- 1School of International Pharmaceutical Business, China Pharmaceutical University, Nanjing, China
- 2Center for Pharmacoeconomics and Outcomes Research, China Pharmaceutical University, Nanjing, China
- 3Sumitomo Pharma (Suzhou) Co., Ltd., Shanghai, China
Background: Schizophrenia is a severe and complex disease with substantial economic and social burdens. Despite multiple treatment choices, adverse events, and impaired social functions are still challenges in clinical therapy. Pharmacoeconomic evaluations could provide evidence to help decision makers improve the utilization of scarce resources. However, there remains some challenges especially in modeling due to uncertainties in progression of schizophrenia. There are limited summaries about the overall methodologies of schizophrenia economic evaluations.
Objective: The aim of this study is to review the existing economic evaluations of antipsychotics for the treatment of schizophrenia and summarize the evidence and methods applied.
Methods: An electronic literature search was performed in PubMed, Web of Science, EBSCO host, The Cochrane Library and ScienceDirect from January 2014 to December 2020. Search terms included “schizophrenia,” “schizophrenic,” “pharmacoeconomic,” “economic evaluation,” “cost-effectiveness,” and “cost-utility.” The Literature was screened and extracted by two researchers independently and assessed with the Quality of Health Economic Studies (QHES) List and Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement.
Results: A total of 25 studies were included in the review. The regions included Europe, North America, Asia and Africa. Most of the studies chose second-generation antipsychotics as comparators and integrated treatment sequences. Time horizons varied from 1 year to lifetime. The healthcare sector was the most common perspective, accordingly, most of the evaluations considered only direct medical costs. The Markov model and decision tree model were the most common choices. Adverse events, compliance and persistence were considered important parameters. Quality-adjusted life-years were the major outcomes applied to the economic evaluations. All utilities for health states and adverse events were collected from published literature. All of the studies applied uncertainty analysis to explore the robustness of the results. The quality of the studies was generally satisfactory. However, improvements were needed in the choice of time horizons, the measurements of outcomes and the descriptions of assumptions.
Conclusions: This study highlights the methodology of economic evaluation of schizophrenia. Recommendations for modeling method and future study are provided.
Introduction
Schizophrenia is a severe and complex mental illness with early onset coupled with behavior or cognitive disorders that have a significant impact on patients' family and society. A systematic review reported that the global age-standardized prevalence of schizophrenia was 0.28% and the prevalence of cases rose from 13.1 million cases in 1990 to 20.9 million cases in 2016 (1). The average annual healthcare costs were estimated to be between $23,887 and $24,988 according to a real-world retrospective study in the US (2). Patients may incur higher expenditures due to comorbidities that are common among them (3). Furthermore, indirect medical costs related to productivity lost or caregiving were 8.5 times higher compared with direct medical costs according to a retrospective study based on medical insurance database in Guangzhou, China (4). Also, schizophrenia has significant impact on caregivers of the patients. The well-being of both patients and caregivers could be affected during their cognitive appraisal processes of the illness, help-seeking experience and the interaction within the families (5) and the burden of caregivers exists in physical and mental health, social relationship, and financial life (6).
Economic evaluations could generate evidence incorporating both costs and consequences for decision makers to clarify different uses for scarce resources (7). Despite the multiple choices of medications in schizophrenia treatment, there still exist substantial burdens and difficulties in clinical therapies due to low adherence and adverse events. Thus, pharmacoeconomic evidence is required to balance the clinical effects with the resources consumed. However, there remain some challenges especially in modeling due to uncertainties in the progression of the diseases, emphasizing the requirements for systematic reviews of the methods applied in the analysis.
Previous systematic reviews have evaluated studies published since 2000 (8–10). However, none of them fully discussed the treatment sequences or methods applied. Furthermore, most of them adopted an extensive range of years of publication, which may not characterize the studies in recent years. Therefore, this study was conducted to review the model-based economic evaluations published recently for antipsychotics and summarize the modeling techniques, including model structures, basic settings, integration and translation of the clinical events, and selection of utility values. In addition, the review also aimed to assess the quality of the studies.
Methods
The systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement developed by Moher et al. (11).
Eligibility Criteria
The inclusion criteria were as follows: (a) economic evaluations adopting cost-effectiveness analysis (CEA) or cost-utility analysis (CUA) approach; (b) patients diagnosed with schizophrenia with no limitation on gender or age; (c) intervention including all antipsychotics; and (d) outcomes presented as incremental cost-effectiveness (ICER). Studies were excluded if they met the any of the following criteria: (a) not reported in English; (b) not related to economic evaluation; (c) cost of illness, health-related quality of life or budget impact analysis studies; (d) abstracts or studies with full-text unavailable; (e) not model-based studies; and (f) chose clinical effect as the only outcome.
Search Strategy
An electronic literature search was performed in PubMed, Web of Science, EBSCO host, The Cochrane Library, ScienceDirect from January 2014 to December 2020. Search items included “schizophrenia,” “schizophrenic,” “pharmacoeconomics,” “economic evaluation,” “cost-effectiveness,” and “cost-utility.” The detailed strategy is provided in Supplementary Material. In addition, references from retrieved studies were searched manually to avoid missing data.
Data Extraction and Analysis
The included studies were screened, extracted and double checked by two researchers independently. Disagreements were resolved by discussion or by consulting a third researcher. General information was collected including title, first author's surname, year of publication, country or region, intervention, and treatment sequences. To summarize the methods applied, characteristics such as perspectives, type of costs, outcomes, model structures, and necessary parameters were recorded. The results and conclusions of studies were included in the extracted form but were not reported as main outcomes due to the arguments regarding the extrapolation of evaluation results (12). All the information was recorded and compared using Microsoft Excel 2016.
Quality Assessment
According to the review of quality assessment tools conducted by Walker et al. (13) in 2012, the Quality of Health Economic Studies (QHES) List was recommended to discriminate the quality of studies as a quantitative measurement. The Consolidated Health Economic Evaluation Reporting Standards (CHEERS) Statement (14) published in 2012 was recommended by the International Society for Pharmacoeconomics and Outcomes research (ISPOR) as a report checklist and for guidance to optimize the quality of reporting. Both QHES and CHEERS were applied to quantitatively and qualitatively assess the quality of studies.
Results
A total of 1,086 citations were retrieved from five electronic databases. After removing duplicates, 610 studies were eligible to enter the screening process and judgements were generated according to the titles and abstracts. Finally, 25 articles published in English were identified and included in the systematic review. A flow of the literature screening was provided in Figure 1 according to the PRISMA statement (11).
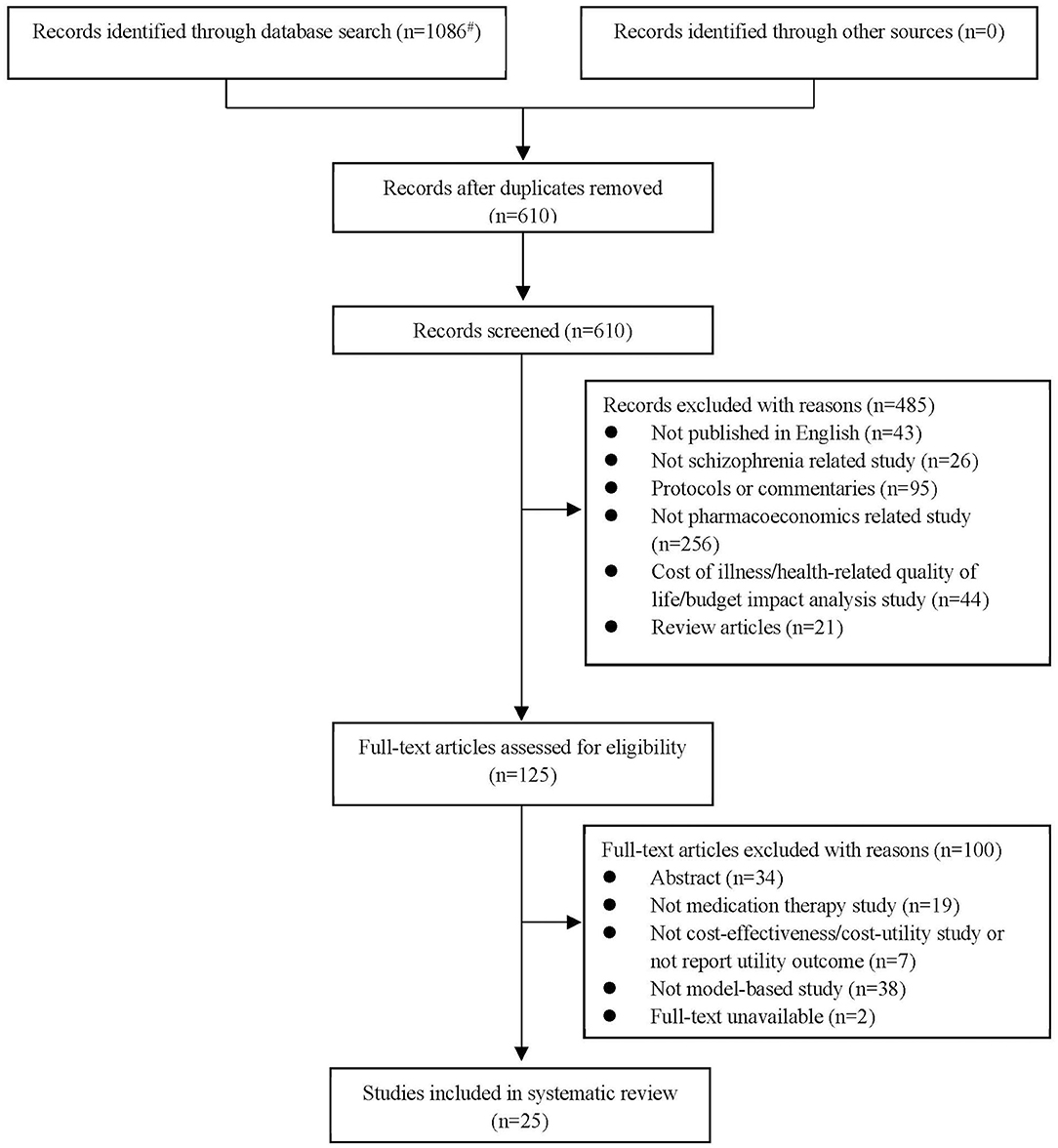
Figure 1. Flow of information through different phases of the systematic review according to the PRISMA statement [#Detailed search results: PubMed Database (n = 202), Web of Science Database (n = 377), EBSCO host Database (n = 113), The Cochrane Library Database (n = 286), ScienceDirect Database (n = 108)].
Basic Characteristics of Included Studies
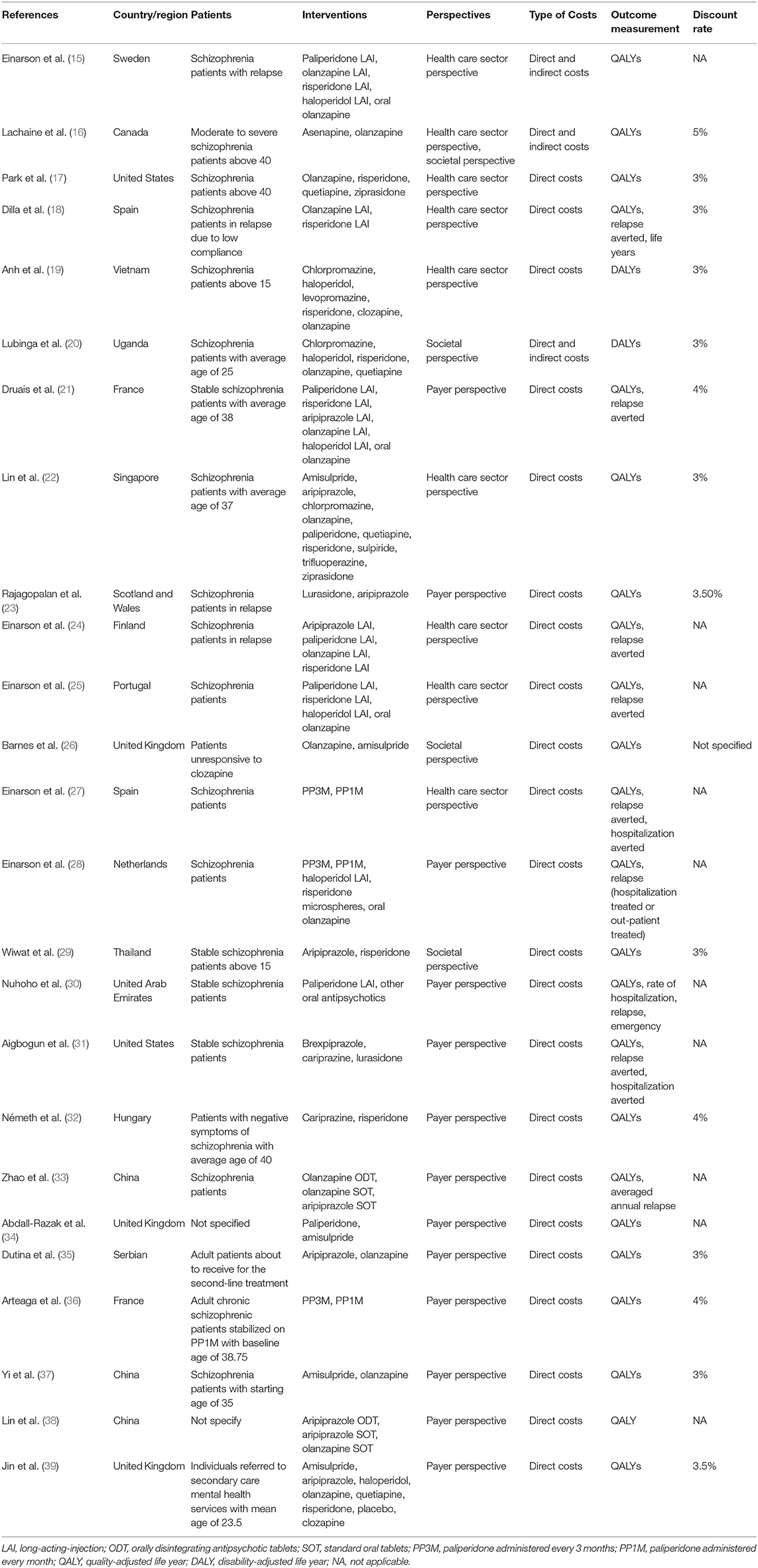
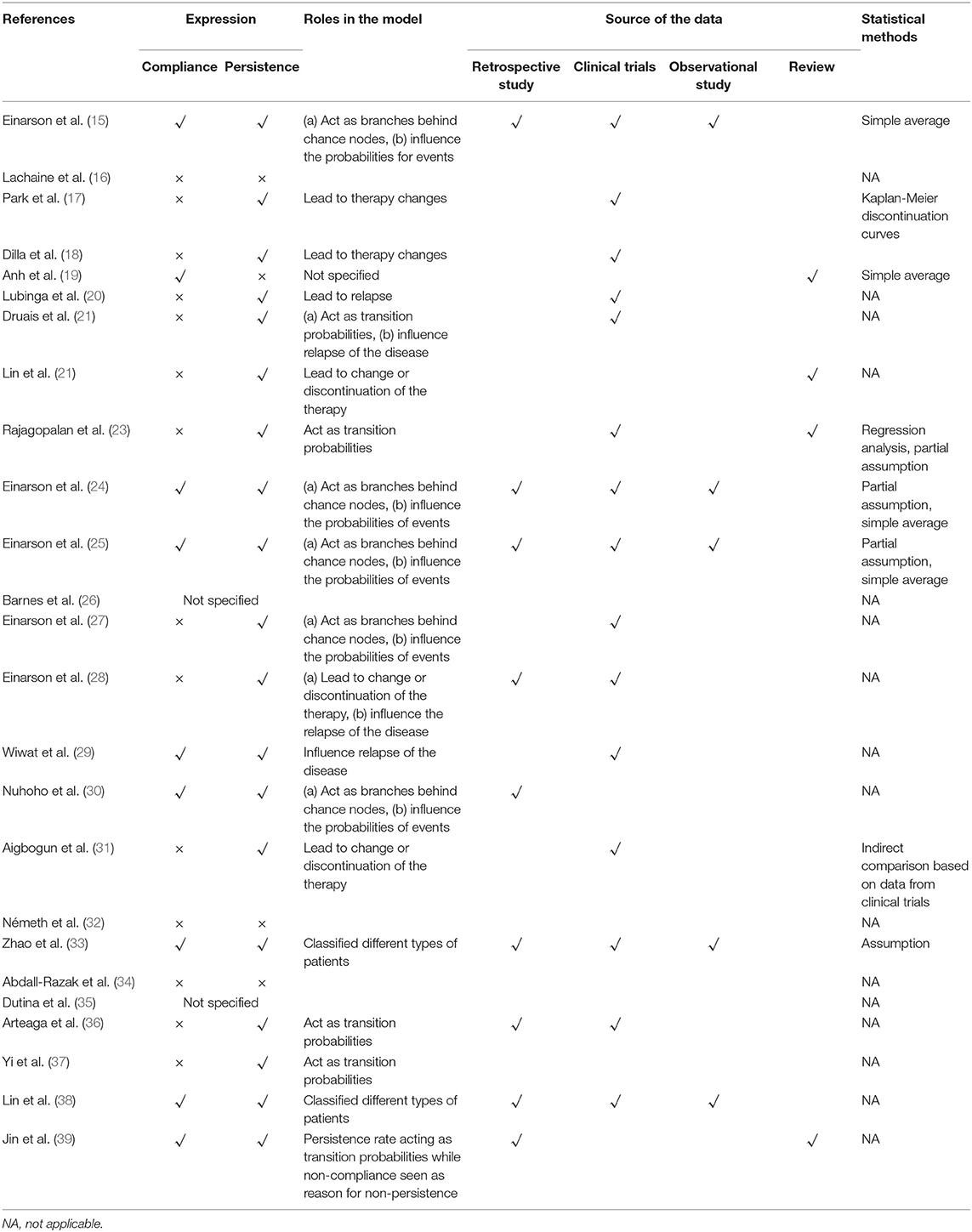
The characteristics of the included studies were summarized in Table 1. The studies covered the regions of Europe (15, 18, 21, 23–28, 32, 34–36, 39), North America (16, 17, 31), Asia (19, 22, 29, 30, 33, 37, 38), and Africa (20). Eleven (44%) (16, 17, 19–22, 29, 32, 36, 37, 39) of the 25 studies specified the onset or averaged ages of patients and four studies (16%) (15, 18, 23, 24) focused on patients in acute or relapsed states. Second-generation antipsychotics (SGAs) also known as atypical antipsychotics were the most common interventions chosen in the analyses, where seven studies (28%) (15, 18, 21, 24, 25, 27, 30) compared the cost-effectiveness of long-acting-injections (LAIs). Eight studies (32%) (18–22, 25, 28, 39) included both first-generation and second-generation antipsychotics as compared interventions where haloperidol was the commonest (n = 7, 87.5%). Most of the studies measured the outcomes using quality-adjusted life-years (QALYs) (n = 23, 92%), while only 2 studies (8%) (19, 20) applied disability-adjusted life-years (DALYs). In addition, nine studies (18, 21, 24, 25, 27, 28, 30, 31, 33) reported both utility and clinical effects, where yearly relapse or relapse avoidance was the most frequent choice.
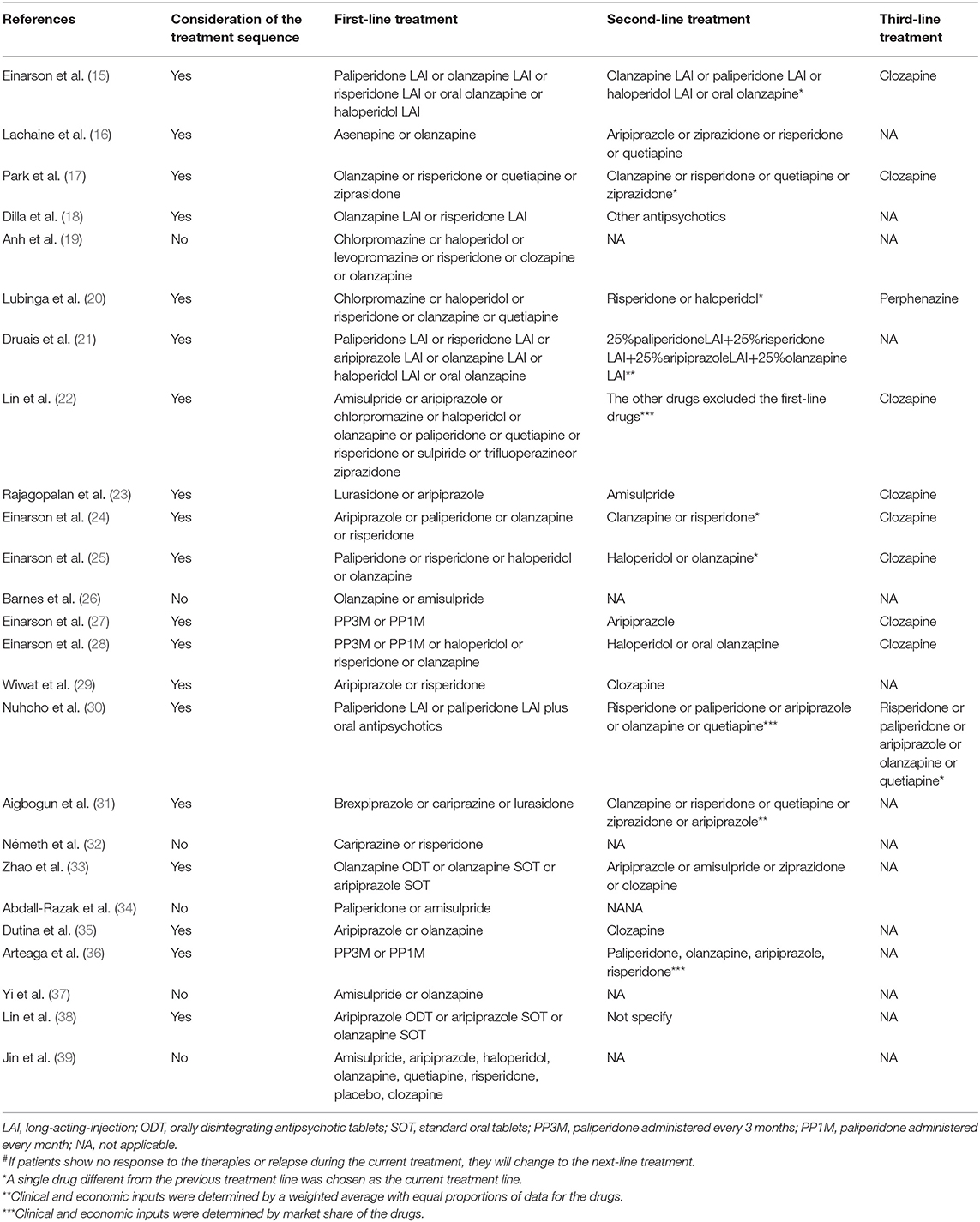
Due to the diverse efficacy and low compliance rate, therapeutic changes are common among schizophrenia patients, which makes treatment sequences worth consideration. Nineteen studies (76%) (15–18, 20–25, 27–31, 33, 35, 36, 38) specified treatment sequences in the models (Table 2). The methods of setting second-line medications were flexible, including changing to drugs that differed from the first-line or applying mixed therapies based on market share (22, 30, 36) or simple averaging (22, 31). Clozapine was the most common last-line therapy.
Results of Methodology Review
Perspectives and Related Costs
Various terms were used to define the study perspectives of the reviewed articles. Therefore, the terms were classified into three categories in this review, which were defined as health care sector perspective (including healthcare system, ministry of health, national health service, and government), payer perspective (including payer, third-party payer, and health insurance), and societal perspective (including societal, modified societal, and broadly societal perspective) based on the report recommended by ISPOR (40). Notably, studies of single-payer health system countries using both the healthcare system and payer were classified into the payer perspective. The perspectives determine the types of costs considered in the analysis (7, 40). Direct medical costs, both direct and indirect costs, and costs paid by payers are most relevant to the health care sector perspective, the societal perspective, and the payer perspective, respectively (7, 41).
Among the eight studies (32%) choosing the health care sector perspective (15, 17–19, 22, 24, 25, 27), seven studies adapted direct medical costs, while one study (15) included indirect costs which is inconsistent with the perspective. The payer perspective was chosen by 13 studies (52%) (21, 23, 28, 30–39) where six studies specified costs paid by payers and seven studies (28, 31, 33, 35–38) merely included direct medical costs. Among the 3 (14.3%) societal perspective-studies (20, 26, 29), only 1 study (20) took both direct and indirect costs into account, indicating that some confusion existed in distinguishing perspectives. One study chose both the healthcare sector and the societal perspective with direct and indirect costs (16).
Types of Models and Health States
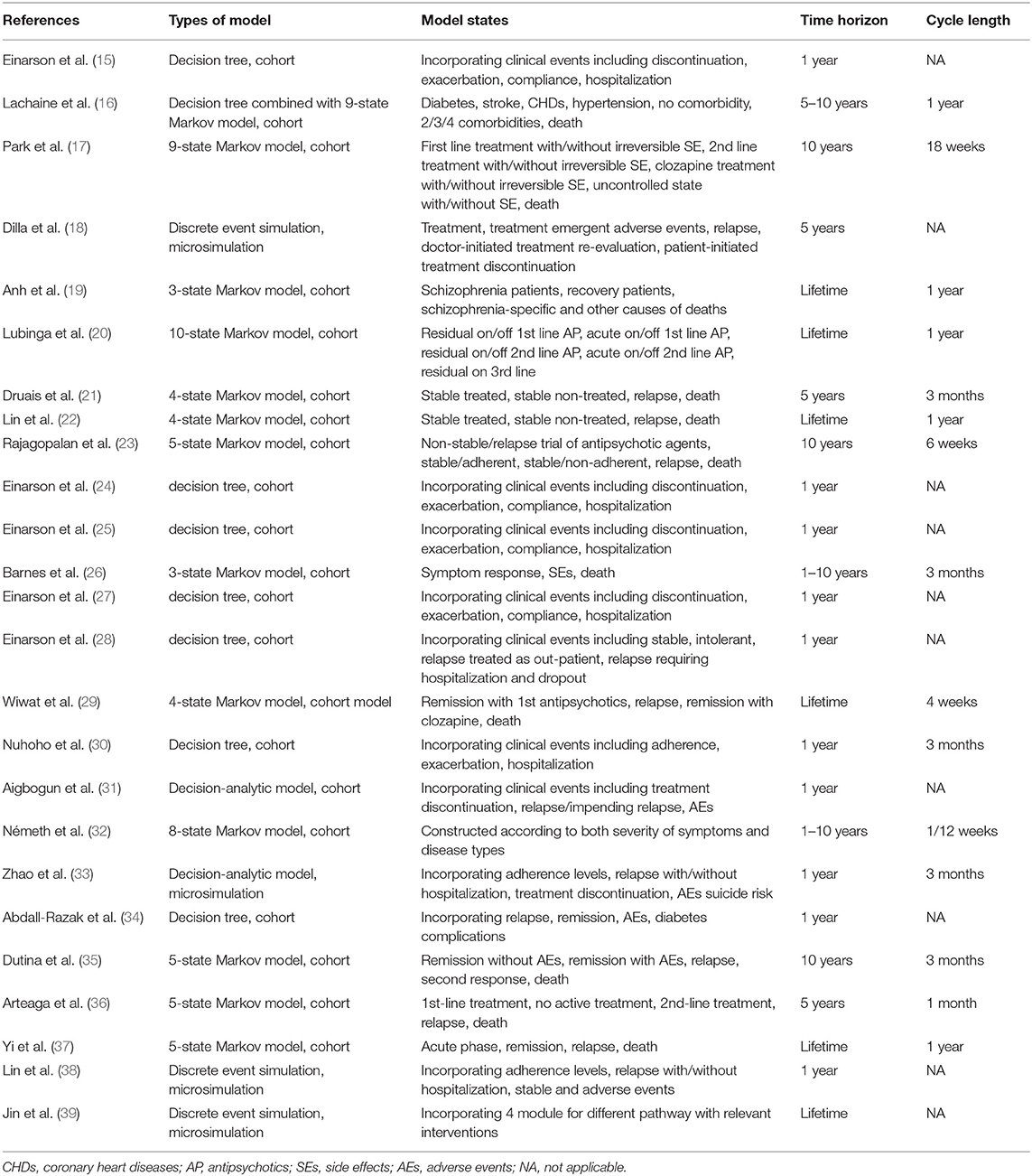
The characteristics of the models and related health states are summarized in Table 3. The Markov model (17, 19, 20, 22, 23, 26, 29, 32, 35–37) and the decision tree model (15, 24, 25, 27, 28, 30, 34) were the most common choices. One study (16) combined the decision tree model with the Markov model to better reflect complication-related treatment switches within the first year of treatment and long-term metabolic complications. Most of the Markov models consisted of a series of health states, which represent treatment sequences, disease progression or related adverse events and were connected by probabilities based on the averaged cohort level (27). Microsimulation models (18, 33, 38, 39) are more flexible and natural for simulating clinical reality by incorporating patient-level characteristics. Due to the differentiation in the choice of therapy and the treatment effect among patients, a patient-level simulation model as well as a Markov model incorporating treatment sequences, relapse, remission, adherence and adverse events could be more suitable for clinical practice.
Time Horizon and Cycle Length
As summarized in Tables 3, 6 (24%) studies (19, 20, 22, 29, 37, 39) chose the lifetime horizon in the model. However, considering the low adherence and frequent changes of medication, a certain number of studies (15, 16, 18, 21, 24–27, 30–33, 35) chose a relatively short time horizon. Among these, most Markov model-based studies explored uncertainty by extending the time horizon as a complementary analysis, while most decision tree model-based or microsimulation model-based studies conducted mere 1-year analyses (15, 24, 25, 27, 30, 31, 33, 34). As a chronic mental disease, schizophrenia should be simulated for a long time period or even lifetime in the model. However, due to the uncertainty in therapy and disease progression, it might be challenging to simulate further into the future as it becomes more unpredictable. Therefore, selecting the appropriate time frame covering events in the near future and then exploring time horizon uncertainty may be a reasonable method for economic evaluations for schizophrenia.
The cycle lengths of Markov models in the included studies varied from 4 weeks to 1 year, where 3 months (21, 26, 30, 32, 33, 35) and 1 year (16, 19, 20, 22, 37) were the most frequently used. The reasons for 3-month cycle selection included appropriate capture the both clinical practice and associated events according to clinical opinion (21, 30), consistent with clinical trials (26), while explanation for 1-year cycle selection was consideration of the realistic treatment management of schizophrenia (37). The length of cycle selection should be depend on both disease and intervention (7), and should be short enough to avoid multiple changes within a single cycle (42). Therefore, a 1-year cycle length may be less preferable compared to a 3-month cycle length.
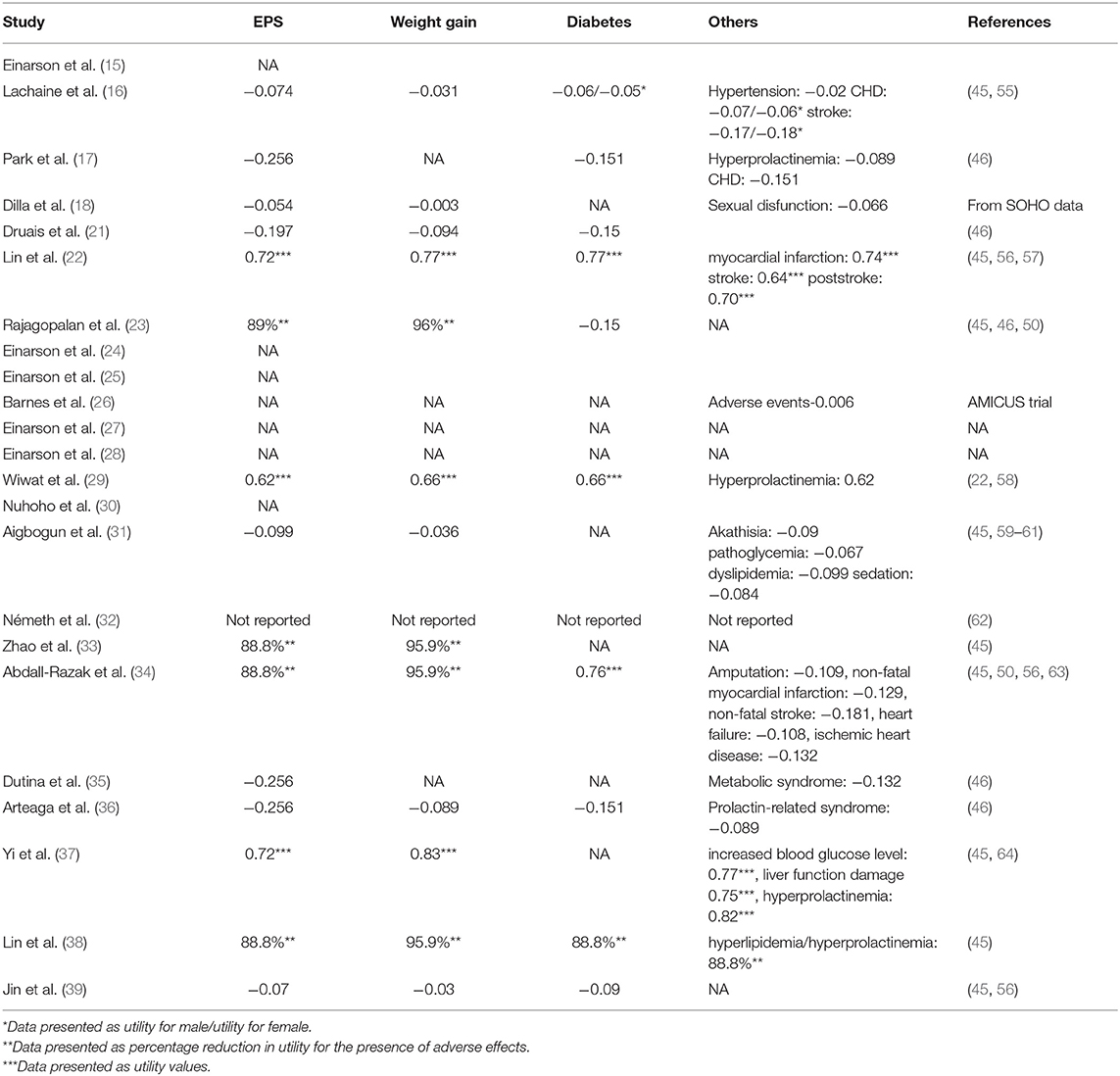
Adverse Events
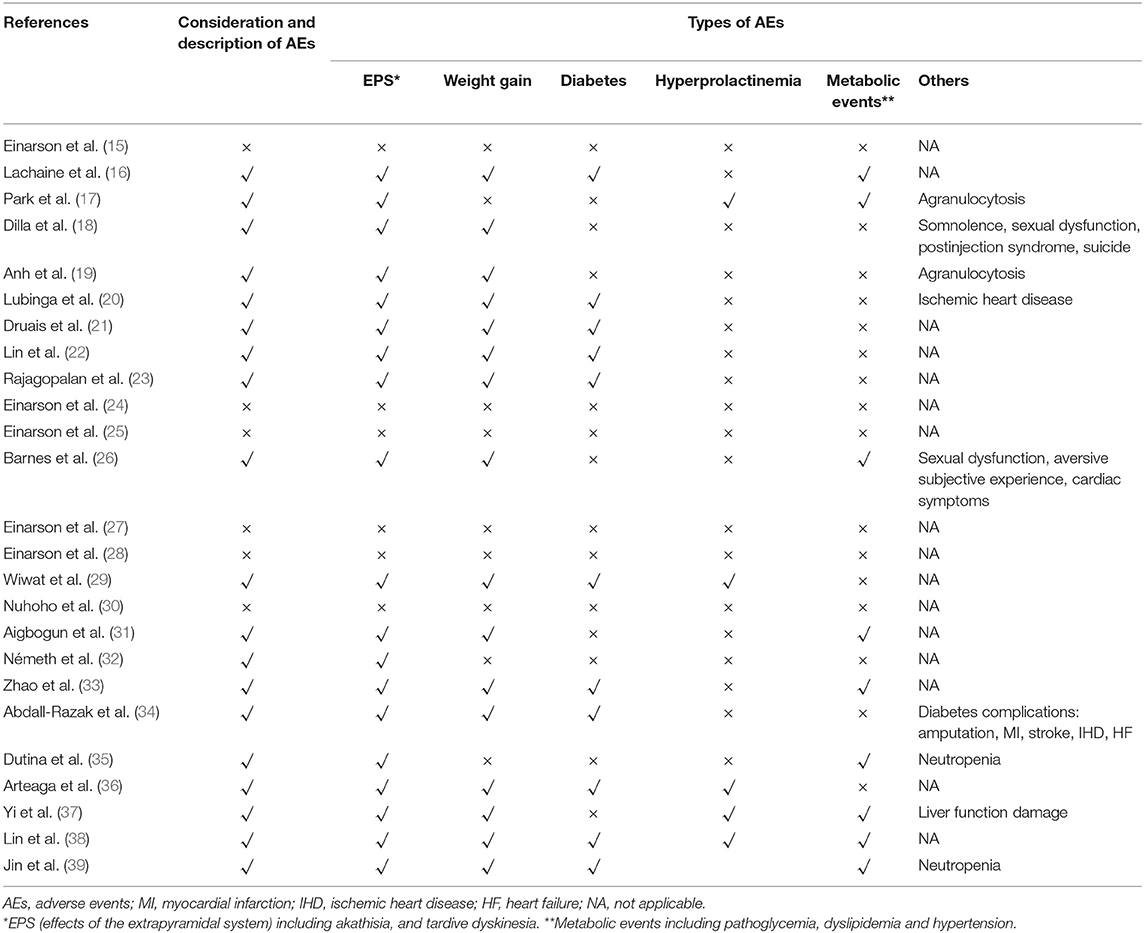
Adverse events (AEs) could impact adherence, efficacy and therapy changes as well as health-related quality of life, especially for schizophrenia. Thus, it is meaningful to take relevant AEs into account in the models. The AEs selected in the evaluations are listed in Table 4. The effect of the extrapyramidal system (EPS), weight gain, and diabetes were the most common adverse events considered. A few studies built separate health states for possible AEs to reflect the influence by applying transition probabilities, costs and utility of the health states (16, 22, 26). The majority of studies incorporated the prevalence, costs and disutility of AEs in each cycle to reflect the impact on disease progression. Both methods were acceptable as long as the choice was made based on proper consideration of the disease progression and available evidence (42).
However, some studies did not fully describe the consideration of AEs (27, 30) or even did not include AEs (15, 24, 25, 28) due to the similar incidence rates, small expenditures or short time horizon. It should be discussed whether the 1-year time horizon was sufficient to capture all important and interesting outcomes, since such a time horizon might fail to capture the impact on both health-related quality of life and costs of relevant clinical events among drugs. It is also notable that AEs with different durations should be clearly described to calculate the respective additional costs and disutilities. Park et al. (17) and Arteaga et al. (36) classified the reversible and irreversible AEs and assumed that reversible AEs would last for 18 weeks or 3 months while irreversible AEs would remain for the rest of the period. Rajagopalan et al. (23) classified the AEs into one-off AEs (such as EPS), persistent AEs (such as weight gain), and cumulatively occurred AEs (such as diabetes). Abdall-Razak et al. (34) clearly described the health-related utilization of the AEs and summarized the calculation of utilities of each AEs. However, more than half of the studies failed to clearly defined both costs and utilities considerations of the AEs.
Compliance and Persistence
Medication compliance (also known as adherence) can be defined as the extent to which the medication-taking of a patient matches that defined by the prescriber while medication persistence (also known as continuous adherence or discontinuation rate) refers to the act confirming to the recommended continuing treatment for the duration of time from the prescriber (43, 44). Both of them could influence the risk of relapse, rehospitalization, costs, quality of life through different aspects, especially for chronic disorders such as schizophrenia (44). Due to the different definitions and roles, it is important to not only take both compliance and persistence into account but also distinguish them when building economic models.
Information about the adoption of compliance or persistence in the included studies is listed in Table 5. The management of medication compliance or medication persistence can be summarized into three types: (1) Patients classified based on the compliance rates (adherence rates) from existing literature and then search for inputs under relevant compliance (15, 24, 25, 30, 33, 38). Such a method is usually applied in decision tree models and microsimulation models. (2) Non-persistence rates (discontinuation or dropout rates) are used rather than compliance to reflect the medication behaviors (17, 18, 20–22, 27, 28, 31, 36, 39). In such studies, non-persistence rates were divided into lack of efficacy, adverse events, intolerance, patients' choice and so on. Patients with treatment withdrawn due to lack of efficacy, adverse events or intolerance would change their therapies according to the treatment sequence. Patients who stopped treatment due to their own choices or for other reasons were defined as not receiving any therapies in the next simulation. Such methods were found in Markov model evaluations (3). Compliance not considered in the analysis. Lachaine et al. (16) assumed that patients remained on their medication continually for 5 years which was chosen as relatively short period to reduce uncertainty. Additionally, Abdall-Razak et al. (34) and Németh et al. (32) did not introduce the non-persistence rate due to a lack of relevant data.
Various sources of reference for compliance or persistence were adopted in the evaluations, including retrospective studies, clinical trial studies, and naturalistic studies. Simple averaging was the major method adopted for multisource data. Most discontinuation rates were collected from clinical trials. Even though there might be differences between the definitions of compliance and persistence, most of the studies did not explain or discuss this issue. Considering the definition of compliance, data from real-world studies might be superior as no intervention has been implemented to influence the medication behaviors of patients.
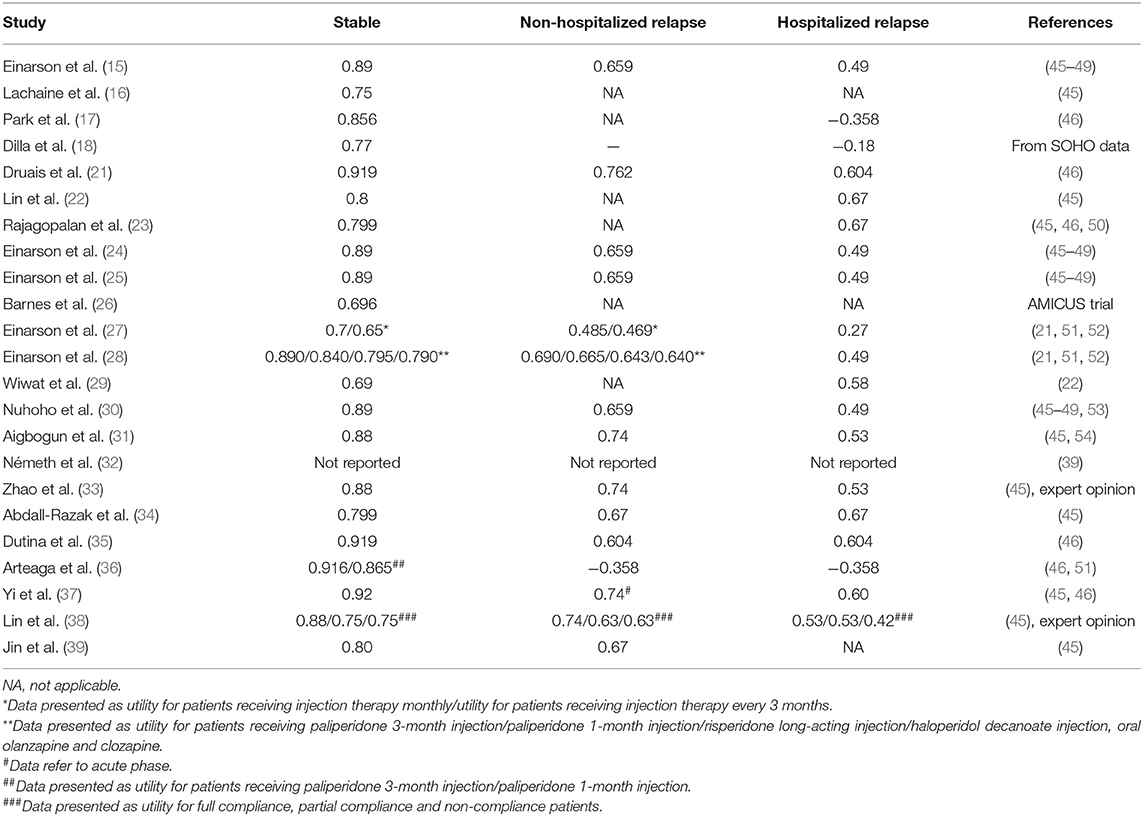
Utility
According to the health states defined in the model, the most commonly used utilities in the studies were those for stable schizophrenia, relapse without hospitalization, relapse with hospitalization and the disutilities of EPS, weight gain, and diabetes. Utilities and references are summarized in Tables 6, 7. Utilities of the stable state, relapse without hospitalization and relapse with hospitalization ranged from 0.65 to 0.919, 0.485 to 0.762, and 0.27 to 0.604, respectively. Apart from EPS, weight gain and diabetes, the utilities or disutilities of hypertension, coronary heart disease, stroke, and hyperprolactinemia were also considered in some studies. Utilities from Lenert et al. (45) and Briggs et al. (46) were the most frequently referenced among the studies.
It was noteworthy that the utilities for the same disease states varied greatly. Possible reasons included varieties in the classification of the researched states, selection of the population, and different methods or instruments applied among referenced quality of life studies.
Definitions or classifications of the health states were usually developed based on a literature review (45, 46), expert opinions (45, 46) and interviews with patients or laypersons (46) rather than through a unified method. Such states are usually framed by several items or descriptions; however, few studies discussed the applicability for states in decision-analytical models.
Layperson, patients with schizophrenia and caregivers are common responders in research on health-related quality of life. The differentiation of responders could induce heterogeneity among the results. For example, Briggs et al. (46) discovered significant differences between laypersons and patients, especially for relapse and EPS states. Regardless of the doubt regarding the response ability of patients with schizophrenia, studies have demonstrated that schizophrenia patients in the stable stage were able to provide valid and reliable answers, indicating the necessity to include such a population (48, 53).
Due to the specialty of mental disease as well as the choice of population such as laypersons, caregivers, or psychiatrists, the majority of the methods generated utilities using the standard gambling or time trade off approaches. The EuroQol-5 dimensions (EQ-5D) questionnaire is preferred by The National Institute for Health and Care Excellence (NICE). However, the sensitivity of the EQ-5D index to capture both social and psychological well-being for patients with schizophrenia is still controversial (65).
Results of the Economic Evaluations
The included studies compared the cost-effectiveness of commonly used SGAs (including long-acting injections). However, due to economic, political, cultural diversities among the different regions, the results of one economic evaluation may not be applicable beyond the defined setting (12). Thus, the results of the evaluation are not to be introduced here, but detailed information would be provided in the Supplementary Material 1. All of the studies adopted sensitivity analysis to verify the robustness of the base-case results. Notably, a change in the time horizon in different scenarios could lead to inverse results (17, 32). Accordingly, discussion about the time horizon may be required for schizophrenia therapy.
Quality Assessment of the Included Studies
The widely used QHES and CHEERS lists economic evaluation checklists were applied for a quantitative and qualitative review.
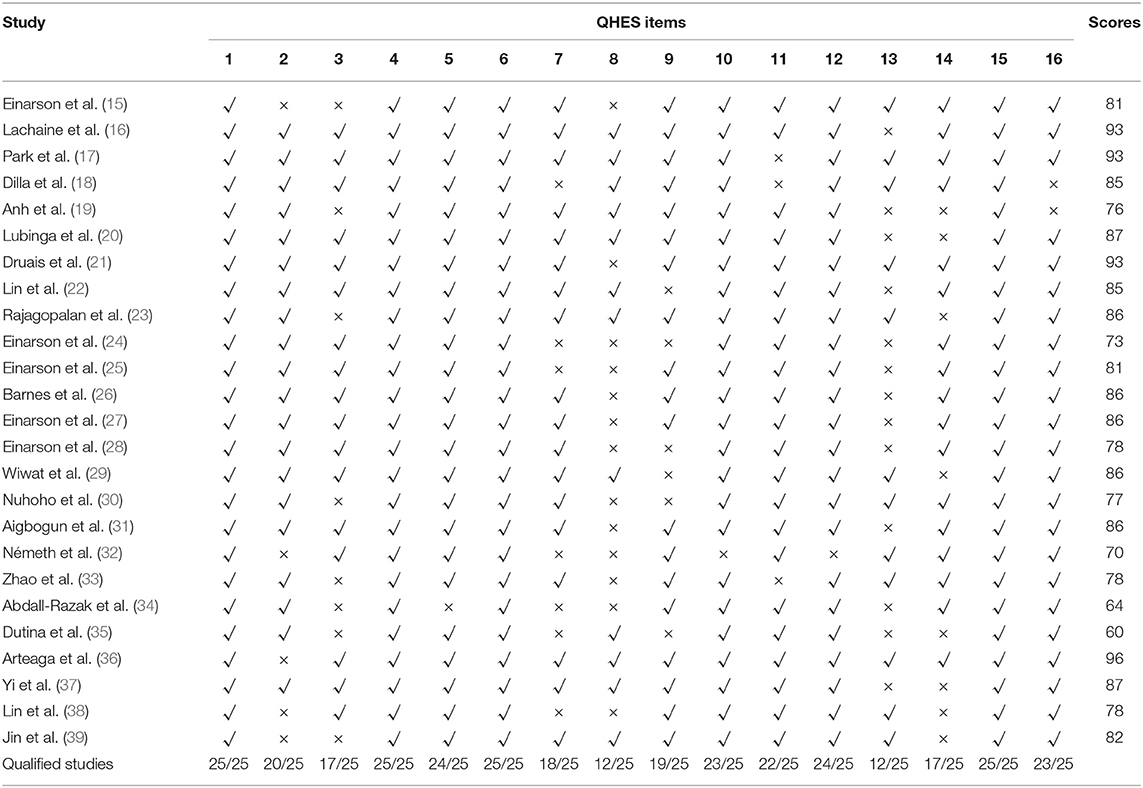
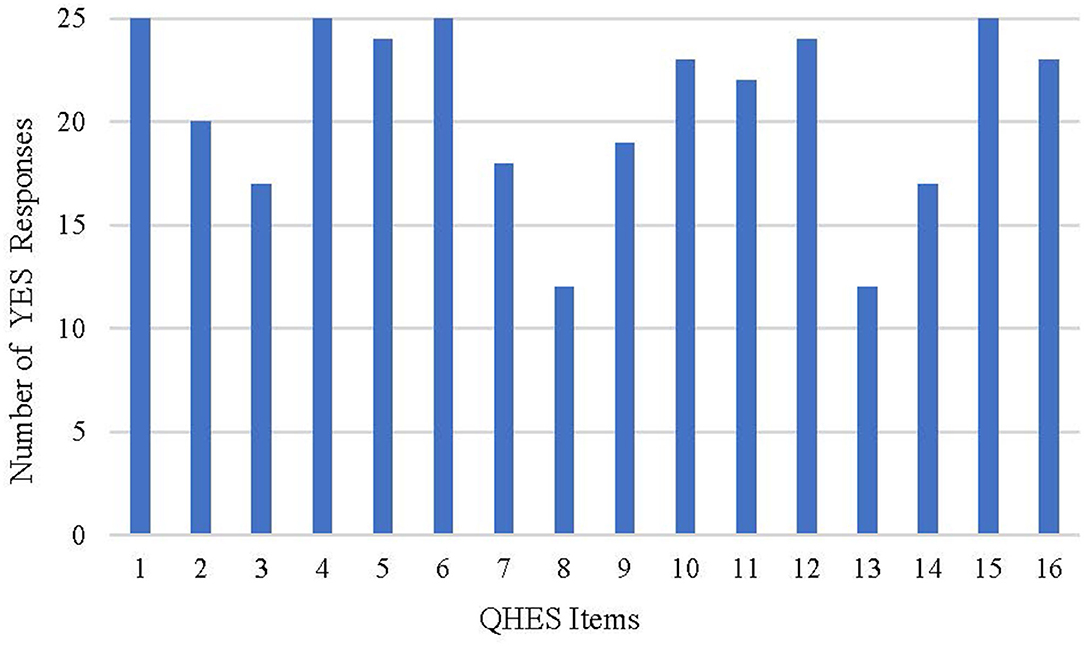
Assessed with the QHES list, scores ranged from 60 to 93 for 25 studies, where 21 (84.0%) studies scored between 75 and 93, and 4 (16.0%) studies scored between 60 and 74, indicating the relatively high quality of the majority studies. As summarized in Table 8 and Figure 2, all of the studies met the requirements of item 1, item 4, item 6, and item 15 representing the descriptions of study objective, subgroup analysis, incremental analysis, and conclusion, respectively. However, no more than half of the studies met the requirements of item 8 (choice of appropriate time horizon and discount rate) and item 13 (statement and justification of the choice of model, assumptions, and limitations).
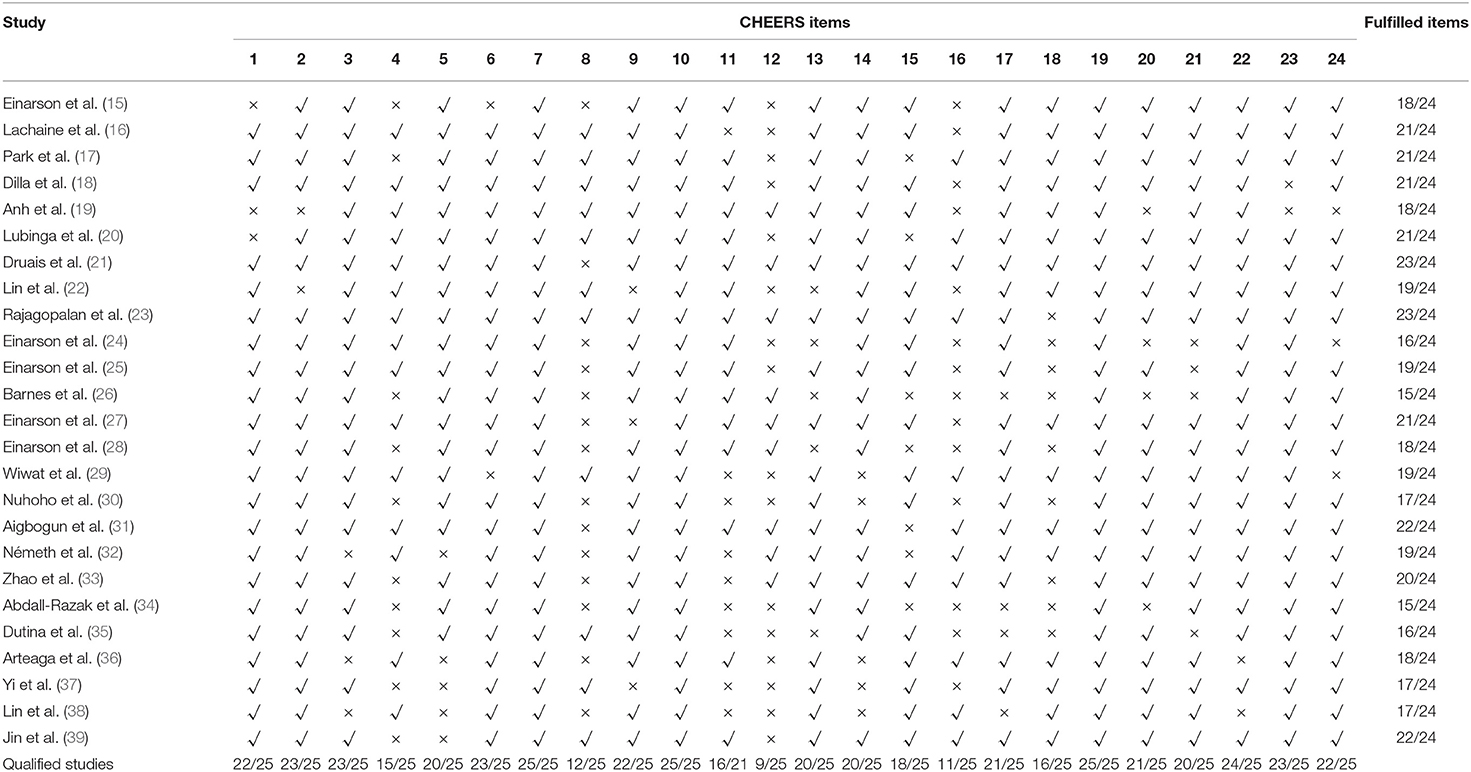
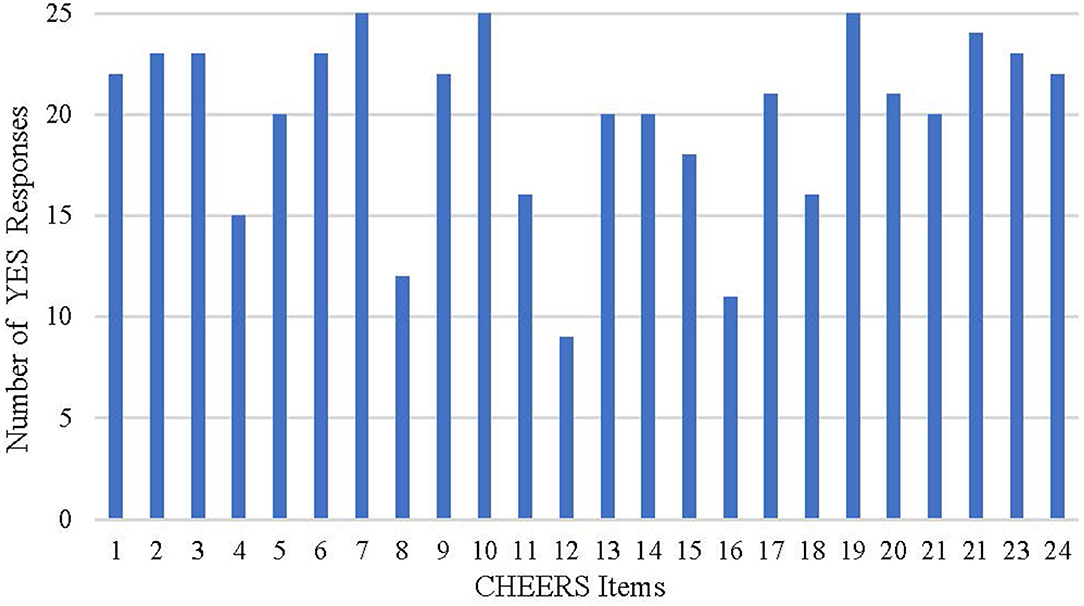
The quality reports evaluated with the CHEERS checklists were showed in Table 9 and Figure 3. The fulfilled items ranged from 16 to 23 for each study. More than half of the studies failed to meet the requirements for time horizon, preference-based outcome measurement, and assumptions. A considerable number of the studies selected a relatively short time horizon, which may be insufficient to capture necessary events. Most of the studies did not fully explain the reasons for time horizon selections. A large number of the studies chose utility values from the published literature, while some studies did not describe the methods used to elicit preference for outcomes. Studies seldom reported or explained the assumptions in single paragraph or table, adding to the difficulty identifying all assumptions underpinning the decision-analytical model. In addition, descriptions were for the parameters included, such as selection of population, measurement of effectiveness, and choice of model type.
Unlike the CHEERS checklist acting as a recommendation of the report format, the QHES list was developed to appraise the quality of economic evaluation (13, 14). According to the assessment of the QHES and CHEERS lists, most of the studies could be identified as relatively high-quality analyses, but certain limitations existed regarding the report quality. The selection of an appropriate time horizon and description of assumptions are frequent limitations for the included studies or even for economic evaluations in other chronic diseases (66, 67).
To compare the quality among the included studies, model types, regions, and time horizons were used as the indicators to classify the studies. The average QHES score of Markov model studies was 84.46 (13 studies) which was higher than that of decision tree model studies (77.14, 7 studies). The average score of the studies applying microsimulation was 80.75. The average numbers of the items consistent with the CHEERS recommendations were 19.23, 17.71, and 20 for Markov model studies, decision tree model studies, and microsimulation model studies. As a result, the Markov model and microsimulation model rather than the decision tree model are more appropriate model types for the study of schizophrenia. The quality of studies among different regions was also slightly different. The average QHES scores of the studies from North America, Asia, and European countries were 90.67, 81.00, and 80.07, respectively. It should be noticed that certain discrepancies exist among the scores of the studies from European countries where the maximum and minimum scores were 96 and 64. The numbers of the items consistent with the CHEERS recommendations (21.3, 18.14, and 18.86, respectively) were similar. However, even though the studies from North America seemed to be of higher quality, the numbers of the studies from the three regions differed a lot (three studies from North America, 7 studies from Asia, and 14 studies from European countries) and this might introduce bias when assessing the qualities.
To analyze the quality differences of studies with different time horizons, we classified the studies into two categories: short-term studies, i.e., the time horizon was 1 year or less, and long-term studies, i.e., the time horizon was longer than 1 year. The averaged QHES scores of the short-term studies (78.2, 10 studies) were lower than that of the longer-term studies (84.3, 15 studies). However, the number of the items consistent with the CHEERS recommendations are similar (18.3 for short-term studies vs. 19.53 for long-term studies). As mentioned above, the QHES list was developed to appraise the quality of economic evaluation while the CHEERS checklist was developed to recommend the report format. It can be inferred from the description of the time horizon from the two lists that the QHES list (Did the analytic horizon allow time for all relevant and important outcomes?) was more subjective and focused on the relationship between the item assessed and model outcomes. While the CHEERS checklist [State the time horizon(s) over which costs and consequences are being evaluated and say why appropriate] highlighted the fact that the statement of relevant aspect was provided and with no requirement to judge the appropriateness for the evaluation. Thus, attention should be paid when choosing checklists and interpreting the results.
Discussion
Purpose of Systematic Review of Economic Evaluations
With the increasing number of publications on health economic studies in recent years, systematic reviews in this filed have caught the attention of decision makers as useful tools to generate evidence (68). However, concerns have been voiced regarding whether cost-effectiveness findings can be transferred from one setting to another (12). Anderson et al. discussed the generalizability of the results of health economic studies and concluded that differences in methods, context, intervention costs, and effects contributed to the limitations on evidence synthesis (12). Gomersall et al. also discussed the debate over futility vs. utility of systematic reviews of economic evidence, emphasizing the inability to compare resources between countries and the differences in context and population (69). Pigone et al. also pointed out the challenges in the presentation of economic systematic reviews due to the large amounts of both synthesized data and generated results (70).
Despite challenges in generating results of economic evaluations via systematic reviews, it was suggested that systematic reviews should focus on methods of model development, sources of both efficacy and utility data, and resources used for specific diseases. It might be valuable for researchers and decision makers to identify the differences among studies (69, 70). For model-based economic evaluations, in particular, model structure selection was recommended based on the summarized existing studies, which could provide relatively comprehensive consideration for the necessary model states and link clinical practice and hypothetical model transitions. Notably, compared with differences in national contexts, it was found that variability in published economic evaluations was related more to the variety of study (71), confirming the importance of summarizing the model methods applied to existing studies. Thus, this systematic review focuses on the methods and study quality of the 25 eligible studies.
Main Findings
Among the included studies, most compared the cost-effectiveness of SGAs or long-acting injections. Fifteen studies considered the treatment sequences in model development, which could enlighten the future model development.
Despite the burden and productivity loss due to schizophrenia, few studies chose the societal perspective covering the indirect costs. Due to the early onset of schizophrenia, the average age of these patients is younger than that of patient with other chronic diseases. It would be necessary to include indirect costs in the evaluation. A retrospective study in the US concluded that indirect and non-health care costs were strong contributors and could be more than 70% of the total burden (72). A systematic review of the indirect costs of schizophrenia in Europe found that the average proportion of indirect costs of total disease expenditure was 44% (73). In China, the per case per annum indirect costs of schizophrenia were approximately US$1723.4 in 2013, accounting for 66.6% of the total costs (74). Therefore, a societal perspective covering both direct and indirect costs is preferable.
Description of the treatment sequences from most of the included studies could improve the model design and reflect the clinical prescription especially for chronic diseases or patients with high rate of therapy change. However, there remains challenges considering treatment sequences in economic evaluation. Medication treatment may vary among individuals due to the genotypes, metabolism, comorbidities, adverse events and so on (75). Also, the followed medication therapy should be impact by the previous medication choices. Thus, it may be questioned to apply a uniform sequency for patients especially in the cohort model. Cost and effectiveness data for the multiple drugs and the analytical methods are other challenges for treatment sequences. Even though choice of single drug based on market share or expert opinions and weighted or unweighted averaged data from multiple drugs are common methods in studies, the appropriateness requires further discussion.
According to the summary of the models applied, the Markov model was the most frequently used and treatment sequences, relapse, remission, and adverse events were the important health state elements in model development. The time horizons varied from 5 years to lifetime for the Markov models. While for the decision tree models, a 1 year time horizon was preferred. Due to the uncertainty in the time frame of treatment, it is recommended the impact of the time horizon be explored in a sensitivity analysis. Adverse events such as EPS, weight gain, and diabetes should be considered in the models for schizophrenia since these factors have a recognized influence on the treatment effect. Also, consideration of different types of AEs should be properly defined to estimate the impact on health outcomes and costs in the economic evaluation.
Based on the criteria studies, compliance and persistence were not clearly classified, thus definition is recommended for economic evaluations since it might determine the choice of appropriate data source. When integrating compliance or persistence, data are required on both health outcomes and costs for patients who are non-compliant or discontinued treatment (76). Evidence for compliance or persistence could be collected from retrospective studies, clinical trials, observational studies, or reviews.
Utility values, derived from the literature may contribute to the heterogeneity among results when they are applied to the same health states in different studies. Differences in the classifications of health states, survey responders, elicitation methods, and regions were the main factors influencing the utility values. Thus, it is recommended that researchers choose proper sources based on the factors above, as well as the publication year or update of methods.
Certain limitations to study quality have been identified, such as the description of appropriate time horizon selection, discount rate, statement, and justification of the choice of model type, assumptions and limitations to the evaluations. For the reporting quality of the studies, time horizon, preference-based outcome measurement, and assumptions were the major missing parts. To improve both the quality of the study and the quality of the report, it is suggested that researchers conduct the evaluation and generate the manuscript under the respective guidance and checklists.
Though there exist studies reviewed the economic evaluation of treatment for schizophrenia (8–10, 77), few fully discussed the treatment sequences, AEs, compliance and persistence of the included studies. Thus, this review provides more comprehensive and detailed information of modeling methodology for economic evaluation.
Limitations
There remain some limitations of this review. First, the review only included studies published after 2014 and does not represent the economic methods used in earlier years. Studies published recently may be more valuable for analysis, considering the relatively high quality, most recent treatment options and updated clinical evidence. Second, this review only included model-based economic evaluations. Even though trial-based economic evaluations for schizophrenia are also important evidence, this study aims to generate summaries and suggestions for model methodology rather than synthesizing economic evidence. In addition, trial-based studies may not provide long-term clinical outcomes and source consumption, especially for chronic diseases.
Suggestions
Based on the results of this review, it is suggested that future research focus on methods to integrate compliance or persistence data for chronic diseases. Due to the diverse utilities cited in the models, characteristics of study groups and measuring approach of preference-based health outcomes from the health-related quality of life research should be explained to provide appropriate options for the studies. Publications of economic evaluations should be designed and reported according to applicable gelines and checklists to improve study quality and provide both scientific and valuable evidence for decision makers. Future research could pay more attention to the economic evaluation of long-acting injection antipsychotics.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
LW and HL contributed to the study design, analysis, and writing. FS, XG, and HX contributed to the review work. JL contributed to the manuscript revise. All authors contributed to the article and approved the submitted version.
Funding
This study received funding from Sumitomo Pharma (Suzhou) Co., Ltd.
Conflict of Interest
This study received funding from Sumitomo Pharma (Suzhou) Co., Ltd. The funder had the following involvement with the study: decision to published. JL was employed by company Sumitomo Pharma.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2021.689123/full#supplementary-material
References
1. Charlson FJ, Ferrari AJ, Santomauro DF, Diminic S, Stockings E, Scott JG, et al. Global epidemiology and burden of schizophrenia: findings from the global burden of disease study 2016. Schizophr Bull. (2018) 44:1195–203. doi: 10.1093/schbul/sby058
2. Shah A, Xie L, Kariburyo F, Zhang Q, Gore M. Treatment patterns, healthcare resource utilization and costs among schizophrenia patients treated with long-acting injectable versus oral antipsychotics. Adv Ther. (2018) 35:1994–2014. doi: 10.1007/s12325-018-0786-x
3. Lafeuille MH, Dean J, Fastenau J, Panish J, Olson W, Markowitz M, et al. Burden of schizophrenia on selected comorbidity costs. Expert Rev Pharmacoecon Outcomes Res. (2014) 14:259–67. doi: 10.1586/14737167.2014.894463
4. Huang Y, Liu G, Liu Y, Wang C, Ren X, Zhang H. Economic burden of schizophrenia: based on medical insurance database from Guangzhou. Chinese Health Econ. (2014) 33:62–5. doi: 10.7664/CHE20140519
5. Caqueo-Urizar A, Rus-Calafell M, Craig TK, Irarrazaval M, Urzua A, Boyer L, et al. Schizophrenia: impact on family dynamics. Curr Psychiatry Rep. (2017) 19:2. doi: 10.1007/s11920-017-0756-z
6. Tamizi Z, Fallahi-Khoshknab M, Dalvandi A, Mohammadi-Shahboulaghi F, Mohammadi E, Bakhshi E. Defining the concept of family caregiver burden in patients with schizophrenia: a systematic review protocol. Syst Rev. (2019) 8:289. doi: 10.1186/s13643-019-1182-6
7. Drummond MF. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press (2002). p. 396.
8. Zhou J, Millier A, Toumi M. Systematic review of pharmacoeconomic models for schizophrenia. J Mark Access Health Policy. (2018) 6:1508272. doi: 10.1080/20016689.2018.1508272
9. Jin H, Tappenden P, Robinson S, Achilla E, MacCabe JH, Aceituno D, et al. A systematic review of economic models across the entire schizophrenia pathway. Pharmacoeconomics. (2020) 38:537–55. doi: 10.1007/s40273-020-00895-6
10. Jin H, Tappenden P, Robinson S, Achilla E, Aceituno D, Byford S. Systematic review of the methods of health economic models assessing antipsychotic medication for schizophrenia. PLoS ONE. (2020) 15:e234996. doi: 10.1371/journal.pone.0234996
11. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
12. Anderson R. Systematic reviews of economic evaluations: utility or futility? Health Econ. (2010) 19:350–64. doi: 10.1002/hec.1486
13. Walker DG, Wilson RF, Sharma R, Bridges J, Niessen L, Bass EB, et al. Best Practices for Conducting Economic Evaluations in Health Care: A Systematic Review of Quality Assessment Tools. Rockville, MD: Agency for Healthcare Research and Quality (2012).
14. Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (cheers)–explanation and elaboration: a report of the ISPOR health economic evaluation publication guidelines good reporting practices task force. Value Health. (2013) 16:231–50. doi: 10.1016/j.jval.2013.02.002
15. Einarson TR, Vicente C, Zilbershtein R, Piwko C, Bø CN, Pudas H, et al. Pharmacoeconomics of depot antipsychotics for treating chronic schizophrenia in Sweden. Nord J Psychiat. (2014) 68:416–27. doi: 10.3109/08039488.2013.852243
16. Lachaine J, Beauchemin C, Mathurin K, Gilbert D, Beillat M. Cost-effectiveness of asenapine in the treatment of schizophrenia in Canada. J Med Econ. (2014) 17:296–304. doi: 10.3111/13696998.2014.897627
17. Park T, Kuntz KM. Cost-effectiveness of second-generation antipsychotics for the treatment of schizophrenia. Value Health. (2014) 17:310–9. doi: 10.1016/j.jval.2014.02.008
18. Dilla T, Möller J, O Donohoe P, Álvarez M, Sacristán JA, Happich M, et al. Long-acting olanzapine versus long-acting risperidone for schizophrenia in Spain – a cost-effectiveness comparison. BMC Psychiatry. (2014) 14:298–309. doi: 10.1186/s12888-014-0298-4
19. Anh NQ, Linh BN, Ha NT, Phanthunane P, Huong NT. Schizophrenia interventions in vietnam: primary results from a cost-effectiveness study. Glob Public Health. (2015) 10(Supppl 1):S21–39. doi: 10.1080/17441692.2014.986158
20. Lubinga SJ, Mutamba BB, Nganizi A, Babigumira JB, A Cost-effectiveness analysis of antipsychotics for treatment of schizophrenia in Uganda. Appl Health Econ Hea. (2015) 13:493–506. doi: 10.1007/s40258-015-0176-3
21. Druais S, Doutriaux A, Cognet M, Godet A, Lancon C, Levy P, et al. Cost effectiveness of paliperidone long-acting injectable versus other antipsychotics for the maintenance treatment of schizophrenia in France. Pharmacoeconomics. (2016) 34:363–91. doi: 10.1007/s40273-015-0348-x
22. Lin L, Zhao YJ, Zhou HJ, Khoo AL, Teng M, Soh LB, et al. Comparative cost-effectiveness of 11 oral antipsychotics for relapse prevention in schizophrenia within singapore using effectiveness estimates from a network meta-analysis. Int Clin Psychopharm. (2016) 31:84–92. doi: 10.1097/YIC.0000000000000111
23. Rajagopalan K, Trueman D, Crowe L, Squirrell D, Loebel A. Cost-utility analysis of lurasidone versus aripiprazole in adults with schizophrenia. Pharmacoeconomics. (2016) 34:709–21. doi: 10.1007/s40273-016-0405-0
24. Einarson TR, Pudas H, Goswami P, van Impe K, Bereza BG. Pharmacoeconomics of long-acting atypical antipsychotics for acutely relapsed chronic schizophrenia in Finland. J Med Econ. (2016) 19:111–20. doi: 10.3111/13696998.2015.1100115
25. Einarson TR, Maia-Lopes S, Goswami P, Bereza BG, Van Impe K. Economic analysis of paliperidone long-acting injectable for chronic schizophrenia in Portugal. J Med Econ. (2016) 19:913–21. doi: 10.1080/13696998.2016.1184156
26. Barnes TRE, Leeson VC, Paton C, Marston L, Davies L, Whittaker W, et al. Amisulpride augmentation in clozapine-unresponsive schizophrenia (AMICUS): a double-blind, placebo-controlled, randomised trial of clinical effectiveness and cost-effectiveness. Health Technol Asses. (2017) 21:1. doi: 10.3310/hta21490
27. Einarson TR, Bereza BG, Garcia LI, Gonzalez MMB, Tedouri F, Van Impe K. Cost-effectiveness of 3-month paliperidone treatment for chronic schizophrenia in Spain. J Med Econ. (2017) 20:1039–47. doi: 10.1080/13696998.2017.1351370
28. Einarson TR, Bereza BG, Tedouri F, Van Impe K, Denee TR, Dries P. Cost-Effectiveness of 3-Month Paliperidone Therapy for Chronic Schizophrenia in the Netherlands. J Med Econ. (2017) 20:1187–99. doi: 10.1080/13696998.2017.1363050
29. Wiwat T, Jadesada L, Natthamon T, Nithiwadee P, Paravee W, Hathaiphat P. Cost-effectiveness analysis of aripiprazole compared with risperidone in the treatment of acute schizophrenia patients in Thailand. Thai J Pharmac Sci. (2018) 42:169–75. Available online at: https://www.researchgate.net/publication/327222600_Cost-effectiveness_analysis_of_aripiprazole_compared_with_risperidone_in_the_treatment_of_acute_schizophrenia_patients_in_Thailand
30. Nuhoho S, Saad A, Saumell G, Ribes D, El Khoury AC. Economic evaluation of paliperidone palmitate once monthly for treating chronic schizophrenia patients in the United Arab Emirates. Curr Med Res Opin. (2018) 34:601–11. doi: 10.1080/03007995.2017.1417246
31. Aigbogun MS, Liu S, Kamat SA, Sapin C, Duhig AM, Citrome L. Relapse prevention: a cost-effectiveness analysis of brexpiprazole treatment in adult patients with schizophrenia in the USA. ClinicoEcon Outcomes Res. (2018) 10:443–56. doi: 10.2147/CEOR.S160252
32. Németh B, Bendes R, Nagy B, Götze Á, Kóczián K, Horváth M, et al. Cost-utility analysis of cariprazine compared to risperidone among patients with negative symptoms of schizophrenia. Health Policy Techn. (2019) 8:84–91. doi: 10.1016/j.hlpt.2019.01.004
33. Zhao J, Jiang K, Li Q, Zhang Y, Cheng Y, Lin Z, et al. Cost-effectiveness of olanzapine in the first-line treatment of schizophrenia in China. J Med Econ. (2019) 22:439–46. doi: 10.1080/13696998.2019.1580714
34. Abdall-Razak A, Macaulay A, Tiefenbach J, Borges K, Mathema S, Zuberi S, et al. Cost-utility analysis of amisulpride and paliperidone in the treatment of schizophrenia. Heliyon. (2019) 5:e2343. doi: 10.1016/j.heliyon.2019.e02343
35. Dutina A, Stasevic-Karlicic I, Pandrc N, Prokic A, Jankovic SM. Cost/effectiveness of aripiprazole vs. olanzapine in the long-term treatment of schizophrenia. Srp Ark Celok Lek. (2019) 147:468–74. doi: 10.2298/SARH181012065D
36. Arteaga DC, Fakra E, Van Gils C, Guillon P. The clinical and economic impact of three-monthly long-acting formulation of paliperidone palmitate versus the one-monthly formulation in the treatment of schizophrenia in France: a cost-utility study. Encephale. (2019) 45:459–67. doi: 10.1016/j.encep.2019.03.001
37. Yi ZM, Men P, Qu S, Li C, Yu X, Zhai S. Comparative cost-effectiveness of amisulpride and olanzapine in the treatment of schizophrenia in China. Expert Rev Pharmacoecon Outcomes Res. (2020) 20:313–20. doi: 10.1080/14737167.2020.1752670
38. Lin Z, Xuan J. Cost-effectiveness of aripiprazole orally disintegrating tablets in the treatment of schizophrenia in China. Expert Rev Pharmacoecon Outcomes Res. (2020) 20:549–57. doi: 10.1080/14737167.2020.1807331
39. Jin H, Tappenden P, MacCabe JH, Robinson S, Byford S. Evaluation of the cost-effectiveness of services for schizophrenia in the UK across the entire care pathway in a single whole-disease model. JAMA Netw Open. (2020) 3:e205888. doi: 10.1001/jamanetworkopen.2020.5888
40. Garrison LJ, Pauly MV, Willke RJ, Neumann PJ. An overview of value, perspective, and decision context-a health economics approach: an ISPOR special task force report. Value Health. (2018) 21:124–30. doi: 10.1016/j.jval.2017.12.006
41. Hay JW, Smeeding J, Carroll NV, Drummond M, Garrison LP, Mansley EC, et al. Good research practices for measuring drug costs in cost effectiveness analyses: issues and recommendations: the ISPOR drug cost task force report–part I. Value Health. (2010) 13:3–7. doi: 10.1111/j.1524-4733.2009.00663.x
42. Weinstein MC, O'Brien B, Hornberger J, Jackson J, Johannesson M, McCabe C, et al. Principles of good practice for decision analytic modeling in health-care evaluation: report of the ISPOR task force on good research practices–modeling studies. Value Health. (2003) 6:9–17. doi: 10.1046/j.1524-4733.2003.00234.x
43. Bright CE. Measuring medication adherence in patients with schizophrenia: an integrative review. Arch Psychiatr Nurs. (2017) 31:99–110. doi: 10.1016/j.apnu.2016.09.003
44. Haddad PM, Brain C, Scott J. Nonadherence with antipsychotic medication in schizophrenia: challenges and management strategies. Patient Relat Outcome Meas. (2014) 5:43–62. doi: 10.2147/PROM.S42735
45. Lenert L, Sturley A, Rapaport M, Chavez S, Mohr P, Rupnow M. Public preferences for health states with schizophrenia and a mapping function to estimate utilities from positive and negative symptom scale scores. Schizophr Res. (2004) 71:155–65. doi: 10.1016/j.schres.2003.10.010
46. Briggs A, Wild D, Lees M, Reaney M, Dursun S, Parry D, et al. Impact of schizophrenia and schizophrenia treatment-related adverse events on quality of life: direct utility elicitation. Health Qual Life Outcomes. (2008) 6:105. doi: 10.1186/1477-7525-6-105
47. Oh PI, Lanctôt KL, Mittmann N, Iskedjian M, Einarson TR. Cost-utility of risperidone compared with standard conventional antipsychotics in chronic schizophrenia. J Med Econ. (2001) 4:137–56. doi: 10.3111/200104137156
48. Revicki DA, Shakespeare A, Kind P. Preferences for schizophrenia-related health states: a comparison of patients, caregivers and psychiatrists. Int Clin Psychopharmacol. (1996) 11:101–8. doi: 10.1097/00004850-199606000-00004
49. Cummins C, Stevens A, Kisely S. The Use of Olanzapine as a First and Second Choice Treatment in Schizophrenia. York: Centre for Reviews and Dissemination (1998).
50. Excellence NIFH. Schizophrenia: Core Interventions in the Treatment and Management of Schizophrenia in Primary and Secondary Care (update) (2010).
51. Osborne RH, Dalton A, Hertel J, Schrover R, Smith DK. Health-related quality of life advantage of long-acting injectable antipsychotic treatment for schizophrenia: a time trade-off study. Health Qual Life Outcomes. (2012) 10:35. doi: 10.1186/1477-7525-10-35
52. Tempest M, Sapin C, Beillat M, Robinson P, Treur M. Cost-effectiveness analysis of aripiprazole once-monthly for the treatment of schizophrenia in the UK. J Ment Health Policy Econ. (2015) 18:185–200. doi: 10.1016/j.jval.2015.09.979
53. Mavranezouli I. A review and critique of studies reporting utility values for schizophrenia-related health states. Pharmacoeconomics. (2010) 28:1109–21. doi: 10.2165/11537300-000000000-00000
54. Furiak NM, Ascher-Svanum H, Klein RW, Smolen LJ, Lawson AH, Montgomery W, et al. Cost-effectiveness of olanzapine long-acting injection in the treatment of patients with schizophrenia in the United States: a micro-simulation economic decision model. Curr Med Res Opin. (2011) 27:713–30. doi: 10.1185/03007995.2011.554533
56. Clarke P, Gray A, Holman R. Estimating utility values for health states of type 2 diabetic patients using the EQ-5D (UKPDS 62). Med Decis Making. (2002) 22:340–9. doi: 10.1177/027298902400448902
57. Luengo-Fernandez R, Gray AM, Bull L, Welch S, Cuthbertson F, Rothwell PM. Quality of life after TIA and stroke: ten-year results of the Oxford vascular study. Neurology. (2013) 81:1588–95. doi: 10.1212/WNL.0b013e3182a9f45f
58. Davies A, Vardeva K, Loze JY. L'Italien GJ, Sennfalt K, Baardewijk M. Cost-effectiveness of atypical antipsychotics for the management of schizophrenia in the UK. Curr Med Res Opin. (2008) 24:3275–85. doi: 10.1185/03007990802507547
59. Levy AR, Christensen TL, Johnson JA. Utility values for symptomatic non-severe hypoglycaemia elicited from persons with and without diabetes in Canada and the United Kingdom. Health Qual Life Outcomes. (2008) 6:73. doi: 10.1186/1477-7525-6-73
60. Ascher-Svanum H, Furiak NM, Lawson AH, Klein TM, Smolen LJ, Conley RR, et al. Cost-effectiveness of several atypical antipsychotics in orally disintegrating tablets compared with standard oral tablets in the treatment of schizophrenia in the United States. J Med Econ. (2012) 15:531–47. doi: 10.3111/13696998.2012.662923
61. Treur M, Baca E, Bobes J, Canas F, Salvador L, Gonzalez B, et al. The cost-effectiveness of paliperidone extended release in Spain. J Med Econ. (2012) 15(Suppl 1):26–34. doi: 10.3111/13696998.2012.734884
62. Mohr PE, Cheng CM, Claxton K, Conley RR, Feldman JJ, Hargreaves WA, et al. The heterogeneity of schizophrenia in disease states. Schizophr Res. (2004) 71:83–95. doi: 10.1016/j.schres.2003.11.008
63. Mata CM, Roset GM, Badia LX, Antonanzas VF, Ragel AJ. Effect of type-2 diabetes mellitus on the quality of life of patients treated at primary care consultations in Spain. Aten Primaria. (2003) 31:493–9. doi: 10.1016/S0212-6567(03)70722-7
64. Lam ET, Lam CL, Lai CL, Yuen MF, Fong DY, So TM. Health-related quality of life of southern Chinese with chronic hepatitis B infection. Health Qual Life Outcomes. (2009) 7:52. doi: 10.1186/1477-7525-7-52
65. van de Willige G, Wiersma D, Nienhuis FJ, Jenner JA. Changes in quality of life in chronic psychiatric patients: a comparison between EuroQol (EQ-5D) and WHOQoL. Qual Life Res. (2005) 14:441–51. doi: 10.1007/s11136-004-0689-y
66. van der Schans S, Goossens L, Boland M, Kocks J, Postma MJ, van Boven J, et al. Systematic review and quality appraisal of cost-effectiveness analyses of pharmacologic maintenance treatment for chronic obstructive pulmonary disease: methodological considerations and recommendations. Pharmacoeconomics. (2017) 35:43–63. doi: 10.1007/s40273-016-0448-2
67. Maru S, Byrnes J, Whitty JA, Carrington MJ, Stewart S, Scuffham PA. Systematic review of model-based analyses reporting the cost-effectiveness and cost-utility of cardiovascular disease management programs. Eur J Cardiovasc Nurs. (2015) 14:26–33. doi: 10.1177/1474515114536093
68. Hutter M, Rodríguez-Ibeas R, Antonanzas F. Methodological reviews of economic evaluations in health care: what do they target? Euro J Health Econ. (2014) 15:829–40. doi: 10.1007/s10198-013-0527-7
69. Gomersall JS, Jadotte YT, Xue Y, Lockwood S, Riddle D, Preda A. Conducting systematic reviews of economic evaluations. Int J Evid Based Healthc. (2015) 13:170–8. doi: 10.1097/XEB.0000000000000063
70. Pignone M, Saha S, Hoerger T, Lohr KN, Teutsch S, Mandelblatt J. Challenges in systematic reviews of economic analyses. Ann Intern Med. (2005) 142:1073–9. doi: 10.7326/0003-4819-142-12_Part_2-200506211-00007
71. Boehler CE, Lord J. Mind the gap! a multilevel analysis of factors related to variation in published cost-effectiveness estimates within and between countries. Med Decis Making. (2016) 36:31–47. doi: 10.1177/0272989X15579173
72. Cloutier M, Aigbogun MS, Guerin A, Nitulescu R, Ramanakumar AV, Kamat SA, et al. The economic burden of schizophrenia in the United States in 2013. J Clin Psychiatry. (2016) 77:764–71. doi: 10.4088/JCP.15m10278
73. Fasseeh A, Nemeth B, Molnar A, Fricke FU, Horvath M, Koczian K, et al. A systematic review of the indirect costs of schizophrenia in Europe. Eur J Public Health. (2018) 28:1043–9. doi: 10.1093/eurpub/cky231
74. Zhai J, Guo X, Chen M, Zhao J, Su Z. An investigation of economic costs of schizophrenia in two areas of China. Int J Ment Health Syst. (2013) 7:26. doi: 10.1186/1752-4458-7-26
75. National Institute for Health and Care Excellence. Psychosis and Schizophrenia in Adults: Prevention and Management. London: National Institute for Health and Care Excellence (2014).
76. Hughes D, Cowell W, Koncz T, Cramer J. Methods for integrating medication compliance and persistence in pharmacoeconomic evaluations. Value Health. (2007) 10:498–509. doi: 10.1111/j.1524-4733.2007.00205.x
Keywords: schizophrenia, economic evaluation, systematic review, modeling, method review
Citation: Wang L, Shi F, Guan X, Xu H, Liu J and Li H (2021) A Systematic Review of Methods and Study Quality of Economic Evaluations for the Treatment of Schizophrenia. Front. Public Health 9:689123. doi: 10.3389/fpubh.2021.689123
Received: 31 March 2021; Accepted: 21 September 2021;
Published: 20 October 2021.
Edited by:
Kevin Lu, University of South Carolina, United StatesCopyright © 2021 Wang, Shi, Guan, Xu, Liu and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hongchao Li, bGlob25nY2hhb0BjcHUuZWR1LmNu
 Luying Wang
Luying Wang Fenghao Shi
Fenghao Shi Xin Guan
Xin Guan He Xu
He Xu Jing Liu
Jing Liu Hongchao Li
Hongchao Li