- 1Department of Epidemiology, School of Public Health, Sun Yat-Sen University, Guangzhou, China
- 2School of Psychology and Counselling, Queensland University of Technology, Brisbane, QLD, Australia
- 3Women’s and Children’s Hospital of Longhua District of Shenzhen, Shenzhen, China
- 4School of Health, Xinhua College of Guangzhou, Guangzhou, China
Objective: Childhood obesity has become a global public health crisis. Previous studies have shown that nutritional supplementation during pregnancy may be protective against offspring obesity. However, the research in this area is still emerging and the impact of moderators, such as birth weight, upon outcomes has not been fully explored. This study aimed to examine the combined effect of maternal supplementation with iron, calcium, folic acid, and multivitamin during pregnancy on the risk of obesity in Chinese preschoolers born macrosomia.
Methods: A total of 6,031 singleton children, born macrosomia, aged 3–6.5 years old were recruited from Longhua District in Shenzhen of China in 2021. Their mothers were asked to complete a structured questionnaire for collecting the sociodemographic characteristics of the child and parents, the child's birth-related characteristics, and maternal supplementation with iron, calcium, folic acid, and multivitamins during pregnancy. The children's weight and height were measured using a standardized method by well-trained medical staff from the Women's and Children's Hospital of Longhua District of Shenzhen.
Results: After controlling for confounding variables, including other nutrients, the results of a series of logistic regressions showed that only iron supplementation (AOR = 0.75, 95% CI = 0.60–0.92) during pregnancy was negatively associated with the presence of obesity in preschoolers born macrosomia in boys. In contrast, there was no independent associations between maternal prenatal ingestion of iron, calcium, folic acid, or multivitamin supplements and obesity in preschool girls born macrosomia. Examination of interaction effects through crossover analyses showed that maternal supplementation with both iron and calcium (AOR = 0.68, 95% CI = 0.49–0.94), and both iron and multivitamins (AOR = 0.64, 95% CI = 0.48–0.86) during pregnancy significantly reduced the risk of obesity in male preschoolers born macrosomia. Furthermore, interaction analysis found a multiplicative interaction between maternal iron and multivitamin supplementation during pregnancy on the risk of obesity in male preschoolers born macrosomia (IOR = 0.52, 95% CI = 0.35–0.79).
Conclusion: Our findings suggest that iron supplementation during pregnancy may reduce the risk of obesity in preschoolers born macrosomia in boys, with this effect enhanced with the combined ingestion of calcium and multivitamin supplementation.
1 Introduction
The worldwide public health crisis of childhood obesity has become a reality. Overweight and obese children had an increase in prevalence from 4.2% in 1990 to 6.7% in 2010, as revealed by cross-sectional studies in 144 countries, which represents a relative increase of 60% (1). In 2016, the number of obese girls and boys worldwide exceeded 50 million and 74 million respectively (2). By 2030, it is expected that the prevalence rate of obesity in Chinese children aged 0–7 will hit 6.0%, meaning that the prevalence of obesity among Chinese children will rise to 6.64 million (3). Wang et al. conducted a survey in Chinese aged 3–7 years and found that there was a 10.38% obesity prevalence rate among preschoolers, with the detection rate of obesity in boys was twice as high as that in girls (4). Overweight or obese children are more prone to psychiatric disorders such as depression, anxiety, and low self-esteem (5, 6), and more susceptible to a variety of metabolic and cardiovascular diseases including hypertension, hyperlipidemia, and type 2 diabetes mellitus (7–10). Moreover, a cohort study found that about three-fourths of overweight or obese children continued to have excess weight into adulthood (11). As such, obesity in childhood results in significant lifetime economic and social costs, including increased spending on health care, reduced wages, and reduced likelihood of employment (12–15). It is therefore critical to identify risk and protective factors for childhood obesity to steer the creation of potent public health measures.
Childhood obesity is the result of a multifactorial combination of genetics, prenatal and postnatal lifestyle, and the environment. In terms of genetic factors, the genes most studied in relation to obesity include the FTO and MC4R genes, while the TCF7l, MTNR1B, ADRB3, INSIG2, APOB48R genes have been related to glucose and lipid metabolism (16). Prenatal factors like mother's pre-pregnancy BMI and father's BMI have been strongly associated with offspring obesity (17). The risk of obesity increases for children born via cesarean section compared to those born via vaginal delivery (18, 19). In addition, rapid maternal weight gain during pregnancy, environmental tobacco exposure and maternal alcohol consumption affect fetal metabolism and growth and further result in increased rates of childhood obesity (20–23). Similarly, excessive catch-up growth of premature infants and low birth weight infants can lead to childhood obesity (24). Furthermore, an earlier meta-analysis demonstrated that macrosomia was an independent risk factor for obesity (25). In addition, postnatal influences on childhood obesity have been well studied. For example, the short duration of breastfeeding, irrational feeding practices, short sleep, low physical activity and long screen time exacerbated the likelihood of obesity in children (26–28).
Macrosomia, a term used to describe a fetus that weighs more than or equal to 4 kg at birth, is associated with gestational diabetes mellitus, pre-pregnancy weight, weight gain during pregnancy, and nutritional factors during pregnancy (29). In recent years, the prevalence of macrosomia has been 8.7% in China (30). A similar incidence rate of 8.07% has recently been found in the United States (31). These high incidence rates are concerning given that macrosomia increases the incidence of cesarean section, perinatal and neonatal complications, compared with normal birth weight fetuses (32, 33). Macrosomia have been exposed to an adverse environment of hyperinsulinemia and hyperglycemia in the uterus, resulting in increased fat and protein storage in the fetus (34). According to the Development Origins of Health and Disease Hypothesis (DOHaD) (35), malnutrition during fetal development can promote obesity through metabolic, genetic and behavioral changes. Studies based in France (36) and China (37) have found that higher birth weight and macrosomia are positively correlated with high BMI growth trajectory patterns of children in the early postnatal period, a prospective cohort study in Asia have suggested that the rapid growth pattern of BMI trajectory in infancy and early childhood will increase the risk of overweight/obesity in children in the future (38). Macrosomia is known to be an independent risk factor for childhood obesity, but not all children born macrosomia will become obese. Early nutrition may influence the incidence of macrosomia and subsequent metabolic patterns through persistent changes in DNA methylation, increasing susceptibility to certain chronic diseases (39, 40). Given that early nutrition can have an impact on the formation of macrosomia and may also have an effect on childhood obesity after birth, it is very necessary to pay attention to the future obesity situation of this special group of macrosomia. So the authors wondered what role nutritional factors play in childhood obesity in macrosomia.
Maternal malnutrition during pregnancy can lead to adverse birth outcomes such as macrosomia and low birth weight infants, and an increased risk of childhood obesity (41–43). Iron is a component of hemoglobin. The decrease in hemoglobin concentration due to increased blood volume during pregnancy leads to maternal iron-deficiency anemia. Prior studies have shown that the correlation between pregnant women's hemoglobin concentration and adverse birth outcomes in offspring is U-shaped (41, 42). The risk of future metabolic diseases, including obesity, could be reduced by taking iron supplementation during pregnancy (44). Calcium supplementation during pregnancy might also be beneficial in reducing the risk of gestational diabetes (GDM) and hypertensive disorders of pregnancy (45, 46). Maternal calcium dysfunction directly affects the synthesis and metabolism of fat in offspring (47). Folic acid during pregnancy can reduce the risk of fetal neural tube malformations, megaloblastic anemia, and pregnancy hypertension (45, 48). Wang et al. found, after adjusting for maternal weight during pregnancy, that children of mothers with adequate folate concentrations had a 43% lower risk of overweight or obesity compared to children of mothers with low folate concentrations (49). Multivitamins refer to products that are synthesized in A specific dose ratio and contain multiple vitamins including A, B, C, D, E and K. Multivitamin supplementation has been shown to effectively reduce the occurrence of complications during pregnancy, the risk of preterm birth, low birth weight, and the birth of macrosomia (50, 51).
Thanks to the extensive health education and free folic acid distribution policy in China, the utilization of nutritional supplements during pregnancy is high. The results of a 2018 survey in China showed that 82.03% of expecting mothers took nutritional supplements during pregnancy, especially with a relatively high supplementation rate of calcium and folic acid (52). Calcium supplementation is mainly due to the burden on bones caused by the weight gain of pregnant women and the demands of the fetus (46, 53). The relatively low iron supplementation rate might be due to the effectiveness of iron-containing diet intervention and the side effects of iron supplements (54, 55), but it is undeniable that iron remains one of the most commonly used nutritional supplements during pregnancy. Due to the significant increase in the demand for a variety of vitamins during pregnancy, single-vitamin supplementation may not always be sufficient to meet the nutritional needs of pregnant women. Therefore, we selected the above four nutritional supplements (iron, calcium, folic acid and multivitamins) as independent variables in this study.
The objective of this study, therefore, was to examine the combined effects of prenatal iron, calcium, folic acid and multivitamin supplementation on obesity in preschoolers born macrosomia in China. To provide a basis for the precise classification and management of obesity in the future.
2 Methods
2.1 Study population
All kindergartens in Longhua District, Shenzhen, China were surveyed for the study population, which comprised 69,639 mother-infant pairs, 7,078 of these children were macrosomia. We used the definition of macrosomia as birth weight greater than or equal to 4 kg, and the preschool age range included 3–6.5 years. Because of missing data (26 pairs due to height information, 89 pairs due to weight information, 528 pairs for mother's age at birth, 183 pairs for father's age at birth, 239 pairs for age at birth, 200 pairs for father's literacy, 157 pairs for mother's pre-pregnancy body mass index, 1 pair for monthly family income status, 1 pair for mode of delivery, and 29 pairs for maternal gestational hypertension), data from a total of 961 pairs were excluded. In addition, data from 86 pairs were excluded due to a combination of either the child or parent's age being outside the inclusion criteria (53 pairs whose children's age was higher or lower than preschool age, and 58 pairs whose parents' age was above or below the childbearing age). The final sample used in the data analysis involved 6,031 mother-child pairs of macrosomia. Written informed consent was obtained from all participants at enrollment. The study protocol was approved by the Ethics Committee of the School of Public Health, Sun Yat-sen University.
2.2 Data collection
Mothers who were enrolled in this study were asked to complete a self-report structured questionnaire for collecting sociodemographic characteristics of the child and parents (such as age, sex, pre-pregnancy maternal BMI, parental marital status, parental education level, household income, and whether or not the child was an only child), prenatal maternal supplementation with iron, calcium, folic acid, and multivitamins, as well as variables related to birth (mode of delivery, gestational age, and birth weight). The obtained data were verified accordingly with the data in the maternal and child health system, and the missing information during the on-site investigation was supplemented as much as possible to ensure the accuracy of the data.
2.3 Measurement of prenatal maternal nutrient supplementation
We collected data on iron, calcium, folic acid, and multivitamin supplementation during pregnancy using the following questions: (1) Did the mother take iron supplements during pregnancy? (2) Did mothers take calcium supplements during pregnancy? (3) Did the mother take folic acid supplements during pregnancy? (4) Did the mother take multivitamins supplements during pregnancy? The answer to each question is 1 for “yes” and 0 for “no”. In the survey, pregnant women were asked to consider whether they took nutritional supplements according to the doctor's recommendation.
2.4 Measurement and definition of obesity
The well-trained medical staffs from Women's and Children's Hospital of Longhua District of Shenzhen were responsible for measuring children's weight and height using a portable electronic scale (cent value = 0.01 kg) by placing the scale on a flat surface and having the preschooler stand in the center of the scale with his/her hat off, bare feet, and wearing tight-fitting lightweight clothing. After the value had stabilized, the medical staff read and recorded the measurement with an accuracy of 0.01 kg. Height was measured using a column-type body altimeter (cent value = 0.1 cm). The column-type body altimeter was placed vertically against the wall on a horizontal floor. Preschoolers were asked to stand on the pedal with heels closed, feet separated at a 60-degree angle, chest raised, abdomen tucked in, eyes looking straight ahead, and the back of the head, buttocks, and heels pressed against the column. The medical staff slid a slider to the top of the skull of the child being measured and read the measurements at a height level with the slider.
In this study, we applied the index of body mass index (BMI) as a criterion of childhood obesity diagnostic criteria, and BMI was calculated by dividing weight (kilograms) by the square of height (meters). Obesity was characterized by a BMI that fell within or exceeds the reference values for gender and age, which were derived from normative data obtained from two representative national cross-sectional surveys in China: the National 2005 Survey of Growth and Development of Children Under 7 Years of Age in Nine Cities of China and the 2005 National Monitoring of Primary and Secondary School Students' Physical Fitness and Health. Based on the above data, Li Hui et al. recommend cut-off points for obesity in Chinese children aged 2∼18 years which involved an age-specific BMI above the 96.3rd percentile for boys and the 98.0th percentile for girls (56). These cut-offs were used in the present study. A systematic review study of children and adolescents aged 2–19 years has supported the use of BMI measured by health professionals as a valid method of assessing body fat in children and adolescents (57).
2.5 Potential confounding variables
Based on previous findings (58, 59), potential confounding variables included in this study were the child's sex, child's age, mode of delivery, parents' age at the childbirth, maternal prepregnancy BMI, marital status, parents' education level, family income, whether the only child, gestational diabetes mellitus, patterns and duration of breastfeeding, frequency of outdoor activities at age 0–1 years, outdoor activities of time 0–1 years of age, nutritional status of 0–1 years old, frequency of outdoor activities at age 1–3 years, outdoor activities of time 1–3 years of age and nutritional status of 1–3 years old. Moreover, previous research results in our group have shown that supplementation of nutrients such as folic acid during pregnancy can affect pre-school obesity in children who are small for gestational age (60), so it is necessary to adjust other nutrients in the model.
2.6 Statistical analyses
Continuous variables were described by mean and standard deviation (SD), and categorical variables were described by frequency and percentage. Comparisons of categorical and numerical variables were performed using chi-square and t-tests, respectively.
A series of binary logistic regression analyses, controlling for potentially confounding variables were conducted to test the association between nutritional supplementation with iron, calcium, folic acid, and multivitamins during pregnancy and obesity in preschoolers born macrosomia. Their multiplicative interactions on obesity were tested by creating multiplicative terms in the logistic regression models, and the strength of the multiplicative interactions was expressed as the interaction of odds ratio (IOR). If the 95% CI of the IOR spanned 1, the multiplicative interaction was considered non-significant. Moreover, crossover analyses were utilized to assess the additive interaction effects and modification effects among the combination of four nutrients. Adjusted odds ratio (AOR), relative excess risk due to interaction (RERI), and attributable proportion due to interaction (AP) were calculated to quantify the magnitude of these effects. If the 95% CIs of RERI and AP did not span 0, the additive interaction was considered statistically significant. Furthermore, after stratifying by sex, the previously mentioned analyses were replicated to assess the sex-specific relationships between prenatal supplementation and obesity in preschoolers born with macrosomia.
Considering the existence of missing data, sensitivity analysis was used to compare with the results of the main analysis content to ensure the reliability of the analysis results.
RStudio version 4.1.2 (Poist, BOSTON, MA, USA) was used for all statistical analyses, and two-tailed p-values < 0.05 were considered statistically significant.
3 Results
3.1 Characteristics of participants
The demographic characteristics of the participants can be found in Table 1, with 62.38% of the children in the study being male and 37.62% being female. The children's mean age was 4.86 years (SD = 0.84), the father's mean age was 31.69 years (SD = 5.10), and the mother's mean age was 29.59 years (SD = 4.46). The mean birth weight of the children at birth was 4.25 kg (SD = 0.42) and the gestational week at delivery was 39.20 weeks (SD = 2.04). Vaginal deliveries (52.33%) were slightly more numerous than other modes of delivery such as cesarean section (47.67%), only 530 mothers (8.79%) admitted to a history of gestational diabetes. The mean height of the children was 111.16 cm (SD = 8.06), the mean weight was 19.83 kg (SD = 4.05), the mean BMI of the children was 15.99 kg/m2 (SD = 2.37), and the mean pre-pregnancy BMI of the mothers was 21.47 kg/m2 (SD = 3.03). Only 32.20% of the children in the study population were reported as having no siblings, and the vast majority of children had married parents (98.64%). The percentage of fathers with a high school education or above was 87.71%, while for mothers, it was 84.88%. More than three-quarters of the participants had a monthly income exceeding 10,000 CNY (86.12%). 60% of the children were exclusively breastfed after birth for an average of 9.19 months. Three quarters of children engaged in outdoor physical activity more than three times per week and more than half spent more than 60 min per time outdoors at the age of 0–1 years. More than 99 percent of parents considered their children's nutritional status to be average or above at age 0–1. By the age of 1–3 years, 69.11% of the children had outdoor exercise more than three times per week, 67.72% of the children had outdoor exercise more than one hour per time, and only 1.11% of the parents thought their children's nutritional status was poor.
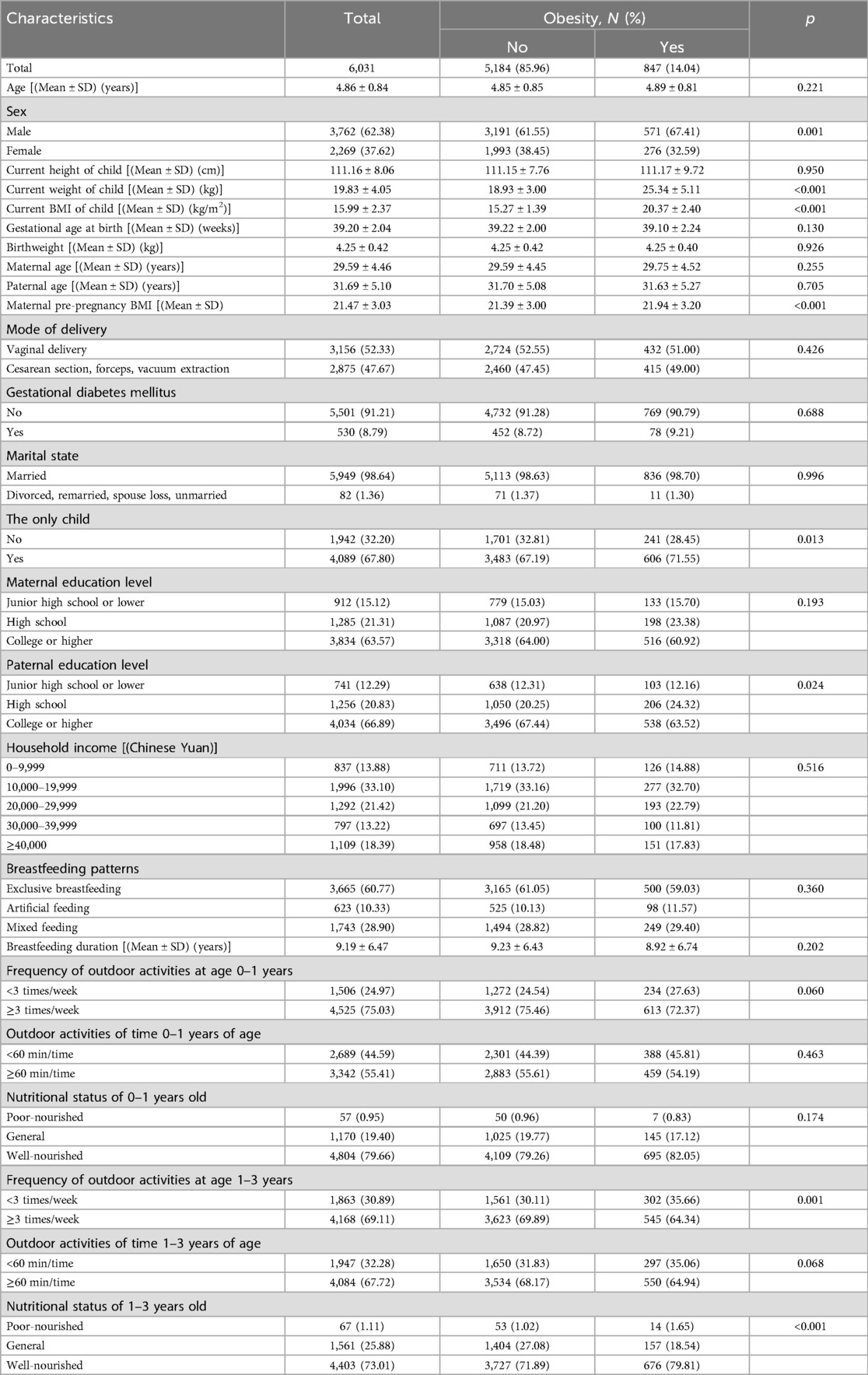
Table 1. Comparison of demographic characteristics between obese and non-obese children born macrosomia.
The prevalence of obesity in preschoolers born macrosomia was 14.04%, and the weight and BMI levels of obese children were significantly higher than those of the normal-weight population. There were significant differences in children's sex, maternal pre-pregnancy BMI, being the only child, father's education level, frequency of outdoor activities at age 1–3 years, and nutritional status of 1–3 years old.
3.2 Associations between the maternal nutrients supplementation during pregnancy and obesity in preschoolers born macrosomia
After controlling for potential confounding variables except nutrients, the results of logistic regression analyses showed that maternal supplementation with iron (AOR = 0.76, 95% CI = 0.65–0.90), calcium (AOR = 0.74, 95% CI = 0.61–0.91), and folic acid (AOR = 0.77, 95% CI = 0.60–0.98) during pregnancy were significantly and negatively associated with obesity in preschoolers born macrosomia. In contrast, no significant association was found between multivitamin supplementation during pregnancy and childhood obesity (Table 2). After further adjustment of other nutrients on this basis, only iron supplementation during pregnancy (AOR = 0.79, 95% CI = 0.67–0.94) was negatively associated with obesity in preschoolers born macrosomia. The adjustment process for single or two nutrients is detailed in Supplementary Tables S1, S2. Sensitivity analysis showed there was a significant effect of calcium supplementation during pregnancy on childhood obesity in girls (AOR = 0.79, 95% CI = 0.64–0.98), as shown in Supplementary Table S10.
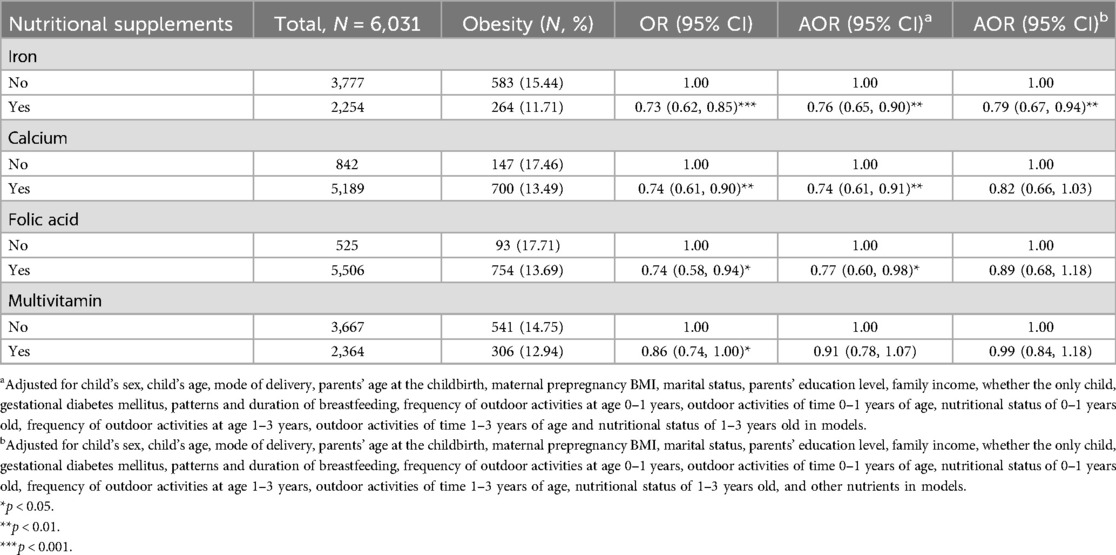
Table 2. Association of maternal nutritional supplements with obesity in preschoolers born macrosomia.
3.3 Combined effects of maternal nutrients supplementation during pregnancy on obesity in preschoolers born macrosomia
Table 3 presents the combined effects of maternal nutritional supplementation during pregnancy on obesity in preschoolers born macrosomia. After adjusting for potential confounding variables, the results of the crossover analyses showed that maternal supplementation with iron and calcium (AOR = 0.68, 95% CI = 0.52–0.88), iron and folic acid (AOR = 0.71, 95% CI = 0.51–0.98), iron and multivitamin (AOR = 0.75, 95% CI = 0.59–0.94) during pregnancy all significantly reduced the likelihood of obesity in preschoolers born macrosomia. There was a multiplication interaction effect between iron and multivitamin supplementation during pregnancy on obesity in preschoolers born macrosomia (IOR = 0.70, 95% CI = 0.50–0.97). The results of the crossover analysis adjusting separately for the other nutrient are detailed in Supplementary Table S3. Sensitivity analyses of iron supplementation showed the same results (Supplementary Table S11).
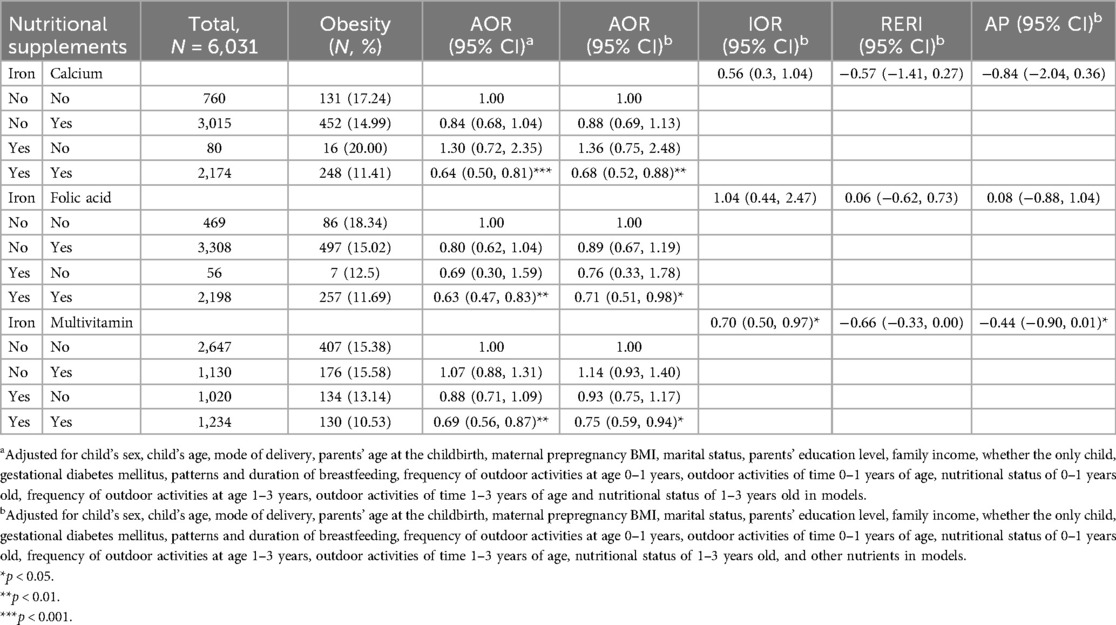
Table 3. Combined effects of maternal nutritional supplements on obesity in preschoolers born macrosomia.
3.4 Combined effects of maternal nutrients supplementation during pregnancy on obesity in preschoolers born macrosomia after stratification by sex
After stratification by sex, the results of the logistic regression indicated that maternal iron supplementation (AOR = 0.75, 95% CI = 0.60–0.92) during pregnancy was significantly negatively associated with obesity in male preschoolers born macrosomia (Table 4). In girls, after controlling for the specified confounding variables except the other nutrients, the results of the logistic regression showed that maternal calcium supplementation (AOR = 0.67, 95% CI = 0.47–0.96) during pregnancy was negatively associated with the presence of obesity in female preschoolers born macrosomia. However, after adjusting for the other three nutrients, no significant results were found, especially after adjusting for folic acid (Supplementary Table S6), with the results showing the protective effect of calcium supplementation during pregnancy against preschool obesity was less significant. The adjustment process for single or two nutrients is detailed in Supplementary Tables S4–S7. Sensitivity analysis showed the same results in boys. There was a marginal effect of calcium supplementation during pregnancy on childhood obesity in girls (AOR = 0.69, 95%CI = 0.46–1.01), as shown in Supplementary Table S12.
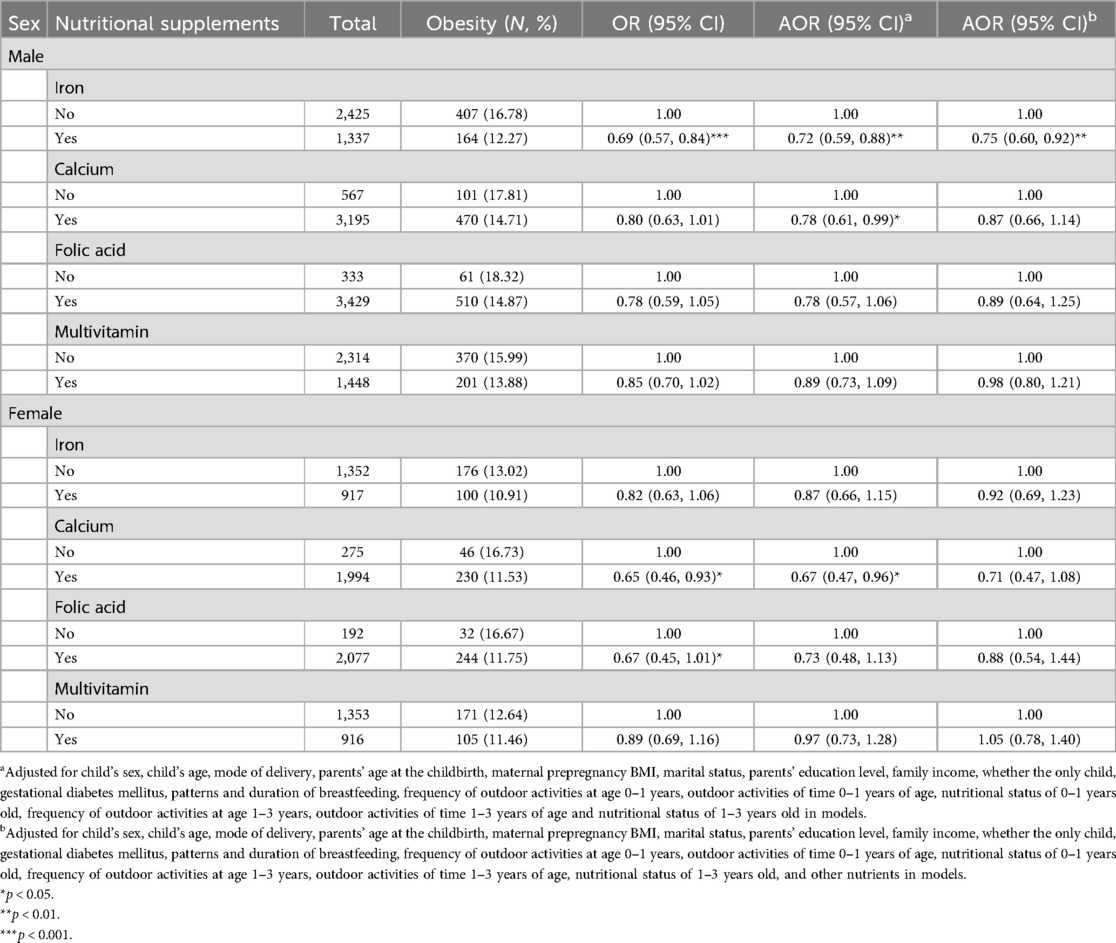
Table 4. Association of maternal nutritional supplements with obesity in preschoolers born macrosomia after stratification by sex.
Table 5 presents the combined effects of maternal supplementation during pregnancy on obesity in preschoolers born macrosomia after stratification by sex. Among boys, the results of the crossover analyses showed that maternal supplementation with iron and calcium (AOR = 0.68, 95% CI = 0.49–0.94), iron and multivitamin (AOR = 0.64, 95% CI = 0.48–0.86) during pregnancy significantly reduced the risk of obesity in male preschoolers born macrosomia. The results from the sensitivity analysis in boys also showed that supplementation of iron and folic acid (AOR = 0.65, 95% CI = 0.44–0.95) during pregnancy significantly reduced the risk of obesity in preschoolers born macrosomia (Supplementary Table S13). A multiplicative interaction between maternal iron and multivitamin supplementation during pregnancy on obesity was found in male preschoolers born macrosomia (IOR = 0.52, 95% CI = 0.35–0.79). Among girls, none of the cross-over analyses were significant. An additive interaction between maternal iron and folic acid supplementation during pregnancy on obesity was observed in female preschoolers born macrosomia (REFI = 0.65, 95% CI = 0.03–1.33, AP = 0.85, 95% CI = −0.18–1.88). The results of the crossover analysis adjusting separately for the other nutrient are detailed in Supplementary Table S8.
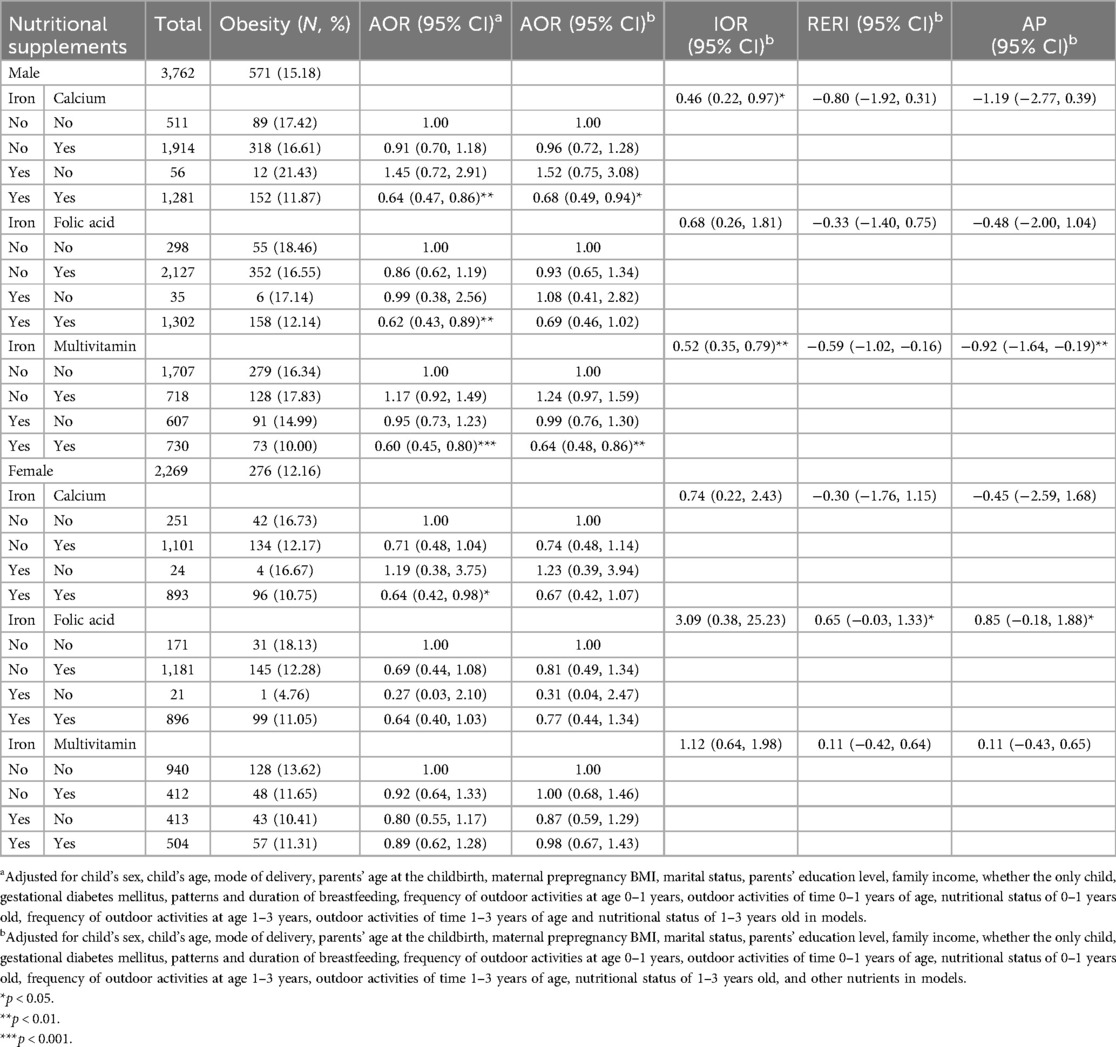
Table 5. Combined effects of maternal nutritional supplements on obesity in preschoolers born macrosomia after stratification by sex.
4 Discussion
To our knowledge, this is the first study to examine the combined effects of maternal supplementation with iron, calcium, folic acid, and multivitamin during pregnancy on obesity in preschoolers born macrosomia in China. The results of supplementary correlation tests showed that the ingestion of any two nutrients were not completely independent, but the correlation coefficients were all small (Supplementary Table S9).
4.1 Associations between the maternal iron supplementation during pregnancy and obesity in preschoolers born macrosomia
Iron supplementation during pregnancy was found to be significantly associated with a reduced risk of obesity in preschoolers born with macrosomia. However, this result was only found in boys. Previous studies on the relationship between maternal iron supplementation during pregnancy have mostly focused on birth weight and the presence of obesity in adulthood, with few studies examining the impact upon obesity during the early childhood period. The Cambridge Baby Growth Study found that at 3 months of age, infants from high-income families, whose mothers had received iron supplementation during pregnancy, had 0.15 mm thicker skinfold than those whose mothers had not received iron supplementation. However, no such differences in obesity indicators were found in subsequent follow-up at 2 years and 9.5 years of age (61). Similarly, previous results from another birth cohort in the United Kingdom did not find an association between maternal iron intake during pregnancy and obesity in children at age 10 years (62). Studies using C282Y of the hemochromatosis gene (HFE) as an instrumental variable of iron status also failed to find an association between iron during pregnancy and obesity in adult offspring (63). The differences in the above results may be due to the differences in different ethnic populations, the ages of the research subjects, and the corresponding obesity screening indicators.
However, the results of experiments with rats show that iron supplementation during pregnancy can reduce plasma triglyceride levels in fetus and adult rats, and reduce the risk of metabolic diseases such as obesity by down-regulating the expression of genes involved in bile acid synthesis and fatty acid synthesis pathways, especially cholesterol 7α-hydroxylase and its upstream regulator liver X receptor α (44). Perinatal iron deficiency in Dawley rats increases the effect of high-fat diet on offspring body weight, and this body weight gain is mainly due to the accumulation of visceral fat (64). Hepcidin is a major inhibitor of intestinal iron absorption. Emanuele et al. found that hepcidin concentration was significantly increased in obese children and showed a clear dose-response relationship (65). Maternal pre-pregnancy BMI, iron supplementation, erythropoiesis-stimulating activity and inflammatory response all affect maternal hepcidin secretion (66). Nicole et al. found that the amount of placental transfer of maternal iron and neonatal iron absorption were related to the levels of inflammatory factors. Although the study did not directly observe the correlation with maternal hepcidin, this may be due to the relative intensity of maternal inflammation and iron depletion, which offset each other (67, 68). Given that iron is highly abundant in the placenta and is an essential cofactor for the formation of reactive oxygen species (ROS), excessive iron accumulation can lead to excessive ROS production and severe autophagy defects in the offspring (69, 70). At the same time, mitochondrial function defects and mitochondrial DNA damage caused by iron deficiency also increase ROS formation (71). The essence of obesity is a systemic chronic inflammatory response. Changes in iron content during pregnancy lead to changes in inflammatory response and oxidative status in offspring, resulting in metabolic or functional disorders, which in turn leads to increased susceptibility to obesity and its related complications (72). These biological mechanisms support our finding, and suggest that the impact of ingesting iron supplements during pregnancy on macrosomia may involve long-term programming effects.
Why did such results only occur in male children? In vitro experiments have found that apoptosis caused by iron-dependent lipid peroxidation induced by the transcription factor BACH1 can stimulate the secretion of FGF21 and inhibit obesity in mice on a high-fat diet (73). A study in the United States found dietary iron deficiency deregulates iron balance in the inguinal White adipose tissue (iWAT) and impairs adaptive thermogenesis, thereby escalating the diet-induced weight gain in male mice (74). An animal experiment in Japan found that iron supplementation regulates obesity and hepatic steatosis induced by a high-fat diet in male mice through mitochondrial signaling (75).A study in Beijing involving school-aged children found that epigenetic modifications of nuclear DNA and mitochondrial DNA were associated with the disorder of serum iron biomarkers in metabolically unhealthy obese children (76). Most of the discussions on the mechanism of iron on male obesity have focused on dietary induction after birth, but the mechanism also provides enlightenment on the effect of maternal iron dose on offspring.
4.2 Combined effects of maternal nutrients supplementation during pregnancy on obesity in preschoolers born macrosomia after stratification
In male macrosomia, we also found that there was a multiplicative interaction effect of maternal iron supplementation and multivitamin supplementation on preschool obesity, but no significant independent effect of maternal multivitamin supplementation on preschool obesity in male. Multivitamin supplementation during pregnancy could enhance the protective effect of iron supplementation on preschool obesity in boys born with macrosomia. In contrast to our findings, several studies in North American laboratories have shown that a high-multivitamin diet during pregnancy increases the development of an obese phenotype in male offspring, involving gut microbiota abundance and epigenetic changes in hypothalamic systems that regulate food intake. The high-vitamin diet group had higher leptin concentrations and leptin receptor expression and decreased proopiomelanocortin (POMC) expression related to appetite suppression (77–79). Multivitamin supplementation during pregnancy may have an obesogenic effect on the offspring Unfortunately, there are no studies on the mechanism of the combined effects of iron and multivitamins supplenmentation during pregnancy. As mentioned earlier, animal experiments have shown that rats with perinatal iron deficiency have increased visceral fat volume (64). In a mother-infant cohort study in Singapore, it was observed that maternal vitamin D levels in the second trimester of pregnancy were negatively correlated with neonatal abdominal subcutaneous adipose tissue volume, especially deep subcutaneous adipose tissue (DSAT), whose metabolism is similar to visceral adipose tissue (80). It is speculated that iron and vitamin D may interact at the binding sites of fat metabolism-related receptors and thus affect the outcome of obesity. However, the mechanisms and pathways of various nutrients on obesity are extremely complex, and further animal experiments and human trials are needed to prove.
The protective effect of iron supplementation on preschool obesity in boys born with macrosomia appeared to be enhanced by incorporating calcium supplementation simultaneously. No research has been conducted on the effects of ingesting iron and calcium supplements simultaneously during pregnancy on childhood obesity. After supplementation, calcium enters the body and is converted into calcium ions (Ca2+). As a second messenger, it mediates multiple non-selective cation channel family Transient Receptor Potential (TRP) channels in adipocytes, regulates the differentiation and maturation of white adipose tissue and the formation of brown adipose tissue (81). The novel tetraspanin MS4A15 interacts with Ca2+ to specifically block ferroptosis by altering the lipid profile of overexpressing cells (82). Iron and calcium can jointly regulate mitophagy and cell metabolism to affect homeostasis. Combined, this evidence supports our finding that prenatal iron and calcium supplementation may have a protective effect against offspring's obesity.
In female children with macrosomia, the results showed there was an additive interaction between folic acid and iron supplementation during pregnancy on preschool childhood obesity, but folic acid and iron supplementation had no significant association with preschool obesity. Therefore, the results should be interpreted with caution. However, in contrast a randomized controlled trial in China found no difference between iron-folic acid supplementation and iron supplementation alone (83). The Boston Birth cohort found a nonlinear L-shaped association between maternal plasma folate concentration in the third trimester and the risk of offspring overweight and obesity (mean age, 6.2 years) (49). The results of a systematic review has also supports that folic acid supplementation during pregnancy had a protective effect on offspring obesity and insulin resistance (84). Similarly, both iron and folic acid supplementation during pregnancy can reduce the risk of adverse pregnancy outcomes such as preterm birth and stillbirth and the mortality of offspring within 5 years of age (85, 86). In a previous study of small for gestational age infants, our research group found that iron supplementation during pregnancy enhanced the protective effect of folic acid supplementation during pregnancy on childhood obesity, but no significant interaction was found (60). As a methyl donor of one-carbon metabolism, folate participates in epigenetic regulation such as DNA methylation, DNA synthesis and repair, and plays an important role in normal fetal growth and development (87). Both in vitro and in mice, folic acid supplementation within the physiological range has been found to induce differential expression of growth genes in placental cells, thereby affecting fetal weight (88, 89). Animal experiments suggest that iron metabolism regulates folate-dependent one-carbon unit cycle metabolism (90), and supplementation of iron and folic acid can promote the transcription of genes encoding folate and ferroportin (91). The literature is still unclear regarding the interaction between iron and folate. For example, on the one hand Joanna et al. found that the combination of iron and folic acid significantly reduced iron levels in the liver and spleen of female rats (92). So far, no studies, outside our research group, have examined the combined effects of the two on obesity.
4.3 Sensitivity analyses
We excluded information from 1,047 mother-infant pairs before analysis. After excluding height, weight, and nutritional supplement missing data, there were 6,936 mother-infant pairs. In the sensitivity analyses based on this population with missing data, similar significant results of iron supplementation on preschool obesity in male macrosomia were found (Supplementary Tables S10–S13). However, in the sensitivity analysis among girls, the results of calcium supplementation showed a significant protective effect against preschool obesity in macrosomia, whereas only a marginal effect was found in our main analyses. In addition, we found that iron and folic acid supplementation during pregnancy could enhance the protective effect of calcium supplementation on preschool obesity in girls born with macrosomia. This indicates that the removal of missing information has a certain bias on the results, which affects the significance of some results. Based on 15 randomized controlled trials worldwide, a meta-analysis revealed that calcium supplementation (500–2,000 mg) can increase fetal birth weight (93). However, in contrast an exploratory analysis carried out by Karakis et al., calcium intake was not found to be related to obesity in children aged 5 years and above (94). This result may be due to the differences in the period, dosage and continuity of calcium supplementation during pregnancy. It has been found in obesity-prone rats that low calcium can lead to an increase in body fat and a decrease in body protein, thereby affecting early body weight (95). Li et al. found that abnormal calcium intake during pregnancy and lactation, such as calcium deficiency, low calcium, and high calcium, may increase adipocytes by encoding the adipogenic differentiation potential of offspring bone marrow mesenchymal stem cells and regulating the significant expression of Wnt/β-catenin signaling pathway, which aggravates obesity induced by high-fat diet in adulthood (96). Unfortunately, these results are based only on male offspring.
4.4 Sex specific differences
As described above, the relationship between supplementation and combined effects of different nutrients during pregnancy and preschool obesity in macrosomia differs between males and females. We propose that there may be a number of possible reasons for sex being a moderator in these associations. First, it is possible that this may be due sex differences in body fat composition, a difference that has been observed as early as the third trimester, suggesting that mesenchymal cells may have sex differences in determining the fate of muscle and adipocyte lineages (97). Second, experiments, by Gallou-Kabani et al., in mice showed that the overall methylation pattern of the placenta was different between males and females, even when they were exposed to the same uterine environment (98). Third, boys develop more rapidly in utero than girls, and boys have longer placenta but lower reserve capacity, increasing their vulnerability to malnutrition (99). Fourth, sex differences in hypothalamic gene expression of pro-melanocortin, leptin receptor (genes that inhibits food intake) and neuropeptide Y, agouti associated protein (genes that promotes food intake), which are involved in the regulation of food intake, were found in the rat study (100).
4.5 Limitation
The results of this study have some limitations as follows. First, this study is a retrospective observational study with all data about the use of nutritional supplementation coming from self-report of mothers 3–6.5 years post-birth. As such, their recall might have been be affected by memory bias. Second, the present study was qualitative and didn't collect information on the dose, frequency, and duration of nutrient use, or the serum levels of the nutrients investigated in this study. In addition to including these measures, in future studies, researchers should consider measuring biomarkers such as the nutritional concentrations in the mothers' or umbilical cord blood to validate self-reported supplementation and obtain quantitative information. Due to the lack of this quantitative information, the conclusions we draw are preliminary and need to be replicated using more precise measures of nutrient supplementation. This is a complicated issue that requires further validation studies given that the timing, dosage and duration of nutrient supplementation recommended by Chinese doctors is personalized for each mother and changes over the course of the pregnancy. Third, the study participants were all from Longhua District, Shenzhen, China. There may be some limitations in extrapolating the results from this sample to other populations due to differences in diet. Fourth, we only evaluated the effect of nutritional supplementation during pregnancy and did not measure maternal nutrient intake through diet during pregnancy. Future studies may also include the use of food frequency questionnaires to derive more accurate associations. Fifth, we used BMI as a measure of obesity. The index of BMI shows obvious racial and regional differences. The screening criteria adopted in this study were different from those of WHO and IOTF. Therefore, it will be important to replicate the findings of this study using other measures of obesity such as skinfold thickness and waist circumference, or utilizing bioelectrical impedance or dual-energy x-ray absorptiometry as measures of body fat. Sixth, obesity is a complicated disease caused by multiple factors, including genetic and behavioral factors, and the confounding factors adjusted for in this study wasn't exhaustive. Other potential covariates need to include in future studies such as paternal obesity, maternal diet during pregnancy and the dietary nutrition of infants or preschoolers. Seventh, we excluded information from 1,047 (14.79%) mother-infant pairs that could have caused selection bias before analysis. Eighth, we did not collect the information on the specific vitamins that comprised the multivitamin used by the mothers during pregnancy, so we could not identify the associations between specific combinations of vitamin supplementation and offspring' obesity.
5 Conclusions
To summarize, our study demonstrates that maternal iron supplementation during pregnancy significantly affects the likelihood of obesity in male preschoolers born with macrosomia. Calcium and multivitamin supplementation during pregnancy could enhance the protective effect of iron supplementation on obesity in male macrosomia. These findings suggest that encouraging maternal iron supplementation during pregnancy may reduce the risk of obesity in male preschoolers born macrosomia. However, these conclusions may be limited by the lack of more detailed quantitative data about the nutrient supplementation engaged in by the participants. Further research is required to explore how timing, dosage and duration of maternal nutrient supplementation during pregnancy may predict childhood obesity in children with macrosomia, and clarify how sex may be a moderator of these associations.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by Ethics Committee of the School of Public Health, Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants' legal guardians/next of kin.
Author contributions
M-FY: Writing – original draft, Formal analysis, Conceptualization, Visualization, Methodology. ES: Writing – review & editing. W-KY: Writing – review & editing, Project administration, Data curation. X-NY: Investigation, Writing – review & editing. G-MW: Investigation, Writing – review & editing. D-LS: Writing – review & editing, Investigation. D-XX: Supervision, Writing – review & editing. Y-FZ: Writing – review & editing, Supervision. W-QC: Resources, Project administration, Supervision, Methodology, Conceptualization, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This research was funded by the Science and Technology Planning Project of Guangdong Province, grant number 2019A1515011915. The funding source was non-profit scientific research management and academic institutions, it had no role in the design of this study, and did not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Acknowledgments
The authors would like to thank all participants in the studies and the clinicians of the Longhua Maternity & Child Healthcare Hospital involved in recruiting participants and collecting data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2025.1608521/full#supplementary-material
References
1. de Onis M, Blössner M, Borghi E. Global prevalence and trends of overweight and obesity among preschool children. Am J Clin Nutr. (2010) 92:1257–64. doi: 10.3945/ajcn.2010.29786
2. NCD-RisC NRFC. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. (2017) 390:2627–42. doi: 10.1016/S0140-6736(17)32129-3
3. Zhang N, Ma GS. Interpretation of report on childhood obesity in China. Acta Nutr Sin. (2017) 39:530–4. doi: 10.13325/j.cnki.acta.nutr.sin.2017.06.005
4. Wang F, Jin X, Jiang J, Yao Y, Yang Q. Situation and effecting factors of preschool children among several cities in China. Chinese J Child Health Care. (2017) 25:346–9. doi: 10.11852/zgetbjzz2017-25-04-07
5. Quek YH, Tam W, Zhang M, Ho R. Exploring the association between childhood and adolescent obesity and depression: a meta-analysis. Obes Rev. (2017) 18:742–54. doi: 10.1111/obr.12535
6. Rankin J, Matthews L, Cobley S, Han A, Sanders R, Wiltshire HD, et al. Psychological consequences of childhood obesity: psychiatric comorbidity and prevention. Adolesc Health Med Ther. (2016) 7:125–46. doi: 10.2147/AHMT.S101631
7. Wühl E. Hypertension in childhood obesity. Acta Paediatr. (2019) 108:37–43. doi: 10.1111/apa.14551
8. Litwin M, Kułaga Z. Obesity, metabolic syndrome, and primary hypertension. Pediatr Nephrol. (2021) 36:825–37. doi: 10.1007/s00467-020-04579-3
9. Brady TM. Obesity-related hypertension in children. Front Pediatr. (2017) 5:197. doi: 10.3389/fped.2017.00197
10. Pulgaron ER, Delamater AM. Obesity and type 2 diabetes in children: epidemiology and treatment. Curr Diab Rep. (2014) 14:508. doi: 10.1007/s11892-014-0508-y
11. Freedman DS, Khan LK, Dietz WH, Srinivasan SR, Berenson GS. Relationship of childhood obesity to coronary heart disease risk factors in adulthood: the Bogalusa heart study. Pediatrics. (2001) 108:712–8. doi: 10.1542/peds.108.3.712
12. Tremmel M, Gerdtham UG, Nilsson PM, Saha S. Economic burden of obesity: a systematic literature review. Int J Environ Res Public Health. (2017) 14:435. doi: 10.3390/ijerph14040435
13. Biener A, Cawley J, Meyerhoefer C. The impact of obesity on medical care costs and labor market outcomes in the US. Clin Chem. (2018) 64:108–17. doi: 10.1373/clinchem.2017.272450
14. Finkelstein EA, Graham WC, Malhotra R. Lifetime direct medical costs of childhood obesity. Pediatrics. (2014) 133:854–62. doi: 10.1542/peds.2014-0063
15. Segal AB, Huerta MC, Aurino E, Sassi F. The impact of childhood obesity on human capital in high-income countries: a systematic review. Obes Rev. (2021) 22:e13104. doi: 10.1111/obr.13104
16. Chen J, Wang H, Wang H, Song J, Xiao W, Liu Z. Research progress on genetic variants’ effects on childhood obesity interventions. Chinese J Sch Health. (2022) 43:1–5. doi: 10.16835/j.cnki.1000-9817.2022.11.034
17. Lobstein T, Baur L, Uauy R. Obesity in children and young people: a crisis in public health. Obes Rev. (2004) 5(Suppl 1):4–104. doi: 10.1111/j.1467-789X.2004.00133.x
18. Wu J, A DLBK, Ge J, Dou YL, Jiang Y, Zhang Z, et al. Study on the correlation between cesarean section and childhood obesity. Matern Child Health Care China. (2014) 29:5125–8. doi: 10.7620/zgfybj.j.issn.1001-4411.2014.31.38
19. Masukume G, McCarthy FP, Russell J, Baker PN, Kenny LC, Morton SM, et al. Caesarean section delivery and childhood obesity: evidence from the growing up in New Zealand cohort. J Epidemiol Community Health. (2019) 73:1063–70. doi: 10.1136/jech-2019-212591
20. Feng Y, Qian J, Song X. The association of pre-pregnancy weight and weight gain during pregnancy with pregnancy complications and delivery outcome. China J Mod Med. (2021) 31:92–6. doi: 10.3969/j.issn.1005-8982.2021.02.018
21. Wojtyla C, Wojtyla-Buciora P, Ciebiera M, Orzechowski S, Wojtyla A. The effect of active and passive maternal smoking before and during pregnancy on neonatal weight at birth. Arch Med Sci. (2021) 17:352–60. doi: 10.5114/aoms.2018.79629
22. Li L, Peters H, Gama A, Carvalhal MI, Nogueira HG, Rosado-Marques V, et al. Maternal smoking in pregnancy association with childhood adiposity and blood pressure. Pediatr Obes. (2016) 11:202–9. doi: 10.1111/ijpo.12046
23. Fuglestad AJ, Boys CJ, Chang PN, Miller BS, Eckerle JK, Deling L, et al. Overweight and obesity among children and adolescents with fetal alcohol spectrum disorders. Alcohol Clin Exp Res. (2014) 38:2502–8. doi: 10.1111/acer.12516
24. Sacco MR, de Castro NP, Euclydes VL, Souza JM, Rondó PH. Birth weight, rapid weight gain in infancy and markers of overweight and obesity in childhood. Eur J Clin Nutr. (2013) 67:1147–53. doi: 10.1038/ejcn.2013.183
25. Yu ZB, Han SP, Zhu GZ, Zhu C, Wang XJ, Cao XG, et al. Birth weight and subsequent risk of obesity: a systematic review and meta-analysis. Obes Rev. (2011) 12:525–42. doi: 10.1111/j.1467-789X.2011.00867.x
26. Carson V, Tremblay MS, Chastin SFM. Cross-sectional associations between sleep duration, sedentary time, physical activity, and adiposity indicators among Canadian preschool-aged children using compositional analyses. BMC Public Health. (2017) 17:848. doi: 10.1186/s12889-017-4852-0
27. Ji M, Tang A, Zhang Y, Zou J, Zhou G, Deng J, et al. The relationship between obesity, sleep and physical activity in Chinese preschool children. Int J Environ Res Public Health. (2018) 15:527. doi: 10.3390/ijerph15030527
28. Kim TV, Pham TND, Nguyen CLD, Nguyen TTD, Okely AD, Tang HK. Prevalence of physical activity, screen time, and sleep, and associations with adiposity and motor development among preschool-age children in Vietnam: the sunrise Vietnam pilot study. Indian J Pediatr. (2022) 89:148–53. doi: 10.1007/s12098-021-03895-2
29. Xie X, Kong B, Duan T. Obstetrics and Gynecology. Beijing: People’s Medical Publishing House (2018). p. 137.
30. Wang D, Hong Y, Zhu L, Wang X, Lv Q, Zhou Q, et al. Risk factors and outcomes of macrosomia in China: a multicentric survey based on birth data. J Matern Fetal Neonatal Med. (2017) 30:623–7. doi: 10.1080/14767058.2016.1252746
31. Salihu HM, Dongarwar D, King LM, Yusuf KK, Ibrahimi S, Salinas-Miranda AA. Trends in the incidence of fetal macrosomia and its phenotypes in the United States, 1971–2017. Arch Gynecol Obstet. (2020) 301:415–26. doi: 10.1007/s00404-019-05400-9
32. Araujo JE, Peixoto AB, Zamarian AC, Elito JJ, Tonni G. Macrosomia. Best Pract Res Clin Obstet Gynaecol. (2017) 38:83–96. doi: 10.1016/j.bpobgyn.2016.08.003
33. Ge J, Wang D, Fan L. Effect of personalized nutrition guidance on the birth rate of fetal macrosomia in Chinese population: a meta-analysis of nine randomized controlled trials. Cell Biochem Biophys. (2015) 72:669–74. doi: 10.1007/s12013-015-0512-0
34. Kc K, Shakya S, Zhang H. Gestational diabetes mellitus and macrosomia: a literature review. Ann Nutr Metab. (2015) 66:14–20. doi: 10.1159/000371628
35. Kemp MW, Kallapur SG, Jobe AH, Newnham JP. Obesity and the developmental origins of health and disease. J Paediatr Child Health. (2012) 48:86–90. doi: 10.1111/j.1440-1754.2010.01940.x
36. Péneau S, Giudici KV, Gusto G, Goxe D, Lantieri O, Hercberg S, et al. Growth trajectories of body mass index during childhood: associated factors and health outcome at adulthood. J Pediatr. (2017) 186:64–71. doi: 10.1016/j.jpeds.2017.02.010
37. Xiong C, Chen K, Zhang Y. Comparison of bmi trajectory pattern between macrosomia and normal birth weight infants aged 0to 24months:a longitudinal study. Acta Med Univ Sci Technol Huazhong. (2022) 51:645–50. doi: 10.3870/j.issn.1672-0741.2022.05.010
38. Aris IM, Chen LW, Tint MT, Pang WW, Soh SE, Saw SM, et al. Body mass index trajectories in the first two years and subsequent childhood cardio-metabolic outcomes: a prospective multi-ethnic Asian cohort study. Sci Rep. (2017) 7:8424. doi: 10.1038/s41598-017-09046-y
39. Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? Am J Hum Genet. (1962) 14:353–62.13937884
40. Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. (2003) 23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003
41. Liu X, An H, Li N, Li Z, Zhang Y, Zhang L, et al. Preconception hemoglobin concentration and risk of low birth weight and small-for-gestational-age: a large prospective cohort study in China. Nutrients. (2022) 14:271. doi: 10.3390/nu14020271
42. Dewey KG, Oaks BM. U-shaped curve for risk associated with maternal hemoglobin, iron status, or iron supplementation. Am J Clin Nutr. (2017) 106:1694S–702. doi: 10.3945/ajcn.117.156075
43. Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. (1998) 351:173–7. doi: 10.1016/s0140-6736(97)07244-9
44. Zhang J, Lewis RM, Wang C, Hales N, Byrne CD. Maternal dietary iron restriction modulates hepatic lipid metabolism in the fetuses. Am J Physiol Regul Integr Comp Physiol. (2005) 288:R104–11. doi: 10.1152/ajpregu.00343.2004
45. Sun G, Yang Y, Liu L, Wang S, Guo C, Zhang Y, et al. Scientific consensus on the use of nutrient supplements. Acta Nutr Sin. (2018) 40:521–5. doi: 10.13325/j.cnki.acta.nutr.sin.2018.06.002
46. Dong C, Yin S. The ten-year retrospect of nutrition and health status of pregnant women in China. Chin J Prev Med. (2018) 52:94–100. doi: 10.3760/cma.j.issn.0253-9624.2018.01.019
47. Fu S, Yang L, Li P, Hofmann O, Dicker L, Hide W, et al. Aberrant lipid metabolism disrupts calcium homeostasis causing liver endoplasmic reticulum stress in obesity. Nature. (2011) 473:528–31. doi: 10.1038/nature09968
48. Varela-Moreiras G, Murphy MM, Scott JM. Cobalamin, folic acid, and homocysteine. Nutr Rev. (2009) 67(Suppl 1):S69–72. doi: 10.1111/j.1753-4887.2009.00163.x
49. Wang G, Hu FB, Mistry KB, Zhang C, Ren F, Huo Y, et al. Association between maternal prepregnancy body mass index and plasma folate concentrations with child metabolic health. JAMA Pediatr. (2016) 170:e160845. doi: 10.1001/jamapediatrics.2016.0845
50. Hou X, Wu L, Zhang R, Wang Z. Relationship between multi-vitamins supplementation during the second and the third trimester and risk of preterm delivery. Chin J Woman Child Health Res. (2018) 29:481–4. doi: 10.3969/j.issn.1673-5293.2018.04.022
51. Shi L, Liu X, Chen P, Gao Y, Sun J, Jin Z. A retrospective cohort study of multivitamin supplementation in pregnancy and pregnancy outcomes. Chin J Clin Obstet Gynecol. (2020) 36:177–81. doi: 10.19538/j.fk2020020120
52. Wang M, Bai A, Li P. Nutrient supplementation during pregnancy and 6 months postpartum among pregnant women in three cities of China: a cross-sectional and follow-up survey. China J Public Health. (2021) 37:1223–7. doi: 10.11847/zgggws1127867
53. Prentice A. Calcium in pregnancy and lactation. Annu Rev Nutr. (2000) 20:249–72. doi: 10.1146/annurev.nutr.20.1.249
54. Skolmowska D, Głąbska D, Kołota A, Guzek D. Effectiveness of dietary interventions to treat iron-deficiency anemia in women: a systematic review of randomized controlled trials. Nutrients. (2022) 14:2724. doi: 10.3390/nu14132724
55. Pathirathna ML, Wimalasiri K, Sekijima K, Sadakata M. Maternal compliance to recommended iron and folic acid supplementation in pregnancy, Sri Lanka: a hospital-based cross-sectional study. Nutrients. (2020) 12:3266. doi: 10.3390/nu12113266
56. Li H, Ji CY, Zong XN, Zhang YQ. Body mass index growth curves for Chinese children and adolescents aged 0 to 18 years. Zhonghua Er Ke Za Zhi. (2009) 47:493–8. doi: 10.3760/cma.j.issn.0578-1310.2009.07.004
57. Alves JC, Mocellin MC, Gonçalves E, Silva DA, Trindade EB. Anthropometric indicators as body fat discriminators in children and adolescents: a systematic review and meta-analysis. Adv Nutr. (2017) 8:718–27. doi: 10.3945/an.117.015446
58. Morgen CS, Ängquist L, Baker JL, Andersen A, Michaelsen KF, Sørensen T. Prenatal risk factors influencing childhood bmi and overweight independent of birth weight and infancy bmi: a path analysis within the Danish national birth cohort. Int J Obes (Lond). (2018) 42:594–602. doi: 10.1038/ijo.2017.217
59. Kunaratnam K, Halaki M, Wen LM, Baur LA, Flood VM. Tracking preschoolers’ lifestyle behaviors and testing maternal sociodemographics and bmi in predicting child obesity risk. J Nutr. (2020) 150:3068–74. doi: 10.1093/jn/nxaa292
60. Lu Q, Strodl E, Liang Y, Huang L, Hu B, Chen W. Joint effects of prenatal folic acid supplement with prenatal multivitamin and iron supplement on obesity in preschoolers born sga: sex specific difference. Nutrients. (2023) 15:380. doi: 10.3390/nu15020380
61. Petry CJ, Olga L, Hughes IA, Ong KK. Associations between maternal iron supplementation in pregnancy and offspring growth and cardiometabolic risk outcomes in infancy and childhood. PLoS One. (2022) 17:e263148. doi: 10.1371/journal.pone.0263148
62. Alwan NA, Cade JE, Greenwood DC, Deanfield J, Lawlor DA. Associations of maternal iron intake and hemoglobin in pregnancy with offspring vascular phenotypes and adiposity at age 10: findings from the avon longitudinal study of parents and children. PLoS One. (2014) 9:e84684. doi: 10.1371/journal.pone.0084684
63. Alwan NA, Lawlor DA, McArdle HJ, Greenwood DC, Cade JE. Exploring the relationship between maternal iron status and offspring’s blood pressure and adiposity: a Mendelian randomization study. Clin Epidemiol. (2012) 4:193–200. doi: 10.2147/CLEP.S33833
64. Bourque SL, Komolova M, McCabe K, Adams MA, Nakatsu K. Perinatal iron deficiency combined with a high-fat diet causes obesity and cardiovascular dysregulation. Endocrinology. (2012) 153:1174–82. doi: 10.1210/en.2011-1700
65. Del Giudice EM, Santoro N, Amato A, Brienza C, Calabrò P, Wiegerinck ET, et al. Hepcidin in obese children as a potential mediator of the association between obesity and iron deficiency. J Clin Endocrinol Metab. (2009) 94:5102–7. doi: 10.1210/jc.2009-1361
66. Fisher AL, Nemeth E. Iron homeostasis during pregnancy. Am J Clin Nutr. (2017) 106:1567S–74. doi: 10.3945/ajcn.117.155812
67. Stoffel NU, Zimmermann MB, Cepeda-Lopez AC, Cervantes-Gracia K, Llanas-Cornejo D, Zeder C, et al. Maternal iron kinetics and maternal–fetal iron transfer in normal-weight and overweight pregnancy. Am J Clin Nutr. (2022) 115:1166–79. doi: 10.1093/ajcn/nqab406
68. Stoffel NU, Lazrak M, Bellitir S, Mir NE, Hamdouchi AE, Barkat A, et al. The opposing effects of acute inflammation and iron deficiency anemia on serum hepcidin and iron absorption in young women. Haematologica. (2019) 104:1143–9. doi: 10.3324/haematol.2018.208645
69. Jahng JWS, Alsaadi RM, Palanivel R, Song E, Hipolito VEB, Sung HK, et al. Iron overload inhibits late stage autophagic flux leading to insulin resistance. Embo Rep. (2019) 20:e47911. doi: 10.15252/embr.201947911
70. Casanueva E, Viteri FE. Iron and oxidative stress in pregnancy. J Nutr. (2003) 133:1700S–8. doi: 10.1093/jn/133.5.1700S
71. Mannaerts D, Faes E, Cos P, Briedé JJ, Gyselaers W, Cornette J, et al. Oxidative stress in healthy pregnancy and preeclampsia is linked to chronic inflammation, iron status and vascular function. PLoS One. (2018) 13:e202919. doi: 10.1371/journal.pone.0202919
72. González-Domínguez Á, Visiedo-García FM, Domínguez-Riscart J, González-Domínguez R, Mateos RM, Lechuga-Sancho AM. Iron metabolism in obesity and metabolic syndrome. Int J Mol Sci. (2020) 21:5529. doi: 10.3390/ijms21155529
73. Nishizawa H, Matsumoto M, Yamanaka M, Irikura R, Nakajima K, Tada K, et al. Bach1 inhibits senescence, obesity, and short lifespan by ferroptotic fgf21 secretion. Cell Rep. (2024) 43:114403. doi: 10.1016/j.celrep.2024.114403
74. Yook JS, Thomas SS, Toney AM, You M, Kim YC, Liu Z, et al. Dietary iron deficiency modulates adipocyte iron homeostasis, adaptive thermogenesis, and obesity in c57bl/6 mice. J Nutr. (2021) 151:2967–75. doi: 10.1093/jn/nxab222
75. Kitamura N, Yokoyama Y, Taoka H, Nagano U, Hosoda S, Taworntawat T, et al. Iron supplementation regulates the progression of high fat diet induced obesity and hepatic steatosis via mitochondrial signaling pathways. Sci Rep. (2021) 11:10753. doi: 10.1038/s41598-021-89673-8
76. Xia L, Luo X, Liang Y, Jiang X, Yang W, Yan J, et al. Epigenetic modifications of nuclear and mitochondrial dna are associated with the disturbance of serum iron biomarkers among the metabolically unhealthy obesity school-age children. Nutr J. (2025) 24:51. doi: 10.1186/s12937-025-01108-6
77. Mjaaseth UN, Norris JC, Aardema NDJ, Bunnell ML, Ward RE, Hintze KJ, et al. Excess vitamins or imbalance of folic acid and choline in the gestational diet alter the gut microbiota and obesogenic effects in wistar rat offspring. Nutrients. (2021) 13:4510. doi: 10.3390/nu13124510
78. Szeto IMY, Aziz A, Das PJ, Taha AY, Okubo N, Reza-Lopez S, et al. High multivitamin intake by wistar rats during pregnancy results in increased food intake and components of the metabolic syndrome in male offspring. Am J Physiol Regul Integr Comp Physiol. (2008) 295:R575–82. doi: 10.1152/ajpregu.90354.2008
79. Cho CE, Pannia E, Huot PSP, Sánchez-Hernández D, Kubant R, Dodington DW, et al. Methyl vitamins contribute to obesogenic effects of a high multivitamin gestational diet and epigenetic alterations in hypothalamic feeding pathways in wistar rat offspring. Mol Nutr Food Res. (2015) 59:476–89. doi: 10.1002/mnfr.201400663
80. Tint MT, Chong MF, Aris IM, Godfrey KM, Quah PL, Kapur J, et al. Association between maternal mid-gestation vitamin d status and neonatal abdominal adiposity. Int J Obes (Lond). (2018) 42:1296–305. doi: 10.1038/s41366-018-0032-2
81. Feng J, Li N. Research progress on zinc, iron and calcium deficiencies and childhood obesity. Chin J Woman Child Health Res. (2021) 32:1234–8. doi: 10.3969/j.issn.1673-5293.2021.08.029
82. Xin S, Mueller C, Pfeiffer S, Kraft V, Merl-Pham J, Bao X, et al. Ms4a15 drives ferroptosis resistance through calcium-restricted lipid remodeling. Cell Death Differ. (2022) 29:670–86. doi: 10.1038/s41418-021-00883-z
83. Serdula MK, Zhou Y, Li H, Liu JM, Mei Z. Prenatal iron containing supplements provided to Chinese women with no or mild anemia had no effect on hemoglobin concentration in post-partum women or their infants at 6 and 12 months of age. Eur J Clin Nutr. (2019) 73:1473–9. doi: 10.1038/s41430-018-0365-x
84. Xie RH, Liu YJ, Retnakaran R, MacFarlane AJ, Hamilton J, Smith G, et al. Maternal folate status and obesity/insulin resistance in the offspring: a systematic review. Int J Obes (Lond). (2016) 40:1–9. doi: 10.1038/ijo.2015.189
85. Caniglia EC, Zash R, Swanson SA, Smith E, Sudfeld C, Finkelstein JL, et al. Iron, folic acid, and multiple micronutrient supplementation strategies during pregnancy and adverse birth outcomes in Botswana. Lancet Glob Health. (2022) 10:e850–61. doi: 10.1016/S2214-109X(22)00126-7
86. Dibley MJ, Titaley CR, D’Este C, Agho K. Iron and folic acid supplements in pregnancy improve child survival in Indonesia. Am J Clin Nutr. (2012) 95:220–30. doi: 10.3945/ajcn.111.022699
87. McGee M, Bainbridge S, Fontaine-Bisson B. A crucial role for maternal dietary methyl donor intake in epigenetic programming and fetal growth outcomes. Nutr Rev. (2018) 76:469–78. doi: 10.1093/nutrit/nuy006
88. Rahat B, Mahajan A, Bagga R, Hamid A, Kaur J. Epigenetic modifications at dmrs of placental genes are subjected to variations in normal gestation, pathological conditions and folate supplementation. Sci Rep. (2017) 7:40774. doi: 10.1038/srep40774
89. McKay JA, Xie L, Adriaens M, Evelo CT, Ford D, Mathers JC. Organ-specific gene expression changes in the fetal liver and placenta in response to maternal folate depletion. Nutrients. (2016) 8:661. doi: 10.3390/nu8100661
90. Oppenheim EW, Adelman C, Liu X, Stover PJ. Heavy chain ferritin enhances serine hydroxymethyltransferase expression and de novo thymidine biosynthesis. J Biol Chem. (2001) 276:19855–61. doi: 10.1074/jbc.M100039200
91. Radziejewska A, Suliburska J, Kołodziejski P, Chmurzynska A. Simultaneous supplementation with iron and folic acid can affect slc11a2 and slc46a1 transcription and metabolite concentrations in rats. Br J Nutr. (2020) 123:264–72. doi: 10.1017/S0007114519002721
92. Suliburska J, Skrypnik K, Chmurzyńska A. Folic acid affects iron status in female rats with deficiency of these micronutrients. Biol Trace Elem Res. (2020) 195:551–8. doi: 10.1007/s12011-019-01888-z
93. Imdad A, Bhutta ZA. Effects of calcium supplementation during pregnancy on maternal, fetal and birth outcomes. Paediatr Perinat Epidemiol. (2012) 26(Suppl 1):138–52. doi: 10.1111/j.1365-3016.2012.01274.x
94. Karakis I, Landau D, Gat R, Shemesh N, Tirosh O, Yitshak-Sade M, et al. Maternal metal concentration during gestation and pediatric morbidity in children: an exploratory analysis. Environ Health Prev Med. (2021) 26:40. doi: 10.1186/s12199-021-00963-z
95. Marotte C, Bryk G, Gonzales Chaves MMS, Lifshitz F, Pita Martín De Portela ML, Zeni SN. Low dietary calcium and obesity: a comparative study in genetically obese and normal rats during early growth. Eur J Nutr. (2014) 53:769–78. doi: 10.1007/s00394-013-0581-z
96. Li P, Wang Y, Li P, Liu YL, Liu WJ, Chen XY, et al. Maternal inappropriate calcium intake aggravates dietary-induced obesity in male offspring by affecting the differentiation potential of mesenchymal stem cells. World J Stem Cells. (2022) 14:756–76. doi: 10.4252/wjsc.v14.i10.756
97. Rodgers A, Sferruzzi-Perri AN. Developmental programming of offspring adipose tissue biology and obesity risk. Int J Obes (Lond). (2021) 45:1170–92. doi: 10.1038/s41366-021-00790-w
98. Gallou-Kabani C, Gabory A, Tost J, Karimi M, Mayeur S, Lesage J, et al. Sex- and diet-specific changes of imprinted gene expression and dna methylation in mouse placenta under a high-fat diet. PLoS One. (2010) 5:e14398. doi: 10.1371/journal.pone.0014398
99. Eriksson JG, Kajantie E, Osmond C, Thornburg K, Barker DJP. Boys live dangerously in the womb. Am J Hum Biol. (2010) 22:330–5. doi: 10.1002/ajhb.20995
100. Huot PSP, Ly A, Szeto IMY, Reza-López SA, Cho D, Kim Y, et al. Maternal and postweaning folic acid supplementation interact to influence body weight, insulin resistance, and food intake regulatory gene expression in rat offspring in a sex-specific manner. Appl Physiol Nutr Metab. (2016) 41:411–20. doi: 10.1139/apnm-2015-0503
Keywords: obesity, supplementation during pregnancy, iron, calcium, folic acid, multivitamin, preschoolers, macrosomia
Citation: Yan M-F, Strodl E, Yang W-K, Yin X-N, Wen G-M, Sun D-L, Xian D-X, Zhao Y-F and Chen W-Q (2025) Combined effects of maternal supplementation of iron, calcium, folic acid, and multivitamin during pregnancy on obesity in Chinese preschoolers born macrosomia. Front. Pediatr. 13:1608521. doi: 10.3389/fped.2025.1608521
Received: 9 April 2025; Accepted: 11 June 2025;
Published: 27 June 2025.
Edited by:
Wenhan Yang, Guangdong Pharmaceutical University, ChinaReviewed by:
Rui Gao, Nanshan Maternity and Child Health Care Hospital, ChinaTianran Shen, Guangdong Pharmaceutical University, China
Wenjing Zhao, Southern University of Science and Technology, China
Copyright: © 2025 Yan, Strodl, Yang, Yin, Wen, Sun, Xian, Zhao and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei-Kang Yang, d3VjYUAxNjMuY29t; Wei-Qing Chen, Y2hlbndxQG1haWwuc3lzdS5lZHUuY24=
 Ming-Fei Yan
Ming-Fei Yan Esben Strodl
Esben Strodl Wei-Kang Yang3*
Wei-Kang Yang3* Dan-Xia Xian
Dan-Xia Xian Wei-Qing Chen
Wei-Qing Chen