- 1UR4391 (ENT Team), Faculty of Health, Paris Est Créteil University, Créteil, France
- 2Department of Clinical Neurophysiology, Henri Mondor University Hospital, AP-HP, Créteil, France
In 1994, the Task Force on Taxonomy of the International Association for the Study of Pain (IASP) published the first classification of chronic pain (1), introducing the concept of “neuropathic pain”, defined as “pain initiated or caused by a primary lesion or dysfunction in the nervous system”. From this date, two mechanisms of chronic pain have been considered, either neuropathic or non-neuropathic (nociceptive) pain. In 2011, the IASP Terminology Working Group (2) updated the definitions of “neuropathic pain”, as “pain caused by a lesion or disease of the somatosensory nervous system”, and “nociceptive pain”, as “pain that arises from actual or threatened damage to non-neural tissue and is due to the activation of nociceptors” occurring with a normally functioning somatosensory nervous system. In 2017, based on a publication by the Terminology Task Force of the IASP (3), the IASP Council adopted the proposal for a third mechanistic descriptor of chronic pain, entitled “nociplastic pain”, defined as “pain that arises from altered nociception despite no clear evidence of actual or threatened tissue damage causing the activation of peripheral nociceptors or evidence for disease or lesion of the somatosensory system causing the pain” (4).
The diagnosis of chronic neuropathic pain, which is now a defined clinical entity (5), remains a challenge. Between 2001 and 2006, the three main clinical questionnaires currently used for the diagnosis of neuropathic pain (6) were developed. These questionnaires are the “Leeds assessment of neuropathic symptoms and signs” pain scale (LANSS) (7), the “douleur neuropathique en 4 questions” questionnaire (DN4) (8), and the painDETECT questionnaire (9). At the time of their publication (2001–2006), the objective of these questionnaires was clearly to distinguish neuropathic pain from nociceptive (non-neuropathic) pain, and the concept of nociplastic pain was not yet introduced and adopted. This history of the progression of knowledge, concepts and definitions of the mechanisms of chronic pain actually constitutes an intrinsic and underestimated limitation to the use of these questionnaires in current clinical practice.
The development and validation of the LANSS, DN4 and painDETECT questionnaires were based on the same design, consisting of the characterization of sensory descriptors whose prevalence differed between two populations of patients with neuropathic pain vs. nociceptive (non-neuropathic) pain. For the three questionnaires, the validation studies included fairly similar clinical conditions both in the neuropathic pain group (peripheral nerve trauma, painful polyneuropathy and postherpetic neuralgia), and in the nociceptive (non-neuropathic) group (osteoarthritis, inflammatory arthropathies and low back pain) (Table 1). However, the most interesting point is to consider the excluded clinical conditions. Fibromyalgia, a typically nociplastic condition, was an exclusion criterion for the DN4 questionnaire study and not mentioned for the validation of the other two questionnaires, but in fact, no patients with fibromyalgia were included in either of these studies. Thus, the three questionnaires were developed without considering nociplastic pain as a condition of exclusion from neuropathic pain, which can be intrinsically explained by the fact that the concept of nociplastic pain is subsequent to the studies that validated these questionnaires.
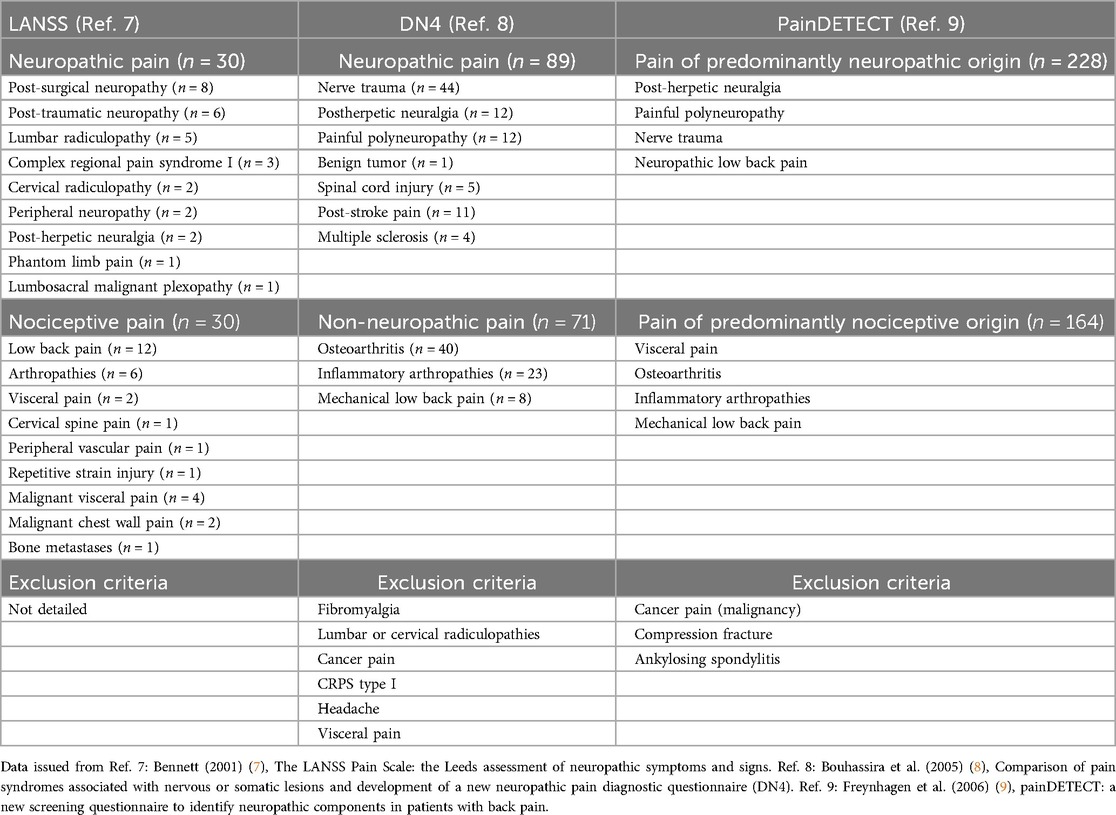
Table 1. Clinical conditions included and excluded in the neuropathic and nociceptive (non-neuropathic) group populations in the LANSS, DN4, and painDETECT studies.
It should also be noted that the characteristics of nociplastic pain go beyond the somatosensory domain and often include the presence of comorbidities, encompassing different cognitive (attentional) and affective (emotional) aspects of chronic pain (10). These additional criteria are not considered by current neuropathic pain questionnaires, further limiting their ability to make a differential diagnosis with nociplastic pain.
Additionally, these questionnaires establish the diagnosis of neuropathic pain on a cumulative score, meaning that the more sensory descriptors the patient presents, the more likely it is that the pain is neuropathic. However, nociplastic pain is characterized by central sensory sensitization (11) that typically results in the patient perceiving a variety of sensory symptoms, including neuropathic-like symptoms. For example, “my pain feels like burns, electric shocks or cramps” and “my pain is accompanied by other unusual sensations throughout my body such as pins and needles, tingling or numbness”, are items of the Fibromyalgia Rapid Screening Tool (FiRST), a validated questionnaire for the diagnosis of fibromyalgia (12). The same sensory descriptors are found in neuropathic pain questionnaires, and therefore, a high score on these questionnaires may well reveal nociplastic rather than neuropathic pain.
Conversely, some typically neuropathic conditions are characterized by very few different sensory descriptors, such as trigeminal neuralgia, which is a recognized cause of neuropathic pain (5), and is associated with symptomatology limited to stabbing or electric shock-like pain attacks (13). This condition is therefore characterized by low scores on the LANSS, DN4, and painDETECT questionnaires, below their cutoff score for the diagnosis of neuropathic pain, but is nevertheless undeniably neuropathic. This inconsistency is explained by the fact that no patients with trigeminal neuralgia were included in the validation studies of these neuropathic pain questionnaires.
Thus, the LANSS, DN4, and painDETECT questionnaires have intrinsic flaws in their development and therefore in their application for the diagnosis of neuropathic pain. The first inaccuracy concerns the patient profile considered in the validation studies, as no nociplastic pain conditions were included for the differential diagnosis, while some neuropathic pain conditions, likely to produce very specific symptoms, such as trigeminal neuralgia, were also not included. Furthermore, the diagnosis of neuropathic pain by these questionnaires is based on a high score, which results from the presence of a variety of different sensory symptoms and therefore favors a central sensitization process rather than specific neuropathic pain. Furthermore, the inclusion of patients in the validation studies was based on an etiological diagnosis, not excluding a secondary central sensitization process. These questionnaires were therefore not developed to differentiate neuropathic pain with secondary central sensitization from nociplastic pain with primary central sensitization, two situations that can present similar clinical sensory descriptors.
Two other questionnaires, the Neuropathic Pain Questionnaire (14) and the IDPain questionnaire (15), have been proposed for the diagnosis of neuropathic pain, but are less emphasized in international guidelines (6). These questionnaires, however, have the same design and limitations as the three questionnaires detailed in this article. They were actually published in the same period (2003 and 2006) as the other questionnaires, before the introduction of the concept of nociplastic pain.
Thus, the LANSS, DN4 and painDETECT questionnaires should be used with caution as screening tools for the diagnosis of neuropathic pain, as they are unable to differentiate neuropathic pain from nociplastic pain. First, because they did not take into account the existence of nociplastic pain in their development (for an obvious historical reason). Second, because high scores that make the diagnosis of neuropathic pain with these tools are more likely to be observed in cases of nociplastic pain or secondary central sensitization process. On the other hand, these questionnaires do not allow for the diagnosis of neuropathic pain in certain neuropathic conditions limited to a single type of sensory descriptors. Thus, these questionnaires could be more useful for monitoring the evolution of a chronic pain syndrome or the efficacy of an intervention aimed at reducing central sensitization. It is necessary to reexamine the use of these questionnaires in clinical practice, and in particular their value in establishing a definite diagnosis of neuropathic pain. There is therefore an implicit justification for developing new standards for the application of these questionnaires and for defining new ones for differential diagnosis.
Author contributions
J-PL: Investigation, Supervision, Conceptualization, Writing – review & editing, Writing – original draft, Validation, Formal analysis.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The author declares that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Merskey H, Bogduk N. Classification of chronic pain. IASP Task Force on Taxonomy. 2nd Ed Seattle: IASP Press (1994).
2. International Association for the Study of Pain. Descriptions of Chronic Pain Syndromes and Definitions of Pain Terms. IASP Terminology (2011). Available online at: https://www.iasp-pain.org/publications/free-ebooks/classification-of-chronic-pain-second-edition-revised/ (Accessed July 30, 2025).
3. Kosek E, Cohen M, Baron R, Gebhart GF, Mico JA, Rice ASC, et al. Do we need a third mechanistic descriptor for chronic pain states? Pain. (2016) 157(7):1382–6. doi: 10.1097/j.pain.0000000000000507
4. International Association for the Study of Pain. Task Force on Taxonomy. IASP Terminology (2017). Available online at: https://www.iasp-pain.org/resources/terminology/ (Accessed July 30, 2025).
5. Scholz J, Finnerup NB, Attal N, Aziz Q, Baron R, Bennett MI, et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain. (2019) 160(1):53–9. doi: 10.1097/j.pain.0000000000001365
6. Truini A, Aleksovska K, Anderson CC, Attal N, Baron R, Bennett DL, et al. Joint European academy of neurology-European pain federation-neuropathic pain special interest group of the international association for the study of pain guidelines on neuropathic pain assessment. Eur J Neurol. (2023) 30(8):2177–96. doi: 10.1111/ene.15831
7. Bennett M. The LANSS pain scale: the Leeds assessment of neuropathic symptoms and signs. Pain. (2001) 92(1–2):147–57. doi: 10.1016/s0304-3959(00)00482-6
8. Bouhassira D, Attal N, Alchaar H, Boureau F, Brochet B, Bruxelle J, et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain. (2005) 114(1-2):29–36. doi: 10.1016/j.pain.2004.12.010
9. Freynhagen R, Baron R, Gockel U, Tölle TR. painDETECT: a new screening questionnaire to identify neuropathic components in patients with back pain. Curr Med Res Opin. (2006) 22(10):1911–20. doi: 10.1185/030079906X132488
10. Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, et al. Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain. (2021) 162(11):2629–34. doi: 10.1097/j.pain.0000000000002324
11. Kaplan CM, Kelleher E, Irani A, Schrepf A, Clauw DJ, Harte SE. Deciphering nociplastic pain: clinical features, risk factors and potential mechanisms. Nat Rev Neurol. (2024) 20(6):347–63. doi: 10.1038/s41582-024-00966-8
12. Perrot S, Bouhassira D, Fermanian J. Development and validation of the fibromyalgia rapid screening tool (FiRST). Pain. (2010) 150(2):250–6. doi: 10.1016/j.pain.2010.03.034
13. Bendtsen L, Zakrzewska JM, Heinskou TB, Hodaie M, Leal PRL, Nurmikko T, et al. Advances in diagnosis, classification, pathophysiology, and management of trigeminal neuralgia. Lancet Neurol. (2020) 19(9):784–96. doi: 10.1016/S1474-4422(20)30233-7
14. Krause SJ, Backonja MM. Development of a neuropathic pain questionnaire. Clin J Pain. (2003) 19(5):306–14. doi: 10.1097/00002508-200309000-00004
Keywords: diagnosis, neuropathic pain, nociceptive pain, nociplastic pain, central sensitization
Citation: Lefaucheur J-P (2025) The intrinsic reason why LANSS, DN4, and PainDETECT questionnaires cannot distinguish neuropathic pain from nociplastic pain. Front. Pain Res. 6:1658126. doi: 10.3389/fpain.2025.1658126
Received: 2 July 2025; Accepted: 7 August 2025;
Published: 21 August 2025.
Edited by:
Yanying Liu, Qingdao Huanghai University, ChinaReviewed by:
Nontawat Chuinsiri, Suranaree University of Technology, ThailandCopyright: © 2025 Lefaucheur. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jean-Pascal Lefaucheur, amVhbi1wYXNjYWwubGVmYXVjaGV1ckBobW4uYXBocC5mcg==
 Jean-Pascal Lefaucheur
Jean-Pascal Lefaucheur