- 1Department of Neurology, Peking University People's Hospital, Beijing, China
- 2Peking University People's Hospital, Beijing, China
- 3Peking University Health Science Center, Beijing, China
Medication Overuse Headache (MOH) can lead to central sensitization (CS), habituation deficits (HD), shortened cortical silent period duration (CSPD), and increased pre-activation levels (PAL), all of which are quantifiable electrophysiological objective indicators related to MOH. Transcranial magnetic stimulation (TMS) is a treatment method for MOH and is primarily divided into three types: single-pulse TMS (sTMS), repetitive TMS (rTMS), and quadruple-pulse TMS (qTMS). Among these, sTMS is convenient for patients of self-administration, qTMS significantly improves the effectiveness of TMS treatment, and rTMS is suitable for widespread use in developing countries. Numerous studies have reported clinical symptom improvements in MOH patients treated with TMS, with statistically significant results. However, only a few studies have observed electrophysiological changes in MOH patients before and after treatment. Whether quantifiable objective indicators can be reversed requires further investigation.
1 Introduction
Medication Overuse Headache (MOH) is a secondary headache that develops as a consequence of acute headache medication(s) overuse in headache sufferers. According to the International Classification of Headache Disorders, MOH is defined as headache occurring on 15 or more days/month for at least three months in a patient with a pre-existing headache disorder as a consequence of overuse of acute symptomatic medication (1). It typically results from the overuse of triptans, the combined use of two or more opioid analgesics for at least 10 days per month for more than 3 months, or the use of nonsteroidal anti-inflammatory drugs (NSAIDs) or acetaminophen (paracetamol) for at least 15 days per month for more than 3 months (1). Specific drugs such as triptans, ergotamine, and non-specific drugs like NSAIDs, codeine, tramadol, and pethidine can all lead to MOH (2). Among these, triptans, ergotamine, and opioid analgesics are more likely to trigger MOH, while the risk of developing MOH with NSAIDs and acetaminophen is the lowest (3, 4).
MOH can arise from, but is not limited to chronic migraine (CM) (5, 6) and tension-type headache (TH) (7). The estimated prevalence of MOH in the general population is 1%–2%, while in chronic migraine (CM) patients, approximately 30%–50% suffer from MOH (8, 9). This figure can rise to as high as 80% in tertiary headache treatment centers (10). Patient education, withdrawal of overused medications, preventive therapy (including oral calcitonin gene-related peptide receptor antagonists or botulinum toxin type A), and occipital nerve blocks are the primary treatments for MOH (3, 4, 11, 12). However, some patients do not respond to medication treatment (11), and more than a quarter of MOH patients relapse within the first year (3). Moreover, pharmacological treatments are associated with numerous adverse effects, including gastrointestinal disturbances, dizziness, drowsiness, fatigue, and memory impairment (13). Alternative therapies, particularly physical therapy, hold significant potential for development (11, 13).
Transcranial Magnetic Stimulation (TMS) may be an excellent option for MOH treatment, offering the advantage of avoiding drug adverse effects and demonstrating a favorable safety profile (13–16). The mechanisms of action and methods of use have gradually been revealed in recent research, demonstrating its unique therapeutic potential.
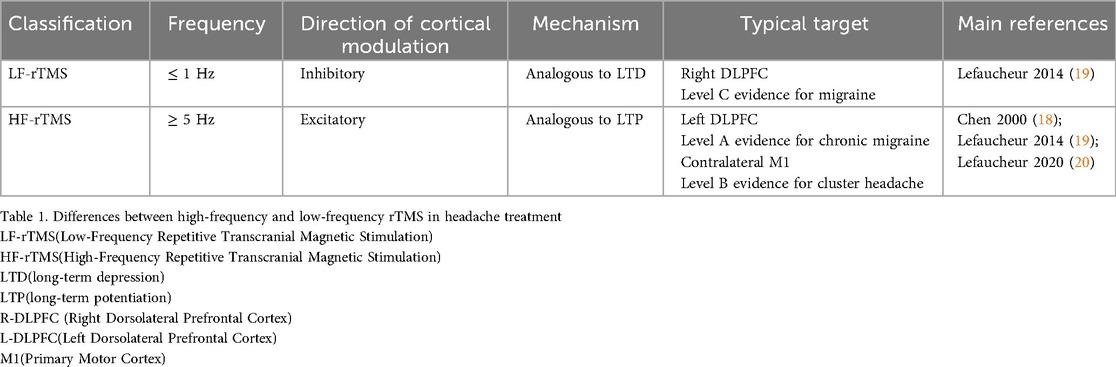
In 1997, Chen first introduced the term “low-frequency” after observing a 20% reduction in the amplitude of motor evoked potentials (MEPs) recorded from muscle following 15 min of 0.9 Hz rTMS applied to the left motor cortex (17). Later, in a 2000 review, Chen formally classified TMS frequencies as “high-frequency” (>5 Hz) and “low-frequency” (≤1 Hz) (18). The distinctions between high- and low-frequency rTMS in headache treatment are summarized in Table 1.
2 TMS treatment targets
The selection of TMS targets is crucial for therapeutic efficacy. Commonly used targets include the primary motor cortex (M1), dorsolateral prefrontal cortex (DLPFC), and occipital lobe cortex (OC). These regions have demonstrated therapeutic potential, although underlying mechanisms require further investigation.
2.1 Primary motor cortex (M1)
The primary motor cortex, also known as the M1 region in TMS literature, is one of the most commonly targeted areas in TMS applications (21). It is in the precentral gyrus of the cerebral cortex and is primarily responsible for motor functions. The motor hotspot method is the most frequently used M1 localization technique in TMS treatment studies. This method involves delivering single-pulse TMS to the left or right M1 region to activate motor neurons, thereby inducing muscle contractions in the target muscle. The corresponding motor-evoked potentials (MEPs) are recorded via electromyography (EMG), and the M1 target cortical region is determined by identifying the site that consistently produces the most stable MEP. Alternative localization approaches, such as functional magnetic resonance imaging (fMRI)-based reconstruction or anatomical markers like the hand knob region (22), have yielded results similar to those obtained through the motor hotspot method. The analgesic effect of the M1 region primarily depends on its action on endogenous opioid neurotransmitters. Positron Emission Tomography (PET) studies show that M1 stimulation directly enhances the inhibitory system mediated by opioid neurotransmitters (23). In 2018, Andre-Obadia applied 20 Hz rTMS to both the hand and facial areas of M1, and found that stimulation of the hand area of M1 produced significantly greater analgesic effects for facial or upper-limb pain compared with stimulation of the facial M1 area or sham rTMS (24). The analgesic mechanism was suggested to depend on affective-cognitive modulation pathways [such as the anterior cingulate cortex (ACC) and periaqueductal gray (PAG)] rather than on a strictly somatotopic effect.
2.2 Dorsolateral prefrontal cortex (DLPFC)
rTMS applied to the left dorsolateral prefrontal cortex (L-DLPFC) at the F3 site of the international 10–20 electrode placement system exerts a broad top-down inhibitory effect along the midbrain-thalamic-cingulate pathway via descending fibers from the prefrontal cortex (25). Consequently, the widespread effects of DLPFC stimulation not only enhance motor cortex function but also modulate affective circuits associated with both pain and depression (26). Two studies directly compared the effects of M1 vs. DLPFC TMS on quantitative sensory testing (QST) measures. During pain treatment with rTMS, high-frequency stimulation is often applied to activate the left DLPFC (27), or low-frequency stimulation to inhibit the right DLPFC (28). This approach may be based on the valence lateralization hypothesis (29), which proposes that the left hemisphere primarily processes positive emotions, whereas the right hemisphere processes negative emotions. Therefore, high-frequency activation of the left DLPFC (27) or low-frequency inhibition of the right DLPFC (28) is selected to modulate emotional and pain-processing networks (20) and thereby achieve an analgesic effect.
2.3 Occipital cortex(OC)
Based on gray matter atrophy coordinate network mapping studies (30–32), large-scale data from 1,000 human resting-state functional connectivity (RSFC) cases have been used to link the anatomical coordinates of gray matter volume reduction with brain networks, identifying disease- and symptom-specific brain networks. The reports indicate that functional abnormalities in the brains of patients with Migraine with Aura (MwA) primarily concentrate in the visual regions (33). EEG studies indicate excessive responsiveness of the OC to visual stimulation (34), and positron emission tomography (PET) studies show an increase in activation in the OC in response to light (35). Mapping of the light-sensitive retinal ganglion cells, which relay to the posterior thalamic dura mater sensitive neurons and subsequently project to the OC, provides a neurobiological pathway that may help explain the hyperactivity of visual processing and photophobia in migraine patients (36). Whether OC could serve as novel targets for MOH is a question worth our consideration and further investigation.
3 MOH electrophysiological indicators
3.1 Amplitude of evoked potentials (Amp), Pre-activation level (PAL), habituation deficit (HD), habituation slope (HS), and central sensitization (CS)
Pre-activation level (PAL) refers to the amplitude (Amp) of the response wave recorded during the first stimulus of an evoked potential. An elevated PAL is defined as central sensitization (CS). In healthy individuals, repeated stimulation of evoked potentials typically results in a gradual decline in response wave amplitude, a characteristic indicative of normal cortical excitability regulation. This adaptive process protects the nervous system from sensory overload and optimizes attentional and memory resources for novel stimuli and this phenomenon is known as habituation. An impairment in this process is referred to as habituation deficit (HD) (37–40).HD is assessed based on the habituation slope (HS), which represents the slope of the change in evoked potential amplitudes or areas (41). Sensory information reaches the cortex through synchronously active thalamic axons, providing a strong excitatory drive to layer 4 (L4) cortical neurons (42). Inefficient thalamocortical drive leads to elevated sensory cortical PAL, resulting in an imbalance in the excitation-inhibition network. This dysfunction can induce hyperresponsiveness of primary sensory cortical neurons, a phenomenon commonly observed in individuals with CM and MOH. A study assessing 29 MOH patients, 64 migraine without aura (MOA) patients, and 42 healthy volunteers (HV) during both attack and interictal phases used N20-HS from median nerve somatosensory evoked potentials (SSEP) to evaluate cortical and subcortical excitability (43). In the MOH group, an increase in response amplitude (Amp) to a small number of repeated stimuli indicates the presence of central sensitization (CS), while the lack of amplitude reduction during subsequent stimulations reflects a habituation deficit (HD). This pattern is similar to the pre-attack phase of episodic migraine (EM), suggesting that the somatosensory cortex has become persistently sensitized. MOH patients appear to be locked in a “sustained attack state,” characterized by hypersensitivity due to high PAL and hyperreactivity due to high HD, which negatively impacts neuronal plasticity (44). This phenomenon may even contribute to widespread cutaneous allodynia (45, 46). Subgroup comparisons in MOH revealed that MOH-NSAIDs patients exhibited increased PAL and the presence of HD, suggesting a potential reduction in inhibitory interneuron function. In contrast, while MOH-triptans patients also showed HD phenomena, there was no increase in PAL, which may be related to the shorter duration of withdrawal headaches in MOH-triptans compared to MOH-NSAIDs patients (47).
3.2 Motor evoked potential (MEP), cortical spreading depression (CSD), and cortical silent period duration (CSPD)
Cortical Spreading Depression (CSD) is a slowly propagating depolarization wave involving neurons and glial cells, which can be seen on the electroencephalogram (EEG) as a high-amplitude negative depolarization wave followed by a high-voltage slow wave or a flat suppression state. CSD exhibits the characteristic of spreading: this depolarization wave accompanied by suppression gradually extends to surrounding cortical areas, with the affected regions slowly returning to normal electrical activity after the suppression phase (48). Cortical spreading depression plays a significant role in aura development (49). Stereotactic electroencephalography in a patient with an attack of MwA demonstrated low-voltage suppression initiating in the left mesial occipital cortex and propagating anteriorly at approximately 3 mm/min, corresponding clinically to a contralateral right superior scintillating scotoma and ipsilateral headache. This is the first definitive electrophysiological demonstration of cortical spreading depression occurring during a migraine headache (50). Silent Period (SP) refers to a period of muscle contraction cessation observed on electromyography (EMG) following stimulation of peripheral nerves, the brainstem, or the cortical area. During this phase, the EMG records a pause in muscle contraction, resulting in a period of electrical silence (51). And the Cortical Silent Period Duration (CSPD) is defined as the sustained period of EMG silence recorded in a target muscle following a single-pulse TMS applied to the M1 region during voluntary muscle contraction. This suppression begins after the motor evoked potential (MEP) and persists until voluntary contraction of the target muscle resumes, reflecting intracortical inhibitory neural mechanisms (52). A study (53) have assessed the characteristics of MEP and CSPD in MOH patients using the orbicularis oris muscle, included 9 MOH-triptans, 9 MOH-NSAIDs, 12 MOH-Bi (MOH patients with overuse of two types of medications), 12 EM, and 13 HV. The CSPD duration among (sub)groups showed the following differences: MOA < MOH-triptans < MOH-Bi < MOH-NSAIDs < HV. A negative correlation was observed between the monthly intake of triptans and CSPD in MOH-triptans. Additionally, an earlier study reported that pain-related cortical evoked potential amplitudes in MOH-triptans were higher than in HV (54). Collectively, these findings suggest that triptans may enhance cortical excitability mechanisms. Although both MOH-triptans and MOH-NSAIDs contribute to migraine chronification, their clinical manifestations do not completely overlap. MOH-triptans tend to experience daily headaches resembling migraine, while MOH-NSAIDs are more prone to typical tension-type daily headaches (55, 56). This difference may be related to the fact that CSPD is relatively prolonged in MOH-NSAIDs compared to MOH-triptans, as the interictal CSPD in CM-MOH-NSAIDs was found to be nearly comparable to that in HV (57). Researchers observed 18 patients with MOH who discontinued triptans and NSAIDs without using preventive medications. After 3 weeks, they re-evaluated CSPD. Among the patients, 10 MOH-Bi patients showed a significant reduction in CSPD after discontinuing the medication, while 8 MOH-triptans patients had similar CSPD before and after discontinuation. Due to the lack of a group of patients who only discontinued NSAIDs, it remains to be determined through further research whether the shorter duration of withdrawal headache in MOH-triptans patients compared to MOH-NSAIDs can be explained by CSPD change.
3.3 Multisensory evoked potential integration
Clinical sensory processing impairments, such as photophobia and phonophobia, are positively correlated with headache intensity (58). Visual or auditory stimuli can trigger migraine attacks in 50%–75% of patients (59). Moreover, migraine patients may experience visual and auditory discomfort even during pain-free periods, which can intensify during attacks (60, 61). A coherent, distinct, and stable perceptual experience arises from the ability to integrate shared sensory information across different modalities (62–64). Multisensory integration occurs when stimuli from different sensory systems are temporally or spatially linked (65–67). Cross-modal illusions serve as a valuable paradigm for assessing how multisensory integration influences perception (68, 69). A well-known example is the sound-induced flash illusion (SIFI), which includes the fission illusion (Fis) and the fusion illusion (Fus), both of which are associated with cross-modal variations in visual cortical excitability. Transcranial electrical stimulation that enhances occipital cortical excitability, or occipital infarcts, can disrupt SIFI, whereas right parietal cortical lesions may cause preserved or even enhanced SIFI effects (70). The Fus and Fis effects represent dissociable phenomena, with Fis being more closely linked to cortical excitability states (70–72). A study investigating SIFI levels in 63 patients with chronic migraine (CM), including 52 medication-overuse headache (MOH) patients (83%) and 24 HV. The results indicated that MOH patients—particularly those in the MOH-triptans subgroup—exhibited excessive excitability in the visual cortex. This disrupted the expected low SIFI effect seen in HV, leading to increased signal resolution in the identification of flash stimuli, potentially due to reduced cortical inhibition within the primary visual areas. These findings suggest that medication-induced maladaptive neuroplasticity may contribute to visual cortical hyperexcitability, increasing susceptibility to MOH triggers. Further exploration of SIFI mechanisms in MOH may help design more targeted preventive treatments.
4 Research status of different TMS paradigms
TMS has been applied in the treatment of MOH, with commonly used types including sTMS (Single-pulse Transcranial Magnetic Stimulation),rTMS (Repetitive Transcranial Magnetic Stimulation), and iTMS (Intermittent Theta Burst Stimulation). Research specifically investigating the therapeutic effects of sTMS, rTMS, and qTMS in MOH populations is limited. Most studies have primarily focused on migraine and chronic headache patients, although some have involved MOH. More targeted research on MOH may be needed in the future.
4.1 Single-pulse TMS (sTMS)
sTMS temporarily disrupts brain activity by interrupting CSD, thereby reducing the occurrence of migraine aura (73). It influences neurotransmitter release by decreasing excitatory glutamate and increasing inhibitory gamma-aminobutyric acid (GABA), which lowers neuronal excitability and helps reduce the frequency and severity of migraines (74, 75). sTMS can also modulate the spontaneous activity of third-order thalamic neurons and trigeminovascular activity induced by C-fibers (73). The first long-term study on sTMS at occipital cortex for adults with CM and high-frequency episodic migraine (HFEM) (76) (≥8 headache days per month) included more than half of the patients with MOH and demonstrated significant efficacy. The proportion of MOH patients decreased from 52% (N = 79/153) at baseline to 19% (N = 29/153) at the third month and 8% (N = 7/87) at the twelfth month (77). Participants underwent self-administered TMS therapy 2–3 times daily for three months. Compared to baseline, the median HIT-6 score decreased by 4 points at the twelfth month. The proportion of patients with severe headache-related disability dropped from 93% at baseline to 63% at both the third and twelfth months. Regardless of MOH status or treatment resistance level, 45% of patients experienced long-term headache improvement. When assessing treatment sustainability using HIT-6 score changes, patients who showed significant score reductions were more likely to continue treatment for 12 months, indicating their recognition of the positive effects of sTMS (77).
4.2 Repetitive TMS (rTMS)
The effects of rTMS on cortical excitability can persist for an extended period, with the direction of modulation primarily depending on the stimulation frequency (78). Since various treatment protocols can be designed to target different pathophysiological aspects of MOH, rTMS theoretically allows for personalized treatment tailored to individual patient needs (79).High-frequency rTMS (HF-rTMS) may promote dendritic spine structural remodeling by reshaping postsynaptic scaffolding proteins and modulating synaptic GABAergic activity. Additionally, it can increase endorphin levels in migraine patients (15). In 2014, Misra (15) studied the effects of left frontal HF-rTMS treatment in 94 migraine patients, including 22 MOH patients. Among them, 56 patients received real stimulation, while 38 received sham stimulation. A reduction in N20 amplitude was found to be associated with decreased headache frequency and severity over one month. Compared to the sham group, the real stimulation group experienced significant HD improvement, which was associated with reduced headache severity but not frequency. That same year, Conforto (14) reported negative results for HF-rTMS targeting the left DLPFC. He randomized 18 CM patients [of whom 14 (77.78%) had MOH] into real and sham stimulation groups (1:1 ratio). Over eight weeks, patients underwent 23 rTMS sessions, with headache days assessed through a headache diary. The study found no significant benefits of HF-rTMS-L-DLPFC and suggested that M1 might be a more promising target than DLPFC. In 2016, Indian researcher Kalita (80) investigated the differences between single-session and three-session HF-rTMS-L-DLPFC for chronic headache treatment. The study included 82 participants, comprising CM and chronic tension-type headache (CTTH) patients, of whom 36 had MOH. After treatment, no significant differences were observed in headache frequency reduction between CM patients with and without MOH at 1, 2, and 3 months post-treatment. Among 10 CTTH patients and 6 mixed chronic daily headache (CDH) patients (including 5 MOH patients), headache frequency showed improvement after rTMS, but without statistically significance. Three years later, Granato (81) conducted a study on 14 MOH patients (10 overusing triptans & NSAIDs, 4 overusing NSAIDs only), administering 20 Hz HF-rTMS-L-DLPFC treatments over two consecutive weeks. The study assessed headache days, duration, intensity, medication use, and disability levels. The results indicated a strong placebo effect, with headache days reduced by 45.5% in the treatment group and 40% in the sham group, failing to confirm the therapeutic efficacy of HF-rTMS-L-DLPFC.
4.3 Quadruple-pulse rTMS (qTMS)
Quadruple-pulse transcranial magnetic stimulation (qTMS) delivers four monophasic magnetic pulses at variable interstimulus intervals (ISI), achieving excitatory effects when ISI < 10 ms and inhibitory effects when ISI > 30 ms on the underlying cortex. Initially, studies on qTMS focused on M1. Researchers recruited 10 HV and assessed motor cortical excitability and plasticity by measuring the peak MEP-Amp from the relaxed right first dorsal interosseous (FDI) muscle. Their findings demonstrated that qTMS induces long-lasting excitability and plasticity changes in M1, suggesting its potential preventive effects on MOH attacks (82, 83). Following qTMS treatment, MEP measurements were conducted every 5 min for 30 min and then every 15 min for 180 min while maintaining a constant output intensity. Results showed that qTMS at short ISIs (1.5–10 ms) enhanced MEPs for over 75 min, resembling long-term potentiation (LTP), whereas long ISIs (30–100 ms) suppressed MEPs, mimicking long-term depression (LTD). Thus, qTMS can bidirectionally modulate synaptic plasticity by altering ISI. Given that sTMS has demonstrated acute analgesic effects on MwA in the visual cortex of EM patients (84) and preventive effects (77), Viganò et al. were the first to apply qTMS to the visual cortex of patients with chronic migraine and medication-overuse headache (CM-MOH). Their inhibitory protocol (qTMS-I) involved delivering 4-pulse sequences every 5 s with an ISI of 50 ms for 30 min (totaling 1,440 pulses). This protocol reduced VEP-Amp and HS in HVs (85). During a one-month treatment period (twice per week), CM-MOH patients also exhibited reductions in VEP-Amp and HS, with the therapeutic effect more pronounced 3 h post-stimulation than immediately after treatment. This qTMS-induced reduction of visual cortical CS led to a nearly 50% reduction in monthly headache days in CM-MOH patients, with HIT-6 score improvements persisting for one month post-treatment. Additionally, half of the patients (n = 6) reverted to an EM pattern (ICHD-3 1.1) (86), allowing for the reintroduction of preventive medications (87). However, severe headache days (Grade 3 intensity), acute medication intake, MIDAS, STAI, and BDI scores did not show significant improvements. A single session of occipital qTMS-I only caused a transient VEP-Amp reduction, with no significant effects on other VEP parameters, suggesting that repetitive rTMS sessions are necessary to induce long-lasting plasticity changes in sensory cortices (88). Building on previous studies (83, 89) and meta-analyses (90), researchers designed a simulated excitatory protocol (qTMS-E), which delivered pre-stimulation pulses to functional MRI (fMRI) of the occipital region V2 and V3 areas every 5 s with an ISI of 50 ms for 10 min, followed by stimulation of V1 area every 5 s with an ISI of 30 ms for 30 min. In the six patients who reverted to EM, VEP-HD significantly improved one month after the qTMS-I treatment phase (39, 91, 92).
5 Placebo effects of rTMS
Placebo-controlled comparisons are essential for evaluating the efficacy of interventions in RCT (randomized controlled trial). The placebo effect arises from various factors, including but not limited to the clinician's demeanor during treatment (enthusiastic, indifferent, or neutral) (93, 94), the patient's awareness of the research process and trial details, and the subjective perception of symptoms, particularly pain. In some studies, placebo interventions have demonstrated clinically relevant response rates of ≥50% (75, 95, 96). A study by Huang et al. (97) on the placebo effects of non-pharmacological therapies found that sham rTMS was significantly more effective than sham CBT(Cognitive Behavioral Therapy), sham nVNS (non-invasive Vagus Nerve Stimulation), sham tDCS (transcranial Direct Current Stimulation), and sham acupuncture in reducing headache days, likely due to the high procedural similarity between sham and real rTMS (14, 75, 81, 98–103). Notably, one study even reported that sham rTMS yielded a higher response rate in reducing headache days than the active intervention, contradicting the classical RCT interpretation that “the most effective treatment is the one with the greatest specificity beyond placebo” (104). This paradox may be attributed to the small sample size in sham rTMS trials, as smaller sample sizes tend to exaggerate treatment effects (105–107). These findings underscore the need for large-scale RCTs on rTMS for MOH, incorporating biomarker-based objective quantification. Such studies would not only elucidate the pathophysiology of MOH but also enhance our understanding of TMS mechanisms, providing strong clinical evidence to support its application in practice.
6 Discussion
Research on TMS for MOH remains limited, and many findings must be extrapolated from studies on CM or EM. The effectiveness of the L-DLPFC target is still debated, while MOH studies targeting the occipital lobe are nearly nonexistent. Additionally, few studies have used objective electrophysiological biomarkers as primary outcome measures, providing an important direction for future MOH treatment research design. Several factors may contribute to variability in the efficacy of TMS for MOH (14, 102, 108): (i) Differences in neuroplasticity and excitability alterations between MOH-NSAIDs and MOH-triptans patients may lead to distinct treatment responses (109–111). (ii) Lack of strict adherence to guidelines (112), including variations in stimulation timing (80, 90), target selection, and stimulation protocols across studies (113). (iii) Inconsistent study designs and measurement parameters, such as differences in baseline characteristics, statistical methods, small sample sizes, uneven group distributions, and lack of long-term follow-up (114, 115). (iv) Placebo effects cannot be ruled out, as the clinician's approach, the participant's understanding of the study, and the inherent placebo effect of device-based therapies (which is generally greater than that of oral medications) may influence outcomes (81, 93, 94, 116).
In the future, multimodal measurements of electrophysiological indicators such as Amp, PAL, HD, HS, CS, MEP, CSD, CSPD, and SIFI, along with TMS-induced high-density electroencephalographic responses (TMS-EEG), will help unravel the underlying mechanisms of CS and HD in MOH patients. These measurements will also allow for more accurate determination of the effects of TMS on the entire brain or other brain regions with abnormal electrical activity, thereby identifying more effective treatment targets and efficacy evaluation indicators for MOH.
7 Conclusion
Future research on TMS treatment for MOH will place greater emphasis on individualized therapy, with electrophysiological assessments providing an objective evaluation metric for this approach. Currently, most studies on TMS treatment for MOH rely on subjective efficacy evaluations based on clinical manifestations, such as pain diaries and pain scales. There is limited research using electrophysiological indicators to assess the efficacy of TMS in treating MOH. By combining objective electrophysiological metrics with subjective clinical symptom indicators, researchers can better understand how TMS modulates neural circuits to achieve pain relief. This will help optimize the clinical application of TMS and enhance our ability to harness this promising treatment method more effectively.
Author contributions
DX: Conceptualization, Data curation, Formal analysis, Investigation, Software, Validation, Writing – original draft. HH: Data curation, Investigation, Software, Validation, Writing – original draft. HG: Conceptualization, Methodology, Resources, Supervision, Writing – review & editing. ZL: Writing – review & editing, Formal analysis, Investigation, Software, Validation. YH: Formal analysis, Investigation, Software, Validation, Writing – review & editing, Data curation, Project administration, Supervision. JM: Data curation, Formal analysis, Investigation, Writing – review & editing. HJ: Data curation, Formal analysis, Investigation, Writing – review & editing, Conceptualization, Funding acquisition, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. Peking University People's Hospital Talent Introduction Scientific Research Start-up Fund Project Grant Amount: 500,000 CNY Project Number: 2022-T-02 Duration: January 1, 2023 – December 31, 2027.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Headache classification committee of the international headache society (IHS) the international classification of headache disorders, 3rd edition. Cephalalgia Int J Headache. (2018) 38(1):1–211. doi: 10.1177/0333102417738202
2. Ong JJY, De Felice M. Migraine treatment: current acute medications and their potential mechanisms of action. Neurotherapeutics. (2018) 15(2):274–90. doi: 10.1007/s13311-017-0592-1
3. Takahashi TT, Ornello R, Quatrosi G, Torrente A, Albanese M, Vigneri S, et al. Medication overuse and drug addiction: a narrative review from addiction perspective. J Headache Pain. (2021) 22(1):32. doi: 10.1186/s10194-021-01224-8
4. Gosalia H, Moreno-Ajona D, Goadsby PJ. Medication-overuse headache: a narrative review. J Headache Pain. (2024) 25(1):89. doi: 10.1186/s10194-024-01755-w
5. Torres-Ferrús M, Ursitti F, Alpuente A, Brunello F, Chiappino D, de Vries T, et al. From transformation to chronification of migraine: pathophysiological and clinical aspects. J Headache Pain. (2020) 21(1):42. doi: 10.1186/s10194-020-01111-8
6. López Martínez MDV, Pareja Román J, Jiménez Hernández MD, Maestu Unturbe C, Ramírez-Castillejo MDC. Chronic migraine with medication overuse: clinical pattern and evolution from a retrospective cohort in Seville, Spain. SN Compr Clin Med. (2020) 2(9):1514–25. doi: 10.1007/s42399-020-00424-8
7. Pan LLH, Ling YH, Wang SJ, Al-Hassany L, Chen WT, Chiang CC, et al. Hallmarks of primary headache: part 2- tension-type headache. J Headache Pain. (2025) 26(1):164. doi: 10.1186/s10194-025-02098-w
8. Katsarava Z, Schneeweiss S, Kurth T, Kroener U, Fritsche G, Eikermann A, et al. Incidence and predictors for chronicity of headache in patients with episodic migraine. Neurology. (2004) 62(5):788–90. doi: 10.1212/01.WNL.0000113747.18760.D2
9. Westergaard ML, Glümer C, Hansen EH, Jensen RH. Prevalence of chronic headache with and without medication overuse: associations with socioeconomic position and physical and mental health status. Pain. (2014) 155(10):2005–13. doi: 10.1016/j.pain.2014.07.002
10. Bigal M, Rapoport A, Sheftell F, Tepper S, Lipton R. Transformed migraine and medication overuse in a tertiary headache centre — clinical characteristics and treatment outcomes. Cephalalgia. (2004) 24(6):483–90. doi: 10.1111/j.1468-2982.2004.00691.x
11. Koonalintip P, Yamutai S, Setthawatcharawanich S, Thongseiratch T, Chichareon P, Wakerley BR. Network meta-analysis comparing efficacy of different strategies on medication-overuse headache. J Headache Pain. (2025) 26(1):43. doi: 10.1186/s10194-025-01982-9
12. Raggi A, Leonardi M, Arruda M, Caponnetto V, Castaldo M, Coppola G, et al. Hallmarks of primary headache: part 1—migraine. J Headache Pain. (2024) 25(1):189. doi: 10.1186/s10194-024-01889-x
13. Hird MA, Sandoe CH. Medication overuse headache: an updated review and clinical recommendations on management. Curr Neurol Neurosci Rep. (2023) 23(7):389–98. doi: 10.1007/s11910-023-01278-y
14. Conforto AB, Amaro E, Gonçalves AL, Mercante JP, Guendler VZ, Ferreira JR, et al. Randomized, proof-of-principle clinical trial of active transcranial magnetic stimulation in chronic migraine. Cephalalgia Int J Headache. (2014) 34(6):464–72. doi: 10.1177/0333102413515340
15. Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: a randomized, placebo-controlled study. J Neurol. (2013) 260(11):2793–801. doi: 10.1007/s00415-013-7072-2
16. Libera D, Colombo B, Nuara A, Spagnolo F, Straffi L, Chieffo R, et al. Repetitive transcranial magnetic stimulation (rTMS) as preventive treatment of chronic migraine: a safe approach with a promising effect (S59.005). Neurology. 80 (7 Suppl):S59.005. doi: 10.1212/WNL.80.7_supplement.S59.005
17. Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, et al. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. (1997) 48(5):1398–403. doi: 10.1212/WNL.48.5.1398
18. Chen R. Studies of human motor physiology with transcranial magnetic stimulation. Muscle Nerve Suppl. (2000) 9:S26–32. doi: 10.1002/1097-4598(2000)999:9%3C::AID-MUS6%3E3.0.CO;2-I
19. Lefaucheur JP, André-Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol Off J Int Fed Clin Neurophysiol. (2014) 125(11):2150–206. doi: 10.1016/j.clinph.2014.05.021
20. Lefaucheur JP, Aleman A, Baeken C, Benninger DH, Brunelin J, Di Lazzaro V, et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol Off J Int Fed Clin Neurophysiol. (2020) 131(2):474–528. doi: 10.1016/j.clinph.2019.11.002
21. Tomeh A, Yusof Khan AHK, Inche Mat LN, Basri H, Wan Sulaiman WA. Repetitive transcranial magnetic stimulation of the primary motor cortex beyond motor rehabilitation: a review of the current evidence. Brain Sci. (2022) 12(6):761. Available online at: https://pubmed.ncbi.nlm.nih.gov/35741646/ (Accessed January 28, 2025). doi: 10.3390/brainsci12060761
22. Rissardo JP, Byroju VV, Mukkamalla S, Caprara ALF. A narrative review of stroke of cortical hand knob area. Med Kaunas Lith. (2024) 60(2):318. doi: 10.3390/medicina60020318
23. Lamusuo S, Hirvonen J, Lindholm P, Martikainen IK, Hagelberg N, Parkkola R, et al. Neurotransmitters behind pain relief with transcranial magnetic stimulation—positron emission tomography evidence for release of endogenous opioids. Eur J Pain. (2017) 21(9):1505–15. doi: 10.1002/ejp.1052
24. Andre-Obadia N, Magnin M, Simon E, Garcia-Larrea L. Somatotopic effects of rTMS in neuropathic pain? A comparison between stimulation over hand and face motor areas. Eur J Pain Lond Engl. (2018) 22(4):707–15. doi: 10.1002/ejp.1156
25. Vaninetti M, Lim M, Khalaf A, Metzger-Smith V, Flowers M, Kunnel A, et al. fMRI findings in MTBI patients with headaches following rTMS. Sci Rep. (2021) 11(1):9573. doi: 10.1038/s41598-021-89118-2
26. De Andrade DC, Mhalla A, Adam F, Texeira MJ, Bouhassira D. Neuropharmacological basis of rTMS-induced analgesia: the role of endogenous opioids. Pain. (2011) 152(2):320–6. doi: 10.1016/j.pain.2010.10.032
27. Leung A, Shirvalkar P, Chen R, Kuluva J, Vaninetti M, Bermudes R, et al. Transcranial magnetic stimulation for pain, headache, and comorbid depression: iNS-NANS expert consensus panel review and recommendation. Neuromodulation Technol Neural Interface. (2020) 23(3):267–90. doi: 10.1111/ner.13094
28. Graff-Guerrero A, González-Olvera J, Fresán A, Gómez-Martín D, Méndez-Núñez JC, Pellicer F. Repetitive transcranial magnetic stimulation of dorsolateral prefrontal cortex increases tolerance to human experimental pain. Brain Res Cogn Brain Res. (2005) 25(1):153–60. doi: 10.1016/j.cogbrainres.2005.05.002
29. Schwartz GE, Davidson RJ, Maer F. Right hemisphere lateralization for emotion in the human brain: interactions with cognition. Science. (1975) 190(4211):286–8. doi: 10.1126/science.1179210
30. Fox MD. Mapping symptoms to brain networks with the human connectome. N Engl J Med. (2018) 379(23):2237–45. doi: 10.1056/NEJMra1706158
31. Darby RR, Joutsa J, Burke MJ, Fox MD. Lesion network localization of free will. Proc Natl Acad Sci. (2018) 115(42):10792–7. doi: 10.1073/pnas.1814117115
32. Darby RR, Joutsa J, Fox MD. Network localization of heterogeneous neuroimaging findings. Brain. (2019) 142(1):70–9. doi: 10.1093/brain/awy292
33. Burke MJ, Joutsa J, Cohen AL, Soussand L, Cooke D, Burstein R, et al. Mapping migraine to a common brain network. Brain. (2020) 143(2):541–53. doi: 10.1093/brain/awz405
34. De Tommaso M, Ambrosini A, Brighina F, Coppola G, Perrotta A, Pierelli F, et al. Altered processing of sensory stimuli in patients with migraine. Nat Rev Neurol. (2014) 10(3):144–55. doi: 10.1038/nrneurol.2014.14
35. Denuelle M, Boulloche N, Payoux P, Fabre N, Trotter Y, Géraud G. A PET study of photophobia during spontaneous migraine attacks. Neurology. (2011) 76(3):213–8. doi: 10.1212/WNL.0b013e3182074a57
36. Noseda R, Kainz V, Jakubowski M, Gooley JJ, Saper CB, Digre K, et al. A neural mechanism for exacerbation of headache by light. Nat Neurosci. (2010) 13(2):239–45. doi: 10.1038/nn.2475
37. Golla FL, Winter AL. Analysis of cerebral responses to flicker in patients complaining of episodic headache. Electroencephalogr Clin Neurophysiol. (1959) 11(3):539–49. doi: 10.1016/0013-4694(59)90052-5
38. Schoenen J, Wang W, Albert A, Delwaide PJ. Potentiation instead of habituation characterizes visual evoked potentials in migraine patients between attacks. Eur J Neurol. (1995) 2(2):115–22. doi: 10.1111/j.1468-1331.1995.tb00103.x
39. Chen WT, Wang SJ, Fuh JL, Ko YC, Lee YC, Hämäläinen MS, et al. Visual cortex excitability and plasticity associated with remission from chronic to episodic migraine. Cephalalgia. (2012) 32(7):537–43. doi: 10.1177/0333102412443337
40. Coppola G, Iacovelli E, Bracaglia M, Serrao M, Di Lorenzo C, Pierelli F. Electrophysiological correlates of episodic migraine chronification: evidence for thalamic involvement. J Headache Pain. (2013) 14(1):76. doi: 10.1186/1129-2377-14-76
41. Abbas Abdulhussein M, Alyasseri ZAA, Mohammed HJ, An X. Lack of habituation in migraine patients based on high-density EEG analysis using the steady state of visual evoked potential. Entropy. (2022) 24(11):1688. doi: 10.3390/e24111688
42. Jia H, Varga Z, Sakmann B, Konnerth A. Linear integration of spine Ca2 + signals in layer 4 cortical neurons in vivo. Proc Natl Acad Sci U S A. (2014) 111(25):9277–82. doi: 10.1073/pnas.1408525111
43. Restuccia D, Vollono C, Virdis D, del Piero I, Martucci L, Zanini S. Patterns of habituation and clinical fluctuations in migraine. Cephalalgia. (2014) 34(3):201–10. doi: 10.1177/0333102413508241
44. Schmidt-Wilcke T, Leinisch E, Straube A, Kämpfe N, Draganski B, Diener HC, et al. Gray matter decrease in patients with chronic tension type headache. Neurology. (2005) 65(9):1483–6. doi: 10.1212/01.wnl.0000183067.94400.80
45. Burstein R. The development of cutaneous allodynia during a migraine attack clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. (2000) 123(8):1703–9. doi: 10.1093/brain/123.8.1703
46. Lovati C, D’Amico D, Bertora P, Rosa S, Suardelli M, Mailland E, et al. Acute and interictal allodynia in patients with different headache forms: an Italian pilot study. Headache J Head Face Pain. (2008) 48(2):272–7. doi: 10.1111/j.1526-4610.2007.00998.x
47. Coppola G, Currà A, Lorenzo D, Parisi C, Gorini V, Sava M, et al. Abnormal cortical responses to somatosensory stimulation in medication-overuse headache. BMC Neurol. (2010) 10(1):126. doi: 10.1186/1471-2377-10-126
48. Leão AAP. The slow voltage variation of cortical spreading depression of activity. Electroencephalogr Clin Neurophysiol. (1951) 3(3):315–21. doi: 10.1016/0013-4694(51)90079-X
49. Grodzka O, Dzagoevi K, Rees T, Cabral G, Chądzyński P, Di Antonio S, et al. Migraine with and without aura-two distinct entities? A narrative review. J Headache Pain. (2025) 26(1):77. doi: 10.1186/s10194-025-01998-1
50. McLeod GA, Josephson CB, Engbers JDT, Cooke LJ, Wiebe S. Mapping the migraine: intracranial recording of cortical spreading depression in migraine with aura. Headache. (2025) 65(4):658–65. doi: 10.1111/head.14907
51. Shahani BT, Young RR. Studies of the normal human silent period. (1973). Available online at: https://karger.com/books/book/860/chapter/5608125/Studies-of-the-Normal-Human-Silent-Period (Accessed January 30, 2025).
52. Lee M, Kim SE, Kim WS, Han J, Kim HJ, Kim BS, et al. Cortico-cortical modulation induced by 1-hz repetitive transcranial magnetic stimulation of the temporal cortex. J Clin Neurol Seoul Korea. (2013) 9(2):75–82. doi: 10.3988/jcn.2013.9.2.75
53. Curra A, Coppola G, Gorini M, Porretta E, Bracaglia M, Di Lorenzo C, et al. Drug-induced changes in cortical inhibition in medication overuse headache. Cephalalgia. (2011) 31(12):1282–90. doi: 10.1177/0333102411415877
54. Ayzenberg I, Obermann M, Nyhuis P, Gastpar M, Limmroth V, Diener HC, et al. Central sensitization of the trigeminal and somatic nociceptive systems in medication overuse headache mainly involves cerebral supraspinal structures. Cephalalgia Int J Headache. (2006) 26(9):1106–14. doi: 10.1111/j.1468-2982.2006.01183.x
55. Mauskop A. Clinical features of withdrawal headache following overuse of triptans and other headache drugs. Neurology. (2002) 58(9):1443–4. doi: 10.1212/WNL.58.9.1443
56. Limmroth V, Katsarava Z, Fritsche G, Przywara S, Diener HC. Features of medication overuse headache following overuse of different acute headache drugs. Neurology. (2002) 59(7):1011–4. doi: 10.1212/WNL.59.7.1011
57. Curra A, Pierelli F, Coppola G, Barbanti P, Buzzi MG, Galeotti F, et al. Shortened cortical silent period in facial muscles of patients with migraine. PAIN. (2007) 132(1):124. doi: 10.1016/j.pain.2007.05.009
58. Kelman L, Tanis D. The relationship between migraine pain and other associated symptoms. Cephalalgia. (2006) 26(5):548–53. doi: 10.1111/j.1468-2982.2006.01075.x
59. Friedman DI, De Ver Dye T. Migraine and the environment. Headache J Head Face Pain. (2009) 49(6):941–52. doi: 10.1111/j.1526-4610.2009.01443.x
60. Woodhouse A, Drummond PD. Mechanisms of increased sensitivity to noise and light in migraine headache. Cephalalgia. (1993) 13(6):417–21. doi: 10.1046/j.1468-2982.1993.1306417.x
61. Wray SH, Mijović-Prelec D, Kosslyn SM. Visual processing in migraineurs. Brain. (1995) 118(1):25–35. doi: 10.1093/brain/118.1.25
62. Calvert GA, Thesen T. Multisensory integration: methodological approaches and emerging principles in the human brain. J Physiol-Paris. (2004) 98(1–3):191–205. doi: 10.1016/j.jphysparis.2004.03.018
63. Ernst MO, Bülthoff HH. Merging the senses into a robust percept. Trends Cogn Sci. (2004) 8(4):162–9. doi: 10.1016/j.tics.2004.02.002
64. Radeau M, Bertelson P. Auditory-visual interaction and the timing of inputs: thomas (1941) revisited. Psychol Res. (1987) 49(1):17–22. doi: 10.1007/BF00309198
65. Bertelson P, Radeau M. Cross-modal bias and perceptual fusion with auditory-visual spatial discordance. Percept Psychophys. (1981) 29(6):578–84. doi: 10.3758/BF03207374
66. Bulkin DA, Groh JM. Seeing sounds: visual and auditory interactions in the brain. Curr Opin Neurobiol. (2006) 16(4):415–9. doi: 10.1016/j.conb.2006.06.008
67. Sekuler R, Sekuler AB, Lau R. Sound alters visual motion perception. Nature. (1997) 385(6614):308–308. doi: 10.1038/385308a0
68. Bolognini N, Russo C, Vallar G. Crossmodal illusions in neurorehabilitation. Front Behav Neurosci. (2015) 9:212. doi: 10.3389/fnbeh.2015.00212
69. Shams L, Kamitani Y, Shimojo S. Visual illusion induced by sound. Cogn Brain Res. (2002) 14(1):147–52. doi: 10.1016/S0926-6410(02)00069-1
70. Bolognini N, Convento S, Casati C, Mancini F, Brighina F, Vallar G. Multisensory integration in hemianopia and unilateral spatial neglect: evidence from the sound induced flash illusion. Neuropsychologia. (2016) 87:134–43. doi: 10.1016/j.neuropsychologia.2016.05.015
71. Brighina F, Bolognini N, Cosentino G, Maccora S, Paladino P, Baschi R, et al. Visual cortex hyperexcitability in migraine in response to sound-induced flash illusions. Neurology. (2015) 84(20):2057–61. doi: 10.1212/WNL.0000000000001584
72. Innes-Brown H, Crewther D. The impact of spatial incongruence on an auditory-visual illusion. PLoS One. (2009) 4(7):e6450. doi: 10.1371/journal.pone.0006450
73. Andreou AP, Holland PR, Akerman S, Summ O, Fredrick J, Goadsby PJ. Transcranial magnetic stimulation and potential cortical and trigeminothalamic mechanisms in migraine. Brain. (2016) 139(7):2002–14. doi: 10.1093/brain/aww118
74. Shen M, Li C, Wei X, Zhang L, Li Y, Wu H, et al. Transcranial magnetic stimulation as a therapy for migraine. An Overview of Systematic Reviews. J Pain Res. (2023) 16:3133–44. doi: 10.2147/JPR.S416993
75. Amin RM, Emara TH, Ashour S, Hemeda M, Eldin NS, Hamed S, et al. The role of left prefrontal transcranial magnetic stimulation in episodic migraine prophylaxis. Egypt J Neurol Psychiatry Neurosurg. (2019) 56:1–6. doi: 10.1186/s41983-019-0140-5
76. Starling AJ, Tepper SJ, Marmura MJ, Shamim EA, Robbins MS, Hindiyeh N, et al. A multicenter, prospective, single arm, open label, observational study of sTMS for migraine prevention (ESPOUSE study). Cephalalgia Int J Headache. (2018) 38(6):1038–48. doi: 10.1177/0333102418762525
77. Lloyd JO, Hill B, Murphy M, Al-Kaisy A, Andreou AP, Lambru G. Single-pulse transcranial magnetic stimulation for the preventive treatment of difficult-to-treat migraine: a 12-month prospective analysis. J Headache Pain. (2022) 23(1):63. doi: 10.1186/s10194-022-01428-6
78. Zis P, Shafique F, Hadjivassiliou M, Blackburn D, Venneri A, Iliodromiti S, et al. Safety, tolerability, and nocebo phenomena during transcranial magnetic stimulation: a systematic review and meta-analysis of placebo-controlled clinical trials. Neuromodulation J Int Neuromodulation Soc. (2020) 23(3):291–300. doi: 10.1111/ner.12946
79. Coppola G, Di Lorenzo C, Serrao M, Parisi V, Schoenen J, Pierelli F. Pathophysiological targets for non-pharmacological treatment of migraine. Cephalalgia. (2016) 36(12):1103–11. doi: 10.1177/0333102415620908
80. Kalita J, Laskar S, Bhoi SK, Misra UK. Efficacy of single versus three sessions of high rate repetitive transcranial magnetic stimulation in chronic migraine and tension-type headache. J Neurol. (2016) 263(11):2238–46. doi: 10.1007/s00415-016-8257-2
81. Granato A, Fantini J, Monti F, Furlanis G, Musho Ilbeh S, Semenic M, et al. Dramatic placebo effect of high frequency repetitive TMS in treatment of chronic migraine and medication overuse headache. J Clin Neurosci. (2019) 60:96–100. doi: 10.1016/j.jocn.2018.09.021
82. Hamada M, Terao Y, Hanajima R, Shirota Y, Nakatani-Enomoto S, Furubayashi T, et al. Bidirectional long-term motor cortical plasticity and metaplasticity induced by quadripulse transcranial magnetic stimulation. J Physiol. (2008) 586(16):3927–47. doi: 10.1113/jphysiol.2008.152793
83. Hamada M, Hanajima R, Terao Y, Okabe S, Nakatani-Enomoto S, Furubayashi T, et al. Primary motor cortical metaplasticity induced by priming over the supplementary motor area. J Physiol. (2009) 587(20):4845–62. doi: 10.1113/jphysiol.2009.179101
84. Lipton RB, Dodick DW, Silberstein SD, Saper JR, Aurora SK, Pearlman SH, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurol. (2010) 9(4):373–80. doi: 10.1016/S1474-4422(10)70054-5
85. Vigano A, D’Elia TS, Sava SL, Colosimo A, Di Piero V, Magis D, et al. Exploring the therapeutic potential of quadripulse rTMS over the visual cortex: a proof-of-concept study in healthy volunteers and chronic migraine patients with medication overuse headache. Biomedicines. (2024) 12(2):288. doi: 10.3390/biomedicines12020288
86. Petrušić I, Messina R, Pellesi L, Azorin DG, Chiang CC, Pietra AD, et al. Application of machine learning in migraine classification: a call for study design standardization and global collaboration. J Headache Pain. (2025) 26(1):200. doi: 10.1186/s10194-025-02134-9
87. Paemeleire K, Louis P, Magis D, Vandenheede M, Versijpt J, Vandersmissen B, et al. Diagnosis, pathophysiology and management of chronic migraine: a proposal of the Belgian headache society. Acta Neurol Belg. (2015) 115(1):1–17. doi: 10.1007/s13760-014-0313-z
88. Fumal A, Coppola G, Bohotin V, Gérardy PY, Seidel L, Donneau AF, et al. Induction of long-lasting changes of visual cortex excitability by five daily sessions of repetitive transcranial magnetic stimulation (rTMS) in healthy volunteers and migraine patients. Cephalalgia. (2006) 26(2):143–9. doi: 10.1111/j.1468-2982.2005.01013.x
89. Hamada M, Ugawa Y. Quadripulse stimulation—a new patterned rTMS. Restor Neurol Neurosci. (2010) 28(4):419–24. doi: 10.3233/RNN-2010-0564
90. Zhong J, Lan W, Feng Y, Yu L, Xiao R, Shen Y, et al. Efficacy of repetitive transcranial magnetic stimulation on chronic migraine: a meta-analysis. Front Neurol. (2022) 13:1050090. doi: 10.3389/fneur.2022.1050090
91. Coppola G, Pierelli F, Schoenen J. Is the cerebral cortex hyperexcitable or hyperresponsive in migraine? Cephalalgia Int J Headache. (2007) 27(12):1427–39. doi: 10.1111/j.1468-2982.2007.01500.x
92. Coppola G, Schoenen J. Cortical excitability in chronic migraine. Curr Pain Headache Rep. (2012) 16(1):93–100. doi: 10.1007/s11916-011-0231-1
93. De Craen AJM, Tijssen JGP, De Gans J, Kleijnen J. Placebo effect in the acute treatment of migraine: subcutaneous placebos are better than oral placebos. J Neurol. (2000) 247(3):183–8. doi: 10.1007/s004150050560
94. Kaptchuk TJ, Stason WB, Davis RB, Legedza ART, Schnyer RN, Kerr CE, et al. Sham device v inert pill: randomised controlled trial of two placebo treatments. Br Med J. (2006) 332(7538):391–7. doi: 10.1136/bmj.38726.603310.55
95. Linde K, Streng A, Jürgens S, Hoppe A, Brinkhaus B, Witt C, et al. Acupuncture for patients with migraine: a randomized controlled trial. JAMA. (2005) 293(17):2118. doi: 10.1001/jama.293.17.2118
96. Bäcker M, Grossman P, Schneider J, Michalsen A, Knoblauch N, Tan L, et al. Acupuncture in migraine: investigation of autonomic effects. Clin J Pain. (2008) 24(2):106–15. doi: 10.1097/AJP.0b013e318159f95e
97. Huang YB, Yuan L, Xiao XY, Wang XY, Feng SJ, Zheng H. Effect of different non-pharmacologic placebo treatments on migraine prevention: a network meta-analysis of randomized controlled trials. Acta Neurol Belg. (2024) 124(4):1125–39. doi: 10.1007/s13760-023-02460-2
98. Leahu P, Bange M, Ciolac D, Scheiter S, Matei A, Gonzalez-Escamilla G, et al. Increased migraine-free intervals with multifocal repetitive transcranial magnetic stimulation. Brain Stimulat. (2021) 14(6):1544–52. doi: 10.1016/j.brs.2021.10.383
99. Teepker M, Hötzel J, Timmesfeld N, Reis J, Mylius V, Haag A, et al. Low-frequency rTMS of the vertex in the prophylactic treatment of migraine. Cephalalgia Int J Headache. (2010) 30(2):137–44. doi: 10.1111/j.1468-2982.2009.01911.x
100. Shah J, Dhull P, Somasekharan M, Soni R, Gupta S. Repetitive transcranial magnetic stimulation for prophylactive treatment of chronic migraine: a randomised, single-blind, parallel-group, sham-controlled trial. Neurol Asia. (2022) 27(1):137–44. doi: 10.54029/2022mau
101. Kumar A, Mattoo B, Bhatia R, Kumaran S, Bhatia R. Neuronavigation based 10 sessions of repetitive transcranial magnetic stimulation therapy in chronic migraine: an exploratory study. Neurol Sci. (2021) 42(1):131–9. doi: 10.1007/s10072-020-04505-3
102. Brighina F, Piazza A, Vitello G, Aloisio A, Palermo A, Daniele O, et al. rTMS of the prefrontal cortex in the treatment of chronic migraine: a pilot study. J Neurol Sci. (2004) 227(1):67–71. doi: 10.1016/j.jns.2004.08.008
103. AbdElkader AA, Fahmy EM, Ahmad AAF, Labib AA, El-Mekkawy LA, El Razek S shaheen MA. The efficacy of repetitive transcranial magnetic stimulation in treating patients with chronic daily headache. Egypt J Neurol Psychiatry Neurosurg. (2021) 57(1):21. doi: 10.1186/s41983-021-00278-4
104. Walach H. The efficacy paradox in randomized controlled trials of CAM and elsewhere: beware of the placebo trap. J Altern Complement Med. (2001) 7(3):213–8. doi: 10.1089/107555301300328070
105. Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JPA, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses? Int J Epidemiol. (2009) 38(1):276–86. doi: 10.1093/ije/dyn179
106. Trikalinos TA, Churchill R, Ferri M, Leucht S, Tuunainen A, Wahlbeck K, et al. Effect sizes in cumulative meta-analyses of mental health randomized trials evolved over time. J Clin Epidemiol. (2004) 57(11):1124–30. doi: 10.1016/j.jclinepi.2004.02.018
107. Ioannidis JPA, Lau J. Evolution of treatment effects over time: empirical insight from recursive cumulative metaanalyses. Proc Natl Acad Sci. (2001) 98(3):831–6. doi: 10.1073/pnas.98.3.831
108. Misra UK, Kalita J, Bhoi SK. High-rate repetitive transcranial magnetic stimulation in migraine prophylaxis: A randomized, placebo-controlled study. J Neurol. (2013) 260(11):2793–801. doi: 10.1007/s00415-013-7072-2
109. Vezzani A, Viviani B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology. (2015) 96:70–82. doi: 10.1016/j.neuropharm.2014.10.027
110. Lim JA, Jung KY, Park B, Kim TJ, Jun JS, Kim KT, et al. Impact of a selective cyclooxygenase-2 inhibitor, celecoxib, on cortical excitability and electrophysiological properties of the brain in healthy volunteers: a randomized, double-blind, placebo-controlled study. PLoS One. (2019) 14(2):e0212689. doi: 10.1371/journal.pone.0212689
111. Patel D, Roy A, Kundu M, Jana M, Luan CH, Gonzalez FJ, et al. Aspirin binds to PPARα to stimulate hippocampal plasticity and protect memory. Proc Natl Acad Sci. (2018) 115(31):E7408–17. doi: 10.1073/pnas.1802021115
112. Reuter U, McClure C, Liebler E, Pozo-Rosich P. Non-invasive neuromodulation for migraine and cluster headache: a systematic review of clinical trials. J Neurol Neurosurg Psychiatry. (2019) 90(7):796–804. doi: 10.1136/jnnp-2018-320113
113. Sahu AK, Sinha VK, Goyal N. Effect of adjunctive intermittent theta-burst repetitive transcranial magnetic stimulation as a prophylactic treatment in migraine patients: a double-blind sham-controlled study. Indian J Psychiatry. (2019) 61(2):139–45. doi: 10.4103/psychiatry.IndianJPsychiatry_472_18
114. Shen M, Li C, Wei X, Zhang L, Li Y, Wu H, et al. Transcranial magnetic stimulation as a therapy for migraine: an overview of systematic reviews. J Pain Res. (2023) 16:3133–44. doi: 10.2147/JPR.S416993
115. Feng Y, Zhang B, Zhang J, Yin Y. Effects of non-invasive brain stimulation on headache intensity and frequency of headache attacks in patients with migraine: a systematic review and meta-analysis. Headache J Head Face Pain. (2019) 59(9):1436–47. doi: 10.1111/head.13645
Keywords: headache, medication overuse headache, electrophysiological indicators, transcranial magnetic stimulation, treatment
Citation: Xiao D, Han H, Guo H, Li Z, He Y, Ma J and Jiang H (2025) The potential application of electrophysiological indicators in TMS treatment for MOH. Front. Pain Res. 6:1689847. doi: 10.3389/fpain.2025.1689847
Received: 21 August 2025; Accepted: 28 October 2025;
Published: 20 November 2025.
Edited by:
David M. Niddam, National Yang Ming Chiao Tung Unviersity, TaiwanReviewed by:
Paolo Martelletti, University of Rome Unitelma Sapienza, ItalyGhada AbdelAzim, Al-Azhar University, Egypt
Copyright: © 2025 Xiao, Han, Guo, Li, He, Ma and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hong Jiang, amgxOTkxQHNpbmEuY29t
†These authors have contributed equally to this work and share first authorship
 Danqing Xiao
Danqing Xiao Haochi Han2,†
Haochi Han2,† Huailian Guo
Huailian Guo Hong Jiang
Hong Jiang