- Department of Ultrasound, Shenzhen Children’s Hospital, Shenzhen, China
Persistent fifth aortic arch (PFAA) is a rare congenital cardiovascular anomaly, often associated with other defects such as interrupted aortic arch (IAA) or patent ductus arteriosus (PDA). We report a 12-day-old male neonate presenting with respiratory distress, edema, and oliguria. Physical examination revealed a grade 2/6 systolic murmur at the left sternal border. Echocardiography identified PFAA with coarctation, IAA, PDA, and an atrial septal defect (ASD), confirmed by cardiac CT. Surgical intervention included aortic arch repair, fifth arch excision, and ASD closure. The postoperative recovery was smooth, and the patient remained clinically well with normal cardiac function during the 1-year follow-up period. This case underscores the critical role of echocardiography in early diagnosis and surgical planning for complex congenital heart disease.
Introduction
Persistent fifth aortic arch (PFAA) arises from incomplete embryonic regression of the fifth aortic arch, often coexisting with cardiovascular anomalies such as PDA, IAA, or pulmonary atresia (1, 2). Gupta et al. (3) defined PFAA as an extrapericardial vessel bridging the ascending and descending aorta. Histologically distinct from normal aortic tissue (4), PFAA results from failed degeneration of the fifth branchial arch, contributing to vascular ring formation and hypoplasia (5–7). Clinical manifestations depend on associated defects and hemodynamic impact (8). While asymptomatic cases may occur incidentally (2), PFAA with coarctation often presents with hypertension, pulse discrepancies, or heart failure (9). This report details a rare case of type B PFAA with IAA, emphasizing the utility of multimodal imaging in diagnosis and management.
Case report
A 12-day-old male was admitted for progressive respiratory distress, edema, and oliguria (urine output: 0.3 mL/kg/h). Born vaginally at 39 + 3 weeks, he had been hospitalized previously for persistent irritability and received respiratory suctioning, monitoring, and antibiotics (amoxicillin) before discharge. Three days later, he developed recurrent respiratory distress, necessitating Nasal Continuous Positive Airway Pressure (NCPAP) and readmission. On admission, physical examination revealed pitting edema over the lower back, a grade 2/6 systolic murmur at the left sternal border (2nd–3rd intercostal spaces), and hepatomegaly (3 cm below the costal margin). Upper limb blood pressure was 85/40 mmHg (lower limbs unmeasured). Oxygen saturation (SpO2) was 99% in all limbs. Laboratory findings included hypoalbuminemia (26.9 g/L; normal range: 35–50 g/L) and elevated N-terminal pro-BNP (>25,000 pg/mL; normal: <300 pg/mL).
Imaging studies included a chest x-ray showing cardiomegaly and pulmonary congestion, followed by echocardiography, which identified a persistent fifth aortic arch (PFAA) with coarctation (narrowest diameter: 5 mm), an interrupted fourth aortic arch (Figure 1). Color Doppler demonstrating turbulent flow at the coarctation site (peak velocity: 4.1 m/s) (Figure 2). A patent ductus arteriosus (PDA; 3 mm) located between the proximal descending aorta and the pulmonary artery bifurcation, and an atrial septal defect (ASD; 6 × 8 mm), accompanied by reduced left ventricular systolic function (ejection fraction: 49%). Cardiac CT (Figures 3, 4) further confirmed the anatomical details, including a 12 mm gap in the fourth aortic arch and the PFAA's aberrant course.
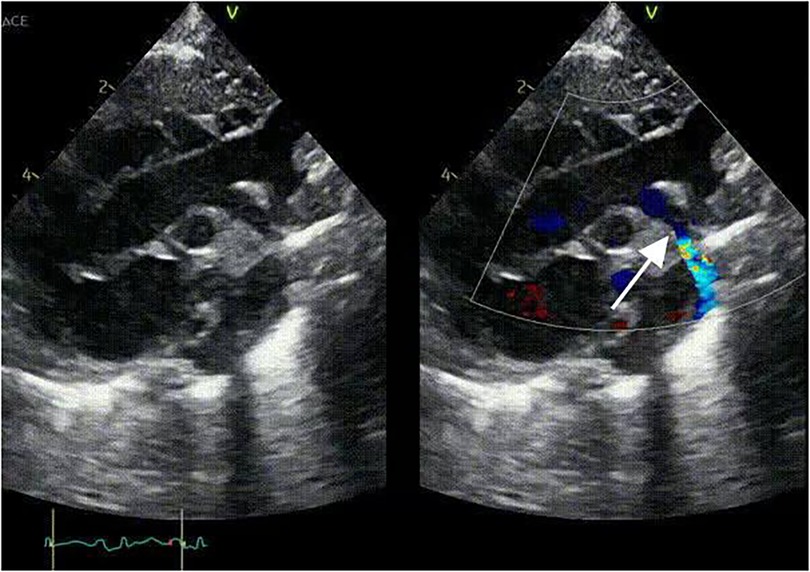
Figure 1. 2D-TTE: persistent fifth aortic arch associated with stenosis (indicated by arrows) and an interrupted fourth aortic arch.
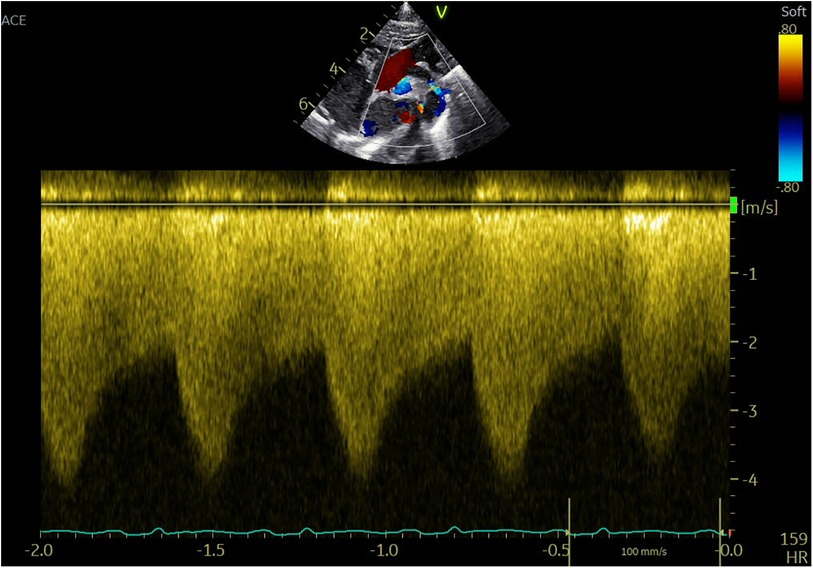
Figure 2. Color Doppler demonstrating turbulent flow at the coarctation site (peak velocity: 4.1 m/s).
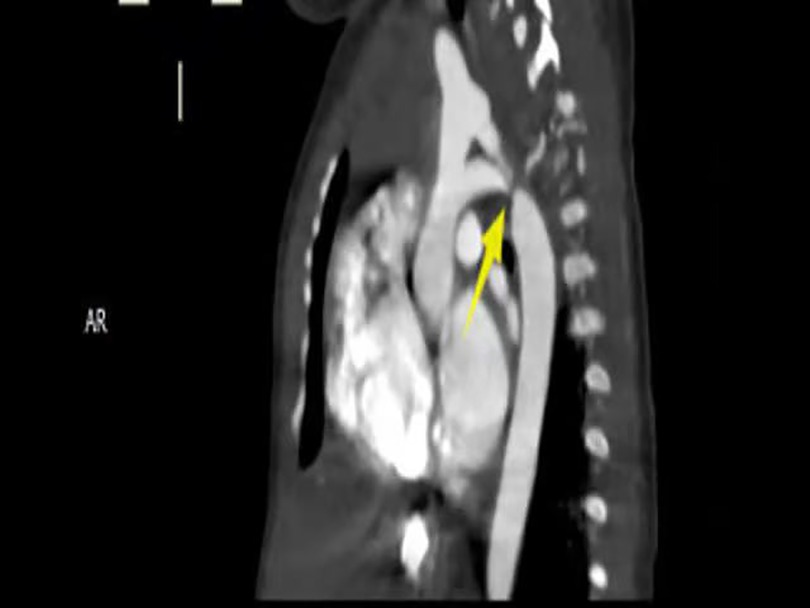
Figure 3. CTA: persistent fifth aortic arch associated with stenosis (indicated by arrows) and an interrupted fourth aortic arch. The fourth arch was interrupted at the distal end of the left subclavian artery, about 12 mm long. The fifth arch continued with the descending aorta, with a length of about 9 mm and an internal diameter of about 5 m at the proximal end.
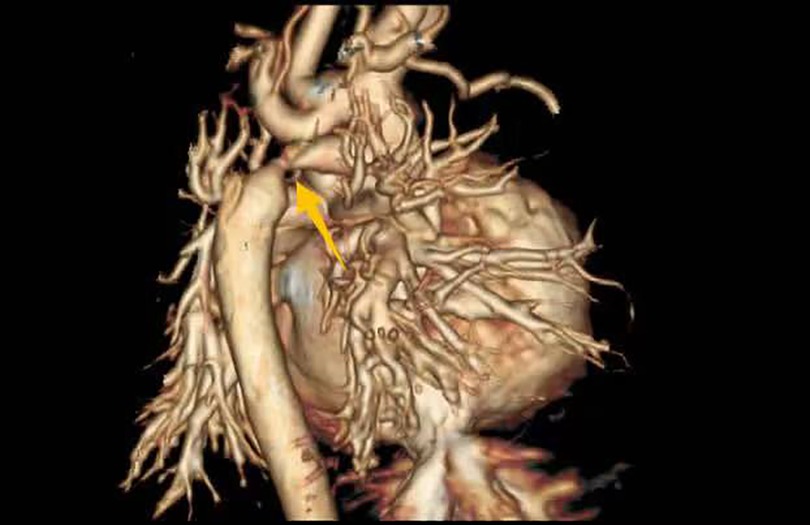
Figure 4. CT 3D reconstruction: persistent fifth aortic arch associated with stenosis (indicated by arrows) and an interrupted fourth aortic arch.
Initial stabilization involved nasal continuous positive airway pressure (NCPAP), intravenous dopamine to address cardiogenic shock, albumin supplementation, and diuretics. Definitive surgical management included aortic arch repair under cardiopulmonary bypass, involving excision of the fifth arch, end-to-end anastomosis between the native arch and descending aorta, PDA ligation, and ASD closure. Postoperative imaging confirmed restored aortic flow and normalized cardiac function (LVEF: 69%), with the patient discharged symptom-free on postoperative day 11.
Discussion
Persistent fifth aortic arch (PFAA) represents an exceptionally rare congenital cardiovascular anomaly with complex embryological origins. During mammalian cardiovascular development, six pairs of aortic arches sequentially form between embryonic weeks 3–8, undergoing precise regression and remodeling processes to establish definitive vascular structures (10). PFAA arises from the failure of fifth branchial arch regression, resulting in persistent vascular channels that exhibit distinct histological characteristics compared to normal aortic tissue (4). This anomaly may manifest as three anatomical subtypes: systemic-to-systemic connections (types A and B) or systemic-to-pulmonary shunts (type C) (11). In type B PFAA, as observed in this case, the fourth aortic arch is interrupted or atretic, leaving the fifth arch as the sole conduit between the ascending and descending aorta (12). The hemodynamic consequences of this configuration depend critically on associated anomalies, such as coarctation or ductal patency, which collectively determine clinical presentation and urgency of intervention.
The neonate in this report exhibited classic manifestations of type B PFAA complicated by coarctation and interrupted fourth aortic arch: respiratory distress, systemic edema, and oliguria. These symptoms reflect the pathophysiological cascade of left ventricular pressure overload, impaired systemic perfusion, and subsequent cardiogenic shock. Notably, the absence of lower limb blood pressure measurement at initial evaluation delayed recognition of coarctation—an oversight that underscores the necessity for systematic four-limb pressure assessment in neonates with suspected aortic arch anomalies. This case aligns with previous reports (8, 9) emphasizing that PFAA-associated coarctation often presents with subtle but critical perfusion discrepancies, requiring heightened clinical suspicion.
Diagnostically, this case illustrates the complementary roles of echocardiography and advanced imaging. While echocardiography provided initial identification of PFAA morphology, coarctation severity, and associated defects (PDA, ASD), cardiac CT offered three-dimensional anatomical clarification of the 12 mm fourth arch interruption and fifth arch course. Current guidelines (13, 14) endorse this multimodal approach, as echocardiography alone may inadequately visualize cervical vasculature or complex arch relationships. Although digital subtraction angiography (DSA) and cardiac catheterization remain gold standards for anatomical delineation (15), their invasive nature and radiation exposure limit utility in critically ill neonates. The noninvasive paradigm demonstrated here—combining echocardiography for functional assessment and CT for anatomical precision—provides a safer diagnostic framework without compromising accuracy.
Surgical management of PFAA with hemodynamically significant anomalies demands meticulous anatomical reconstruction. In this patient, the surgical strategy addressed three critical components: (1) excision of the hypoplastic fifth arch to eliminate turbulent flow, (2) end-to-end anastomosis restoring aortic continuity, and (3) concurrent closure of PDA and ASD to normalize circulatory physiology. This approach aligns with Zhao et al.'s principles (10) for PFAA repair, prioritizing anatomical correction while minimizing residual gradients. The postoperative ejection fraction improvement (49%–69%) and symptom resolution validate the intervention's success, though long-term surveillance remains essential to monitor for recoarctation or ventricular remodeling.
Two broader implications emerge from this case. First, PFAA's rarity and phenotypic variability necessitate high-volume center management, where multidisciplinary teams can integrate advanced imaging, neonatal critical care, and specialized surgical expertise. Second, the evolving role of fetal echocardiography warrants emphasis—earlier prenatal detection could enable delivery planning and immediate postnatal stabilization, potentially mitigating complications like renal hypoperfusion observed here. Future studies should explore optimal timing for surgical intervention in asymptomatic PFAA cases, balancing the risks of early intervention against the consequences of delayed treatment.
In conclusion, this case reinforces PFAA as a diagnostically and therapeutically challenging entity within congenital heart disease. It highlights the critical interplay between meticulous clinical examination, strategic imaging utilization, and tailored surgical correction. As neonatal care advances, continued refinement of diagnostic protocols and surgical techniques will be paramount to improving outcomes for this rare patient population.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Ethics statement
Written informed consent was obtained from the individual(s), and minor(s)' legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
GY: Writing – original draft. HZ: Writing – original draft. FS: Writing – review & editing.
Funding
The author(s) declare that no financial support was received for the research and/or publication of this article.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Generative AI statement
The author(s) declare that no Generative AI was used in the creation of this manuscript.
Any alternative text (alt text) provided alongside figures in this article has been generated by Frontiers with the support of artificial intelligence and reasonable efforts have been made to ensure accuracy, including review by the authors wherever possible. If you identify any issues, please contact us.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Van Praagh R, Van Praagh S. Persistent fifth arterial arch in man. Am J Cardiol. (1969) 24(2):279–82. doi: 10.1016/0002-9149(69)90417-2
2. Shan H, Du X, Zheng G, Ke T, Liao C, Yang H. Persistent fifth aortic arch: a comprehensive literature review. Front Pediatr. (2023) 11:1183345. doi: 10.3389/fped.2023.1183345
3. Gupta SK, Gulati GS, Anderson RH. Clarifying the anatomy of the fifth arch artery. Ann Pediatr Cardiol. (2016) 9(1):62–7. doi: 10.4103/0974-2069.171392
4. Atsumi N, Moriki N, Sakakibara Y, Mitsui T, Horigome H, Kamma H. Persistent fifth aortic arch associated with type A aortic arch interruption. Jpn J Thorac Cardiovasc Surg. (2001) 49:509–12. doi: 10.1007/BF02919546
5. Rajagopal R, Garg PK, Khera PS, Sharma S. “Double-lumen” aortic arch with “double-lumen” brachiocephalic artery. Ann Pediatr Cardiol. (2019) 12(2):141–3. doi: 10.4103/apc.APC_106_18
6. Yang H, Zhu X, Wu C, Zhao X, Ji X. Assessment of persistent fifth aortic arch by echocardiography and computed tomography angiography. Medicine (Baltimore). (2020) 99(9):e19297. doi: 10.1097/MD.0000000000019297
7. Naimo PS, Vazquez-Alvarez MC, d’Udekem Y, Jones B, Konstantinov IE. Double-lumen aortic arch: persistence of the fifth aortic arch. Ann Thorac Surg. (2016) 101(5):e155–56. doi: 10.1016/j.athoracsur.2015.10.014
8. Marcus BS, Rubio A, Deen JF. Transcatheter relief of coarctation of the aorta in a persistent fifth aortic arch anatomy. Prog Pediatr Cardiol. (2020) 57:101200. doi: 10.1016/j.ppedcard.2020.101200
9. Weinberg PM. Aortic arch anomalies. J Cardiovasc Magn Reson. (2006) 8:633–43. doi: 10.1080/10976640600713756
10. Zhao YH, Su Z-K, Liu J-F, Cao D-F, Ding W-X. Surgical treatment of persistent fifth aortic arch associated with interrupted aortic arch. Ann Thorac Surg. (2007) 84:1016–9. doi: 10.1016/j.athoracsur.2007.04.030
11. Valderrama P, Álvarez T, Ballesteros F, Rodríguez A, Zunzunegui JL. Coarctation of persistent fifth aortic arch with interrupted fourth arch. Rev Esp Cardiol. (2016) 69(3):337–8. doi: 10.1016/j.recesp.2015.10.024
12. Nakashima K, Oka N, Hayashi H, Shibata M, Kitamura T, Itatani K, et al. A case report of persistent fifth aortic arch presenting with severe left ventricular dysfunction. Int Heart J. (2014) 55:87–8. doi: 10.1536/ihj.13-167
13. Bernheimer J, Friedberg M, Chan F, Silverman N. Echocardiographic diagnosis of persistent fifth aortic arch. Echocardiography. (2007) 24(3):258–62. doi: 10.1111/j.1540-8175.2007.00383.x
14. Kusano N, Marutani S, Masumi H, Ueshima K, Takada N, Nishino T, et al. A case of persistent fifth aortic arch with an interrupted fourth aortic arch. Ann Thorac Surg. (2022) 114(3):e173–5. doi: 10.1016/j.athoracsur.2021.11.049
Keywords: congenital heart disease, persistent fifth arch, aortic arch interruption, echocardiography, children
Citation: Yi G, Zehang H and Shumin F (2025) Case Report: A case of persistent fifth aortic arch associated with stenosis and interrupted aortic arch. Front. Pediatr. 13:1676467. doi: 10.3389/fped.2025.1676467
Received: 16 September 2025; Accepted: 10 November 2025;
Published: 27 November 2025.
Edited by:
Osman Al-Radi, King Abdulaziz University, Saudi ArabiaReviewed by:
Fabrizio De Rita, ARNAS Ospedali Civico Di Cristina Benfratelli, ItalyVladislav Vukomanović, University of Belgrade, Serbia
Copyright: © 2025 Yi, Zehang and Shumin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fan Shumin, ZnNtZG9jdG9yQDE2My5jb20=
 Gao Yi
Gao Yi Hu Zehang
Hu Zehang Fan Shumin
Fan Shumin