- Department of Clinical Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, China
Background: This study aimed to investigate the Aspergillus species distribution, antifungal sensitivities, clinical characteristics, and risk factors of patients with invasive aspergillosis (IA) in a tertiary teaching hospital in Anhui Province.
Methods: In the present study, 156 Aspergillus isolates were collected from patients admitted to a 2,800-bed comprehensive hospital between January 2019 and April 2021. The epidemiology of Aspergillus species was well-examined, and its antifungal susceptibility was specifically measured by the microbroth dilution method. The risk factors of patients with IA were documented and analyzed intensively. In addition, gene sequencing was employed to determine gene mutations of cytochrome P450 14-α sterol demethylase-Aspergillus (cyp51A) associated with azole resistance among Aspergillus fumigatus.
Results: The Aspergillus species distribution was dominated by A. fumigatus (56.41%), Aspergillus flavus (20.51%), and Aspergillus niger (15.38%) locally. In particular, all Aspergillus species showed very low minimum inhibitory concentrations (MICs, ≤ 0.5 μg/ml) for azoles and echinocandins, slightly high MICs (1.66–2.91 μg/ml) for amphotericin B, and exceptionally high MICs (>64 μg/ml) for flucytosine. Azole-resistant rate of Aspergillus species in this local region reached up to 5.79%. Correlation analyses of multiple antifungals indicate a significant MIC relevance between isavuconazole and voriconazole (Pearson correlation coefficient was 0.81, P < 0.0001). The clinical risk factors for patients with IA were found primarily to be pulmonary diseases (P = 0.007) and patients' age (P < 0.001). Notably, three mutant loci (TR46/Y121F/T289A) of the cyp51A gene were identified in azole-resistant A. fumigatus.
Conclusions: The Aspergillus species emerged increasingly, of which A. fumigatus and A. flavus remained the main pathogens for invasive Aspergillus infections in the local region. The vast majority of Aspergillus species exhibited good susceptibility to all the antifungals, except flucytosine. The local occurrence of azole-resistant Aspergillus species grew gradually and needed monitoring in time. Pulmonary diseases and age were likely considered as highly associated risk factors for IA. To our knowledge, the clinically isolated azole-resistant A. fumigatus with TR46/Y121F/T289A mutations identified here were rarely reported in the area of China.
Introduction
Invasive aspergillosis (IA) is clinically one of the most serious invasive fungal infections, with high morbidity, mortality, and costs of care (1). Over 200,000 cases of IA are reported to be infected with Aspergillus species each year (2). Aspergillus fumigatus is the most frequently encountered Aspergillus spp. that causes IA and allergic diseases, although other species, such as Aspergillus flavus, Aspergillus niger, Aspergillus terreus, Aspergillus versicolor, and Aspergillus nidulans, can also induce diseases (3, 4). Immunocompromised patients and those with underlying lung diseases are susceptible to IA, ranging from acute IA to chronic pulmonary aspergillosis (CPA) (5, 6). However, early diagnosis of IA is difficult and its misdiagnosis happens readily since Aspergillus infections seldom have characteristic manifestations and the specific pathogenic agents regularly take a long time to be detected (7).
Current antifungal resistance situations are of serious concern (8–10), although azole antifungals prove critical in the treatment of IA, including voriconazole, isavuconazole, posaconazole, itraconazole, etc. for first-line or remedial regimens (11). As the incidence of aspergillosis is increasing year by year, the resistance rate of Aspergillus spp. to azoles also gradually rises, and particularly the resistance of A. fumigatus to azoles in different countries/regions varies diversely. To date, studies have identified alterations in cytochrome P450 sterol 14α-demethylase (CYP51), a target protein within many azole-resistant A. fumigatus, which likely impair antifungal binding ability as a result of amino acid substitutions due to single nucleotide polymorphisms of the gene encoding CYP51-Aspergillus (CYP51A) protein (12). Therefore, clinical awareness and assessment of prevailing strains, epidemiological characteristics, and drug sensitivity profiles of invasive Aspergillus infections in local regions are key to early clinical treatment and improvement of clinical outcomes of patients.
In this study, we investigated the species distribution and drug sensitivities of Aspergillus spp. from patients with IA and their clinical characteristics and risk factors in combination with corresponding clinical data in the local Anhui Province of China. Notably, the gene encoding CYP51A in azole-resistant A. fumigatus was sequenced to potentially explore a possible molecular reason for the antifungal resistance.
Materials and Methods
Patient Data Collection
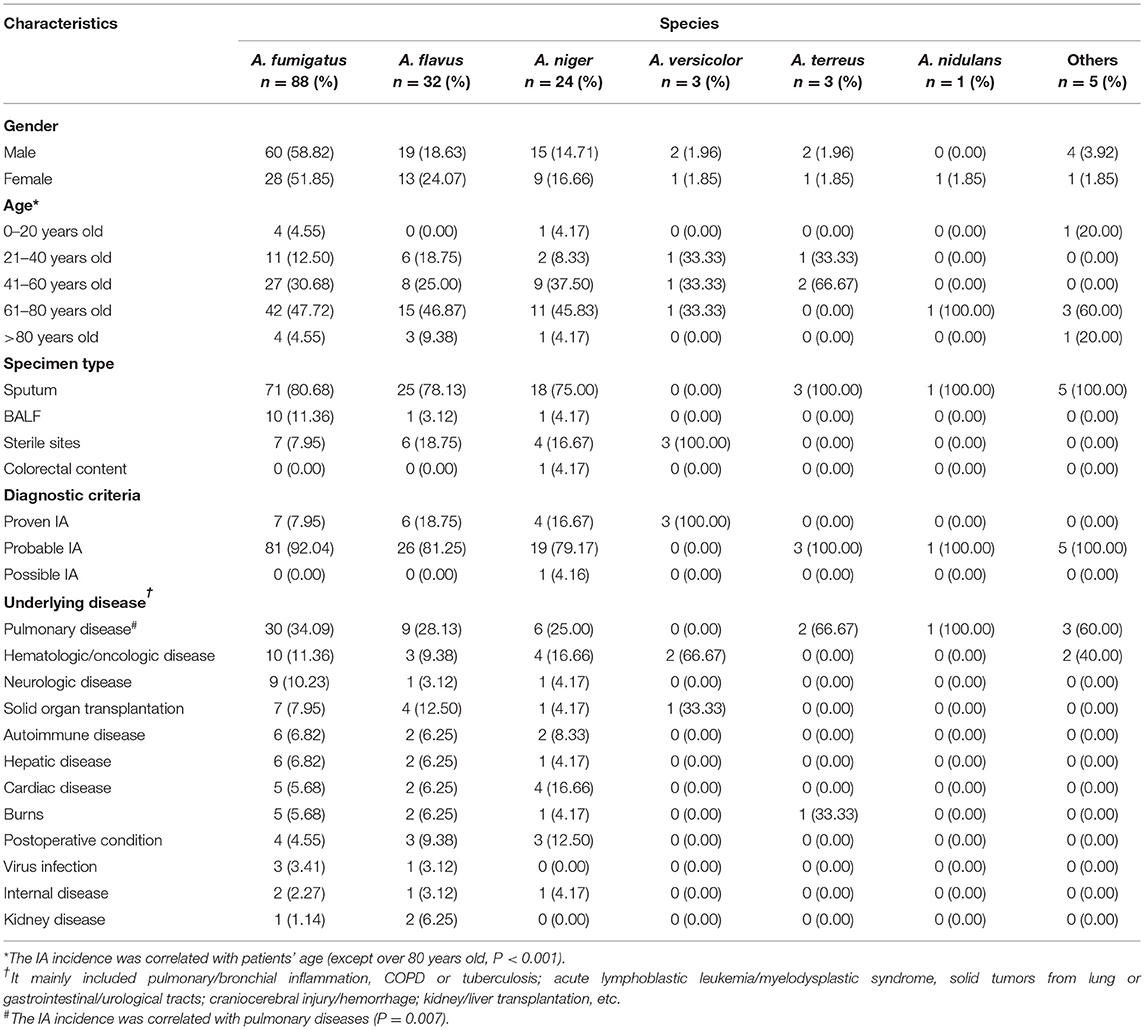
We routinely harvested fungal isolates from patients who developed IA in a tertiary hospital containing a comprehensive 2,800-bed facility affiliated to a medical university of Anhui Province in central China between January 2019 and April 2021. Demographic information and clinical data were retrieved from a computerized hospital data system. Specimens from both sterile and non-sterile body sites were carefully collected, among which samples from the conventionally non-sterile body sites were obtained aseptically by vesicopuncture, or from deep lower respiratory tracts of patients with IA primarily by bronchofiberscopy, for direct examination under microscopy and mycological cultures. All the cases were categorized as proven, probable, or possible IA according to the revised definitions of invasive fungal disease (IFD) from the European Organization for the Research and Treatment of Cancer/Mycoses Study Group (EORTC/MSG) consensus group (13), and based on the Chinese expert consensus statement issued by the Chinese Medical Association (14, 15). Briefly, the proven IA requires Aspergillus identification in pathological tissues or fungal cultures of specimens obtained by aseptic procedures from sterile normal or clinically/radiologically abnormal sites consistent with infectious disease processes. The probable IA is mainly clinically probable invasive pulmonary aspergillosis, which was diagnosed by host factors, clinical features, and mycological evidence. For the host factors, our subjects were principally cancer and/or organ transplant patients with long-term use of hormones or immunosuppressants within the past 60–90 days. The clinical features revealed that part of the patients presented the following patterns on CT images: dense, well-circumscribed lesions with/without halo signs, air crescent signs, cavities, wedge-shaped, or segmental/lobar consolidations. In other patients, tracheobronchial ulcers, nodules, pseudo-membranes, plaques, or eschars were visible via bronchoscopy. For the mycological evidence, Aspergillus spp. were recovered by culture and/or microscopic detection of fungal elements from deep lower respiratory tracts, bronchoalveolar lavage fluid (BALF), bronchial brush biopsy, or aspirates. Only one strain of Aspergillus spp. was isolated from colorectal contents of a patient with Crohn's disease that was considered as the possible IA, with the fact that the absence of fungi and blood culture-negative results were observed after antifungal treatment. The present study ruled out the repeatedly isolated fungal species from the same patients. Multiple risk factors were collected for the analysis of IA incidence in the local region.
Microorganism Identification
All clinical specimens were inoculated to plates of Sabouraud Dextrose agar and CHROMagar Candida agar(Hefei Tianda Biotech Co., Ltd., Anhui, China) for culture at 35°C for 48 h, except blood samples which were processed using a BacT/ALERT 3D automated microbial detection system (BacT/ALERT 3D, bioMérieux, Marcy l'Étoile, France). Aspergillus spp. were identified by morphological identifications combined with matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) on a Vitek MS system (bioMérieux, Marcy l'Étoile, France), and DNA sequencing (Sangon Biotech Co., Ltd., Shanghai, China) if ambiguity with MS occurs. Sample preparations for MS analysis were conducted as described previously (16). It was considered acceptable for the identification results when the MS confidence value was 99.9%. The DNA sequencing for Aspergillus species identification was conducted using two universal fungal primers for internal transcribed spacer (ITS1-forward: 5′-TCC GTA GGT GAA CCT GCG G-3′, ITS4-reverse: 5′-TCC TCC GCT TAT TGA TAT GC-3′) (17).
Antifungal Susceptibility Testing
The antifungal susceptibility test for Aspergillus spp. via Sensititre YeastOne (SYO) kit (Zhuhai DL Biotech Co., Ltd., Shenzhen, China) was carried out in accordance with the manufacturer's instructions. Briefly, after being cultured on potato dextrose agar at 35°C for 7 days, conidia were harvested using sterile saline containing Tween-20. The conidial inoculum suspension was prepared at a turbidity of 0.5 McFarland units for the assay. With 48-h of culturing (except echinocandins which were cultured for 24-h), the antifungal drug sensitivity profiles of these inocula were finally available according to the interpretations of the kit instructions. For those initially tested as resistant isolates, we repeated the assays to confirm the testing results. The present study did not involve fluconazole that is not active against Aspergillus spp. due to its inherent resistance. Quality control strains included Candida krusei ATCC6258 and Candida parapsilosis ATCC22019. Specifically, the minimum inhibitory concentrations (MICs) for amphotericin B (AMB), flucytosine (FC), and four azoles, including isavuconazole (ISA), voriconazole (VRC), posaconazole (POS), and itraconazole (ITR), and the minimum effective concentrations (MECs) for micafungin (MIF) and caspofungin (CAS) were determined for all the Aspergillus inocula. The interpretative criteria for susceptibility and breakpoints for antifungal drugs were referenced to the European Committee on Antibiotic Susceptibility Testing (EUCAST), and the Clinical and Laboratory Standards Institute (CLSI) M38-A3 microbroth dilution method.
DNA Sequencing of Azole-Resistance Related Gene
The promoter and coding regions of the cyp51A gene were amplified by the PCR and separately sequenced using previously published primers (18). Briefly, the primers for the cyp51A promoter region were the following: PA7F 5′-TCATATGTTGCTCAGCGG-3′ and PA5R 5′-TCTCTGCACGCAAAGAAGAAC-3′ (19). PCR conditions included as follows: 95°C for 10 min, 35 cycles of 94°C for 1 min, 43°C for 45 s, and 72°C for 1 min, followed by a final extension at 72°C for 7 min. The primers for the coding region of cyp51A were the following: P450A1 5′-ATGGTGCCGATGCTATGG-3′ and P450A2 5′-CTGTCTCACTTGGATGTG-3′ (20). PCR conditions were as follows: 95°C for 5 min, 35 cycles of 94°C for 3 min, 47.4°C for 45 s, and 72°C for 2 min, with a final extension at 72°C for 7 min. The PCR products were semi-quantified by agarose gel electrophoresis and used as templates for sequencing (Sangon Biotech Co., Ltd., Shanghai, China). The resultant fragment sequences of promoter and coding regions of the cyp51A gene were aligned with the ones deposited in GenBank (Registration number: AF338659) (19) and were thoroughly analyzed using the Meg Align software (DNA Star, Inc., Lasergene, Madison, WI, USA).
Statistical Analysis
The data of geometric mean of MICs, MIC50, MIC90, and Pearson χ2 test were processed with IBM SPSS Statistics 21 (SPSS Inc., Chicago, IL, USA), and P < 0.05 was considered significantly different. Correlation analyses of MICs for azoles were performed using GraphPad Prism software version 5 (GraphPad Software Inc., San Diego, CA, USA).
Ethics Statement
The protocols of the study were approved by and carried out following the recommendations of the Life Ethics Committee of Anhui Medical University. All subjects gave their written informed consents as per the Declaration of Helsinki.
Results
Clinical Distribution Characteristics of Invasive Aspergillus Infections
During the study period, 156 Aspergillus isolates were collected and identified to species level using MALDI-TOF MS and/or DNA sequencing, together with macroscopic/microscopic observations of cell morphology, galactomannan, and 1,3-β-D glucan tests (data not shown), chest CT imaging, and/or bronchoscopic analyses (Figure 1 and Supplementary Figure 1). The duplicate isolates from the same patients with similar susceptibility profiles were removed from the analysis. There were 20 patients with proven IA (12.82%), 135 patients with probable IA (i.e., probable invasive pulmonary aspergillosis, 86.54%), and one patient with possible IA (0.64%). Among those with probable invasive pulmonary aspergillosis, 30 cases were available with typical pulmonary aspergillosis CT images, including 14 of inflammatory/pulmonary interstitial/well-circumscribed lesions, 4 of fibrous foci, and 12 of distinctive characteristics (6 cases with patchy nodules, 4 cases with cavities, and 2 cases with air crescent signs). The remaining cases were examined via bronchoscopy or microscopic observation of Aspergillus elements, and they presented typical clinical features as described earlier (Supplementary Figure 2). Additionally, 14 cases were tested serologically positive in galactomannan assays.
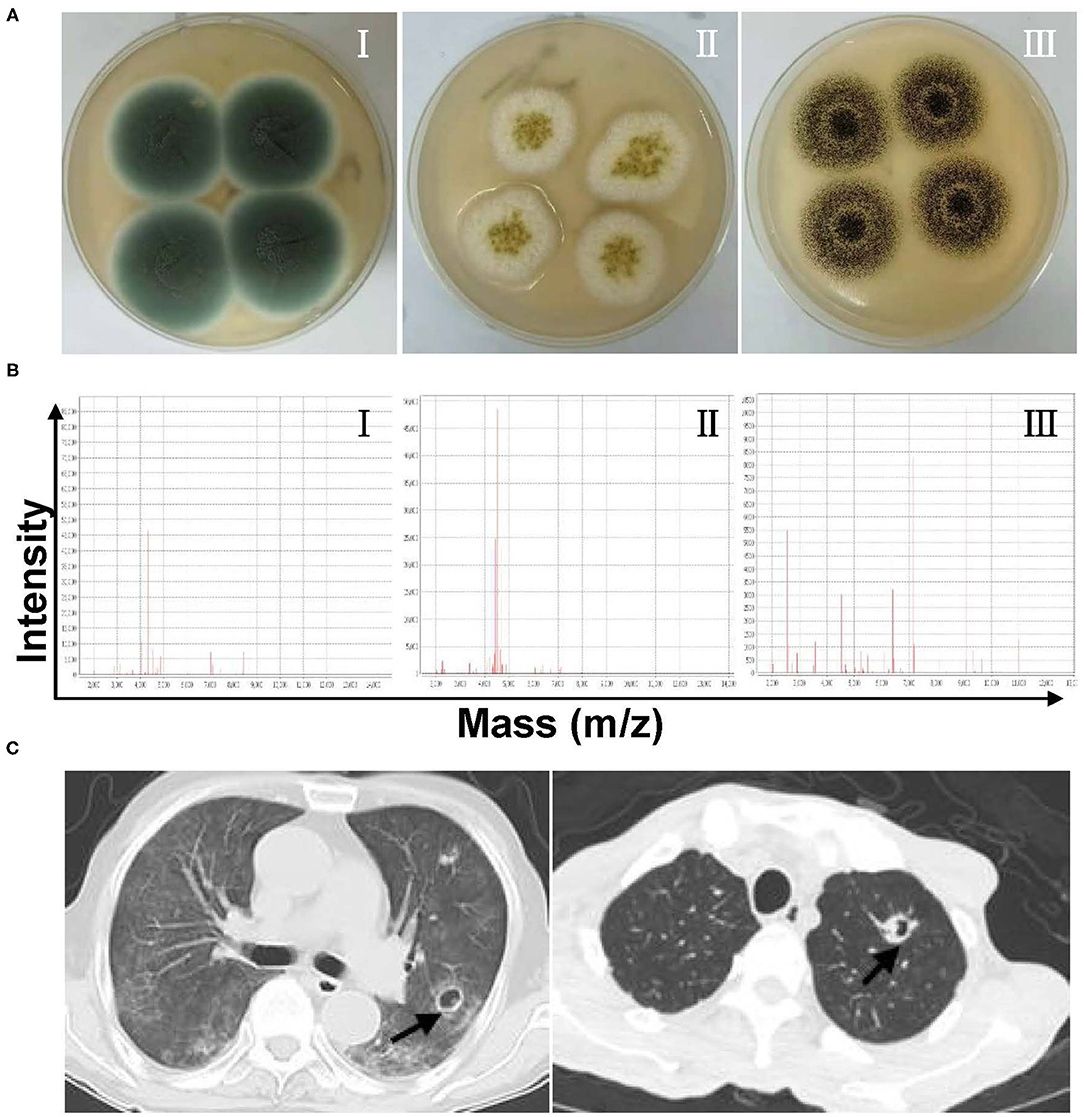
Figure 1. (A) Representative culturing macroscopic results of Aspergillus spp. (Sabouraud Dextrose agar medium). I–III: Aspergillus fumigatus, Aspergillus flavus, and Aspergillus niger. (B) Representative identification information of Aspergillus spp. by matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS). I–III: A. fumigatus, A. flavus, and A. niger. (C) Chest CT imaging features of patients with invasive pulmonary aspergillosis.
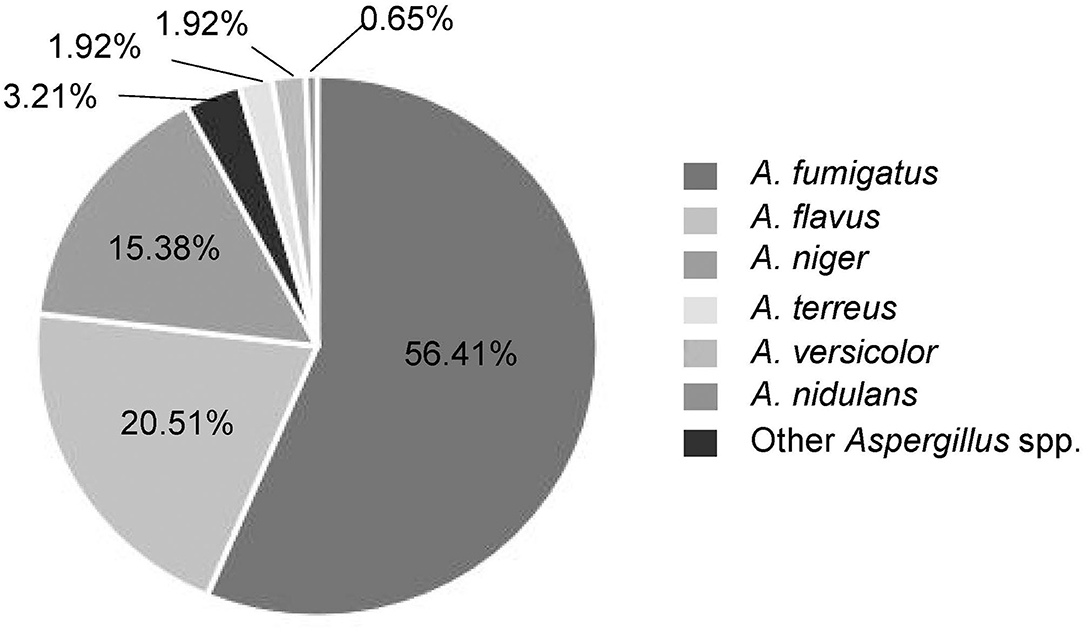
Among these Aspergillus spp., A. fumigatus was the predominant species isolated from patients with invasive Aspergillus infections which accounted for 56.41%, followed by A. flavus (20.51%), A. niger (15.38%), A. terreus (1.92%), A. versicolor (1.92%), and others (Figure 2). The specimens were of different sources. Most of Aspergillus isolates (n = 135, 86.54%) were recovered from respiratory specimens, including sputum from deep lower respiratory tracts (n = 123, 78.85%) and BALF (n = 12, 7.69%). Other specimen sources included sterile sites (mainly blood, ascites, fine-needle aspirates, etc.; n = 20, 12.82%) and colorectal content (n = 1, 0.64%) (Table 1). In the local region, A. fumigatus was the main infectious agent for the respiratory system which accounts for 60% (81/135), followed by A. flavus 19.26% (26/135), A. niger 14.07% (19/135), and A. terreus 2.22% (3/135). However, in non-respiratory systems, non-A. fumigatus Aspergillus species were dominant (66.67%, 14/21), including all the A. versicolor isolates.
For all the Aspergillus spp. isolated here, Department of Respiratory Medicine contributed the most (34.62%, 54/156), followed by intensive care unit (ICU; 22.44%, 35/156), infection unit (7.69%, 12/156), and burns unit (5.77%, 9/156) (Figure 3). As listed in Table 1, the male to female sex ratio was 1.89 (102 vs. 54), and the most susceptible age was between 61 and 80 years among patients with IA (with a mean age of 58.39 years). In general, the IA incidence grew with age except for patients over 80 years old (correlation analysis, P < 0.001). The most prevalent underlying diseases were pulmonary diseases (including pulmonary/bronchial inflammation, chronic obstructive pulmonary disease (COPD), tuberculosis, lung cancer, among others (32.69%, 51/156; correlation analysis, P = 0.007). These diseases were followed by hematological/oncological diseases (13.46%, 21/156), solid organ transplantations (8.33%, 13/156), neurological diseases (7.05%, 11/156), and cardiac diseases (7.05%, 11/156), among others.
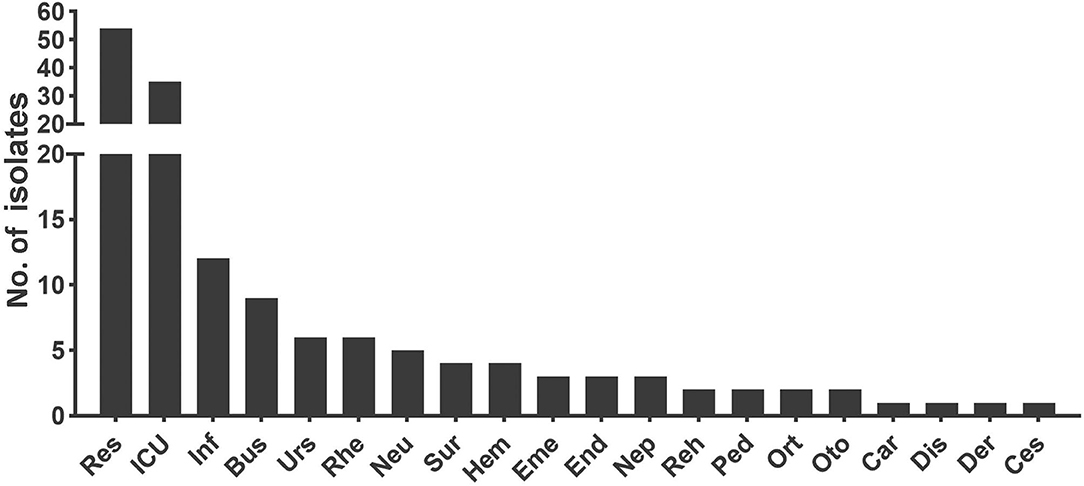
Figure 3. The department distribution of patients infected with invasive Aspergillus isolates. Res, Department of Respiratory Medicine; ICU, Intensive Care Unit; Inf, Infection Unit; Bus, Burns Unit; Urs, Department of Urology; Rhe, Department of Rheumatology and Immunology; Neu, Department of Neurology; Sur, Department of Surgery including organ transplantation, hepatobiliary and pancreatic surgery, gastrointestinal surgery, and joint surgery; Hem, Department of Hematology; Eme, Emergency Room; End, Department of Endocrinology; Nep, Department of Nephrology; Reh, Department of Rehabilitation; Ped, Department of Pediatrics; Ort, Department of Orthopedics; Oto, Department of Otolaryngology; Car, Department of Cardiology; Dis, Department of Gastroenterology; Der, Department of Dermatological Venereology; Ces, Department of Chest Surgery.
Antifungal Susceptibility Patterns
Among the 156 Aspergillus isolates, apart from those improperly preserved and not applicable for testing, 121 strains were available to undergo antifungal susceptibility assays. The MIC/MEC value, range, and geometric mean (GM) are summarized in Table 2 and are specifically shown in Figure 4. To be noted, except intrinsic resistance, there were 39.73% (29/73) of A. fumigatus, 54.17% (13/24) of A. flavus, and 13.64% (3/22) of A. niger isolates with MICs of more than 2 μg/ml for AMB. The Aspergillus spp. showed high MICs (>64 μg/ml) for FC, but not A. niger (10.62 μg/ml). On the contrary, they all presented very low MICs for MIF (≤ 0.008 to 0.02 μg/ml) and CAS (≤ 0.008 to 0.03 μg/ml). For azoles, most of the tested isolates had MICs of ≤ 0.5 μg/ml for POS (n = 120, 99.17%), ITR (n = 117, 96.69%), VRC (n = 110, 90.91%), and ISA (n = 107, 88.43%). A large proportion of A. fumigatus was susceptible to POS (100%), ITR (100%), ISA (95.89%), and VRC (95.89%), among which POS presented the lowest MICs (0.09 μg/ml). The A. flavus also displayed a high in vitro sensitivity to azoles, albeit 8.33% of resistance to ISA and VRC. Nearly all the other Aspergillus spp. (i.e., A. niger, A. terreus, and A. nidulans) harbored sensitive phenotypes to all azole drugs tested.
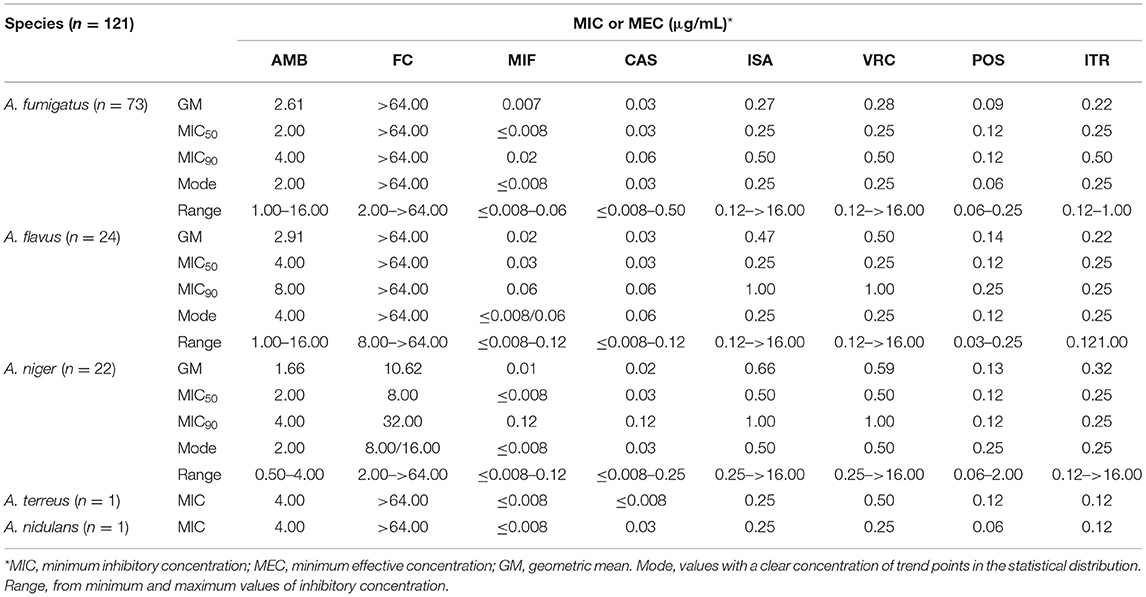
Table 2. Antifungal susceptibility patterns and characteristics in the five common Aspergillus species.
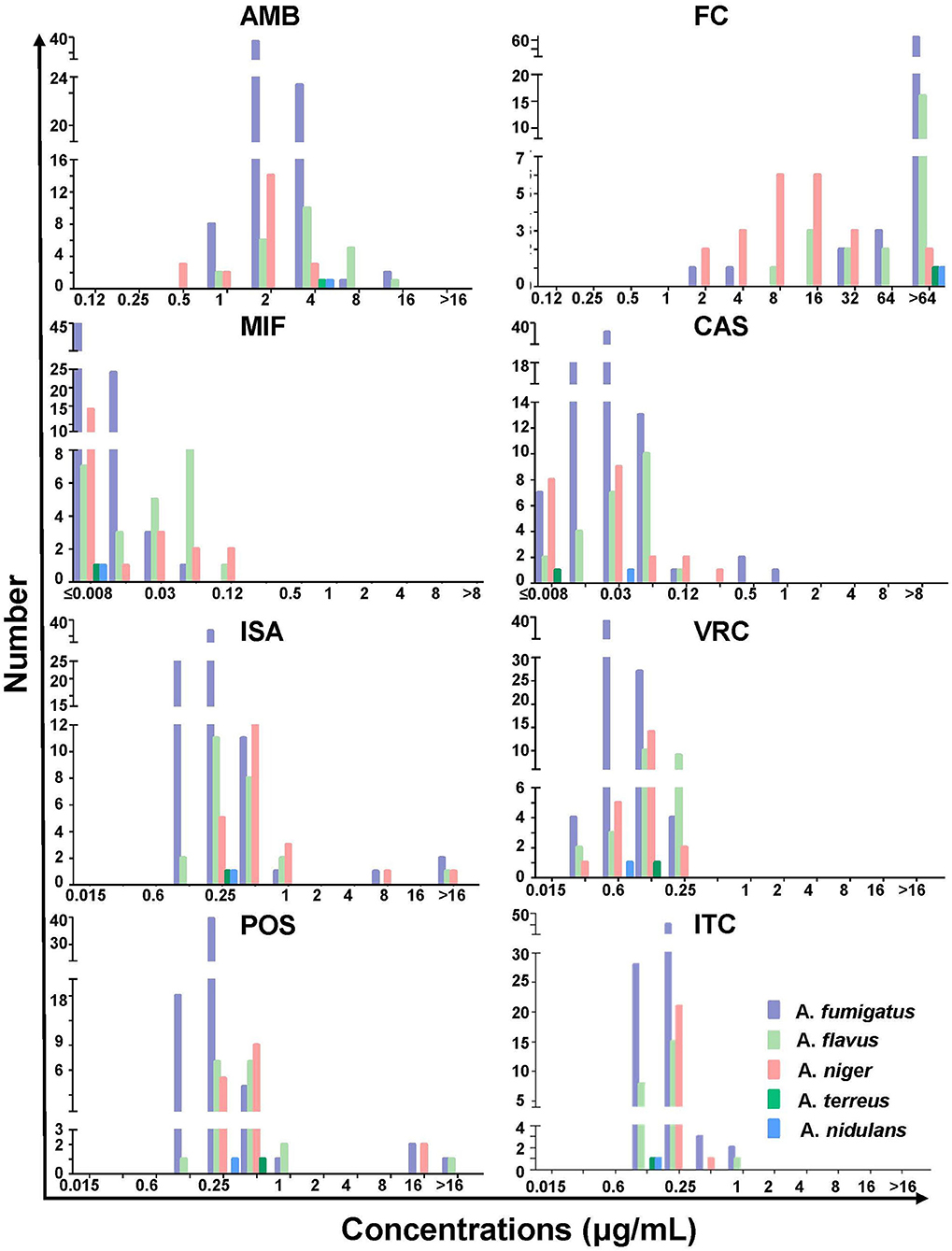
Figure 4. The minimum inhibitory or effective concentration distribution of amphotericin B (AMB), flucytosine (FC), micafungin (MIF), caspofungin (CAS), isavuconazole (ISA), voriconazole (VRC), posaconazole (POS), and itraconazole (ITR) for the indicated Aspergillus spp.
It is worth noting that three A. fumigatus, two A. flavus, and two A. niger was screened out to be azole-resistant according to the antifungal susceptibility data and epidemiological cutoff values (ECVs). The relevance of MICs between different azoles was assessed through correlation analysis using scatter plots, which was able to indicate a considerable cross-resistance if there was a significant MIC correlation between two azoles. As shown in Figure 5, a significant MIC correlation was identified between ISA and VRC (Pearson correlation coefficient was 0.81, P < 0.0001).
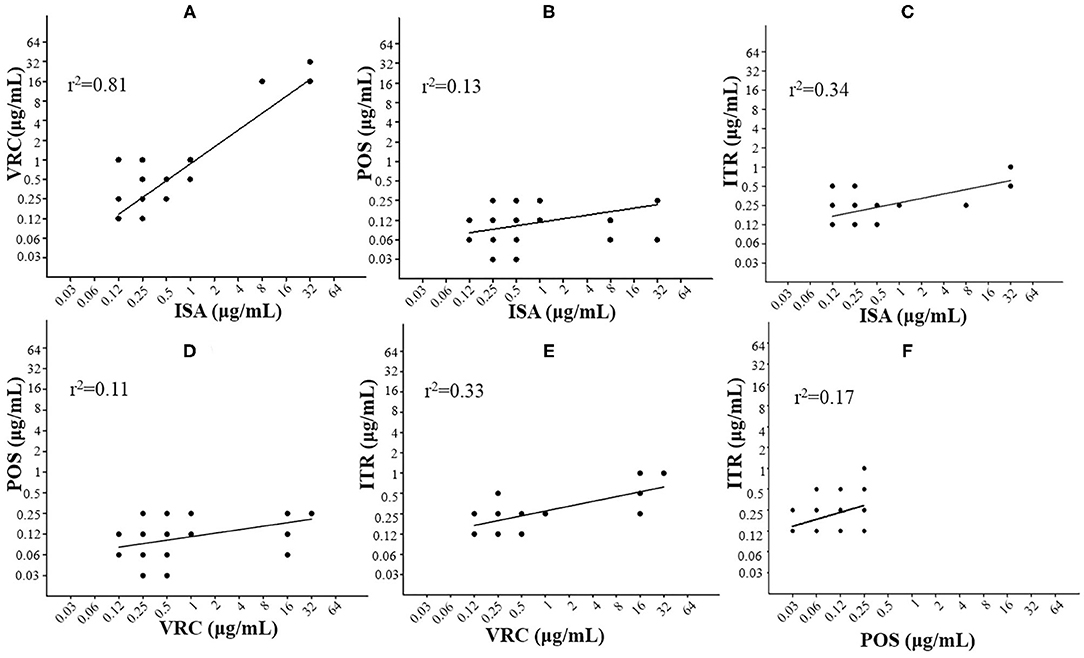
Figure 5. Correlation analyses with scatter plots to compare multiple antifungal minimum inhibitory concentrations (MICs) as indicated. The significant correlation of ISA and VRC (R2 = 0.81) was shown for Aspergillus spp. A strong correlation suggests considerable cross-resistance. (A) ISA vs VRC; (B) ISA vs POS; (C) ISA vs ITR; (D) VRC vs POS; (E) VRC vs ITR; and (F) POS vs ITR.
Gene Mutations of Cyp51A Associated With Azole Resistance in A. fumigatus
To further investigate a possible mechanism of azole resistance in A. fumigatus, all three were azole-resistant, and typically azole-sensitive A. fumigatus isolates were recovered for the purpose. Both the promoter fragment and coding region in the cyp51A gene were determined by PCR and sequencing. The full-lengths and nucleotide sequences of the promoter and coding region of cyp51A in A. fumigatus (GenBank Registration number: AF338659) were used as reference sequences, respectively. As summarized in Table 3, all the azole-resistant A. fumigatus possessed a TR46 repeat insertion (TCT AGA ATC ACG CGG TCC GGA TGT GTG CTG AGC CGA ATG AAA GTT G) in the 5'-end upstream of cyp51A (GenBank accession number: OL396581). Meanwhile, the sequencing analysis spotted 2 single missense mutations (TAT to TTT and ACC to GCC) in all the azole-resistant A. fumigatus isolates, leading to amino acid substitutions of Y121F and T289A, respectively. In addition, almost all the azole-resistant and typically sensitive A. fumigatus were identified with 5 missense mutations (F46Y, M172V, N248T, D255E, and E427K) at the amino acid level (GenBank accession number: OL388442 and OL388443).

Table 3. Mutations of cyp51A gene or its coding protein and cross-resistance patterns in azole-resistant and typical wild-type Aspergillus fumigatus.
Discussion
Currently, more than 30 Aspergillus spp. have been confirmed to correlate with IA, the most common ones are A. fumigatus, A. flavus, A. niger, A. terreus, and A. nidulans (21). The prevalence of IA and its associated fungal spectrum widely vary from country to country and even among different regions within a country, due to infection sites, age, climate, geographic conditions, agricultural activities, and others factors (22).
In China, A. fumigatus was the most commonly isolated Aspergillus spp., followed by A. flavus, A. niger, and others (23–25). In addition, the incidence of IA caused by non-A. fumigatus Aspergillus species has increased in China in recent years. Wang et al. reported that the incidence of invasive pulmonary aspergillosis (IPA) caused by A. flavus was greater than that by A. fumigatus in patients with hepatitis B virus-related liver failures (26). Li et al. also found an increase in the number of IA cases with A. niger and A. tuberculosis in clinical and environmental samples from China (27). In this study, more than half of the strains we identified were A. fumigatus, then mainly followed by A. flavus and A. niger. Previous studies revealed that IA was identified primarily in respiratory and urinary tracts (28, 29). In the present study, the clinical specimens of invasive Aspergillus infections were mainly from deep lower respiratory tracts out of various clinical departments/units, especially including the Department of Respiratory Medicine, infection unit, ICU, and burns unit. Patients from these units often experience serious illnesses and long-term hospitalization and are subject to extensive use of antibiotics, hormones, immunosuppressants, and/or invasive operations. Thus, these patients usually with a high rate of IA should draw special attention during clinical treatments.
Many patients with IA in our study were men over 40 years of age, similar to previous studies (30). IA is often involved in patients with preexisting pulmonary disorders, such as COPD, active or previous tuberculosis, and previously treated lung cancers (31, 32). In the present study, the most common underlying systemic condition was pulmonary diseases, mainly including pulmonary/bronchial inflammation and COPD. Thus, pulmonary diseases were identified as important risk factors for invasive Aspergillus infections.
In clinical settings, antifungal susceptibility patterns are constantly compelling concerns. The antifungal agents are generally categorized into different groups including polyenes, fluoropyrimidine analogs, azoles, echinocandins, etc. (33). This study suggests that the AMB susceptibility to Aspergillus spp. in local China would be similar to other studies (34–36), which should be under close surveillance. Resistance to AMB is usually caused by decreased ergosterol or changed lipids of the target in plasma membrane due to alterations of ergosterol biosynthetic pathways which lead to decreased abilities to binding AMB (37), germination of conidia by UV irradiation (38), and mutations of genes encoding sphingolipids FEN1 and SUR4 (39). In our study, 38.84% of Aspergillus spp. were resistant to AMB, for which the specific resistance mechanisms need further investigations. The efficacy of FC in treating IA is controversial (40, 41). Our findings demonstrated that Aspergillus spp. showed high MIC values to FC, indicating that administration of FC may be an unwise treatment option.
Over the past decades, the frequency of azole resistance has dramatically increased globally (8–10). Resistance of Aspergillus spp. to azoles is growing and has become a global health problem. New resistance mechanisms continue to emerge and jeopardize the role of azoles in the treatment of aspergillosis, directly affecting clinical outcomes. The data from the global antifungal surveillance program showed that 5.79% of A. fumigatus elevated MICs for one or more azoles (21). The reported frequency of azole resistance differs remarkably between countries/regions, and the antifungal resistance makes the diagnosis and treatment of aspergillosis more complicated (42). The recent Aspergillus guidelines from the European Society for Clinical Microbiology and Infectious Diseases (ESCMID), the European Confederation of Medical Mycology (ECMM), and the European Respiratory Society (ERS) recommend that first-line treatment with liposomal AMB or azole plus echinocandins should be considered if the resistance rate exceeds 10% (42). Due to the lack of clinical breakpoints or epidemiological thresholds, the CLSI epidemiological thresholds currently established in A. fumigatus species complex are carefully referenced in the present study. Our local rate of resistance to azoles in Aspergillus spp. was 5.79% (6/121), with 4.11% (3/73) in A. fumigatus, similar to the report by Deng et al. (8) that azole-resistant A. fumigatus was prevalent in East and Southeast China (8, 23, 34).
Azoles are able to target the ergosterol biosynthesis process by inhibiting the fungal cytochrome P450-dependent enzyme wool sterol 14-α-demethylase, leading to altered cell membrane function and cell death. The reliable safety and therapeutic efficacy of azoles make them considerately suitable for prophylactic and empirical treatment to severely immunosuppressed patients (43). Notably, ISA, a broad-spectrum azole, showed substantial bioavailability (about 98%) and potent activities in animal models of IA, trichomoniasis, invasive candidiasis, and cryptococcosis (44). Though ISA has not been approved for clinical use in China, A. fumigatus isolates presented reduced in vitro activities to it, likely due to the cross-resistance of the azole family (45). The azole resistance in Aspergillus spp. is associated with multiple distinct adaptive strategies (46–48), among which overexpression and/or open reading frame (ORF) mutation of cyp51A gene can alter the binding affinity of azoles to the enzyme, wool sterol 14-α-demethylase (48). In 2011, ARTEMIS DISK Global Antifungal Surveillance Project first reported that TR34/L98H mutation could be found in A. fumigatus collected in China (21). Subsequently, Liu et al. declared that the TR34/L98H mutation was the predominant mutation in China and also was quite common in Europe and some other Asian countries (25, 49). Further studies from China have identified more mutations in A. fumigatus including TR34/L98H/S297T/F495I, TR34/L98H, G432A, M220I, and TR46/Y121F/T289A (21, 25, 43, 50). To date, azole-resistant A. fumigatus has spread mainly in southeastern and northern China (51). In this study, we reported that TR46/Y121F/T289A mutations were found in two-thirds of azole-resistant A. fumigatus isolates and they exhibited very high MIC values to ISA and VRC. We seldom observed mutant strains with TR34/L98H mutation and/or TR53, thus the TR46/Y121F/T289A mutation pattern is likely the dominant phenotype in the local region. The extensive use of sterol demethylation inhibitors (DMIs) in agriculture may contribute to the increasing emergence of azole-resistant A. fumigatus. The presence of tandem repeats upstream of the 14-α-demethylase gene is an important mechanism found in phytopathogenic mycorrhizal fungi that can develop resistance to azoles through DMI fungicides exposure and function as a transcriptional enhancer, allowing significant overexpression of cyp51A gene (52–55). Invasive Aspergillus infections in immuno-competent hosts are uncommon, though an immuno-competent adult in our study was infected with A. fumigatus after a drowning event, and the isolated strains showed azole resistance in an in vitro drug susceptibility test, supporting the possibility that the resistant strains might be of environmental origin (56). Other mutation-harboring A. fumigatus strains found in our research, including F46Y, M172V, N248T, D255E, and/or E427K, are scarcely related to azole resistance (12, 57). In addition, in one azole-resistant A. fumigatus isolate, none of TR46/Y121F/T289A mutations were found, suggesting that other mechanisms may be involved and need to be further investigated (8, 58).
Conclusions
In summary, this study reported the Aspergillus species distribution and antifungal sensitivities, and clinical characteristics and risk factors of patients with IA of a local region in central China. The A. fumigatus and A. flavus were still the major pathogens for invasive Aspergillus infections here. The vast majority of Aspergillus spp. exhibited good susceptibility to almost all the antifungals commonly used in clinics. The findings implied that pulmonary diseases and age are likely considered as the main risk factors for such infections. The polymorphism of the cyp51A gene in A. fumigatus may be closely associated with azole resistance, contributing to treatment failures in patients with IA. Their important clinical implications emphasize the need for antifungal susceptibility surveillance and screening out mutations in resistance-linked genes.
Data Availability Statement
The data presented in the study are available in the Genbank repository, accession number OL396581, OL388442 and OL388443s.
Ethics Statement
The studies involving human participants were reviewed and approved by the Life Ethics Committee of Anhui Medical University. Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author Contributions
YW, MZ, and YX conceived and designed the experiments, contributed to the interpretation of results, and assisted in writing the manuscript. YW, LZho, MZ, and YX designed the research protocol and performed the experiments. YW, LZha, LZho, MZ, and YX performed data acquisition and analysis. All authors read and approved the final manuscript.
Funding
This study was supported by the Foundation of the Anhui Science and Technology Department (201904a07020049) (YX). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.835092/full#supplementary-material
Abbreviations
IA, invasive aspergillosis; CPA, chronic pulmonary aspergillosis; IFD, invasive fungal disease; EORTC/MSG, the European Organization for the Research and Treatment of Cancer/Mycoses Study Group; BALF, bronchoalveolar lavage fluid; MALDI-TOF MS, matrix-assisted laser desorption ionization-time of flight mass spectrometry; SYO, Sensititre YeastOne; MIC, minimum inhibitory concentration; MEC, minimum effective concentration; GM, geometric mean; MIC50, MIC/MEC at which 50% of the isolates tested were inhibited; MIC90, MIC/MEC at which 90% of the isolates tested were inhibited; AMB, amphotericin B; FC, flucytosine; MIF, micafungin; CAS, caspofungin; ISA, isavuconazole; VRC, voriconazole; POS, posaconazole; ITR, itraconazole; EUCAST, the European Committee on Antibiotic Susceptibility Testing; CLSI, the Clinical and Laboratory Standards Institute; PCR, polymerase chain reaction; ICU, intensive care unit; COPD, chronic obstructive pulmonary disease; IPA, invasive pulmonary aspergillosis; ESCMID, the European Society for Clinical Microbiology and Infectious Diseases; ECMM, the European Confederation of Medical Mycology; ERS, the European Respiratory Society; ORF, open reading frame.
References
1. Kim A, Nicolau DP, Kuti JL. Hospital costs and outcomes among intravenous antifungal therapies for patients with invasive aspergillosis in the United States. Mycoses. (2011) 54:e301–12. doi: 10.1111/j.1439-0507.2010.01903.x
2. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. (2012) 4:165rv13. doi: 10.1126/scitranslmed.3004404
3. van de Veerdonk FL, Gresnigt MS, Romani L, Netea MG, Latge JP. Aspergillus fumigatus morphology and dynamic host interactions. Nat Rev Microbiol. (2017) 15:661–74. doi: 10.1038/nrmicro.2017.90
4. Sugui JA, Kwon-Chung KJ, Juvvadi PR, Latge JP, Steinbach WJ. Aspergillus fumigatus and related species. Cold Spring Harb Perspect Med. (2014) 5:a019786. doi: 10.1101/cshperspect.a019786
5. Bongomin F, Gago S, Oladele RO, Denning DW. Global and multi-national prevalence of fungal diseases-estimate precision. J Fungi. (2017) 3:57. doi: 10.3390/jof3040057
6. Soubani AO, Chandrasekar PH. The clinical spectrum of pulmonary aspergillosis. Chest. (2002) 121:1988–99. doi: 10.1378/chest.121.6.1988
7. Zhang R, Wang S, Lu H, Wang Z, Xu X. Misdiagnosis of invasive pulmonary aspergillosis: a clinical analysis of 26 immunocompetent patients. Int J Clin Exp Med. (2014) 7:5075–82.
8. Deng S, Zhang L, Ji Y, Verweij PE, Tsui KM, Hagen F, et al. Triazole phenotypes and genotypic characterization of clinical Aspergillus fumigatus isolates in China. Emerg Microbes Infect. (2017) 6:e109. doi: 10.1038/emi.2017.97
9. Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J Antimicrob Chemother. (2012) 67:362–6. doi: 10.1093/jac/dkr443
10. Jensen RH, Hagen F, Astvad KM, Tyron A, Meis JF, Arendrup MC. Azole-resistant Aspergillus fumigatus in Denmark: a laboratory-based study on resistance mechanisms and genotypes. Clin Microbiol Infect. (2016) 22:570 e1–9. doi: 10.1016/j.cmi.2016.04.001
11. Rybak JM, Ge W, Wiederhold NP, Parker JE, Kelly SL, Rogers PD, et al. Mutations in hmg1, challenging the paradigm of clinical triazole resistance in Aspergillus fumigatus. mBio. (2019) 10:e00437–19. doi: 10.1128/mBio.00437-19
12. Howard SJ, Arendrup MC. Acquired antifungal drug resistance in Aspergillus fumigatus: epidemiology and detection. Med Mycol. (2011) 49(Suppl. 1):S90–5. doi: 10.3109/13693786.2010.508469
13. Donnelly JP, Chen SC, Kauffman CA, Steinbach WJ, Baddley JW, Verweij PE, et al. Revision and Update of the Consensus Definitions of Invasive Fungal Disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. (2020) 71:1367–76. doi: 10.1093/cid/ciz1008
14. Society of Critical Care Medicine CMA. Guidelines for the diagnostic and treatment of invasive fungal infections in critically ill patients. Chin J Intern Med. (2007) 46:960–6.
15. Editorial Board of Chinese Journal of Internal medicine. Diagnostic criteria and treatment principles for invasive pulmonary fungal infections (Draft). Chin J Intern Med. (2006) 45:697–700.
16. Zhou L, Chen Y, Xu Y. Performance of VITEK mass spectrometry V3.0 for rapid identification of clinical Aspergillus fumigatus in different culture conditions based on ribosomal proteins. Infect Drug Resist. (2017) 10:499–506. doi: 10.2147/IDR.S148121
17. Gardes M WT, Fortin JA, Bruns TD, Taylor JW. Identification of indigenous and introduced symbioticfungi in ectomycorrhizaeby amplification of nuclear and mitochondrial ribosomal DNA. Can J Bot. (1991) 69:180–90. doi: 10.1139/b91-026
18. Gonzalez-Lara MF, Roman-Montes CM, Diaz-Lomeli P, Rangel-Cordero A, Valenzuela MO, Ponce-de-Leon A, et al. Azole resistance and cyp51A mutation screening in Aspergillus fumigatus in Mexico. J Antimicrob Chemother. (2019) 74:2047–50. doi: 10.1093/jac/dkz121
19. Diaz-Guerra TM, Mellado E, Cuenca-Estrella M, Rodriguez-Tudela JL. A point mutation in the 14alpha-sterol demethylase gene cyp51A contributes to itraconazole resistance in Aspergillus fumigatus. Antimicrob Agents Chemother. (2003) 47:1120–4. doi: 10.1128/AAC.47.3.1120-1124.2003
20. Mellado E, Diaz-Guerra TM, Cuenca-Estrella M, Rodriguez-Tudela JL. Identification of two different 14-alpha sterol demethylase-related genes (cyp51A and cyp51B) in Aspergillus fumigatus and other Aspergillus species. J Clin Microbiol. (2001) 39:2431–8. doi: 10.1128/JCM.39.7.2431-2438.2001
21. Lockhart SR, Frade JP, Etienne KA, Pfaller MA, Diekema DJ, Balajee SA. Azole resistance in Aspergillus fumigatus isolates from the ARTEMIS global surveillance study is primarily due to the TR/L98H mutation in the cyp51A gene. Antimicrob Agents Chemother. (2011) 55:4465–8. doi: 10.1128/AAC.00185-11
22. Chen M, Xu Y, Hong N, Yang Y, Lei W, Du L, et al. Epidemiology of fungal infections in China. Front Med. (2018) 12:58–75. doi: 10.1007/s11684-017-0601-0
23. Yang X, Chen W, Liang T, Tan J, Liu W, Sun Y, et al. A 20-year antifungal susceptibility surveillance (From 1999 to 2019) for Aspergillus spp. and proposed epidemiological cutoff values for Aspergillus fumigatus and Aspergillus flavus: a study in a tertiary hospital in China. Front Microbiol. (2021) 12:680884. doi: 10.3389/fmicb.2021.680884
24. Gao L, Yu J, Li R. Epidemiology of aspergillosis in mainland China. Chin J Mycol. (2010) 5:247–51.
25. Liu M, Zeng R, Zhang L, Li D, Lv G, Shen Y, et al. Multiple cyp51A-based mechanisms identified in azole-resistant isolates of Aspergillus fumigatus from China. Antimicrob Agents Chemother. (2015) 59:4321–5. doi: 10.1128/AAC.00003-15
26. Wang W, Zhao CY, Zhou JY, Wang YD, Shen C, Zhou DF, et al. Invasive pulmonary aspergillosis in patients with HBV-related liver failure. Eur J Clin Microbiol Infect Dis. (2011) 30:661–7. doi: 10.1007/s10096-010-1137-2
27. Li Y, Wan Z, Liu W, Li R. Identification and susceptibility of Aspergillus section nigri in china: prevalence of species and paradoxical growth in response to echinocandins. J Clin Microbiol. (2015) 53:702–5. doi: 10.1128/JCM.03233-14
28. Zeng X, Peng M, Liu G, Huang Y, Zhang T, Wen J, et al. Strain distribution and drug susceptibility of invasive fungal infection in clinical patients with systemic internal diseases. Front Bioeng Biotechnol. (2020) 8:625024. doi: 10.3389/fbioe.2020.625024
29. Smith JA, Kauffman CA. Pulmonary fungal infections. Respirology. (2012) 17:913–26. doi: 10.1111/j.1440-1843.2012.02150.x
30. Iqbal N, Irfan M, Zubairi AB, Jabeen K, Awan S, Khan JA. Clinical manifestations and outcomes of pulmonary aspergillosis: experience from Pakistan. BMJ Open Respir Res. (2016) 3:e000155. doi: 10.1136/bmjresp-2016-000155
31. Smith NL, Denning DW. Underlying conditions in chronic pulmonary aspergillosis including simple aspergilloma. Eur Respir J. (2011) 37:865–72. doi: 10.1183/09031936.00054810
32. Engel TGP, Slabbers L, de Jong C, Melchers WJG, Hagen F, Verweij PE, et al. Prevalence and diversity of filamentous fungi in the airways of cystic fibrosis patients - a Dutch, multicentre study. J Cyst Fibros. (2019) 18:221–6. doi: 10.1016/j.jcf.2018.11.012
33. Robbins N, Caplan T, Cowen LE. Molecular evolution of antifungal drug resistance. Annu Rev Microbiol. (2017) 71:753–75. doi: 10.1146/annurev-micro-030117-020345
34. Xu Y, Chen M, Zhu J, Gerrits van den Ende B, Chen AJ, Al-Hatmi AMS, et al. Aspergillus species in lower respiratory tract of hospitalized patients from Shanghai, China: species diversity and emerging azole resistance. Infect Drug Resist. (2020) 13:4663–72. doi: 10.2147/IDR.S281288
35. Hermida-Alava K, Brito Devoto T, Sautua F, Gordo M, Scandiani M, Formento N, et al. Antifungal susceptibility profile and molecular identification of cyp51C mutations in clinical and environmental isolates of Aspergillus flavus from Argentina. Mycoses. (2021) 64:95–101. doi: 10.1111/myc.13193
36. Trevino-Rangel RJ, Villanueva-Lozano H, Bonifaz A, Castanon-Olivares LR, Andrade A, Becerril-Garcia MA, et al. Species distribution and antifungal susceptibility patterns of Aspergillus isolates from clinical specimens and soil samples in Mexico. Med Mycol. (2021) 59:1006–14. doi: 10.1093/mmy/myab031
37. Ellis D. Amphotericin B: spectrum and resistance. J Antimicrob Chemother. (2002) 49(Suppl. 1):7–10. doi: 10.1093/jac/49.suppl_1.7
38. Manavathu EK, Alangaden GJ, Chandrasekar PH. In-vitro isolation and antifungal susceptibility of amphotericin B-resistant mutants of Aspergillus fumigatus. J Antimicrob Chemother. (1998) 41:615–9. doi: 10.1093/jac/41.6.615
39. Sharma S, Alfatah M, Bari VK, Rawal Y, Paul S, Ganesan K. Sphingolipid biosynthetic pathway genes FEN1 and SUR4 modulate amphotericin B resistance. Antimicrob Agents Chemother. (2014) 58:2409–14. doi: 10.1128/AAC.02130-13
40. Verweij PE, Donnelly JP, Kullberg BJ, Meis JF, De Pauw BE. Amphotericin B versus amphotericin B plus 5-flucytosine: poor results in the treatment of proven systemic mycoses in neutropenic patients. Infection. (1994) 22:81–5. doi: 10.1007/BF01739009
41. Vermes A, Guchelaar HJ, Dankert J. Flucytosine: a review of its pharmacology, clinical indications, pharmacokinetics, toxicity and drug interactions. J Antimicrob Chemother. (2000) 46:171–9. doi: 10.1093/jac/46.2.171
42. Lestrade PPA, Meis JF, Melchers WJG, Verweij PE. Triazole resistance in Aspergillus fumigatus: recent insights and challenges for patient management. Clin Microbiol and Infect. (2019) 25:799–806. doi: 10.1016/j.cmi.2018.11.027
43. Chen J, Li H, Li R, Bu D, Wan Z. Mutations in the cyp51A gene and susceptibility to itraconazole in Aspergillus fumigatus serially isolated from a patient with lung aspergilloma. J Antimicrob Chemother. (2005) 55:31–7. doi: 10.1093/jac/dkh507
44. Maertens JA, Raad II, Marr KA, Patterson TF, Kontoyiannis DP, Cornely OA, et al. Isavuconazole versus voriconazole for primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): a phase 3, randomised-controlled, non-inferiority trial. Lancet. (2016) 387:760–69. doi: 10.1016/S0140-6736(15)01159-9
45. Wiederhold NP, Gil VG, Gutierrez F, Lindner JR, Albataineh MT, McCarthy DI, et al. First detection of TR34 L98H and TR46 Y121F T289A Cyp51 mutations in Aspergillus fumigatus isolates in the United States. J Clin Microbiol. (2016) 54:168–71. doi: 10.1128/JCM.02478-15
46. Chamilos G, Kontoyiannis DP. Update on antifungal drug resistance mechanisms of Aspergillus fumigatus. Drug Resist Updat. (2005) 8:344–58. doi: 10.1016/j.drup.2006.01.001
47. Li Y, Zhang Y, Lu L. Calcium signaling pathway is involved in non-CYP51 azole resistance in Aspergillus fumigatus. Med Mycol. (2019) 57:S233–8. doi: 10.1093/mmy/myy075
48. Nywening AV, Rybak JM, Rogers PD, Fortwendel JR. Mechanisms of triazole resistance in Aspergillus fumigatus. Environ Microbiol. (2020) 22:4934–52. doi: 10.1111/1462-2920.15274
49. Chen Y, Lu Z, Zhao J, Zou Z, Gong Y, Qu F, et al. Epidemiology and molecular characterizations of azole resistance in clinical and environmental Aspergillus fumigatus isolates from China. Antimicrob Agents Chemother. (2016) 60:5878–84. doi: 10.1128/AAC.01005-16
50. Chen Y, Wang H, Lu Z, Li P, Zhang Q, Jia T, et al. Emergence of TR46/Y121F/T289A in an Aspergillus fumigatus isolate from a Chinese patient. Antimicrob Agents Chemother. (2015) 59:7148–50. doi: 10.1128/AAC.00887-15
51. Chen Y, Lu Z, Han L, Huang L. Progress of research on azole resistance in Aspergillus fumigatus. Chin J Epidemiol. (2016) 31:1687–92. doi: 10.3760/cma.j.issn.0254-6450.2016.12.025
52. Mellado E, Garcia-Effron G, Alcazar-Fuoli L, Melchers WJ, Verweij PE, Cuenca-Estrella M, et al. A new Aspergillus fumigatus resistance mechanism conferring in vitro cross-resistance to azole antifungals involves a combination of cyp51A alterations. Antimicrob Agents Chemother. (2007) 51:1897–904. doi: 10.1128/AAC.01092-06
53. Verweij PE, Chowdhary A, Melchers WJG, Meis JF. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin Infect Dis. (2016) 62:362–8. doi: 10.1093/cid/civ885
54. Verweij PE, Snelders E, Kema GH, Mellado E, Melchers WJ. Azole resistance in Aspergillus fumigatus: a side-effect of environmental fungicide use? Lancet Infect Dis. (2009) 9:789–95. doi: 10.1016/S1473-3099(09)70265-8
55. Chowdhary A, Sharma C, Hagen F, Meis JF. Exploring azole antifungal drug resistance in Aspergillus fumigatus with special reference to resistance mechanisms. Future Microbiol. (2014) 9:697–711. doi: 10.2217/fmb.14.27
56. Ratermann KL, Ereshefsky BJ, Fleishaker EL, Thornton AC, Buch KP, Martin CA. Fulminant invasive pulmonary aspergillosis after a near-drowning accident in an immunocompetent patient. Ann Pharmacother. (2014) 48:1225–9. doi: 10.1177/1060028014537611
57. Escribano P, Rodriguez-Sanchez B, Diaz-Garcia J, Martin-Gomez MT, Ibanez-Martinez E, Rodriguez-Mayo M, et al. Azole resistance survey on clinical Aspergillus fumigatus isolates in Spain. Clin Microbiol Infect. (2021) 27:1170 e1–7. doi: 10.1016/j.cmi.2020.09.042
Keywords: Aspergillus spp., invasive aspergillosis, antifungal sensitivity, risk factors, azole resistance
Citation: Wang Y, Zhang L, Zhou L, Zhang M and Xu Y (2022) Epidemiology, Drug Susceptibility, and Clinical Risk Factors in Patients With Invasive Aspergillosis. Front. Public Health 10:835092. doi: 10.3389/fpubh.2022.835092
Received: 14 December 2021; Accepted: 04 March 2022;
Published: 15 April 2022.
Edited by:
Marc Thilo Figge, Leibniz Institute for Natural Product Research and Infection Biology, GermanyReviewed by:
Guanzhao Liang, Chinese Academy of Medical Sciences and Peking Union Medical College, ChinaSadegh Khodavaisy, Tehran University of Medical Sciences, Iran
Copyright © 2022 Wang, Zhang, Zhou, Zhang and Xu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhang, emhhbmdtaW42OTEyQDE2My5jb20=; Yuanhong Xu, eHlob25nMTk2NEAxNjMuY29t
 Yuerong Wang
Yuerong Wang Luwen Zhang
Luwen Zhang Min Zhang
Min Zhang Yuanhong Xu
Yuanhong Xu