- 1Center for Translational Medicine, Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Department of Obstetrics and Gynecology, West China Second University Hospital, Sichuan University, Chengdu, China
- 3West China School of Basic Medical Sciences and Forensic Medicine, Sichuan University, Chengdu, China
- 4Office for West China Institute of Women and Children's Health, West China Second University Hospital, Sichuan University, Chengdu, China
Background: Infertility is a nationwide public health priority in the U.S. However, few studies have investigated the effects of dietary intake of polyunsaturated fatty acids (PUFAs) on female infertility. This study explored the association between PUFA intake and risk of infertility.
Methods: A total of 1,785 women aged 20–44 years from three National Health and Nutrition Examination Survey cycles (2013–2018) were included in this cross-sectional study. The intake of PUFAs was obtained from a 24-h dietary interview on two separate days with a 3–10-day interval, and nutrient residue models were used. Fertility status was assessed by positive response to two relative questions via a questionnaire. Logistic regression models were used and some covariates were adjusted.
Results: Among all the participants, 340 (19.05%) women suffered from infertility. The intake of docosahexaenoic acid (DHA) (OR = 0.998, 95% CI 0.998, 0.009) was slightly related to the risk of infertility. In contrast, women with higher α-linolenic acid (ALA) (OR = 1.416, 95% CI 1.138, 1.763) and linoleic acid (LA) intake (OR = 1.020, 95% CI 1.002, 1.038) presented with a relatively higher risk of primary infertility. Furthermore, in 20–34-year-old women, higher omega-6/omega-3 was significant associated with the risk of infertility (OR = 1.002, 95%CI 1.000, 1.005).
Conclusions: Our results suggest that PUFA intake is only slightly associated with infertility. The higher the DHA intake, the lower the risk of infertility regardless of age. In women with primary infertility, ALA and LA has negative effect.
Introduction
Infertility is characterized by the failure to establish a clinical pregnancy after 12 months of regular, unprotected sexual intercourse or impairment of a person's capacity to reproduce, either as an individual or with his/her partner (1). According to the U.S. National Center for Health Statistics, it was estimated that ~6.7–19.4% of reproductive-aged women in the U.S. suffered from infertility between 2011 and 2019 (2, 3). The pathogenesis of infertility is complex, involving male and female factors, and a combination of both. In females, some common diseases such as polycystic ovary syndrome (4), endometriosis (5), and uterine fibroids (6) may cause infertility. To date, the U.S. Centers for Disease Control (CDC) declared the diagnosis and treatment of infertility as a national public health priority (7).
Diet is a modifiable lifestyle factor that plays an essential role in influencing human fertility (8). Various dietary components can have different effects on human physiological processes. Polyunsaturated fatty acids (PUFAs), a type of fatty acid containing multiple double bonds in their structures, mainly omega-3 and omega-6 fatty acids, are considered essential fatty acids because they cannot be produced by the human body. These fatty acids must be included in the daily diet. The most common sources of PUFAs are crop seeds, vegetable oils and cereal products, whereas for some types of PUFAs, such as eicosapentaenoic acid (EPA), docosapentaenoic acid (DPA), and docosahexaenoic acid (DHA), fish is the main source (9).
PUFAs play a significant role in the regulation of body homeostasis. Omega-3 fatty acids, especially EPA and DHA, exert anti-inflammatory, vasodilatory, bronchodilatory, and platelet anti-aggregation effects (10), whereas omega-6 fatty acids have opposing effects related to inflammation, vasoconstriction, and platelet aggregation (11). Persistent chronic inflammation leads to high risk of cancer and atherosclerosis, which can contribute to acute cardiovascular diseases (12, 13). In contrast, omega-3 fatty acids not only alter the functions of vascular and carcinogen biomarkers but also offer substantial protection against many chronic and metabolic diseases (14, 15). Thus, a proportionally higher intake of omega-3 fatty acids can protect against inflammatory diseases, cancer, cardiovascular diseases, diabetes, obesity, osteoporosis, neurological degeneration, and bone fractures (10, 13, 16, 17).
Furthermore, PUFAs are considered vital for reproductive health. In males, PUFAs can affect fertility (18). Previous researchers have found that DHA accumulated during sperm maturation in the sperm membrane (19) and was associated with higher sperm motility (20, 21), normal morphology (20, 22), and concentration (20, 23). Sperm membrane DHA can also affect key fertilization events such as capacitation, acrosome reaction, and sperm-oocyte fusion (24). In females, PUFAs are essential substrates that can affect female fertility during early reproductive phase, including oocyte maturation, embryo implantation, and oocyte quality (25–28).
There has been growing concern about the effect of dietary intake of fatty acids, especially omega-3 and omega-6 fatty acids, on the prevalence of female infertility. However, clinical research on dietary intake of PUFA and female infertility is still lacking. Associations between omega-3 and omega-6 fatty acids and body health have been explored mainly in in-vitro and animal studies, but the potential impact on female fertility needs attention. The objective of this study was to determine whether omega-3 and omega-6 fatty acids in the diet are associated with infertility using a national population-based survey of three National Health and Nutrition Examination Survey (NHANES) cycles (2013–2018) in representative American civilian women.
Methods
Data source and sample
A cross-sectional study was conducted based on data from 2013–2018 cycles of the NHANES, which aimed to assess the health and nutritional status of adults and children in the United States. Combining interviews and physical examinations, NHANES is a multistage study that uses complex stratified sampling methods to collect a representative sample of the U.S. population in every 2-year cycle. The NCHS Research Ethics Review Board (ERB) approved all the NHANES protocols.
All female respondents between the ages of 20 and 44 years were eligible for this study (n = 3,707) because we can obtain available data on reproductive health from these age groups. Women with a history of hysterectomy or removal of both ovaries were excluded from the study. Women with kilocalorie intake per day <600 or >6,000 (29), or lack of information about PUFA intake and weight were excluded. Considering the possibility that some of these women may not have had the experience of trying pregnancy, we also excluded them from the study. A total of 1,785 women aged 20–44 years were included. The sample selection process is illustrated in Figure 1.
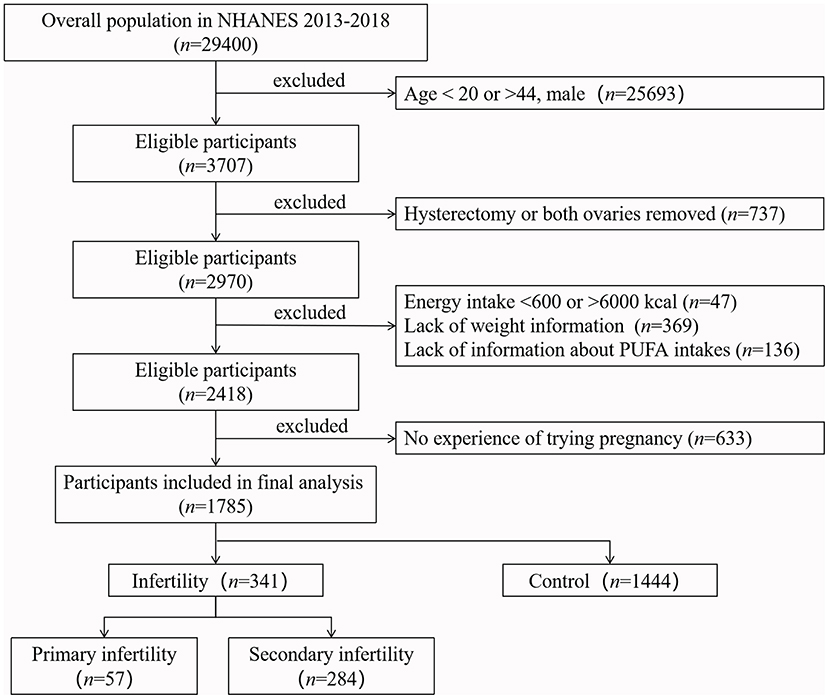
Figure 1. Flow chart of the sample selection from NEANES 2013–2018. According to the inclusion and exclusion criteria, 1,785 eligible participants were finally included in our study.
Independent variables
The dietary data in NHANES was obtained from the dietary interview, which contained 24-h dietary recall interviews estimating the intake of energy, nutrients, and other food components from food and beverage consumption on two separate days with a 3–10-day interval. To minimize possible bias, all interviewers were required to complete a 1-week training and conduct supervised practice interviews before qualifying. In addition, a set of measuring guides (various glasses, bowls, and so on) was used, and additional foods not remembered earlier were asked repeatedly to ensure that the outcome was more reliable.
The Multiple Source Method (MSM) (https://msm.dife.de/) was used to estimate the usual dietary PUFA intakes of all participants from 24 h recall information. According to the User Guide of MSM, everyone was assumed as a habitual consumer. Age, race and BMI were included in MSM regression models as explanatory variables. There were five types of omega-3 fatty acids, including α-linolenic acid (ALA), stearidonic acid (SDA), EPA, DPA, and DHA, and two types of omega-6 fatty acids, including linoleic acid (LA) and arachidonic acid (AA). Nutrient residual models were used to estimate calorie-adjusted values for intake of each nutrient (30). In addition, we calculated the total omega-3 and omega-6 fatty acid intake, as well as omega-6/omega-3. Intakes of each PUFA were analyzed as a continuous variable as well as tertiles by dividing participants into three groups.
Dependent variable
According to the definition of infertility (1), women whose response to either of two questions “Have you ever attempted to become pregnant over a period of at least a year without becoming pregnant?” “Have you ever been to a doctor or other medical provider because you have been unable to become pregnant?” was “yes” were considered ever infertile (31), which were further divided into primary infertility group and secondary infertility group based on the answer from another question “Ever been pregnant?”. Furthermore, if a person reported not ever infertile yet never had children, we considered that they might not have been trying pregnancy and excluded them (Figure 1).
Covariates
Covariates were selected based on known associations between infertility and PUFA intake based on previous studies (31–33). Demographic variables such as age, race, educational level, marital status, ratio of family income to poverty (RIP), and smoking status were included in the analysis. In addition, reproductive factors such as age at the first menstrual period, regular periods, treatment for pelvic infection/pelvic inflammatory disease (PID), birth control pills, and female hormones were also included in the study.
Statistical analysis
All statistical analyses were conducted according to the NHANES analytic guidelines (34). The appropriate dietary two-day sample weight was used to produce national and representative estimates, and all nutrients were adjusted by energy intake using nutrient residual models. Continuous variables with normally distributed were presented as mean and standard deviation (S. D.), otherwise median, P25 (25 percentile) and P75 (75 percentile). Categorical variables were presented as percentages. χ2 (for categorical variables), two independent sample t-tests (for normally distributed variables) and Wilcoxon rank sum test (for not normally distributed variables) were conducted to test the significance of the differences between the infertility and control groups.
The association between PUFA intake and infertility was assessed using two logistic regression models: a crude model (no covariate adjusted) and an adjusted model containing all covariates. Considering the effect of age on infertility, we conducted a subgroup analysis stratified by age (35 years). In addition, because of the possible differences between primary and secondary infertility, we further analyzed data from women with primary and secondary infertility separately. Odds ratio (OR) and 95% confidence intervals (CI) were used to assess the strength of the association. All statistical analyses were performed using SAS software version 9.4.
Results
Population characteristics of participants
A total of 1,785 women aged 20–44 years were included in this analysis, of which 340 (19.05%) suffered from infertility. The characteristics of all the participants are shown in Table 1. The results revealed that the infertile population in our study had higher education levels and family income, and they tended to be married. However, women with infertility showed higher BMI values. Among the reproductive factors related to infertility, pelvic infection and female hormones were more common in infertile population.
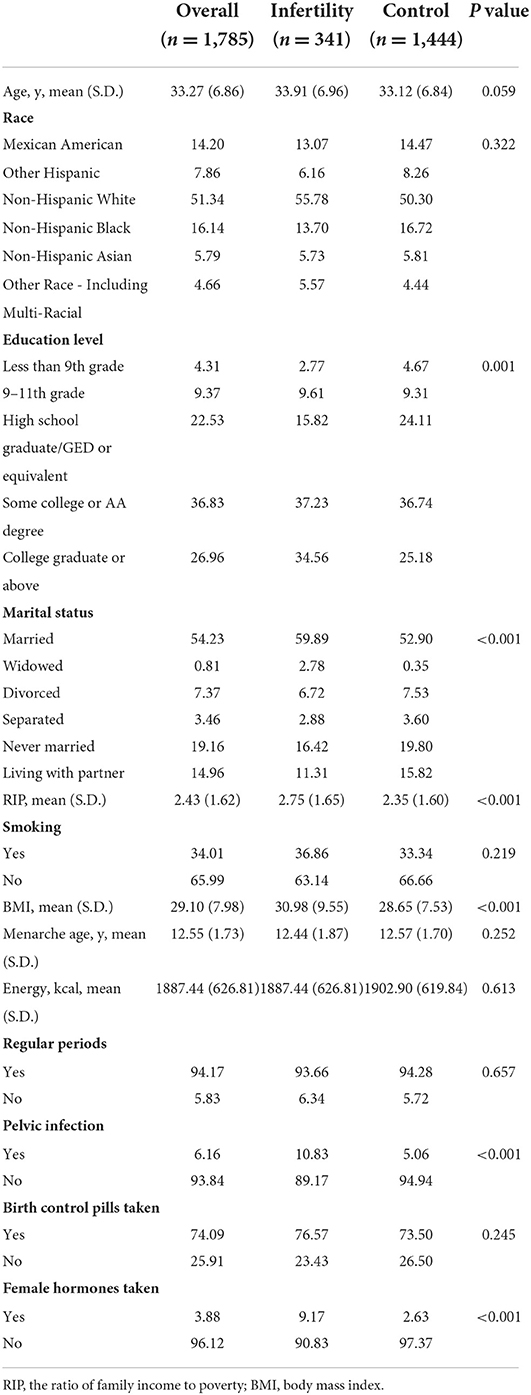
Table 1. Population characteristics of U.S. women aged 20–44 y, National Health and Nutrition Examination Survey, 2013–2018, weighted.
Furthermore, the comparisons between eligible participants (n = 3,707) and participants included in the final analysis (n = 1,785) regarding PUFA consumption, infertility and baseline information were presented in Supplementary Tables 1, 2. According to the results, age, education level, marital status, RIP, smoking status, BMI, regular period, acceptance of pelvic infection and infertility rate in eligible participants were significant different from women in the final analysis.
Overview of omega-3 and omega-6 fatty acids intake
The average intakes of omega-3 and omega-6 fatty acids are shown in Table 2. Wilcoxon rank sum test showed that the average intake of DPA, LA, AA, total omega-6 fatty acid and omega-6/omega-3 in infertile women were significantly higher than those in the control group. In addition, the intakes of each PUFA were divided into three groups, and the ranges of tertiles were presented in Supplementary Table 3.
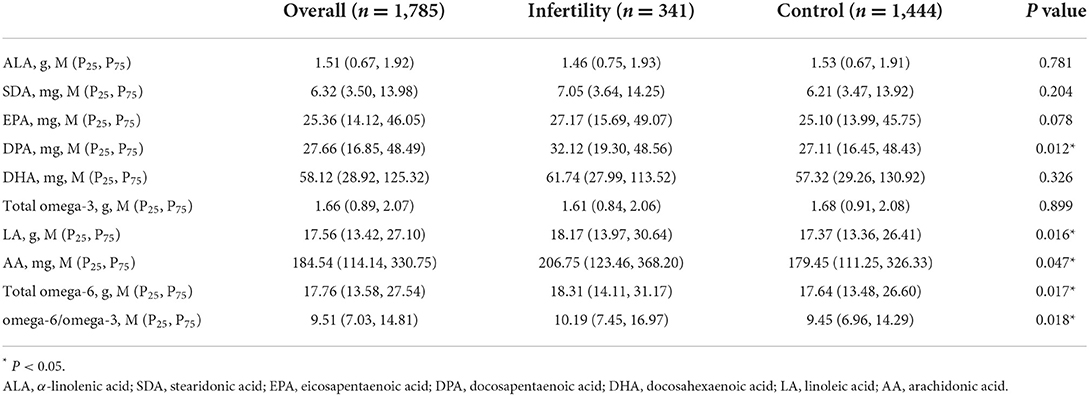
Table 2. Overview of omega-3 and omega-6 fatty acids intakes for U.S. women aged 20–44 years, weighted.
Associations between PUFA intakes and the risk of infertility
Table 3 demonstrates the association between PUFA intake and the risk of infertility in the participants. As for omega-3 fatty acids, in the crude model, those who consumed fewer DHA (OR = 0.999, 95% CI 0.998, 1.000) had a higher risk of infertility. When adjusted for all possible influential factors, the associations with DHA (OR = 0.998, 95% CI 0.998, 0.999) remained significant. When ALA was grouped into tertiles, compared with those in the lowest tertile, those in the middle tertile had a 33.9% (95% CI 0.434, 0.932) lower risk of infertility. In the population aged 20–34 years, when analyzed as continuous variables, only SDA presented protective effects of 1.4% (95% CI 0.973, 0.999). Furthermore, the upper tertile of DHA intake had a 38.9% (95%CI 0.386, 0.967) lower risk than the lowest tertile. In women over 35, DHA was still a slight protective effect (OR = 0.998, 95%CI 0.996, 0.999). For omega-6 fatty acids, in the overall population, LA (OR = 1.001, 95%CI 1.003, 1.018), AA (OR = 1.000, 95%CI 1.000, 1.001) and total omega-6 fatty acid (OR = 1.001, 95%CI 1.003, 1.018) presented the risk effects in the crude model, while in the adjusted model, only slight risk effects of AA (OR = 1.000, 95% CI 1.000, 1.001) were observed. Due to the strong collinearity between LA and total omega-6 fatty acids (data not shown), their effects remained the same. However, when stratified by age, no significant differences were found. In addition, we witnessed significant difference in the omega-6/omega-3 in 20–34-year-old women and controls, revealing a risk effect of 0.2% (95%CI 1.000, 1.005).
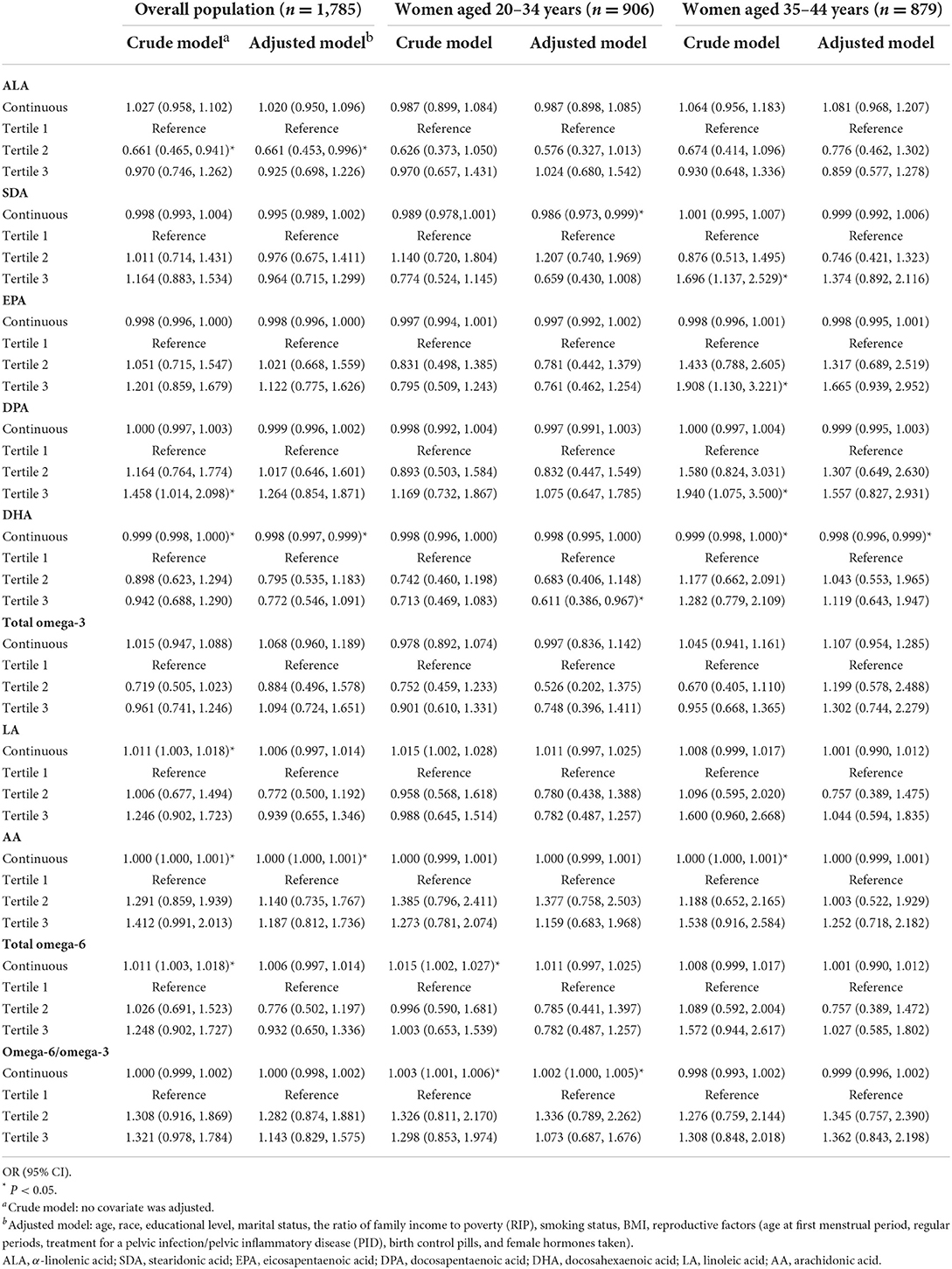
Table 3. Associations between PUFA intakes and the risk of infertility for U.S. women aged 20–34 years and 35–44 year, weighted.
Associations between PUFA intakes and the risk of primary and secondary infertility
Considering the possible differences in factors between primary and secondary infertility, we further conducted a separate analysis in these two groups, and the results are displayed in Table 4. In this part, as for omega-3 fatty acid, ALA (OR = 1.416, 95% CI 1.138, 1.763) presented a relatively high-risk function in the primary population. Interestingly, when analyzed as tertiles, women with SDA intake in the second tertile had a 1.46-fold (95%CI 1.004, 6.063) higher risk of primary infertility than those in the first tertile. Similar to the results in the overall population, DHA was a slightly protective factor against the prevalence of secondary infertility (OR = 0.998, 95%CI 0.997, 0.999). However, when grouped as tertiles, significance can be found neither in primary infertility women nor in the secondary infertility population. The total omega-3 fatty acids only showed a positive effect in the primary infertility population in the crude model. With respect to omega-6 fatty acids, in the primary infertility population, relatively slight risk functions of LA (OR = 1.020, 95% CI 1.002, 1.038) and total omega-6 fatty acid (OR = 1.020, 95% CI 1.002, 1.038) intake were observed, whereas no significance was detected in women with secondary infertility. Furthermore, omega-6/omega-3 was not significant in either woman with primary or secondary infertility.
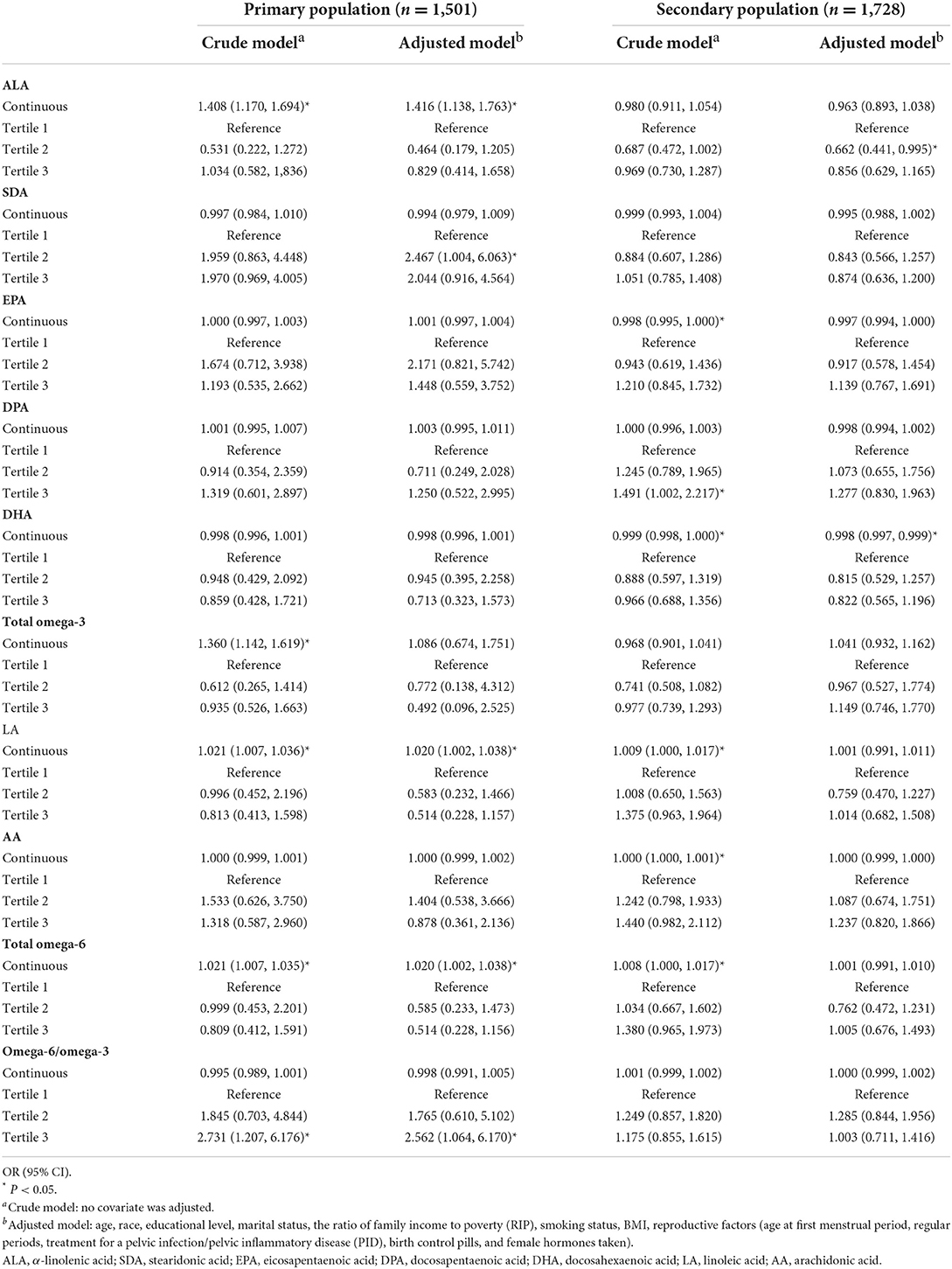
Table 4. Associations between PUFA intakes and the risk of primary and secondary infertility for U.S. women aged 20–44 years, weighted.
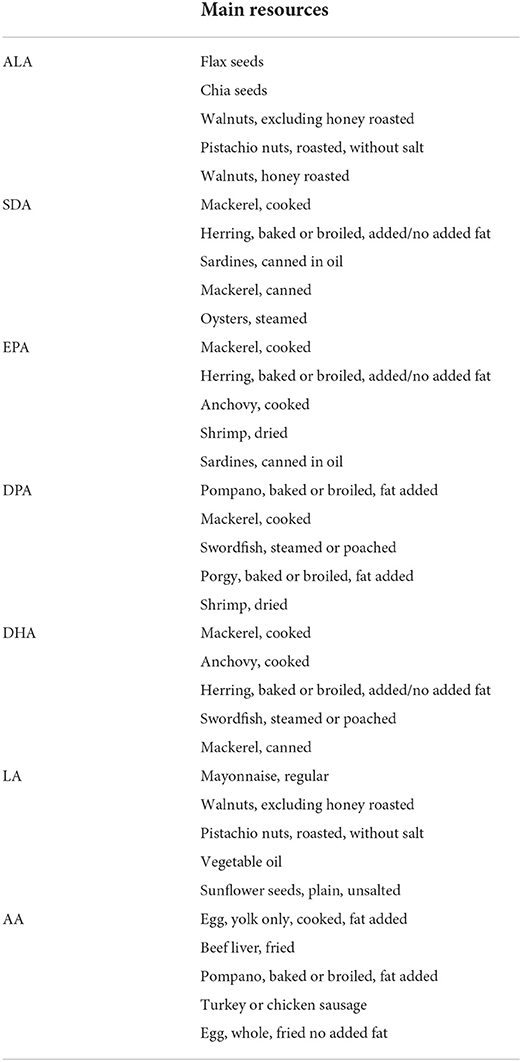
The main resources of PUFA
Further, according to the food codes provided by NHANES (https://wwwn.cdc.gov/Nchs/Nhanes/2017-2018/DRXFCD_J.htm), we summarized and listed the top five foods that contributed to PUFA intakes in Table 5. As for omega-3 fatty acids, the main resource was fish, while for omega-6 fatty acids, crop seeds, vegetable oils and some meat products were major sources.
Discussion
In this national cross-sectional study of associations between PUFA intake and infertility, we found that women with lower DHA intake had a higher risk of infertility, and ALA and LA was a risk factor for primary infertility.
As for the effects of total PUFA intakes to infertility in the overall population of infertile women aged 20–44 years, previous studies have reported inconsistent results regarding these relationships. A nested prospective case-control study conducted by Stanhiser et al. (35) suggested that there was no association between serum omega-3 or omega-6 fatty acids and infertility, even though they only tested the serum levels and not the dietary intake of fatty acids. Another study, similar to our results, showed that higher levels of serum omega-3 fatty acids, not omega-6 fatty acids, were significantly associated with a higher probability of clinical pregnancy in women undergoing assisted reproductive technology (ART) cycles (36). The average ages in these two studies were 33.3 and 34.8 years, respectively. Considering the various bioavailabilities of PUFAs, the intake of omega-3 fatty acids cannot perfectly represent their serum levels (9). This may be one of the reasons why researchers have obtained inconsistent results. When evaluating the intake of PUFAs rather than circulating levels, the latest prospective cohort study revealed that women aged 30–44 years taking omega-3 supplements had a 1.51 (95% CI 1.12–2.04) time higher probability of conceiving compared to women who did not (37). Another prospective cohort study in 2018 suggested that low omega-3 fatty acid intake was associated with reduced fertility, using time to pregnancy (TTP) as the outcome index (38). Nevertheless, the studies mentioned above only tested the total levels of omega-3 and omega-6 fatty acids. When evaluated separately, due to the complexity of the pathogenesis of infertility, our results only revealed a very slight significant association between DHA and infertility: the lower the DHA intake, the higher the risk of infertility, regardless of age. In women aged >35 years, the effects were weakened, which may be mainly caused by the decline of ovarian function or any other possible decline in the reproductive system. Women aged >35 years always face the challenge of declining ovarian reserve, oocyte number, and quality (39), all of which are the main causes of infertility, and the effects of diet may be weakened. To date, no clinical trial has evaluated the relationship between DHA and infertility, and only a few basic studies have revealed their potential effects. Hohos et al. (40) found a strong positive correlation between EPA, DPA, and DHA and primordial follicle numbers in mice, suggesting potential associations with ovarian reserve. Another study revealed that DHA can stimulate the proliferation and steroidogenesis of bovine granulosa cells (41), which may consequently promote reproduction. In women with polycystic ovary syndrome (PCOS), which is a vital factor of infertility, previous studies found that EPA and DHA may improve PCOS symptoms in rats by decreasing lipids and reducing weight and metabolic anomalies (42). A case-control study of Chinese women (43) also indicated the protective effects of consuming long-chain omega-3 PUFA, especially EPA and DHA, on PCOS. Nevertheless, according to our results, EPA and DPA may not be as efficient as expected in preventing infertility.
As omega-6 fatty acids may compete for enzymes in the metabolism of fatty acids with omega-3 fatty acids, affecting the conversion of ALA to EPA and DPA, and eventually to DHA, it was recommended that omega-6/omega-3 consumption should be reduced to 4 or lower (35). We also tested the effect of the omega-6/omega-3 intake ratio on infertility, revealing the risk effects in women under 35. Two independent prospective cohort studies conducted on American women who underwent IVF cycles did not obtain consistent results. Chiu et al. (36) found that the serum omega-6/omega-3 was not associated with ART outcomes. In contrast, Jungheim et al. (44) suggested that the increased LA/ALA ratio was related to incremental implantation and pregnancy rates. Notably, both cohort studies focused only on the serum levels of PUFAs. Generally, omega-3 fatty acids are thought to be anti-inflammatory, whereas omega-6 fatty acids are pro-inflammatory (45). Many chronic diseases, such as cardiovascular diseases, cancer, and autoimmune diseases, are related to increased levels of inflammatory factors such as IL-1β, IL-6, tumor necrosis factor (TNF), and C-reactive protein (CPR). Moreover, these factors may be induced by higher omega-6 fatty acid intake and lower omega-3 fatty acid intake (46). Inflammation is associated with many gynecologic diseases, including endometriosis (47), adenomyosis (48), PCOS (49), and uterine fibroids (48), all of which lead to decreased fertility. However, since the results of clinical studies are inconsistent, further RCT or larger-sample prospective cohort studies may be needed to determine the exact effect of the omega-6/omega-3 on infertility or ART outcomes.
Secondary infertility, the most common form of female infertility worldwide several years ago (50), is thought to have a pathogenesis distinct from primary infertility. Reproductive tract infections, which may cause secondary fallopian tube obstruction, are the main cause of infertility after primiparity, especially in regions with high unsafe abortion rates and poor maternity care (50). A case-control study conducted in Rwanda on women with secondary infertility revealed that obstetric events (such as a history of no prenatal care during the last pregnancy, early age of the first pregnancy, unwanted pregnancies, and stillbirths), HIV, and other sexually transmitted infections could all contribute to secondary infertility (51). Unlike the complex pathogenesis of primary infertility, secondary infertility seems much simpler, and some cannot be reversed by dietary adjustments. Consequently, when only secondary infertility participants were included, we found only a slight positive effect of DHA. Indeed, the positive function of DHA is generally attributed to its impact on oocyte quality and steroidogenesis (36). Women with a birth history always have relatively more normal ovarian function, which can be easily reversed by dietary adjustment.
In contrast, when excluding participants with secondary infertility, we observed relatively strong effects of ALA, LA, and total omega-6 fatty acids. A previous study indicated potential negative correlations between serum ALA levels and implantation rates in women undergoing IVF cycles (52). In this study, researchers found a positive association between elevated serum ALA levels and the presence of endometriosis, which is a known factor in infertility (52). Another prospective cohort study observed negative correlations between metaphase II oocytes and ALA levels in follicular fluid (53). Nevertheless, owing to the inconsistent results of ALA in primary infertility when analyzed as continuous variables and tertiles, the results should be interpreted cautiously. Similar to ALA, as a main source of omega-6 fatty acids, LA had adverse effects on oocyte development. A basic study found a decline in LA concentration when the follicle size increased (54). Further studies revealed that the negative effect of LA on oocyte maturation may be caused by the inhibition of the development of metaphase II oocytes and cumulus cell expansion. Treatment of cumulus-oocyte complexes with LA decreased the cleaved embryo rate and blastocyst yield (55). Meanwhile, an elevated level of LA causes a higher omega-6/omega-3, which may also be a risk factor for infertility.
To identify the external validation, we compared the baseline information, PUFA intakes and infertility information between all eligible participants and the final participates, suggesting some differences. As the participants included in the final analysis were selected via the inclusive and exclusive criteria, the final results may be more applicable for the population satisfying these criteria. For example, women with kilocalorie intake per day <600 or >6,000, or with a history of hysterectomy or removal of both ovaries may not suitable.
The present study has some limitations. First, owing to the cross-sectional study design of the NHANES, it is difficult to provide evidence of a causal relationship between PUFA intake and the risk of infertility. Therefore, it is impossible for us to determine which event, the intake of PUFAs or infertility, comes first. Some participants may have consciously changed their dietary patterns after being diagnosed with infertility. In addition, the assessment of PUFA intakes may cause bias. Because of the non-prospective study design, dietary data could only be collected based on memory, leading to recall bias. However, efforts were made to minimize this kind of bias: to use a set of measuring guides (various glasses, bowls) or ask for additional foods not remembered earlier, repeatedly, to ensure that the outcome was more reliable. Also, we did not include dietary supplements in that we can solely get the available information about the total PUFA supplement intake, not separated fatty acid supplement intakes, and very few people in the final analysis had PUFA supplement consumption.
Despite these limitations, our study had several strengths. First, we had a large sample size with nationwide representative samples, which can increase the strength of the evidence. Furthermore, the usual dietary intakes of PUFAs were estimated by MSM, which was a new statistical method for calculating intakes combining dietary intake data with supporting data such as age and sex via regression models. In this way, the estimation of usual dietary intake became more accurate. Additionally, the importance and functions of PUFAs nowadays are always considered in males, not females, and clinical studies are regarding the same are absent. Existing studies on PUFAs and female infertility have only focused on serum levels of PUFAs, or solely carried out basic research on animal models. However, it is obvious that with regard to dietary advice for women in their daily lives, the intake matters.
Infertility is a multifactorial disease with various etiologies. Our study concluded that DHA was a protective factor against infertility, and LA and total omega-6 fatty acids were risk factors for primary infertility in women. For women aiming to improve fertility via a daily diet or dietary supplements, these results may provide evidence and instruction. Despite these limitations, the clinical implications and conclusions of this study should be interpreted carefully.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found at: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The studies involving human participants were reviewed and approved by the NCHS Research Ethics Review Board (ERB). The patients/participants provided their written informed consent to participate in this study.
Author contributions
RW, YF, and FM contributed to conception and design of the study. RW and JC organized the database and wrote the first draft of the manuscript. RW performed the statistical analysis. RW, YF, JC, and YC wrote sections of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China, China (31771662 and 31470797).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpubh.2022.938343/full#supplementary-material
References
1. Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. (2017) 108:393–406. doi: 10.1016/j.fertnstert.2017.06.005
2. Key Statistics from the National Survey of Family Growth - 2015-2019. (2021). Available online at: https://www.cdc.gov/nchs/nsfg/key_statistics/i-keystat.htm#infertility
3. Key Statistics from the National Survey of Family Growth - 2011-2015. (2021). Available online at: https://www.cdc.gov/nchs/nsfg/key_statistics/i.htm#infertility
4. Hart RJ. Physiological aspects of female fertility: Role of the environment, modern lifestyle, and genetics. Physiol. Rev. (2016) 96:873–909. doi: 10.1152/physrev.00023.2015
5. Tanbo T, Fedorcsak P. Endometriosis-associated infertility: Aspects of pathophysiological mechanisms and treatment options. Acta obstetricia et gynecologica Scandinavica. (2017) 96:659–67. doi: 10.1111/aogs.13082
6. Jacoby VL, Fujimoto VY, Giudice LC, Kuppermann M, Washington AE. Racial and ethnic disparities in benign gynecologic conditions and associated surgeries. Am J Obstet Gynecol. (2010) 202:514–21. doi: 10.1016/j.ajog.2010.02.039
7. Macaluso M, Wright-Schnapp TJ, Chandra A, Johnson R, Satterwhite CL, Pulver A, et al. A public health focus on infertility prevention, detection, and management. Fertil Steril. (2010) 93:16.e1-0. doi: 10.1016/j.fertnstert.2008.09.046
8. Gaskins AJ, Chavarro JE. Diet and fertility: A review. Am J Obstet Gynecol. (2018) 218:379–89. doi: 10.1016/j.ajog.2017.08.010
9. Cholewski M, Tomczykowa M, Tomczyk M. A comprehensive review of chemistry, sources and bioavailability of omega-3 fatty acids. Nutrients. (2018) 10. doi: 10.3390/nu10111662
10. Kang JX, Liu A. The role of the tissue omega-6/omega-3 fatty acid ratio in regulating tumor angiogenesis. Cancer Met Rev. (2013) 32:201–10. doi: 10.1007/s10555-012-9401-9
11. Dennis EA, Norris PC. Eicosanoid Storm in Infection and Inflammation. Nat Rev Immunol. (2015) 15:511–23. doi: 10.1038/nri3859
12. Serhan CN, Savill J. Resolution of inflammation: The beginning programs the end. Nat. Immunol. (2005) 6:1191–7. doi: 10.1038/ni1276
13. Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. (2008) 269:363–77. doi: 10.1016/j.canlet.2008.03.044
14. Adkins Y, Kelley DS. Mechanisms underlying the cardioprotective effects of omega-3 polyunsaturated fatty acids. J Nutr Biochem. (2010) 21:781–92. doi: 10.1016/j.jnutbio.2009.12.004
15. Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, et al. Ω-3 Polyunsaturated fatty acid biomarkers and coronary heart disease: Pooling project of 19 cohort studies. JAMA Intern Med. (2016) 176:1155–66. doi: 10.1001/jamainternmed.2016.2925
16. Innis SM. Dietary omega 3 fatty acids and the developing brain. Brain Res. (2008) 1237:35–43. doi: 10.1016/j.brainres.2008.08.078
17. Bazinet RP, Layé S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. (2014) 15:771–85. doi: 10.1038/nrn3820
18. Nassan FL, Chavarro JE, Tanrikut C. Diet and men's fertility: Does diet affect sperm quality? Fertil Steril. (2018) 110:570–7. doi: 10.1016/j.fertnstert.2018.05.025
19. Lenzi A, Gandini L, Maresca V, Rago R, Sgrò P, Dondero F, et al. fatty acid composition of spermatozoa and immature germ cells. Mol Hum Reprod. (2000) 6:226–31. doi: 10.1093/molehr/6.3.226
20. Safarinejad MR. Effect of omega-3 polyunsaturated fatty acid supplementation on semen profile and enzymatic anti-oxidant capacity of seminal plasma in infertile men with idiopathic oligoasthenoteratospermia: A double-blind, placebo-controlled, randomised study. Andrologia. (2011) 43:38–47. doi: 10.1111/j.1439-0272.2009.01013.x
21. Conquer JA, Martin JB, Tummon I, Watson L, Tekpetey F. Fatty acid analysis of blood serum, seminal plasma, and spermatozoa of normozoospermic vs. asthenozoospermic males. Lipids. (1999) 34:793–9. doi: 10.1007/s11745-999-0425-1
22. Tavilani H, Doosti M, Nourmohammadi I, Mahjub H, Vaisiraygani A, Salimi S, et al. Lipid composition of spermatozoa in normozoospermic and asthenozoospermic males. Prostaglandins Leukotrienes Essen Fatty Acid. (2007) 77:45–50. doi: 10.1016/j.plefa.2007.07.001
23. Zalata AA, Christophe AB, Depuydt CE, Schoonjans F, Comhaire FH. The fatty acid composition of phospholipids of spermatozoa from infertile patients. Mol Hum Reprod. (1998) 4:111–8. doi: 10.1093/molehr/4.2.111
24. Flesch FM, Gadella BM. Dynamics of the mammalian sperm plasma membrane in the process of fertilization. Biochimica et biophysica acta. (2000) 1469:197–235. doi: 10.1016/s0304-4157(00)00018-6
25. Sturmey RG, Reis A, Leese HJ, McEvoy TG. Role of fatty acids in energy provision during oocyte maturation and early embryo development. Zuchthygiene. (2009) 44:50–8. doi: 10.1111/j.1439-0531.2009.01402.x
26. Norwitz ER, Schust DJ, Fisher SJ. Implantation and the survival of early pregnancy. New Eng J Med. (2001) 345:1400–8. doi: 10.1056/NEJMra000763
27. Nehra D, Le HD, Fallon EM, Carlson SJ, Woods D, White YA, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging cell. (2012) 11:1046–54. doi: 10.1111/acel.12006
28. Hammiche F, Vujkovic M, Wijburg W, de Vries JH, Macklon NS, Laven JS, et al. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril. (2011) 95:1820–3. doi: 10.1016/j.fertnstert.2010.11.021
29. Young IE, Parker HM, Cook RL, O'Dwyer NJ, Garg ML, Steinbeck KS, et al. Association between obesity and omega-3 status in healthy young women. Nutrients. (2020) 12. doi: 10.3390/nu12051480
30. Willett WC, Howe GR, Kushi LH. Adjustment for total energy intake in epidemiologic studies. Am J Clin Nutr. (1997) 65(4 Suppl):1220S−8S; discussion 9S−31S. doi: 10.1093/ajcn/65.4.1220S
31. Trnka B, Polan M, Zigmont VA. Exposure to di-2-ethylhexyl phthalate (DEHP) and infertility in Women, NHANES 2013–2016. Reprod Toxicol. (Elmsford, NY). (2021) 103:46–50. doi: 10.1016/j.reprotox.2021.05.010
32. Gleason JL, Shenassa ED, Thoma ME. Self-reported infertility, metabolic dysfunction, and cardiovascular events: a cross-sectional analysis among US women. Fertil Steril. (2019) 111:138–46. doi: 10.1016/j.fertnstert.2018.10.009
33. Cave C, Hein N, Smith LM, Anderson-Berry A, Richter CK, Bisselou KS, et al. Omega-3 Long-chain polyunsaturated fatty acids intake by ethnicity, income, and education level in the United States: NHANES 2003–2014. Nutrients. (2020) 12:2045. doi: 10.3390/nu12072045
34. NHANES. NHANES Survey Methods and Analytic Guidelines. (2022). Available online at: https://wwwn.cdc.gov/nchs/nhanes/analyticguidelines.aspx (accessed June 3, 2022).
35. Stanhiser J, Jukic AMZ, Steiner AZ. Serum Omega-3 and Omega-6 fatty acid concentrations and natural fertility. Hum Reprod. (2020) 35:950–7. doi: 10.1093/humrep/dez305
36. Chiu YH, Karmon AE, Gaskins AJ, Arvizu M, Williams PL, Souter I, et al. Serum omega-3 fatty acids and treatment outcomes among women undergoing assisted reproduction. Hum Reprod. (2018) 33:156–65. doi: 10.1093/humrep/dex335
37. Stanhiser J, Jukic AMZ, McConnaughey DR, Steiner AZ. Omega-3 fatty acid supplementation and fecundability. Hum Reprod. (2022) 37:1037–46. doi: 10.1093/humrep/deac027
38. Wise LA, Wesselink AK, Tucker KL, Saklani S, Mikkelsen EM, Cueto H, et al. Dietary fat intake and fecundability in 2 preconception cohort studies. Am J Epidemiol. (2018) 187:60–74. doi: 10.1093/aje/kwx204
39. Crawford NM, Steiner AZ. Age-related infertility. Obstet Gynecol Clin North Am. (2015) 42:15–25. doi: 10.1016/j.ogc.2014.09.005
40. Hohos NM, Cho KJ, Swindle DC, Allshouse AA, Rudolph MC, Skaznik-Wikiel ME. Fat-1 transgene is associated with improved reproductive outcomes. Endocrinology. (2018) 159:3981–92. doi: 10.1210/en.2018-00723
41. Maillard V, Desmarchais A, Durcin M, Uzbekova S, Elis S. Docosahexaenoic acid (DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod Biol Endocrinol. (2018) 16:40. doi: 10.1186/s12958-018-0357-7
42. Komal F, Khan MK, Imran M, Ahmad MH, Anwar H, Ashfaq UA, et al. Impact of different omega-3 fatty acid sources on lipid, hormonal, blood glucose, weight gain and histopathological damages profile in pcos rat model. J Transl Med. (2020) 18:349. doi: 10.1186/s12967-020-02519-1
43. Lu L, Li X, Lv L, Xu Y, Wu B, Huang C. Dietary and serum N-3 pufa and polycystic ovary syndrome: a matched case-control study. Br J Nutr. (2021) 2021:1–10. doi: 10.1017/s0007114521003007
44. Jungheim ES, Frolova AI, Jiang H, Riley JK. Relationship between serum polyunsaturated fatty acids and pregnancy in women undergoing in vitro fertilization. J Clin Endocrinol Metab. (2013) 98:E1364–8. doi: 10.1210/jc.2012-4115
45. Calder PC. Omega-3 fatty acids and inflammatory processes. Nutrients. (2010) 2:355–74. doi: 10.3390/nu2030355
46. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. (2002) 56:365–79. doi: 10.1016/s0753-3322(02)00253-6
47. Kolanska K, Alijotas-Reig J, Cohen J, Cheloufi M, Selleret L, d'Argent E, et al. Endometriosis with infertility: a comprehensive review on the role of immune deregulation and immunomodulation Therapy. Am J Reprod Immunol. (2021) 85:e13384. doi: 10.1111/aji.13384
48. Vannuccini S, Clifton VL, Fraser IS, Taylor HS, Critchley H, Giudice LC, et al. Infertility and reproductive disorders: impact of hormonal and inflammatory mechanisms on pregnancy outcome. Hum Reprod Update. (2016) 22:104–15. doi: 10.1093/humupd/dmv044
49. Piltonen TT, Chen J, Erikson DW, Spitzer TL, Barragan F, Rabban JT, et al. Mesenchymal stem/progenitors and other endometrial cell types from women with polycystic ovary syndrome (PCOS) display inflammatory and oncogenic potential. J Clin Endocrinol Metab. (2013) 98:3765–75. doi: 10.1210/jc.2013-1923
50. Breckenridge A. Angiotensin converting enzyme inhibition in clinical practice. Re-examination of step care: choice of first drug. J Cardiovasc Pharmacol. (1985) 7 Suppl 1:S117–20.
51. Dhont N, Luchters S, Muvunyi C, Vyankandondera J, De Naeyer L, Temmerman M, et al. The risk factor profile of women with secondary infertility: an unmatched case-control study in Kigali, Rwanda. BMC Women's Health. (2011) 11:32. doi: 10.1186/1472-6874-11-32
52. Jungheim ES, Macones GA, Odem RR, Patterson BW, Moley KH. Elevated serum A-linolenic acid levels are associated with decreased chance of pregnancy after in vitro fertilization. Fertil Steril. (2011) 96:880–3. doi: 10.1016/j.fertnstert.2011.07.1115
53. Mirabi P, Chaichi MJ, Esmaeilzadeh S, Ali Jorsaraei SG, Bijani A, Ehsani M, et al. The role of fatty acids on ICSI outcomes: a prospective cohort study. Lipids Health Dis. (2017) 16:18. doi: 10.1186/s12944-016-0396-z
54. Homa ST, Brown CA. Changes in linoleic acid during follicular development and inhibition of spontaneous breakdown of germinal vesicles in cumulus-free bovine oocytes. J Reprod Fertil. (1992) 94:153–60. doi: 10.1530/jrf.0.0940153
Keywords: infertility, docosapentaenoic acid, linoleic acid, polyunsaturated fatty acids, NHANES
Citation: Wang R, Feng Y, Chen J, Chen Y and Ma F (2022) Association between polyunsaturated fatty acid intake and infertility among American women aged 20–44 years. Front. Public Health 10:938343. doi: 10.3389/fpubh.2022.938343
Received: 07 May 2022; Accepted: 28 July 2022;
Published: 17 August 2022.
Edited by:
Bartira Gorgulho, Federal University of Mato Grosso, BrazilReviewed by:
Antonio Augusto Carioca, University of Fortaleza, BrazilAlexsandro Macedo Silva, University of São Paulo, Brazil
Copyright © 2022 Wang, Feng, Chen, Chen and Ma. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fang Ma, bWFmYW5nbWVkQDEyNi5jb20=; Yingjiao Chen, bGltb2dlczUyNEAxNjMuY29t
†These authors have contributed equally to this work
 Ruohan Wang
Ruohan Wang Ying Feng3†
Ying Feng3† Jiahe Chen
Jiahe Chen Fang Ma
Fang Ma