- 1Organ Transplant Center, First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
- 2Sun Yat-sen University, Guangzhou, China
- 3Department of Clinical Laboratory, The First Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
Cytomegalovirus (CMV) infections are commonly observed in immunocompromised patients. However, hemorrhagic cystitis (HC) is an exceptionally rare manifestation. Here, we report a pediatric kidney transplant recipient who developed CMV-related HC, presenting with acute painful macrohematuria, bladder wall thickening, and graft hydronephrosis during the early posttransplant period.
Introduction
Hemorrhagic cystitis (HC) is defined as a diffuse inflammatory condition of the urinary bladder characterized by bleeding from the bladder mucosa (1). HC may result from both infectious and non-infectious etiologies. Infectious factors include bacteria, viruses [such as adenovirus, cytomegalovirus (CMV), and BK virus], fungi, or parasites, and non-infectious factors include chemotherapy (such as cyclophosphamide and ifosfamide), environmental toxins, and radiation (1–3). Notably, CMV is an exceptionally rare cause of HC (4). A previous report described an unusual case of late-onset CMV-related HC in a kidney transplant recipient, emphasizing its atypical clinical manifestations and the therapeutic role of intravenous ganciclovir (5). However, early-onset cases have not yet been reported. Here, we report a pediatric kidney transplant recipient who developed CMV-related hemorrhagic cystitis, presenting with acute painful macrohematuria, bladder wall thickening, and graft hydronephrosis during the early posttransplant period.
Case
A 10-year-old boy underwent kidney transplantation from a brain-dead donor. The recipient was CMV seropositive prior to transplantation. Ureter–bladder anastomosis was performed with placement of a double-J stent, which was removed 29 days later. Induction immunosuppressive therapy consisted of basiliximab, followed by maintenance therapy with tacrolimus, mycophenolate mofetil (MMF), and prednisone. The allograft function remained stable at creatinine levels ranging from 60 to 80 µmol/L. Oral valganciclovir was administered at hospital discharge for viral prophylaxis.
On postoperative day 18, he developed acute painful macrohematuria with white, purulent flocculent deposits, accompanied by pain in the lower abdomen, perineal, and scrotal regions. He also presented with noticeable overactive bladder syndrome and recurrent high fever (up to 38°C), while no other systemic abnormalities were noted. Notably, 5 days before the onset of symptoms, the tacrolimus trough concentration reached 17.7 µg/L (normal therapeutic range: 5–15 µg/L). Laboratory tests showed a maximum urine red blood cell count of 1,119 cells/µL, a maximum urine white blood cell count of 286 cells/µL, and a maximum 24 h urine protein quantification of 3.422 g/24 h. The changes in urine erythrocytes, urine protein, and urine leukocytes are shown in Figure 1. Repeated urine cultures were negative for bacterial and fungal pathogens. However, both CMV IgM and IgG were positive. The highest CMV DNA loads in blood and urine were 1.42 × 104 and 1.06 × 106 copies/mL, respectively (Figure 1).
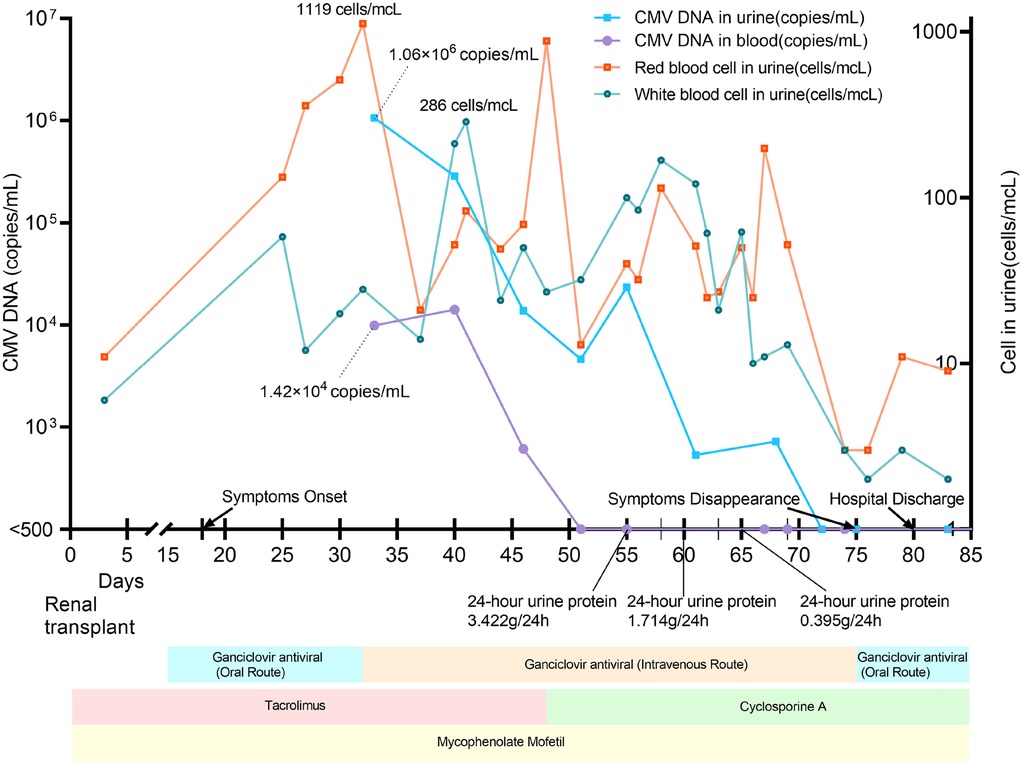
Figure 1. Treatments, clinical and laboratory findings according to posttransplant days. The local laboratory reference value was <500 copies/mL for both urine and plasma samples.
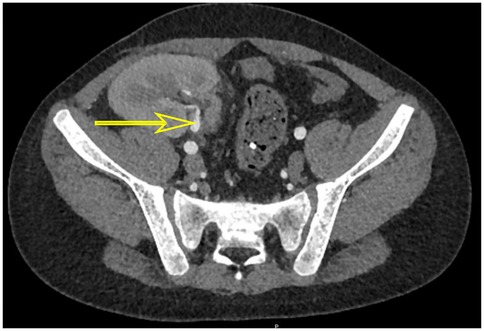
On posttransplant days 39 and 60, he underwent cystoscopy, and both procedures revealed HC features of clots in the bladder. The first bladder mucosa biopsy pathology revealed a suspicious nuclear pseudoinclusion. However, immunohistochemistry staining was negative for CMV. The second bladder mucosa biopsy showed no evidence of viral inclusion bodies, as did urine pathology. Although these findings did not provide a clear diagnosis of CMV infection, his metagenomic next-generation (mNGS) sequencing of bladder tissue indicated the presence of CMV and Torque teno virus. Furthermore, polymerase chain reaction (PCR) testing of the bladder tissue revealed a peak CMV DNA load of 1.06 × 103 copies/mL, indicating active viral replication. Computed tomography (CT) demonstrated bladder wall thickening and graft hydronephrosis (Figures 2, 3).

Figure 2. CT scan showed thickened bladder wall (yellow arrow) before treatment (A) and after treatment (B) and graft kidney hydronephrosis (blue arrow) before treatment (C) and after treatment (D).
After ruling out other possible causes of hematuria and identifying CMV in bladder tissue biopsies by mNGS and PCR testing, the patient was diagnosed with CMV-related HC. At treatment initiation, intravenous ganciclovir was administered, tacrolimus was switched to cyclosporine, and prednisone was temporarily withheld to restore antiviral immune competence. Once the viral DNA load began to decline, intravenous ganciclovir was transitioned to oral administration. Due to the adjustment of the immunosuppressive protocol and antiviral drug regimen, CMV viral load declined. On posttransplant day 75, his symptoms generally disappeared. Laboratory tests showed the disappearance of urine protein, a blood creatinine level of 53 µmol/mL, and negative results for CMV detection in urine and blood. On posttransplant day 80, he was discharged from the hospital without recurrence of CMV-related HC. Follow-up examinations conducted 17 months after transplantation revealed chronic inflammatory changes of the bladder wall with marked improvement and resolution of graft hydronephrosis (Figure 2).
Discussion
CMV-related hemorrhagic cystitis is more commonly observed in pediatric and immunocompromised patients. Viral infections represent an important cause of hemorrhagic cystitis, with CMV being one of the implicated pathogens (1). Due to the disproportion between the high prevalence of CMV-seronegative recipients and the increased proportion of CMV-seropositive donors in pediatric transplantation, this population is at a particularly elevated risk of CMV infection (6). However, in our case, the recipient was pretransplant CMV seropositive, whereas donor serology was not performed. In addition, tacrolimus overexposure may lead to an excessive immunosuppressive burden, potentially contributing to CMV-related HC. Notably, all but one of the previously reported CMV-related HC cases occurred in immunosuppressed patients (7). Regrettably, lymphocyte subset counting was not performed during the onset of HC. In addition, no other risk factors for HC (1) were identified in this patient. These findings highlight the importance of prophylactic antiviral therapy, particularly in pediatric recipients at increased risk of CMV-related HC.
PCR and mNGS tests are important diagnostic tools for CMV-related HC. Cystoscopy can help identify inflammatory changes in the bladder and pinpoint bleeding sites, while CMV infection can be confirmed through bladder biopsy specimens using culture, immunohistochemical staining, or in situ hybridization. However, cystoscopy and bladder mucosal biopsy are invasive tests and should be chosen carefully. Furthermore, it has been reported that CMV can only be detected in deep biopsies (8), meaning that the diagnostic yield can be limited by biopsy depth. The bladder tissue is bulging due to chronic inflammation, making it difficult to retrieve the CMV virus from the lesion in our case. Given the difficulties in diagnosing CMV-related HC, a combination of mNGS and PCR tests is necessary.
Hemorrhagic cystitis has been well described in four grades (9) and may present with serious complications. In addition to manifesting as urinary tract symptoms such as painful hematuria, CMV-related HC also produces systemic symptoms of CMV infection. In patients who have undergone kidney transplantation, CMV-related HC may lead to hydronephrosis of the graft kidney. Bladder spasm due to CMV-related HC and bladder wall thickening contributed to the hydronephrosis in our study. In addition, CMV ureteritis as a cause of ureteral obstruction may also lead to hydronephrosis (10, 11). In our case, the presence of proteinuria, which was not related to the primary disease, improved with effective CMV treatment. We consider that this may have been caused by kidney injury resulting from the CMV infection.
Timely adjustment of immune status and antiviral drug regimen was crucial for the successful treatment of CMV-related HC. In our study, we promptly switched from tacrolimus to cyclosporine and discontinued prednisone, which together reduced immunosuppressive intensity and helped control viral replication (12). Our study also confirms the effectiveness of changing tacrolimus to cyclosporine for CMV-related HC. In addition, timely intravenous administration of antiviral drugs was essential to virus elimination. Despite the improved symptoms, laboratory parameters fluctuated repeatedly in this case; this separation phenomenon suggests that early treatment is crucial to avoid the transition to chronic infection.
Conclusions
We present a case study of a pediatric kidney transplant recipient who experienced acute painful macrohematuria, bladder wall thickening, and graft kidney hydronephrosis soon after transplantation. The detection of CMV through bladder mucosal biopsy was challenging, and combining mNGS and PCR tests can better detect microorganisms. Moreover, prompt adjustments to the immunosuppressive protocol and antiviral drug regimen resulted in improved bladder symptoms and graft kidney hydronephrosis, with no recurrence.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving humans were approved by the research ethics committee of the First Affiliated Hospital of Sun Yat-sen University. The studies were conducted in accordance with the local legislation and institutional requirements. Written informed consent for participation in this study was provided by the participants’ legal guardians/next of kin. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
JiL: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Software, Validation, Visualization, Writing – original draft, Writing – review & editing. CW: Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing – original draft, Writing – review & editing. MW: Data curation, Formal analysis, Visualization, Writing – original draft, Writing – review & editing. PC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. XS: Conceptualization, Data curation, Formal analysis, Writing – original draft, Writing – review & editing. MH: Conceptualization, Investigation, Methodology, Validation, Writing – original draft, Writing – review & editing. QF: Formal analysis, Investigation, Project administration, Software, Writing – original draft, Writing – review & editing. JuL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. LL: Conceptualization, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Writing – original draft, Writing – review & editing. CW: Data curation, Funding acquisition, Investigation, Project administration, Validation, Writing – original draft, Writing – review & editing.
Funding
The author(s) declare that financial support was received for the research and/or publication of this article. This study was supported by Key Scientific and Technological Program of Guangzhou City (201903010058, 201803040011); Science and Technology Planning Project of Guangdong Province, China (2015B020226002); Guangdong Basic and Applied Basic Research Foundation (2020A1515010884); National Natural Science Foundation of China (81870511, 82170770); Guangdong Provincial Key Laboratory on Organ Donation and Transplant Immunology (2017B030314018, 2020B1212060026); Guangdong Provincial International Cooperation Base of Science and Technology (Organ Transplantation) (2020A0505020003); and Major Clinical Technology Project, Municipal Health Commission, Guangzhou, China (2023P-ZD15).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence, and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Manikandan R, Kumar S, Dorairajan LN. Hemorrhagic cystitis: a challenge to the urologist. Indian J Urol. (2010) 26(2):159–66. doi: 10.4103/0970-1591.65380
2. Khan AM, Ajmal Z, Tuz Zahra F, Ramani A, Zackon I. Hemorrhagic cystitis secondary to adenovirus and BK virus infection in a diffuse large B-cell lymphoma patient with recent CAR T-cell therapy. Case Rep Hematol. (2020) 2020:6621967. doi: 10.1155/2020/6621967
3. Haldar S, Dru C, Bhowmick NA. Mechanisms of hemorrhagic cystitis. Am J Clin Exp Urol. (2014) 2(3):199–208.25374922
4. Padayachee WPR, Sadhwani S, Doherty SW, Mukendi AM, Van den Berg E, Botha AR. Haemorrhagic cystitis due to cytomegalovirus in a patient with AIDS. Afr J Urol. (2020) 26:30. doi: 10.1186/s12301-020-00039-4
5. Ersan S, Yorukoglu K, Sert M, Atila K, Celik A, Gulcu A, et al. Unusual case of severe late-onset cytomegalovirus-induced hemorrhagic cystitis and ureteritis in a renal transplant patient. Ren Fail. (2012) 34(2):247–50. doi: 10.3109/0886022X.2011.647209
6. Höcker B, Zencke S, Krupka K, Fichtner A, Pape L, Dello Strologo L, et al. Cytomegalovirus infection in pediatric renal transplantation and the impact of chemoprophylaxis with (val-)ganciclovir. Transplantation. (2016) 100(4):862–70. doi: 10.1097/TP.0000000000000888
7. Taktak A, Acar B, Gür G, Tiryaki T, Karakuş E, Çaycı FŞ, et al. Cytomegalovirus-related hemorrhagic cystitis in an immunocompetent child. Ren Fail. (2014) 36(7):1148–50. doi: 10.3109/0886022X.2014.926757
8. Whitaker JA, Jacob JT, Little JV, DiazGranados CA. Cytomegalovirus cystitis with bladder wall dehiscence in a patient with AIDS. Aids. (2008) 22(6):795–6. doi: 10.1097/QAD.0b013e3282f56103
9. Cipe FE, Soygür T, Doğu F, Erdoğan O, Bozdoğan G, Ikincioğullari A. Late onset hemorrhagic cystitis in a hematopoietic stem cell recipient: treatment with intravesical hyaluronic acid. Pediatr Transplant. (2010) 14(6):E79–82. doi: 10.1111/j.1399-3046.2009.01169.x
10. Ardalan M. Rare presentations of cytomegalovirus infection in renal allograft recipients. Nephrourol Mon. (2012) 4(2):431–6. doi: 10.5812/numonthly.1844
11. Rico JE, Cardona X, Rodelo J, Reino A, Arias LF, Arbeláez M. Ureterostomy cytomegalovirus infection presenting as stoma ulceration in a kidney allograft receptor: a case report. Actas Urol Esp. (2008) 32(6):649–52. doi: 10.1016/S0210-4806(08)73903-2
12. Kizilbash SJ, Rheault MN, Bangdiwala A, Matas A, Chinnakotla S, Chavers BM. Infection rates in tacrolimus versus cyclosporine-treated pediatric kidney transplant recipients on a rapid discontinuation of prednisone protocol: 1-year analysis. Pediatr Transplant. (2017) 21(4):e12919. doi: 10.1111/petr.12919
Keywords: cytomegalovirus infections, hemorrhagic cystitis, pediatrics, kidney transplantation, infection
Citation: Li J, Wu C, Wei M, Chen P, Su X, Hong M, Fu Q, Li J, Liu L and Wang C (2025) Case Report: A case of cytomegalovirus-related hemorrhagic cystitis early after pediatric kidney transplantation. Front. Pediatr. 13:1665675. doi: 10.3389/fped.2025.1665675
Received: 14 July 2025; Accepted: 4 November 2025;
Published: 24 November 2025.
Edited by:
Silviu Grisaru, University of Calgary, CanadaReviewed by:
Nicole Hayde, Children’s Hospital at Montefiore, United StatesOlle Thor,Hans Ringden, Karolinska Institutet (KI), Sweden
Copyright: © 2025 Li, Wu, Wei, Chen, Su, Hong, Fu, Li, Liu and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, cmV4bGVlMDEwMjA3QDE2My5jb20=; Longshan Liu, bGl1bHNoYW5AbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work
 Jianming Li
Jianming Li Chenglin Wu
Chenglin Wu Mengxun Wei2
Mengxun Wei2 Xiaojun Su
Xiaojun Su Mengzhi Hong
Mengzhi Hong Jun Li
Jun Li Longshan Liu
Longshan Liu Changxi Wang
Changxi Wang