- 1Department of Information Engineering, University of Brescia, Brescia, Italy
- 2Department of Design, Politecnico di Milano, Milan, Italy
- 3Medical and Surgical Specialties, Radiological Sciences and PublicHealth (DSMC), University of Brescia, Brescia, Italy
- 4Department of Electronics, Information and Bioengineering, Politecnico di Milano, Milano, Italy
- 5IRCCS Istituto Auxologico Italiano, San Giuseppe Hospital, Piancavallo, Italy
Background: Non-specific low back pain (NS-LBP) is a is a highly prevalent musculoskeletal condition, with an estimated 619 million prevalent cases worldwide in 2020. Alterations in spinal and lower limb dynamics are considered as potential factors directly involved in this condition, thus we carried out a systematic review to summarize the evidence regarding walking kinematics in NS-LBP.
Methods: The reporting of this review followed the “2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA 2020 checklist) and the protocol was preliminary registered in PROSPERO (ID: CRD42023431380). A search strategy was implemented in Medline, Embase, Scopus, Web of Science, and IEEE Xplore databases, up to March 2024. Inclusion criteria were: any analytical observational research instrumentally assessing the trunk and lower limbs kinematics of spontaneous walking in NS-LBP, in a comparison with healthy people. Study selection and data extraction were performed by two blinded reviewers, the methodological quality was evaluated by the Joanna Briggs Institute (JBI) Critical Appraisal Checklist and the quality of the evidence was rated through GRADE criteria.
Results: Overall, a total of 19 cross-sectional studies were included in this review and none of those was found without methodological issues. The meta-analysis showed a lower gait velocity [−15.42 (−22.78, −8.06) cm/s; p ≤ 0.0001], a lower cadence [−9.85 (−18.72, −0.99) steps/min; p = 0.03] and a lower step length [−6.30 (−11.83; −0.77) cm; p = 0.03] in NS-LBP. Regarding motion analysis, a few authors observed a less and asymmetrical motion of the lower spine in the frontal and in the transverse plane.
Conclusion: There is very-low quality evidence that gait speed, cadence and step length are reduced in patients with NS-LBP. There is proof of a movement reduction in the lower lumbar spine and in the pelvis, both in the transverse and in the frontal plane. No differences in the lower limb kinematics was consistent over the studies.
Systematic Review Registration: https://www.crd.york.ac.uk/prospero/, identifier CRD42023431380.
1 Introduction
Low back pain (LBP) is a highly prevalent musculoskeletal condition, with an estimated 619 million prevalent cases worldwide in 2020 (1). Beyond its clinical impact, LBP represents a major social and economic burden, with total costs reaching billions of dollars annually in high-income countries (2) However, in a vast majority of the cases (57%–89%), no specific etiology is identifiable so the expression “non-specific LBP” (NS-LBP) is commonly used (3).
In this context, several underlying components have been hypothesized to contribute to LBP development, including age, gender, socio-demographic features, psychological characteristics, lifestyle, and mechanical issues. Therefore, LBP etiology can be properly defined as multifactorial (4). Mechanical factors, are suggested to be related to repetitive and prolonged stresses on the spine. These factors can be associated with posture and movement issues, and seem to be related to the development and persistence of the pain (5). More in detail, perturbations in spinal and lower limb dynamics have been considered as potential factors directly involved in NS-LBP, including joint rigidity, muscle stiffness or weakness, and poor neuromuscular function (e.g., altered timing of activation, incoordination); in fact, all these factors could lead to asymmetrical or abnormal mechanical loading of the lumbar spine (6–8).
Since walking represents one of the human activities that is frequently and routinely repeated throughout the whole day, it can be affected and contribute to pain, activity limitations, and disability in subjects presenting NS-LBP (9). For this reason, a quantitative and reliable evaluation of walking is of paramount importance when assessing subjects with NS-LBP. According to the main findings present in scientific literature, there is proof that the speed of gait in NS-LBP is, in fact, lower than in healthy people, whereas there is no consensus regarding other spatio-temporal parameters (10, 11). In the same way, there are conflicting results concerning how NS-LBP can affect the movement observed in different planes of motion; several authors found in fact that NS-LBP subjects showed a reduced range of motion in the axial plane, while others reported wider ranges of spinal and pelvic rotations (9–13).
Despite the large number of studies carried out over the years, to the best of our knowledge, no systematic review exists on this topic, so far; this lack presents certain implications since the absence of shared evidence and/or common guidelines prevents investigators to address future research and clinicians to develop tailored rehabilitative treatments for specific subgroups of NS-LBP subjects (14).
Therefore, we hypothesized that there is a potential associationbetween NS-LBP and modifications of the spinal and lower limb kinematics during walking when compared to healthy people. In this frame we carried out a systematic review focused to provide a structured summary of the actual evidence on this specific topic, specifically addressing the use of quantitative and instrumental assessing methods.
2 Methods
The reporting of this systematic review followed the “2020 Preferred Reporting Items for Systematic Reviews and Meta-Analysis” (PRISMA 2020 checklist) (15). A “PECO” strategy was used to state the research question (P: non-specific low back pain; E: exposure to changes in kinematics during walking; C: kinematics in healthy people; O: each kinematic indicator measured through instrumental assessment) (16). The protocol was regularly approved and published in the international prospective register of systematic reviews (PROSPERO, https://www.crd.york.ac.uk/prospero/, registration ID: CRD42023431380).
2.1 Search strategy and eligibility criteria
A systematic search was conducted to establish if kinematics is altered in NS-LBP subjects. Medline (PubMed), Embase, Scopus, Web of Science, and IEEE Xplore databases were consulted up to January 2024 and monthly updates were carried out until January 2025. In addition, we performed cross-referencing to retrieve any possible missing studies, and grey literature was also considered through Google web searching and Google Semantic Scholar. Different search terms and keywords were used, such as “low back pain”, “spinal pain”, “backache”, “kinematics”, “biomechanics”, “gait”, “walking”, “locomotor”, “sensor” and “optoelectronics”. These words were combined differently according to database search criteria. Inclusion criteria for this review were the following: any kind of analytical observation research including case-control, cohort and cross-sectional studies, assessing the trunk and lower limbs kinematics of spontaneous walking (preferred speed) in NS-LBP subjects, and comparing it with that of healthy people. Randomized controlled trials were excluded, as the purpose of this review was not to assess the effectiveness of interventions, but to synthetize evidence on kinematic characteristics in NS-LBP.
We included only studies that evaluated kinematics by using instrumental assessing methods, including—for instance—marker-based optoelectronic systems and wearable sensors. Further considered criteria were adult population (18–70 years old), and English as the main language to ensure homogeneity of data extraction and interpretation, and to allow reproducibility by the scientific community. As for the exclusion criteria, we did not consider studies where walking was assessed on the treadmill or studies dealing with subjects affected by other conditions which could affect the walking performance (e.g., neurological, orthopedic, and rheumatic comorbidities). Due to the intrinsic variability of gait assessment, no restriction in terms of protocol was applied, except for the spontaneity of walking (preferred speed) which had to be present.
2.2 Study selection and data collection
All the records obtained from the search process were managed using “Rayyan, Intelligent Systematic Review” (https://www.rayyan.ai) (17). Title, abstract, and full texts were screened independently by two blinded reviewers (FDF, MF) to identify eligible studies. Any discrepancy was resolved through a consensus with another reviewer (VC). At the time the first screening procedure was conducted (2023), this software had not yet implemented any artificial intelligence-based based features. Details of the study selection stage are reported in the PRISMA 2020 flow diagram (Figure 1). Main characteristics and the major findings of the included studies were extracted in a standardized form and summarized in a table (Table 1) reporting first author name and year of publication, study design, main objective, outcomes, sample size, the technology employed in the gait assessment and the main results obtained according to the aim of this review. The data extraction form was developed and approved by all the authors involved in this study, and a 6-h consensus training was implemented before starting. Once again, the same reviewers independently screened the included studies and resolved any disagreement through a discussion.
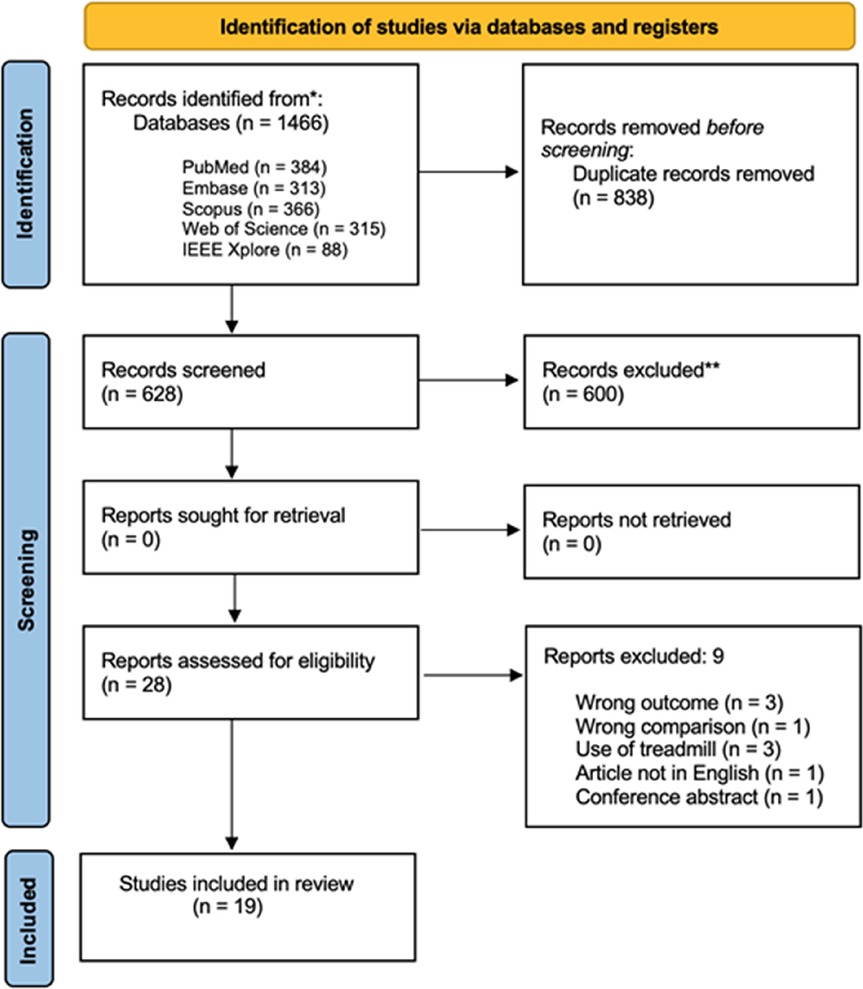
Figure 1. Flow diagram based on PRISMA statement (https://www.prisma-statement.org).
In case of missing data, investigators of the included studies were contacted via email.
2.3 Methodological quality assessment
Considering that all the included studies are attributable to a cross-sectional design (see Results section), the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for Analytical Cross-Sectional Studies was embraced to assess the methodological quality of the included studies (18). Two blinded reviewers (FDF, MF) independently made their assessment by reading the full-texts and a final discussion with a third reviewer (VC) resolved each discrepancy. This tool includes eight questions considering selection criteria, subjects and setting description, exposure and condition measurement, confounding factors identification and management, outcome measures, and statistical analysis. Assessors can give their response considering four possible answers: yes, no, not clear, not applicable.
2.4 Measures and synthesis of results
The primary outcome of this review was the difference in spatial-temporal parameters (e.g., velocity, cadence, stride length, step width, duration of gait cycle), and in trunk and lower limb kinematics (e.g., ROM in frontal, sagittal and transverse plane, motion patterns, coordination, variability and symmetry indexes) during walking between NS-LBP and healthy people.
As measurements of the kinematics parameters, we reported results and differences among groups in a descriptive way, by using mean ± standard deviation (SD) and mean and 95% confidence interval (CI). Results were considered statistically significant when the p-value was reported lower than 0.05.
The meta-analysis was performed using “Review Manager 5.4” (The Nordic Cochrane Center, https://training.cochrane.org/online-learning/core-software-cochrane-reviews/revman). We performed the meta-analysis only when at least two studies—comparable in terms of PECO parameters—investigated at least one of the considered outcomes. We considered the mean difference (MD) along with its 95% confidence interval (CI), calculated using a random-effects model. An effect size ranging from 0.2 to 0.49 is to be considered “small,” from 0.5 to 0.79 “moderate,” and if greater than 0.8, it is “large”. I2 statistics was calculated to measure heterogeneity, explaining how much the variations among studies are attributable to heterogeneity rather than chance. The interpretation of I2 values was realized as follows: 0%–40% “no importance”, range: 30%–60% “moderate”, range: 50%–90% “substantial”, and 75% or above “considerable” (19).
The quality of evidence was rated through the Grades of Recommendation, Assessment, Development and Evaluation (GRADE) method, as suggested by the Updated Cochrane Back Review Group method guidelines. This specific framework provides that it is possible to downgrade the quality of evidence from “high” to “moderate”, “low” or “very low” based on 5 key-domains: risk of bias, inconsistency, indirectness, imprecision, and publication bias (20).
3 Results
3.1 Study selection
Overall, the search strategy identified 1,466 results, 838 of which were duplicates and were consequently removed. An additional 600 records were excluded after screening for title and abstract. A total of 28 full-text articles were evaluated for eligibility and 9 studies were finally rejected with reasons (for further details see Figure 1). Additionally, 10 studies (9, 21–29) were also considered in the meta-analysis, the remaining 9 only in the qualitative summary (11, 30–37).
3.2 Description of the studies
All the included studies presented a cross-sectional design with at least one direct comparison between NS-LBP subjects and healthy people. In 5 studies (26%) the authors implemented three groups, by dividing their sample into specific classes (i.e., obese subjects, subjects presenting lumbar disc herniation, subjects with pronated feet, dancers, and subjects referring leg pain); however, the main comparison focused on the differences between NS-LBP and healthy subjects was always present. The aggregate number of participants was 725 and 410 of these were female (57%); the subjects were almost equally distributed across the studies (sample size median: 38, average: 38.15, IQR: 22–55, range: 20–70). The mean age of the participants was 36.33 ± 9.01 (median: 37, IQR: 32–41, range: 22.4–55.4).
Fourteen (74%) out of the 19 included studies reported data regarding pain intensity or disability levels at baseline. In particular, four studies (21%) only assessed pain intensity through a 0–10 or a 0–100 visual analog scale (VAS) or a 0–10 numeric rating scale (NRS); two studies (11%) investigated the functional status by using the “Oswestry disability index” (ODI) or the “Roland and Morris disability questionnaire” (RMDQ); eight studies (42%) assessed both of these outcomes. The mean pain intensity score of the NS-LBP population recruited in the studies was 4.14 ± 1.33 [range: 2.1–5.7], and the mean disability score was 24.37 ± 9.07 [range: 12.5–40.5].
Seventeen studies (89%) assessed kinematics through the optoelectronic motion analysis system, while two studies (11%) adopted wearable inertial measurement unit (IMU) sensors.
Further details are shown in Table 1.
3.3 Outcomes
All the included studies assessed at least one of the following walking kinematic indicators: spatial-temporal parameters, range of motion, qualitative indexes and motion patterns; each of these variables were considered as the primary outcomes in the current review.
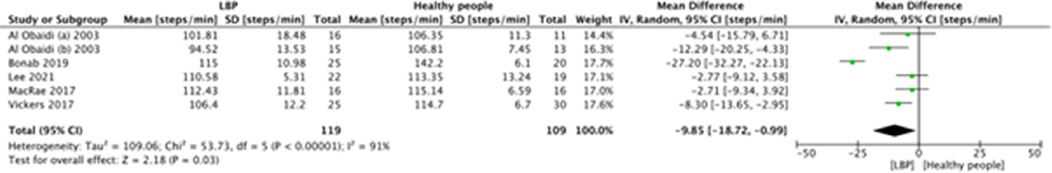
In detail, twelve studies (63%) investigated spatial-temporal parameters; all of these considered the “gait speed” and the “cadence”, five (26%) assessed the “step length” and three (16%) measured the “stride length”. In this context, other considered indicators were step time, step width, single and double support time, and percentages of gait cycle.
Thoracic and lumbar kinematics was investigated in eight works (42%), whereas in eleven studies (58%) the lower limbs’ kinematics was measured for at least one of the following joint complexes: pelvis, hip, knee, and ankle.
Additionally, in eleven studies (58%) only quantitative parameters were retrieved, mostly peak angles and range of motion measurements. Conversely, six authors (26%) qualitatively assessed the walking kinematics by considering coordination, asymmetry, variability, and differences in motion patterns.
3.4 Methodological quality of the included studies
None of the included studies was completely judged free from methodological issues. Four (21%) out of the 19 included studies did not correctly state the inclusion criteria for their sample, and in the other two works (11%) this aspect was not clearly defined (risk of selection and reporting bias). In three studies (16%), we did not find any complete description regarding the sample characteristics or the research setting, and in five studies (26%) we judged this item as “not clear” (risk of selection and reporting bias). In addition, nine studies (47%) did not openly specify to have used objective and standard criteria for the participants’ selection, and in one case (5%) the procedure the authors adopted was judged as critical (risk of selection bias). Then, just one study (5%) identified all the potential confounding factors and only six studies (32%) used sample matching or multivariate analyses in order to manage some possible confounders. Finally, in two studies (11%) not enough details were provided regarding the outcome measurement (risk of detection bias) and in just one study (5%) an appropriate statistical analysis was not used, since only descriptive statistics were performed.
Further details are presented in Table 2.
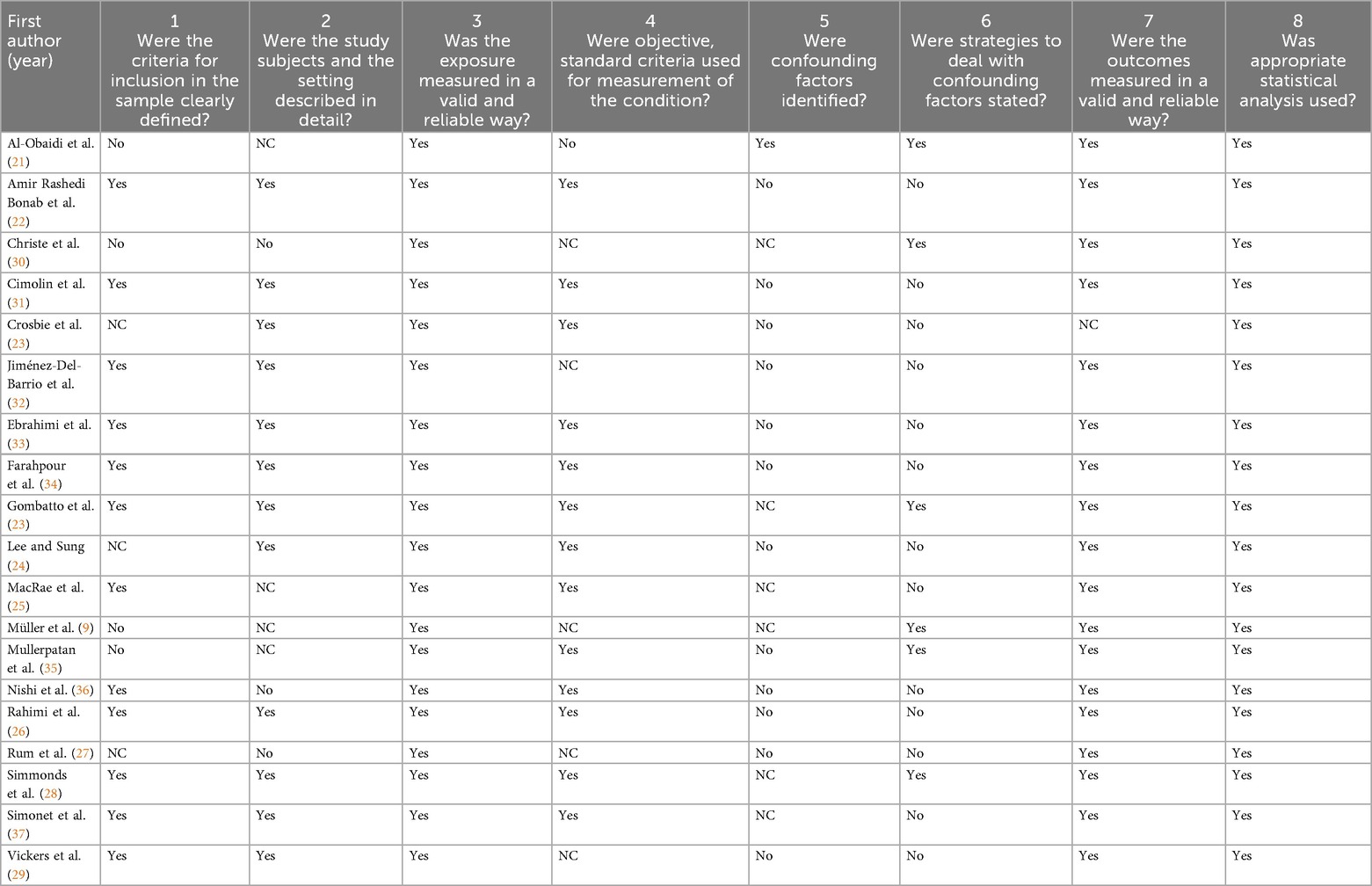
Table 2. Methodological quality of the included studies assessed through the Joanna Briggs Institute (JBI) critical appraisal checklist for analytical cross-sectional studies.
3.5 Description of results
3.5.1 Spatial-temporal parameters
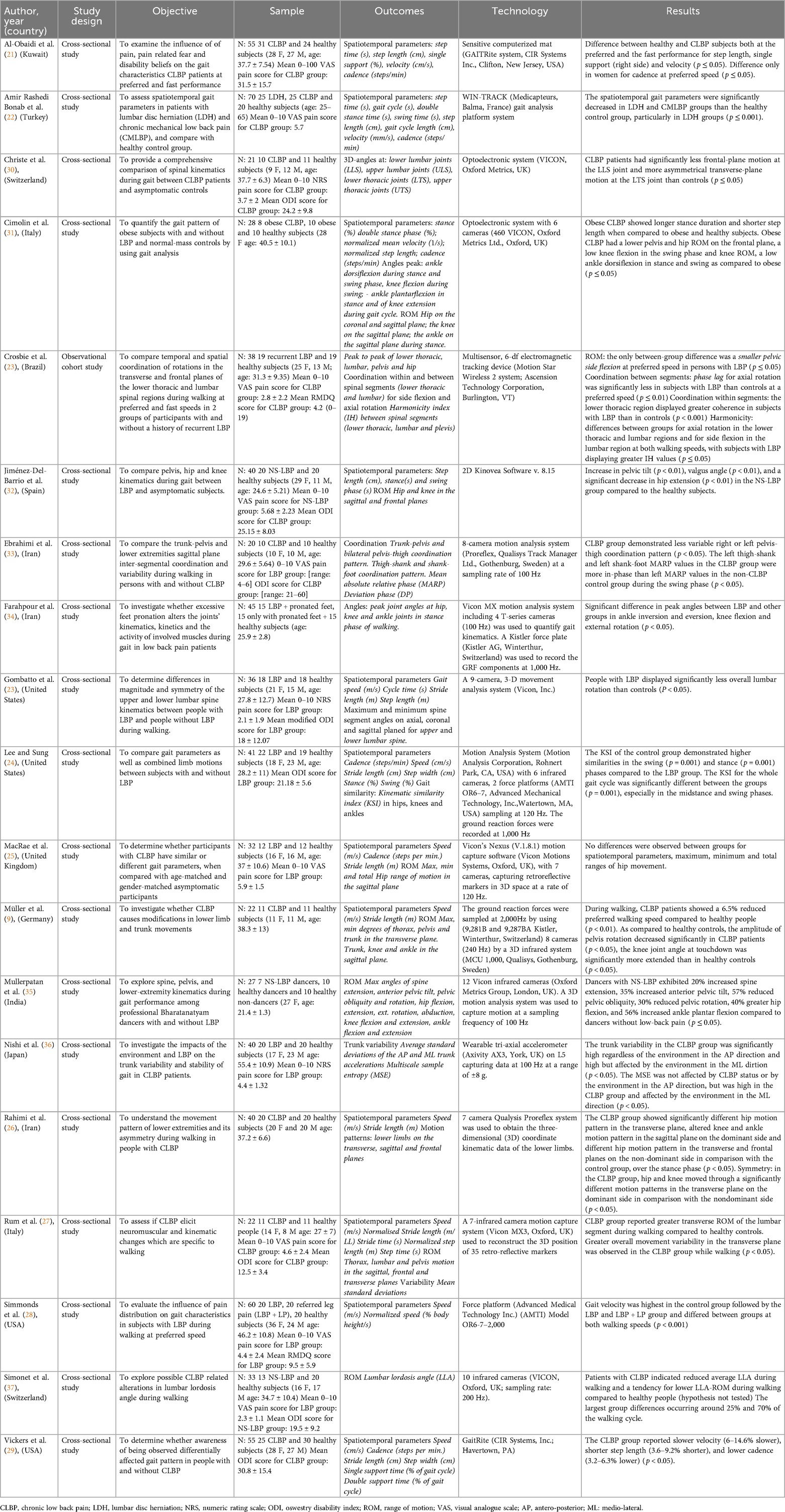
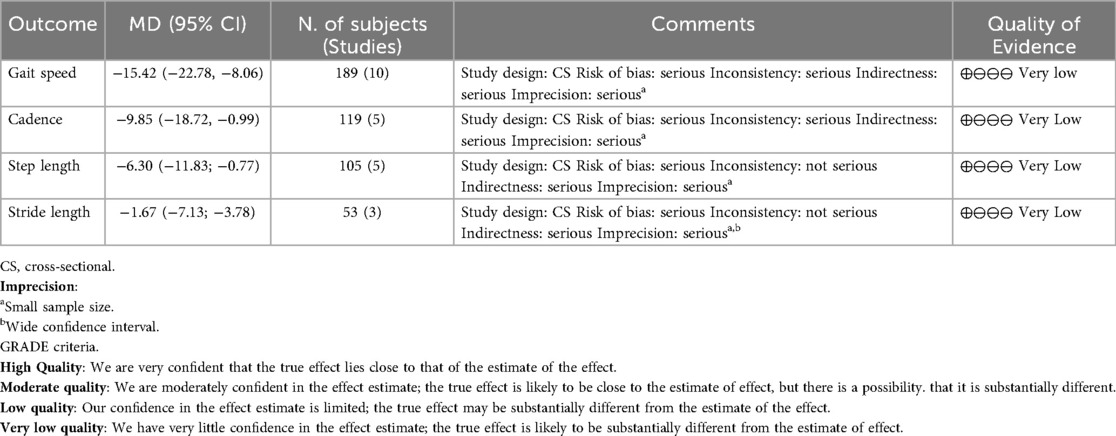
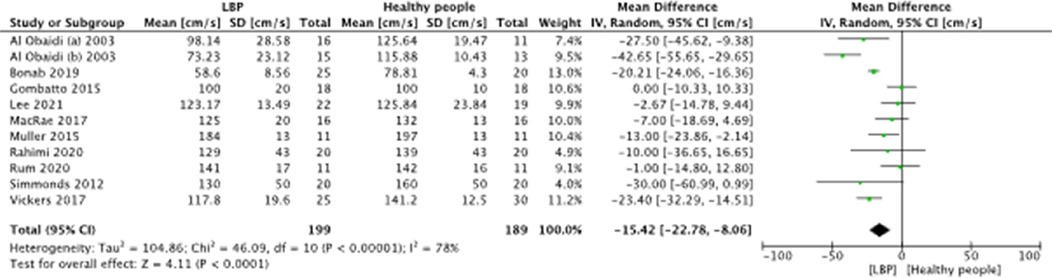
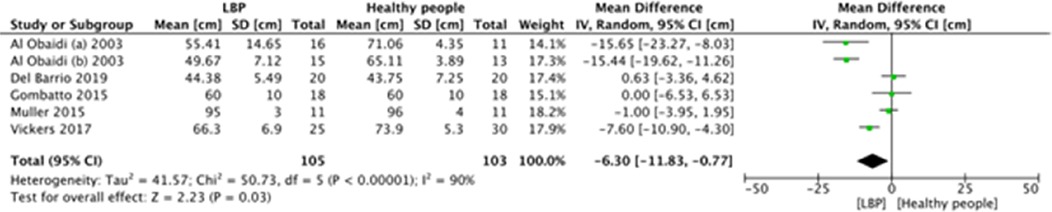
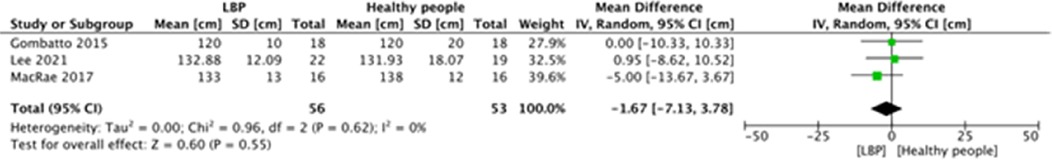
A meta-analysis was possible for the following spatial-temporal parameters: gait speed, cadence, step length, and stride length; the corresponding forest plots are detailed in Figures 2–6. The analyses showed how people with LBP present a lower value of gait velocity with respect to healthy subjects [−15.42 (−22.78, −8.06) cm/s; p ≤ 0.0001], a lower value of cadence [−9.85 (−18.72, −0.99) steps/min; p = 0.03] and a lower value of step length [−6.30 (−11.83; −0.77) cm; p = 0.03]. No significant difference was observed in stride length [ −1.67 (−7.13; 3.78) cm; p = 0.55]. A sensitivity analysis (Figure 3) was performed for gait speed by excluding studies that had not controlled confounding factors, showing similar results to the general comparison [−18.74 (−32.59, −4.88) cm/s; p = 0.008]. The quality of evidence for each of these parameters was rated as “very low”. Further details are reported in Table 3.
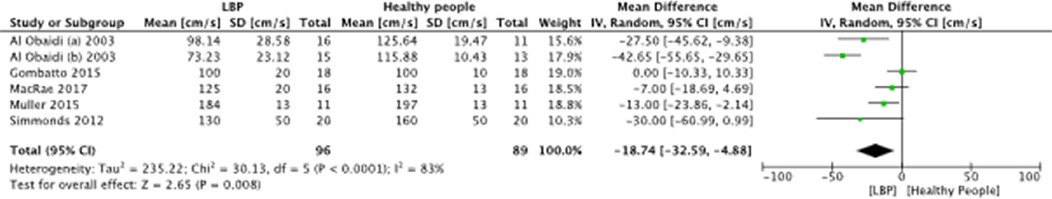
Figure 3. Forest plot of comparison between LBP subjects and healthy people (sensitivity analysis). Gait speed (cm/s.).
Other parameters such as gait cycle time, single and double support time (stance and swing duration) and step width were measured occasionally and in different modalities (in seconds or in percentage of gait cycle), thus a quantitative summary was not possible. To summarize, in two studies (11%) a significant reduction in the single support duration was found in LBP people, only one of these (22) registered a decrease in step time and in gait cycle duration. In the same way, Cimolin et al. (31) detected a longer stance duration and a shorter step length in a population of obese LBP.
Other five studies (26%) assessed gait parameters without finding any significant difference with healthy people; among those, only Lee et al. (25) and Vickers et al. (29) measured step width, and no differences were observed as well.
3.5.2 Quantitative kinematic parameters
As regards motion, a wide heterogeneity in the outcome measures was retrieved and a meta-analysis was not possible. Most of the authors (11 studies, 58%) investigated the range of motion (ROM) on the three different planes, by assessing the total range, minimum or maximum angles, or the difference in the peak angles during specific gait phases. Seven studies (37%) considered these endpoints on the lumbar or the thorax segments of the spine. Christe et al. found an overall less frontal-plane motion at the lower-lumbar segment (p ≤ 0.05) and a more asymmetrical motion in the transverse plane (30), while Gombatto et al. reported less overall rotation (p ≤ 0.05) (23). Conversely, Rum's study found a significant increase in the lumbar motion on the transverse plane (p ≤ 0.05) (27). Mullerpatan et al. reported a 20% increased spine extension in a population of NS-LBP dancers (35), while Simonet et al. pointed out a lower lumbar lordosis angle if compared to healthy subjects (hypotheses not tested by the authors) (37).
Eight studies (42%) assessed the lower limb kinematics, including pelvis, hip, knee, and ankle joints. Among those, two authors found an increased anterior pelvic tilt (p < 0.01) in NS-LBP subjects (32, 35), Muller et al. identified a decrease in pelvis rotation (p ≤ 0.05) (9), while Crosbie detected a smaller pelvic sidebending (p ≤ 0.05) (11). Mullerpatan et al. assessed hip ROM finding greater overall hip flexion (40%) (35), whereas Jiménez-Del-Barrio focused on a reduction in the hip extension (p < 0.01) (32). Farahpour et al. reported significant differences in peak angles in ankle inversion and eversion, and in knee flexion and external rotation (p ≤ 0.05) in a sample of NS-LBP with pronated-feet (34). Muller found a major knee extension at touchdown in NS-LBP people (p ≤ 0.05) (9). Cimolin et al. examined obese NS-LBP subjects, reporting an overall lower pelvis and hip ROM on the frontal plane, a lower knee flexion in the swing phase and a low ankle dorsiflexion in stance and swing, as compared to non-LBP obese people (p ≤ 0.05) (31).
Two studies (11%) did not find any significant difference in the lower limb kinematics (11, 25).
3.5.3 Qualitative parameters for motion assessment
Six studies (32%) qualitatively assessed kinematics, by using different outcome measures; two of those assessed inter-segmental coordination. In detail, Crosbie et al. reported that “phase lag” for axial rotation was significantly less in subjects with LBP than controls at a preferred speed (p ≤ 0.01) and that lower thoracic regions displayed greater coherence (p < 0.001); the same author also calculated the harmonicity index (IH), obtaining differences between groups for axial rotation in the lower thoracic and lumbar regions and for side flexion in the lumbar region, with subjects with LBP displaying greater IH values (p ≤ 0.05) (11). In the same way, Ebrahimi et al. found that LBP subjects demonstrated a less variable pelvis-thigh coordination pattern (p ≤ 0.05) (33); in addition, in LBP subjects the thigh-shank and shank-foot “mean absolute relative phase” (MARP) values were more in-phase than MARP values in healthy people during the swing phase (p ≤ 0.05).
One study (5%) considered the “kinematic similarity index” to assess the lower limb motion asymmetry during gait, finding lower values of similarities in the swing and stance phases in LBP people (p = 0.001). Two studies (11%) reported a greater trunk variability in LBP; in particular, Rum et al. reported an overall increase in the transverse plane (p ≤ 0.05) (27) and Nishi et al. obtained significantly higher values of “average standard deviation” of the antero-posterior and medio-lateral trunk accelerations (p ≤ 0.05) (36). Finally, Rahimi et al. assessed motion patterns, reporting significantly different hip motion patterns in the transverse plane, altered knee and ankle motion patterns in the sagittal plane, and different hip motion patterns in the transverse and frontal planes over the stance phase in the LBP group (p ≤ 0.05) (26).
4 Discussion
This systematic review with meta-analysis is, to our knowledge, the first to investigate gait kinematics in subjects with NS-LBP. In order to present the findings more clearly, we grouped them into two main categories: spatial-temporal parameters and quantitative kinematics.
When compared with healthy individuals, people with NS-LBP consistently showed alterations in the spatial-temporal domain, mainly walking at slower speeds, with reduced cadence and shorter step length. These results were confirmed by sensitivity analysis, where the exclusion of studies not controlling for confounders still supported a reduction in gait speed. Other parameters were less frequently reported, but there are consistent hints of reduced step time and gait cycle duration, findings that seem to extend also to obese NS-LBP subgroups. This suggests that temporal adaptations during gait may be a robust feature of this condition.
In the field of quantitative motion analysis, heterogeneity across outcome measures remains a limiting factor, preventing firm generalizations. Nevertheless, several studies converge in reporting lumbar and pelvic kinematic alterations during walking, especially in the transverse and frontal planes, with additional changes also observed at hip and knee levels. Obese and pronated-feet NS-LBP individuals also displayed ankle motion modifications. Furthermore, gait patterns in NS-LBP appear less coordinated, more asymmetric, and more variable than those of healthy subjects, even though these findings were not always consistent across the literature.
Although some studies did not detect substantial differences (23–28), the majority of available evidence points to altered spatial-temporal parameters in NS-LBP, probably driven by shorter step length and reduced cadence (21). Pain could be a primary driver of these adaptations, as there is evidence that individuals with LBP often take shorter steps on the affected side (38–40), and that pain intensity correlates with temporal gait parameters (22). However, non-physical factors may also play a relevant role, as highlighted by studies reporting the influence of fear-avoidance beliefs, anticipation of pain, anxiety, depression, awareness of being observed, and disability levels (29, 41–45). These results underline the multifactorial nature of gait adaptations in NS-LBP and suggest that future observational research should better disentangle the physical and psycho-behavioral contributors, as well as their interaction.
As discussed above, the most consistent quantitative kinematic finding is the reduction in lumbar spine ROM, particularly in the transverse and frontal planes. This has also been confirmed by studies excluded from our review for methodological reasons (14, 46, 47). The extent of this restriction varies across studies, but focusing on axial rotation, the reported loss ranges between one and four degrees. Such values are biomechanically meaningful (23), since reduced lumbar mobility can compromise dynamic stability and overall gait efficiency. One possible explanation is that NS-LBP subjects stiffen their lower lumbar segments during functional activities such as walking (48). Abnormal EMG activity of the extensor muscles may also contribute (49). Notably, both Christe et al. and Gombatto et al. reported greater restriction in the lower lumbar spine compared with the upper, consistent with the more frequent localization of pain in these segments (23, 30). However, contradictory findings were also observed: Mullerpatan et al. described increased lumbar extension (35), while Simonet et al. reported a lower lordosis angle (37). These discrepancies may reflect specific sample characteristics; for example, Mullerpatan's study involved dancers with LBP, a population known for hyperextension patterns, higher risk of spondylolysis (50), and motor control issues (51).
Regarding lower limb kinematics, results were highly inconsistent, likely due to the heterogeneity of sub-populations (e.g., obese, pronated feet, professional dancers). Nonetheless, decreased pelvic tilt, altered hip and knee excursions, and reduced inter-segmental coordination emerged as recurring patterns, often accompanied by greater asymmetry and variability. These findings may be related to muscle imbalances (52), as weakness, shortening, or elongation of certain muscle groups can affect joint motion and lead to compensatory kinematic changes (53, 54). It remains debated whether such alterations are direct consequences of pain or the result of long-term adaptations over time (55, 56). Clinicians should therefore adopt a comprehensive physical assessment when dealing with NS-LBP patients, considering both segmental deficits and global functional interactions.
Concerning the quality of evidence, only the meta-analyzed outcomes (gait speed, cadence, step length, and stride length) were assessed. All were rated as “very low” because of the observational design, inconsistency, indirectness, and imprecision. Specifically, heterogeneity was evident in gait speed, cadence, and step length, likely due to sample differences in age, BMI, pain intensity, and disability levels, as only a minority of studies matched participants for confounders. Consequently, downgrading for inconsistency and indirectness was required. Finally, sample sizes were generally small (below 400), and for stride length the confidence interval crossed the line of no effect, justifying further downgrading for imprecision.
Taken together, the findings suggest that NS-LBP patients display measurable modifications in both spatial-temporal and lumbo-pelvic kinematics during gait. These changes highlight the need for careful clinical assessment (57) and open the possibility for targeted therapeutic interventions. Specific training programs may help restore mobility and coordination (58), with gait assessment serving as a valuable tool to monitor progress.
Our findings complement previous meta-analyses addressing spinal kinematics across multiple tasks in LBP (59), by narrowing the scope to gait in non-specific LBP and providing a quantitative synthesis of spatio-temporal parameters. This focused approach refines the clinical interpretation of lumbopelvic motion during walking and its implications for assessment and rehabilitation.
Future studies should continue along this research line, both from a technological and clinical perspective. On the technological side, greater efforts should be made to validate and apply wearable solutions, such as IMU-based systems, which could represent a feasible alternative to marker-based optoelectronic devices still predominant in the field. On the clinical side, investigations should focus on clarifying the relative contributions of pain, physical adaptations, and psycho-behavioral factors (e.g., fear-avoidance, anxiety, avoidance strategies) (55). Additionally, more rigorous study designs are needed, including matching for confounders or focusing on specific subgroups of NS-LBP patients, to increase the robustness of findings.
Finally, the relevance of objective motion analysis in NS-LBP should also be acknowledged at the healthcare policy level, to ensure broader access and integration of these methods in clinical practice.
As with any study, this review has limitations. Despite a blinded and comprehensive search strategy, the breadth of the field may have led to some omissions. Moreover, the wide variability of kinematic parameters across studies prevented us from conducting meta-analyses in several domains, resulting in a qualitative synthesis with limited generalizability. As in all systematic reviews, publication bias remains a possibility and cannot be entirely ruled out (60).
5 Conclusions
There is very-low-quality evidence indicating that individuals with NS-LBP walk with reduced gait speed, cadence, and step length, while stride length appears comparable to healthy controls. A decrease in lower lumbar and pelvic motion, particularly in the transverse and frontal planes, is also observed.
Further high-quality studies are needed to strengthen the evidence and clarify whether these kinematic alterations are primarily driven by pain, physical adaptations, or psycho-behavioral factors.
Data availability statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding author.
Author contributions
FDF: Writing – original draft, Visualization, Writing – review & editing, Investigation, Conceptualization, Methodology, Formal analysis, Data curation. NL: Project administration, Supervision, Methodology, Conceptualization, Writing – review & editing, Writing – original draft, Visualization. MF: Formal analysis, Writing – original draft, Data curation, Visualization, Investigation. ES: Visualization, Methodology, Writing – review & editing, Writing – original draft. SC: Visualization, Writing – original draft, Data curation. VC: Supervision, Writing – review & editing, Project administration, Writing – original draft, Methodology, Visualization, Conceptualization.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. GBD 2021 Low Back Pain Collaborators, Ferreira ML, de Luca K, Haile LM, Steinmetz JD, Culbreth GT, Cross M, et al. Global, regional, and national burden of low back pain, 1990–2020, its attributable risk factors, and projections to 2050: a systematic analysis of the global burden of disease study 2021. Lancet Rheumatol. (2023) 5(6):e316–29. doi: 10.1016/S2665-9913(23)00098-X
2. Fatoye F, Gebrye T, Ryan CG, Useh U, Mbada C. Global and regional estimates of clinical and economic burden of low back pain in high-income countries: a systematic review and meta-analysis. Front Public Health. (2023) 11:1098100. doi: 10.3389/fpubh.2023.1098100
3. Hart LG, Deyo RA, Cherkin DC. Physician office visits for low back pain. Frequency, clinical evaluation, and treatment patterns from a U.S. national survey. Spine. (1995) 20:11–9. doi: 10.1097/00007632-199501000-00003
4. Manchikanti L, Singh V, Falco FJ, Benyamin RM, Hirsch JA. Epidemiology of low back pain in adults. Neuromodulation. (2014) 17(Suppl 2):3–10. doi: 10.1111/ner.12018
5. Karayannis NV, Jull GA, Hodges PW. Physiotherapy movement based classification approaches to low back pain: comparison of subgroups through review and developer/expert survey. BMC Musculoskeletal Disord. (2012) 13:24. doi: 10.1186/1471-2474-13-24
6. Christe G, Redhead L, Legrand T, Jolles BM, Favre J. Multi-segment analysis of spinal kinematics during sit-to-stand in patients with chronic low back pain. J Biomechan. (2016) 49(10):2060–7. doi: 10.1016/j.jbiomech.2016.05.015
7. Chaléat-Valayer E, Mac-Thiong JM, Paquet J, Berthonnaud E, Siani F, Roussouly P. Sagittal spino-pelvic alignment in chronic low back pain. Eur Spine J. (2011) 20(Suppl 5):634–40. doi: 10.1007/s00586-011-1931-2
8. Van Dillen LR, Gombatto SP, Collins DR, Engsberg JR, Sahrmann SA. Symmetry of timing of hip and lumbopelvic rotation motion in 2 different subgroups of people with low back pain. Arch Phys Med Rehabil. (2007) 88(3):351–60. doi: 10.1016/j.apmr.2006.12.021
9. Müller R, Ertelt T, Blickhan R. Low back pain affects trunk as well as lower limb movements during walking and running. J Biomech. (2015) 48(6):1009–14. doi: 10.1016/j.jbiomech.2015.01.042
10. Lamoth CJ, Stins JF, Pont M, Kerckhoff F, Beek PJ. Effects of attention on the control of locomotion in individuals with chronic low back pain. J Neuroeng Rehabil. (2008) 5:13. doi: 10.1186/1743-0003-5-13
11. Crosbie J, de Faria Negrão Filho R, Nascimento DP, Ferreira P. Coordination of spinal motion in the transverse and frontal planes during walking in people with and without recurrent low back pain. Spine (Phila Pa 1976). (2013) 38(5):E286–92. doi: 10.1097/BRS.0b013e318281de28
12. van den Hoorn W, Bruijn SM, Meijer OG, Hodges PW, van Dieën JH. Mechanical coupling between transverse plane pelvis and thorax rotations during gait is higher in people with low back pain. J Biomech. (2012) 45(2):342–7. doi: 10.1016/j.jbiomech.2011.10.024
13. Seay JF, Van Emmerik RE, Hamill J. Influence of low back pain status on pelvis-trunk coordination during walking and running. Spine (Phila Pa 1976). (2011) 36(16):E1070–9. doi: 10.1097/BRS.0b013e3182015f7c
14. Dankaerts W, O’Sullivan P, Burnett A, Straker L, Davey P, Gupta R. Discriminating healthy controls and two clinical subgroups of nonspecific chronic low back pain patients using trunk muscle activation and lumbosacral kinematics of postures and movements: a statistical classification model. Spine (Phila Pa 1976). (2009) 34(15):1610–8. doi: 10.1097/BRS.0b013e3181aa6175
15. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J (Clin Res Ed). (2009) 339:b2700. doi: 10.1136/bmj.b2700
16. Morgan RL, Whaley P, Thayer KA, Schünemann HJ. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int. (2018) 121:1027–31. doi: 10.1016/j.envint.2018.07.015
17. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
18. Moola S, Munn Z, Sears K, Sfetcu R, Currie M, Lisy K, et al. Conducting systematic reviews of association (etiology): the joanna briggs institute’s approach. Int J Evid Based Healthcare. (2015) 13(3):163–9. doi: 10.1097/XEB.0000000000000064
19. Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al., editors. Cochrane Handbook for Systematic Reviews of Interventions. 2nd ed. Chichester: John Wiley & Sons (2019).
20. Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. (2008) 336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD
21. Al-Obaidi SM, Al-Zoabi B, Al-Shuwaie N, Al-Zaabie N, Nelson RM. The influence of pain and pain-related fear and disability beliefs on walking velocity in chronic low back pain. Int J Rehabil Res. (2003) 26(2):101–8. doi: 10.1097/00004356-200306000-00004
22. Amir Rashedi Bonab M, Kuru Colak TK, Toktas ZO, Konya D. Assessment of spatiotemporal gait parameters in patients with lumbar disc herniation and patients with chronic mechanical low back pain. Turk Neurosurg. (2020) 30(2):277–84. doi: 10.5137/1019-5149.JTN.27499-19.2
23. Gombatto SP, Brock T, DeLork A, Jones G, Madden E, Rinere C. Lumbar spine kinematics during walking in people with and people without low back pain. Gait Posture. (2015) 42(4):539–44. doi: 10.1016/j.gaitpost.2015.08.010
24. Lee D, Sung P. Gait asymmetry comparison between subjects with and without nonspecific chronic low back pain. Symmetry (Basel). (2021) 13(11):2129. doi: 10.3390/sym13112129
25. MacRae CS, Critchley D, Lewis JS, Shortland A. Comparison of standing postural control and gait parameters in people with and without chronic low back pain: a cross-sectional case-control study. BMJ Open Sport Exerc Med. (2018) 4(1):e000286. doi: 10.1136/bmjsem-2017-000286
26. Rahimi A, Arab AM, Nourbakhsh MR, Hosseini SM, Forghany S. Lower limb kinematics in individuals with chronic low back pain during walking. J Electromyogr Kinesiol. (2020) 51:102404. doi: 10.1016/j.jelekin.2020.102404
27. Rum L, Brasiliano P, Vannozzi G, Laudani L, Macaluso A. Non-specific chronic low back pain elicits kinematic and neuromuscular changes in walking and gait termination. Gait Posture. (2021) 84:238–44. doi: 10.1016/j.gaitpost.2020.12.005
28. Simmonds MJ, Lee CE, Etnyre BR, Morris GS. The influence of pain distribution on walking velocity and horizontal ground reaction forces in patients with low back pain. Pain Res Treat. (2012) 2012:214980. doi: 10.1155/2012/214980
29. Vickers J, Reed A, Decker R, Conrad BP, Olegario-Nebel M, Vincent HK. Effect of investigator observation on gait parameters in individuals with and without chronic low back pain. Gait Posture. (2017) 53:35–40. doi: 10.1016/j.gaitpost.2017.01.002
30. Christe G, Kade F, Jolles BM, Favre J. Chronic low back pain patients walk with locally altered spinal kinematics. J Biomech. (2017) 60:211–8. doi: 10.1016/j.jbiomech.2017.06.042
31. Cimolin V, Vismara L, Galli M, Zaina F, Negrini S, Capodaglio P. Effects of obesity and chronic low back pain on gait. J Neuroeng Rehabil. (2011) 8:55. doi: 10.1186/1743-0003-8-55
32. Jiménez-Del-Barrio S, Mingo-Gómez MT, Estébanez-de-Miguel E, Saiz-Cantero E, Del-Salvador-Miguélez AI, Ceballos-Laita L. Adaptations in pelvis, hip and knee kinematics during gait and muscle extensibility in low back pain patients: a cross-sectional study. J Back Musculoskelet Rehabil. (2020) 33(1):49–56. doi: 10.3233/BMR-191528
33. Ebrahimi S, Kamali F, Razeghi M, Haghpanah SA. Comparison of the trunk-pelvis and lower extremities sagittal plane inter-segmental coordination and variability during walking in persons with and without chronic low back pain. Hum Mov Sci. (2017) 52:55–66. doi: 10.1016/j.humov.2017.01.004
34. Farahpour N, Jafarnezhadgero A, Allard P, Majlesi M. Muscle activity and kinetics of lower limbs during walking in pronated feet individuals with and without low back pain. J Electromyogr Kinesiol. (2018) 39:35–41. doi: 10.1016/j.jelekin.2018.01.006
35. Mullerpatan R, Bharnuke JK, Hiller CE. Gait kinematics of bharatanatyam dancers with and without low back pain. Crit Rev Phys Rehabil Med. (2019) 31:1–10. doi: 10.1615/CritRevPhysRehabilMed.2019030243
36. Nishi Y, Shigetoh H, Fujii R, Osumi M, Morioka S. Changes in trunk variability and stability of gait in patients with chronic low back pain: impact of laboratory versus daily-living environments. J Pain Res. (2021) 14:1675–86. doi: 10.2147/JPR.S310775
37. Simonet E, Winteler B, Frangi J, Suter M, Meier ML, Eichelberger P, et al. Walking and running with non-specific chronic low back pain: what about the lumbar lordosis angle? J Biomech. (2020) 108:109883. doi: 10.1016/j.jbiomech.2020.109883
38. Khodadadeh S, Eisenstein SM. Gait analysis of patients with low back pain before and after surgery. Spine (Phila Pa 1976). (1993) 18(11):1451–5. doi: 10.1097/00007632-199318110-00008
39. Simmonds Maureen J, Claveau Y. Measures of pain and physical function in patients with low back pain Physiother Theory Pract. (1997) 13:53–65. doi: 10.3109/09593989709036448
40. Taylor S, Frost H, Taylor A, Barker K. Reliability and responsiveness of the shuttle walking test in patients with chronic low back pain. Physiother Res Int. (2001) 6(3):170–8. doi: 10.1002/pri.225
41. Al-Obaidi SM, Nelson RM, Al-Awadhi S, Al-Shuwaie N. The role of anticipation and fear of pain in the persistence of avoidance behavior in patients with chronic low back pain. Spine. (2000) 25(9):1126–31. doi: 10.1097/00007632-200005010-00014
42. Crombez G, Vervaet L, Baeyens F, Lysens R, Eelen P. Do pain expectancies cause pain in chronic low back patients? A clinical investigation. Behav Res Ther. (1996) 34(11–12):919–25. doi: 10.1016/s0005-7967(96)00049-6
43. Crombez G, Vlaeyen JW, Heuts PH, Lysens R. Pain-related fear is more disabling than pain itself: evidence on the role of pain-related fear in chronic back pain disability. Pain. (1999) 80(1–2):329–39. doi: 10.1016/s0304-3959(98)00229-2
44. Etemadi Y, Salavati M, Arab AM, Ghanavati T. Balance recovery reactions in individuals with recurrent nonspecific low back pain: effect of attention. Gait Posture. (2016) 44:123–7. doi: 10.1016/j.gaitpost.2015.11.017
45. Hamacher D, Hamacher D, Schega L. A cognitive dual task affects gait variability in patients suffering from chronic low back pain. Exp Brain Res. (2014) 232(11):3509–13. doi: 10.1007/s00221-014-4039-1
46. Mitchell T, O’Sullivan PB, Burnett AF, Straker L, Smith A. Regional differences in lumbar spinal posture and the influence of low back pain. BMC Musculoskeletal Disord. (2008) 9:152. doi: 10.1186/1471-2474-9-152
47. Gombatto SP, Collins DR, Sahrmann SA, Engsberg JR, Van Dillen LR. Patterns of lumbar region movement during trunk lateral bending in 2 subgroups of people with low back pain. Phys Ther. (2007) 87(4):441–54. doi: 10.2522/ptj.20050370
48. van der Hulst M, Vollenbroek-Hutten MM, Rietman JS, Schaake L, Groothuis-Oudshoorn KG, Hermens HJ. Back muscle activation patterns in chronic low back pain during walking: a “guarding” hypothesis. Clin J Pain. (2010) 26(1):30–7. doi: 10.1097/AJP.0b013e3181b40eca
49. Steele J, Bruce-Low S, Smith D, Jessop D, Osborne N. Lumbar kinematic variability during gait in chronic low back pain and associations with pain, disability and isolated lumbar extension strength. Clin Biomech. (2014) 29(10):1131–8. doi: 10.1016/j.clinbiomech.2014.09.013
50. Gottschlich LM, Young CC. Spine injuries in dancers. Curr Sports Med Rep. (2011) 10(1):40–4. doi: 10.1249/JSR.0b013e318205e08b
51. Roussel N, De Kooning M, Schutt A, Mottram S, Truijen S, Nijs J, et al. Motor control and low back pain in dancers. Int J Sports Med. (2013) 34(2):138–43. doi: 10.1055/s-0032-1321722
52. Nadler SF, Malanga GA, Feinberg JH, Prybicien M, Stitik TP, DePrince M. Relationship between hip muscle imbalance and occurrence of low back pain in collegiate athletes: a prospective study. Am J Phys Med Rehabil. (2001) 80(8):572–7. doi: 10.1097/00002060-200108000-00005
53. Pizol GZ, Ferro Moura Franco K, Cristiane Miyamoto G, Maria Nunes Cabral C. Is there hip muscle weakness in adults with chronic non-specific low back pain? A cross-sectional study. BMC Musculoskeletal Disord. (2023) 24(1):798. doi: 10.1186/s12891-023-06920-x
54. Vatovec R, Voglar M. Changes of trunk muscle stiffness in individuals with low back pain: a systematic review with meta-analysis. BMC Musculoskeletal Disord. (2024) 25(1):155. doi: 10.1186/s12891-024-07241-3
55. Waddell G, Newton M, Henderson I, Somerville D, Main CJ. A fear-avoidance beliefs questionnaire (FABQ) and the role of fear-avoidance beliefs in chronic low back pain and disability. Pain. (1993) 52(2):157–68. doi: 10.1016/0304-3959(93)90127-B
56. Goubert D, Oosterwijck JV, Meeus M, Danneels L. Structural changes of lumbar muscles in non-specific low back pain: a systematic review. Pain Physician. (2016) 19(7):E985–1000.27676689
57. Knezevic NN, Candido KD, Vlaeyen JWS, Van Zundert J, Cohen SP. Low back pain. Lancet. (2021) 398(10294):78–92. doi: 10.1016/S0140-6736(21)00733-9
58. Wirth B, Schweinhardt P. Personalized assessment and management of non-specific low back pain. Eur J Pain. (2024) 28(2):181–98. doi: 10.1002/ejp.2190
59. Errabity A, Calmels P, Han WS, Bonnaire R, Pannetier R, Convert R, et al. The effect of low back pain on spine kinematics: a systematic review and meta-analysis. Clinical Biomech. (2023) 108:106070. doi: 10.1016/j.clinbiomech.2023.106070
Keywords: low back pain, kinematics, gait analysis, gait speed, sensors, walking, IMU, optoelectronic
Citation: Dal Farra F, Lopomo NF, Fascia M, Scalona E, Cerfoglio S and Cimolin V (2025) How non-specific low back pain affects gait kinematics: a systematic review and meta-analysis. Front. Pain Res. 6:1693068. doi: 10.3389/fpain.2025.1693068
Received: 26 August 2025; Accepted: 28 October 2025;
Published: 20 November 2025.
Edited by:
paola Sandroni, Mayo Clinic, United StatesReviewed by:
Jerome Molimard, Centre Ingénierie et Santé, Ecole des Mines de Saint-Étienne, FranceOnur Seçgin Nişanci, Kafkas Universitesi Tip Fakultesi, Türkiye
Copyright: © 2025 Dal Farra, Lopomo, Fascia, Scalona, Cerfoglio and Cimolin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Serena Cerfoglio, c2VyZW5hLmNlcmZvZ2xpb0Bwb2xpbWkuaXQ=
 Fulvio Dal Farra
Fulvio Dal Farra Nicola Francesco Lopomo
Nicola Francesco Lopomo Matteo Fascia1
Matteo Fascia1 Emilia Scalona
Emilia Scalona Serena Cerfoglio
Serena Cerfoglio Veronica Cimolin
Veronica Cimolin