- 1Shanghai Key Laboratory of Magnetic Resonance, Department of Physics, School of Physics and Materials Science, East China Normal University, Shanghai, China
- 2Department of Developmental and Behavioral Pediatrics, Shanghai Institute of Pediatric Translational Medicine, Shanghai Children’s Medical Center, Shanghai Jiao Tong University School of Medicine, Shanghai, China
- 3MOE-Shanghai Key Laboratory of Children’s Environmental Health, Shanghai Jiao Tong University School of Medicine, Shanghai, China
Objective: Primary monosymptomatic nocturnal enuresis (PMNE) refers to bed-wetting in children who have no other lower urinary tract symptoms and are never dry for more than 6 months. Our previous studies demonstrated that children with PMNE exhibited brain functional abnormalities compared with healthy controls; however, researches on the abnormalities in gray matter were limited. This study aimed to investigate brain structural changes in gray matter of children with PMNE using magnetic resonance imaging (MRI).
Methods: Gray matter volumes (GMVs) and gyrification indices (GIs) were calculated using voxel-based and surface-based morphometry analyses of structural MRI data acquired from 26 children with PMNE and 28 healthy children. To identify between-group differences in gray matter, two-sample t-tests were conducted on GMV and GI images separately.
Results: Compared with the controls, children with PMNE showed significantly increased GMVs in the supplementary motor area and medial prefrontal cortex regions (mean GMV in PMNE: 0.54 ± 0.07 l; mean GMV in controls: 0.50 ± 0.06 l) and reduced GIs in the right precuneus (mean GI in PMNE: 25.74° ± 2.34°; mean GI in controls: 27.97° ± 1.79°).
Conclusion: Children with PMNE showed abnormal GMVs in frontal lobe and GIs in precuneus, and these changes might be involved in the pathological mechanism of PMNE.
Introduction
Enuresis, defined as children aged 5 years or older who wet the bed at least twice a week for three consecutive months, is not induced by any substance/polyuria/general medical condition according to the Diagnostic and Statistical Manual of Mental Disorders (5th edition). Enuresis is common in childhood, and its prevalence was reported as nearly 10% in 7-year-old children and 2% in 12-year-old children (1–3). As the dominant subtype, primary monosymptomatic nocturnal enuresis (PMNE) refers to bed-wetting in children who have no other lower urinary tract symptoms and are never dry for more than 6 months (4, 5). In addition to bed-wetting, children with enuresis usually suffer from low self-esteem, poor school performance, and low life quality, which affect their development (6–9).
The causes of enuresis are mainly related to genetics, nocturnal polyuria, nocturnal detrusor overactivity, high arousal thresholds, and disturbed central nervous system mechanisms (10, 11). Enuretic children can more easily fill their bladder due to increased urine output and decreased bladder available capacity but are more difficult to wake up; involuntary voiding is inhibited mainly by the brainstem and is controlled by some superior regions such as the thalamus, supplementary motor area (SMA), anterior cingulate cortex, and prefrontal cortex (PFC) (12, 13).
In recent years, magnetic resonance imaging (MRI) has been used to study enuresis, revealing significant functional changes between children with and without enuresis. Yu et al. demonstrated that the elevated oxygen extraction fraction values in primary nocturnal enuresis were positively correlated with the difficulty of arousal (14). Furthermore, studies using task functional MRI associated with working memory and response inhibition, respectively, identified that enuretic children exhibited different patterns (15–17). In addition, resting-state functional MRI studies have found that children with PMNE exhibit not only local spontaneous brain activity changes but also brain network alterations, compared with the controls (4, 18, 19).
Furthermore, a few studies focusing on structural changes in enuresis have also been performed. By using diffusion tensor images of children with PMNE, we previously demonstrated that some brain regions related to micturition control, such as the thalamus, medial frontal cortex, and anterior cingulate cortex, exhibited significant microstructural abnormalities (20). Yu et al. found that decreased gray matter (GM) densities may be involved in memory/attention deficits in children with primary nocturnal enuresis (21).
Considering that the function of the human brain is always associated with its structure and researches on the GM abnormalities of PMNE are limited, we hypothesize that these children may have brain structural abnormalities related to micturition control or arousal mechanisms. Examples of these changes include altered gray matter volume (GMV) and folding, and they may be associated with the pathological mechanisms of enuresis. Voxel-based morphometry (VBM) and surface-based morphometry (SBM) analyses, for example, calculating GMV and gyrification index (GI; based on the absolute values of mean curvature of the cerebral cortical surface), are beneficial for measuring structural abnormalities (specifically in GM) in patients without a prior hypothesis (22, 23). Thus, this study used VBM and SBM analyses to evaluate GM brain structural alterations in children with PMNE.
Materials and Methods
Participants
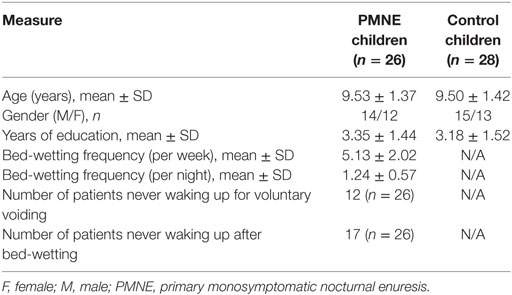
In total, 26 children with PMNE and 28 generally age- and gender-matched healthy controls participated in this study (Table 1). All the participants were from primary and junior middle schools in Shanghai and the surrounding areas. The patients were outpatients from Shanghai Children Medical Center who were diagnosed by Doctor Ma. The PMNE inclusion criteria were as follows: bed-wetting during the night but asymptomatic during the day time; frequency of twice per week or more for three consecutive months; never dry for more than 6 months; symptoms not caused by any related diseases or medicine; and no other lower urinary tract diseases. Healthy volunteers, recruited using advertisements, had not wet the bed since 5 years of age. Children with any other neurological diseases (such as attention deficit/hyperactivity disorder and autism), internal implant metals, or claustrophobia were excluded prior to MR scanning. All children achieved IQ test scores greater than 80 (Wechsler Intelligence Scale for Children-Revised) and were right handed. This study was carried out in accordance with the recommendations from the East China Normal University Committee on Human Research Protection. Both the children and their parents signed informed consent forms.
MRI Protocols
Magnetic resonance imaging data were collected at the Shanghai Key Laboratory of Magnetic Resonance (East China Normal University, Shanghai, China) using a 3.0 Tesla Siemens Trio Tim system (Siemens, Erlangen, Germany). Custom-fit foam pads were placed around the participants’ heads to minimize motion, and a 12-channel head coil was used to scan. Whole-brain anatomical volumes were obtained using a high-resolution T1-weighted three-dimensional magnetization-prepared rapid acquisition gradient echo pulse sequence (repetition time = 1,900 ms, echo time = 3.42 ms, inversion time = 900 ms, field of view = 240 mm2 × 240 mm2, acquisition matrix = 256 × 256, slice thickness = 1 mm, 192 slices).
VBM and SBM Analyses
Voxel-based morphometry and SBM analyses were performed using the Computational Anatomy Toolbox (CAT121; Structural Brain Mapping Group, Jena University Hospital, Jena, Germany), which is an extension of Statistical Parametric Mapping software (SPM122; Wellcome Trust Centre for Neuroimaging, University College London, United Kingdom). Briefly, preprocessing included bias correction, normalization to the standard MRI space using the DARTEL-algorithm, and segmentation into GM/whiter matter/cerebrospinal fluid. Considering that the subjects were children rather than adults, preprocessing was optimized based on recommendations in the CAT12 toolbox manual. All the T1-weighted images were initially segmented using a customized tissue probability map (TPM) created with Template-O-Matic Toolbox (TOM83; Imaging Research Center, Cincinnati Children’s Hospital Medical Center, OH, USA) and normalized to a customized DARTEL-template created with SPM12. Customized DARTEL template and TPM can reflect the age and gender of a specific population. Next, data quality was evaluated by displaying one slice for all the images and calculating the sample homogeneity. Finally, the modulated normalized GM images were smoothed with a Gaussian filter [8-mm full width at half maximum (FWHM)]. In addition, total intracranial volume was estimated for further analyses.
The central cortical surface for left and right hemisphere in each participant was created on the basis of the tissue segmentation using a fully automated SBM method provided by the CAT12 toolbox (23). GIs were extracted based on the absolute mean curvature from each vertex of the cortical surface mesh, and then GI images were smoothed with a FWHM kernel of 15 mm.
Statistics Analyses
Smoothed GM and GI images across all the participants (26 patients and 28 controls) were entered into group whole-brain analysis. Two-sample t-tests based on general linear model implemented in SPM12 were conducted to detect which brain regions showed between-group differences of GMV and cortical folding separately, using age and gender as covariates. In GMV analysis, total intracranial volume was also included as a covariate to avoid different brain sizes confounding the results, and threshold masking was set to an absolute value of 0.2. All the statistical results were assigned thresholds at voxel-level uncorrected p < 0.005 and cluster-level FWE corrected p < 0.05.
In addition, the GMV and the GI in the peak coordinates across all the participants were extracted from the GM images and the GI images, respectively, and the peak coordinates were acquired from the results of whole-brain analysis. The mean GMV and GI of each group were calculated.
Results
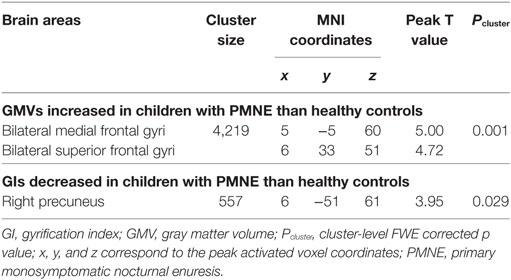
Whole-brain GMV analysis revealed a cluster of higher GMVs in children with PMNE compared to controls. The cluster included the SMA and medial PFC regions. GIs in the right precuneus of children with PMNE were significantly reduced compared with those in healthy controls. Only clusters surpassing thresholds at voxel-level uncorrected p < 0.005 and cluster-level FWE corrected p < 0.05 are shown in Table 2 and Figure 1. The mean GMVs of SMA/PFC cluster were 0.54 ± 0.07 l (PMNE) and 0.50 ± 0.06 l (controls); the mean GIs of precuneus were 25.74° ± 2.34° (PMNE) and 27.97° ± 1.79° (controls).
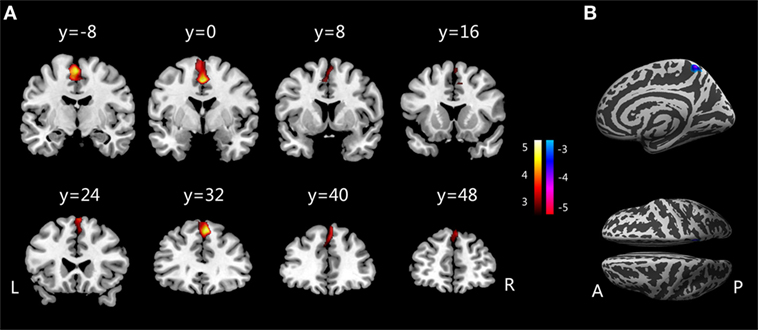
Figure 1. (A) Increased gray matter volumes in children with PMNE compared with healthy controls. (B) Decreased gyrification indices in children with PMNE compared with healthy controls. A, anterior; L, left; P, posterior; PMNE, primary monosymptomatic nocturnal enuresis; R, right. The color scale indicates the t-values.
Discussion
This study assessed changes in brain GM in children with PMNE using VBM and SBM analyses, revealing that these children exhibited significantly altered GMVs and GIs in their SMA/medial PFC and precuneus regions.
Compared with the healthy controls, children with PMNE showed increased GMVs in their SMA and medial PFC regions. The SMA plays important roles in controlling movement, especially in preparation for voluntary actions (24), and contributes to pelvic floor and urethral sphincter contraction (25, 26). The SMA was also found to be strongly connected with the thalamus (27), which is essential for switching between sleep and wakefulness and relaying sensory and motor signals to the cerebral cortex for further processing and is activated during bladder voiding initiation and filling (12, 28). Bladder filling or voiding is controlled not only by the midbrain and brainstem but also by superior regions. The SMA, as reviewed by Griffiths, is associated with the emotional aspects of bladder control during the storage phase (29). When a person had an urgent need to void during contraction of the urethral sphincter to delay leakage, the SMA would receive signals relayed via the thalamus to evoke “urgency” and relax the bladder to prevent incontinence (12, 24). The SMA in enuretic children was activated with urgency during bladder filling in their sleep, but these children failed in their attempt to tighten the urethral sphincter, ultimately wetting the bed.
The PFC is a part of the frontal lobe located anterior to the SMA and has been associated with executive control, decision-making, and controlling voluntary micturition (12). The medial PFC was deactivated upon bladder filling and activated during voiding (26, 30), and people with GM lesions in their medial frontal cortex suffered from micturition disturbances (31). Our results showed that GMVs in children with PMNE were increased in the medial PFC. This region predominantly has an inhibitory effect on controlling micturition in the brain–bladder network, as proposed by Griffiths and Fowler (12, 32). In the normal continence mechanism, the medial PFC connects with the periaqueductal gray directly and indirectly and may enhance inhibition of the brainstem switch. Thus, abnormalities in the medial PFC in children with PMNE may influence their decision to void at night. Furthermore, this result was supported by our previous finding using resting-state functional MRI that decreased amplitude of low-frequency fluctuation and regional homogeneity were present in the medial frontal lobe in children with PMNE (18). Yu et al. had reported that the GM density of enuretic children changed when compared with healthy children in the dorsolateral PFC as well. Therefore, the altered SMA and medial PFC volumes in children with PMNE were in accordance with reported deficits in urinary continence and may be involved in the pathological mechanism of enuresis (18, 26, 29, 31).
Children with PMNE showed decreased GIs in their precuneus, which was consistent with our previous finding that PMNE children exhibited altered regional homogeneity in their precuneus compared with healthy controls (18). Children with PMNE are considered “deep but poor” sleepers due to their high arousal thresholds and frequently disturbed sleep (10). These children are very difficult to wake from sleep and do not void involuntarily during the day. Thus, it is apparent that disturbed sleep and arousal mechanisms exist in PMNE children, and they may not be switched between sleep and wakefulness conscious states (14, 33). The precuneus is regarded as a key brain area for conscious information processing (34, 35). Previous studies found that the precuneus became less active in decreased conscious states, such as sleep and vegetative states (36), and a study on vegetation also reported that the precuneus was reactivated once consciousness returned (37). In response to full bladder sensations, normal children awaken from sleep to avoid wetting bed, while enuretic children often fail to do so, which is attributed to their arousal deficit. Therefore, we considered that structural alterations in the precuneus of children with PMNE might implicate their inability to switch from sleep to wakefulness when confronted with a full bladder at night. Although there might be arousal deficits in the patients of this study, considering the lack of support from sleep/arousal studies, it needs more evidence to demonstrate the connections between abnormal precuneus and their difficult arousal.
While we found significant structural alterations in children with PMNE, our study has several limitations. First, the sample size of children with PMNE was relatively small, and our results should be verified using a larger sample in a subsequent study. Furthermore, it is known that the prevalence rate of PMNE decreases with age (1), and 2–3 years following study would be useful to investigate abnormal development in the brains of children with PMNE (38, 39).
In conclusion, this study evaluated the structural abnormalities of GM in children with PMNE using VBM and SBM, and the alterations in children with PMNE were mainly located in the SMA/medial PFC and precuneus regions. The underlying pathophysiological relations between these alterations in GM and clinical symptoms in PMNE (e.g., deficits in urinary continence, high arousal threshold) need to be further clarified.
Ethics Statement
This study was carried out in accordance with the recommendations from the East China Normal University Committee on Human Research Protection. Both the children and their parents signed informed consent forms.
Author Contributions
XD, MW, and JM designed the study. MW, AZ, HL, SX, and ZQ acquired the data. MW and JZ analyzed the data. MW and XD interpreted the data and drafted the manuscript. XD and JM revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This research was supported by grants from the National Natural Science Foundation of China (81571658), the Social Science Foundation of China (15ZDB016), and the Science and Technology Committee of Shanghai City (14411969200 and 16411952800).
Footnotes
- ^http://dbm.neuro.uni-jena.de/cat/ (Accessed: May 27, 2017).
- ^http://www.fil.ion.ucl.ac.uk/spm/software/spm12 (Accessed: May 27, 2017).
- ^http://dbm.neuro.uni-jena.de/software/tom (Accessed: May 27, 2017).
References
1. Wen JG, Wang QW, Chen Y, Wen JJ, Liu K. An epidemiological study of primary nocturnal enuresis in Chinese children and adolescents. Eur Urol (2006) 49:1107–13. doi:10.1016/j.eururo.2005.11.011
2. Vande Walle J, Rittig S, Bauer S, Eggert P, Marschall-Kehrel D, Tekgul S, et al. Practical consensus guidelines for the management of enuresis. Eur J Pediatr (2012) 171:971–83. doi:10.1007/s00431-012-1687-7
3. Buckley BS, Lapitan MC; Epidemiology Committee of the Fourth International Consultation on Incontinence, Paris, 2008. Prevalence of urinary incontinence in men, women, and children – current evidence: findings of the Fourth International Consultation on Incontinence. Urology (2010) 76:265–70. doi:10.1016/j.urology.2009.11.078
4. Lei D, Ma J, Zhang J, Wang M, Zhang K, Chen F, et al. Connectome-scale assessments of functional connectivity in children with primary monosymptomatic nocturnal enuresis. Biomed Res Int (2015) 2015:463708. doi:10.1155/2015/463708
5. Austin PF, Bauer SB, Bower W, Chase J, Franco I, Hoebeke P, et al. The standardization of terminology of lower urinary tract function in children and adolescents: update report from the standardization committee of the International Children’s Continence Society. Neurourol Urodyn (2016) 35:471–81. doi:10.1002/nau.22751
6. Bachmann C, Lehr D, Janhsen E, Sambach H, Muehlan H, von Gontard A, et al. Health related quality of life of a tertiary referral center population with urinary incontinence using the DCGM-10 questionnaire. J Urol (2009) 182:2000–6. doi:10.1016/j.juro.2009.03.065
7. Kuwertz-Broking E, von Gontard A. Clinical management of nocturnal enuresis. Pediatr Nephrol (2017) 1–10. doi:10.1007/s00467-017-3778-1
8. Arda E, Cakiroglu B, Thomas DT. Primary nocturnal enuresis: a review. Nephrourol Mon (2016) 8:e35809. doi:10.5812/numonthly.35809
9. Sa CA, Gusmao Paiva AC, de Menezes MC, de Oliveira LF, Gomes CA, de Figueiredo AA, et al. Increased risk of physical punishment among enuretic children with family history of enuresis. J Urol (2016) 195:1227–30. doi:10.1016/j.juro.2015.11.022
10. Neveus T. Pathogenesis of enuresis: towards a new understanding. Int J Urol (2017) 24:174–82. doi:10.1111/iju.13310
11. Von Gontard A, Schmelzer D, Seifen S, Pukrop R. Central nervous system involvement in nocturnal enuresis: evidence of general neuromotor delay and specific brainstem dysfunction. J Urol (2001) 166:2448–51. doi:10.1016/S0022-5347(05)65613-3
12. Griffiths DJ, Fowler CJ. The micturition switch and its forebrain influences. Acta Physiol (Oxf) (2013) 207:93–109. doi:10.1111/apha.12019
13. Kitta T, Mitsui T, Kanno Y, Chiba H, Moriya K, Shinohara N. Brain-bladder control network: the unsolved 21st century urological mystery. Int J Urol (2015) 22:342–8. doi:10.1111/iju.12721
14. Yu B, Huang M, Zhang X, Ma H, Peng M, Guo Q. Noninvasive imaging of brain oxygen metabolism in children with primary nocturnal enuresis during natural sleep. Hum Brain Mapp (2017) 38:2532–9. doi:10.1002/hbm.23538
15. Zhang K, Ma J, Lei D, Wang M, Zhang J, Du X. Task positive and default mode networks during a working memory in children with primary monosymptomatic nocturnal enuresis and healthy controls. Pediatr Res (2015) 78:422–9. doi:10.1038/pr.2015.120
16. Yu B, Guo Q, Fan G, Ma H, Wang L, Liu N. Evaluation of working memory impairment in children with primary nocturnal enuresis: evidence from event-related functional magnetic resonance imaging. J Paediatr Child Health (2011) 47:429–35. doi:10.1111/j.1440-1754.2010.02000.x
17. Lei D, Ma J, Du X, Shen G, Tian M, Li G. Altered brain activation during response inhibition in children with primary nocturnal enuresis: an fMRI study. Hum Brain Mapp (2012) 33:2913–9. doi:10.1002/hbm.21411
18. Lei D, Ma J, Du X, Shen G, Tian M, Li G. Spontaneous brain activity changes in children with primary monosymptomatic nocturnal enuresis: a resting-state fMRI study. Neurourol Urodyn (2012) 31:99–104. doi:10.1002/nau.21205
19. Yu B, Sun H, Ma H, Peng M, Kong F, Meng F, et al. Aberrant whole-brain functional connectivity and intelligence structure in children with primary nocturnal enuresis. PLoS One (2013) 8:e51924. doi:10.1371/journal.pone.0051924
20. Lei D, Ma J, Shen X, Du X, Shen G, Liu W, et al. Changes in the brain microstructure of children with primary monosymptomatic nocturnal enuresis: a diffusion tensor imaging study. PLoS One (2012) 7:e31023. doi:10.1371/journal.pone.0031023
21. Yu B, Kong F, Peng M, Ma H, Liu N, Guo Q. Assessment of memory/attention impairment in children with primary nocturnal enuresis: a voxel-based morphometry study. Eur J Radiol (2012) 81:4119–22. doi:10.1016/j.ejrad.2012.01.006
22. Zhang J, Wu YL, Su J, Yao Q, Wang M, Li GF, et al. Assessment of gray and white matter structural alterations in migraineurs without aura. J Headache Pain (2017) 18:74. doi:10.1186/s10194-017-0783-5
23. Luders E, Thompson PM, Narr KL, Toga AW, Jancke L, Gaser C. A curvature-based approach to estimate local gyrification on the cortical surface. Neuroimage (2006) 29:1224–30. doi:10.1016/j.neuroimage.2005.08.049
24. Nguyen VT, Breakspear M, Cunnington R. Reciprocal interactions of the SMA and cingulate cortex sustain premovement activity for voluntary actions. J Neurosci (2014) 34:16397–407. doi:10.1523/JNEUROSCI.2571-14.2014
25. Schrum A, Wolff S, van der Horst C, Kuhtz-Buschbeck JP. Motor cortical representation of the pelvic floor muscles. J Urol (2011) 186:185–90. doi:10.1016/j.juro.2011.03.001
26. Tadic SD, Griffiths D, Schaefer W, Murrin A, Clarkson B, Resnick NM. Brain activity underlying impaired continence control in older women with overactive bladder. Neurourol Urodyn (2012) 31:652–8. doi:10.1002/nau.21240
27. Zhang S, Ide JS, Li CS. Resting-state functional connectivity of the medial superior frontal cortex. Cereb Cortex (2012) 22:99–111. doi:10.1093/cercor/bhr088
28. Jan JE, Reiter RJ, Wasdell MB, Bax M. The role of the thalamus in sleep, pineal melatonin production, and circadian rhythm sleep disorders. J Pineal Res (2009) 46:1–7. doi:10.1111/j.1600-079X.2008.00628.x
29. Griffiths D. Functional imaging of structures involved in neural control of the lower urinary tract. Handb Clin Neurol (2015) 130:121–33. doi:10.1016/B978-0-444-63247-0.00007-9
30. Sugaya K, Nishijima S, Kadekawa K, Ashitomi K, Ueda T, Yamamoto H. Intravenous or local injections of flavoxate in the rostral pontine reticular formation inhibit urinary frequency induced by activation of medial frontal lobe neurons in rats. J Urol (2014) 192:1278–85. doi:10.1016/j.juro.2014.04.092
31. Andrew J, Nathan PW. Lesions on the anterior frontal lobes and disturbances of micturition and defaecation. Brain (1964) 87:233–62. doi:10.1093/brain/87.2.233
32. Griffiths D. Neural control of micturition in humans: a working model. Nat Rev Urol (2015) 12:695–705. doi:10.1038/nrurol.2015.266
33. Casali AG, Gosseries O, Rosanova M, Boly M, Sarasso S, Casali KR, et al. A theoretically based index of consciousness independent of sensory processing and behavior. Sci Transl Med (2013) 5:198ra105. doi:10.1126/scitranslmed.3006294
34. Xie G, Deschamps A, Backman SB, Fiset P, Chartrand D, Dagher A, et al. Critical involvement of the thalamus and precuneus during restoration of consciousness with physostigmine in humans during propofol anaesthesia: a positron emission tomography study. Br J Anaesth (2011) 106:548–57. doi:10.1093/bja/aeq415
35. Vogt BA, Laureys S. Posterior cingulate, precuneal and retrosplenial cortices: cytology and components of the neural network correlates of consciousness. Prog Brain Res (2005) 150:205–17. doi:10.1016/S0079-6123(05)50015-3
36. Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain (2006) 129:564–83. doi:10.1093/brain/awl004
37. Laureys S, Owen AM, Schiff ND. Brain function in coma, vegetative state, and related disorders. Lancet Neurol (2004) 3:537–46. doi:10.1016/S1474-4422(04)00852-X
38. McCaskey U, von Aster M, Maurer U, Martin E, Tuura RO, Kucian K. Longitudinal brain development of numerical skills in typically developing children and children with developmental dyscalculia. Front Hum Neurosci (2018) 11:629. doi:10.3389/Fnhum.2017.00629
Keywords: nocturnal enuresis, magnetic resonance imaging, structural change, supplementary motor area, precuneus
Citation: Wang M, Zhang A, Zhang J, Lu H, Xu S, Qin Z, Ma J and Du X (2018) Morphometric Magnetic Resonance Imaging Study in Children With Primary Monosymptomatic Nocturnal Enuresis. Front. Pediatr. 6:103. doi: 10.3389/fped.2018.00103
Received: 20 December 2017; Accepted: 28 March 2018;
Published: 13 April 2018
Edited by:
Ubirajara Barroso Jr., Universidade Federal da Bahia, BrazilReviewed by:
Cristian Roberto Sager, Garrahan Hospital, ArgentinaTiago Elias Rosito, Federal University of Rio Grande do Sul (UFRGS), Brazil
Copyright: © 2018 Wang, Zhang, Zhang, Lu, Xu, Qin, Ma and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Ma, bWFqdW5Ac2hzbXUuZWR1LmNu;
Xiaoxia Du, eHhkdUBwaHkuZWNudS5lZHUuY24=
 Mengxing Wang
Mengxing Wang Anyi Zhang
Anyi Zhang Jilei Zhang1
Jilei Zhang1 Jun Ma
Jun Ma Xiaoxia Du
Xiaoxia Du