- Department of Pediatric Surgery, Baoan Women’s and Children’s Hospital, Shenzhen, China
Although most cases of neonatal sacrococcygeal teratoma are benign, some can grow into very large lesions, potentially causing high-output heart failure, massive bleeding, disseminated intravascular coagulation, and even fatal outcomes. We report three cases of giant sacrococcygeal teratomas, detailing their diagnostic and therapeutic management. We emphasize that clinicians should be prepared with comprehensive contingency plans and that interdisciplinary collaboration is essential to enhance patient survival rates and minimize potential complications.
Introduction
Teratoma is the most common germ cell tumor in children, characterized by the abnormal development of tissues derived from at least two of the three germ layers. Teratomas can occur in both the gonads and extra-gonadal sites, with a predilection for midline locations, among which the sacrococcygeal region is the most common site in neonates. Sacrococcygeal teratoma (SCT) is a tumor that arises in the sacrococcygeal region, characterized by the presence of tissue components from multiple embryonic germ layers (1).
Several factors have been identified as predictors of a poor prognosis, including tumor volume and the proportion of its vascular and/or solid components, changes in placental size and morphology, histological features of the tumor, intrapelvic extension of the tumor, a tumor diagnosis before 20 weeks of gestation, fetal hydrops, polyhydramnios, low Apgar score at birth, low birth weight, and preterm delivery before 30 weeks of gestation (2). Most fatalities are associated with high-output cardiac failure, which leads to fetal hydrops from increased vascular flow to the solid components of the tumor, or with severe hemorrhage due to tumor rupture during delivery (3). Although most sacrococcygeal teratomas in neonates are benign, some may grow to a large size and cause serious complications, including high-output heart failure and massive hemorrhage. More than 80% of fetuses with a tumor-to-fetal weight ratio (TFR) greater than 0.12 before 24 weeks of gestation suffer from poor outcomes (4). Even after surgical resection of the tumor, recurrence, malignant transformation, and other conditions may still occur in some cases during long-term follow-up (5).
There are limited reports in the literature on giant sacrococcygeal teratomas. Therefore, we report the diagnostic and treatment experiences from three cases of giant sacrococcygeal teratomas.
Case presentation
The mothers in cases 1, 2, and 3 were 35, 32, and 34 years old, respectively. Both parents of each child were in good health, with no history of tumors or mental illness, and there was no consanguinity. The families maintained harmonious relationships. In case 1, the mother received dexamethasone to promote fetal lung maturity and magnesium sulfate for pregnancy maintenance prior to a cesarean section. The mothers in cases 2 and 3 did not receive any special medications during pregnancy. The newborn in case 1 presented with weak spontaneous respiration and hypotonia at birth. Endotracheal intubation and resuscitation using a T-piece resuscitator were performed. His Apgar scores were 8, 9, and 9 at 1, 5, and 10 min, respectively. The newborns in cases 1 and 2 exhibited stable vital signs at birth, with no signs of respiratory distress, cyanosis, grunting, or apnea. The Apgar scores were 10 at 1, 5, and 10 min.
The treatment protocol followed in case 1 is provided here as an example of the treatment protocol for a large teratoma. After a prenatal color Doppler ultrasound revealed a large fetal teratoma, obstetricians, neonatologists, and pediatric surgeons performed assessments and monitored the pregnancy to determine the optimal delivery time. Following birth, neonatologists and pediatric surgeons promptly conducted a brief assessment and transferred the infant to the neonatal intensive care unit. Prior to surgery, neonatologists, pediatric surgeons, anesthesiologists, and radiologists held a multidisciplinary consultation and completed thorough preparations. Postoperatively, the pediatric surgeon and neonatologist jointly formulated a rehabilitation plan for the infant.
The patient was placed in the prone position during the surgery. One of the challenges associated with this positioning under anesthesia is ensuring adequate respiratory support. To address this, reinforced endotracheal tubes were utilized. Throughout the procedure, the patient's vital signs and respiratory parameters were continuously monitored to allow for the prompt detection of and adjustment for any abnormalities. Immediate interventions were undertaken if any issues arose. In addition, due to the fragility of neonatal skin, a surgical posture mat and artificial skin were used to protect all areas in contact with the operating surface. The patient's head was rotated regularly, and their skin condition was assessed periodically throughout the procedure. Due to the presence of a significant lesion in the sacrococcygeal region, nerve blocks were not feasible. Therefore, postoperative pain management was primarily achieved through local infiltration anesthesia at the incision site and patient-controlled intravenous analgesia (PCIA). Fentanyl was used as the primary analgesic agent. PCIA is programmed by the anesthesiologist at a specific rate to continuously deliver pain-relieving medication, thereby achieving effective analgesia. Postoperatively, if medical staff observe signs of discomfort in the neonatal patient and believe the discomfort is due to surgical wound pain, they may activate the patient-controlled analgesia (PCA) pump to administer an additional dose of medication.
To prevent surgical site infection, prophylactic antibiotics were administered 30 min prior to the operation. Operative time was minimized, and intraoperative normothermia was maintained. Postoperatively, the patient was placed in alternating prone and lateral positions. A rectal tube was inserted after surgery and is typically removed after the first bowel movement, usually on postoperative day 2. A urinary catheter was also placed and is generally removed between postoperative days 3–5. Perianal hygiene was maintained, with care taken to keep the area clean and dry. All surgical incisions healed without complications.
Case 1
Two months before the child's birth, a three-dimensional ultrasound revealed a predominantly cystic-solid mass in the sacrococcygeal region of the fetus. There was vaginal bleeding observed prenatally. An ultrasound showed a sacrococcygeal teratoma measuring approximately 22.3 cm × 15.7 cm × 15.7 cm, with complete anterior placenta previa. After a multidisciplinary discussion, a cesarean section was performed. A large mass was observed in the sacrococcygeal region (Figure 1a). A CT scan showed a large cystic-solid mass in the pelvic floor, predominantly cystic in nature, with internal septations. The lesion's edge contained solid components, with punctate calcifications visible within the solid parts. The mass appeared to connect to the right side of the pelvis, measuring approximately 24.0 cm × 16.9 cm × 17.5 cm. After a multidisciplinary consultation, a resection of the sacrococcygeal teratoma was performed on the first day after birth.
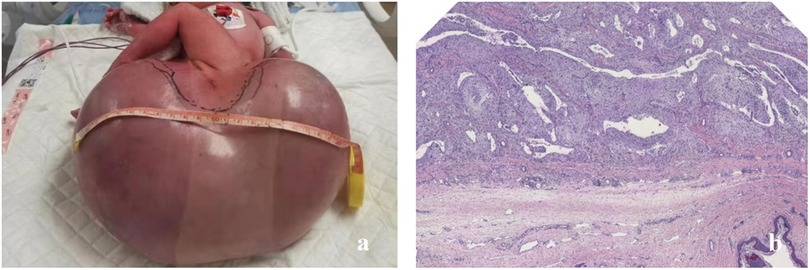
Figure 1. (a) A neonate in case 1 presenting with a giant sacrococcygeal teratoma. (b) The postoperative histopathological examination confirmed it to be a giant mature teratoma.
A circumferential incision was made along the transverse axis of the mass, and the skin was incised to expose the lesion. The mass appeared cystic with a thin wall, containing fluid, gelatinous material, and firm tissue components. A resection was carried out along the subcutaneous plane to the tumor base. On the right side, the lesion extended near the right hip joint, and the joint capsule was identified. Anteriorly, the mass extended to the rectal wall. A careful resection was performed, guided by the anal canal. Posteriorly, the mass extended superiorly along the anterior aspect of the coccyx. The coccyx tip was resected. The upper pole of the mass was located approximately 3 cm from the coccyx tip, near the peritoneum. Both blunt and sharp resection techniques were used to separate the tumor from surrounding tissues, and the lesion was completely excised. The postoperative pathology results showed that it was a giant mature teratoma (Figure 1b).
Before the operation, the patient’s alpha-fetoprotein (AFP) levels were >3,000.0 µg/L. The patient’s AFP levels remained >3,000.0 µg/L 17 days after surgery, and 1 year later, their AFP levels decreased to 3.5 µg/L. However, a rectal examination revealed a palpable, cystic mass measuring approximately 3.0 cm × 4.0 cm in the anterior sacrococcygeal area, with a smooth surface and no tenderness. Both plain and enhanced magnetic resonance imaging (MRI) scans showed a cystic lesion anterior to and to the left of the sacrococcygeal area (Figure 2a).
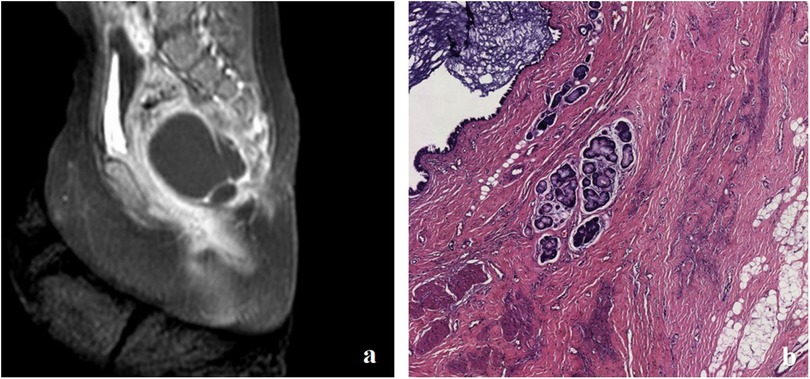
Figure 2. (a) MRI showing a cystic lesion located anterolaterally to the sacrococcygeal area in case 1. (b) Postoperative pathology confirmed the diagnosis of a mature cystic teratoma.
Follow-up MRI scans, both plain and enhanced, conducted 1.5 years after surgery, showed a slight shrinkage of the tumor; a second surgical intervention was performed to complete the excision of the cystic mass, which measured approximately 3 cm × 3 cm × 3 cm and had a thin wall. Upon opening the cystic mass, clear yellow-green fluid mixed with a milky white substance was observed. No solid tissue was found. Postoperative pathology confirmed a cystic mature teratoma without immature components (Figure 2b). Pelvic MRI scans 2 years after the second surgery showed that the cystic lesion in the left anterior sacrococcygeal area essentially disappeared.
Case 2
The infant was born with a gestational age of 32 weeks and 6 days, and a birth weight of 3,680 g. A mass measuring approximately 20 cm × 16 cm × 11 cm was observed in the sacrococcygeal region on imaging (Figure 3a). The patient’s AFP levels were >192,000.0 µg/L. MRI of the sacrococcygeal region showed a large cystic-solid mass in the buttocks and perineum, extending into the pelvic cavity with a rich blood supply. A resection of the sacrococcygeal teratoma and partial coccygectomy with drainage was performed on the second day after birth.
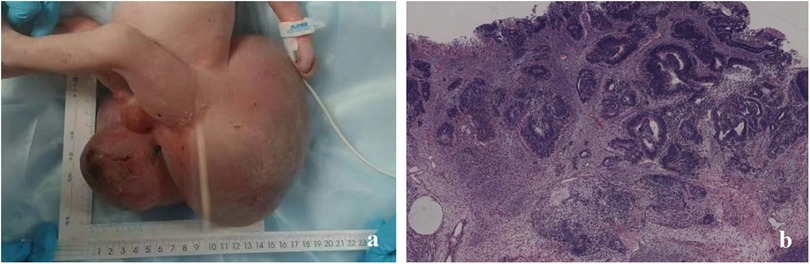
Figure 3. In case 2, (a) a neonate presented with a giant sacrococcygeal teratoma. (b) Postoperative pathology confirmed it to be an immature teratoma.
The patient’s preoperative hemoglobin concentration (HGB) was 125 g/L, and 170 mL of washed red blood cell suspension was transfused. During surgery, significant bleeding from the wound occurred, with an estimated intraoperative blood loss of approximately 300 mL. In total, 200 mL of red blood cell suspension and 100 mL of plasma were transfused intraoperatively. On the first postoperative day, a small amount of persistent wound bleeding was observed. Laboratory tests showed a prothrombin time (PT) of 20.3 s, an international normalized ratio (PT-INR) of 1.78, an activated partial thromboplastin time (APTT) of 64.9 s, a fibrinogen (FIB) level of 1.03 g/L, and a HGB of 119 g/L. Subsequently, an additional 85 mL of washed red blood cells, 43 mL of platelets, and 42 mL of plasma were transfused.
The postoperative pathological result was immature teratoma, grade 3 (the immature teratoma grading was based on the amount of neuroepithelial tissue) (Figure 3b). The patient’s postoperative AFP level gradually decreased. No abnormalities were observed in the sacrococcygeal ultrasound performed at 4 months postoperatively.
Case 3
The infant was born at 38 weeks and 4 days, with a birth weight of 2,900 g, and a mass of approximately 12 cm × 12 cm × 10 cm was observed in the sacrococcygeal region (Figure 4a). Intraoperatively, two prominent vessels connecting to the tumor, measuring approximately 0.3 cm in diameter, were identified. These vessels were ligated, and the tumor was completely excised (Figure 4b). No abnormalities were observed in the sacrococcygeal ultrasound performed at 4 months postoperatively.
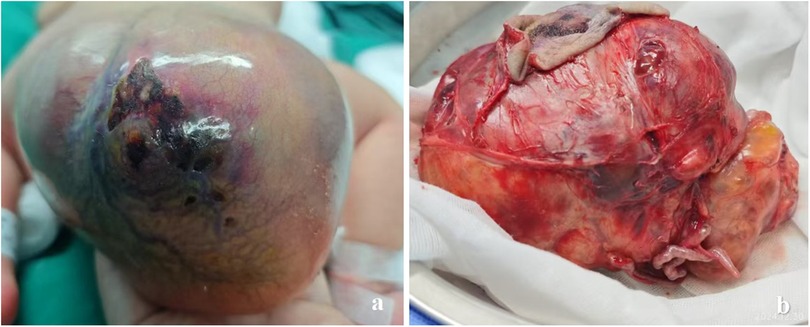
Figure 4. In case 3, (a) a neonate presented with a giant sacrococcygeal teratoma, with the mass resembling a head in appearance. (b) The appearance of the sacrococcygeal teratoma after complete resection, with two prominent vessels connected to the tumor tissue.
Discussion
SCT is the most common tumor in neonates. The reported perinatal mortality rate varies widely in the literature, ranging from 13% to 50% (3). In prenatal diagnosis, beyond evaluating the placenta, amniotic fluid volume, and intrauterine fetal development, particular emphasis should be placed on identifying perinatal complications associated with fetal SCT (6). Prenatal complications such as hydrops with high-output cardiac failure, tumor necrosis and hemorrhage, polyhydramnios, preterm labor, and, in some cases, fetal demise can occur (7, 8). The risk of mortality increases significantly under these conditions; thus, serial monitoring with an ultrasound and echocardiography during pregnancy is essential (8, 9). On an ultrasound, signs such as placentomegaly, reversed diastolic blood flow in the umbilical arteries, and fluid accumulation in body cavities may indicate the onset of fetal hydrops (8, 9). These complications require prompt intervention, with a careful balance between urgent and planned management (8). It is crucial to assess the tumor size, composition, and vascularity to facilitate the formulation of an early surgical intervention plan (6). To mitigate the risk of hemorrhage, cesarean section should be considered for infants with large or highly vascularized tumors (10, 11).
The prognosis of a fetus or newborn diagnosed with a sacrococcygeal teratoma is determined by three key factors: the surgical resectability of the teratoma, the timing of the diagnosis, and the tumor's tissue composition (12, 13). Large and rapidly growing sacrococcygeal teratomas with substantial vascularity are linked to higher perinatal mortality and morbidity when compared to smaller, less vascularized lesions (14). The solid component of a sacrococcygeal teratoma typically demonstrates significant vascularity and a propensity for rapid expansion, thereby increasing the risk of massive hemorrhage and high-output cardiac failure (15). Prenatal surgical procedures for SCT lack evidence from randomized clinical trials, so patients should be selected carefully, with a postnatal intervention favored (16). Large sacrococcygeal tumors should be removed as soon as possible after birth to prevent rupture or intratumoral bleeding (16). In our cases, we performed surgery on the children within 48 h.
When evaluating a sacrococcygeal teratoma, the patient’s preoperative serum AFP level is a valuable diagnostic tool (17, 18). However, caution must be exercised when interpreting AFP levels, particularly in neonates and infants, as their natural hepatic production results in elevated concentrations. Therefore, relying solely on AFP levels cannot unequivocally confirm or exclude the presence of sacrococcygeal teratoma. Following the surgical resection of a sacrococcygeal teratoma, monitoring serum AFP levels can assist in detecting potential recurrences. It is crucial to interpret AFP levels in conjunction with other clinical findings and imaging studies for an accurate assessment (19). In our case series, one case demonstrated a gradual normalization of AFP levels following surgery but was found to have a recurrence during follow-up. Follow-up ultrasonography in the other two children showed no evidence of recurrence after surgery. Their AFP levels gradually decreased but remained elevated, which may be attributed to the relatively short duration of postoperative follow-up (currently only 4 months).
Coccygectomy is considered the optimal approach to prevent benign teratoma recurrence while minimizing damage to the tumor cyst wall. However, sacrococcygeal teratomas can recur years after complete excision, necessitating lifelong monitoring. Although survival rates are generally excellent, mortality for tumors larger than 10 cm can reach 18%. Even with complete coccygectomy, recurrence rates range from 11% to 22%, mandating close follow-up—consisting of a physical examination (including a rectal examination), diagnostic imaging, and AFP measurements—every 3–6 months for at least 3 years (20). In our case series, one patient experienced recurrence 1 year after the initial surgery and was successfully treated with a subsequent operation, showing no recurrence at the 2-year follow-up. The second patient remained recurrence-free at the 4-month follow-up.
Given the tumor's distinctive location, both bladder and rectal functions are widely recognized as being vulnerable to impairment from the tumor itself, surgical intervention, or both. Existing data indicate that bladder dysfunction may be underreported in individuals with benign sacrococcygeal teratoma (21). In our case series, one patient was found to have persistent congenital megacolon after surgery, without any observed bladder dysfunction. The other two patients showed no evidence of rectal or bladder dysfunction following surgery.
Neonatal hypothermia is also a concern during surgical procedures due to neonates’ low subcutaneous fat and immature thermoregulation, which makes them especially susceptible to intraoperative hypothermia. Before the neonate enters the operating room, it is essential to appropriately raise the room temperature and place an inflatable warming blanket on the surgical bed. Intraoperatively, the neonate's vital signs should be closely monitored. In all three of our cases, no intraoperative hypothermia was observed.
Prenatal ultrasound plays a crucial role in the management of sacrococcygeal teratomas, not only in the early detection and evaluation of the tumor's nature but also in the continuous monitoring of tumor growth and prenatal multidisciplinary team (MDT) interventions (22). Regular prenatal ultrasound examinations allow for precise measurement of the tumor's location and size (22). Based on the findings from a prenatal ultrasound and fetal MRI monitoring, an MDT consisting of pediatric surgeons, fetal medicine specialists, neonatologists, ultrasonographers, and obstetricians can assess each case individually, decide on the mode and timing of delivery, and formulate a postnatal treatment plan. This approach shortens the time required for a postnatal diagnosis and treatment in affected infants (22). The involvement of an experienced multidisciplinary team is crucial for providing comprehensive care, as their collective expertise enables them to evaluate the risks and benefits of various management options and develop a tailored treatment plan (19).
Conclusion
Giant neonatal sacrococcygeal teratomas are rare, high-risk tumors associated with significant operative morbidity and mortality. We presented three neonatal cases, outlining our diagnostic and management strategies. Given the disease's rapid progression, clinicians must prepare comprehensive contingency plans; interdisciplinary collaboration is vital to improve survival outcomes and reduce complications.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
This study involving humans was approved by the Baoan Women's and Children's Hospital. The study was conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
LW: Conceptualization, Writing – review & editing, Supervision, Investigation, Writing – original draft, Data curation, Formal analysis, Methodology. YN: Supervision, Writing – review & editing. JW: Writing – review & editing, Methodology. XL: Conceptualization, Writing – review & editing. BZ: Writing – review & editing, Supervision. ZL: Writing – original draft, Writing – review & editing, Formal analysis, Funding acquisition, Investigation, Methodology.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that Generative AI was used in the creation of this manuscript. ChatGPT and Kimi were used to assist in reading the literature and article translation.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sheng QS, Xu XM, Cheng XB, Wang WB, Chen WB, Lin JJ, et al. Multi-stage resection and repair for the treatment of adult giant sacrococcygeal teratoma: a case report and literature review. Oncol Lett. (2015) 10:425–9. doi: 10.3892/ol.2015.3249
2. Maneira-Sousa P, Rocha G, Soares P, Arnet V, Guimarães S, Fragoso AC, et al. Massive sacrococcygeal teratoma in a preterm infant. Clin Case Rep. (2021) 9:1183–6. doi: 10.1002/ccr3.3722
3. van Heurn LJ, Coumans A, Haak MC, van der Kaaij A, van Heurn LWE, Pajkrt E, et al. Prognostic accuracy of factors associated with poor outcome in prenatally diagnosed sacrococcygeal teratoma: a systematic review and meta-analysis. Prenat Diagn. (2023) 43:1495–505. doi: 10.1002/pd.6457
4. Ding Y, Yang M, Lv M, Jiang Y, Dong T, Zhao B, et al. The ex-utero intrapartum treatment (EXIT) strategy for fetal giant sacrococcygeal teratoma with cardiac insufficiency: a case report and review of the literature. Front Oncol. (2022) 12:1035058. doi: 10.3389/fonc.2022.1035058
5. Fumino S, Tajiri T, Usui N, Tamura M, Sago H, Ono S, et al. Japanese clinical practice guidelines for sacrococcygeal teratoma, 2017. Pediatr Int. (2019) 61:672–8. doi: 10.1111/ped.13844
6. Yan W, Pan W, Chen K, Wu W, Huang S, Wang J, et al. Diagnoses, managements and outcomes of sacrococcygeal teratoma in neonates: a report of 104 cases at a single center. Chin J Pediatr Surg. (2024) 45:237–41. doi: 10.3760/cma.j.cn421158-20231113-00482
7. Rios LT, Araujo Júnior E, Nardozza LM, Moron AF, Martins Mda G. Prenatal diagnosis of sacrococcygeal teratoma using two and three-dimensional ultrasonography. Case Rep Obstet Gynecol. (2012) 2012:131369. doi: 10.1155/2012/131369
8. Rattan KN, Singh J. Neonatal sacrococcygeal teratoma: our 20-year experience from a tertiary care centre in north India. Trop Doct. (2021) 51:209–12. doi: 10.1177/0049475520973616
9. Wilson RD, Hedrick H, Flake AW, Johnson MP, Bebbington MW, Mann S, et al. Sacrococcygeal teratomas: prenatal surveillance, growth and pregnancy outcome. Fetal Diagn Ther. (2009) 25:15–20. doi: 10.1159/000188056
10. Kum CK, Wong YC, Prabhakaran K. Management of fetal sacroccocygeal teratoma. Ann Acad Med Singap. (1993) 22:377–80.8373123
11. Gross SJ, Benzie RJ, Sermer M, Skidmore MB, Wilson SR. Sacrococcygeal teratoma: prenatal diagnosis and management. Am J Obstet Gynecol. (1987) 156:393–6. doi: 10.1016/0002-9378(87)90290-0
12. Neupane D, Dahal A, Lageju N, Jaiswal L, Bista N, Sapkota A. Giant sacrococcygeal teratoma in a neonate: illustrative case. J Neurosurg Case Lessons. (2022) 3:1–4. doi: 10.3171/CASE22125
13. Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, Wilson RD, et al. Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J Pediatr Surg. (2004) 39:430–8; discussion 438. doi: 10.1016/j.jpedsurg.2003.11.005
14. Kremer ME, Wellens LM, Derikx JP, van Baren R, Heij HA, Wijnen MH, et al. Hemorrhage is the most common cause of neonatal mortality in patients with sacrococcygeal teratoma. J Pediatr Surg. (2016) 51:1826–9. doi: 10.1016/j.jpedsurg.2016.07.005
15. Usui N, Kitano Y, Sago H, Kanamori Y, Yoneda A, Nakamura T, et al. Outcomes of prenatally diagnosed sacrococcygeal teratomas: the results of a Japanese nationwide survey. J Pediatr Surg. (2012) 47:441–7. doi: 10.1016/j.jpedsurg.2011.08.020
16. Grammatikopoulou I, Kontomanolis EN, Chatzaki E, Chouridou E, Pavlidis P, Papadopoulos EM, et al. Immature malignant sacrococcygeal teratoma: case report and review of the literature. Clin Exp Obstet Gynecol. (2013) 40:437–9.24283184
17. Peiró JL, Sbragia L, Scorletti F, Lim FY, Shaaban A. Management of fetal teratomas. Pediatr Surg Int. (2016) 32:635–47. doi: 10.1007/s00383-016-3892-3
18. Phi JH. Sacrococcygeal teratoma: a tumor at the center of embryogenesis. J Korean Neurosurg Soc. (2021) 64:406–13. doi: 10.3340/jkns.2021.0015
19. Kornete A, Bokucava D, Vedmedovska N. Fetal sacrococcygeal teratoma: a case report of a giant tumor with an excellent outcome. Acta Med Litu. (2023) 30:206–15. doi: 10.15388/Amed.2023.30.2.13
20. Sabir WN, Ahmed SM, Hasan KM, Mohammed BA, Kareem HO, Najmadden ZB, et al. Giant sacrococcygeal teratoma in an infant: a case report with a literature review. Ann Med Surg (Lond). (2023) 85:5666–9. doi: 10.1097/ms9.0000000000001274
21. Berger M, Heinrich M, Lacher M, Hubertus J, Stehr M, von Schweinitz D. Postoperative bladder and rectal function in children with sacrococcygeal teratoma. Pediatr Blood Cancer. (2011) 56:397–402. doi: 10.1002/pbc.22887
Keywords: sacrococcygeal teratoma, neonates, case report, comprehensive contingency plans, interdisciplinary collaboration
Citation: Wu Ri Ni L, Nong Y, Wang J, Liang X, Zhang B and Lin Z (2025) Case Report: Giant sacrococcygeal teratoma in neonates—a report of three cases. Front. Pediatr. 13:1600920. doi: 10.3389/fped.2025.1600920
Received: 27 March 2025; Accepted: 25 June 2025;
Published: 22 July 2025.
Edited by:
Maria Marcela Bailez, Garrahan Hospital, ArgentinaReviewed by:
José Estevão-Costa, University of Porto, PortugalBruno Martinez-Leo, Momentum Foundation for Congenital Colorectal Diseases, AC, Mexico
Anup Bista, NHS Ayrshire and Arran, United Kingdom
Copyright: © 2025 Wu Ri Ni, Nong, Wang, Liang, Zhang and Lin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhenying Lin, MTk2MTMyNzQ3MEBxcS5jb20=
 Letu Wu Ri Ni
Letu Wu Ri Ni Baoxin Zhang
Baoxin Zhang Zhenying Lin
Zhenying Lin