Abstract
Background:
The effects of high-flow nasal cannula (HFNC) compared to non-invasive positive pressure ventilation (NIPPV) on children with bronchiolitis remain unclear.
Methods:
This meta-analysis was performed following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement. Randomized controlled trials (RCTs) were identified from a comprehensive search in PubMed, EMBASE, Cochrane Library, and Web of Science without time and language limitations. Primary endpoints include the rate of treatment failure, the rate of need for intubation, and the pediatric intensive care unit (PICU) length of stay.
Results:
Five RCTs including 541 children of less than 24 months were enrolled in the meta-analysis. Compared to the NIPPV group, the rate of treatment failure was significantly higher in the HFNC treatment group (I2 = 0.0%, P = 0.574; RR 1.523, 95% CI 1.205 to 1.924, P < 0.001). No significant difference was noted in the need for intubation (I2 = 0.0%, P = 0.431; RR 0.874, 95% CI 0.598 to 1.276, P = 0.485) and the PICU length of stay (I2 = 0.0%, P = 0.568; WMD = –0.097, 95% CI = –0.480 to 0.285, P = 0.618) between the HFNC group and the NIPPV treatment.
Conclusion:
Compared to the NIPPV group, HFNC therapy was associated with a significantly higher treatment failure rate in children suffering from bronchiolitis. The intubation rate and the PICU length of stay were comparable between the two approaches.
Introduction
Bronchiolitis is an acute infection of the lower respiratory tract and one of the significant causes of illness and hospitalization in young children (1, 2). It is usually caused by the respiratory syncytial virus (RSV), which almost all children will be infected by 2 years of age (3, 4). Severe bronchiolitis is featured with airway obstruction, hypoxemia, increased work of breathing, and respiratory distress, which need advanced supportive management, including hydration, oxygen support, or assisted ventilation (5–7). Given the complications of mechanical ventilation, non-invasive positive pressure ventilation (NIPPV), such as continuous positive airway pressure (CPAP) and nasal positive pressure ventilation (NPPV), has been widely used and proved to be effective in the treatment of bronchiolitis (8–12).
The high-flow nasal cannula (HFNC) is another choice (13, 14). Compared to a simple nasal cannula, HFNC can reduce the dead space in the nasopharynx, decrease breathing work, and provide proper humidity and temperature (15, 16). Moreover, it is staff-friendly and more comfortable for children than CPAP since there is no need for close monitoring and a stressful tight-fitting interface (17, 18). Several trials and systematic reviews have compared the effects of HFNC and NIPPV; however, the results were inconsistent or inconclusive for the shortage of evidence. Therefore, to clarify this issue, we conducted this updated meta-analysis of HFNC versus NIPPV in infants with bronchiolitis.
Methods
Search strategy
This meta-analysis was performed following the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement (19). All published RCTs investigating the effects of HFNC compared with NIPPV (including CPAP and NPPV) were sought out by a comprehensive search in PubMed, EMBASE, Cochrane Library, and Web of Science from the establishment to May 2022 without language restriction. Search formula was performed as (high flow nasal cannula) AND (bronchiolitis) AND (children) AND (randomized). Two authors (ZZ and LZ) performed the selection independently and resolved disagreements by referring to the third author (SX).
Inclusion and exclusion criteria
Trials were enrolled if they met the following inclusion criteria: (1) RCT; (2) HFNC treatment was applied and compared with the NIPPV method, and (3) reported at least one of the following outcomes: the rate of treatment failure (defined as the author of each trial), the rate of need for intubation, and the pediatric intensive care unit (PICU) length of stay. Duplicated literature, reviews, conference abstracts, and case reports were excluded.
Data extraction and quality assessment
Two independent authors (ZZ and LZ) evaluated each trial’s eligibility and methodological quality according to the Modified Jadad scale (20). Data were extracted using a pre-designed structured form, including data elements: (1) general information, such as the name of the author, population size, year of publication, and study design; (2) patient characteristics, such as age, weight, the proportion of RSV positive, the baseline value of heart rate, respiratory rate, and SPO2; (3) outcomes as mentioned above. The disagreements were resolved by consensus or referring to the third author (SX).
Statistical analysis
The pooled risk ratios (RRs) with 95% confidence intervals (CI) and the weighted mean difference (WMD) with 95% CI were calculated for dichotomous outcomes and continuous outcomes respectively. Inter-study heterogeneity was measured by the I2 test, and a random-effects (RE) model was used for all pooled outcomes (21). In the case of high heterogeneity, the sensitivity or subgroup analysis would be considered. Publication bias was evaluated using a funnel plot with Begg’s test (22). Results with a two-sided P-value < 0.05 indicated a statistical significance. All statistical analyses were performed using Stata v12.0 (Stata Corp., College Station, TX, United States) with the metan function.
Results
Search results and study characteristics
A total of 231 titles and abstracts were identified by the search strategy, in which 83 duplicate records were excluded. Another 142 citations were removed as reviews, conference abstracts, case reports, or irrelevant studies by screening the titles and abstracts. In the six articles retrieved for full-text review, one was excluded for retrospective design. Eventually, five RCTs (23–27) involving a total of 541 children of less than 24 months were included in the present meta-analysis. The detailed literature selection process is illustrated in Figure 1. The baseline characteristics of the enrolled studies are presented in Table 1, and more detailed information (such as treatment failure criteria, timing of failure, the predictors of treatment failure, and the initial settings) is summarized in online Supplementary Table 1.
FIGURE 1
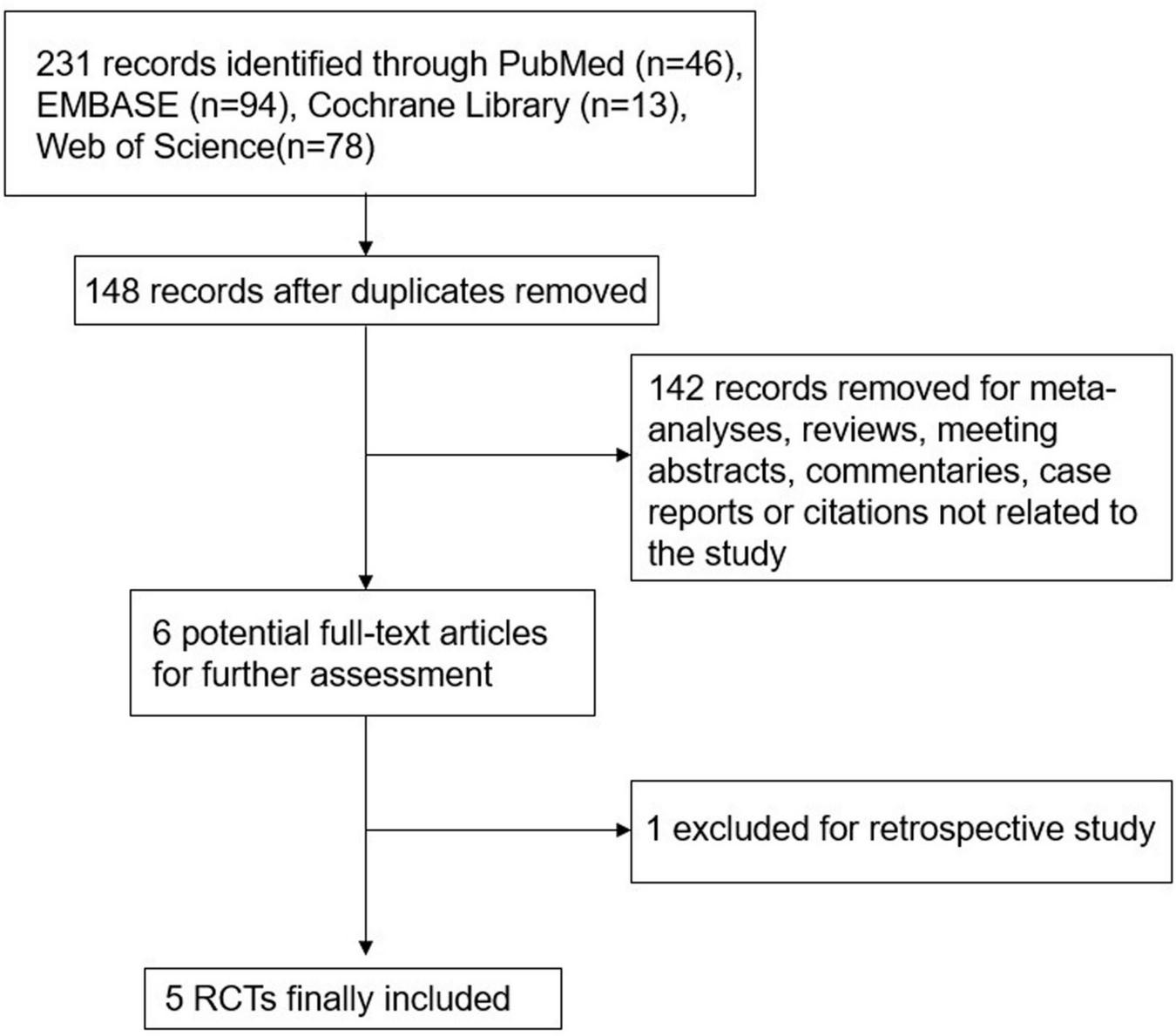
Flow chart of study selection.
TABLE 1
| Study | Year | NIPPV strategy | NIPPV interface | HG/NG | Sample size, n | Age, m | Weight, kg | RSV positive, n (%) | RR | HR | SPO2(%) |
| Borgi et al. (23) | 2021 | CPAP/NPPV | Nasal mask or nasal prong | HG | 130 | 1.8 ± 1.2 | 4.6 ± 1.3 | 46 (35.4) | 68 ± 14.8 | 170.2 ± 16.5 | 90.3 ± 6.9 |
| NG | 125 | 1.6 ± 1.1 | 4.4 ± 1.2 | 44 (35.2) | 65.9 ± 14.5 | 170.4 ± 19.0 | 91.2 ± 6.8 | ||||
| Vahlkvist et al. (24) | 2020 | CPAP | Nasal prong | HG | 22 | 2.1 (0.5–8.8) | 5.2 (3.3–8.6) | 20 (90) | 56 ± 12 | 156 ± 21 | NR |
| NG | 28 | 2.8 (0.3–11.3) | 5.2 (2.8–9.7) | 25 (89) | 60 ± 15 | 155 ± 22 | |||||
| Cesar et al. (25) | 2020 | CPAP | Nasal prong | HG | 35 | 3.4 (1.4–5.4) | 5.9 ± 1.8 | 30 (85.7) | 49.2 ± 10.3 | 147.6 ± 22.6 | 97.5 (95–99) |
| NG | 28 | 2.4 (0.9–3.3) | 5.5 ± 1.5 | 26 (92.9) | 49.2 ± 10.7 | 152.4 ± 18.1 | 98 (96–99) | ||||
| Sarkar et al. (26) | 2018 | CPAP | Nasal mask or nasal prong | HG | 15 | 4.1 ± 2.9 | NR | NR | 73.6 ± 3.6 | 164 ± 8.8 | 88.3 ± 2.5 |
| NG | 16 | 2.8 ± 1.0 | 72.8 ± 3.7 | 168.5 ± 5.7 | 88.8 ± 1.4 | ||||||
| Milési et al. (27) | 2017 | CPAP | NR | HG | 71 | 1.4 ± 1.3 | 4.1 ± 1.3 | 125 (88) | 52 ± 18 | 166 ± 20 | 95 ± 5 |
| NG | 71 | 1.3 ± 1.1 | 4.1 ± 1.1 | 54 ± 18 | 165 ± 19 | 95 ± 4 |
Characteristics of included studies.
HG high flow nasal cannula group, NG non-invasive positive pressure ventilation group, CPAP continuous positive airway pressure, NPPV nasal positive pressure ventilation, NIPPV non-invasive positive pressure ventilation, RSV respiratory syncytial virus, RR respiratory rate, HR heart rate, SPO2 oxygen saturation, NR not reported.
Quality assessment and publication bias
The methodological quality was evaluated by the Modified Jadad scale, including randomization, double-blinding, withdrawals and dropouts, and allocation concealment (20). The scores of the enrolled trials are summarized in Table 2, ranging from 4 to 5. No publication bias was found using a symmetrical funnel plot based on the outcome of treatment failure (Begg’s test, P = 0.462) (Figure 2).
TABLE 2
| Author | Randomization | Double blinding | Allocation concealment | Withdrawals/dropouts | Scores |
| Borgi et al. (23) | Yes | No | Unclear | Yes | 4 |
| Vahlkvist et al. (24) | Yes | No | Yes | Yes | 5 |
| Cesar et al. (25) | Yes | No | Unclear | Yes | 4 |
| Sarkar et al. (26) | Yes | No | Unclear | Yes | 4 |
| Milési et al. (27) | Yes | No | Yes | Yes | 5 |
Assessment of methodological quality of included studies.
FIGURE 2
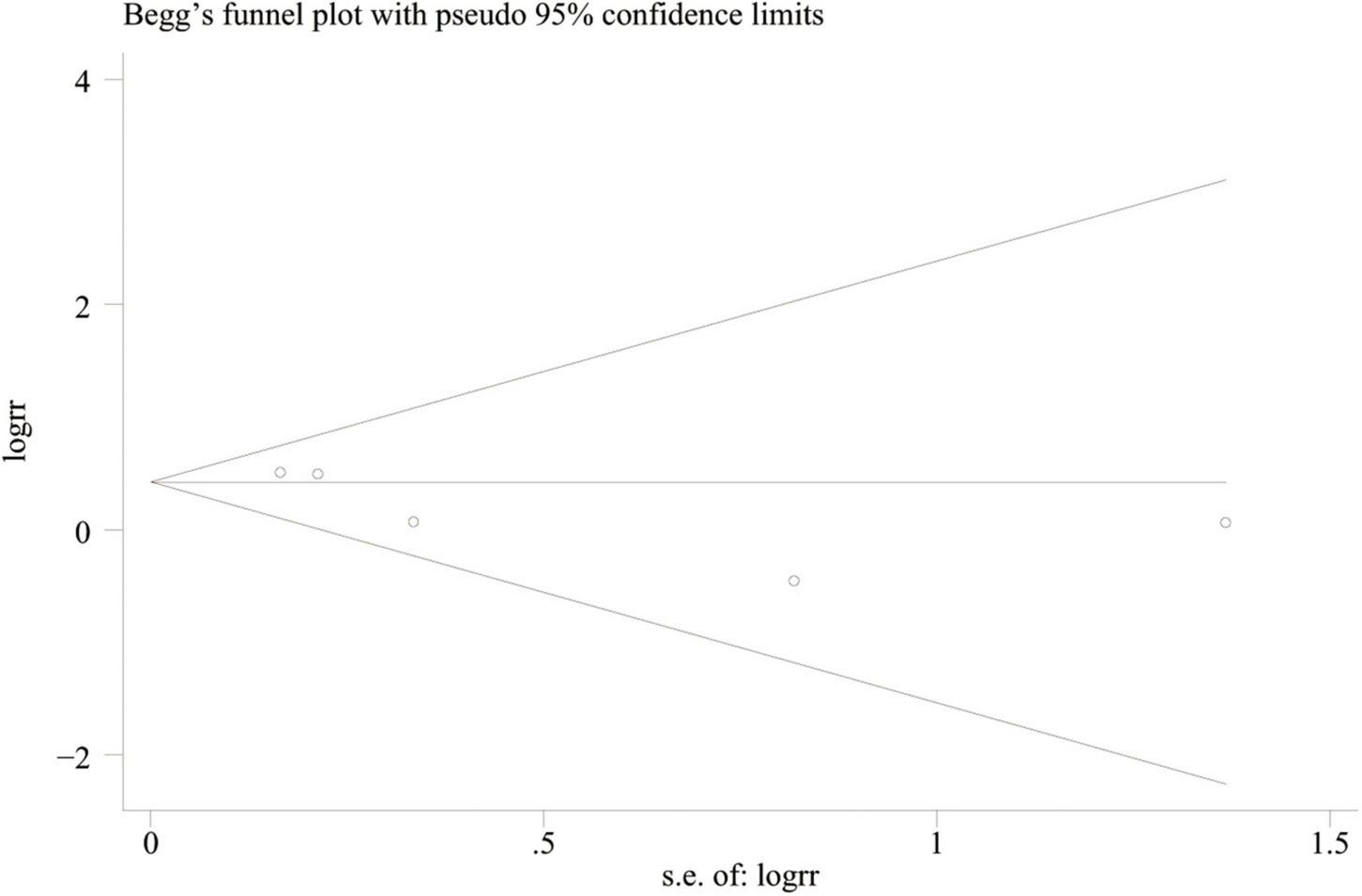
Funnel plot for treatment failure rate.
Meta-analysis results
Treatment failure
All five studies (23–27) involving 541 cases reported the rate of treatment failure. Compared to the NIPPV group, the rate of treatment failure was significantly higher in the HFNC treatment group (I2 = 0.0%, P = 0.574; RR 1.523, 95% CI 1.205 to 1.924, P < 0.001) (Figure 3).
FIGURE 3
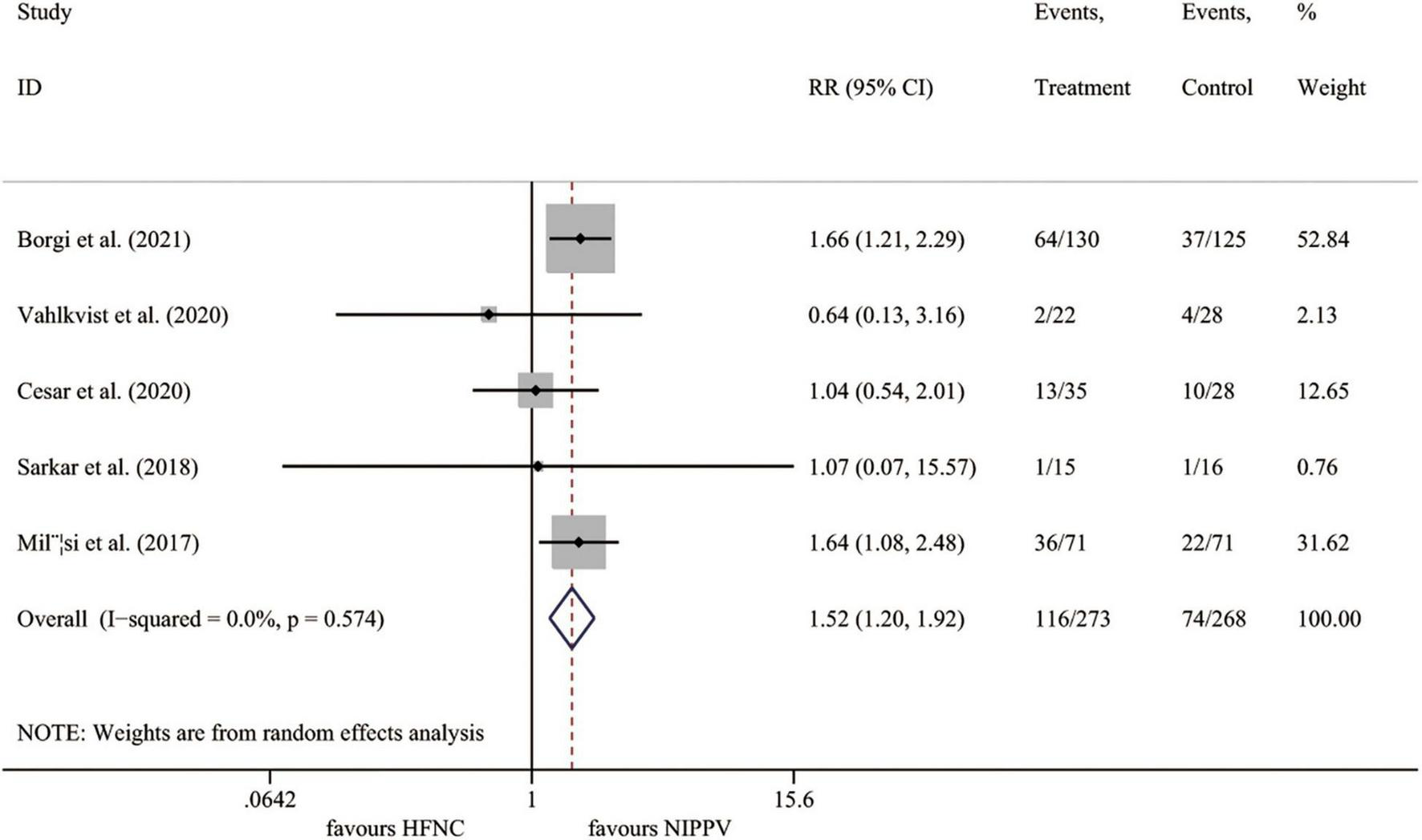
Forest plot for treatment failure rate. RR, relative risk; HFNC, high flow nasal cannula; NIPPV, non-invasive positive pressure ventilation.
Need for intubation
Four trials (23, 25–27) reported the intubation rate, including 491 cases. Compared to the NIPPV group, the HFNC therapy showed no benefits on the incidence of intubation (I2 = 0.0%, P = 0.431; RR 0.874, 95% CI 0.598 to 1.276, P = 0.485) (Figure 4).
FIGURE 4
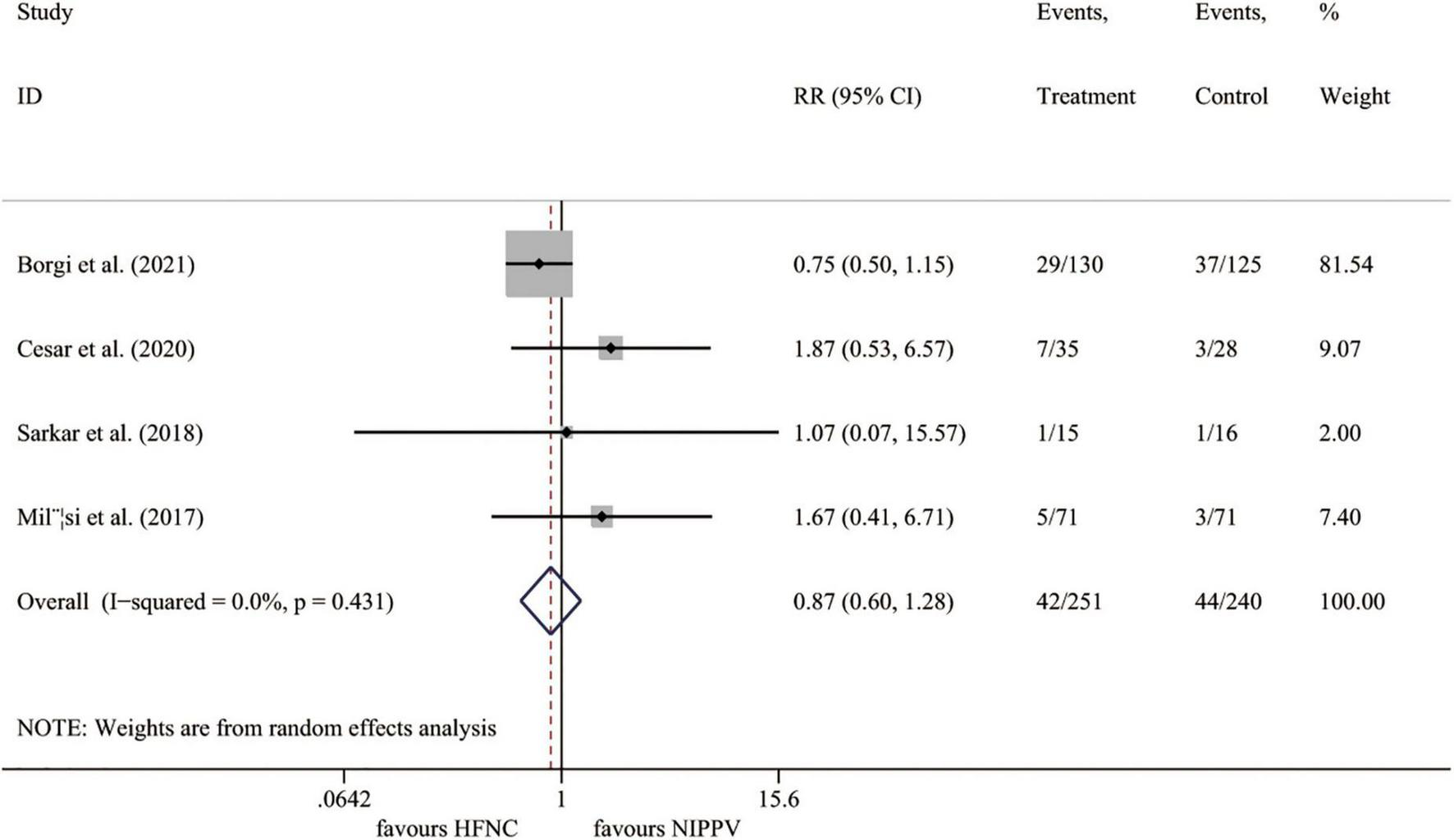
Forest plot for intubation rate. RR, relative risk; HFNC, high flow nasal cannula; NIPPV, non-invasive positive pressure ventilation.
Pediatric intensive care unit length of stay
Four RCTs (23, 25–27) presented the outcome of PICU stay length. The results demonstrated no significant difference between the HFNC group and the NIPPV approach (I2 = 0.0%, P = 0.568; WMD = –0.097, 95% CI = –0.480 to 0.285, P = 0.618) (Figure 5).
FIGURE 5
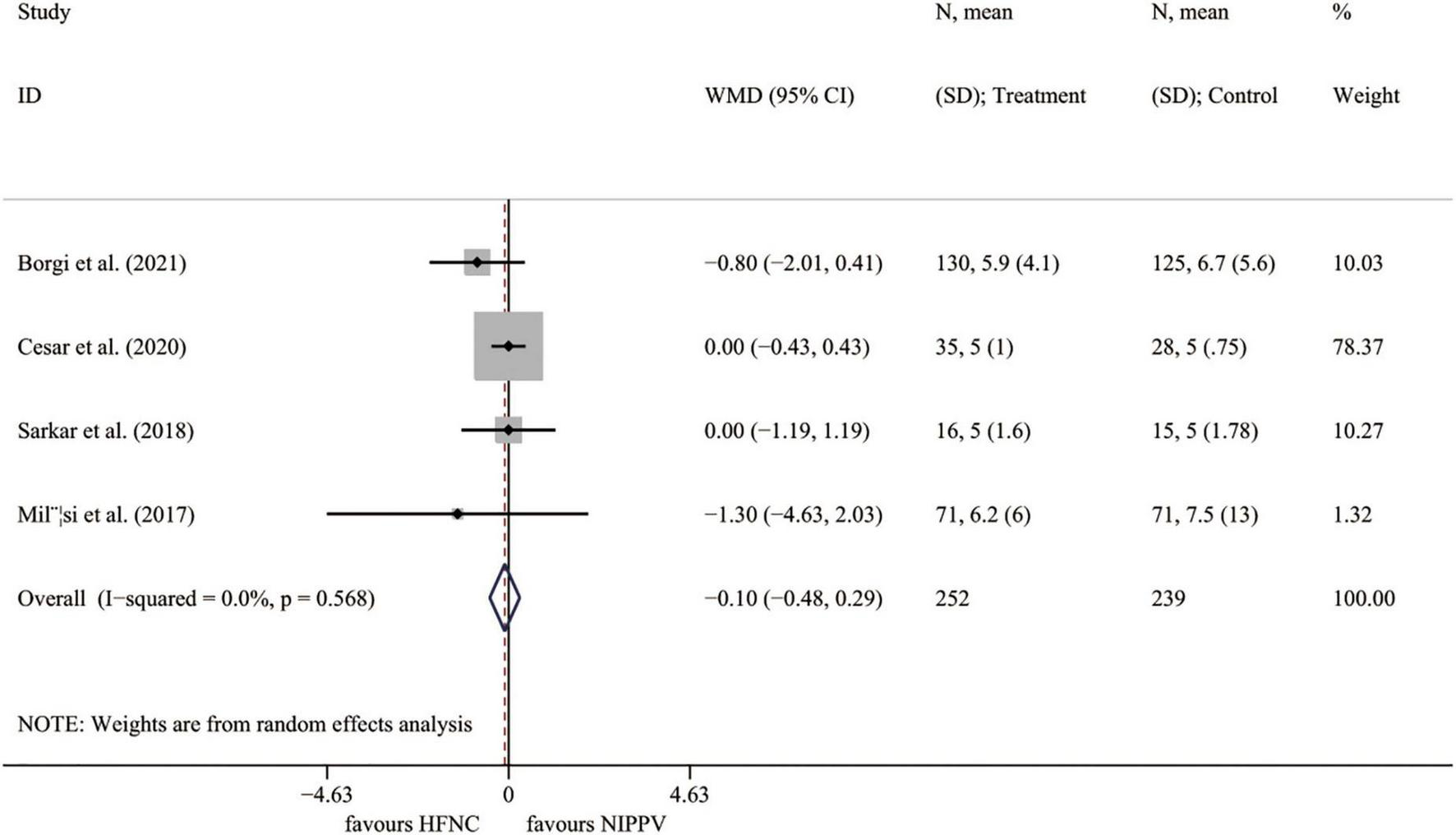
Forest plot for PICU length of stay. PICU, pediatric intensive care unit; WMD, weighted mean difference; HFNC, high flow nasal cannula; NIPPV, non-invasive positive pressure ventilation.
Discussion
In the present meta-analysis, we investigated the effects of HFNC compared to NIPPV in children with bronchiolitis. The main finding was that the rate of treatment failure was significantly higher in the HFNC group. For other outcomes, the rate of intubation and the PICU length of stay were comparable between the two groups.
In previous studies, HFNC was associated with a lower rate of treatment failure and intubation in young children with bronchiolitis (28, 29). Similarly, several studies investigated the effects of NIPPV in the treatment of bronchiolitis and proved that NIPPV, especially for CPAP, was superior to the standard treatment (8, 30). Compared to the NIPPV, HFNC was more comfortable and acceptable for the children and was associated with a lower rate of adverse events such as nasal injury (26, 27). However, though studies have compared the effects of HFNC and NIPPV, the results were inconsistent or inconclusive. Milesi et al. reported that in young infants with moderate to severe bronchiolitis, initial management with HFNC had a higher treatment failure rate than CPAP (27). On the contrary, Vahlkvist et al. (24) and Cesar et al. (25) found that treatment with HFNC led to a rate of treatment failure comparable to CPAP. Cataño-Jaramillo et al. conducted a systematic review including three RCTs to investigate this issue (31). Although they found a trend favoring CPAP over HFNC for the outcome of treatment failure, there was a lack of statistical significance (P = 0.05). Our study, including five RCTs and 541 cases, almost doubles the previous research, making the conclusion clearer than ever.
Non-invasive positive pressure ventilation was widely used in the treatment of respiratory illnesses. As investigated by the previous publications, the benefits might mainly be driven by providing positive end-expiratory pressure (PEEP), resulting in distending airway pressure on the distal airway. This effect may decrease the airways’ resistance and help prevent alveolar collapse and obstructive apnea (8). HFNC is suggested to deliver a warm and humidified gas and reduce the airway dead space and resistance (15, 32). As reported, HFNC may also generate a potential PEEP in the airways (33). Flow rates of ≥ 6 L/min appear to provide positive pressure throughout the respiratory cycle, with a PEEP range from 2 to 5 cm H2O (34, 35); however, the pressure is variable and unmonitored, depending on the weight of infants, flow rate, and leaks through the mouth and nares. All these factors may limit its effects in young children with bronchiolitis (14). In the present study, we did not notice any significant difference between the two approaches for the rate of intubation and PICU length of stay, which might be affected by the restricted study number and population, as well as the fact that for infants treated with HFNC a switch toward NIPPV group was allowed before intubation in some studies (23). Besides, most of the adverse effects were minor and comparable between the two groups.
As far as we know, the present study is the first meta-analysis to ascertain the superiority of NIPPV compared to HFNC on the primary outcome of treatment failure in children with bronchiolitis. However, the potential limitations of the study should not be ignored. First, though RCTs were enrolled, the total patient number of the study was only 541 cases, and the analysis power might be affected. Second, since the devices were clinically widely used and recognizable, none of the studies was blinded, which may lead to a performance bias. Similarly, only three trials reported the method of concealment, which means selection bias may affect the results. Third, because only five trials were included for assessment, the power of the funnel plot asymmetry test for publication bias might be restricted. Finally, the meta-analysis contained studies with inconsistent intervention regimens and children’s clinical features, which may also affect the results. For example, Borgi et al. included both CPAP and NPPV in the same group, and the success rate could have been influenced by using NPPV with variable pressure compared to CPAP with constant pressure. Therefore, the study results should be interpreted cautiously, and more RCTs with a larger population were expected.
Conclusion
Compared to the NIPPV approach, HFNC therapy was associated with a significantly higher treatment failure rate in children suffering from bronchiolitis. The intubation rate and the PICU length of stay were comparable between the two groups. Given the study’s limitations, the results should be interpreted cautiously, and further investigation is warranted.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Statements
Data availability statement
The original contributions presented in this study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author contributions
ZZ and SX conceived and designed the study. ZZ, LZ, and SX performed the study and wrote the main manuscript text. LZ and YZ contributed analysis tools and prepared figures. All authors reviewed the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2022.947667/full#supplementary-material
References
1.
Meissner HC . Viral bronchiolitis in children.N Engl J Med. (2016) 374:62–72. 10.1056/NEJMra1413456
2.
Ralston SL Lieberthal AS Meissner HC Alverson BK Baley JE Gadomski AM et al Clinical practice guideline: the diagnosis, management, and prevention of bronchiolitis. Pediatrics. (2014) 134:e1474–502.
3.
Fleming DM Pannell RS Cross KW . Mortality in children from influenza and respiratory syncytial virus.J Epidemiol Commun Health. (2005) 59:586–90. 10.1136/jech.2004.026450
4.
Thompson WW Shay DK Weintraub E Brammer L Cox N Anderson LJ et al Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA. (2003) 289:179–86. 10.1001/jama.289.2.179
5.
Oymar K Skjerven HO Mikalsen IB . Acute bronchiolitis in infants, a review.Scand J Trauma Resusc Emerg Med. (2014) 22:23. 10.1186/1757-7241-22-23
6.
Oakley E Borland M Neutze J Acworth J Krieser D Dalziel S et al Nasogastric hydration versus intravenous hydration for infants with bronchiolitis: a randomised trial. Lancet Respir Med. (2013) 1:113–20. 10.1016/S2213-2600(12)70053-X
7.
Lazner MR Basu AP Klonin H . Non-invasive ventilation for severe bronchiolitis: analysis and evidence.Pediatr Pulmonol. (2012) 47:909–16. 10.1002/ppul.22513
8.
Jat KR Mathew JL . Continuous positive airway pressure (CPAP) for acute bronchiolitis in children.Cochrane Database Syst Rev. (2015) 1:CD010473. 10.1002/14651858.CD010473.pub2
9.
Ganu SS Gautam A Wilkins B Egan J . Increase in use of non-invasive ventilation for infants with severe bronchiolitis is associated with decline in intubation rates over a decade.Intensive Care Med. (2012) 38:1177–83. 10.1007/s00134-012-2566-4
10.
Donlan M Fontela PS Puligandla PS . Use of continuous positive airway pressure (CPAP) in acute viral bronchiolitis: a systematic review.Pediatr Pulmonol. (2011) 46:736–46. 10.1002/ppul.21483
11.
Essouri S Laurent M Chevret L Durand P Ecochard E Gajdos V et al Improved clinical and economic outcomes in severe bronchiolitis with pre-emptive nCPAP ventilatory strategy. Intensive Care Med. (2014) 40:84–91. 10.1007/s00134-013-3129-z
12.
Ducharme-Crevier L Essouri S Emeriaud G . Noninvasive ventilation in pediatric intensive care: from a promising to an established therapy, but for whom, when, why, and how?Pediatr Crit Care Med. (2015) 16:481–2. 10.1097/PCC.0000000000000390
13.
Milesi C Baleine J Matecki S Durand S Combes C Novais AR et al Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. (2013) 39:1088–94. 10.1007/s00134-013-2879-y
14.
Mikalsen IB Davis P Oymar K . High flow nasal cannula in children: a literature review.Scand J Trauma Resusc Emerg Med. (2016) 24:93. 10.1186/s13049-016-0278-4
15.
Pham TM O’Malley L Mayfield S Martin S Schibler A . The effect of high flow nasal cannula therapy on the work of breathing in infants with bronchiolitis.Pediatr Pulmonol. (2015) 50:713–20. 10.1002/ppul.23060
16.
Beggs S Wong ZH Kaul S Ogden KJ Walters JA . High-flow nasal cannula therapy for infants with bronchiolitis. Cochrane Database Syst Rev. (2014) 1:CD009609.
17.
Mayfield S Jauncey-Cooke J Hough JL Schibler A Gibbons K Bogossian F . High-flow nasal cannula therapy for respiratory support in children.Cochrane Database Syst Rev. (2014) 2014:CD009850. 10.1002/14651858.CD009850.pub2
18.
Ramnarayan P Schibler A . Glass half empty or half full? The story of high-flow nasal cannula therapy in critically ill children.Intensive Care Med. (2017) 43:246–9. 10.1007/s00134-016-4663-2
19.
Moher D Liberati A Tetzlaff J Altman DG Group P . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement.Ann Intern Med. (2009) 151:264–9, W264. 10.7326/0003-4819-151-4-200908180-00135
20.
Oremus M Wolfson C Perrault A Demers L Momoli F Moride Y . Interrater reliability of the modified Jadad quality scale for systematic reviews of Alzheimer’s disease drug trials.Dement Geriatr Cogn Disord. (2001) 12:232–6. 10.1159/000051263
21.
Cumpston M Li T Page MJ Chandler J Welch VA Higgins JP et al Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. (2019) 10:ED000142. 10.1002/14651858.ED000142
22.
Begg CB Mazumdar M . Operating characteristics of a rank correlation test for publication bias.Biometrics. (1994) 50:1088–101. 10.2307/2533446
23.
Borgi A Louati A Ghali N Hajji A Ayari A Bouziri A et al High flow nasal cannula therapy versus continuous positive airway pressure and nasal positive pressure ventilation in infants with severe bronchiolitis: a randomized controlled trial. Pan Afr Med J. (2021) 40:133. 10.11604/pamj.2021.40.133.30350
24.
Vahlkvist S Jürgensen L la Cour A Markoew S Petersen TH Kofoed PE . High flow nasal cannula and continuous positive airway pressure therapy in treatment of viral bronchiolitis: a randomized clinical trial.Eur J Pediatr. (2020) 179:513–8. 10.1007/s00431-019-03533-2
25.
Cesar RG Bispo BRP Felix PHCA Modolo MCC Souza AAF Horigoshi NK et al High-flow nasal cannula versus continuous positive airway pressure in critical bronchiolitis: a randomized controlled pilot. J Pediatric Intens Care. (2020) 9:248–55. 10.1055/s-0040-1709656
26.
Sarkar M Sinha R Roychowdhoury S Mukhopadhyay S Ghosh P Dutta K et al Comparative study between noninvasive continuous positive airway pressure and hot humidified high-flow nasal cannulae as a mode of respiratory support in infants with acute bronchiolitis in pediatric intensive care unit of a Tertiary Care Hospital. Ind J Crit Care Med. (2018) 22:85–90. 10.4103/ijccm.IJCCM_274_17
27.
Milési C Essouri S Pouyau R Liet JM Afanetti M Portefaix A et al High flow nasal cannula (HFNC) versus nasal continuous positive airway pressure (nCPAP) for the initial respiratory management of acute viral bronchiolitis in young infants: a multicenter randomized controlled trial (TRAMONTANE study). Intens Care Med. (2017) 43:209–16. 10.1007/s00134-016-4617-8
28.
Kepreotes E Whitehead B Attia J Oldmeadow C Collison A Searles A et al High-flow warm humidified oxygen versus standard low-flow nasal cannula oxygen for moderate bronchiolitis (HFWHO RCT): an open, phase 4, randomised controlled trial. Lancet. (2017) 389:930–9. 10.1016/S0140-6736(17)30061-2
29.
Guillot C Le Reun C Behal H Labreuche J Recher M Duhamel A et al First-line treatment using high-flow nasal cannula for children with severe bronchiolitis: applicability and risk factors for failure. Arch Pediatr. (2018) 25:213–8. 10.1016/j.arcped.2018.01.003
30.
Oymar K Bardsen K . Continuous positive airway pressure for bronchiolitis in a general paediatric ward; a feasibility study.BMC Pediatr. (2014) 14:122. 10.1186/1471-2431-14-122
31.
Catano-Jaramillo ML Jaramillo-Bustamante JC Florez ID . Continuous Positive Airway Pressure vs. High Flow Nasal Cannula in children with acute severe or moderate bronchiolitis. A systematic review and Meta-analysis.Med Intensiva (Engl Ed). (2022) 46:72–80. 10.1016/j.medin.2020.09.008
32.
Dysart K Miller TL Wolfson MR Shaffer TH . Research in high flow therapy: mechanisms of action.Respir Med. (2009) 103:1400–5. 10.1016/j.rmed.2009.04.007
33.
Lee JH Rehder KJ Williford L Cheifetz IM Turner DA . Use of high flow nasal cannula in critically ill infants, children, and adults: a critical review of the literature.Intensive Care Med. (2013) 39:247–57. 10.1007/s00134-012-2743-5
34.
Milési C Baleine J Matecki S Durand S Combes C Novais AR et al Is treatment with a high flow nasal cannula effective in acute viral bronchiolitis? A physiologic study. Intensive Care Med. (2013) 39:1088–94.
35.
Hough JL Pham TM Schibler A . Physiologic effect of high-flow nasal cannula in infants with bronchiolitis.Pediatr Crit Care Med. (2014) 15:e214–9. 10.1097/PCC.0000000000000112
Summary
Keywords
high-flow nasal cannula, non-invasive positive pressure ventilation, bronchiolitis, children, CPAP (continuous positive air pressure)
Citation
Zhong Z, Zhao L, Zhao Y and Xia S (2022) Comparison of high flow nasal cannula and non-invasive positive pressure ventilation in children with bronchiolitis: A meta-analysis of randomized controlled trials. Front. Pediatr. 10:947667. doi: 10.3389/fped.2022.947667
Received
19 May 2022
Accepted
27 June 2022
Published
15 July 2022
Volume
10 - 2022
Edited by
Renato Cutrera, Bambino Gesù Children’s Hospital (IRCCS), Italy
Reviewed by
Camilla Gizzi, Sandro Pertini Hospital, Italy; Elena Caresta, Umberto I Hospital, Italy
Updates
Copyright
© 2022 Zhong, Zhao, Zhao and Xia.
This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shuyue Xia, dr_syxia@qq.com
This article was submitted to Pediatric Pulmonology, a section of the journal Frontiers in Pediatrics
Disclaimer
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.