multiple personalized features were likely to be more effective in improving self-management outcomes (24). Also, a previous systematic review investigating the evidence supporting the use of digital mental health interventions suggested four factors contributing to the success of digital mental health interventions: (1) ease of use; (2) opportunities for social interactions; (3) having human support; and (4) having the digital mental health interventions tailored to the participants’ needs (24). Also, daily email reminders and feedback were considered motivating factors for individuals with osteoarthrosis to perform the exercises (24).
The overall finding of the current review that digital pain interventions may have similar or greater effects on pain intensity and disability than other interventions align well with previous reviews, even though we were more stringent in the inclusion criteria, as the digital component needed to correspond to at least 75% of the intervention for a study to be included in the present review. It has been found that remote exercise programs were not less effective than in-person physical therapy for pain intensity in patients with osteoarthritis (45–47). Telerehabilitation was comparable to conventional in-person rehabilitation in improving clinical outcomes following total knee replacement (48). Despite the diversity of digital solutions used, a subgroup analysis of a previous systematic review indicated no significant difference among the different digital modes of delivery for pain intensity and physical function (47).
Very few studies explored the effect of digital interventions on self-efficacy, catastrophizing, fear of movement, and anxiety. These variables need to be included in future trials evaluating the effectiveness of digital interventions for older adults, considering their impact and relevance on pain intensity and pain-associated disability. Higher self-efficacy is associated with higher outcome expectations (49) and protects against decreased disability and performance at follow-up (49). Higher kinesiophobia is associated with decreased self-reported physical function and performance (50), and both higher catastrophizing and anxiety are associated with higher pain disability (51).
This systematic review also suggests that digital interventions are safe with few non-serious adverse events, most often similar to those occurring in the group receiving other interventions, suggesting that it is safe to use digital interventions with older adults with both chronic and post-surgical painful conditions. However, caution should be taken when interpreting these data and a systematic assessment of serious and non-serious adverse events is recommended in all future trials. More than 40% of included trials did not report on adverse events, and among those that reported on adverse events, the methodology used for their assessment was not always clear.
Study limitations
The low quality of included studies, as assessed using Rob2, mostly resulted from the inability to blind outcome assessors for self-reported (participant-reported) measures, which are potentially influenced by the participants' knowledge of the intervention received. This led to the downgrading of the evidence when applying the GRADE. The small sample sizes (n < 400) of a few meta-analyses (pain intensity at 6-month follow-up when the comparison were other interventions; pain intensity at post-intervention when the comparison was no intervention; self-reported disability at 6 and 12-month follow up when the comparison were other intervention, self-reported disability when the comparison was no intervention; performance for all comparisons, and self-efficacy when the comparison were other interventions), also led to the downgrading of evidence for imprecision when applying the GRADE. These meta-analyses might have lacked sufficient statistical power to detect between-group differences, which was reflected in the certainty of evidence for the effect estimate. The included studies varied in terms of intervention duration (ranging from 3 to 48 weeks) and frequency (from twice a week to unrestricted use). This diversity might have affected results, as a dose-response result might be expected in interventions targeting pain and disability, with a minimal dose of intervention being needed to achieve meaningful improvement (52). Future studies can explore whether a dose-response relationship exists for digital interventions and whether this varies depending on clinical conditions or intervention content. Few studies were included in the follow-up meta-analysis, weakening any conclusion on the medium and long-term effectiveness of digital interventions. Data extraction was performed by a single reviewer and reviewed for correctness and completeness by a second reviewer who was not blind to the data extracted by the first reviewer and could have been unintentionally influenced by it.
Research and clinical practice recommendations
The apparent safety of digital interventions and the potential for a positive impact on pain and disability cautiously suggest that digital interventions can be used in clinical practice to decrease pain and self-reported disability and improve performance. The choice between a face-to-face intervention and a digital intervention might be left to patients' preferences and ability to safely and correctly use the digital means needed for the intervention. This is particularly relevant as older adults have less access to digital means and lower digital literacy skills than younger groups (53). Therefore, ensuring that the older adult has access to and can use the digital means necessary for the digital intervention is crucial (53), both before and during the intervention. Choosing technology that allows some degree of personalization or adjustment to the individual's needs and preferences, that is inexpensive, and that is simple to use, may facilitate the use of digital means for healthcare by older adults. Furthermore, when choosing the digital intervention, the clinician might want to give preference to interventions allowing for a synchronous component and personalization features.
Future trials, in addition to employing more methodologically robust designs that overcome the limitations identified in this review, can compare digital interventions with different degrees of personalization and the synchronous and asynchronous administration of the same intervention. Furthermore, most existing trials use older adults with knee and hip osteoarthritis or patients who have undergone hip or knee replacement. Therefore, there is a need to investigate the effectiveness of digital interventions for other painful conditions that are prevalent in older adults, such as low back pain (54), pain in the shoulder and foot (55) and also for multiple painful body sites, as the majority of older adults have at least 3 painful body sites (3).
Conclusion
Our results suggest that digital interventions are at least as good as other interventions at decreasing pain and self-reported disability and improving performance. Furthermore, for older adults with painful chronic conditions, they may reduce pain intensity and pain disability, at post-intervention, slightly more. The evidence is very uncertain on the effect of digital interventions on pain intensity and disability at follow-up, and on the effect of digital interventions on self-efficacy. Further studies are needed to investigate digital pain management for currently under-investigated clinical conditions, such as low back pain and multisite pain, and to investigate which aspects of digital pain management (e.g., interaction) are likely to have a higher impact on the intervention effect.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
AGS: Funding acquisition, Project administration, Writing – review & editing, Formal analysis, Methodology, Writing – original draft, Data curation, Conceptualization, Investigation. AJS: Methodology, Data curation, Investigation, Writing – review & editing. RA: Writing – review & editing, Investigation, Data curation. NR: Writing – review & editing, Data curation, Conceptualization.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This work was funded by FEDER - Fundo Europeu de Desenvolvimento Regional funds through the COMPETE 2030 and by Portuguese funds through FCT- Fundação para a Ciência e a Tecnologia in the framework of the project COMPETE2030-FEDER-00781500.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpain.2025.1657014/full#supplementary-material
References
1. Lehti TE, Rinkinen M-O, Aalto U, Roitto HM, Knuutila M, Öhman H, et al. Prevalence of musculoskeletal pain and analgesic treatment among community-dwelling older adults: changes from 1999 to 2019. Drugs Aging. (2021) 38:931–7. doi: 10.1007/s40266-021-00888-w
2. Domenichiello AF, Ramsden CE. The silent epidemic of chronic pain in older adults. Prog Neuro Psychopharmacol Biol Psychiatry. (2019) 93:284–90. doi: 10.1016/j.pnpbp.2019.04.006
3. Silva A, Queirós A, Sa-Couto P, Rocha N. Self-reported disability: association with lower extremity performance and other determinants in older adults attending primary care. Phy Ther. (2015) 95:1628–37. doi: 10.2522/ptj.20140323
4. Niknejad B, Bolier R, Henderson CR, Delgado D, Kozlov E, Löckenhoff CE, et al. Association between psychological interventions and chronic pain outcomes in older adults. JAMA Intern Med. (2018) 178:830. doi: 10.1001/jamainternmed.2018.0756
5. Alpalhão V, Cordeiro N, Pezarat-Correia P. Kinesiophobia and fear avoidance in older adults: a scoping review on the state of research activity. J Aging Phys Act. (2022) 30:1075–84. doi: 10.1123/japa.2021-0409
6. Zhaoyang R, Martire LM, Darnall BD. Daily pain catastrophizing predicts less physical activity and more sedentary behavior in older adults with osteoarthritis. Pain. (2020) 161:2603–10. doi: 10.1097/j.pain.0000000000001959
7. Azevedo LF, Costa-Pereira A, Mendonça L, Dias CC, Castro-Lopes JM. Chronic pain and health services utilization. Med Care. (2013) 51:859–69. doi: 10.1097/MLR.0b013e3182a53e4e
8. Mäntyselkä P, Kumpusalo E, Ahonen R, Kumpusalo A, Kauhanen J, Viinamäki H, et al. Pain as a reason to visit the doctor: a study in Finnish primary health care. Pain. (2001) 89:175–80. doi: 10.1016/S0304-3959(00)00361-4
9. Torres JL, da Silva SLA, Ferreira FR, Mendes LPS, Machado LA. Chronic pain is associated with increased health care use among community-dwelling older adults in Brazil: the pain in the elderly (PAINEL) study. Fam Pract. (2019) 36:594–9. doi: 10.1093/fampra/cmy123
10. Silva AG, Queiros A, Rocha NP. Functioning and primary healthcare utilization in older adults: a 1-year follow-up study. Physiother Theory Pr. (2018) 35:1–10. doi: 10.1080/09593985.2018.1442536
11. Ferreira ML, Machado G, Latimer J, Maher C, Ferreira PH, Smeets RJ. Factors defining care-seeking in low back pain—a meta-analysis of population based surveys. Eur J Pain. (2010) 14(7):747.e1–7. doi: 10.1016/j.ejpain.2009.11.005
12. Suntai Z, Won CR, Noh H. Access barrier in rural older adults’ use of pain management and palliative care services: a systematic review. Am J Hosp Palliat Med. (2021) 38:494–502. doi: 10.1177/1049909120959634
13. Fatoye F, Gebrye T, Mbada C, Useh U. Economic evaluations of digital health interventions for the management of musculoskeletal disorders: systematic review and meta-analysis. J Med Internet Res. (2023) 25:e41113. doi: 10.2196/41113
15. Culmer N, Smith TB, Stager C, Wright A, Fickel A, Tan J, et al. Asynchronous telemedicine: a systematic literature review. Telemed Rep. (2023) 4:366–86. doi: 10.1089/tmr.2023.0052
16. Ghanvatkar S, Kankanhalli A, Rajan V. User models for personalized physical activity interventions: scoping review. JMIR Mhealth Uhealth. (2019) 7:e11098. doi: 10.2196/11098
17. Mapinduzi J, Ndacayisaba G, Verbrugghe J, Timmermans A, Kossi O, Bonnechère B. Effectiveness of mHealth interventions to improve pain intensity and functional disability in individuals with hip or knee osteoarthritis: a systematic review and meta-analysis. Arch Phys Med Rehabil. (2025) 106:280–91. doi: 10.1016/j.apmr.2024.06.008
18. Edward H, Nicolau D, Wu J, Paramanantharajah N, Wojkowski S, Macedo L, et al. Effectiveness of physiotherapist-led tele-rehabilitation for older adults with chronic conditions: a systematic review and meta-analysis. Disabil Rehabil. (2024) 106:1–15.
19. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Br Med J. (2021) 372:n71. doi: 10.1136/bmj.n71
20. Treede R-D, Rief W, Barke A, Aziz Q, Bennett MI, Benoliel R, et al. A classification of chronic pain for ICD-11. Pain. (2015) 156:1003–7. doi: 10.1097/j.pain.0000000000000160
21. Treede R, Rief W, Barke A, Aziz Q, Bennett M, Benoliel R, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the International Classification of Diseases (ICD-11). Pain. (2019) 160(1):19–27. doi: 10.1097/j.pain.0000000000001384
22. Latham NK, Mehta V, Nguyen AM, Jette AM, Olarsch S, Papanicolaou D, et al. Performance-based or self-report measures of physical function: which should be used in clinical trials of hip fracture patients? Arch Phys Med Rehabil. (2008) 89:2146–55. doi: 10.1016/j.apmr.2008.04.016
23. Büchter RB, Weise A, Pieper D. Development, testing and use of data extraction forms in systematic reviews: a review of methodological guidance. BMC Med Res Methodol. (2020) 20:259. doi: 10.1186/s12874-020-01143-3
24. Hwang M, Jiang Y. Personalization in digital health interventions for older adults with cancer: a scoping review. J Geriatr Oncol. (2023) 14:101652. doi: 10.1016/j.jgo.2023.101652
25. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I, et al. Rob 2: a revised tool for assessing risk of bias in randomised trials. Br Med J. (2019) 366:l4898. doi: 10.1136/bmj.l4898
26. Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. The GRADE Working Group (2013). Available online at: https://gdt.gradepro.org/app/handbook/handbook.html
27. Higgins J, Thomas J, Chandler J, Cumpston M, Li T, Page M, et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.5. (2024). (Accessed August 2024).
28. Santesso N, Glenton C, Dahm P, Garner P, Akl EA, Alper B, et al. GRADE guidelines 26: informative statements to communicate the findings of systematic reviews of interventions. J Clin Epidemiol. (2020) 119:126–35. doi: 10.1016/j.jclinepi.2019.10.014
29. Zhao R, Cheng L, Zheng Q, Lv Y, Wang Y-M, Ni M, et al. A smartphone application-based remote rehabilitation system for post-total knee arthroplasty rehabilitation: a randomized controlled trial. J Arthroplast. (2024) 39(3):575–81.e8. doi: 10.1016/j.arth.2023.08.019
30. Barrett PC, Hackley DT, Yu-Shan AA, Shumate TG, Larson KG, Deneault CR, et al. Provision of a home-based video-assisted therapy program is noninferior to in-person hand therapy after thumb carpometacarpal arthroplasty. J Bone Jt Surg. (2024) 106(8):674–80. doi: 10.2106/JBJS.23.00597
31. Bettger JP, Green CL, Holmes DN, Chokshi A, Mather RC, Hoch BT, et al. Effects of virtual exercise rehabilitation in-home therapy compared with traditional care after total knee arthroplasty: vERITAS, a randomized controlled trial. J Bone Jt Surg. (2020) 102(2):101–9. doi: 10.2106/JBJS.19.00695
32. Bennell KL, Nelligan R, Dobson F, Rini C, Keefe F, Kasza J, et al. Effectiveness of an internet-delivered exercise and pain-coping skills training intervention for persons with chronic knee pain: a randomized trial. Ann Intern Med (2017) 166(7):453–62. doi: 10.7326/M16-1714
33. Duong V, Robbins SR, Dennis S, Venkatesha V, Ferreira ML, Hunter DJ. Combined digital interventions for pain reduction in patients undergoing knee replacement. JAMA Netw OPEN. (2023) 6(9):e2333172. doi: 10.1001/jamanetworkopen.2023.33172
34. Crawford DA, Duwelius PJ, Sneller MA, Morris MJ, Hurst JM, Berend KR, et al. 2021 Mark coventry award: use of a smartphone-based care platform after primary partial and total knee arthroplasty: a prospective randomized controlled trial. Bone Jt J. (2021) 103-B:3–12. doi: 10.1302/0301-620X.103B6.BJJ-2020-2352.R1
35. Lo HHM, Ng MSN, Fong HPY, Lai HH-K, Wang B, Wong SY, et al. Examining the feasibility, acceptability and preliminary efficacy of an immersive virtual reality-assisted lower limb strength training for knee osteoarthritis: a mixed-method pilot randomized controlled trial. JMIR Serious Games. (2024) 12:1–15. doi: 10.2196/52563
36. Wang H-N, Luo P, Liu S, Liu Y, Zhang X, Li J. Effectiveness of internet-based telehealth programs in patients with hip or knee osteoarthritis: systematic review and meta-analysis. J Med Internet Res. (2024) 26:e55576. doi: 10.2196/55576
37. Xiang X-N, Wang Z-Z, Hu J, Zhang J-Y, Li K, Chen Q-X, et al. Telehealth-supported exercise or physical activity programs for knee osteoarthritis: systematic review and meta-analysis. J Med Internet Res. (2024) 26:e54876. doi: 10.2196/54876
38. Conner-Spady BL, Bohm E, Loucks L, Dunbar MJ, Marshall DA, Noseworthy TW. Patient expectations and satisfaction 6 and 12 months following total hip and knee replacement. Qual Life Res. (2020) 29:705–19. doi: 10.1007/s11136-019-02359-7
39. Laukka E, Lakoma S, Harjumaa M, Hiltunen S, Härkönen H, Jansson M, et al. Older adults’ preferences in the utilization of digital health and social services: a qualitative analysis of responses to open-ended questions. BMC Health Serv Res. (2024) 24:1184. doi: 10.1186/s12913-024-11564-1
40. Sturm J, Dierick A, Christianen M, van Gelder M, Wouters E. Possibilities patience, and perseverance: a preliminary analysis of the needs and experiences of ten older adults regarding their use of digital health technology. Healthcare. (2023) 11:1612. doi: 10.3390/healthcare11111612
41. Judson TJ, Subash M, Harrison JD, Yeager J, Williams AM, Grouse CK, et al. Patient perceptions of e-visits: qualitative study of older adults to inform health system implementation. JMIR Aging. (2023) 6:e45641. doi: 10.2196/45641
42. Timurtaş E, Hüzmeli İ, demirbüken İ, Polat MG. Clinical outcomes of asynchronous telerehabilitation through a mobile app are equivalent to synchronous telerehabilitation in patients with fibromyalgia: a randomized control study. BMC Musculoskelet Disord. (2025) 26:118. doi: 10.1186/s12891-025-08377-6
43. Zou J, Wang F, Yang X, Wang H, Niswander L, Zhang T, et al. Association between rare variants in specific functional pathways and human neural tube defects multiple subphenotypes. Neural Dev. (2020) 15:8. doi: 10.1186/s13064-020-00145-7
44. Villalobos-Zúñiga G, Cherubini M. Apps that motivate: a taxonomy of app features based on self-determination theory. Int J Hum Comput Stud. (2020) 140:102449. doi: 10.1016/j.ijhcs.2020.102449
45. McHugh CG, Kostic AM, Katz JN, Losina E. Effectiveness of remote exercise programs in reducing pain for patients with knee osteoarthritis: a systematic review of randomized trials. Osteoarthr Cartil Open. (2022) 4:100264. doi: 10.1016/j.ocarto.2022.100264
46. Xie S-H, Wang Q, Wang L-Q, Wang L, Song K-P, He C-Q. Effect of internet-based rehabilitation programs on improvement of pain and physical function in patients with knee osteoarthritis: systematic review and meta-analysis of randomized controlled trials. J Med Internet Res. (2021) 23(1):e21542. doi: 10.2196/21542
47. Safari R, Jackson J, Sheffield D. Digital self-management interventions for people with osteoarthritis: systematic review with meta-analysis. J Med Internet Res. (2020) 22(7):e15365. doi: 10.2196/15365
48. Tsang MP, Man GCW, Xin H, Chong YC, Ong MT-Y, Yung PS-H. The effectiveness of telerehabilitation in patients after total knee replacement: a systematic review and meta-analysis of randomized controlled trials. J Telemed Telecare. (2024) 30:795–808. doi: 10.1177/1357633X221097469
49. Marszalek J, Price LL, Harvey WF, Driban JB, Wang C. Outcome expectations and osteoarthritis: association of perceived benefits of exercise with self-efficacy and depression. Arthritis Care Res (Hoboken). (2017) 69:491–8. doi: 10.1002/acr.22969
50. Naugle KM, Blythe C, Naugle KE, Keith N, Riley ZA. Kinesiophobia predicts physical function and physical activity levels in chronic pain-free older adults. Front Pain Res. (2022) 3:874205. doi: 10.3389/fpain.2022.874205
51. LaRowe LR, Bakhshaie J, Vranceanu A-M, Greenberg J. Anxiety, pain catastrophizing, and pain outcomes among older adults with chronic orofacial pain. J Behav Med. (2024) 47:537–43. doi: 10.1007/s10865-024-00473-7
52. Liang Z, Tian S, Wang C, Zhang M, Guo H, Yu Y, et al. The best exercise modality and dose for reducing pain in adults with low back pain: a systematic review with model-based Bayesian network meta-analysis. J Orthop Sport Phys Ther. (2024) 54:315–27. doi: 10.2519/jospt.2024.12153
53. Lythreatis S, Singh SK, El-Kassar A-N. The digital divide: a review and future research agenda. Technol Forecast Soc Change. (2022) 175:121359. doi: 10.1016/j.techfore.2021.121359
54. Wong CK, Mak RY, Kwok TS, Tsang JS, Leung MY, Funabashi M, et al. Prevalence, incidence, and factors associated with non-specific chronic low back pain in community-dwelling older adults aged 60 years and older: a systematic review and meta-analysis. J Pain. (2022) 23:509–34. doi: 10.1016/j.jpain.2021.07.012
55. Cai Y, Liu F, Wanigatunga AA, Urbanek JK, Simonsick EM, Ferrucci L, et al. Musculoskeletal pain characteristics and objectively measured physical activity in older adults. J Gerontol A Biol Sci Med Sci. (2024) 79(4):glae039. doi: 10.1093/gerona/glae039
56. Weber F, Kloek C, Stuhrmann S, Blum Y, Grüneberg C, Veenhof C. Usability and preliminary effectiveness of an app-based physical activity and education program for people with hip or knee osteoarthritis—a pilot randomized controlled trial. Arthritis Res Ther (2024) 26:83. doi: 10.1186/s13075-024-03291-z
57. Moutzouri M, Koumantakis GA, Hurley M, Kladouchou AG, Gioftsos G. Effectiveness of a web-guided self-managed telerehabilitation program enhanced with outdoor physical activity on physical function, physical activity levels and pain in patients with knee osteoarthritis: a randomized controlled trial. J Clin Med (2024) 13(4):934. doi: 10.3390/jcm13040934
58. Master H, Coronado RA, Whitaker S, Block S, Vanston SW, Pennings JS, et al. Combining wearable technology and telehealth counseling for rehabilitation after lumbar spine surgery: feasibility and acceptability of a physical activity intervention. Phys Ther (2024) 104(2):pzad096. doi: 10.1093/ptj/pzad096
59. Shim GY, Kim EH, Lee SJ, Chang CB, Lee YS, Lee JI, et al. Postoperative rehabilitation using a digital healthcare system in patients with total knee arthroplasty: a randomized controlled trial. Arch Orthop Trauma Surg. (2023) 143:6361–70. doi: 10.1007/s00402-023-04894-y
60. Lee E-L, Jang MH, Lee B-J, Han SH, Lee HM, Choi SU, et al. Home-based remote rehabilitation leads to superior outcomes for older women with knee osteoarthritis: a randomized controlled trial. J Am Med Dir Assoc. (2023) 24(10):1555–61. doi: 10.1016/j.jamda.2023.08.013
61. Janhunen M, Katajapuu N, Paloneva J, Pamilo K, Oksanen A, Keemu H, et al. Effects of a home-based, exergaming intervention on physical function and pain after total knee replacement in older adults: a randomised controlled trial. BMJ Open Sport Exerc Med. (2023) 9:e001416. doi: 10.1136/bmjsem-2022-001416
62. Sánchez-Laulhé PR, Biscarri-Carbonero Á, Suero-Pineda A, Luque-Romero LG, Barrerogarcía FJ, Blanquero J, et al. The effects of a mobile app-delivered intervention in people with symptomatic hand osteoarthritis: a pragmatic randomized controlled trial. Eur J Phys Rehabil Med. (2023) 59(1):54–64. doi: 10.23736/S1973-9087.22.07744-9
63. Thiengwittayaporn S, Wattanapreechanon P, Sakon P, Peethong A, Ratisoontorn N, Charoenphandhu N, et al. Development of a mobile application to improve exercise accuracy and quality of life in knee osteoarthritis patients: a randomized controlled trial. Arch Orthop Trauma Surg. (2023) 143:729–38. doi: 10.1007/s00402-021-04149-8
64. Nuevo M, Rodríguez-Rodríguez D, Jauregui R, Fabrellas N, Zabalegui A, Conti M, et al. Telerehabilitation following fast-track total knee arthroplasty is effective and safe: a randomized controlled trial with the ReHub® platform. Disabil Rehabil. (2023) 46:2629–39. doi: 10.1080/09638288.2023.2228689
65. Cheng KC, Lau KMK, Cheng ASK, Lau TSK, Lau FOT, Lau MCH, et al. Use of mobile app to enhance functional outcomes and adherence of home-based rehabilitation program for elderly with hip fracture: a randomized controlled trial. Hong Kong Physiother J. (2022) 42(02):99–110. doi: 10.1142/S101370252250010X
66. An J, Ryu H-K, Lyu S-J, Yi H-J, Lee B-H. Effects of preoperative telerehabilitation on muscle strength, range of motion, and functional outcomes in candidates for total knee arthroplasty: a single-blind randomized controlled trial. Int J Environ Res Public Health. (2021) 18(11):6071. doi: 10.3390/ijerph18116071
67. Fanning J, Brooks AK, Ip E, Nicklas BJ, Rejeski WJ, Nesbit B, et al. A mobile health behavior intervention to reduce pain and improve health in older adults with obesity and chronic pain: the MORPH pilot trial. Front Digit Heal. (2020) 2:598456. doi: 10.3389/fdgth.2020.598456
68. Doiron-Cadrin P, Kairy D, Vendittoli P-A, Lowry V, Poitras S, Desmeules F. Feasibility and preliminary effects of a tele-prehabilitation program and an in-person prehablitation program compared to usual care for total hip or knee arthroplasty candidates: a pilot randomized controlled trial. Disabil Rehabil. (2020) 42(7):989–98. doi: 10.1080/09638288.2018.1515992
69. Pronk Y, Peters M, Sheombar MCW, Brinkman JM. Effectiveness of a mobile eHealth app in guiding patients in pain control and opiate use after total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth. (2020) 8:1–14. doi: 10.2196/16415
70. Pelle T, Bevers K, van der Palen J, van den Hoogen FHJ, van den Ende CHM. Effect of the Dr. Bart application on healthcare use and clinical outcomes in people with osteoarthritis of the knee and/or hip in The Netherlands; a randomized controlled trial. Osteoarthr Cartil. (2020) 28(4):418–27. doi: 10.1016/j.joca.2020.02.831
71. Zadro JR, Shirley D, Simic M, Mousavi SJ, Ceprnja D, Maka K, et al. Video-game-based exercises for older people with chronic low back pain: a randomized controlled table trial (GAMEBACK). Phys Ther. (2019) 99(1):14–27. doi: 10.1093/ptj/pzy112
72. Kloek CJJ, Bossen D, Spreeuwenberg PM, Dekker J, de Bakker DH, Veenhof C. Effectiveness of a blended physical therapist intervention in people with hip osteoarthritis, knee osteoarthritis, or both: a cluster- randomized controlled trial. Phys Ther. (2018) 98(7):560–70. doi: 10.1093/ptj/pzy045
73. Rini C, Porter LS, Somers TJ, McKee DC, DeVellis RF, Smith M, et al. Automated internet-based pain coping skills training to manage osteoarthritis pain: a randomized controlled trial. Pain. (2015) 156(5):837–48. doi: 10.1097/j.pain.0000000000000121
74. Bossen D, Veenhof C, Van Beek KE, Spreeuwenberg PM, Dekker J, Bakker D, et al. Effectiveness of a web-based physical activity intervention in patients with knee and/or hip osteoarthritis: randomized controlled trial. J Med Internet Res. (2013) 15:e257. doi: 10.2196/jmir.2662
75. Gohir SA, Eek F, Kelly A, Abhishek A, Valdes AM. Effectiveness of internet-based exercises aimed at treating knee osteoarthritis the iBEAT-OA randomized clinical trial. JAMA Netw Open. (2021) 4(2):e210012. doi: 10.1001/jamanetworkopen.2021.0012
76. Charlton JM, Krowchuk NM, Eng JJ, Li LC, Hunt MA. Remotely delivered, individualized, and self-directed gait modification for knee osteoarthritis: a pilot trial. Clin Biomech (Bristol, Avon). (2023) 106:105981. doi: 10.1016/j.clinbiomech.2023.105981
77. Moffet H, Tousignant M, Nadeau S, Mérette C, Boissy P, Corriveau H, et al. In-home telerehabilitation compared with face to-face rehabilitation after total knee arthroplasty: a noninferiority randomized controlled trial. J Bone Jt Surg Am. (2015) 97(14):1129–41. doi: 10.2106/JBJS.N.01066
78. Osterloh J, Knaack F, Bader R, Behrens M, Peschers J, Nawrath L, et al. The effect of a digital-assisted group rehabilitation on clinical and functional outcomes after total hip and knee arthroplasty—a prospective randomized controlled pilot study. BMC Musculoskelet Disord. (2023) 24:1–11. doi: 10.1186/s12891-023-06270-8
79. Timmers T, Janssen L, van der Weegen W, Das D, Marijnissen WJ, Hannink G, et al. The effect of an app for day-to-day postoperative care education on patients with total knee replacement: randomized controlled trial. JMIR Mhealth Uhealth. (2019) 7:1–16. doi: 10.2196/15323
80. Tousignant M, Moffet H, Boissy P, Corriveau H, Cabana F, Marquis F. A randomized controlled trial of home telerehabilitation for post-knee arthroplasty. J Telemed Telecare. (2011) 17:195–8. doi: 10.1258/jtt.2010.100602
81. Kane LT, Thakar O, Jamgochian G, Lazarus MD, Abboud JA, Namdari S, et al. The role of telehealth as a platform for postoperative visits following rotator cuff repair: a prospective, randomized controlled trial. J Shoulder Elb Surg. (2020) 29:775–83. doi: 10.1016/j.jse.2019.12.004
82. Nelson M, Bourke M, Crossley K, Russell T. Telerehabilitation is non-inferior to usual care following total hip replacement—a randomized controlled non-inferiority trial. Physiother (United Kingdom). (2020) 107:19–27. doi: 10.1016/j.physio.2019.06.006
83. Allen KD, Arbeeva L, Callahan LF, Golightly YM, Goode AP, Heiderscheit BC, et al. Physical therapy vs internet-based exercise training for patients with knee osteoarthritis: results of a randomized controlled trial. Osteoarthr Cartil. (2018) 26:383–96. doi: 10.1016/j.joca.2017.12.008
Keywords: pain, older adults, digital health, telerehabilitation, mobile health
Citation: Silva AG, Santos AJ, Andias R and Rocha NP (2025) Effectiveness of digital pain management for older adults with musculoskeletal pain: systematic review with meta-analysis. Front. Pain Res. 6:1657014. doi: 10.3389/fpain.2025.1657014
Received: 30 June 2025; Accepted: 2 September 2025;
Published: 17 September 2025.
Edited by:
Janet H. Van Cleave, University of Texas Health Science Center at Houston, United StatesReviewed by:
Hayk Petrosyan, JFK Johnson Rehabilitation Institute, United StatesHao-Nan Wang, Sichuan University, China
Copyright: © 2025 Silva, Santos, Andias and Rocha. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Anabela G. Silva, YXNpbHZhQHVhLnB0
 Anabela G. Silva
Anabela G. Silva Ana J. Santos
Ana J. Santos Rosa Andias
Rosa Andias Nelson P. Rocha
Nelson P. Rocha